Adsorption/Desorption Performances of Simulated Radioactive Nuclide Cs+ on the Zeolite-Rich Geopolymer from the Hydrothermal Synthesis of Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation Methods
2.3. Isothermal Adsorption Experiment
2.4. Desorption Kinetics Test
2.5. Characterization
3. Results and Discussion
3.1. Products Analysis
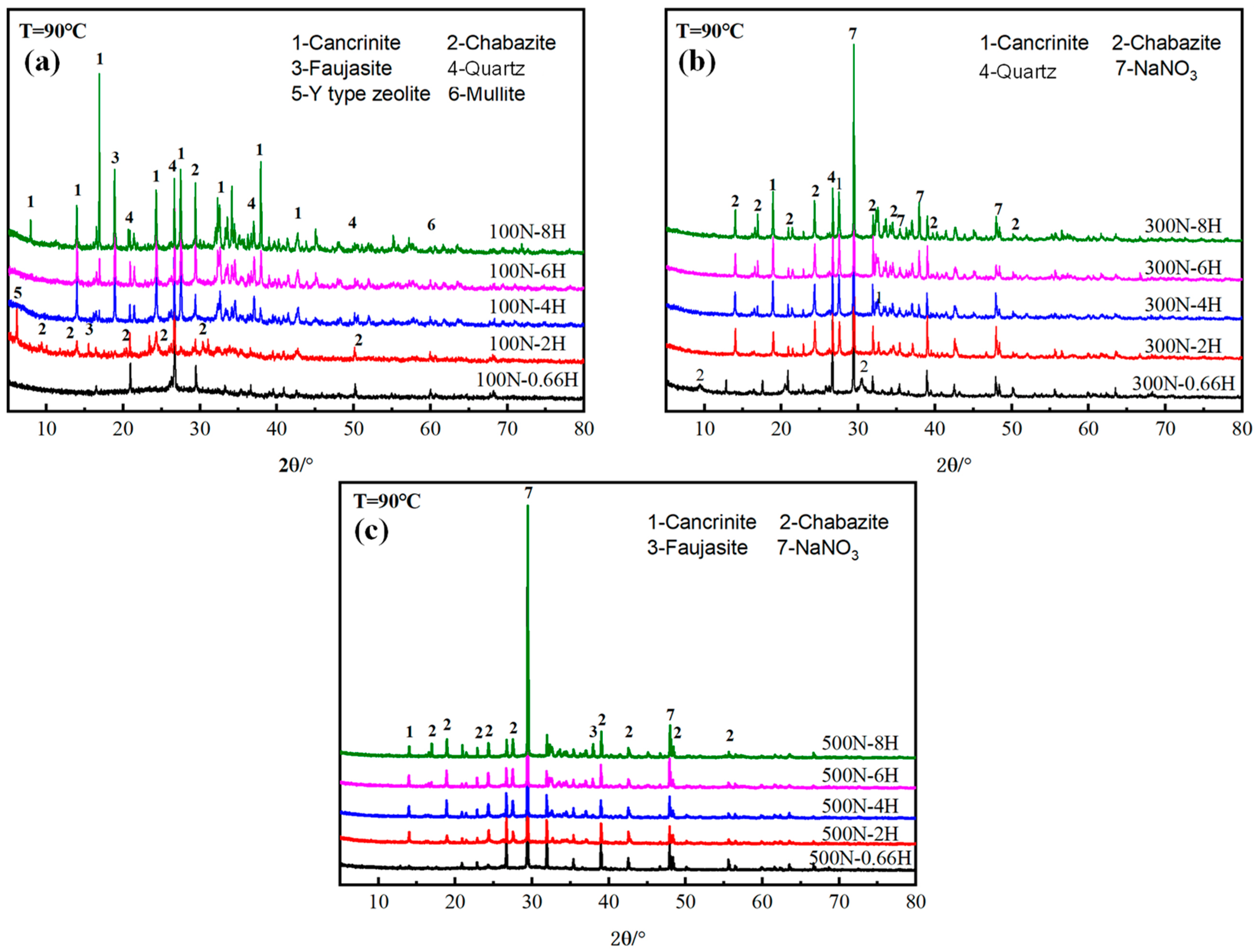
3.1.1. XRD
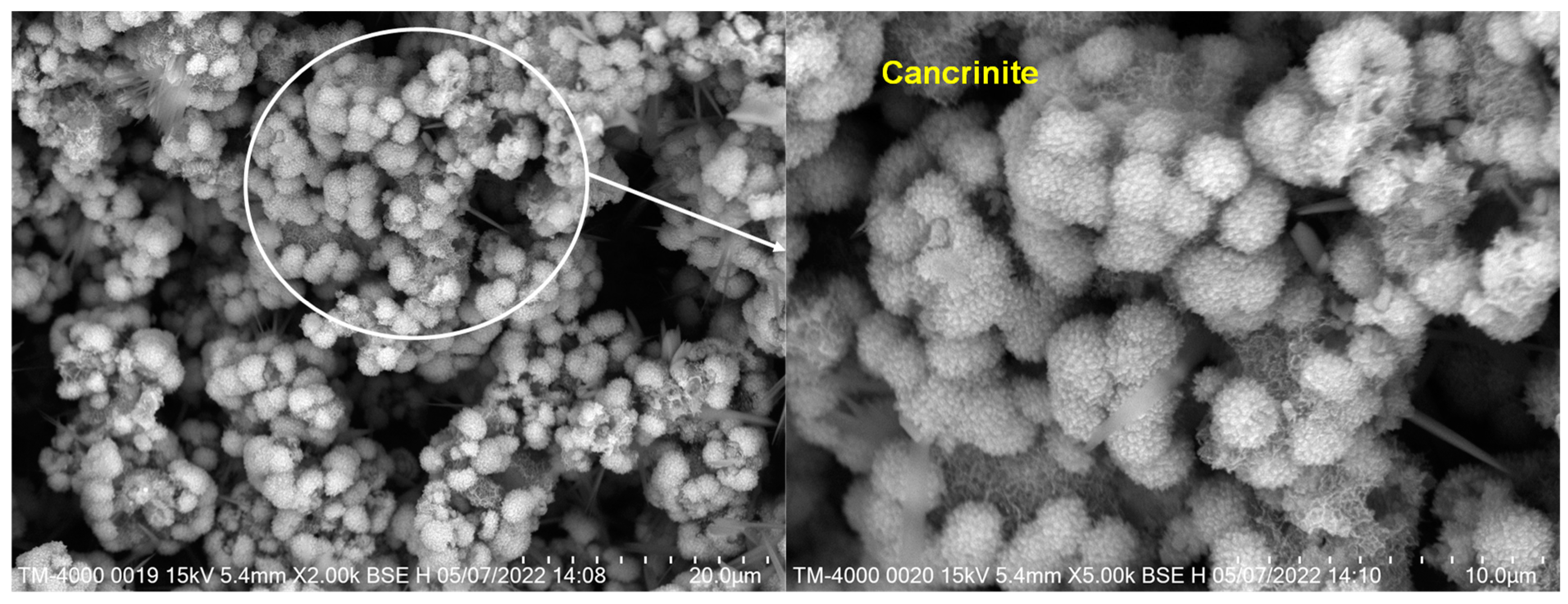
3.1.2. SEM
3.1.3. FTIR
3.2. Adsorption Performances
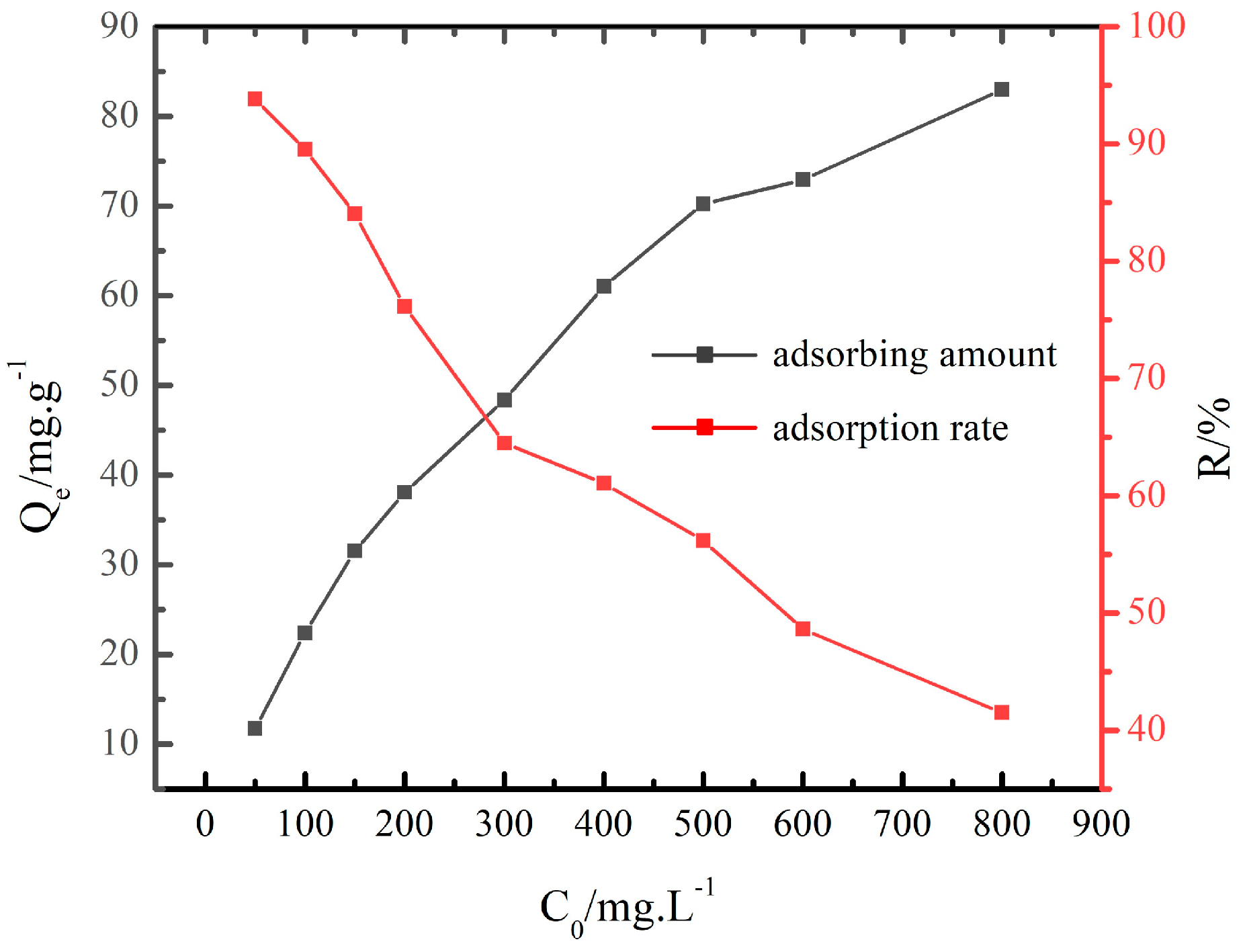
3.2.1. Adsorption Capacity and Adsorption Rate
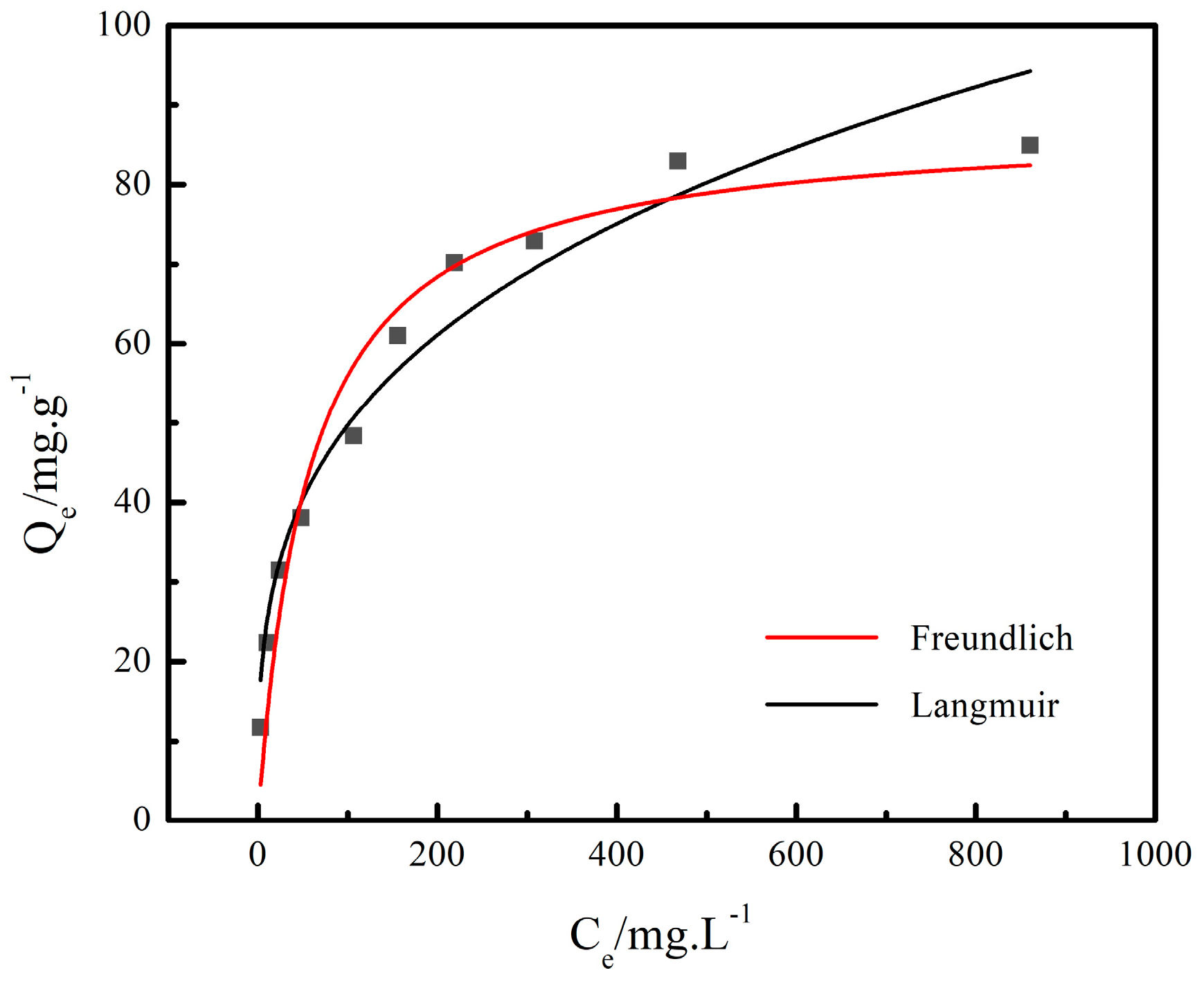
3.2.2. Adsorption Isotherm Model
3.3. Desorption Performances
4. Conclusions
- (1)
- In the low NaNO3 system (100 g/L), an increase in the NaOH concentration from 0.66 M to 2 M promoted the formation of Y-type zeolites and chabazite, while an increase in the NaOH concentration from 2 M to 8 M led to the transformation of zeolites into cancrinite. In the high NaNO3 (300~500 g/L) system, the increase in NaOH concentrations above 2 M had no obvious effect on the product transformation, and the products were mainly cancrinite and chabazite.
- (2)
- In the low NaNO3 system (100 g/L) with NaOH concentrations of 2 M, Y-type zeolite was formed at 90 °C. With the increase in curing temperatures (90~150 °C), the Y-type zeolite was firstly transformed into garronite and chabazite, and then cancrinite at last. It can be concluded that NaNO3 concentrations, NaOH concentrations, and curing temperatures all promote the crystallization of cancrinite and chabazite.
- (3)
- At the NaNO3 concentration of 100 g/L and 120 °C, the adsorption capacity of Cs+ decreased with the increase in the initial concentration of Cs+, and the adsorption rate of Cs+ increased with the increase in the initial concentration of Cs+ initial. The adsorption of Cs+ in the low initial concentration range was more suitable for the Freundlich equation, while the Langmuir equation fit the adsorption process at the high initial concentration range. The adsorption of Cs+ on the chabazite/garronite-rich geopolymer was dominated by physical and chemical adsorption at the same time.
- (4)
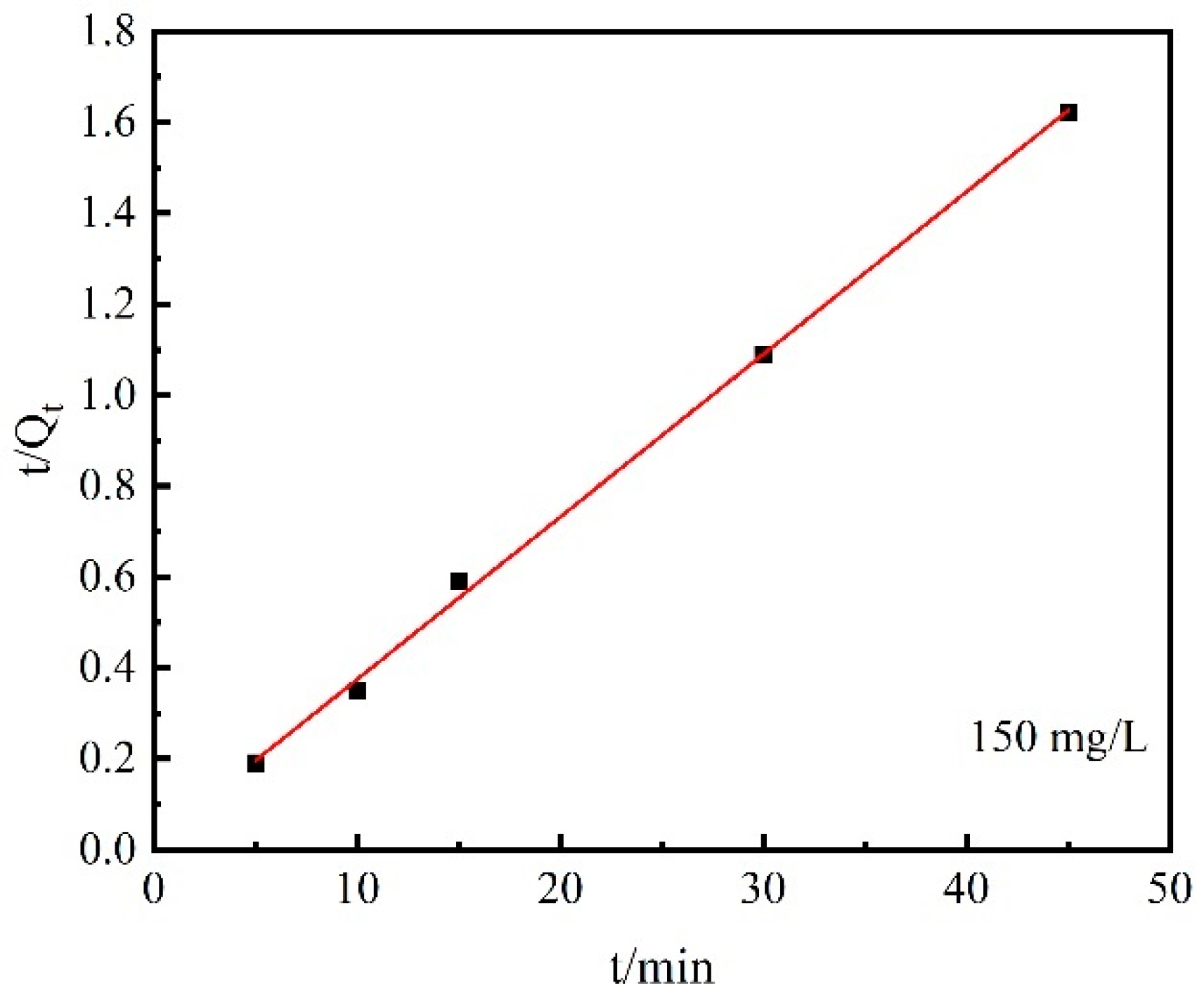
- In the range of 5~45 min, the desorption kinetic process of Cs+ on the chabazite/garronite-rich geopolymer was in good agreement with the pseudo-second-order equation. Indeed, the desorption of Cs+ on the chabazite/garronite-rich geopolymer was a chemical desorption.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kosowski, P.; Kosowska, K.; Janiga, D. Primary Energy Consumption Patterns in Selected European Countries from 1990 to 2021: A Cluster Analysis Approach. Energies 2023, 16, 6941. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; Macphee, D.; Lachowski, E.; Palomo, A. Immobilization of cesium in alkaline activated fly ash matrix. J. Nucl. Mater. 2005, 346, 185–193. [Google Scholar] [CrossRef]
- Fu, S.; He, P.; Wang, M.; Cui, J.; Wang, M.; Duan, X.; Yang, Z.; Jia, D.; Zhou, Y. Hydrothermal synthesis of pollucite from metakaolin-based geopolymer for hazardous wastes storage. J. Clean. Prod. 2019, 248, 119240. [Google Scholar] [CrossRef]
- Tyupina, E.A.; Kozlov, P.P.; Krupskaya, V.V. Application of Cement-Based Materials as a Component of an Engineered Barrier System at Geological Disposal Facilities for Radioactive Waste—A Review. Energies 2023, 16, 605. [Google Scholar] [CrossRef]
- He, P.; Wang, Q.; Fu, S.; Wang, M.; Zhao, S.; Liu, X.; Jiang, Y.; Jia, D.; Zhou, Y. Hydrothermal transformation of geopolymers to bulk zeolite structures for efficient hazardous elements adsorption. Sci. Total. Environ. 2021, 767, 144973. [Google Scholar] [CrossRef]
- Wang, C.F.; Li, J.S.; Wang, L.J.; Sun, X.Y. Influence of NaOH concentrations on synthesis of pure-form zeolite A from fly ash using two-stage method. J. Hazard. Mater. 2008, 155, 58–64. [Google Scholar] [CrossRef]
- Khalid, H.R.; Lee, N.K.; Park, S.M.; Abbas, N.; Lee, H.K. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard. Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef]
- Huseien, G.F.; Mirza, J.; Ismail, M.; Ghoshal, S.; Hussein, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Liu, Y.; Zhou, B. Synthesis and characterization of fly ash geopolymer paste for goaf backfill: Reuse of soda residue. J. Clean. Prod. 2020, 260, 121045. [Google Scholar] [CrossRef]
- Yarusova, S.; Shichalin, O.; Belov, A.; Azon, S.; Buravlev, I.Y.; Golub, A.; Mayorov, V.Y.; Gerasimenko, A.; Papynov, E.; Ivanets, A.; et al. Synthesis of amorphous KAlSi3O8 for cesium radionuclide immobilization into solid matrices using spark plasma sintering technique. Ceram. Int. 2021, 48, 3808–3817. [Google Scholar] [CrossRef]
- Shichalin, O.; Papynov, E.; Nepomnyushchaya, V.; Ivanets, A.; Belov, A.; Dran’kov, A.; Yarusova, S.; Buravlev, I.; Tarabanova, A.; Fedorets, A.; et al. Hydrothermal synthesis and spark plasma sintering of NaY zeolite as solid-state matrices for cesium-137 immobilization. J. Eur. Ceram. Soc. 2022, 42, 3004–3014. [Google Scholar] [CrossRef]
- Penilla, R.P.; Bustos, A.G.; Elizalde, S.G. Immobilization of Cs, Cd, Pb and Cr by synthetic zeolites from Spanish low-calcium coal fly ash. Fuel 2006, 85, 823–832. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Paprocki, A.; Ferret, L.S.; Azevedo, C.M.; Pires, M. Synthesis of zeolite Na-P1 under mild conditions using Brazilian coal fly ash and its application in wastewater treatment. Fuel 2015, 139, 59–67. [Google Scholar] [CrossRef]
- Wu, D.; Sui, Y.; Chen, X.; He, S.; Wang, X.; Kong, H. Changes of mineralogical–chemical composition, cation exchange capacity, and phosphate immobilization capacity during the hydrothermal conversion process of coal fly ash into zeolite. Fuel 2008, 87, 2194–2200. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Mu, J. Solidification/stabilization mechanism of Pb (II), Cd (II), Mn (II) and Cr (III) in fly ash based geopolymers. Constr. Build. Mater. 2018, 160, 818–827. [Google Scholar] [CrossRef]
- Wdowin, M.; Wiatros-Motyka, M.M.; Panek, R.; Stevens, L.A.; Franus, W.; Snape, C.E. Experimental study of mercury removal from exhaust gases. Fuel 2014, 128, 451–457. [Google Scholar] [CrossRef]
- Lei, H.; Muhammad, Y.; Wang, K.; Yi, M.; He, C.; Wei, Y.; Fujita, T. Facile fabrication of metakaolin/slag-based zeolite microspheres (M/SZMs) geopolymer for the efficient remediation of Cs+ and Sr2+ from aqueous media. J. Hazard. Mater. 2021, 406, 124292. [Google Scholar] [CrossRef]
- Lee, N.K.; Khalid, H.R.; Lee, H.K. Adsorption characteristics of cesium onto mesoporous geopolymers containing nano-crystalline zeolites. Microporous Mesoporous Mater. 2017, 242, 238–244. [Google Scholar] [CrossRef]
- Hui, K.S.; Chao CY, H.; Kot, S.C. Removal of mixed heavy metal ions in wastewater by zeolite 4A and residual products from recycled coal fly ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef]
- Querol, X.; Moreno, N.; Umaña, J.C.; Alastuey, A.; Hernández, E.; López-Soler, A.; Plana, F. Synthesis of zeolites from coal fly ash: An overview. Int. J. Coal Geol. 2002, 50, 413–423. [Google Scholar] [CrossRef]
- Kumar, M.M.; Jena, H. Direct single-step synthesis of phase pure zeolite Na-P1, hydroxy sodalite and analcime from coal fly ash and assessment of their Cs+ and Sr2+ removal efficiencies. Microporous Mesoporous Mater. 2022, 333, 111738. [Google Scholar] [CrossRef]
- Palomo, Á.; Alonso, S.; Fernandez-Jiménez, A.; Sobrados, I.; Sanz, J. Alkaline Activation of Fly Ashes: NMR Study of the Reaction Products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Zheng, Z.; Ma, X.; Zhang, Z.; Li, Y. In-situ transition of amorphous gels to Na-P1 zeolite in geopolymer: Mechanical and adsorption properties. Constr. Build. Mater. 2019, 202, 851–860. [Google Scholar] [CrossRef]
- Ren, X.; Liu, S.; Qu, R.; Xiao, L.; Hu, P.; Song, H.; Wu, W.; Zheng, C.; Wu, X.; Gao, X. Synthesis and characterization of single-phase submicron zeolite Y from coal fly ash and its potential application for acetone adsorption. Microporous Mesoporous Mater. 2019, 295, 109940. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, Y.; Sun, H.; Zhang, Z.; Ma, X. Coupling effect of NaOH and NaNO3 on the solidified fly ash-cement matrices containing Cs+: Reaction products, microstructure and leachability. J. Nucl. Mater. 2020, 539, 152252. [Google Scholar] [CrossRef]
- Aono, H.; Takeuchi, Y.; Itagaki, Y.; Johan, E. Synthesis of chabazite and merlinoite for Cs+ adsorption and immobilization properties by heat-treatment. Solid State Sci. 2019, 100, 106094. [Google Scholar] [CrossRef]
- Munthali, M.W.; Johan, E.; Aono, H.; Matsue, N. Cs+ and Sr2+ adsorption selectivity of zeolites in relation to radioactive decontamination. J. Asian Ceram. Soc. 2015, 3, 245–250. [Google Scholar] [CrossRef]
- Perná, I.; Šupová, M.; Hanzlíček, T.; Špaldoňová, A. The synthesis and characterization of geopolymers based on metakaolin and high LOI straw ash. Constr. Build. Mater. 2019, 228, 116765. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.S.; Zhan, B.J.; Sharma, U.; Poon, C.S. Compressive strength and microstructural properties of dry-mixed geopolymer pastes synthesized from GGBS and sewage sludge ash. Constr. Build. Mater. 2018, 182, 597–607. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Zhao, J.; Zhang, Z.; Wang, H.; Zhou, S.; Wu, L. Synthesis of zeolite P1 from fly ash under solvent-free conditions for ammonium removal from water. J. Clean. Prod. 2018, 202, 11–22. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Majd, M.M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption isotherm models: A comprehensive and systematic review (2010−2020). Sci. Total. Environ. 2021, 812, 151334. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Hou, L.; Liu, J.; Li, J.; Lu, Z.; Niu, Y. Adsorption and enrichment of simulated 137Cs in geopolymer foams. J. Environ. Chem. Eng. 2021, 9, 105733. [Google Scholar] [CrossRef]
- Allaoui, M.; Berradi, M.; Bensalah, J.; Es-Sahbany, H.; Dagdag, O.; Ibn Ahmed, S. Study of the adsorption of nickel ions on the sea shells of Mehdia: Kinetic and thermodynamic study and mathematical modelling of experimental data. Mater. Today Proc. 2021, 45, 7494–7500. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, Y.; Wang, Q.; Chang, Q.; Li, C. Rapid removal of methylene blue and nickel ions and adsorption/desorption mechanism based on geopolymer adsorbent. Colloid Interface Sci. Commun. 2021, 45, 100551. [Google Scholar] [CrossRef]
- Deng, H.; Li, Y.; Huang, Y.; Ma, X.; Wu, L.; Cheng, T. An efficient composite ion exchanger of silica matrix impregnated with ammonium molybdophosphate for cesium uptake from aqueous solution. Chem. Eng. J. 2016, 286, 25–35. [Google Scholar] [CrossRef]
- Deng, H.; Li, Y.; Wu, L.; Ma, X. The novel composite mechanism of ammonium molybdophosphate loaded on silica matrix and its ion exchange breakthrough curves for cesium. J. Hazard. Mater. 2017, 324, 348–356. [Google Scholar] [CrossRef]




| Oxide Composition | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | Other |
|---|---|---|---|---|---|---|---|
| Content (%) | 59.79 | 21.99 | 5.55 | 5.13 | 3.21 | 1.35 | 2.98 |
| 60 °C Water Bath for 8 h; Curing at 90 °C for 24 h | |||||
|---|---|---|---|---|---|
| Sample Number | NaNO3 (g/L) | NaNO3 (g) | W/F | NaOH (g) | Fly Ash (g) |
| 100N-0.66H | 100 | 4.15 | 8 | 1.06 | 5 |
| 100N-2H | 100 | 4.15 | 8 | 3.23 | 5 |
| 100N-4H | 100 | 4.15 | 8 | 6.45 | 5 |
| 100N-6H | 100 | 4.15 | 8 | 9.68 | 5 |
| 100N-8H | 100 | 4.15 | 8 | 12.90 | 5 |
| 300N-0.66H | 300 | 13.55 | 8 | 1.06 | 5 |
| 300N-2H | 300 | 13.55 | 8 | 3.23 | 5 |
| 300N-4H | 300 | 13.55 | 8 | 6.45 | 5 |
| 300N-6H | 300 | 13.55 | 8 | 9.68 | 5 |
| 300N-8H | 300 | 13.55 | 8 | 12.90 | 5 |
| 500N-0.66H | 500 | 24.90 | 8 | 1.06 | 5 |
| 500N-2H | 500 | 24.90 | 8 | 3.23 | 5 |
| 500N-4H | 500 | 24.90 | 8 | 6.45 | 5 |
| 500N-6H | 500 | 24.90 | 8 | 9.68 | 5 |
| 500N-8H | 500 | 24.90 | 8 | 12.90 | 5 |
| Stirring in Water Bath at 60 °C for 8 h, Curing at Different Temperatures for 24 h | |||||
|---|---|---|---|---|---|
| Sample Number | NaNO3 (g/L) | NaNO3/g | W/F | NaOH/g | Fly Ash/g |
| 100N-2H-60 °C | 100 | 4.15 | 8 | 3.23 | 5 |
| 100N-2H-90 °C | 100 | 4.15 | 8 | 3.23 | 5 |
| 100N-2H-120 °C | 100 | 4.15 | 8 | 3.23 | 5 |
| 100N-2H-150 °C | 100 | 4.15 | 8 | 3.23 | 5 |
| Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL | R2 | n | KF (mg/g) | R2 | |
| T120 | 87.91 | 1.75 × 10−2 | 0.95 | 3.36 | 12.64 | 0.96 |
| Desorption Time (min) | 5 | 10 | 15 | 30 | 45 | 60 |
|---|---|---|---|---|---|---|
| Desorption rate (%) | 83.38 | 90.63 | 80.52 | 87.73 | 88.25 | 101.74 |
| Desorption capacity (mg/g) | 26.28 | 28.56 | 25.38 | 27.65 | 27.81 | 32.06 |
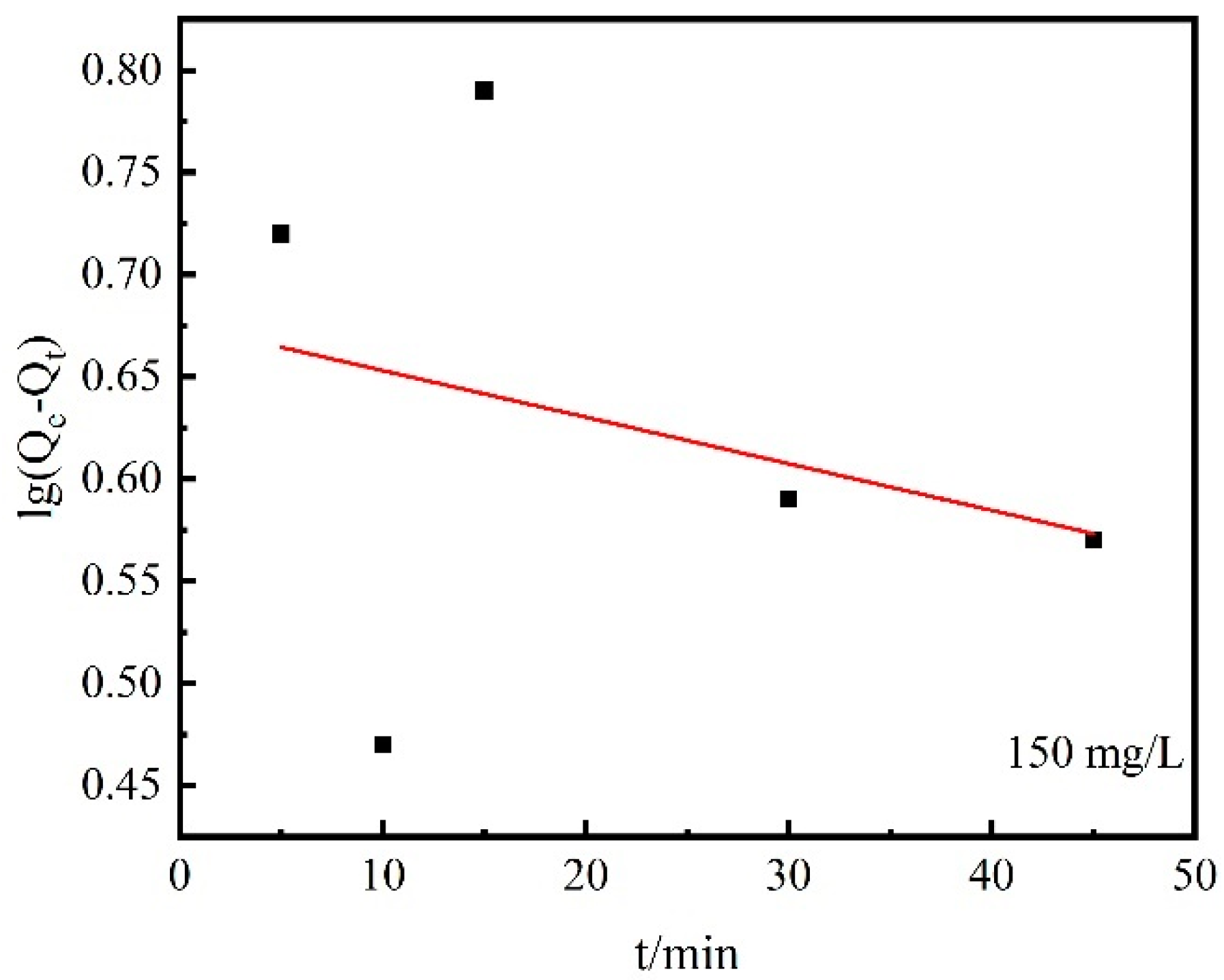
| Kinetic Equation | Desorption Kinetic Equation | Desorption Rate | Theoretical Equilibrium Desorption Capacity/mg·g−1 | R2 |
|---|---|---|---|---|
| Pseudo-first-order | lg(Qe − Qt) = 0.68 − 2.28 × 10−3 t | 5.25 × 10−3 min−1 | 4.74 | 0.09 |
| Pseudo-second-order | t/Qt =1.71 × 10−2 + 3.576 × 10−2 t | 7.48 × 10−2 mg/(g·min−1) | 27.96 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Yang, J.; Cui, M.; Yang, K.; Shang, H.; Ma, X.; Li, Y. Adsorption/Desorption Performances of Simulated Radioactive Nuclide Cs+ on the Zeolite-Rich Geopolymer from the Hydrothermal Synthesis of Fly Ash. Energies 2023, 16, 7815. https://doi.org/10.3390/en16237815
Zheng Z, Yang J, Cui M, Yang K, Shang H, Ma X, Li Y. Adsorption/Desorption Performances of Simulated Radioactive Nuclide Cs+ on the Zeolite-Rich Geopolymer from the Hydrothermal Synthesis of Fly Ash. Energies. 2023; 16(23):7815. https://doi.org/10.3390/en16237815
Chicago/Turabian StyleZheng, Zhao, Jun Yang, Maoxuan Cui, Kui Yang, Hui Shang, Xue Ma, and Yuxiang Li. 2023. "Adsorption/Desorption Performances of Simulated Radioactive Nuclide Cs+ on the Zeolite-Rich Geopolymer from the Hydrothermal Synthesis of Fly Ash" Energies 16, no. 23: 7815. https://doi.org/10.3390/en16237815
APA StyleZheng, Z., Yang, J., Cui, M., Yang, K., Shang, H., Ma, X., & Li, Y. (2023). Adsorption/Desorption Performances of Simulated Radioactive Nuclide Cs+ on the Zeolite-Rich Geopolymer from the Hydrothermal Synthesis of Fly Ash. Energies, 16(23), 7815. https://doi.org/10.3390/en16237815






