The Origin and Occurrence of Natural Hydrogen
Abstract
1. Introduction
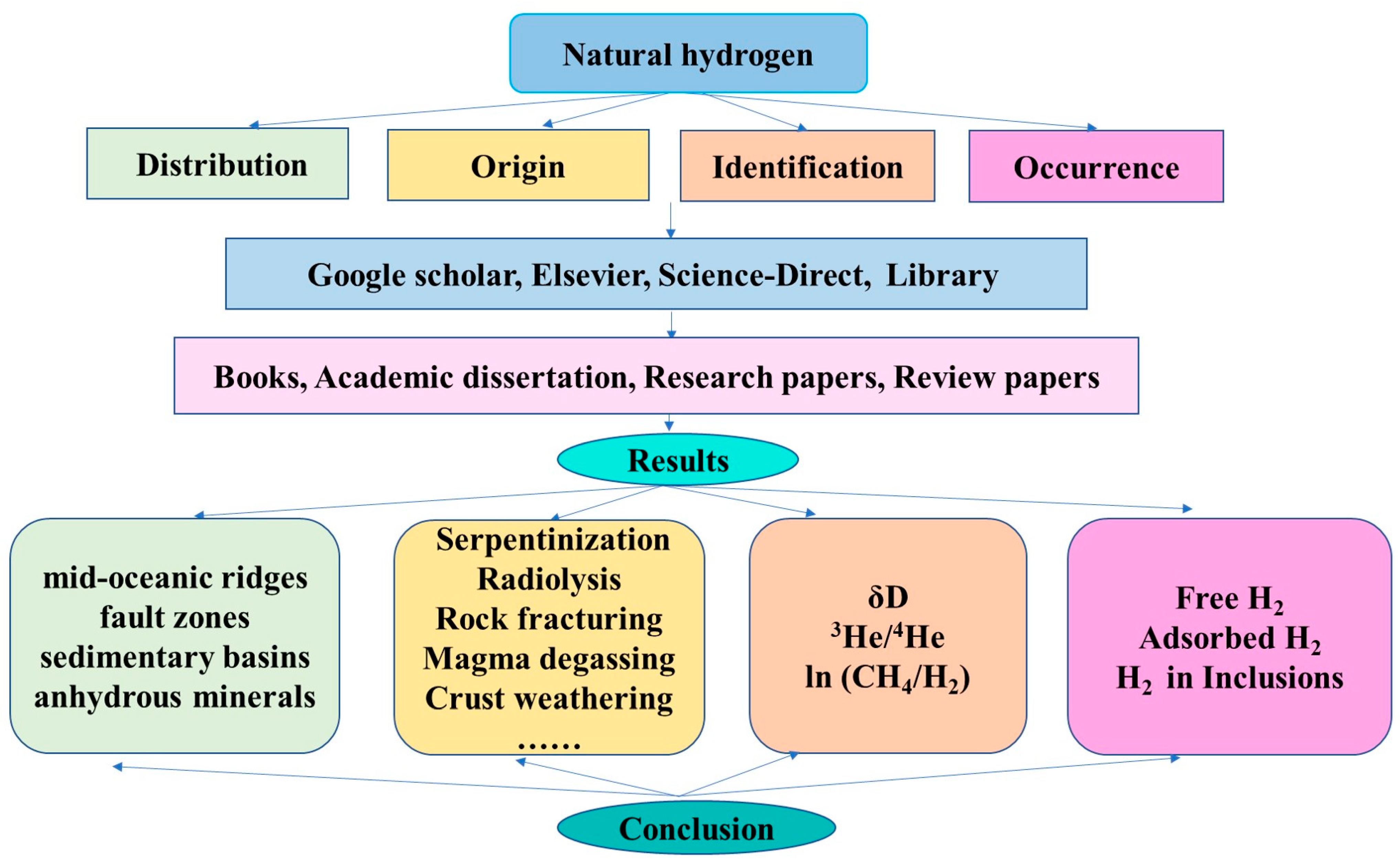
2. Methodology
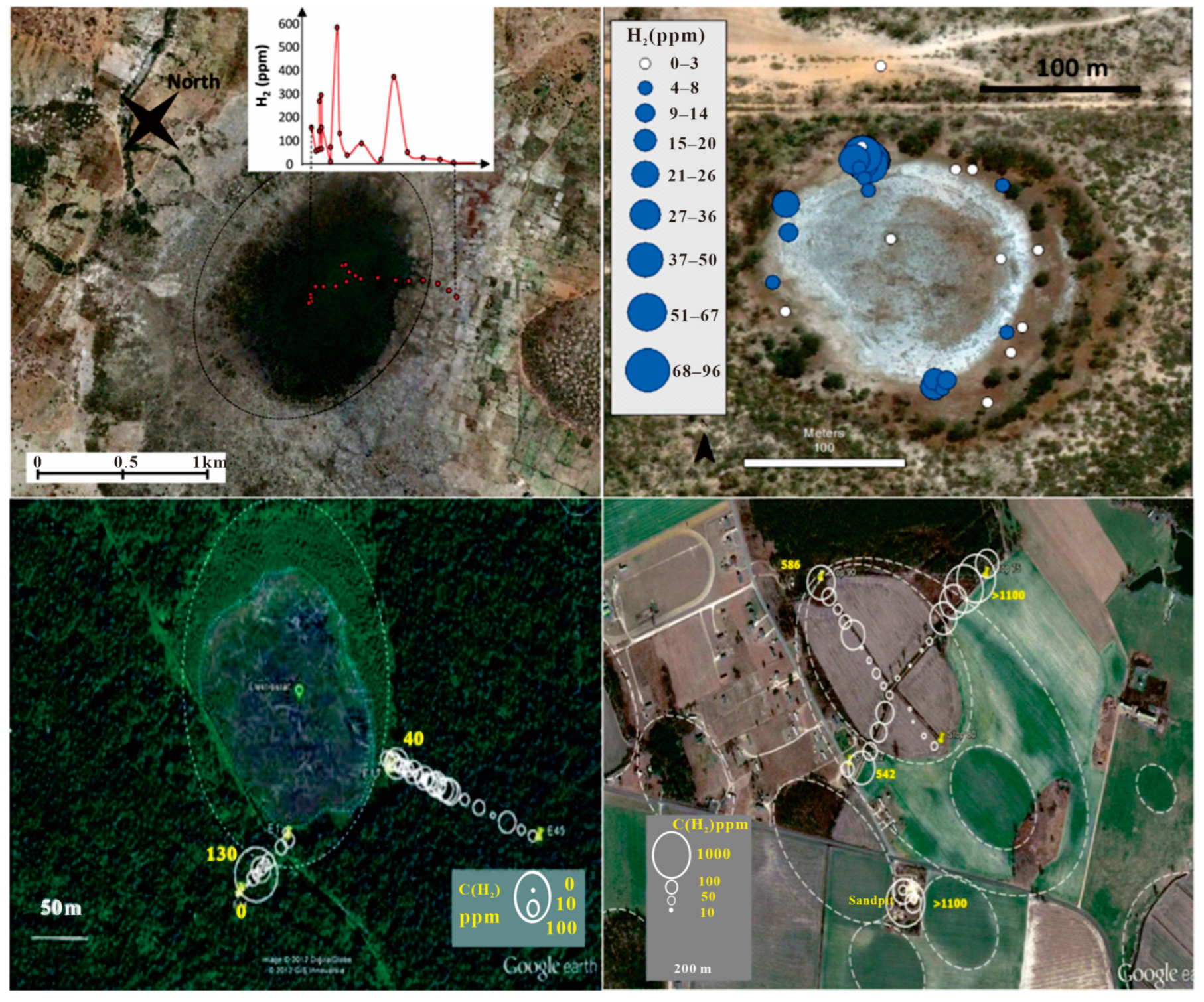
3. Hydrogen Distribution
4. Origin and Identification of Natural Hydrogen
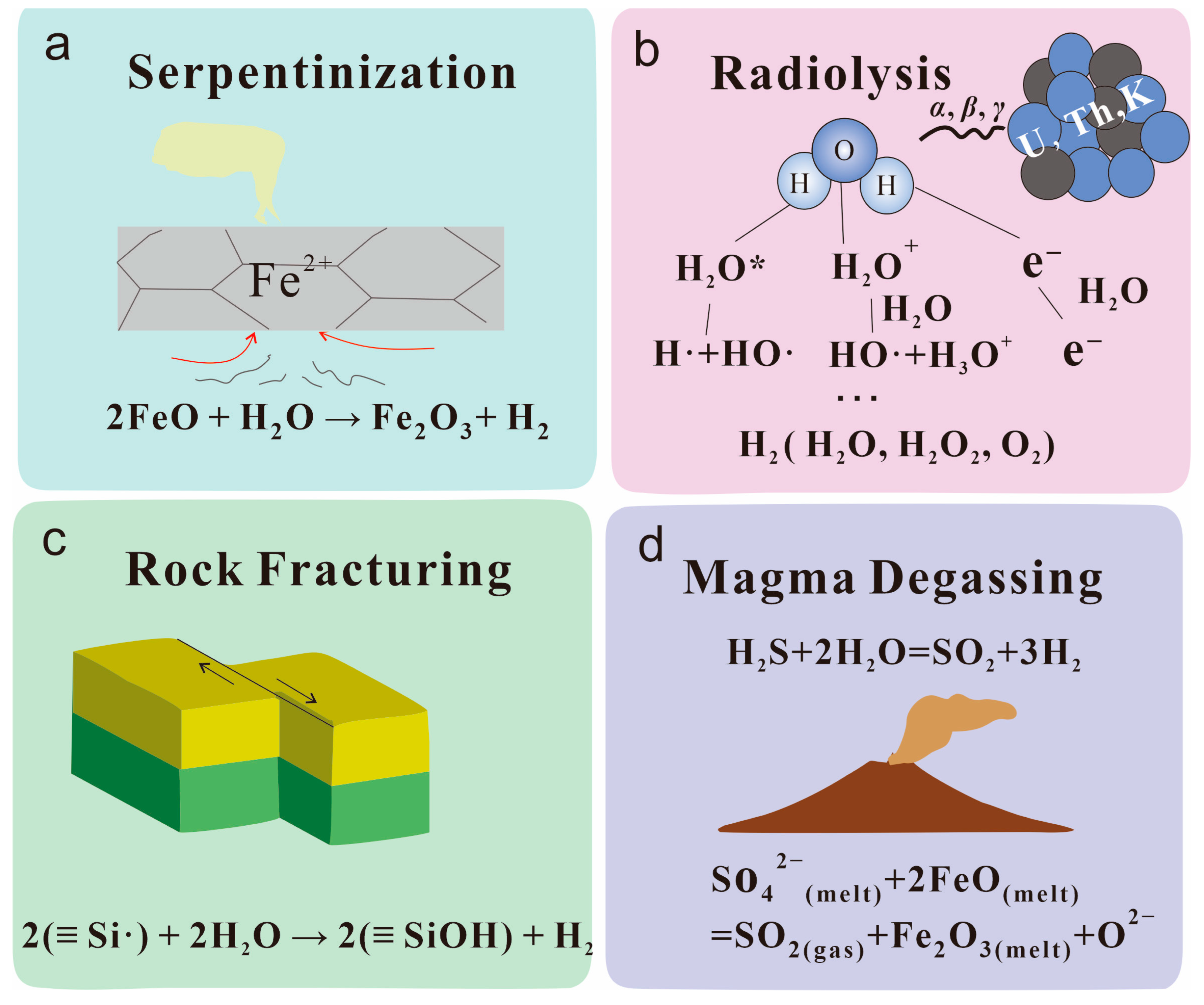
4.1. Origin
4.1.1. Serpentinization
4.1.2. Radiolysis
4.1.3. Rock Fracturing
4.1.4. Magma Degassing
4.1.5. Crust Weathering
4.1.6. High-Temperature Basalt Alteration
4.1.7. Lava–Seawater Interaction
4.1.8. Crystallization
4.1.9. Pyrite Formation
4.2. Identification
5. Hydrogen Occurrence
5.1. Free Hydrogen
5.2. Adsorbed Hydrogen
5.3. Hydrogen in Inclusions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| H2 | Hydrogen |
| H2S | Hydrogen sulfide |
| CH4 | Methane |
| FeO | Ferrous oxide |
| Fe2O3 | Ferric oxide |
| FeOOH | Hydroxyl oxidize iron |
| Fe3O4 | Ferroferric oxide |
| FeS2 | Ferrous disulfide |
| N2 | Nitrogen |
| COH | Carbon–oxygen–hydrogen |
| MORBs | Midocean ridge basalts |
| MAR | Mid-Atlantic Ridge |
| H2O2 | Hydrogen peroxide |
| O2 | Oxygen |
| SiO2 | Silicon dioxide |
References
- Çelik, D.; Yıldız, M. Investigation of hydrogen production methods in accordance with green chemistry principles. Int. J. Hydrog. Energy 2017, 42, 23395–23401. [Google Scholar] [CrossRef]
- Da Silva Veras, T.; Mozer, T.S.; da Silva César, A. Hydrogen: Trends, production and characterization of the main process worldwide. Int. J. Hydrog. Energy 2017, 42, 2018–2033. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Borowski, P.F.; Karlikowska, B. Clean Hydrogen Is a Challenge for Enterprises in the Era of Low-Emission and Zero-Emission Economy. Energies 2023, 16, 1171. [Google Scholar] [CrossRef]
- Kumar, S.; Baalisampang, T.; Arzaghi, E.; Garaniya, V.; Abbassi, R.; Salehi, F. Synergy of green hydrogen sector with offshore industries: Opportunities and challenges for a safe and sustainable hydrogen economy. J. Clean. Prod. 2022, 384, 135545. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Babic, U.; Suermann, M.; Büchi, F.N.; Gubler, L.; Schmidt, T.J. Critical review—Identifying critical gaps for polymer electrolyte water electrolysis development. J. Electrochem. Soc. 2017, 164, F387. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Chalk, S.G.; Miller, J.F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for clean energy systems. J. Power Sources 2006, 159, 73–80. [Google Scholar] [CrossRef]
- Ruse, E.; Buzaglo, M.; Pri-Bar, I.; Shunak, L.; Nadiv, R.; Pevzner, S.; Siton-Mendelson, O.; Skripnyuk, V.M.; Rabkin, E.; Regev, O. Hydrogen storage kinetics: The graphene nanoplatelet size effect. Carbon 2018, 130, 369–376. [Google Scholar] [CrossRef]
- Prabhukhot Prachi, R.; Wagh Mahesh, M.; Gangal Aneesh, C. A review on solid state hydrogen storage material. Adv. Energy Power 2016, 4, 11–22. [Google Scholar] [CrossRef]
- Ozturk, T.; Demirbas, A. Boron compounds as hydrogen storage materials. Energy Sources Part A 2007, 29, 1415–1423. [Google Scholar] [CrossRef]
- Raza, A.; Glatz, G.; Alafnan, S.; Mahmoud, M.; Gholami, R. Depleted shale gas formations as naturally-occurring storage compartments for hydrogen: A molecular-level assessment. Fuel 2023, 334, 126695. [Google Scholar] [CrossRef]
- Midilli, A.; Ay, M.; Dincer, I.; Rosen, M.A. On hydrogen and hydrogen energy strategies: I: Current status and needs. Renew. Sustain. Energy Rev. 2005, 9, 255–271. [Google Scholar] [CrossRef]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Principi, G.; Agresti, F.; Maddalena, A.; Lo Russo, S. The problem of solid state hydrogen storage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Smith, N.J.P.; Shepherd, T.J.; Styles, M.T.; Williams, G.M. Hydrogen exploration: A review of global hydrogen accumulations and implications for prospective areas in NW Europe. In Geological Society, London, Petroleum Geology Conference Series; The Geological Society of London: London, UK, 2005; Volume 6, pp. 349–358. [Google Scholar]
- Prinzhofer, A.; Cissé, C.S.T.; Diallo, A.B. Discovery of a large accumulation of natural hydrogen in Bourakebougou (Mali). Int. J. Hydrogen Energy 2018, 43, 19315–19326. [Google Scholar] [CrossRef]
- Gaucher, E.C. New perspectives in the industrial exploration for native hydrogen. Elem. Int. Mag. Mineral. Geochem. Petrol. 2020, 16, 8–9. [Google Scholar] [CrossRef]
- Murray, J.; Clément, A.; Fritz, B.; Schmittbuhl, J.; Bordmann, V.; Fleury, J.M. Abiotic hydrogen generation from biotite-rich granite: A case study of the Soultz-sous-Forêts geothermal site, France. Appl. Geochem. 2020, 119, 104631. [Google Scholar] [CrossRef]
- Johnsgard, S.K. The Fracture Pattern of North-Central Kansas and Its Relation to Hydrogen Soil Gas Anomalies over the Midcontinent Rift System; University of Kansas, Geology: Lawrence, KS, USA, 1988. [Google Scholar]
- Smith, N.J.P. It’s time for explorationists to take hydrogen more seriously. First Break 2002, 20. [Google Scholar] [CrossRef]
- Truche, L.; Bazarkina, E.F. Natural hydrogen the fuel of the 21st century. EDP Sci. 2019, 98, 03006. [Google Scholar] [CrossRef]
- Sleep, N.H.; Meibom, A.; Fridriksson, T.; Coleman, R.G.; Bird, D.K. H2-rich fluids from serpentinization: Geochemical and biotic implications. Proc. Natl. Acad. Sci. USA 2004, 101, 12818–12823. [Google Scholar] [CrossRef] [PubMed]
- Ménez, B. Abiotic hydrogen and methane: Fuels for life. Elem. Int. Mag. Mineral. Geochem. Petrol. 2020, 16, 39–46. [Google Scholar] [CrossRef]
- Truche, L.; McCollom, T.M.; Martinez, I. Hydrogen and abiotic hydrocarbons: Molecules that change the world. Elem. Int. Mag. Mineral. Geochem. Petrol. 2020, 16, 13–18. [Google Scholar] [CrossRef]
- Zgonnik, V. The occurrence and geoscience of natural hydrogen: A comprehensive review. Earth-Sci. Rev. 2020, 203, 103140. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Xu, H. Genesis and energy significance of natural hydrogen. Unconv. Resour. 2023. [Google Scholar] [CrossRef]
- Welhan, J.T.; Craig, H. Methane and hydrogen in East Pacific Rise hydrothermal fluids. Geophys. Res. Lett. 1979, 6, 829–831. [Google Scholar] [CrossRef]
- Charlou, J.L.; Donval, J.P. Hydrothermal methane venting between 12 °N and 26 °N along the Mid-Atlantic Ridge. J. Geophys. Res. Solid Earth 1993, 98, 9625–9642. [Google Scholar] [CrossRef]
- Vacquand, C.; Deville, E.; Beaumont, V.; Guyot, F.; Sissmann, O.; Pillot, D.; Arcilla, C.; Prinzhofe, A. Reduced gas seepages in ophiolitic complexes: Evidences for multiple origins of the H2-CH4-N2 gas mixtures. Geochim. Et Cosmochim. Acta 2018, 223, 437–461. [Google Scholar] [CrossRef]
- Vacquand, C. Genesis and Mobility of Natural Hydrogen: Energy Source or Storable Energy Carrier? Institut de Physique du Globe de Paris: Paris, France, 2011. [Google Scholar]
- Etiope, G.; Samardžić, N.; Grassa, F.; Hrvatović, H.; Miošić, N.; Skopljak, F. Methane and hydrogen in hyperalkaline groundwaters of the serpentinized Dinaride ophiolite belt, Bosnia and Herzegovina. Appl. Geochem. 2017, 84, 286–296. [Google Scholar] [CrossRef]
- Sugisaki, R.; Ido, M.; Takeda, H.; Isobe, Y.; Hayashi, Y.; Nakamura, N.; Satake, H.; Mizutani, Y. Origin of hydrogen and carbon dioxide in fault gases and its relation to fault activity. J. Geol. 1983, 91, 239–258. [Google Scholar] [CrossRef]
- Wiersberg, T.; Erzinger, J. Origin and spatial distribution of gas at seismogenic depths of the San Andreas Fault from drill-mud gas analysis. Appl. Geochem. 2008, 23, 1675–1690. [Google Scholar] [CrossRef]
- Wakita, H.; Nakamura, Y.; Kita, I.; Fujii, N.; Notsu, K. Hydrogen release: New indicator of fault activity. Science 1980, 210, 188–190. [Google Scholar] [CrossRef]
- Sato, M.; Sutton, A.J.; McGee, K.A.; Russell-Robinson, S. Monitoring of hydrogen along the San Andreas and Calaveras faults in central California in 1980–1984. J. Geophys. Res. Solid Earth 1986, 91, 12315–12326. [Google Scholar] [CrossRef]
- McCarthy, J.H., Jr.; Cunningham, K.I.; Roberts, A.A.; Dietrich, J.A. Soil Gas Studies around Hydrogen-Rich Natural Gas Wells in Northern Kansas; US Geological Survey: Reston, VA, USA, 1986. [Google Scholar]
- Potter, J.; Salvi, S.; Longstaffe, F.J. Abiogenic hydrocarbon isotopic signatures in granitic rocks: Identifying pathways of formation. Lithos 2013, 182, 114–124. [Google Scholar] [CrossRef]
- Rogozhin, E.A.; Gorbatikov, A.V.; Larin, N.V.; Stepanova, M.Y. Deep structure of the Moscow Aulacogene in the western part of Moscow. Izvestiya. Atmos. Ocean. Phys. 2010, 46, 973. [Google Scholar] [CrossRef]
- Li, X.; Krooss, B.M.; Weniger, P.; Littke, R. Liberation of molecular hydrogen (H2) and methane (CH4) during non-isothermal pyrolysis of shales and coals: Systematics and quantification. Int. J. Coal Geol. 2015, 137, 152–164. [Google Scholar] [CrossRef]
- Suzuki, N.; Saito, H.; Hoshino, T. Hydrogen gas of organic origin in shales and metapelites. Int. J. Coal Geol. 2017, 173, 227–236. [Google Scholar] [CrossRef]
- Schimmelmann, A.; Sessions, A.L.; Mastalerz, M. Hydrogen isotopic (D/H) composition of organic matter during diagenesis and thermal maturation. Annu. Rev. Earth Planet. Sci. 2006, 34, 501–533. [Google Scholar] [CrossRef]
- Kita, D.; Stedman, D.H. Kinetic studies of reactions of hydrogen atoms with HCl, Cl2 and NOCl, and chlorine atoms with H2 and NOCl. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1982, 78, 1249–1259. [Google Scholar] [CrossRef]
- Kita, I.; Matsuo, S.; Wakita, H. H2 generation by reaction between H2O and crushed rock: An experimental study on H2 degassing from the active fault zone. J. Geophys. Res. Solid Earth 1982, 87, 10789–10795. [Google Scholar] [CrossRef]
- Lollar, B.S.; Onstott, T.C.; Lacrampe-Couloume, G.; Ballentine, C.J. The contribution of the Precambrian continental lithosphere to global H2 production. Nature 2014, 516, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Freund, F.; Dickinson, J.T.; Cash, M. Hydrogen in rocks: An energy source for deep microbial communities. Astrobiology 2002, 2, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Stevens, T.O.; McKinley, J.P. Lithoautotrophic microbial ecosystems in deep basalt aquifers. Science 1995, 270, 450–455. [Google Scholar] [CrossRef]
- Bjornstad, B.N.; McKinley, J.P.; Stevens, T.O.; Rawson, S.A.; Fredrickson, J.K.; Long, P.E. Generation of hydrogen gas as a result of drilling within the saturated zone. Groundw. Monit. Remediat. 1994, 14, 140–147. [Google Scholar] [CrossRef]
- Goebel, E.D.; Coveney, R.M., Jr.; Angino, E.E.; Zeller, E.J. Naturally occurring hydrogen gas from a borehole on the western flank of Nemaha anticline in Kansas. AAPG Bull. 1983, 67, 1324. [Google Scholar]
- Charlou, J.L.; Donval, J.P.; Fouquet, Y.; Jean-Baptiste, P.; Holmc, N. Geochemistry of high H2 and CH4 vent fluids issuing from ultramafic rocks at the Rainbow hydrothermal field (36 14′ N, MAR). Chem. Geol. 2022, 191, 345–359. [Google Scholar] [CrossRef]
- Frieder, K.; Jesse, D.T.; Wolfgang, B. Abiotic Sources of Molecular Hydrogen on Earth. Elements 2020, 16, 19–24. [Google Scholar] [CrossRef]
- Toft, P.B.; Arkani-Hamed, J.; Haggerty, S.E. The effects of serpentinization on density and magnetic susceptibility: A petrophysical model. Phys. Earth Planet. Inter. 1990, 65, 137–157. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; McCollom, T.M. Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 2013, 178, 55–69. [Google Scholar] [CrossRef]
- Jørgensen, B.B.; D’Hondt, S. A starving majority deep beneath the seafloor. Science 2006, 314, 932–934. [Google Scholar] [CrossRef] [PubMed]
- Dzaugis, M.E.; Spivack, A.J.; Dunlea, A.G.; Murray, R.W.; D’Hondt, S. Radiolytic hydrogen production in the subseafloor basaltic aquifer. Front. Microbiol. 2016, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Slater, G.F.; Lollar, B.S.; Lacrampe-Couloume, G.; Onstott, T.C. The yield and isotopic composition of radiolytic H2, a potential energy source for the deep subsurface biosphere. Geochim. Cosmochim. Acta 2005, 69, 893–903. [Google Scholar] [CrossRef]
- Lin, H.T.; Cowen, J.P.; Olson, E.J.; Lilley, M.D.; Jungbluth, S.P.; Wilson, S.T.; Rappé, M.S. Dissolved hydrogen and methane in the oceanic basaltic biosphere. Earth Planet. Sci. Lett. 2014, 405, 62–73. [Google Scholar] [CrossRef]
- Hirose, T.; Kawagucci, S.; Suzuki, K. Mechanoradical H2 generation during simulated faulting: Implications for an earthquake-driven subsurface biosphere. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef]
- Telling, J.; Boyd, E.S.; Bone, N.; Jones, E.L.; Tranter, M.; MacFarlane, J.W.; Martin, P.G.; Wadham, J.L.; Lamarche-Gagnon, G.; Skidmore, M.L.; et al. Rock comminution as a source of hydrogen for subglacial ecosystems. Nat. Geosci. 2015, 8, 851–855. [Google Scholar] [CrossRef]
- Apps, J.A.; van de Kamp, P.C. Energy gases of abiogenic origin in the Earth’s crust. US Geol. Surv. Prof. Pap. 1993, 1570, 81–132. [Google Scholar]
- Hekinian, R.; Chaigneau, M.; Cheminée, J.L. Popping rocks and lava tubes from the Mid-Atlantic Rift Valley at 36 °N. Nature 1973, 245, 371–373. [Google Scholar] [CrossRef]
- Sarda, P.; Graham, D. Mid-ocean ridge popping rocks: Implications for degassing at ridge crests. Earth Planet. Sci. Lett. 1990, 97, 268–289. [Google Scholar] [CrossRef]
- Bach, W.; Edwards, K.J. Iron and sulfide oxidation within the basaltic ocean crust: Implications for chemolithoautotrophic microbial biomass production. Geochim. Et Cosmochim. Acta 2003, 67, 3871–3887. [Google Scholar] [CrossRef]
- Coogan, L.A. The lower oceanic crust. Treatise Geochem 2007, 4, 497–541. [Google Scholar] [CrossRef]
- Karson, J.A.; Kelley, D.S.; Fornari, D.J.; Perfit, M.R.; Shank, T.M. Discovering the Deep: A Photographic Atlas of the Seafloor and Ocean Crust; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Soule, S.A.; Fornari, D.J.; Perfit, M.R.; Ridley, W.I.; Reed, M.H.; Cann, J.R. Incorporation of seawater into mid-ocean ridge lava flows during emplacement. Earth Planet. Sci. Lett. 2006, 252, 289–307. [Google Scholar] [CrossRef]
- Pasquet, G.; Houssein, H.R.; Sissmann, O.; Varet, J.; Moretti, I. An Attempt to Study Natural H2 Resources across an Oceanic Ridge Penetrating a Continent: The Asal–Ghoubbet Rift (Republic of Djibouti). Geosciences 2021, 12, 16. [Google Scholar] [CrossRef]
- Perfit, M.R.; Cann, J.R.; Fornari, D.J.; Engels, J.; Smith, D.K.; Ian, R.W.; Edwards, M.H. Interaction of sea water and lava during submarine eruptions at mid-ocean ridges. Nature 2003, 426, 62–65. [Google Scholar] [CrossRef]
- Baker, E.T.; Lupton, J.E.; Resing, J.A.; Baumberger, T.; Lilley, M.D.; Walker, S.L.; Rubin, K.H. Unique event plumes from a 2008 eruption on the Northeast Lau Spreading Center. Geochem. Geophys. Geosyst. 2011, 12. [Google Scholar] [CrossRef]
- Drobner, E.; Huber, H.; Wächtershäuser, G.; Rose, D.; Stetter, K.O. Pyrite formation linked with hydrogen evolution under anaerobic conditions. Nature 1990, 346, 742–744. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W., III. Kinetics of pyrite formation by the H2S oxidation of iron (II) monosulfide in aqueous solutions between 25 and 125 °C: The mechanism. Geochim. Cosmochim. Acta 1997, 61, 135–147. [Google Scholar] [CrossRef]
- Gallant, R.M.; Von Damm, K.L. Geochemical controls on hydrothermal fluids from the Kairei and Edmond vent fields, 23–25 S, Central Indian Ridge. Geochem. Geophys. Geosyst. 2006, 7. [Google Scholar] [CrossRef]
- Jeffrey, A.W.A.; Kaplan, I.R. Hydrocarbons and inorganic gases in the Gravberg-1 well, Siljan Ring, Sweden. Chem. Geol. 1988, 71, 237–255. [Google Scholar] [CrossRef]
- Guélard, J.; Beaumont, V.; Rouchon, V.; Guyot, F.; Pillot, D.; Jézéquel, D.; Ader, M.; Newell, K.D.; Deville, E. Natural H2 in Kansas: Deep or shallow origin? Geochem. Geophys. Geosyst. 2017, 18, 1841–1865. [Google Scholar] [CrossRef]
- Meng, Q.; Jin, Z.; Sun, D.; Liu, Q.; Zhu, D.; Liu, J.; Huang, X.; Wang, L. Geological background and exploration prospects for the occurrence of high-content hydrogen. Pet. Geol. Exp. 2021, 43, 208–216. [Google Scholar] [CrossRef]
- Angino, E.E.; Zeller, E.J.; Dreschhoff, G.A.M.; Goebel, E.D.; Coveney, R.M., Jr. Spatial distribution of hydrogen in soil gas in central Kansas, USA. In Geochemistry of Gaseous Elements and Compounds; Theophrastus Publ: Athens, Greece, 1990; pp. 485–493. [Google Scholar]
- Newell, K.D.; Doveton, J.H.; Merriam, D.F.; Lollar, B.S.; Waggoner, W.M.; Magnuson, L.M. H2-rich and Hydrocarbon Gas Recovered in a Deep Precambrian Well in Northeastern Kansas. Nat. Resour. Res. 2007, 16, 277–292. [Google Scholar] [CrossRef]
- Goebel, E.D.; Coveney, R.M.J.; Angino, E.E.; Zeller, E.J.; Dreschhoff, K. Geology, composition, isotopes of naturally occurring H2/N2 rich gas from wells near Junction City, Kansas. Oil Gas J. 1984, 82, 215–222. [Google Scholar]
- Angino, E.E.; Coveney, R.M.; Goebel, E.D.; Zeller, E.J.; Dreschhoff, G.A.M. Hydrogen and nitrogen—Origin, distribution, and abundance, a followup. Oil Gas J. 1984, 82, 142–146. [Google Scholar]
- Abrajano, T.A.; Sturchio, N.C.; Bohlke, J.K.; Lyon, G.L.; Poreda, R.J.; Stevens, C.M. Methane-hydrogen gas seeps, Zambales ophiolite, Philippines: Deep or shallow origin? Chem. Geol. 1988, 71, 211–222. [Google Scholar] [CrossRef]
- Kelley, D.S.; Karson, J.A.; Blackman, D.K.; FruÈh-Green, G.L.; Butterfield, D.A.; Lilley, M.D.; Olson, E.J.; Schrenk, M.O.; Roe, K.K.; Lebon, G.T.; et al. An off-axis hydrothermal vent field near the Mid-Atlantic Ridge at 30 °N. Nature 2001, 412, 145–149. [Google Scholar] [CrossRef]
- Neal, C.; Stanger, G. Hydrogen generation from mantle source rocks in Oman. Earth Planet. Sci. Lett. 1983, 66, 315–320. [Google Scholar] [CrossRef]
- Mayhew, L.E.; Ellison, E.T.; McCollom, T.M.; Trainor, T.P.; Templeton, A.S. Hydrogen generation from low-temperature water–rock reactions. Nat. Geosci. 2013, 6, 478–484. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, Y.; Tong, J.; Fu, Q.; Zhu, J.; Zhu, D.; Jin, Z. Distribution and geochemical characteristics of hydrogen in natural gas from the Jiyang Depression, Eastern China. Acta Geol. Sin.-Engl. Ed. 2015, 89, 1616–1624. [Google Scholar]
- Abrajano, T.A.; Sturchio, N.C.; Kennedy, B.M.; Lyon, G.L.; Muehlenbachs, K.; Bohlke, J.K. Geochemistry of reduced gas related to serpentinization of the Zambales ophiolite, Philippines. Appl. Geochem. 1990, 5, 625–630. [Google Scholar] [CrossRef]
- Coveney, R.M., Jr.; Goebel, E.D.; Zeller, E.J.; Dreschhoff, G.A.M.; Angino, E.E. Serpentinization and the Origin of Hydrogen Gas in Kansas. AAPG Bull. 1987, 71, 39–48. [Google Scholar] [CrossRef]
- Morrill, P.L.; Kuenen, J.G.; Johnson, O.J.; Suzuki, S.; Rietze, A.; Sessions, A.L.; Fogel, M.L.; Nealson, K.H. Geochemistry and geobiology of a present-day serpentinization site in California: The Cedars. Geochim. Et Cosmochim. Acta 2013, 109, 222–240. [Google Scholar] [CrossRef]
- Yuce, G.; Italiano, F.; D’Alessandro, W.; Yalcin, T.H.; Yasin, D.U.; Gulbay, A.H.; Ozyurt, N.N.; Rojay, B.; Karabacak, V.; Bellomo, S.; et al. Origin and interactions of fluids circulating over the Amik Basin (Hatay, Turkey) and relationships with the hydrologic, geologic and tectonic settings. Chem. Geol. 2014, 388, 23–39. [Google Scholar] [CrossRef]
- Canfield, D.E.; Rosing, M.T.; Bjerrum, C. Early anaerobic metabolisms. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1819–1836. [Google Scholar] [CrossRef]
- Symonds, R.B.; Poreda, R.J.; Evans, W.C.; Janik, C.J.; Ritchie, B.E. Mantle and crustal sources of carbon, nitrogen, and noble gases in Cascade-Range and Aleutian-Arc volcanic gases. US Geol. Surv. Open-File Rep. 2003, 03-436, 26. [Google Scholar]
- Hao, Y.; Pang, Z.; Tian, J.; Wang, Y.; Li, Z.; Li, L.; Xing, L. Origin and evolution of hydrogen-rich gas discharges from a hot spring in the eastern coastal area of China. Chem. Geol. 2020, 538, 119477. [Google Scholar] [CrossRef]
- Zgonnik, V.; Beaumont, V.; Deville, E.; Larin, N.; Pillot, D.; Farrell, K.M. Evidence for natural molecular hydrogen seepage associated with Carolina bays (surficial, ovoid depressions on the Atlantic Coastal Plain, Province of the USA). Prog. Earth Planet. Sci. 2015, 2, 1–15. [Google Scholar] [CrossRef]
- Zgonnik, V.; Beaumont, V.; Larin, N.; Pillot, D.; Deville, E. Diffused flow of molecular hydrogen through the Western Hajar mountains, Northern Oman. Arab. J. Geosci. 2019, 12, 1–10. [Google Scholar] [CrossRef]
- Scott, E. Exploring for native hydrogen. Nat. Rev. Earth Environ. 2021, 2, 589. [Google Scholar] [CrossRef]
- Halas, P.; Dupuy, A.; Franceschi, M.; Bordmann, V.; Fleury, J.M.; Duclerc, D. Hydrogen gas in circular depressions in South Gironde, France: Flux, stock, or artefact? Appl. Geochem. 2021, 127, 104928. [Google Scholar] [CrossRef]
- Frery, E.; Langhi, L.; Maison, M.; Moretti, I. Natural hydrogen seeps identified in the North Perth Basin, Western Australia. Int. J. Hydrogen Energy 2021, 46, 31158–31173. [Google Scholar] [CrossRef]
- Prinzhofer, A.; Moretti, I.; Françolin, J.; Pacheco, C.; d’Agostino, A.; Werly, J.; Rupin, F. Natural hydrogen continuous emission from sedimentary basins: The example of a Brazilian H2-emitting structure. Int. J. Hydrogen Energy 2019, 44, 5676–5685. [Google Scholar] [CrossRef]
- Truche, L.; Joubert, G.; Dargent, M.; Martz, P.; Cathelineau, M.; Rigaudier, T.; Quirt, D. Clay minerals trap hydrogen in the Earth’s crust: Evidence from the Cigar Lake uranium deposit, Athabasca. Earth Planet. Sci. Lett. 2018, 493, 186–197. [Google Scholar] [CrossRef]
- Багрій, І.; Гoжик, П.; Павлюк, М.; Забулoнoв, Ю.; Рудькo, Г.; Мальчевський, І. Обґрунтування пoшукoвoї технoлoгії вoдневих скупчень та геoдинамічних явищ. Фoліант 2019, 2, 18–28. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals radii of hydrogen in gas-phase and condensed molecules. Struct. Chem. 1999, 10, 395–400. [Google Scholar] [CrossRef]
- Giardini, A.A.; Subbarayudu, G.V.; Melton, C.E. The emission of occluded gas from rocks as a function of stress: Its possible use as a tool for predicting earthquakes. Geophys. Res. Lett. 1976, 3, 355–358. [Google Scholar] [CrossRef]
- Grozeva, N.G.; Klein, F.; Seewald, J.S.; Sylva, S.P. Chemical and isotopic analyses of hydrocarbon-bearing fluid inclusions in olivine-rich rocks. Philos. Trans. R. Soc. A 2020, 378, 20180431. [Google Scholar] [CrossRef]
- Klein, F.; Grozeva, N.G.; Seewald, J.S. Abiotic methane synthesis and serpentinization in olivine-hosted fluid inclusions. Proc. Natl. Acad. Sci. USA 2019, 116, 17666–17672. [Google Scholar] [CrossRef]
- Konnerup-Madsen, J.; Rose-Hansen, J. Volatiles associated with alkaline igneous rift activity: Fluid inclusions in the Ilímaussaq intrusion and the Gardar granitic complexes (South Greenland). Chem. Geol. 1982, 37, 79–93. [Google Scholar] [CrossRef]
- Chapelle, F.H.; O’Neill, K.; Bradley, P.M.; Methé, B.A.; Ciufo, S.A.; Knobel, L.L.; Lovley, D.R. A hydrogen-based subsurface microbial community dominated by methanogens. Nature 2002, 415, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Takai, K.; Gamo, T.; Tsunogai, U.; Nakayama, N.; Hirayama, H.; Nealson, K.H.; Horikoshi, K. Geochemical and microbiological evidence for a hydrogen-based, hyperthermophilic subsurface lithoautotrophic microbial ecosystem (HyperSLiME) beneath an active deep-sea hydrothermal field. Extremophiles 2004, 8, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Nealson, K.H.; Inagaki, F.; Takai, K. Hydrogen-driven subsurface lithoautotrophic microbial ecosystems (SLiMEs): Do they exist and why should we care? Trends Microbiol. 2005, 13, 405–410. [Google Scholar] [CrossRef]

| Free gas | Oil and gas fields |
| Orebodies | |
| Kimberlite pipes | |
| Volcanic gas | |
| Hot springs | |
| Faults | |
| Adsorbed | Sedimentary basins |
| In inclusions | In ultrabasic rocks |
| In precambrian rocks | |
| In igneous rocks | |
| In volcanic rocks | |
| Dissolve in water | Hydrocarbon fields |
| Ground water | |
| In waters from fractured Precambrian Shield rocks |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jin, Z.; Chen, X.; Su, Y.; Huang, X. The Origin and Occurrence of Natural Hydrogen. Energies 2023, 16, 2400. https://doi.org/10.3390/en16052400
Wang L, Jin Z, Chen X, Su Y, Huang X. The Origin and Occurrence of Natural Hydrogen. Energies. 2023; 16(5):2400. https://doi.org/10.3390/en16052400
Chicago/Turabian StyleWang, Lu, Zhijun Jin, Xiao Chen, Yutong Su, and Xiaowei Huang. 2023. "The Origin and Occurrence of Natural Hydrogen" Energies 16, no. 5: 2400. https://doi.org/10.3390/en16052400
APA StyleWang, L., Jin, Z., Chen, X., Su, Y., & Huang, X. (2023). The Origin and Occurrence of Natural Hydrogen. Energies, 16(5), 2400. https://doi.org/10.3390/en16052400






