Recent Progress in the Use of Perovskites for Electrochemical, Photoelectrochemical, and Photovoltaic–Electrochemical CO2 Reduction
Abstract
:1. Introduction
2. Structure of Perovskites
2.1. Perovskite Oxides
2.2. Metal–Halide Perovskites
3. Fundamentals of CO2 Reduction
4. Perovskites for CO2 Reduction
4.1. Direct Electrochemical CO2 Reduction
4.2. Photoelectrochemical CO2 Reduction
4.3. Photovoltaic–Electrochemical CO2 Reduction
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Jing, X.; Wang, Y.; Li, F. Towards Carbon-Neutral Methanol Production from Carbon Dioxide Electroreduction. ChemNanoMat 2021, 7, 728–736. [Google Scholar] [CrossRef]
- Wu, H.; Yuan, Y.; Chen, Y.; Lv, D.; Tu, S.; Wu, Y.; Li, Z.; Xia, Q. Highly Efficient Capture of Postcombustion Generated CO2 through a Copper-Based Metal-Organic Framework. Energy Fuels 2021, 35, 610–617. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured tin catalysts for selective electrochemical reduction of carbon dioxide to formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, S.; Pan, Z.; Yin, B. Electrochemical CO2 reduction to CO using solid oxide electrolysis cells with high-performance Ta-doped bismuth strontium ferrite air electrode. Energy 2021, 228, 120579. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, Q.; Xia, R.; Meyer, T.J. CO2 Reduction: From Homogeneous to Heterogeneous Electrocatalysis. Acc. Chem. Res. 2020, 53, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Q. Recent Advances in Breaking Scaling Relations for Effective Electrochemical Conversion of CO2. Adv. Energy Mater 2016, 6, 1600463. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalt. Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Creissen, C.E.; Fontecave, M. Solar-Driven Electrochemical CO2 Reduction with Heterogeneous Catalysts. Adv. Energy Mater. 2021, 11, 2002652. [Google Scholar] [CrossRef]
- Bin, A.R.; Yusoff, M.; Jang, J. Highly efficient photoelectrochemical water splitting by a hybrid tandem perovskite solar cell. Chem. Commun. 2016, 52, 5824–5827. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Atla, V.; Brian, J.P.; Kumari, S.; Nguyen, T.Q.; Sunkara, M.; Spurgeon, J.M. Reduced SnO2 Porous Nanowires with a High Density of Grain Boundaries as Catalysts for Efficient Electrochemical CO2-into-HCOOH Conversion. Angew. Chem. Int. Ed. 2017, 56, 3645–3649. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Héroguel, F.; Luterbacher, J.; Hu, X. Densely Packed, Ultra Small SnO Nanoparticles for Enhanced Activity and Selectivity in Electrochemical CO2 Reduction. Angew. Chem. 2018, 130, 2993–2997. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H. Efficient Electrochemical Reduction of Carbon Dioxide to Acetate on Nitrogen-Doped Nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yadav, R.M.; Liu, M.; Sharma, P.P.; Tiwary, C.S.; Ma, L.; Zou, X.; Zhou, X.D.; Yakobson, B.I.; Lou, J.; et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes. ACS Nano 2015, 9, 5364–5371. [Google Scholar] [CrossRef] [PubMed]
- Zendehdel, M.; Yaghoobi Nia, N.; Paci, B.; Generosi, A.; Di Carlo, A. Zero-Waste Scalable Blade-Spin Coating as Universal Approach for Layer-By-Layer Deposition of 3D/2D Perovskite Films in High Efficiency Perovskite Solar Modules. Sol. RRL 2021, 6, 2100637. [Google Scholar] [CrossRef]
- Yaghoobi Nia, N.; Saranin, D.; Palma, A.L.; Di Carlo, A. Perovskite solar cells. In Solar Cells and Light Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–228. [Google Scholar]
- Zhang, H.T.; Park, T.J.; Islam, A.N.M.N.; Tran, D.S.J.; Manna, S.; Wang, Q.; Mondal, S.; Yu, H.; Banik, S.; Cheng, S.; et al. Reconfigurable perovskite nickelate electronics for artificial intelligence. Science 2022, 375, 533–539. [Google Scholar] [CrossRef]
- Sutherland, B.R.; Sargent, E.H. Perovskite photonic sources. Nat. Photonics 2016, 10, 295–302. [Google Scholar] [CrossRef]
- Tofanello, A.; Freitas, A.L.M.; de Queiroz, T.B.; Bonadio, A.; Martinho, H.; Souza, J.A. Magnetism in a 2D Hybrid Ruddlesden–Popper Perovskite through Charge Redistribution Driven by an Organic Functional Spacer. J. Phys. Chem. Lett. 2022, 13, 1406–1415. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef]
- Zendehdel, M.; Yaghoobi Nia, N.; Yaghoubinia, M. Emerging Thin Film Solar Panels. In Reliability and Ecological Aspects of Photovoltaic Modules; IntechOpen: London, UK, 2020. [Google Scholar]
- Sun, H.; Dai, J.; Zhou, W.; Shao, Z. Emerging Strategies for Developing High-Performance Perovskite-Based Materials for Electrochemical Water Splitting. Energy Fuels 2020, 34, 10547–10567. [Google Scholar] [CrossRef]
- Liang, J.; Chen, D.; Yao, X.; Zhang, K.; Qu, F.; Qin, L.; Huang, Y.; Li, J. Recent Progress and Development in Inorganic Halide Perovskite Quantum Dots for Photoelectrochemical Applications. Small 2020, 16, 1903398. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yan, B.; Jiang, Z.; Yao, S.; Liu, Z.; Wu, Q.; Ran, R.; Senanayake, S.D.; Weng, D.; Chen, J.G. High selectivity of CO2 hydrogenation to CO by controlling the valence state of nickel using perovskite. Chem. Commun. 2018, 54, 7354–7357. [Google Scholar] [CrossRef]
- Goel, P.; Sundriyal, S.; Shrivastav, V.; Mishra, S.; Dubal, D.P.; Kim, K.H.; Deep, A. Perovskite materials as superior and powerful platforms for energy conversion and storage applications. Nano Energy 2021, 80, 105552. [Google Scholar] [CrossRef]
- Yin, W.J.; Weng, B.; Ge, J.; Sun, Q.; Li, Z.; Yan, Y. Oxide perovskites, double perovskites and derivatives for electrocatalysis, photocatalysis, and photovoltaics. Energy Environ. Sci. 2019, 12, 442–462. [Google Scholar] [CrossRef]
- Li, X.; Zhao, H.; Liang, J.; Luo, Y.; Chen, G.; Shi, X.; Lu, S.; Gao, S.; Hu, J.; Liu, Q.; et al. A-site perovskite oxides: An emerging functional material for electrocatalysis and photocatalysis. J. Mater. Chem. A 2021, 9, 6650–6670. [Google Scholar] [CrossRef]
- Porta, P.; De Rossi, S.; Faticanti, M.; Minelli, G.; Pettiti, I.; Lisi, L.; Turco, M. Perovskite-Type Oxides: I. Structural, Magnetic, and Morphological Properties of LaMn1−xCuxO3 and LaCo1−xCuxO3 Solid Solutions with Large Surface Area. J. Solid State Chem. 1999, 146, 291–304. [Google Scholar] [CrossRef]
- Wei, W.J.; Li, C.; Li, L.S.; Tang, Y.Z.; Jiang, X.X.; Lin, Z.S. Phase transition, optical and dielectric properties regulated by anion-substitution in a homologous series of 2D hybrid organic–inorganic perovskites. J. Mater. Chem. C 2019, 7, 11964–11971. [Google Scholar] [CrossRef]
- Song, K.W.; Lee, K.T. Characterization of Ba0.5Sr0.5M1−xFexO3−δ (M = Co and Cu) perovskite oxide cathode materials for intermediate temperature solid oxide fuel cells. Ceram. Int. 2012, 38, 5123–5131. [Google Scholar] [CrossRef]
- Devi, S.; Taxak, V.B.; Chahar, S.; Dalal, M.; Dalal, J.; Hooda, A.; Khatkar, A.; Malik, R.K.; Khatkar, S.P. Crystal chemistry and optical analysis of a novel perovskite type SrLa2Al2O7:Sm3+ nanophosphor for white LEDs. Ceram. Int. 2019, 45, 15571–15579. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Song, P.; Qin, H.; Jiang, M. Electrical properties and ethanol-sensing characteristics of perovskite La1 − xPbxFeO3. Sens. Actuators B Chem. 2006, 114, 836–840. [Google Scholar] [CrossRef]
- Bhat, A.A.; Khandy, S.A.; Islam, I.; Tomar, R. Optical, electrochemical and photocatalytic properties of cobalt doped CsPbCl3 nanostructures: A one-pot synthesis approach. Sci. Rep. 2021, 11, 16473. [Google Scholar] [CrossRef]
- Islam, I.; Khandy, S.A.; Zaman, M.B.; Hafiz, A.K.; Siddiqui, A.M.; Chai, J.-D. Growth and characterization of crystalline BaSnO3 perovskite nanostructures and the influence of heavy Mn doping on its properties. J. Alloys Compd. 2021, 867, 158900. [Google Scholar] [CrossRef]
- Khandy, S.A.; Vaid, S.G.; Islam, I.; Hafiz, A.K.; Chai, J.-D. Understanding the stability concerns and electronic structure of CsYbX3 (X=Cl,Br) halidoperovskites for optoelectronic applications. J. Alloys Compd. 2021, 867, 158966. [Google Scholar] [CrossRef]
- Babu, R.; Giribabu, L.; Singh, S.P. Recent Advances in Halide-Based Perovskite Crystals and Their Optoelectronic Applications. Cryst. Growth Des. 2018, 18, 2645–2664. [Google Scholar] [CrossRef]
- Yaghubinia, M.; Ebnali, M.; Zendehdel, M.; Yaghoubinia, M. Improvement of perovskite solar cells photovoltaic performance by localized surface plasmon effect of silver-alumina core-shell nanoparticles. In Proceedings of the PHOTOPTICS 2016-Proceedings of the 4th International Conference on Photonics, Optics and Laser Technology, Rome, Italy, 27–29 February 2016. [Google Scholar]
- Di Carlo, A.; Yaghoobi Nia, N.; Zendehdel, M.; Agresti, A.; Pescetelli, S.; Vesce, L.; Castriotta, L.A.; Matteocci, F.; Di Giacomo, F.; Saranin, D.; et al. Halide perovskite modules and panels. In Proceedings of the nanoGe Spring Meeting 2022; Fundació Scito: València, Spain, 2022. [Google Scholar]
- Yaghoobi Nia, N.; Zendehdel, M.; Abdi-Jalebi, M.; Castriotta, L.A.; Kosasih, F.U.; Lamanna, E.; Abolhasani, M.M.; Zheng, Z.; Andaji-Garmaroudi, Z.; Asadi, K.; et al. Beyond 17% stable perovskite solar module via polaron arrangement of tuned polymeric hole transport layer. Nano Energy 2021, 82, 105685. [Google Scholar] [CrossRef]
- Schmidt, L.C.; Pertegás, A.; González-Carrero, S.; Malinkiewicz, O.; Agouram, S.; Espallargas, G.M.; Bolink, H.J.; Galian, R.E.; Pérez-Prieto, J. Nontemplate synthesis of CH3NH3PbBr3 perovskite nanoparticles. J. Am. Chem. Soc. 2014, 136, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Yaghoobi Nia, N.; Lamanna, E.; Zendehdel, M.; Palma, A.L.; Zurlo, F.; Castriotta, L.A.; Di Carlo, A. Doping Strategy for Efficient and Stable Triple Cation Hybrid Perovskite Solar Cells and Module Based on Poly(3-hexylthiophene) Hole Transport Layer. Small 2019, 15, 1904399. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, C.C.; Cao, D.H.; Chang, R.P.H.; Kanatzidis, M.G. Lead-free solid-state organic–inorganic halide perovskite solar cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Sardashti, M.K.; Zendehdel, M.; Yaghoobi Nia, N.; Karimian, D.; Sheikhi, M. High Efficiency MAPbI 3 Perovskite Solar Cell Using a Pure Thin Film of Polyoxometalate as Scaffold Layer. ChemSusChem 2017, 10, 3773–3779. [Google Scholar] [CrossRef]
- Li, Y.F.; Chou, S.Y.; Huang, P.; Xiao, C.; Liu, X.; Xie, Y.; Zhao, F.; Huang, Y.; Feng, J.; Zhong, H.; et al. Stretchable Organometal-Halide-Perovskite Quantum-Dot Light-Emitting Diodes. Adv. Mater. 2019, 31, 1807516. [Google Scholar] [CrossRef] [PubMed]
- Navazani, S.; Yaghoobi Nia, N.; Zendehdel, M.; Shokuhfar, A.; Di Carlo, A. Fabrication of high efficiency, low-temperature planar perovskite solar cells via scalable double-step crystal engineering deposition method fully out of glove box. Sol. Energy 2020, 206, 181–187. [Google Scholar] [CrossRef]
- Ermanova, I.; Yaghoobi Nia, N.; Lamanna, E.; Di Bartolomeo, E.; Kolesnikov, E.; Luchnikov, L.; Di Carlo, A. Crystal Engineering Approach for Fabrication of Inverted Perovskite Solar Cell in Ambient Conditions. Energies 2021, 14, 1751. [Google Scholar] [CrossRef]
- Castriotta, L.A.; Zendehdel, M.; Yaghoobi Nia, N.; Leonardi, E.; Löffler, M.; Paci, B.; Generosi, A.; Rellinghaus, B.; Di Carlo, A. Reducing Losses in Perovskite Large Area Solar Technology: Laser Design Optimization for Highly Efficient Modules and Mini Panels. Adv. Energy Mater 2022, 12, 2103420. [Google Scholar] [CrossRef]
- Zhang, H.; Darabi, K.; Yaghoobi Nia, N.; Krishna, A.; Ahlawat, P.; Guo, B.; Almalki, M.H.S.; Su, T.S.; Ren, D.; Bolnykh, V.; et al. A universal co-solvent dilution strategy enables facile and cost-effective fabrication of perovskite photovoltaics. Nat. Commun. 2022, 13, 89. [Google Scholar] [CrossRef]
- Yaghoobi Nia, N.; Giordano, F.; Zendehdel, M.; Cinà, L.; Palma, A.L.; Medaglia, P.G.; Zakeeruddin, S.M.; Grätzel, M.; Di Carlo, A. Solution-based heteroepitaxial growth of stable mixed cation/anion hybrid perovskite thin film under ambient condition via a scalable crystal engineering approach. Nano Energy 2020, 69, 104441. [Google Scholar] [CrossRef]
- Teo, S.H.; Ng, C.H.; Ng, Y.H.; Islam, A.; Hayase, S.; Taufiq-Yap, Y.H. Resolve deep-rooted challenges of halide perovskite for sustainable energy development and environmental remediation. Nano Energy 2022, 99, 107401. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Liu, D.; Yu, J.; Hussain, A.; Liu, Y. Photocatalytic, electrocatalytic and photoelectrocatalytic conversion of carbon dioxide: A review. Environ. Chem. Lett. 2021, 19, 941–967. [Google Scholar] [CrossRef]
- Mao, X.; Hatton, T.A. Recent Advances in Electrocatalytic Reduction of Carbon Dioxide Using Metal-Free Catalysts. Ind. Eng. Chem. Res. 2015, 16, 4033–4042. [Google Scholar] [CrossRef]
- Kumaravel, V.; Bartlett, J.; Pillai, S.C. Photoelectrochemical Conversion of Carbon Dioxide (CO2) into Fuels and Value-Added Products. ACS Energy Lett. 2020, 5, 486–519. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, S.; Beak, M.; Park, J.; Kwon, K. Performance of Photovoltaic-Driven Electrochemical Cell Systems for CO2 Reduction. Chem. Eng. J. 2022, 15, 130259. [Google Scholar] [CrossRef]
- Chen, K.; Qi, K.; Zhou, T.; Yang, T.; Zhang, Y.; Guo, Z.; Lim, C.K.; Zhang, J.; Žutic, I.; Zhang, H.; et al. Water-Dispersible CsPbBr3 Perovskite Nanocrystals with Ultra-Stability and its Application in Electrochemical CO2 Reduction. Nano-Micro Lett. 2021, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, X.; Yang, Y.; Jiang, Y.; Xia, C. Mixed-Conductor Sr2Fe1.5Mo0.5O6-δ as Robust Fuel Electrode for Pure CO2 Reduction in Solid Oxide Electrolysis Cell. ACS Sustain. Chem. Eng. 2017, 5, 11403–11412. [Google Scholar]
- Pi, Y.; Guo, J.; Shao, Q.; Huang, X. All-inorganic SrSnO3 perovskite nanowires for efficient CO2 electroreduction. Nano Energy 2019, 62, 861–868. [Google Scholar] [CrossRef]
- Hou, P.; Wang, X.; Wang, Z.; Kang, P. Gas Phase Electrolysis of Carbon Dioxide to Carbon Monoxide Using Nickel Nitride as the Carbon Enrichment Catalyst. ACS Appl. Mater. Interfaces 2018, 10, 38024–38031. [Google Scholar] [CrossRef]
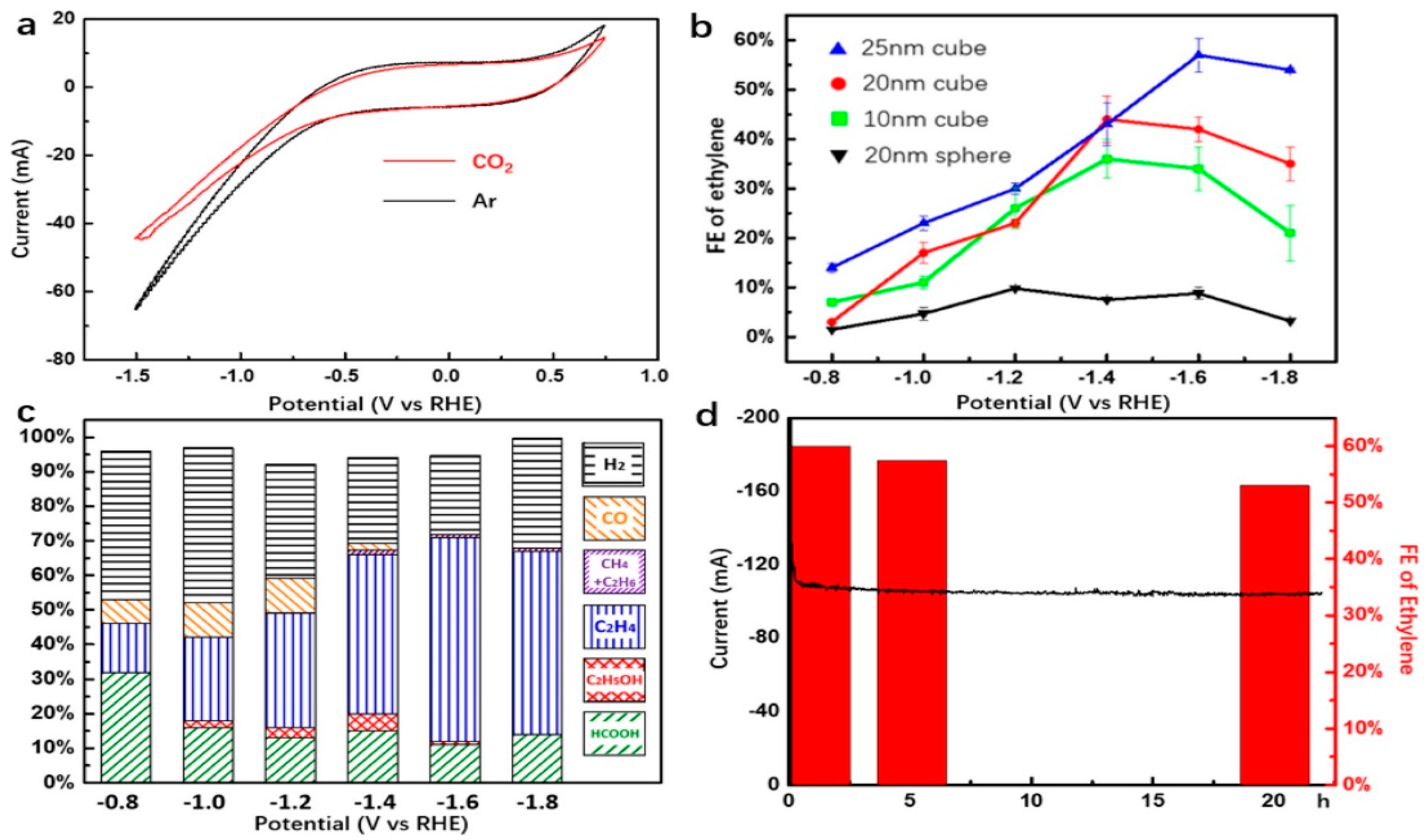
- Yin, Z.; Yu, C.; Zhao, Z.; Guo, X.; Shen, M.; Li, N.; Muzzio, M.; Li, J.; Liu, H.; Lin, H.; et al. Cu3N nanocubes for selective electrochemical reduction of CO2 to ethylene. Nano Lett. 2019, 19, 8658–8663. [Google Scholar] [CrossRef]
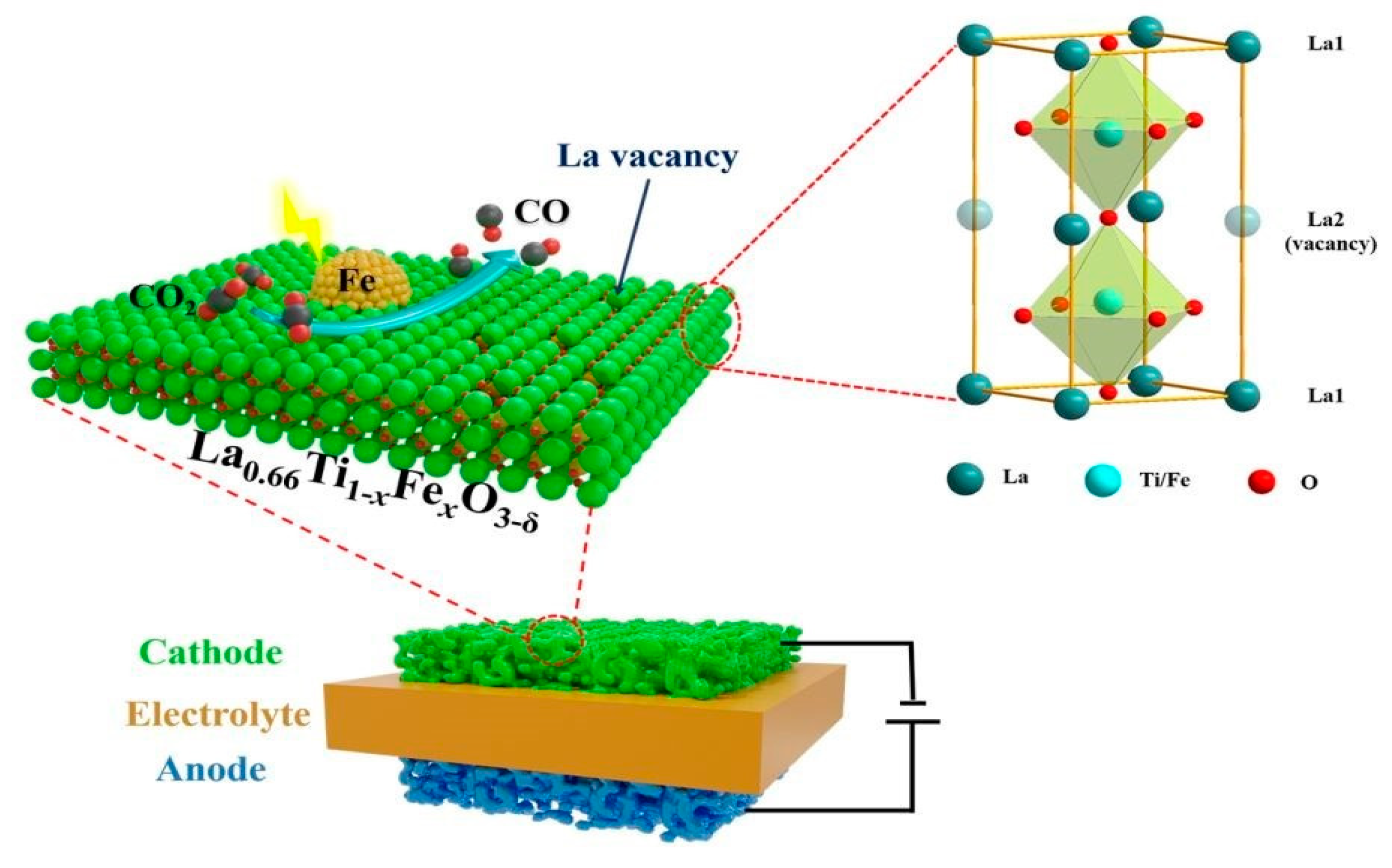
- Hu, S.; Zhang, L.; Cai, L.; Cao, Z.; Jiang, Q.; Yu, W.; Wu, Y.; Zhu, X.; Yang, W. Iron stabilized 1/3 A-site deficient La–Ti–O perovskite cathodes for efficient CO2 electroreduction. J. Mater. Chem. A 2020, 8, 21053–21061. [Google Scholar] [CrossRef]
- Chen, S.; Su, Y.; Deng, P.; Qi, R.; Zhu, J.; Chen, J.; Wang, Z.; Zhou, L.; Guo, X.; Xia, B.Y. Highly Selective Carbon Dioxide Electroreduction on Structure-Evolved Copper Perovskite Oxide toward Methane Production. ACS Catal. 2020, 10, 4640–4646. [Google Scholar] [CrossRef]
- Hu, S.; Zhang, L.; Liu, H.; Li, W.; Cao, Z.; Cai, L.; Zhu, Y.; Zhu, X.; Yang, W. Detrimental phase evolution triggered by Ni in perovskite-type cathodes for CO2 electroreduction. J. Energy Chem. 2019, 36, 87–94. [Google Scholar] [CrossRef]
- Ma, M.; Yang, X.; Xu, C.; Ren, R.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Constructing highly active alloy-perovskite interfaces for efficient electrochemical CO2 reduction reaction. Sep. Purif. Technol. 2022, 296, 121411. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Wei, Y.; Wei, F.; Kong, L.; Feng, J.; Lu, J.-Q.; Zhou, X.; Yang, F. Efficient and Selective Electroreduction of CO2 to HCOOH over Bismuth-Based Bromide Perovskites in Acidic Electrolytes. Chem. Eur. J. 2022, 28, e202201832. [Google Scholar] [CrossRef] [PubMed]
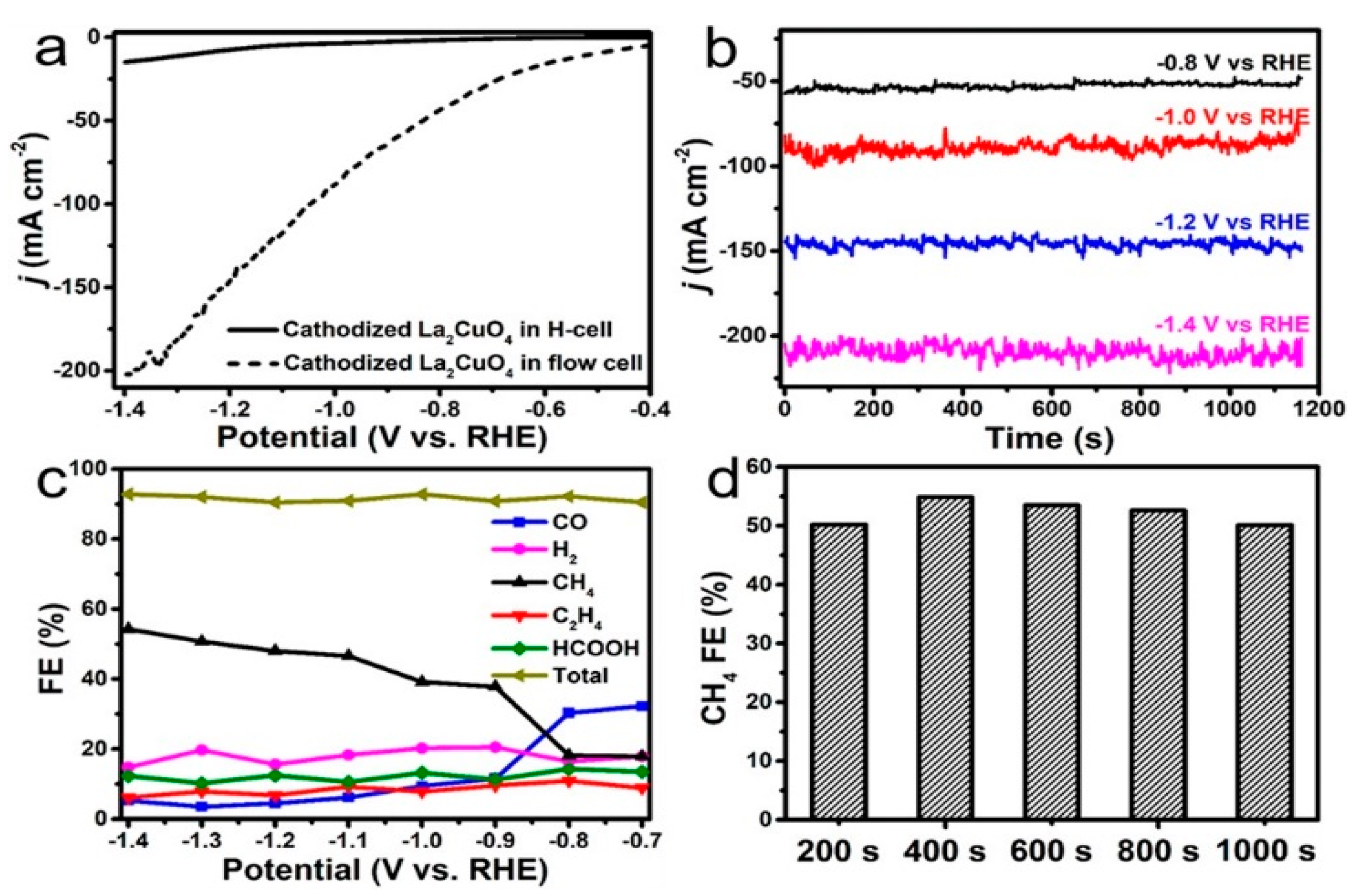
- Zhu, Y.; Zhou, W.; Dong, Z.; Zhang, X.; Chen, Z.; Liu, Z.; Li, F.; Fan, J.; Jiao, M.; Liu, L. Nanosized LaInO3 perovskite for efficient electrocatalytic reduction of CO2 to formate. J. CO2 Util. 2023, 68, 102342. [Google Scholar] [CrossRef]
- Bae, K.T.; Jeong, I.; Akromjon, A.; Im, H.N.; Lee, K.T. Robust and efficient Fe/Mn bimetal doped Pr4/3Ba2/3Co2/3Fe2/3Mn2/3O5+δ double perovskite catalysts for direct CO2 electrolysis. Chem. Eng. J. 2023, 472, 145015. [Google Scholar] [CrossRef]
- Sivula, K.; Van De Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Sun, H.; Song, S.; Xu, X.; Dai, J.; Yu, J.; Zhou, W.; Shao, Z.; Jung, W. Recent Progress on Structurally Ordered Materials for Electrocatalysis. Adv. Energy Mater. 2021, 11, 2101937. [Google Scholar] [CrossRef]
- Arandiyan, H.; Mofarah, S.S.; Sorrell, C.C.; Doustkhah, E.; Sajjadi, B.; Hao, D.; Wang, Y.; Sun, H.; Ni, B.J.; Rezaei, M.; et al. Defect engineering of oxide perovskites for catalysis and energy storage: Synthesis of chemistry and materials science. Chem. Soc. Rev. 2021, 50, 10116–10211. [Google Scholar] [CrossRef]
- Chen, J.; Yin, J.; Zheng, X.; Ait Ahsaine, H.; Zhou, Y.; Dong, C.; Mohammed, O.F.; Takanabe, K.; Bakr, O.M. Compositionally Screened Eutectic Catalytic Coatings on Halide Perovskite Photocathodes for Photoassisted Selective CO2 Reduction. ACS Energy Lett. 2019, 4, 1279–1286. [Google Scholar] [CrossRef]
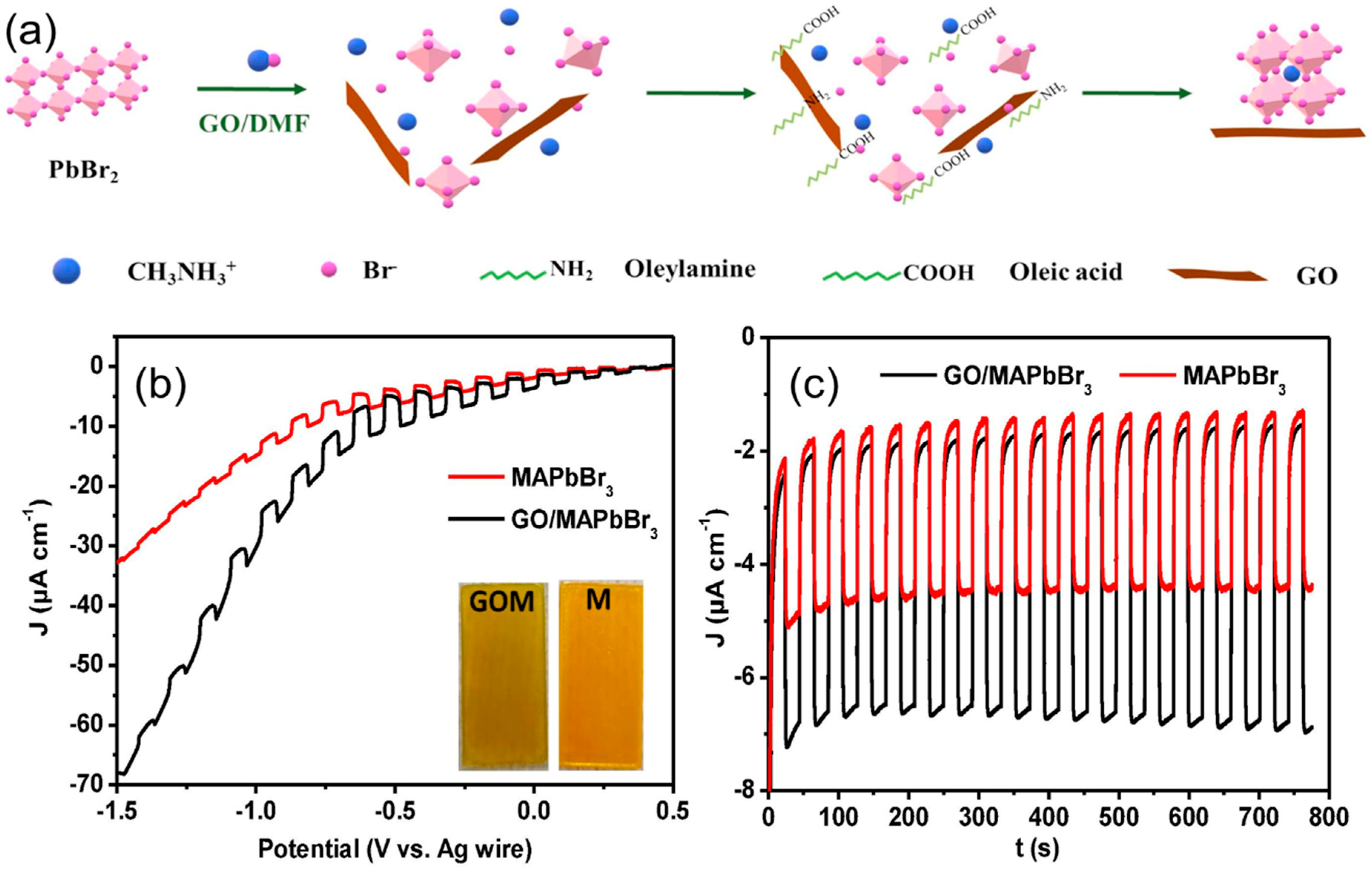
- Wang, Q.; Tao, L.; Jiang, X.; Wang, M.; Shen, Y. Graphene oxide wrapped CH3NH3PbBr3 perovskite quantum dots hybrid for photoelectrochemical CO2 reduction in organic solvents. Appl. Surf. Sci. 2019, 465, 607–613. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, H.; Wang, H.; Ma, W.; Zong, X.; Li, C. Carbon Encapsulation of Organic–Inorganic Hybrid Perovskite toward Efficient and Stable Photo-Electrochemical Carbon Dioxide Reduction. Adv. Energy Mater. 2020, 10, 2002105. [Google Scholar] [CrossRef]
- Jang, Y.J.; Jeong, I.; Lee, J.; Lee, J.; Ko, M.J.; Lee, J.S. Unbiased Sunlight-Driven Artificial Photosynthesis of Carbon Monoxide from CO2 Using a ZnTe-Based Photocathode and a Perovskite Solar Cell in Tandem. ACS Nano. 2016, 10, 6980–6987. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, R.; Sun, H.; Yang, W.; Liang, W.; Li, F.; Zheng, R.; Huang, J. Synergistically Interface-Engineered Inorganic Halide Perovskite Photocathodes for Photoelectrochemical CO2 Reduction. Energy Fuels 2023. [Google Scholar] [CrossRef]
- Schreier, M.; Héroguel, F.; Steier, L.; Ahmad, S.; Luterbacher, J.S.; Mayer, M.T.; Luo, J.; Grätzel, M. Solar conversion of CO2 to CO using Earth-abundant electrocatalysts prepared by atomic layer modification of CuO. Nat. Energy 2017, 2, 17087. [Google Scholar] [CrossRef]
- Huan, T.N.; Dalla Corte, D.A.; Lamaison, S.; Karapinar, D.; Lutz, L.; Menguy, N.; Foldyna, M.; Turren-Cruz, S.H.; Hagfeldt, A.; Bella, F.; et al. Low-cost high-efficiency system for solar-driven conversion of CO2 to hydrocarbons. Proc. Natl. Acad. Sci. USA 2019, 116, 9735–9740. [Google Scholar] [CrossRef] [PubMed]
- Schreier, M.; Curvat, L.; Giordano, F.; Steier, L.; Abate, A.; Zakeeruddin, S.M.; Luo, J.; Mayer, M.T.; Grätzel, M. Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics. Nat. Commun. 2015, 6, 7326. [Google Scholar] [CrossRef] [PubMed]
- Esiner, S.; Wang, J.; Janssen, R.A.J. Light-Driven Electrochemical Carbon Dioxide Reduction to Carbon Monoxide and Methane Using Perovskite Photovoltaics. Cell Rep. Phys. Sci. 2020, 1, 100058. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, Y.; Chen, S.; Hu, Y.; Yang, S.; Tie, Z.; Jin, Z. Single-Atom Metal Anchored Zr6-Cluster-Porphyrin Framework Hollow Nanocapsules with Ultrahigh Active-Center Density for Electrocatalytic CO2 Reduction. Nano Lett. 2022, 22, 3340–3348. [Google Scholar] [CrossRef]
- Khandy, S.A.; Islam, I.; Kaur, K.; Ali, A.M.; El-Rehim, A.F.A. Electronic structure, stability, photocatalytic and optical properties of new lead-free double perovskites Tl2PtX6 (X = Cl, Br) for light-harvesting applications. Mater. Chem. Phys. 2023, 297, 127293. [Google Scholar] [CrossRef]
- Bhat, A.A.; Khandy, S.A.; Ali, A.M.; Tomar, R. Photoluminescence Emission Studies on a Lanthanum-Doped Lead Free Double Halide Perovskite, La:Cs2SnCl6. J. Phys. Chem. Lett. 2023, 14, 5004–5012. [Google Scholar] [CrossRef]
- Zhang, M.; Jeerh, G.; Zou, P.; Lan, R.; Wang, M.; Wang, H.; Tao, S. Recent development of perovskiteoxide-based electrocatalysts and their applications in low to intermediate temperature electrochemical devices. Mater. Today 2021, 49, 351–377. [Google Scholar] [CrossRef]


| Electrochemical Thermodynamic Half-Reactions | Standard Potentials (V) vs. SHE |
|---|---|
| CO2(g) + 4H+ + 4e− = C(s) + 2 H2O(l) | 0.210 |
| CO2(g) + 2H+ + 2e− = HCOOH(l) | −0.250 |
| CO2(g) + 2H+ + 2e− = CO(g) + H2O(l) | −0.106 |
| CO2(g) + 4H+ + 4e− = CH2O(l) + H2O(l) | −0.070 |
| CO2(g) + 6H+ + 6e− = CH3OH(l) + H2O(l) | 0.016 |
| CO2(g) + 8H+ + 8e− = CH4(g) + 2H2O(l) | 0.169 |
| 2CO2(g) + 2H+ + 2e− = H2C2O4(aq) | −0.500 |
| 2CO2(g) + 12H+ + 12e− = CH2CH2(g) + 4H2O(l) | 0.064 |
| Cathode/Photocathode | Anode/Photoanode | PV Absorber | Method | CO2RR Product | FE% | Solar-to-Product Efficiency | References |
|---|---|---|---|---|---|---|---|
| Sr2Fe1.5Mo0.5O6−δ | Pt plate | - | EC | CO | 95 | - | [57] |
| SrSnO3 nanowires | Pt wire | - | EC | HCO2− | 80 | - | [58] |
| Cu3N nanocubes | - | - | EC | C2H4 | 60 | - | [60] |
| La0.66Ti1-xFexO3δ | Gd0.2Ce0.8O2-δ | - | EC | CO | ∼100 | - | [61] |
| La2CuO4 | - | - | EC | CH4 | 56.3 | - | [62] |
| Sr2 Fe 1.5-x NixMo0.5O6-δ | (La0.6Sr0.4)0.98 Co0.2Fe0.8O3-δ, Gd0.2Ce0.8O2-δ | - | EC | CO | 99.9 | - | [63] |
| (PrBa)0.95Fe1.6Ni0.2Nb0.2O5+δ | La0.6Sr0.4 Co0.2Fe0.8O3-δ | - | EC | CO | 99.3 | - | [64] |
| Cs3Bi2Br9/C | Pt foil | - | EC | HCOOH | 92 | - | [65] |
| LaInO3 | Ni foam | - | EC | HCOO— | 91.4 | - | [66] |
| Pr4/3Ba2/3Co2/3Fe2/3Mn2/3O5+δ/ Gd-doped ceria | - | - | EC | CO | 90 | - | [67] |
| In0.4Bi0.6 alloy-coated CH3NH3PbI3 | Pt | - | PEC | HCOOH | ~100 | 7.2 | [71] |
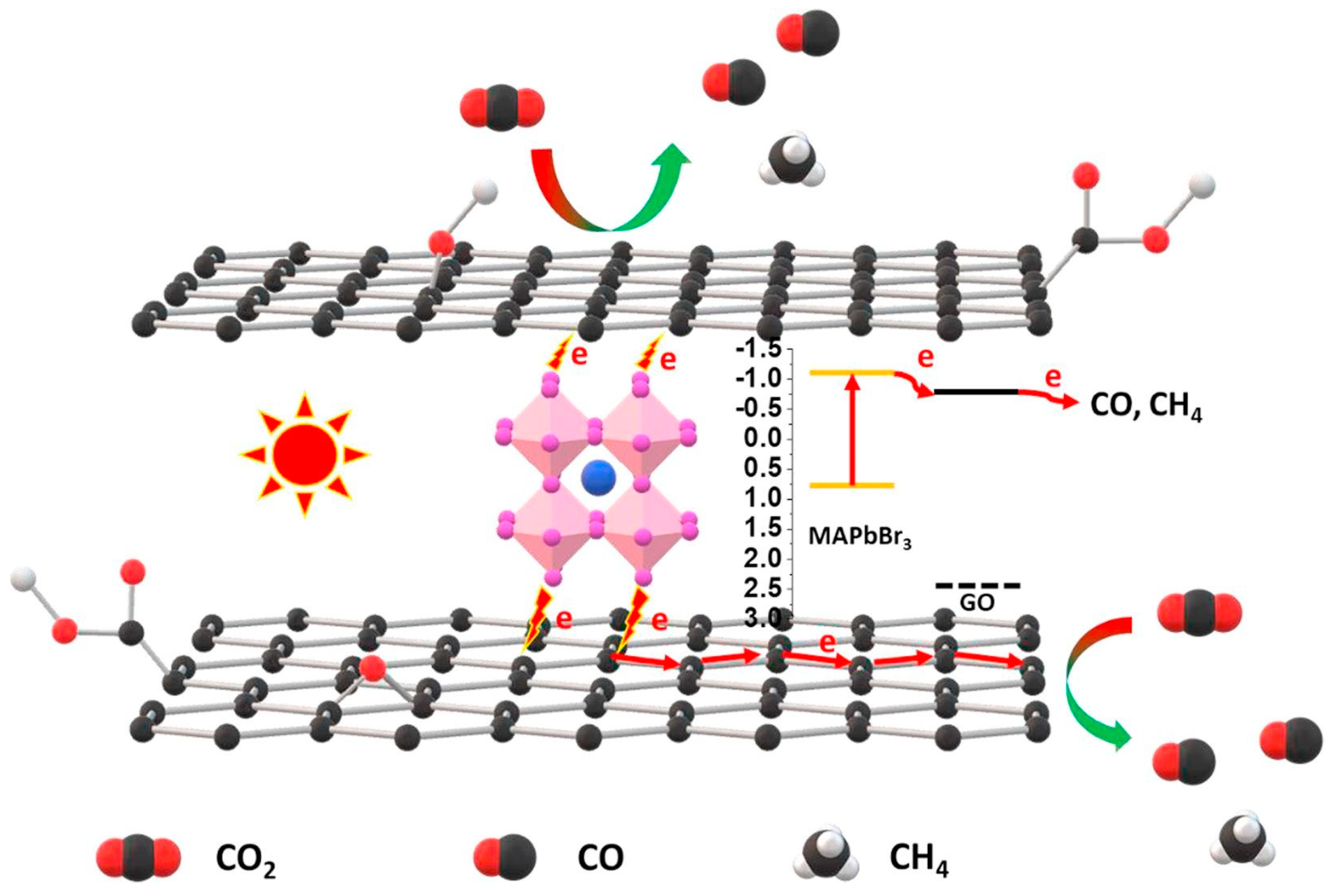
| GO/CH3NH3PbBr3 | Pt wire | - | PEC | CO and CH4 | - | - | [72] |
| (Cs0.15FA0.85)Pb(I0.9Br0.1)3 | Si photoanode | - | PEC | CO | 88 | 3.85 | [73] |
| ZnO@ZnTe@CdTe /CH3NH3PbI3 perovskite tandem cell | Cobalt–bicarbonate | - | PEC | CO | ~80% | 0.43% | [74] |
| CsPbBr3-F-N-Au | Pt foil | - | PEC | CO | - | - | [75] |
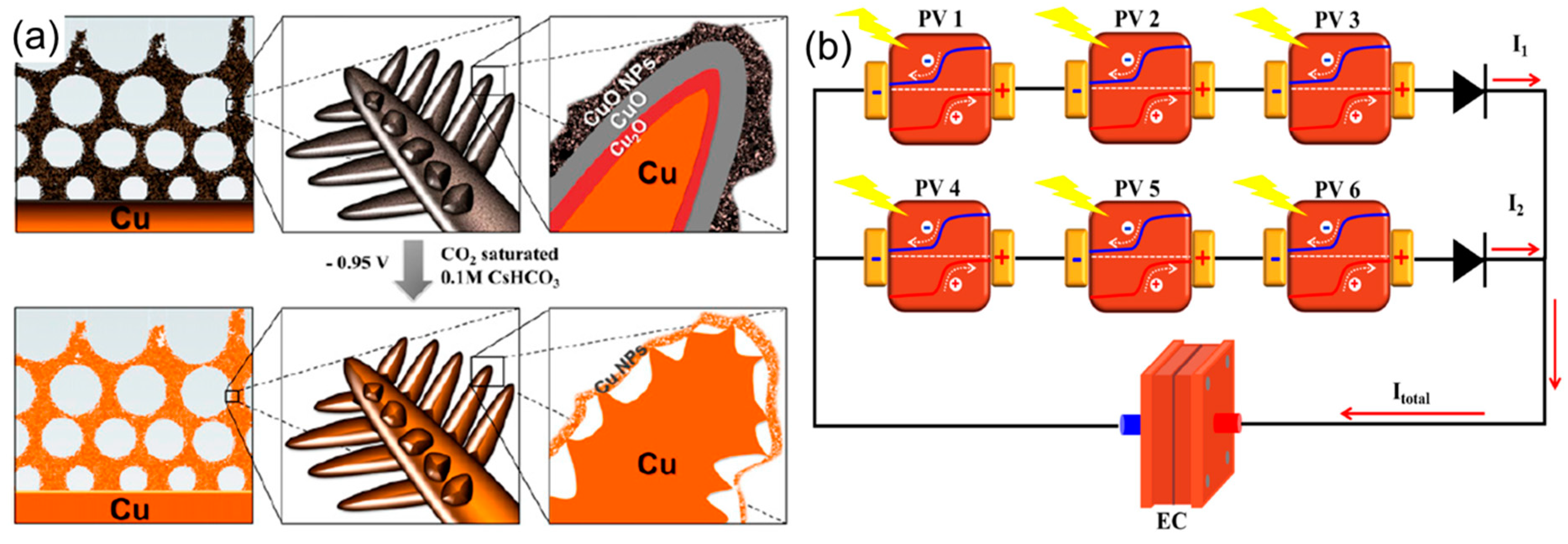
| Dendritic-nanostructured CuO | Dendritic-nanostructured CuO | GaInP/GaInAs/Ge | PV-EC | hydrocarbons | 62 | 2.3 | [77] |
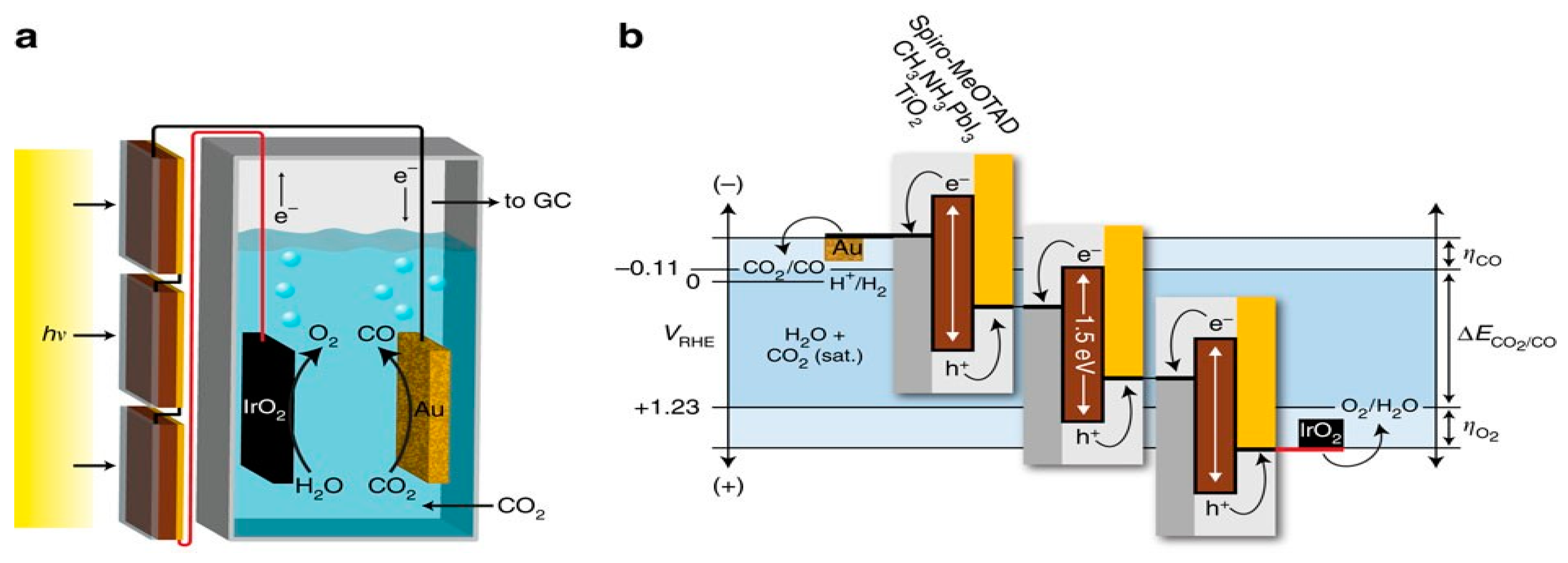
| Oxidized Au | IrO2 | CH3NH3PbI3 | PV-EC | CO | 90 | 6.5 | [78] |
| Au wire | RuO2 | (HC(NH2)2)0.66(CH3NH3)0.34PbI2.85Br0.15 | PV-EC | CO | 80 | >8% | [79] |
| Co-SAs/Zr-CPF | RuO2/C | Cs0.05(FA0.85MA0.15)0.95]Pb0.9(I0.85Br0.15)3 | PV-EC | CO | >50 | 12.5 | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmadi-Kashani, M.; Zendehdel, M.; Schirone, L.; Abolhasani, M.M.; Yaghoobi Nia, N. Recent Progress in the Use of Perovskites for Electrochemical, Photoelectrochemical, and Photovoltaic–Electrochemical CO2 Reduction. Energies 2023, 16, 7632. https://doi.org/10.3390/en16227632
Ahmadi-Kashani M, Zendehdel M, Schirone L, Abolhasani MM, Yaghoobi Nia N. Recent Progress in the Use of Perovskites for Electrochemical, Photoelectrochemical, and Photovoltaic–Electrochemical CO2 Reduction. Energies. 2023; 16(22):7632. https://doi.org/10.3390/en16227632
Chicago/Turabian StyleAhmadi-Kashani, Mina, Mahmoud Zendehdel, Luigi Schirone, Mohammad Mahdi Abolhasani, and Narges Yaghoobi Nia. 2023. "Recent Progress in the Use of Perovskites for Electrochemical, Photoelectrochemical, and Photovoltaic–Electrochemical CO2 Reduction" Energies 16, no. 22: 7632. https://doi.org/10.3390/en16227632
APA StyleAhmadi-Kashani, M., Zendehdel, M., Schirone, L., Abolhasani, M. M., & Yaghoobi Nia, N. (2023). Recent Progress in the Use of Perovskites for Electrochemical, Photoelectrochemical, and Photovoltaic–Electrochemical CO2 Reduction. Energies, 16(22), 7632. https://doi.org/10.3390/en16227632








