An Assessment of the Conversion of Biomass and Industrial Waste Products to Activated Carbon
Abstract
:1. Introduction
1.1. Biochar Production
1.1.1. Pyrolysis
1.1.2. Hydrothermal Carbonization
1.1.3. Torrefaction
1.1.4. Gasification
1.2. Biochar Modification
1.2.1. Physical Activation
1.2.2. Chemical Activation
1.2.3. Surface Oxidation/Acidification
1.2.4. Magnetization
1.3. Biochar Characterization
2. Material and Methods
3. Results and Discussion
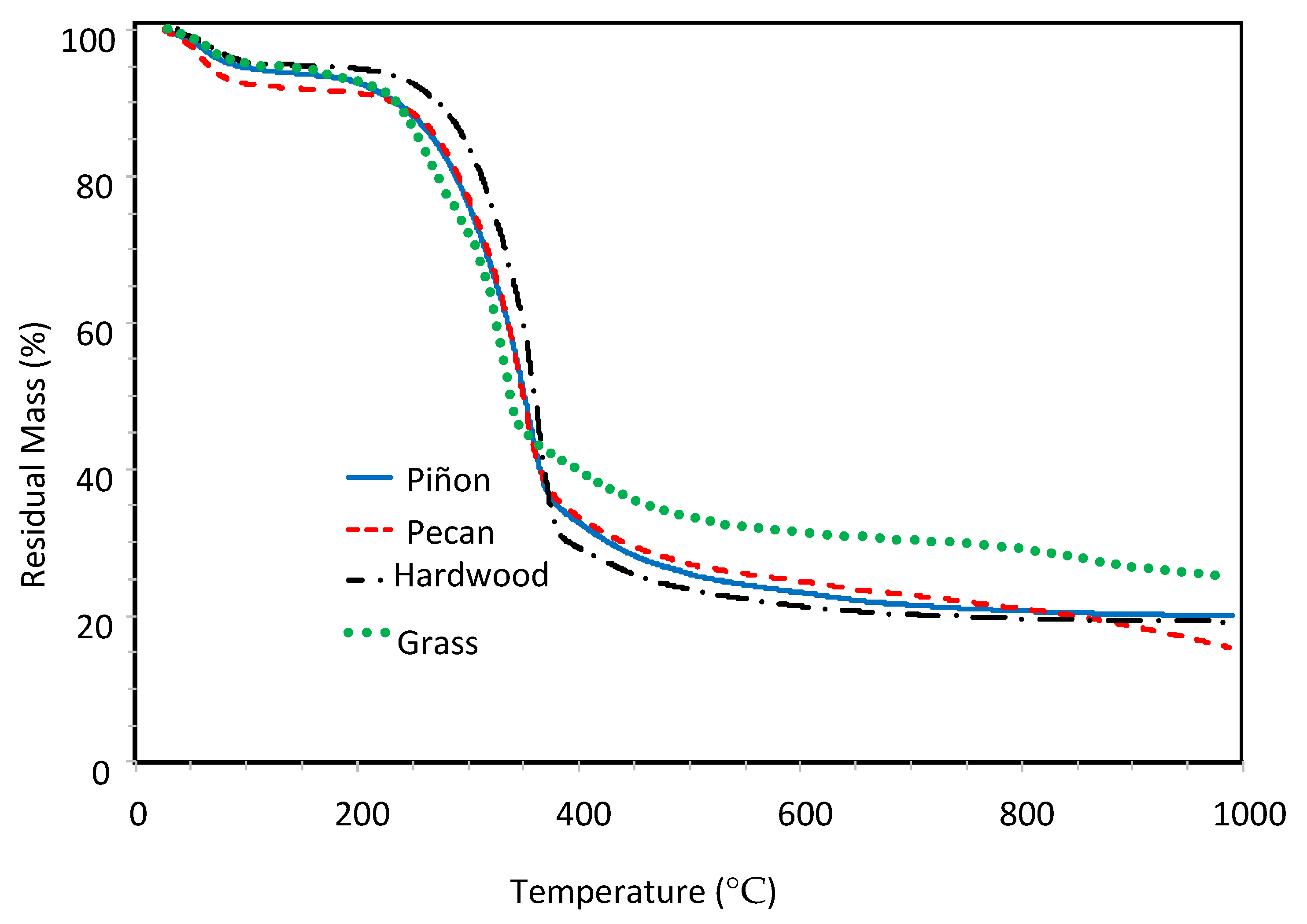
3.1. Initial Thermogravimetric Analysis
3.2. Isothermal TGA Tests and Tube Furnace Runs
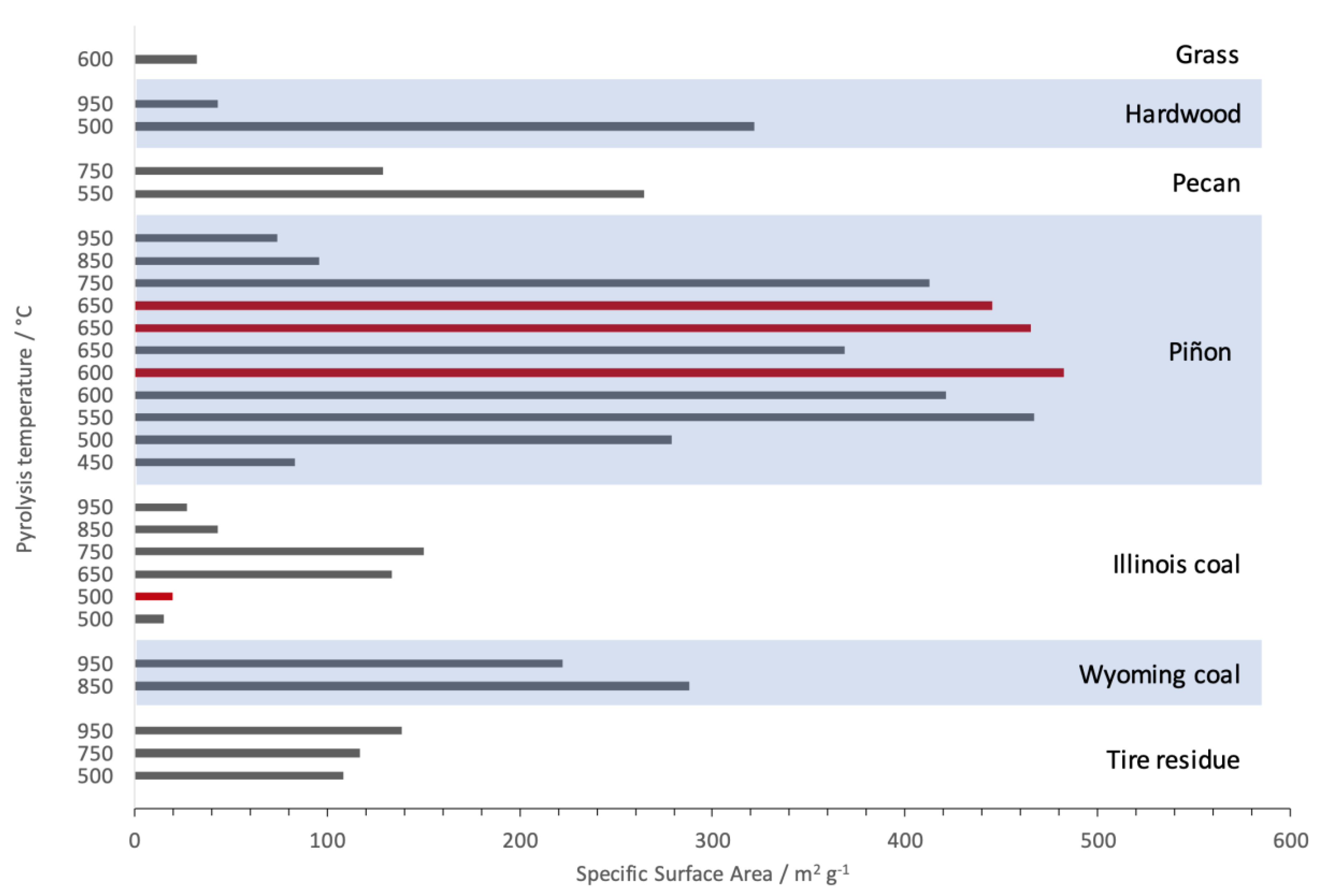
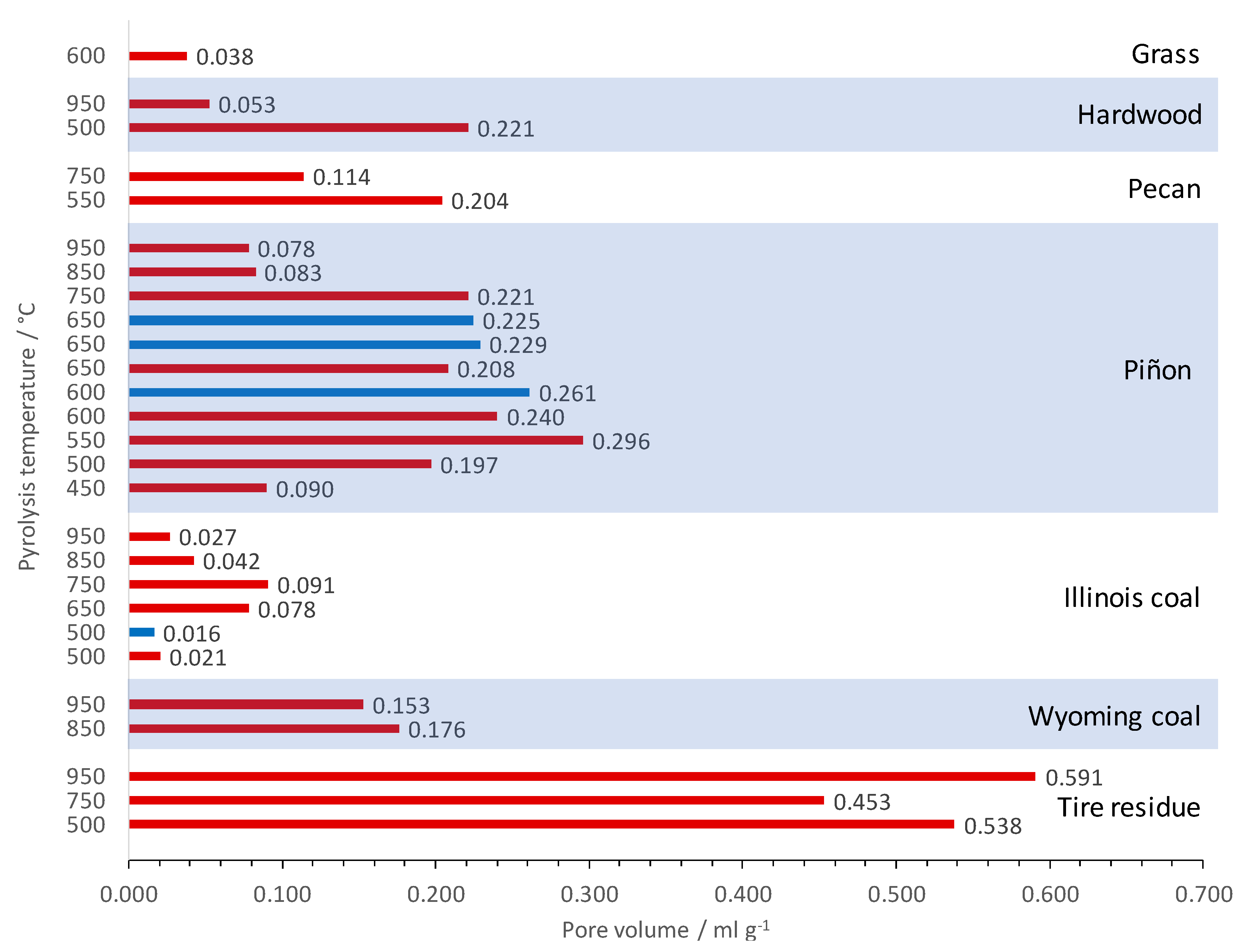
3.3. Textural Properties of the Produced Activated Carbon Materials
4. Conclusions
- (1)
- For applications such as municipal water treatment, in particular, the biomass feedstocks are most desirable in comparison to hydrocarbon feedstocks because of their significantly higher yield and because they are a more socially acceptable source;
- (2)
- While piñon wood gave the best results, this and some of the other biomass feedstocks have a negative value, so a double benefit is realized: both the production of a valuable and useful product, activated carbon, and the disposal of plant fiber without incineration;
- (3)
- In addition, the optimal process variables are indicated by the results. Pyrolysis temperatures in the range 600–650 °C give the highest yield, and an atmosphere with trace oxygen gives the best results.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sohi, S.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- Rosa, J.; Paneque, M.; Miller, A.Z.; Knicker, H. Relating physical and chemical properties of four different biochars and their application rate to biomass production of Lolium perenne on a Calcic Cambisol during a pot experiment of 79 days. Sci. Total Environ. 2014, 499, 175–184. [Google Scholar] [CrossRef]
- Erickson, B. Regenerating degraded dirt. CEN Glob. Enterp. 2016, 94, 40–43. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Niazi, N.K.; Hassan, N.E.E.; Bibi, I.; Wang, H.; Tsang, D.C.W.; Ok, Y.S.; Bolan, N.; Rinklebe, J. Wood-based biochar for the removal of potentially toxic elements in water and wastewater: A critical review. Int. Mater. Rev. 2018, 64, 216–247. [Google Scholar] [CrossRef]
- Cheng, F.; Li, X. Preparation and Application of Biochar-Based Catalysts for Biofuel Production. Catalysts 2018, 8, 346. [Google Scholar] [CrossRef]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Zhao, X.; Wang, S.; Xing, G. Comparisons of Biochar Properties from Wood Material and Crop Residues at Different Temperatures and Residence Times. Energy Fuels 2013, 27, 5890–5899. [Google Scholar] [CrossRef]
- Wu, W.; Yang, M.; Feng, Q.; McGrouther, K.; Wang, H.; Lu, H.; Chen, Y. Chemical characterization of rice straw-derived biochar for soil amendment. Biomass Bioenergy 2012, 47, 268–276. [Google Scholar] [CrossRef]
- Byrne, C.E.; Nagle, D.C. Carbonization of Wood for Advanced Materials Applications. Carbon 1997, 35, 259–266. [Google Scholar] [CrossRef]
- Mok, W.S.L.; Antal, M.J.; Szabo, P.; Varhegyi, G.; Zelei, B. Formation of Charcoal from Biomass in a Sealed Reactor. Ind. Eng. Chem. Res. 1992, 31, 1162–1166. [Google Scholar] [CrossRef]
- Brewer, C.E. Biochar Characterization and Engineering. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2012; pp. 12–84. [Google Scholar]
- Titirici, M.M.; White, R.J.; Falco, C.; Sevilla, M. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796–6822. [Google Scholar] [CrossRef]
- Buentello-Montoya, D.; Zhang, X.; Li, J. The use of gasification solid products as catalysts for tar reforming. Renew. Sustain. Energy Rev. 2019, 107, 399–412. [Google Scholar] [CrossRef]
- González, J.F.; Román, S.; Encinar, J.M.; Martínez, G. Pyrolysis of various biomass residues and char utilization for the production of activated carbons. J. Anal. Appl. Pyrolysis 2009, 85, 134–141. [Google Scholar] [CrossRef]
- Cha, J.S.; Choi, J.C.; Ko, J.H.; Park, Y.K.; Park, S.H.; Jeong, K.-E.; Kim, S.-S.; Jeon, J.-K. The low-temperature scr of no over rice straw and sewage sludge derived char. Chem. Eng. J. 2010, 156, 321–327. [Google Scholar] [CrossRef]
- Ros, A.; Lillo-Ródenas, M.; Fuente, E.; Montes-Morán, M.; Martín, M.; Linares-Solano, A. High surface area materials prepared from sewage sludge-based precursors. Chemosphere 2006, 65, 132–140. [Google Scholar] [CrossRef]
- Son, E.; Poo, K.; Chang, J.; Chae, K.-J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci Total Environ. 2018, 615, 161–168. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Parthasarathy, P.; Abdelaal, A.H.; Mackey, H.; McKay, G.; Al-Ansari, T. Investigation of biomass components on the slow pyrolysis products yield using Aspen Plus for techno-economic analysis. Biomass Convers. Biorefin. 2022, 12, 669–681. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, K. Environmental application of biochar: Current status and perspectives. Bioresour. Techno. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Z.; Bolan, N.S.; Wang, H.; Wang, Y.; Chen, W. Visualizing the development trend and research frontiers of biochar in 2020: A scientometric perspective. Biochar 2021, 3, 419–436. [Google Scholar] [CrossRef]
- Chen, Y.D.; Wang, R.; Duan, X.; Wang, S.; Ren, N.Q.; Ho, S.H. Production, properties, and catalytic applications of sludge derived biochar for environmental remediation. Water Res 2020, 187, 116390. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Biomass and Bioenergy Insight into biochar properties and its cost analysis. Biomass Bioenergy 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Djellabi, R.; Aboagye, D.; Galloni, M.G.; Andhalkar, V.V.; Nouacer, S.; Nabgan, W.; Rtimi, S.; Constantí, M.; Cabello, F.M.; Contreras, S. Combined conversion of lignocellulosic biomass into high-value products with ultrasonic cavitation and photocatalytic produced reactive oxygen species—A review. Bioresour. Technol. 2022, 368, 128333. [Google Scholar] [CrossRef] [PubMed]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Malyan, S.K.; Kumar, S.S.; Fagodiya, R.K.; Ghosh, P.; Kumar, A.; Singh, R.; Singh, L. Biochar for environmental sustainability in the energy-water-agroecosystem. nexus. Renew. Sustain. Energy Rev. 2021, 149, 11137. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, W.A.; Nasrullah, M. Advanced techniques in the production of biochar from lignocellulosic biomass and environmental applications. Clean. Mater. 2022, 6, 100137. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.; Nasrullah, M. A comprehensive assessment of the method for producing biochar, its characterization, stability, and potential applications in regenerative economic sustainability—A review. Clean. Mater. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Shabir, R.; Li, Y.; Zhang, L.; Chen, C. Biochar surface properties and chemical composition determine the rhizobial survival rate. J. Environ. Manag. 2023, 326, 116594. [Google Scholar] [CrossRef]
- Liu, P.; Zhuang, H.; Qian, Y.; Yang, J.; Pan, Y.; Zhou, Z.; Jia, L.; Qi, F. Recent advances in mass spectrometric studies on the reaction process of biomass pyrolysis. Fuel Process. Technol. 2022, 237, 107473. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef] [PubMed]







| Thermochemical Process | Range (°C) | Heating Rate | Pressure | Residence Time | Primary Product |
|---|---|---|---|---|---|
| Slow Pyrolysis | 350–800 | Slow <10 °C/min | Atmospheric | Hours Days | Char |
| Torrefaction | 200–300 | Slow <10 °C/min | Atmospheric | Minutes Hours | Stabilized, friable biomass |
| Fast Pyrolysis | 400–600 | Very Fast ~1000 °C/s | Vacuum-Atmospheric | Seconds | Bio-oil |
| Flash Pyrolysis | 300–800 | Fast | Elevated | Minutes | Biocarbon/Char |
| Gasification | 700–1500 | Moderate-Very Fast | Atmospheric-Elevated | Seconds Minutes | Syngas/Producer gas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coker, E.N.; Lujan-Flores, X.; Donaldson, B.; Yilmaz, N.; Atmanli, A. An Assessment of the Conversion of Biomass and Industrial Waste Products to Activated Carbon. Energies 2023, 16, 1606. https://doi.org/10.3390/en16041606
Coker EN, Lujan-Flores X, Donaldson B, Yilmaz N, Atmanli A. An Assessment of the Conversion of Biomass and Industrial Waste Products to Activated Carbon. Energies. 2023; 16(4):1606. https://doi.org/10.3390/en16041606
Chicago/Turabian StyleCoker, Eric N., Xavier Lujan-Flores, Burl Donaldson, Nadir Yilmaz, and Alpaslan Atmanli. 2023. "An Assessment of the Conversion of Biomass and Industrial Waste Products to Activated Carbon" Energies 16, no. 4: 1606. https://doi.org/10.3390/en16041606
APA StyleCoker, E. N., Lujan-Flores, X., Donaldson, B., Yilmaz, N., & Atmanli, A. (2023). An Assessment of the Conversion of Biomass and Industrial Waste Products to Activated Carbon. Energies, 16(4), 1606. https://doi.org/10.3390/en16041606









