Control of Cement Timing, Mineralogy, and Texture on Hydro-chemo-mechanical Coupling from CO2 Injection into Sandstone: A Synthesis
Abstract
:1. Introduction
2. Geology Background of Morrow B Sandstone
3. Methods
3.1. Core Sample Selection and Preparation
3.2. Sample Characterizations
3.2.1. Petrography
3.2.2. Microprobe Analysis
3.2.3. Porosity
3.2.4. X-ray Micro-computed Tomography (X-ray μCT)
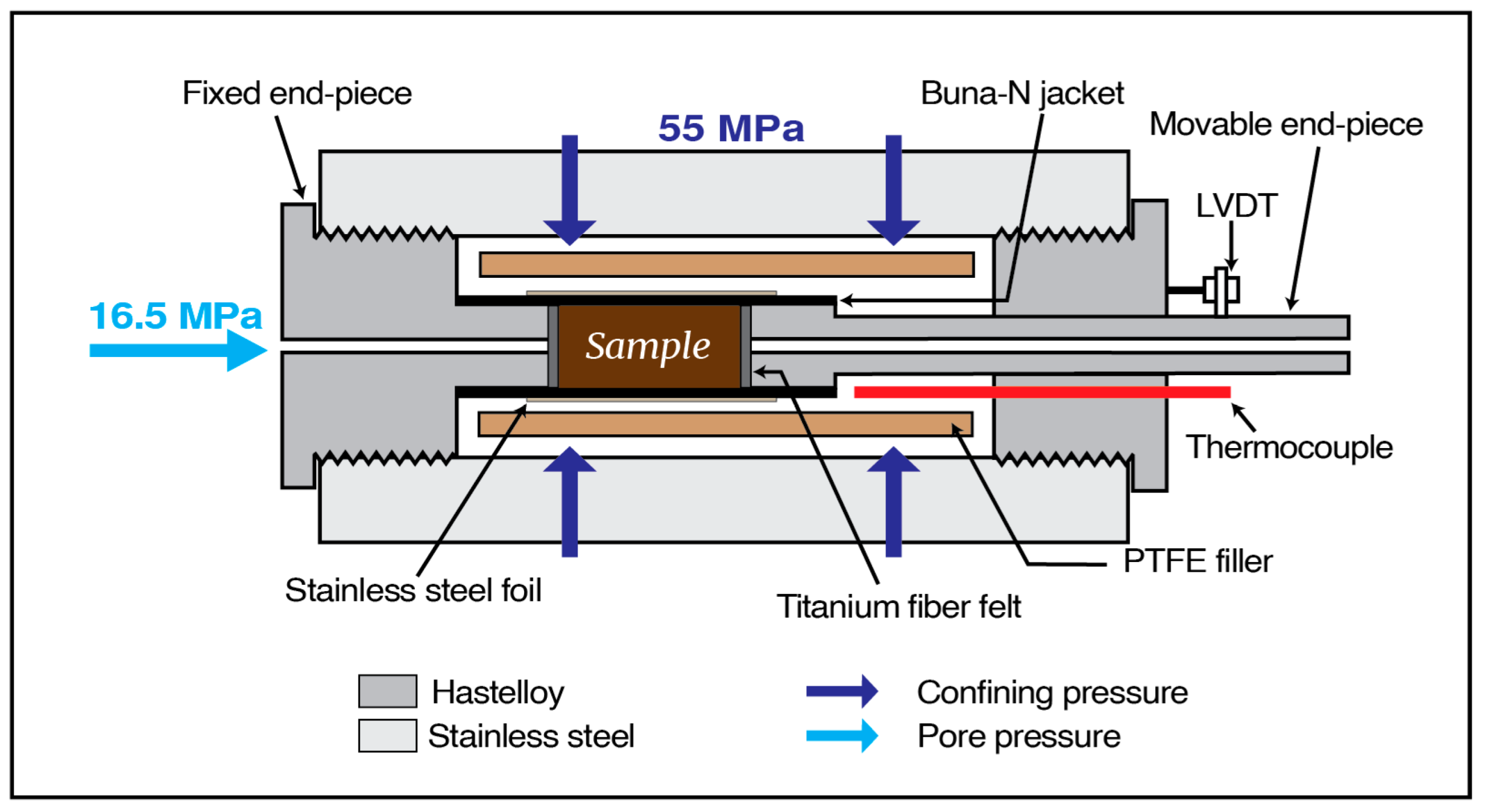
3.3. Hydro-chemo-mechanical (HCM) Coupling Experiments
3.3.1. Flow-through Experiment
3.3.2. Geomechanical Tests
3.3.3. Creep/Flow-through Experiment
4. Results
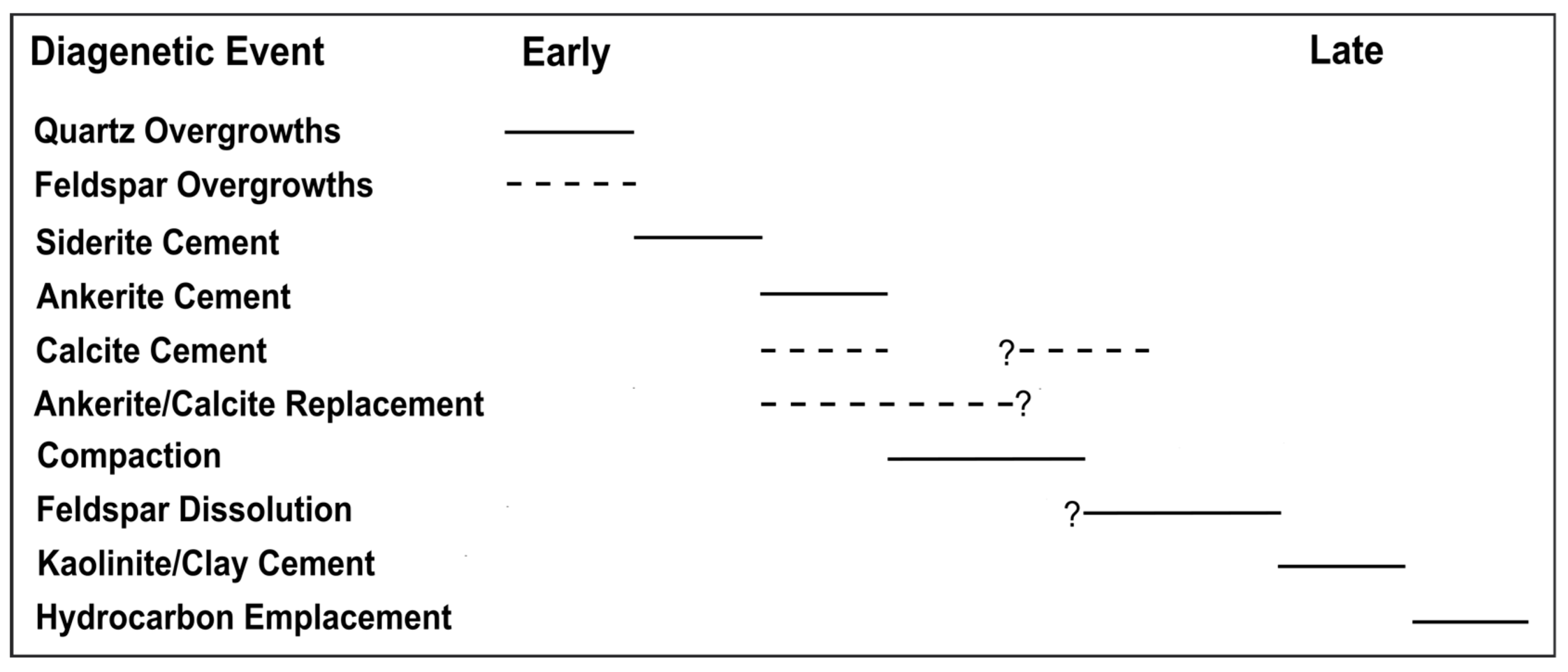
4.1. Paragenetic Sequence
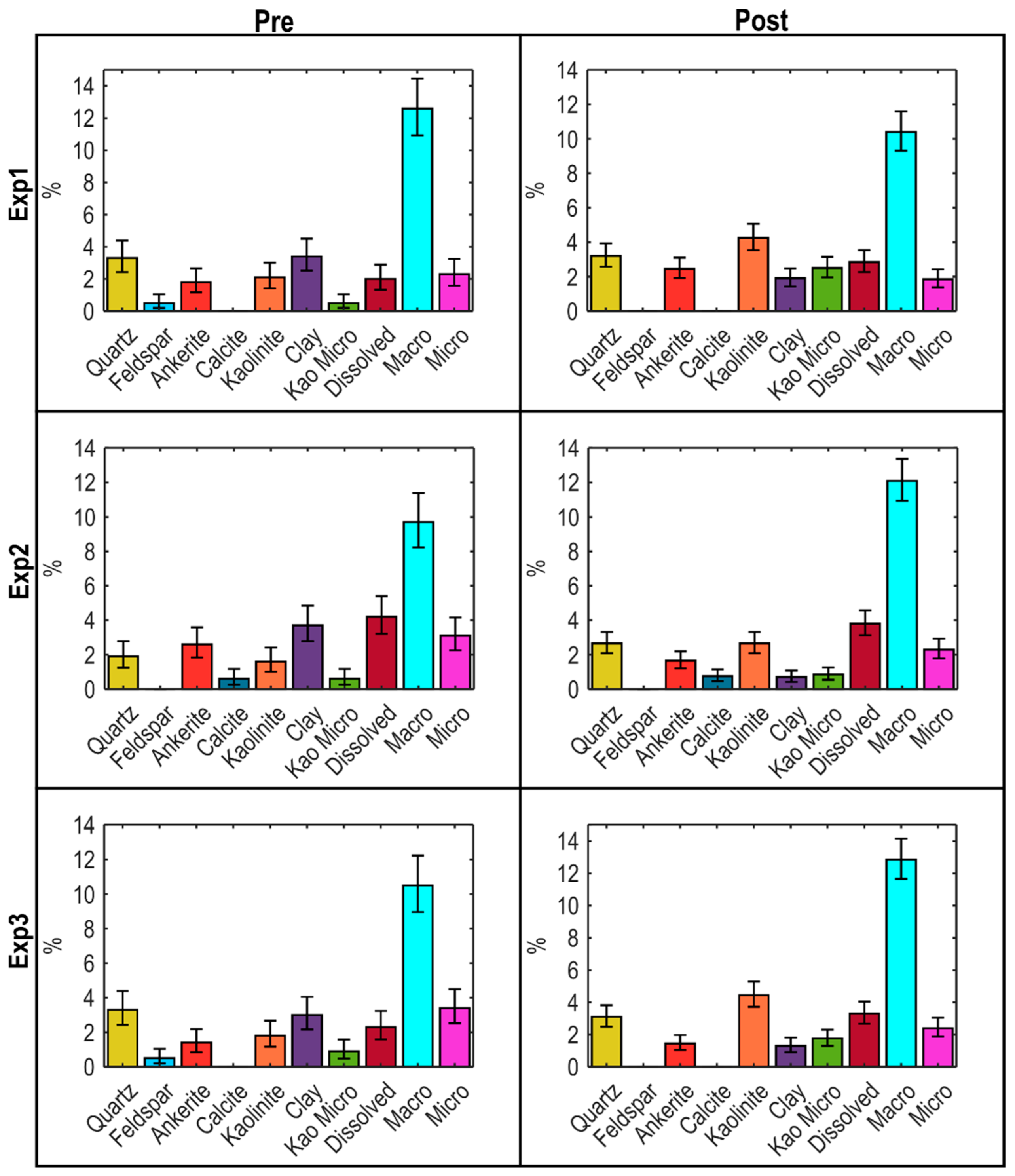
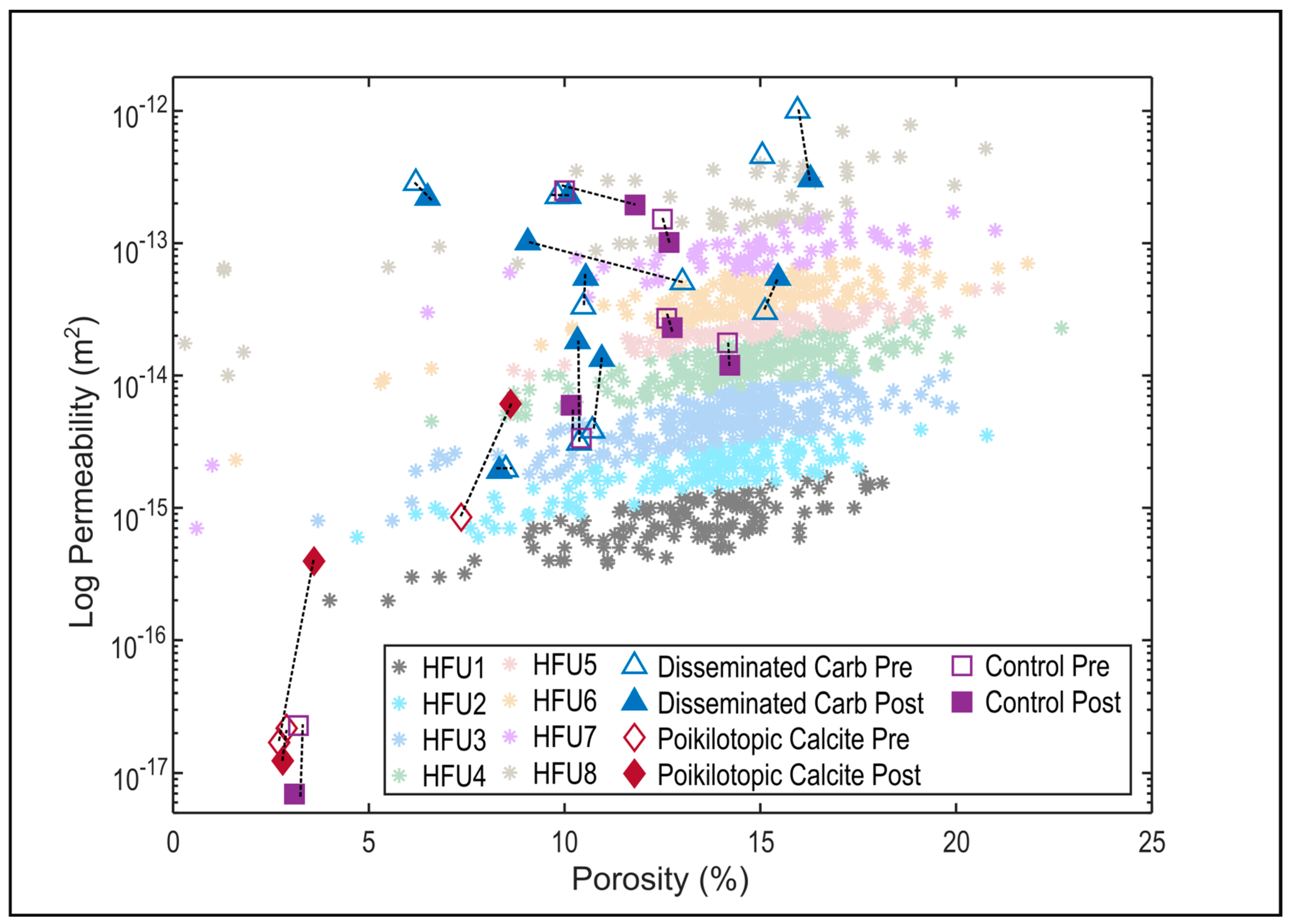
4.2. Changes in Hydraulic Properties
4.3. Changes in Mineralogy and Fluid Composition
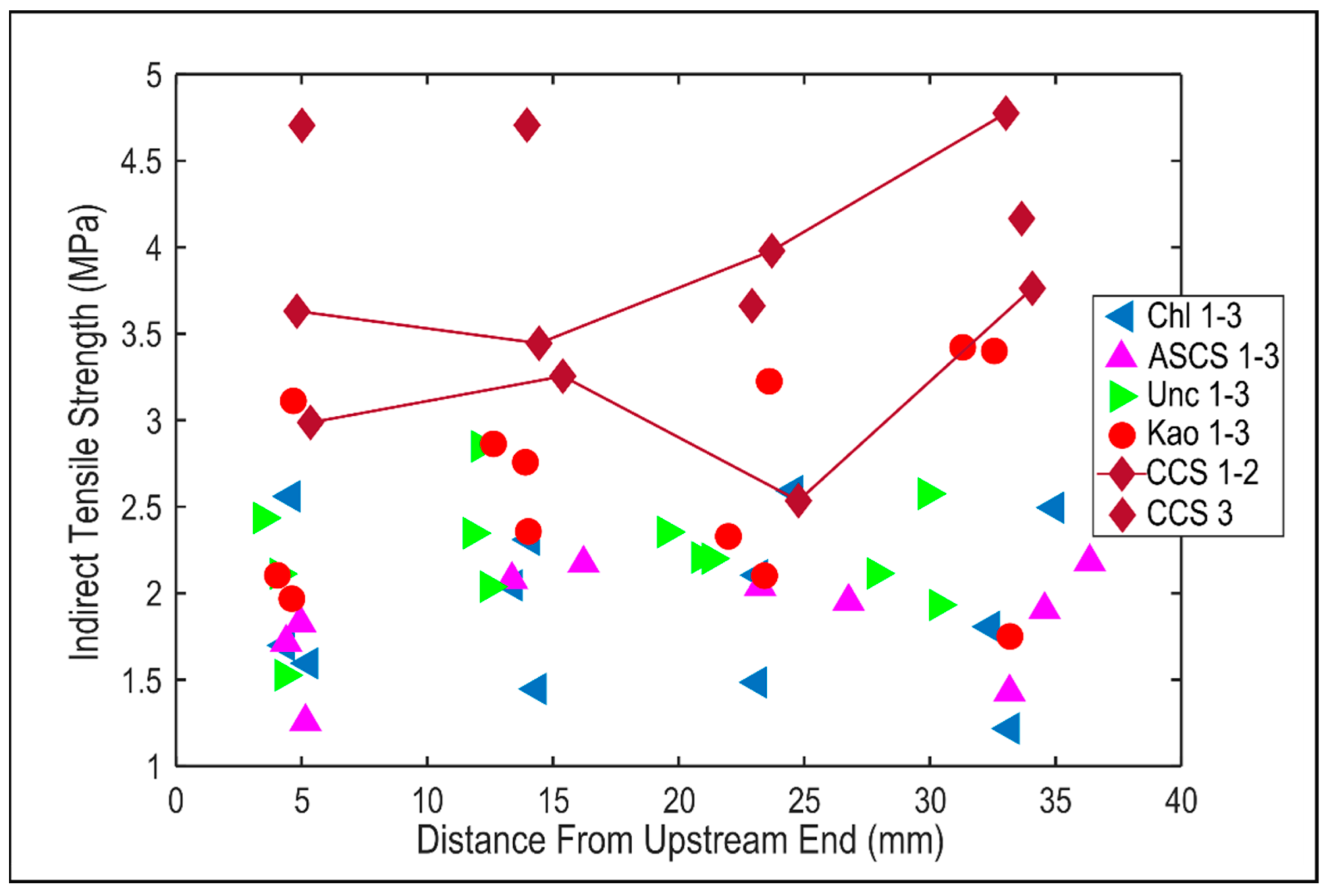
4.4. Changes in Geomechanical Properties
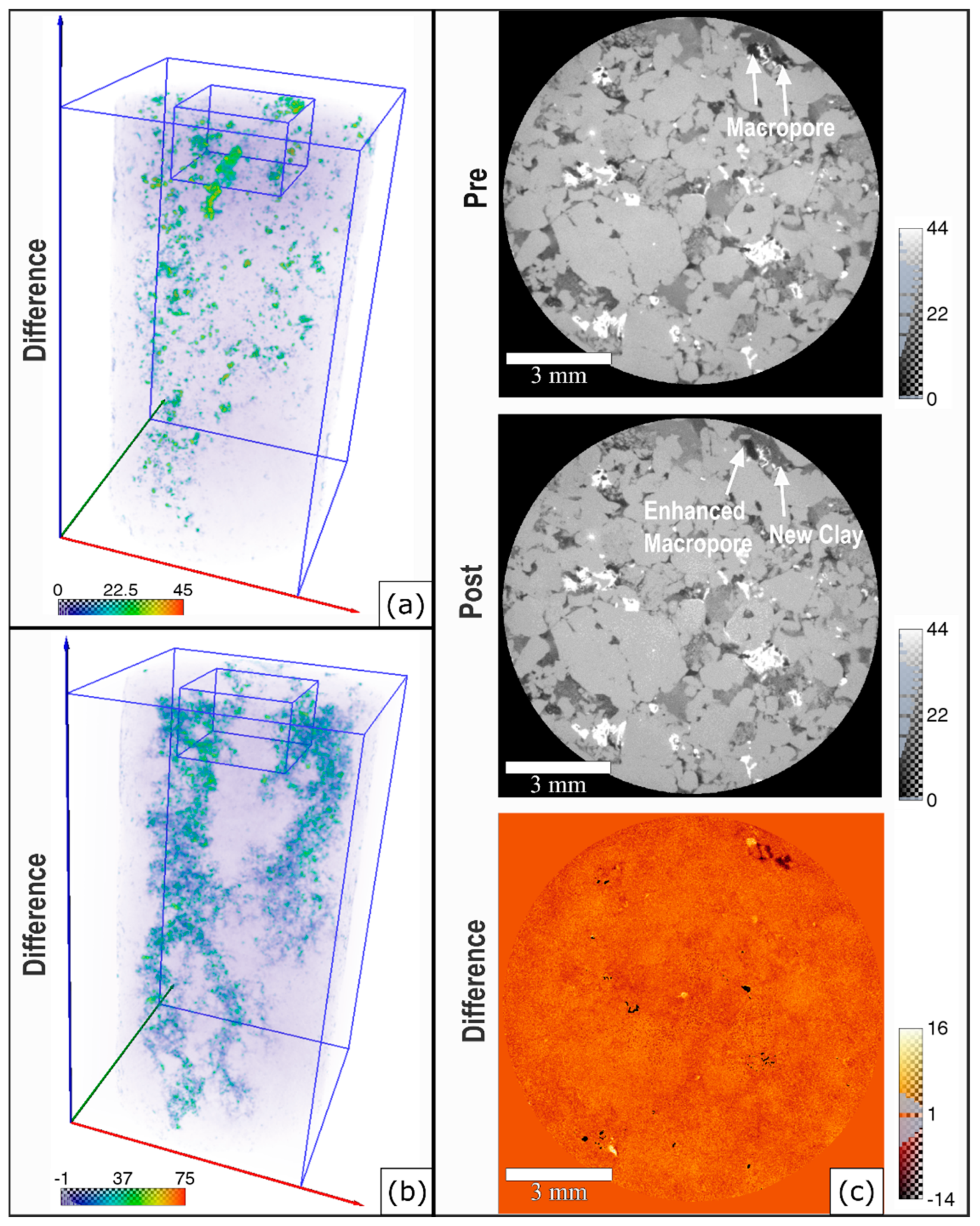
4.5. Changes in X-ray μCT Images
4.6. Additional Analyses
5. Discussion
6. Conclusions
- Primary mineralogy of sandstones should be dominated by felsic minerals to limit reactivity.
- Preference should be given to early-stage, non-reactive cements such as quartz overgrowths. Given its limited solubility in CO2-enriched saline waters, anhydrite may also be a reasonable early cement.
- If reactive cements (i.e., carbonate and Fe-rich chlorite cements) are present, it is best if they formed late in the diagenetic sequence and are not load-bearing.
- If non-reactive, load-bearing cements and late-stage, reactive cements are present, reservoir performance (injectivity) may significantly improve without losing reservoir mechanical integrity.
- For long-term injection programs, it appears that CO2 will inhibit subcritical fractures and, thus, mechanical weakening of the reservoir.
- Minor dissolution of cements decreases ultrasonic velocities by up to 300 m/s, which may create ambiguity in monitoring the plume of supercritical CO2.
- Degradation of seismic velocities during injection may suggest a loss of strength but is not a strong indicator; local rock texture controls whether changes in velocities will correspond to changes in strength.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rezk, M.G.; Foroozesh, J.; Zivar, D.; Mumtaz, M. CO2 storage potential during CO2 enhanced oil recovery in sandstone reservoirs. J. Nat. Gas Sci. Eng. 2019, 66, 233–243. [Google Scholar] [CrossRef]
- Celia, M.A. Geological storage of captured carbon dioxide as a large-scale carbon mitigation option. Water Resour. Res. 2017, 53, 3527–3533. [Google Scholar] [CrossRef]
- Dai, Z.; Viswanathan, H.; Xiao, T.; Middleton, R.; Pan, F.; Ampomah, W.; Yang, C.; Zhou, Y.; Jia, W.; Lee, S.-Y.; et al. CO2 Sequestration and Enhanced Oil Recovery at Depleted Oil/Gas Reservoirs. Energy Procedia 2017, 114, 6957–6967. [Google Scholar] [CrossRef]
- Dai, Z.; Middleton, R.; Viswanathan, H.; Fessenden-Rahn, J.; Bauman, J.; Pawar, R.; Lee, S.-Y.; McPherson, B. An Integrated Framework for Optimizing CO2 Sequestration and Enhanced Oil Recovery. Environ. Sci. Technol. Lett. 2014, 1, 49–54. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Elangovan, D. A Brief Review of Hydrogen Production Methods and Their Challenges. Energies 2023, 16, 1141. [Google Scholar] [CrossRef]
- Godin, J.; Liu, W.; Ren, S.; Xu, C.C. Advances in recovery and utilization of carbon dioxide: A brief review. J. Environ. Chem. Eng. 2021, 9, 105644. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Wellman, T.P.; Grigg, R.B.; McPherson, B.J.; Svec, R.K.; Lichtner, P.C. Evaluation of CO2-brine-reservoir rock interaction with laboratory flow tests and reactive transport modeling. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar]
- Dai, Z.; Xu, L.; Xiao, T.; McPherson, B.; Zhang, X.; Zheng, L.; Dong, S.; Yang, Z.; Soltanian, M.R.; Yang, C.; et al. Reactive chemical transport simulations of geologic carbon sequestration: Methods and applications. Earth-Sci. Rev. 2020, 208, 103265. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rabiei, M.; Rasouli, V.; Rezaee, R.; Fakhari, N. Impact of geochemical and geomechanical changes on CO2 sequestration potential in sandstone and limestone aquifers. Greenh. Gases Sci. Technol. 2019, 9, 905–923. [Google Scholar] [CrossRef]
- Zheng, H.; Feng, X.-T.; Li, S.; Qiu, S.; Wang, B. Coupled chemo-mechanical behavior of CO2 mineral trapping in the reservoir sandstones during CO2–EWR. Environ. Earth Sci. 2019, 78, 481. [Google Scholar] [CrossRef]
- Gaus, I.; Audigane, P.; André, L.; Lions, J.; Jacquemet, N.; Durst, P.; Czernichowski-Lauriol, I.; Azaroual, M. Geochemical and solute transport modelling for CO2 storage, what to expect from it? Int. J. Greenh. Gas Control. 2008, 2, 605–625. [Google Scholar] [CrossRef]
- Roded, R.; Paredes, X.; Holtzman, R. Reactive transport under stress: Permeability evolution in deformable porous media. Earth Planet. Sci. Lett. 2018, 493, 198–207. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.; Grigg, R.B.; Cather, M.; Gragg, E.; Will, R.A.; White, M.; Moodie, N.; Dai, Z. Performance assessment of CO2-enhanced oil recovery and storage in the Morrow reservoir. Géoméch. Geophys. Geo-Energy Geo-Resour. 2017, 3, 245–263. [Google Scholar] [CrossRef]
- Ampomah, W.; Balch, R.S.; Cathar, M.; Will, R.; Lee, S.Y.; Dai, Z. Performance of CO2-EOR and Storage Processes Under Uncertainty. In Proceedings of the SPE Europec featured at 78th EAGE Conference and Exhibition, Vienna, Austria, 30 May–2 June 2016. [Google Scholar]
- Pan, F.; McPherson, B.J.; Dai, Z.; Jia, W.; Lee, S.-Y.; Ampomah, W.; Viswanathan, H.; Esser, R. Uncertainty analysis of carbon sequestration in an active CO2-EOR field. Int. J. Greenh. Gas Control 2016, 51, 18–28. [Google Scholar] [CrossRef]
- William Ampomah, R.S.B.; Grigg, R.B.; McPherson, B.; Will, R.A.; Lee, S.-Y.; Dai, Z.; Pan, F. Co-optimization of CO2-EOR and storage processes in mature oil reservoirs. Greenh. Gases Sci. Technol. 2017, 7, 128–142. [Google Scholar] [CrossRef]
- Dai, Z.; Viswanathan, H.; Middleton, R.; Pan, F.; Ampomah, W.; Yang, C.; Jia, W.; Xiao, T.; Lee, S.-Y.; McPherson, B.; et al. CO2 Accounting and Risk Analysis for CO2 Sequestration at Enhanced Oil Recovery Sites. Environ. Sci. Technol. 2016, 50, 7546–7554. [Google Scholar] [CrossRef]
- Khosravi, M.H.; Kheirollahi, M.; Liu, B.; Gentzis, T.; Liu, K.; Morta, H.B.; Ostadhassan, M. Physico-chemo-mechanical impact of sc-CO2 on shale formations: The Bakken. Gas Sci. Eng. 2023, 112, 204945. [Google Scholar] [CrossRef]
- Luquot, L.; Gouze, P. Experimental determination of porosity and permeability changes induced by injection of CO2 into carbonate rocks. Chem. Geol. 2009, 265, 148–159. [Google Scholar] [CrossRef]
- Rinehart, A.J.; Dewers, T.A.; Broome, S.T.; Eichhubl, P. Effects of CO2 on mechanical variability and constitutive behavior of the Lower Tuscaloosa Formation, Cranfield Injection Site, USA. Int. J. Greenh. Gas Control 2016, 53, 305–318. [Google Scholar] [CrossRef]
- Wang, K.; Ma, L.; Taylor, K.G. Microstructure changes as a response to CO2 storage in sedimentary rocks: Recent developments and future challenges. Fuel 2023, 333, 126403. [Google Scholar] [CrossRef]
- Rathnaweera, T.D.; Ranjith, P.G.; Perera, M.S.A.; Ranathunga, A.S.; Wanniarachchi, W.A.M.; Yang, S.Q.; Lashin, A.; Arifi, N.A. An experimental investigation of coupled chemico-mineralogical and mechanical changes in varyingly-cemented sandstones upon CO2 injection in deep saline aquifer environments. Energy 2017, 133, 404–414. [Google Scholar] [CrossRef]
- Carroll, S.A.; McNab, W.W.; Dai, Z.; Torres, S.C. Reactivity of Mount Simon Sandstone and the Eau Claire Shale Under CO2 Storage Conditions. Environ. Sci. Technol. 2013, 47, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xiao, T.; Wu, Z.; Dai, Z.; McPherson, B. Impact of Mineral Reactive Surface Area on Forecasting Geological Carbon Sequestration in a CO2-EOR Field. Energies 2021, 14, 1608. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Luhmann, A.J.; Kong, X.-Z.; Saar, M.O.; Seyfried, W.E. CO2 sequestration in feldspar-rich sandstone: Coupled evolution of fluid chemistry, mineral reaction rates, and hydrogeochemical properties. Geochim. Cosmochim. Acta 2015, 160, 132–154. [Google Scholar] [CrossRef]
- Pearce, J.K.; Dawson, G.K.W.; Sommacal, S.; Golding, S.D. Micro CT and Experimental Study of Carbonate Precipitation from CO2 and Produced Water Co-Injection into Sandstone. Energies 2021, 14, 6998. [Google Scholar] [CrossRef]
- Kaufmann, G.; Dreybrodt, W. Calcite dissolution kinetics in the system CaCO3–H2O–CO2 at high undersaturation. Geochim. Cosmochim. Acta 2007, 71, 1398–1410. [Google Scholar] [CrossRef]
- Pandey, S.N.; Chaudhuri, A.; Kelkar, S.; Sandeep, V.R.; Rajaram, H. Investigation of permeability alteration of fractured limestone reservoir due to geothermal heat extraction using three-dimensional thermo-hydro-chemical (THC) model. Geothermics 2014, 51, 46–62. [Google Scholar] [CrossRef]
- Zhou, X.P.; Du, E.B.; Wang, Y.T. Thermo-hydro-chemo-mechanical coupling peridynamic model of fractured rock mass and its application in geothermal extraction. Comput. Geotech. 2022, 148, 104837. [Google Scholar] [CrossRef]
- Tian, S.; Zhou, J.; Xian, X.; Gan, Q.; Yang, K.; Zheng, Y.; Deng, G.; Zhang, F. Impact of supercritical CO2 exposure time on the porosity and permeability of dry and wet shale: The influence of chemo-mechanical coupling effects. Energy 2023, 270, 126905. [Google Scholar] [CrossRef]
- Espinoza, D.N.; Jung, H.; Major, J.R.; Sun, Z.; Ramos, M.J.; Eichhubl, P.; Balhoff, M.T.; Choens, R.C.; Dewers, T.A. CO2 charged brines changed rock strength and stiffness at Crystal Geyser, Utah: Implications for leaking subsurface CO2 storage reservoirs. Int. J. Greenh. Gas Control 2018, 73, 16–28. [Google Scholar] [CrossRef]
- Rathnaweera, T.; Ranjith, P.; Perera, M.; Haque, A.; Lashin, A.; Al Arifi, N.; Chandrasekharam, D.; Yang, S.; Xu, T.; Wang, S.; et al. CO2-induced mechanical behaviour of Hawkesbury sandstone in the Gosford basin: An experimental study. Mater. Sci. Eng. A 2015, 641, 123–137. [Google Scholar] [CrossRef]
- Rohmer, J.; Pluymakers, A.; Renard, F. Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth-Sci. Rev. 2016, 157, 86–110. [Google Scholar] [CrossRef]
- Lamy-Chappuis, B.; Angus, D.; Fisher, Q.J.; Yardley, B.W. The effect of CO2-enriched brine injection on the mechanical properties of calcite-bearing sandstone. Int. J. Greenh. Gas Control. 2016, 52, 84–95. [Google Scholar] [CrossRef]
- Hangx, S.; van der Linden, A.; Marcelis, F.; Bauer, A. The effect of CO2 on the mechanical properties of the Captain Sandstone: Geological storage of CO2 at the Goldeneye field (UK). Int. J. Greenh. Gas Control 2013, 19, 609–619. [Google Scholar] [CrossRef]
- Otu, S.; Rinehart, A.J.; Luhmann, A.J.; Simmons, J.; Mozley, P. Effects of CO2 on creep deformation in sandstones at carbon sequestration reservoir conditions: An experimental study. Int. J. Greenh. Gas Control. 2023, 129, 103970. [Google Scholar] [CrossRef]
- Simmons, J.; Rinehart, A.; Luhmann, A.; Mozley, P.; Heath, J.; Majumdar, B. Using petrographically observable microstructure to predict hydromechanical changes in a complex siliciclastic storage site during CO2 injection. Int. J. Greenh. Gas Control. 2022, 119, 103724. [Google Scholar] [CrossRef]
- Simmons, J.D.; Wang, S.; Luhmann, A.J.; Rinehart, A.J.; Heath, J.E.; Majumdar, B.S. Paragenetic controls on CO2-fluid-rock interaction and weakening in a macroporous-dominated sandstone. Appl. Geochem. 2023, 156, 105744. [Google Scholar] [CrossRef]
- Wu, Z.; Luhmann, A.J.; Rinehart, A.J.; Mozley, P.S.; Dewers, T.A.; Heath, J.E.; Majumdar, B.S. Chemo-mechanical Alterations Induced from CO2 Injection in Carbonate-Cemented Sandstone: An Experimental Study at 71 degrees C and 29 MPa. J. Geophys. Res. Solid Earth 2020, 125, e2019JB019096. [Google Scholar] [CrossRef]
- Gallagher, S. Depositional and Diagenetic Controls on Reservoir Heterogeneity: Upper Morrow Sandstone, Farnsworth Unit, Ochiltree County, Texas. Master’s Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2014. [Google Scholar]
- Rose-Coss, D. A refined depositional sequence stratographic and structural model for the reservoir and caprok intervals at the Farnsworth Unit, Ochilltree County, TX. Master’s Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2016. [Google Scholar]
- Krystinik, L.F.; Blakeney, B.A. Sedimentology of the Upper Morrow Formation in Eastern Colorado and Western Kansas; Rocky Mountain Association of Geologists: Denver, CO, USA, 1990. [Google Scholar]
- Gragg, E. Petroleum System Modeling of the Northwest Anadarko Basin: Implications for Carbon Storage. Master’s Thesis, New Mexico Institute of Mining and Technology, Socorro, NM, USA, 2016. [Google Scholar]
- Nelson, P.H.; Gianoutsos, N.J.; Ii, R.M.D. Underpressure in Mesozoic and Paleozoic rock units in the Midcontinent of the United States. AAPG Bull. 2015, 99, 1861–1892. [Google Scholar] [CrossRef]
- Sorenson, R.P. A dynamic model for the Permian Panhandle and Hugoton fields, western Anadarko basin. AAPG Bull. 2005, 89, 921–938. [Google Scholar] [CrossRef]
- Rasmussen, L.; Fan, T.; Rinehart, A.; Luhmann, A.; Ampomah, W.; Dewers, T.; Heath, J.; Cather, M.; Grigg, R. Carbon Storage and Enhanced Oil Recovery in Pennsylvanian Morrow Formation Clastic Reservoirs: Controls on Oil–Brine and Oil–CO2 Relative Permeability from Diagenetic Heterogeneity and Evolving Wettability. Energies 2019, 12, 3663. [Google Scholar] [CrossRef]
- Pettijohn, F.J.; Potter, P.E.; Siever, R. Sand and Sandstone; Springer: New York, NY, USA, 1987. [Google Scholar]
- Howarth, R.J. Improved estimators of uncertainty in proportions, point-counting, and pass-fail test results. Am. J. Sci. 1998, 298, 594–607. [Google Scholar] [CrossRef]
- Duan, Z.; Sun, R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar. Chem. Geol. 2003, 193, 257–271. [Google Scholar] [CrossRef]
- Darcy, H. Les Fontaines Publiques de la Ville de Dijon: Exposition et Application des Principes à Suivre et des Formules à Employer dans les Questions de Distribution d’Eau; Victor Dalmont: Paris, France, 1856; Volume 1. [Google Scholar]
- Bethke, C.M.; Yeakel, S. The Geochemists Workbench: Reference Manual; Aqueous Solutions, LLC: Champaign, IL, USA, 2013. [Google Scholar]
- Fjaer, E.; Holt, R.M.; Horsrud, P.; Raaen, A.M.; Risnes, R. (Eds.) Petroleum Related Rock Mechanics; Developments in Petroleum Science; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- D3967-95a; Standard Test Method for Splitting Tensile Strength of Intact Rock Core Specimens. ASTM International: West Conshohocken, PA, USA, 1995.
- Atkinson, B.K. Subcritical Crack Growth in Geological Materials. J. Geophys. Res. 1984, 89, 4077–4114. [Google Scholar] [CrossRef]
- Dove, P.M. Geochemical controls on the kinetics of quartz fracture at subcritical tensile stresses. J. Geophys. Res. Solid Earth 1995, 100, 22349–22359. [Google Scholar] [CrossRef]
- Ilgen, A.G.; Mook, W.M.; Tigges, A.B.; Choens, R.C.; Artyushkova, K.; Jungjohann, K.L. Chemical Controls on the Propagation Rate of Fracture in Calcite. Sci. Rep. 2018, 8, 16465. [Google Scholar] [CrossRef]
- Brzesowsky, R.H.; Hangx, S.J.T.; Brantut, N.; Spiers, C.J. Compaction Creep of Sands Due to Time-Dependent Grain Failure: Effects of Chemical Environment, Applied Stress, and Grain Size. J. Geophys. Res. Solid Earth 2014, 119, 7521–7541. [Google Scholar] [CrossRef]
- Hangx, S.J.T.; Spiers, C.J.; Peach, C.J. Creep of simulated reservoir sands and coupled chemical-mechanical effects of CO2 injection. J. Geophys. Res. Solid Earth 2010, 115, B09205. [Google Scholar] [CrossRef]
- Taifan, W.; Boily, J.F.; Baltrusaitis, J. Surface Chemistry of Carbon Dioxide Revisited. Surf. Sci. Rep. 2016, 71, 595–671. [Google Scholar] [CrossRef]
- Scholle, P.A.; Ulmer-Scholle, D.S. A Color Guide to the Petrography of Carbonate Rocks: Grains, Textures, Porosity, Diagenesis; American Association of Petroleum Geologists: Tulsa, OK, USA, 2003. [Google Scholar]




| Morrow B Sandstone Sample | Well | Depth (m) | HFU | Ave. Diameter (cm) | Ave. Length (cm) | Number of Samples |
|---|---|---|---|---|---|---|
| (MP1, MP2, MP3, MP4) | 13-14 | 2354.7–2355.8 | 5 | 3.71 | 7.20 | 4 |
| (Unc1, Unc2, Unc3) | 13-10A | 2340.7–2341.1 | 5 | 2.55 | 5.51 | 3 |
| Siderite-chlorite-cemented (Chl1, Chl2, Chl3) | 13-10A | 2337.45–2338.33 | 4 | 2.54 | 5.24 | 3 |
| Ankerite-siderite-cemented (ASCS1, ASCS2, ASCS3) | 13-10A | 2339.4–2339.5 | 4 | 2.61 | 5.42 | 3 |
| Kaolinite-cemented (Kao1, Cal/Kao2, Kao3) | 13-10A | 2342.28–2342.57 | 3 | 2.56 | 5.22 | 3 |
| Calcite-cemented (CCS1, CCS2, CCS3) | 13-10A | 2348.1–2348.2 | 1 | 2.60 | 5.20 | 3 |
| Sample ID | Fluid | Flow Rate (mL/min) | Duration (Days) | Depth (m) | Diameter (cm) | Length (cm) | Fluid Volume (mL) | Pore Volume |
|---|---|---|---|---|---|---|---|---|
| Chl1 | Brackish + CO2 | 0.01 | 35 | 2337.5–2338.3 | 2.57 | 5.32 | 500 | 130–260 |
| Chl2 | Brackish + CO2 | 0.1 | 3.5 | 2.49 | 5.21 | 500 | ||
| Chl3 | Brackish | 0.01 | 35 | 2.55 | 5.20 | 500 | ||
| ASCS1 | Brackish + CO2 | 0.01 | 35 | 2339.4–2339.5 | 2.61 | 5.48 | 500 | 267 |
| ASCS2 | Brackish + CO2 | 0.1 | 3.5 | 2.61 | 5.32 | 500 | 176 | |
| ASCS3 | Brackish | 0.01 | 35 | 2.61 | 5.45 | 500 | 147 | |
| Unc1 | Brackish + CO2 | 0.01 | 35 | 2340.7–2341.1 | 2.55 | 5.42 | 500 | 176 |
| Unc2 | Brackish + CO2 | 0.1 | 3.5 | 2.56 | 5.54 | 500 | 166 | |
| Unc3 | Brackish | 0.01 | 35 | 2.55 | 5.57 | 500 | 172 | |
| Kao1 | Brackish + CO2 | 0.01 | 35 | 2342.3–2342.6 | 2.58 | 5.11 | 500 | 130–260 |
| Cal/Kao2 | Brackish + CO2 | 0.1 | 3.5 | 2.57 | 5.11 | 500 | ||
| Kao3 | Brackish | 0.01 | 35 | 2.54 | 5.44 | 500 | ||
| CCS1 | Brackish + CO2 | 0.01 | 35 | 2348.1–2348.2 | 2.61 | 5.30 | 500 | 499 |
| CCS2 | Brackish + CO2 | 0.02 + 0.01 | 32 | 2.59 | 5.09 | 500 | 675 | |
| CCS3 | Brackish | 0.01 | 35 | 2.59 | 5.20 | 500 | 597 |
| Sample ID | Fluid | Flow Rate (mL/min) | Duration (Days) | Depth (m) | Diameter (cm) | Length (cm) | Fluid Volume (mL) | Pore Volume |
|---|---|---|---|---|---|---|---|---|
| MP1 | Brackish + CO2 | 0.01 | 24 | 2354.7–2355.8 | 3.63 | 7.12 | 272 | 24.4 |
| MP2 | Brackish | 0.01 | 28 | 3.67 | 7.14 | 388 | 38.0 | |
| MP3 * | Brackish|Brackish + CO2 | 0.02 | 38 | 3.72 | 7.52 | 509|508 | 42.1|42.0 | |
| MP4 * | Brackish|Brackish + CO2 | 0.1 | 8 | 3.82 | 7.05 | 509|513 | 48.2|48.6 |
| Paragenetic Event | Evidence | Photomicrographs |
|---|---|---|
| Quartz (Qtz) overgrowths occurred before carbonate cementation/replacement | Siderite cement always surrounds quartz overgrowths |  |
| Siderite cementation occurred before ankerite cementation | Ankerite always surrounds siderite cement when both minerals are present | 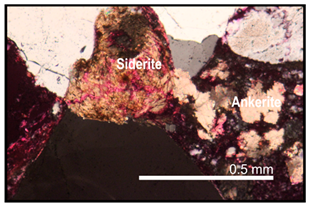 |
| Siderite cementation occurred before calcite cementation | Calcite always surrounds siderite cement |  |
| Carbonate cementation occurred before compaction | Ankerite stops compaction by forming long contacts with framework grains in areas where loss of intergranular volume (IGV) is apparent | 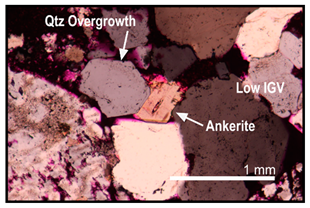 |
| Carbonate replacement occurred before feldspar dissolution | Feldspar relics are observed within undissolved calcite that occurs inside dissolved feldspar grains | 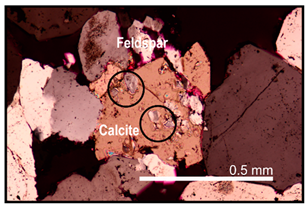 |
| Compaction | Numerous low IGV areas and long, sutured, and concavo-convex grain contacts | 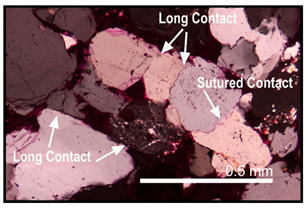 |
| Some calcite cementation occurred during/after compaction | Calcite cement is sometimes found outside heavily compacted low IGV areas and formed a poikilotopic texture | 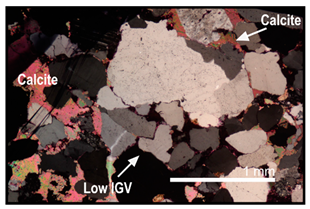 |
| Feldspar dissolution primarily occurred after compaction but before clay cementation | Intragranular porosity in dissolved feldspar is well preserved in low IGV areas while being filled with secondary clays | 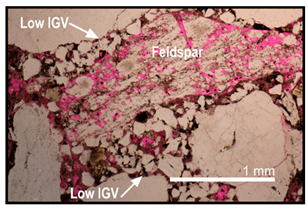 |
| Clay cementation occurred before hydrocarbon emplacement | Clays are oil stained, as shown by the dark coatings | 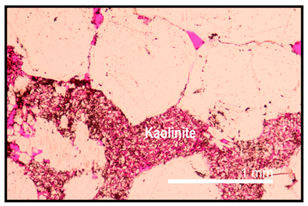 |
| Sample ID | Pre/Post | Porosity (%) | Abs. Change (%) | Permeability (m2) | Abs. Change (m2) |
|---|---|---|---|---|---|
| Chl1 | pre | 10.48 | 0.06 | 6.54 × 10−15 | 4.25 × 10−15 |
| post | 10.54 | 1.08 × 10−14 | |||
| Chl2 | pre | 15.12 | 0.33 | 5.96 × 10−15 | 4.84 × 10−15 |
| post | 15.45 | 1.08 × 10−14 | |||
| Chl3 (control) | pre | 14.16 | 0.06 | 3.48 × 10−15 | −1.13 × 10−15 |
| post | 14.22 | 2.36 × 10−15 | |||
| ASCS1 | pre | 6.20 * | 0.30 * | 5.62 × 10−14 | −1.27 × 10−14 |
| post | 6.50 | 4.35 × 10−14 | |||
| ASCS2 | pre | 9.82 * | 0.28 * | 4.47 × 10−14 | 1.00 × 10−16 |
| post | 10.10 | 4.48 × 10−14 | |||
| ASCS3 (control) | pre | 10.00 | 1.80 | 4.92 × 10−14 | −1.08 × 10−14 |
| post | 11.80 | 3.84 × 10−14 | |||
| Unc1 | pre | 10.37 | −0.04 | 6.12 × 10−16 | 2.97 × 10−15 |
| post | 10.33 | 3.58 × 10−15 | |||
| Unc2 | pre | 10.71 | 0.24 | 7.60 × 10−16 | 1.88 × 10−15 |
| post | 10.95 | 2.64 × 10−15 | |||
| Unc3 (control) | pre | 10.43 | −0.26 | 6.61 × 10−16 | 5.13 × 10−16 |
| post | 10.17 | 1.17 × 10−15 | |||
| Kao1 | pre | 8.50 | −0.17 | 3.85 × 10−16 | −9.87 × 10−18 |
| post | 8.33 | 3.75 × 10−16 | |||
| Cal/Kao2 | pre | 7.36 | 1.26 | 1.68 × 10−16 | 1.04 × 10−15 |
| post | 8.62 | 1.20 × 10−15 | |||
| Kao3 (control) | pre | 12.61 | 0.14 | 5.34 × 10−15 | −7.99 × 10−16 |
| post | 12.75 | 4.54 × 10−15 | |||
| CCS1 | pre | 2.71 * | 0.89 * | 3.35 × 10−18 | 7.47 × 10−17 |
| post | 3.60 | 7.80 × 10−17 | |||
| CCS2 | pre | 2.90 | −0.10 | 4.29 × 10−18 | −1.85 × 10−18 |
| post | 2.80 | 2.44 × 10−18 | |||
| CCS3 (control) | pre | 3.20 | −0.10 | 4.50 × 10−18 | −3.13× 10−18 |
| post | 3.10 | 1.37 × 10−18 | |||
| MP1 | pre | 15.05 | - | 9.00 × 10−14 | −3.00 × 10−14 |
| post | - | 6.00 × 10−14 | |||
| MP2 | pre | 12.50 | 0.17 | 3.00 × 10−14 | −9.97 × 10−15 |
| post | 12.67 | 2.00 × 10−14 | |||
| MP3 | pre | 15.95 | 0.33 | 1.98 × 10−13 | −1.38 × 10−13 |
| post | 16.28 | 6.00 × 10−14 | |||
| MP4 | pre | 13.01 | −3.95 | 1.00 × 10−14 | 1.00 × 10−14 |
| post | 9.06 | 2.00 × 10−14 |
| Alteration | Photomicrograph of Evidence |
|---|---|
| Siderite alteration occurred as noted by the precipitation of new iron oxide (Fe oxide) coatings |  |
| Ankerite dissolution indicated by crystal deterioration and dissolved edges |  |
| Calcite was often completely dissolved, but sometimes only partially dissolved |  |
| Chlorite dissolution resulted in the release of iron which quickly reprecipitated as iron oxide | 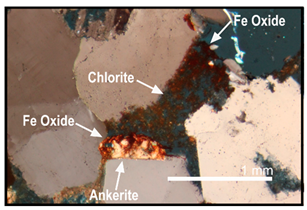 |
| Microcracking occurred along the bottom length of sample MP2 resulting in a large zone of highly deformed framework grains | 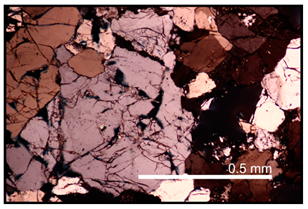 |
| Higher resolution of the shattered quartz grain from the image above | 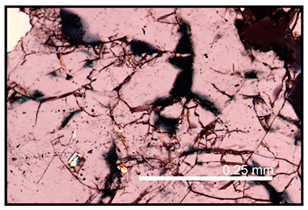 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Simmons, J.D.; Otu, S.; Rinehart, A.; Luhmann, A.; Heath, J.; Mozley, P.; Majumdar, B.S. Control of Cement Timing, Mineralogy, and Texture on Hydro-chemo-mechanical Coupling from CO2 Injection into Sandstone: A Synthesis. Energies 2023, 16, 7949. https://doi.org/10.3390/en16247949
Wu Z, Simmons JD, Otu S, Rinehart A, Luhmann A, Heath J, Mozley P, Majumdar BS. Control of Cement Timing, Mineralogy, and Texture on Hydro-chemo-mechanical Coupling from CO2 Injection into Sandstone: A Synthesis. Energies. 2023; 16(24):7949. https://doi.org/10.3390/en16247949
Chicago/Turabian StyleWu, Zhidi, Jason D. Simmons, Samuel Otu, Alex Rinehart, Andrew Luhmann, Jason Heath, Peter Mozley, and Bhaskar S. Majumdar. 2023. "Control of Cement Timing, Mineralogy, and Texture on Hydro-chemo-mechanical Coupling from CO2 Injection into Sandstone: A Synthesis" Energies 16, no. 24: 7949. https://doi.org/10.3390/en16247949
APA StyleWu, Z., Simmons, J. D., Otu, S., Rinehart, A., Luhmann, A., Heath, J., Mozley, P., & Majumdar, B. S. (2023). Control of Cement Timing, Mineralogy, and Texture on Hydro-chemo-mechanical Coupling from CO2 Injection into Sandstone: A Synthesis. Energies, 16(24), 7949. https://doi.org/10.3390/en16247949






