Abstract
Thermal-driven refrigeration technologies, e.g., absorption- or adsorption-type, are gathering momentum since they can utilize low-grade heat from industrial, solar or geothermal energy. However, heat sources and end users are usually mismatched, which could lead to potential heat pollution and increased carbon emissions. Long-distance thermal energy transportation is good for district heating and cooling, which is of great significance if it can achieve a high energy-transportation density and low heat loss. In this paper, a compression-assisted chemisorption chiller driven by a low-temperature heat source for cold transportation is initially proposed, which aims to transport liquid ammonia with chemical potential and generate a cooling effect for end users. A feasibility analysis of the compression-assisted chemisorption chiller is preliminarily performed for 2 km cold transportation. The results show that the highest theoretical coefficient of performance and the energy efficiency of the compression-assisted adsorption chiller using a sodium bromide–ammonia working pair can reach 0.46 and 0.25, respectively, when the evaporation temperature is 20 °C. Among the three selected low-temperature salts, ammonium chloride–ammonia shows the best performance, which is up to about 40% higher than those of sodium bromide–ammonia and barium chloride–ammonia. It is demonstrated that compared with common absorption chillers, a compression-assisted adsorption system has a reasonable working efficiency to transport cold energy when the low- or ultralow-temperature heat source, e.g., lower than 60 °C, is required to be utilized.
1. Introduction
Efficient heat or cold transportation, especially for low-grade thermal energy at a long distance, is of great importance, but it still poses several challenges when considering the working temperature, pressure or heat loss [1,2]. The conventional methods for heat or cold transportation aim to use the sensible or latent heat of water [3]. But, the effective transportation distance by using these methods is quite limited; otherwise, thermal energy will have an inevitable loss for the end users [4]. Thus, waste heat from the centralized source [5], for example, power plants, iron and steel or paper mills away from cities, would also cause a strong heat burden to the local environment [6]. To deal with local thermal energy for potential domestic cooling and heating well, various thermal-energy-transportation technologies including chemical reactions [7], thermal energy storage using phase-change material [8], etc., have been widely developed [9] in the past few decades.
Among them, chemical reactions have been gathering momentum since they have a higher energy density in the transportation processes [10]. The reverse chemisorption or decomposition processes can be used for thermal energy storage and transportation in terms of different working pairs, e.g., lithium bromide–water (LiBr-H2O) [11], ammonia–water (NH3-H2O) [12], etc. Various working temperatures for sorption heat utilization can be achieved due to unique working characteristics. It is acknowledged that liquid–gas absorption technology was first introduced into the study of low-grade thermal energy transportation in the 1990s [13]. The LiBr-H2O working pair is commonly used for heat transportation and desiccant cooling, and the chemical solution could be transported at an ambient temperature [14]. Thus, the costs of pump, tube and other auxiliary equipment are able to be reduced when compared with other energy-transportation technologies at a low temperature [7]. Another typical absorption using a working pair of NH3-H2O is attractive and has a longer transportation distance with a lower mass flow rate than that of LiBr-H2O. Lin et al. [15] established a set of experimental NH3-H2O absorption systems that could be used for more than 100 °C waste-heat recovery. The system may be operated as an absorption heat pump and absorption chiller across the seasons. Akisawa et al. [16] established an absorption system in terms of various thermal-energy-transportation distances. It was indicated that the transportation distance had little influence on the liquid-solution concentration and the overall cooling performance. To gain the scale-up system performance of the absorption chiller, a system with large cooling power is simulated based on a real system design. The COP of the system could reach about 0.64 when the cooling power increases from 90 kW to 3517 kW. Later, a novel combined cooling heating and power system integrated with a NH3-H2O system was proposed by Han et al. [17]. Driven by low-grade heat, the Kalina cycle was used for the power generation of 641.3 kW, and the NH3-H2O working pair also produced a cooling power of 642.9 kW in the evaporator. The operation mode could be switched based on different heat demands and supplies. It demonstrates that absorption technologies have been widely investigated and could be applied for larger-scale heat or cold transportation.
As a comparable heat-driven technology, adsorption based on a solid–gas reaction has been gathering momentum to produce cooling effects in recent years, which is characterized by its simple structure, easiness to control, etc. [18,19]. Adsorption refrigeration technologies could be generally classified into physical adsorption, e.g., silica gel–water; activated carbon–ammonia and chemical adsorption, e.g., metal chloride–ammonia [20]; and metal hydride–hydrogen [21]. The silica gel–water sorption chiller was commercialized in the 1980s [22]. This adsorption refrigeration system can match low-temperature waste-heat recovery well even when its driving temperature is as low as 50 °C [23]. However, physical adsorption can only use chilled water for sensible heat transportation, which has no difference from common cold transportation using water. Comparably, a chemical-adsorption refrigeration system could have a similar working temperature while it could have the advantage of working at the lower evaporation temperature below 0 °C by using metal chloride–ammonia working pairs [24]. For long-distance thermal energy transportation, it is still a challenge for adsorption cooling systems in real working situations. The main barrier is to transport the liquid ammonia at the ambient temperature and keep the transportation pressure. Thus, the common sorption chiller is not enough to meet the requirement for long-distance transportation when various energy sources and demands also need to be considered, e.g., the low- and ultralow-temperature heat source. Thus, the compression-assisted adsorption chiller is a method that could produce cooling power by flexibly adjusting the internal working pressure of working pairs. A compressor is introduced into a basic adsorption type, which aims to utilize a relatively low heat source [25,26]. Gao et al. [27] investigated a pressure-boost thermochemical-adsorption cooling system to achieve continuous output. However, the cooling temperature of the proposed cycle is limited. Godefroy et al. [28] comprehensively compared three ways of coupling a solid–gas sorption refrigeration cycle with a Rankine cycle to create innovative hybrid cycles for power and refrigeration cogeneration. The most favorable configuration and adsorbent were screened and selected to meet the requirements in various applications. Later, their team developed the criteria to provide a complete overview of the energy and exergy performance of two novel hybrid sorption cycles, which was regarded as the basis for future experimental investigation [29].
To the authors’ best knowledge, research on compression-assisted adsorption chillers (CAACs) for thermal energy transportation has been rarely reported, and it usually has a low heat-source temperature when compared with common absorption systems. This work aims to investigate the technical feasibility of CAACs for cold transportation. The common low-temperature chloride–ammonia working pairs are used for the analysis of low- or ultralow-temperature heat recovery. The compression processes are used to reduce the desorption temperature due to the limited heat-source temperature or pressure loss during transportation, which results in a failure to drive adsorption working processes. The framework of this paper is illustrated as follows. The concept of a CAAC system for cold transportation is proposed and described in Section 2. The methodologies based on the adsorption model are indicated in Section 3. The results and discussions for the performance analyses on the system are indicated in Section 4, followed by the conclusions in Section 5.
2. Concept of Adsorption Chiller for Cold Transportation
2.1. Working Processes and Thermal Cycles
The schematic diagram of the CAAC using metal chloride–ammonia for cold transportation is shown in Figure 1. It generally looks like a typical adsorption chiller, which is mainly composed of an adsorber, a condenser, an evaporator, a pump, a compressor and several ammonia valves. A pump is used to transport liquid ammonia from the heat source to the places of the end users. The compressor is used to adjust the working pressure when considering the gas ammonia returning back and adsorbed in the adsorber. The general working processes of the CAAC for cold transportation are illustrated as follows. In the desorption process, the adsorber is heated by the centralized low-grade heat source, i.e., hot water since the low-temperature heat source is assumed to be used in this work. The ammonia is gradually desorbed from the adsorption reactor to the condenser (point 1 to point 4). Then, the high-temperature ammonia in the condenser is condensed and releases heat to the ambient. For cold transportation, a pump is used for the liquid refrigerant through the pipeline, which usually could be arranged underground (point 4 to point 6). After transportation, the ammonia goes through the throttle valve and evaporates at an evaporating temperature, and thus it produces a cooling effect for end users (point 6 to point 8). In the sorption process, the ammonia gas is sorbed by the adsorber. If the gas pressure is not enough for transportation, a compressor is used to maintain the sorption pressure (point 8 to point 1). The thermal cycle of the adsorption cooling system for cold transportation is correspondingly shown in Figure 2. When hot water flows into the adsorber with a heat input of Qheat, the desorption process happens from point A at Th to point B at Tc. The condensed liquid ammonia is transported to places to provide cooling effects to end users in the evaporator at point C. The sorption process happens from point C to point D’. If sorption processes cannot happen from point C to D’, e.g., from point C to point D, the compressor can pressurize the internal reaction pressure for the sorption process.
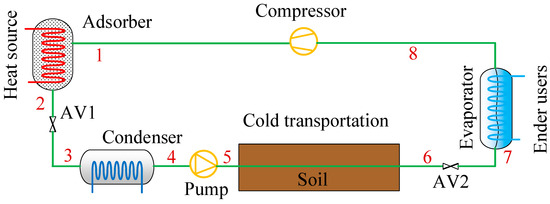
Figure 1.
Concept of compression-assisted adsorption chiller for cold transportation.
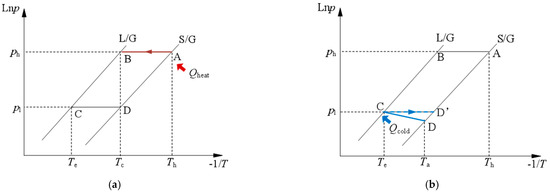
Figure 2.
Thermal cycles of compression-assisted adsorption chiller for cold transportation (a) desorption process; (b) adsorption process.
2.2. Solid Sorbent Selection
The sorbent selection of the CAAC for cold transportation is of great importance due to the different working requirements of the heat source and end users [30]. The equilibrium lines of metal chlorides reacting with ammonia can be found in Figure 3. The temperature–pressure Clausius–Clapeyron reaction equation of the chemical working pairs could refer to Equation (1), which indicates the relationship between the equilibrium reaction pressure and temperature based on the reaction enthalpy and entropy. It is acknowledged that the metal chlorides can be generally classified into high-temperature salts (HTSs), middle-temperature salts (MTSs) and low-temperature salts (LTSs) when considering the equilibrium reaction pressure of 1.5 MPa. The reaction temperature is then accordingly classified as above 150 °C, between 90 and 150 °C and below 90 °C. Thus, the common HTSs are MnCl2 and NiCl2. Common MTSs are SrCl2 and CaCl2, while common LTSs are BaCl2, NH4Cl and NaBr. The basic selection criteria are mainly dependent on three aspects. The safety problem of the CAAC is the first target of the adsorption cooling system. The second is for a high working efficiency, i.e., the coefficient of performance (COP) of the adsorption cycle, which is mainly related to the sorption capacity and reaction heat. Finally, the operation condition is also the key for adsorption cycles. The heat source in this work is hot water, which is set below 60 °C. Under this scenario, it can be found from Figure 3 that BaCl2, NH4Cl and NaBr are then screened out due to the suitable working-temperature ranges. The reaction equations of NaBr, NH4Cl and BaCl2 are shown in Equations (2)–(4), respectively, based on the reacted ammonia amount. Table 1 shows the main parameters of the selected chemical sorbents. The equilibrium desorption temperature is evaluated at a heat-sink temperature of 25 °C. The composite sorbent with expanded natural graphite (ENG), i.e., an 80% mass ratio of salt and 500 kg·m−3, is applied for the analysis due to its stability in the real system. Thermal conductivities, permeability and more information can be found in our previous research [31].
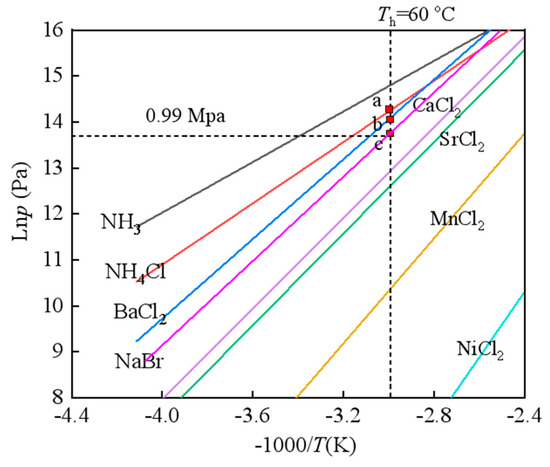
Figure 3.
The P-T equilibrium lines of typical metal chlorides reacting with ammonia (Points a, b, c depict the equilibrium state when heat source temperature is 25 °C).

Table 1.
The main thermal parameters of selected chemical adsorbents [32].
3. Methodology
The following assumptions are made for the simulation of the CAAC for cold transportation in this work:
- (1)
- The CAAC system is operated at steady conditions;
- (2)
- The isentropic efficiency of the pump is 0.8;
- (3)
- The isentropic efficiency of the compressor is 0.75;
- (4)
- The heat and pressure losses in the components (except for the pipelines) are neglected;
- (5)
- Ammonia liquid is transported by a straight pipe without pipe rise. Only the frictional head loss is considered. The length of the pipelines is preliminarily set as 2 km for a performance comparison with absorption systems [7]. The absolute roughness is 0.06 mm.
- (6)
- The heat-source temperatures range from 60 °C to 85 °C while the evaporation temperature is selected from 0 °C to 20 °C.
The temperature and pressure of the ammonia CAAC using NH4Cl-NH3 are presented in Table 2 as an example to illustrate the working processes. Since the thermodynamics analysis of the thermal cycle of the CAAC is targeted, 1 mol of ammonia is used for the calculation. The reaction enthalpy in Table 1 is used for the analysis, and the exergy is then calculated based on the enthalpy and evaporation latent heat of ammonia. It is indicated that the working pressure ranges from 11.51 bar to 6.07 bar when the selected working temperature increases from 60 °C to 10 °C.

Table 2.
The temperature and pressure of 1 mol ammonia of CAAC using NH4Cl-NH3 [35].
Energy and Exergy of CAAC
In the proposed CAAC system for cold transportation, the adsorber is driven by the sensible heat of hot water. The heat input of the adsorber of the CAAC is calculated as Equation (5), which is composed of the reaction heat and sensible heat:
The sensible heat part of the heat input in Equation (2) is then expressed as Equation (6):
where Th is the hot water temperature and Tref is the condensation temperature.
The heat-exergy input of the CAAC can be expressed as Equation (7):
where Tref is the reference temperature.
The power of the compressor is consumed for the CAAC with cold transportation, which can be expressed as Equation (8) in terms of the different working processes:
where k is the adiabatic index of ammonia and ηcom is the efficiency of the used compressor.
The pump consumption for the CAAC can be expressed as Equation (9):
where ηpu is the isentropic efficiency of the ammonia pump; ρam is the density of the ammonia; Htot is the hydraulic head, which is related to the height of the liquid and pressure resistance; and Vflux is the flow rate of the liquid ammonia.
It is admitted that the sorbent and sorbate cannot be fully reacted due to the working conditions in the real working condition. Thus, the global conversion rate (ψcon) is used to indicate the incompleteness of the reaction, which could be defined according to Equation (10):
where Xrea and Xi are the sorption capacity under real and ideal working conditions, respectively.
The cold output amount and exergy of the CAAC can be expressed as Equations (11) and (12), respectively:
where Te is the evaporation temperature.
The energy and exergy efficiencies of the CAAC for cold transportation can be expressed as Equations (13) and (14):
4. Results and Discussion
4.1. Thermodynamic Analysis
To generally understand the thermal performance of the CAAC with cold transportation, the NaBr-NH3 working pair is first used for the thermal analysis. Figure 4 indicates the theoretical thermal performance of the CAAC when the evaporation temperature increases from 0 °C to 5 °C, in which Figure 4a,b present the COP and exergy efficiency, respectively. The performance of the CAAC with or without transportation is compared to indicate the difference in using or not using cold transportation technology. It is indicated that the COP and exergy efficiency increase with the increase in the evaporation temperature due to the reduction in the internal working-pressure potential of the working pairs. The COP of the CAAC ranges from 0.425 to 0.459 without considering cold transportation, which is 4.7% higher than that with cold transportation. This is mainly because the energy consumption of the pump is not that high when compared with that without cold transportation in the selected 2 km. Additionally, the exergy efficiency of the CAAC without cold transportation increases from 0.22 to 0.31. It is demonstrated that cold transportation has a larger influence on the exergy loss of the CAAC, which leads to a 30% decrement in the exergy efficiency. This could be mainly attributed to the fact that electricity consumption accounts for a larger part of the heat-exergy input. Then, the thermal performance of the CAAC with cold transportation is compared in terms of different chemical working pairs, i.e., NaBr-NH3, NH4Cl-NH3 and BaCl2-NH3, which are shown in Figure 5. Among these working pairs, NH4Cl-NH3 shows the highest COP and exergy efficiency due to its relatively high specific reaction heat and high sorption capacity. Comparably, the CAAC by using the NaBr-NH3 working pair shows the lowest working performance since its working capacity is relatively low. It reveals that by using different chemical working pairs, the COP of the CAAC with cold transportation ranges from 0.447 to 0.436 while the exergy efficiency increases from 0.42 to 0.546.
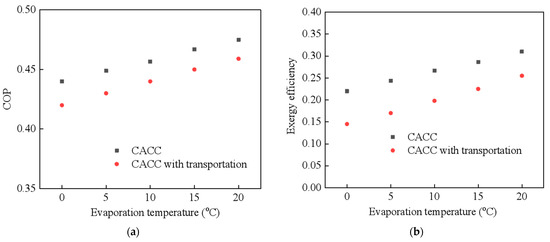
Figure 4.
Thermal performance of compression-assisted adsorption chiller vs. various ambient temperatures (a) COP; (b) exergy efficiency.
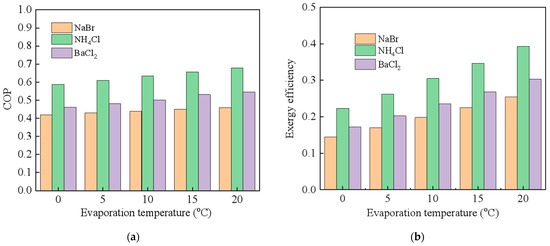
Figure 5.
Thermal performance of compression-assisted adsorption chiller for cold transportation in terms of different chemical working pairs (a) COP; (b) exergy efficiency.
4.2. Thermal Analysis Based on the Tested Sorption Capacity
The composite sorbent with expanded natural graphite (ENG) is then applied for the thermal analysis due to its improved heat and mass-transfer performance. It can lead to high thermal stability in the real system and thus prevent the sorption capacity from degrading due to swelling and agglomeration phenomena. A consolidation density of 500 kg·m−3 and a mass ratio of 80% are selected for the composite sorbents to test the sorption characteristics in this section [36]. Figure 6 shows a photo of the sorption-performance test bench for composite sorbents. The unit is mainly composed of a reactor; a refrigerant vessel; a differential-pressure transmitter; two pressure sensors; two cryostats, i.e., thermostat 1 and thermostat 2; etc. The refrigerant vessel acts as a condenser or evaporator, which is determined by the working conditions. The temperature of the sorption reactor is controlled by thermostat 1 while the refrigerant vessel is controlled by thermostat 2. The mass of the composite sorbents is measured by the balance (BSA124s), and its measuring error is 0.1 mg. The pressure difference between the liquid end and vapor end of the evaporator/condenser is measured by the smart differential-pressure transmitter with a testing error of 0.2%. The relative error of the sorption quantity of the composite sorbents is 0.38%. More details of the testing can be found in previous work [37]. The testing sorption capacity is used as the value of Xrea in Equation (10), which is similar to the sorption capacity under real working conditions.
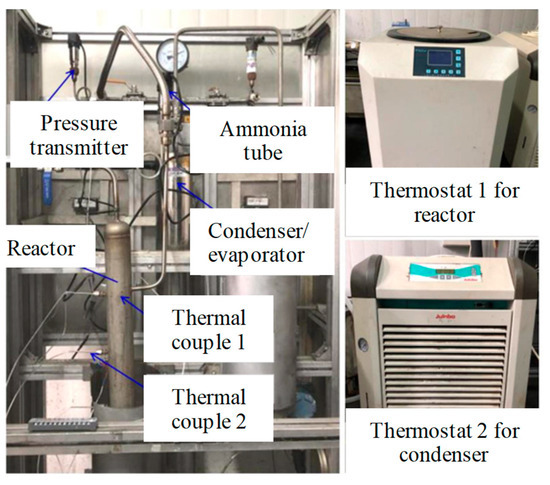
Figure 6.
Photo of sorption-performance test bench for composite sorbent [38].
Figure 7 indicates the sorption capacity of the NH4Cl-NH3 working pairs under different working conditions for the cooling output; Figure 7a shows the sorption capacity at evaporation temperatures from −10 °C to 20 °C while Figure 7b indicates the capacity at various heat-source temperatures from 50 °C to 80 °C. Temperature ranges for the testing are wider than those used for the theoretical analysis. This is mainly because the transportation processes for the CAAC would cause pressure loss for the analysis of the thermal performance. For NH4Cl-NH3, the maximum equilibrium sorption capacity is 0.867 kg·kg−1 when the reaction completely proceeds. It is indicated that the sorption capacity increases with the increase in the evaporation temperature and heat-source temperature. The highest global conversion rate and sorption capacity of the NH4Cl composite sorbent can reach up to 100% and 0.867 kg·kg−1. When the heat-source temperature is 60 °C, the testing sorption capacity ranges from 0.6 to 0.867 with an increase in the evaporation temperature from −10 °C to 20 °C. The increment in the sorption capacity could reach 44.5%. Additionally, when the evaporation temperature is 5 °C, the sorption capacity ranges from 0.62 to 0.867 with the increase in the heat-source temperature from 55 °C to 80 °C. The increment in the sorption capacity can reach 41.1%, which indicates that the evaporation temperature has a stronger influence on the sorption capacity when compared to that of the heat-source temperature. This is mainly due to the fact that the reaction potential could be much improved by increasing the evaporation temperature from the freezing condition to the air conditioning condition. Based on the testing sorption capacity, the COP of the CAAC using NH4Cl-NH3 for cold transportation is revealed in terms of various evaporation temperatures and heat-source temperatures, as shown in Figure 8. It is evident that the COP of the CAAC for cold transportation in a real testing performance is decreased when compared with the aforementioned theoretical thermal performance. This could be attributed to the fact that the composite NH4Cl has a higher heat input when considering the ENG and metal part of the reactor. Additionally, the COP can be slightly improved when the heat source is higher than 70 °C, which could also be attributed to its sorption characteristics. Additionally, the COP can be greatly improved by increasing the evaporation temperature. For various evaporation temperatures and heat-source temperatures, the COP of the CAAC for cold transportation ranges from 0.4 to 0.56.
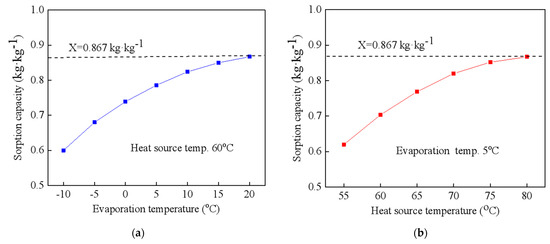
Figure 7.
Sorption quantity of NH4Cl-NH3 working pairs vs. (a) evaporation temperatures and (b) heat-source temperature.
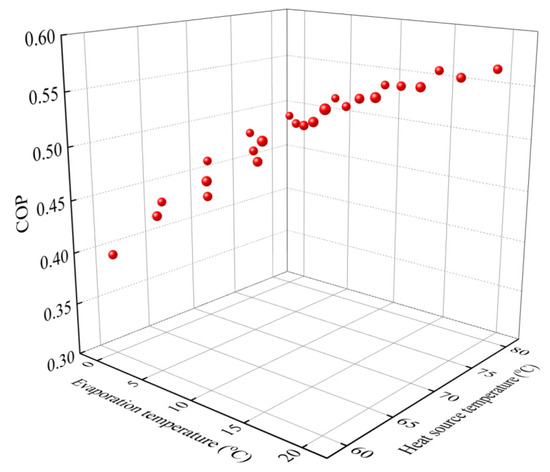
Figure 8.
COP of compression-assisted adsorption chiller using NH4Cl-NH3 vs. various evaporation and heat-source temperatures.
To have a general understanding of the CAAC for cold transportation, the CAAC using NH4Cl-NH3 is compared with other common LiBr-H2O and NH3-H2O absorption chillers, which are shown in Table 3. It is demonstrated that the NH4Cl-NH3 working pair has the lowest heat-source driving temperature, which could be even lower than 60 °C. Comparably, the NH3-H2O absorption chiller has the highest driving temperature, i.e., about 120 °C. For the thermal performance, the NH3-H2O absorption chiller may have the highest COP when compared with the other two types. However, with the increase in the heat-source temperature above 100 °C, the COP of the LiBr-H2O and NH4Cl-NH3 sorption type could be higher than that of the NH3-H2O absorption type, which is about 0.5. Additionally, LiBr-water could have the working modes of the air conditioning condition and dehumidification, which can work under normal system pressure while NH3-H2O absorption and NH4Cl-NH3 adsorption operate under both freezing and air conditioning conditions with high system pressure. Additionally, due to the cost of the chemical adsorbents, the initial system investment of NH4Cl-NH3 would be 1.5–2 times higher than that of the LiBr-H2O absorption system. To conclude, LiBr-H2O absorption could be the most effective way for cold and heat transportation to occur if the heat source is appropriate within its working range. Ammonia–water absorption is better to be used for freezing working conditions and domestic heating but has a high thermal-driving temperature. For the adsorption type, it would be suitable for a situation when the heat-source temperature is low, e.g., lower than 60 °C, and there is a need for freezing applications.

Table 3.
Comparison between CAAC and common absorption chillers for cold transportation.
5. Conclusions
In order to preliminarily analyze the potential working feasibility of the adsorption system for thermal energy transportation, a compression-assisted adsorption chiller is initially proposed for cold transportation, which aims to use low-grade heat. Various chemical working pairs are used for comparison. The conclusion can be yielded as follows:
- (1)
- The coefficient of performance of the compression-assisted adsorption chiller using sodium bromide–ammonia ranges from 0.425 to 0.459 without considering cold transportation. Additionally, the exergy efficiency of the compression-assisted adsorption chiller without cold transportation increases from 0.22 to 0.31. For different working pairs, the coefficient of performance and exergy efficiency of the compression-assisted adsorption chiller with cold transportation ranges from 0.447 to 0.436 and from 0.42 to 0.546, respectively.
- (2)
- For various operation conditions, the testing sorption capacity of ammonium chloride–ammonia ranges from 0.6 to 0.867. The coefficient of performance of the compression-assisted adsorption chiller for cold transportation in the real testing performance is decreased when compared with the aforementioned theoretical thermal performance. For various working conditions, the coefficient of performance of the compression-assisted adsorption chiller with cold transportation ranges from 0.4 to 0.56.
- (3)
- Lithium bromide–water absorption could be the most effective way for cold and heat transportation to occur if the heat source is appropriate. Ammonia–water absorption is better to be used for freezing working conditions and domestic heating but has a high thermal-driving temperature. For the adsorption type, it would be suitable for the situation when the heat-source temperature is low, e.g., lower than 60 °C, and there is a need for freezing applications.
Thermal-energy-transportation technology could be a potential tool in the near future. The development of adsorption technology would be effective for low- or ultralow-temperature heat-source utilization. Also, the liquid ammonia transportation for cooling could be integrated with next-generation hydrogen transportation and conversion technologies, which would be a more cost-effective way to gain a double-win solution.
Author Contributions
Conceptualization, S.J.; methodology, W.Z.; software, L.J.; validation, M.Y., B.L. and L.J.; formal analysis, M.Y. and G.X.; investigation, M.Y., S.J., W.Z. and L.J.; data curation, S.J.; writing—original draft, L.J.; project administration, L.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the National Natural Science Foundation of China (no. 52276022), the Science and Technology Project of the Jiangsu Province Market Supervision Administration (KJ2023001), the Open-sharing and Independent Research Project for Large-scale Scientific Instruments of Jiangsu Province (TC2023A062) and the Basic Research Funds for the Central University ‘Innovative Team of Zhejiang University’ under contract number (2-2050205-22-68).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
Authors Guangyue Xia and Baoqin Liu were employed by the company Jinan Energy Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Nomenclature
| Nomenclature | |
| Cp | Specific heat (kJ·kg−1·K−1) |
| CAAC | Compression-assisted adsorption chiller |
| COP | Coefficient of performance |
| E | Exergy (kJ) |
| ENG | Expanded natural graphite |
| HTS | High-temperature salt |
| h | Enthalpy (kJ·kg−1) |
| k | The adiabatic index |
| LTS | Low-temperature salt |
| M | Mass of composite sorbent (kg) |
| MTS | Middle-temperature salt |
| m | Mass of ammonia (kg) |
| P | Pressure (Pa) |
| Q | Heat (kg) |
| R | Gas constant (J·mol−1·K−1) |
| T | Temperature (°C) |
| V | Flow rate (kg·s−1) |
| w | Work input (kJ·kg−1) |
| X | Sorption capacity (kg·kg−1) |
| Greek symbols | |
| ΔH | Reaction enthalpy of sorbent (J·mol−1) |
| ∆S | Reaction entropy of sorbent (J·mol−1·K−1) |
| Ψ | Global conversion rate |
| Subscripts | |
| a | Adsorbent |
| am | Ammonia |
| c | Condensation |
| com | Compression |
| en | Energy |
| eva | Evaporation |
| ex | Exergy |
| h | Heat |
| i | Ideal |
| in | Input |
| ma | Mass |
| s | Sensible |
| out | Output |
| p | Pump |
| r | Reaction |
| re | Reactor |
| rea | Real |
| tot | Total |
References
- Zhang, W.Y.; Mehari, A.; Zhang, X.J.; Roskilly, A.; Jiang, L. Ammonia-based sorption thermal battery: Concepts, thermal cycles, applications, and perspectives. Energy Storage Mater. 2023, 62, 102930. [Google Scholar] [CrossRef]
- Hirsch, P.; Grochowski, M.; Duzinkiewicz, K. Decision support system for design of long distance heat transportation system. Energy Build. 2018, 173, 378–388. [Google Scholar] [CrossRef]
- Chiu, J.N.; Flores, J.C.; Martin, V.; Lacarrière, B. Industrial surplus heat transportation for use in district heating. Energy 2016, 110, 139–147. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Xu, X.; Zhang, S. Research progress of phase change cold storage materials used in cold chain transportation and their different cold storage packaging structures. J. Mol. Liq. 2020, 319, 114360. [Google Scholar] [CrossRef]
- Nakul, S.; Arunachala, U.C. Status, trends and significance of parabolic trough technology in the changing heat transportation scenario. Sol. Energy 2019, 187, 57–81. [Google Scholar] [CrossRef]
- Feng, T.; Ji, J.; Zhang, X. Research progress of phase change cold energy storage materials used in cold chain logistics of aquatic products. J. Energy Storage 2023, 60, 106568. [Google Scholar] [CrossRef]
- Gao, J.T.; Xu, Z.Y.; Chiu, J.N.W.; Su, C.; Wang, R.Z. Feasibility and economic analysis of solution transportation absorption system for long-distance thermal transportation under low ambient temperature. Energy Convers. Manag. 2019, 196, 793–806. [Google Scholar] [CrossRef]
- Şahan, N.; Mazman, M.; Kaypmaz, C.; Beyhan, B.; Paksoy, H. Flexible phase change material packages for green transportation vehicles. J. Power Sources 2023, 576, 233147. [Google Scholar] [CrossRef]
- Qi, T.; Ji, J.; Zhang, X.; Liu, L.; Xu, X.; Ma, K.; Gao, Y. Research progress of cold chain transport technology for storage fruits and vegetables. J. Energy Storage 2022, 56, 105958. [Google Scholar] [CrossRef]
- Lovegrove, K.; Luzzi, A.; Soldiani, I.; Kreetz, H. Developing ammonia based thermochemical energy storage for dish power plants. Sol. Energy 2004, 76, 331–337. [Google Scholar] [CrossRef]
- Jo, Y.K.; Kim, J.-K.; Lee, S.G.; Kang, Y.T. Development of type 2 solution transportation absorption system for utilizing LNG cold energy. Int. J. Refrig. 2007, 30, 978–985. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, R.; Luo, L.; Xia, Z.; Lin, P. Transportation of low-grade thermal energy over long distance by ammonia-water absorption. Chin. Sci. Bull. 2009, 54, 948–957. [Google Scholar] [CrossRef]
- Kang, Y.; Akisawa, A.; Sambe, Y.; Kashiwagi, T. Absorption heat pump systems for solution transportation at ambient temperature—STA cycle. Energy 2000, 25, 355–370. [Google Scholar] [CrossRef]
- Dou, P.; Jia, T.; Chu, P.; Dai, Y.; Shou, C. Performance analysis of no-insulation long distance thermal transportation system based on single-stage absorption-resorption cycle. Energy 2022, 243, 123125. [Google Scholar] [CrossRef]
- Lin, P.; Wang, R.Z.; Xia, Z.Z.; Ma, Q. Experimental investigation on heat transportation over long distance by ammonia–water absorption cycle. Energy Convers. Manag. 2009, 50, 2331–2339. [Google Scholar] [CrossRef]
- Akisawa, A.; Watanabe, F.; Enoki, K.; Takei, T. Performance of thermal energy transportation based on absorption heat pump cycle over 200m distance—Solution transportation absorption chiller. Appl. Therm. Eng. 2017, 127, 1200–1205. [Google Scholar] [CrossRef]
- Han, B.-C.; Cheng, W.-L.; Li, Y.-Y.; Nian, Y.-L. Thermodynamic analysis of heat driven Combined Cooling Heating and Power system (CCHP) with energy storage for long distance transmission. Energy Convers. Manag. 2017, 154, 102–117. [Google Scholar] [CrossRef]
- Jiang, L.; Ji, Y.; Shi, W.; Fan, Y.; Wang, R.; Zhang, X.; Roskilly, A. Compression-assisted adsorption thermal battery based on composite sorbent for heat supply in alpine cold region. J. Energy Storage 2023, 63, 107033. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, T. Energy and exergy analyses of ammoniated salts based thermochemical sorption heat storage system. J. Energy Storage 2022, 52, 104670. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, T.; Yu, N.; Wang, C.Y. Experimental investigation on a strontium chloride/ammonia sorption heat storage system. Appl. Therm. Eng. 2023, 219, 119478. [Google Scholar] [CrossRef]
- Bao, H.; Wang, Y.; Charalambous, C.; Lu, Z.; Wang, L.; Wang, R.; Roskilly, A.P. Chemisorption cooling and electric power cogeneration system driven by low grade heat. Energy 2014, 72, 590–598. [Google Scholar] [CrossRef]
- El-Ghetany, H.H.; Omara, M.A.; Abdelhady, R.G.; Abdelaziz, G.B. Design of silica gel/water adsorption chiller powered by solar energy for air conditioning applications. J. Energy Storage 2023, 63, 107055. [Google Scholar] [CrossRef]
- Pan, Q.W.; Liu, L.; Wang, B.; Xu, J.; Ge, T.S. Design and experimental study on a small-scale silica gel/water adsorption chiller with heat and mass recovery scheme for solar energy use. Sol. Energy 2023, 252, 91–100. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, D.H.; Yoon, S.H. Transient modeling of a chemisorption heat pump using ammonia with expanded graphite–NaBr. Appl. Therm. Eng. 2023, 234, 121233. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, W. A hybrid compression-assisted absorption thermal battery with high energy storage density/efficiency and low charging temperature. Appl. Energy 2021, 282, 116068. [Google Scholar] [CrossRef]
- Wu, W.; Ding, Z.; Sui, Y.; Leung, M. Comparative dynamic performance of hybrid absorption thermal batteries using H2O/1,3-dimethylimidazolium dimethylphosphate. Energy Convers. Manag. 2021, 228, 113690. [Google Scholar] [CrossRef]
- Gao, P.; Hu, H.; Jin, S.; Wang, S.; Chen, Y.; Wu, W.; Yang, Q.; Zhu, F.; Wang, L. Solar-driven compression-assisted desorption chemisorption refrigeration/cold energy storage system. Energy Convers. Manag. 2022, 258, 115474. [Google Scholar] [CrossRef]
- Godefroy, A.; Perier-Muzet, M.; Mazet, N. Thermodynamic analyses on hybrid sorption cycles for low-grade heat storage and cogeneration of power and refrigeration. Appl. Energy 2019, 255, 113751. [Google Scholar] [CrossRef]
- Godefroy, A.; Perier-Muzet, M.; Mazet, N. Novel hybrid thermochemical cycles for low-grade heat storage and autothermal power generation: A thermodynamic study. Appl. Energy 2020, 270, 115111. [Google Scholar] [CrossRef]
- Jiang, L.; Roskilly, A.P. Thermal conductivity, permeability and reaction characteristic enhancement of ammonia solid sorbents: A review. Int. J. Heat Mass Transf. 2019, 130, 1206–1225. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.W.; Jin, Z.Q.; Wang, R.Z.; Dai, Y.J. Effective thermal conductivity and permeability of compact compound ammoniated salts in the adsorption/desorption process. Int. J. Therm. Sci. 2013, 71, 103–110. [Google Scholar] [CrossRef]
- Wang, R.Z.; Wang, L.W.; Wu, J.Y. Adsorption Refrigeration Technology—Theory and Application; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Yu, Y.; Wang, L.W.; An, G.L. Experimental study on sorption and heat transfer performance of NaBr-NH3 for solid sorption heat pipe. Int. J. Heat Mass Transf. 2018, 117, 125–131. [Google Scholar] [CrossRef]
- Jiang, L.; Roskilly, A.P.; Wang, R.Z.; Wang, L.W.; Lu, Y. Analysis on innovative modular sorption and resorption thermal cell for cold and heat cogeneration. Appl. Energy 2017, 204, 767–779. [Google Scholar] [CrossRef]
- Sajid, M.U.; Bicer, Y. Performance Assessment of Spectrum Selective Nanofluid-Based Cooling for a Self-Sustaining Greenhouse. Energy Technol. 2021, 9, 2000875. [Google Scholar] [CrossRef]
- Yan, T.; Wang, R.Z.; Li, T.X. Experimental investigation on thermochemical heat storage using manganese chloride/ammonia. Energy 2018, 143, 562–574. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, L.W.; Wang, R.Z. Investigation on thermal conductive consolidated composite CaCl2 for adsorption refrigeration. Int. J. Therm. Sci. 2014, 81, 68–75. [Google Scholar] [CrossRef]
- Xie, X.; Jin, S.; Gao, P.; Wu, W.; Yang, Q.; Wang, L. Ammonia-based hybrid chemisorption-compression heat pump for high-temperature heating. Appl. Therm. Eng. 2023, 232, 121081. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).