Process Optimization and CO2 Emission Analysis of Coal/Biomass Gasification Integrated with a Chemical Looping Process
Abstract
:1. Introduction
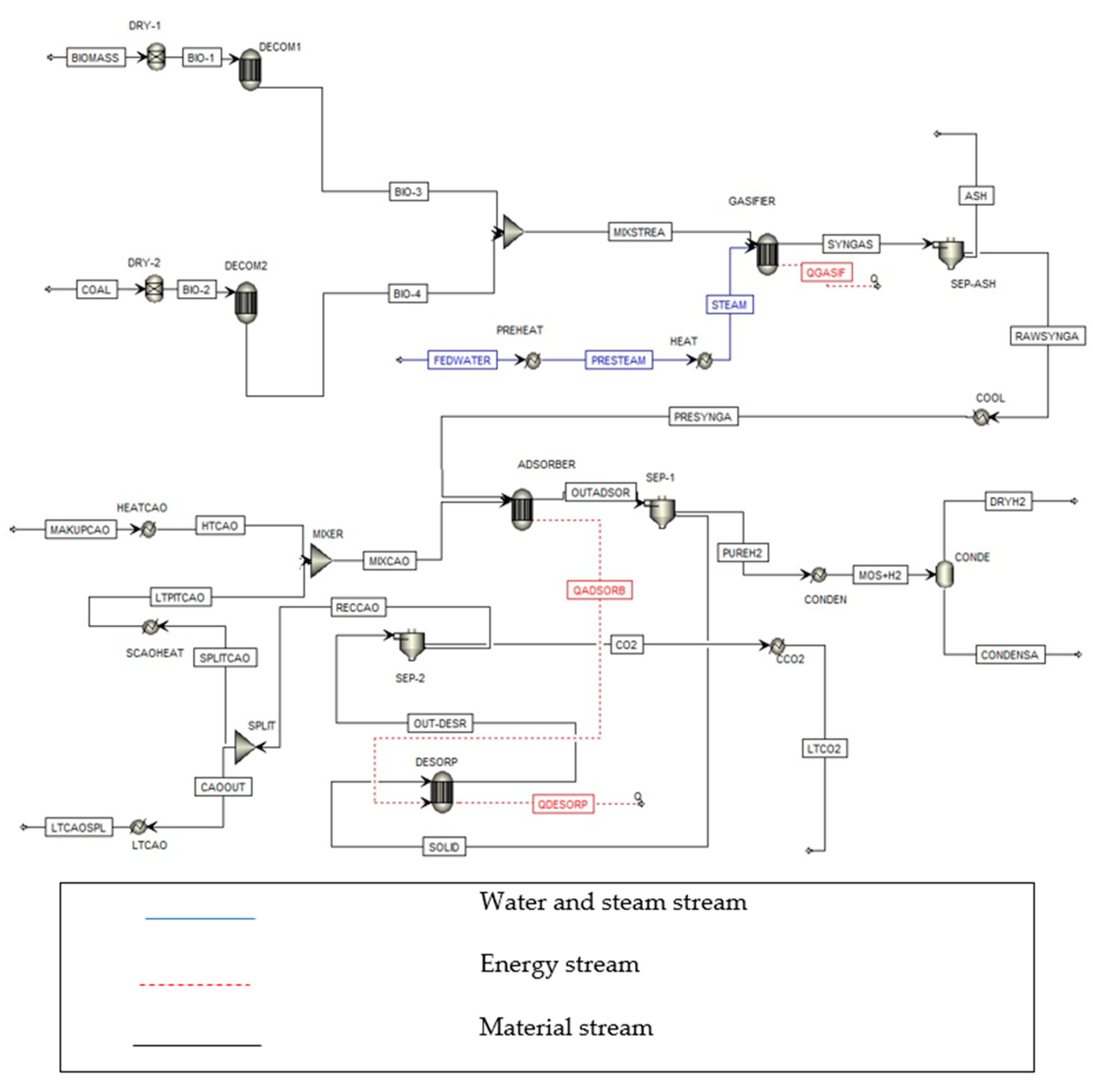
2. Process Flow Diagram Description
3. Methodology
3.1. Parametric Analysis
3.2. Experimental Design and Optimization
3.3. Energy Efficiency Analysis
3.4. Environmental Impact Analysis
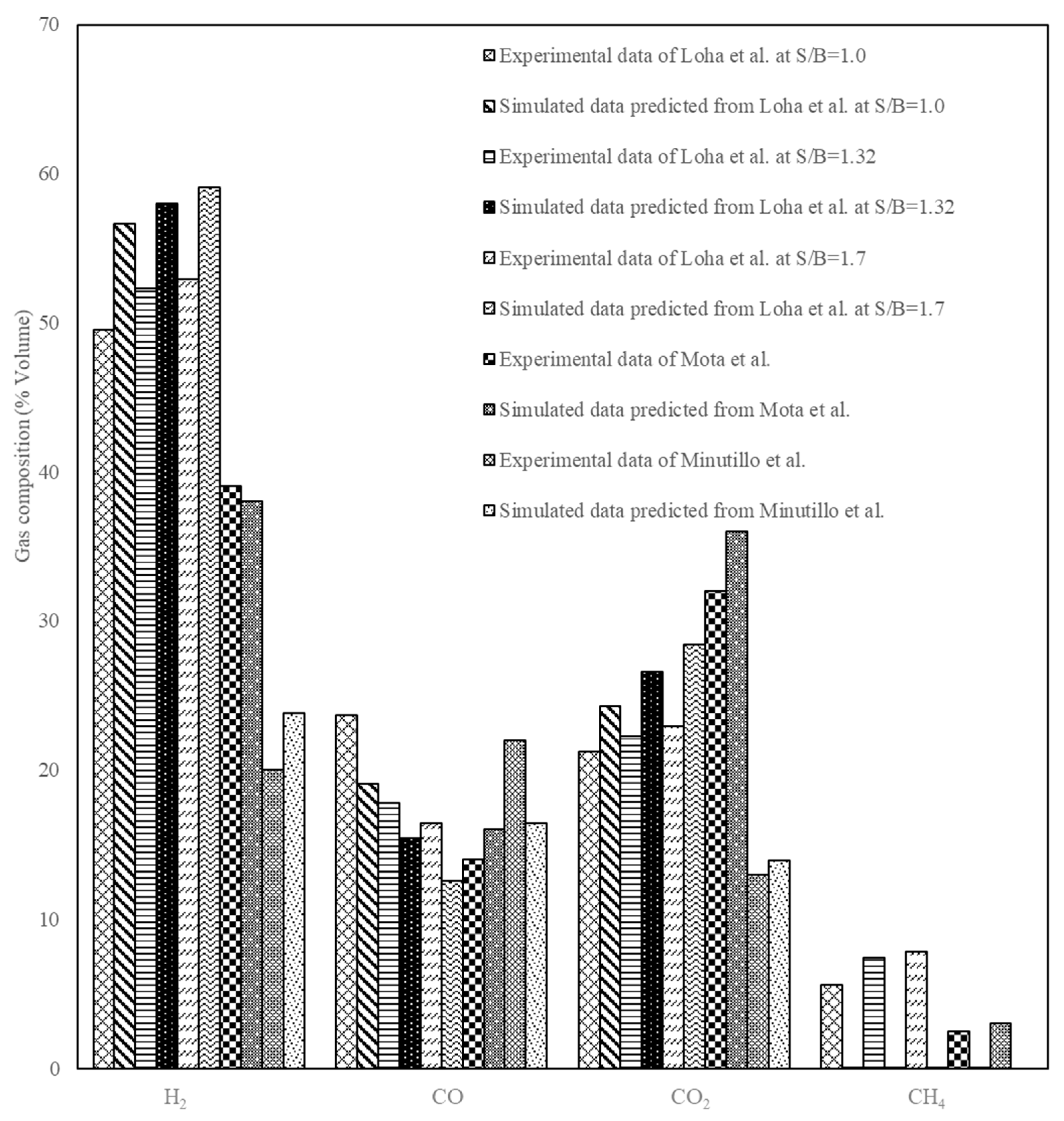
4. Model Validation
5. Result and Discussion
5.1. Sensitivity Analysis
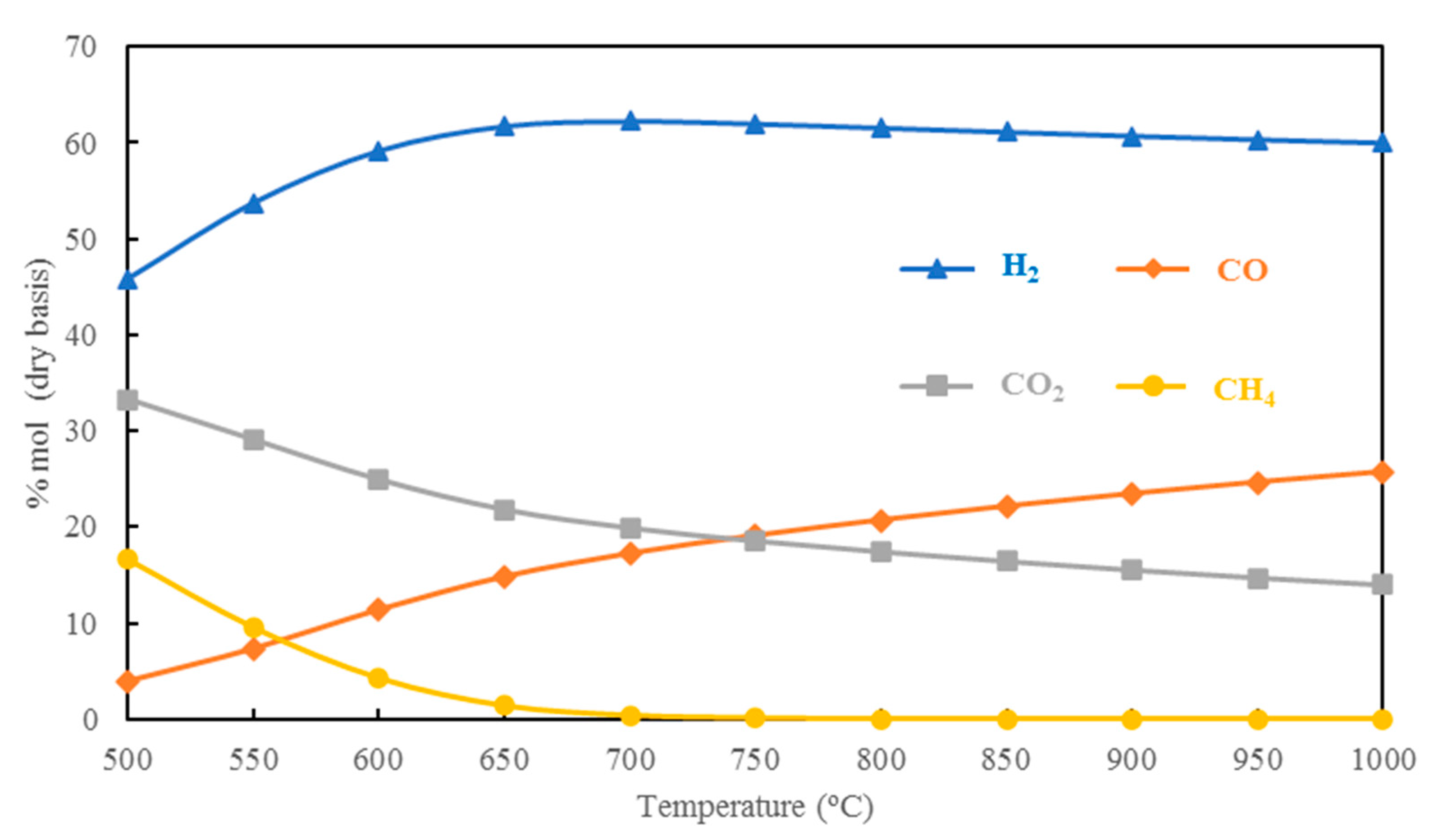
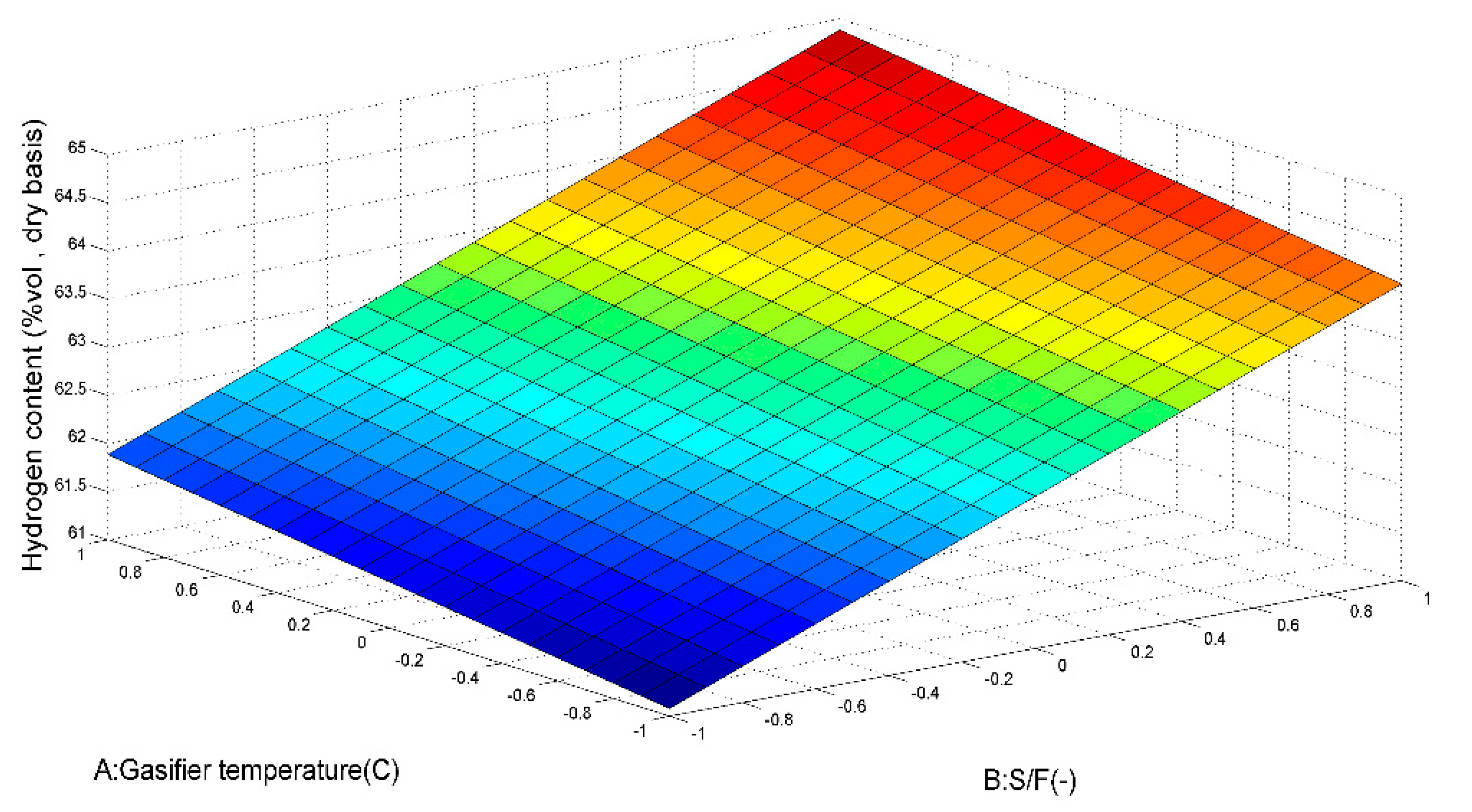
5.1.1. Effect of Gasification Temperature on H2 Production
5.1.2. Effect of S/F Mass Ratio on H2 Production
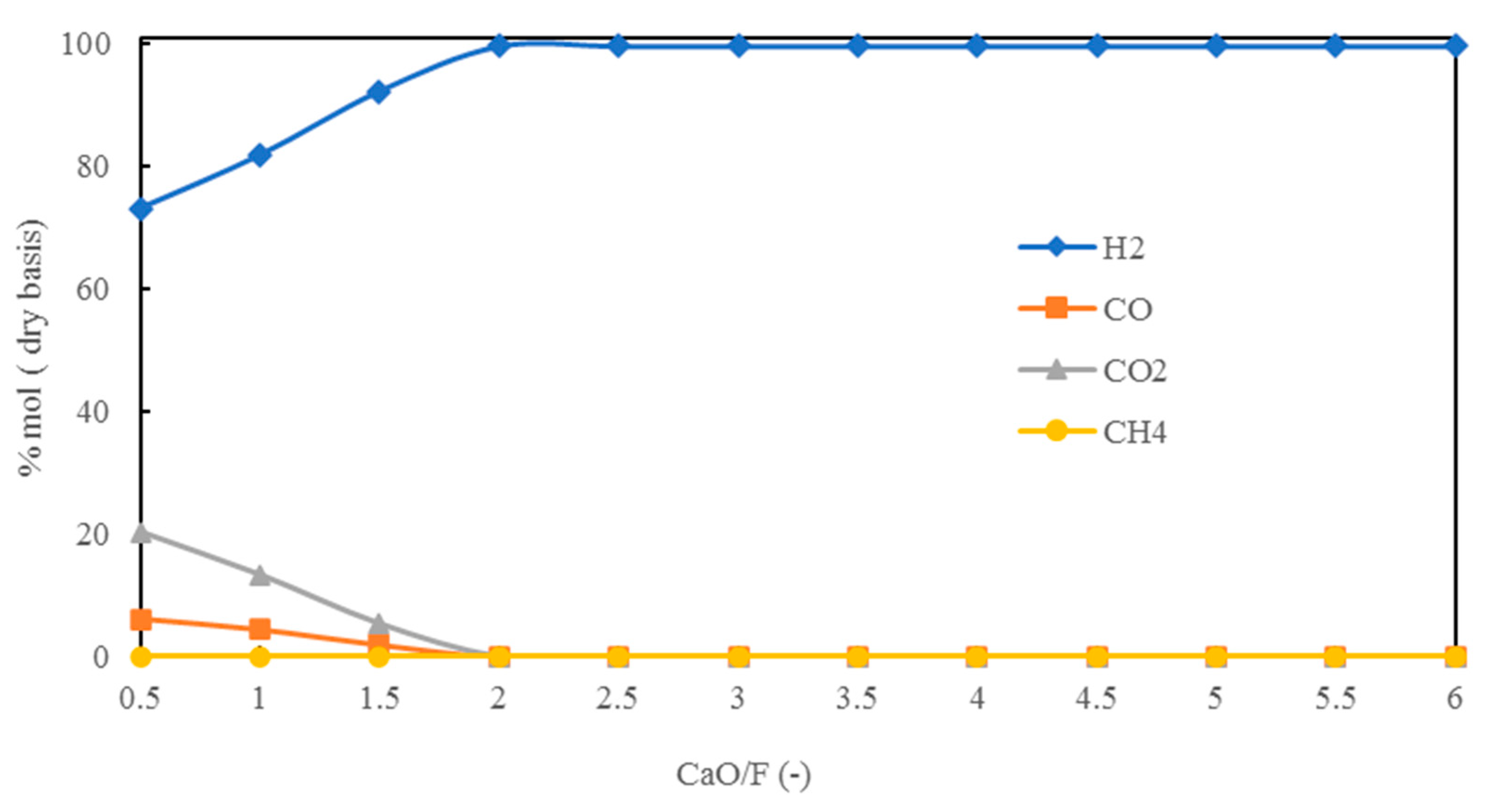
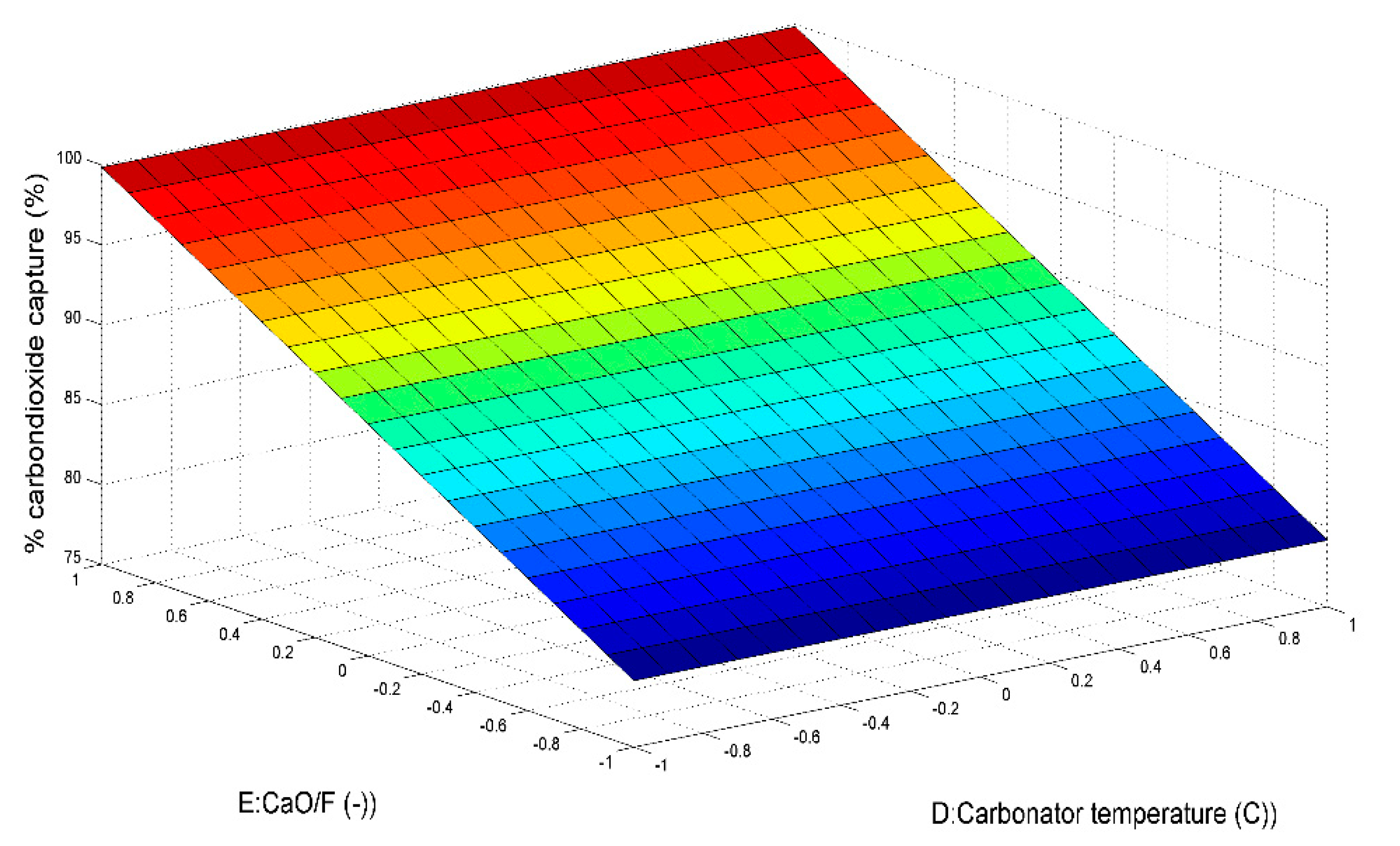
5.1.3. Effect of CaO/F Mass Ratio on H2 Production
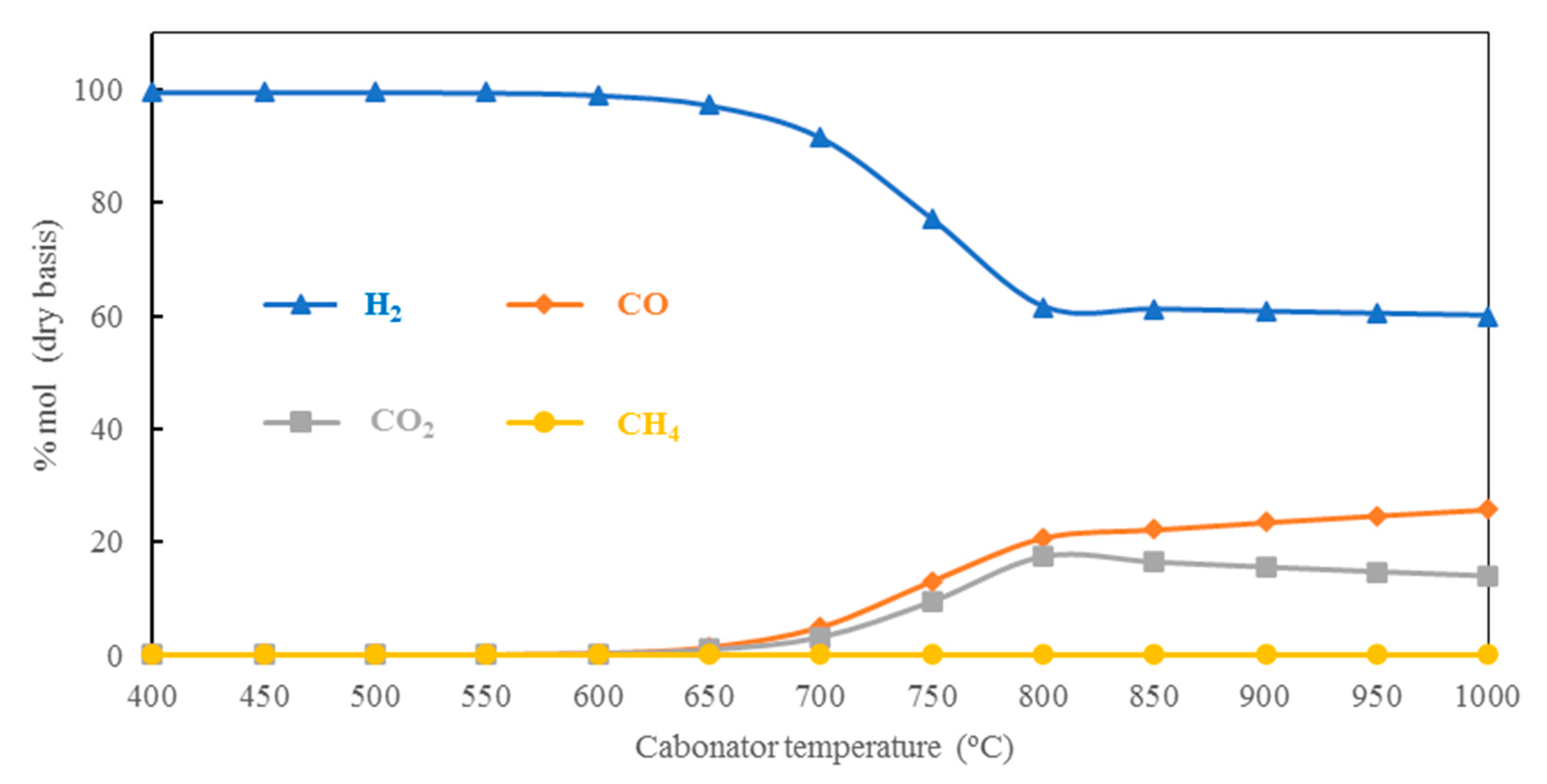
5.1.4. Effect of Carbonator Temperature on H2 Production
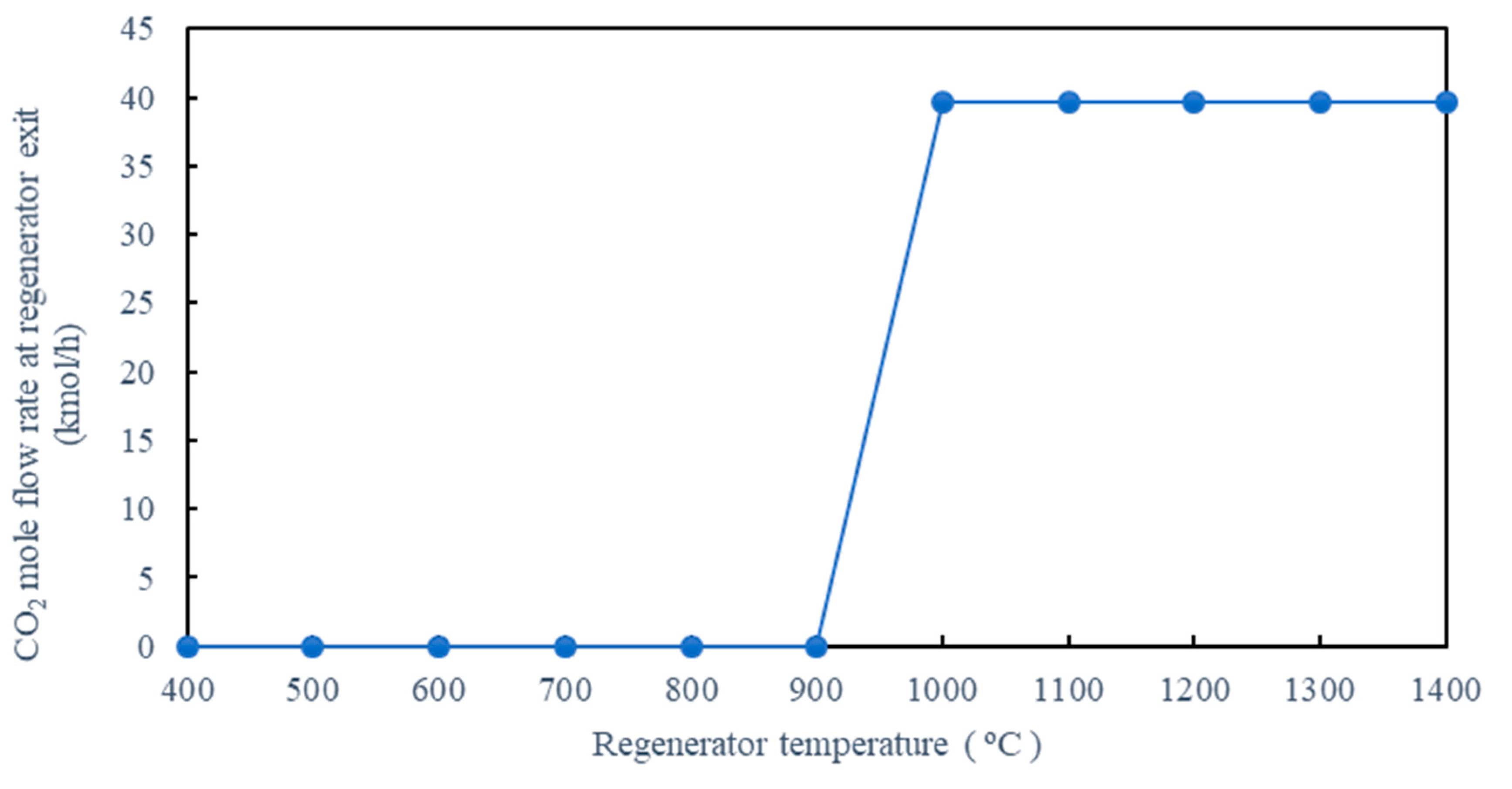
5.1.5. Effect of Regenerator Temperature on CO2 Removal
5.2. Design of Experimental Procedure
5.2.1. Effect of Process Parameters on the Gasification Process
5.2.2. Effect of Process Parameters on the CO2 Capture Process
5.3. The Energy Efficiency of Co-Gasification of Biomass and Coal Integrated with Calcium Looping Carbon Dioxide Capture Process
5.4. The Environmental Impact Assessment
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage. Mater. Chem. Phys. 2020, 239, 122102. [Google Scholar] [CrossRef]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; TRUFIN, C.N.; Filote, C. Hydrogen Fuel Cell Technology for the Sustainable Future of Stationary Applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef] [Green Version]
- Prestipino, M.; Piccolo, A.; Polito, M.F.; Galvagno, A. Combined Bio-Hydrogen, Heat, and Power Production Based on Residual Biomass Gasification: Energy, Exergy, and Renewability Assessment of an Alternative Process Configuration. Energies 2022, 15, 5524. [Google Scholar] [CrossRef]
- Ulejczyk, B.; Józwik, P.; Nogal, Ł.; Młotek, M.; Krawczyk, K. Efficient Conversion of Ethanol to Hydrogen in a Hybrid Plasma-Catalytic Reactor. Energies 2022, 15, 3050. [Google Scholar] [CrossRef]
- Matus, E.; Sukhova, O.; Ismagilov, I.; Kerzhentsev, M.; Stonkus, O.; Ismagilov, Z. Hydrogen Production through Autothermal Reforming of Ethanol: Enhancement of Ni Catalyst Performance via Promotion. Energies 2021, 14, 5176. [Google Scholar] [CrossRef]
- Lin, K.-W.; Wu, H.-W. Thermodynamic analysis and experimental study of partial oxidation reforming of biodiesel and hydrotreated vegetable oil for hydrogen-rich syngas production. Fuel 2019, 236, 1146–1155. [Google Scholar] [CrossRef]
- Rekleitis, G.; Haralambous, K.-J.; Loizidou, M.; Aravossis, K. Utilization of Agricultural and Livestock Waste in Anaerobic Digestion (A.D): Applying the Biorefinery Concept in a Circular Economy. Energies 2020, 13, 4428. [Google Scholar] [CrossRef]
- Arteaga-Pérez, L.E.; Casas-Ledón, Y.; Pérez-Bermúdez, R.; Peralta, L.M.; Dewulf, J.; Prins, W. Energy and exergy analysis of a sugar cane bagasse gasifier integrated to a solid oxide fuel cell based on a quasi-equilibrium approach. Chem. Eng. J. 2013, 228, 1121–1132. [Google Scholar] [CrossRef]
- Fernandez-Lopez, M.; Pedroche, J.; Valverde, J.L.; Sánchez-Silva, L. Simulation of the gasification of animal wastes in a dual gasifier using Aspen Plus®. Energy Convers. Manag. 2017, 140, 211–217. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Yao, Z.; Cui, X.; Wang, C. CO2 gasification of woody biomass: Experimental study from a lab-scale reactor to a small-scale autothermal gasifier. Energy 2019, 170, 497–506. [Google Scholar] [CrossRef]
- Gao, N.; Śliz, M.; Quan, C.; Bieniek, A.; Magdziarz, A. Biomass CO2 gasification with CaO looping for syngas production in a fixed-bed reactor. Renew. Energy 2021, 167, 652–661. [Google Scholar] [CrossRef]
- Mongkolsiri, P.; Jitkeaw, S.; Patcharavorachot, Y.; Arpornwichanop, A.; Authayanun, S. Comparative analysis of biomass and coal based co-gasification processes with and without CO2 capture for HT-PEMFCs. Int. J. Hydrog. Energy 2019, 44, 2216–2229. [Google Scholar] [CrossRef]
- Hu, J.; Shao, J.; Yang, H.; Lin, G.; Chen, Y.; Wang, X.; Zhang, W.; Chen, H. Co-gasification of coal and biomass: Synergy, characterization and reactivity of the residual char. Bioresour. Technol. 2017, 244, 1–7. [Google Scholar] [CrossRef]
- Xiao, Y.; Xu, S.; Liu, Y.; Qiao, C. Catalytic steam co-gasification of biomass and coal in a dual loop gasification system with olivine catalysts. J. Energy Inst. 2020, 93, 1074–1082. [Google Scholar] [CrossRef]
- Shahabuddin, M.; Bhattacharya, S. Co-Gasification Characteristics of Coal and Biomass Using CO2 Reactant under Thermodynamic Equilibrium Modelling. Energies 2021, 14, 7384. [Google Scholar] [CrossRef]
- Howaniec, N.; Smolinski, A.; Stańczyk, K.; Pichlak, M. Steam co-gasification of coal and biomass derived chars with synergy effect as an innovative way of hydrogen-rich gas production. Int. J. Hydrog. Energy 2011, 36, 14455–14463. [Google Scholar] [CrossRef]
- Seçer, A.; Fakı, E.; Üzden, S.T.; Hasanoğlu, A. Hydrothermal co-gasification of sorghum biomass and çan lignite in mild conditions: An optimization study for high yield hydrogen production. Int. J. Hydrog. Energy 2020, 45, 2668–2680. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Wang, Q.; Han, L.; Feng, Y.; Sharif, Z.; Li, Z.; Cen, J.; Kumar, S. Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. Sustainability 2022, 14, 2189. [Google Scholar] [CrossRef]
- Teixeira, P.; Bacariza, C.; Correia, P.; Pinheiro, C.I.C.; Cabrita, I. Hydrogen Production with In Situ CO2 Capture at High and Medium Temperatures Using Solid Sorbents. Energies 2022, 15, 4039. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yang, L.; Fan, X.; Chu, L. Revealing the effects of Ni on sorption-enhanced water-gas shift reaction of CaO for H2 production by density functional theory. Process Saf. Environ. Prot. 2022, 157, 254–265. [Google Scholar] [CrossRef]
- Stadler, T.J.; Barbig, P.; Kiehl, J.; Schulz, R.; Klövekorn, T.; Pfeifer, P. Sorption-Enhanced Water-Gas Shift Reaction for Synthesis Gas Production from Pure CO: Investigation of Sorption Parameters and Reactor Configurations. Energies 2021, 14, 355. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, Z.; Zhou, Z. High-purity H2 production by sorption-enhanced water gas shift on a K2CO3-promoted Cu/MgO–Al2O3 difunctional material. Sustain. Energy Fuels 2021, 5, 3340–3350. [Google Scholar] [CrossRef]
- Ojha, B.; Schober, M.; Turad, S.; Jochum, J.; Kohler, H. Gasification of Biomass: The Very Sensitive Monitoring of Tar in Syngas by the Determination of the Oxygen Demand—A Proof of Concept. Processes 2022, 10, 1270. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons Ltd.: New York, NY, USA, 2001. [Google Scholar]
- Bet Sarkis, R.; Zare, V. Proposal and analysis of two novel integrated configurations for hybrid solar-biomass power generation systems: Thermodynamic and economic evaluation. Energy Convers. Manag. 2018, 160, 411–425. [Google Scholar] [CrossRef]
- Loha, C.; Chatterjee, P.; Chattopadhyay, H. Performance of fluidized bed steam gasification of biomass–Modeling and experiment. Energy Convers. Manag. 2011, 52, 1583–1588. [Google Scholar] [CrossRef]
- Minutillo, M.; Alessandra, P.; Elio, J.; Viviana, C.; Suk, W.N.; Sung, P.Y. Coupling of Biomass Gasification and SOFC–Gas Turbine Hybrid System for Small Scale Cogeneration Applications. Energy Procedia 2017, 105, 730–737. [Google Scholar] [CrossRef]
- Mota, R.; Krishnamoorthy, G.; Dada, O.; Benson, S.A. Hydrogen Rich Syngas Production from Oxy-Steam Gasification of a Lignite Coal–A Design and Optimization Study. Appl. Therm. Eng. 2015, 90, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Atsonios, K.; Zeneli, M.; Nikolopoulos, A.; Nikolopoulos, N.; Grammelis, P.; Kakaras, E. Calcium looping process simulation based on an advanced thermodynamic model combined with CFD analysis. Fuel 2015, 153, 370–381. [Google Scholar] [CrossRef]
- Krerkkaiwan, S.; Fushimi, C.; Tsutsumi, A.; Kuchonthara, P. Synergetic effect during copyrolysis/gasification of biomass and sub-bituminous coal. Fuel Process. Technol. 2013, 115, 11–18. [Google Scholar] [CrossRef]
- Ongkabin, T. Syngas from Coal and Biomass Gasification Integrated with Combustion in Circulating Fluidized Bed. Master’s Thesis, Chulalongkorn University, Krung Thep Maha Nakhon, Thailand, 2017. Available online: http://cuir.car.chula.ac.th/bitstream/123456789/38326/1/Tassanai_on.pdf (accessed on 10 April 2022).

| Fuel Type | |||
|---|---|---|---|
| Pellet Pine Wood | Lignite Coal | Rice Husk | |
| Proximate analysis (wt.%, wet basis) | |||
| Fixed Carbon | 14.85 | 33.60 | 14.99 |
| Volatile matter | 74.94 | 28.82 | 55.54 |
| Moisture | 10.00 | 31.20 | 9.95 |
| Ash | 0.18 | 6.38 | 19.52 |
| Ultimate analysis (wt.%, dry basis) | |||
| C | 50.50 | 47.83 | 39.44 |
| H | 5.90 | 6.67 | 3.22 |
| N | 0.30 | 0.52 | 0.08 |
| O | 43.00 | 37.95 | 37.74 |
| S | 0.20 | 0.65 | 0.01 |
| LHV (kJ/g) | 17.30 | 19.29 | 13.52 |
| CO2-emission factors (kg/MJ) | 0 | 0.0946 | 0 |
| Parameters | Value |
|---|---|
| Atmospheric condition (atm) | 1 |
| Biomass flow rate (kg/h) | 1000 |
| Coal flow rate (kg/h) | 1000 |
| Coal: Biomass | 1:1 |
| Steam flow rate (kg/h) | 2000 |
| Steam temperature (°C) | 800 |
| Calcium oxide (kg/h) | 2000 |
| Gasifier temperature (°C) | 700 |
| Carbonator temperature (°C) | 450 |
| Regenerator temperature (°C) | 900 |
| This Study | Atsonios et al. [30] | |
|---|---|---|
| Parameters | ||
| Wet gas composition (mole fraction) CO2, out | 0.00456 | 0.018 |
| CO2 capture efficiency | 88.06 | 85.57 |
| Run | Gasification Temperature (°C) | S/F Mass Ratio | C/B Mass Ratio | H2 Content (%vol., Dry Basis) |
|---|---|---|---|---|
| 1 | 700 | 1 | 0.5:0.5 | 61.12 |
| 2 | 700 | 1 | 0.75:0.25 | 61.96 |
| 3 | 700 | 2 | 0.5:0.5 | 64.09 |
| 4 | 750 | 1 | 0.75:0.25 | 61.81 |
| 5 | 750 | 2 | 0.75:0.25 | 64.68 |
| 6 | 750 | 1 | 0.5:0.5 | 61.04 |
| 7 | 700 | 2 | 0.75:0.25 | 65.09 |
| 8 | 750 | 2 | 0.5:0.5 | 64.09 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 19.34 | 2 | 9.67 | 494.63 | <0.0001 | significant |
| B-S/F | 18.05 | 1 | 18.05 | 923.32 | <0.0001 | |
| C-C/B | 1.29 | 1 | 1.29 | 65.95 | 0.0005 | |
| Residual | 0.0978 | 5 | 0.0196 | |||
| Cor Total | 19.44 | 7 | ||||
| Std. Dev. | 0.1398 | R² | 0.995 | |||
| Mean | 62.98 | Adjusted R² | 0.993 | |||
| C.V. % | 0.222 | Predicted R² | 0.987 | |||
| Adeq Precision | 44.464 |
| Run | Carbonator Temperature (°C) | CaO/F Mass Ratio | CO2 Capture (%) | H2 Content (%vol., Dry Basis) |
|---|---|---|---|---|
| 1 | 550 | 1 | 79.87 | 98.47 |
| 2 | 450 | 3 | 99.99 | 99.59 |
| 3 | 550 | 3 | 99.65 | 99.53 |
| 4 | 450 | 1 | 78.42 | 99.01 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 427.22 | 1 | 427.22 | 769.02 | 0.0013 | significant |
| E-CaO/F | 427.22 | 1 | 427.22 | 769.02 | 0.0013 | |
| Residual | 1.11 | 2 | 0.555 | |||
| Cor Total | 3151.42 | 3 | ||||
| Std. Dev. | 0.745 | R² | 0.997 | |||
| Mean | 89.49 | Adjusted R² | 0.996 | |||
| C.V. % | 0.832 | Predicted R² | 0.9896 | |||
| Adeq Precision | 39.217 |
| Heat Duty of Each Unit (kW) | Values |
|---|---|
| Gasifier unit | 3332.32 |
| Regenerator unit | 3207.00 |
| Carbonator unit | −3023.59 |
| Parameters | Case No. | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| CaO/F mass ratio (−) | 1 | 1 | 3 | 3 |
| Carbonator temperature (°C) | 450 | 550 | 450 | 550 |
| Hydrogen production (kg/h) | 183.82 | 182.92 | 184.75 | 184.72 |
| Hydrogen content (%vol., dry basis) | 99.01 | 98.47 | 99.59 | 99.53 |
| % CO2 capture (%) | 78.42 | 79.87 | 99.99 | 99.65 |
| Energy efficiency of hydrogen production (%) | 42.64 | 42.43 | 42.86 | 42.85 |
| Total mass flow rate of CO2 equivalent emission (kg/h) | 1788.15 | 1768.59 | 1808.62 | 1807.86 |
| Specific emission of released CO2 (g CO2/MJ) | 80.26 | 79.77 | 80.77 | 80.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sornumpol, R.; Saebea, D.; Arpornwichanop, A.; Patcharavorachot, Y. Process Optimization and CO2 Emission Analysis of Coal/Biomass Gasification Integrated with a Chemical Looping Process. Energies 2023, 16, 2728. https://doi.org/10.3390/en16062728
Sornumpol R, Saebea D, Arpornwichanop A, Patcharavorachot Y. Process Optimization and CO2 Emission Analysis of Coal/Biomass Gasification Integrated with a Chemical Looping Process. Energies. 2023; 16(6):2728. https://doi.org/10.3390/en16062728
Chicago/Turabian StyleSornumpol, Ratikorn, Dang Saebea, Amornchai Arpornwichanop, and Yaneeporn Patcharavorachot. 2023. "Process Optimization and CO2 Emission Analysis of Coal/Biomass Gasification Integrated with a Chemical Looping Process" Energies 16, no. 6: 2728. https://doi.org/10.3390/en16062728
APA StyleSornumpol, R., Saebea, D., Arpornwichanop, A., & Patcharavorachot, Y. (2023). Process Optimization and CO2 Emission Analysis of Coal/Biomass Gasification Integrated with a Chemical Looping Process. Energies, 16(6), 2728. https://doi.org/10.3390/en16062728








