CO2 Adsorption Performance of Activated Coke Prepared from Biomass and Coal
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
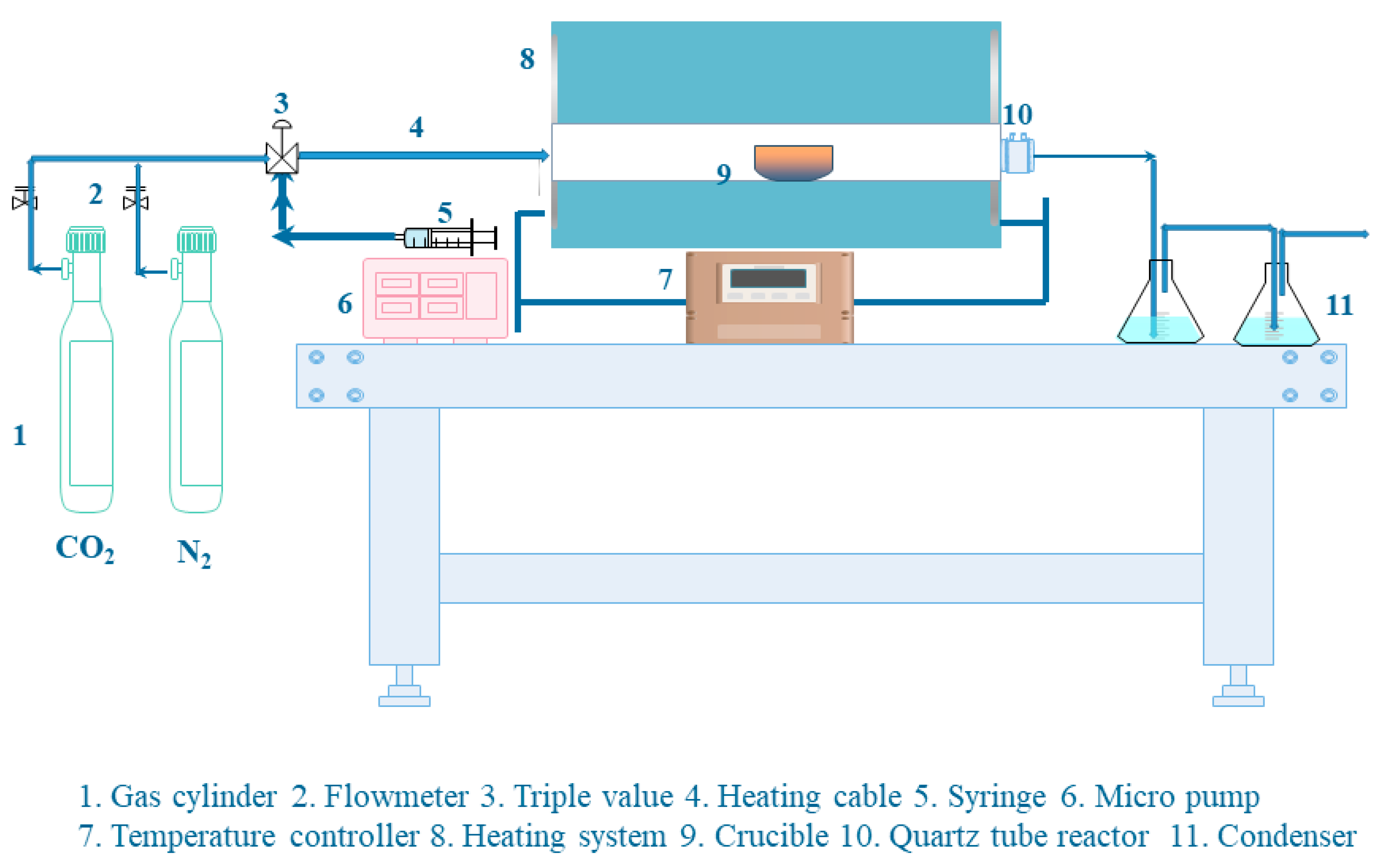
2.2. Preparation of Activated Cokes
2.3. Characterization
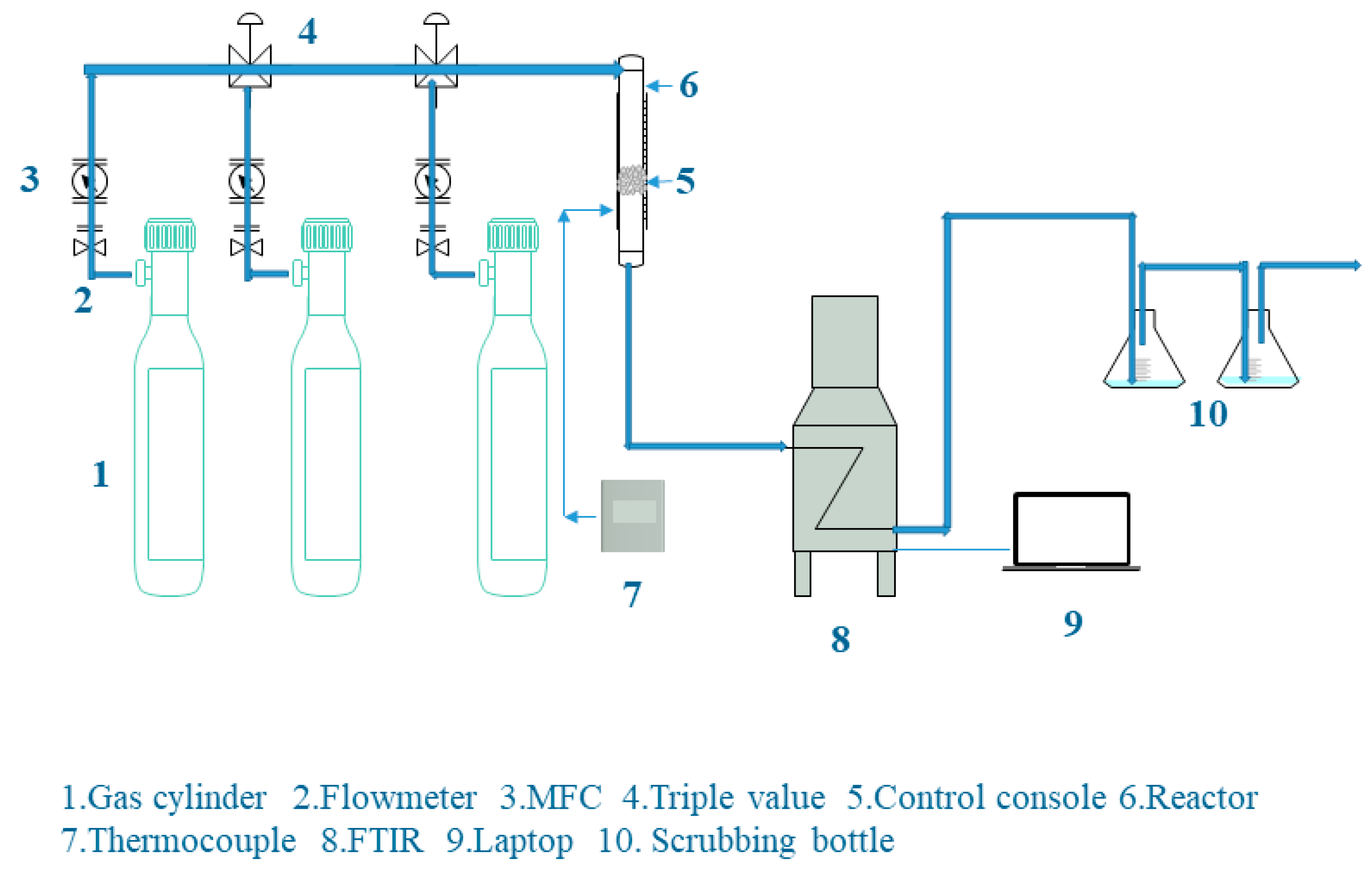
2.4. Carbon Dioxide Adsorption Experiment
2.5. Model Construction and Calculation Methods
3. Results and Discussion
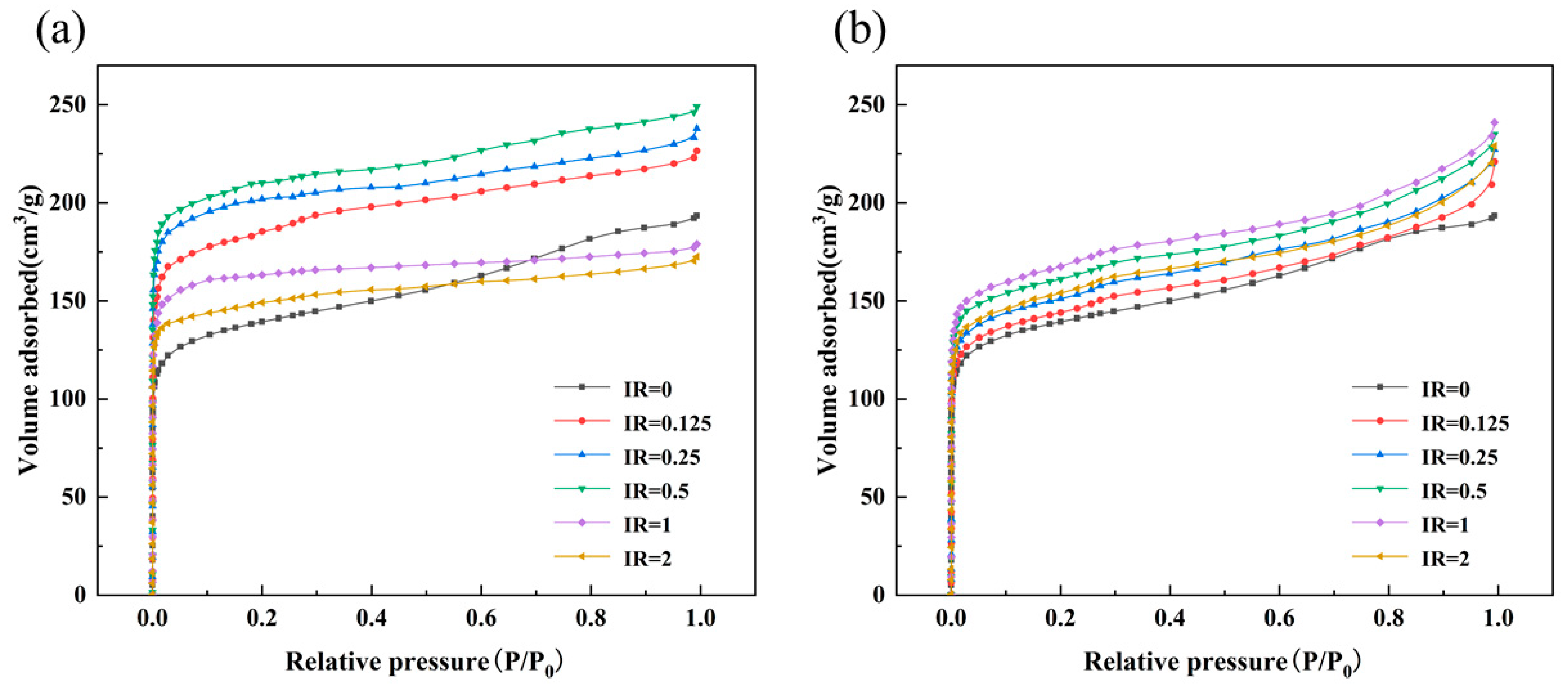
3.1. Effect of the Activation Conditions on the Physical Properties of Activated Coke
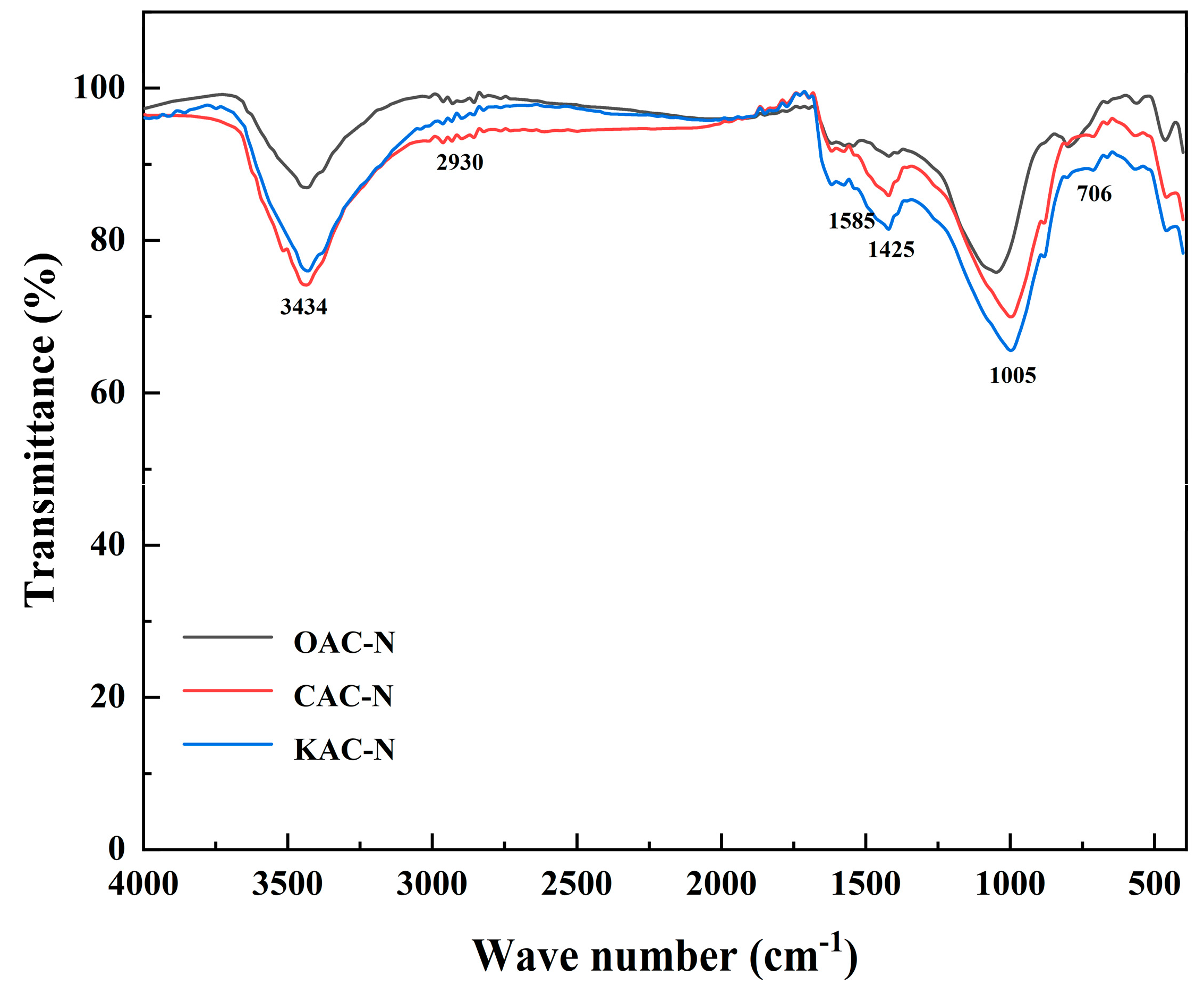
3.2. Evolution of Surface Functional Groups during the Activation of Activated Coke
3.3. Evolution of the Surface Morphology during the Activation of Coke
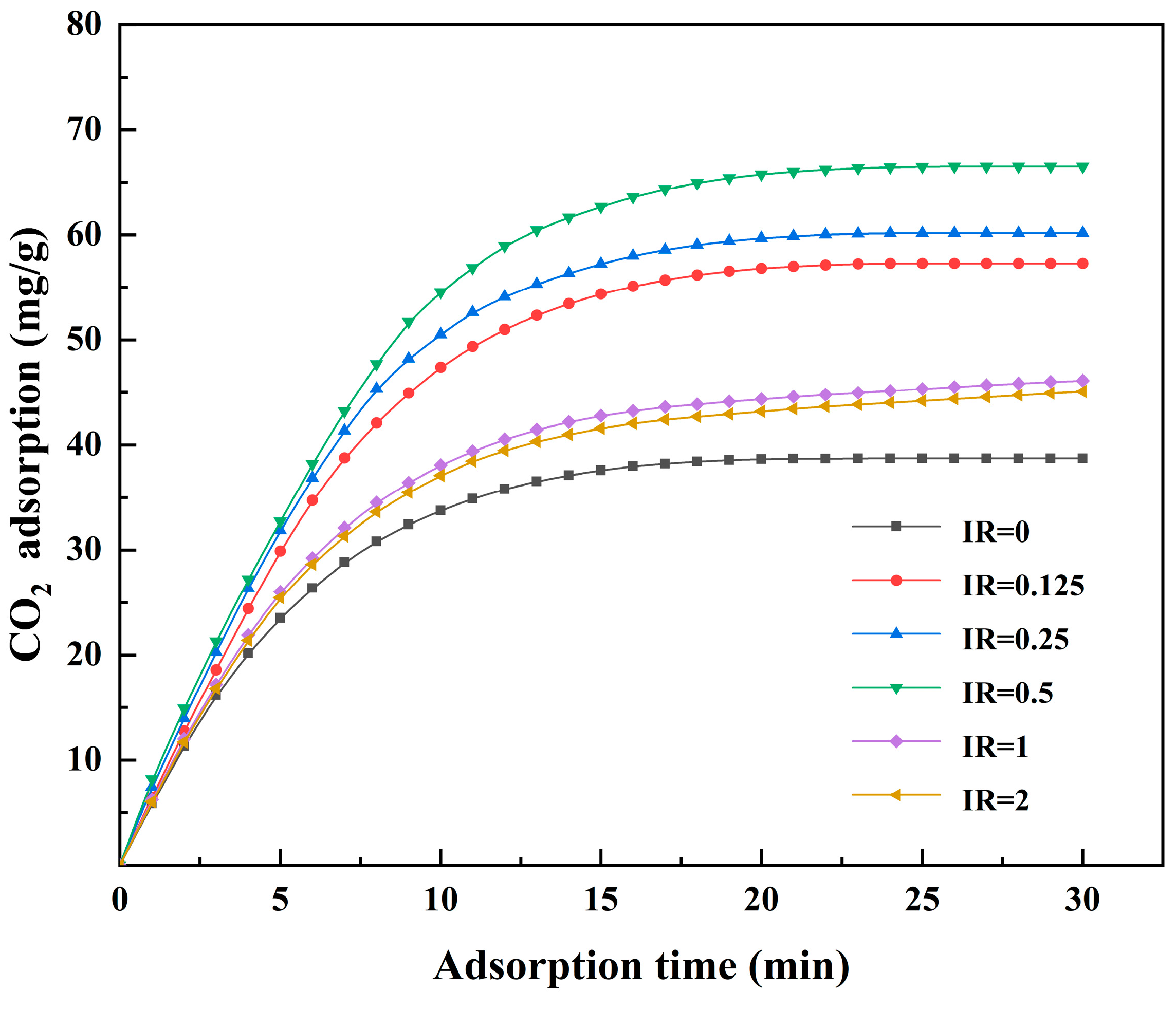
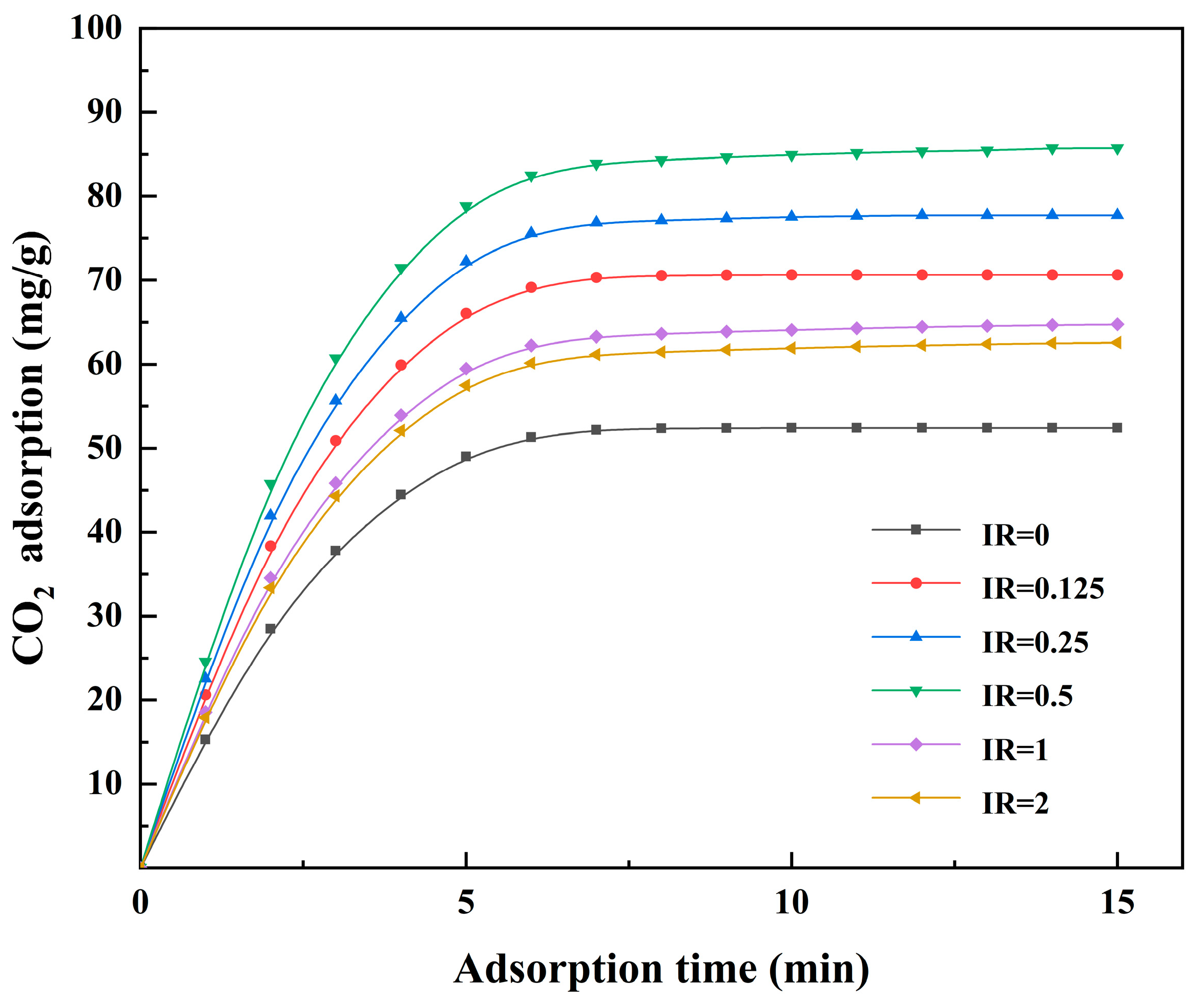
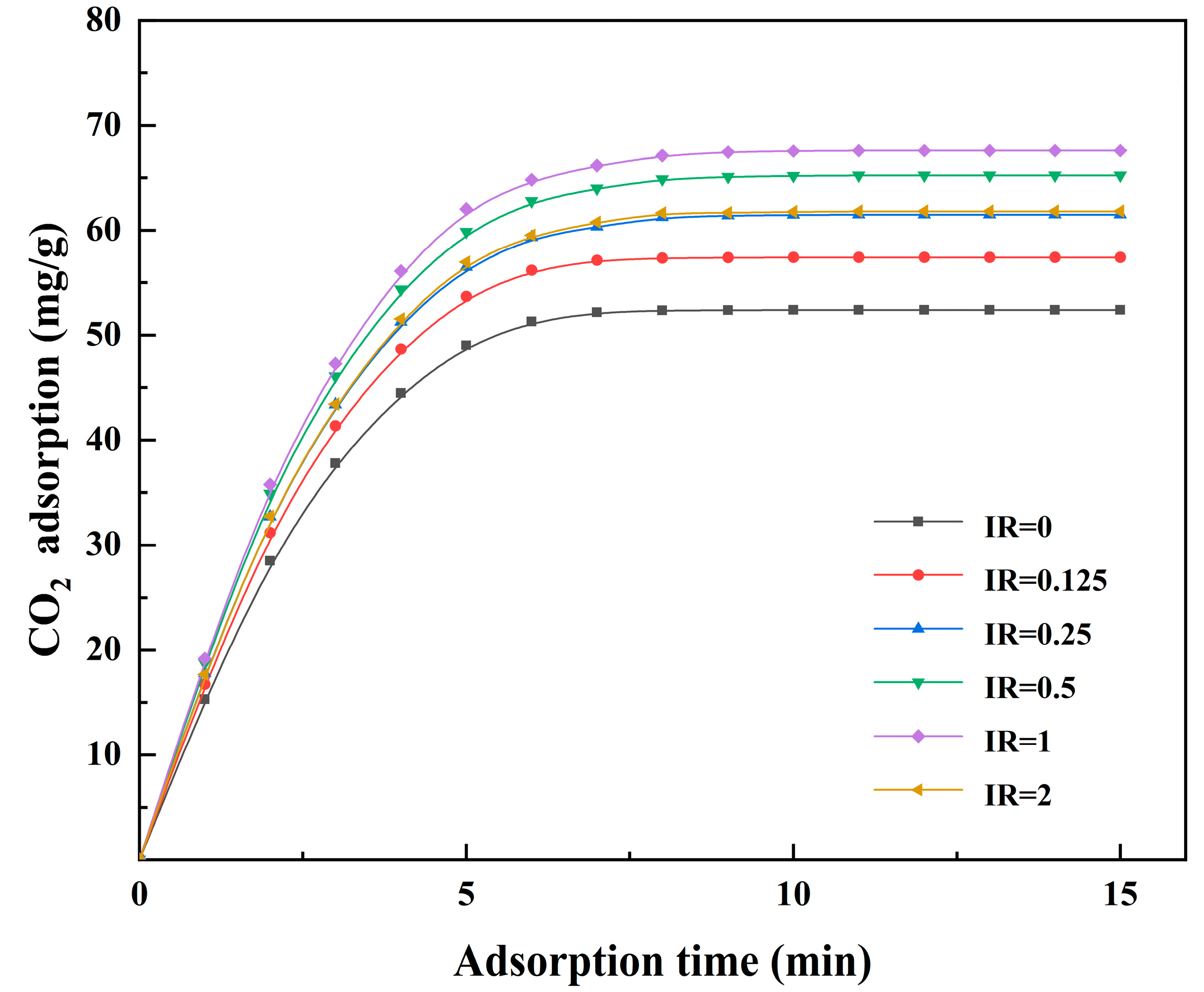
3.4. Effect of the Activation Conditions of Activated Coke on CO2 Adsorption
3.4.1. Adsorption of CO2 in the Flue Gas of Conventional Power Plants
3.4.2. Adsorption of CO2 in the Flue Gas of Oxy–Fuel Combustion
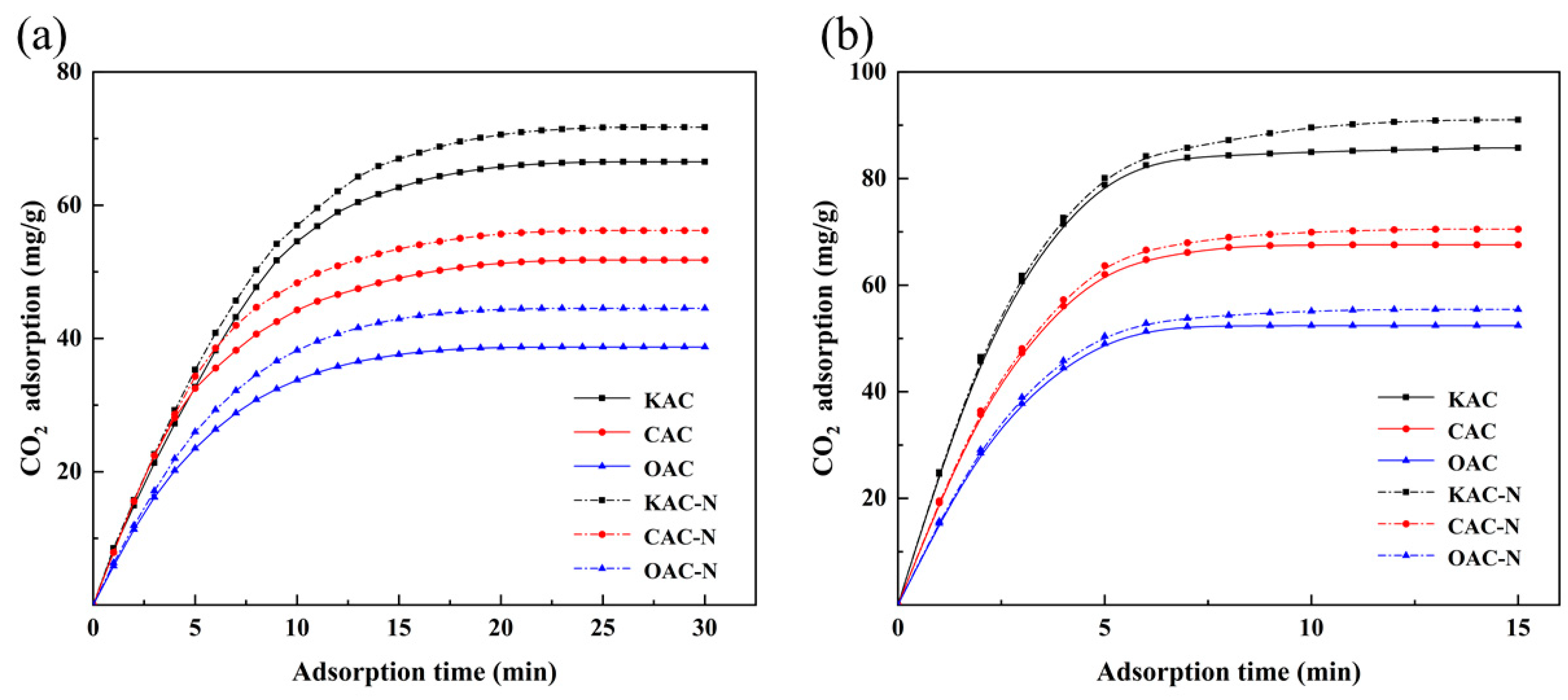
3.5. Adsorption Experiments of Modified Activated Coke
3.6. Effect of Nitrogen-Containing Functional Groups on the Adsorption of CO2 by Activated Coke
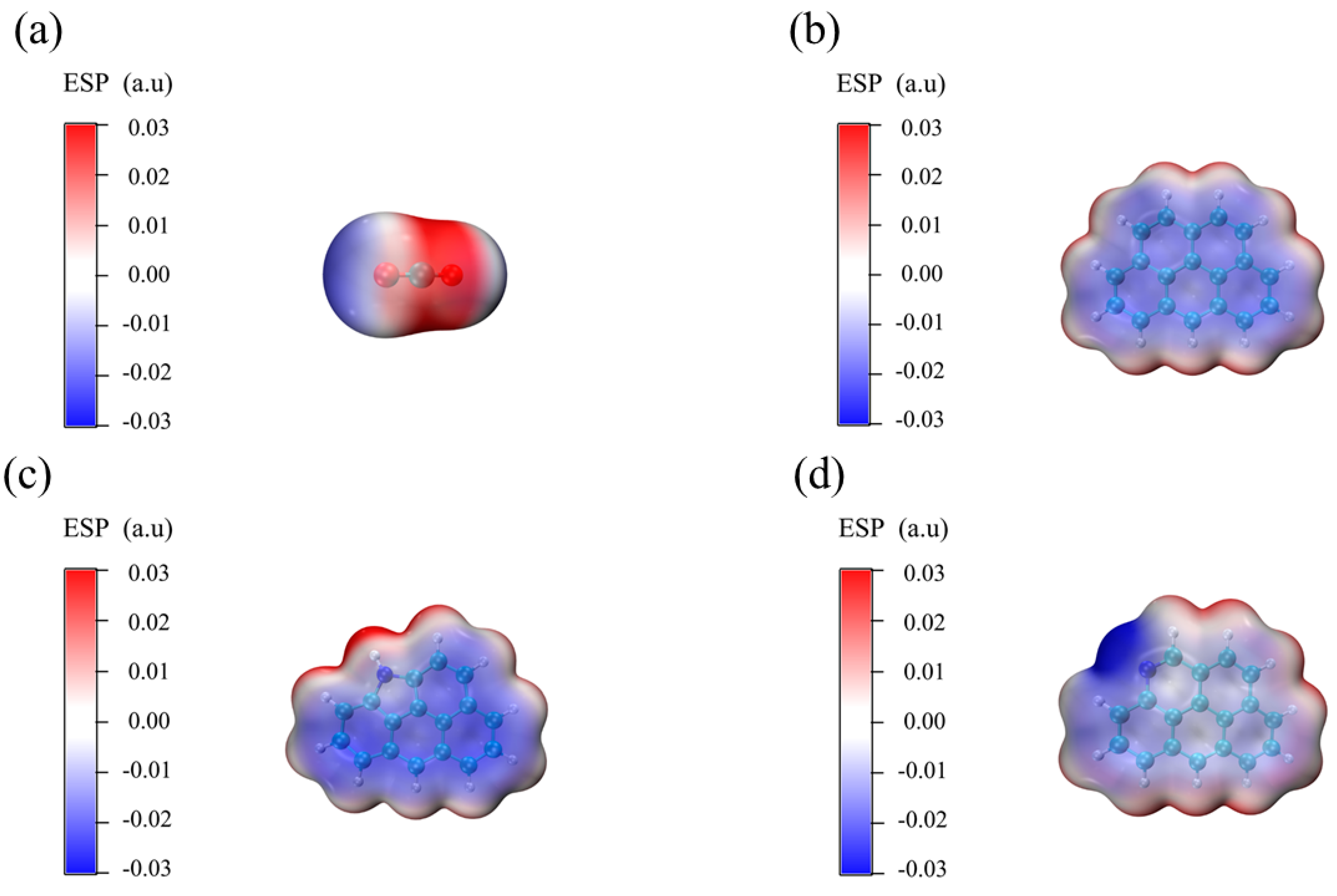
3.6.1. Electrostatic Potential Analysis
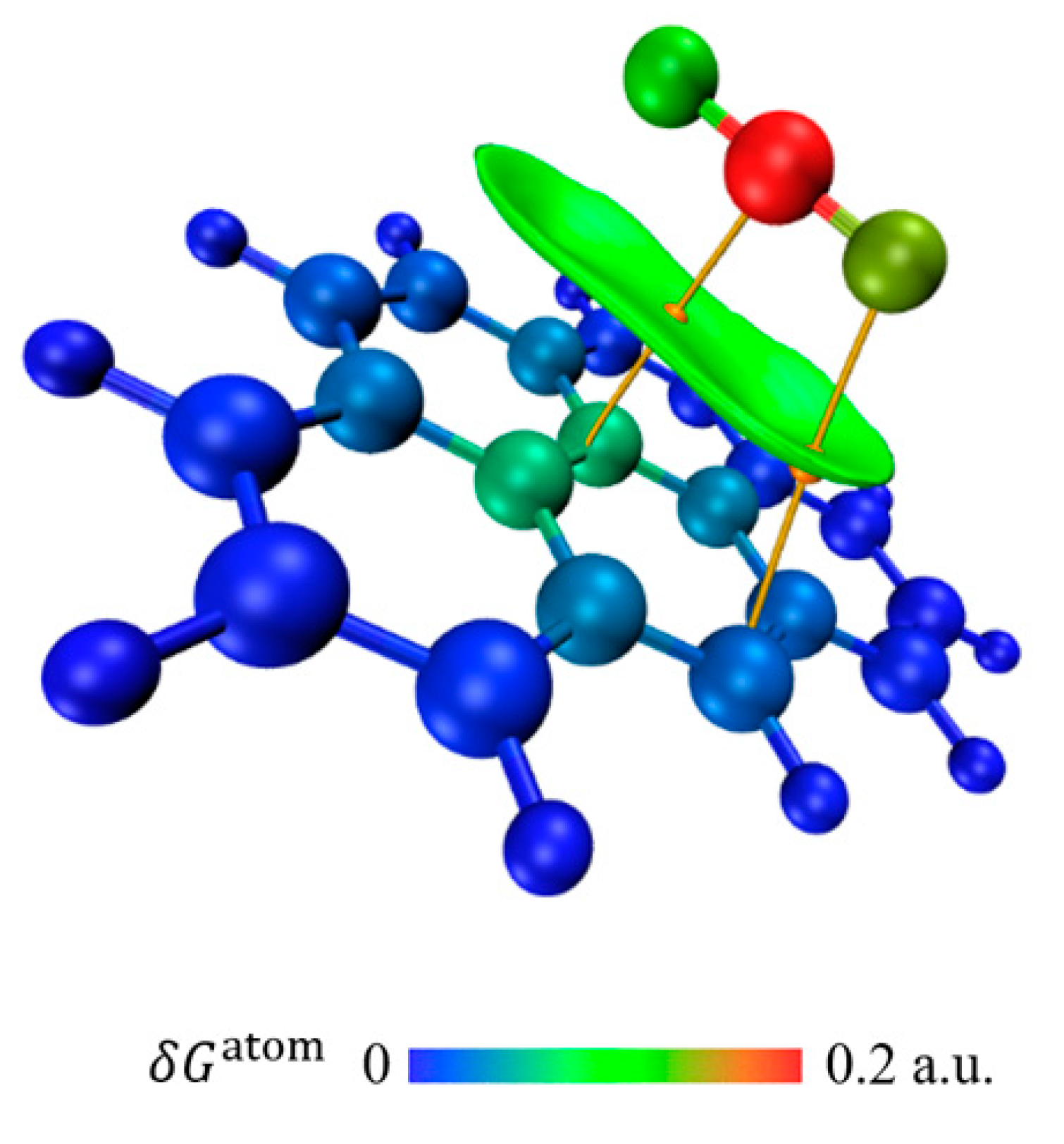
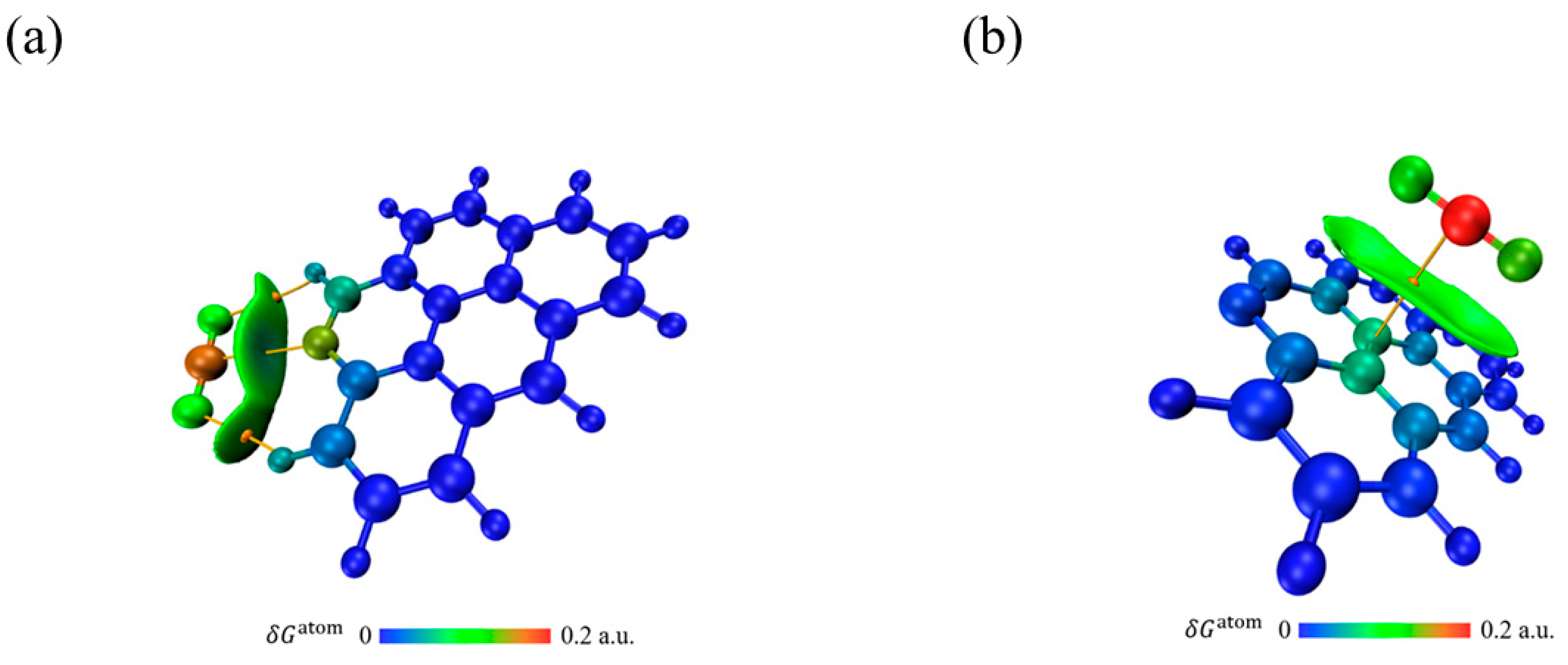
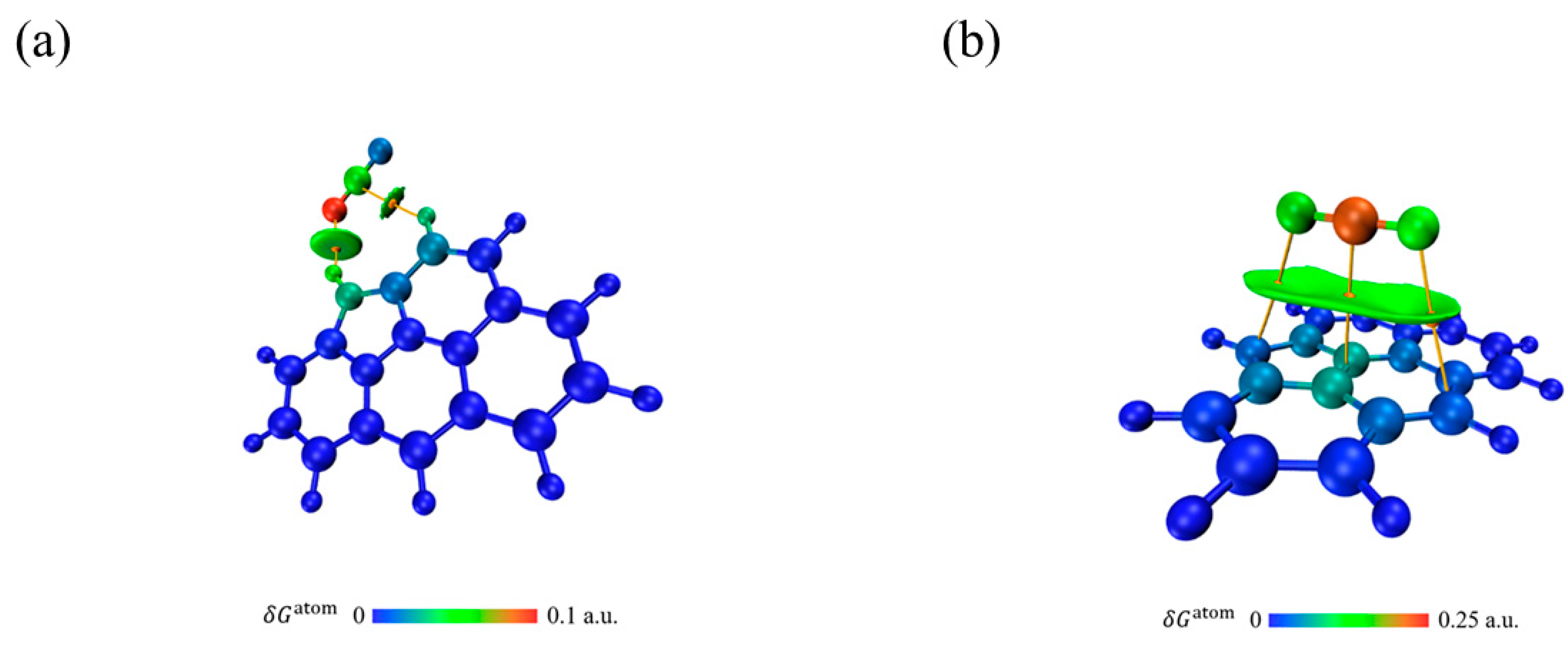
3.6.2. Adsorption Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peters, G.P.; Andrew, R.M.; Canadell, J.G.; Friedlingstein, P.; Jackson, R.B.; Korsbakken, J.I.; Le Quéré, C.; Peregon, A. Carbon dioxide emissions continue to grow amidst slowly emerging climate policies. Nat. Clim. Chang. 2020, 10, 3–6. [Google Scholar] [CrossRef]
- Kielbasa, K.; Bayar, S.; Varol, E.A.; Srenscek-Nazzal, J.; Bosacka, M.; Miadlicki, P.; Serafin, J.; Wrobel, R.J.; Michalkiewicz, B. Carbon Dioxide Adsorption over Activated Carbons Produced from Molasses Using H2SO4, H3PO4, HCl, NaOH, and KOH as Activating Agents. Molecules 2022, 27, 7467. [Google Scholar] [CrossRef]
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials, Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Zuo, H.; Wu, X.; E, J.; Wang, T.; Zhang, F.; Lu, N. System development and environmental performance analysis of a solar-driven supercritical water gasification pilot plant for hydrogen production using life cycle assessment approach. Energy Convers. Manag. 2019, 184, 60–73. [Google Scholar] [CrossRef]
- Carapellucci, R.; Di Battista, D.; Cipollone, R. The retrofitting of a coal-fired subcritical steam power plant for carbon dioxide capture: A comparison between MCFC-based active systems and conventional MEA. Energy Convers. Manag. 2019, 194, 124–139. [Google Scholar] [CrossRef]
- Spigarelli, B.P.; Kawatra, S.K. Opportunities and challenges in carbon dioxide capture. J. CO2 Util. 2013, 1, 69–87. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Bhavsar, S.; Najera, M.; Solunke, R.; Veser, G. Chemical looping: To combustion and beyond. Catal. Today 2014, 228, 96–105. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Plant derived porous graphene nanosheets for efficient CO2 capture. RSC Adv. 2014, 4, 44634–44643. [Google Scholar] [CrossRef]
- Tian, Z.; Huang, J.; Zhang, X.; Shao, G.; He, Q.; Cao, S.; Yuan, S. Ultra-microporous N-doped carbon from polycondensed framework precursor for CO2 adsorption. Microporous Mesoporous Mater. 2018, 257, 19–26. [Google Scholar] [CrossRef]
- Majchrzak, A.; Nowak, W. Separation characteristics as a selection criteria of CO2 adsorbents. J. CO2 Util. 2017, 17, 69–79. [Google Scholar] [CrossRef]
- Jang, E.; Choi, S.W.; Hong, S.-M.; Shin, S.; Lee, K.B. Development of a cost-effective CO2 adsorbent from petroleum coke via KOH activation. Appl. Surf. Sci. 2018, 429, 62–71. [Google Scholar] [CrossRef]
- Zanella, O.; Tessaro, I.C.; Féris, L.A. Desorption- and decomposition-based techniques for the regeneration of activated carbon. Chem. Eng. Technol. 2014, 37, 1447–1459. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, T.; Vellingiri, K.; Tsang, D.C.; Shon, J.; Kim, K.-H.; Kumar, S. Recycling and regeneration of carbonaceous and porous materials through thermal or solvent treatment. Chem. Eng. J. 2019, 364, 514–529. [Google Scholar] [CrossRef]
- Reza, M.S.; Yun, C.S.; Afroze, S.; Radenahmad, N.; Bakar, M.S.A.; Saidur, R.; Taweekun, J.; Azad, A.K. Preparation of activated carbon from biomass and its’ applications in water and gas purification, a review. Arab J. Basic Appl. Sci. 2020, 27, 208–238. [Google Scholar] [CrossRef]
- Din, M.I.; Ashraf, S.; Intisar, A. Comparative study of different activation treatments for the preparation of activated carbon: A mini-review. Sci. Prog. 2017, 100, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Bubanale, S.; Shivashankar, M. History, method of production, structure and applications of activated carbon. Int. J. Eng. Res 2017, 6, 495–498. [Google Scholar]
- Sarwar, A.; Ali, M.; Khoja, A.H.; Nawar, A.; Waqas, A.; Liaquat, R.; Naqvi, S.R.; Asjid, M. Synthesis and characterization of biomass-derived surface-modified activated carbon for enhanced CO2 adsorption. J. CO2 Util. 2021, 46, 101476. [Google Scholar] [CrossRef]
- Zhang, C.; Song, W.; Sun, G.; Xie, L.; Wang, J.; Li, K.; Sun, C.; Liu, H.; Snape, C.E.; Drage, T. CO2 capture with activated carbon grafted by nitrogenous functional groups. Energy Fuels 2013, 27, 4818–4823. [Google Scholar] [CrossRef]
- Fu, J.; Zhou, B.; Zhang, Z.; Wang, T.; Cheng, X.; Lin, L.; Ma, C. One-step rapid pyrolysis activation method to prepare nanostructured activated coke powder. Fuel 2020, 262, 116514. [Google Scholar] [CrossRef]
- Zhao, Y.; Mu, J.; Wang, Y.; Liu, Y.; Wang, H.; Song, H. Preparation of hierarchical porous carbon through one-step KOH activation of coconut shell biomass for high-performance supercapacitor. J. Mater. Sci. Mater. Electron. 2023, 34, 527. [Google Scholar] [CrossRef]
- Li, Y.; Lu, L.; Lyu, S.; Xu, H.; Ren, X.; Levendis, Y.A. Activated coke preparation by physical activation of coal and biomass co-carbonized chars. J. Anal. Appl. Pyrolysis 2021, 156, 105137. [Google Scholar] [CrossRef]
- Khalil, H.; Jawaid, M.; Firoozian, P.; Rashid, U.; Islam, A.; Akil, H.M. Activated carbon from various agricultural wastes by chemical activation with KOH: Preparation and characterization. J. Biobased Mater. Bioenergy 2013, 7, 708–714. [Google Scholar] [CrossRef]
- Kılıç, M.; Apaydın-Varol, E.; Pütün, A.E. Preparation and surface characterization of activated carbons from Euphorbia rigida by chemical activation with ZnCl2, K2CO3, NaOH and H3PO4. Appl. Surf. Sci. 2012, 261, 247–254. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, S.; Li, H.; Pan, D.; Patil, R.R.; Guo, Z.; Seok, I. Modification of coconut shell-based activated carbon and purification of wastewater. Adv. Compos. Hybrid Mater. 2021, 4, 65–73. [Google Scholar] [CrossRef]
- Zhang, C.; Song, W.; Ma, Q.; Xie, L.; Zhang, X.; Guo, H. Enhancement of CO2 capture on biomass-based carbon from black locust by KOH activation and ammonia modification. Energy Fuels 2016, 30, 4181–4190. [Google Scholar] [CrossRef]
- Wang, L.; Sha, L.; Zhang, S.; Cao, F.; Ren, X.; Levendis, Y.A. Preparation of activated coke by carbonization, activation, ammonization and thermal treatment of sewage sludge and waste biomass for SO2 absorption applications. Fuel Process. Technol. 2022, 231, 107233. [Google Scholar] [CrossRef]
- Sun, F.; Liu, X.; Gao, J.; Pi, X.; Wang, L.; Qu, Z.; Qin, Y. Highlighting the role of nitrogen doping in enhancing CO2 uptake onto carbon surfaces: A combined experimental and computational analysis. J. Mater. Chem. A 2016, 4, 18248–18252. [Google Scholar] [CrossRef]
- Chen, H.; Guo, Y.; Du, Y.; Xu, X.; Su, C.; Zeng, Z.; Li, L. The synergistic effects of surface functional groups and pore sizes on CO2 adsorption by GCMC and DFT simulations. Chem. Eng. J. 2021, 415, 128824. [Google Scholar] [CrossRef]
- Saha, D.; Kienbaum, M.J. Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: A critical review. Microporous Mesoporous Mater. 2019, 287, 29–55. [Google Scholar] [CrossRef]
- Ma, X.; Li, L.; Chen, R.; Wang, C.; Zhou, K.; Li, H. Doping of alkali metals in carbon frameworks for enhancing CO2 capture: A theoretical study. Fuel 2019, 236, 942–948. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Soltani, S.M. Impact of surface functional groups and their introduction methods on the mechanisms of CO2 adsorption on porous carbonaceous adsorbents. Carbon Capture Sci. Technol. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Bardestani, R.; Patience, G.S.; Kaliaguine, S. Experimental methods in chemical engineering: Specific surface area and pore size distribution measurements—BET, BJH, and DFT. Can. J. Chem. Eng. 2019, 97, 2781–2791. [Google Scholar] [CrossRef]
- Rehman, A.; Baek, J.W.; Rene, E.R.; Sergienko, N.; Behera, S.K.; Park, H.-S. Effect of process parameters influencing the chemical modification of activated carbon fiber for carbon dioxide removal. Process Saf. Environ. Prot. 2018, 118, 384–396. [Google Scholar] [CrossRef]
- Pi, X.; Sun, F.; Gao, J.; Qu, Z.; Wang, A.; Qie, Z.; Wang, L.; Liu, H. A new insight into the SO2 adsorption behavior of oxidized carbon materials using model adsorbents and DFT calculations. Phys. Chem. Chem. Phys. 2019, 21, 9181–9188. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, L.; Zhang, Y.; Cao, F.; Sun, Q.; Ren, X.; Wennersten, R. Effect of hydroxyl functional groups on SO2 adsorption by activated carbon. J. Environ. Chem. Eng. 2022, 10, 108727. [Google Scholar] [CrossRef]
- Liu, J.; Deng, Y.; Li, X.; Wang, L. Promising nitrogen-rich porous carbons derived from one-step calcium chloride activation of biomass-based waste for high performance supercapacitors. ACS Sustain. Chem. Eng. 2016, 4, 177–187. [Google Scholar] [CrossRef]
- Caicedo-Salcedo, O.D.; Vargas-Delgadillo, D.P.; Giraldo, L.; Moreno-Piraján, J.C. Data of preparation and characterization of activated carbon using two activant agents and mango seed as precursor material. Data Brief 2019, 27, 104769. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yue, Q.; Gao, B.; Li, A. Insight into activated carbon from different kinds of chemical activating agents: A review. Sci. Total Environ. 2020, 746, 141094. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, C.; Li, C. CO2 adsorption kinetics of K2CO3/activated carbon for low-concentration CO2 removal from confined spaces. Chem. Eng. Technol. 2015, 38, 891–899. [Google Scholar] [CrossRef]
- Huang, G.-G.; Liu, Y.-F.; Wu, X.-X.; Cai, J.-J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Wu, W.; Wu, C.; Zhang, G.; Liu, J. Preparation of microporous carbonaceous CO2 adsorbents by activating bamboo shoot shells with different chlorides: Experimental and theoretical calculations. J. Anal. Appl. Pyrolysis 2022, 168, 105742. [Google Scholar] [CrossRef]
- Dehkordi, S.S.R.; Delavar, Q.; Ebrahim, H.A.; Partash, S.S. CO2 adsorption by coal-based activated carbon modified with sodium hydroxide. Mater. Today Commun. 2022, 33, 104776. [Google Scholar] [CrossRef]
- Tan, Y.; Islam, M.A.; Asif, M.; Hameed, B. Adsorption of carbon dioxide by sodium hydroxide-modified granular coconut shell activated carbon in a fixed bed. Energy 2014, 77, 926–931. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Choi, S.W.; Shang, J.; Hanif, A.; Dissanayake, P.D.; Tsang, D.C.; Kwon, J.-H.; Lee, K.B.; Ok, Y.S. Carbon dioxide capture in biochar produced from pine sawdust and paper mill sludge: Effect of porous structure and surface chemistry. Sci. Total Environ. 2020, 739, 139845. [Google Scholar] [CrossRef]
- Yaumi, A.; Bakar, M.A.; Hameed, B. Reusable nitrogen-doped mesoporous carbon adsorbent for carbon dioxide adsorption in fixed-bed. Energy 2017, 138, 776–784. [Google Scholar] [CrossRef]
- Zhai, Y.; Pang, D.; Chen, H.; Xiang, B.; Chen, J.; Li, C.; Zeng, G.; Qiu, L. Effects of ammonization on the surface physico-chemical properties of sludge-based activated carbon. Appl. Surf. Sci. 2013, 280, 590–597. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Jang, D.-I.; Bae, S.-T.; Park, S.-J. Facile synthesis of nitrogen-enriched mesoporous carbon for carbon dioxide capture. Int. J. Hydrogen Energy 2014, 39, 12347–12352. [Google Scholar] [CrossRef]
- Lim, G.; Lee, K.B.; Ham, H.C. Effect of N-containing functional groups on CO2 adsorption of carbonaceous materials: A density functional theory approach. J. Phys. Chem. C 2016, 120, 8087–8095. [Google Scholar] [CrossRef]
- Jiang, D.; Li, H.; Wang, S.; Cheng, X.; Bartocci, P.; Fantozzi, F. Insight the CO2 adsorption onto biomass-pyrolysis derived char via experimental analysis coupled with DFT calculation. Fuel 2023, 332, 125948. [Google Scholar] [CrossRef]






| Samples | Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | V | A | FC | C | H | S | N | O | |
| Zhundong coal | 10.28 | 29.54 | 4.45 | 55.73 | 67.10 | 3.50 | 0.61 | 0.47 | 28.32 |
| Coconut shell | 6.53 | 73.87 | 0.85 | 18.75 | 49.56 | 6.56 | 0.03 | 0.15 | 42.47 |
| Active Agent | IR | SBET (m2/g) | Smic (m2/g) | Micropore Ratio (%) | Yield (%) |
|---|---|---|---|---|---|
| None | 0 | 528.26 | 334.70 | 63.36 | 43.51 |
| KOH | 0.125 | 574.35 | 451.10 | 78.54 | 32.52 |
| KOH | 0.25 | 600.46 | 493.04 | 82.11 | 28.24 |
| KOH | 0.5 | 629.81 | 538.17 | 85.44 | 20.42 |
| KOH | 1 | 450.21 | 405.91 | 90.16 | 21.98 |
| KOH | 2 | 412.87 | 392.43 | 95.04 | 22.11 |
| CaCl2 | 0.125 | 560.10 | 366.59 | 65.45 | 34.25 |
| CaCl2 | 0.25 | 575.63 | 384.41 | 66.78 | 29.11 |
| CaCl2 | 0.5 | 595.44 | 410.20 | 68.89 | 27.45 |
| CaCl2 | 1 | 610.66 | 424.24 | 69.54 | 26.27 |
| CaCl2 | 2 | 580.45 | 390.76 | 67.32 | 25.47 |
| Samples | Raw Materials | T (°C) | W (%) | V (mg/g) |
|---|---|---|---|---|
| KAC | bituminous coal and coconut shell | 25 | 10 | 66.50 |
| CAC | bituminous coal and coconut shell | 25 | 10 | 51.78 |
| Activated carbon | coal | 25 | 12 | 51.41 [48] |
| Activated carbon | coconut shell | 35 | 10 | 27.10 [49] |
| Sludge biochar | sludge | 25 | 15 | 32.12 [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Wang, S.; Hao, M.; Shao, W.; Zhang, S.; Zhang, L.; Ren, X. CO2 Adsorption Performance of Activated Coke Prepared from Biomass and Coal. Energies 2023, 16, 3872. https://doi.org/10.3390/en16093872
Gao H, Wang S, Hao M, Shao W, Zhang S, Zhang L, Ren X. CO2 Adsorption Performance of Activated Coke Prepared from Biomass and Coal. Energies. 2023; 16(9):3872. https://doi.org/10.3390/en16093872
Chicago/Turabian StyleGao, He, Shaohua Wang, Miaomiao Hao, Wei Shao, Shuhui Zhang, Lei Zhang, and Xiaohan Ren. 2023. "CO2 Adsorption Performance of Activated Coke Prepared from Biomass and Coal" Energies 16, no. 9: 3872. https://doi.org/10.3390/en16093872
APA StyleGao, H., Wang, S., Hao, M., Shao, W., Zhang, S., Zhang, L., & Ren, X. (2023). CO2 Adsorption Performance of Activated Coke Prepared from Biomass and Coal. Energies, 16(9), 3872. https://doi.org/10.3390/en16093872








