Abstract
In response to the escalating global energy requests and the need to address them in a sustainable manner, researchers have identified hydrogen as an energy vector that provides a practical way to store and use energy from renewable sources. To make a step forward, the electrocatalytic properties for the hydrogen evolution reaction in an alkaline medium of graphite electrodes modified with combinations of nickel phosphite and free-base porphyrins were investigated voltammetrically. The sample obtained by combining the respective phosphite with 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin exhibited the highest catalytic activity, surpassing that observed for the specimens manufactured using the individual catalysts and thus providing enhanced water-splitting performance. The best electrode displayed an overpotential of 0.43 V (at i = −10 mA/cm2) and a Tafel slope of 0.14 V/dec. Since the catalytic activity of the compositions containing the metal salt and the porphyrins has not been previously studied, the investigation and its outcome constitute an original contribution to the growing water-splitting literature, providing new opportunities for obtaining better results than the ones reported for electrodes modified solely with the phosphite.
1. Introduction
As the global energy crisis becomes an ever more pressing concern due to the rapid depletion of fossil fuels accompanied by acute environmental pollution, there is an urgent need to develop eco-friendly renewable energy sources and promote clean energy carriers [1,2,3]. Hydrogen has the potential to be such a carrier, but not all strategies for obtaining it are non-polluting. For example, H2 production via natural gas reforming creates undesired CO2 [4], but electrocatalytic water splitting is an encouraging approach for the large-scale generation of green H2 [5]. However, because of the sluggish kinetics of the two half-cell main reactions of the process, namely the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER), only <5% of the globally produced amount of H2 originates from electrocatalytic water splitting [1]. To address this issue, researchers have been continuously trying to find more efficient catalytic materials, and as a result, the current benchmark electrocatalysts are RuO2 and IrO2 for the OER and Pt for the HER [6]. Since noble metals are both rare and expensive, they cannot ensure large-scale electrochemical H2 production, so the search for electrocatalysts has become focused on transition metal-based materials [7,8]. Studies performed on these cheaper catalysts can be aimed at evaluating the water-splitting properties of each single material or of a combination thereof [9,10,11,12]. In the case of combinations, it should be pointed out that not all of the catalysts have to be metal-based, and there are reported studies in which one of the components is metal-free [13,14]. Among the metal-free compounds that have been shown to exhibit water-splitting catalytic properties are free-base porphyrins [15,16]. Porphyrins are extended aromatic heterocycles, diversely substituted, that share the central porphine structure comprised of four pyrrole rings bonded in the α position with four methine moieties [17].
Some basic notions regarding this class of macromolecules are in order [17,18,19,20]. For one thing, their macrocycle is an aromatic system with 18 delocalized π electrons, and as is usually the case with extended aromaticity-possessing compounds, porphyrins exhibit increased thermodynamic stability. For another, they are amphoteric—a behavior for which the four N atoms located in the center of the macrocycle are responsible. Because of its size, the macrocycle can bind nearly all metal cations. However, when metallated, porphyrins no longer possess both acidic and basic properties. They are known to occur naturally but are also products of laboratorial synthesis. Concerning their applicative potential, based on their optical properties, porphyrin derivatives are being used to manufacture functional devices, used as dyes for dye-sensitized solar cells and used in the medical field—specifically in the photodynamic therapy of cancer.
One feature evidenced and shared by all members of the porphyrin class is their ability to assemble into self-organized structures of different complexity, that are stable and display clearly distinguishable features and boundaries [21]. The formation of the new architectures occurs spontaneously when the appropriate conditions are fulfilled, and the interactions between the molecules serving as their building blocks are non-covalent [22]. The simplest arrangements are typically J-type (or side-by-side) and H-type (or face-to-face, or sandwich) stackings. The more complex ones result either from the interactions between tokens of one of these types or both [23]. The porphyrin assemblies develop properties of their own, and in some reported cases, they preserve the ability to catalyze the decomposition of water [24,25].
Porphyrins that have a metal cation in the center of their macrocycle are called metalloporphyrins, and they have also been shown to display catalytic activity for H2 production via electrochemical water splitting [26,27]. The same can be said about compounds with a similar chemical structure, such as metallophthalocyanines [28,29].
Due to the major advantages of a stable structure and high designability allowing to modify the molecule with various functional groups [30], porphyrins and their large plethora of metalloporphyrin derivatives are among the main candidates to catalyze the HER and the OER. For instance, GaIII chloride tetrakis(pentafluorophenyl)porphyrin demonstrated to be a properly active and very stable electrocatalyst for the HER, involving the macrocycle ligand in electron transfer and the GaIII ion in the hydride-binding process [31]. The study reported by Wang et al. [32] is another example of an investigation relevant to the water splitting field in which a porphyrin-based catalyst was employed for the electrochemical decomposition of water. The researchers obtained porphyrin coordination polymer supported transition-metal sulfides and identified the catalyst with the highest activity for both half-cell main reactions in acidic electrolyte solution. Its behavior was attributed to a synergistic effect and to its particular structural characteristics. In yet another fitting example of a porphyrin derivative-based electrocatalyst for water splitting [33], a covalent metalloporphyrin polymer was coated on carbon nanotubes and revealed to have high catalytic activity for overall water splitting in basic medium following an optimization procedure. Ge et al. [15]. found that a newly synthesized 2D crystalline covalent organic polymer based on 5,10,15,20-tetrakis(4-aminophenyl)porphyrin can be used as an efficient HER electrocatalyst in strong acidic medium and OER electrocatalyst in strong alkaline environment. Furthermore, it was able to catalyze both reactions in neutral phosphate buffer solution. When the researchers replaced the free-base porphyrin with its Zn-derivative, the activity of the catalytic material depreciated for both reactions. This observation indicated the center of the metal-free porphyrin macrocycle as the catalytically active site. Lastly, an investigation focusing on polymers electrosynthesized from cobalt metalloporphyrins and corroles, showed that the electropolymer formed on carbon cloth from cobalt tetrakis(p-N-pyrrolylphenyl)porphyrin behaved as the most active catalyst for the oxygen evolution reaction in alkaline medium [34]. The study also evidenced the stability of the catalytically active films.
The present study is an original contribution to the expanding scientific literature relevant to the water-splitting domain. It evaluates the HER electrocatalytic properties in an alkaline medium of graphite electrodes modified with combinations between a transition metal-based compound—Ni11(HPO3)8(OH)6—and each member from a series of four metal-free porphyrins. Previous reports have shown that nickel phosphite (NiPh) exhibits water-splitting catalytic activity [35,36,37,38]. As for the porphyrins, namely 5-(4-pyridyl)-10,15,20-tris(4-phenoxyphenyl)porphyrin (P1), 5,10,15,20-tetrakis(4-allyloxyphenyl)porphyrin (P2), 5,10,15,20-tetrakis(p-tolyl)porphyrin (P3) and 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin (P4), they have been the focus of various studies reported by Fagadar et al. [23,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53], during which they were thoroughly characterized by physical–chemical and electrochemical methods, and their potential applications were evidenced together with their HER and OER electrocatalytic activity in basic medium. However, the alkaline HER (A-HER) catalytic activity of compositions containing NiPh and each of the porphyrins has not been previously reported. In the current work, the new compositions based on the specified materials were employed to remedy this situation. Their A-HER catalytic activity was evaluated for the first time, but the originality of the investigation is also provided by the fact that, to the best of our knowledge, the electrochemical water-splitting activity of any combination between a porphyrin and nickel phosphite has not been the focus of previously published studies.
2. Materials and Methods
2.1. Materials and Reagents
NiPh was synthesized hydrothermally using high temperature and high pressure, as previously reported [37,54]. The free-base porphyrins with the chemical structures shown in Scheme 1 were also synthesized based on published procedures—P1 [39], P2 [48,55], P3 [56] and P4 [57].
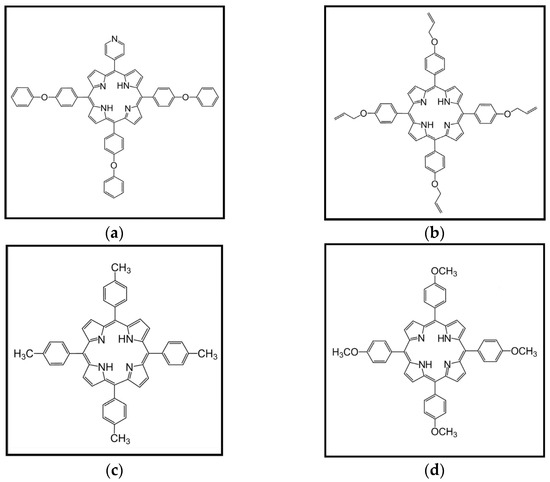
Scheme 1.
Chemical structures of: (a) 5-(4-pyridyl)-10,15,20-tris(4-phenoxyphenyl)porphyrin; (b) 5,10,15,20-tetrakis(4-allyloxyphenyl)porphyrin; (c) 5,10,15,20-tetrakis(p-tolyl)porphyrin; (d) 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin.
Porphyrin solutions were obtained with different solvents, as follows: N,N-dimethylformamide (DMF), benzonitrile (PhCN), tetrahydrofuran (THF) and dichloromethane (DCM), purchased from Sigma Aldrich (Saint Louis, MO, USA) and Merck (Darmstadt, Germany). The subsequent reagents were also used in the study: KOH (Merck), KNO3 (Merck), K3[Fe(CN)6] (Sigma Aldrich), C2H5OH (Honeywell, Charlotte, NC, USA) and C3H6O (Chimreactiv, Bucharest, Romania). Bi-distilled water was employed to prepare all aqueous solutions. Spectroscopic graphite rods of SW.114 type (“Kablo Bratislava”, National Corporation “Electrocarbon Topolcany” Factory, Bratislava, Slovakia) were utilized as electroconductive substrate in the electrode manufacturing process.
2.2. Electrode Manufacturing Procedure
After inserting the graphite rods (ø = 6 mm) into polyethylene tubes, a heat treatment (of 180 °C) was applied to ensure the sealing between the tubes and the graphite rods. The two ends of each rod served different purposes during the study. One was modified with the catalytic materials and immersed into the alkaline electrolyte, while the other was coupled to the potentiostat. The modification procedure involved several steps: (a) the surface of the rod end was polished with silicon carbide paper and felt; (b) the polished surface was washed with bi-distilled water, ethanol and acetone; (c) after drying at 23 ± 2 °C, a volume of 10 µL from a suspension of NiPh in porphyrin solution was drop-casted on the graphite support and (d) the modified substrate dried at 40 °C for 4 h and at 23 ± 2 °C for 20 h. Appropriate porphyrin amounts were dissolved in the previously specified organic solvents with different polarities—DMF > PhCN > THF > DCM [58,59]—to obtain 0.15 mM solutions. Subsequently, 1.5 mg NiPh were added to each (0.5 mL) solution, and the resulting suspensions were ultrasonicated for 40 min. The manufactured modified electrodes were designated as shown in Table 1.

Table 1.
Electrode designations.
2.3. Electrochemical Experiments
The electrochemical setup consisted of a glass cell with three electrodes coupled to a Voltalab PGZ 402 potentiostat (Radiometer Analytical, Lyon, France). G0 and each modified sample were used as working electrodes (Sgeom = 0.28 cm2), a Pt plate (Sgeom = 0.8 cm2) served as an auxiliary electrode and the Ag/AgCl (sat. KCl) electrode was employed as reference. The electrolyte solutions utilized during the A-HER experiments were 0.1 M and 1 M KOH aqueous solutions. The selection of these alkaline media, as opposed to acidic or neural environments, had to do with the reactivity and stability of the non-noble metal-based electrocatalysts for water splitting. The studies reported in the scientific literature [60,61] indicate the following tendency for the respective catalysts: their stability decreases from neutral to alkaline and to acidic medium, while their catalytic activity decreases from acidic to alkaline and to neutral environment. Thus, to avoid the issues of low stability and poor activity, the investigations were performed in basic electrolyte solutions. The four porphyrin-base compounds are very stable in alkaline media.
The iR-corrected cathodic polarization curves were traced at the scan rate (v) of 5 mV/s, in agreement with reported studies [36,45]. Before each A-HER experiment, the electrolyte solution was subjected to high-purity nitrogen bubbling for deaerating. The electrochemical potential (E) was expressed vs. the reversible hydrogen electrode (RHE) using Equation (1) [62]. Unless otherwise specified, the values of the current density (i) refer to the geometric current density. The hydrogen evolution overpotential was obtained with Equation (2), and the Tafel slope values were determined with Equation (3) [63,64].
where: ERHE is the reversible hydrogen electrode potential (V), EAg/AgCl(sat. KCl) is the potential vs. the Ag/AgCl (sat. KCl) reference electrode (V), ηH2 is the hydrogen evolution overpotential (V), i is the current density (mA/cm2) and b is the Tafel slope.
ERHE = EAg/AgCl(sat. KCl) + 0.059 × pH + 0.197
ηH2 = |ERHE|
ηH2 = b × log(i) + a
To estimate the electroactive surface area (EASA) of the most catalytically active electrode, cyclic voltammograms were traced in the 0–0.8 V potential range, at increasing scan rate values (v = 0.05, 0.1, 0.15, 0.2, 0.25, 0.3 and 0.35 V/s), in 1 M KNO3 electrolyte solution as such and in the presence of 4 mM K3[Fe(CN)6]. The voltammetry data were then used to calculate the EASA with the Randles–Sevcik equation [65,66]—Equation (4).
where: Ip = the peak current (A); n = the number of electrons involved in the redox process at T = 298 K; A = the surface area of the working electrode (cm2); D = the diffusion coefficient of the electroactive species (cm2/s); C = the bulk concentration of the electroactive species (M) and v = the scan rate (V/s). For the ferrocyanide/ferricyanide redox couple, n = 1 and the theoretical value of the diffusion coefficient is 6.7 × 10−6 cm2/s [67].
Ip = (2.69 × 105) × n3/2 × A × D1/2 × C × v1/2
2.4. Morphostructural Characterization
A scanning electron microscope—Inspect S model (FEI Company, Hillsboro, OR, USA)—equipped with an energy-dispersive X-ray spectroscopy (EDS) module and operated in high-vacuum mode was used to record scanning electron microscopy (SEM) images and elemental maps on the surface of the modified electrodes.
3. Results
3.1. A-HER Electrocatalytic Activity of the Modified Electrodes
The polarization curves recorded in a 0.1 M KOH medium on the electrodes manufactured using compositions made of NiPh and each of the four porphyrins are presented in Figure 1. The overpotential (ηH2) was determined at the current density of −10 mA/cm2, in agreement with other studies [61,68], and the smallest value was found for GP4-NiPh-THF (ηH2 = 0.65 V). To better understand the electrocatalytic properties of this electrode, it was further investigated in a strong alkaline solution (1 M KOH) and compared with the sample obtained by applying 1.5 mg NiPh from THF on the graphite support, designated GNiPh-THF. Based on the experimental results shown in Figure 2, the electrocatalytic activity of GP4-NiPh-THF is superior to that of GNiPh-THF. The ηH2 values for the former electrode were smaller than those for the latter at all the current density values observed in the investigated potential range in which H2 evolved. At i = −10 mA/cm2, ηH2 = 0.43 V for GP4-NiPh-THF and 0.47 V for GNiPh-THF. A comparison between the results obtained on GP4-NiPh-THF in the two electrolyte solutions reveals that the increase in KOH concentration led to the improvement of the catalytic activity—a previously reported phenomenon [62,64].
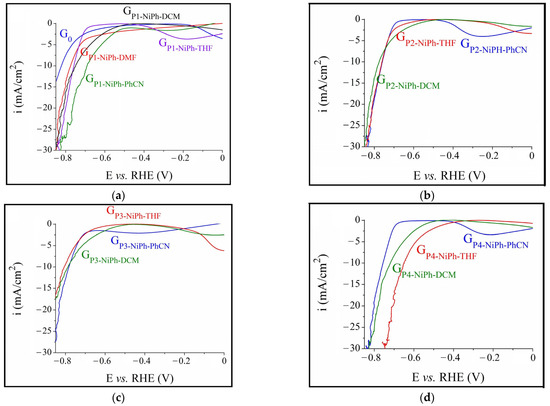
Figure 1.
Cathodic polarization curves obtained in 0.1 M KOH solution on G0 and on the specimens modified with compositions based on NiPh and (a) P1, (b) P2, (c) P3 and (d) P4.
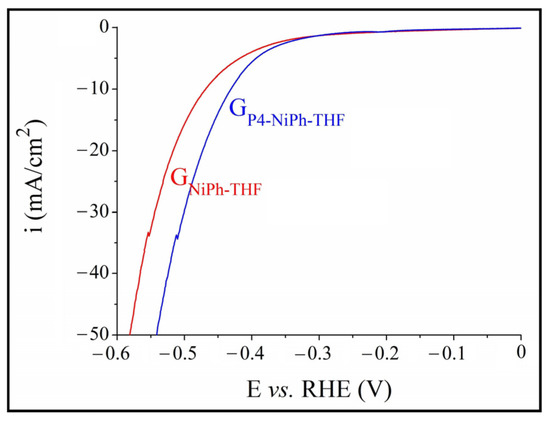
Figure 2.
Cathodic polarization curves recorded in 1 M KOH solution on the GP4-NiPh-THF and GNiPh-THF modified electrodes.
The EASA value was estimated for both specimens, GP4-NiPh-THF and GNiPh-THF, following the procedure described in the Experimental Section and it was found to be 0.511 cm2 and 0.427 cm2, respectively. The cyclic voltammograms traced at increasing scan rates in 1 M KNO3 solution with 4 mM K3[Fe(CN)6] revealed the anodic and cathodic peaks characteristic of the ferrocyanide/ferricyanide redox couple. The anodic and cathodic peak current densities were represented graphically vs. the square root of the scan rate (Figure 3). For both electrodes, the absolute values of the peak current densities increased directly proportional to the scan rate. The linear dependence between the two parameters indicates that the oxidation and reduction reactions were diffusion controlled [65,69].
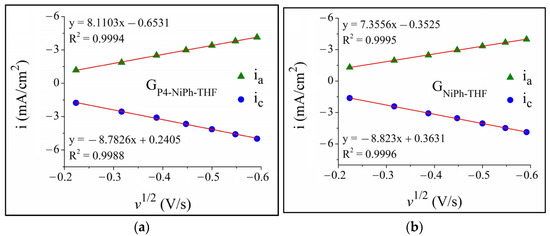
Figure 3.
The plots of the anodic and cathodic peak current densities vs. the square root of the scan rate (ia and ic) for the (a) GP4-NiPh-THF and (b) GNiPh-THF modified electrodes.
The HER kinetics at the GNiPh-THF/electrolyte and GP4-NiPh-THF/electrolyte interfaces were investigated in a 1 M KOH solution. To obtain the Tafel plots shown in Figure 4a the current density values were normalized by the EASA values (iEASA). The Tafel slope can be determined based on the Tafel plots and it constitutes an indicator of the electrocatalytic activity of electrodes, with the smaller value representing the more rapid chemical reaction kinetics [70]. In the case of the GNiPh-THF and GP4-NiPh-THF samples, the determined Tafel slope values were 0.16 and 0.14 V/dec, respectively. The smaller overpotential and Tafel slope for GP4-NiPh-THF outline its superior electrocatalytic properties.
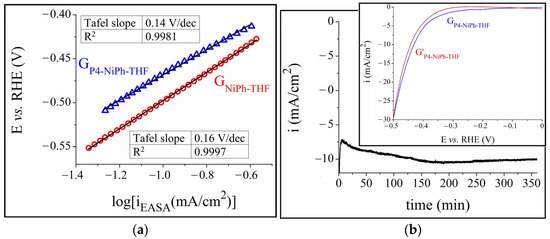
Figure 4.
(a) The Tafel plots obtained for the GP4-NiPh-THF and GNiPh-THF modified electrodes in 1 M KOH solution. (b) The i-time curve recorded on the GP4-NiPh-THF modified electrode in 1 M KOH solution and inset with the linear sweep voltammograms recorded on the same electrode before (GP4-NiPh-THF) and after (G’P4-NiPh-THF) the stability test.
Since an electrode’s electrochemical stability is also important when considering its potential application in the water-splitting field, a chronoamperometric test was performed on the specified sample and the recorded current density vs. time curve is presented in Figure 4b. The experiment lasted 6 h in 1 M KOH solution and at E = −0.43 V. The current density increased to −7.2 mA/cm2 in the first minutes, gradually decreased to i = −10.5 mA/cm2 at t = 190 min, then it increased to ~−10 mA/cm2 and remained nearly constant until the test ended. The inset displays the curves traced on the GP4-NiPh-THF electrode before and after the stability study. A small increase in overpotential, of ~10 mV, was observed at i = −10 mA/cm2, indicating that the sample was relatively stable in the strong alkaline medium.
The electrochemical evaluation of the specimen’s catalytic properties for the H2 evolution reaction in basic electrolyte solutions was followed by its morphostructural investigation.
3.2. Morphostructural Study
SEM analysis was performed on the GNiPh-THF and GP4-NiPh-THF samples, as well as on an electrode manufactured by drop-casting only P4 from THF (designated GP4-THF). Figure 5a presents a SEM micrograph obtained on GP4-THF. The porphyrin molecules self-assembled as bundles comprised of smaller aggregates with different shapes, including square-like, rectangle-like and irregular architectures. The investigation of the GNiPh-THF specimen revealed a preponderance of elongated structures resembling ribbons, rods and needles (Figure 5b). Figure 5c,d show the EDS elemental maps of Ni and P obtained on the same area displayed in Figure 5b that confirm the presence of these atoms from the nickel phosphite sample.
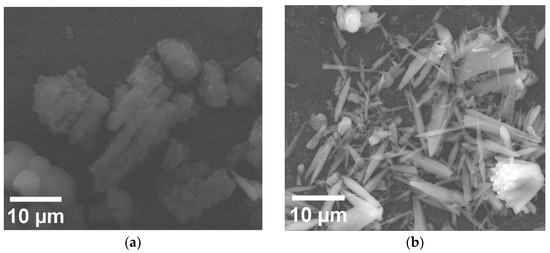
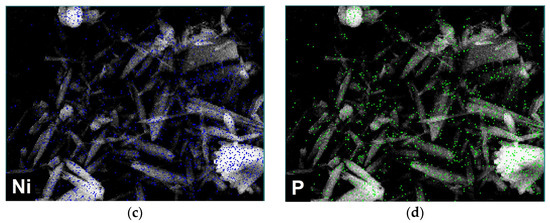
Figure 5.
(a) SEM micrograph recorded on the GP4-THF modified electrode. (b) SEM micrograph obtained on the GNiPh-THF specimen. (c,d) EDS elemental maps of Ni and P recorded on GNiPh-THF.
The SEM characterization of the electrode manufactured using P4 and NiPh outlined a mixture between the porphyrin bundles and the previously specified elongated structures (Figure 6a). The SEM image from Figure 6b was obtained on the same specimen after performing the chronoamperometric stability test. Very similar architectures can be distinguished, indicating that the surface of the GP4-NiPh-THF electrode did not suffer any notable changes due to the electrochemical experiment. Furthermore, the recorded EDS maps of Ni and P (Figure 6c,d) show the presence of these atoms after the test, evidencing the electrode’s stability.
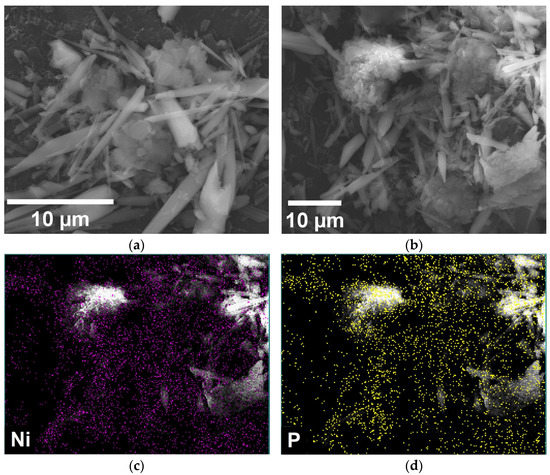
Figure 6.
(a) SEM micrograph recorded on the GP4-NiPh-THF electrode before the stability test. (b) SEM micrograph obtained on GP4-NiPh-THF after the stability test. (c,d) EDS elemental maps of Ni and P recorded on GP4-NiPh-THF after the test.
3.3. Additional Considerations
As mentioned, GP4-NiPh-THF displayed smaller overpotential and Tafel slope values than GNiPh-THF. Furthermore, considering the previously reported results by Fagadar et al. [45], it was more electrocatalytically active than GP4-THF and also than the most catalytically active specimen identified in the respective investigation. The latter exhibited an A-HER overpotential value of 0.5 V (at i = −10 mA/cm2) and a Tafel slope of 0.19 V/dec. In other words, combining the two catalytic materials led to enhanced electrocatalytic activity.
It is known that porphyrin molecules self-assemble via non-covalent interactions and form well-defined aggregates [24]. The SEM analysis of GP4-THF revealed inhomogeneities represented by the disordered distribution of the P4 aggregates, as well as by their fringes and faults. Inhomogeneities increase the electrocatalytically active sites exposed to the electrolyte and consequentially improve the electrode’s catalytic activity. For free-base porphyrins, the catalytically active site is constituted by the central N4 site of the macrocycle [16].
It should also be pointed out that when the electrode substrate is made from atoms that are less electronegative than the N atoms of the metal-free porphyrin, as is the case with graphite, charge transfer effects unfold between the organic compound and the support that induced positive charges on the surface of the latter. These effects further contribute to the electrode’s catalytic activity [16] together with similar transport effects, as well as structural and π-resonance phenomena [45,71].
NiPh can catalyse the HER with its own active sites. Menezes et al. [36] performed a detailed study of this material and evidenced the participation in a strong basic medium of the phosphite anions as catalytically active sites for H2 evolution with the assistance of the Ni2+ cations.
In the case of the GP4-NiPh-THF electrode, the estimated EASA value was higher than the one calculated for GNiPh-THF. Because of the direct proportionality relationship between EASA and the active sites implicated in an electrochemical process [72], it can be concluded that by combining P4 and NiPh the number of active sites exposed to the electrolyte increased and led to improved electrocatalytic activity. Furthermore, the mixture of the two materials might also have enhanced the charge transfer and regulated the electron density at the active sites [73,74]. This result could be even more clearly explained by the fact that although the methoxy-grafted group can be considered to exert an electron-withdrawing inductive effect due to the inductive effect of the oxygen atom, the lone pairs on the oxygen determine the opposite effect. Consequently, the methoxy substituent can also be considered an electron-donating group, but by resonance. This versatile behaviour could contribute to controlling the electron density on the active sites, eventually improving the catalytic activity.
The A-HER electrocatalytic properties of GP4-NiPh-THF are inferior to those of the commercial Pt/C on glassy carbon substrate (ηH2 = 0.051 V at i = −10 mA/cm2 and Tafel slope = 0.024 V/dec) [75] and to those reported by Menezes et al. [36], who used electrophoretic deposition to manufacture a NiPh-modified nickel foam electrode (ηH2 = 0.121 V at i = −10 mA/cm2 and Tafel slope = 0.102 V/dec). However, the conclusion that the combination between NiPh and P4 is more electrocatalytically active for the HER in the basic solution than NiPh alone opens up new opportunities for obtaining even better results than those already found in the literature for electrodes modified solely with the metal salt.
Of the published work focusing on materials with HER catalytic activity in a 1 M KOH electrolyte solution, results comparable with the ones obtained in this study were found for the electrodes specified in what follows. A glassy carbon electrode modified with Co-embedded N-rich carbon nanotubes manufactured by Zou et al. [76] exhibited an overpotential of 0.37 V. In another example, Lai et al. [77] prepared a tri-doped porous graphite carbon on oxidized carbon cloth electrode for which ηH2 = 0.446 V, and the Tafel slope was 0.154 V/dec. Values of 0.427 V and 0.213 V/dec for the same parameters were communicated for a graphite electrode modified with hydrothermally synthesized MnO2 and Nafion [66]. An electrode prepared by modifying glassy carbon with N-doped graphene microtubes was investigated by Zhang et al. [78], and the determined ηH2 and Tafel slope values were 0.432 V and 0.117 V/dec, respectively. Further examples include metalloporphyrin-based modified electrodes manufactured and reported by Cai et al. [79], Wang et al. [80] and Jia et al. [81].
4. Conclusions
The HER electrocatalytic activity of graphite electrodes modified with combinations between nickel phosphite and free-base porphyrins in alkaline electrolyte solutions was successfully evaluated. The most active sample was found to be GP4-NiPh-THF, it displayed an overpotential of 0.43 V (at i = −10 mA/cm2) and a Tafel slope value of 0.14 V/dec. Its catalytic activity surpassed that of the electrodes modified with the individual components. The electrochemical stability of the GP4-NiPh-THF specimen in a strong alkaline environment was also investigated and revealed good results. The morphostructural study performed on the GNiPh-THF, GP4-THF and GP4-NiPh-THF electrodes provided a deeper understanding of the way in which NiPh and P4 organize on the graphite support, individually and combined.
Regarding future research directions, replacing the drop-casting method with more advanced electrode modification techniques, such as pulsed laser deposition, is a promising approach for obtaining a specimen with improved A-HER electrocatalytic properties.
Author Contributions
Conceptualization, B.-O.T. and E.F.-C.; Methodology, B.-O.T.; Software, B.-O.T.; Validation, E.F.-C.; Formal analysis, B.-O.T. and E.F.-C.; Investigation, B.-O.T. and P.S.; Resources, E.F.-C. and M.P.; Data curation, B.-O.T., P.S. and M.P.; Writing—original draft preparation, B.-O.T. and E.F.-C.; Writing—review and editing, B.-O.T. and E.F.-C.; Visualization, B.-O.T.; Supervision, E.F.-C.; Project administration, P.S. and M.P.; Funding acquisition, E.F.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Romanian Academy, Programme number 3/2023 from ICT.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, M.; Liu, D.; Zi, B.; Chen, Y.; Liu, D.; Du, X.; Li, F.; Zhou, P.; Ke, Y.; Li, J.; et al. Remarkable Synergistic Effect in Cobalt-Iron Nitride/Alloy Nanosheets for Robust Electrochemical Water Splitting. J. Energy Chem. 2022, 65, 405–414. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, P.; Lin, J.; Cao, J.; Qi, J. Modification Strategies on Transition Metal-Based Electrocatalysts for Efficient Water Splitting. J. Energy Chem. 2021, 58, 446–462. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Qin, Y.; Zhang, W.; Wang, L.; Luo, M.; Yang, H.; Guo, S. Recent Advances on Water-Splitting Electrocatalysis Mediated by Noble-Metal-Based Nanostructured Materials. Adv. Energy Mater. 2020, 10, 1903120. [Google Scholar] [CrossRef]
- Crabtree, G.W.; Dresselhaus, M.S. The Hydrogen Fuel Alternative. MRS Bull. 2008, 33, 421–428. [Google Scholar] [CrossRef]
- Hota, P.; Das, A.; Maiti, D.K. A Short Review on Generation of Green Fuel Hydrogen through Water Splitting. Int. J. Hydrog. Energy 2023, 48, 523–541. [Google Scholar] [CrossRef]
- Chakraborty, B.; Beltrán-Suito, R.; Hausmann, J.N.; Garai, S.; Driess, M.; Menezes, P.W. Enabling Iron-Based Highly Effective Electrochemical Water-Splitting and Selective Oxygenation of Organic Substrates through In Situ Surface Modification of Intermetallic Iron Stannide Precatalyst. Adv. Energy Mater. 2020, 10, 2001377. [Google Scholar] [CrossRef]
- Li, X.P.; Huang, C.; Han, W.K.; Ouyang, T.; Liu, Z.Q. Transition Metal-Based Electrocatalysts for Overall Water Splitting. Chin. Chem. Lett. 2021, 32, 2597–2616. [Google Scholar] [CrossRef]
- Li, S.; Li, E.; An, X.; Hao, X.; Jiang, Z.; Guan, G. Transition Metal-Based Catalysts for Electrochemical Water Splitting at High Current Density: Current Status and Perspectives. Nanoscale 2021, 13, 12788–12817. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Li, X.; Mu, S.; Verpoort, F.; Xue, J.; Kou, Z.; Wang, J. Nurturing the Marriages of Single Atoms with Atomic Clusters and Nanoparticles for Better Heterogeneous Electrocatalysis. Interdiscip. Mater. 2022, 1, 51–87. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Zang, W.; Li, X.; Chen, D.; Kou, Z.; Mu, S.; Wang, J. Nanoframes of Co3O4–Mo2N Heterointerfaces Enable High-Performance Bifunctionality toward Both Electrocatalytic HER and OER. Adv. Funct. Mater. 2022, 32, 2107382. [Google Scholar] [CrossRef]
- Si, C.; Zhang, W.; Lu, Q.; Guo, E.; Yang, Z.; Chen, J.; He, X.; Luo, J. Recent Advances in Perovskite Catalysts for Efficient Overall Water Splitting. Catalysts 2022, 12, 601. [Google Scholar] [CrossRef]
- Raja, D.S.; Lin, H.W.; Lu, S.Y. Synergistically Well-Mixed MOFs Grown on Nickel Foam as Highly Efficient Durable Bifunctional Electrocatalysts for Overall Water Splitting at High Current Densities. Nano Energy 2019, 57, 1–13. [Google Scholar] [CrossRef]
- Wan, W.; Wei, S.; Li, J.; Triana, C.A.; Zhou, Y.; Patzke, G.R. Transition Metal Electrocatalysts Encapsulated into N-Doped Carbon Nanotubes on Reduced Graphene Oxide Nanosheets: Efficient Water Splitting through Synergistic Effects. J. Mater. Chem. A 2019, 7, 15145–15155. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.; Lu, X. Integrated Transition Metal and Compounds with Carbon Nanomaterials for Electrochemical Water Splitting. J. Mater. Chem. A 2021, 9, 3786–3827. [Google Scholar] [CrossRef]
- Ge, Y.; Lyu, Z.; Marcos-Hernández, M.; Villagrán, D. Free-Base Porphyrin Polymer for Bifunctional Electrochemical Water Splitting. Chem. Sci. 2022, 13, 8597–8604. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Lee, K.; Min, M.; Cho, Y.; Kim, M.; Lee, H. A Molecular Approach to an Electrocatalytic Hydrogen Evolution Reaction on Single-Layer Graphene. Nanoscale 2017, 9, 3969–3979. [Google Scholar] [CrossRef] [PubMed]
- Fagadar-Cosma, E.; Vlascici, D.; Fagadar-Cosma, G. Porfirinele de la Sinteză la Aplicații; Eurostampa: Timisoara, Romania, 2008; ISBN 978-973-687-680-6. [Google Scholar]
- Král, V.; Králová, J.; Kaplánek, R.; Bříza, T.; Martásek, P. Quo Vadis Porphyrin Chemistry? Physiol. Res. 2006, 55, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, F.; Linhardt, R.J. Porphyrin-Based Compounds and Their Applications in Materials and Medicine. Dye. Pigment. 2021, 188, 109136. [Google Scholar] [CrossRef]
- Wei, W.; Zhao, Z.X.; Xia, B.H.; Li, W. Theoretical Analysis of Expanded Porphyrins: Aromaticity, Stability, and Optoelectronic Properties. Front. Chem. 2022, 10, 1–10. [Google Scholar] [CrossRef]
- Magna, G.; Monti, D.; Di Natale, C.; Paolesse, R.; Stefanelli, M. The Assembly of Porphyrin Systems in Well-Defined Nanostructures: An Update. Molecules 2019, 24, 4307. [Google Scholar] [CrossRef]
- Martin, K.E.; Wang, Z.; Busani, T.; Garcia, R.M.; Chen, Z.; Jiang, Y.; Song, Y.; Jacobsen, J.L.; Vu, T.T.; Schore, N.E.; et al. Donor-Acceptor Biomorphs from the Ionic Self-Assembly of Porphyrins. J. Am. Chem. Soc. 2010, 132, 8194–8201. [Google Scholar] [CrossRef] [PubMed]
- Birdeanu, M.; Fagadar-Cosma, E. The Self-Assembly of Porphyrin Derivatives into 2D and 3D Architectures. In Quantum Nanosystems: Structure, Properties and Interactions; Putz, M.V., Ed.; Apple Academic Press: Toronto, ON, Canada, 2014; pp. 173–206. ISBN 9781774633144. [Google Scholar]
- Zhang, W.; Lai, W.; Cao, R. Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems. Chem. Rev. 2017, 117, 3717–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Wang, L.; Wang, H.; Cao, R.; Wang, J.; Bai, F.; Fan, H. Self-Assembled One-Dimensional Porphyrin Nanostructures with Enhanced Photocatalytic Hydrogen Generation. Nano Lett. 2018, 18, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Beyene, B.B.; Hung, C.H. Recent Progress on Metalloporphyrin-Based Hydrogen Evolution Catalysis. Coord. Chem. Rev. 2020, 410, 213234. [Google Scholar] [CrossRef]
- Yao, B.; He, Y.; Wang, S.; Sun, H.; Liu, X. Recent Advances in Porphyrin-Based Systems for Electrochemical Oxygen Evolution Reaction. Int. J. Mol. Sci. 2022, 23, 6036. [Google Scholar] [CrossRef] [PubMed]
- Keshipour, S.; Asghari, A. A Review on Hydrogen Generation by Phthalocyanines. Int. J. Hydrog. Energy 2022, 47, 12865–12881. [Google Scholar] [CrossRef]
- Chen, L.; Sagar, R.U.R.; Chen, J.; Liu, J.; Aslam, S.; Nosheen, F.; Anwar, T.; Hussain, N.; Hou, X.; Liang, T. Cobalt Phthalocyanine as an Efficient Catalyst for Hydrogen Evolution Reaction. Int. J. Hydrog. Energy 2021, 46, 19338–19346. [Google Scholar] [CrossRef]
- Li, Q.; Bao, Y.; Bai, F. Porphyrin and Macrocycle Derivatives for Electrochemical Water Splitting. MRS Bull. 2020, 45, 569–573. [Google Scholar] [CrossRef]
- Wang, N.; Lei, H.; Zhang, Z.; Li, J.; Zhang, W.; Cao, R. Electrocatalytic Hydrogen Evolution with Gallium Hydride and Ligand-Centered Reduction. Chem. Sci. 2019, 10, 2308–2314. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Q.; Dou, Y.; Sudi, M.S.; Zhu, W.; Shang, D.; Li, L. Porphyrin Coordination Polymer Supported Transition-Metal Sulfides as Precious-Metal-Free Electrocatalysts for Efficient Overall Water Splitting. Dye. Pigment. 2022, 206, 110620. [Google Scholar] [CrossRef]
- Wang, Y.; Song, D.; Li, J.; Shi, Q.; Zhao, J.; Hu, Y.; Zeng, F.; Wang, N. Covalent Metalloporphyrin Polymer Coated on Carbon Nanotubes as Bifunctional Electrocatalysts for Water Splitting. Inorg. Chem. 2022, 61, 10198–10204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Wang, Y.; Yang, S.; Wu, X.; Lv, B.; Wang, N.; Gao, Y.; Xu, X.; Lei, H.; et al. Electropolymerization of Cobalt Porphyrins and Corroles for the Oxygen Evolution Reaction. Chin. Chem. Lett. 2021, 32, 3807–3810. [Google Scholar] [CrossRef]
- Menezes, P.W.; Indra, A.; Das, C.; Walter, C.; Göbel, C.; Gutkin, V.; Schmeißer, D.; Driess, M. Uncovering the Nature of Active Species of Nickel Phosphide Catalysts in High-Performance Electrochemical Overall Water Splitting. ACS Catal. 2017, 7, 103–109. [Google Scholar] [CrossRef]
- Menezes, P.W.; Panda, C.; Loos, S.; Bunschei-Bruns, F.; Walter, C.; Schwarze, M.; Deng, X.; Dau, H.; Driess, M. A Structurally Versatile Nickel Phosphite Acting as a Robust Bifunctional Electrocatalyst for Overall Water Splitting. Energy Environ. Sci. 2018, 11, 1287–1298. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Ivanovici, M.G.; Svera, P.; Vlazan, P.; Sfirloaga, P.; Poienar, M. Ni11□ (HPO3)8(OH)6 Multifunctional Materials: Electrodes for Oxygen Evolution Reaction and Potential Visible-Light Active Photocatalysts. J. Alloy. Compd. 2020, 848, 156595. [Google Scholar] [CrossRef]
- Poienar, M.; Svera, P.; Taranu, B.-O.; Ianasi, C.; Sfirloaga, P.; Buse, G.; Veber, P.; Vlazan, P. Electrochemical Investigation of the OER Activity for Nickel Phosphite-Based Compositions and Its Morphology-Dependent Fluorescence Properties. Crystals 2022, 12, 1803. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Fagadar-Cosma, G.; Vasile, M.; Enache, C. Synthesis, Spectroscopic and Self-Assembling Characterization of Novel Photoactive Mixed Aryl-Substituted Porphyrin. Curr. Org. Chem. 2012, 16, 931–941. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Lascu, A.; Palade, A.; Creanga, I.; Birdeanu, M. Hybrid Material Based on 5-(4-Pyridyl)-10,15,20-Tris(4-Phenoxyphenyl)-Porphyrin and Gold Colloid for CO2 Detection. Dig. J. Nanomater. Biostruct. 2016, 11, 419–424. [Google Scholar]
- Birdeanu, A.V.; Birdeanu, M.; Fagadar-Cosma, E. Corrosion Protection Characteristics of Ceramics, Porphyrins and Hybrid Ceramics/Porphyrins, Deposited as Single and Sandwich Layers, by Pulsed Laser Deposition (PLD). J. Alloy. Compd. 2017, 706, 220–226. [Google Scholar] [CrossRef]
- Alexandrova, R.; Kalfin, R.; Tudose, R.; Fagadar-Cosma, E. Comparative Cytotoxicity Assays Performed Using a Free Porphyrin and Its Zn(II), Co(II) and Cu(II) Complexes. Influence of Optical and Aggregation Properties. Stud. Univ. Babes-Bolyai Chem. 2018, 63, 65–77. [Google Scholar] [CrossRef]
- Salageanu, L.; Muntean, D.; George, H.F.; Lascu, A.; Anghel, D.; Bagiu, I.C.; Fagadar-Cosma, E. Antimicrobial Activity of Different Substituted Meso-Porphyrin Derivatives. Rev. Romana Med. Lab. 2020, 28, 205–216. [Google Scholar] [CrossRef]
- Anghel, D.; Lascu, A.; Epuran, C.; Fratilescu, I.; Ianasi, C.; Birdeanu, M.; Fagadar-Cosma, E. Hybrid Materials Based on Silica Matrices Impregnated with Pt-Porphyrin or PtNPs Destined for CO2 Gas Detection or for Wastewaters Color Removal. Int. J. Mol. Sci. 2020, 21, 4262. [Google Scholar] [CrossRef] [PubMed]
- Taranu, B.O.; Fagadar-Cosma, E. Catalytic Properties of Free-Base Porphyrin Modified Graphite Electrodes for Electrochemical Water Splitting in Alkaline Medium. Processes 2022, 10, 611. [Google Scholar] [CrossRef]
- Vlascici, D.; Fagadar-Cosma, E.; Popa, I.; Chiriac, V.; Gil-Agusti, M. A Novel Sensor for Monitoring of Iron(III) Ions Based on Porphyrins. Sensors 2012, 12, 8193–8203. [Google Scholar] [CrossRef]
- Vlascici, D.; Popa, I.; Chiriac, V.A.; Fagadar-Cosma, G.; Popovici, H.; Fagadar-Cosma, E. Potentiometric Detection and Removal of Copper Using Porphyrins. Chem. Cent. J. 2013, 7, 111. [Google Scholar] [CrossRef]
- Taranu, B.O.; Fagadar-Cosma, E.; Popa, I.; Plesu, N.; Taranu, I. Adsorbed Functionalized Porphyrins on Polyaniline Modified Platinum Electrodes. Comparative Electrochemical Properties. Dig. J. Nanomater. Biostruct. 2014, 9, 667–679. [Google Scholar]
- Popa, I.; Fagadar-Cosma, G.; Taranu, B.O.; Birdeanu, A.V.; Taranu, I.; Vlascici, D.; Birdeanu, M.; Fagadar-Cosma, E. Electrochemical Behavior of Tetra(4-Methoxyphenyl)Porphyrin Thin Films Obtained by Laser Deposition on Graphite Electrode. Dig. J. Nanomater. Biostruct. 2014, 9, 1277–1287. [Google Scholar]
- Popa, I.; Fagadar-Cosma, E.; Taranu, B.-O.; Birdeanu, M.; Fagadar-Cosma, G.; Taranu, I. Corrosion Protection Efficiency of Bilayer Porphyrin-Polyaniline Film Deposited on Carbon Steel. Macromol. Symp. 2015, 352, 16–24. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Tarabukina, E.; Zakharova, N.; Birdeanu, M.; Taranu, B.; Palade, A.; Creanga, I.; Lascu, A.; Fagadar-Cosma, G. Hybrids Formed between Polyvinylpyrrolidone and an A3B Porphyrin Dye: Behaviour in Aqueous Solutions and Chemical Response to CO2 Presence. Polym. Int. 2016, 65, 200–209. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Vlascici, D.; Sebarchievici, I.; Fagadar-Cosma, E. The Aggregation Behavior of an A3B Free Base Porphyrin and Its Application as Chromium(III)-Selective Membrane Sensor. Stud. Univ. Babes-Bolyai Chem. 2016, 61, 199–212. [Google Scholar]
- Taranu, B.O.; Sebarchievici, L.; Taranu, I.; Birdeanu, M.; Cosma, E.F. Electrochemical and Microscopic Characterization of Two Meso-Substituted A3B and A4 Porphyrins. Rev. Chim. 2016, 67, 892–896. [Google Scholar]
- Poienar, M.; Maignan, A.; Sfirloaga, P.; Malo, S.; Vlazan, P.; Guesdon, A.; Lainé, F.; Rouquette, J.; Martin, C. Polar Space Group and Complex Magnetism in Ni11□(HPO3)8(OH)6: Towards a New Multiferroic Material? Solid State Sci. 2015, 39, 92–96. [Google Scholar] [CrossRef]
- Dudas, Z.; Enache, C.; Fagadar-Cosma, G.; Armeanu, I.; Fagadar-Cosma, E. Hybrid Silica-Porphyrin Materials with Tailored Pore Sizes. Mater. Res. Bull. 2010, 45, 1150–1156. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Armeanu, I.; Dascalu, D.; Fagadar-Cosma, G.; Vasile, M.; Grozescu, I. The Influence of PH over Topography and Spectroscopic Properties of Silica Hybrid Materials Embedding Meso-Tetratolylporphyrin. Mater. Res. Bull. 2009, 44, 426–431. [Google Scholar] [CrossRef]
- Fagadar-Cosma, E.; Enache, C.; Tudose, R.; Armeanu, I.; Mosoarca, E.; Vlascici, D.; Costisor, O. UV-VIS and Fluorescence Spectra of Meso-Tetraphenylporphyrin and Meso-Tetrakis-(4-Methoxyphenyl) Porphyrin in THF and THF-Water Systems. The Influence of PH. Rev. Chim. 2007, 58, 451–455. [Google Scholar]
- Snyder, L.R.; Kirkland, J.J.; Glajch, J.L. Practical HPLC Method Development, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; Volume 3, ISBN 978-0-471-00703-6. [Google Scholar]
- Szabadai, Z.; Sbarcea, L.; Udrescu, L. Analiza Fizică Și Chimică a Medicamentului; Victor Babes Publishing House: Timisoara, Romania, 2016; ISBN 978-606-786-020-7. [Google Scholar]
- Wang, S.; Lu, A.; Zhong, C.J. Hydrogen Production from Water Electrolysis: Role of Catalysts. Nano Converg. 2021, 8, 4. [Google Scholar] [CrossRef]
- Durovic, M.; Hnat, J.; Bouzek, K. Electrocatalysts for the Hydrogen Evolution Reaction in Alkaline and Neutral Media. A Comparative Review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Poienar, M.; Taranu, B.-O.; Svera, P.; Sfirloaga, P.; Vlazan, P. Disclosing the Thermal Behaviour, Electrochemical and Optical Properties of Synthetic Fe3(PO4)2(OH)2 Materials. J. Therm. Anal. Calorim. 2022, 147, 11839–11855. [Google Scholar] [CrossRef]
- Fratilescu, I.; Lascu, A.; Taranu, B.O.; Epuran, C.; Birdeanu, M.; Macsim, A.-M.; Tanasa, E.; Vasile, E.; Fagadar-Cosma, E. One A3B Porphyrin Structure—Three Successful Applications. Nanomaterials 2022, 12, 1930. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Fagadar-Cosma, E. The PH Influence on the Water-Splitting Electrocatalytic Activity of Graphite Electrodes Modified with Symmetrically Substituted Metalloporphyrins. Nanomaterials 2022, 12, 3788. [Google Scholar] [CrossRef]
- Sebarchievici, I.; Taranu, B.O.; Birdeanu, M.; Rus, S.F.; Fagadar-Cosma, E. Electrocatalytic Behaviour and Application of Manganese Porphyrin/Gold Nanoparticle-Surface Modified Glassy Carbon Electrodes. Appl. Surf. Sci. 2016, 390, 131–140. [Google Scholar] [CrossRef]
- Taranu, B.O.; Vlazan, P.; Racu, A. Water Splitting Studies in Alkaline Medium Using Graphite Electrodes Modified with Transition Metal Oxides and Compositions Containing Them. Stud. Univ. Babes-Bolyai Chem. 2022, 67, 79–95. [Google Scholar] [CrossRef]
- Yang, M.; Yang, Y.; Liu, Y.; Shen, G.; Yu, R. Platinum Nanoparticles-Doped Sol-Gel/Carbon Nanotubes Composite Electrochemical Sensors and Biosensors. Biosens. Bioelectron. 2006, 21, 1125–1131. [Google Scholar] [CrossRef]
- Taranu, B.-O.; Vlazan, P.; Svera, P.; Poienar, M.; Sfirloaga, P. New Functional Hybrid Materials Based on Clay Minerals for Enhanced Electrocatalytic Activity. J. Alloy. Compd. 2022, 892, 162239. [Google Scholar] [CrossRef]
- Sebarchievici, I.; Lascu, A.; Fagadar-Cosma, G.; Palade, A.; Fringu, I.; Birdeanu, M.; Taranu, B.; Fagadar-Cosma, E. Optical and Electrochemical-Mediated Detection of Ascorbic Acid Using Manganese Porphyrin and Its Gold Hybrids. Comptes Rendus Chim. 2018, 21, 327–338. [Google Scholar] [CrossRef]
- Gu, Z.; Zhang, Y.; Wei, X.; Duan, Z.; Ren, L.; Ji, J.; Zhang, X.; Zhang, Y.; Gong, Q.; Wu, H.; et al. Unveiling the Accelerated Water Electrolysis Kinetics of Heterostructural Iron-Cobalt-Nickel Sulfides by Probing into Crystalline/Amorphous Interfaces in Stepwise Catalytic Reactions. Adv. Sci. 2022, 9, 2201903. [Google Scholar] [CrossRef]
- Paul, R.; Zhai, Q.; Roy, A.K.; Dai, L. Charge Transfer of Carbon Nanomaterials for Efficient Metal-free Electrocatalysis. Interdiscip. Mater. 2022, 1, 28–50. [Google Scholar] [CrossRef]
- Zhou, Z.; Zaman, W.Q.; Sun, W.; Cao, L.M.; Tariq, M.; Yang, J. Cultivating Crystal Lattice Distortion in IrO2: Via Coupling with MnO2 to Boost the Oxygen Evolution Reaction with High Intrinsic Activity. Chem. Commun. 2018, 54, 4959–4962. [Google Scholar] [CrossRef]
- Liu, Y.; Xing, Y.; Zheng, X.; Xu, S.; Li, D.; Jiang, D. Synergistically Enhancing Electrocatalytic Activity of Co2P by Cr Doping and P Vacancies for Overall Water Splitting. Appl. Surf. Sci. 2022, 600, 154099. [Google Scholar] [CrossRef]
- Wang, S.; Xu, L.; Lu, W. Synergistic Effect: Hierarchical Ni3S2@Co(OH)2 Heterostructure as Efficient Bifunctional Electrocatalyst for Overall Water Splitting. Appl. Surf. Sci. 2018, 457, 156–163. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Yang, L.; Cheng, D.; Cao, D. Single-Atom Ru Doping Induced Phase Transition of MoS2 and S Vacancy for Hydrogen Evolution Reaction. Small Methods 2019, 3, 1900653. [Google Scholar] [CrossRef]
- Zou, X.; Huang, X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmeková, E.; Asefa, T. Cobalt-Embedded Nitrogen-Rich Carbon Nanotubes Efficiently Catalyze Hydrogen Evolution Reaction at All PH Values. Angew. Chem.—Int. Ed. 2014, 53, 4372–4376. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Li, S.; Wu, F.; Saqib, M.; Luque, R.; Xu, G. Unprecedented Metal-Free 3D Porous Carbonaceous Electrodes for Full Water Splitting. Energy Environ. Sci. 2016, 9, 1210–1214. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.H.; Su, H.; Lv, L.B.; Zhao, T.J.; Ge, J.M.; Wei, X.; Wang, K.X.; Li, X.H.; Chen, J.S. Nitrogen-Doped Graphene Microtubes with Opened Inner Voids: Highly Efficient Metal-Free Electrocatalysts for Alkaline Hydrogen Evolution Reaction. Nano Res. 2016, 9, 2606–2615. [Google Scholar] [CrossRef]
- Cai, G.; Zeng, L.; He, L.; Sun, S.; Tong, Y.; Zhang, J. Imine Gels Based on Ferrocene and Porphyrin and Their Electrocatalytic Property. Chem.—Asian J. 2020, 15, 1963–1969. [Google Scholar] [CrossRef]
- Wang, A.; Cheng, L.; Zhao, W.; Shen, X.; Zhu, W. Electrochemical Hydrogen and Oxygen Evolution Reactions from a Cobalt-Porphyrin-Based Covalent Organic Polymer. J. Colloid Interface Sci. 2020, 579, 598–606. [Google Scholar] [CrossRef]
- Jia, H.; Yao, Y.; Gao, Y.; Lu, D.; Du, P. Pyrolyzed Cobalt Porphyrin-Based Conjugated Mesoporous Polymers as Bifunctional Catalysts for Hydrogen Production and Oxygen Evolution in Water. Chem. Commun. 2016, 52, 13483–13486. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).