Abstract
Life is dependent on water. However, in terms of the potential effects, water scarcity is quickly emerging as one of the most critical problems in the world. To access more fresh water for drinking, sanitation, and irrigation, water can be harvested from different forms of water on earth. Atmospheric harvesting is the best alternative for producing fresh water for everyday life and reducing global water shortages. To date, many modern technologies have been introduced for this application, with several prototypes being demonstrated. Thus, this study explores the potential benefits of the current atmospheric water harvesting systems in terms of their modes, atmospheric conditions, and production rate and examines the key factors that affect the efficiency of atmospheric water harvesting, such as temperature and humidity. According to the studies, there has been a significant advancement in energy harvesting and conversion technology, along with atmospheric water harvesting, over the past few years, including new mechanisms and technical paths. However, there are still many obstacles; in particular, most of the technologies depend on outdoor conditions. In order to overcome this issue, new directions need to be investigated. Here, we discuss the principles, advantages, limitations, and potential applications of these technologies.
1. Introduction
Background
In recent times, the human necessity for safe and sufficient water resources and distribution has become a fundamental problem for the rising population, climatic changes, industrialization, and improper water managemen. As a result, severe water shortages have occurred worldwide and have placed further stress on the scarce freshwater resources [1]. World water consumption will increase by 55%, and approximately 25% of large cities will face water shortages [2]. Droplets and vapor in the atmosphere are estimated to exist in 13 sextillions (1021) litters. Approximately 10% of freshwater sources come from atmospheric water; therefore, it can be used as an alternative [3]. Air contains 14,000 km3 of water, but rivers and lakes contain only 1200 km2 of fresh water [4]. Globally, approximately 2.2 billion people lack access to safe clean water [5]. According to the United Nations (UN), 70% of the world’s water is used for cultivation, 22% is used for industries, and the remaining 8% for domestic purposes [6]. The rapid decline of freshwater resources due to global warming, the increase in the population and the aging infrastructure of various water utilities has made addressing the regular water demand more difficult in many regions [1]. A combination of population growth, socioeconomic development, and changing consumption patterns has driven the global water consumption trend upward by about 1% per year since the 1980s. Over 1 billion people lack access to water today, and the demand will increase by 30% by 2030, according to the United Nations Convention to Combat Desertification (UNCCD) report from 2014. Globally, up to 2.4 billion people may be living in water-scarce areas by 2025 [7].
There are three different types of water in the atmosphere: gaseous, liquid, and solid. Solid and liquid water have long been used directly by humans, but gaseous water has not been captured as often [8]. Currently, the water conservation techniques in deserts and wastelands, such as active dew collection (condensers powered by electricity), water transfer infrastructure (dams, reservoirs, diversions of rivers, and water pipelines), and seawater desalination plants, are typically expensive to construct and maintain, and the operational costs are very high [9,10,11,12]. On the other hand, atmospheric water harvesting (AWH) could prove to be a key to solving the water shortage challenges in landlocked regions, particularly those with arid and semiarid climate conditions, as it provides potable water to a small group of people without harming the environment [13]. Technologies for “passive” water production have received a lot of attention recently because they do not use additional energy. For instance, researchers have developed several solar vapor generators (SVGs) made of photothermal materials (metallic nanostructures, inorganic semiconductor materials, carbon-based light absorbing materials, and polymeric materials) and a hydrophilic matrix [14,15]. The SVG gadgets can float on the water’s surface and generate clean water using solar energy. The use of passive technologies for directly harvesting atmospheric water using synthetic materials as an alternative to conventional methods is considered promising. This is because rapid water harvesting is required and electricity is insufficient. These technologies are affordable, energy efficient, easy to install, and have high harvesting performances compared to the conventional methods [16]. In contrast to traditional water harvesting processes, such as desalination, reverse osmosis, and condensation, chemical-based AWH does not require additional energy, particularly the metal organic frame (MOF) and hygroscopic inorganic compounds. Chemically constructed AWH materials rely on their properties, such as Laplace pressure gradients, polymer chain changes, and adjustable intermolecular space, to harvest water.
The purpose of this review is to discuss the various technologies used for AWH, including their production rate, climatic conditions, and technology used. At the end of the review, we provide a comprehensive discussion about the different techniques and their drawbacks.
2. Materials and Methods
An overview of the atmospheric water harvesting technologies is provided in this section. The performance evaluating indexes, which are different depending on the technology, are summarized first. The working principles, arrangements, and test setups are outlined for each technology. Figure 1 shows most recognized and most popular AWH technologies used: sorption-based, condensation, fog capturing, and dew water harvesting [17,18,19].
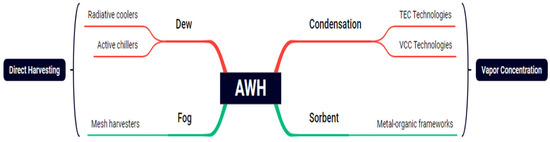
Figure 1.
Different methods of AWH systems and process categories [20,21,22].
2.1. Dew Water Harvesting
Dew water harvesting is a method of collecting and storing water from dew, which forms on the surface during cool and humid nights. In the current market, several companies are developing or producing large-scale dewing systems. The military, victims of natural disasters, and other situations dealing with limited liquid water supply have proposed these systems for water harvesting [23,24]. As dews are collected by manipulating natural processes, they can be considered a typical water source. Passive dew harvesting has resulted in a maximum water harvesting rate (WHR) of 0.3–0.6 L/m2/day [24]. Radiative condensers will be able to achieve an optimal WHR by improving their properties, such as hydrophilicity, size, and shape. It is still not reliable to use this technique in areas with poor weather conditions, even with improvements, because weather conditions can influence the condenser performance and limit its applicability. This makes it unreliable as an AWH source [25]. The energy requirement for dew collection is very high due to the high usage of energy required to overcome the latent heat used for the evaporation and cooling, which are also needed for atmospheric water harvesting [26]. Table 1 compares a few dew water harvesting studies that demonstrate the potential of dew water harvesting as a sustainable source of water for communities in arid regions. However, the amount of dew that forms and the ease of collecting it are limiting factors. Additionally, it is important to consider that dew can contain pollutants, so proper filtration and treatment may be necessary before use.

Table 1.
Comparison of several key research on dew water harvesting.
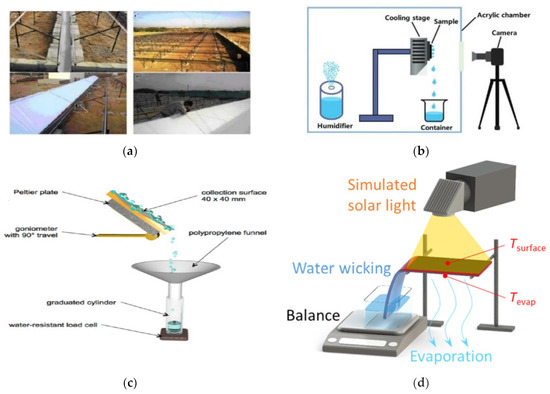
Figure 2.
Different types of dew water harvesting technology. (a) Water Harvesting Plant for Dew Bottling [27]. (b) Hybrid Surfaces for Improved Dew Collection [29]. (c) Dew water collection [22]. (d) Solar driven freshwater harvesting [30].
Table 1.
Comparison of several key research on dew water harvesting.
| SN | Surface Material | Relative Humidity RH (%) | Production (L/m2 h) | Literature |
|---|---|---|---|---|
| 1 | Styrene foam panels (Figure 2a) | - | 0.6 | [27] |
| 2 | Epoxy/SiO2 grooves | - | 0.2 | [19] |
| 3 | Polymer flat surfaces | 100 | 0.2 | [28] |
| 4 | Patterned copper (Figure 2b) | 50 | 1.1 | [29] |
| 5 | Nanostructured Teflon AF and PTFE surfaces (Figure 2c) | 98 | 0.72 | [22] |
| 6 | Porous coating layer (Figure 2d) | 0.13 | [30] | |
| 7 | Rough Duralumin Alloy Plates | 70 | 0.15 | [31] |
| 8 | Needle-Shaped Black Silicon | 70 | 0.1–0.27 | [32] |
2.2. Fog Water Harvesting
Fog harvesting is preferable, especially in coastal areas with no availability of fresh water. Fog harvesting is a method of collecting water by placing mesh perpendicular to the direction of the wind to catch fog droplets. Water is collected in a tank by gravity after successive impacts, in which droplets coalesce and grow large enough to fall. The first experiment using the net was conducted in 1956 in northern Chile by a Catholic university [33]. Since 1987, Schemenauer and colleagues have conducted several experiments following similar concepts, but on a larger scale (Figure 3a) [34]. There are several places around the world in which fog collection experiments have been successfully conducted, including Namibia, Saudi Arabia, Oman, Chile, and Peru. The above successful experiment inspired several parts of the world for researching fog collection [35]. The normal condition for the liquid to water is 0.1–0.5 gm−3, with a fog immersion time of 40%, and a collection rate of 50% with a 3 m/s wind speed [36]. Using high-elevation fog can produce up to 3–7 kg/day/m2 water [34]. The major limitation of fog water harvesting is carrying the collected water to residents, as the fog collectors are mostly away from the resident area. Thus, it is required to install a pipeline for water transfer. The installation of pipelines is costly, and therefore makes this system uneconomic [37]. Examine the possibility of fog collectors around the residence area. To be successful and efficient, a fog-extraction project must meet the following criteria [38]:
- (1)
- There should be continuous fog throughout the year and the duration should be long.
- (2)
- High-attitude fogs and a high amount of water content are the major criteria for fog water harvesting in arid lands.
- (3)
- Fog collection is more efficient when the wind is present.
The main disadvantage of fog collection is that the efficiency of the water flowing through the collector meshes is proportional to the amount of water reaching the collector gutter [39]. Two main factors affect the efficiency of collection based on surface wettability: the mesh becomes clogged with pinned droplets after the recapture of deposited droplets. At present, wire mesh is used for fog harvesting, with two limitations. Microscopic fog droplets cannot be captured by coarse meshes. In contrast, fine meshes are prone to clogging; to avoid clogging, a superhydrophobic surface treatment can be used, but they cannot be used for a long period. Table 2 shows the different types of fog water harvesting systems and their production.

Table 2.
Comparison of different types of Fog water harvesting.
Table 2.
Comparison of different types of Fog water harvesting.
| SN | Material | Condition | Production | Literature |
|---|---|---|---|---|
| 1 | 35 LFCs | Winter dry season | 6300 L of water on average per day throughout the course of 4–6 months during the winter dry season. | [40] |
| 2 | Four LFCs were added in the 2000s, and four more in 2011. | - | - | [41] |
| 3 | 60 fog nets | 100 L of water each day, a reduction in water use of over 60%. | [42] | |
| 4 | Locally created collector for fog | 100 L/day | [43] | |
| 5 | SFC-fog collector | 6 L/day | [43] | |
| 6 | 35 large fog collectors Figure 3c | Winter | 200 L | [44] |

Figure 3.
Different types of fog harvesting systems. (a) LFC fog collector situated at Mount Machos (Spain) [34]. (b) LFC fog collector situated at Guatemala [44].
Based on the preceding Table 2, a significant study on fog collectors was carried out. The production rate will be high in areas with high levels of fog and low rainfall. This is because fog is most likely to form in areas with cool, moist air and high humidity, and water harvesting systems rely on the condensation of fog droplets to collect water. Some of the best regions for fog water harvesting include coastal areas near the ocean, mountainous regions, and certain desserts. Despite the huge water collection, it only happens during the winter dry season, which happens around once a year. As a result of this restriction, fog collectors could only be utilized in specific locations.
2.3. Sorption Based
Sorption-based atmospheric water harvesting is a method of collecting water from the atmosphere using materials that can absorb water vapor. This process takes advantage of the humidity in the air to extract water that can be used for drinking, irrigation, or other purposes. When compared to dew and fog, in which water is already present in the form of liquid, but in the sorption-based system, it does not need to be a liquid form for production. In adsorption, the forces or interactions between the adsorbate and sorbent determine whether the adsorption is physical or chemical [45]. The process of absorption occurs when molecules, atoms, and ions from a substance and are taken up by the bulk phase (liquid, gas, or solid) of the material [46]. The use of solid adsorbents for water harvesting has been proposed in numerous studies, mainly based on silica gel, hygroscopic salts (LiCl, LiBr, CaCl), and zeolites [46,47]. During adsorption, the adsorbent structure remains the same, at a relative humidity below 100%, and the hygroscopic salts deliquesce or dissolve into an aqueous solution. When the partial pressure of the vapor in the solution is less than that in the air, repetitive cycling can lead to particle agglomeration and reduced vapor permeability, thus decreasing the performance [48,49]. Hygroscopic salts can be placed in the pores of an adsorbent, such as SiO2 or activated carbon, to help to prevent this problem, and desiccants are recommended for dehumidification [50,51]. The formation of porous carbon or silica gel host matrices contains hygroscopic salt adsorbents using CaCl2 and libr2 [52]. According to Wang et al., the experimental results in silica gel Licl and CaCl2 are active carbon fiber and matrixes [53]. In practice, composite salt absorbents are still challenging due to the salt loss caused by crystallization and hysteresis at high RH [50,51]. The rest of the discussion is mainly about water harvesting from absorption. Water vapor from an ambient air stream can easily contact a desiccant. After isolating the desiccant, the heat is passed for the vapor to release. Then, the desiccant becomes a local humidity source at 100% (RH). As a result, the condenser requires less sensible cooling, and condensation occurs on the collected water vapor. Compared to a dew system, in which water must freeze from the air to harvest water, the adsorption-based approaches can harvest water even at zero degrees celsius [26]. For the release of water vapor from a material, heat must be provided for both cooling and overcoming adsorption enthalpy and lowering the RH (which is determined by the material isotherm). Water vapor is released from the material as a result of this process; however, it can take a large amount of energy to desorb. Thermal energy from low-grade sources can be used to power the system. As a result, the high local relative humidity produced by desiccant adsorption can cause condensation at an ambient temperature due to an increase in the vapor concentration [54,55]. Table 3 shows the different materials used and their production.
2.3.1. Physical Adsorbents
There are nanometre-scale voids and pores in silica gel, which is an amorphous and porous form of silicon dioxide. Amorphous silicon dioxide consists of an irregular three-dimensional framework of silicon and oxygen atoms. Silicon dioxide gel is amorphous and porous, consists of alternating silicon and oxygen atoms, and various substances can be adsorbents from silica gel, such as alcohols, phenols, and water. In terms of the International Union of Pure and Applied Chemistry (IUPAC), the classification of different grades of silica gel have been produced so far. In adsorption technology, silica gel is most commonly used due to its availability, cheaper cost, lack of pollution production, and requirement of a lower temperature for regeneration [56]. At low RH, the adsorption capacity of silica gel is low, and its thermal conductivity is also not sufficient. Zeolites have been used for decades as an adsorbent and are still important. There are various grades of zeolite, both minerals and synthetic, that are made up of alumina silicate crystals and alkaline or alkaline earth elements. There are six groups of pores on the aluminosilicate structure that can adsorb large amounts of molecules. In recent years, aluminophosphate has been introduced as a promising version of zeolites with a high regeneration temperature, low adsorption capacity, and poor thermal conductivity [57].
2.3.2. Polymeric Adsorbents
A MOF is a coordination of polymer made up of metal ions or a cluster of metal ions combined with organic ligands to form a single molecule, two-dimensional structure, or three-dimensional structure. There have been several studies conducted due to the retention property of MOFs and it is found to be highly effective and requires less energy [58]. In particular, zirconium MOFs with carboxylate organic linkers and Zr6O4(OH)4(-CO2)n secondary building blocks showed highly intriguing water adsorption characteristics [59]. Over 80 adsorption-desorption cycles, MOF-841 demonstrated the best uptake of water and retained its structure [58]. These results led to the development and testing of MOF-801-based devices in the Arizona desert as a result of similar results observed in another zirconium MOF [60]. There are two parts for the device, with the inner box containing the MOF material and the outer box containing the lid. During the night, the MOF-801 was exposed to ambient air to hold the water molecules, and during the day, the lid is closed, allowing the device to be exposed to sunlight to regenerate. In a desert environment with temperatures ranging between 20 and 40 degrees and humidity levels between 5 and 40%, this device delivered 200–300 mL of water per kg of MOF/day.
2.3.3. Chemical Sorbent
There are two types of hygroscopic salt: anhydrous salts are a hydrothermal hydration reaction that occurs in solution because of the vapor pressure gradient that causes anhydrous salts to sorb water vapor [61,62]. Salt crystals dissolve in water when they have a high ability to sorb water [63]. As salt changes from a solid phase to a saturated solution, it is called deliquescence relative humidity (DRH). MgSO4 and CuCl2 are salts capable of transforming from solid phases to saturated solutions at high RH [64]. It is known that liquid salts such as LiCl and CaCl2 are more efficient at sorbing moisture in the solution phase, as well as being able to continue sorbing moisture even if their concentration in the solution is reduced. As a result of the difference in the concentration between a deliquescence salt solution and a liquid, water vapor molecules can be captured and transferred into the solution. Continuous water production can be achieved through this spontaneous mass transfer [61]. Liquid sorbents face challenges such as contamination, corrosion, and limited exposure to fresh air, especially in the case of spontaneous mass transfer [65].
The recently created MOFs are promising, according to the literature from Table 3, xbecause of their adaptability and flexible topologies, which allow them to capture water from the air at low relative humidity levels. Some recently created hygroscopic gels have also demonstrated tremendous promise for use in AWH systems. It was discovered that by choosing the right adsorbents, the time and space constraints for AWH, as well as the energy needs, may all be decreased. The adsorption-based AWH systems guarantee no bulky equipment, are more cost-effective, and are environmentally benign. As a result, this study provides thorough information on AWH using adsorbent materials.

Table 3.
Comparison of different types of sorption-based atmospheric water harvesting.
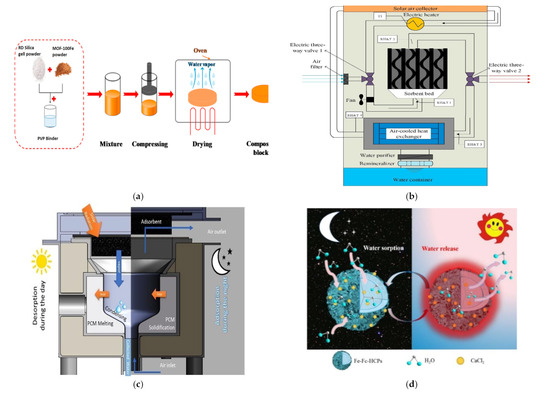
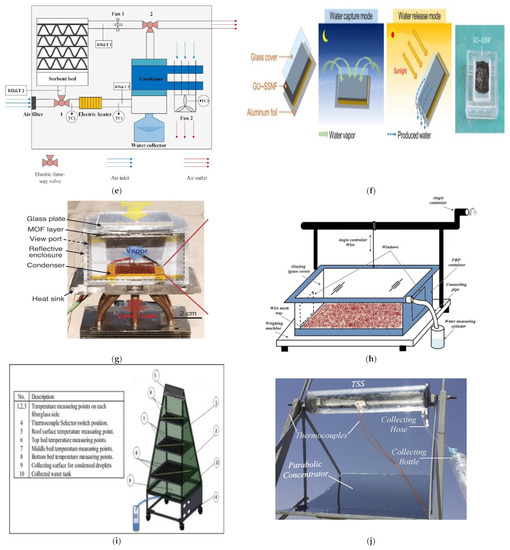

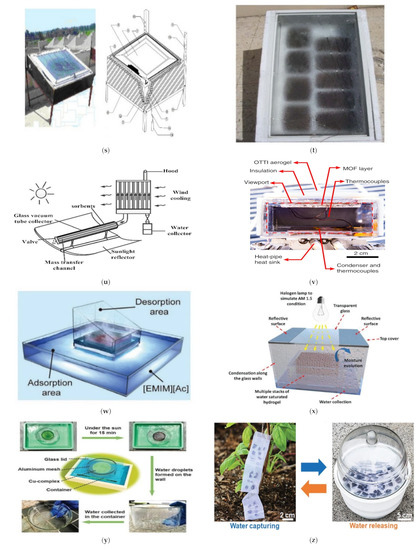
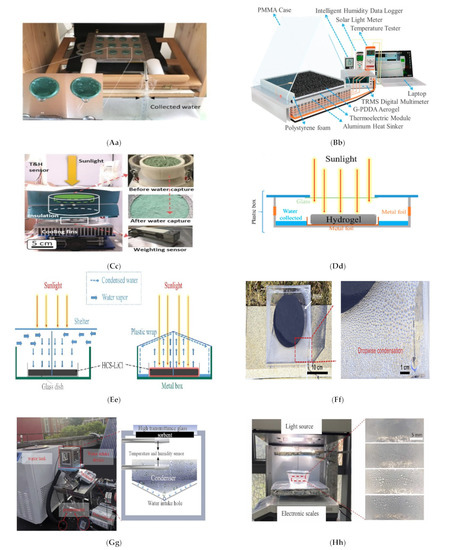
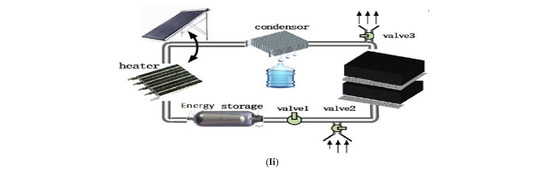
Figure 4.
(a) Heat-driven atmospheric water harvester [66]. (b) Air-cooled adsorption water harvesting system [69]. (c) Solar powered atmospheric water harvesting (LiCl/MgSO4/ACF) [70]. (d) Hollowmicrospheres for the harvesting of atmospheric water [74]. (e) Multicyclic sorption-based water harvesting system [72]. (f) Atmospheric water harvesting with nanofibrous membranes [73]. (g) Water harvesting powered by natural sunlight [75]. (h) Atmospheric water harvesting based on (CaCl2/Vermiculite/Saw wood) [79]. (i) Water production from humid air using solar energy [80]. (j) Atmospheric water harvesting powered by a parabolic concentrator system [82]. (k) Composite desiccant is used to generate water from atmospheric air [83]. (l) Water extraction from atmospheric air using a foldable solar-powered apparatus [84]. (m) Portable solar-powered equipment for desiccant-based water extraction from the atmosphere [85]. (n) A highly effective semi-open system for atmospheric freshwater generation [86]. (o) Using Manganese Dioxide to Harvest Water from the Atmosphere in Dry Regions [87]. (p) Water harvesting with nano sorbent [89]. (q) Three phase sorption air water harvesting [90]. (r) Atmospheric water harvesting (zwitterionic polymer hydrogel’s salting-in effect) [91]. (s) A solar collector with a sand bed to harvest water from the air [92]. (t) Utilizing Solar Energy to Recover Water from air in the Atmosphere [93]. (u) Fresh water extraction from the atmosphere using two solar driven sorption devices [94]. (v) For arid climates, an atmospheric water harvesting device based on adsorption [95]. (w) An Interfacial Solar Driven Atmospheric Water Generator [115]. (x) Hydrology of a Hydrogel with Ultrahigh Hygroscopicity, facilitated by solar energy, for the harvesting of clean water from the humidity above the surface of the sea [97]. (y) The Copper Complex for Autonomous Urban Agriculture and the Harvesting of Air Moisture [98]. Different types of sorption-based atmospheric water harvesting. (z) Atmospheric water harvesting with super-absorbent gels [101]. (Aa) MOF matrix with autonomous atmospheric water seepage [102]. (Bb) Using a hygroscopic aerogel and fast sorption kinetics to generate atmospheric water and electricity simultaneously [103]. (Cc) The use of hygroscopic salts as water harvesting agent in the arid air [104]. (Dd) An atmospheric water generator using a hybrid hydrogel with high water vapor harvesting capability [106]. (Ee) Enables multiple water collection cycles with nano sorbents [89]. (Ff) An efficient atmospheric water harvesting method based on deliquescent sorbations with heterogeneous wettability and radiative cooling [110]. (Gg) Collecting water in large quantities and efficiently using a honeycomb-shaped polymer that absorbs moisture, powered solely by sunlight [111]. (Hh) Designing a highly efficient and cost-effective system for collecting atmospheric water based on the natural patterns of a superabsorbent material [112]. (Ii) An expandable and efficient method for collecting water from the atmosphere using the process of absorption [113].
Table 3.
Comparison of different types of sorption-based atmospheric water harvesting.
| S No. | Composite Material | Conditions | Production | Literature |
|---|---|---|---|---|
| 1 | Silica gel-MIL 100(Fe) (Figure 4a) | Temperature 70 and 50 °C | - | [66] |
| 2 | MIL-100(Fe) | Temperature 423 K | 86.8 L/day. | [67] |
| 3 | P-SG and P-SG-L | Temperature 40 degree RH 10% | 1.70 g/g | [68] |
| 4 | carbon fibre felt (ACFF)–silica sol–LiCl3 (Figure 4b) | Temperature 31 degree RH 63% | 7.7 kg | [69] |
| 5 | MgSO4 and LiCl (Figure 4c) | RH 35% | 0.92 g/water/adsorbent | [70] |
| 6 | CaCl2 nanocrystals (Figure 4d) | 80% RH, 15 °C | 2.685 gH2O/gCaCl2 | [71] |
| 7 | ASLi30 (Figure 4e) | Temperature 51 degree | 0.42 kgwater/kg adsorbent | [72] |
| 8 | GO–SSNF (Figure 4f) | RH 30% | 0.96 g/g | [73] |
| 9 | MIL-125(Ti)_NH2 | Temperature range between 283–323 K | 320 L/day/ton. | [74] |
| 10 | Zr6O4(OH)4(fumarate)6 (Figure 4g) | RH 20% | 2.8 L | [75] |
| 11 | Cr-soc-MOF-1 | RH 70% | 1.95 g/g | [76] |
| 12 | Aluminum Fumarate | Temperature 25% | 0.33 g/g | [77] |
| 13 | AQSOA-Z01 zeolites | Temperature 298 K and 333 K | 0.23 g/g | [78] |
| 14 | AlPO4-LTA | Temperature 10 to 15 degree | 0.37 g/g | [57] |
| 15 | Calciumchloride + vermiculite- saw wood (Figure 4h) | - | 195 mL/kg/day | [79] |
| 16 | Calcium chloride and cloth (Figure 4i) | - | 3.02 L/day/m2 | [80] |
| 17 | Calcium chloride and black cloth | 12%RH | Minimum 230 mL/m2/day | [81] |
| 18 | 30% Calcium chloride and black cloth (Figure 4j) | RH 16% | 0.51 L/kg | [82] |
| 19 | LITHIUM BROMIDE ANHYDROUS (LiBr) (Figure 4k) | - | 73 mL/day | [83] |
| 20 | Calcium chloride and black cloth (Figure 4l) | - | 272–750 g/day | [84] |
| 21 | Calcium chloride and cloth (Figure 4m) | - | 0.3295 kg/m2/day to 0.6310 kg/m2/day | [85] |
| 22 | carbon felt + LiCl (Figure 4n) | 85% | 14.7 kg | [86] |
| 23 | Layered structure MnO2 (Figure 4o) | RH 23% Dew point temperature 11 °C. | 0.07 kgwater/kgsorbant | [87] |
| 24 | Functionalized carbon nanotube (FCNT) | RH 40% | 5.6 gwater/gsorbent | [88] |
| 25 | LiCl is contained inside a hollow nanocarbon capsule’s void core. (Figure 4p) | RH 60% | 1.6 kgwater/kgsorbant | [89] |
| 26 | ACF (LiCl, CaCl2, and LiNO3) (Figure 4q) | Temperature 25 °C RH 20% | 1.18 gwater/gsorbant-LiCl | [90] |
| 28 | PDMAPS (Figure 4r) | Temperature 65 °C | 28 g | [91] |
| 29 | Alginate-chained hydrogel that has been altered using binary salts | - | 5.6 g | [88] |
| 30 | pyramidal solar still for CaCl2 (4-shelves) (Figure 4s) | Temperature 15 degree | 2-1 L | [92] |
| 31 | CaCl2 single-basin solar still (Figure 4t) | RH 40% | 1.0 L/m2/day | [93] |
| 32 | CaCl2 ACF sorbent (Figure 4u) | 70–80 °C | 0.32 kg water, 2.25 kgACFCA, and a surface area of 0.77 m2 | [94] |
| 33 | [ZrO4(OH)2(fumarate)6] air-cooled sorbent-based device- (MOF)-801(Figure 4v) | RH 40% | 0.25 L | [95] |
| 34 | Adsorption-Desorption in Liquid Sorbent (Figure 4w) | RH 80% | 2.8 L/m2/day | [57] |
| 35 | ALPO4-LTA | 15% RH | 0.37 g/g | [57] |
| 36 | COF-432 | At 30 °C (40% relative humidity, RH) and 35 °C (30% RH), respectively, adsorption and desorption are carried out. | 0.33 g/g | [96] |
| 37 | Cr-soc-MOF-1 | T 25 °C, 70% RH | 1.95 g/g | [76] |
| 38 | A hydrogel that is super hygroscopic (Figure 4x) | T 25 °C, 90% RH | 4.20 g/g | [97] |
| 39 | Cu (II)-ethanolamine hydrogel (Figure 4y) | T 25 °C, 30% RH | 0.14 g/g | [98] |
| 40 | Co-SHM | T 25 °C, 30% RH | 0.11 g/g | [99] |
| 41 | IPN- interpenetrating polymer network | T 25 °C, 30% RH | 0.18 g/g | [100] |
| 42 | SMAG (Figure 4z) | T 25 °C, 30% RH | 0.70 g/g | [101] |
| 43 | PC-MOF (Figure 4Aa) | T 25 °C, 30% RH | 0.76 g/g | [102] |
| 44 | G-PDDA (Figure 4Bb) | T 25 °C, 30% RH | 0.13 g/g | [103] |
| 45 | LiCl@MIL-101(Cr)_51 (Figure 4Cc) | T 30 °C, 30% RH | 0.82 g/g | [104] |
| 46 | LiCl@HS-200 | T 25 °C, 30% RH | 0.76 g/g | [105] |
| 47 | PAM-CNT-CaCl2 (Figure 4Dd) | T 25 °C, 35% RH | 0.69 g/g | [106] |
| 48 | HCS-LiCl (Figure 4Ee) | T 25 °C, 35% RH | 0.69 g/g | [89] |
| 49 | Alg-CaCl | T 28 °C, 33% RH | 1.18 g/g | [48,49] |
| 50 | ACFP30 | T 25 °C, 70% RH | 1.62 g/g | [107] |
| 51 | saturated LiBr solution | T 25 °C, 30% RH | 0.34 g/g | [108] |
| 52 | saturated LiCl solution | T 25 °C, 60% RH | 1.79 g/g | [105] |
| 53 | [Pyrrol][Ac] [DEA][Fo] [DEA][Ac] | T 29 °C, 57% RH T 29 °C, 57% RH T 29 °C, 57% RH | 0.45 g/g 0.4945 g/g 0.6545 g/g | [109] |
| 54 | Effective atmospheric water collection with deliquescent sorbents requires heterogeneous wettability and radiative cooling. (Figure 4Ff) | 60% RH | 2.62 g/g/day | [110] |
| 55 | PCLG (Figure 4Gg) | RH 55% | 2.9 L/m2 | [111] |
| 56 | ILCA (Figure 4Hh) | 23 °C and 50% RH | 2.791 g | [112] |
| 57 | multicyclic sorption | T 51 °C | 0.42 kgwater/kgsorbant | [72] |
| 58 | Three-phase sorption | RH 70% | 3.18 g | [90] |
| 59 | CoCl2 (BTDD) | RH 30% | 0.82 gH2O/gMOF | [54,55] |
| 60 | Universal scalable sorption-base (Figure 4Ii) | RH 75% | 38.5 kg/day | [113] |
| 61 | Hygroscopic nanostructured biopolymer aerogels | T 26.8–36.3 °C | 88.3 g | [114] |
2.4. Condensation Based
In most cases, condensation technologies are used. Condensation-based AWH is a refrigerant-based system. Through a condenser, the refrigerant is circulated by using a compressor. Water starts condensing when the temperature reaches the dew point. The coil is blown over by filtered air. Purification systems ensure that water is clean and free from the microorganisms found in the environment [116]. The capacity to cool the coil, ambient temperature, humidity, and the volume of air passing over it determines how fast it produces water. The performance of atmospheric water harvesting is more efficient when the relative humidity and air temperature are high. If the ambient temperature or relative humidity falls below 18.3 °C (65 °F), cooling condensation AWGs will not function efficiently. The main limitation is that it requires a lot of energy for working the compressor. The Water Harvest Rate (WHR) is the weight per gram of water harvested per hour and it represents the power consumption per unit mass of water harvested, expressed in kg/h. To harvest the same amount of water more quickly, a higher WHR and lower UPC are desirable.
2.4.1. Condensation-Based VCC (Vapor Compression Cycle) Technologies
VCC technologies are used for water harvesting, as well as for air conditioning. Several studies show that VCC technology is used for water harvesting [117,118]. Normally, condensed water is produced through an air conditioning system, and it is used as a by-product. An atmospheric water harvesting system was proposed by Luo et al. by using VCC technology for military purposes. Evaporators are used to cool and dehumidify the ambient air, while the condenser is used despite the heat from the dehumidifier. In the above system, when the ambient temperature is 26.7 degrees and 19.4 °C for wet bulbs, respectively, the water harvesting rate is 1.50 kg/h [119]. A device with two parallel evaporators was proposed by Zhang et al. and Liu. The main intention behind this study is to evaluate the performance of the heat transfer area under the humid climate of Tianjin, which lies on the northeast coast of China; the temperature, water harvesting rate, and energy consumption of evaporators were tested. The results show that the amount of water harvested changes during dry months in which the average water production is 1.50 to 4.20 kg/h, and it varies in dry weather, between 50 to 1.80 kg/h. Water harvesting with VCC is more effective with higher humidity ratios in the air [120].
2.4.2. Condensation Technologies Using Thermo-Electric Cooler (TEC)
In contrast to VCC, a Thermo-Electric Cooler (TEC) utilizes the direct current to achieve refrigeration at the cold end by utilizing the Peltier effect. As a result of the hot end dissipating heat, it is cooled. Semiconductors range between 130 °C and 90 °C in temperature [121]. Water can be harvested from the air using this method. In comparison to VCC systems, semiconductor chips have a small volume, making them more portable and enabling the water harvesting devices to be smaller. TEC-powered devices have been investigated for military use in remote areas with abundant solar radiation and scarce water by Zhang et al. For water harvesting from the air, TECs are continuously powered by solar panels, which generate electricity that is stored in batteries [122]. TEC processes were studied experimentally and numerically by Cao et al. In this study, TEC1-12706 was selected, the working temperature of which ranged between 50 and 80 °C. The voltage and current maximums were 15.2 V and 6 A, with a maximum cooling capacity of 56.5 W and a maximum heating capacity of 92.4 W. A prototype consisting of 127 pairs of TEC was used for the study. According to the results, the temperature cools quicker due to the heat dissipating fans and the large current. The fan can lower the cooling temperature from 20.5 to 2 within 5 min by using a current of 2.5 A and a voltage of 7.5. When the current and voltage are reduced to 1.5 A and 5 V, within 5 min, the temperature will reduce from 19.5 °C to 8 °C. The amount of water harvested was 7 × 10−3 kg/h under the condition of 25 °C temperature and 60% RH [123]. Table 4 shows the different types of condensation-based water harvesting systems and their production.

Table 4.
Comparison of various types of fog water harvesting.
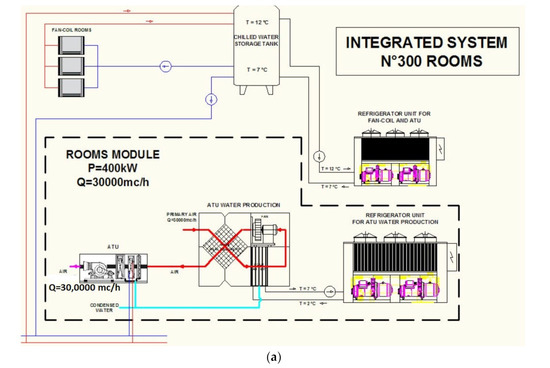
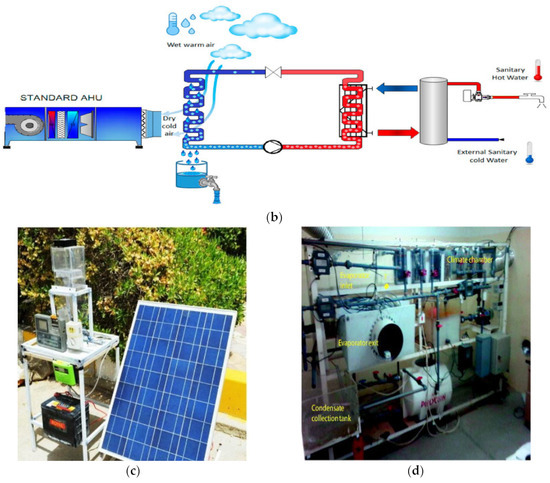
Figure 5.
Various types of fog water harvesting. (a) Combining air conditioning and drinking water production into a single integrated system [124]. (b) Collecting water from the air using refrigeration techniques [127]. (c) Harvesting water from the atmosphere using solar energy and the Peltier effect [129]. (d) Extracting water from the condensation produced by vapor compression systems in hot and humid regions as a source of water [117].
Table 4.
Comparison of various types of fog water harvesting.
| SN | System Information | Condition | Production | Reference |
|---|---|---|---|---|
| 1 | The rated air flow rate is 578 m3 h−1, and the VCC-Rated compressor power is 1035 W. | dry bulb wet 26.7 °C wet bulb temperature: 19.4 °C | 1.5 kg/h | [124] |
| 2 | VCC- Uses R22, weighs 39 kg, and has a rated compressor output of 370 W in addition to 1.5 kW of rated cooling capacity. | ambient condition: 35 °C 20–40% RH | 0.13 kg/h | [125] |
| 3 | VCC-AFR ranges between 300 and 1000 m3 per hour. | - | 1.5 kg/h to 4.2 kg/h. | [118] |
| 4 | VCC | Velocity 2–5 ms−1 | 22-26 L/day | [120] |
| 5 | HVAC system (Figure 5a) | RH = 60%, Temperature= 35 °C | 425 L/h | [124,126] |
| 6 | HVAC | Temperature = 25~50 °C, RH = 15~90%, | 2.33 L/h | [117] |
| 7 | HVAC condensate recovery (Figure 5b) | T = 27.3 °C RH = 86% | 78.3 L/h | [127] |
| 8 | TEC (portable water generator) | T = 22 °C, RH = 100% | 24 mL/h | [128] |
| 9 | TEC (Figure 5c) | T = 24.29 °C, RH = 67.8%, | 20 mL/h | [129] |
| 10 | TEC (solar energy is used to power water collection) | T = 24~31 °C, RH = 60~80%, | 20 mL/h | [130] |
| 11 | TEC (thermoelectric dehumidifier system for air ducts) | T = 35 °C, RH = 60–90% | 0.82 L/h | [131] |
| 12 | VCC-Frontal AFR is around 650 m3 h−1 and area is 0.04 m2 (Figure 5d) | - | 0.92 kg/h to 1.08 kg/h. | [117] |
As shown in Table 4, the main characteristics of the papers are summarized in terms of the atmospheric condition and performance. In dry air, condensation-based systems are inefficient, a lot of electricity is required for the system to run, and the production rate depends on the outdoor conditions.
3. Performance Assessment Indicators
It is evident that the cold channel temperature is the same as that on the cold side of the TEC. Cold and hot airflows were considered to be one-dimensional. In the hot channel, the outlet’s moist air has the following temperature:
- temperature of the cooling coil.
- temperature of the air flow into the cooling coil.
- heat transfer coefficient for dry air.
- A surface area of the cooling coil.
- outdoor dry bulb temperature.
- m mass flow rate of the air through the cooling coil.
- Cp specific heat capacity of the air.
Using the heat transfer coefficient in the cold channel requires the temperature of the inlet air (°C), the temperature of the output air (°C), the temperature of the cold side of the TEC (°C), and (w/m2/K), where A is the cooling area (m2), mass flow rate is (kg/s), and volumetric heat capacity is (J/kg·K). In the hot channel, the heat transfer coefficient is:
The flow in the tubes, according to Gnielinski’s correlation, are:
According to the Petukhovs correlation with the moody chart, f is the Darcy friction factor [121]. The hydraulic diameter is Dh (m). The heat transmission coefficient in the cold channel is denoted hdry (w/m2/K), K represents the air’s thermal conductivity coefficient, the Nusselt number is Nu, the Prandtl number is Pr, and the Reynolds number is Re. Re is used to recognize and forecast the various laminar or turbulent flow regimes. Where Re = 3000 to 5 × 106 Equation (5) [132].
where (in J/s) Qc represents the heat transported from the cold side, the volumetric heat capacity (CP) (J·kg−1 K1), Tin in is the air’s inlet temperature (°C). The outflow air’s temperature is known as Tout (°C). As soon as the air temperature hits the dew point, the heat transfer coefficient and quantity of the exchanged energy in the cold channel will change:
where hwet is the wet air enthalpy (J/s), the enthalpy of the dry air is hdry (J/s) and the water vapor’s specific heat is C (kJ/kgC).
There is an inlet air enthalpy named hin (J/s), an outlet air enthalpy named hout (J/s), and a saturated air enthalpy named hsurface (J/s).
where Tamb is the temperature of the inlet air in the hot channel and Qc is the heat transfer rate from the cold side (J/s) (C). The inlet air temperature is Th, heat is transferred from the hot side by Qh (J/s), where hot air (hh) represents the enthalpy of the hot air, and saturated water (hf) represents the enthalpy of the saturated water at the outlet temperature (C)
stands for the amount of water produced, for the intake air’s absolute humidity, for the output air’s absolute humidity, and t for the passage of time (s).
4. Parameters of Atmospheric Water Vapor
For the atmosphere to be used as a source of water, certain properties are essential:
- ➢
- Relative humidity (Φ)
- ➢
- Absolute humidity (ω)
- ➢
- Dew-point temperature (Td)
Air is a gas mixture, composed of nitrogen, oxygen, and argon gas as its major components (in this order), as well as water vapor in variable contents. The relative humidity measures the amount of moisture present in the atmosphere compared to the saturation process [133].
- Φ is the relative humidity.
- Pw is the partial pressure of water vapor in the air.
- Ps is the saturation vapor pressure of water.
The dewpoint (Td) is the temperature at the onset of condensation and at which the relative humidity reaches 100%. The water content in the air is determined by the RH and T, which is denoted as the absolute humidity (ω). The maximum amount of water that can be extracted is the absolute humidity. In Figure 6, the relationship between the absolute humidity and temperature is plotted at different RH. Under 100% RH, the upper curve represents saturation, where condensation occurs. During cooling or humidification, unsaturated air can become saturated [133].
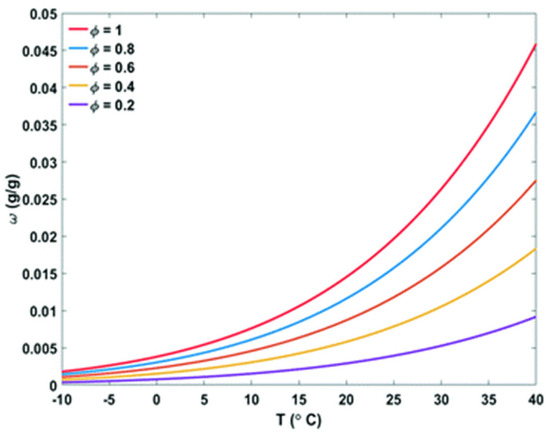
Figure 6.
Relative and absolute humidity in the air depends on temperature (T). Saturated air is created by either reducing the temperature or raising the absolute humidity level in unsaturated air [134].
The mathematical relation between the relative humidity (Φ), absolute humidity (ω), total air pressure (P), and temperature (T) is given in equation ii. This equation can be solved for T for a given absolute humidity and air pressure and can be used to calculate the dew-point Td [135].
The empirical AERK Magnus formulation can be used mathematically to describe the saturation pressure of the water vapor Ps at temperature T (°C) [135].
(Example in the range of T = −40 °C to 50 °C) by using equation iii
In Equation (4), the total air pressure P can be calculated using the partial pressures of the dry air Pa and water vapor pressure Pw [136]. For example, Pa can be 100,000 Pa.
P = Pa + Pw
Air enthalpy consists of three terms:
- Dry air—Ha
- Water vapor—Hwv
- Heat capacity—Cp,a
In the temperature range [10 °C, 50 °C], it is safe to assume that the heat capacity of the air Cp,a is 1.005 kJ/kgC. Water vapor has a heat capacity of 1.82 kJ/kgC, on average, between the temperatures of 10 °C and 50 °C, and its enthalpy is 2500.9 kJ/kg at 0 °C [136].
H = Ha + ωHwv
Ha = Cp,aT
Hwv = Hwv(0 °C) + Cp,wvT
At an atmospheric pressure and normal boiling temperature (100 °C), the heat of the condensation of the water (ΔHvap) is equal to 2257 kJ/kg, which is a significant amount of energy. To allow for the phase shift of water from vapor to liquid, this significant quantity of energy must be eliminated [137].
5. Conclusions
Atmospheric water harvesting has great potential to address water scarcity in arid and semi-arid regions. There have been various experimental studies, laboratory studies, and theoretical research conducted in the field of AWH on the basis of the technologies, materials, and climatic conditions. This review has described various technologies and their climatic condition and production rate. Some are more suitable for specific situations than others, and all have advantages and disadvantages. Fog harvesting systems are relatively simple and inexpensive to construct, as the necessary materials can usually be found in the local environment. Unfortunately, fog only occurs in certain areas with low rainfall, so it can only provide a limited solution to water shortages. Dew water harvesting is an environmentally friendly and sustainable source of water, as it does not require the use of energy or chemicals. However, it is limited by the amount of dew that forms on surfaces and the size of the collection area. Therefore, dew harvesting can only be used in certain areas, where the conditions are right for dew to form. Despite this limitation, dew harvesting is still a viable option for obtaining water in areas where it is available. Condensation Atmospheric Water Harvesting is a technology that can provide a reliable source of clean water, especially in areas where other water sources are contaminated or inaccessible. This technology can be scaled up or down to suit different environments and community needs. The amount of water that can be collected through Condensation Atmospheric Water Harvesting is limited by the surface area of the collection system and the amount of humidity in the air. Therefore, it is important to consider the local environmental conditions when planning a Condensation Atmospheric Water Harvesting system. With the right design, Condensation Atmospheric Water Harvesting can be a useful tool for providing clean water to communities in need. From the above studies, it is clear that most of the technologies mainly depend on the outdoor conditions and rely on the humidity level. However, there are other atmospheric water harvesting technologies, such as dew, that can be used to produce water even in areas with low humidity but low production. Based on the current knowledge, it seems that atmospheric water harvesting could be a promising approach to increasing access to water in areas with limited water resources. While atmospheric water harvesting shows promise as a solution to water scarcity, its feasibility and effectiveness in outdoor conditions with varying humidity levels still require further research. To improve our understanding of this approach, studies can focus on optimizing the design of atmospheric water harvesting systems to maximize the water collection under different humidity and temperature conditions. Additionally, research can explore the potential impacts of atmospheric water harvesting on local ecosystems and the availability of water resources for other uses. Further investigation is necessary to fully assess the viability of atmospheric water harvesting as a sustainable water management strategy.
Funding
The authors would like to thank Northumbria University UK for funding under reference (POC Solar2Water grant), RDF20/EE/MCE/SHAHZAD, and Northern Accelerator Proof-of-Concept award for AD4DCs (NACCF-232) Awarded to Muhammad Wakil Shahzad.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AWH | Atmospheric water harvesting |
| HVAC | heating, ventilation, and air conditioning |
| MOF | metal organic frame |
| SVG | Solar vapor generators |
| TEC | Thermo-Electric Cooler |
| UNCCD | United Nations Convention to Combat Desertification |
| UN | United Nations |
| VCC | vapor compression cycle |
| WHR | water harvesting rate |
| Subscripts | |
| temperature of the cooling coil | |
| temperature of air flow into the cooling coil | |
| heat transfer coefficient for dry air | |
| A | surface area of the cooling coil |
| outdoor dry bulb temperature | |
| m | mass flow rate of air through the cooling coil |
| Cp | specific heat capacity of air |
| Qc | heat transported from the cold side |
| Tin | air’s inlet temperature (C) |
| Tout | outflow air’s temperature |
| hwet | enthalpy of moist air |
| ℎDry | enthalpy of dry air |
| C | specific humidity ratio of the air |
| hsurface | Enthalpy of the surface of the evaporator (J/kg) |
| hert | Enthalpy of the refrigerant flow into the evaporator (J/kg) |
| hin | Enthalpy of the refrigerant entering the condenser (J/kg) |
| hwet | Enthalpy of the air at the evaporator inlet (J/kg) |
| A | Area of the heat transfer surface in m2 |
| ṁ | Mass flow rate of air (kg/s) |
| Cp | Specific heat capacity of air at constant pressure (J/(kg.K)) |
| e | Effectiveness of the heat exchanger |
| Qc | Cooling capacity in watts (W) |
| ṁ air | Mass flow rate of air in kilograms per second (kg/s) |
| hin | Enthalpy of the air entering the cooling coil in joules per kilogram (J/kg) |
| hout | Enthalpy of the air leaving the cooling coil in joules per kilogram (J/kg) |
| win | Humidity ratio of the air entering the cooling coil in kilograms of water vapor per kilogram of dry air (kg/kg) |
| wout | Humidity ratio of the air leaving the cooling coil in kilograms of water vapor per kilogram of dry air (kg/kg) |
| Hf | Latent heat of vaporization of water at the coil temperature in joules per kilogram (J/kg) |
| Qh | Heating capacity in watts (W) |
| hh | Enthalpy of the hot fluidin joules per kilogram (J/kg) |
| Th | Temperature of the hot fluid (°C) |
| Tamb | Ambient temperature (°C) |
| amount of water produced | |
| intake air’s absolute humidity | |
| output air’s absolute humidity, and t for the passage of time (s) | |
| Φ | Relative humidity |
| ω | Absolute humidity |
| Td | Dew-point temperature |
| Pw | partial pressure of water vapor in the air |
| Ps | saturation vapor pressure of water |
| P | total air pressure |
References
- Salehi, M. Global Water Shortage and Potable Water Safety; Today’s Concern and Tomorrow’s Crisis. Env. Int. 2022, 158, 106936. [Google Scholar] [CrossRef] [PubMed]
- Lund Schlamovitz, J.; Becker, P. Differentiated Vulnerabilities and Capacities for Adaptation to Water Shortage in Gaborone, Botswana. Int. J. Water Resour. Dev. 2020, 37, 278–299. [Google Scholar] [CrossRef]
- Water Facts—Worldwide Water Supply. ARWEC|CCAO|Area Offices|California-Great Basin|Bureau of Reclamation. Available online: https://www.usbr.gov/mp/arwec/water-facts-ww-water-sup.html (accessed on 27 September 2022).
- Al-Shalabi, H.; Andraws, S.; Alrabea, A.I.; Kumar, A.V.S. V Model of E-Learning Using Gagne Nine Steps of Education. J. Softw. Eng. Appl. 2012, 5, 850–854. [Google Scholar] [CrossRef]
- Progress on Household Drinking Water, Sanitation and Hygiene, 2000–2017—UNICEF DATA. Available online: https://data.unicef.org/resources/progress-drinking-water-sanitation-hygiene-2019/ (accessed on 4 May 2022).
- Impacts of Water Scarcity on the Social Welfare of Citizens in the Middle East. Middle East Institute. Available online: https://www.mei.edu/publications/impacts-water-scarcity-social-welfare-citizens-middle-east (accessed on 10 May 2022).
- UNCCD. Desertification the Invisible Frontline; UNCCD: Bonn, Germany, 2014. [Google Scholar]
- Zhu, X.; Zhang, C.; Yin, J.; Zhou, H. Optimization of Water Diversion Based on Reservoir Operating Rules: Analysis of the Biliu River Reservoir, China. J. Hydrol. Eng. 2014, 19, 411–421. [Google Scholar] [CrossRef]
- Al-Sahlawi, M.A. Seawater Desalination in Saudi Arabia: Economic Review and Demand Projections. In Desalination; Elsevier: Amsterdam, The Netherlands, 1999; Volume 123, pp. 143–147. [Google Scholar]
- Rodriguez, C.A.; Flessa, K.W.; Dettman, D.L. Effects of Upstream Diversion of Colorado River Water on the Estuarine Bivalve Mollusc Mulinia Coloradoensis. Conserv. Biol. 2001, 15, 249–258. [Google Scholar] [CrossRef]
- Micale, G.; Cipollina, A.; Rizzuti, L. Seawater Desalination for Freshwater Production. In Green Energy and Technology; Springer Science and Business Media Deutschland GmbH: Berlin/Heidelberg, Germany, 2009; pp. 1–15. [Google Scholar]
- Karagiannis, I.C.; Soldatos, P.G. Water Desalination Cost Literature: Review and Assessment. Desalination 2008, 223, 448–456. [Google Scholar] [CrossRef]
- Wahlgren, R.V. Atmospheric Water Vapour Processor Designs for Potable Water Production: A Review. Water Res. 2001, 35, 101–108. [Google Scholar] [CrossRef]
- Hao, L.; Liu, N.; Bai, H.; He, P.; Niu, R.; Gong, J. High-Performance Solar-Driven Interfacial Evaporation through Molecular Design of Antibacterial, Biomass-Derived Hydrogels. J. Colloid Interface Sci. 2022, 608, 840–852. [Google Scholar] [CrossRef]
- Li, R.; Wu, M.; Shi, Y.; Aleid, S.; Wang, W.; Zhang, C.; Wang, P. Hybrid Water Vapor Sorbent Design with Pollution Shielding Properties: Extracting Clean Water from Polluted Bulk Water Sources. J. Mater. Chem. A Mater. 2021, 9, 14731–14740. [Google Scholar] [CrossRef]
- Sultan Irshad, M.; Wang, X.; Sehar Abbasi, M.; Arshad, N.; Chen, Z.; Guo, Z.; Yu, L.; Qian, J.; You, J.; Mei, T. Semiconductive, Flexible MnO2 NWs/Chitosan Hydrogels for Efficient Solar Steam Generation. ACS Publ. 2021, 9, 3887–3900. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A Multi-Structural and Multi-Functional Integrated Fog Collection System in Cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef] [PubMed]
- Gido, B.; Friedler, E.; Broday, D.M. Assessment of Atmospheric Moisture Harvesting by Direct Cooling. Atmos Res. 2016, 182, 156–162. [Google Scholar] [CrossRef]
- Bintein, P.B.; Lhuissier, H.; Mongruel, A.; Royon, L.; Beysens, D. Grooves Accelerate Dew Shedding. Phys. Rev. Lett. 2019, 122, 098005. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.Y.; Liu, Y.; Jiang, H.B.; Li, S.Y.; Kaya, C.; Stegmaier, T.; Han, Z.W.; Ren, L.Q. A Bioinspired Structured Graphene Surface with Tunable Wetting and High Wearable Properties for Efficient Fog Collection. Nanoscale 2018, 10, 16127–16137. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M. Research UC Berkeley. Available online: https://vcresearch.berkeley.edu/faculty/omar-m-yaghi (accessed on 29 September 2022).
- Gerasopoulos, K.; Luedeman, W.L.; Ölçeroglu, E.; McCarthy, M.; Benkoski, J.J. Effects of Engineered Wettability on the Efficiency of Dew Collection. ACS Appl. Mater. Interfaces 2018, 10, 4066–4076. [Google Scholar] [CrossRef]
- Water Abundance XPRIZE | XPRIZE Foundation. Available online: https://www.xprize.org/prizes/water-abundance (accessed on 5 September 2022).
- Watergen|Water from Air. Available online: https://www.watergen.com/ (accessed on 5 September 2022).
- Khalil, B.; Adamowski, J.; Shabbir, A.; Jang, C.; Rojas, M.; Reilly, K.; Ozga-Zielinski, B. A Review: Dew Water Collection from Radiative Passive Collectors to Recent Developments of Active Collectors. Sustain. Water Resour. Manag. 2016, 2, 71–86. [Google Scholar] [CrossRef]
- LaPotin, A.; Kim, H.; Rao, S.R.; Wang, E.N. Adsorption-Based Atmospheric Water Harvesting: Impact of Material and Component Properties on System-Level Performance. Acc. Chem. Res. 2019, 52, 1588–1597. [Google Scholar] [CrossRef]
- Sharan, G.; Roy, A.K.; Royon, L.; Mongruel, A.; Beysens, D. Dew Plant for Bottling Water. J. Clean Prod. 2017, 155, 83–92. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, L.; Wang, P.; Jin, Y.; Wang, P.; Zhang, L. Atmospheric Water Harvesting: Role of Surface Wettability and Edge Effect. Glob. Chall. 2017, 1, 1700019. [Google Scholar] [CrossRef]
- Hou, K.; Li, X.; Li, Q.; Chen, X. Tunable Wetting Patterns on Superhydrophilic/Superhydrophobic Hybrid Surfaces for Enhanced Dew-Harvesting Efficacy. Adv. Mater. Interfaces 2020, 7, 1901683. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Fu, B.; Song, C.; Shang, W.; Tao, P.; Deng, T. All-Day Freshwater Harvesting through Combined Solar-Driven Interfacial Desalination and Passive Radiative Cooling. ACS Appl. Mater. Interfaces 2020, 12, 47612–47622. [Google Scholar] [CrossRef] [PubMed]
- Trosseille, J.; Mongruel, A.; Royon, L.; Medici, M.G.; Beysens, D. Roughness-Enhanced Collection of Condensed Droplets. Eur. Phys. J. 2019, 42, 144. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Trosseille, J.; Mongruel, A.; Marty, F.; Basset, P.; Laurent, J.; Royon, L.; Cui, T.; Beysens, D.; Bourouina, T. Tailoring Silicon for Dew Water Harvesting Panels. iScience 2021, 24, 102814. [Google Scholar] [CrossRef]
- Gultepe, I.; Tardif, R.; Michaelides, S.C.; Cermak, J.; Bott, A.; Bendix, J.; Müller, M.D.; Pagowski, M.; Hansen, B.; Ellrod, G.; et al. Fog Research: A Review of Past Achievements and Future Perspectives. Pure Appl. Geophys. 2007, 164, 1121–1159. [Google Scholar] [CrossRef]
- Klemm, O.; Schemenauer, R.S.; Lummerich, A.; Cereceda, P.; Marzol, V.; Corell, D.; van Heerden, J.; Reinhard, D.; Gherezghiher, T.; Olivier, J.; et al. Fog as a Fresh-Water Resource: Overview and Perspectives. Ambio 2012, 41, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Batisha, A.F. Feasibility and Sustainability of Fog Harvesting. Sustain. Water Qual. Ecol. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Montecinos, S.; Carvajal, D.; Cereceda, P.; Concha, M. Collection Efficiency of Fog Events. Atmos. Res. 2018, 209, 163–169. [Google Scholar] [CrossRef]
- Abdul-Wahab, S.A.; Al-Hinai, H.; Al-Najar, K.A.; Al-Kalbani, M.S. Feasibility of Fog Water Collection: A Case Study from Oman. J. Water Supply Res. Technol. Aqua 2007, 56, 275–280. [Google Scholar] [CrossRef]
- Schemenauer, R.S.; Cereceda, P.; Osses, P. The complementary aspects of projects to collect rain, fog and dew. In Proceedings of the XI th International Conference on Rainwater Catchment System, Mexico City, Mexico, 25–29 August 2003. [Google Scholar]
- Tu, Y.; Wang, R.; Zhang, Y.; Wang, J. Progress and Expectation of Atmospheric Water Harvesting. Joule 2018, 2, 1452–1475. [Google Scholar] [CrossRef]
- Hu, Y.; Fang, Z.; Wan, X.; Ma, X.; Wang, Y.; Dong, M.; Ye, Z.; Peng, X. Ferrocene Dicarboxylic Acid Ligand-Exchanged Hollow MIL-101(Cr) Nanospheres for Solar-Driven Atmospheric Water Harvesting. ACS Sustain. Chem. Eng. 2022, 10, 6446–6455. [Google Scholar] [CrossRef]
- Marzol Jaén, M.V. Fog Water Collection in a Rural Park in the Canary Islands (Spain). Atmos. Res. 2002, 64, 239–250. [Google Scholar] [CrossRef]
- The Fog Harvesters of Lima, Peru–NaturPhilosophie. Available online: https://www.naturphilosophie.co.uk/fog-harvesters-lima-peru/ (accessed on 4 July 2022).
- Larrain, H.; Velásquez, F.; Cereceda, P.; Espejo, R.; Pinto, R.; Osses, P.; Schemenauer, R.S. Fog Measurements at the Site “Falda Verde” North of Chañaral Compared with Other Fog Stations of Chile. Atmos. Res. 2002, 64, 273–284. [Google Scholar] [CrossRef]
- Latest News. FogQuest: Sustainable Water Solutions. Fog Collection|Rainwater Collection|Rural Water Projects. Available online: http://www.fogquest.org/latest-news/ (accessed on 21 September 2022).
- Butt, H.-J.; Graf, K.; Kappl, M. Physics and Chemistry of Interfaces; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Shan, H.; Pan, Q.; Xiang, C.; Poredoš, P.; Ma, Q.; Ye, Z.; Hou, G.; Wang, R. High-Yield Solar-Driven Atmospheric Water Harvesting with Ultra-High Salt Content Composites Encapsulated in Porous Membrane. Cell Rep. Phys. Sci. 2021, 2, 100664. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Diercks, C.S.; Yaghi, O.M. Metal–Organic Frameworks for Water Harvesting from Air. Adv. Mater. 2018, 30, 1704304. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.X.; Dai, Y.J.; Wu, J.Y.; Wang, R.Z. Experimental Comparison of Two Honeycombed Desiccant Wheels Fabricated with Silica Gel and Composite Desiccant Material. Energy Convers. Manag. 2006, 47, 2523–2534. [Google Scholar] [CrossRef]
- Kallenberger, P.A.; Fröba, M. Water Harvesting from Air with a Hygroscopic Salt in a Hydrogel–Derived Matrix. Commun. Chem. 2018, 1, 28. [Google Scholar] [CrossRef]
- Wang, W.; Wu, L.; Li, Z.; Fang, Y.; Ding, J.; Xiao, J. An Overview of Adsorbents in the Rotary Desiccant Dehumidifier for Air Dehumidification. Dry. Technol. 2013, 31, 1334–1345. [Google Scholar] [CrossRef]
- La, D.; Dai, Y.; Li, Y.; Ge, T.; Wang, R. Case Study and Theoretical Analysis of a Solar Driven Two-Stage Rotary Desiccant Cooling System Assisted by Vapor Compression Air-Conditioning. Sol. Energy 2011, 85, 2997–3009. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Tokarev, M.M.; Parmon, V.N.; Aristov, Y.I. Selective Water Sorbents for Multiple Application, 6. Freshwater Production from the Atmosphere. React. Kinet. Catal. Lett. 1998, 65, 153–159. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, J.Y.; Wang, R.Z.; Wang, L.W. Experimental Research of Composite Solid Sorbents for Fresh Water Production Driven by Solar Energy. Appl. Eng. 2017, 121, 941–950. [Google Scholar] [CrossRef]
- Rieth, A.J.; Yang, S.; Wang, E.N.; Dincǎ, M. Record Atmospheric Fresh Water Capture and Heat Transfer with a Material Operating at the Water Uptake Reversibility Limit. ACS Cent. Sci. 2017, 3, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Rao, S.R.; Kapustin, E.A.; Narayanan, S.; Yang, S.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Response to Comment on “Water Harvesting from Air with Metal-Organic Frameworks Powered by Natural Sunlight. Science 2017, 358, eaao3139. [Google Scholar] [CrossRef]
- Heidari, A.; Roshandel, R.; Vakiloroaya, V. An Innovative Solar Assisted Desiccant-Based Evaporative Cooling System for Co-Production of Water and Cooling in Hot and Humid Climates. Energy Convers. Manag. 2019, 185, 396–409. [Google Scholar] [CrossRef]
- Krajnc, A.; Varlec, J.; Mazaj, M.; Ristić, A.; Logar, N.Z.; Mali, G. Superior Performance of Microporous Aluminophosphate with LTA Topology in Solar-Energy Storage and Heat Reallocation. Adv. Energy Mater. 2017, 7, 1601815. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal-Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yaghi, O.M. Metal-Organic Frameworks for Water Harvesting from Air, Anywhere, Anytime. ACS Cent. Sci. 2020, 6, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J.; Yaghi, O.M. Practical Water Production from Desert Air. Sci. Adv. 2018, 4, eaat3198. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Liu, G.; Li, J.; Hu, X.; Xu, N.; Zhao, W.; Zhu, B.; Zhu, J. An Interfacial Solar Heating Assisted Liquid Sorbent Atmospheric Water Generator. Angew. Chem. Int. Ed. 2019, 58, 12054–12058. [Google Scholar] [CrossRef]
- Feng, A.; Akther, N.; Duan, X.; Peng, S.; Onggowarsito, C.; Mao, S.; Fu, Q.; Kolev, S.D. Recent Development of Atmospheric Water Harvesting Materials: A Review. ACS Mater. Au 2022, 2, 576–595. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Wu, J. Adsorption Refrigeration Technology: Theory and Application; John Wiley & Sons: New York, NY, USA, 2014; ISBN 9781118197479. [Google Scholar]
- Li, R.; Shi, Y.; Shi, L.; Alsaedi, M.; Wang, P. Harvesting Water from Air: Using Anhydrous Salt with Sunlight. Envon. Sci. Technol. 2018, 52, 5398–5406. [Google Scholar] [CrossRef]
- Salehi, A.A.; Ghannadi-Maragheh, M.; Torab-Mostaedi, M.; Torkaman, R.; Asadollahzadeh, M. A Review on the Water-Energy Nexus for Drinking Water Production from Humid Air. Renew. Sustain. Energy Rev. 2020, 120, 109627. [Google Scholar] [CrossRef]
- Maher, H.; Rupam, T.H.; Rocky, K.A.; Bassiouny, R.; Saha, B.B. Silica Gel-MIL 100(Fe) Composite Adsorbents for Ultra-Low Heat-Driven Atmospheric Water Harvester. Energy 2022, 238, 121741. [Google Scholar] [CrossRef]
- Silva, M.P.; Ribeiro, A.M.; Silva, C.G.; Ho Cho, K.; Lee, U.H.; Faria, J.L.; Loureiro, J.M.; Chang, J.S.; Rodrigues, A.E.; Ferreira, A. Atmospheric Water Harvesting on MIL-100(Fe) upon a Cyclic Adsorption Process. Sep. Purif. Technol. 2022, 290, 120803. [Google Scholar] [CrossRef]
- Ma, Q.; Zheng, X. Preparation and Characterization of Thermo-Responsive Composite for Adsorption-Based Dehumidification and Water Harvesting. Chem. Eng. J. 2022, 429, 132498. [Google Scholar] [CrossRef]
- Wang, W.; Xie, S.; Pan, Q.; Dai, Y.; Wang, R.; Ge, T. Air-Cooled Adsorption-Based Device for Harvesting Water from Island Air. Renew. Sustain. Energy Rev. 2021, 141, 110802. [Google Scholar] [CrossRef]
- Ejeian, M.; Entezari, A.; Wang, R.Z. Solar Powered Atmospheric Water Harvesting with Enhanced LiCl /MgSO4/ACF Composite. Appl. Eng. 2020, 176, 115396. [Google Scholar] [CrossRef]
- Hu, Y.; Fang, Z.; Ma, X.; Wan, X.; Wang, S.; Fan, S.; Ye, Z.; Peng, X. CaCl2 Nanocrystals Decorated Photothermal Fe-Ferrocene MOFs Hollow Microspheres for Atmospheric Water Harvesting. Appl. Mater. Today 2021, 23, 101076. [Google Scholar] [CrossRef]
- Wang, W.; Pan, Q.; Xing, Z.; Liu, X.; Dai, Y.; Wang, R.; Ge, T. Viability of a Practical Multicyclic Sorption-Based Water Harvester with Improved Water Yield. Water Res. 2022, 211, 118029. [Google Scholar] [CrossRef]
- Kim, S.; Liang, Y.; Kang, S.; Choi, H. Solar-Assisted Smart Nanofibrous Membranes for Atmospheric Water Harvesting. Chem. Eng. J. 2021, 425, 131601. [Google Scholar] [CrossRef]
- Silva, M.P.; Ribeiro, A.M.; Silva, C.G.; Narin, G.; Nogueira, I.B.R.; Lee, U.H.; Faria, J.L.; Loureiro, J.M.; Chang, J.S.; Rodrigues, A.E.; et al. Water Vapor Harvesting by a (P)TSA Process with MIL-125(Ti)_NH2 as Adsorbent. Sep. Purif. Technol. 2020, 237, 116336. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water Harvesting from Air with Metal-Organic Frameworks Powered by Natural Sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Towsif Abtab, S.M.; Alezi, D.; Bhatt, P.M.; Shkurenko, A.; Belmabkhout, Y.; Aggarwal, H.; Weseliński, Ł.J.; Alsadun, N.; Samin, U.; Hedhili, M.N.; et al. Reticular Chemistry in Action: A Hydrolytically Stable MOF Capturing Twice Its Weight in Adsorbed Water. Chem 2018, 4, 94–105. [Google Scholar] [CrossRef]
- Teo, H.W.B.; Chakraborty, A.; Kitagawa, Y.; Kayal, S. Experimental Study of Isotherms and Kinetics for Adsorption of Water on Aluminium Fumarate. Int. J. Heat Mass. Transf. 2017, 114, 621–627. [Google Scholar] [CrossRef]
- Kayal, S.; Baichuan, S.; Saha, B.B. Adsorption Characteristics of AQSOA Zeolites and Water for Adsorption Chillers. Int. J. Heat Mass Transf. 2016, 92, 1120–1127. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, A. Composite Desiccant Material “CaCl2/Vermiculite/Saw Wood”: A New Material for Fresh Water Production from Atmospheric Air. Appl. Water Sci. 2017, 7, 2103–2111. [Google Scholar] [CrossRef]
- Mohamed, M.H.; William, G.E.; Fatouh, M. Solar Energy Utilization in Water Production from Humid Air. Sol. Energy 2017, 148, 98–109. [Google Scholar] [CrossRef]
- Elashmawy, M. Experimental Study on Water Extraction from Atmospheric Air Using Tubular Solar Still. J. Clean Prod. 2020, 249, 119322. [Google Scholar] [CrossRef]
- Elashmawy, M.; Alshammari, F. Atmospheric Water Harvesting from Low Humid Regions Using Tubular Solar Still Powered by a Parabolic Concentrator System. J. Clean Prod. 2020, 256, 120329. [Google Scholar] [CrossRef]
- Srivastava, S.; Yadav, A. Water Generation from Atmospheric Air by Using Composite Desiccant Material through Fixed Focus Concentrating Solar Thermal Power. Sol. Energy 2018, 169, 302–315. [Google Scholar] [CrossRef]
- Fathy, M.H.; Awad, M.M.; Zeidan, E.S.B.; Hamed, A.M. Solar Powered Foldable Apparatus for Extracting Water from Atmospheric Air. Renew. Energy 2020, 162, 1462–1489. [Google Scholar] [CrossRef]
- Talaat, M.A.; Awad, M.M.; Zeidan, E.B.; Hamed, A.M. Solar-Powered Portable Apparatus for Extracting Water from Air Using Desiccant Solution. Renew. Energy 2018, 119, 662–674. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, R.Z.; Wang, L.W.; Liu, J.Y. A High Efficient Semi-Open System for Fresh Water Production from Atmosphere. Energy 2017, 138, 542–551. [Google Scholar] [CrossRef]
- Wang, J.; Dang, Y.; Meguerdichian, A.G.; Dissanayake, S.; Kankanam-Kapuge, T.; Bamonte, S.; Tobin, Z.M.; Achola, L.A.; Suib, S.L. Water Harvesting from the Atmosphere in Arid Areas with Manganese Dioxide. Environ. Sci. Technol. Lett. 2019, 7, 48–53. [Google Scholar] [CrossRef]
- Entezari, A.; Ejeian, M.; Wang, R. Super Atmospheric Water Harvesting Hydrogel with Alginate Chains Modified with Binary Salts. ACS Mater. Lett. 2020, 2, 471–477. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Wu, M.; Hong, S.; Wang, P. Improving Atmospheric Water Production Yield: Enabling Multiple Water Harvesting Cycles with Nano Sorbent. Nano Energy 2020, 67, 104255. [Google Scholar] [CrossRef]
- Entezari, A.; Ejeian, M.; Wang, R.Z. Extraordinary Air Water Harvesting Performance with Three Phase Sorption. Mater. Today Energy 2019, 13, 362–373. [Google Scholar] [CrossRef]
- Aleid, S.; Wu, M.; Li, R.; Wang, W.; Zhang, C.; Zhang, L.; Wang, P. Salting-in Effect of Zwitterionic Polymer Hydrogel Facilitates Atmospheric Water Harvesting. ACS Mater. Lett. 2022, 4, 511–520. [Google Scholar] [CrossRef]
- Kabeel, A.E. Application of Sandy Bed Solar Collector System for Water Extraction from Air. Int. J. Energy Res. 2006, 30, 381–394. [Google Scholar] [CrossRef]
- Hamed, A.M.; Aly, A.A.; Zeidan, E.-S.B. Application of Solar Energy for Recovery of Water from Atmospheric Air in Climatic Zones of Saudi Arabia. Nat. Resour. 2011, 2, 8–17. [Google Scholar] [CrossRef]
- Wang, J.Y.; Liu, J.Y.; Wang, R.Z.; Wang, L.W. Experimental Investigation on Two Solar-Driven Sorption Based Devices to Extract Fresh Water from Atmosphere. Appl. Eng. 2017, 127, 1608–1616. [Google Scholar] [CrossRef]
- Kim, H.; Rao, S.R.; Kapustin, E.A.; Zhao, L.; Yang, S.; Yaghi, O.M.; Wang, E.N. Adsorption-Based Atmospheric Water Harvesting Device for Arid Climates. Nat. Commun. 2018, 9, 1191. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L.; Hanikel, N.; Lyle, S.J.; Zhu, C.; Proserpio, D.M.; Yaghi, O.M. A Porous Covalent Organic Framework with Voided Square Grid Topology for Atmospheric Water Harvesting. J. Am. Chem. Soc. 2020, 142, 2218–2221. [Google Scholar] [CrossRef] [PubMed]
- Krishna Nandakumar, D.; Zhang, Y.; Kishore Ravi, S.; Guo, N.; Zhang, C.; Ching Tan, S.; Nandakumar, D.K.; Zhang, Y.; Ravi, S.K.; Tan, S.C.; et al. Solar Energy Triggered Clean Water Harvesting from Humid Air Existing above Sea Surface Enabled by a Hydrogel with Ultrahigh Hygroscopicity. Adv. Mater. 2019, 31, 1806730. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, X.; Qu, H.; Gen Yu, Z.; Zhang, Y.; Jie Eey, T.; Zhang, Y.-W.; Ching Tan, S.; Yang, J.; Zhang, X.; et al. A Moisture-Hungry Copper Complex Harvesting Air Moisture for Potable Water and Autonomous Urban Agriculture. Adv. Mater. 2020, 32, 2002936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, J.; Borayek, R.; Qu, H.; Nandakumar, D.K.; Zhang, Q.; Ding, J.; Tan, S.C. Super-Hygroscopic Film for Wearables with Dual Functions of Expediting Sweat Evaporation and Energy Harvesting. Nano Energy 2020, 75, 104873. [Google Scholar] [CrossRef]
- Matsumoto, K.; Sakikawa, N.; Miyata, T. Thermo-Responsive Gels That Absorb Moisture and Ooze Water. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Liu, Y.; Shi, Y.; Dai, Y.; Yu, G. Super Moisture-Absorbent Gels for All-Weather Atmospheric Water Harvesting. Adv. Mater. 2019, 31, 1806446. [Google Scholar] [CrossRef]
- Yilmaz, G.; Meng, F.L.; Lu, W.; Abed, J.; Peh, C.K.N.; Gao, M.; Sargent, E.H.; Ho, G.W. Autonomous Atmospheric Water Seeping MOF Matrix. Sci. Adv. 2020, 6, 8605–8621. [Google Scholar] [CrossRef]
- Yang, K.; Pan, T.; Pinnau, I.; Shi, Z.; Han, Y. Simultaneous Generation of Atmospheric Water and Electricity Using a Hygroscopic Aerogel with Fast Sorption Kinetics. Nano Energy 2020, 78, 105326. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Chao, J.; Wu, S.; Yan, T.; Li, W.; Cao, B.; Wang, R. Efficient Solar-Driven Water Harvesting from Arid Air with Metal–Organic Frameworks Modified by Hygroscopic Salt. Angew. Chem. Int. Ed. 2020, 59, 5202–5210. [Google Scholar] [CrossRef]
- Yang, K.; Shi, Y.; Wu, M.; Wang, W.; Jin, Y.; Li, R.; Shahzad, M.W.; Ng, K.C.; Wang, P. Hollow Spherical SiO2 Micro-Container Encapsulation of LiCl for High-Performance Simultaneous Heat Reallocation and Seawater Desalination. J. Mater. Chem. A 2020, 8, 1887–1895. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Alsaedi, M.; Wu, M.; Shi, L.; Wang, P. Hybrid Hydrogel with High Water Vapor Harvesting Capacity for Deployable Solar-Driven Atmospheric Water Generator. Envon. Sci. Technol. 2018, 52, 11367–11377. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, R.Z.; Wang, L.W. Water Vapor Sorption Performance of ACF-CaCl2 and Silica Gel-CaCl2 Composite Adsorbents. Appl. Eng. 2016, 100, 893–901. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Restuccia, G.; Freni, A.; Aristov, Y.I. Water Sorption on Composites “LiBr in a Porous Carbon”. Fuel Process. Technol. 2002, 79, 225–231. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Lu, X.; Zhao, C.; Yan, C.; Mu, T. Water Sorption in Protic Ionic Liquids: Correlation between Hygroscopicity and Polarity. New J. Chem. 2013, 37, 1959–1967. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, S.; Zhong, H.; Zhang, B.; Cui, M.; Jiang, M.; Wang, S.; Wang, Z. Heterogeneous Wettability and Radiative Cooling for Efficient Deliquescent Sorbents-Based Atmospheric Water Harvesting. Cell Rep. Phys. Sci. 2022, 3, 100879. [Google Scholar] [CrossRef]
- Wang, J.; Deng, C.; Zhong, G.; Ying, W.; Li, C.; Wang, S.; Liu, Y.; Wang, R.; Zhang, H. High-Yield and Scalable Water Harvesting of Honeycomb Hygroscopic Polymer Driven by Natural Sunlight. Cell Rep. Phys. Sci. 2022, 3, 100954. [Google Scholar] [CrossRef]
- Deng, F.; Wang, C.; Xiang, C.; Wang, R. Bioinspired Topological Design of Super Hygroscopic Complex for Cost-Effective Atmospheric Water Harvesting. Nano Energy 2021, 90, 106642. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, R.Z.; Tu, Y.D.; Wang, L.W. Universal Scalable Sorption-Based Atmosphere Water Harvesting. Energy 2018, 165, 387–395. [Google Scholar] [CrossRef]
- Wang, M.; Sun, T.; Wan, D.; Dai, M.; Ling, S.; Wang, J.; Liu, Y.; Fang, Y.; Xu, S.; Yeo, J.; et al. Solar-Powered Nanostructured Biopolymer Hygroscopic Aerogels for Atmospheric Water Harvesting. Nano Energy 2021, 80, 105569. [Google Scholar] [CrossRef]
- Qi, H.; Wei, T.; Zhao, W.; Zhu, B.; Liu, G.; Wang, P.; Lin, Z.; Wang, X.; Li, X.; Zhang, X.; et al. An Interfacial Solar-Driven Atmospheric Water Generator Based on a Liquid Sorbent with Simultaneous Adsorption–Desorption. Adv. Mater. 2019, 31, 1903378. [Google Scholar] [CrossRef] [PubMed]
- Harish; Dhanraj, S.; Gopi, R.; Loganathan, G.B. An Efficiency Study on Water Extraction from Air Using Thermophoresis Method. IOP Conf. Ser. Mater. Sci. Eng. 2019, 574, 012003. [Google Scholar] [CrossRef]
- Al-Farayedhi, A.A.; Ibrahim, N.I.; Gandhidasan, P. Condensate as a Water Source from Vapor Compression Systems in Hot and Humid Regions. Desalination 2014, 349, 60–67. [Google Scholar] [CrossRef]
- Elattar, H.F.; Fouda, A.; Nada, S.A. Performance Investigation of a Novel Solar Hybrid Air Conditioning and Humidification–Dehumidification Water Desalination System. Desalination 2016, 382, 28–42. [Google Scholar] [CrossRef]
- Groendijk, L.; de Vries, H.E. Development of a Mobile Water Maker, a Sustainable Way to Produce Safe Drinking Water in Developing Countries. Desalination 2009, 248, 106–113. [Google Scholar] [CrossRef]
- Zolfagharkhani, S.; Zamen, M.; Shahmardan, M.M. Thermodynamic Analysis and Evaluation of a Gas Compression Refrigeration Cycle for Fresh Water Production from Atmospheric Air. Energy Convers. Manag. 2018, 170, 97–107. [Google Scholar] [CrossRef]
- Van Wylen, G.J.; Sonntag, R.E.; Borgnakke, C. Fundamentals of Classical Thermodynamics; Wiley: New York, NY, USA, 1985; ISBN 9780471829331. [Google Scholar]
- Zhang, T.; Wan, R.H.; Yan, T.; Wang, L.; Lin, C.H. Development of Portable Air-Based Water Collector for Field Operations under Conditions of Severe Shortage. Chin. Med. Equip. 2010, 8, 70–72. [Google Scholar]
- Cao, D.; Zou, Y. System Optimization of Water Exaction from Air by Semiconductor Cooling Air. Build. Energy Environ. 2022, 35, 71–73. [Google Scholar]
- Magrini, A.; Cattani, L.; Cartesegna, M.; Magnani, L. Integrated Systems for Air Conditioning and Production of Drinking Water—Preliminary Considerations. Energy Procedia 2015, 75, 1659–1665. [Google Scholar] [CrossRef]
- Habeebullah, B.A. Potential Use of Evaporator Coils for Water Extraction in Hot and Humid Areas. Desalination 2009, 237, 330–345. [Google Scholar] [CrossRef]
- Magrini, A.; Cattani, L.; Cartesegna, M.; Magnani, L. Water Production from Air Conditioning Systems: Some Evaluations about a Sustainable Use of Resources. Sustainability 2017, 9, 1309. [Google Scholar] [CrossRef]
- Cattani, L.; Magrini, A.; Cattani, P. Water Extraction from Air by Refrigeration—Experimental Results from an Integrated System Application. Appl. Sci. 2018, 8, 2262. [Google Scholar] [CrossRef]
- He, W.; Yu, P.; Hu, Z.; Lv, S.; Qin, M.; Yu, C. Experimental Study and Performance Analysis of a Portable Atmospheric Water Generator. Energies 2019, 13, 73. [Google Scholar] [CrossRef]
- Liu, S.; He, W.; Hu, D.; Lv, S.; Chen, D.; Wu, X.; Xu, F.; Li, S. Experimental Analysis of a Portable Atmospheric Water Generator by Thermoelectric Cooling Method. Energy Procedia 2017, 142, 1609–1614. [Google Scholar] [CrossRef]
- Robertson, B.I.; Shin, Y.-H.; Choi, J.-W.; Kadhim, T.J.; Abbas, A.K.; Kadhim, H.J. Experimental Study of Atmospheric Water Collection Powered by Solar Energy Using the Peltier Effect. IOP Conf. Ser. Mater. Sci. Eng. 2019, 671, 012155. [Google Scholar] [CrossRef]
- Irshad, K.; Almalawi, A.; Habib, K.; Zahir, M.H.; Ali, A.; Islam, S.; Saha, B.B. Experimental Study of a Thermoelectric Air Duct Dehumidification System for Tropical Climate. Heat Transf. Eng. 2020, 42, 1159–1171. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Cimbala, J.M. Fluids Mechanics—Fundaments and Applications. J. Chem. Inf. Model 2017, 53, 1024–1051. [Google Scholar]
- Gabler, R.E. Physical Geography; Wiley: New York, NY, USA, 2017; ISBN 9780495555063. [Google Scholar]
- Peeters, R.; Vanderschaeghe, H.; Rongé, J.; Martens, J.A. Energy Performance and Climate Dependency of Technologies for Fresh Water Production from Atmospheric Water Vapour. Env. Sci. 2020, 6, 2016–2034. [Google Scholar] [CrossRef]
- Huang, J. A Simple Accurate Formula for Calculating Saturation Vapor Pressure of Water and Ice. J. Appl. Meteorol. Clim. 2018, 57, 1265–1272. [Google Scholar] [CrossRef]
- Moonaliza, M. Introduction and Basic Concepts. In Thermodynamics an Engineering Approach, 5th ed.; McGraw-Hill: New York, NY, USA, 2011; Available online: https://www.academia.edu/41069675/Introduction_and_Basic_Concepts_Thermodynamics_An_Engineering_Approach_5th_edition_ (accessed on 21 September 2022).
- Incropera, F.; DeWitt, D.; Bergman, T.; Lavine, A. The Lumped Capacitance Method. In Principles of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 279–286. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).