Recent Progress and Challenges in MXene-Based Phase Change Material for Solar and Thermal Energy Applications
Abstract
1. Introduction
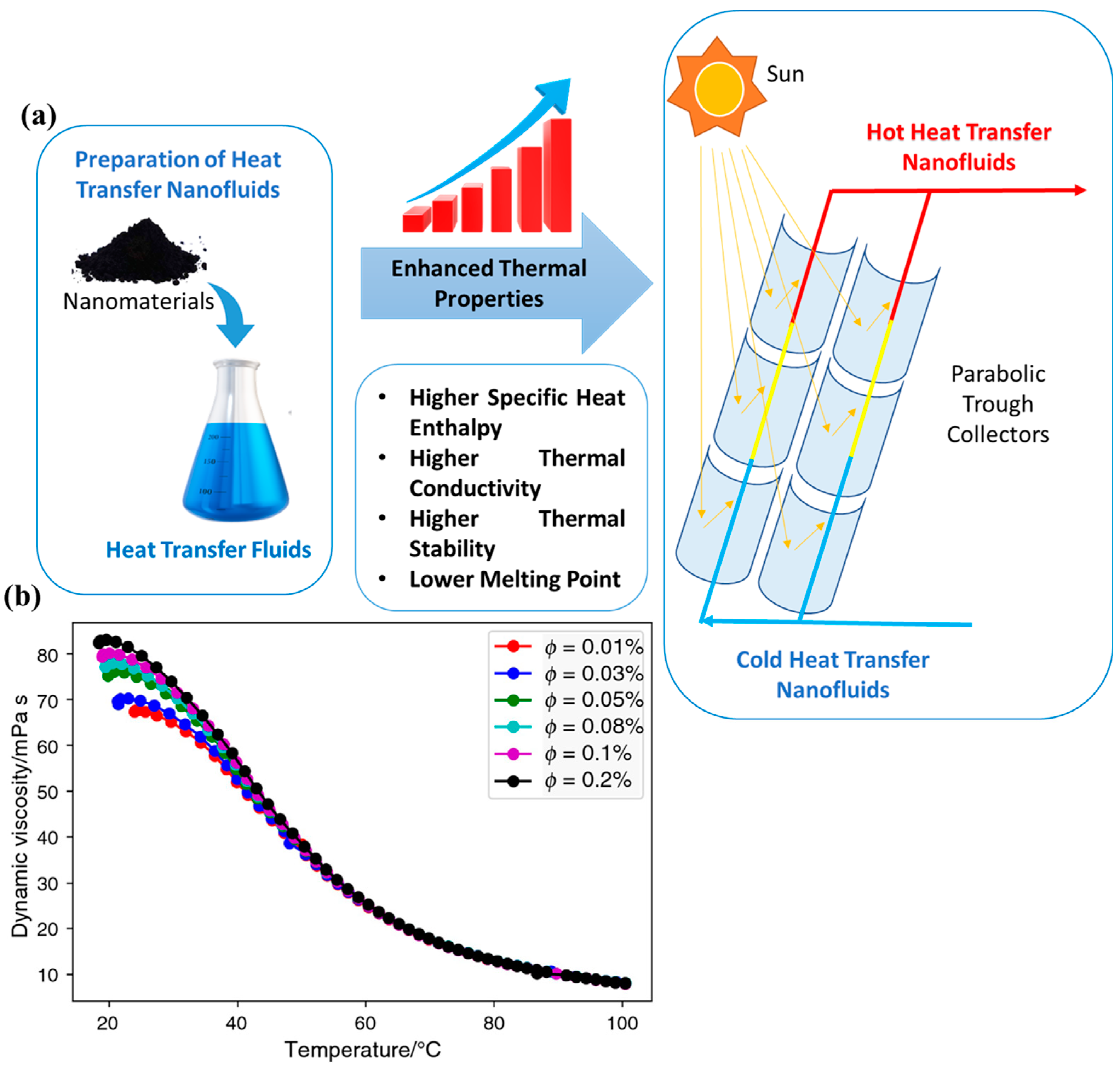
2. Preparation of MXene
2.1. Top-Down Synthesis Approach
2.1.1. Precursor
2.1.2. Etching Strategy
2.1.3. Exfoliation Route
2.2. Bottom-Up Processing Techniques
2.2.1. Chemical Vapor Deposition (CVD) Route
2.2.2. Other Fabrication Routes
3. Global Recent Progress on Phase Change Materials (PCM)
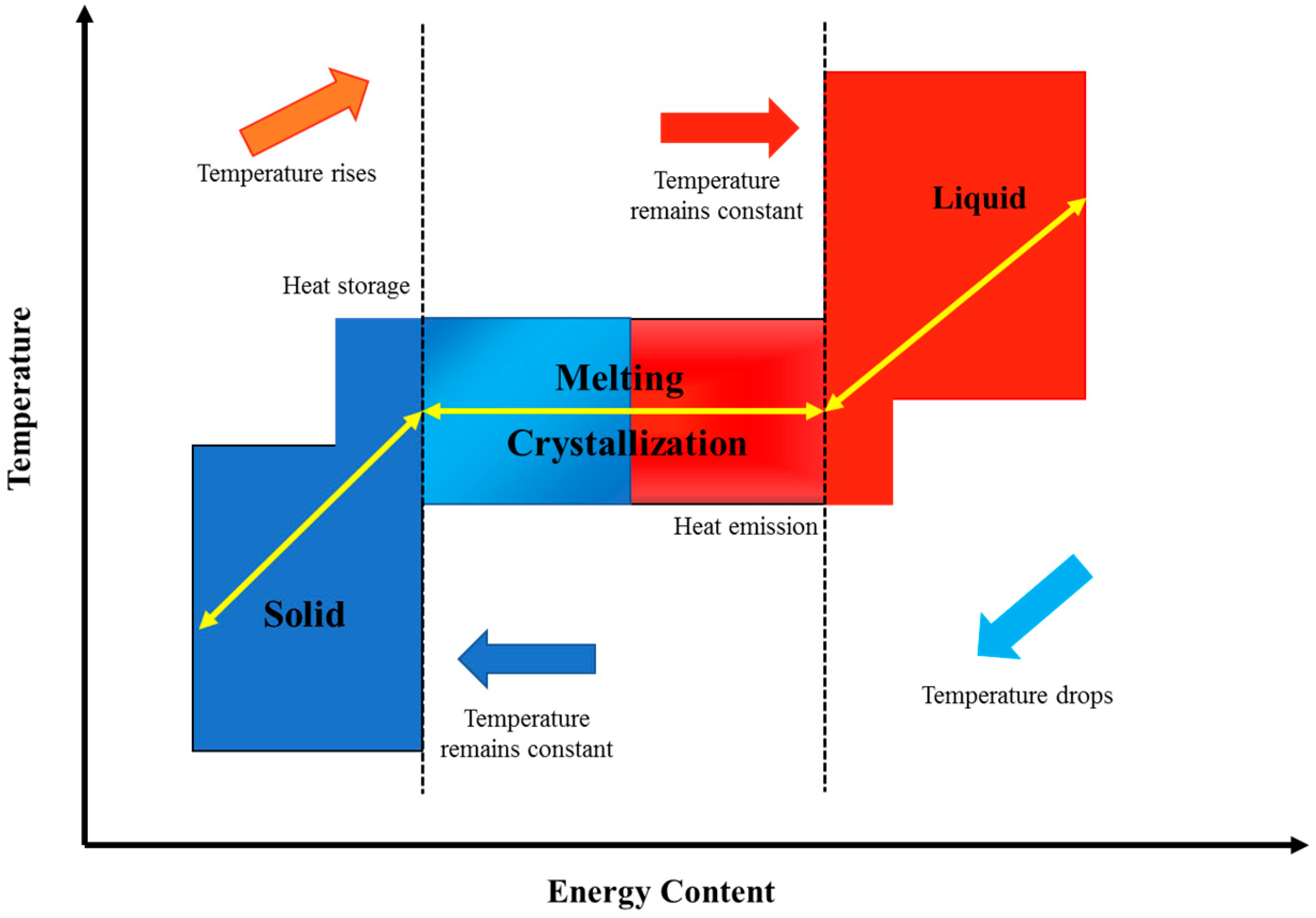
3.1. Phase Change Material and Its Classification
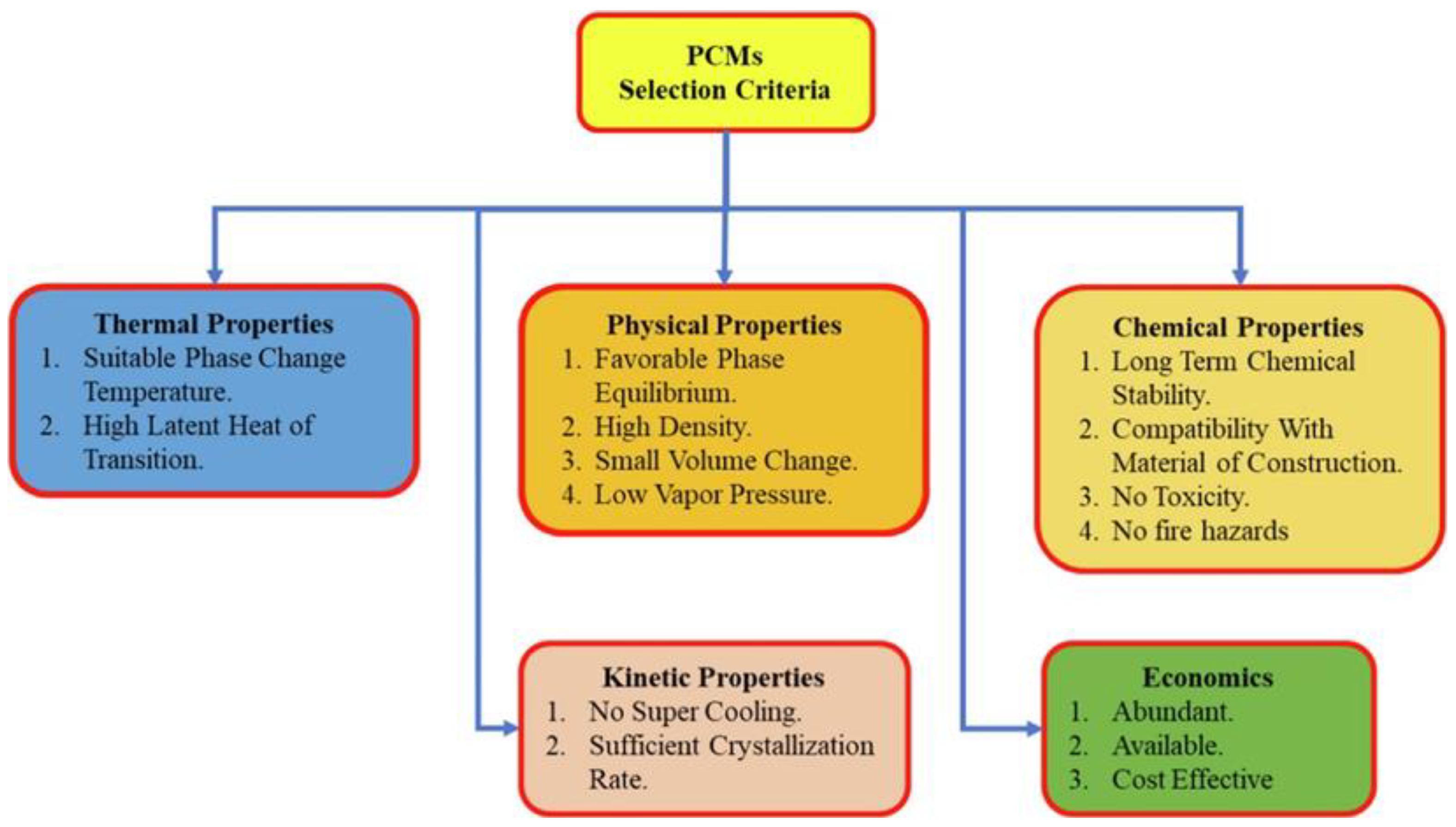
3.2. PCM Selection
4. Phase Change Materials under Thermal Regulation of Solar Thermal Energy Storage Applications
4.1. Selection of Nanoparticles and MXene
4.2. MXene-Based Phase Change Materials
5. Solar Thermal Energy Application
5.1. MXene Architect PCM for Solar Energy (SE) Applications
5.2. Photothermal Conversion Applications
5.3. Electrothermal Conversion Applications
5.4. MXene Phase Change Materials in Electronic Applications
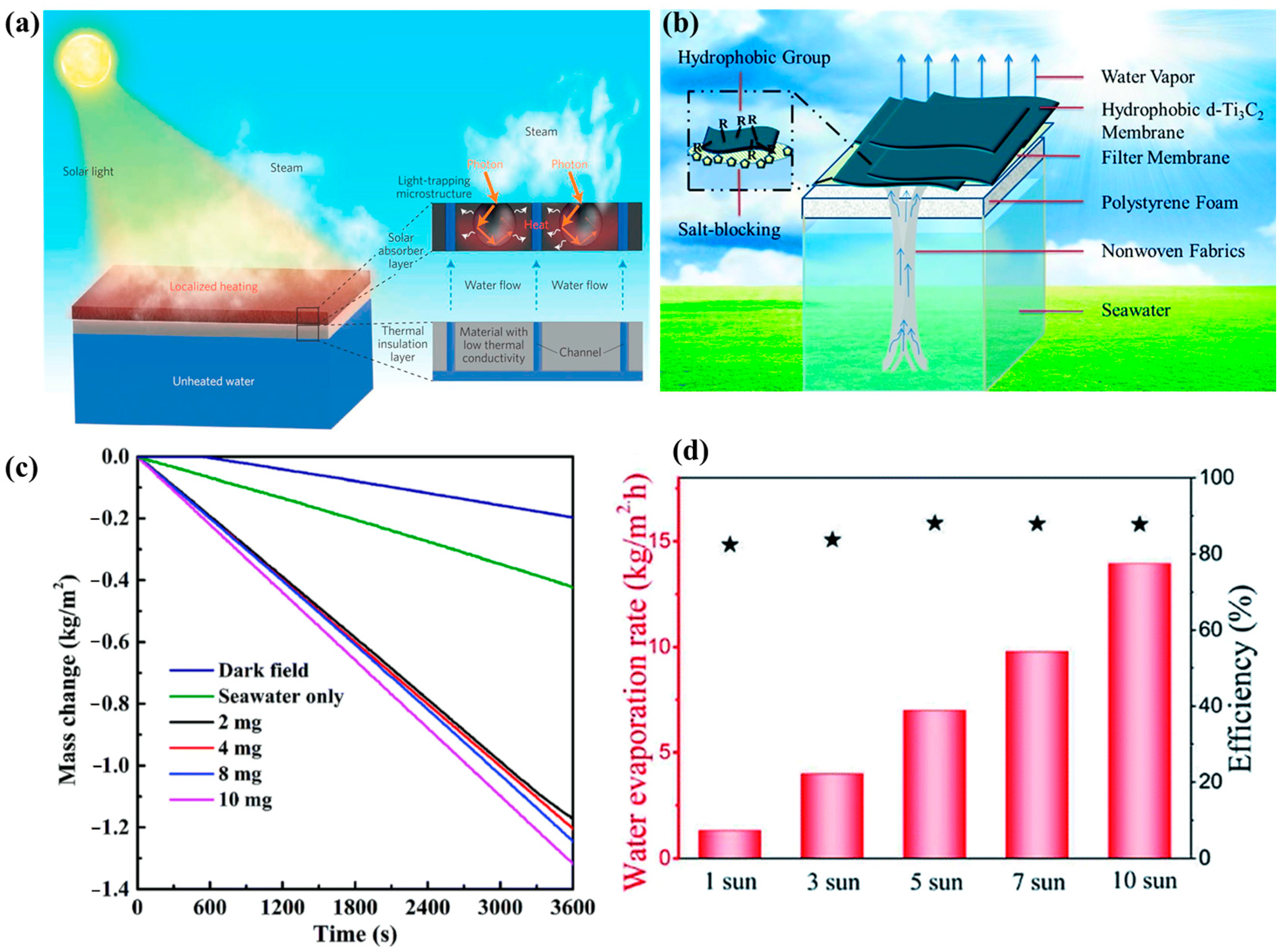
5.5. Solar Water Desalination Applications
6. Challenges and Future Perspectives
- Due to technical difficulties and limitations in the synthesis and etching process, only 20 out of 70 MAX phases have been successfully etched into 2D MXene materials. Their inherent properties influence the success and ease of converting MAX phases into 2D MXene materials. Additionally, a lack of understanding or limited research on some MAX phases may impede their successful transformation into 2D MXene materials. The research focus and priority in this area may also be directed towards certain MAX phases due to their specific applications or desired properties.
- Different methods have been used to determine the properties of MXene, and numerous characterization methods have been used to test its stability, strength, and surface characteristics. Phase change materials and nanofluids have effectively used these 2D materials to boost system performance.
- Nanofluids and PCMs with MXene bases showed exceptional optical characteristics and a higher value of “k”. Researchers have noted great outcomes from the practical use of these materials in seawater desalination.
- MXene materials exhibit thermal conductivity properties similar to those of ceramics and metals, both known for their high heat conductivity. Theoretical calculations have substantiated the high thermal conductivity potential of MXene materials. However, when considering macroscopic MXene materials such as films, their actual thermal conductivity is lower than expected. To realize the full potential of MXene materials in real-world applications, significant progress must be made in overcoming the challenge of producing macroscopic MXene materials with high thermal conductivity. This requires addressing two crucial scientific issues: controlling the surface termination of MXene and reducing the thermal resistance at the interface between MXene nanosheets during the construction process of macroscopic materials.
- The majority of MXene-based transition metal materials exhibit inadequate mechanical properties, which significantly hinders their utilization in various domains. The viability of MXene-based TM materials as a practical material solution is contingent on enhancing their mechanical properties, including tensile and compressive strength, as well as toughness.
- Although MXene has been widely developed recently, and its applications in the energy sector, particularly in the field of thermal energy, have expanded, it still has some problems that need to be resolved by researchers in the future. Among the principal obstacles are that the processing of MXene requires secure synthesis pathways that lead to effective, affordable, high-quality, and recurring MXene. Future research must examine the identification of new MAX phases and the related 2D materials. Therefore, future 2D materials with low economic cost and good stability must be considered.
- MXene’s limited electrochemical stability restricts its applicability and necessitates more research. However, the high price of MXene-based energy storage materials still poses a significant barrier to progress. Roadblocks related to cost and scale-up can be removed to bring about an energy sector revolution.
- There is a limited understanding of the thermal behavior of MXene-based PCMs. While it is known that MXene-based PCMs have the potential to store and release large amounts of energy, the mechanisms behind this behavior are not yet fully understood. This knowledge gap makes it difficult to predict the performance of MXene-based PCMs under different conditions and to design materials with specific properties.
- MXene-based PCMs have shown great potential for solar energy storage, where they can store excess energy generated by solar panels during the day and release it at night. This can help to reduce the dependence on grid-connected power and improve the overall efficiency of solar energy systems. In addition, MXene-based PCMs can be used to regulate the temperature of solar panels, increasing their efficiency and lifespan.
- In thermal energy storage, MXene-based PCMs can be used to store and release heat generated by industrial processes, heating and cooling systems, and renewable energy sources. They can provide stable and efficient temperature control, reducing energy waste and increasing the efficiency of energy systems. In addition, the high thermal stability of MXene-based PCMs allows for multiple phase transitions without degradation, making them suitable for long-term energy storage.
- Its application areas should be increased in order to increase the use of MXene in research and development fields, particularly thermal management.
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, X.; Yang, R.; Tan, G.; Fan, S. Terrestrial radiative cooling: Using the cold universe as a renewable and sustainable energy source. Science 2020, 370, 786–791. [Google Scholar] [CrossRef]
- Ying, J.; Tan, X.; Lv, L.; Wang, X.; Gao, J.; Yan, Q.; Ma, H.; Nishimura, K.; Li, H.; Yu, J. Tailoring highly ordered graphene framework in epoxy for high-performance polymer-based heat dissipation plates. ACS Nano 2021, 15, 12922–12934. [Google Scholar] [CrossRef]
- Ye, Y.; Chou, L.-Y.; Liu, Y.; Wang, H.; Lee, H.K.; Huang, W.; Wan, J.; Liu, K.; Zhou, G.; Yang, Y. Ultralight and fire-extinguishing current collectors for high-energy and high-safety lithium-ion batteries. Nat. Energy 2020, 5, 786–793. [Google Scholar] [CrossRef]
- Hu, R.; Liu, Y.; Shin, S.; Huang, S.; Ren, X.; Shu, W.; Cheng, J.; Tao, G.; Xu, W.; Chen, R. Emerging materials and strategies for personal thermal management. Adv. Energy Mater. 2020, 10, 1903921. [Google Scholar] [CrossRef]
- Han, C.-G.; Qian, X.; Li, Q.; Deng, B.; Zhu, Y.; Han, Z.; Zhang, W.; Wang, W.; Feng, S.-P.; Chen, G. Giant thermopower of ionic gelatin near room temperature. Science 2020, 368, 1091–1098. [Google Scholar] [CrossRef]
- Benyettou, F.; Das, G.; Nair, A.R.; Prakasam, T.; Shinde, D.B.; Sharma, S.K.; Whelan, J.; Lalatonne, Y.; Traboulsi, H.; Pasricha, R. Covalent organic framework embedded with magnetic nanoparticles for MRI and chemo-thermotherapy. J. Am. Chem. Soc. 2020, 142, 18782–18794. [Google Scholar] [CrossRef]
- Ding, T.; Zhou, Y.; Ong, W.L.; Ho, G.W. Hybrid solar-driven interfacial evaporation systems: Beyond water production towards high solar energy utilization. Mater. Today 2021, 42, 178–191. [Google Scholar] [CrossRef]
- Hu, R.; Xi, W.; Liu, Y.; Tang, K.; Song, J.; Luo, X.; Wu, J.; Qiu, C.-W. Thermal camouflaging metamaterials. Mater. Today 2021, 45, 120–141. [Google Scholar] [CrossRef]
- Bark, H.; Tan, M.W.M.; Thangavel, G.; Lee, P.S. Deformable high loading liquid metal nanoparticles composites for thermal energy management. Adv. Energy Mater. 2021, 11, 2101387. [Google Scholar] [CrossRef]
- Xu, Y.; Kraemer, D.; Song, B.; Jiang, Z.; Zhou, J.; Loomis, J.; Wang, J.; Li, M.; Ghasemi, H.; Huang, X. Nanostructured polymer films with metal-like thermal conductivity. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Yan, Q.; Dai, W.; Gao, J.; Tan, X.; Lv, L.; Ying, J.; Lu, X.; Lu, J.; Yao, Y.; Wei, Q. Ultrahigh-aspect-ratio boron nitride nanosheets leading to superhigh in-plane thermal conductivity of foldable heat spreader. ACS Nano 2021, 15, 6489–6498. [Google Scholar] [CrossRef]
- He, S.; Li, Y.; Liu, L.; Jiang, Y.; Feng, J.; Zhu, W.; Zhang, J.; Dong, Z.; Deng, Y.; Luo, J. Semiconductor glass with superior flexibility and high room temperature thermoelectric performance. Sci. Adv. 2020, 6, eaaz8423. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zheng, K.; Cao, X.; Fan, Q.; Ye, G.; Lu, J.; Zhang, J.; Ma, Y. Flexible graphene nanocomposites with simultaneous highly anisotropic thermal and electrical conductivities prepared by engineered graphene with flat morphology. ACS Nano 2020, 14, 11733–11742. [Google Scholar] [CrossRef]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.; Saini, J. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Kaygusuz, K. The viability of thermal energy storage. Energy Sources 1999, 21, 745–755. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Telkes, M.; Raymond, E. Storing solar heat in chemicals. Heat. Vent. 1949, 46, 80–86. [Google Scholar]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Kenisarin, M.; Mahkamov, K. Solar energy storage using phase change materials. Renew. Sustain. Energy Rev. 2007, 11, 1913–1965. [Google Scholar] [CrossRef]
- Fernandes, D.; Pitié, F.; Cáceres, G.; Baeyens, J. Thermal energy storage: “How previous findings determine current research priorities”. Energy 2012, 39, 246–257. [Google Scholar] [CrossRef]
- Garg, H.; Mullick, S.; Bhargava, A. Latent heat or phase change thermal energy storage. In Solar Thermal Energy Storage; Springer: Berlin/Heidelberg, Germany, 1985; pp. 154–291. [Google Scholar]
- Hasnain, S. Review on sustainable thermal energy storage technologies, Part I: Heat storage materials and techniques. Energy Convers. Manag. 1998, 39, 1127–1138. [Google Scholar] [CrossRef]
- Selamat, U.; Osman, K.; Rahim, A.H.A. Heat and Flow Analysis of a Chilled Water Storage System using Computational Fluid Dynamics. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 57, 131–140. [Google Scholar]
- Rashid, F.L.; Shareef, A.S.; Alwan, H.F. Enhancement of fresh water production in solar still using new phase change materials. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 61, 63–72. [Google Scholar]
- Krishna, Y.; Faizal, M.; Saidur, R.; Ng, K.; Aslfattahi, N. State-of-the-art heat transfer fluids for parabolic trough collector. Int. J. Heat Mass Transf. 2020, 152, 119541. [Google Scholar] [CrossRef]
- Krishna, Y.; Aslfattahi, N.; Saidur, R.; Faizal, M.; Ng, K. Fatty acid/metal ion composite as thermal energy storage materials. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Zendehboudi, A.; Aslfattahi, N.; Rahman, S.; Sabri, M.F.M.; Said, S.M.; Arifutzzaman, A.; Sidik, N.A.C. Optimization of thermal conductivity of NanoPCM-based graphene by response surface methodology. J. Adv. Res. Fluid Mech. Therm. Sci. 2020, 75, 108–125. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.-e.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Pacile, D.; Meyer, J.; Girit, Ç.; Zettl, A. The two-dimensional phase of boron nitride: Few-atomic-layer sheets and suspended membranes. Appl. Phys. Lett. 2008, 92, 133107. [Google Scholar] [CrossRef]
- Ataca, C.; Sahin, H.; Ciraci, S. Stable, Single-Layer MX2 Transition-Metal Oxides and Dichalcogenides in a Honeycomb-Like Structure. J. Phys. Chem. C 2012, 116, 8983–8999. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Naguib, M.; Gogotsi, Y. Synthesis of two-dimensional materials by selective extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Parashar, N.; Aslfattahi, N.; Yahya, S.M.; Saidur, R. An artificial neural network approach for the prediction of dynamic viscosity of MXene-palm oil nanofluid using experimental data. J. Therm. Anal. Calorim. 2021, 144, 1175–1186. [Google Scholar] [CrossRef]
- Aslfattahi, N.; Saidur, R.; Arifutzzaman, A.; Sadri, R.; Bimbo, N.; Sabri, M.F.M.; Maughan, P.A.; Bouscarrat, L.; Dawson, R.J.; Said, S.M. Experimental investigation of energy storage properties and thermal conductivity of a novel organic phase change material/MXene as A new class of nanocomposites. J. Energy Storage 2020, 27, 101115. [Google Scholar] [CrossRef]
- Numan, A.; Rafique, S.; Khalid, M.; Zaharin, H.A.; Radwan, A.; Mokri, N.A.; Ching, O.P.; Walvekar, R. Microwave-assisted rapid MAX phase etching and delamination: A paradigm shift in MXene synthesis. Mater. Chem. Phys. 2022, 288, 126429. [Google Scholar] [CrossRef]
- Khosla, A.; Awan, H.T.A.; Singh, K.; Walvekar, R.; Zhao, Z.; Kaushik, A.; Khalid, M.; Chaudhary, V. Emergence of MXene and MXene–Polymer Hybrid Membranes as Future-Environmental Remediation Strategies. Adv. Sci. 2022, 9, 2203527. [Google Scholar] [CrossRef]
- Rasool, K.; Pandey, R.P.; Rasheed, P.A.; Buczek, S.; Gogotsi, Y.; Mahmoud, K.A. Water treatment and environmental remediation applications of two-dimensional metal carbides (MXenes). Mater. Today 2019, 30, 80–102. [Google Scholar] [CrossRef]
- Huang, K.; Li, Z.; Lin, J.; Han, G.; Huang, P. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem. Soc. Rev. 2018, 47, 5109–5124. [Google Scholar] [CrossRef]
- Chaudhary, V.; Khanna, V.; Awan, H.T.A.; Singh, K.; Khalid, M.; Mishra, Y.; Bhansali, S.; Li, C.-Z.; Kaushik, A. Towards hospital-on-chip supported by 2D MXenes-based 5th generation intelligent biosensors. Biosens. Bioelectron. 2022, 220, 114847. [Google Scholar] [CrossRef]
- Barsoum, M.W. MAX Phases: Properties of Machinable Ternary Carbides and Nitrides; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Verger, L.; Xu, C.; Natu, V.; Cheng, H.-M.; Ren, W.; Barsoum, M.W. Overview of the synthesis of MXenes and other ultrathin 2D transition metal carbides and nitrides. Curr. Opin. Solid State Mater. Sci. 2019, 23, 149–163. [Google Scholar] [CrossRef]
- Nowotny, V.H. Strukturchemie einiger verbindungen der übergangsmetalle mit den elementen C, Si, Ge, Sn. Prog. Solid State Chem. 1971, 5, 27–70. [Google Scholar] [CrossRef]
- Anasori, B.; Halim, J.; Lu, J.; Voigt, C.A.; Hultman, L.; Barsoum, M.W. Mo2TiAlC2: A new ordered layered ternary carbide. Scr. Mater. 2015, 101, 5–7. [Google Scholar] [CrossRef]
- Tao, Q.; Dahlqvist, M.; Lu, J.; Kota, S.; Meshkian, R.; Halim, J.; Palisaitis, J.; Hultman, L.; Barsoum, M.W.; Persson, P.O. Two-dimensional Mo1. 33C MXene with divacancy ordering prepared from parent 3D laminate with in-plane chemical ordering. Nat. Commun. 2017, 8, 14949. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Lu, J.; Dahlqvist, M.; Mockute, A.; Calder, S.; Petruhins, A.; Meshkian, R.; Rivin, O.; Potashnikov, D.; Caspi, E.a.N. Atomically layered and ordered rare-earth i-MAX phases: A new class of magnetic quaternary compounds. Chem. Mater. 2019, 31, 2476–2485. [Google Scholar] [CrossRef]
- Anasori, B.; Lukatskaya, M.R.; Gogotsi, Y. 2D metal carbides and nitrides (MXenes) for energy storage. Nat. Rev. Mater. 2017, 2, 1–17. [Google Scholar] [CrossRef]
- Cambaz, G.Z.; Yushin, G.N.; Gogotsi, Y.; Lutsenko, V.G. Anisotropic etching of SiC whiskers. Nano Lett. 2006, 6, 548–551. [Google Scholar] [CrossRef]
- Seh, Z.W.; Fredrickson, K.D.; Anasori, B.; Kibsgaard, J.; Strickler, A.L.; Lukatskaya, M.R.; Gogotsi, Y.; Jaramillo, T.F.; Vojvodic, A. Two-dimensional molybdenum carbide (MXene) as an efficient electrocatalyst for hydrogen evolution. ACS Energy Lett. 2016, 1, 589–594. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.-Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Alhabeb, M.; Maleski, K.; Anasori, B.; Lelyukh, P.; Clark, L.; Sin, S.; Gogotsi, Y. Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2Tx MXene). Chem. Mater. 2017, 29, 7633–7644. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Xu, W.; Zhang, J.; Zhou, Y.; Cheng, Z. Rapid preparation, thermal stability and electromagnetic interference shielding properties of two-dimensional Ti3C2 MXene. Ceram. Int. 2019, 45, 19902–19909. [Google Scholar] [CrossRef]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Halim, J.; Kota, S.; Bish, D.; Gogotsi, Y.; Barsoum, M.W. Ion-exchange and cation solvation reactions in Ti3C2 MXene. Chem. Mater. 2016, 28, 3507–3514. [Google Scholar] [CrossRef]
- Natu, V.; Sokol, M.; Verger, L.; Barsoum, M.W. Effect of Edge Charges on Stability and Aggregation of Ti3C2Tz MXene Colloidal Suspensions. J. Phys. Chem. C 2018, 122, 27745–27753. [Google Scholar] [CrossRef]
- Omomo, Y.; Sasaki, T.; Wang, L.; Watanabe, M. Redoxable nanosheet crystallites of MnO2 derived via delamination of a layered manganese oxide. J. Am. Chem. Soc. 2003, 125, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Nickl, J.J.; Schweitzer, K.K.; Luxenberg, P. Gasphasenabscheidung im system Ti Si C. J. Less Common Met. 1972, 26, 335–353. [Google Scholar] [CrossRef]
- Yin, X.; Travitzky, N.; Greil, P. Three-dimensional printing of nanolaminated Ti3AlC2 toughened TiAl3–Al2O3 composites. J. Am. Ceram. Soc. 2007, 90, 2128–2134. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Liu, Z.; Chen, L.; Guo, J.; Kang, N.; Ma, X.-L.; Cheng, H.-M.; Ren, W. Large-area high-quality 2D ultrathin Mo2C superconducting crystals. Nat. Mater. 2015, 14, 1135–1141. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q.; Li, W.; He, H.; Yang, L.; Lu, Z.; Huang, H. Ultrafine Pt nanoparticle-decorated 3D hybrid architectures built from reduced graphene oxide and MXene nanosheets for methanol oxidation. Chem. Mater. 2019, 31, 9277–9287. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, Q.; Huang, H.; He, H.; Yang, L.; Li, W. Polyelectrolyte-Induced Stereoassembly of Grain Boundary-Enriched Platinum Nanoworms on Ti3C2Tx MXene Nanosheets for Efficient Methanol Oxidation. ACS Appl. Mater. Interfaces 2020, 12, 23822–23830. [Google Scholar] [CrossRef]
- Wang, Z.; Kochat, V.; Pandey, P.; Kashyap, S.; Chattopadhyay, S.; Samanta, A.; Sarkar, S.; Manimunda, P.; Zhang, X.; Asif, S. Metal immiscibility route to synthesis of ultrathin carbides, borides, and nitrides. Adv. Mater. 2017, 29, 1700364. [Google Scholar] [CrossRef]
- Jia, J.; Xiong, T.; Zhao, L.; Wang, F.; Liu, H.; Hu, R.; Zhou, J.; Zhou, W.; Chen, S. Ultrathin N-doped Mo2C nanosheets with exposed active sites as efficient electrocatalyst for hydrogen evolution reactions. ACS Nano 2017, 11, 12509–12518. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Liu, L.; Jin, X.; Wang, W.; Zhang, S.; Tang, B. MXene Ti3C2Tx for phase change composite with superior photothermal storage capability. J. Mater. Chem. A 2019, 7, 14319–14327. [Google Scholar] [CrossRef]
- Joshi, S.; Wang, Q.; Puntambekar, A.; Chakrapani, V. Facile synthesis of large area two-dimensional layers of transition-metal nitride and their use as insertion electrodes. ACS Energy Lett. 2017, 2, 1257–1262. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Wang, H.; Chan, C.H.; Chan, N.Y.; Chen, X.X.; Dai, J.-Y. Plasma-enhanced pulsed-laser deposition of single-crystalline Mo2C ultrathin superconducting films. Phys. Rev. Mater. 2017, 1, 034002. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, F.; Wang, H.; Chan, C.H.; Lu, W.; Dai, J.-y. Substrate orientation-induced epitaxial growth of face centered cubic Mo2C superconductive thin film. J. Mater. Chem. C 2017, 5, 10822–10827. [Google Scholar] [CrossRef]
- Zalba, B.; Marín, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castell, A.; Barreneche, C.; De Gracia, A.; Fernández, A.I. Materials used as PCM in thermal energy storage in buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1675–1695. [Google Scholar] [CrossRef]
- Oró, E.; Barreneche, C.; Farid, M.M.; Cabeza, L.F. Experimental study on the selection of phase change materials for low temperature applications. Renew. Energy 2013, 57, 130–136. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Phase change materials for advanced cooling packaging. Environ. Chem. Lett. 2018, 16, 845–859. [Google Scholar] [CrossRef]
- Hasan, A.; McCormack, S.J.; Huang, M.J.; Norton, B. Evaluation of phase change materials for thermal regulation enhancement of building integrated photovoltaics. Sol. Energy 2010, 84, 1601–1612. [Google Scholar] [CrossRef]
- Huang, M.J. The effect of using two PCMs on the thermal regulation performance of BIPV systems. Sol. Energy Mater. Sol. Cells 2011, 95, 957–963. [Google Scholar] [CrossRef]
- Biwole, P.H.; Eclache, P.; Kuznik, F. Phase-change materials to improve solar panel’s performance. Energy Build. 2013, 62, 59–67. [Google Scholar] [CrossRef]
- Lo, B.V.; Ciulla, G.; Piacentino, A.; Cardona, F. On the efficacy of PCM to shave peak temperature of crystalline photovoltaic panels: An FDM model and field validation. Energies 2013, 6, 6188–6210. [Google Scholar]
- Huang, M.J.; Eames, P.C.; Norton, B.; Hewitt, N.J. Natural convection in an internally finned phase change material heat sink for the thermal management of photovoltaics. Sol. Energy Mater. Sol. Cells 2011, 95, 1598–1603. [Google Scholar] [CrossRef]
- Hendricks, J.H.C.; Sark, W.G.J.H.M. Annual performance enhancement of building integrated photovoltaic modules by applying phase change materials. Prog. Photovolt. 2013, 21, 620–630. [Google Scholar] [CrossRef]
- Sharma, S.; Tahir, A.; Reddy, K.S.; Mallick, T.K. Performance enhancement of a Building-Integrated Concentrating Photovoltaic system using phase change material. Sol. Energy Mater. Sol. Cells 2016, 149, 29–39. [Google Scholar] [CrossRef]
- Pakrouh, R.; Hosseini, M.J.; Ranjbar, A.A. A parametric investigation of a PCM-based pin fin heat sink. Mech. Sci. 2015, 6, 65–73. [Google Scholar] [CrossRef]
- Machniewicz, A.; Knera, D.; Heim, D. Effect of Transition Temperature on Efficiency of PV/PCM Panels. Energy Procedia 2015, 78, 1684–1689. [Google Scholar] [CrossRef]
- Javani, N.; Dincer, I.; Naterer, G.F. New latent heat storage system with nanoparticles for thermal management of electric vehicles. J. Power Sources 2014, 268, 718–727. [Google Scholar] [CrossRef]
- Khodadadi, J.M.; Hosseinizadeh, S.F. Nanoparticle-enhanced phase change materials (NEPCM) with great potential for improved thermal energy storage. Int. Commun. Heat Mass Transf. 2007, 34, 534–543. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A.; Henry Biwole, P. Heat transfer study of phase change materials with graphene nano particle for thermal energy storage. Sol. Energy 2017, 146, 453–463. [Google Scholar] [CrossRef]
- Ho, C.J.; Gao, J.Y. Preparation and thermophysical properties of nanoparticle-in-paraffin emulsion as phase change material. Int. Commun. Heat Mass Transf. 2009, 36, 467–470. [Google Scholar] [CrossRef]
- Hajare, V.; Gawali, B. Experimental study of latent heat storage system using nano-mixed phase change material. Int. J. Eng. Technol. Manag. Ment Appl. Sci. 2015, 3, 37–44. [Google Scholar]
- Hajare, V.; Narode, A.; Gawali, B.; Bamane, S. Technology. Experimental investigation of enhancement in heat transfer using nano-mixed PCM. Int. J. Eng. Res. 2014, 3, 843–848. [Google Scholar]
- Ab Latif, F.E.; Numan, A.; Mubarak, N.M.; Khalid, M.; Abdullah, E.C.; Manaf, N.A. Walvekar, REvolution of MXene and its 2D heterostructure in electrochemical sensor applications. Co-Ord. Chem. Rev. 2022, 471, 214755. [Google Scholar] [CrossRef]
- Raheem, I.; Mubarak, N.M.; Karri, R.R.; Solangi, N.H.; Jatoi, A.S.; Mazari, S.A.; Khalid, M.; Tan, Y.H.; Koduru, J.R.; Malafaia, G.J.C. Rapid growth of MXene-based membranes for sustainable environmental pollution remediation. Chemosphere 2022, 311, 137056. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Joshi, D.J.; Koduru, J.R.; Malek, N.I. Review on MXenes-based nanomaterials for sustainable opportunities in energy storage, sensing and electrocatalytic reactions. J. Mol. Liq. 2021, 342, 117524. [Google Scholar] [CrossRef]
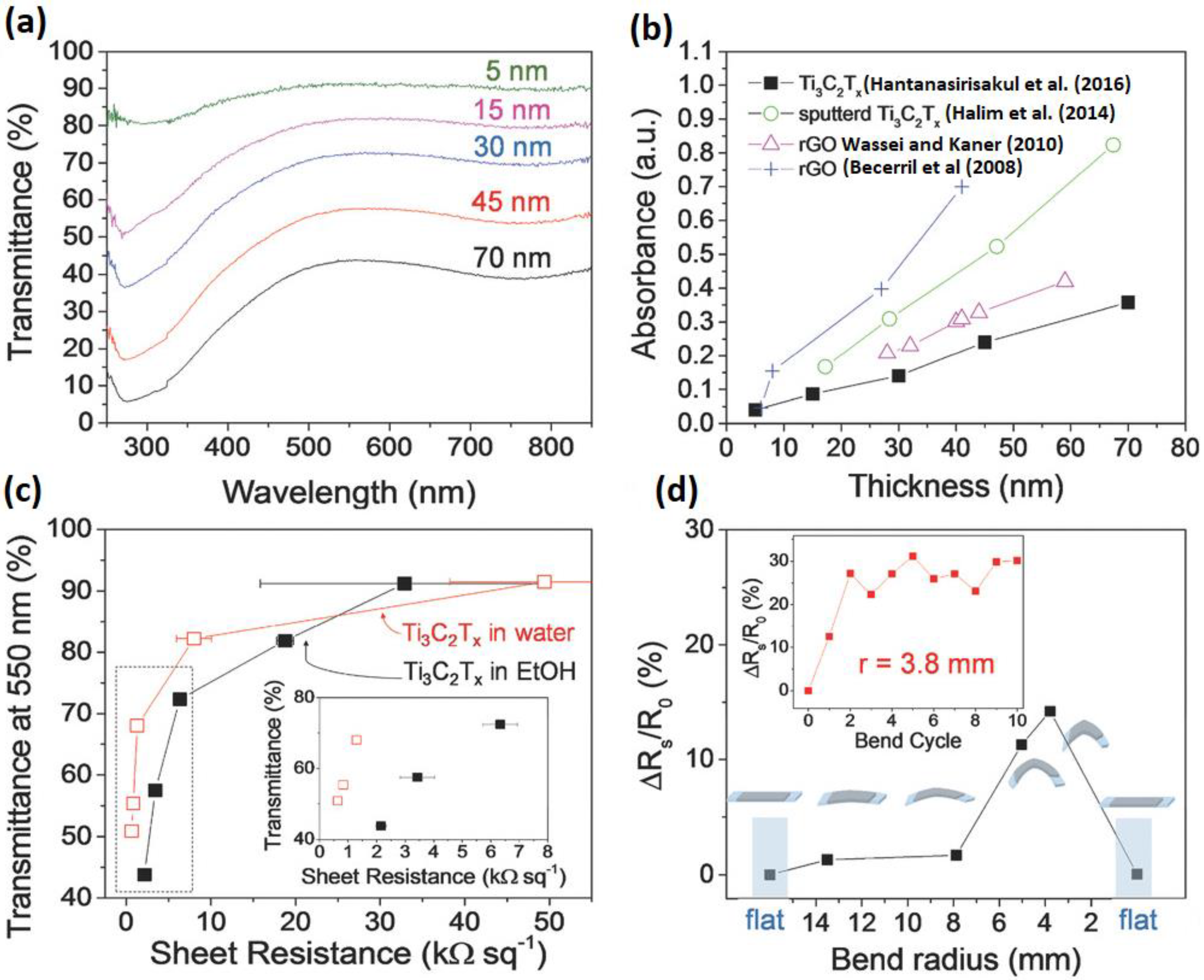
- Hantanasirisakul, K.; Zhao, M.Q.; Urbankowski, P.; Halim, J.; Anasori, B.; Kota, S.; Ren, C.E.; Barsoum, M.W.; Gogotsi, Y. Fabrication of Ti3C2Tx MXene transparent thin films with tunable optoelectronic properties. Adv. Eletron. Mater. 2016, 2, 1600050. [Google Scholar] [CrossRef]
- Halim, J.; Lukatskaya, M.R.; Cook, K.M.; Lu, J.; Smith, C.R.; Näslund, L.-Å.; May, S.J.; Hultman, L.; Gogotsi, Y.; Eklund, P.; et al. Transparent Conductive Two-Dimensional Titanium Carbide Epitaxial Thin Films. Chem. Mater. 2014, 26, 2374–2381. [Google Scholar] [CrossRef]
- Wassei, J.K.; Kaner, R.B. Graphene, a promising transparent conductor. Mater. Today 2010, 13, 52–59. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, W.; Wang, L.; Xie, H. Vertical orientation graphene/MXene hybrid phase change materials with anisotropic properties, high enthalpy, and photothermal conversion. Sci. China Technol. Sci. 2022, 65, 882–892. [Google Scholar] [CrossRef]
- Liu, H.; Fu, R.; Su, X.; Wu, B.; Wang, H.; Xu, Y.; Liu, X. MXene confined in shape-stabilized phase change material combining enhanced electromagnetic interference shielding and thermal management capability. Compos. Sci. Technol. 2021, 210, 108835. [Google Scholar] [CrossRef]
- Du, Y.; Huang, H.; Hu, X.; Liu, S.; Sheng, X.; Li, X.; Lu, X.; Qu, J. Melamine foam/polyethylene glycol composite phase change material synergistically modified by polydopamine/MXene with enhanced solar-to-thermal conversion. Renew. Energy 2021, 171, 1–10. [Google Scholar] [CrossRef]
- Wang, F.; Guo, J.; Li, S.; Wang, Y.; Hu, X.; Li, Z.; Shen, Y.; Li, C. Facile self-assembly approach to construct a novel MXene-decorated nano-sized phase change material emulsion for thermal energy storage. Ceram. Int. 2022, 48, 4722–4731. [Google Scholar] [CrossRef]
- Sheng, X.; Dong, D.; Lu, X.; Zhang, L.; Chen, Y. MXene-wrapped bio-based pomelo peel foam/polyethylene glycol composite phase change material with enhanced light-to-thermal conversion efficiency, thermal energy storage capability and thermal conductivity. Compos. Part A Appl. Sci. Manuf. 2020, 138, 106067. [Google Scholar] [CrossRef]
- Khan, A.A.; Yahya, S.M.; Ali, M.A. Synthesis and Characterization of Titania–MXene-Based Phase Change Material for Sustainable Thermal Energy Storage. Sustainability 2023, 15, 516. [Google Scholar] [CrossRef]
- Luo, Y.; Xie, Y.; Jiang, H.; Chen, Y.; Zhang, L.; Sheng, X.; Xie, D.; Wu, H.; Mei, Y. Flame-retardant and form-stable phase change composites based on MXene with high thermostability and thermal conductivity for thermal energy storage. Chem. Eng. J. 2021, 420, 130466. [Google Scholar] [CrossRef]
- Cheng, H.; Xing, L.; Zuo, Y.; Pan, Y.; Huang, M.; Alhadhrami, A.; Ibrahim, M.M.; El-Bahy, Z.M.; Liu, C.; Shen, C.; et al. Constructing nickel chain/MXene networks in melamine foam towards phase change materials for thermal energy management and absorption-dominated electromagnetic interference shielding. Adv. Compos. Hybrid Mater. 2022, 5, 755–765. [Google Scholar] [CrossRef]
- Shao, Y.-W.; Hu, W.-W.; Gao, M.-H.; Xiao, Y.-Y.; Huang, T.; Zhang, N.; Yang, J.-H.; Qi, X.-D.; Wang, Y. Flexible MXene-coated melamine foam based phase change material composites for integrated solar-thermal energy conversion/storage, shape memory and thermal therapy functions. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106291. [Google Scholar] [CrossRef]
- Gao, S.; Ding, J.; Wang, W.; Lu, J. MXene based flexible composite phase change material with shape memory, self-healing and flame retardant for thermal management. Compos. Sci. Technol. 2023, 234, 109945. [Google Scholar] [CrossRef]
- Krishna, Y.; Saidur, R.; Aslfattahi, N.; Faizal, M.; Ng, K. Enhancing the thermal properties of organic phase change material (palmitic acid) by doping MXene nanoflakes. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2020. [Google Scholar]
- Wang, L.; Liang, W.; Wang, C.; Fan, Y.; Liu, Y.; Xiao, C.; Sun, H.; Zhu, Z.; Li, A. Dodecylamine/Ti3C2-pectin form-stable phase change composites with enhanced light-to-thermal conversion and mechanical properties. Renew. Energy 2021, 176, 663–674. [Google Scholar] [CrossRef]
- Du, X.; Qiu, J.; Deng, S.; Du, Z.; Cheng, X.; Wang, H. Ti3C2Tx@PDA-Integrated Polyurethane Phase Change Composites with Superior Solar-Thermal Conversion Efficiency and Improved Thermal Conductivity. ACS Sustain. Chem. Eng. 2020, 8, 5799–5806. [Google Scholar] [CrossRef]
- Ji, X.; Jiang, Y.; Liu, T.; Lin, S.; Du, A. MXene aerogel-based phase change film for synergistic thermal management inspired by antifreeze beetles. Cell Rep. Phys. Sci. 2022, 3, 100815. [Google Scholar] [CrossRef]
- Mo, Z.; Mo, P.; Yi, M.; Hu, Z.; Tan, G.; Selim, M.S.; Chen, Y.; Chen, X.; Hao, Z.; Wei, X.; et al. Ti3C2Tx@ polyvinyl alcohol foam-supported phase change materials with simultaneous enhanced thermal conductivity and solar-thermal conversion performance. Sol. Energy Mater. Sol. Cells 2021, 219, 110813. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, S.; Liang, W.; Luo, S.; He, Z.; Ge, Y.; Wang, H.; Cao, R.; Zhang, F.; Wen, Q. Broadband nonlinear photonics in few-layer MXene Ti3C2Tx (T = F, O, or OH). Laser Photonics Rev. 2018, 12, 1700229. [Google Scholar] [CrossRef]
- Lin, P.; Xie, J.; He, Y.; Lu, X.; Li, W.; Fang, J.; Yan, S.; Zhang, L.; Sheng, X.; Chen, Y. MXene aerogel-based phase change materials toward solar energy conversion. Sol. Energy Mater. Sol. Cells 2020, 206, 110229. [Google Scholar] [CrossRef]
- Lu, X.; Huang, H.; Zhang, X.; Lin, P.; Huang, J.; Sheng, X.; Zhang, L.; Qu, J.-p. Novel light-driven and electro-driven polyethylene glycol/two-dimensional MXene form-stable phase change material with enhanced thermal conductivity and electrical conductivity for thermal energy storage. Compos. Part B Eng. 2019, 177, 107372. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Jiang, X.; Xu, J.; Huang, W.; Zhang, F.; Liu, J.; Yang, F.; Song, Y.; Ge, Y. MXene Ti3C2Tx: A promising photothermal conversion material and application in all-optical modulation and all-optical information loading. Adv. Opt. Mater. 2019, 7, 1900060. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.; Shang, W.; Wu, J.; Zhu, J.; Chen, G.; Deng, T. Solar-driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Lü, B.; Chen, Y.; Li, P.; Wang, B.; Müllen, K.; Yin, M. Stable radical anions generated from a porous perylenediimide metal-organic framework for boosting near-infrared photothermal conversion. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef]
- Lin, K.-T.; Lin, H.; Yang, T.; Jia, B. Structured graphene metamaterial selective absorbers for high efficiency and omnidirectional solar thermal energy conversion. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Sanchez Casalongue, H.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Iqbal, A.; Shahzad, F.; Hantanasirisakul, K.; Kim, M.-K.; Kwon, J.; Hong, J.; Kim, H.; Kim, D.; Gogotsi, Y.; Koo, C.M. Anomalous absorption of electromagnetic waves by 2D transition metal carbonitride Ti3CNTx (MXene). Science 2020, 369, 446–450. [Google Scholar] [CrossRef]
- Li, R.; Zhang, L.; Shi, L.; Wang, P. MXene Ti3C2: An effective 2D light-to-heat conversion material. ACS Nano 2017, 11, 3752–3759. [Google Scholar] [CrossRef]
- Wang, Q.W.; Zhang, H.B.; Liu, J.; Zhao, S.; Xie, X.; Liu, L.; Yang, R.; Koratkar, N.; Yu, Z.Z. Multifunctional and water-resistant MXene-decorated polyester textiles with outstanding electromagnetic interference shielding and joule heating performances. Adv. Funct. Mater. 2019, 29, 1806819. [Google Scholar] [CrossRef]
- Park, T.H.; Yu, S.; Koo, M.; Kim, H.; Kim, E.H.; Park, J.-E.; Ok, B.; Kim, B.; Noh, S.H.; Park, C. Shape-adaptable 2D titanium carbide (MXene) heater. ACS Nano 2019, 13, 6835–6844. [Google Scholar] [CrossRef]
- Zhao, X.; Peng, L.-M.; Tang, C.-Y.; Pu, J.-H.; Zha, X.-J.; Ke, K.; Bao, R.-Y.; Yang, M.-B.; Yang, W. All-weather-available, continuous steam generation based on the synergistic photo-thermal and electro-thermal conversion by MXene-based aerogels. Mater. Horiz. 2020, 7, 855–865. [Google Scholar] [CrossRef]
- Mazhar, S.; Qarni, A.A.; Haq, Y.U.; Haq, Z.U.; Murtaza, I. Promising PVC/MXene based flexible thin film nanocomposites with excellent dielectric, thermal and mechanical properties. Ceram. Int. 2020, 46, 12593–12605. [Google Scholar] [CrossRef]
- Borysiuk, V.; Mochalin, V.N. Thermal stability of two-dimensional titanium carbides Tin+1Cn (MXenes) from classical molecular dynamics simulations. MRS Commun. 2019, 9, 203–208. [Google Scholar] [CrossRef]
- Jin, X.; Wang, J.; Dai, L.; Liu, X.; Li, L.; Yang, Y.; Cao, Y.; Wang, W.; Wu, H.; Guo, S. Flame-retardant poly (vinyl alcohol)/MXene multilayered films with outstanding electromagnetic interference shielding and thermal conductive performances. Chem. Eng. J. 2020, 380, 122475. [Google Scholar] [CrossRef]
- Zhang, P.; Liao, Q.; Yao, H.; Huang, Y.; Cheng, H.; Qu, L. Direct solar steam generation system for clean water production. Energy Storage Mater. 2019, 18, 429–446. [Google Scholar] [CrossRef]
- Zhao, X.; Zha, X.-J.; Pu, J.-H.; Bai, L.; Bao, R.-Y.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Macroporous three-dimensional MXene architectures for highly efficient solar steam generation. J. Mater. Chem. A 2019, 7, 10446–10455. [Google Scholar] [CrossRef]
- Zha, X.-J.; Zhao, X.; Pu, J.-H.; Tang, L.-S.; Ke, K.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, M.-B.; Yang, W. Macroporous three-dimensional MXene architectures for highly efficient solar steam generation. J. Mater. Chem. A ACS Appl. Mater. Interfaces 2019, 11, 36589–36597. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Yang, C.; Tian, Y.; Han, Y.; Liu, J.; Yin, X.; Que, W. A hydrophobic surface enabled salt-blocking 2D Ti3 C2 MXene membrane for efficient and stable solar desalination. J. Mater. Chem. A 2018, 6, 16196–16204. [Google Scholar] [CrossRef]


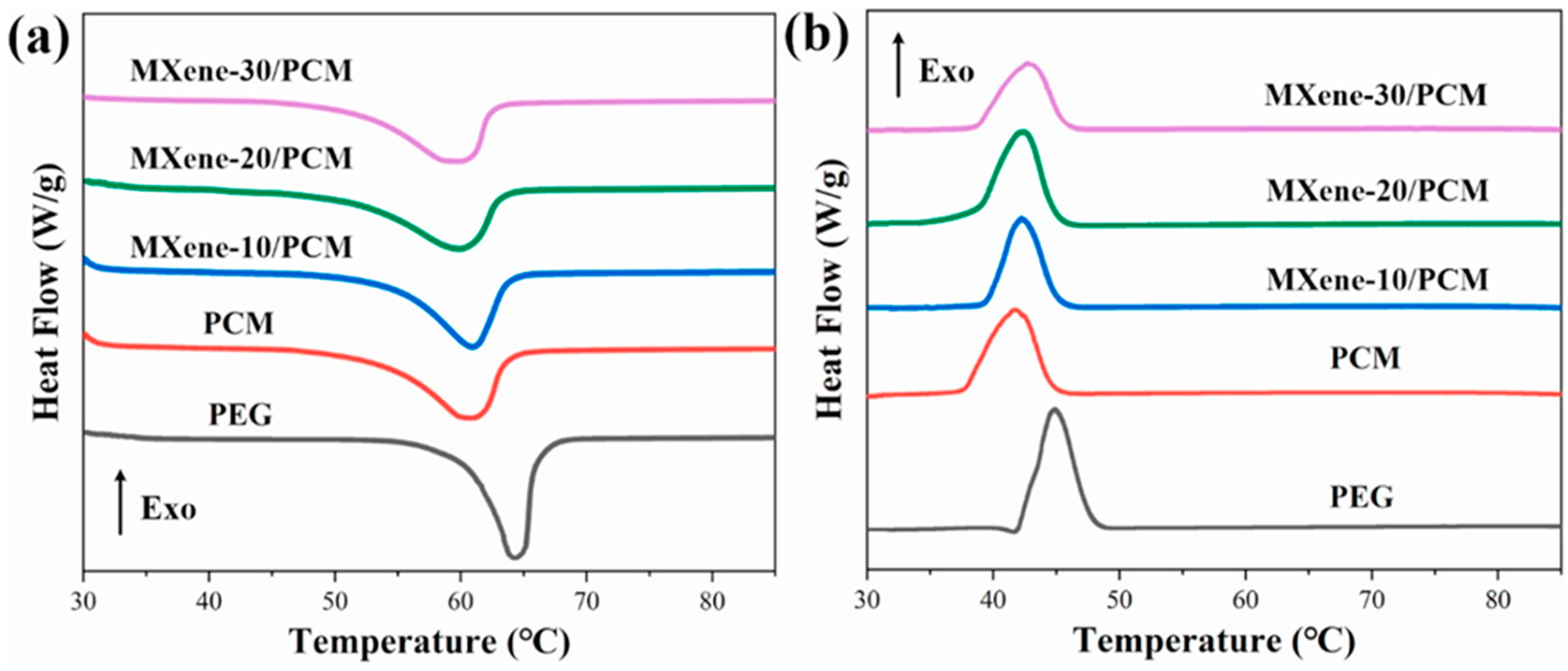


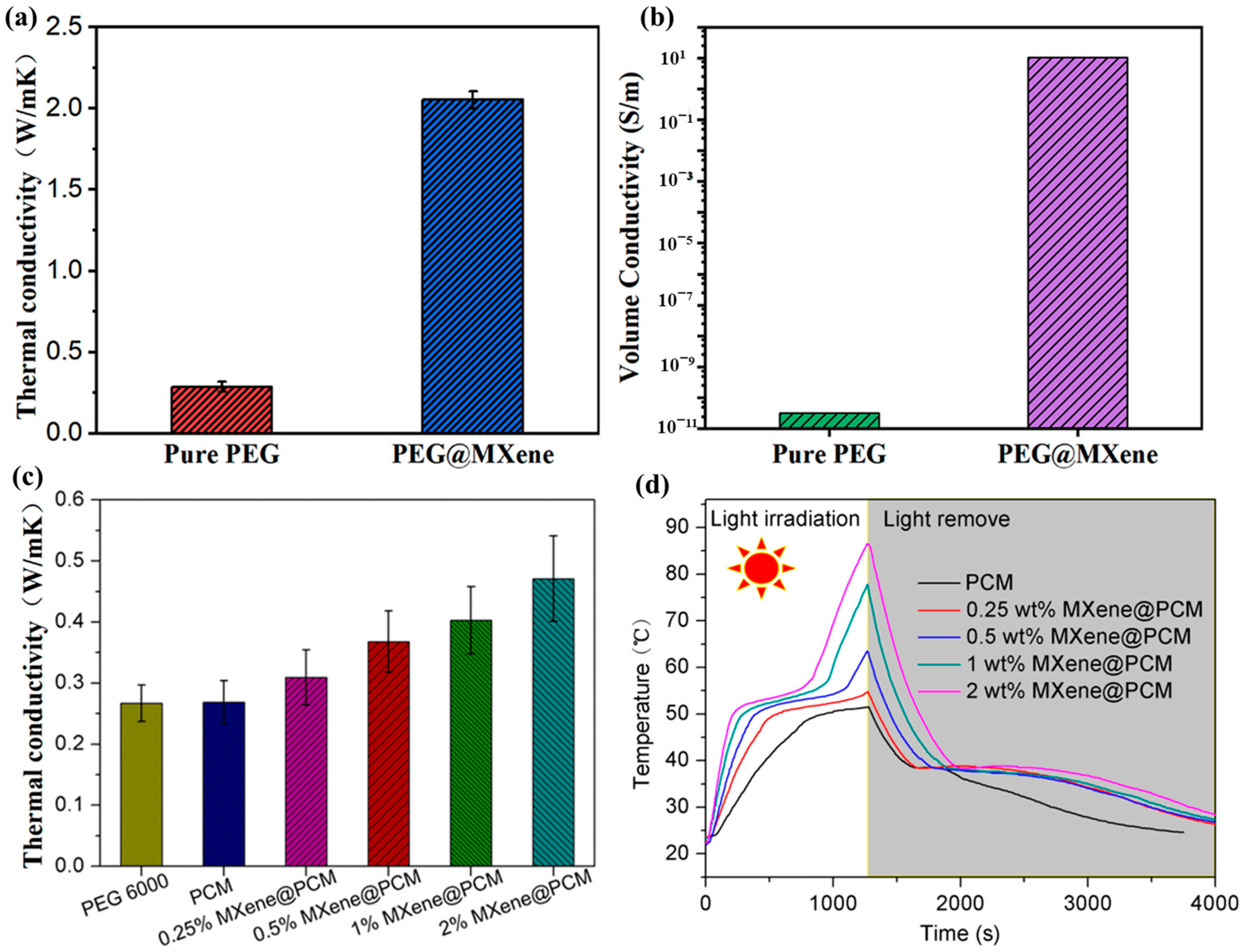
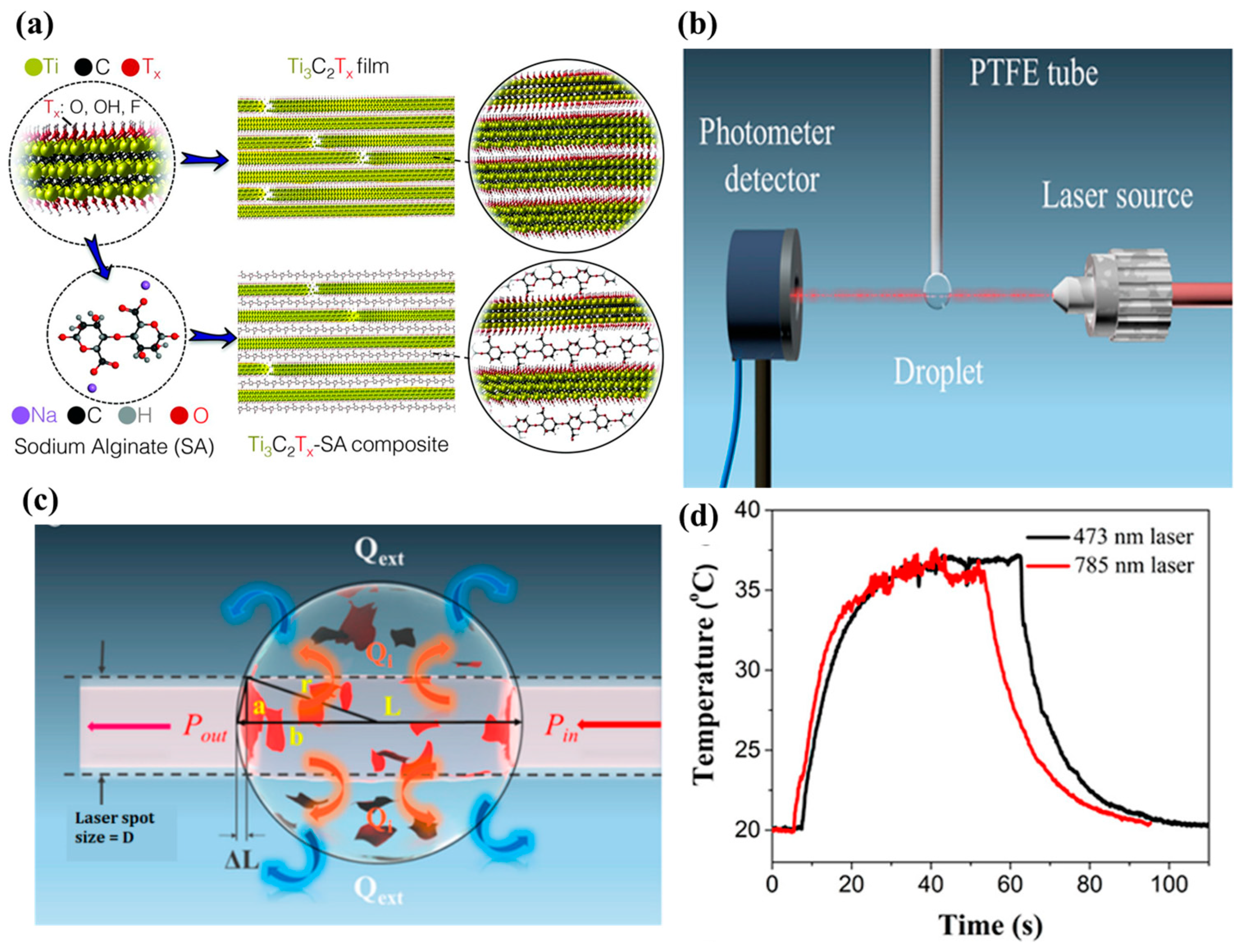
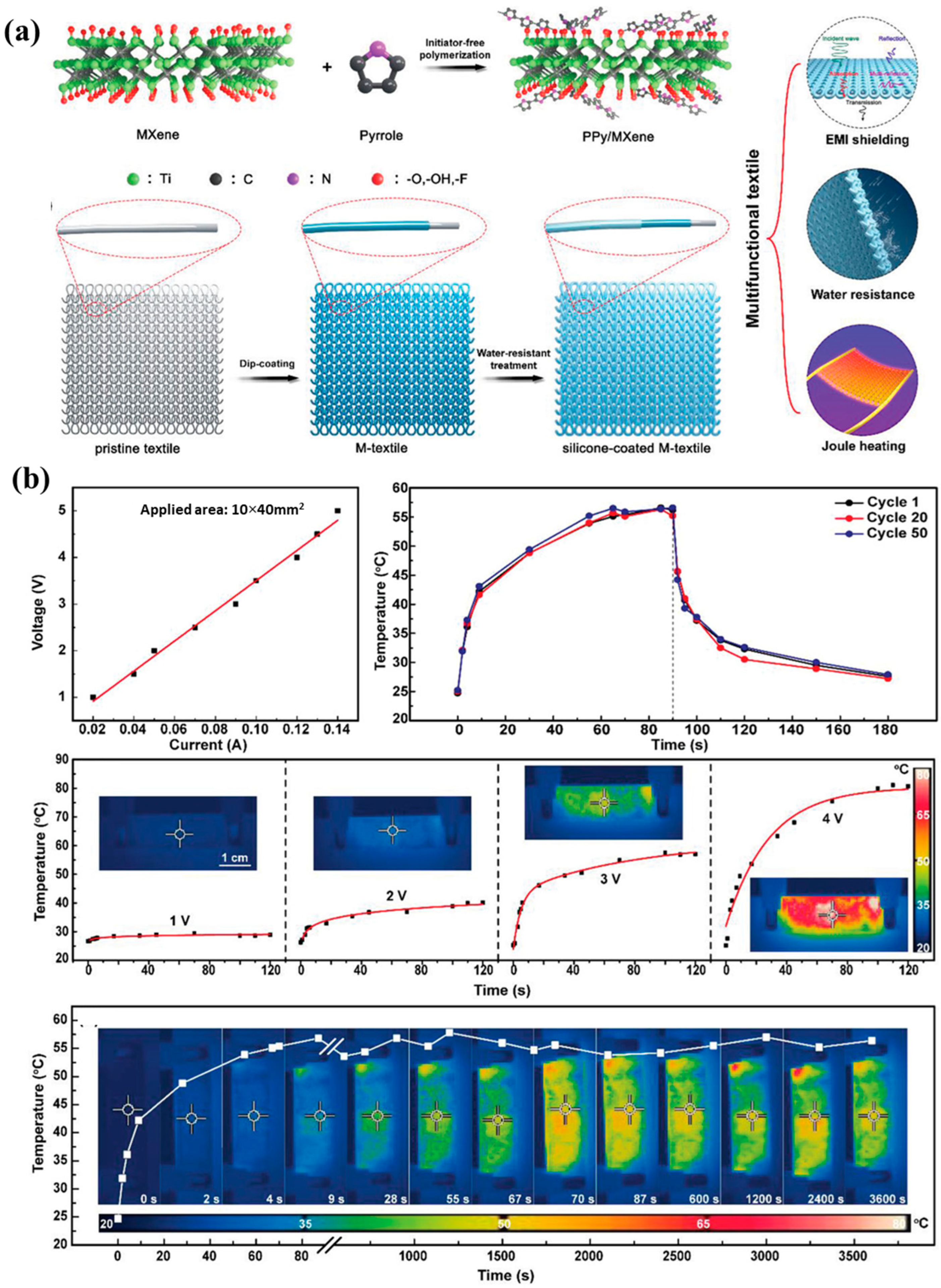
| Base PCM | Melting Temperature (°C) | Latent Heat of Fusion (kJ/kg) | Specific Heat Capacity (kJ/kgK) | Thermal Conductivity (W/mK) | Reference |
|---|---|---|---|---|---|
| RT20 | 21.23–25.73 | 140.3 | 1.8–2.4 | 0.2 | [72] |
| RT21 | 21 | 134 | n.a. | 0.2 | [73] |
| RT25 | 26.6 | 232 | 1.8–2.4 | 0.19 | [74] |
| RT27 | 26–28 | 184 | 1.8–2.4 | 0.2 | [75,76,77] |
| RT31 | 29 | 169 | 1.82 | 0.2 | [73] |
| RT35 | 35 | 157 | 1.78–2.37 | 0.2 | [76] |
| RT42 | 38–43 | 174 | 2.0 | 0.2 | [78] |
| RT44 | 41–45 | 255 | 2.0 | 0.2 | [79] |
| RT60 | 60 | 144 | n.a. | 0.2 | [73] |
| RT10HC | 9 | 126 | 2 | 0.2 | [80] |
| RT18HC | 17 | 222 | 2 | 0.2 | [80] |
| RT25HC | 22 | 177 | 2 | 0.2 | [80] |
| RT35HC | 34 | 197 | 2 | 0.2 | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awan, H.T.A.; Kumar, L.; Wong, W.P.; Walvekar, R.; Khalid, M. Recent Progress and Challenges in MXene-Based Phase Change Material for Solar and Thermal Energy Applications. Energies 2023, 16, 1977. https://doi.org/10.3390/en16041977
Awan HTA, Kumar L, Wong WP, Walvekar R, Khalid M. Recent Progress and Challenges in MXene-Based Phase Change Material for Solar and Thermal Energy Applications. Energies. 2023; 16(4):1977. https://doi.org/10.3390/en16041977
Chicago/Turabian StyleAwan, Hafiz Taimoor Ahmed, Laveet Kumar, Weng Pin Wong, Rashmi Walvekar, and Mohammad Khalid. 2023. "Recent Progress and Challenges in MXene-Based Phase Change Material for Solar and Thermal Energy Applications" Energies 16, no. 4: 1977. https://doi.org/10.3390/en16041977
APA StyleAwan, H. T. A., Kumar, L., Wong, W. P., Walvekar, R., & Khalid, M. (2023). Recent Progress and Challenges in MXene-Based Phase Change Material for Solar and Thermal Energy Applications. Energies, 16(4), 1977. https://doi.org/10.3390/en16041977









