Management of Fly Ash to Synthesise Geopolymers and Zeolites
Abstract
1. Introduction
2. Fly Ash—Production, Characteristics, Properties
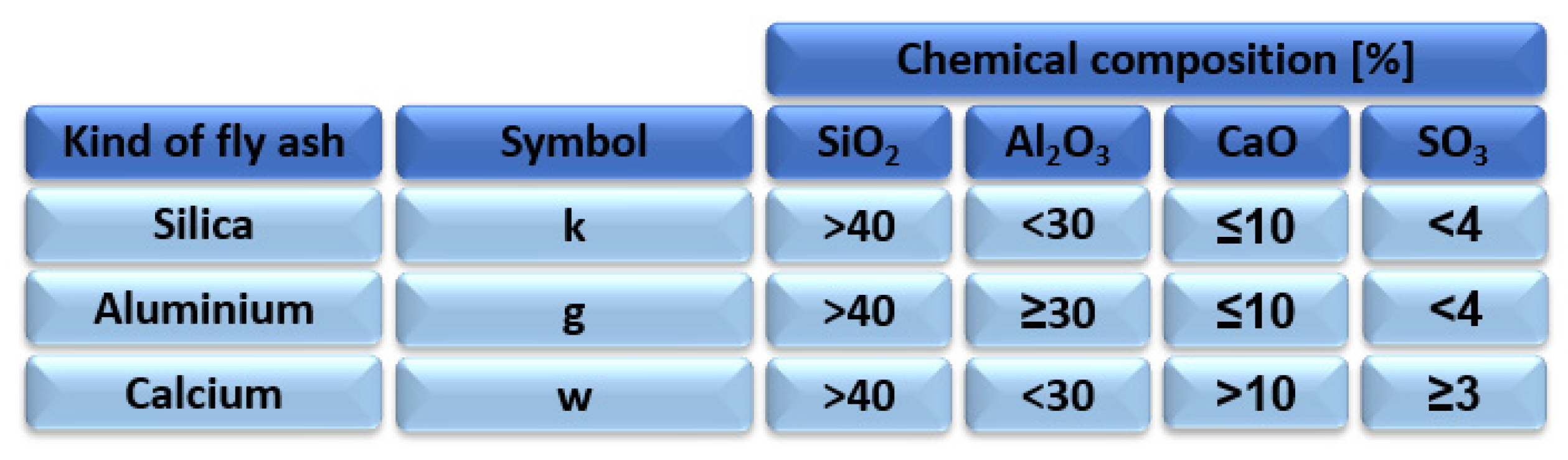
2.1. Polish Fly Ash
- Fine-grained—where the grain size is <0.075 mm and its content is less than 25%;
- Medium-grained—where the grain size is <0.075 mm and the total content in the in the fraction is between 40 and 75%;
- Coarse-grained—the size of a single grain is <0.075 mm and the amount of particles of this size particle size does not exceed 40% of the total volume.
- Bottom ash—by-products of combustion, characterised by irregular grain shape and varying physical and chemical properties. They show very pozzulanic properties, high resistance to external forces. They contain a large amount of alkaline compounds in their structure and have a high hydrophilicity. They are mainly used in the mining, energy, and construction industries [26].
- Slag—the main chemical compounds forming the crystalline structure are silicon oxide and aluminium oxide. Slag can be divided into two varieties. The first includes unburned slag, its characteristic feature is its dark grey colour, and the carbon in it is only partially burnt, while the grains are glassy. The second variety of slag includes burnt slag, which has a brick-red colour. Coal firing results in a large amount of sinter; the grain fraction is relatively small. In addition to the enamel content, the substances included in the chemical composition of the slag are mullite crystals, fused quartz, anorthite, melilite, burnt clay rock and clayey ironstone, magnetite, and gypsum [27,28].
- Flue gases, the resulting by-products of combustion in the gaseous state. They include primarily CO2 and also SO2, NOx [29].
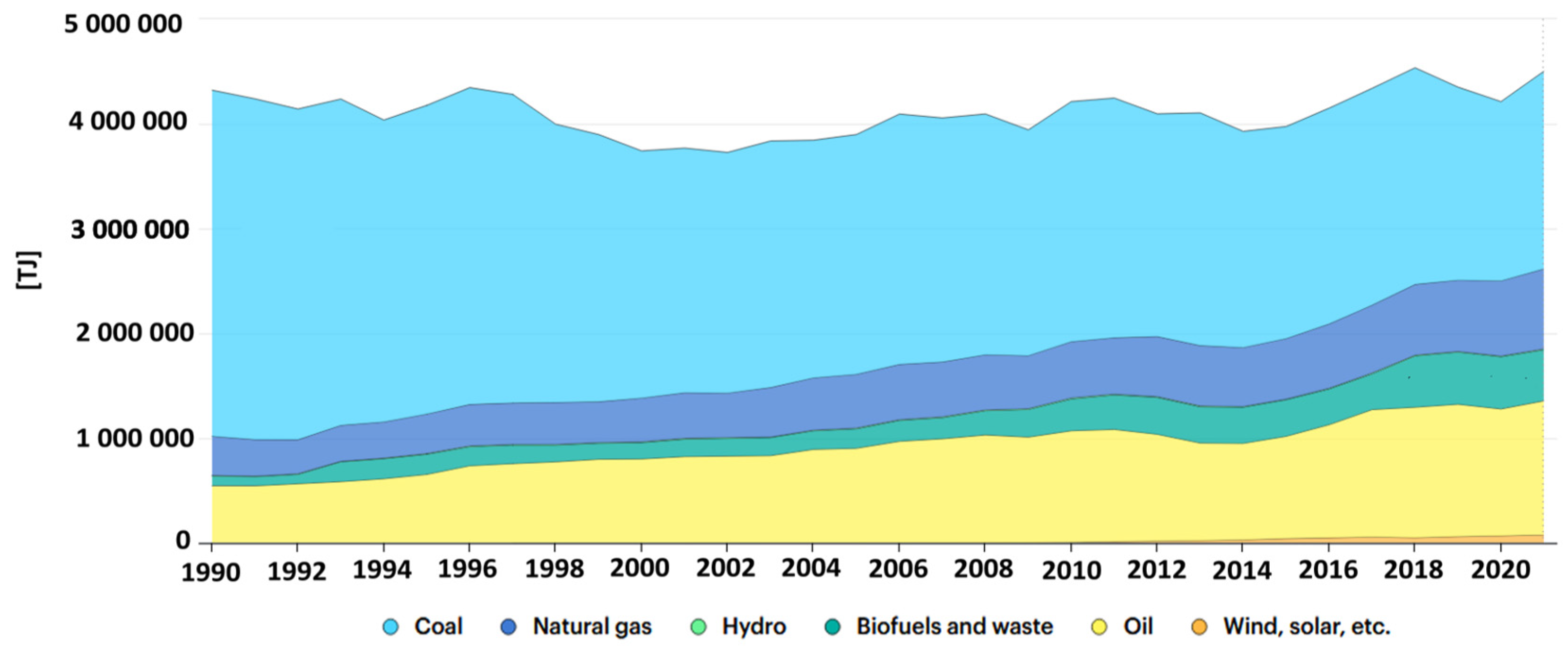
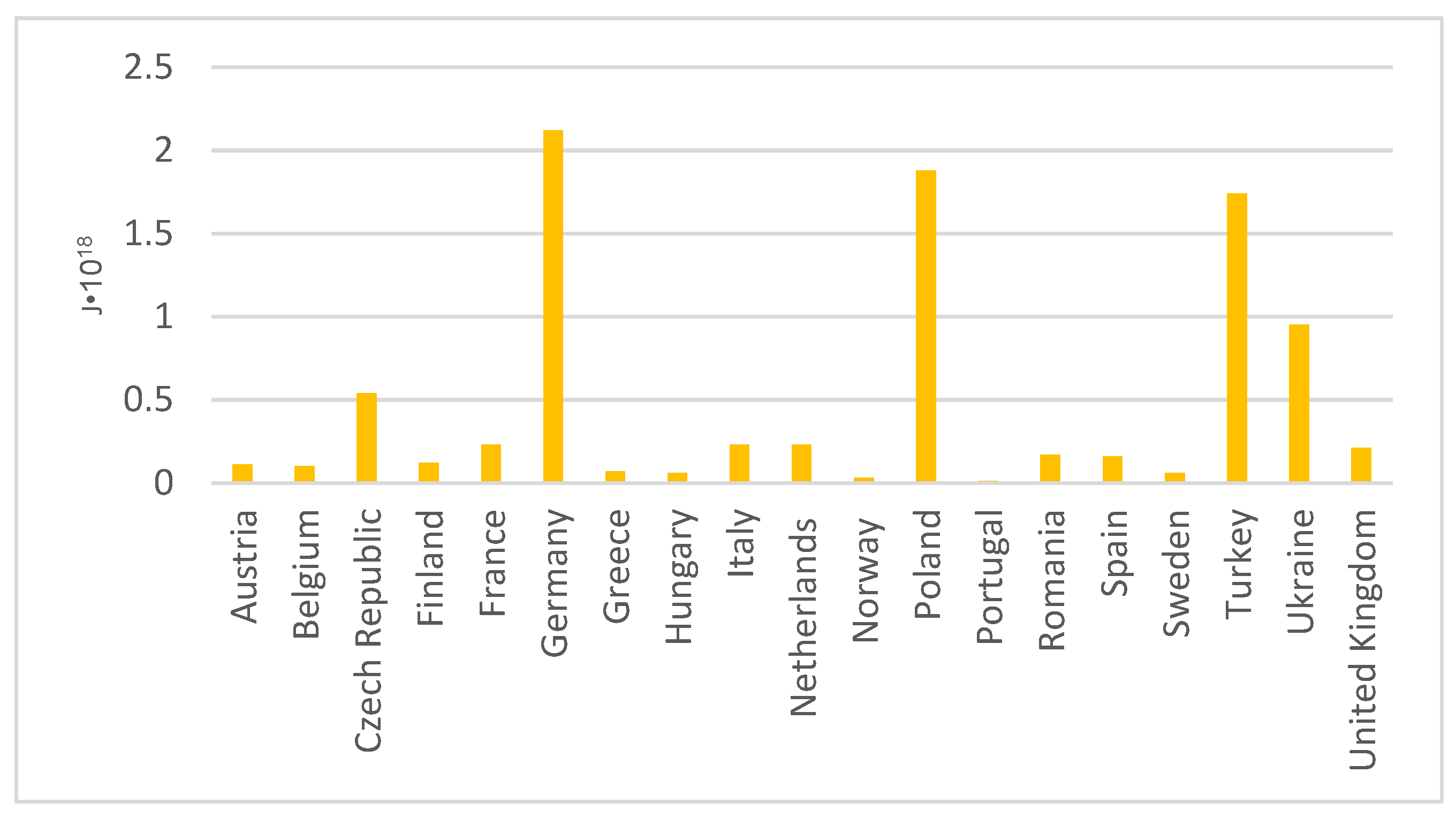
2.2. Energy Sector in Europe—Fly Ash
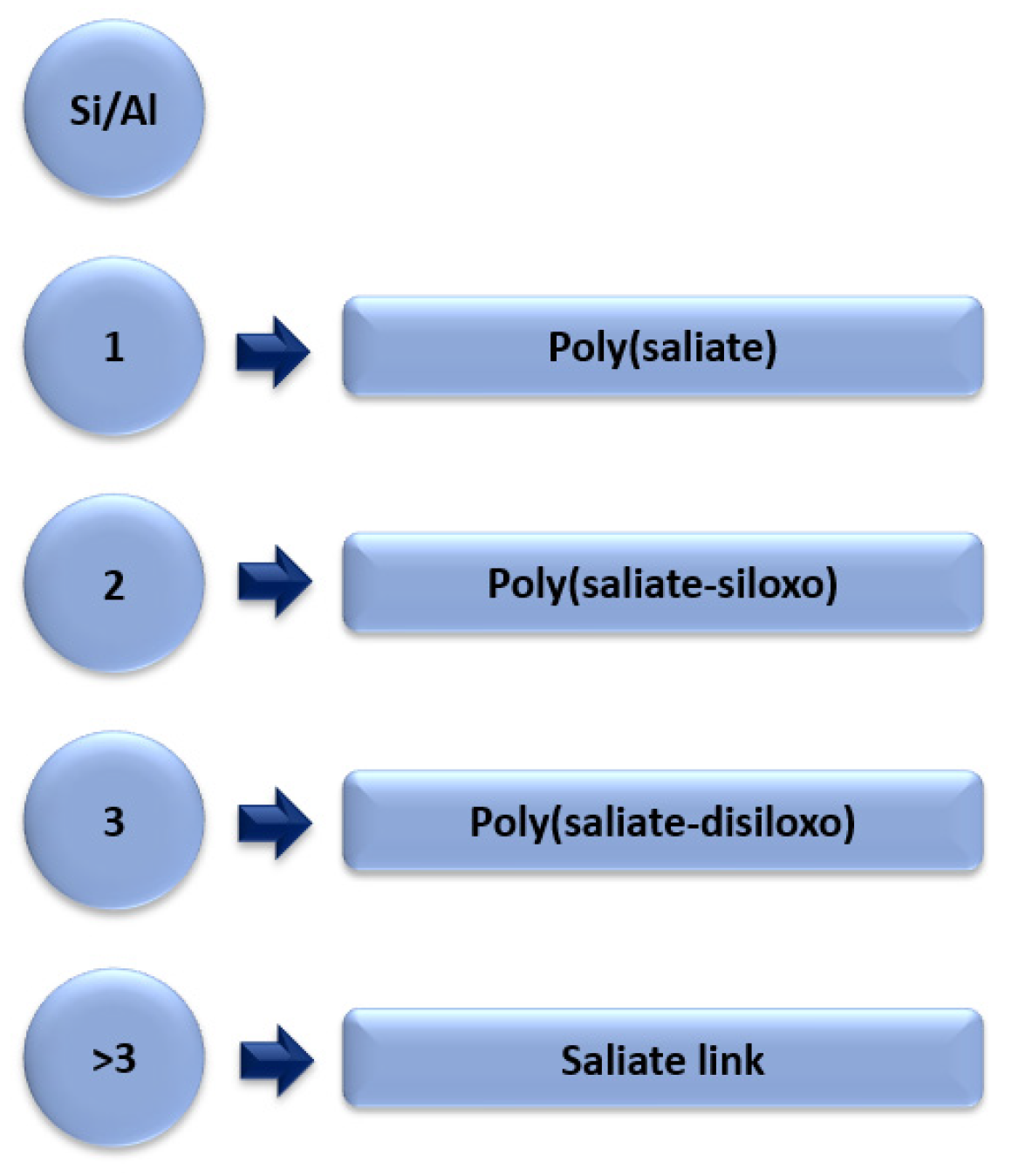
3. The Process of the Synthesis of Geopolymers Using Different Fly Ashes
4. Zeolites as Part of a Circular Economy
Physicochemical Properties of Synthetic Zeolites
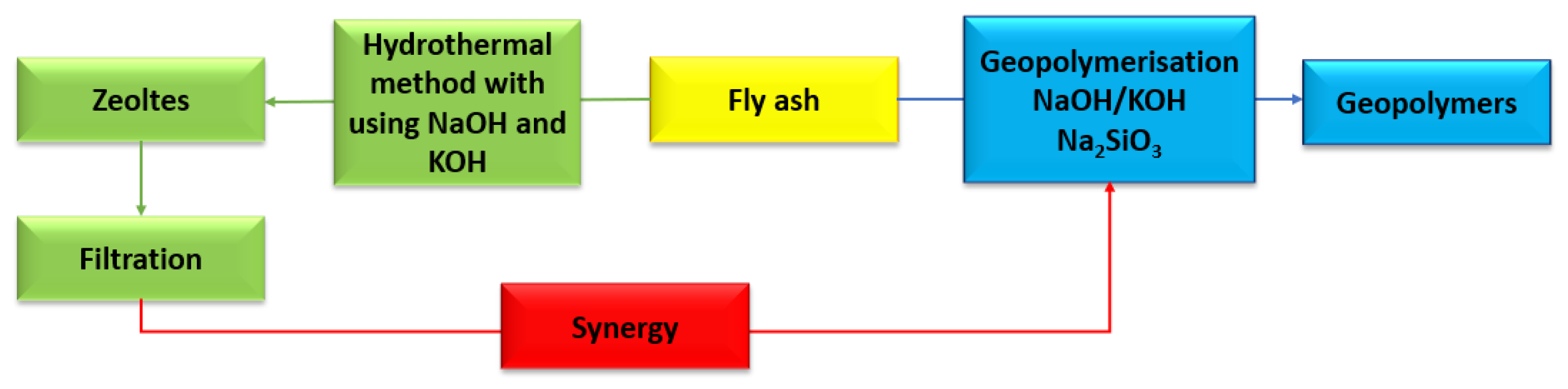
5. Synergy of Zeolite and Geopolymer Synthesis Using Fly Ash
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Ju, T.; Meng, Y.; Han, S.; Lin, L.; Jiang, J. On the State of the Art of Crystalline Structure Reconstruction of Coal Fly Ash: A Focus on Zeolites. Chemosphere 2021, 283, 131010. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, H.; Zhang, J. Understanding the Effect of High-Volume Fly Ash on Micro-Structure and Mechanical Properties of Cemented Coal Gangue Paste Backfill. Constr. Build. Mater. 2023, 378, 131202. [Google Scholar] [CrossRef]
- Zhang, S.; Dai, S.; Finkelman, R.B.; Graham, I.T.; French, D.; Hower, J.C.; Li, X. Leaching Characteristics of Alkaline Coal Combustion By-Products: A Case Study from a Coal-Fired Power Plant, Hebei Province, China. Fuel 2019, 255, 115710. [Google Scholar] [CrossRef]
- Zimar, Z.; Robert, D.; Zhou, A.; Giustozzi, F.; Setunge, S.; Kodikara, J. Application of Coal Fly Ash in Pavement Subgrade Stabilisation: A Review. J. Environ. Manag. 2022, 312, 114926. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, M.; Lv, Y.; Ting, Z.J.; Zhao, S.; Liu, Z.; Zhang, X.; Yang, Y.; You, Y.; Yuan, W. Utilization of Municipal Solid Waste Incineration Fly Ash as Construction Materials Based on Geopolymerization. Resour. Conserv. Recycl. Adv. 2023, 19, 200162. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.S.; Poon, C.S.; Jiang, W.H.; Ma, Z.H.; Chen, X.; Lu, J.X.; Dong, H.X. Physicochemical and Pozzolanic Properties of Municipal Solid Waste Incineration Fly Ash with Different Pretreatments. Waste Manag. 2023, 160, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Liu, X. Detoxification, Solidification and Recycling of Municipal Solid Waste Incineration Fly Ash: A Review. Chem. Eng. J. 2021, 420, 130349. [Google Scholar] [CrossRef]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the Management of MSW Incineration Ashes from Gas Cleaning: New Perspectives on Recovery of Secondary Raw Materials and Circular Economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Liu, B. Degradation Technologies and Mechanisms of Dioxins in Municipal Solid Waste Incineration Fly Ash: A Review. J. Clean Prod. 2020, 250, 119507. [Google Scholar] [CrossRef]
- Wang, C.Q.; Zeng, Z.Y.; Wang, A.M.; Gao, S.H.; Huang, J.S. Basic Properties, Characteristic Heavy Metals Leaching and Migration of Coal Incineration Fly Ash-Based Mortar. Structures 2023, 54, 1179–1195. [Google Scholar] [CrossRef]
- Ustabaş, İ.; Kaya, A. Comparing the Pozzolanic Activity Properties of Obsidian to Those of Fly Ash and Blast Furnace Slag. Constr. Build. Mater. 2018, 164, 297–307. [Google Scholar] [CrossRef]
- Borowski, G.; Ozga, M. Comparison of the Processing Conditions and the Properties of Granules Made from Fly Ash of Lignite and Coal. Waste Manag. 2020, 104, 192–197. [Google Scholar] [CrossRef]
- Barros, M.V.; Salvador, R.; de Francisco, A.C.; Piekarski, C.M. Mapping of Research Lines on Circular Economy Practices in Agriculture: From Waste to Energy. Renew. Sustain. Energy Rev. 2020, 131, 109958. [Google Scholar] [CrossRef]
- Opferkuch, K.; Caeiro, S.; Salomone, R.; Ramos, T.B. Circular Economy Disclosure in Corporate Sustainability Reports: The Case of European Companies in Sustainability Rankings. Sustain. Prod. Consum. 2022, 32, 436–456. [Google Scholar] [CrossRef]
- Nayak, D.K.; Abhilash, P.P.; Singh, R.; Kumar, R.; Kumar, V. Fly Ash for Sustainable Construction: A Review of Fly Ash Concrete and Its Beneficial Use Case Studies. Clean. Mater. 2022, 6, 100143. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Calero-Rodríguez, A.; Bonet-Martínez, E.; Pérez-Villarejo, L.; Sánchez-Soto, P.J. Geopolymers Made from Metakaolin Sources, Partially Replaced by Spanish Clays and Biomass Bottom Ash. J. Build. Eng. 2021, 40, 102761. [Google Scholar] [CrossRef]
- Stefańska, A.; Łach, M.; Mikuła, J. Geopolimery Jako Przykład Możliwości Zagospodarowania Odpadów. In Nowoczesne technologie XXI w. – przegląd, trendy i badania. Tom 1; Wydawnictwo Naukowe TYGIEL sp. z o.o: Lublin, Poland, 2019. [Google Scholar]
- Belviso, C. State-of-the-Art Applications of Fly Ash from Coal and Biomass: A Focus on Zeolite Synthesis Processes and Issues. Prog. Energy Combust. Sci. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Mushtaq, F.; Zahid, M.; Bhatti, I.A.; Nasir, S.; Hussain, T. Possible Applications of Coal Fly Ash in Wastewater Treatment. J. Environ. Manag. 2019, 240, 27–46. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Fedyna, M.; Marzec, M.; Panek, R.; Szerement, J.; Marcińska-Mazur, L.; Jarosz, R.; Bajda, T.; Franus, W.; Mierzwa-Hersztek, M. The Influence of Zeolite X Ion-Exchangeable Forms and Impregnation with Copper Nitrate on the Adsorption of Phosphate Ions from Aqueous Solutions. J. Water Process Eng. 2022, 50, 103299. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S.J. The Geopolymerisation of Alumino-Silicate Minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Strzałkowska, E. Rare Earth Elements and Other Critical Elements in the Magnetic Fraction of Fly Ash from Several Polish Power Plants. Int. J. Coal Geol. 2022, 258, 104015. [Google Scholar] [CrossRef]
- Chmielniak, T.; Stelmach, S. Współczesne technologie zgazowania węgla. Probl. Ekol. 2009, 13, 69–76. [Google Scholar]
- Łuczak-Wilamowska, B. Możliwość Zastosowania Popiołów-odpadów Przemysłu energetycznego-do uszczelniania i rekultywacji składowisk odpadów. Biuletyn Państwowego Instytutu Geologicznego 2011, 446, 477–482. [Google Scholar]
- Kudełko, M. Modeling of Polish Energy Sector—Tool Specification and Results. Energy 2021, 215, 119149. [Google Scholar] [CrossRef]
- Tamanna, K.; Raman, S.N.; Jamil, M.; Hamid, R. Coal Bottom Ash as Supplementary Material for Sustainable Construction: A Comprehensive Review. Constr. Build. Mater. 2023, 389, 131679. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Harat, A.; Jaguś, A.; Porszke, A. Environmental Aspects of the Leaching of Mineral components from Power Plant’s Fly Ashes and Slags. Pol. J. Mater. Environ. Eng. 2022, 4, 21–29. [Google Scholar] [CrossRef]
- Piotr Smarzewski, D.B.-H. Mechanical and Microstructural Properties of High Performance Concrete with Burnt Coal Cinder. Available online: https://yadda.icm.edu.pl/yadda/element/bwmeta1.element.baztech-89ece729-bee0-4391-a0d5-def18c3572e8 (accessed on 20 October 2023).
- Mu, L.; Wang, S.; Lu, J.; Liu, G.; Zhao, L.; Lan, Y. Effect of Flue Gas Condensing Waste Heat Recovery and Its Pressure Drop on Energy Saving and Carbon Reduction for Refinery Heating Furnace. Energy 2023, 279, 128081. [Google Scholar] [CrossRef]
- Zhang, C.; Fu, J.; Song, W. Mechanical Model and Strength Development Evolution of High Content Fly Ash–Cement Grouting Material. Constr. Build. Mater. 2023, 398, 132492. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Komorek, J.; Białecka, B.; Nowak, J.; Klupa, A. Assessment of the Potential of Polish Fly Ashes as a Source of Rare Earth Elements. Ore Geol. Rev. 2020, 124, 103638. [Google Scholar] [CrossRef]
- Shon, C.-S.; Kim, Y.-S. Evaluation of West Texas Natural Zeolite as an Alternative of ASTM Class F Fly Ash. Constr. Build. Mater. 2013, 47, 389–396. [Google Scholar] [CrossRef]
- Franus, W.; Wiatros-Motyka, M.M.; Wdowin, M. Coal Fly Ash as a Resource for Rare Earth Elements. Environ. Sci. Pollut. Res. 2015, 22, 9464–9474. [Google Scholar] [CrossRef] [PubMed]
- Claudia, B.; Darina, B.; Andrea, B.G.; Constantin, C.; Jo, D.; Patricia, D.; Yildirim, K.; Latunussa, C.E.L.; Lucia, M.; Simone, M.; et al. Methodology for Establishing the EU List of Critical Raw Materials: Guidelines; EU Publications: Luxembourg, 2017; ISBN 9789279680519. [Google Scholar]
- Jarosinski, A.; Kulczycka, J. Possibilities of Obtaining Certain Critical Raw Materials in Poland in the Contex of Circular Economy Implementation. Inz. Miner. 2018, 19, 315–324. [Google Scholar]
- Pgdn, P.; Suárez-Ruiz, I.; Valentim, B. Atlas of Fly Ash Occurrences Identification and Petrographic Classification of Fly Ash Components Working Group. In Commission III-ICCP International Committee for Coal and Organic Petrology-ICCP Go to “Main Menu of Contents”; Porto University: Portugal, 2015; ISBN 978-84-608-1416-0. [Google Scholar]
- Suárez-Ruiz, I.; Valentim, B.; Borrego, A.G.; Bouzinos, A.; Flores, D.; Kalaitzidis, S.; Malinconico, M.L.; Marques, M.; Misz-Kennan, M.; Predeanu, G.; et al. Development of a Petrographic Classification of Fly-Ash Components from Coal Combustion and Co-Combustion. (An ICCP Classification System, Fly-Ash Working Group—Commission III.). Int. J. Coal Geol. 2017, 183, 188–203. [Google Scholar] [CrossRef]
- IEA. Putting CO2 to Use Creating Value from Emissions; IEA: Paris, France, 2019. [Google Scholar]
- The Statistical Review of World Energy Analysesdata on World Energy Markets from the Prior Year. The Review Has Been Providing Timely, Comprehensive and Objective Data to the Energy Community since 1952. In Bp Statistical Review of World Energy; Energy Institute (EI): London, UK, 2022.
- Commision to the European Parlament; The European Council; The Council; The European Economic and Social Committee; Committe of the Regions. The European Green Deal; Office of the European Union: Brussels, Belgium, 2020. [Google Scholar]
- Sanjuán, M.Á.; Argiz, C. Fineness of Coal Fly Ash for Use in Cement and Concrete. Fuels 2021, 2, 471–486. [Google Scholar] [CrossRef]
- Lu, L.; Liu, C.; Qu, S.; Zhang, M. Experimental Study on the Mechanical and Hydraulic Behaviour of Fibre-Reinforced Cemented Soil with Fly Ash. Constr. Build. Mater. 2022, 321, 126374. [Google Scholar] [CrossRef]
- Gazdič, D.; Kulísek, K.; Fridrichová, M.; Dvořák, K. Fired Hydraulic Binder Based on Fluidized Combustion Fly Ash. Procedia Eng. 2017, 172, 319–324. [Google Scholar] [CrossRef]
- Gao, K.; Iliuta, M.C. Trends and Advances in the Development of Coal Fly Ash-Based Materials for Application in Hydrogen-Rich Gas Production: A Review. J. Energy Chem. 2022, 73, 485–512. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Proffen, T.; van Deventer, J.S.J. Molecular Mechanisms Responsible for the Structural Changes Occurring during Geopolymerization: Multiscale Simulation. AIChE J. 2012, 58, 2241–2253. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly Ash-Based Geopolymer: Clean Production, Properties and Applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernández-Jiménez, A.; Macphee, D.E. Compatibility Studies between N-A-S-H and C-A-S-H Gels. Study in the Ternary Diagram Na2O–CaO–Al2O3–SiO2–H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Mallicoat, S.W.; Kriven, W.M.; van Deventer, J.S.J. Understanding the Relationship between Geopolymer Composition, Microstructure and Mechanical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2005, 269, 47–58. [Google Scholar] [CrossRef]
- Palomo, A.; Krivenko, P.; Garcia-Lodeiro, I.; Kavalerova, E.; Maltseva, O.; Fernández-Jiménez, A. A Review on Alkaline Activation: New Analytical Perspectives. Mater. Constr. 2014, 64, e022. [Google Scholar] [CrossRef]
- Ettahiri, Y.; Bouargane, B.; Fritah, K.; Akhsassi, B.; Pérez-Villarejo, L.; Aziz, A.; Bouna, L.; Benlhachemi, A.; Novais, R.M. A State-of-the-Art Review of Recent Advances in Porous Geopolymer: Applications in Adsorption of Inorganic and Organic Contaminants in Water. Constr. Build. Mater. 2023, 395, 132269. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Davaabal, B.; Bayarzul, U.; Ankhtuya, A.; Jadambaa, T.; Mackenzie, K.J.D. Utilization of Radioactive High-Calcium Mongolian Flyash for the Preparation of Alkali-Activated Geopolymers for Safe Use as Construction Materials. Ceram. Int. 2014, 40, 16475–16483. [Google Scholar] [CrossRef]
- Kozhukhova, N.I.; Zhernovskaya, I.V.; Danakin, D.N.; Teslya, A.Y.; Kozhukhova, M.I. Effect of Structure and Stereochemistry on Metakaolin Reactivity When Geopolymerization. In XIII General Meeting of the Russian Mineralogical Society and the Fedorov Session; Marin, Y., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 484–491. [Google Scholar]
- Kozhukhova, N.; Strokova, V.; Zhernovsky, I.; Sobolev, K. Geopolymerization and Structure Formation in Alkali Activated Aluminosilicates with Different Crystallinity Degree. In Proceedings of the 14th International Congress for Applied Mineralogy (ICAM2019), Belgorod, Russia, 23–27 September 2019. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, J.; Jiang, Y.; Zang, J.; Wu, T.; Ma, F.; Qian, B.; Wang, L.; Hu, Y.; Ma, B. The Performance of Micropore-Foamed Geopolymers Produced from Industrial Wastes. Constr. Build. Mater. 2021, 304, 124636. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.B.; Ming, L.Y.; Yong, H.C.; Tahir, M.F.M. Clay-Based Materials in Geopolymer Technology. In Cement Based Materials; InTech: Gandhinagar, India, 2018. [Google Scholar]
- Mikuła, J.S. Rozwiązania Proekologiczne w Zakresie Produkcji: Praca Zbiorowa. T. 1, Nowoczesne Materiały Kompozytowe Przyjazne Środowisku; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2014; ISBN 9788372427809. [Google Scholar]
- Raza, M.H.; Zhong, R.Y. A Sustainable Roadmap for Additive Manufacturing Using Geopolymers in Construction Industry. Resour. Conserv. Recycl. 2022, 186, 106592. [Google Scholar] [CrossRef]
- Ge, X.; Hu, X.; Shi, C. The Effect of Different Types of Class F Fly Ashes on the Mechanical Properties of Geopolymers Cured at Ambient Environment. Cem. Concr. Compos. 2022, 130, 104528. [Google Scholar] [CrossRef]
- Bezerra, B.P.; Morelli, M.R.; Luz, A.P. Effect of Reactive Silica Sources on the Properties of Na-Metakaolin-Based Geopolymer Binder. Constr. Build. Mater. 2023, 364, 129989. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Zhang, Y.; Ma, J.; Xiao, R.; Guo, F.; Bai, Y.; Huang, B. Influence of Size Effect on the Properties of Slag and Waste Glass-Based Geopolymer Paste. J. Clean. Prod. 2023, 383, 135428. [Google Scholar] [CrossRef]
- Tang, J.; Ji, X.; Liu, X.; Zhou, W.; Chang, X.; Zhang, S. Mechanical and Microstructural Properties of Phosphate-Based Geopolymers with Varying Si/Al Molar Ratios Based on the Sol-Gel Method. Mater. Lett. 2022, 308, 131178. [Google Scholar] [CrossRef]
- Rattanasak, U.; Pankhet, K.; Chindaprasirt, P. Effect of Chemical Admixtures on Properties of High-Calcium Fly Ash Geopolymer. Int. J. Miner. Metall. Mater. 2011, 18, 364–369. [Google Scholar] [CrossRef]
- Somna, K.; Jaturapitakkul, C.; Kajitvichyanukul, P.; Chindaprasirt, P. NaOH-Activated Ground Fly Ash Geopolymer Cured at Ambient Temperature. Fuel 2011, 90, 2118–2124. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jaturapitakkul, C.; Chalee, W.; Rattanasak, U. Comparative Study on the Characteristics of Fly Ash and Bottom Ash Geopolymers. Waste Manag. 2009, 29, 539–543. [Google Scholar] [CrossRef]
- Bhutta, M.A.R.; Hussin, W.M.; Azreen, M.; Tahir, M.M. Sulphate Resistance of Geopolymer Concrete Prepared from Blended Waste Fuel Ash. J. Mater. Civ. Eng. 2014, 26, 04014080. [Google Scholar] [CrossRef]
- Long, T.; Wang, Q.; Guan, Z.; Chen, Y.; Shi, X. Deterioration and Microstructural Evolution of the Fly Ash Geopolymer Concrete against MgSO4 Solution. Adv. Mater. Sci. Eng. 2017, 2017. [Google Scholar] [CrossRef]
- Lakhssassi, M.Z.; Alehyen, S.; Alouani, M.E.; Taibi, M. The Effect of Aggressive Environments on the Properties of a Low Calcium Fly Ash Based Geopolymer and the Ordinary Portland Cement Pastes. Mater. Today Proc. 2019, 13, 1169–1177. [Google Scholar] [CrossRef]
- Huang, Q.; Shi, X.S.; Wang, Q.Y.; Tang, L. The Influence of Carbonization on the Performances of Fly Ash Geopolymeric Concrete. Appl. Mech. Mater. 2015, 744–746, 1519–1526. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Castel, A.; Sanjayan, J.; Pathmanathan, R. Carbonation of a Blended Slag-Fly Ash Geopolymer Concrete in Field Conditions after 8 Years. Constr. Build. Mater. 2016, 125, 661–669. [Google Scholar] [CrossRef]
- Czuma, N.; Zarębska, K.; Motak, M.; Gálvez, M.E.; Da Costa, P. Ni/Zeolite X Derived from Fly Ash as Catalysts for CO2 Methanation. Fuel 2020, 267, 117139. [Google Scholar] [CrossRef]
- Murayama, N.; Takahashi, T.; Shuku, K.; Lee, H.H.; Shibata, J. Effect of Reaction Temperature on Hydrothermal Syntheses of Potassium Type Zeolites from Coal Fly Ash. Int. J. Miner. Process. 2008, 87, 129–133. [Google Scholar] [CrossRef]
- Derkowski, A.; Franus, W.; Beran, E.; Czímerová, A. Properties and Potential Applications of Zeolitic Materials Produced from Fly Ash Using Simple Method of Synthesis. Powder Technol. 2006, 166, 47–54. [Google Scholar] [CrossRef]
- Kirdeciler, S.K.; Akata, B. One Pot Fusion Route for the Synthesis of Zeolite 4A Using Kaolin. Adv. Powder Technol. 2020, 31, 4336–4343. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, H.; Xu, D.; Han, L.; Niu, D.; Tian, B.; Zhang, J.; Zhang, L.; Wu, W. Removal of Ammonium from Aqueous Solutions Using Zeolite Synthesized from Fly Ash by a Fusion Method. Desalination 2011, 271, 111–121. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.C.; Alastuey, A.; Hernández, E.; López-Soler, A.; Plana, F. Synthesis of Zeolites from Coal Fly Ash: An Overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Park, M.; Choi, L.; Lim, W.T.; Kim, M.C.; Choi, J.; Ho Heo, N. Molten-Salt Method for the Synthesis of Zeolitic Materials II. Charact. Zeolitic Mater. 2000, 37, 91–98. [Google Scholar]
- Hollman, G.G.; Steenbruggen, G.; Janssen-Jurkovičová, M. A Two-Step Process for the Synthesis of Zeolites from Coal Fly Ash. Fuel 1999, 78, 1225–1230. [Google Scholar]
- Mimura, H.; Yokota, K.; Akiba, K.; Onodera, Y. Alkali Hydrothermal Synthesis of Zeolites from Coal Fly Ash and Their Uptake Properties of Cesium Ion. J. Nucl. Sci. Technol. 2001, 38, 766–772. [Google Scholar] [CrossRef][Green Version]
- Steenbruggen, G.; Hollman, G.G. The Synthesis of Zeolites from Fly Ash and the Properties of the Zeolite Products. J. Geochem. Explor. 1998, 62, 305–309. [Google Scholar] [CrossRef]
- Querol, X.; Uman Äa, J.C.; Plana, F.; Alastuey, A.; Lopez-Soler, A.; Medinaceli, A.; Valero, A.; Domingo, M.J.; Garcia-Rojo, E. Synthesis of Zeolites from fly Ash at Pilot Plant Scale. Examples of Potential Applications. Fuel 2001, 80, 857–865. [Google Scholar] [CrossRef]
- Wdowin, M.; Franus, M.; Panek, R.; Badura, L.; Franus, W. The Conversion Technology of Fly Ash into Zeolites. Clean Technol. Environ. Policy 2014, 16, 1217–1223. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, F.S.; Zhu, J.; Liu, Z. Effective Utilization of Waste Ash from MSW and Coal Co-Combustion Power Plant-Zeolite Synthesis. J. Hazard Mater. 2008, 153, 382–388. [Google Scholar] [CrossRef]
- Deng, L.; Xu, Q.; Wu, H. Synthesis of Zeolite-like Material by Hydrothermal and Fusion Methods Using Municipal Solid Waste Fly Ash. Procedia Environ. Sci. 2016, 31, 662–667. [Google Scholar] [CrossRef]
- Tanaka, H.; Fujii, A. Effect of Stirring on the Dissolution of Coal Fly Ash and Synthesis of Pure-Form Na-A and -X Zeolites by Two-Step Process. Adv. Powder Technol. 2009, 20, 473–479. [Google Scholar] [CrossRef]
- Feng, W.; Wan, Z.; Daniels, J.; Li, Z.; Xiao, G.; Yu, J.; Xu, D.; Guo, H.; Zhang, D.; May, E.F.; et al. Synthesis of High Quality Zeolites from Coal Fly Ash: Mobility of Hazardous Elements and Environmental Applications. J. Clean. Prod. 2018, 202, 390–400. [Google Scholar] [CrossRef]
- Czuma, N.; Zarębska, K.; Baran, P. Analysis of the Influence of Fusion Synthesis Parameters on the SO2 Sorption Properties of Zeolites Produced out of Fly Ash. In Proceedings of the E3S Web of Conferences, Kraków, Poland, 17–19 May 2016. [Google Scholar]
- Pedrolo, D.R.S.; De Menezes Quines, L.K.; De Souza, G.; Marcilio, N.R. Synthesis of Zeolites from Brazilian Coal Ash and Its Application in SO2 Adsorption. J. Environ. Chem. Eng. 2017, 5, 4788–4794. [Google Scholar] [CrossRef]
- Siddharth; Maiti, S.; Raj, H.; Bisht, R.S.; Minocha, A.K.; Panigrahi, S.K.; Alexander, S.; Sameer; Singh, M. X-Ray Photoelectron Spectroscopy Study on Adsorption Property of Harmful Air Pollutants on Zeolite Prepared from Fly Ash. Mater. Res. Express 2018, 5, 085507. [Google Scholar] [CrossRef]
- Czarna-Juszkiewicz, D.; Cader, J.; Wdowin, M. From Coal Ashes to Solid Sorbents for Hydrogen Storage. J. Clean. Prod. 2020, 270, 122355. [Google Scholar] [CrossRef]
- Yang, T.; Han, C.; Liu, H.; Yang, L.; Liu, D.; Tang, J.; Luo, Y. Synthesis of Na-X Zeolite from Low Aluminum Coal Fly Ash: Characterization and High Efficient As (V) Removal. Adv. Powder Technol. 2019, 30, 199–206. [Google Scholar] [CrossRef]
- Kunecki, P.; Wdowin, M.; Hanc, E. Fly Ash-Derived Zeolites and Their Sorption Abilities in Relation to Elemental Mercury in a Simulated Gas Stream. J. Clean. Prod. 2023, 391, 136181. [Google Scholar] [CrossRef]
- Czarna, D.; Baran, P.; Kunecki, P.; Panek, R.; Żmuda, R.; Wdowin, M. Synthetic Zeolites as Potential Sorbents of Mercury from Wastewater Occurring during Wet FGD Processes of Flue Gas. J. Clean. Prod. 2016, 172, 2636–2645. [Google Scholar] [CrossRef]
- Czuma, N.; Casanova, I.; Baran, P.; Szczurowski, J.; Zarębska, K. CO2 Sorption and Regeneration Properties of Fly Ash Zeolites Synthesized with the Use of Differentiated Methods. Sci. Rep. 2020, 10, 1825. [Google Scholar] [CrossRef] [PubMed]
- Zarębska, K.; Szczurowski, J.; Gazda-Grzywacz, M.; Wróbel, W.; Bator, J.; Baran, P. Geopolymer Building Materials Based on Fly Ash in Terms of Removing SO2, CO2, and Water Vapor. Energies 2023, 16, 5188. [Google Scholar] [CrossRef]
- Baran, P.; Nazarko, M.; Włosińska, E.; Kanciruk, A.; Zarębska, K. Synthesis of Geopolymers Derived from Fly Ash with an Addition of Perlite. J. Clean. Prod. 2021, 293, 126112. [Google Scholar] [CrossRef]
- Rożek, P.; Król, M.; Mozgawa, W. Geopolymer-Zeolite Composites: A Review. J. Clean. Prod. 2019, 230, 557–579. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, S.; Qiu, X.; Meng, Y.; Wang, H.; Zhou, S.; Qiao, Q.; Yan, C. Clinoptilolite Based Zeolite-Geopolymer Hybrid Foams: Potential Application as Low-Cost Sorbents for Heavy Metals. J. Environ. Manag. 2023, 330, 117167. [Google Scholar] [CrossRef]
- Yang, S.; Yang, L.; Gao, M.; Bai, H.; Nagasaka, T. Synthesis of Zeolite-Geopolymer Composites with High Zeolite Content for Pb(II) Removal by a Simple Two-Step Method Using Fly Ash and Metakaolin. J. Clean. Prod. 2022, 378, 134528. [Google Scholar] [CrossRef]
- Papa, E.; Minelli, M.; Marchioni, M.C.; Landi, E.; Miccio, F.; Natali Murri, A.; Benito, P.; Vaccari, A.; Medri, V. Metakaolin-Based Geopolymer—Zeolite NaA Composites as CO2 Adsorbents. Appl. Clay Sci. 2023, 237, 106900. [Google Scholar] [CrossRef]

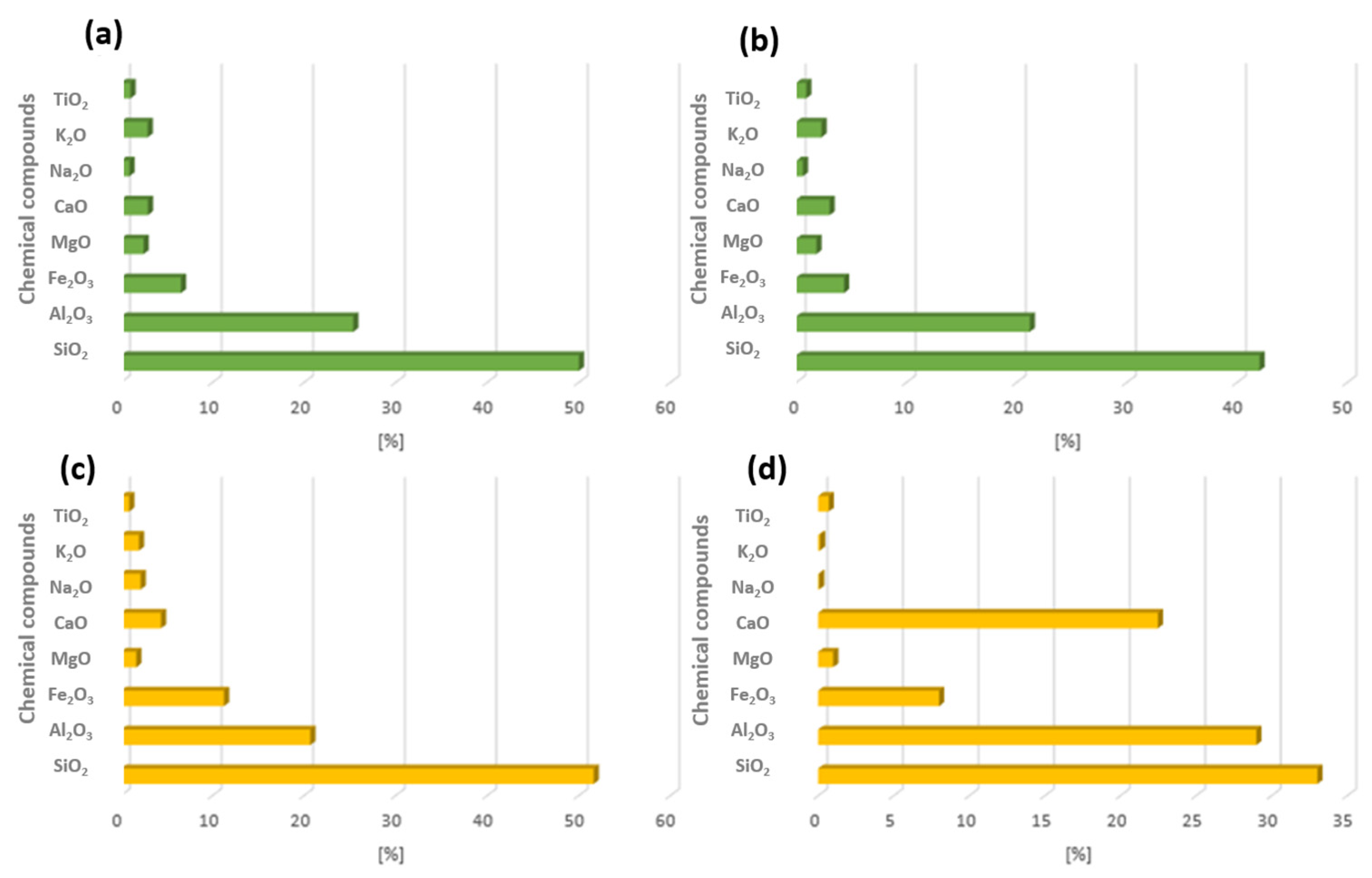








| Power Plant in Poland | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O |
|---|---|---|---|---|---|---|---|
| Łagisza | 49.00 | 27.72 | 6.92 | 2.41 | 3.24 | 1.44 | 2.65 |
| Jaworzno | 49.33 | 26.35 | 9.62 | 2.47 | 3.93 | 1.51 | 2.67 |
| Siersza | 47.54 | 24.71 | 7.30 | 3.40 | 5.17 | 2.86 | 2.60 |
| Łaziska | 49.58 | 28.08 | 6.69 | 2.70 | 3.28 | 1.08 | 3.36 |
| Rybnik | 49.12 | 26.73 | 5.52 | 2.19 | 3.43 | 1.14 | 3.11 |
| Stalowa Wola | 52.91 | 21.72 | 5.83 | 2.29 | 3.01 | 0.90 | 2.77 |
| Power Plant | SiO2 | Al2O3 | Fe2O3 | CaO | Na2O | K2O | MgO | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Britain | 44.00–55.80 | 17.70–32.80 | 4.90–15.00 | 1.10–5.40 | 0.20–2.60 | 1.00–4.50 | 1.20–4.40 | 0.90–1.10 |
| France | 47.00–53.00 | 26.00–34.00 | 4.34–7.27 | 2.30–6.66 | 0.04–6.40 | 1.04–1.33 | 0.90–2.44 | 1.32–1.81 |
| Germany | 20.00–80.00 | 1.00–22.70 | 1.00–22.00 | 2.00–52.00 | 0.00–4.21 | 0.00–4.40 | 0.50–11.00 | 0.10–1.08 |
| Greece | 21.00–70.10 | 4.17–22.00 | 2.50–10.90 | 5.00–45.00 | 0.04–4.50 | 0.30–3.00 | 1.21–6.00 | 0.12–1.20 |
| Portugal | 48.00–59.10 | 19.90–29.60 | 4.46–7.40 | 1.38–4.65 | 0.41–1.25 | 1.01–2.25 | 1.00–1.75 | 0.90–1.40 |
| Italy | 33.80–54.00 | 10.90–33.40 | 3.00–8.80 | 2.00–39.59 | 0.00–1.16 | 0.00–2.60 | 0.00–2.40 | 0.59–2.60 |
| The Netherlands | 45.10–59.70 | 24.80–28.90 | 3.30–9.00 | 0.50–6.80 | 0.10–1.20 | 0.60–2.90 | 0.60–3.70 | 0.90–1.80 |
| Poland | 32.20–56.50 | 3.97–32.20 | 3.97–9.00 | 1.16–29.90 | 0.00–3.11 | 0.19–3.34 | 0.52–5.94 | 0.20–2.22 |
| Spain | 41.10–58.60 | 17.60–45.40 | 2.60–16.20 | 0.30–11.80 | 0.20–4.50 | 0.20–4.05 | 0.30–3.20 | 0.50–1.80 |
| Denmark | 48.00–65.00 | 26.00–33.00 | 3.30–8.30 | 2.20–7.80 | 1.10–2.80 | - | - | - |
| Turkey | 18.10–60.30 | 7.63–30.80 | 4.10–11.30 | 0.20–38.20 | 0.10–2.57 | 0.32–5.62 | 0.43–8.98 | 0.57–1.50 |
| Bulgaria | 12.50–59.00 | 8.36–29.50 | 4.36–45.90 | 1.50–28.90 | 0.00–1.90 | 0.00–6.46 | 0.49–5.11 | 0.00–2.30 |
| Czech Republic | 51.90–53.80 | 25.50–33.00 | 5.51–8.00 | 1.84–5.80 | 0.25–0.70 | 1.75–2.74 | 0.92–1.94 | 0.96–2.10 |
| Romania | 40.80–54.30 | 15.70–26.20 | 7.58–9.93 | 2.42–13.80 | 0.19–0.83 | 1.35–2.66 | 1.49–2.48 | 0.06–1.07 |
| Serbia | 50.20–70.00 | 11.00–27.00 | 5.30–10.40 | 1.06–8.19 | 0.24–0.70 | 0.44–1.60 | 1.28–3.12 | 0.30–1.00 |
| Materials | Synthesis Conditions | Aim of the Research | Reference |
|---|---|---|---|
| Fly ash | NaOH, Na2SiO3, CaCl2, CaSO4, Na2SO4, Temperature: 65 °C Time: 48 h | The use of high-calcium fly ash for geopolymer synthesis, together with the use of admixtures, improves the bonding performance of geopolymer chains. Determination of the effect of the calcium chloride admixture on the setting time of the geopolymer paste. | [63] |
| Fly ash | NaOH 4.5–16.5 mol/dm3, Temperature: 25–28 °C, Time: 7, 14, 28, 42, 60 days, | Activation of fly ash with sodium hydroxide in a concentration range of 4.5 to 16.5 mol/dm3 and testing the compressive strength. | [64] |
| Fly ash, Bottom ash | NaOH 5, 10, 15 mol/dm3, Na2SiO3, Temperature: 65 °C, Time: 48 h, | Use of fly ash and bottom ash for mechanical activation with sodium hydroxide of different concentrations, comparison of mechanical properties of the obtained products (compressive strength). | [65] |
| Fly ash | NaOH, Na2SiO3, Na2SO4(aq), Temperature: 28 °C, Time: 28 days, | A geopolymer synthesis process using a fly ash blend consisting of: thermal fuel fly ash, palm oil from municipal solid waste, together with a solution of Na2SO4 solution. Comparison of the properties of the resulting product with classical Portland cement: sulphate resistance, compressive strength. | [66] |
| Fly ash | NaOH 10 mol/dm3, Na2SiO3, 5%MgSO4(aq), | Comparison of the effect of magnesium sulphate solution on the geopolymer obtained with sodium hydroxide solution and sodium silicate to classic Portland cement. | [67] |
| Fly ash | NaOH, Na2SiO3, Temperature: 70 °C, Time: 24 h, | Interaction of solutions of sulphuric acid (VI), sodium sulphate, and sodium chloride on synthetic geopolymer material and Portland cement. Use of analytical methods to calculate the weight loss of the materials tested. | [68] |
| Fly ash | NaOH, Na2SiO3, | Effects of carbon dioxide on geopolymers and Portland cement. Carrying out the carbonation process at a temperature of 20 ± 2 °C, a carbon dioxide concentration of 20 ± 3%, and a relative humidity of 70 ± 5%. | [69] |
| Fly ash slag | NaOH 7 mol/dm3 KOH 7 mol/dm3 Na2SiO3 | The in situ natural mineral carbonation process of a geopolymer obtained by mechanical activation of fly ash with slag in ratios of 75:25% and 70:30%. Analysis of carbonation reaction products by TGA and FT-IR analysis after 8 years. | [70] |
| Method of Synthesis | Fly Ash | Parameters | Product | Reference |
|---|---|---|---|---|
| Hydrothermal | Coal | Activation solution: 2.0 mol/dm3 NaOH Ratio liquid/solid: 25 cm3/g Temperature: 90 °C Time: 6–48 h | Na-P1 Na-X Na-A | [78] |
| Hydrothermal | Coal | Activation solution: 0.5–2.0 mol/dm3 KOH Ratio liquid/solid: 5–50 cm3/g Temperature: 80–160 °C Time: 12–72 h | K-H | [79] |
| Hydrothermal | Coal | Activation solution: 2.0 mol/dm3 KOH Ratio liquid/solid: 2.5 Temperature: 90–150 °C Time: 12 h | Na-P1 | [80] |
| Hydrothermal | Coal | Activation solution: 0.5–3.0 mol/dm3 NaOH Ratio liquid/solid: 18 cm3/g Temperature: <175 °C Time: 24 h Activation solution: 5.0 mol/dm3 NaOH Ratio liquid/solid: 18 cm3/g Temperature: <175 °C Time: 24 h | Na-P1 F zeolite Kalsilite Tobermorite | [81] |
| Hydrothermal | Coal | Activation solution: 0.5–3.0 mol/dm3 NaOH Ratio liquid/solid: 10–18 cm3/g Temperature: 90–175 °C Time: 3–48 h Activation solution: 5.0 mol/dm3 NaOH Ratio liquid/solid: 10–18 cm3/g Temperature: <150 °C Time: 3–48 h | Na-P1 Linde F Kalsilite Tobermorite | [76] |
| Hydrothermal | Coal | Activator: 1200 g NaOH Mass of the FA sample: 2000 g Water: 9000 cm3 Temperature: 80 °C Time: 36 h | Na-P1 | [82] |
| Hydrothermal | Coal | Activation solution: 3.0 mol/dm3 NaOH Ratio liquid/solid: 20 cm3/g Temperature: 75 °C Time: 24 h Activation solution: 1.0 mol/dm3 NaOH Ratio liquid/solid: 20 cm3/g Admixtures: 100 cm3 3.0 mol/dm3 NaCl Temperature: 105 °C Time: 24 h Activation solution: 5.0 mol/dm3 NaOH Ratio liquid/solid: 40 cm3/g Admixtures: 200 cm3 3.0 mol/dm3 NaCl Temperature: 105 °C Time: 24 h | NA-X Na-P1 Sodalite | [73] |
| Fushion | MSW | Ratio FA/NaOH: 0.6–2.0 Heat time: 1 h Temperature of heated: 550 °C Water to dissolving: 45 cm3 Time of crystallized: 0.5–26 h Temperature of crystalized: 40–180 °C | X HS | [83] |
| Fushion | MSW | Ratio FA/NaOH: 1.2 Time of heated: 1 h Temperature of heated: 550 °C Water to dissolving: 250 cm3 Admixtures: 12 g glass powder, 7 g Al2O3 Time of crystallisation: 24 h Temperature of crystalized: 90 °C | Y A | [84] |
| Hydrothermal | Coal | Activation solution: 2.2 mol/dm3 NaOH Ratio liquid/solid: 50 cm3/g Admixtures: 20 cm3 NaAlO2 Temperature: 85 °C Time: 24 h | Na-A Na-X | [85] |
| Fushion | Coal | Ratio FA/NaOH: 0.9–2.0 Time of heated: 1–2 h Temperature of heated: 300–600 °C Water to dissolving: 12 cm3/1 g Time of crystallized: 24 h Temperature of crystalized: 80 °C | A X | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, P.; Sobala, J.; Szczurowski, J.; Zarębska, K. Management of Fly Ash to Synthesise Geopolymers and Zeolites. Energies 2023, 16, 7888. https://doi.org/10.3390/en16237888
Baran P, Sobala J, Szczurowski J, Zarębska K. Management of Fly Ash to Synthesise Geopolymers and Zeolites. Energies. 2023; 16(23):7888. https://doi.org/10.3390/en16237888
Chicago/Turabian StyleBaran, Paweł, Jakub Sobala, Jakub Szczurowski, and Katarzyna Zarębska. 2023. "Management of Fly Ash to Synthesise Geopolymers and Zeolites" Energies 16, no. 23: 7888. https://doi.org/10.3390/en16237888
APA StyleBaran, P., Sobala, J., Szczurowski, J., & Zarębska, K. (2023). Management of Fly Ash to Synthesise Geopolymers and Zeolites. Energies, 16(23), 7888. https://doi.org/10.3390/en16237888





