1. Introduction
Some agencies, such as the World Meteorological Organization, have formally declared that July 2023 was the month with the highest global mean temperature since the inception of meteorological data archives, and it may be the hottest month over the time span of 120,000 years. Seasoned experts in this field assert that in the context of global warming, record-breaking high temperatures should be regarded as a foreseeable consequence, and extreme weather is the grave reality caused by climate change. It is, therefore, imperative to reduce greenhouse gas emissions.
Since the ratification of the Paris Agreement during the 21st United Nations Climate Change Conference in 2015, nations across the globe have actively sought to develop robust strategies to usher in the era of CO
2 emission peak and ultimate neutrality [
1,
2,
3]. Among all the means available for achieving the goal of carbon neutrality, negative emission technologies, as a pivotal and indispensable component, will ensure the realization of carbon neutrality, in the period of profound decarbonization [
4,
5,
6,
7,
8,
9].
Carbon dioxide capture, utilization, and storage (CCUS) is the most potential negative emission technology. This technology serves as a cornerstone to support the goal of carbon neutrality, and it can contribute to 15% of total CO
2 emission reduction. Deep saline aquifer storage is the most potential type of CO
2 geological storage. Applying the highly precise CSLF calculation method, some scholars predict that deep saline aquifers in the principal sedimentary basins of China can store 119.2 billion tons of CO
2 [
10,
11,
12]. Based on the ideal assumption that the exploitation space of oil and gas or coalbed methane is occupied by the same amount of CO
2, the method converts the recoverable oil/gas resources into the space volume under the in situ conditions of the reservoir and converts the CO
2 density under the reservoir conditions into the geological sequestration of CO
2.
The sequestration mechanisms of saline aquifers include tectonic sequestration, residual gas sequestration, dissolution sequestration, and mineralization sequestration. Mineralization sequestration enables the dissolution of CO
2 in the formation water, thus inducing a reduction in its pH. As a result, a series of water–rock geochemical reactions occur, leading to the dissolution of some minerals associated with the precipitation of secondary minerals (mostly carbonate minerals). In this process, a fraction of metal cations, such as Ca
2+ and Mg
2+, enter into reactions with carbonates to precipitate, and thus permanent CO
2 sequestration is realized. Significantly, mineralization sequestration is the most ideal storage state, representing the ultimate transformational outcome of the first three sequestration mechanisms. However, it should be noted that the temporal cycle inherent to mineralization sequestration is the longest, spanning timeframes ranging from centuries to millennia [
13,
14,
15].
Moreover, CO
2 can interact with rock minerals after it is injected into reservoirs, which may cause leakage and other untoward incidents in reservoirs with poor caprock sealing capacity, so as to damage the safety of long-term storage [
16,
17]. Thus, it is necessary to conduct in-depth research on the geochemical reaction mechanisms between CO
2 and formation minerals, which is of great significance for the accurate prediction of sequestration volumes and the safety evaluation of long-term storage.
Owing to earlier studies on CCS/CCUS in North American countries, some research results have been obtained with respect to the mechanisms underlying CO
2 mineralization storage within diverse geological reservoirs such as oil reservoirs, oil–water transition zones, and deep saline aquifers. Ueda et al. [
18] experimentally investigated the effects of rock mineral composition, pressure, and temperature on CO
2–water–rock (mineral) interactions, which demonstrated the feasibility and substantial potential inherent in CO
2 mineralization storage. Ketzer et al. [
19] probed the CO
2–water–rock (mineral) interaction during CO
2 sequestration within the Rio Bonito Formation of Brazil. Their findings unveiled the dissolution of calcite cement within sandstone, along with observations regarding calcite and iron calcite precipitation. Farquhar et al. [
20] completed a 16-day indoor simulation experiment to research the geochemical dynamics of sandstone reservoirs located on Queen’s Island, Australia, by subjecting these formations to CO
2 dissolution. This investigation reveals the dissolution of various sandstone minerals, encompassing carbonate minerals, chlorite, and a minor fraction of feldspars. Li et al. [
21] employed modeling techniques involving PHREEQC calculations to investigate the interaction dynamics between the liquid phase and rock strata. Their analyses indicated that with the increase in burial depth (pressure), Ca
2+ can form calcite to facilitate precipitation. Balashov et al. [
22] researched the dissolution reaction of sandstone minerals by establishing a diffusion transport model. Their research revealed that calcite acts as the preeminent carbon-fixing mineral, and the precipitation of secondary minerals is influenced by the initial mineral dissolution. These pioneering investigations collectively contribute to a deeper comprehension of the intricate realm of CO
2 mineralization storage within geological reservoirs, further confirming the salience of these processes in the context of carbon management strategies.
In general, however, research outcomes have predominantly centered on the characteristics of marine sedimentary basins. Yet, for the widely distributed terrestrial sedimentary saline aquifer basins in China, substantial disparities arise due to differences in mineral composition, structural maturity, and related factors in comparison with marine sedimentary basins. Additionally, the stratigraphic environment in these terrestrial basins is notably more complex. Consequently, significant gaps and deficiencies exist in our understanding of reaction mechanisms of CO
2–water–rock (minerals) [
23,
24,
25,
26] within these contexts, necessitating further in-depth investigations.
In the realm of experimental investigations into CO
2–water–rock interactions, emphasis is commonly placed on indoor experiments conducted under elevated temperature and pressure conditions. These experiments are often facilitated using high-temperature, high-pressure reactors or core displacement methods. The primary distinction between these approaches lies in the presence or absence of fluid transport within the experimental setup. While these experiments have significantly contributed to our understanding of CO
2–water–rock interactions, they do have inherent limitations. One notable limitation is the substitution of pure water for authentic formation water in the experimental design, leading to deviations in reaction outcomes compared with those encountered in real geological formations. Additionally, the intricate mineral composition of the selected geological cores poses challenges in precisely studying the dissolution mechanisms of individual minerals involved in the reactions. Notably, significant disparities in mineral composition can result in more pronounced variations in reaction outcomes [
27,
28,
29].
To comprehensively analyze the complex mechanisms involved in CO2–water–rock (mineral) reactions within terrestrial deep saline aquifers, in this study, the Daqing Oilfield in China is used as a representative example. The research involves core sampling to investigate the mineral composition of saline aquifer rocks, including sandstone minerals and clay minerals, within three distinct blocks. Four specific minerals—potassium feldspar, plagioclase feldspar, calcite, and kaolinite—were selected for detailed examination due to their significance within the geological context. These minerals were subjected to simulated conditions of temperature, pressure, and mineralization typical of saline aquifers in the Daqing Oilfield. Under these controlled conditions, we explored the dissolution and erosion processes affecting these minerals, as well as the resulting changes in the ionic composition and pH of the formation water throughout the geochemical reaction process.
2. Experiments
2.1. Experimental Samples
Table 1 and
Table 2 present the composition data of the main sandstone minerals and clay minerals in three distinct blocks of the Daqing Oilfield. A cursory examination of the mineral content data reveals that quartz predominates in abundance. However, it is noteworthy that quartz exhibits remarkable chemical stability, even when subjected to the temperature and pressure conditions encountered within saline aquifers. Consequently, in short-term indoor experiments, discerning significant dissolution phenomena in quartz proves challenging. Therefore, our experimental focus narrowed down to three primary minerals: potassium feldspar, plagioclase feldspar, and calcite. Among these, potassium feldspar and plagioclase feldspar hold a significant position due to their substantial content within the feldspar mineral category. These minerals are particularly susceptible to dissolution and erosion when subjected to CO
2 injection conditions. On the other hand, calcite, a quintessential carbonate mineral, while relatively less abundant in terrestrial sedimentary strata (comprising less than 10% of the mineral content), exhibits a comparatively higher dissolution rate than feldspar minerals. This property renders calcite influential in the context of CO
2 mineralization and storage. Kaolinite, a clay mineral, emerges as an additional subject of interest, as it is an outcome of feldspar dissolution. The intensity of the dissolution reaction involving kaolinite holds significance, as it directly impacts the stratum’s stability over protracted durations under CO
2 injection conditions.
It is worth noting that acquiring high-purity feldspar minerals and clay minerals under natural conditions proves challenging. Consequently, the mineral groups utilized in the experiments unavoidably have trace impurities. However, the precision of the experiments was diligently maintained through meticulous analysis, including monitoring changes in the ion composition of water during the reaction process. The experimental fluid employed in the study was meticulously prepared in the laboratory to align with the composition of the formation water in a designated block within Aonan. This fluid exhibited a mineralization level of 11,636.3 mg/L. For reference,
Table 3 presents a comprehensive breakdown of the ionic composition of the formation water and pertinent details concerning the prepared reagents utilized in the experiments.
2.2. Experimental Setups
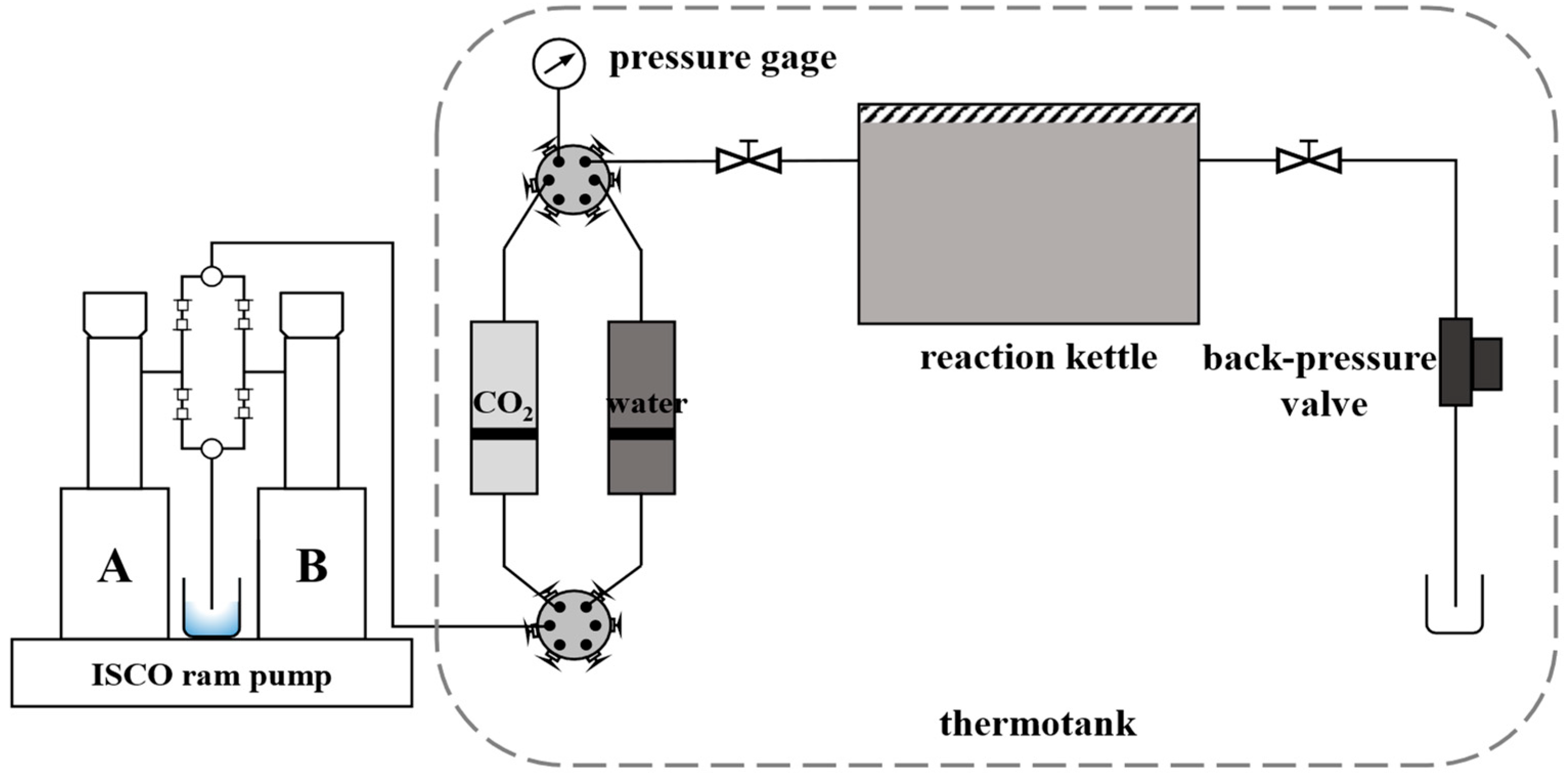
The experimental device consisted of a stainless steel high-temperature and high-pressure reactor, an ISCO constant-speed ram pump, a thermotank, and a return valve. The experimental system device is shown in
Figure 1. In the experiment, CO
2 was injected into the reactor through the ISCO ram pump to simulate the stratum pressure, and the thermostat box was used to control the temperature of the whole reaction system, which was considered to be the simulated stratum temperature for the entire experiment. The experimental parameters were used to simulate the temperature and pressure conditions of formation water and ionic composition in a block of Aonan. The partial pressure of CO
2 in the reactor was 16 MPa, and the temperature of the thermotank was 60 °C.
2.3. Experimental Process
Prior to the commencement of dissolution reactions, it is necessary to crush and mill the minerals. This process enables the minerals to undergo a more pronounced dissolution reaction within a shorter timeframe, which is obviously different from the real ore-forming process, but the laboratory test period is short, so only by increasing the reaction contact surface area in this way can more obvious changes be observed. The minerals before and after the reaction were sampled for comparative analysis through compositional analysis, surface morphology examination, and specific surface area testing. For the mineral composition analysis, we employed X-ray diffraction (XRD), while the mineral surface morphology analysis was performed using scanning electron microscopy (SEM). The specific surface area of the minerals was determined using a specific surface area adsorption instrument. The mass of the four groups of reacted minerals equaled 20 g.
To prevent clogging the pipeline and affecting fluid sampling, a layer of stainless steel mesh was placed at the bottom of the reactor. Mineral powder was added, followed by injecting 400 mL of prepared formation water into the reactor. CO2 was then injected into the reactor using the ISCO ram pump until the pressure reached 16 MPa. Once the preset pressure was reached, the valve was closed, and the thermostat temperature was set to 60 °C to initiate the reaction. Over the course of 15 days, liquid samples were taken at five different time points, namely 1, 3, 6, 10, and 15 days after the start of the reaction. The sampling interval gradually increased, in accordance with the decreasing rate of mineral dissolution. After the five sampling events, the pH and ionic concentration were measured along with the initial preparation of the formation water. Following the reaction, the mineral powder was removed, rinsed with deionized water, and then dried in an oven at 60 °C for 24 h. Subsequently, mineral composition, surface morphology analysis, and specific surface area measurements were conducted.
Equations (1)–(4) represent the primary reaction equations governing the dissolution of potassium feldspar, sodium feldspar, calcite, and kaolinite, respectively.
In the actual stratigraphic environment, various factors such as the complex composition of reservoir minerals and the differences in temperature, pressure, and other environmental parameters contribute to the generation of different products from similar minerals. For instance, sodium feldspar can generate different secondary minerals like kaolinite and sodalite (NaAlCO3(OH)2) through dissolution, depending on the ionic composition of the formation water, as well as the temperature and pressure conditions. Consequently, the reaction formula for these minerals is not unique. To understand the mechanism of mineral erosion, it is necessary to consider the reaction environment and the time span involved. Furthermore, in the mineralization sequestration process, which occurs over an extended period, the mechanism of action varies at different stages.
3. Results and Discussion
3.1. pH Change in the Reaction System
Under experimental conditions, CO
2 is in a supercritical state, making it challenging to determine pH in situ due to high temperature, pressure, and a closed system. To overcome this, we chose to analyze liquid samples taken after CO
2 dissolution equilibrium to determine pH. Additionally, we utilized the PR-HV equation of state [
30] to estimate CO
2 solubility under the reaction conditions. This approach helps to minimize experimental error by allowing for comparative analysis.
The Peng–Robinson equation of state is a useful tool for calculating CO
2 solubility under varying temperatures, pressures, and formation water salinity. The equation is expressed as follows:
In the given equation, represents the pressure of the reaction system in MPa. denotes the ideal gas constant, while signifies the temperature of the reaction system in Kelvin. represents the molar volume in cm3/mol. The variables and correspond to the gravitational and repulsive constants of the mixing system, respectively. The variable represents the number of moles of the total components in the mixing system, and denotes the mole fraction of component i in the liquid phase. The parameters and represent the equation of state for the i-component of the pure substance. Additionally, is a temperature-dependent function. represents the excess Gibbs free energy as the system’s pressure approaches infinity. and denote the critical temperature and pressure of component i, respectively. is defined as the ratio of to , and lastly, is a function of the eccentricity factor , which is determined as follows: 0.37646 + 1.5426 − 0.26992; .
Many researchers have found that the Peng–Robinson calculation model is not entirely suitable for strong polar material water conditions in numerous experiments and practical applications. As a result, a new PR-HV prediction model was subsequently proposed based on it, in conjunction with the Huron–Vidal fugacity coefficient model.
In the above equation, represents the mixture fugacity coefficient, denotes the mixture deviation factor, signifies the mixture molar volume in cubic meters per mole, and represents the activity coefficient of the component.
The fugacity coefficient of the gas phase within the mixed system can be determined utilizing the PR-HV model and the provided conditions. Subsequently, the fugacity coefficient of the liquid phase and the H
2CO
3 content can be iterated to calculate the solubility of CO
2. It is important to note that in the CO
2 solubility calculation model used in this paper, it is assumed that NaCl contributes to the total salinity of the formation water, because the solubility of CO
2 is more affected by the total salinity rather than the mineral composition, and NaCl accounts for a relatively high proportion of the mineral composition, which can simplify the calculation and has little impact on the accuracy. Moreover, we did not consider the hydrolysis of cations in our model. The parameters used in the model are presented in
Table 4.
Based on the given conditions, the solubility of CO
2 in water was calculated to be 1.26 mol/kg when CO
2 dissolution reached equilibrium under the simulated stratigraphic conditions in the experiment. Since carbonic acid is a weak acid, its ionization determines the concentration of H
+ in the solution. This concentration can be obtained using the ionization equilibrium constant and the solubility.
The H+ concentration was calculated to be 10–3.8 mol/L, resulting in a pH of 3.8. During the experiment, however, the measured pH of the solution after dissolution equilibrium was found to be 4.1, slightly higher than the calculated value. The authors attributed this discrepancy to two main factors. Firstly, the experiment used NaHCO3 water; this type of water contains , which inhibits the ionization of H2CO3 to some extent. Additionally, the rapid decompression of the solution during the test caused some CO2 to escape, leading to a slightly higher pH.
By comparing and analyzing the two methods, it is evident that the type of the formation water has an impact on the dissolution of CO2 during the process of CO2 sequestration in saline aquifers. Additionally, the presence of NaHCO3 in the water has a negative effect on the dissolution and potential mineralization of CO2.
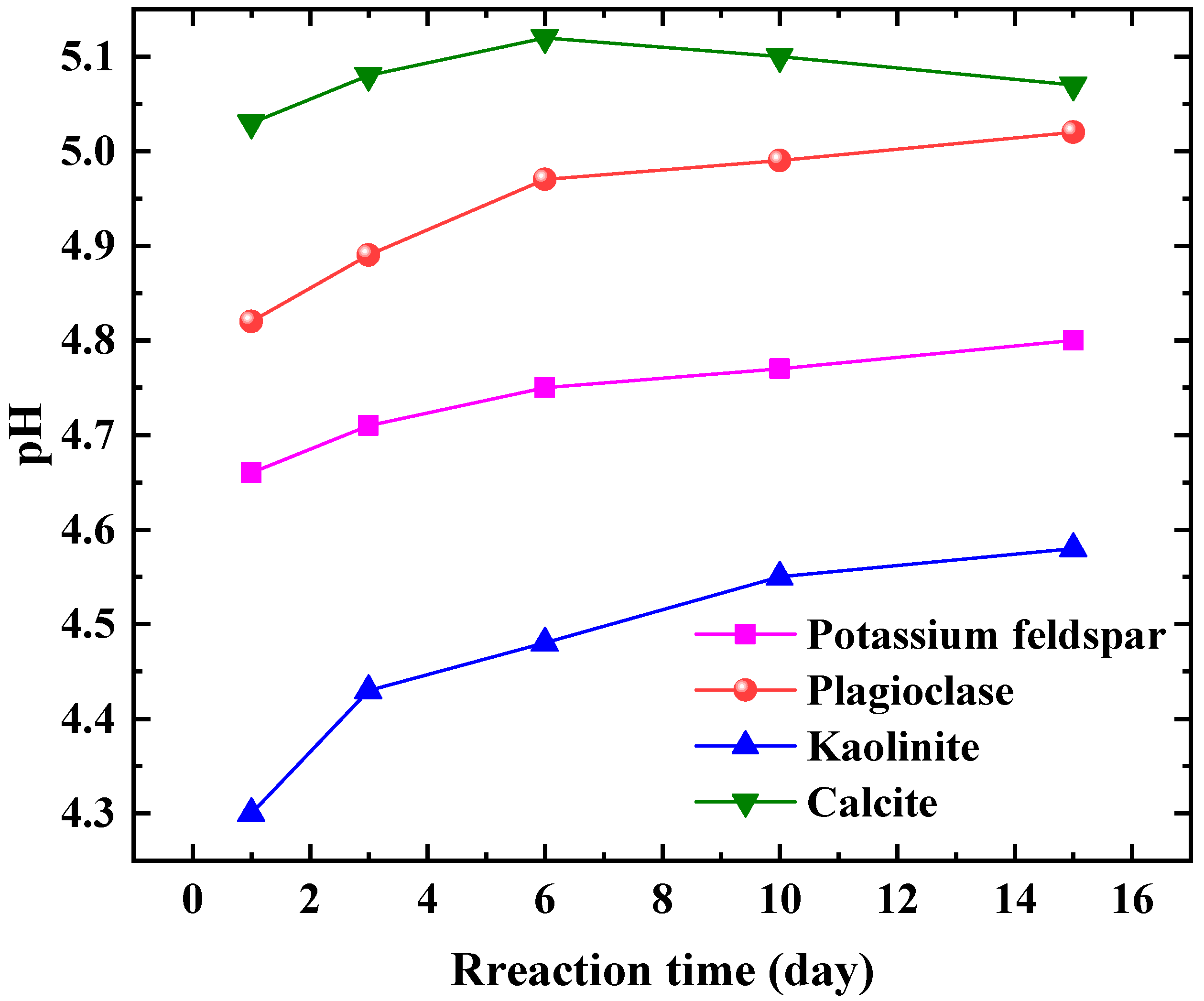
The pH changes in the four reaction systems are depicted in
Figure 2. The curves, from highest to lowest, correspond to the calcite group, plagioclase group, potassium feldspar group, and kaolinite group. The pH of the calcite group remained stable between 5.0 and 5.2 during the first 1st to 15th day after the reaction, suggesting a relatively quick attainment of dissolution–precipitation equilibrium. On the other hand, both the plagioclase feldspar group and the potassium feldspar group exhibited an increasing pH trend throughout the reaction process, with values lower than those of the calcite group. This implies that feldspar minerals have a slower reaction rate than carbonate minerals, requiring more time to reach equilibrium. Furthermore, the pH of the plagioclase feldspar group exceeded that of the potassium feldspar group. The higher pH of the plagioclase group indicates a faster reaction rate and a more intense dissolution process. Conversely, the kaolinite group displayed the lowest pH, indicating its ability to remain stable in weakly acidic environments. This finding holds significant implications for studying the stability of strata under long-term CO
2 injection conditions, as kaolinite is the primary product of dissolved and eroded feldspar minerals, and it aids in determining the chemical properties of kaolinite under weakly acidic conditions. It is worth noting that feldspar minerals may contain trace amounts of impurity minerals under realistic conditions; however, the concentration is negligible and does not significantly impact the pH test results. In line with previous studies [
31], it has been observed that these impurity minerals are often overlooked. Unlike feldspar, kaolinite and calcite do not encounter this issue as they exhibit nearly 100% purity.
When comparing the pH changes during the reaction process of various mineral groups, it becomes apparent that there is a significant difference in the sensitivity of different types of minerals to CO2 fluids. The sensitivity rankings, from highest to lowest, are carbonate minerals, feldspar minerals, and clay minerals. In China, terrestrial sedimentary basins are widely distributed, with the formation minerals primarily consisting of feldspar and clay minerals. The content of carbonate minerals is relatively low in these basins. As a result, the interaction between CO2, water, and rock (minerals) is a long and intricate process, leading to slow changes in the pH of the formation water.
3.2. Changes in Cation Mass Concentrations
The relationship between the mass concentration of major cations and the time during the reaction process of each mineral group is illustrated in
Figure 3. Specifically,
Figure 3a depicts the changes in the concentration of major cations during the dissolution process of potassium feldspar group minerals. The concentration of K
+ gradually increased during the reaction, albeit at a slow rate, and did not exceed 5 ppm. This indicates that potassium feldspar underwent dissolution, but the rate of dissolution was slow. The concentration of Na
+ initially increased and then slightly decreased as the reaction progressed. This can be attributed to the presence of plagioclase feldspar in the mineral group, where the increase in Na
+ concentration may result from the dissolution of plagioclase feldspar. The slight decrease in concentration toward the end of the reaction suggested that the minerals approached equilibrium, leading to a decrease in the rate of dissolution. The concentration of Ca
2+ and Mg
2+ exhibited a small decrease overall, indicating their involvement in carbonate minerals such as CaCO
3, MgCO
3, CaMg(CO
3)
2, etc. Additionally, XRD analysis conducted before and after the reaction of the minerals (refer to
Section 3.4 for more details) confirms their participation in the transformation process from illite to ilmenite.
Figure 3b illustrates the changes in the concentration of main cations during the dissolution process of the plagioclase feldspar group. The Na
+ concentration in this group was significantly higher than the other groups, primarily due to the dissolution of sodium feldspar. The overall Na
+ concentration exhibited an initial rapid increase followed by a gradual decline. This trend can be attributed to several factors. Firstly, the sodium feldspar gradually reached a saturated state, leading to a decrease in the reaction rate. Additionally, this may be attributed to the albitization of calcium feldspar or the precipitation of Na-containing minerals. Conversely, the dissolution of sodium feldspar may also result in the precipitation of Na-containing minerals. The Ca
2+ concentration was noticeably higher than that of the potassium feldspar group, primarily due to the dissolution of calcium feldspar. In the first fifteen days after the reaction, the Ca
2+ concentration exhibited small fluctuations with no significant trend. This suggests that calcium feldspar had a higher dissolution rate than sodium feldspar and reached the saturation state more quickly. The concentration of Mg
2+ showed no obvious trend and remained largely unaffected by any reaction.
Figure 3c illustrates the variations in the concentration of major cations during the dissolution of minerals belonging to the kaolinite group. It shows that there are minimal changes in the concentration of major cations, suggesting a weak reaction and chemical stability of kaolinite in a weakly acidic stratigraphic environment. Specifically, the concentration of Na
+ slightly decreased in the later stages of the reaction, although to a lesser extent than the first two groups of feldspar minerals, and therefore we posit that limited secondary mineral precipitation, such as dawsonite, may have occurred; however, the extent of this reaction was too minute to be discerned through SEM or XRD analysis. Additionally, the concentrations of Ca
2+ and Mg
2+ initially decreased, then increased, and finally stabilized in the later stages of the reaction. Kaolinite, being the primary product of feldspar minerals undergoing dissolution, is formed in an acidic environment with low cation concentration. The main factor contributing to its chemical stability in acidic conditions is the presence of hydrogen bonding as an intermolecular force. Furthermore, the edges of its crystals serve as the primary areas of charge distribution, and the cation exchange effect is weak.
Figure 3d illustrates the variations in the concentration of major cations during the dissolution process of minerals in the calcite group. The concentration of Ca
2+ was notably higher than other groups, averaging around 400 ppm. There was a slight change in concentration within 1 to 15 days after the reaction, followed by a gradual decrease in the later stages. This indicates that calcite achieves dissolution equilibrium relatively quickly compared with feldspar minerals and clay minerals. The equilibrium time for calcite was an order of magnitude shorter than that of the other two minerals. The slight decrease in concentration in the later stages can be attributed to the reprecipitation effect of calcite. On the other hand, the concentration of Na
+ and Mg
2+ exhibited minimal variation and had a negligible impact on the dissolution of calcite.
By comparing the changes in the mass concentration of major cations during the dissolution of various mineral groups, it is evident that the time to reach equilibrium varies significantly among different classes of minerals. Carbonate minerals exhibit the highest reaction rate, and the equilibrium can be achieved within a day, while feldspar minerals and clay minerals have lower rates. Based on the literature analysis, the dissolution of these two mineral types takes between 5 and 20 years to reach equilibrium, indicating a slow dissolution process. By comparing
Figure 3a,b, it can be observed that plagioclase feldspar reacts at a higher rate than potassium feldspar, resulting in a shorter time required to reach saturation. In order to enhance the reliability of the research results, we refer to some scholars’ research results. Zhu et al. [
31] analyzed the changes in cation composition in the fluid during the reaction of potassium feldspar and Plagioclase in an experimental study of CO
2–fluid–feldspar interaction, which was basically consistent with our study; the concentrations of Na
+ and K
+ increased, and the rates gradually decreased, while the concentrations of Ca
2+ and Mg
2+ did not change significantly. In their experimental study of the reaction between kaolinite and CO
2 solution, Tang et al. [
32] found that the reaction of kaolinite was weak, and the crystal surface was slightly dissolved. Gui et al. [
33] studied the genetic relationship between kaolinite and dawsonite and found that kaolinite can transform into dawsonite in a weakly acidic environment rich in CO
2, which seems to explain the obvious downward trend of Na
+ concentration in
Figure 3c.
3.3. Changes in Mineral Surface Morphology
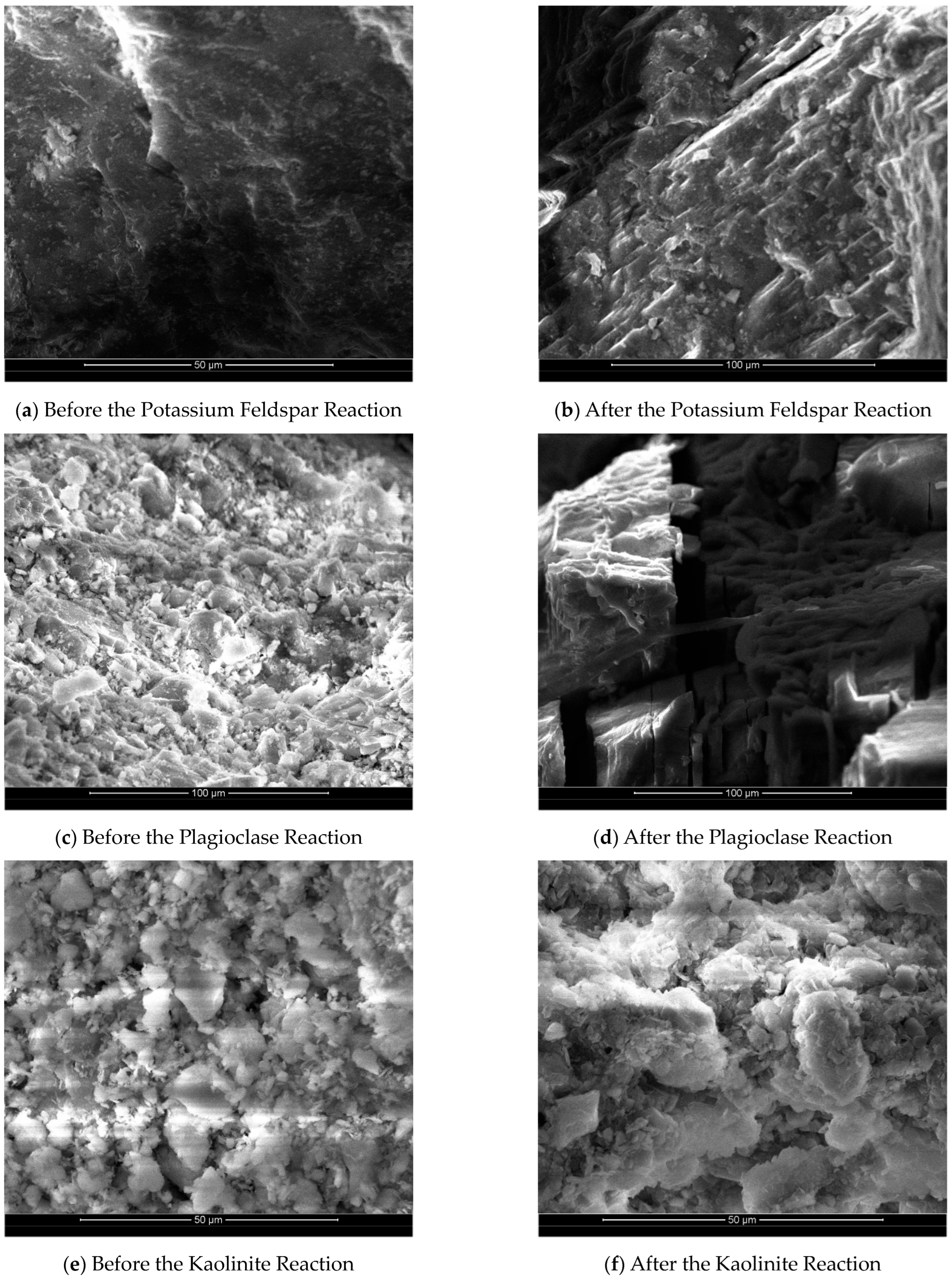
The SEM images in
Figure 4 reveal the variations in the surface dissolution of different mineral groups before and after the reaction. Since SEM analysis is a local analysis, in order to ensure the reliability of observation results, we conducted scanning on multiple points of the sample and found that the overall change trend was consistent, and the most easily observed scanning image was selected in the manuscript. Specifically,
Figure 4a,b provide a comparison of the surface images of the potassium feldspar group before and after the reaction. Prior to the reaction, the mineral surface of the potassium feldspar group appeared relatively smooth and flat, with only a small portion showing depression. After the reaction, however, the mineral surface became rougher, exhibiting more pronounced dissolution steps and unevenness. This indicates that the minerals underwent partial dissolution. Additionally, the reaction led to an increase in the specific surface area of the minerals, further confirming their partial dissolution.
Figure 4c,d depict a comparison of SEM images illustrating the surface of plagioclase group minerals before and after the reaction. It is worth noting that, prior to the experiments discussed in this paper, the plagioclase feldspar had large particles, necessitating some grinding. As a result, the SEM image of the plagioclase group minerals before the reaction exhibits poor homogeneity and numerous small crystalline grains on the surface. However, the SEM image after the reaction reveals a more pronounced dissolution of the plagioclase group. Almost all of the small crystal grains present on the surface before the reaction are dissolved, leaving a fish-scale-like surface with secondary mineral precipitates attached. This observation, combined with the analysis of the change in Na
+ concentration during the reaction and the XRD analysis (for more details, refer to
Section 3.4), indicates the formation of secondary sodium alumina (NaAlCO
3(OH)
2) precipitates on the surface.
Figure 4e,f depict a comparison of SEM images of the surface of kaolinite group minerals before and after the reaction. Overall, the surface morphology of kaolinite remains relatively unchanged, indicating a weak dissolution reaction. However, there is a slight degree of dissolution observed at the crystal’s edges, resembling petals. The majority of the crystal’s middle section remains unaltered. The weak etching effect on kaolinite is primarily attributed to its molecular structure. In the presence of a high concentration of H
+ ions, Al-OH readily combines with H
+ ions, resulting in a positive charge. This charge is evenly distributed in the aluminum–oxygen octahedron, enhancing the stability of the silica–oxygen tetrahedron compared with the aluminum–oxygen octahedron. The chemical stability of kaolinite in an acidic environment suggests that various clay minerals may convert to kaolinite under the influence of CO
2 dissolution.
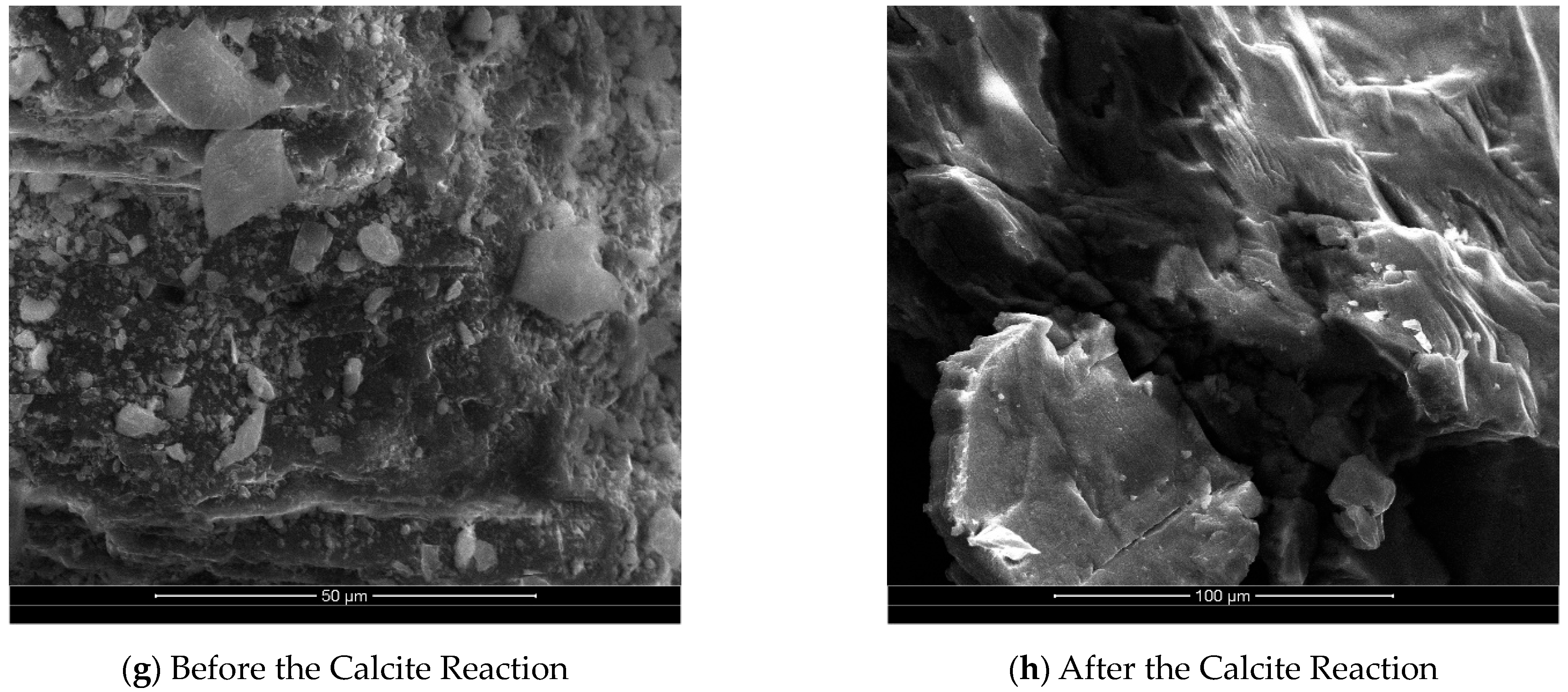
Figure 4g,h illustrate a comparison of SEM images of the surface of calcite group minerals before and after the reaction. The dissolution effect on calcite is more pronounced, as evidenced by the transformation of intact large grains into fine microcrystals or complete dissolution after the reaction. The surface of calcite becomes rough, exhibiting dissolution steps. The analysis of the change in Ca
2+ concentration during the reaction process reveals that most of the Ca
2+ in the dissolving grains exists in a free state within the solution. A small portion of Ca
2+ participates in reprecipitation and reconverts into CaCO
3, which attaches to the mineral surface.
By comparing the SEM images of the surface corrosion changes before and after the reaction between different mineral groups, it is evident that the corrosion intensity varies significantly among different mineral types. Feldspar and calcite minerals exhibit more noticeable corrosion, while kaolinite shows minimal corrosion. Additionally, the surfaces of all mineral groups become rougher after the reaction. The determination of the specific surface area of the minerals before and after the reaction corroborates these observations, indicating a gradual increase in the specific surface area with the mineral reaction. This increase in surface area provides favorable conditions for further mineral corrosion by CO2.
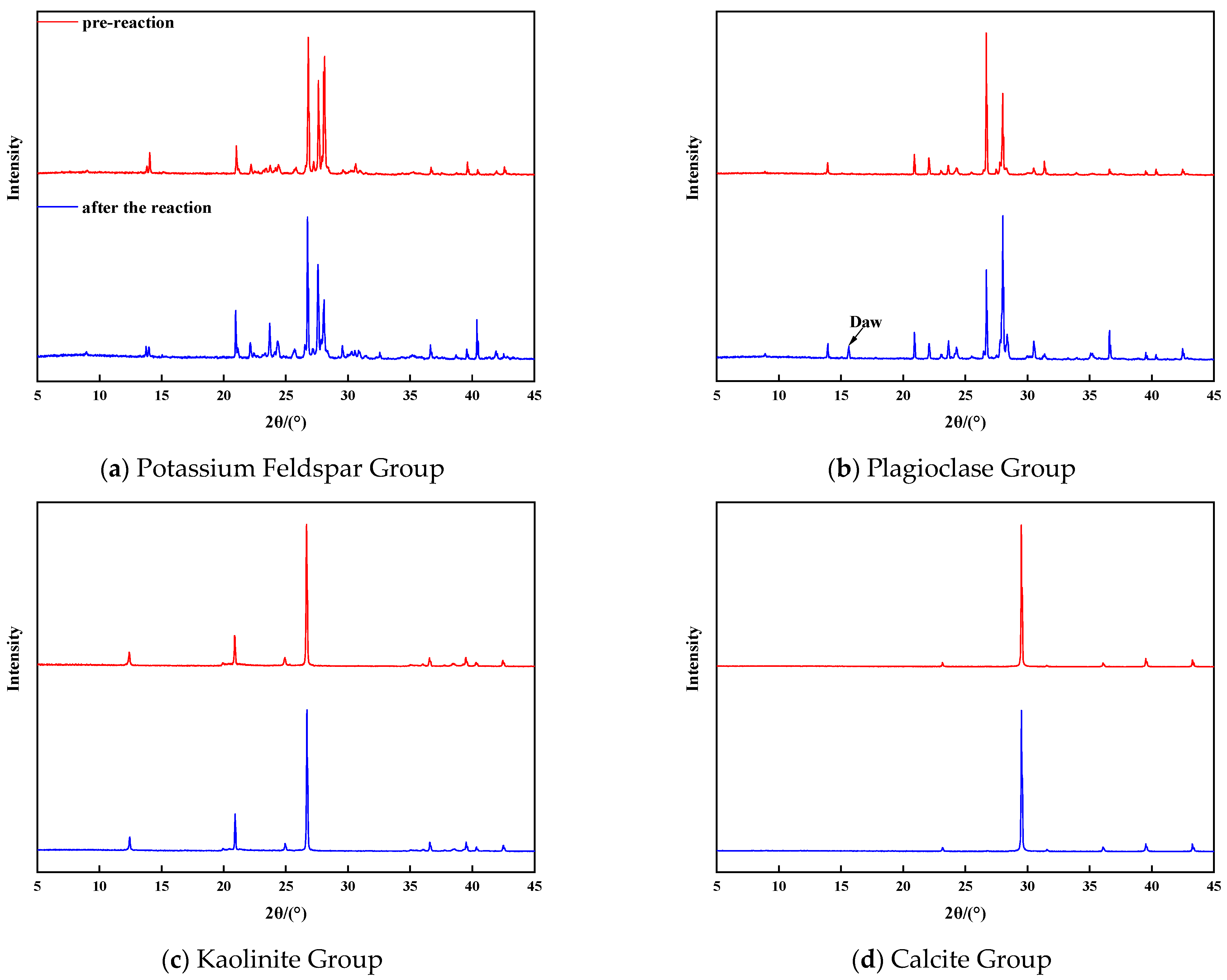
3.4. Changes in Mineral Composition
XRD diffraction analysis was conducted to examine the changes in the mineral composition of each group before and after the reaction. The results reveal that the feldspar minerals experienced significant alterations in mineral composition, while the changes in kaolinite and calcite were relatively minor. Specifically,
Figure 5a illustrates the XRD diffraction characteristics of potassium feldspar group minerals before and after the reaction. The spectrum changes, combined with quantitative analysis, indicate a decrease in potassium feldspar content and an increase in quartz content. This suggests that the dissolution of potassium feldspar resulted in the formation of secondary quartz. Additionally, there was a slight increase in clay minerals. The quantitative analysis of clay minerals revealed a small amount of illite in the potassium feldspar group before the reaction, which decreased by 43% after the reaction and transformed into an illite–illite mixed layer with a mixed layer ratio of 45%.
Figure 5b displays the XRD diffraction characteristics of plagioclase group minerals before and after the reaction. A new peak appears in the diffraction pattern after the reaction, identified as sodium alumina (NaAlCO
3(OH)
2) through comparison with the standard card of mineral diffraction. This finding demonstrates that plagioclase feldspar can generate secondary carbonate minerals when exposed to CO
2 fluid, thus providing a new direction for CO
2 mineralization and storage. Regarding the final form of carbon sequestration in the brackish water layer, most scholars believe that CO
32− combines with divalent metal cations to produce precipitates such as CaCO
3 and MgCO
3. However, the concentration of divalent metal cations in the brackish water layer is not very high, which limits the efficiency of this process. Sodalite, a secondary carbonate mineral, contains Na+ and Al
3+ as its metal cations. The dissolution of feldspar minerals, particularly sodium feldspar, and the high Na
+ concentration in the stratigraphic fluids of the brackish water layer can provide ample Na
+ and Al
3+. Therefore, sodalite holds great promise as a carbon-sequestration mineral. The reaction equation for the production of sodalumite through the CO
2 dissolution of sodium feldspar is as follows:
The generation of sodalite requires high temperature, high CO2 partial pressure, a weak acidic fluid environment rich in Na+ and Al3+, and the injection of supercritical CO2 into the feldspar-rich brackish water layer. These conditions facilitate the mineralization and sequestration of CO2.
Figure 5c depicts the XRD diffraction characteristics of kaolinite group minerals before and after the reaction. No significant changes were observed after the reaction. Quantitative analysis revealed a 0.5% dissolution of kaolinite and a corresponding 0.5% increase in quartz content, indicating that kaolinite dissolved and thus generated secondary quartz. The stability of kaolinite in an acidic environment was further confirmed by comparing the XRD diffraction patterns of the kaolinite group before and after the reaction. The reaction equation is as follows:
Figure 5d displays the XRD diffraction features of calcite group minerals before and after the reaction. A comparison of the maps revealed almost no change in the mineral composition of the calcite group. Although calcite exhibited the highest reaction rate and intensity in the early stage of the reaction, its mineral composition remained largely unchanged. This can be attributed to the high purity of minerals in the calcite group, with few other cations available to combine with CO
32− and form precipitates.
The analysis of XRD profiles before and after the reaction indicated that feldspar minerals underwent more complex composition changes upon dissolution, particularly in the plagioclase group. The abundance of Na+ and Al3+ in plagioclase feldspar makes it highly suitable for CO2 mineralization and sequestration.
3.5. Mineral Specific Surface Area Variations
The mineral dissolution rate is significantly influenced by the size of the mineral-specific surface area. The equation for the mineral dissolution rate, based on the classical transition state theory, can be expressed as follows:
In this equation, represents the mineral dissolution rate, represents the mineral-specific surface area, is the mineral dissolution rate constant at room temperature, is the activation energy of the mineral dissolution reaction, is the ideal gas constant, is the reaction temperature, is the activity of a specific component in the solution, represents the number of reaction stages, is the mineral saturation, and and are experimental fitting parameters.
When other conditions remain constant, the specific surface area of minerals has a linear relationship with the mineral dissolution rate. However, the reaction rate varies during the mineral dissolution process due to changes in the specific surface area. Various reports in the literature show significant variations in the specific surface area of the same mineral, attributed to differences in mineral structure maturity and determination methods. In this paper, we employed the liquid nitrogen adsorption method using a specific surface area adsorption meter (BET) to measure the changes in the specific surface area before and after the mineral dissolution reaction. The results of these tests are presented in
Table 5.
The test results indicate that the specific surface area of each mineral group increased after the reaction. This increase is primarily due to the minerals’ rougher surface resulting from dissolution, which is beneficial for long-term CO
2 mineralization and sequestration. However, the formation of secondary mineral precipitates after primary mineral dissolution may diminish this advantage if they adhere to the surface. The specific surface area of similar minerals varies significantly due to the low maturity and poor homogeneity of mineral structure in terrestrial sedimentary strata. In future studies, it is crucial to thoroughly investigate and analyze the homogeneity of the strata and the maturity of minerals in the target area. Additionally, the specific surface area of minerals should be analyzed using various methods to minimize errors. This will provide valuable references for predicting the amount and efficiency of CO
2 mineralization storage. In order to enhance the reliability of our research findings, we referred to the research conducted by other scholars. In their study on water–rock interactions in reservoirs for CO
2 formation, Wang et al. [
34] found that the specific surface area of rock samples rich in feldspar and kaolinite increased after undergoing reactions at different temperature conditions, with a change rate ranging from 25.65% to 53.39%, which aligns with our research result.