Characterisation of Novel and High Performing Double-Sided Microporous-Layers-Coated Gas Diffusion Layers for Polymer Electrolyte Membrane Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
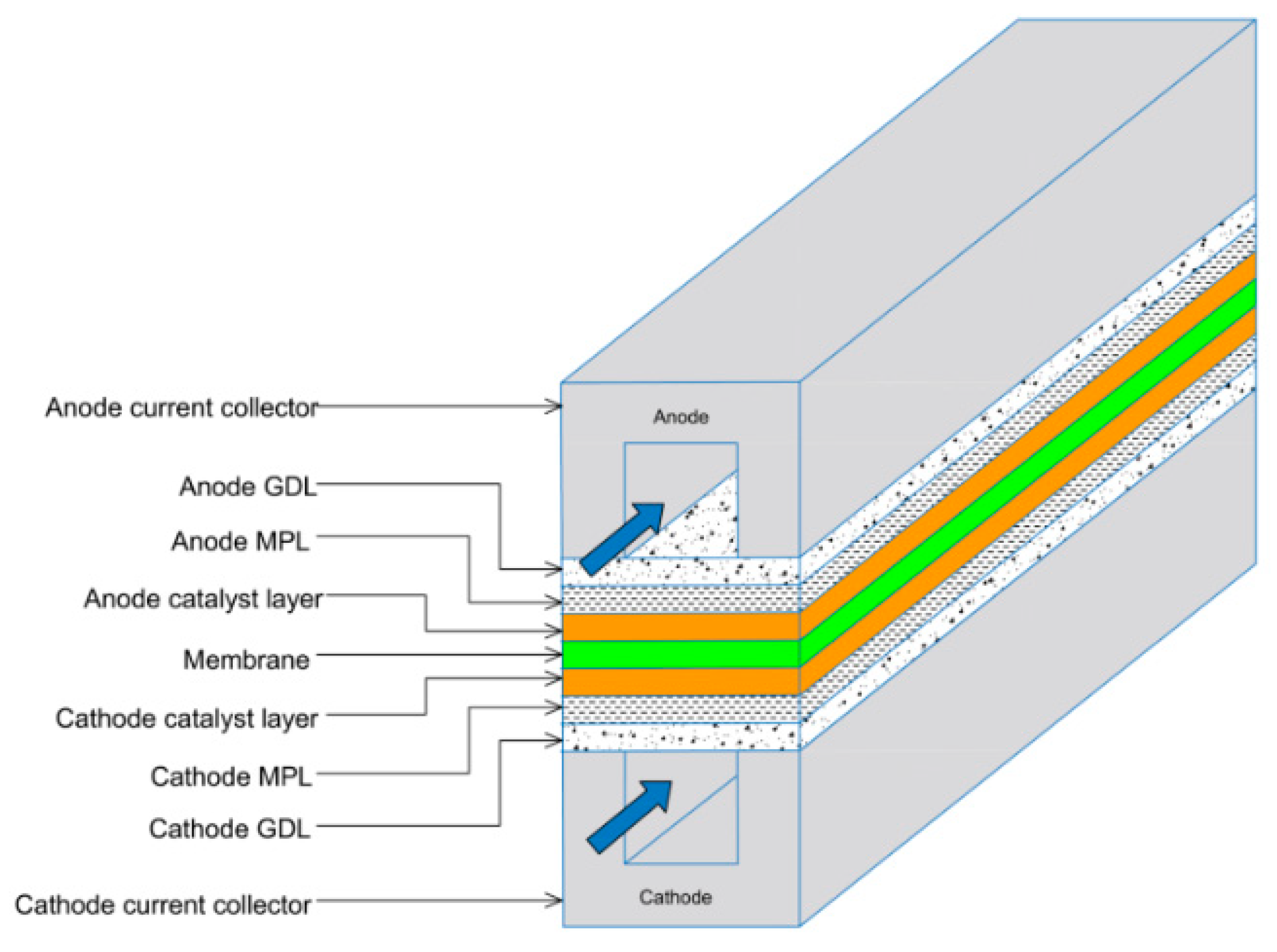
2.1. Fabrication Procedure
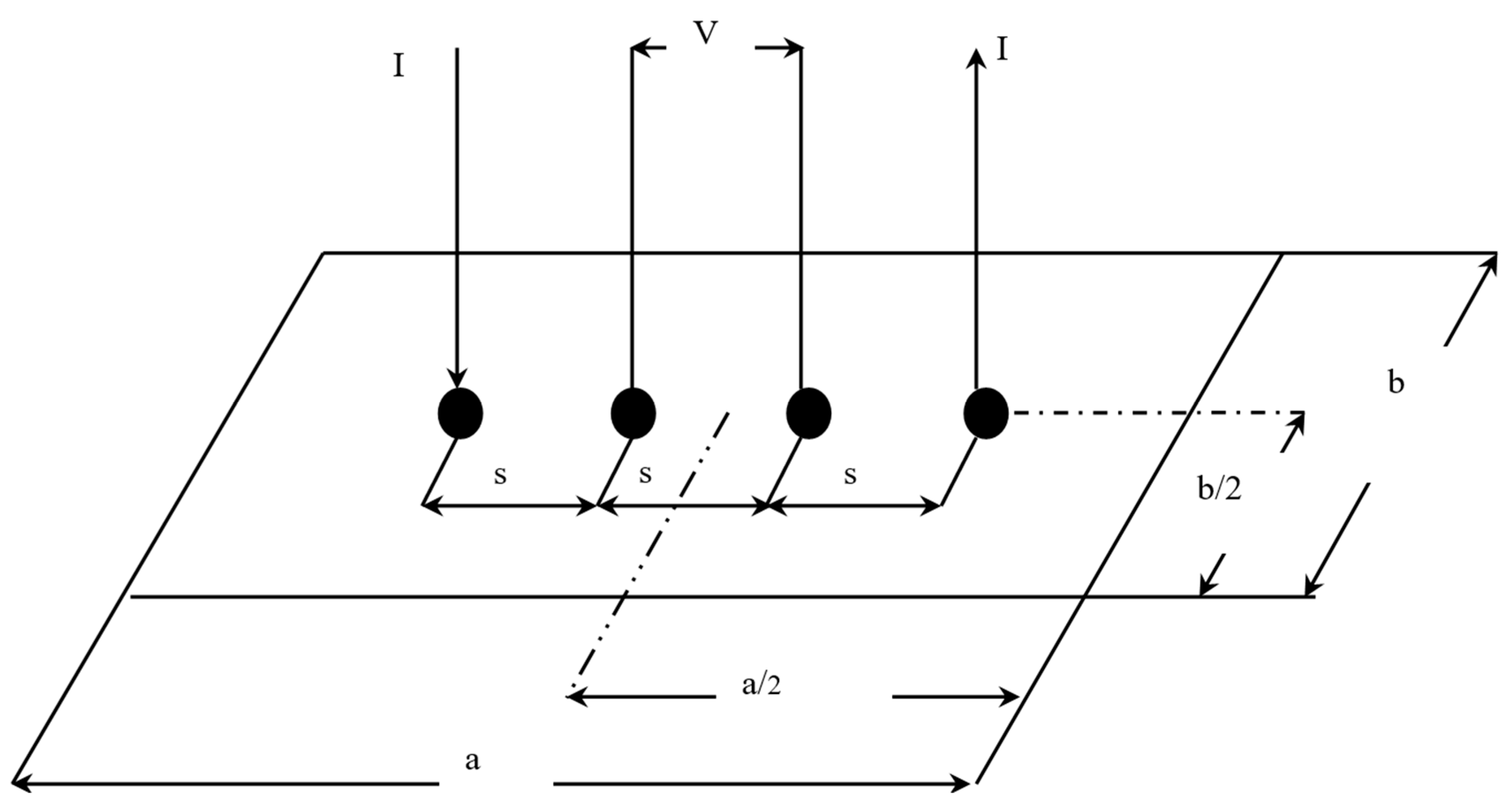
2.2. In-Plane Electrical Conductivity
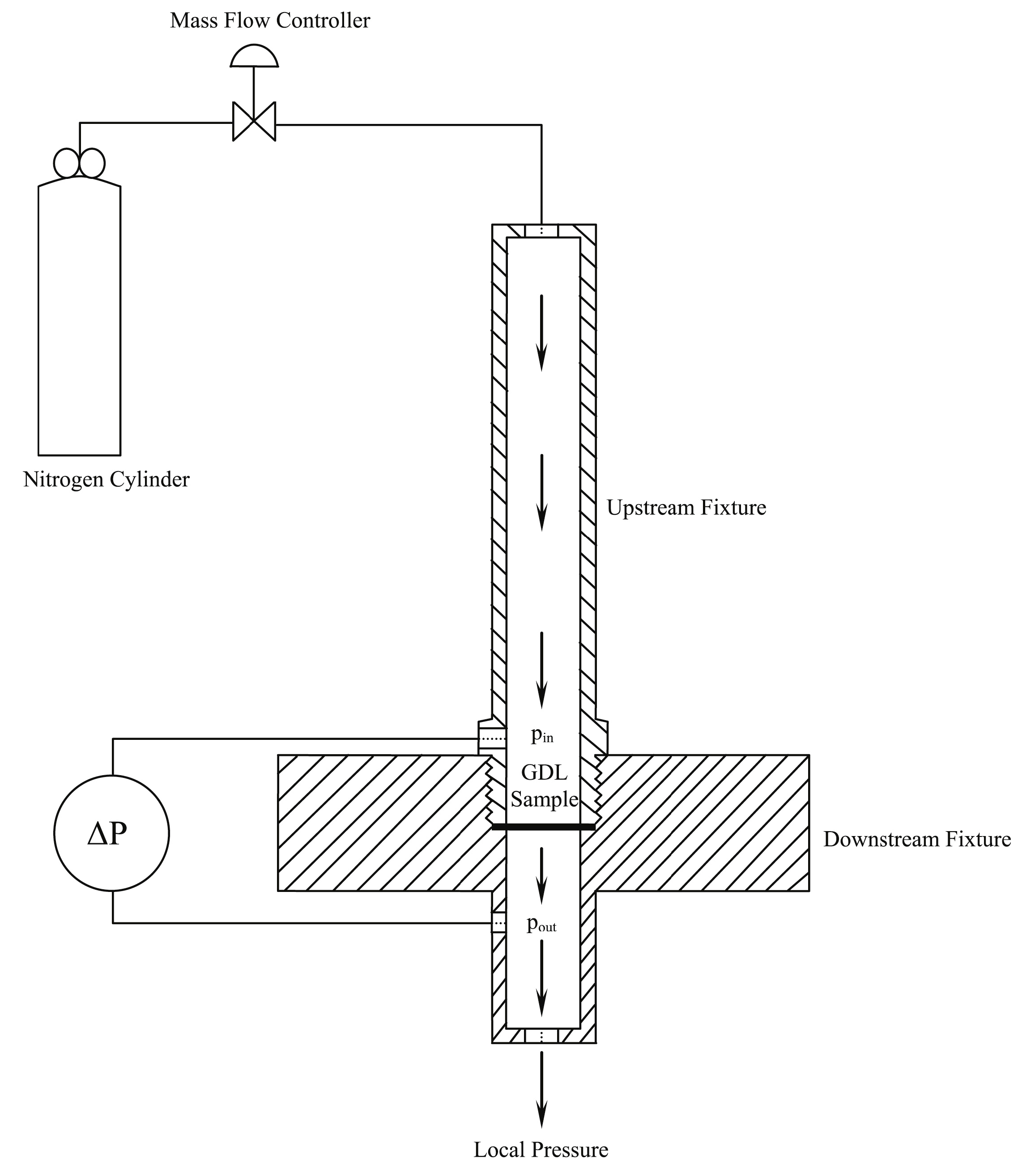
2.3. Permeability
2.4. Pore Size Distribution
2.5. Contact Angle
2.6. Morphology
2.7. In Situ Fuel Cell Testing
3. Results and Discussion
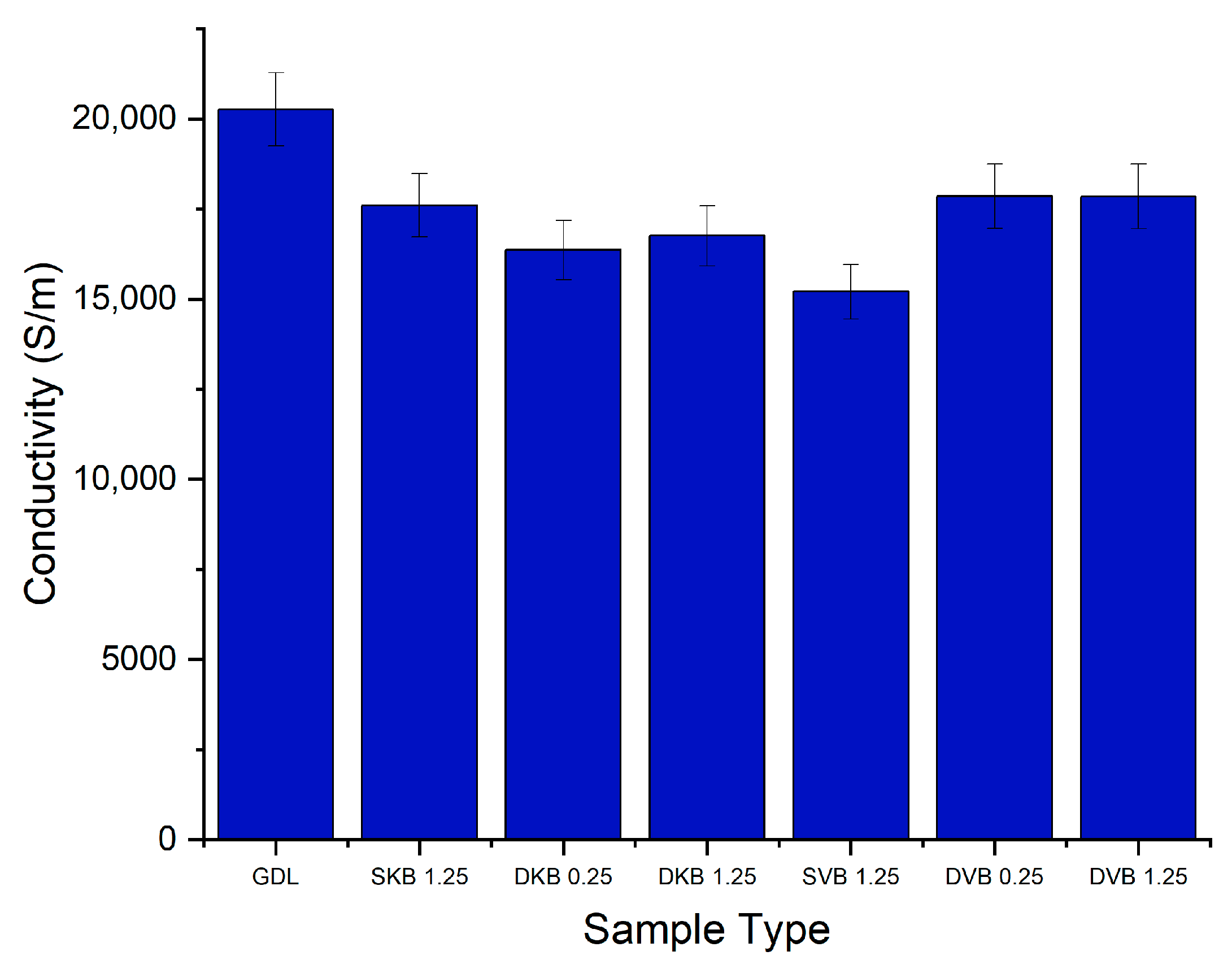
3.1. In-Plane Electrical Conductivity
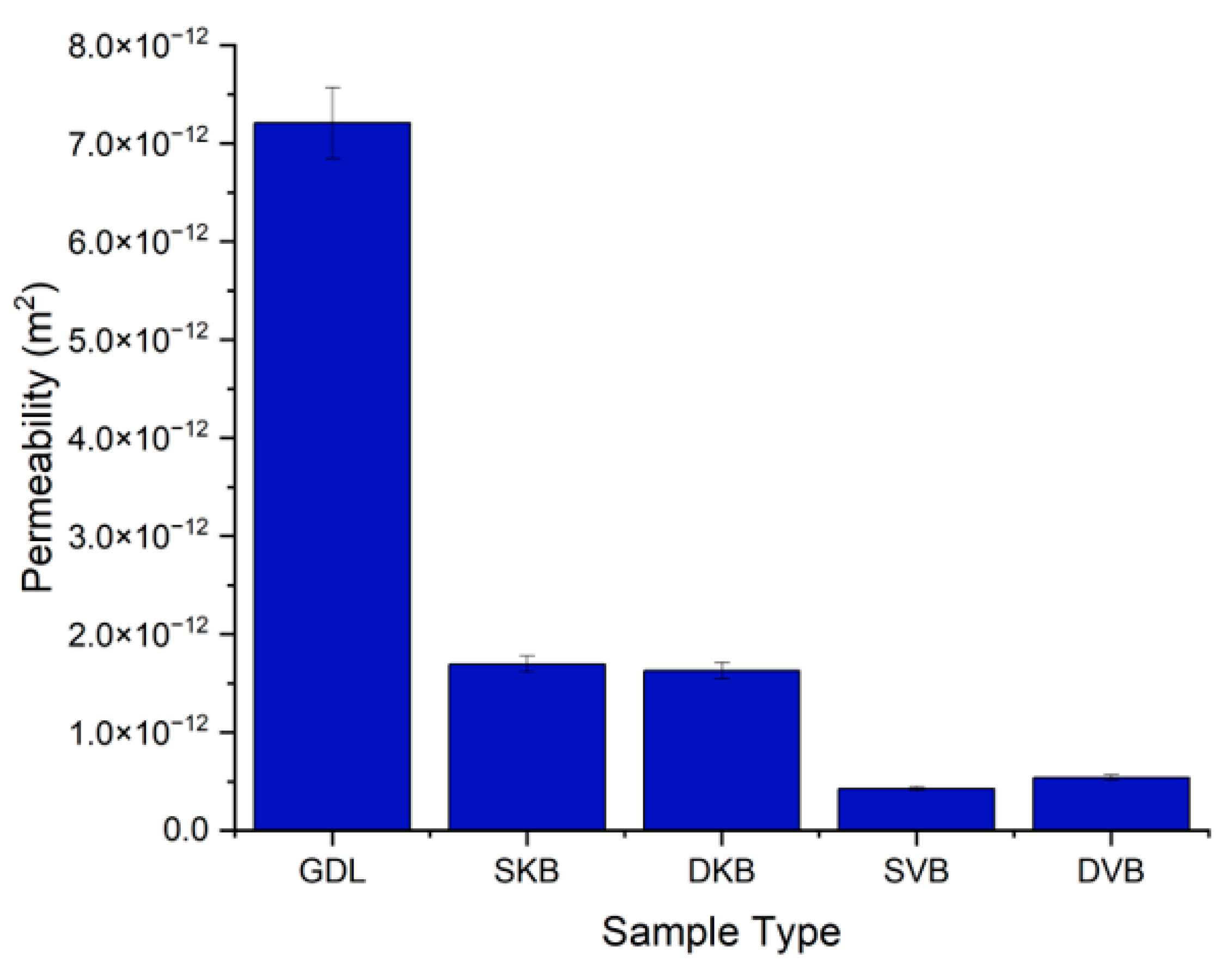
3.2. Gas Permeability
3.3. Pore Size Distribution
3.4. Contact Angle
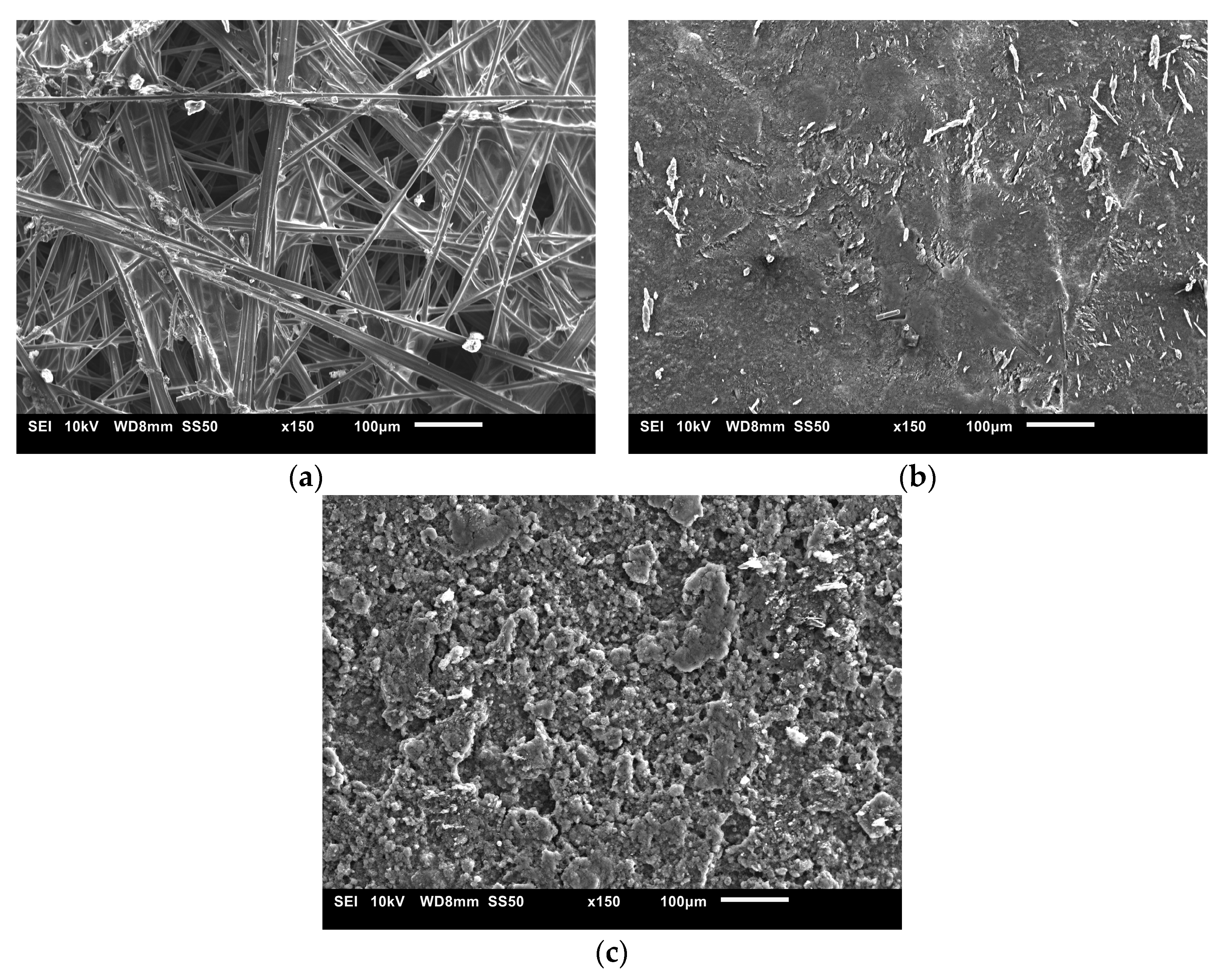
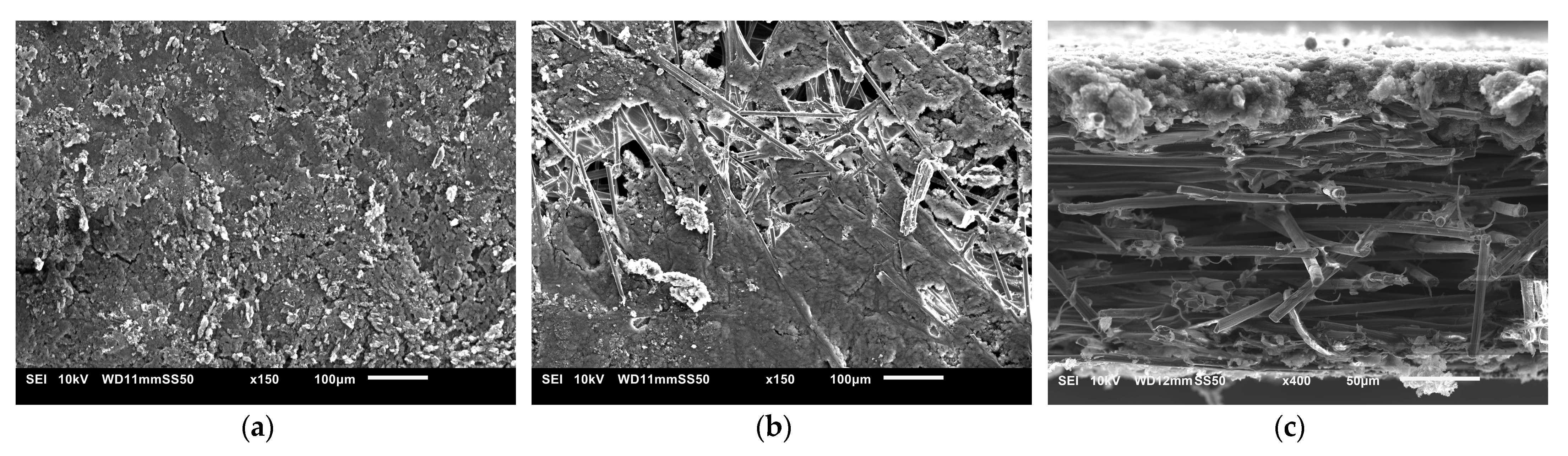
3.5. Morphology
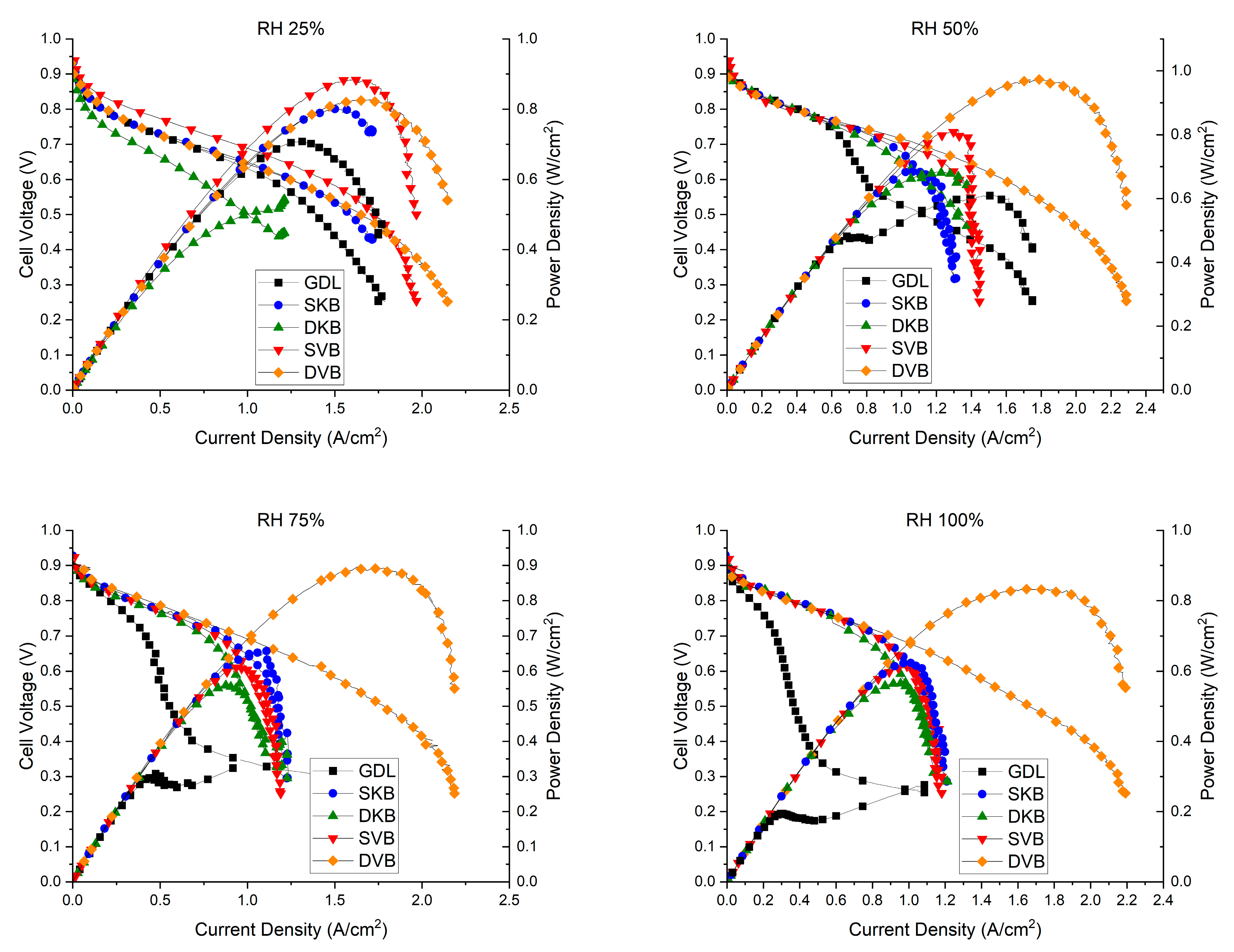
3.6. Fuel Cell Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Cross Sectional Area (m2) |
| C | Correction factor |
| d | Thickness (m) |
| i | Current density (A/m2) |
| I | Current (A) |
| k | Permeability (m2) |
| L | Thickness (m) |
| P | Pressure (Pa) |
| R | Electrical Resistance (Ω) |
| T | Temperature (K) |
| t | Thickness (m) |
| µ | Fluid Viscosity (Pa·s) |
| ρ | Electrical Resistivity (Ω/m) |
| σ | Electrical Conductivity (S/m) |
References
- Aldakheel, F.; Ismail, M.; Hughes, K.; Ingham, D.; Ma, L.; Pourkashanian, M.; Cumming, D.; Smith, R. Gas permeability, wettability and morphology of gas diffusion layers before and after performing a realistic ex-situ compression test. Renew. Energy 2020, 151, 1082–1091. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Elsevier Science & Technology: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Hoogers, G. Fuel Cell Technology Handbook; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Park, S.; Lee, J.-W.; Popov, B.N. Effect of carbon loading in microporous layer on PEM fuel cell performance. J. Power Sources 2006, 163, 357–363. [Google Scholar] [CrossRef]
- Kitahara, T.; Konomi, T.; Nakajima, H. Microporous layer coated gas diffusion layers for enhanced performance of polymer electrolyte fuel cells. J. Power Sources 2010, 195, 2202–2211. [Google Scholar] [CrossRef]
- Weber, A.Z.; Newman, J. Effects of Microporous Layers in Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 2005, 152, A677–A688. [Google Scholar] [CrossRef]
- Ismail, M.; Damjanovic, T.; Ingham, D.; Pourkashanian, M.; Westwood, A. Effect of polytetrafluoroethylene-treatment and microporous layer-coating on the electrical conductivity of gas diffusion layers used in proton exchange membrane fuel cells. J. Power Sources 2010, 195, 2700–2708. [Google Scholar] [CrossRef]
- Gostick, J.T.; Ioannidis, M.A.; Fowler, M.W.; Pritzker, M.D. On the role of the microporous layer in PEMFC operation. Electrochem. Commun. 2009, 11, 576–579. [Google Scholar] [CrossRef]
- Okereke, I.C.; Ismail, M.S.; Ingham, D.B.; Hughes, K.; Ma, L.; Pourkashanian, M. Single- and Double-Sided Coated Gas Diffusion Layers Used in Polymer Electrolyte Fuel Cells: A Numerical Study. Energies 2023, 16, 4363. [Google Scholar] [CrossRef]
- Bednarek, T.; Tsotridis, G. Issues associated with modelling of proton exchange membrane fuel cell by computational fluid dynamics. J. Power Sources 2017, 343, 550–563. [Google Scholar] [CrossRef]
- Netwall, C.J.; Gould, B.D.; Rodgers, J.A.; Nasello, N.J.; Swider-Lyons, K.E. Decreasing contact resistance in proton-exchange membrane fuel cells with metal bipolar plates. J. Power Sources 2013, 227, 137–144. [Google Scholar] [CrossRef]
- Schmittinger, W.; Vahidi, A. A review of the main parameters influencing long-term performance and durability of PEM fuel cells. J. Power Sources 2008, 180, 1–14. [Google Scholar] [CrossRef]
- Makharia, R.; Mathias, M.F.; Baker, D.R. Measurement of Catalyst Layer Electrolyte Resistance in PEFCs Using Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2005, 152, A970–A977. [Google Scholar] [CrossRef]
- Owejan, J.P.; Owejan, J.E.; Gu, W.; Trabold, T.A.; Tighe, T.W.; Mathias, M.F. Water Transport Mechanisms in PEMFC Gas Diffusion Layers. J. Electrochem. Soc. 2010, 157, B1456–B1464. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Zhang, J.; Xu, H.; Zhu, X.; Chen, J.; Yi, B. A bi-functional micro-porous layer with composite carbon black for PEM fuel cells. J. Power Sources 2006, 162, 474–479. [Google Scholar] [CrossRef]
- Chang, H.-M.; Chang, M.-H. Effect of gas diffusion layer with double-side microporous layer coating on polymer electrolyte membrane fuel cell performance. J. Fuel Cell Sci. Technol. 2013, 10, 021005. [Google Scholar] [CrossRef]
- Huang, G.-M.; Chang, M.-H. Effect of Gas Diffusion Layer with Double-Side Microporous Layer Coating on Proton Exchange Membrane Fuel Cell Performance Under Different Air Inlet Relative Humidity. Int. J. Electrochem. Sci. 2014, 9, 7819–7831. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement of Sheet Resistivities with the Four-Point Probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Mench, M. Fuel Cell Engines; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Nitta, I.; Himanen, O.; Mikkola, M. Contact resistance between gas diffusion layer and catalyst layer of PEM fuel cell. Electrochem. Commun. 2008, 10, 47–51. [Google Scholar] [CrossRef]
- Orogbemi, O.; Ingham, D.; Ismail, M.; Hughes, K.; Ma, L.; Pourkashanian, M. Through-plane gas permeability of gas diffusion layers and microporous layer: Effects of carbon loading and sintering. J. Energy Inst. 2018, 91, 270–278. [Google Scholar] [CrossRef]
- Ozden, A.; Alaefour, I.E.; Shahgaldi, S.; Li, X.; Colpan, C.O.; Hamdullahpur, F. Gas Diffusion Layers for PEM Fuel Cells: Ex- and In-Situ Characterization. In Exergetic, Energetic and Environmental Dimensions; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Giesche, H. Mercury Porosimetry: A General (Practical) Overview. Part. Part. Syst. Charact. 2006, 23, 9–19. [Google Scholar] [CrossRef]
- El-Kharouf, A.; Mason, T.J.; Brett, D.J.; Pollet, B.G. Ex-situ characterisation of gas diffusion layers for proton exchange membrane fuel cells. J. Power Sources 2012, 218, 393–404. [Google Scholar] [CrossRef]
- Lee, H.-K.; Park, J.-H.; Kim, D.-Y.; Lee, T.-H. A study on the characteristics of the diffusion layer thickness and porosity of the PEMFC. J. Power Sources 2004, 131, 200–206. [Google Scholar] [CrossRef]
- Fang, Z.; Star, A.G.; Fuller, T.F. Effect of Carbon Corrosion on Wettability of PEM Fuel Cell Electrodes. J. Electrochem. Soc. 2019, 166, F709–F715. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, F. Impact of PTFE content and distribution on liquid–gas flow in PEMFC carbon paper gas distribution layer: 3D lattice Boltzmann simulations. Int. J. Hydrogen Energy 2016, 41, 8550–8562. [Google Scholar] [CrossRef]
- Mench, M.M.; Kumbur, E.C.; Veziroglu, T.N. Polymer Electrolyte Fuel Cell Degradation; Academic Press: Amsterdam, The Netherlands, 2012; ISBN 9780123869364. [Google Scholar]
- Lee, F.; Ismail, M.; Ingham, D.; Hughes, K.; Ma, L.; Lyth, S.; Pourkashanian, M. Alternative architectures and materials for PEMFC gas diffusion layers: A review and outlook. Renew. Sustain. Energy Rev. 2022, 166, 112640. [Google Scholar] [CrossRef]
- Litster, S.; Sinton, D.; Djilali, N. Ex situ visualization of liquid water transport in PEM fuel cell gas diffusion layers. J. Power Sources 2006, 154, 95–105. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.; Wang, Z.; Shi, Z.; Wu, S.; Song, D.; Zhang, J.; Fatih, K.; Zhang, J.; Wang, H.; et al. A review of water flooding issues in the proton exchange membrane fuel cell. J. Power Sources 2008, 178, 103–117. [Google Scholar] [CrossRef]
- Chun, J.H.; Park, K.T.; Jo, D.H.; Lee, J.Y.; Kim, S.G.; Lee, E.S.; Jyoung, J.-Y.; Kim, S.H. Determination of the pore size distribution of micro porous layer in PEMFC using pore forming agents under various drying conditions. Int. J. Hydrogen Energy 2010, 35, 11148–11153. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhang, H.; Hu, J.; Yi, B. Preparation and characterization of sulfated zirconia (SO42−/ZrO2)/Nafion composite membranes for PEMFC operation at high temperature/low humidity. J. Membr. Sci. 2006, 280, 148–155. [Google Scholar] [CrossRef]
- Kumar, G.G.; Kim, A.; Nahm, K.S.; Elizabeth, R. Nafion membranes modified with silica sulfuric acid for the elevated temperature and lower humidity operation of PEMFC. Int. J. Hydrogen Energy 2009, 34, 9788–9794. [Google Scholar] [CrossRef]






| Sample Type | Abbreviation | GDL Substrate | MPL Material | MPL Loading Side 1 (mg/cm2) | MPL Loading Side 2 (mg/cm2) |
|---|---|---|---|---|---|
| Uncoated GDL | GDL | Toray Carbon Paper 060 PTFE 10 wt.% | - | - | - |
| Single-Sided MPL-coated GDL | SVB | Toray Carbon Paper 060 PTFE 10 wt.% | Vulcan Black PTFE 20 wt.% | 1.25 | - |
| Single-Sided MPL-coated GDL | SKB | Toray Carbon Paper 060 PTFE 10 wt.% | Ketjenblack PTFE 20 wt.% | 1.25 | - |
| Double-Sided MPL-Coated GDL | DVB | Toray Carbon Paper 060 PTFE 10 wt.% | Vulcan Black PTFE 20 wt.% | 1.25 | 0.25 |
| Double-Sided MPL-Coated GDL | DKB | Toray Carbon Paper 060 PTFE 10 wt.% | Ketjenblack PTFE 20 wt.% | 1.25 | 0.25 |
| Sample Type | Porosity (%) | Average Pore Diameter (nm) |
|---|---|---|
| GDL | 76.4 | 287.8 |
| SKB | 75.1 | 195.8 |
| SVB | 74.9 | 246.7 |
| DKB | 71.3 | 175.9 |
| DVB | 72.8 | 213.0 |
| Relative Humidity | Sample Type | Peak Power Density (W/cm2) | Maximum Current Density (A/cm2) |
|---|---|---|---|
| 25% | GDL | 0.71 | 1.33 |
| SVB | 0.88 | 1.62 | |
| SKB | 0.79 | 1.52 | |
| DVB | 0.82 | 1.67 | |
| DKB | 0.51 | 1.13 | |
| 50% | GDL | 0.61 | 1.51 |
| SVB | 0.79 | 1.36 | |
| SKB | 0.67 | 1.11 | |
| DVB | 0.96 | 1.85 | |
| DKB | 0.68 | 1.22 | |
| 75% | GDL | 0.32 | 0.91 |
| SVB | 0.61 | 0.96 | |
| SKB | 0.66 | 1.10 | |
| DVB | 0.89 | 1.76 | |
| DKB | 0.55 | 0.89 | |
| 100% | GDL | 0.27 | 1.08 |
| SVB | 0.62 | 1.01 | |
| SKB | 0.62 | 1.02 | |
| DVB | 0.83 | 1.74 | |
| DKB | 0.56 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruscillo, F.; Zhang, K.; Ismail, M.S.; Hughes, K.J.; Ingham, D.B.; Ma, L.; Pourkashanian, M. Characterisation of Novel and High Performing Double-Sided Microporous-Layers-Coated Gas Diffusion Layers for Polymer Electrolyte Membrane Fuel Cells. Energies 2023, 16, 7601. https://doi.org/10.3390/en16227601
Ruscillo F, Zhang K, Ismail MS, Hughes KJ, Ingham DB, Ma L, Pourkashanian M. Characterisation of Novel and High Performing Double-Sided Microporous-Layers-Coated Gas Diffusion Layers for Polymer Electrolyte Membrane Fuel Cells. Energies. 2023; 16(22):7601. https://doi.org/10.3390/en16227601
Chicago/Turabian StyleRuscillo, Fernando, Kun Zhang, Mohammed S. Ismail, Kevin J. Hughes, Derek B. Ingham, Lin Ma, and Mohamed Pourkashanian. 2023. "Characterisation of Novel and High Performing Double-Sided Microporous-Layers-Coated Gas Diffusion Layers for Polymer Electrolyte Membrane Fuel Cells" Energies 16, no. 22: 7601. https://doi.org/10.3390/en16227601
APA StyleRuscillo, F., Zhang, K., Ismail, M. S., Hughes, K. J., Ingham, D. B., Ma, L., & Pourkashanian, M. (2023). Characterisation of Novel and High Performing Double-Sided Microporous-Layers-Coated Gas Diffusion Layers for Polymer Electrolyte Membrane Fuel Cells. Energies, 16(22), 7601. https://doi.org/10.3390/en16227601













