Abstract
The management of wastewater from soilless tomato cultivation poses a technological and economic challenge. Given the above, the aim of this study was to determine the treatment efficiency of wastewater from soilless tomato cultivation in a bio-electrochemical reactor under conditions of direct electric current flow. The treatment efficiency was tested in three time variants of wastewater exposure to the electric current: V1—24 h exposure phase; V2—12 h exposure phase/12 h no exposure phase; and V3—12 h no exposure phase/12 h exposure phase. Experiments were conducted with two organic substrates, sodium acetate and acetic acid, at the C/N ratio of 1.25, with a direct current intensity of 1.25 A·m−2 and hydraulic retention time of 24 h. The study results show the feasibility of achieving a satisfactory technological effect in a bio-electrochemical reactor without the need for electric current flow throughout the 24 h treatment cycle. From the energy consumption and technological standpoints, the most viable approach, ensuring 90.4 ± 1.6% and 94.9 ± 0.7% efficiencies of nitrogen and phosphorus removal, respectively, turned out to be feeding the reactor with sodium acetate and wastewater exposure to the electric current flow only during the first 12 h of the treatment cycle. The scope of the conducted research justifies its continuation in order to determine the optimal time for supplying electricity to the bio-electrochemical reactor and the impact of the C/N value on the nitrogen and COD effluent concentrations.
1. Introduction
Due to the specific composition of drainage water from the soilless cultivation of crops, including tomatoes, that have high concentrations of nitrates and phosphorus and a low concentration of organic compounds [1], its treatment by means of conventional biological methods based on activated sludge is extremely difficult [2]. For this reason, alternative treatment methods are sought, like bio-treatment aided with an external organic substrate [3,4], using microalgae [5] and wetlands [6], and deploying hydrogenotrophic denitrification aided by direct electric current [7] or alternating electric current [8]. Owing to the combination of biological and electrochemical processes and the supply of carbon from various sources, it is feasible to provide appropriate conditions for hydrogenotrophic denitrification, heterotrophic and electrochemical nitrate reduction, and the electrocoagulation of phosphorus in one reactor. This has also been confirmed in a study by Rodziewicz et al. [7], who showed that the use of an electrobiological disc contactor enabled the removal of 99.8% of phosphorus and 53.4% of nitrogen from synthetic greenhouse wastewater. In the cited study, sodium acetate served as the external source of carbon and the electric current density used was 10.0 A·m−2, whereas the hydraulic wastewater retention time (HRT) was 24 h. A not very high reduction in nitrogen concentration was achieved in the aerobic reactor, namely a bio-electrochemical disc contactor, which showed that there is a need to search for a technological solution that would ensure better control of aerobic conditions. Such a reactor can be a Sequential Batch Biofilm Reactor (SBBR) with a biological membrane.
In the study by Bryszewski et al. [9], in view of the conclusions drawn from the work of Rodziewicz et al. [7], the SBBR was used to treat drainage from soilless tomato cultivation, with sodium acetate as the external carbon source. Depending on the aerobic conditions applied in the reactor, the efficiency of the removal of total nitrogen, total phosphorus, and organic compounds expressed by chemical oxygen demand (COD) reached 81% (anoxic reactor), 91% (aerobic reactor), and 63% (anoxic-aerobic reactor), respectively. In the experimental variant with HRT = 24 h, the electric current flew between the reactor’s electrodes throughout the entire treatment cycle.
In the reactors with the current flow, nitrogen removal proceeded via three processes, i.e., the commonly known and implemented heterotrophic denitrification, autotrophic denitrification (in this case, hydrogenotrophic denitrification), and electrochemical nitrate reduction. The efficiency of hydrogenotrophic denitrification, which takes place upon the activity of hydrogenotrophs that use gaseous hydrogen (electron donor) produced during water electrolysis on the cathode or anode, results from the current flow [10,11].
The course of hydrogenotrophic denitrification depends on such factors as pH, temperature, HRT, and amounts of inorganic carbon and dissolved hydrogen. Its efficiency is also largely determined by the electric current density. The literature describes investigations addressing the removal of nitrogen in bio-electrical reactors conducted in a broad range of electric current densities. Safari et al. [12] tested densities ranging from 2 mA·cm−2 to 32 mA·cm−2, and found that nitrogen was most efficiently removed at 8 mA·cm−2. According to Park et al. [13], the highest rate of nitrate reduction in a bio-electrical reactor, reaching 0.17 mg N-NO3·cm−2·d−1, was achieved under the flow of electric current with a density of 19 A·m−2. In turn, Tong et al. [14] applied current densities of 0–1.6 A·m−2 and achieved the highest nitrate removal rate at 400 mA·m−2. Investigations conducted by Kłodowska et al. [15] demonstrated a high total nitrogen removal efficiency, reaching 85%, at a current density of 210 mA·m−2. Unfortunately, too-high current density affected the population of microorganisms in the reactor [16]. In the study conducted by Wei et al. [17], the number of live bacteria decreased by 10%, 15%, and 29% at current densities of 6.7 A·m−2, 12.3 A·m−2, and 24.7 A·m−2, respectively, with reactors operating at a continuous current flow.
The flow of electric current through the electrodes contributes not only to the course of nitrate reduction but also to the electrocoagulation process [18]. Ghazouani et al. [19] treated synthetic sewage using an electric current with densities from 1 mA·cm−2 to 40 mA·cm−2. The initial nitrate nitrogen concentration was 350 mg N·L−1 and the phosphate concentration was 50 mg P·L−1. The efficiencies of removing nitrogen and phosphorus compounds in the studied range of current densities were 37–99% and 51–99%, respectively. Despite the high nitrate nitrogen removal efficiency, the process produced ammonium ion, which at the highest current density tested accounted for about 90% of total nitrogen. Electric current density is also a factor that can directly affect the efficiency of electrocoagulation, as it allows the amount of coagulant that enters the solution to be controlled. The equivalent mass of aluminum and iron resulting from anode dissolution is 335.6 mg·A−1·h−1 and 1.014 mg·A−1·h−1, respectively. It should be noted that the current density directly affects the pH and temperature of the process [20]. The range of densities applied is broad. Depending on the source, the density of the current applied in phosphorus removal has been reported to range from 1 A·m−2 to 50 A·m−2 [21]. Omwene et al. [22] managed to remove 99% of phosphorus from household sewage upon the use of an electric current with densities ranging from 10 to 40 A·m−2. However, the time needed to achieve such a high efficiency varied and was 100 min for the current density of 10 A·m−2 and 50 min for the current density of 40 A·m−2. This is due to the fact that the amount of coagulant produced depends not only on the current density but also on the duration of the current flow.
In periodic bio-electrochemical reactors, a whole series of processes takes place within one treatment cycle, both in parallel and in series. The course and efficiency of many of them depends on the presence of intermediate or final products of processes which commenced at the beginning of the cycle. Most of these processes are affected by the current flow between the electrodes [23]. The literature presents data on the operation of bio-electrochemical reactors, in which the current is supplied during the entire cycle [9,12,13,14,15,16,17]. However, there are no reports on the effect of the length of the current flow phase, which is a fraction of the cycle duration and phase sequence in the cycle (phase with current flow, phase without current flow), on the removal efficiency of biogenic and organic compounds.
Given the above, the aim of this study was to determine the treatment efficiency of wastewater from soilless tomato cultivation in a bio-electrochemical reactor under conditions of direct electric current flow, including effects of flow phase duration and flow phase sequence. The scope of the research included determining the efficiency of removing nitrogen, phosphorus and organic compounds in the bio-electrochemical reactor depending on the duration and mode of reactor supply with electric current and on the type of external carbon source used.
2. Materials and Methods
2.1. Reactors
Six sequencing batch biofilm reactors (SBBR) were used for the treatment of wastewater from soilless tomato cultivation. A single SBBR had an anode made of iron and a cathode made of stainless-steel discs mounted on a rotating vertical shaft. The electrodes were connected to a direct current power supply (Rohde & Schwarz HMP 4040, Munich, Germany). The technological parameters of the reactor were as follows: number of discs—5; diameter of a single disc—12 cm; disc thickness—1 mm; distance between discs—1.5 cm; total disc surface area—0.113 m2; active volume of the reactor—2 L; number of disc revolutions—14 rpm; and immersion of discs—100%. The construction of the reactor allowed for the exchange of the entire volume of the treated wastewater. Iron anodes were used in the study because they are less susceptible to digestion and, as a result, allow less sludge to be generated than aluminum electrodes [24]. Activated sludge from the denitrification chamber of the Municipal Sewage Treatment Plant “Łyna” in Olsztyn was used as the inoculum. The adaptation period lasted 3 months, after which the appropriate tests began. During the adaptation period, the same reactor operating parameters were used as during the actual tests.
2.2. Organization of Experiments
The experiment aimed to determine the influence of direct electric current flow on the efficiency of wastewater treatment in a bio-electrochemical reactor in three time variants of wastewater exposure to the electric current: V1—24 h exposure phase; V2—12 h exposure phase/12 h no exposure phase; and V3—12 h no exposure phase/12 h exposure phase.
The electric current density chosen based on the results of previous studies was 1.25 A·m2. At this density, the dephosphatation efficiency exceeded 92% and, at the same time, the specific energy consumption per unit load of removed biogenic compounds was significantly lower than at densities of 2.5 and 5.0 A·m2 [23].
Two types of organic substrate were fed into the reactors, i.e., sodium acetate in reactors R1–R3 and acetic acid in reactors R4–R6 (Figure 1). The literature indicates the technological advantages of these types of external carbon sources [8,23]. A carbon dose of C/N = 1.5 was applied in the experiments, with 100% of the organic substrate dose delivered at the beginning of the cycle, which according to previous studies guarantees a very high removal efficiency of both nitrogen and phosphorus compounds and at the same time does not cause high concentrations of organic compounds in the effluent [8].
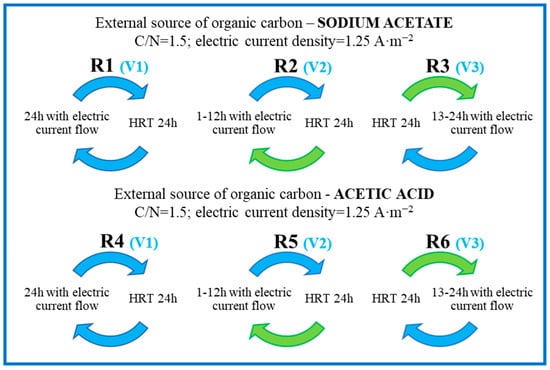
Figure 1.
Study design.
It was assumed that the concentrations of contaminants would vary in the effluent depending on which phase of the treatment cycle was being conducted with direct electric current flow.
2.3. Drainage Water
Experiments were conducted with real horticultural drainage from a greenhouse (surface area of approximately 200,000 m2), wherein tomatoes are grown on a substratum from mineral wool [1]. The physicochemical indicators of the drainage water were as follows: total organic carbon (TOC) 15.6 ± 3.1 mg C∙L−1; total nitrogen (TN) 536.9 ± 10.7 mg N∙L−1; nitrates 535.71 ± 7.40 mg N∙L−1; nitrites 0.090 ± 0.010 mg N∙L−1; ammonia nitrogen 1.1 ± 0.2 mg N∙L−1; total phosphorus (TP) 103.8 ± 5.3 mg P∙L−1; pH 6.21; and electrolytic conductivity (EC) 6.213 ± 0.202 mS∙cm−1.
Table 1 shows the TOC, pH, and EC values in the effluent after organic substrate addition.

Table 1.
The pH value, electrolytic conductivity, and total organic carbon concentration after organic substrate addition to the wastewater.
2.4. Analytical Methods
The following physicochemical analyses were conducted for the wastewater inflow to the reactors and for the treated effluent: total organic carbon (TOC) concentration using the TOC-L CPH/TNM-L analyzer (Shimadzu Corporation, Kioto, Japan) with the method of “oxidative combustion with infrared analysis”; total nitrogen concentration using the TOC-L CPH/TNM-L analyzer (Shimadzu Corporation, Japan) with the “oxidizing combustion–chemiluminescence” method; ammonia nitrogen concentration with the HACH Lange LCK 303–304 method; nitrite concentration with the HACH Lange LCK 341–342 method; nitrate concentration with the HACH Lange LCK 339–340 method; total phosphorus concentration with the HACH Lange LCK 348–350 method; iron concentration with the HACH Lange LCK 321 and LCK 521 methods; pH value, electrolytic conductivity and temperature using the HQ 4300 multi-parameter meter (Hach, Germany).
Statistical analysis of the results obtained was performed using the STATISTICA 13.3 PL software, at a significance level of α = 0.05.
3. Results and Discussion
3.1. pH, EC, and Iron Content
The pH value of the effluent after organic substrate addition was 7.13 and 3.88 in the experimental variants, with sodium acetate and acetic acid, respectively, used as substrates (Figure 2). In the sodium acetate variant, the effluent pH ranged from 8.00 in R1 to 8.60 in R2, whereas in the acetic acid variant the lowest pH reached 3.65 (R4) and the highest one reached 7.16 (R5). A high pH value (6.89) was also noted in the R6 reactor with acetic acid fed as the substrate. The increase in pH values measured in the reactors was due to an increase in alkalinity caused by denitrification. In the case of bio-electrochemical reactors fed with acetic acid, the consumption of this substrate by microorganisms was also of great importance. The final pH of the treated wastewater was also affected by the formation of H+ ions upon water electrolysis and the resultant pH decrease [25]. Therefore, there was no pH increase in R4 despite denitrification (low efficiency of the acid substrate consumption and H+ ion production throughout the cycle).
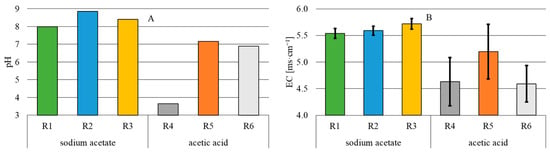
Figure 2.
pH value (A) and electrolytic conductivity (B) of the effluent.
The electrolytic conductivity of the wastewater fed to the reactor was 6.213 ± 0.202 mS·cm−1, whereas after organic substrate addition it reached 6.101 ± 0.247 mS·cm−1 in the acetic acid variant and 7.421 ± 0.229 mS·cm−1 in the sodium acetate variant. The EC of the effluent treated in the sodium acetate variant ranged from 5.539 ± 0.093 mS·cm−1 to 5.717 ± 0.098 mS·cm−1 (Figure 2B). In the acetic acid variant, the highest EC value was recorded in R5, where it reached 5.196 ± 0.515 mS·cm−1. The reactors with 24 h current flow and those with 12 h current flow in the second phase of the treatment cycle had similar electrolytic conductivity, reaching 4.631 ± 0.451 mS·cm−1 (in R4) and 4.592 ± 0.343 mS·cm−1 (in R6), respectively. The EC in the sodium acetate variant was higher compared to the variants with acetic acid used as the substrate. This was due to EC values of the solutions used as carbon sources (30.900 mS·cm−1 sodium acetate solution; 1.106 mS·cm−1 acetic acid solution). The presented results show that, despite providing an additional load of organic compounds, the effluent had a lower EC value compared to the influent. The decrease in electrolytic conductivity is due to the consumption of organic substrate and the removal of some of the anions and cations present in wastewater [7,8,26], as well as dilution caused by the introduction of organic substrate (R4–R6).
The total iron concentration in the influent was 0.080 ± 0.010 mg Fe·L−1 and did not increase in the effluent after the electrochemical dissolution of electrodes only in R3, where it reached 0.058 ± 0.010 mg Fe·L−1 (Figure 3). In the sodium acetate variant, the highest total iron concentration was recorded in R1 with a 24 h flow of electric current, i.e., 0.210 ± 0.130 mg Fe·L−1. Similarly, in the acetic acid variant, the highest concentration of total iron, reaching 0.860 ± 0.370 mg Fe·L−1, was determined in R4 with 24 h electric current exposure. The lowest concentration of total iron in the acetic acid variant was recorded in reactor R6 (0.170 ± 0.080 mg Fe·L−1).
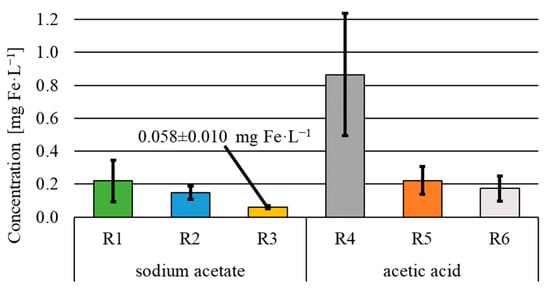
Figure 3.
Concentration of total iron in the effluent.
The increase in the total iron concentration in the treated wastewater was due to the iron anode dissolution. The increase in wastewater contamination with iron observed in the present study was, however, small compared to the literature data. Tejera et al. [27], who treated leachate from a municipal landfill at current densities of 5 mA·cm−2 and 10 mA·cm−2, determined iron concentration in the treated effluent at 100 mg·L−1 and 200 mg·L−1, respectively. In turn, Krystynik et al. [28] removed chromium and nickel ions during the treatment of groundwater from a metallurgical plant and found a residual iron concentration in the effluent between 0.2 and 2.2 mg Fe·L−1 at a current density of 11.42 mA·cm−2. In their study, they used a flow-through electrocoagulation cell with a flow rate of 350 L·h−1.
3.2. Removal of Organic Compounds
Experiments were conducted with an organic carbon dose of C/N 1.5, 100% of which was fed to the reactors at the beginning of the treatment cycle. This mode of organic carbon feeding caused the concentration of organic compounds (Table 1) to increase to mean values of 804.3 ± 7.9 mg C·L−1 (R1–R3) and 806.2 ± 26.5 mg C·L−1 (R4–R6).
The efficiency of sodium acetate consumption (Figure 4) was at a very similar level in all variants of electric current flow and ranged from 96.7 ± 0.6% (R1) to 97.3 ± 1.0% (R3). The efficiency of acetic acid consumption was significantly lower and amounted to 65.6 ± 1.0% under a 24 h flow of electric current. In the case of a 12 h current flow, the efficiency of organic substrate consumption in R5 and R6 was similar and reached 80.4 ± 2.3% and 82.5 ± 1.8%, respectively. The concentration of organic compounds in the effluent corresponding to the above efficiencies ranged from 21.7 ± 8.2 mg C·L−1 (R3) to 26.3 ± 4.9 mg C·L−1 (R1) in the sodium acetate variant and from 141.1 ± 14.7 mg C·L−1 (R6) to 277.2 ± 28.7 mg C·L−1 (R4) in the acetic acid variant. The statistical analysis did not show any significant effect of the electric current flow on the concentration of organic compounds in the treated wastewater in the case of sodium acetate used as the substrate and a statistically significant effect of the electric current in the case of reactors fed with acetic acid. Statistical differences were found between reactor R4 (current flow through the whole treatment cycle—24 h) and reactors R5 and R6 (current flow in the first and second phase of the cycle, respectively). The conducted study demonstrated a reduction in the consumption of organic compounds upon the flow of electric current, when acetic acid was used as the organic substrate. No negative effect (α = 0.05) of the electric current flow was found in the reactors fed with sodium acetate.
Figure 4.
Efficiency of the consumption of organic compounds.
As a result of the electric current flow in the reactor fed with acetic acid, the efficiency of organic substrate consumption was lower because acetic acid causes a pH decrease. At the same time, the pH value drops due to the flow of electric current [25]. The appropriate pH for heterotrophic and autotrophic denitrification is within the ranges of 7.5–9.5 and 6.5–8.6 [23,24,25], respectively. The pH of wastewater in reactor R4 was 3.65. According to the literature data, autotrophic and heterotrophic denitrification is slowed down and even arrested at pH levels below 6 [29]. In the bio-electrochemical reactors (R1–R6), some organic compounds may also have been oxidized via electrochemical oxidation (oxygen production on the anode) and electrocoagulation [30].
Benhadji et al. [31] removed from 73% to 89% of organic compounds expressed by COD from leather industry wastewater using an iron anode and applying an electric current with a density of 150 A·m−2. The initial COD was 7.680 mg O2·L−1, and the current flow duration was 15 to 75 min. In turn, Eyvaz [32] removed 83% of organic compounds during the electrocoagulation of brewing wastewater (at the initial COD concentration reaching 3.440 mg O2·L−1). The electric current density applied was 150 A·m−2, the process spanned 15 min, and the initial wastewater pH was 7.
3.3. Removal of Nitrogen Compounds
The mean concentration of total nitrogen in the influent was 536.9 ± 10.7 mg N·L−1. The lowest removal efficiencies (Figure 5) determined in the experiments with sodium acetate and acetic acid used as substrates and with a 24 h current flow were 81.8 ± 1.0% (R1) and 39.7 ± 5.5% (R4), respectively. Similarly and at the same time, the highest removal efficiencies were obtained using sodium acetate in R2 and R3, i.e., 90.4 ± 1.6% (R2) and 89.9 ± 1.0% (R3), respectively.
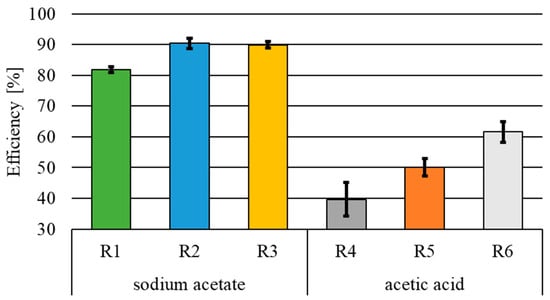
Figure 5.
Total nitrogen removal efficiency.
The main form of nitrogen present in the influent was nitrate nitrogen, which accounted for 99.78% of total nitrogen. The highest concentration of total nitrogen in the effluent from the reactor fed with sodium acetate was determined in R1, where it reached 97.7 ± 5.4 mg N·L−1. In the case of effluents from R2 and R3, it was 51.8 ± 8.8 mg N·L−1 and 54.2 ± 5.2 mg N·L−1, respectively. In the case of R1, the main form of nitrogen, accounting for 98.40%, was nitrate nitrogen. In reactors R2 and R3, nitrogen occurred mainly in the form of nitrite nitrogen, the percentages of which were 74.28% and 86.63%, respectively. In the acetic acid variant, the concentration of total nitrogen ranged from 206.4 ± 18.3 mg N·L−1 (R6) to 323.8 ± 29.5 mg N·L−1 (R4), and the main form of nitrogen in the effluent was nitrate nitrogen, accounting for 68.00% in R6 to 99.94% in R4.
The statistical analysis showed a significant influence of wastewater exposure to the electric current on the concentration of total nitrogen in the effluent from the reactors with both sodium acetate and acetic acid used as substrates. In the case of the sodium acetate variant, the 12 h electric current flow in R2 and R3 had no statistically significant effect on the total nitrogen concentration in the effluent. At the same time, this parameter was statistically significantly (α = 0.05) affected by the type of organic carbon source used.
The obtained efficiency of total nitrogen removal was mainly due to the biological processes. At the applied C/N ratio of 1.5, heterotrophic and autotrophic denitrification turned out to be responsible for the removal of nitrogenous compounds. A carbon/nitrogen ratio above 0.5 increases the contribution of heterotrophic denitrification in the treatment process. Below this value (C/N < 0.5), autotrophic processes are superior in bio-electrochemical systems [33]. As reported by Hao et al. [34], nitrogen removal as a result of simultaneous heterotrophic and autotrophic denitrification is possible at C/N from 1 to 3. In this C/N range, at a current intensity of 40 mA, the cited researchers removed from 62.2% to 98.3% of nitrates from synthetic domestic sewage with an initial nitrogen concentration of 30 mg N·L−1.
During 24 h exposure of wastewater to the electric current, the lowest nitrogen removal efficiencies were recorded among all determined values in the reactors fed with both acetic acid (R4) and sodium acetate (R1). These results suggest that a 24 h flow of electric current with a density of 1.2 A·m−2 inhibited the heterotrophic denitrification, which was the main process responsible for nitrogen removal. This is due to the fact that during the electric current flow, water electrolysis generates hydrogen, which is an electron donor during hydrogenotrophic denitrification. However, its too-high concentration can be harmful to biofilm growth and metabolism. In addition, the formation of excess amounts of hydrogen can destroy biofilm structures [35]. According to Lin et al. [36], the high concentration of hydrogen causes its adhesion to the surface of microorganisms, thereby negatively affecting the transport of nitrates to the deeper layers of the biofilm. In turn, as Tong et al. [14] claimed, the stimulation of heterotrophic bacteria with electric current has a positive effect on the viability of microorganisms and allows the efficiency of nitrate removal to be increased. However, the too-high current density applied, which according to these authors was 1.6 A·m−2, resulted in the inhibition of denitrification. In the study conducted by Zhu et al. [37], despite applying an electric current with densities of 10.5 A·m−2 to 315.8 A·m−2, no negative effect of hydrogen was observed in the treatment of synthetic groundwater at C/N = 1.5 and a nitrate nitrogen content of 35 mg N·L−1. The nitrogen removal efficiency in the above-mentioned current density range was similar, ranging from 92.4% (315.8 A·m−2) to 93.2% (10.5 A·m−2). The low nitrogen removal efficiency noted in R4 may also be due to the low pH, which in this reactor was in the range of 3.04–4.58, which is well below optimal for both autotrophic (6.5–8.6) and heterotrophic (7.5–9.5) denitrification. At the same time, the analysis of the literature data suggests that the effectiveness of aiding nitrogen removal through the use of electric current may be determined by the type and associated quantitative and qualitative composition of the treated wastewater and other factors, such as the type of material the cathode and anode are made of.
In the bio-electrochemical reactors with 12 h electric current flow in both the first and second phases of the treatment cycle, an increase was recorded in the concentration of nitrites (Figure 6). The literature data indicates that the reasons for nitrite accumulation during heterotrophic denitrification may include a high nitrate concentration, a high oxygen concentration, inadequate wastewater retention time, and both low and high pH, as well as the composition of the microbiological community [38,39]. In addition, during autotrophic denitrification, the accumulation of nitrites is influenced by the concentration of hydrogen [40]. Both too high and too low H2 levels increase the share of nitrites during autotrophic denitrification. Probably, at the 12 h current flow, the amount of hydrogen produced may have been too low to ensure efficient hydrogenotrophic denitrification. Also, Chang et al. [41] demonstrated a significant effect of hydrogen concentration on nitrite accumulation during hydrogenotrophic denitrification. They showed the inhibition of nitrite reductase at a hydrogen concentration lower than 0.2 mg H2·L−1, and that of nitrate reductase at a hydrogen concentration below 0.1 mg H2·L−1. In addition, according to these authors, nitrite reductase is more sensitive to changes in hydrogen concentration, thus causing the accumulation of nitrites. The optimal hydrogen concentration for effective hydrogenotrophic denitrification is 0.2 mg H2·L−1. In turn, Tong et al. [42] investigated the treatment process of wastewater with parameters corresponding to groundwater contaminated with nitrogen compounds (the initial concentration of nitrates and nitrites and ammonia was 16.316 mg N·L−1, 2.715 mg N·L−1, and 1.088 mg N·L−1, respectively) via simultaneous autotrophic and heterotrophic denitrification (C/N = 0.6). Nitrite accumulation at the current densities range of 0–280.4 mA·m−2 decreased from 3.73 mg N·L−1 to 0 mg N·L−1. A current density above 310.4 mA·m−2 increased nitrite concentration. According to these authors, changes in nitrite concentration in the treated wastewater were caused by fluctuations in H2 concentration as a result of electric current flow. Probably, various concentrations of H2 (high within the electrode) might have also affected the course of denitrification in the aforementioned study [43].
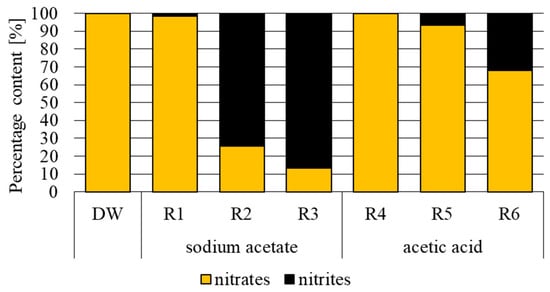
Figure 6.
Percentage content of nitrogen forms in the influents and effluents (R1–R6) (DW-influent).
3.4. Removal of Phosphorus Compounds
The mean concentration of phosphorus in the wastewater inflowing to the bio-electrochemical reactors was 103.8 ± 5.3 mg P·L−1. The highest efficiency of phosphorus removal (Figure 7), reaching 94.9 ± 0.7% (R2) and 97.1 ± 0.6% (R5), was recorded in the reactors, where the electric current flew through the first 12 h of the cycle. In the sodium acetate variant, the lowest total phosphorus removal efficiency, reaching 90.4 ± 1.6%, was determined in R3 (electric current flow in the second phase of the cycle). In contrast, in the acetic acid variant, the lowest efficiency of 93.9 ± 0.9% was determined at 24 h current flow.
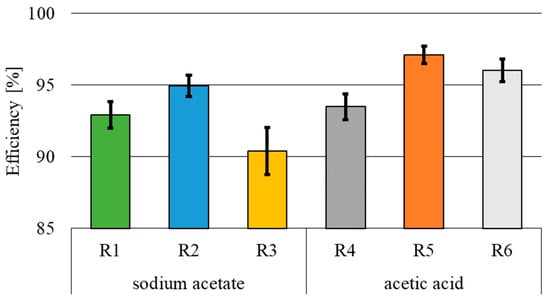
Figure 7.
Total phosphorus removal efficiency.
The concentration of total phosphorus in the effluent from the reactors fed with sodium acetate was between 5.3 ± 0.8 mg P·L−1 (R2) and 10.0 ± 1.7 mg P·L−1 (R3), whereas respective values determined in the acetic acid variant ranged from 3.0 ± 0.6 mg P·L−1 (R3) to 6.8 ± 0.9 mg P·L−1 (R5). The statistical analysis showed a significant effect of the mode of bio-electrochemical reactors fed with a direct electric current on the concentration of total phosphorus in the effluent in the variant with acetic acid used as the substrate. There were no statistically significant differences in the concentration of total phosphorus in the treated wastewater between the variant with sodium acetate used as the source of organic carbon and the variants where wastewater was exposed to the electric current for 24 h (R1) and for the first 12 h of the treatment cycle (R2). Statistically significant differences in the quality of wastewater were shown between reactor R3 (electric current flow for 12 h in the second phase of the cycle) and reactors R1 and R2 (α = 0.05). The type of organic carbon source had a statistically significant effect on the total phosphorus concentration in the effluent at the 12 h electric current flow in both the first and second phase of the cycle. Such an effect was not observed in the variants with the electric current flow for 24 h.
Analyzing the obtained efficiencies of total phosphorus removal (Figure 7) and wastewater pH values (Figure 3), it may be speculated that the main process responsible for phosphorus removal in reactors R1–R3 (pH > 8) and R5 (pH = 7.16) was precipitation with calcium and magnesium ions. According to Mielcarek et al. [43], phosphorus precipitation with Ca2+ and Mg2+ ions from greenhouse wastewater occurs at pH > 7. The pH levels recorded in R6 (6.89) and R4 (pH 3.65) indicate that phosphorus removal was mainly driven by electrocoagulation. The higher efficiency of phosphorus removal in the reactors fed with acetic acid (R4–R6) may be due to the enhanced electrocoagulation at the beginning of the cycle at acidic pH (pH approximated 3.88 after organic substrate addition). According to the literature data, the removal of phosphorus by electrocoagulation occurs more efficiently at the initial acidic pH [44,45]. Irdemez et al. [45] treated synthetic wastewater with a phosphorus concentration of 100 mg P·L−1 using electrocoagulation (current intensity of 0.75A and experiment duration of 20 min) and achieved total phosphorus removal efficiencies of 86%, 71%, and 64% at the initial pH values of 3.0, 6.0, and 9.0, respectively. In turn, Kuokkanen et al. [46] removed from 70% to 94% of total phosphorous at pH values of from 5 to 9, respectively. The initial concentration of total phosphorus in the cited study was 30 mg P·L−1, the electric current density was 100 A·m−2, and the current flow time was 15 min. The concentration of phosphorus in the effluent from the reactors was also determined by the consumption of part of the total phosphorus during biomass synthesis.
The present study results show that it is possible to achieve a satisfactory technological effect in a bio-electrochemical reactor without the need for electric current flow throughout the 24 h treatment cycle. In reactor R2, under the presence of sodium acetate and electric current flow within the first 12 h of the cycle, the nitrogen and phosphorus removal efficiencies were 90.4 ± 1.6% and 94.9 ± 0.7%, respectively, while the efficiency of organic compounds consumption during wastewater treatment was 97.1 ± 1.0%.
The treatment of wastewater from soilless tomato cultivation in bio-electrochemical reactors involves supplying an external source of carbon to the treated wastewater. This was due to the too-low efficiency of hydrogenotrophic denitrification. It was necessary to supply heterotrophic denitrifiers with sodium acetate and acetic acid to the reactor. Only thanks to this was it possible to reduce the nitrogen concentration in treated sewage to below 55.0 mg N·L−1, which does not allow it to be released into the environment. It is therefore necessary to continue research to determine the impact of the time for supplying electricity to the reactor and the C/N ratios at which it will be possible to reduce the nitrogen concentration to levels below 40.0 and 30.0 mg N·L−1. At the same time, it will be necessary to bear in mind that the introduction of an external carbon source cannot contribute to an increase in the concentration of carbon compounds in the reactor outflow to a level unacceptable under applicable regulations. Moreover, it should be remembered that extending the duration of the phase in which electricity is supplied and increasing the dose of the external carbon source is associated with increased treatment costs, larger amounts of sewage sludge and, therefore, an increase in the costs of sludge management.
4. Conclusions
So far, the results of research on the operation of bio-electrochemical reactors have clearly indicated that the efficiency of the processes of removing organic compounds, phosphorus, and nitrogen depends on the current density and the duration of the current flow in the reactor. Increasing the values of these parameters contributed to the increase in the efficiency of the mentioned processes. The research that is the subject of this article proves that in a sequencing batch biofilm reactor, electric current does not have to flow during the entire cycle, and the duration of the phase with current flow does not have to be equal to the hydraulic retention time of the sewage in the reactor. Reducing the duration of the phase with current flow did not reduce the efficiency of nitrogen and phosphorus compound removal and ensured a more effective use of the external carbon source and, consequently, lower concentrations of organic compounds in treated sewage. This issue is important not only from the technological but also from the economic point of view, because shortening the duration of the current flow will reduce the energy costs of wastewater treatment, inhibit electrode digestion, prolong electrode life, and reduce the concentration of the dissolving metal in the treated wastewater. The research results allow us to formulate the following detailed conclusions:
- Regardless of carbon source type, the 12 h period of current flow ensured a more efficient consumption of carbon and a lower concentration of organic compounds in the effluent than the 24 h current flow.
- A significant impact of the supply mode and the duration of the electric current flow on the removal efficiency and concentration of total nitrogen in the treated wastewater was found for both external carbon sources.
- For both carbon sources and for all supply modes and durations of the electric current flow, the removal efficiency of phosphorus exceeded 90%. The lowest concentration of phosphorus in the effluent was determined in the bio-electrochemical reactor with 12 h current flow in the first phase of the cycle and in the presence of acetic acid, whereas the highest one was in the reactor with 12 h current flow in the second phase of the cycle and the presence of sodium acetate.
- The flow of electric current in the reactors resulted in an increased concentration of total iron in the effluent.
- From the energy consumption and technological standpoints, the most viable approach turned out to be feeding the reactor with sodium acetate and wastewater exposure to the electric current flow only during the first 12 h of the treatment cycle. It ensured 90.4 ± 1.6% and 94.9 ± 0.7% efficiencies of nitrogen and phosphorus removal, respectively. In addition, it resulted in a low concentration of carbon compounds in the effluent, reaching 23.2 ± 7.1 mg C·L−1, due to the high consumption of organic compounds during wastewater treatment (97.1 ± 1.0%).
- The nitrogen removal efficiency in the bio-electrochemical reactor was higher in the presence of sodium acetate.
- A higher consumption of organic substrate and, consequently, the lowest concentration of organic compounds in the treated wastewater among all analytical variants were determined in the reactors with sodium acetate, regardless of the electric power supply mode.
- In the case of the reactors fed with sodium acetate, the highest carbon removal efficiencies (above 97.0%) were recorded under the 12 h current flow, whereas the lowest ones were under the 24 h current flow.
- The lowest efficiency of removing organic compounds, amounting to 65.6 ± 3.6%, was recorded in the bio-electrochemical reactor with acetic acid used as a substrate and electric current flow for 24 h. In the reactors with a 12 h current flow, the removal efficiency of organic compounds exceeded 80%.
- The lowest efficiency of total nitrogen removal, regardless of the carbon source, was recorded in the reactors with a 24 h current flow and reached 81.8 ± 1.0% and 39.7 ± 5.5% in the sodium acetate and acetic acid variant, respectively.
- The highest nitrogen removal efficiency (90.4 ± 1.6% and 89.9 ± 1.0%) was recorded in the reactors with sodium acetate and 12 h wastewater exposure to the electric current.
- The concentration of nitrites was higher in the effluent from the reactors with 12 h than in those with 24 h current flow.
Author Contributions
Conceptualization, A.M., K.Ł.B. and J.R.; methodology, A.M. and J.R.; software, K.K.; validation, W.J., J.R. and M.K.; formal analysis, M.K. and J.R.; investigation, K.Ł.B. and K.K.; resources, A.M.; data curation, K.Ł.B. and J.S.-S.; writing—original draft preparation, W.J., A.M. and J.R.; writing—review and editing, A.M., W.J. and J.S.-S.; visualization, K.Ł.B. and J.S.-S.; supervision, A.M. and J.R.; project administration, A.M. and J.R.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The study was part of the project “Development a precise treatment of wastewater from soilless tomato cultivation technology using electro biological hybrid reactor” as part of the LIDER X program, financed by The National Centre for Research and Development No. LIDER/4/0019/L-10/18/NCBR/2019. Co-financing amount: PLN 1,492,500.00.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mielcarek, A.; Rodziewicz, J.; Janczukowicz, W.; Dobrowolski, A. Analysis of Wastewater Generated in Greenhouse Soilless Tomato Cultivation in Central Europe. Water 2019, 11, 2538. [Google Scholar] [CrossRef]
- Bugajski, P.; Kaczor, G.; Bergel, T. The Removal of Reliability Nitrogen in Wastewater Treatment Plant with Sequencing Biological Reactor. Acta Sci. Pol. Form. Circumiectus 2015, 14, 19–27. [Google Scholar] [CrossRef]
- Park, J.B.K.; Craggs, R.J.; Sukias, J.P.S. Removal of Nitrate and Phosphorus from Hydroponic Wastewater Using a Hybrid Denitrification Filter (HDF). Bioresour. Technol. 2009, 100, 3175–3179. [Google Scholar] [CrossRef]
- Kwon, M.J.; Hwang, Y.; Lee, J.; Ham, B.; Rahman, A.; Azam, H.; Yang, J.S. Waste Nutrient Solutions from Full-Scale Open Hydroponic Cultivation: Dynamics of Effluent Quality and Removal of Nitrogen and Phosphorus Using a Pilot-Scale Sequencing Batch Reactor. J. Environ. Manag. 2021, 281, 111893. [Google Scholar] [CrossRef] [PubMed]
- Ajeng, A.A.; Rosli, N.S.M.; Abdullah, R.; Yaacob, J.S.; Qi, N.C.; Loke, S.P. Resource Recovery from Hydroponic Wastewaters Using Microalgae-Based Biorefineries: A Circular Bioeconomy Perspective. J. Biotechnol. 2022, 360, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, J.; Lie, Z.; Lai, D.Y.F. Effects of Applying Different Carbon Substrates on Nutrient Removal and Greenhouse Gas Emissions by Constructed Wetlands Treating Carbon-Depleted Hydroponic Wastewater. Bioresour. Technol. 2022, 357, 127312. [Google Scholar] [CrossRef] [PubMed]
- Rodziewicz, J.; Mielcarek, A.; Janczukowicz, W.; Jóźwiak, T.; Struk-Sokołowska, J.; Bryszewski, K. The Share of Electrochemical Reduction, Hydrogenotrophic and Heterotrophic Denitrification in Nitrogen Removal in Rotating Electrobiological Contactor (REBC) Treating Wastewater from Soilless Cultivation Systems. Sci. Total Environ. 2019, 683, 21–28. [Google Scholar] [CrossRef]
- Mielcarek, A.; Bryszewski, K.Ł.; Rodziewicz, J.; Janczukowicz, W. Single-Stage or Two-Stages Bio-Electrochemical Treatment Process of Drainage from Soilless Tomato Cultivation with Alternating Current. Sep. Purif. Technol. 2022, 299, 121762. [Google Scholar] [CrossRef]
- Bryszewski, K.; Rodziewicz, J.; Mielcarek, A. Usuwanie w Reaktorze Typu Sequencing Batch Biofilm Reactor (SBBR) Azotu i Fosforu Ze Ścieków Pochodzących z Bezglebowej Uprawy Pomidorów. Gaz Woda I Tech. Sanit. 2018, Nr 5, 184–186. [Google Scholar]
- Di Capua, F.; Papirio, S.; Lens, P.N.L.; Esposito, G. Chemolithotrophic Denitrification in Biofilm Reactors. Chem. Eng. J. 2015, 280, 643–657. [Google Scholar] [CrossRef]
- Kong, F.; Ren, H.Y.; Liu, D.; Wang, Z.; Nan, J.; Ren, N.Q.; Fu, Q. Improved Decolorization and Mineralization of Azo Dye in an Integrated System of Anaerobic Bioelectrochemical Modules and Aerobic Moving Bed Biofilm Reactor. Bioresour. Technol. 2022, 353, 127147. [Google Scholar] [CrossRef]
- Safari, M.; Rezaee, A.; Ayati, B.; Jafari, A.J. Autohydrogenotrophic Denitrification by a Bioelectrochemical Process: A Viability Study. Iran J. Health Saf. Environ. 2014, 1, 53–58. [Google Scholar]
- Park, H., Il; Kim, D.K.; Choi, Y.J.; Pak, D. Nitrate Reduction Using an Electrode as Direct Electron Donor in a Biofilm-Electrode Reactor. Process. Biochem. 2005, 40, 3383–3388. [Google Scholar] [CrossRef]
- Tong, S.; Liu, H.; Feng, C.; Chen, N.; Zhao, Y.; Xu, B.; Zhao, J.; Zhu, M. Stimulation Impact of Electric Currents on Heterotrophic Denitrifying Microbial Viability and Denitrification Performance in High Concentration Nitrate-Contaminated Wastewater. J. Env. Sci. 2019, 77, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Kłodowska, I.; Rodziewicz, J.; Janczukowicz, W.; Cydzik-Kwiatkowska, A.; Rusanowska, P. Influence of Carbon Source on the Efficiency of Nitrogen Removal and Denitrifying Bacteria in Biofilm from Bioelectrochemical SBBRs. Water 2018, 10, 393. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, J.; Li, W. Shifts of Biomass and Microbial Community Structure in Response to Current Densities in a Biofilm Electrode Reactor for NOx Removal. Energy Fuels 2019, 33, 5415–5421. [Google Scholar] [CrossRef]
- Wei, V.; Elektorowicz, M.; Oleszkiewicz, J.A. Influence of Electric Current on Bacterial Viability in Wastewater Treatment. Water Res. 2011, 45, 5058–5062. [Google Scholar] [CrossRef]
- Mohammadi, A.; Khadir, A.; Tehrani, R.M.A. Optimization of Nitrogen Removal from an Anaerobic Digester Effluent by Electrocoagulation Process. J. Environ. Chem. Eng. 2019, 7, 103195. [Google Scholar] [CrossRef]
- Ghazouani, M.; Bousselmi, L.; Akrout, H. Combined Electrocoagulation and Electrochemical Treatment on BDD Electrodes for Simultaneous Removal of Nitrates and Phosphates. J. Environ. Chem. Eng. 2020, 8, 104509. [Google Scholar] [CrossRef]
- Sahu, O.; Mazumdar, B.; Chaudhari, P.K. Treatment of Wastewater by Electrocoagulation: A Review. Environ. Sci. Pollut. Res. 2014, 21, 2397–2413. [Google Scholar] [CrossRef]
- Attour, A.; Touati, M.; Tlili, M.; Ben Amor, M.; Lapicque, F.; Leclerc, J.P. Influence of Operating Parameters on Phosphate Removal from Water by Electrocoagulation Using Aluminum Electrodes. Sep. Purif. Technol. 2014, 123, 124–129. [Google Scholar] [CrossRef]
- Omwene, P.I.; Kobya, M.; Can, O.T. Phosphorus Removal from Domestic Wastewater in Electrocoagulation Reactor Using Aluminium and Iron Plate Hybrid Anodes. Ecol. Eng. 2018, 123, 65–73. [Google Scholar] [CrossRef]
- Rodziewicz, J.; Mielcarek, A.; Bryszewski, K.; Janczukowicz, W.; Kłobukowska, K. Energy Consumption for Nutrient Removal from High-Nitrate and High-Phosphorus Wastewater in Aerobic and Anaerobic Bioelectrochemical Reactors. Energies 2022, 15, 7251. [Google Scholar] [CrossRef]
- Rajaniemi, K.; Tuomikoski, S.; Lassi, U. Electrocoagulation Sludge Valorization—A Review. Resources 2021, 10, 127. [Google Scholar] [CrossRef]
- Lei, Y.; Hidayat, I.; Saakes, M.; van der Weijden, R.; Buisman, C.J.N. Fate of Calcium, Magnesium and Inorganic Carbon in Electrochemical Phosphorus Recovery from Domestic Wastewater. Chem. Eng. J. 2019, 362, 453–459. [Google Scholar] [CrossRef]
- Lee, J.Y.; Rahman, A.; Azam, H.; Kim, H.S.; Kwon, M.J. Characterizing Nutrient Uptake Kinetics for Efficient Crop Production during Solanum Lycopersicum Var. Cerasiforme Alef. Growth in a Closed Indoor Hydroponic System. PLoS ONE 2017, 12, e0177041. [Google Scholar] [CrossRef] [PubMed]
- Tejera, J.; Hermosilla, D.; Gascó, A.; Miranda, R.; Alonso, V.; Negro, C.; Blanco, Á. Treatment of Mature Landfill Leachate by Electrocoagulation Followed by Fenton or UVA-LED Photo-Fenton Processes. J. Taiwan Inst. Chem. Eng. 2021, 119, 33–44. [Google Scholar] [CrossRef]
- Krystynik, P.; Masin, P.; Krusinova, Z.; Kluson, P. Ecologically Non-Invasive Decontamination of Natura 2000 Locality from Old Deposits of Hexavalent Chromium and Bivalent Nickel by Modular Electrocoagulation Combined with Ca(OH)2 Addition. Water 2020, 12, 2894. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Song, X. High-Effective Denitrification of Low C/N Wastewater by Combined Constructed Wetland and Biofilm-Electrode Reactor (CW–BER). Bioresour. Technol. 2016, 203, 245–251. [Google Scholar] [CrossRef]
- Moreno-Casillas, H.A.; Cocke, D.L.; Gomes, J.A.G.; Morkovsky, P.; Parga, J.R.; Peterson, E. Electrocoagulation Mechanism for COD Removal. Sep. Purif. Technol. 2007, 56, 204–211. [Google Scholar] [CrossRef]
- Benhadji, A.; Taleb Ahmed, M.; Maachi, R. Electrocoagulation and Effect of Cathode Materials on the Removal of Pollutants from Tannery Wastewater of Rouïba. Desalination 2011, 277, 128–134. [Google Scholar] [CrossRef]
- Eyvaz, M. Treatment of Brewery Wastewater with Electrocoagulation: Improving the Process Performance by Using Alternating Pulse Current. Int. J. Electrochem. Sci. 2016, 11, 4988–5008. [Google Scholar] [CrossRef]
- Zhao, Y.; Feng, C.; Wang, Q.; Yang, Y.; Zhang, Z.; Sugiura, N. Nitrate Removal from Groundwater by Cooperating Heterotrophic with Autotrophic Denitrification in a Biofilm–Electrode Reactor. J. Hazard. Mater. 2011, 192, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Li, S.; Li, J.; Meng, C. Denitrification of Simulated Municipal Wastewater Treatment Plant Effluent Using a Three-Dimensional Biofilm-Electrode Reactor: Operating Performance and Bacterial Community. Bioresour. Technol. 2013, 143, 178–186. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, J.; Ge, Y.; Zhang, Y.; Feng, H.; Cong, Y. Efficient Nitrogen Removal by Simultaneous Photoelectrocatalytic Oxidation and Electrochemically Active Biofilm Denitrification. Electrochim. Acta 2016, 198, 165–173. [Google Scholar] [CrossRef]
- Lin, X.; Yin, H.; Wang, L.; Chen, Y.; Zhao, F.; Pu, Y.; Tang, X. Study of a Three-Dimensional Biofilm-Electrode Reactor (3D-BER) That Combined Heterotrophic and Autotrophic Denitrification (HAD) to Remove Nitrate from Water. RSC Adv. 2023, 13, 14675–14684. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fan, J.; Zhang, M.; Li, Z.; Yang, J.; Liu, X.; Wang, X. Current Intensities Altered the Performance and Microbial Community Structure of a Bio-Electrochemical System. Chemosphere 2021, 265, 129069. [Google Scholar] [CrossRef]
- Du, R.; Peng, Y.; Cao, S.; Li, B.; Wang, S.; Niu, M. Mechanisms and Microbial Structure of Partial Denitrification with High Nitrite Accumulation. Appl. Microbiol. Biotechnol. 2016, 100, 2011–2021. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Biological Nitrate Removal from Water and Wastewater by Solid-Phase Denitrification Process. Biotechnol. Adv. 2016, 34, 1103–1112. [Google Scholar] [CrossRef]
- Di Capua, F.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Electron Donors for Autotrophic Denitrification. Chem. Eng. J. 2019, 362, 922–937. [Google Scholar] [CrossRef]
- Chang, C.C.; Szu, K.T.; Hsien, K.H. Hydrogenotrophic Denitrification with Immobilized Alcaligenes Eutrophus for Drinking Water Treatment. Bioresour. Technol. 1999, 69, 53–58. [Google Scholar] [CrossRef]
- Tong, S.; Chen, N.; Wang, H.; Liu, H.; Tao, C.; Feng, C.; Zhang, B.; Hao, C.; Pu, J.; Zhao, J. Optimization of C/N and Current Density in a Heterotrophic/Biofilm-Electrode Autotrophic Denitrification Reactor (HAD-BER). Bioresour. Technol. 2014, 171, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Mielcarek, A.; Jóźwiak, T.; Rodziewicz, J.; Bryszewski, K.; Janczukowicz, W.; Kalisz, B.; Tavares, J.M.R. Recovery of Phosphorus and Other Minerals from Greenhouse Wastewater Generated during Soilless Tomato Cultivation by Means of Alkalizing Agents. Sci. Total Environ. 2023, 892, 164757. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, M.; Moghaddam, M.R.A.; Arami, M. A Comparison between Aluminum and Iron Electrodes on Removal of Phosphate from Aqueous Solutions by Electrocoagulation Process. Int. J. Environ. Res. 2011, 5, 403–412. [Google Scholar]
- Irdemez, Ş.; Demircioǧlu, N.; Yildiz, Y.Ş.; Bingül, Z. The Effects of Current Density and Phosphate Concentration on Phosphate Removal from Wastewater by Electrocoagulation Using Aluminum and Iron Plate Electrodes. Sep. Purif. Technol. 2006, 52, 218–223. [Google Scholar] [CrossRef]
- Kuokkanen, V.; Kuokkanen, T.; Rämö, J.; Lassi, U.; Roininen, J. Removal of Phosphate from Wastewaters for Further Utilization Using Electrocoagulation with Hybrid Electrodes—Techno-Economic Studies. J. Water Process Eng. 2015, 8, e50–e57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).