State-of-Art Review on Chemical Indicators for Monitoring the Aging Status of Oil-Immersed Transformer Paper Insulation
Abstract
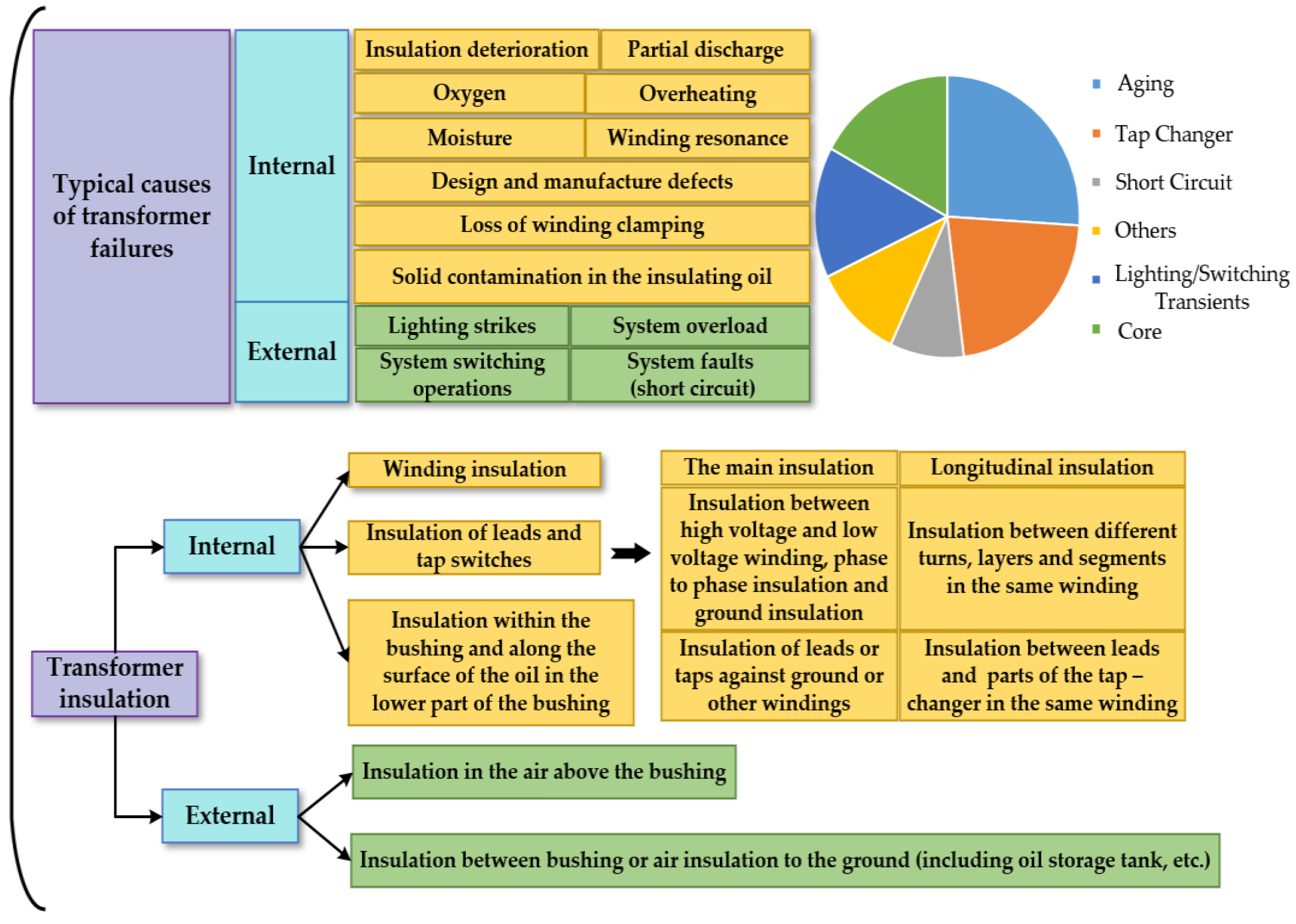
1. Introduction
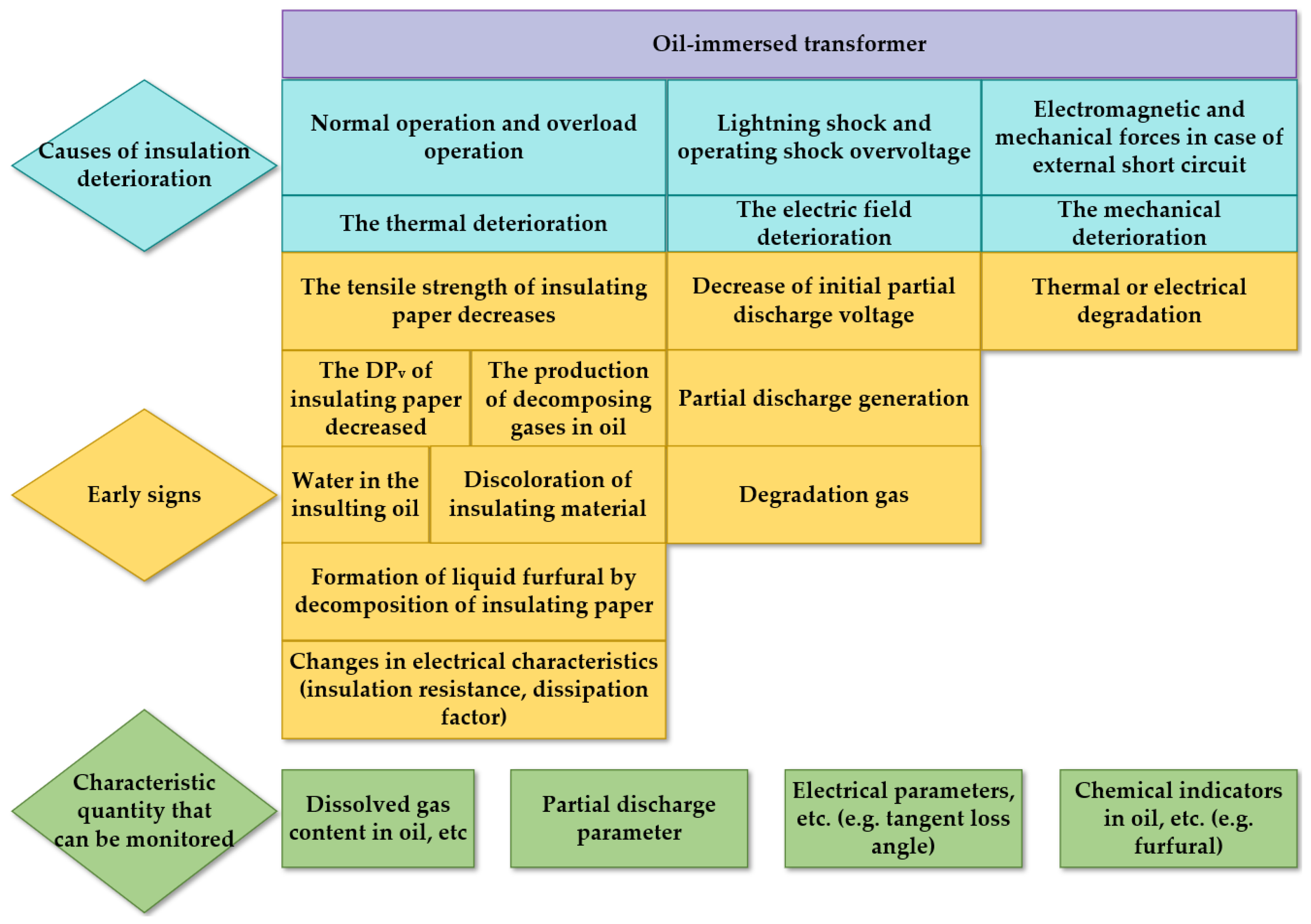
2. Foundational Principles of Transformer Paper Insulation Aging
2.1. Influence Factors of Aging for Transformer Oil-Impregnated Insulating Paper System
2.2. Aging Mechanism of Transformer Paper Insulation
- (i)
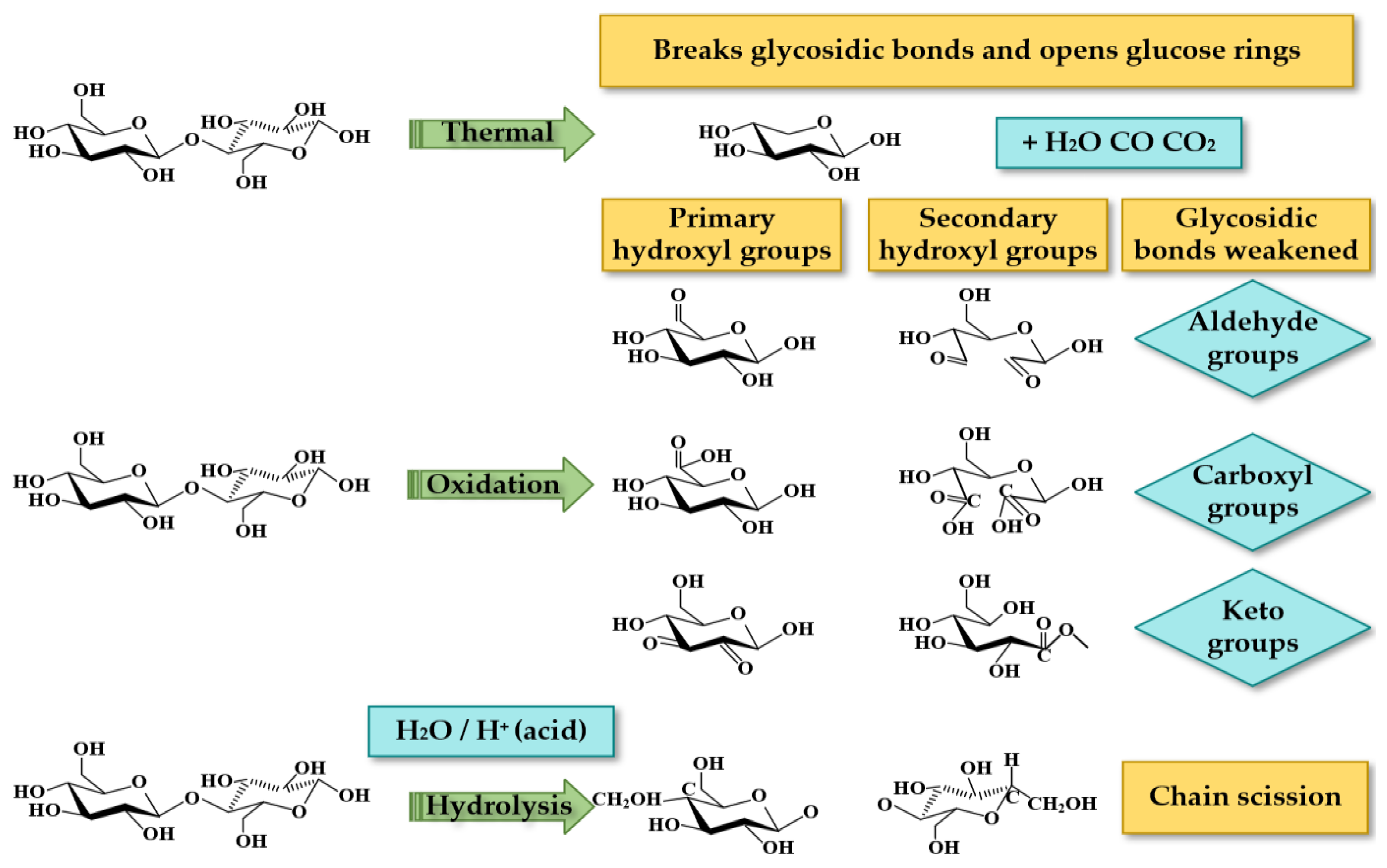
- Pyrolysis [36,37,38] is the main degradation process above 130 °C. The direct degradation of cellulose caused by pyrolysis is generally more obvious above 200 °C. Below 200 °C, pyrolysis mainly accelerates other forms of degradation, similar to the normal aging of cellulose, but faster than normal aging. From the microscopic mechanism, the thermal stability of cellulose C-O bonds is much weaker than the C-H bonds of insulating oil. Under the effect of temperature, C-O bonds can be broken, the degree of polymerization of cellulose will be reduced, and the mechanical strength will be continuously reduced. Because insulating paper has a low thermal conductivity, heat easily accumulates in the paper. With the accumulation of heat, local chemical bonds are cleavaged, and products such as aldehydes, carboxyl groups, and carbon dioxide are produced. Macroscopically, the rate of pyrolysis is determined by the interaction of oxygen, water, acid concentration, and temperature.
- (ii)
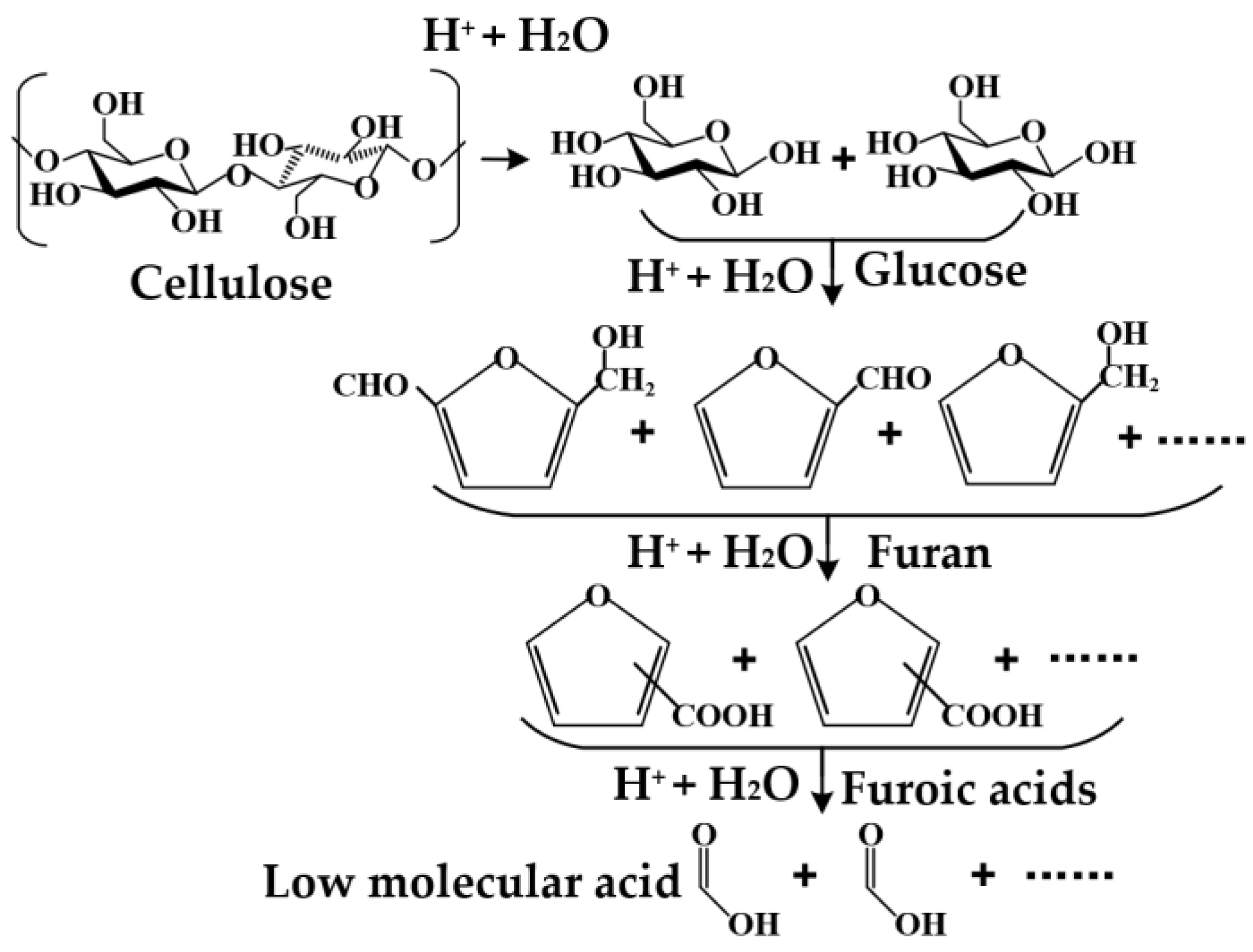
- Hydrolysis [17,26,34,39] is the main degradation process in the range of 70 °C –130 °C. Cellulose is hygroscopic in nature, and not only water molecules can accumulate in the cellulose chains, but the pyrolysis of cellulose can also produce water. The hydrolysis reaction between water and cellulose is the main form of aging of insulating paper. The hydrolysis reaction causes the 1,4-β-glycosidic bond between glucose groups to be broken to produce short-chain molecules, which are further hydrolyzed to form low-molecular carboxylic acids and water. Lundgaard et al. believed that each break of the cellulose glycoside bond absorbed one water molecule and then released three water molecules, so a total of two water molecules are generated each time the bond was broken. In addition, chain fission can indicate the rate of hydrolytic degradation.
- (iii)
- Oxidation [17,39,40] is mainly dominant at temperatures below 75 °C. Cellulose is relatively sensitive to oxidation, and its hydroxyl groups are easily oxidized to carbonyl and carboxyl groups, resulting in secondary chain cleavage reactions. However, free oxygen atoms were not formed inside the transformer during the aging process of transformer oil and cellulose paper. Some scholars have proposed that transition metal ions such as Cu+/Cu2+ and Fe2+/Fe3+ in transformers catalyze the reaction of oxygen and water to generate hydrogen peroxide and that the oxidation process can be catalyzed by hydroxyl radicals (·OH) produced by the decomposition of hydrogen peroxide.
2.3. On the Kinetics of Degradation of Cellulose
3. Chemical Indicators for Monitoring the Aging Condition
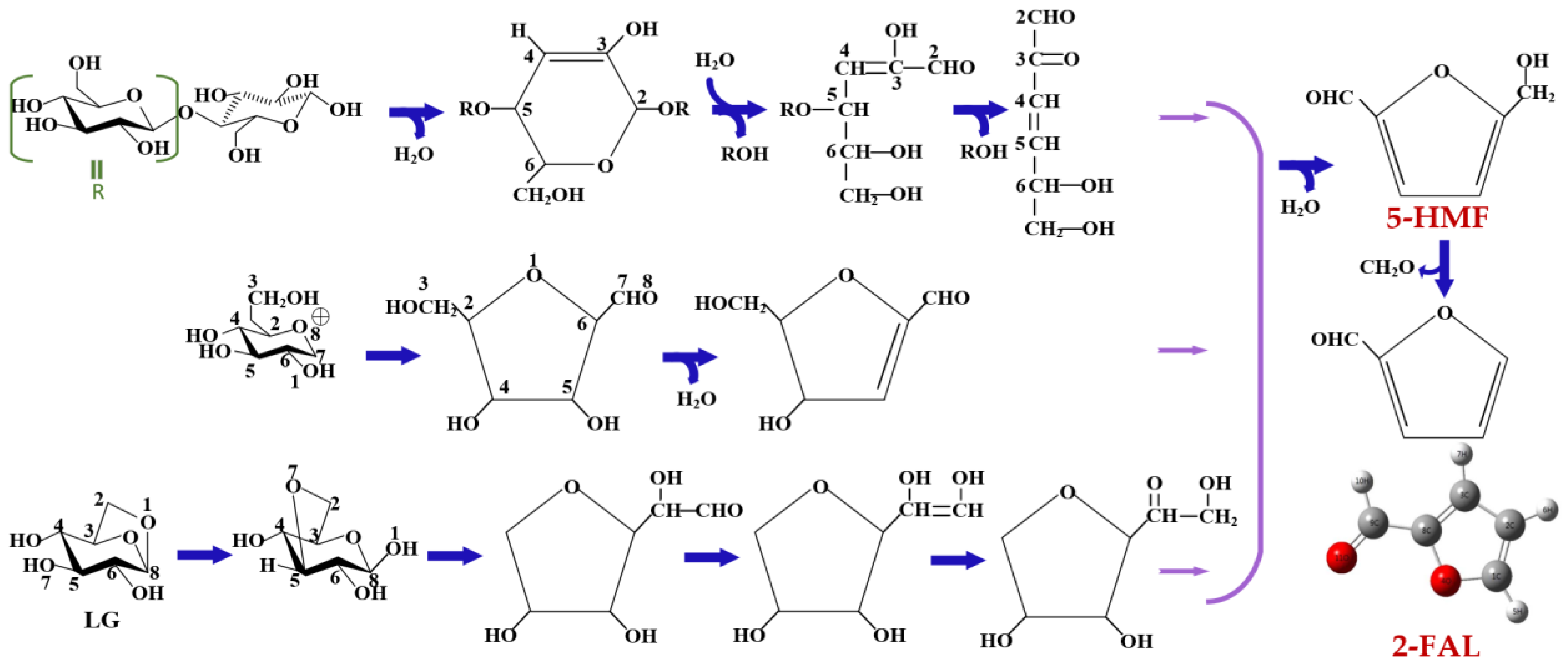
3.1. Furan Compounds Analysis
3.1.1. Research on Furan Compounds
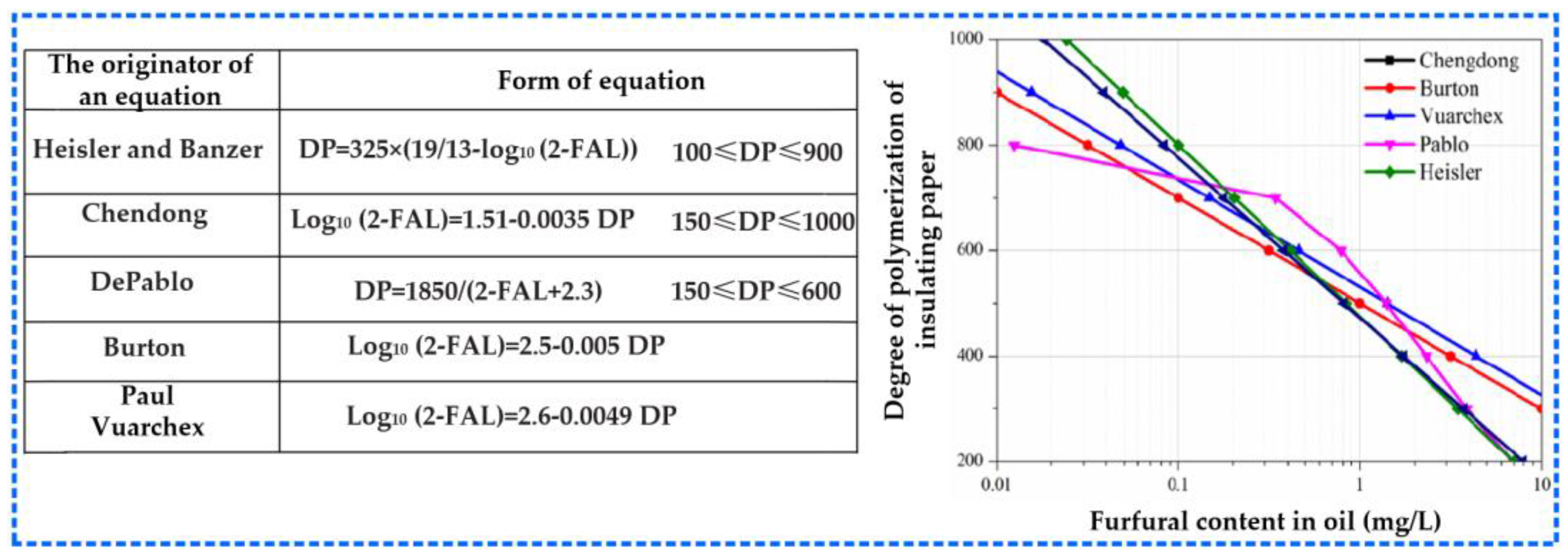
3.1.2. Characteristics of Furan Compounds
3.2. Carbon-Oxygen Gases Analysis
3.2.1. Research on Carbon-Oxygen Gases
3.2.2. Characteristics of Carbon-Oxygen Gases
3.3. Low Molecular Alcohols Analysis
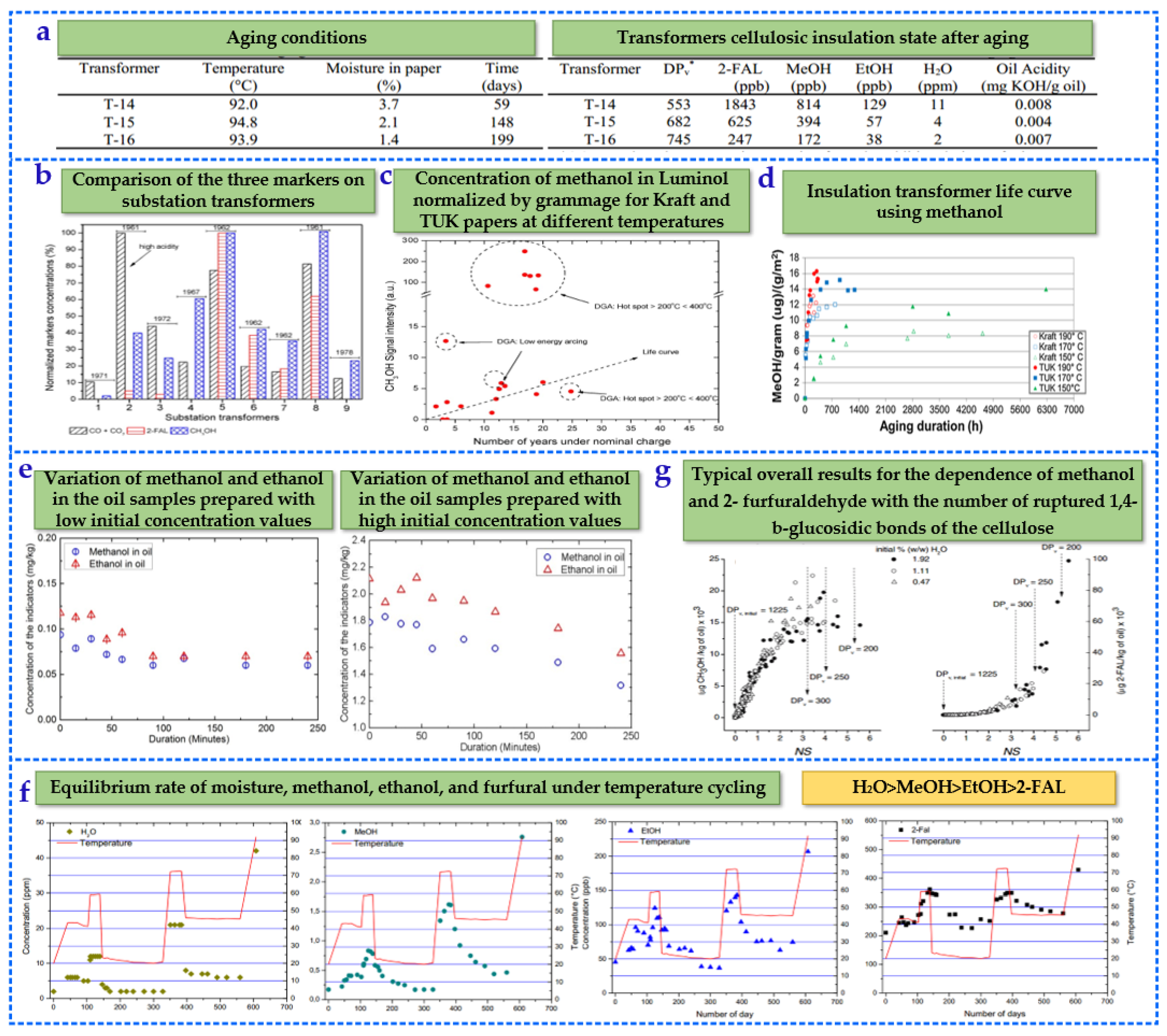
3.3.1. Research on Low Molecular Alcohol Compounds
3.3.2. Characteristics of Low Molecular Alcohol Compounds
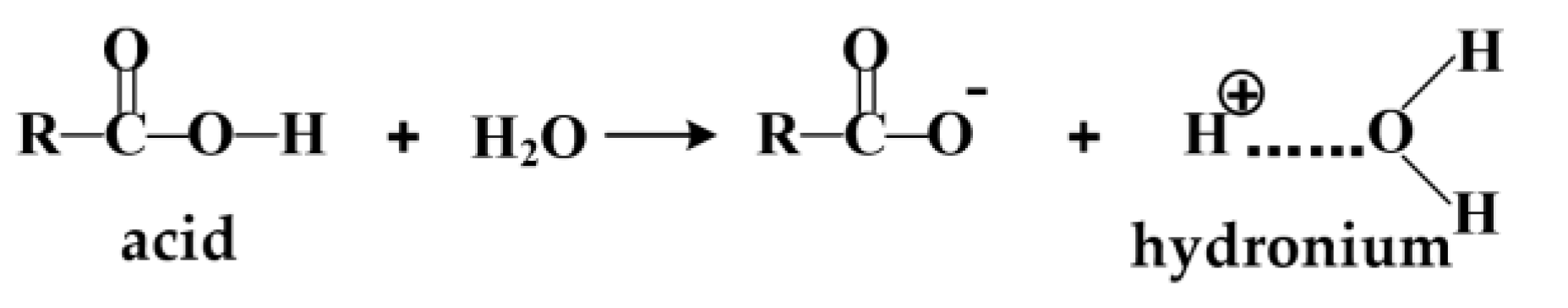
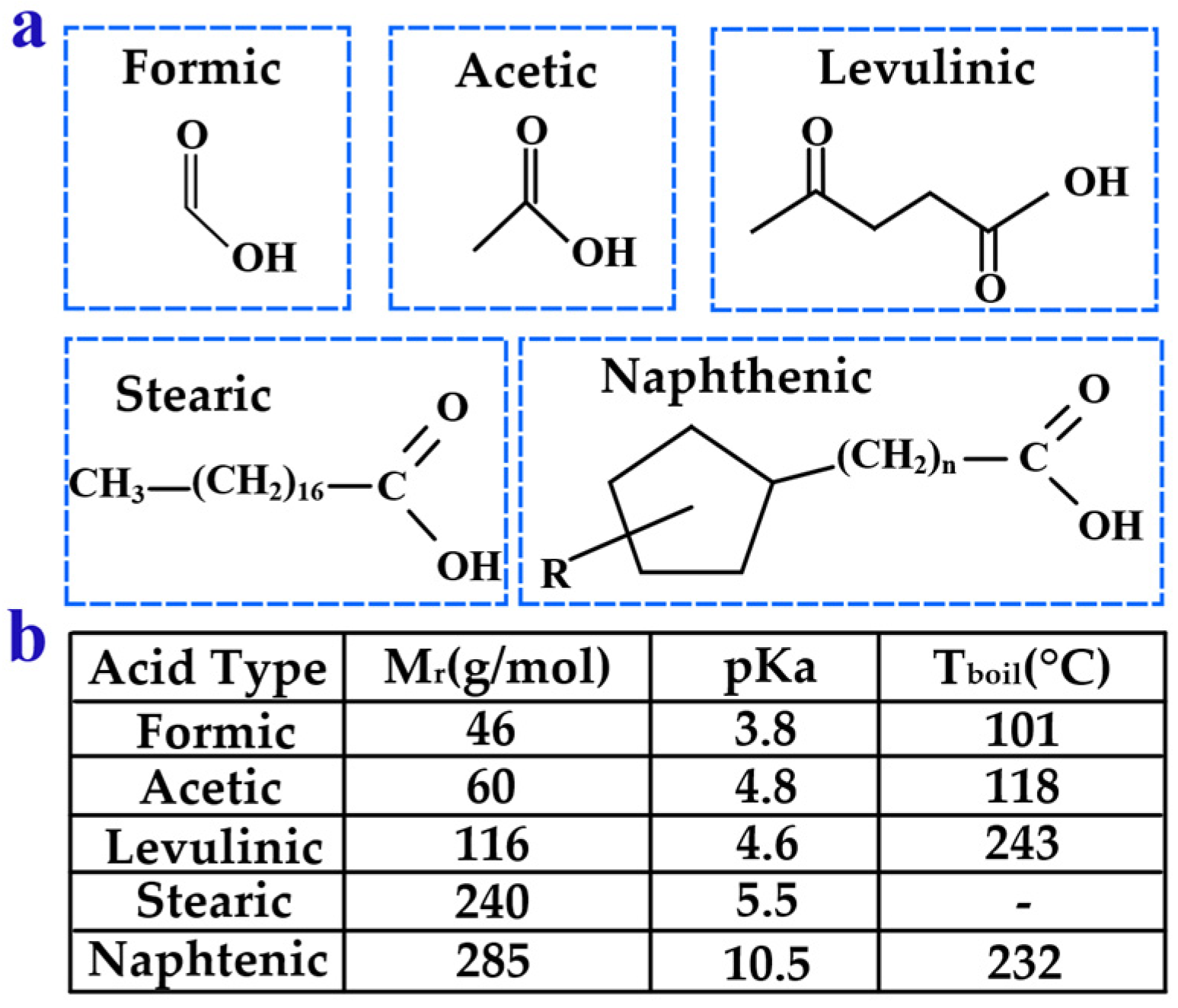
3.4. Acid Compounds Analysis
3.4.1. Research on Organic Acids
3.4.2. Characteristics of Organic Acids
3.5. Ketone Compounds Analysis
3.5.1. Characteristics and Research on Acetone
3.5.2. Research on Methyl Ethyl Ketone
3.6. Sugar Analysis
3.6.1. Classification and Interrelationship of Sugars
3.6.2. Characteristics and Research on Sugars
3.7. Others
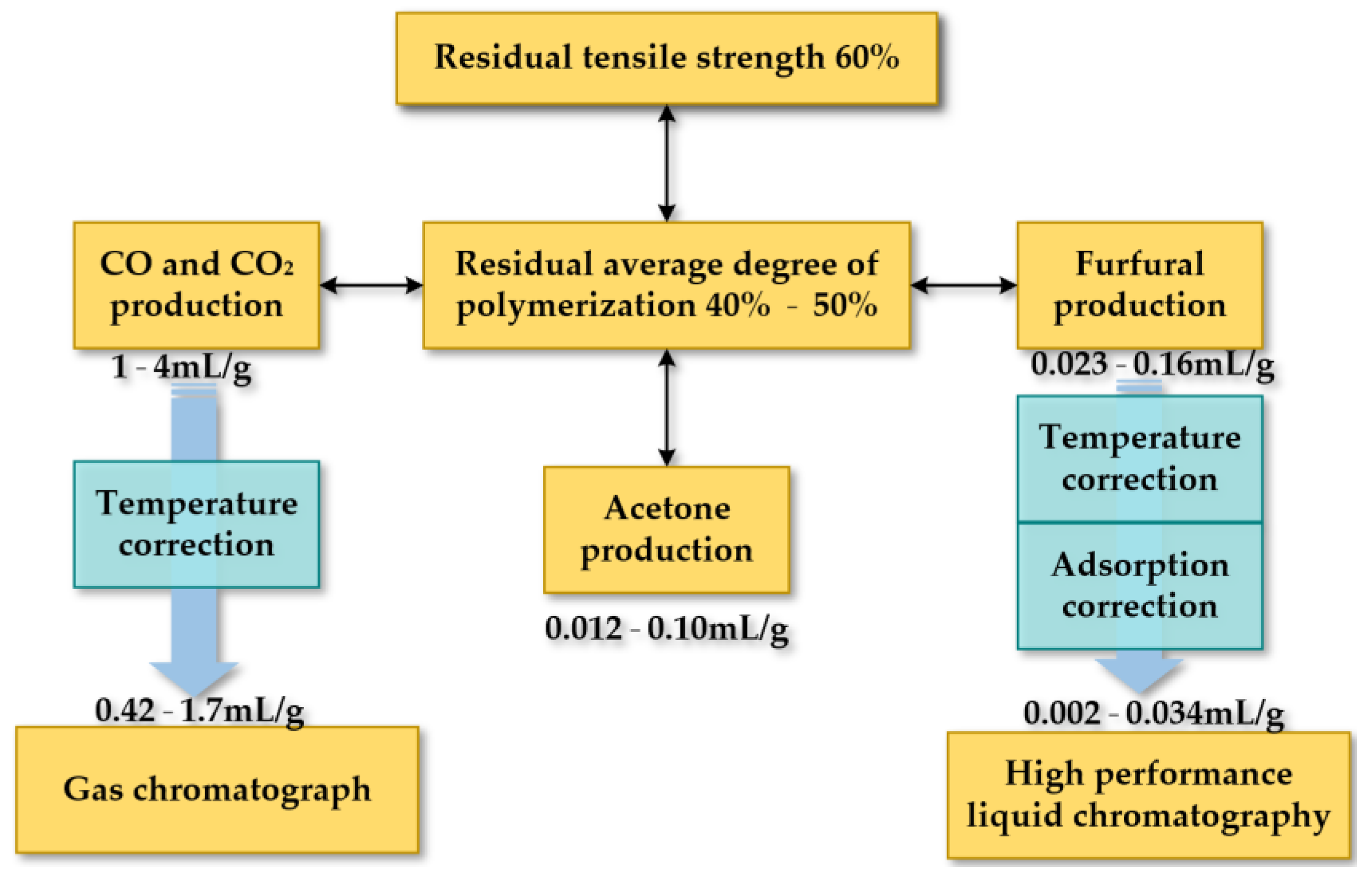
4. Monitoring the Aging Condition of Paper Insulation
4.1. Study on Production Mechanism of Indicators from the Microscopic Perspective
4.2. Monitoring Technology of Chemical Indicators
4.2.1. Monitoring of Furan Compounds
4.2.2. Monitoring of Carbon-Oxygen Gases
4.2.3. Monitoring of Low Molecular Alcohols
4.2.4. Monitoring of Acid Compounds
4.2.5. Monitoring of Ketone Compounds
4.2.6. Monitoring of Sugar Compounds
5. Perspectives
- (i)
- The dynamic equilibrium and distribution law of chemical indicators in oil-paper are also changed as a result of the complex operating conditions and diverse influencing factors. Therefore, the equilibrium/diffusion mechanism of chemical indicators is the key to aging evaluation and the main focus to determine the accuracy of field detection [36,59,99,105,115]. After a change to the oil, the oil filter, and the oil supplement, it is crucial to modify the chemical indicator’s concentration. In addition, it is well known that the concentration of the chemical indicator in oil that can be detected is related to its distribution ratio between oil and paper, as well as to the chemical indicator’s solubility in oil and the insulating paper’s adsorption capacity for the chemical indicator. So, it is essential to establish the kinetic equilibrium model of the chemical indicator to the oil-paper system in the following three aspects: (1) The adsorption and diffusion mechanism of the chemical indicators in the insulating paper; (2) the diffusion principle of chemical indicators between the oil-paper interface; (3) The solubility and diffusion ability of chemical indicators in insulating oil.
- (ii)
- With the help of the basic theory of interdisciplinary mature platforms (such as classical mechanics, relativity, quantum mechanics and electromagnetism, molecular chemistry, surface science, and so on), the mechanism of cellulose degradation is further explored and the relationship between various chemical indicators and transformer operating faults can be revealed [137,138]. In addition, the development of detection technology for chemical indicators should also be taken into account [154,165,167,168], in order to design the phase analysis platform which is simple to operate and easy to carry for the field transformer to measure chemical indicators in oil.
- (iii)
- Existing studies to analyze the aging degradation mechanism of insulating paper cellulosic mostly depend on molecular reaction dynamics simulation software [136,145,148]. However, current molecular reaction simulation studies are only carried out under the influence of a single environmental factor. In the future, we can further integrate multiple factors for analysis, such as superposition temperature, electric field, and environmental factors. Secondly, the molecular reaction dynamics model is analyzed from the micro level, and the interaction between molecules in the insulating paper cannot be comprehensively simulated. The research direction can be extended to the mesoscopic scale. The process of insulating paper to generate chemical indicators can be simulated from the micro, mesoscopic and macro multi-scale levels.
- (iv)
- A common trend for the present is the development of new, environmentally friendly insulating oil and modified insulating paper. The chemical indicator of aging degradation of the improved oil-paper insulation system is different from that of the traditional oil-paper insulation system. (1) It is found by comparing the fault gas of traditional mineral oil and synthetic ester that the propane content in the synthetic ester is significant and can be used as the key gas for thermal faults, whereas the role of propane in mineral oil as an indicator is minimal [55]. (2) Under the same aging conditions, the gas yield and acid content of vegetable oil are higher than that of mineral oil, but the furfural content of vegetable oil is lower than that of mineral oil [31]. (3) The chemical indicators of modified paper and traditional paper are also different. The concentration of furfural degraded by the modified insulating paper is much lower than that of the traditional insulating paper under the same aging state [98]. However, there is no difference in the content of low-molecular alcohols produced during the aging process regardless of the type of insulating paper. Therefore, it is necessary to study the chemical indicators generated during the deterioration process of the improved insulating oil and paper as well as the analysis of the formation path and the equilibrium diffusion mechanism of chemical indicators.
- (v)
- Considering the influence of multiple factors (temperature, water, aging degree, etc.) on cellulose degradation, the kinetic Equation, the Arrhenius Equation and the existing aging evaluation equation based on chemical indicators can be further modified [45,46,47]. In addition, the chemical indicator between oil and paper in turn accelerates the deterioration process of insulating paper and needs further research. At present, due to the differences in experimental models and environments adopted by many researchers, there are no universally acknowledged results in this field.
- (vi)
- The transformer winding hotspot temperature is generally 80–140 °C, which is much higher than the normal oil temperature. Therefore, the aging state of the paper insulation in the hotspot area is serious, which is the ‘weak area’ of the whole transformer paper insulation, and its operation risk is the greatest. However, the traditional electrical detection method has the disadvantage of power failure of the hanging hood. So, it is interesting to explore the relationship between chemical indicators in oil and the winding hotspot’s aging state. The existing research also found that the formation of the ethanol indicator is closely related to the winding hotspot area [99]. The authors also observe that the methanol concentration rises gradually along the axial height of the winding (from the top to the bottom) through non-uniform thermal aging experiments [173].
- (vii)
- There are many kinds of chemical indicators dissolved in oil, so it is possible to determine the weight coefficient of each chemical indicator to represent the aging degree of insulating paper through the weighting method. On the basis of the above, a model for the coupling of multiple chemical indicators to characterize the aging state of insulating paper can be established [8]. The establishment of a multiple chemical indicator model can greatly improve the transformer insulation status’s evaluation method. This is a crucial direction for our future development.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, L.; Kim, D.; Abu-Siada, A.; Kumar, S. Oil-Immersed Power Transformer Condition Monitoring Methodologies: A Review. Energies 2022, 15, 3379. [Google Scholar] [CrossRef]
- Bracale, A.; Carpinelli, G.; Pagano, M.; De Falco, P. A Probabilistic Approach for Forecasting the Allowable Current of Oil-Immersed Transformers. IEEE Trans. Power Del. 2018, 33, 1825–1834. [Google Scholar] [CrossRef]
- Christina, A.J.; Salam, M.A.; Rahman, Q.M.; Wen, F.; Ang, S.P.; Voon, W. Causes of transformer failures and diagnostic methods—A review. Renew. Sustain. Energy Rev. 2018, 82, 1442–1456. [Google Scholar]
- Foros, J.; Istad, M. Health Index, Risk and Remaining Lifetime Estimation of Power Transformers. IEEE Trans. Power Del. 2020, 35, 2612–2620. [Google Scholar] [CrossRef]
- N’cho, J.S.; Fofana, I.; Hadjadj, Y.; Beroual, A. Review of Physicochemical-Based Diagnostic Techniques for Assessing Insulation Condition in Aged Transformers. Energies 2016, 9, 367. [Google Scholar] [CrossRef]
- Area, M.C.; Ceradame, H. Paper aging and degradation: Recent findings and research methods. Bioresources 2011, 6, 5307–5337. [Google Scholar]
- Okabe, S.; Ueta, G.; Tsuboi, T. Investigation of aging degradation status of insulating elements in oil-immersed transformer and its diagnostic method based on field measurement data. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 346–355. [Google Scholar] [CrossRef]
- Zhang, E.; Zheng, H.; Zhang, C.; Wang, J.; Shi, K.; Guo, J.; Schwarz, H.; Zhang, C. Aging state assessment of transformer cellulosic paper insulation using multivariate chemical indicators. Cellulose 2021, 28, 2445–2460. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Wang, F.; Huang, Z.; Zhou, Q. A novel aging indicator of transformer paper insulation based on dispersion staining colors of cellulose fibers in oil. IEEE Electr. Insul. Mag. 2018, 34, 8–16. [Google Scholar] [CrossRef]
- Emsley, A.M.; Stevens, G.C. Review of chemical indicators of degradation of cellulosic electrical paper insulation in oil-filled transformers. IEE Proc. Sci. Meas. Techol. 1994, 141, 324–334. [Google Scholar] [CrossRef]
- Du, D.; Tang, C.; Zhang, J.; Hu, D. Effects of hydrogen sulfide on the mechanical and thermal properties of cellulose insulation paper: A molecular dynamics simulation. Mater. Chem. Phys. 2020, 240, 122153. [Google Scholar] [CrossRef]
- Wise, L.D. Wood Chemistry; Reinhold Publishing Co.: New York, NY, USA, 1946. [Google Scholar]
- Zhou, Y.; Chen, W.; Yang, D.; Zhang, R. Raman spectrum characteristics and aging diagnosis of oil-paper insulation with different oil-paper ratios. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1587–1594. [Google Scholar] [CrossRef]
- Emsley, A.M.; Heywood, R.J.; Ali, M.; Xiao, X. Degradation of cellulosic insulation in power transformers. Part 4: Effects of ageing on the tensile strength of paper. IEE Proc. Sci. Meas. Technol. 2000, 147, 285–290. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Le, T.T.; Darveniza, M.; Saha, T. A study of degradation of cellulosic insulation materials in a power transformer. Part 2: Tensile strength of cellulose insulation paper. Polym. Degrada. Stabil. 1995, 49, 429–435. [Google Scholar] [CrossRef]
- Ariannik, M.; Razi-Kazemi, A.A.; Lehtonen, M. An approach on lifetime estimation of distribution transformers based on degree of polymerization. Reliab. Eng. Syst. Saf. 2020, 198, 106881. [Google Scholar] [CrossRef]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D.; Painter, T.J. Aging of oil-impregnated paper in power transformers. IEEE Trans. Power Deliv. 2004, 19, 230–239. [Google Scholar] [CrossRef]
- Shroff, D.H.; Stannett, A.W. A review of paper aging in power transformers. Gener. Transm. Distrib. IEE Proc. C 1985, 132, 312–319. [Google Scholar] [CrossRef]
- Force, C.T. Ageing of Cellulose in Mineral-Oil Insulated Transformers; CIGRE: Paris, France, 2007. [Google Scholar]
- Sana, T.K. Review of modem diagnostic techniques for assessing insulation condition in aged transformers. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 903–917. [Google Scholar]
- Saha, T.K.; Purkait, P. Investigations of Temperature Effects on the Dielectric Response Measurements of Transformer Oil-Paper Insulation System. IEEE Trans. Power Deliv. 2008, 23, 252–260. [Google Scholar] [CrossRef]
- Emsley, A.M. The kinetics and mechanisms of degradation of cellulosic insulation in power transformers. Polym. Degrada. Stabil. 1994, 44, 343–349. [Google Scholar] [CrossRef]
- Emsley, A.M.; Xiao, X.; Heywood, R.J.; Ali, M. Degradation of cellulosic insulation in power transformers. Part 2: Formation of furan products in insulating oil. IEE Proc. Sci. Meas. Techol. 2000, 147, 110–114. [Google Scholar] [CrossRef]
- Behjat, V.; Emadifar, R.; Pourhossein, M.; Rao, U.M.; Fofana, I.; Najjar, R. Improved Monitoring and Diagnosis of Transformer Solid Insulation Using Pertinent Chemical Indicators. Energies 2021, 14, 3977. [Google Scholar] [CrossRef]
- Matharage, S.Y.; Liu, Q.; Wang, Z.D. Aging assessment of kraft paper insulation through methanol in oil measurement. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1589–1596. [Google Scholar] [CrossRef]
- Oommen, T.V.; Prevost, T.A. Cellulose insulation in oil-filled power transformers: Part II maintaining insulation integrity and life. IEEE Electr. Insul. Mag. 2006, 22, 5–14. [Google Scholar] [CrossRef]
- Burton, P.J.; Graham, J.; Hall, A.C.; Laver, J.A.; Oliver, A.J. Recent Developments by CEGB to Improve the Prediction and Monitoring of Transformer Performance; CIGRE: Paris, France, 1984; Volume 30, p. 1209. [Google Scholar]
- Oria, C.; Ortiz, A.; Ferreño, D.; Carrascal, I.; Fernández, I. State-of-the-art review on the performance of cellulosic dielectric materials in power transformers: Mechanical response and ageing. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 939–954. [Google Scholar] [CrossRef]
- Rao, U.M.; Fofana, I.; Jaya, T.; Rodriguez-Celis, E.M.; Jalbert, J.; Picher, P. Alternative Dielectric Fluids for Transformer Insulation System: Progress, Challenges, and Future Prospects. IEEE Access 2019, 7, 184552–184571. [Google Scholar]
- Ueta, G.; Tsuboi, T.; Okabe, S.; Amimoto, T. Study on degradation causing components of various characteristics of transformer insulating oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 2216–2224. [Google Scholar] [CrossRef]
- Feng, D.; Hao, J.; Liao, R.; Chen, X.; Cheng, L.; Liu, M. Comparative Study on the Thermal-Aging Characteristics of Cellulose Insulation Polymer Immersed in New Three-Element Mixed Oil and Mineral Oil. Polymers 2019, 11, 1292. [Google Scholar] [CrossRef]
- Łojewska, J.; Miśkowiec, P.; Łojewski, T.; Proniewicz, L.M. Cellulose oxidative and hydrolytic degradation: In situ FTIR approach. Polym. Degrada. Stabil. 2005, 88, 512–520. [Google Scholar] [CrossRef]
- CIGRE Working Group. Ageing of Liquid Impregnated Cellulose for Power Transformers; CIGRE: Paris, France, 2018; Volume 1, p. 53. [Google Scholar]
- Lelekakis, N.; Wenyu, G.; Martin, D.; Wijaya, J.; Susa, D. A field study of aging in paper-oil insulation systems. IEEE Electr. Insul. Mag. 2012, 28, 12–19. [Google Scholar] [CrossRef]
- Cheim, L.; Platts, D.; Prevost, T.; Xu, S. Furan analysis for liquid power transformers. IEEE Electr. Insul. Mag. 2012, 28, 8–21. [Google Scholar] [CrossRef]
- Chen, L.; Liao, Y.; Guo, Z.; Cao, Y.; Ma, X. Products distribution and generation pathway of cellulose pyrolysis. J. Clean. Prod. 2019, 232, 1309–1320. [Google Scholar] [CrossRef]
- Usino, D.O.; Ylitervo, P.; Pettersson, A.; Richards, T. Influence of temperature and time on initial pyrolysis of cellulose and xylan. J. Anal. Appl. Pyrol. 2020, 147, 104782. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cho, J.; Tompsett, G.A.; Westmoreland, P.R.; Huber, G.W. Kinetics and mechanism of cellulose pyrolysis. J. Phys. Chem. C 2009, 113, 20097–20107. [Google Scholar] [CrossRef]
- Margutti, S.; Conio, G.; Calvini, P.; Pedemonte, E. Hydrolytic and oxidative degradation of paper. Restaurator 2001, 22, 67–83. [Google Scholar] [CrossRef]
- Ese, M.H.G.; Liland, K.B.; Lundgaard, L.E. Oxidation of paper insulation in transformers. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 939–946. [Google Scholar] [CrossRef]
- Kuhn, W. On the kinetics of depolymerisation of high molecular chains. Ber. Deut. Chem. Ges 1930, 63, 503. [Google Scholar]
- Ekenstam, A. The behaviour of cellulose in mineral acid solutions: Kinetic study of the decomposition of cellulose in acid solutions. Ber. Deut. Chem. Ges 1936, 69, 553–559. [Google Scholar]
- Emsley, A.M.; Stevens, G.C. Kinetics and mechanisms of the low-temperature degradation of cellulose. Cellulose 1994, 1, 26–56. [Google Scholar] [CrossRef]
- Calvini, P.; Gorassini, A.; Merlani, A. On the kinetics of cellulose degradation: Looking beyond the pseudo zero order rate equation. Cellulose 2008, 15, 193–203. [Google Scholar] [CrossRef]
- Gilbert, R.; Jalbert, J.; Tétreault, P.; Morin, B.; Denos, Y. Kinetics of the production of chain-end groups and methanol from the depolymerization of cellulose during the ageing of paper/oil systems. Part 1: Standard wood kraft insulation. Cellulose 2009, 16, 327–338. [Google Scholar] [CrossRef]
- Emsley, A.M.; Heywood, R.J.; Ali, M.; Eley, C.M. On the kinetics of degradation of cellulose. Cellulose 1997, 4, 1–5. [Google Scholar] [CrossRef]
- Calvini, P. The influence of levelling-off degree of polymerisation on the kinetics of cellulose degradation. Cellulose 2005, 12, 445–447. [Google Scholar] [CrossRef]
- Calvini, P.; Gorassini, A. On the rate of paper degradation: Lessons from the past. Restaurator 2006, 27, 275–290. [Google Scholar] [CrossRef]
- Pablo, A. Furfural and ageing: How are they related. In Proceedings of the IEE Colloquium on Insulating Liquids, Leatherhead, UK, 27 May 1999; p. 5. [Google Scholar]
- Scheirs, J.; Camino, G.; Avidano, M.; Tumiatti, W. Origin of franic compounds in thermal degradation of cellulosic insulating paper. J. Appl. Polym. Sci. 1998, 69, 2541–2547. [Google Scholar] [CrossRef]
- Furanic Compounds for Diagnosis; CIGRE Brochure; CIGRE: Paris, France, 2012; p. 494.
- Stebbins, R.D.; Myers, D.S.; Shkolnik, A.B. Furanic compounds in dielectric liquid samples: Review and update of diagnostic interpretation and estimation of insulation ageing. In Proceedings of the 2003 IEEE International Conference Properties and Applications of Dielectric Materials, Nagoya, Japan, 1–5 June 2003; pp. 921–926. [Google Scholar]
- Urquiza, D.; Garcia, B.; Burgos, J.C. Statistical study on the reference values of furanic compounds in power transformers. IEEE Electr. Insul. Mag. 2015, 4, 15–23. [Google Scholar] [CrossRef]
- Sun, W.; Yang, L.; Zare, F.; Lin, Y.; Cheng, Z. Improved method for aging assessment of winding hot-spot insulation of transformer based on the 2-FAL concentration in oil. Int. J. Electr. Power Energy Syst. 2019, 112, 191–198. [Google Scholar] [CrossRef]
- Teymouri, A.; Vahidi, B. Power transformer cellulosic insulation destruction assessment using a calculated index composed of CO, CO2, 2-Furfural, and Acetylene. Cellulose 2020, 28, 489–502. [Google Scholar] [CrossRef]
- Zhang, C.H.; Macalpine, J.M.K. Furfural concentration in transformer oil as an indicator of paper ageing, part 1: A review. In Proceedings of the 2006 IEEE Power Systems Conference Exposition, Atlanta, GA, USA, 29 October–1 November 2006; pp. 1088–1091. [Google Scholar]
- Liao, R.; Lin, Y.; Guo, P.; Liu, H. The effects of insulating oil replacement upon power transformer condition assessment. Electr. Power Compon. Syst. 2015, 43, 1971–1979. [Google Scholar] [CrossRef]
- Sans, J.R.; Bilgin, K.M.; Kelly, J.J. Large-scale survey of furanic compounds in operating transformers and implications for estimating service life. In Proceedings of the 1998 IEEE International Conference Symposium on Electrical Insulation, Arlington, VA, USA, 7–10 June 1998; pp. 543–553. [Google Scholar]
- Lin, Y.; Yang, L.; Liao, R.; Sun, W.; Zhang, Y. Effect of oil replacement on furfural analysis and aging assessment of power transformers. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2611–2619. [Google Scholar] [CrossRef]
- Unsworth, J.; Mitchell, F. Degradation of electrical insulating paper monitored with high performance liquid chromatography. IEEE Trans. Dielectr. Electr. Insul. 1990, 25, 737–746. [Google Scholar] [CrossRef]
- Vasovic, V.; Lukic, J.; Mihajlovic, D.; Pejovic, B.; Radakovic, Z.; Radoman, U.; Orlovic, A. Aging of transformer insulation—Experimental transformers and laboratory models with different moisture contents: Part I—DP and furans aging profiles. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 1840–1846. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Chen, Z.; Jiang, D.; Yu, H.; Wang, X. Highly selective conversion of glucose into furfural over modified zeolites. Chem. Eng. J. 2017, 307, 868–876. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Lai, Y.Z. Thermal degradation of 1,6-anhydro-beta-Dglucopyranose. J. Org. Chem. 1972, 37, 278–284. [Google Scholar] [CrossRef]
- Šivec, R.; Grilc, M.; Huš, M.; Likozar, B. Multiscale modeling of (Hemi) cellulose hydrolysis and cascade hydrotreatment of 5-hydroxymethylfurfural, furfural, and levulinic acid. Ind. Eng. Chem. Res. 2019, 58, 16018–16032. [Google Scholar] [CrossRef]
- Allan, D.M.; Jones, C.F. Thermal-oxidative stability and oil-paper partition coeffcients of selected model furan compounds at practical temperature. In Proceedings of the International Symposium on High Voltage Engineering, Graz, Austria, 28 August–1 September 1995; p. 1004. [Google Scholar]
- Pahlavanpour, B.; Heywood, R.; Wilson, G. Power transformer ageing. In Proceedings of the CIG&, SC15 Meeting, Sydney, Australia, 1998. [Google Scholar]
- Feng, D.; Wang, Z.; Jarman, P. Transmission Power Transformer Assessment Using Furan Measurement with the aid of Thermal Model. In Proceedings of the 2012 IEEE International Conference Condition Monitoring and Diagnosis, Bali, Indonesia, 23–27 September 2012; pp. 521–524. [Google Scholar]
- Łojewski, T.; Sawoszczuk, T.; Łagan, J.M.; Zięba, K.; Barański, A.; Łojewska, J. Furfural as a marker of cellulose degradation. A quantitative approach. Appl. Phys. A 2010, 100, 873–884. [Google Scholar] [CrossRef]
- Liao, R.; Liang, S.; Yang, L.; Hao, J.; Li, J. Comparison of Ageing Results for Transformer Oil-paper Insulation Subjected to Thermal Ageing in Mineral Oil and Ageing in Retardant Oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 225–232. [Google Scholar] [CrossRef]
- Lin, Y.; Liao, R.; Tao, F.; Wei, C. Effects of Moisture on Furfural Partitioning in Oil-Paper Insulation System and Aging Assessment of Power Transformers. Electr. Power Compon. Syst. 2019, 47, 192–199. [Google Scholar] [CrossRef]
- Kan, H.; Miyamoto, T.; Makino, Y.; Namba, S.; Hara, T. Absorption of CO2 and CO gases and furfural in insulating oil into paper insulation in oil-immersed transformers. In Proceedings of the 1994 IEEE International Conference Symposium on Electrical Insulation, Pittsburgh, PA, USA, 5–8 June 1994; pp. 41–44. [Google Scholar]
- Jalbert, J.; Lessard, M. Cellulose chemical markers in transformer oil insulation, part 1: Temperature correction factors. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 2287–2291. [Google Scholar] [CrossRef]
- Pahlavanpour, P.; Martins, M. Insulating Paper Ageing and Furfural Formation. In Proceedings of the IEEE Electrical Insulation Conference Electrical Manufacturing & Coil Winding Technology Conference, Indianapolis, IN, USA, 23–25 September 2003; pp. 283–288. [Google Scholar]
- Feng, D.; Yang, L.; Zhou, L.; Liao, R. Influential factors and correction method of furfural content in transformer oil. IEEE Access 2019, 7, 53487–53495. [Google Scholar] [CrossRef]
- Hao, J.; Feng, D.; Liao, R.; Yang, L.; Lin, Y. Effect of temperature on the production and diffusion behaviour of furfural in oil–paper insulation systems. IET Gener. Transm. Dis. 2018, 12, 3124–3129. [Google Scholar] [CrossRef]
- Griffin, P.J.; Lewand, L.R.; Pahlavanpour, B. The analysis of paper degradation by-products as a tool for monitoring fault conditions in oil-filled electric apparatus. In Proceedings of the 1995 International Conference the Reliability of Transmission and Distribution Equipment, Coventry, UK, 29–31 March 1995; pp. 79–84. [Google Scholar]
- Liu, J.; Zhang, H.; Geng, C.; Fan, X.; Zhang, Y. Aging Assessment Model of Transformer Insulation Based on Furfural Indicator under Different Oil/Pressboard Ratios and Oil Change. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1061–1069. [Google Scholar] [CrossRef]
- Chen, C.; Li, S.; He, T.; Liu, Y. Concentration of 5-hydroxymethylfurfural produced by insulating paper in an oil–paper insulation system. IEEJ Trans. Electr. Electr. Eng. 2020, 15, 828–832. [Google Scholar] [CrossRef]
- Batista, D.A.; Patriarca, P.A.; Trindade, E.M.; Wilhelm, H.M. Colorimetric methodology for monitoring the cellulose insulating paper degradation in electrical equipments filled with mineral oil. Cellulose 2008, 15, 497–505. [Google Scholar] [CrossRef]
- Hill, D.J.T.; Le, T.T.; Darveniza, M.; Saha, T. A study of the degradation of cellulosic insulation materials in a power transformer. Part III: Degradation products of cellulose insulation paper. Polym. Degrada. Stabil. 1996, 51, 211–218. [Google Scholar] [CrossRef]
- Zhuang, X.; Yuan, Z.; Wu, C. Analysis of ultra-low acid hydrolysate of cellulose. Trans. Chin. Soc. Agric. Eng. 2007, 23, 177–182. (In Chinese) [Google Scholar]
- Burton, P.J. Applications of liquid chromatography to the analysis of electrical insulating materials. In Proceedings of the CIGRE Conference, Paris, France, 1988; pp. 15–28. [Google Scholar]
- Tamura, R.; Anetai, H.; Ishii, T.; Kawamura, T. The diagnostic of aging deterioration of insulating paper. JIEE Proc. A 1981, 101, 30–36. [Google Scholar]
- Teymouri, A.; Vahidi, B. CO2/CO concentration ratio: A complementary method for determining the degree of polymerization of power transformer paper insulation. IEEE Electr. Insul. Mag. 2017, 33, 24–30. [Google Scholar] [CrossRef]
- Abu-Siada, A.; Islam, S. A new approach to identify power transformer criticality and asset management decision based on dissolved gas-in-oil analysis. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1007–1012. [Google Scholar] [CrossRef]
- Sun, C.; Ohodnicki, P.R.; Stewart, E.M. Chemical sensing strategies for real-time monitoring of transformer oil: A review. IEEE Sens. J. 2017, 17, 5786–5806. [Google Scholar] [CrossRef]
- Bakar, N.A.; Abu-Siada, A.; Islam, S. A review of dissolved gas analysis measurement and interpretation techniques. IEEE Electr. Insul. Mag. 2014, 30, 39–49. [Google Scholar] [CrossRef]
- Hohlein-Atanasova, I.; Frotscher, R. Carbon oxides in the interpretation of dissolved gas analysis in transformers and tap changers. IEEE Electr. Insul. Mag. 2010, 26, 22–26. [Google Scholar] [CrossRef]
- The Institute of Electrical and Electronics Engineers Standard. IEEE Guide for the Interpretation of Gases Generated in Oil-Immersed Transformers—Section 1.2 Limitations; IEEE Power & Energy Society: New York, NY, USA, 1991; p. C57.104. [Google Scholar]
- Gilbert, R.; Jalbert, J.; Duchesne, S.; Tétreault, P.; Morin, B.; Denos, Y. Kinetics of the production of chain-end groups and methanol from the depolymerization of cellulose during the ageing of paper/oil systems. Part 2: Thermally-upgraded insulating papers. Cellulose 2010, 17, 253–269. [Google Scholar] [CrossRef]
- Faiz, J.; Soleimani, M. Dissolved gas analysis evaluation in electric power transformers using conventional methods a review. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1239–1248. [Google Scholar] [CrossRef]
- Jalbert, J.; Gilbert, R.; Denos, Y.; Gervais, P. Methanol: A Novel Approach to Power Transformer Asset Management. IEEE Trans. Power Deliv. 2012, 27, 514–520. [Google Scholar] [CrossRef]
- Schaut, A.; Autru, S.; Eeckhoudt, S. Applicability of methanol as new marker for paper degradation in power transformers. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 533–540. [Google Scholar] [CrossRef]
- Jalbert, J.; Gilbert, R.; Tétreault, P.; Morin, B.; Lessard-Déziel, L. Identification of a chemical indicator of the rupture of 1,4-β-glycosidic bonds of cellulose in an oil-impregnated insulating paper system. Cellulose 2007, 14, 295–309. [Google Scholar] [CrossRef]
- Jalbert, J.; Rodriguez-Celis, E.; Duchesne, S.; Morin, B.; Mohamed, R.; Gilbert, R. Kinetics of the production of chain-end groups and methanol from the depolymerization of cellulose during the ageing of paper/oil systems. Part 3: Extension of the study under temperature conditions over 120 degrees C. Cellulose 2015, 22, 829–848. [Google Scholar] [CrossRef]
- Arroyo, O.H.; Fofana, I.; Jalbert, J.; Ryadi, M. Relationships between methanol marker and mechanical performance of electrical insulation papers for power transformers under accelerated thermal aging. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3625–3632. [Google Scholar] [CrossRef]
- Arroyo, O.H.; Jalbert, J.; Fofana, I.; Ryadi, M. Temperature dependence of methanol and the tensile strength of insulation paper: Kinetics of the changes of mechanical properties during ageing. Cellulose 2017, 24, 1031–1039. [Google Scholar] [CrossRef]
- Yamagata, N.; Katsunori, M.; Etsuo, O. Diagnosis of thermal degradation for thermally upgraded paper in mineral oil. Condition monitoring and diagnosis. In Proceedings of the 2008 IEEE International Conference Condition Monitoring and Diagnosis, Beijing, China, 21–24 April 2008; pp. 1000–1004. [Google Scholar]
- Rodriguez-Celis, E.M.; Duchesne, S.; Jalbert, J.; Ryadi, M. Understanding ethanol versus methanol formation from insulating paper in power transformers. Cellulose 2015, 22, 3225–3236. [Google Scholar] [CrossRef]
- Jalbert, J.; Rodriguez-Celis, E.M.; Arroyo-Fernández, O.H.; Duchesne, S.; Morin, B. Methanol Marker for the Detection of Insulating Paper Degradation in Transformer Insulating Oil. Energies 2019, 12, 3969. [Google Scholar] [CrossRef]
- Zhang, E.; Zheng, H.; Zhang, Y.; Liu, J.; Shi, Z.; Shi, K.; Zhang, C.; Shao, G.; Zhang, C.; Schwarz, H. Lifespan Model of the Relationships between Ethanol Indicator and Degree of Polymerization of Transformer Paper Insulation. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1859–1866. [Google Scholar] [CrossRef]
- Jalbert, J.; Duchesne, S.; Rodriguez-Celis, E.; Tétreault, P.; Collin, P. Robust and sensitive analysis of methanol and ethanol from cellulose degradation in mineral oils. J. Chromatogr. A 2012, 1256, 240–245. [Google Scholar] [CrossRef]
- Bruzzoniti, M.C.; Maina, R.; De-Carlo, R.M.; Sarzanini, C.; Tumiatti, V. GC methods for the determination of methanol and ethanol in insulating mineral oils as markers of cellulose degradation in power transformers. Chromatographia 2014, 77, 1081–1089. [Google Scholar] [CrossRef]
- Rodriguez-Celis, E.; Jalbert, J.; Duchesne, S.; Noirhomme, B.; Lessard, M.C.; Ryadi, M. Chemical markers use for the diagnosis and life estimation of power transformers: A preliminary study of their origins. In Proceedings of the CIGRE Canada Conference, Montreal, QC, Canada, 1–5 October 2012. [Google Scholar]
- Zhang, E.; Liu, J.; Fan, X.; Zhang, Y.; Zhang, C. Reduction Mechanism of Alcohols Contents Caused by Acids During Oil-Paper Insulation Aging. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1867–1874. [Google Scholar] [CrossRef]
- PEDIAA. Difference between Ethanol and Methanol. 2016. Available online: http://pediaa.com/difference-between-ethanol-and-methanol/ (accessed on 4 January 2016).
- Przybylek, P. A New Concept of Applying Methanol to Dry Cellulose Insulation at the Stage of Manufacturing a Transformer. Energies 2018, 11, 1658. [Google Scholar] [CrossRef]
- Mander, L.; Liu, H.W. Comprehensive Natural Products II: Chemistry and Biology; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Lundgaard, L.E.; Hansenm, W.; Ingebrigtsen, S. Aging of mineral oil impregnated cellulose by acid catalysis. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 540–546. [Google Scholar] [CrossRef]
- Xiang, Q.; Lee, Y.; Pettersson, P.O.; Torget, R.W. Heterogeneous aspects of acid hydrolysis of α-cellulose. In Biotechnology for Fuels and Chemicals; Springer: Berlin/Heidelberg, Germany, 2003; pp. 505–514. [Google Scholar]
- Zheng, H.; Yang, E.; Li, X.; Liu, C.; Wang, Z.; Yana, T.; Yao, W. Microscopic Reaction Mechanisms of Formic Acid Generated During Pyrolysis of Cellulosic Insulating Paper. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1661–1668. [Google Scholar] [CrossRef]
- Lelekakis, N.; Wijaya, J.; Martin, D.; Susa, D. The effect of acid accumulation in power-transformer oil on the aging rate of paper insulation. IEEE Electr. Insul. Mag. 2014, 30, 19–26. [Google Scholar] [CrossRef]
- Ivanov, K.I.; Panfilova, E.S.; Kullkovskaya, T.N.; Zhakhovskaya, V.P.; Savinova, V.K.; Seminova, M.G. Influence of the Products of Oxidation of Mineral Oils on Ageing of Paper Insulation in Transformers. In Proceedings of the Zh. Prikl. Khim, Leningrad, Russia, 1974; pp. 2705–2711. [Google Scholar]
- Lundgaard, L.E.; Hansen, W.; Ingebrigtsen, S.; Linhjell, D.; Dahlund, M. Aging of Kraft paper by acid catalyzed hydrolysis. In Proceedings of the IEEE International Conference Dielectric Liquids, Coimbra, Portugal, 26 June–1 July 2005; pp. 381–384. [Google Scholar]
- Kouassi, K.; Fofana, I.; Cissé, L.; Hadjadj, Y.; Yapi, K.M.L.; Diby, K.A. Impact of Low Molecular Weight Acids on Oil Impregnated Paper Insulation Degradation. Energies 2018, 11, 1465. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Wang, J.; Tian, W.; Hu, D. Analysis and mechanism of adsorption of naphthenic mineral oil, water, formic acid, carbon dioxide, and methane on meta-aramid insulation paper. J. Mate. Sci. 2019, 54, 8556–8570. [Google Scholar] [CrossRef]
- Yang, E.; Zheng, H.; Yang, T.; Yao, W.; Wang, Z.; Li, X.; Liu, C.; Feng, Y. Investigation on Formation and Solubility of Formic Acid, Acetic Acid and Levulinic Acid in Insulating Oil Using COSMO-RS. J. Mol. Liq. 2021, 346, 118256. [Google Scholar] [CrossRef]
- Awata, M.; Mizuno, K.; Ueda, T.; Ohta, N.; Ishii, T.; Tsukioka, H. Diagnosis by Acetone for Deterioration of Breathing Transformers Containing an Adsorbent in the Insulating Oil. IEEJ Trans. Power Energy 1997, 117, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Kaneko, S.; Kohtoh, M.; Amimoto, T. Analysis results for insulating oil components in field transformers. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 302–311. [Google Scholar] [CrossRef]
- Kabyemela, B.M.; Adschiri, T.; Malaluan, R.M.; Arai, K. Kinetics of glucose epimerization and decomposition in subcritical and supercritical water. Ind. Eng. Chem. Res. 1997, 36, 1552–1558. [Google Scholar] [CrossRef]
- Goto, K. Reaction mechanism of sugar derivatives in supercritical water. Kobunshi Ronbunshu 2001, 58, 685–691. [Google Scholar] [CrossRef]
- Jumppanen, J.; Nurmi, J.; Pastinen, O. High Purity Production of L-Ribose from L-Arabinose. U.S. Patent EP1131329B1, 2006. [Google Scholar]
- Scheirs, J.; Camino, G.; Tumiatti, W.; Avidano, M. Study of the mechanism of thermal degradation of cellulosic paper insulation in electrical transformer oil. Angew. Makromol. Chem. 1998, 259, 19–24. [Google Scholar] [CrossRef]
- Lessard, M.C.; Van, N.L.; Masse, M.; Penneau, J.F.; Grob, R. Thermal aging study of insulating papers used in power transformers. In Proceedings of the 1996 IEEE Conference Electrical Insulation and Dielectric Phenomena, Millbrae, CA, USA, 23–23 October 1996; pp. 854–859. [Google Scholar]
- Lessard, M.G.; Masst, M. Prediction of remaining life of the paper insulation by the analysis of new oil-soluble compounds in power transformers. In Proceedings of the 2003 IEEE Conference Electrical Insulation and Dielectric Phenomena, Albuquerque, NM, USA, 19–22 October 2003; pp. 129–132. [Google Scholar]
- Lessard, M.C.; Van, N.L.; Penneau, J.F.; Grob, R.; Masse, M. Physicochemical characterization of the thermal aging of insulating paper in power transformers. In Proceedings of the 1996 IEEE International Conference Symposium on Electrical Insulation, Montreal, QC, Canada, 16–19 June 1996; pp. 533–537. [Google Scholar]
- Peng, D.; Yang, D.; Wang, C.; Li, M. The Influence of Transformer Oil Aging to Dielectric Dissipation Factor and Its Insulating Lifetime. In Proceedings of the Asia-Pacific Power and Energy Engineering Conference, Wuhan, China, 27–31 March 2009; pp. 1–4. [Google Scholar]
- Emsley, A.M.; Xiao, X.; Heywood, R.J.; Ali, M. Degradation of cellulosic insulation in power transformers. Part 3: Effects of oxygen and water on ageing in oil. IEE Proc. Sci. Meas. Technol. 2000, 147, 115–119. [Google Scholar] [CrossRef]
- Hohlein, I.; Kachler, A.J. Aging of cellulose at transformer service temperatures. Part 2. Influence of moisture and temperature on degree of polymerization and formation of furanic compounds in free-breathing systems. IEEE Electr. Insul. Mag. 2005, 21, 20–24. [Google Scholar] [CrossRef]
- Fofana, I.; Borsi, H.; Gockenbach, E. Results on aging of cellulose paper under selective conditions. In Proceedings of the Annual Report Conference on IEEE Electrical Insulation and Dielectric Phenomena, Kitchener, ON, Canada, 14–17 October 2001; pp. 205–208. [Google Scholar]
- Liu, J.; Fan, X.; Zhang, C.; Lai, C.; Zhang, Y.; Zheng, H.; Lai, L.; Zhang, E. Moisture Diagnosis of Transformer Oil-Immersed Insulation with Intelligent Technique and Frequency-Domain Spectroscopy. IEEE Trans. Ind. Inform. 2021, 17, 4624–4634. [Google Scholar] [CrossRef]
- Jiang, J.; Du, B.; Cavallini, A. Effect of moisture migration on surface discharge on oil-pressboard of power transformers under cooling. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 1743–1751. [Google Scholar] [CrossRef]
- Nishikawa, S.; Ono, S. Transmission of X-rays through fibrous, lamellar and granular substances. Tokyo Sugaku-Buturigakkwai Kizi Dai Ki 1913, 7, 131–138. [Google Scholar]
- Meyer, K.H.; Mark, H. Über den Bau des krystallisierten Anteils der Cellulose. Ber. Der Dtsch. Chem. Ges. 1928, 61, 593–614. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, S.; Xie, J.; Lv, C. Molecular simulation and experimental analysis of Al2O3-nanoparticle-modified insulation paper cellulose. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1018–1026. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, S.; Wang, Q.; Wang, X.; Hao, J. Thermal stability of modified insulation paper cellulose based on molecular dynamics simulation. Energies 2017, 10, 397. [Google Scholar] [CrossRef]
- Van-Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A reactive force field for hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Rasmussen, H.; Sørensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef]
- Qian, X.; Nimlos, M.R.; Davis, M.; Johnson, D.K.; Himmel, M.E. Ab initio molecular dynamics simulations of β-D-glucose and β-D-xylose degradation mechanisms in acidic aqueous solution. Carbohydr. Res. 2005, 340, 2319–2327. [Google Scholar] [CrossRef]
- Nimlos, M.R.; Qian, X.; Davis, M.; Himmel, M.E.; Johnson, D.K. Energetics of xylose decomposition as determined using quantum mechanics modelling. J. Phys. Chem. A 2006, 110, 11824–11838. [Google Scholar] [CrossRef]
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. Mechanism of Brønsted acid-catalyzed conversion of carbohydrates. J. Catal. 2012, 295, 122. [Google Scholar] [CrossRef]
- Qian, X. Mechanisms and energetics for Brønsted acid-catalyzed glucose condensation, dehydration and isomerization reactions. Top. Catal. 2012, 55, 218–226. [Google Scholar] [CrossRef]
- Tian, M. Molecular Simulation Study on the Influence of Moisture and Acid on the Microscopic Properties of Oil-Impregnated Insulation Paper. Master’s Thesis, Chongqing University, Chongqing, China, 2014. (In Chinese). [Google Scholar]
- Davydov, V.; Roizman, O. Transformer Operating Risk Assessment: Development of Models. In Proceedings of the Electric Power Research Institute EPRI, Palo Alto, CA, USA, 2005; pp. 13–27. [Google Scholar]
- Liao, R.; Zhu, M.; Zhou, X.; Yang, L.; Yan, J.; Sun, C. Molecular dynamics simulation of the diffusion behavior of water molecules in oil and cellulose composite media. Acta Phys. Chim. Sin. 2011, 27, 815–824. [Google Scholar]
- Tanaka, F.; Fukui, N. The behavior of cellulose molecules in aqueous environments. Cellulose 2004, 11, 33–38. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Zhu, M.; Zhang, H. Research on pyrolysis mechanism of transformer oil-paper insulation based on reaction molecular dynamics simulation. Insul. Mater. 2019, 52, 79–85. (In Chinese) [Google Scholar]
- Zhang, Y.; Li, Y.; Zheng, H.; Zhu, M.; Liu, J.; Yang, T.; Zhang, C.; Li, Y. Microscopic reaction mechanism of the production of methanol during the thermal aging of cellulosic insulating paper. Cellulose 2020, 27, 2455–2467. [Google Scholar] [CrossRef]
- Vahidi, B.; Teymouri, A. Quality Confirmation Tests for Power Transformer Insulation Systems; Springer International Publishing: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Abu-Siada, A. Correlation of furan concentration and spectral response of transformer oil-using expert systems. IET Sci. Meas. Technol. 2011, 5, 183–188. [Google Scholar] [CrossRef]
- Wang, R.; Huang, X.; Wang, L. Facile electrochemical method and corresponding automated instrument for the detection of furfural in insulation oil. Talanta 2016, 148, 412–418. [Google Scholar] [CrossRef]
- Abu-Siada, A.; Lai, S.P.; Islam, S. Remnant life estimation of power transformer using oil UV-Vis spectral response. In Proceedings of the IEEE/PES Power Systems Conference Exposition, Seattle, WA, USA, 15–18 March 2009; pp. 1–5. [Google Scholar]
- Peng, L.; Fu, Q.; Lin, M.; Zhao, Y.; Qian, Y.; Li, S. A novel furfural-detection-method for the aging prediction of paper insulation in power transformer. In Proceedings of the 2018 IEEE Conference Electrical Insulation and Dielectric Phenomena, Cancun, Mexico, 21–24 October 2018; pp. 630–633. [Google Scholar]
- Chen, W.; Gu, Z.; Zou, J.; Wan, F.; Xiang, Y. Analysis of furfural dissolved in transformer oil based on confocal laser Raman spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 915–921. [Google Scholar] [CrossRef]
- Chun, C.; Xiao-Jian, M.A.; Pei-Lin, C. Spectrophotometric Determination of 5-Hydroxyfurfural and Furfural in the Hydrolyzed Liquor of Celulose. Phys. Test. Chem. Anal. 2008, 44, 223–225. [Google Scholar]
- Zhang, Y.; Song, Y.; Hu, X.; Liao, X.; Ni, Y.; Li, Q. Determination of 5-Hydroxymethylfurfural and Furfuralin Soft Beverages by HPLC. Adv. Mater. Res. 2012, 550–553, 1959–1966. [Google Scholar] [CrossRef]
- Q/CSG 114002-2011; China Southern Power Grid Corporation Preventive Test Procedures for Power Equipment. China Southern Power Grid Co., Ltd.: Guangzhou, China, 2011. (In Chinese)
- Xue, C. Monitoring Paper Insulation Aging by Measuring Furfural Contents in Oil. In Proceedings of the Technical Report of State Electric Power Research Institute EPRI, August 1990. [Google Scholar]
- Jalbert, J.; Lessard, M.C. Cellulose chemical markers relationship with insulating paper post-mortem investigations. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 3550–3554. [Google Scholar] [CrossRef]
- Singh, S.; Bandyopadhyay, M.N. Dissolved gas analysis technique for incipient fault diagnosis in power transformers: A bibliographic survey. IEEE Electr. Insul. Mag. 2010, 26, 41–46. [Google Scholar] [CrossRef]
- IE Commission. Mineral Oil-Filled Electrical Equipment in Service—Guidance on the Interpretation of Dissolved and Free Gases Analysis; IE Commission: Dublin, Ireland, 2015; IEC 60599-2015. [Google Scholar]
- Mcshane, C.P.; Rapp, K.J.; Corkran, J.L.; Gauger, J.A.; Luksich, J. Aging of paper insulation in natural ester dielectric fluid. In Proceedings of the IEEE/PES Transmission and Distribution Conference Exposition, Atlanta, GA, USA, 2 November 2001; pp. 675–679. [Google Scholar]
- Dettmer-Wilde, K.; Engewald, W. Practical Gas Chromatography; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Schaut, A.; Eeckhoudt, S. Identification of Early-Stage Paper Degradation by Methanol; CIGRE: Paris, France, 2012; p. A2-107. [Google Scholar]
- Zheng, H.; Zhang, C.; Zhang, Y.; Liu, J.; Zhang, E.; Shi, Z.; Shao, G.; Shi, K.; Guo, J.; Zhang, C. Optimization of ethanol detection by automatic headspace method for cellulose insulation aging of oil-immersed transformers. Polymers 2020, 12, 1567. [Google Scholar] [CrossRef] [PubMed]
- Molavi, H.; Yousefpour, A.; Mirmostafa, A.; Sabzi, A.; Hamedi, S.; Narimani, M.; Abdi, N. Static headspace GC/MS method for determination of methanol and ethanol contents, as the degradation markers of solid insulation systems of power transformers. Chromatographia 2017, 80, 1129–1135. [Google Scholar] [CrossRef]
- Matharage, S.Y.; Liu, Q.; Davenport, E. Methanol Detection in Transformer Oils using Gas Chromatography and Ion Trap Mass Spectrometer. In Proceedings of the 2014 IEEE International Conference Dielectric Liquids, Bled, Slovenia, 29 June–3 July 2014; pp. 1–4. [Google Scholar]
- Fu, Q.; Peng, L.; Li, L.; Lin, M.; Zhao, Y.; Li, S.; Chen, C. Detection of Methanol in Power Transformer Oil Using Spectroscopy. J. Electr. Eng. Technol. 2019, 14, 861–867. [Google Scholar] [CrossRef]
- Ingebrigtsen, S.; Dahlund, M.; Hansen, W.; Linhjell, D.; Lundgaard, L.E. Solubility of carboxylic acids in paper (Kraft)-oil insulationsystems. In Proceedings of the 2004 IEEE Conference Electrical Insulation and Dielectric Phenomena, Boulder, CO, USA, 20 October 2004; pp. 253–257. [Google Scholar]
- Wang, H.; Liu, X.; Wang, Z.; Xue, K.; Chen, G. Study on the analysis method of acetone volume fraction in power transformer oil. High Volt. Apparatus. 2008, 5, 395–398. (In Chinese) [Google Scholar]
- Gu, Z.; Chen, W.; Du, L.; Shi, H.; Wan, F. Application of Raman Spectroscopy for the Detection of Acetone Dissolved in Transformer Oil. J. Appl. Spectrosco. 2018, 85, 225–231. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, J.; Chai, X.; Kong, H. A new method for determining the content of mixed sugar by ultraviolet spectroscopy. Acta Chim. Sin. 2008, 5, 1233–1237. (In Chinese) [Google Scholar]
- Zhang, E.; Liu, J.; Song, B.; Zhang, H.; Fan, X.; Zhan, Y.; Fu, Q. Influence of Operational Defects and Hotspot Temperature on Methanol Concentration in Transformer Oil. IEEE Trans. Power Deliv. 2023. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, F.; Wang, Z.; Li, J. A critical review of plant-based insulating fluids for transformer: 30-year development. Renew. Sust. Energ. Rev. 2021, 141, 110783. [Google Scholar] [CrossRef]



| Characteristic Faults | |||
|---|---|---|---|
| Thermal | Electrical | ||
| Oil | Paper | Partial Discharge | Arcing |
| 150–300 °C H2, CH4, C2H4, C2H6 | CO, CO2 | H2, CH4 | H2, C2H2 |
| 300–700 °C H2, CH4, C2H4, C2H6 | |||
| >700 °C H2, C2H2, C2H4 | |||
| Sugars | Retention Time (min) | DPv Appearance | Maximum Concentration Level Attained (ppb) |
|---|---|---|---|
| Levoglucosan | 2.2 | 1300 | 1000 |
| Cellobiose | 12.4 | 1300 | 10,000 |
| Mannose | 5.4 | 1300 | 13,500 |
| Rhamnose | 4.2 | 728 | 3000 |
| Research Scholars | Method | EtOH/MeOH MDL (ng g−1) | EtOH/MeOH ML (ng g−1) |
|---|---|---|---|
| J. Jalbert [102] | GC-MS | 3.6/4 | 13/14 |
| M. Bruzzoniti [103] | GC-MS | 3.1/1.3 | 9.3/3.9 |
| M. Bruzzoniti [103] | GC-FID | 26.8/12.1 | 79.6/36 |
| H. Molavi [166] | GC-MS | 155/144 | 495/458 |
| Z. Wang [167] | GC-MS | 21.1/19.5 | 67.1/62.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, E.; Liu, J.; Zhang, C.; Zheng, P.; Nakanishi, Y.; Wu, T. State-of-Art Review on Chemical Indicators for Monitoring the Aging Status of Oil-Immersed Transformer Paper Insulation. Energies 2023, 16, 1396. https://doi.org/10.3390/en16031396
Zhang E, Liu J, Zhang C, Zheng P, Nakanishi Y, Wu T. State-of-Art Review on Chemical Indicators for Monitoring the Aging Status of Oil-Immersed Transformer Paper Insulation. Energies. 2023; 16(3):1396. https://doi.org/10.3390/en16031396
Chicago/Turabian StyleZhang, Enze, Jiang Liu, Chaohai Zhang, Peijun Zheng, Yosuke Nakanishi, and Thomas Wu. 2023. "State-of-Art Review on Chemical Indicators for Monitoring the Aging Status of Oil-Immersed Transformer Paper Insulation" Energies 16, no. 3: 1396. https://doi.org/10.3390/en16031396
APA StyleZhang, E., Liu, J., Zhang, C., Zheng, P., Nakanishi, Y., & Wu, T. (2023). State-of-Art Review on Chemical Indicators for Monitoring the Aging Status of Oil-Immersed Transformer Paper Insulation. Energies, 16(3), 1396. https://doi.org/10.3390/en16031396






