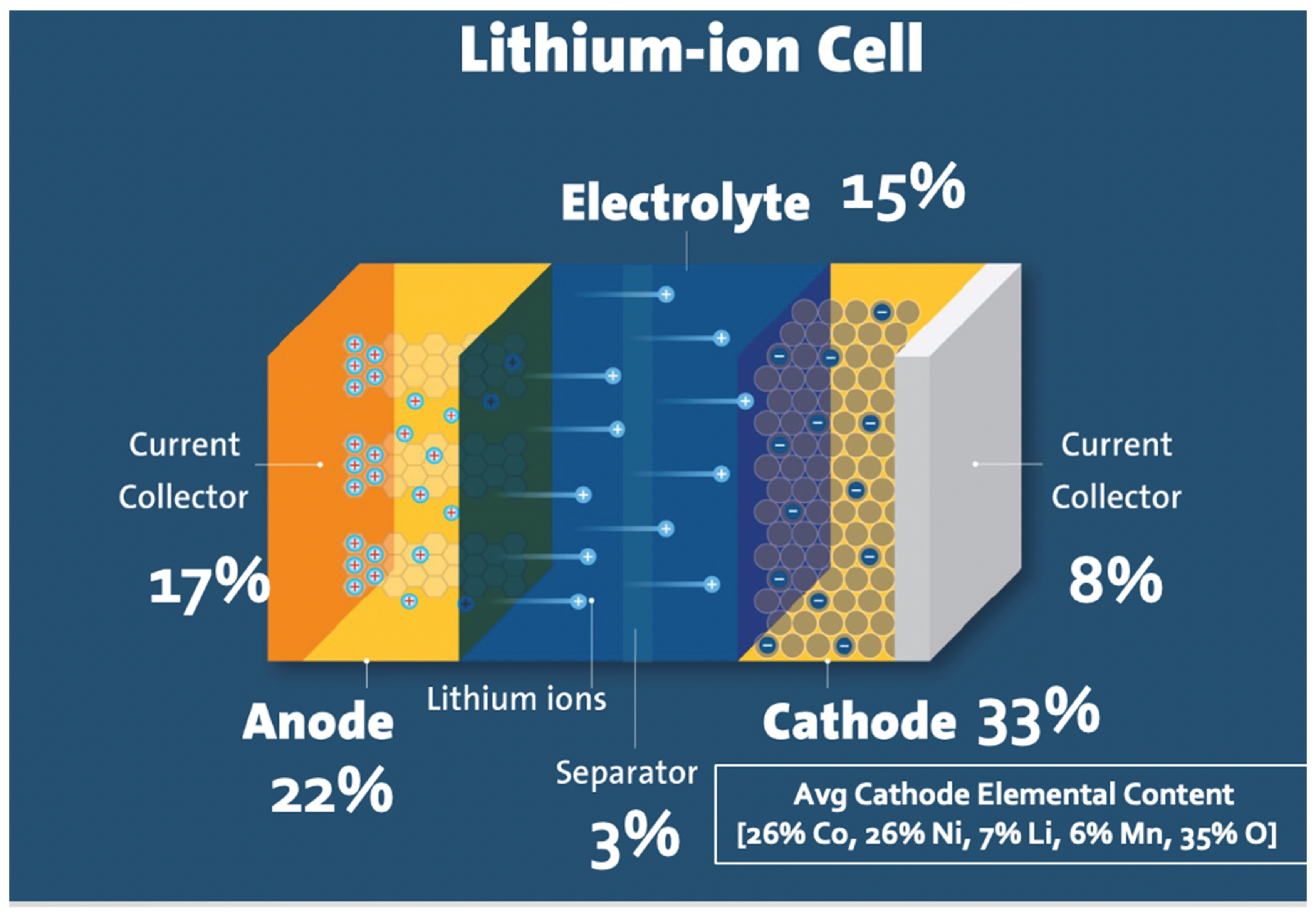
Recovery and Recycling of Valuable Metals from Spent Lithium-Ion Batteries: A Comprehensive Review and Analysis
Abstract
1. Introduction
2. Lithium-Ion Development Batteries over the Years
3. Why Recycle Lithium-Ion Batteries?
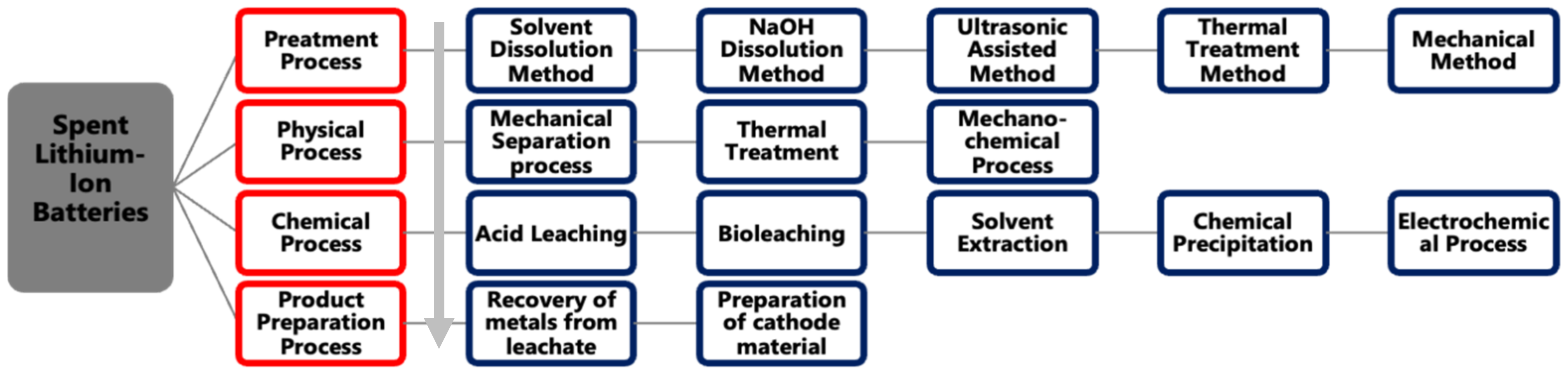
4. Conventional Recycling Methodologies
4.1. Overview
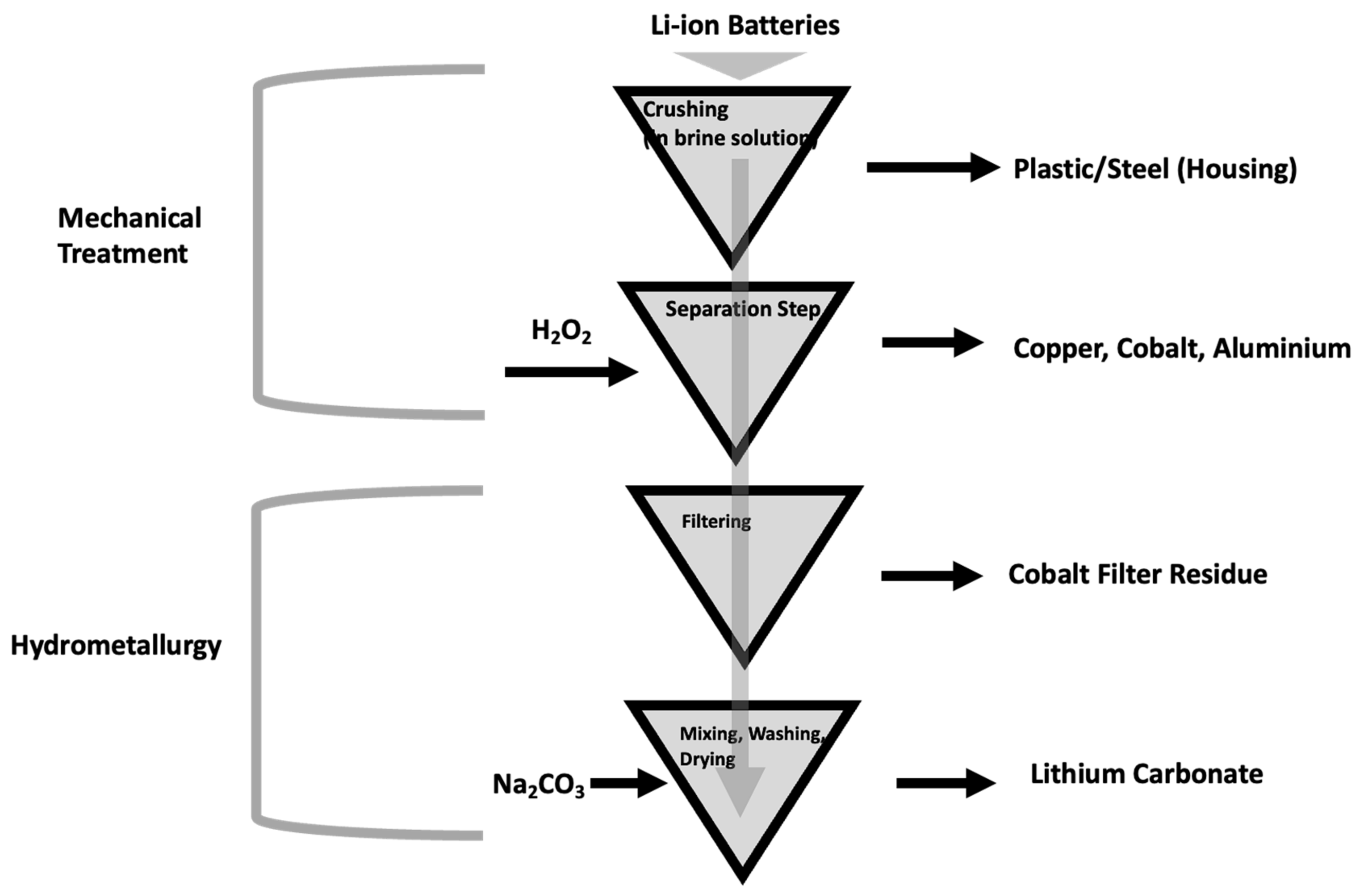
4.2. Pre-Treatment Process
4.3. Solvent Dissolution Method
4.4. NaOH Dissolution Method
4.5. Ultrasonic-Assisted Separation
4.6. Thermal Treatment Method
4.7. Mechanical Method
4.8. Physical Processes
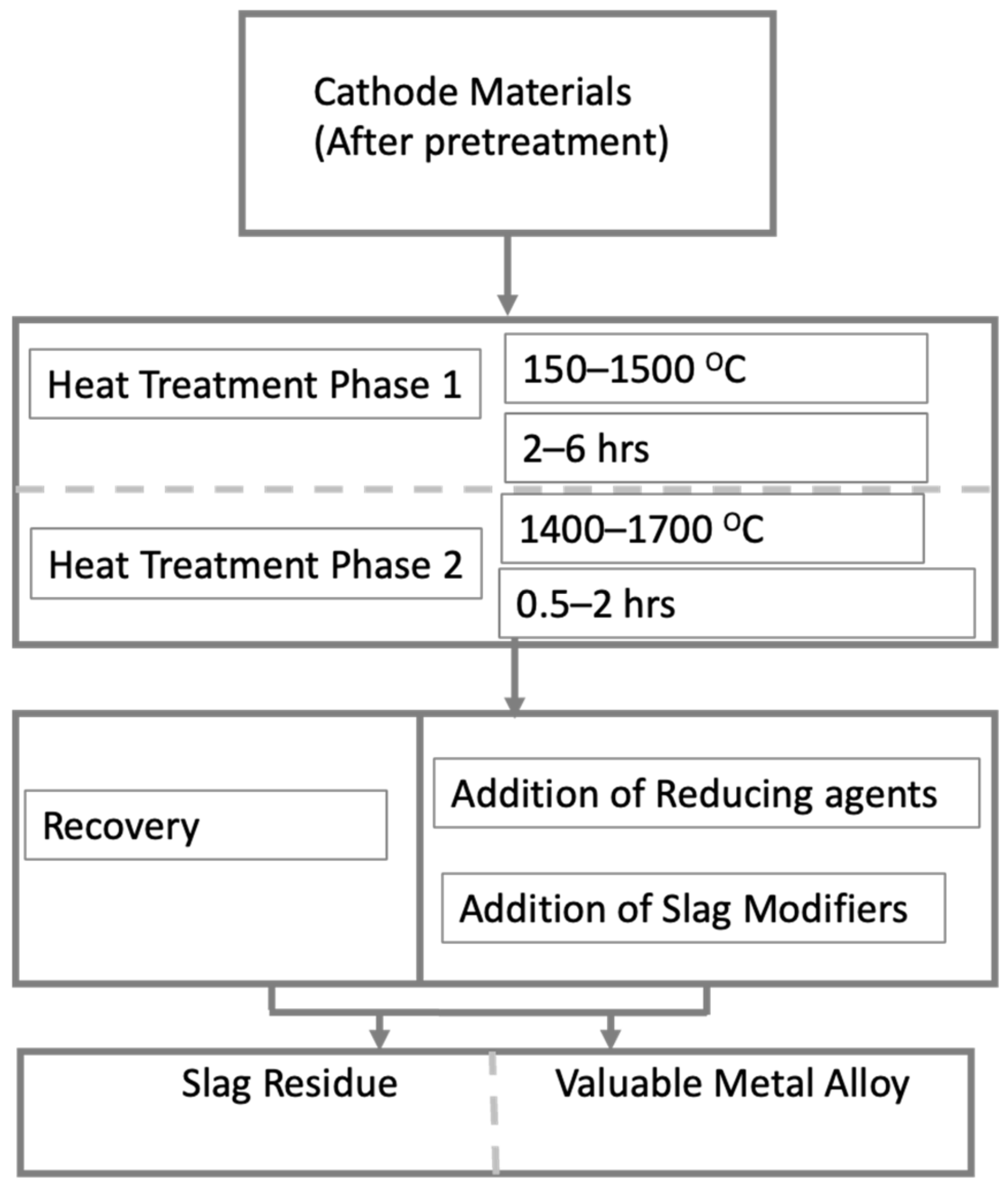
4.8.1. Pyrometallurgical Process
4.8.2. Hydrometallurgical Process
4.9. Chemical Processes
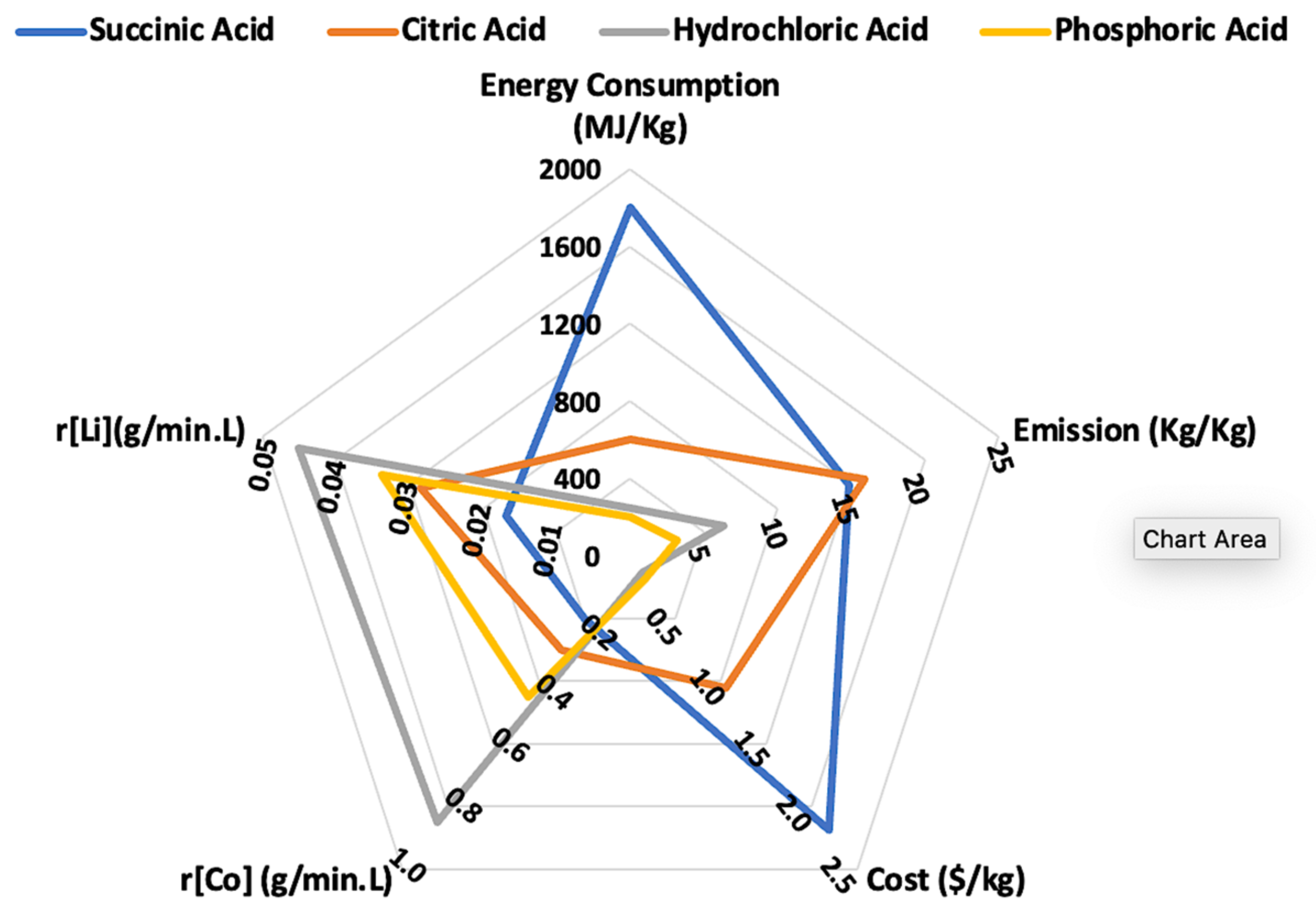
4.9.1. Conventional Leaching
4.9.2. Bio-Metallurgical Process
4.9.3. Solvent Extraction
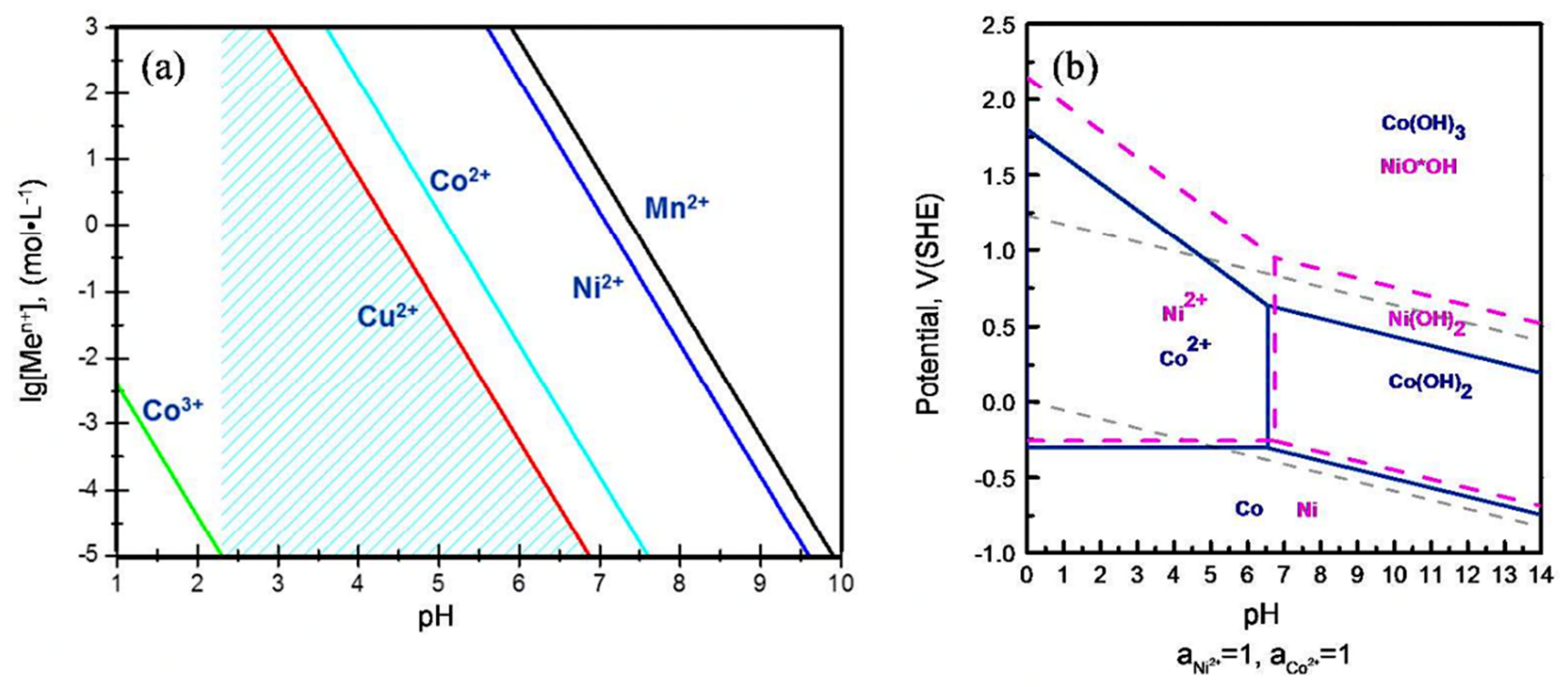
4.9.4. Chemical Precipitation
4.9.5. Active Cathode Material Resynthesis
4.10. Electrochemical Process
5. Valuable Metal Recovery and Preparation
5.1. Metal Recovery
5.2. Metal Preparation
6. Industrial Developed Processes
6.1. Umicore Process
6.2. Toxco Process
6.3. INMETCO Process
7. Summary of Recycling Spent Li-Ion Batteries
8. Conclusions and Outlook
- The key issues are whether the lab-scale innovations have the potential for industrial applications and how to scale them incrementally.
- The subprocesses underlying the leaching process are yet unclear. To help in choosing the ideal leaching reagent and operation circumstances, a lot of work still has to be done. A deep investigation is required, for instance, on changes in the crystallography induced by the leaching process. Insight into the response mechanism during leaching will be made possible by this, and it will be very beneficial.
- The majority of the valuable metals in the spent Li-ion batteries have not yet been selectively leached. Future comparative research investigations ought to be improved.
- The majority of investigative research and studies on the recycling of spent Li-ion batteries have only considered the kinetics and the impact of operational parameters on the leaching process; however, more work needs to be put into developing a comprehensive evaluation system so that more important factors, like the overall process energy consumption, can be taken into account.
- Additionally, since spent Li-ion batteries are harmful to the environment, efforts also need to be focused on improving collection efficiency and minimizing landfilling.
- There should be no restrictions on how spent Li-ion batteries are recycled, and designing, producing, and recycling Li-ion batteries need to take a complete, all-encompassing life cycle approach.
Funding
Data Availability Statement
Conflicts of Interest
References
- al Shaqsi, A.Z.; Sopian, K.; Al-Hinai, A. Review of Energy Storage Services, Applications, Limitations, and Benefits. Energy Rep. 2020, 6, 288–306. [Google Scholar] [CrossRef]
- ESMAP. Reuse and Recycling: Environmental Sustainability of Lithium-Ion Battery Energy Storage Systems an Energy Storage Partnership Report; ESMAP: Washington, DC, USA, 2020. [Google Scholar]
- Phuc Anh, L.E. The Recyclability of Lithium-Ion Battery Materials; The University of Queensland: St Lucia, Australia, 2019. [Google Scholar]
- European Union Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on Batteries and Accumulators and Waste Batteries and Accumulators and Repealing Directive 91/157/EEC (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32006L0066 (accessed on 22 November 2022).
- Zheng, X.; Zhu, Z.; Lin, X.; Zhang, Y.; He, Y.; Cao, H.; Sun, Z. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 2018, 4, 361–370. [Google Scholar] [CrossRef]
- de Oliveira Demarco, J.; Stefanello Cadore, J.; da Silveira de Oliveira, F.; Hiromitsu Tanabe, E.; Assumpção Bertuol, D. Recovery of Metals from Spent Lithium-Ion Batteries Using Organic Acids. Hydrometallurgy 2019, 190, 105169. [Google Scholar] [CrossRef]
- Bloomberg Precious and Industrial Metals Markets. Available online: https://www.bloomberg.com/markets/commodities/futures/metals (accessed on 28 April 2022).
- Unites States Geological Survey. Mineral Commodity Summaries 2020; ESMAP: Reston, VA, USA, 2020. [Google Scholar]
- Georgi-Maschler, T.; Friedrich, B.; Weyhe, R.; Heegn, H.; Rutz, M. Development of a Recycling Process for Li-Ion Batteries. J. Power Sources 2012, 207, 173–182. [Google Scholar] [CrossRef]
- Othman, E.A.; van der Ham, A.G.J.; Miedema, H.; Kersten, S.R.A. Recovery of Metals from Spent Lithium-Ion Batteries Using Ionic Liquid [P8888][Oleate]. Sep. Purif. Technol. 2020, 252, 117435. [Google Scholar] [CrossRef]
- Bertuol, D.A.; Machado, C.M.; Silva, M.L.; Calgaro, C.O.; Dotto, G.L.; Tanabe, E.H. Recovery of Cobalt from Spent Lithium-Ion Batteries Using Supercritical Carbon Dioxide Extraction. Waste Manag. 2016, 51, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Pai, K.V.; Manjanna, J.; Keny, S.J. Use of Mild Organic Acid Reagents to Recover the Co and Li from Spent Li-Ion Batteries. Waste Manag. 2016, 51, 234–238. [Google Scholar] [CrossRef]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Dissolution of Active cathode Material of Spent Li-Ion Batteries Using Tartaric Acid and Ascorbic Acid Mixture to Recover Co. Hydrometallurgy 2016, 161, 54–57. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.; Yoo, J.; Shin, Y.; Bae, J.; Jeong, J.; Kim, K.; Dong, P.; Kwon, K. A Comprehensive Review on the Pretreatment Process in Lithium-Ion Battery Recycling. J. Clean. Prod. 2021, 294, 126329. [Google Scholar] [CrossRef]
- Seyed, M.; Khosh Koo, S. Optimisation of Influential Factors in Electrowinning of Tellurium by Means of PLS Modelling; Lulea University of Technology: Lulea, Sweden, 2009. [Google Scholar]
- Halli, P.; Wilson, B.P.; Hailemariam, T.; Latostenmaa, P.; Yliniemi, K.; Lundström, M. Electrochemical Recovery of Tellurium from Metallurgical Industrial Waste. J. Appl. Electrochem. 2020, 50, 1–14. [Google Scholar] [CrossRef]
- Werner, D.; Peuker, U.A.; Mütze, T. Recycling Chain for Spent Lithium-Ion Batteries. Metals 2020, 10, 316. [Google Scholar] [CrossRef]
- Chandran, V.; Ghosh, A.; Patil, C.K.; Mohanavel, V.; Priya, A.K.; Rahim, R.; Madavan, R.; Muthuraman, U.; Karthick, A. Comprehensive Review on Recycling of Spent Lithium-Ion Batteries. Mater. Today Proc. 2021, 47, 167–180. [Google Scholar] [CrossRef]
- Nishi, Y. Lithium Ion Secondary Batteries; Past 10 Years and the Future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Reddy, M.v.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Piątek, J.; Afyon, S.; Budnyak, T.M.; Budnyk, S.; Sipponen, M.H.; Slabon, A. Sustainable Li-Ion Batteries: Chemistry and Recycling. Adv. Energy Mater. 2021, 11, 2003456. [Google Scholar] [CrossRef]
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521. [Google Scholar] [CrossRef]
- CSIRO CSIRO Annual Report 2019-20; CSIRO: Canberra, Australia, 2020.
- Zhao, Y.; Pohl, O.; Bhatt, A.I.; Collis, G.E.; Mahon, P.J.; Rüther, T.; Hollenkamp, A.F. A Review on Battery Market Trends, Second-Life Reuse, and Recycling. Sustain. Chem. 2021, 2, 167–205. [Google Scholar] [CrossRef]
- Global Battery Alliance A Vision for a Sustainable Battery Value Chain in 2030 Unlocking the Full Potential to Power Sustainable Development and Climate Change Mitigation; Global Battery Alliance: Geneva, Switzerland, 2020.
- Steward, D.; Mayyas, A.; Mann, M. Economics and Challenges of Li-Ion Battery Recycling from End-of-Life Vehicles. Procedia Manuf. 2019, 33, 272–279. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-Ion Battery Materials: Present and Future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Phadke, S.; Cao, M.; Anouti, M. Approaches to Electrolyte Solvent Selection for Poly-Anthraquinone Sulfide Organic Electrode Material. ChemSusChem 2018, 11, 965–974. [Google Scholar] [CrossRef]
- Harper, G.; Sommerville, R.; Kendrick, E.; Driscoll, L.; Slater, P.; Stolkin, R.; Walton, A.; Christensen, P.; Heidrich, O.; Lambert, S.; et al. Recycling Lithium-Ion Batteries from Electric Vehicles. Nature 2019, 575, 75–86. [Google Scholar] [CrossRef]
- Zhu, S.; He, W.; Li, G.; Zhou, X.; Huang, J.; Zhang, X. Recovering Copper from Spent Lithium Ion Battery by a Mechanical Separation Process. In Proceedings of the 2011 International Conference on Materials for Renewable Energy & Environment, Shanghai, China, 20–22 May 2011; pp. 1008–1012. [Google Scholar] [CrossRef]
- Shin, S.M.; Kim, N.H.; Sohn, J.S.; Yang, D.H.; Kim, Y.H. Development of a Metal Recovery Process from Li-Ion Battery Wastes. Hydrometallurgy 2005, 79, 172–181. [Google Scholar] [CrossRef]
- Granata, G.; Moscardini, E.; Pagnanelli, F.; Trabucco, F.; Toro, L. Product Recovery from Li-Ion Battery Wastes Coming from an Industrial Pre-Treatment Plant: Lab Scale Tests and Process Simulations. J. Power Sources 2012, 206, 393–401. [Google Scholar] [CrossRef]
- He, L.-P.; Sun, S.-Y.; Mu, Y.-Y.; Song, X.-F.; Yu, J.-G. Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Using L-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 2017, 5, 714–721. [Google Scholar] [CrossRef]
- Song, D.; Wang, X.; Nie, H.; Shi, H.; Wang, D.; Guo, F.; Shi, X.; Zhang, L. Heat Treatment of LiCoO2 Recovered from Cathode Scraps with Solvent Method. J. Power Sources 2014, 249, 137–141. [Google Scholar] [CrossRef]
- Pant, D.; Dolker, T. Green and Facile Method for the Recovery of Spent Lithium Nickel Manganese Cobalt Oxide (NMC) Based Lithium Ion Batteries. Waste Manag. 2017, 60, 689–695. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Cao, H.; Zhao, C.; Lin, X.; Ning, P.; Zhang, Y.; Jin, W.; Sun, Z. A Closed-Loop Process for Selective Metal Recovery from Spent Lithium Iron Phosphate Batteries through Mechanochemical Activation. ACS Sustain. Chem. Eng. 2017, 5, 9972–9980. [Google Scholar] [CrossRef]
- Wang, F.; Sun, R.; Xu, J.; Chen, Z.; Kang, M. Recovery of Cobalt from Spent Lithium Ion Batteries Using Sulphuric Acid Leaching Followed by Solid–Liquid Separation and Solvent Extraction. RSC Adv. 2016, 6, 85303–85311. [Google Scholar] [CrossRef]
- Guan, J.; Li, Y.; Guo, Y.; Su, R.; Gao, G.; Song, H.; Yuan, H.; Liang, B.; Guo, Z. Mechanochemical Process Enhanced Cobalt and Lithium Recycling from Wasted Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2017, 5, 1026–1032. [Google Scholar] [CrossRef]
- Saeki, S.; Lee, J.; Zhang, Q.; Saito, F. Co-Grinding LiCoO2 with PVC and Water Leaching of Metal Chlorides Formed in Ground Product. Int. J. Miner. Process. 2004, 74, S373–S378. [Google Scholar] [CrossRef]
- Zhang, G.; Yuan, X.; He, Y.; Wang, H.; Zhang, T.; Xie, W. Recent Advances in Pretreating Technology for Recycling Valuable Metals from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2021, 406, 124332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, W.; Li, G.; Zhang, X.; Huang, J.; Zhu, S. Recycling of Electrode Materials from Spent Lithium-Ion Batteries. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, Y.; Cao, H.; Nawaz, F.; Zhang, Y. A Novel Process for Recycling and Resynthesizing LiNi1/3Co1/3Mn1/3O2 from the Cathode Scraps Intended for Lithium-Ion Batteries. Waste Manag. 2014, 34, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Essehli, R.; Jafta, C.J.; Livingston, K.M.; Belharouak, I. Recovery of Cathode Materials and Aluminum Foil Using a Green Solvent. ACS Sustain. Chem. Eng. 2021, 9, 6048–6055. [Google Scholar] [CrossRef]
- Dong, M.; Xue, Z.; Wang, L.; Xia, Y. NaOH Induced the Complete Dissolution of ι-Carrageenan and the Corresponding Mechanism. Polymer 2018, 151, 334–339. [Google Scholar] [CrossRef]
- Nan, J.; Han, D.; Zuo, X. Recovery of Metal Values from Spent Lithium-Ion Batteries with Chemical Deposition and Solvent Extraction. J. Power Sources 2005, 152, 278–284. [Google Scholar] [CrossRef]
- Li, L.; Zhai, L.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Recovery of Valuable Metals from Spent Lithium-Ion Batteries by Ultrasonic-Assisted Leaching Process. J Power Sources 2014, 262, 380–385. [Google Scholar] [CrossRef]
- Li, J.; Shi, P.; Wang, Z.; Chen, Y.; Chang, C.-C. A Combined Recovery Process of Metals in Spent Lithium-Ion Batteries. Chemosphere 2009, 77, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- He, L.-P.; Sun, S.-Y.; Song, X.-F.; Yu, J.-G. Recovery of Cathode Materials and Al from Spent Lithium-Ion Batteries by Ultrasonic Cleaning. Waste Manag. 2015, 46, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Qiu, K. Vacuum Pyrolysis and Hydrometallurgical Process for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xu, S.; He, Y.; Liu, X. Thermal Treatment Process for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Hydrometallurgy 2016, 165, 390–396. [Google Scholar] [CrossRef]
- Hanisch, C.; Loellhoeffel, T.; Diekmann, J.; Markley, K.J.; Haselrieder, W.; Kwade, A. Recycling of Lithium-Ion Batteries: A Novel Method to Separate Coating and Foil of Electrodes. J. Clean. Prod. 2015, 108, 301–311. [Google Scholar] [CrossRef]
- Xu, Y.; Song, D.; Li, L.; An, C.; Wang, Y.; Jiao, L.; Yuan, H. A Simple Solvent Method for the Recovery of LixCoO2 and Its Applications in Alkaline Rechargeable Batteries. J. Power Sources 2014, 252, 286–291. [Google Scholar] [CrossRef]
- Makuza, B.; Tian, Q.; Guo, X.; Chattopadhyay, K.; Yu, D. Pyrometallurgical Options for Recycling Spent Lithium-Ion Batteries: A Comprehensive Review. J. Power Sources 2021, 491, 229622. [Google Scholar] [CrossRef]
- Espinosa, D.C.R.; Bernardes, A.M.; Tenório, J.A.S. An Overview on the Current Processes for the Recycling of Batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Hydrometallurgical Processing of Spent Lithium Ion Batteries (LIBs) in the Presence of a Reducing Agent with Emphasis on Kinetics of Leaching. Chem. Eng. J. 2015, 281, 418–427. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of Lithium from Primary and Secondary Sources by Pre-Treatment, Leaching and Separation: A Comprehensive Review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Ren, G.; Xiao, S.; Xie, M.; Pan, B.; Fan, Y.; Wang, F.; Xia, X. Advances in Molten Slags, Fluxes, and Salts: Proceedings of the 10th International Conference on Molten Slags, Fluxes and Salts 2016; Reddy, R.G., Chaubal, P., Pistorius, P.C., Pal, U., Eds.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-48625-3. [Google Scholar]
- Sun, Z.; Cao, H.; Xiao, Y.; Sietsma, J.; Jin, W.; Agterhuis, H.; Yang, Y. Toward Sustainability for Recovery of Critical Metals from Electronic Waste: The Hydrochemistry Processes. ACS Sustain. Chem. Eng. 2017, 5, 21–40. [Google Scholar] [CrossRef]
- Xiao, J.; Li, J.; Xu, Z. Novel Approach for in Situ Recovery of Lithium Carbonate from Spent Lithium Ion Batteries Using Vacuum Metallurgy. Environ. Sci. Technol. 2017, 51, 11960–11966. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, G.; Xu, Z. Environmentally-Friendly Oxygen-Free Roasting/Wet Magnetic Separation Technology for in Situ Recycling Cobalt, Lithium Carbonate and Graphite from Spent LiCoO2/Graphite Lithium Batteries. J. Hazard. Mater. 2016, 302, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.M.; Santos, J.S.; Pereira, E.C.; Freitas, M.B.J.G. Electrodeposition of Cobalt from Spent Li-Ion Battery Cathodes by the Electrochemistry Quartz Crystal Microbalance Technique. J Power Sources 2008, 185, 549–553. [Google Scholar] [CrossRef]
- Garcia, E.M.; Tarôco, H.A.; Matencio, T.; Domingues, R.Z.; dos Santos, J.A.F.; de Freitas, M.B.J.G. Electrochemical Recycling of Cobalt from Spent Cathodes of Lithium–Ion Batteries: Its Application as Coating on SOFC Interconnects. J. Appl. Electrochem. 2011, 41, 1373–1379. [Google Scholar] [CrossRef]
- Garcia, E.M.; Tarôco, H.A.; Matencio, T.; Domingues, R.Z.; dos Santos, J.A.F.; Ferreira, R.v.; Lorençon, E.; Lima, D.Q.; de Freitas, M.B.J.G. Electrochemical Recycling of Cobalt from Spent Cathodes of Lithium-Ion Batteries: Its Application as Supercapacitor. J. Appl. Electrochem. 2012, 42, 361–366. [Google Scholar] [CrossRef]
- Ferreira, D.A.; Prados, L.M.Z.; Majuste, D.; Mansur, M.B. Hydrometallurgical Separation of Aluminium, Cobalt, Copper and Lithium from Spent Li-Ion Batteries. J. Power Sources 2009, 187, 238–246. [Google Scholar] [CrossRef]
- Joulié, M.; Laucournet, R.; Billy, E. Hydrometallurgical Process for the Recovery of High Value Metals from Spent Lithium Nickel Cobalt Aluminum Oxide Based Lithium-Ion Batteries. J. Power Sources 2014, 247, 551–555. [Google Scholar] [CrossRef]
- Li, L.; Dunn, J.B.; Zhang, X.X.; Gaines, L.; Chen, R.J.; Wu, F.; Amine, K. Recovery of Metals from Spent Lithium-Ion Batteries with Organic Acids as Leaching Reagents and Environmental Assessment. J. Power Sources 2013, 233, 180–189. [Google Scholar] [CrossRef]
- Zeng, X.; Li, J.; Shen, B. Novel Approach to Recover Cobalt and Lithium from Spent Lithium-Ion Battery Using Oxalic Acid. J. Hazard. Mater. 2015, 295, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Nayaka, G.P.; Pai, K.V.; Santhosh, G.; Manjanna, J. Recovery of Cobalt as Cobalt Oxalate from Spent Lithium Ion Batteries by Using Glycine as Leaching Agent. J. Environ. Chem. Eng. 2016, 4, 2378–2383. [Google Scholar] [CrossRef]
- Zheng, X.; Gao, W.; Zhang, X.; He, M.; Lin, X.; Cao, H.; Zhang, Y.; Sun, Z. Spent Lithium-Ion Battery Recycling—Reductive Ammonia Leaching of Metals from Cathode Scrap by Sodium Sulphite. Waste Manag. 2017, 60, 680–688. [Google Scholar] [CrossRef]
- Ku, H.; Jung, Y.; Jo, M.; Park, S.; Kim, S.; Yang, D.; Rhee, K.; An, E.-M.; Sohn, J.; Kwon, K. Recycling of Spent Lithium-Ion Battery Cathode Materials by Ammoniacal Leaching. J. Hazard. Mater. 2016, 313, 138–146. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, F.-S. Innovative Leaching of Cobalt and Lithium from Spent Lithium-Ion Batteries and Simultaneous Dechlorination of Polyvinyl Chloride in Subcritical Water. J. Hazard. Mater. 2016, 316, 19–25. [Google Scholar] [CrossRef]
- Takacova, Z.; Havlik, T.; Kukurugya, F.; Orac, D. Cobalt and Lithium Recovery from Active Mass of Spent Li-Ion Batteries: Theoretical and Experimental Approach. Hydrometallurgy 2016, 163, 9–17. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.; Chen, H.; Luo, T.; Xu, Z. Leaching Study of Spent Li-Ion Batteries. Procedia Environ. Sci. 2012, 16, 443–450. [Google Scholar] [CrossRef]
- Chen, X.; Ma, H.; Luo, C.; Zhou, T. Recovery of Valuable Metals from Waste Cathode Materials of Spent Lithium-Ion Batteries Using Mild Phosphoric Acid. J. Hazard. Mater. 2017, 326, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Rhee, K.-I. Preparation of LiCoO2 from Spent Lithium-Ion Batteries. J. Power Sources 2002, 109, 17–21. [Google Scholar] [CrossRef]
- Barik, S.P.; Prabaharan, G.; Kumar, L. Leaching and Separation of Co and Mn from Electrode Materials of Spent Lithium-Ion Batteries Using Hydrochloric Acid: Laboratory and Pilot Scale Study. J. Clean. Prod. 2017, 147, 37–43. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Jha, A.K.; Kumar, V.; Hait, J.; Pandey, B.D. Recovery of Lithium and Cobalt from Waste Lithium Ion Batteries of Mobile Phone. Waste Manag. 2013, 33, 1890–1897. [Google Scholar] [CrossRef]
- Swain, B.; Jeong, J.; Lee, J.; Lee, G.-H.; Sohn, J.-S. Hydrometallurgical Process for Recovery of Cobalt from Waste Cathodic Active Material Generated during Manufacturing of Lithium Ion Batteries. J. Power Sources 2007, 167, 536–544. [Google Scholar] [CrossRef]
- Zhu, S.; He, W.; Li, G.; Zhou, X.; Zhang, X.; Huang, J. Recovery of Co and Li from Spent Lithium-Ion Batteries by Combination Method of Acid Leaching and Chemical Precipitation. Trans. Nonferr. Met. Soc. China 2012, 22, 2274–2281. [Google Scholar] [CrossRef]
- Chen, X.; Guo, C.; Ma, H.; Li, J.; Zhou, T.; Cao, L.; Kang, D. Organic Reductants Based Leaching: A Sustainable Process for the Recovery of Valuable Metals from Spent Lithium Ion Batteries. Waste Manag. 2018, 75, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Pinna, E.G.; Ruiz, M.C.; Ojeda, M.W.; Rodriguez, M.H. Cathodes of Spent Li-Ion Batteries: Dissolution with Phosphoric Acid and Recovery of Lithium and Cobalt from Leach Liquors. Hydrometallurgy 2017, 167, 66–71. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A Closed Loop Process for Recycling Spent Lithium Ion Batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Suzuki, T.M.; Inoue, K. Hydrometallurgical Process for Recovery of Metal Values from Spent Lithium-Ion Secondary Batteries. Hydrometallurgy 1998, 47, 259–271. [Google Scholar] [CrossRef]
- Huang, Y.; Han, G.; Liu, J.; Chai, W.; Wang, W.; Yang, S.; Su, S. A Stepwise Recovery of Metals from Hybrid Cathodes of Spent Li-Ion Batteries with Leaching-Flotation-Precipitation Process. J. Power Sources 2016, 325, 555–564. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Organic Oxalate as Leachant and Precipitant for the Recovery of Valuable Metals from Spent Lithium-Ion Batteries. Waste Manag. 2012, 32, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qu, W.; Zhang, X.; Lu, J.; Chen, R.; Wu, F.; Amine, K. Succinic Acid-Based Leaching System: A Sustainable Process for Recovery of Valuable Metals from Spent Li-Ion Batteries. J. Power Sources 2015, 282, 544–551. [Google Scholar] [CrossRef]
- Chen, X.; Luo, C.; Zhang, J.; Kong, J.; Zhou, T. Sustainable Recovery of Metals from Spent Lithium-Ion Batteries: A Green Process. ACS Sustain. Chem. Eng. 2015, 3, 3104–3113. [Google Scholar] [CrossRef]
- Golmohammadzadeh, R.; Rashchi, F.; Vahidi, E. Recovery of Lithium and Cobalt from Spent Lithium-Ion Batteries Using Organic Acids: Process Optimization and Kinetic Aspects. Waste Manag. 2017, 64, 244–254. [Google Scholar] [CrossRef]
- Chen, X.; Kang, D.; Cao, L.; Li, J.; Zhou, T.; Ma, H. Separation and Recovery of Valuable Metals from Spent Lithium Ion Batteries: Simultaneous Recovery of Li and Co in a Single Step. Sep. Purif. Technol. 2019, 210, 690–697. [Google Scholar] [CrossRef]
- Ijadi Bajestani, M.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of Heavy Metals from Spent Household Batteries Using Acidithiobacillus Ferrooxidans: Statistical Evaluation and Optimization. Sep. Purif. Technol. 2014, 132, 309–316. [Google Scholar] [CrossRef]
- Dominguez-Benetton, X.; Varia, J.C.; Pozo, G.; Modin, O.; ter Heijne, A.; Fransaer, J.; Rabaey, K. Metal Recovery by Microbial Electro-Metallurgy. Prog. Mater. Sci. 2018, 94, 435–461. [Google Scholar] [CrossRef]
- Mishra, D.; Kim, D.-J.; Ralph, D.E.; Ahn, J.-G.; Rhee, Y.-H. Bioleaching of Metals from Spent Lithium Ion Secondary Batteries Using Acidithiobacillus Ferrooxidans. Waste Manag. 2008, 28, 333–338. [Google Scholar] [CrossRef]
- Ren, W.-X.; Li, P.-J.; Geng, Y.; Li, X.-J. Biological Leaching of Heavy Metals from a Contaminated Soil by Aspergillus Niger. J. Hazard. Mater. 2009, 167, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Luo, S.; Deng, X.; Li, L.; Au, C. Influence of Silver Ions on Bioleaching of Cobalt from Spent Lithium Batteries. Miner. Eng. 2013, 49, 40–44. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Lin, J.-G. Enhancement of Metal Bioleaching from Contaminated Sediment Using Silver Ion. J. Hazard. Mater. 2009, 161, 893–899. [Google Scholar] [CrossRef]
- Xin, B.; Zhang, D.; Zhang, X.; Xia, Y.; Wu, F.; Chen, S.; Li, L. Bioleaching Mechanism of Co and Li from Spent Lithium-Ion Battery by the Mixed Culture of Acidophilic Sulfur-Oxidizing and Iron-Oxidizing Bacteria. Bioresour. Technol. 2009, 100, 6163–6169. [Google Scholar] [CrossRef]
- Xin, Y.; Guo, X.; Chen, S.; Wang, J.; Wu, F.; Xin, B. Bioleaching of Valuable Metals Li, Co, Ni and Mn from Spent Electric Vehicle Li-Ion Batteries for the Purpose of Recovery. J. Clean. Prod. 2016, 116, 249–258. [Google Scholar] [CrossRef]
- Bahaloo-Horeh, N.; Mousavi, S.M. Enhanced Recovery of Valuable Metals from Spent Lithium-Ion Batteries through Optimization of Organic Acids Produced by Aspergillus Niger. Waste Manag. 2017, 60, 666–679. [Google Scholar] [CrossRef] [PubMed]
- Horeh, N.B.; Mousavi, S.M.; Shojaosadati, S.A. Bioleaching of Valuable Metals from Spent Lithium-Ion Mobile Phone Batteries Using Aspergillus Niger. J. Power Sources 2016, 320, 257–266. [Google Scholar] [CrossRef]
- Niu, Z.; Zou, Y.; Xin, B.; Chen, S.; Liu, C.; Li, Y. Process Controls for Improving Bioleaching Performance of Both Li and Co from Spent Lithium Ion Batteries at High Pulp Density and Its Thermodynamics and Kinetics Exploration. Chemosphere 2014, 109, 92–98. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Lee, M.S. A Review on the Separation of Molybdenum, Tungsten, and Vanadium from Leach Liquors of Diverse Resources by Solvent Extraction. Geosyst. Eng. 2016, 19, 247–259. [Google Scholar] [CrossRef]
- Whitworth, A.J.; Forbes, E.; Verster, I.; Jokovic, V.; Awatey, B.; Parbhakar-Fox, A. Review on Advances in Mineral Processing Technologies Suitable for Critical Metal Recovery from Mining and Processing Wastes. Clean. Eng. Technol. 2022, 7, 100451. [Google Scholar] [CrossRef]
- Shakibania, S.; Mahmoudi, A.; Mokmeli, M. Separation of Vanadium and Iron from the Steelmaking Slag Convertor Using Aliquat 336 and D2EHPA: Effect of the Aqueous Species and the Extractant Type. Miner. Eng. 2022, 181, 107521. [Google Scholar] [CrossRef]
- Zhao, J.M.; Shen, X.Y.; Deng, F.L.; Wang, F.C.; Wu, Y.; Liu, H.Z. Synergistic Extraction and Separation of Valuable Metals from Waste Cathodic Material of Lithium Ion Batteries Using Cyanex272 and PC-88A. Sep. Purif. Technol. 2011, 78, 345–351. [Google Scholar] [CrossRef]
- Jha, A.K.; Jha, M.K.; Kumari, A.; Sahu, S.K.; Kumar, V.; Pandey, B.D. Selective Separation and Recovery of Cobalt from Leach Liquor of Discarded Li-Ion Batteries Using Thiophosphinic Extractant. Sep. Purif. Technol. 2013, 104, 160–166. [Google Scholar] [CrossRef]
- Joo, S.-H.; Shin, D.J.; Oh, C.; Wang, J.-P.; Senanayake, G.; Shin, S.M. Selective Extraction and Separation of Nickel from Cobalt, Manganese and Lithium in Pre-Treated Leach Liquors of Ternary Cathode Material of Spent Lithium-Ion Batteries Using Synergism Caused by Versatic 10 Acid and LIX 84-I. Hydrometallurgy 2016, 159, 65–74. [Google Scholar] [CrossRef]
- Darvishi, D.; Haghshenas, D.F.; Alamdari, E.K.; Sadrnezhaad, S.K.; Halali, M. Synergistic Effect of Cyanex 272 and Cyanex 302 on Separation of Cobalt and Nickel by D2EHPA. Hydrometallurgy 2005, 77, 227–238. [Google Scholar] [CrossRef]
- Virolainen, S.; Fallah Fini, M.; Laitinen, A.; Sainio, T. Solvent Extraction Fractionation of Li-Ion Battery Leachate Containing Li, Ni, and Co. Sep. Purif. Technol. 2017, 179, 274–282. [Google Scholar] [CrossRef]
- Joo, S.-H.; Shin, D.; Oh, C.; Wang, J.-P.; Shin, S.M. Extraction of Manganese by Alkyl Monocarboxylic Acid in a Mixed Extractant from a Leaching Solution of Spent Lithium-Ion Battery Ternary Cathodic Material. J. Power Sources 2016, 305, 175–181. [Google Scholar] [CrossRef]
- Chen, X.; Fan, B.; Xu, L.; Zhou, T.; Kong, J. An Atom-Economic Process for the Recovery of High Value-Added Metals from Spent Lithium-Ion Batteries. J. Clean. Prod. 2016, 112, 3562–3570. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, G.; Xie, M.; Xu, S.; He, Y. Synthesis and Performance of Spherical LiNixCoyMn1-x-YO2 Regenerated from Nickel and Cobalt Scraps. Hydrometallurgy 2016, 165, 358–369. [Google Scholar] [CrossRef]
- Sa, Q.; Gratz, E.; He, M.; Lu, W.; Apelian, D.; Wang, Y. Synthesis of High Performance LiNi1/3Mn1/3Co1/3O2 from Lithium Ion Battery Recovery Stream. J. Power Sources 2015, 282, 140–145. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.X.; Chen, R.J.; Wu, F.; Amine, K. Ascorbic-Acid-Assisted Recovery of Cobalt and Lithium from Spent Li-Ion Batteries. J. Power Sources 2012, 218, 21–27. [Google Scholar] [CrossRef]
- Zou, H.; Gratz, E.; Apelian, D.; Wang, Y. A Novel Method to Recycle Mixed Cathode Materials for Lithium Ion Batteries. Green Chem. 2013, 15, 1183. [Google Scholar] [CrossRef]
- Yao, L.; Yao, H.; Xi, G.; Feng, Y. Recycling and Synthesis of LiNi1/3Co1/3Mn1/3O2 from Waste Lithium-Ion Batteries Using d,l-Malic Acid. RSC Adv. 2016, 6, 17947–17954. [Google Scholar] [CrossRef]
- Yao, L.; Xi, Y.; Xi, G.; Feng, Y. Synthesis of Cobalt Ferrite with Enhanced Magnetostriction Properties by the Sol−gel−hydrothermal Route Using Spent Li-Ion Battery. J. Alloys Compd. 2016, 680, 73–79. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, X.; Cao, H.; Lin, X.; Liu, C.; Sun, Y.; Zhang, Y.; Sun, Z. Selective Recovery of Lithium from Spent Lithium Iron Phosphate Batteries: A Sustainable Process. Green Chem. 2018, 20, 3121–3133. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-F.; Yang, D.; Du, T.; Gong, H.; Luo, W.-B. The Current Process for the Recycling of Spent Lithium Ion Batteries. Front. Chem. 2020, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Du, Z.; He, Y.; Wang, H.; Xie, W.; Zhang, T. A Sustainable Process for the Recovery of Anode and Cathode Materials Derived from Spent Lithium-Ion Batteries. Sustainability 2019, 11, 2363. [Google Scholar] [CrossRef]
- Huang, B.; Pan, Z.; Su, X.; An, L. Recycling of Lithium-Ion Batteries: Recent Advances and Perspectives. J. Power Sources 2018, 399, 274–286. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Song, J.; Song, D.; Zhang, L.; Shi, X. Environmentally Friendly Recycling and Effective Repairing of Cathode Powders from Spent LiFePO 4 Batteries. Green Chem. 2016, 18, 2500–2506. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of Gel-Type Polymer Electrolytes for Lithium-Ion Batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Myoung, J.; Jung, Y.; Lee, J.; Tak, Y. Cobalt Oxide Preparation from Waste LiCoO2 by Electrochemical–Hydrothermal Method. J. Power Sources 2002, 112, 639–642. [Google Scholar] [CrossRef]
- Sharma, A.; Das, J. Small Molecules Derived Carbon Dots: Synthesis and Applications in Sensing, Catalysis, Imaging, and Biomedicine. J. Nanobiotechnol. 2019, 17, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, M.I.; Choo, W.L.; Breuer, P.L. The effect of additives and impurities on the cobalt electrowinning process. Miner. Eng. 2000, 13, 1231–1241. [Google Scholar] [CrossRef]
- Sharma, I.G.; Alex, P.; Bidaye, A.C.; Suri, A.K. Electrowinning of Cobalt from Sulphate Solutions. Hydrometallurgy 2005, 80, 132–138. [Google Scholar] [CrossRef]
- Mulaudzi, N.; Kotze, M.H. Direct Cobalt Electrowinning as an Alternative to Intermediate Cobalt Mixed Hydroxide Product. In Proceedings of the Southern African Institute of Mining and Metallurgy Base Metals Conference, White River, South Africa, 2–4 September 2013. [Google Scholar]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and Technologies for the Recycling and Recovery of Spent Lithium-Ion Batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- ESI-Africa The Growing Lithium-Ion Battery Recycling Market. Available online: https://www.esi-africa.com/regional-news/international/the-growing-lithium-ion-battery-recycling-market/ (accessed on 22 November 2022).
- Cheng, Q. Effect of Different Reductants on Leaching Lithium and Cobalt from Lithium Ion Batteries in Tartaric Acid Solution. IOP Conf. Ser. Earth Environ. Sci. 2018, 192, 012007. [Google Scholar] [CrossRef]
- Yan, J. Handbook of Clean Energy Systems; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 6, ISBN 978-1-118-38858-7. [Google Scholar]
- Lander, L.; Cleaver, T.; Rajaeifar, M.A.; Nguyen-Tien, V.; Elliott, R.J.R.; Heidrich, O.; Kendrick, E.; Edge, J.S.; Offer, G. Financial Viability of Electric Vehicle Lithium-Ion Battery Recycling. iScience 2021, 24, 102787. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Iyer-Raniga, U. Lithium-Ion Battery Recycling in the Circular Economy: A Review. Recycling 2022, 7, 33. [Google Scholar] [CrossRef]
- Velázquez-Martínez, O.; Valio, J.; Santasalo-Aarnio, A.; Reuter, M.; Serna-Guerrero, R. A Critical Review of Lithium-Ion Battery Recycling Processes from a Circular Economy Perspective. Batteries 2019, 5, 68. [Google Scholar] [CrossRef]
- Aboagye, E.A.; Chea, J.D.; Yenkie, K.M. Systems Level Roadmap for Solvent Recovery and Reuse in Industries. iScience 2021, 24, 103114. [Google Scholar] [CrossRef] [PubMed]
- Zou, H. Development of a Recycling Process for Li-Ion Batteries; Worcester Polytechnic Institute: Worcester, MA, USA, 2012. [Google Scholar]
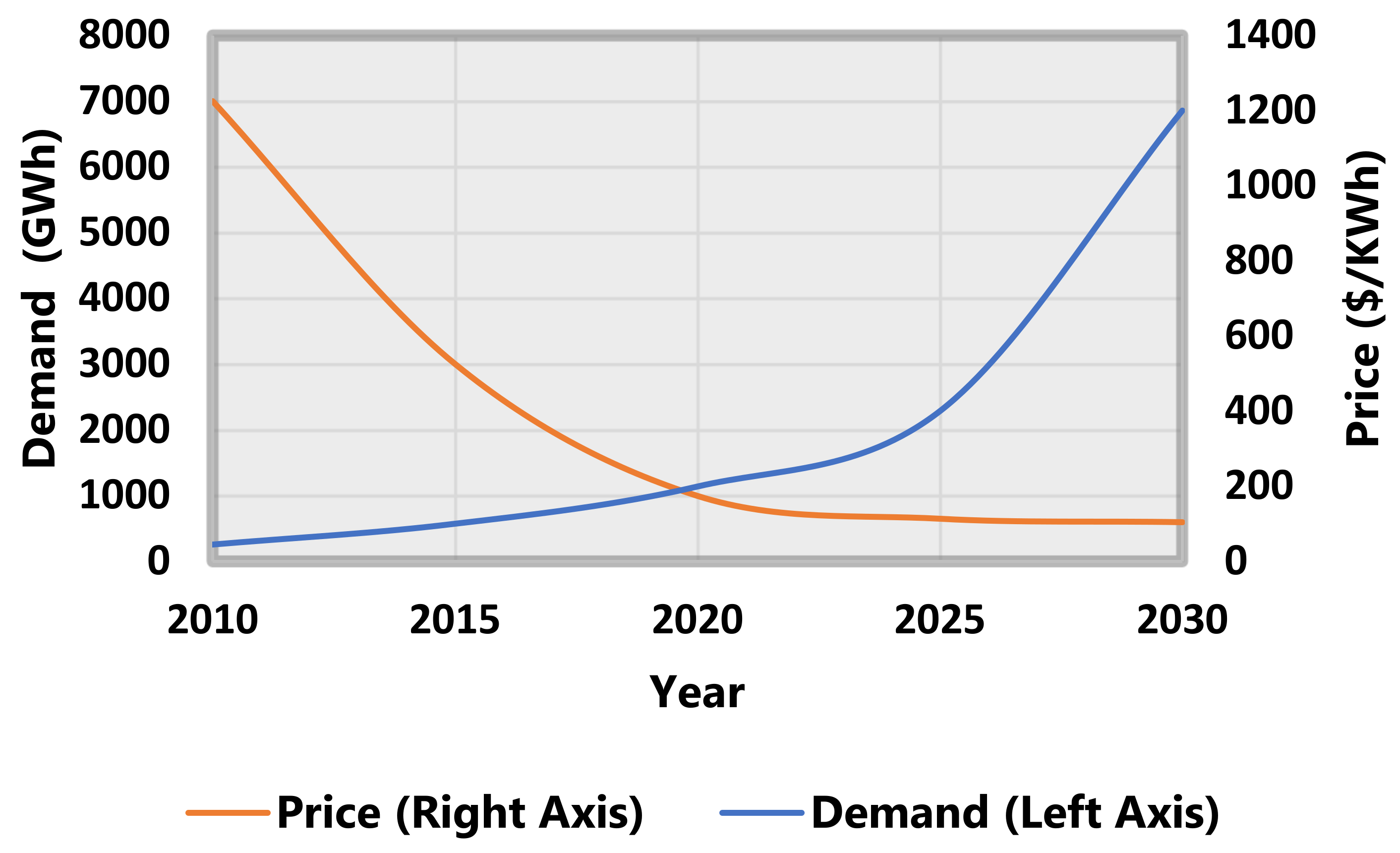

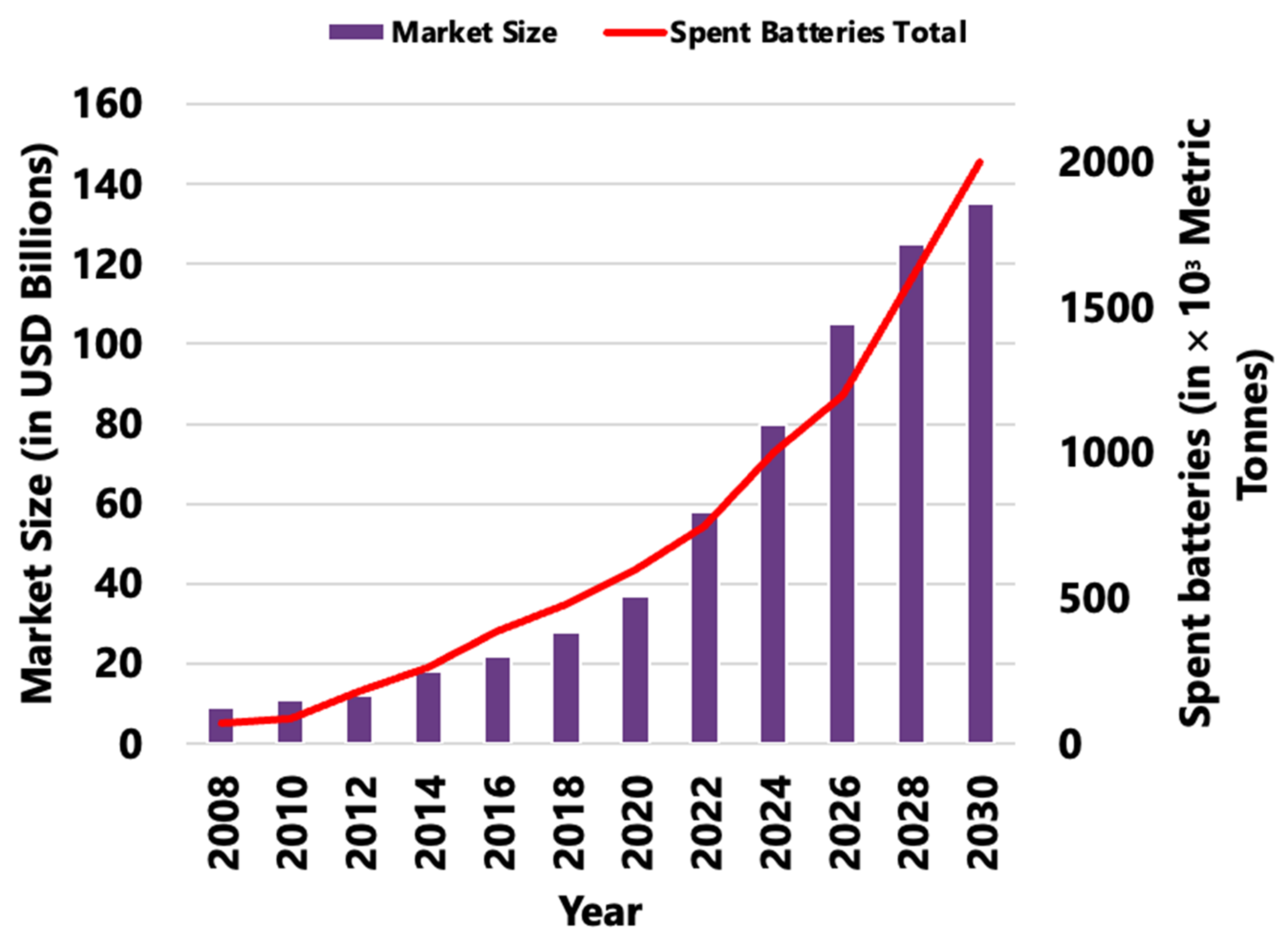
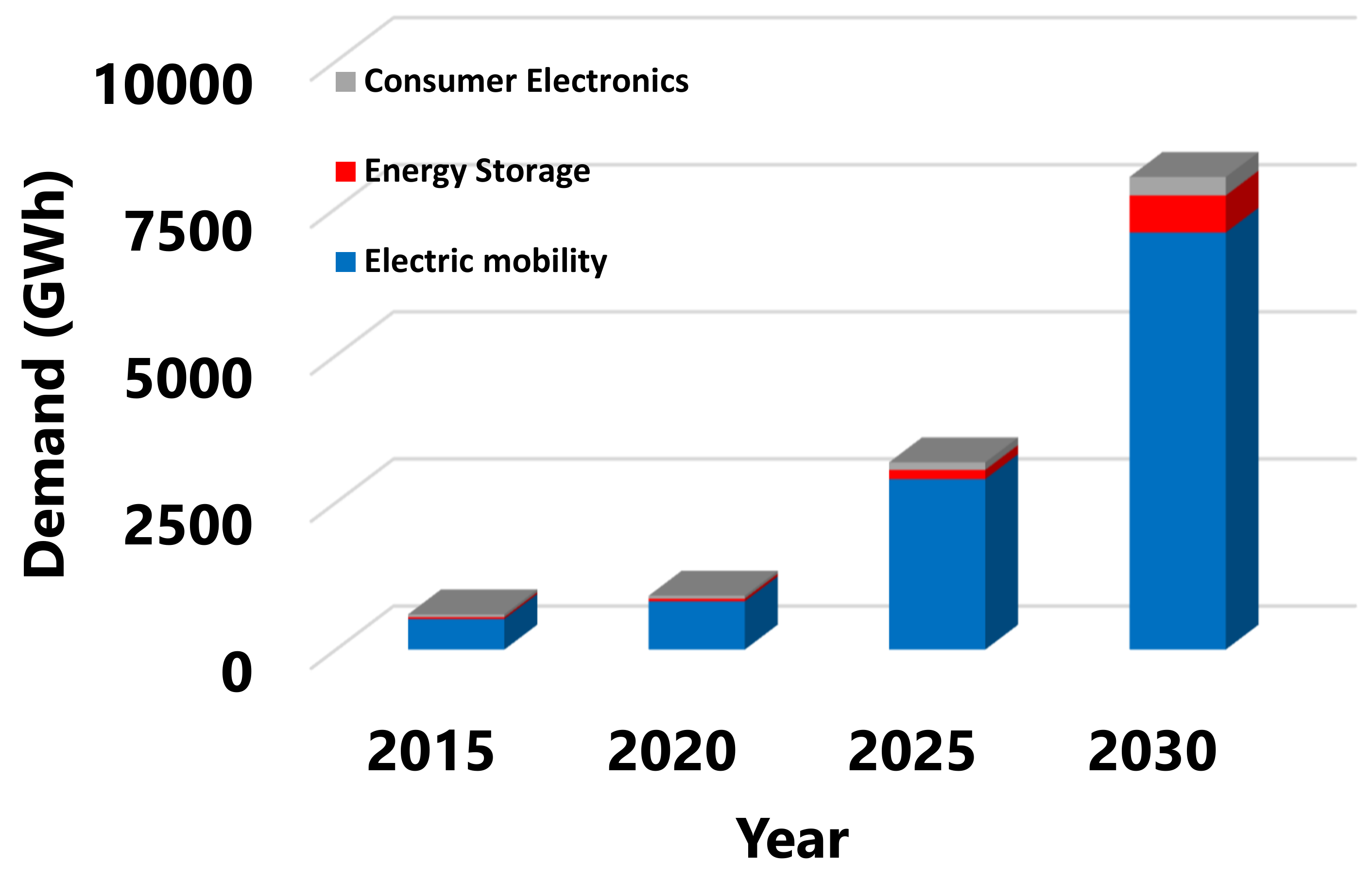




| Component | Approximate Value |
|---|---|
| US$/Ton | |
| Cobalt | 87,633 |
| Aluminium | 2753 |
| Nickel | 28,370 |
| Manganese | 2000 |
| Iron | 300 |
| Electrolyte | 1500 |
| Copper | 9219 |
| Lithium | 59,720 |
| Method | Advantages | Disadvantages |
|---|---|---|
| Solvent Dissolution method | High separation efficiency | The costlier the solution, the high degree of toxicity |
| NaOH method | Simple operation with high separation efficiency | Alkali wastewater is harmful to the ecosystem, and Al extraction is challenging since it is in an ionic state |
| Ultrasonic assisted separation | The operation method is simple, with no hazardous or toxic traits | High capital cost, noise pollution |
| Thermal treatment | Simple operation, high-efficiency process | Capital cost is the high, high toxic gas emission |
| Mechanical methods | Operation method that is simple to employ | High levels of hazardous gas emissions and incomplete metal removal from spent Li-ion batteries |
| Temp | Time | Leaching Efficiency (%) | ||||
|---|---|---|---|---|---|---|
| Leaching Process and Active Cathode Material Leached | Reagent | (°C) | (min) | Co | Li | Refs. |
| Inorganic Acid Leaching | ||||||
| Spent Li-ion batteries (LCO) | 0.7 M H3PO4 + 4 vol % H2O2 | 40 | 60 | 98 | 96 | [74] |
| Spent Li-ion batteries (LCO + NMC) | 1 M H2SO4 + 0.075 M NaHSO3 | 95 | 240 | 92 | 97 | [55] |
| Spent Li-ion batteries (LCO) | 1 M HNO3 + 1.7 vol % H2O2 | 75 | 60 | 96 | 96 | [75] |
| LCO | 1.75 M HCl | 50 | 90 | 98 | 97 | [76] |
| Spent Li-ion batteries (LCO) (from e-gadgets) | 2 M H2SO4 + 5 vol % H2O2 | 75 | 60 | 71 | 98 | [77] |
| LCO | 2 M H2SO4+5 vol % H2O2 | 75 | 30 | 94 | 96 | [78] |
| LCO | 2 M H2SO4+2 vol % H2O2 | 60 | 120 | 97 | 87 | [79] |
| LCO | 2 M H2SO4+ 0.4 g/g Sucrose | 95 | 120 | 96 | 99 | [80] |
| Spent Li-ion batteries (LCO) (from cell phones) | 2% vol % H3PO4 + 2 vol % H2O2 | 90 | 60 | 98 | 89 | [81] |
| LiNixMnyCozO (NMC) compounds | 4 M H2SO4 + 5 vol % H2O2 | 65–70 | 120 | 97 | [82] | |
| LCO | 4 M HCl | 80 | 30 | 91 | 94 | [83] |
| LFP and LMO | 6.5 M HCl + 5 vol % H2O2 | 30 | 60 | 76 | [84] | |
| Alkaline Leaching | ||||||
| Spent Li-ion batteries (Li(Ni1/3Co1/3Mn1/3)O2) | 4 M NH3-1.5 mol/L (NH4)2SO4 + 0.5 M Na2SO4 | 80 | 300 | 81 | 96 | [69] |
| Organic Acid Leaching | ||||||
| Spent Li-ion batteries (LCO) | 1.5 M citric acid + 0.2 M salicylic acid (15 g L−1 ), 6 vol % H2O2 | 90 | 90 | 99 | 97 | |
| Spent Li-ion batteries (LCO) | 0.4 M tartaric acid + 0.02 M ascorbic acid | 80 | 60 | 94 | 96 | [13] |
| Spent Li-ion batteries (LCO) | 1.5 M Citric Acid + 0.2 M Salicylic Acid+6 vol % H2O2 | 90 | 90 | 99 | 97 | [85] |
| Spent Li-ion batteries (LCO) | 0.5 M glycine + 0.02 M ascorbic acid | 80 | 120 | 92 | [13] | |
| Spent Li-ion batteries (LCO) | 1 M iminodiacetic acid + 0.02 M ascorbic acid | 80 | 120 | 98 | 90 | [12] |
| Spent Li-ion batteries (LCO) | 1 M maleic acid + 0.02 M ascorbic acid | 80 | 120 | 98 | 96 | [12] |
| LCO | 1 M oxalate + 5 vol % H2O2 | 80 | 120 | 97 | [85] | |
| Spent Li-ion batteries (LCO) | 1 M oxalic acid | 95 | 150 | 97 | 98 | [67] |
| Spent Li-ion batteries (LCO) | 1.5 M succinic acid + 4 vol % H2O2 | 70 | 40 | 98 | 95 | [86] |
| Spent Li-ion batteries (LCO) | 2 M citric acid + 0.6 g/g H2O2 (H2O2/spent Li-ion batteries) | 70 | 80 | 96 | 98 | [87] |
| Spent Li-ion batteries, (LCO & LiNi0.5Co0.2Mn0.3O2 (NMC)) | 2 M L-tartaric acid + 4 vol % H2O2 | 70 | 30 | 97 | 99 | [88,89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tawonezvi, T.; Nomnqa, M.; Petrik, L.; Bladergroen, B.J. Recovery and Recycling of Valuable Metals from Spent Lithium-Ion Batteries: A Comprehensive Review and Analysis. Energies 2023, 16, 1365. https://doi.org/10.3390/en16031365
Tawonezvi T, Nomnqa M, Petrik L, Bladergroen BJ. Recovery and Recycling of Valuable Metals from Spent Lithium-Ion Batteries: A Comprehensive Review and Analysis. Energies. 2023; 16(3):1365. https://doi.org/10.3390/en16031365
Chicago/Turabian StyleTawonezvi, Tendai, Myalelo Nomnqa, Leslie Petrik, and Bernard Jan Bladergroen. 2023. "Recovery and Recycling of Valuable Metals from Spent Lithium-Ion Batteries: A Comprehensive Review and Analysis" Energies 16, no. 3: 1365. https://doi.org/10.3390/en16031365
APA StyleTawonezvi, T., Nomnqa, M., Petrik, L., & Bladergroen, B. J. (2023). Recovery and Recycling of Valuable Metals from Spent Lithium-Ion Batteries: A Comprehensive Review and Analysis. Energies, 16(3), 1365. https://doi.org/10.3390/en16031365