Utilization of Organic Waste in a Direct Carbon Fuel Cell for Sustainable Electricity Generation
Abstract
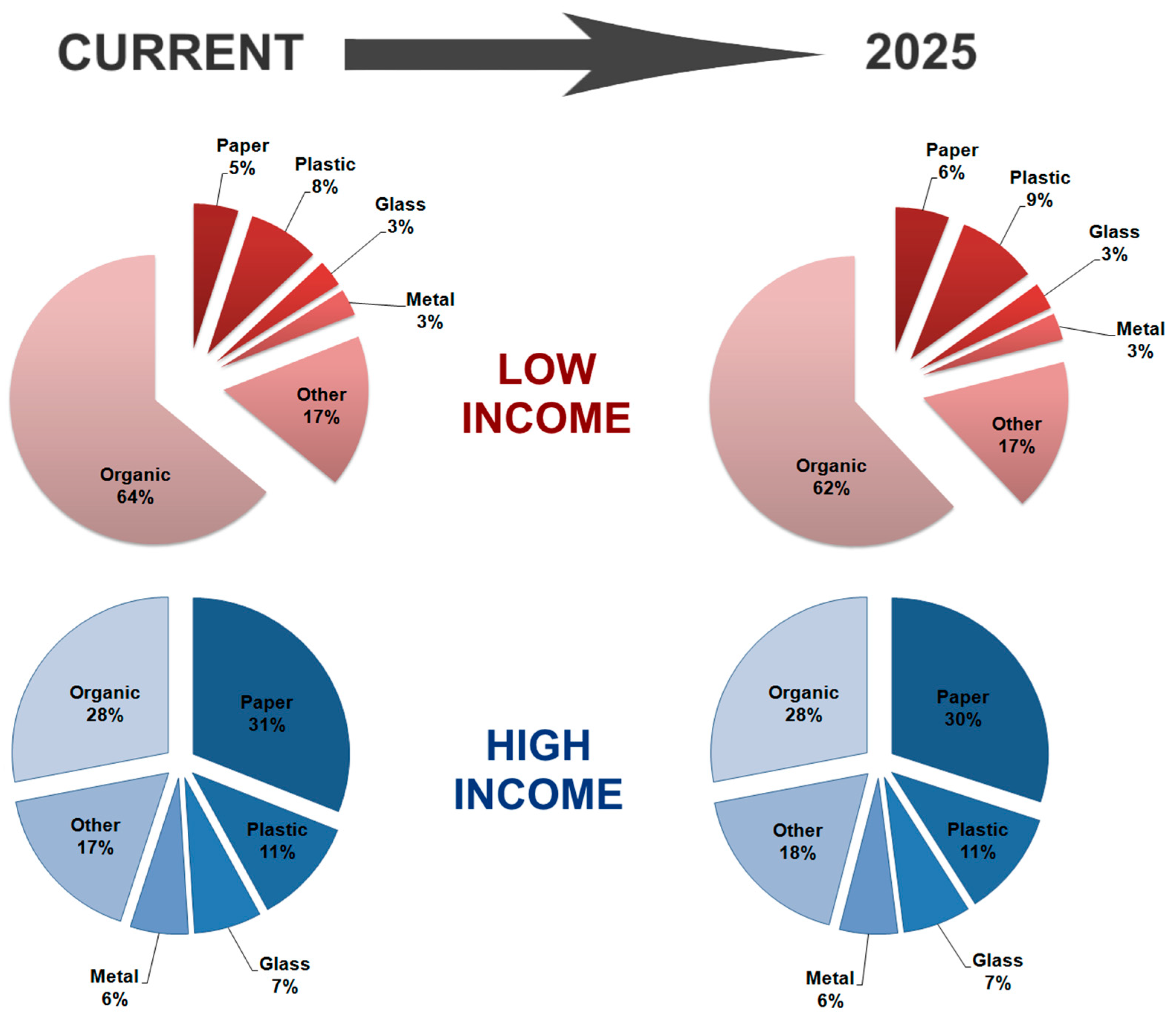
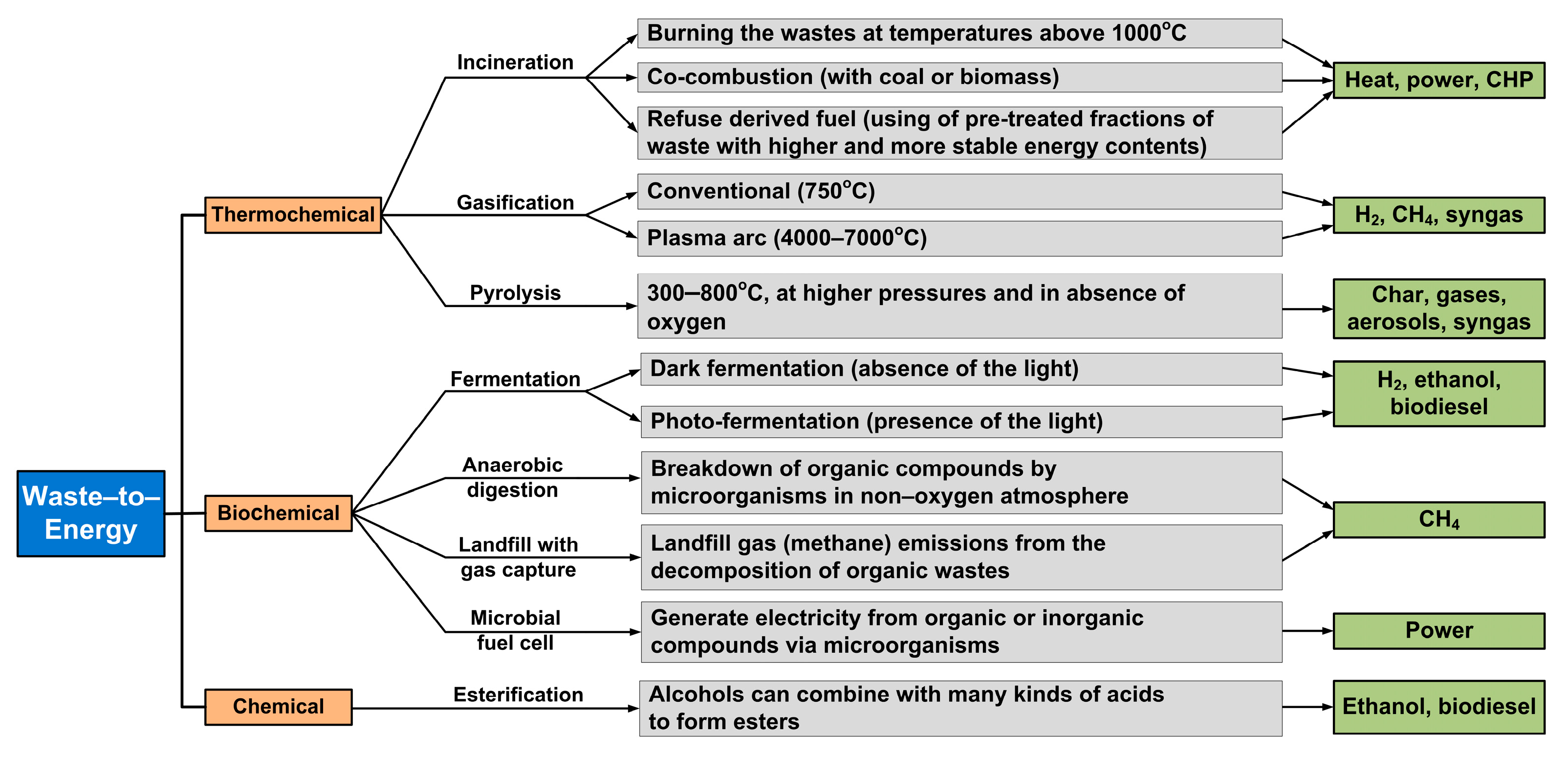
:1. Introduction
2. Materials and Methods
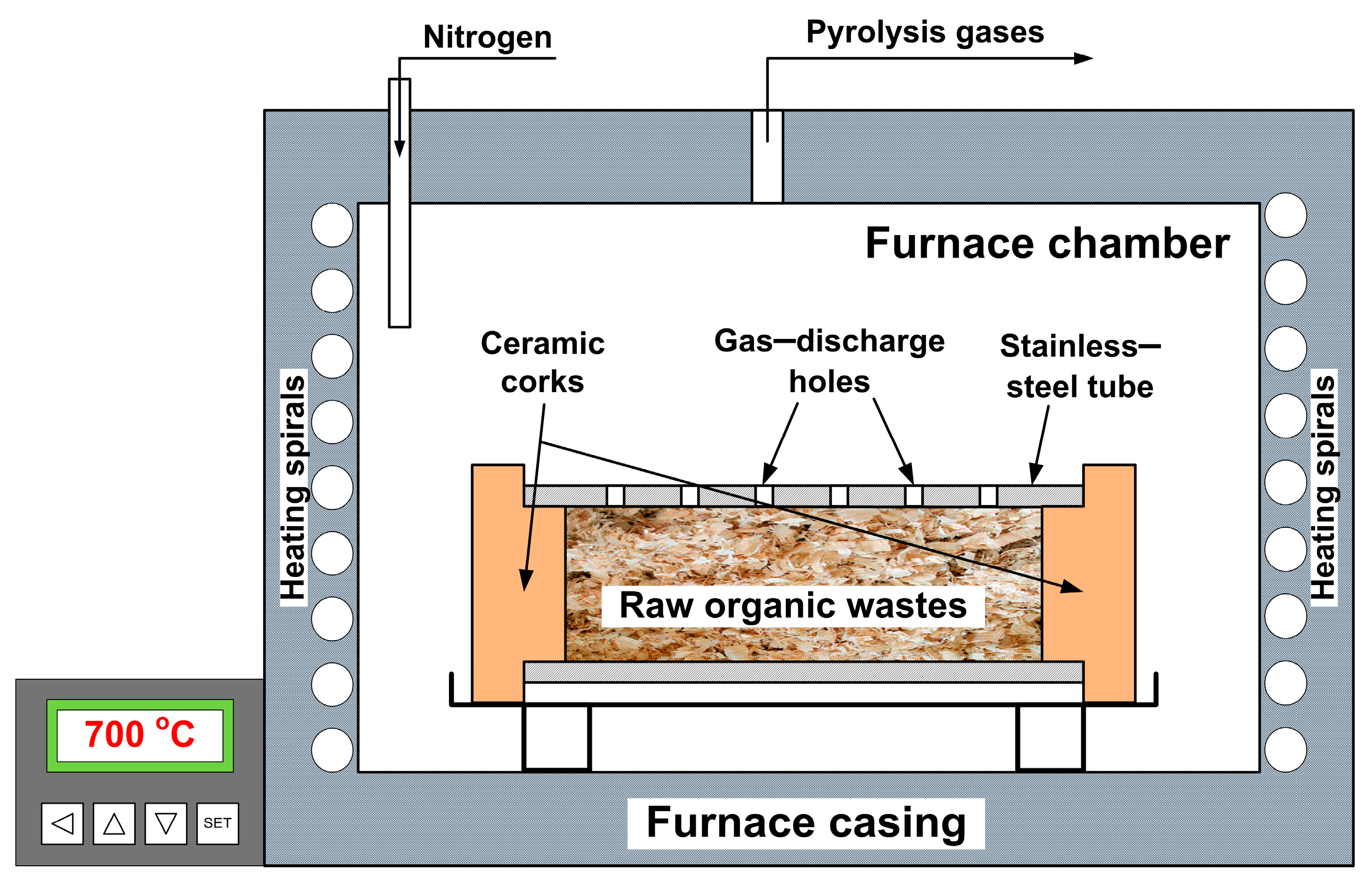
2.1. Samples Preparation
2.2. Samples Preparation
2.3. MH-DCFC Performance Test
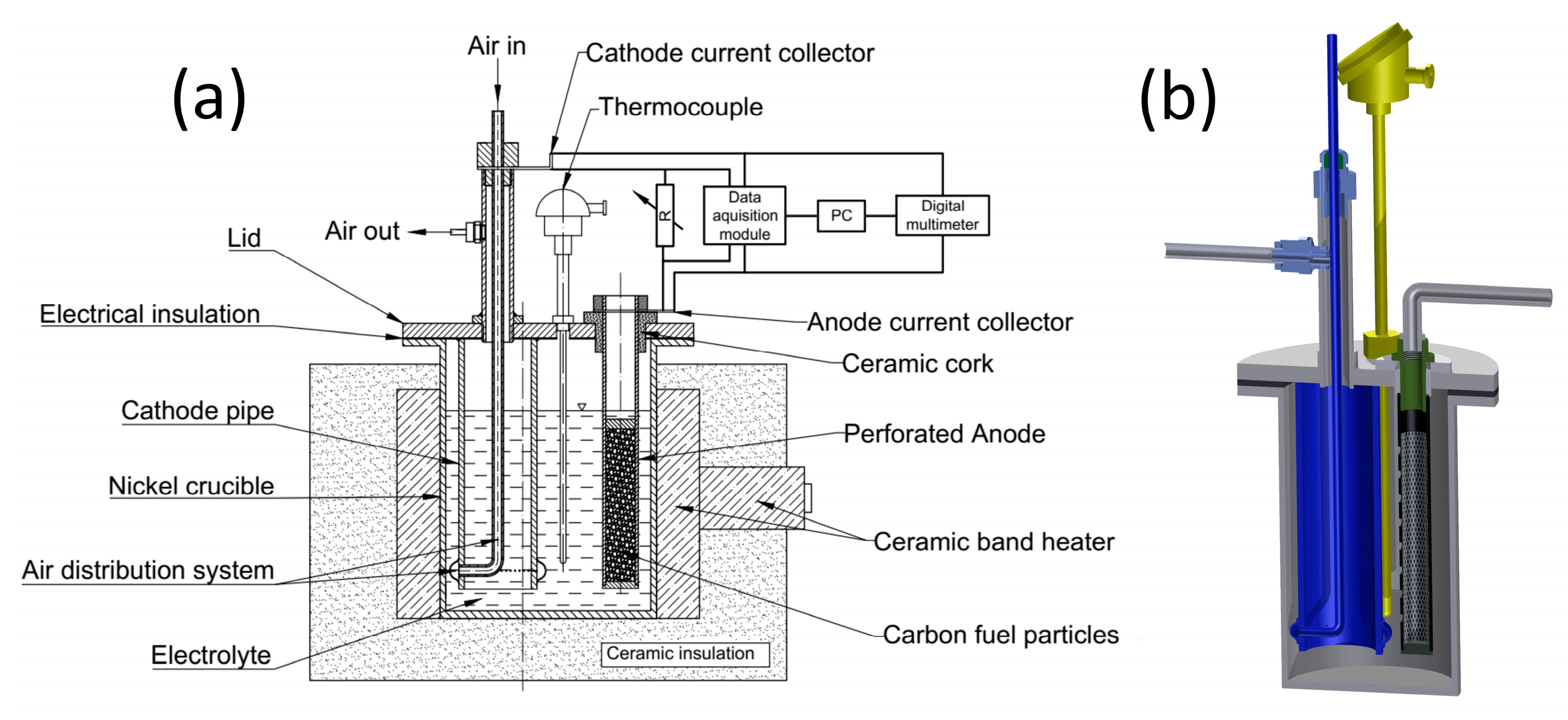
2.3.1. MH-DCFC Construction Details
2.3.2. Test Methodology
3. Results and Discussion
3.1. Physicochemical Fuel Samples Characterization
3.1.1. Proximate and Ultimate Analysis
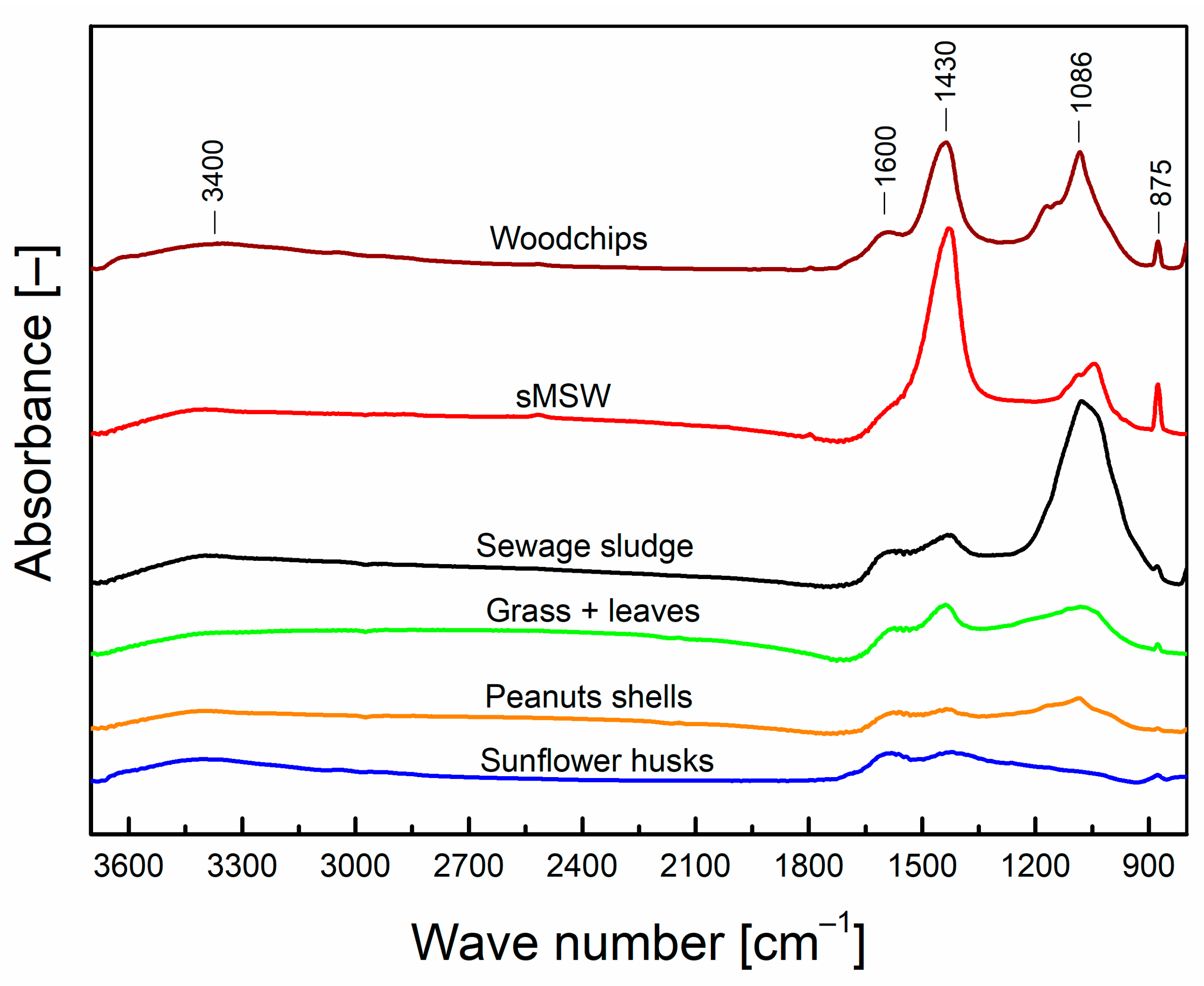
3.1.2. FTIR Analysis
3.1.3. SEM/EDX Analysis
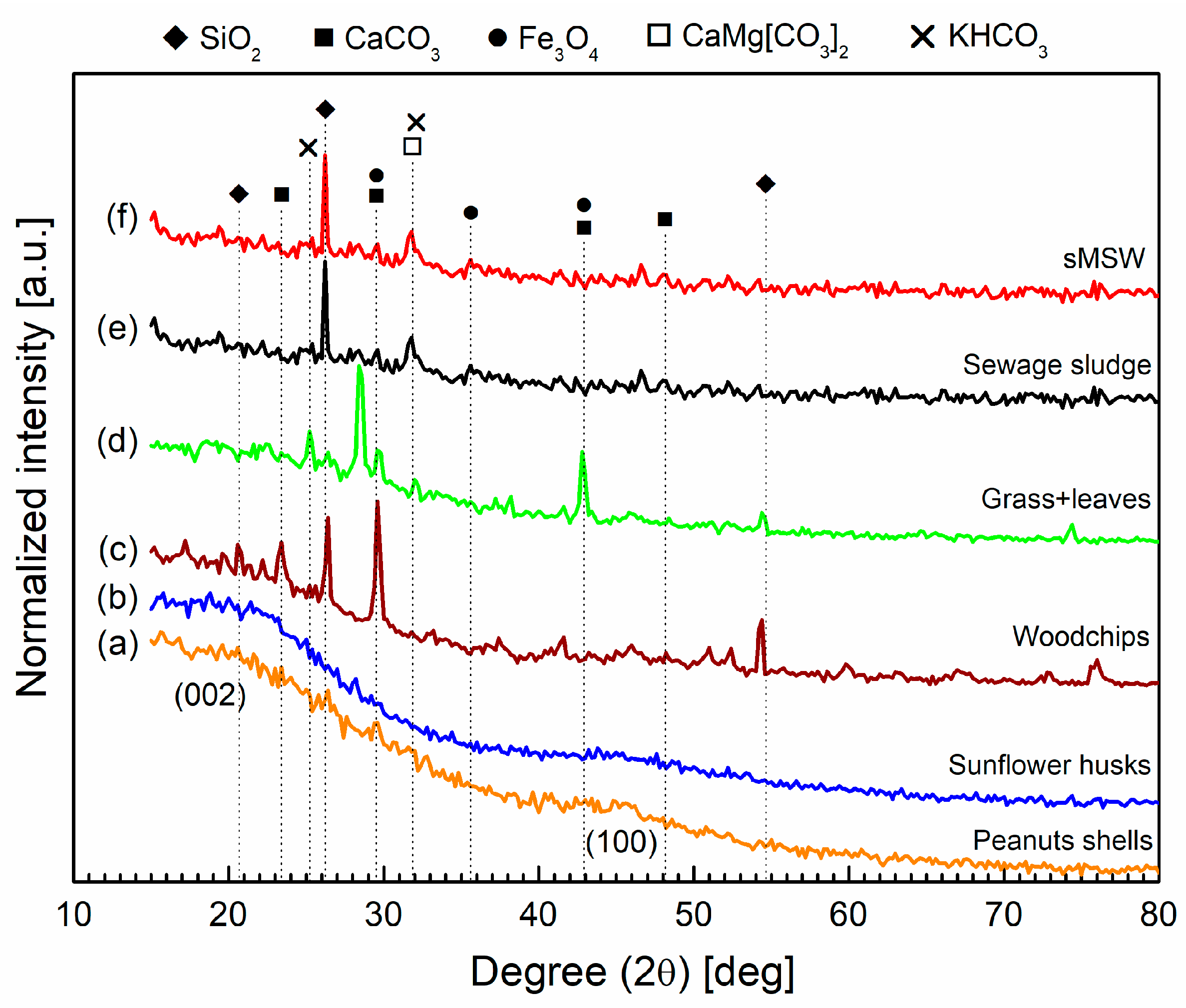
3.1.4. XRD Analysis
3.2. Evaluation of Organic Waste Delivered Fuels (Biochar) in the MH-DCFC
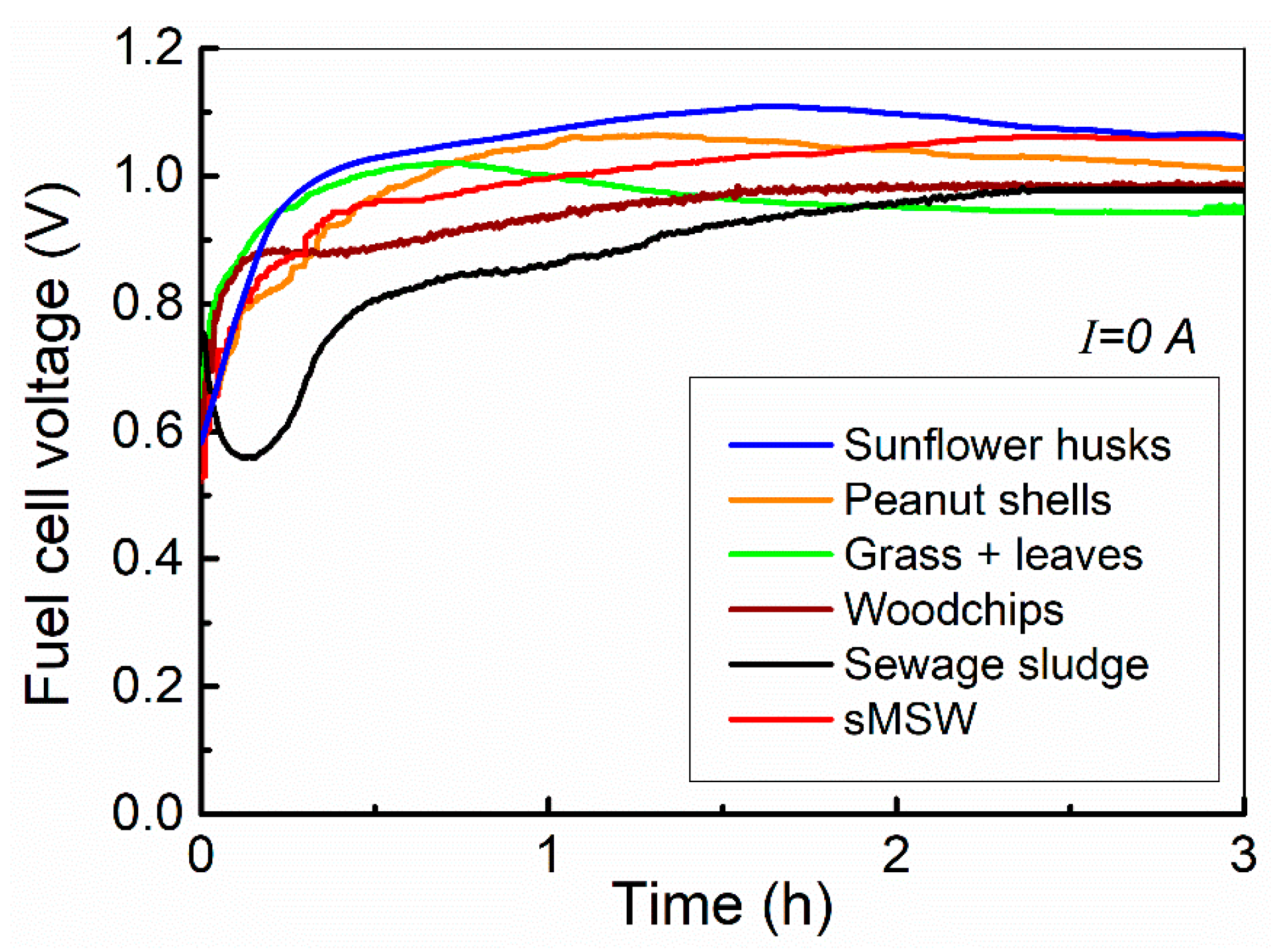
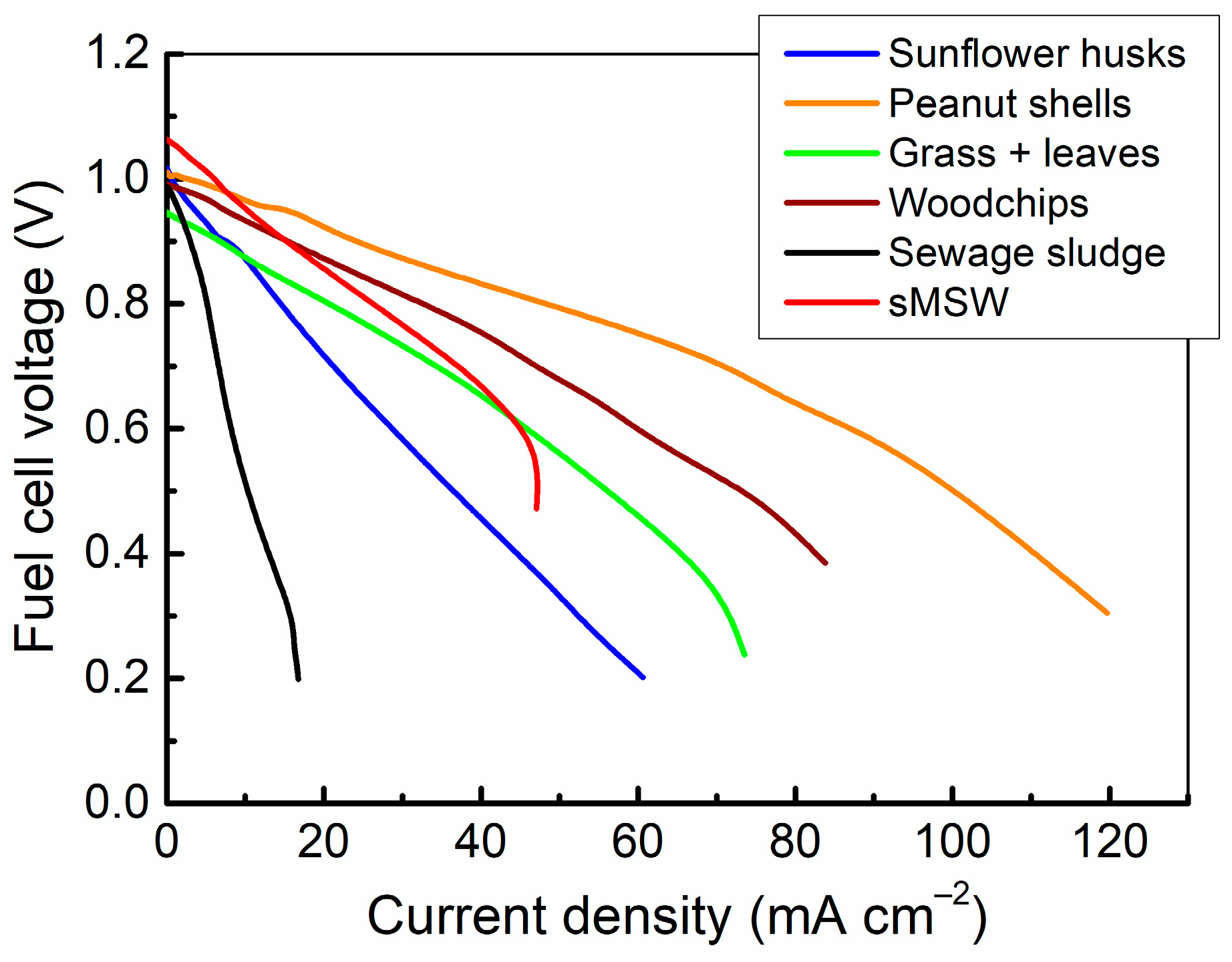
3.2.1. The Effect of Organic Waste Type on Cell Voltage
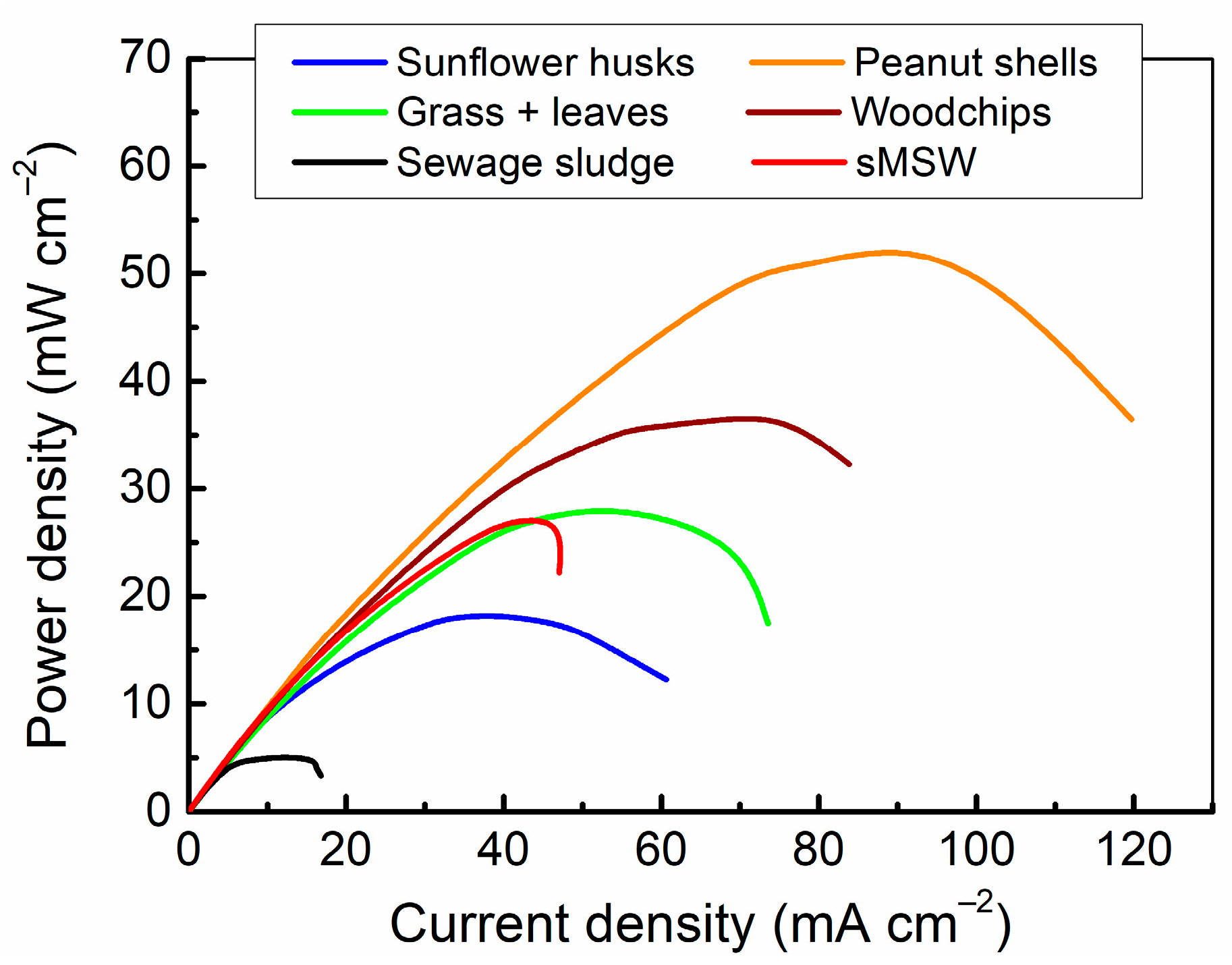
3.2.2. The Effect of Organic Waste Type on Current and Power Densities
3.3. Comparison Results and Discussion
4. Conclusions
- The highest OCV values were observed for sMSW, while the least favourable outcomes were obtained for Grass+ leaves. The OCV values for sunflower husks and peanut shells were approximately 1.0 V. It is noteworthy that the experimental OCV values measured for sMSW surpassed the theoretical standard potential of 1.025 V. A higher OCV value suggests the occurrence of additional reactions beyond the direct electrochemical oxidation of carbon to CO2. One potential explanation may be linked to the chemical composition of the waste and the presence of certain impurities within the ash, which could also undergo chemical oxidation within the cell. Alternatively, the elevated OCV might be attributed to an increased number of available active sites, indicating a higher chemical reactivity of the fuel.
- Among the tested fuels, carbonized peanut shells provided the highest power density of 53.1 mW cm−2 because of the low ash and high carbon content. Despite the high carbon and the low ash content, the power density value for carbonized sunflower husks was not as high as expected because of the low content of reactive oxygen-containing groups present at the biochar surface. Moreover, MH-DCFC directly fuelled with sewage sludge achieved the lowest current and power density values.
- Generally, the higher elemental carbon, lower ash content, and the presence of a reactive surface oxygen functional group in examined pyrolyzed organic waste might contribute to the better cell performance.
- The research study establishes the potential of carbonized organic waste as a prospective alternative fuel source for power generation in a MH-DCFC.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allevi, E.; Gnudi, A.; Konnov, I.V.; Oggioni, G. Municipal solid waste management in circular economy: A sequential optimization model. Energy Econ. 2021, 100, 105383. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Hoornweg, D.A.; Bhada-Tata, P. What a Waste? A Global Review of Solid Waste Management; World Bank: Washington, DC, USA, 2012. [Google Scholar]
- Vaverkova, M.D. Landfill impacts on the environments—Review. Geosciences 2019, 9, 431. [Google Scholar] [CrossRef]
- Landfill Directive. Council Directive 1999/31/EC of 26 April 1999 on the landfill of waste. Off. J. 1999, L 182, 1–19. [Google Scholar]
- Indochoodan, T.G.; Haq, I.; Kalamdhad, A.S. 14-Factors Affecting Anaerobic Digestion for Biogas Production: A Review. In Advanced Organic Waste Management-Sustainable Practices and Approaches, 1st ed.; Hussain, C.M., Hait, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 223–233. [Google Scholar]
- World Energy Resources: Waste to Energy, World Energy Council, London. 2016. Available online: https://www.worldenergy.org/assets/images/imported/2016/10/World-Energy-Resources-Full-report-2016.10.03.pdf (accessed on 15 August 2023).
- Young, G.C. Municipal Solid Waste to Energy Conversion Processes: Economic, Technical, and Renewable Comparisons; John Wiley & Sons: Hoboken, NJ, Canada, 2010. [Google Scholar]
- Lanzini, A.; Madi, H.; Chiodo, V.; Papurello, D.; Maisano, S.; Santarelli, M. Dealing with fuel contaminants in biogas-fed solid oxide fuel cell (SOFC) and molten carbonate fuel cell (MCFC) plants: Degradation of catalytic and electro-catalytic active surfaces and related gas purification methods. Prog. Energy Combust. Sci. 2017, 61, 150–188. [Google Scholar] [CrossRef]
- Rabou, L.P.L.M.; Van Leijenhorst, R.J.C.; Hazewinkel, J.H.O. High Efficiency Power Production from Biomass and Waste. Technical Report ECN-E-08-086. 2008. Available online: https://publications.tno.nl/publication/34628934/63x0fV/e08086.pdf (accessed on 15 August 2023).
- Papurello, D.; Lanzini, A.; Tognana, L.; Silvestri, S.; Santarelli, M. Waste to energy: Exploitation of biogas from organic waste in a 500 Wel solid oxide fuel cell (SOFC) stack. Energy 2015, 85, 145–158. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sust. Energ. Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.C.; Ryu, C.; Jeon, J.K.; Shin, M.C.; Park, Y.K. Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Dudek, M. On the utilization of coal samples in direct carbon solid oxide fuel cell technology. Solid State Ion. 2015, 271, 121–127. [Google Scholar] [CrossRef]
- Jewulski, J.; Skrzypkiewicz, M.; Struzik, M.; Lubarska-Radziejewska, I. Lignite as a fuel for direct carbon fuel cell system. Int. J. Hydrogen Energy 2014, 39, 21778–21785. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, A.; Qiu, J.; Zhang, Y.; Cai, J.; Dang, Y. Utilization of bituminous coal in a direct carbon fuel cell. Int. J. Hydrogen Energy 2016, 41, 8576–8582. [Google Scholar] [CrossRef]
- Kacprzak, A.; Kobyłecki, R.; Włodarczyk, R.; Bis, Z. The effect of fuel type on the performance of a direct carbon fuel cell with molten alkaline electrolyte. J. Power Sour. 2014, 255, 179–186. [Google Scholar] [CrossRef]
- Munnings, C.; Kulkarni, A.; Giddey, S.; Badwal, S.P.S. Biomass to power conversion in a direct carbon fuel cell. Int. J. Hydrogen Energy 2014, 39, 12377–12385. [Google Scholar] [CrossRef]
- Dudek, M.; Tomczyk, P.; Socha, R.; Skrzypkiewicz, M.; Jewulski, J. Biomass fuels for direct carbon fuel cell with solid oxide electrolyte. Int. J. Electrochem. Sci. 2013, 8, 3229–3253. [Google Scholar] [CrossRef]
- Jang, H.; Ocon, J.D.; Lee, S.; Lee, J.K.; Lee, J. Direct power generation from waste coffee grounds in a biomass fuel cell. J. Power Sour. 2015, 296, 433–439. [Google Scholar] [CrossRef]
- Yu, J.; Zhao, Y.; Li, Y. Utilization of corn cob biochar in a direct carbon fuel cell. J. Power Sour. 2014, 270, 312–317. [Google Scholar] [CrossRef]
- Elleuch, A.; Yu, J.; Boussetta, A.; Yu, J.; Halouani, K.; Li, Y. Experimental investigation of direct carbon fuel cell fueled by almond shell biochar: Part I. Physico-chemical characterization of the biochar fuel and cell performance examination. Int. J. Hydrogen Energy 2013, 38, 16590–16604. [Google Scholar] [CrossRef]
- Sean, M.; Dosh, V. Palm Oil Waste as Fuel Source in a Direct Carbon Fuel Cell. In Proceedings of the 2nd Engineering Undergraduate Research Catalyst Conference, Kuala Lumpur, Malaysia, 2 July 2014; Taylor’s University: Selangor, Malaysia, 2014. [Google Scholar]
- Dudek, M.; Socha, R. Direct electrochemical conversion of the chemical energy of raw waste wood to electrical energy in tubular direct carbon solid oxide fuel cells. Int. J. Electrochem. Sci. 2014, 9, 7414–7430. [Google Scholar] [CrossRef]
- Hao, W.; He, X.; Mi, Y. Achieving high performance in intermediate temperature direct carbon fuel cells with renewable carbon as a fuel source. Appl. Energy 2014, 135, 174–181. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Eom, S.Y.; Rhie, Y.H.; Sung, Y.M.; Moon, C.E.; Choi, G.M.; Kim, D.J. Application of refuse fuels in a direct carbon fuel cell system. Energy 2013, 51, 447–456. [Google Scholar] [CrossRef]
- Predtechensky, M.R.; Varlamov, Y.D.; Bobrenok, O.F.; Ulyankin, S.N. Solid hydrocarbon conversion in a fuel cell with molten carbonate electrolyte. J. Eng. Thermophys. 2009, 18, 93–98. [Google Scholar] [CrossRef]
- Dudek, M.; Adamczyk, B.; Sitarz, M.; Śliwa, M.; Lach, R.; Skrzypkiewicz, M.; Raźniak, A.; Ziąbka, M.; Zuwała, J.; Grzywacz, P. The usefulness of walnut shells as waste biomass fuels in direct carbon solid oxide fuel cells. Biomass Bioenergy 2018, 19, 144–154. [Google Scholar] [CrossRef]
- Li, J.; Wei, B.; Wang, C.; Zhou, Z.; Lü, Z. High-performance and stable La0.8Sr0.2Fe0.9Nb0.1O3-δ anode for direct carbon solid oxide fuel cells fueled by activated carbon and corn straw derived carbon. Int. J. Hydrogen Energy 2018, 43, 12358–12367. [Google Scholar] [CrossRef]
- Adeniyi, O.D. Solid Oxide Direct Carbon Fuel Cell Electrochemical Performance Using Wheat and Spruce Carbon Fuels. In Energy Sources, Part A: Recovery, Utilization, and Environmental Effects; Taylor & Francis Group: London, UK, 2015; Volume 37, pp. 2401–2407. [Google Scholar]
- Kacprzak, A.; Włodarczyk, R.; Kobyłecki, R.; Ścisłowska, M.; Bis, Z. Fuel Cell as Part of Clean Technologies. In Environmental Engineering IV, 1st ed.; Pawlowski, A., Dudzinska, M.R., Pawlowski, L., Eds.; Taylor & Francis Group: London, UK, 2013; pp. 443–450. [Google Scholar]
- Kacprzak, A.; Kobyłecki, R.; Bis, Z. The effects of operating conditions on the performance of a direct carbon fuel cell. Arch. Thermodyn. 2013, 34, 187–197. [Google Scholar] [CrossRef]
- Kacprzak, A.; Kobyłecki, R.; Bis, Z. Influence of temperature and composition of NaOH-KOH and NaOH-LiOH electrolytes on the performance of a direct carbon fuel cell. J. Power Sour. 2013, 239, 409–414. [Google Scholar] [CrossRef]
- Tulloch, J.; Allen, J.; Wibberley, L.; Donne, S. Influence of selected coal contaminants on graphitic carbon electro-oxidation for application to the direct carbon fuel cell. J. Power Sour. 2014, 260, 140–149. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; De Marco, R.; Bradley, J.; Dicks, A. Evaluation of Raw Coals as Fuels for Direct Carbon Fuel Cell. J. Power Sour. 2010, 195, 4051–4068. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; De Marco, R.; Bradley, J.; Dicks, A. Modification of coal as a fuel for the direct carbon fuel cell. J. Phys. Chem. A 2010, 114, 3855–3862. [Google Scholar] [CrossRef]
- Liu, G.Y.; Zhang, Y.T.; Cai, J.T.; Zhang, X.Q.; Qiu, J.S. Fuels for direct carbon fuel cells: Present status and development prospects. Carbon 2015, 86, 371. [Google Scholar] [CrossRef]
- Elleuch, A.; Halouani, K.; Li, Y. Investigation of chemical and electrochemical reactions mechanisms in a direct carbon fuel cell using olive wood charcoal as sustainable fuel. J. Power Sour. 2015, 281, 350–361. [Google Scholar] [CrossRef]
- Jafri, N.; Yoon, L.W.; Wong, W.Y.; Cheah, K.H. Power generation from palm kernel shell biochar in a direct carbon fuel cell. SN Appl. Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Jafri, N.; Wong, W.Y.; Yoon, L.W.; Cheah, K.H. Pretreated mesocarp fibre biochars as carbon fuel for direct carbon fuel cells. Int. J. Hydrogen Energy 2021, 46, 16762–16775. [Google Scholar] [CrossRef]
- Cai, W.; Tong, X.; Yan, X.; Li, H.; Li, Y.; Gao, X.; Wang, H. Direct carbon solid oxide fuel cells powered by rice husk biochar. Int. J. Energy Res. 2022, 46, 4965–4974. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Krueger, R.; Fiet, K.J.; Jankowski, A.F.; Cooper, J.F. Direct conversion of carbon fuels in a molten carbonate fuel cell. J. Electrochem. Soc. 2004, 152, A80. [Google Scholar] [CrossRef]

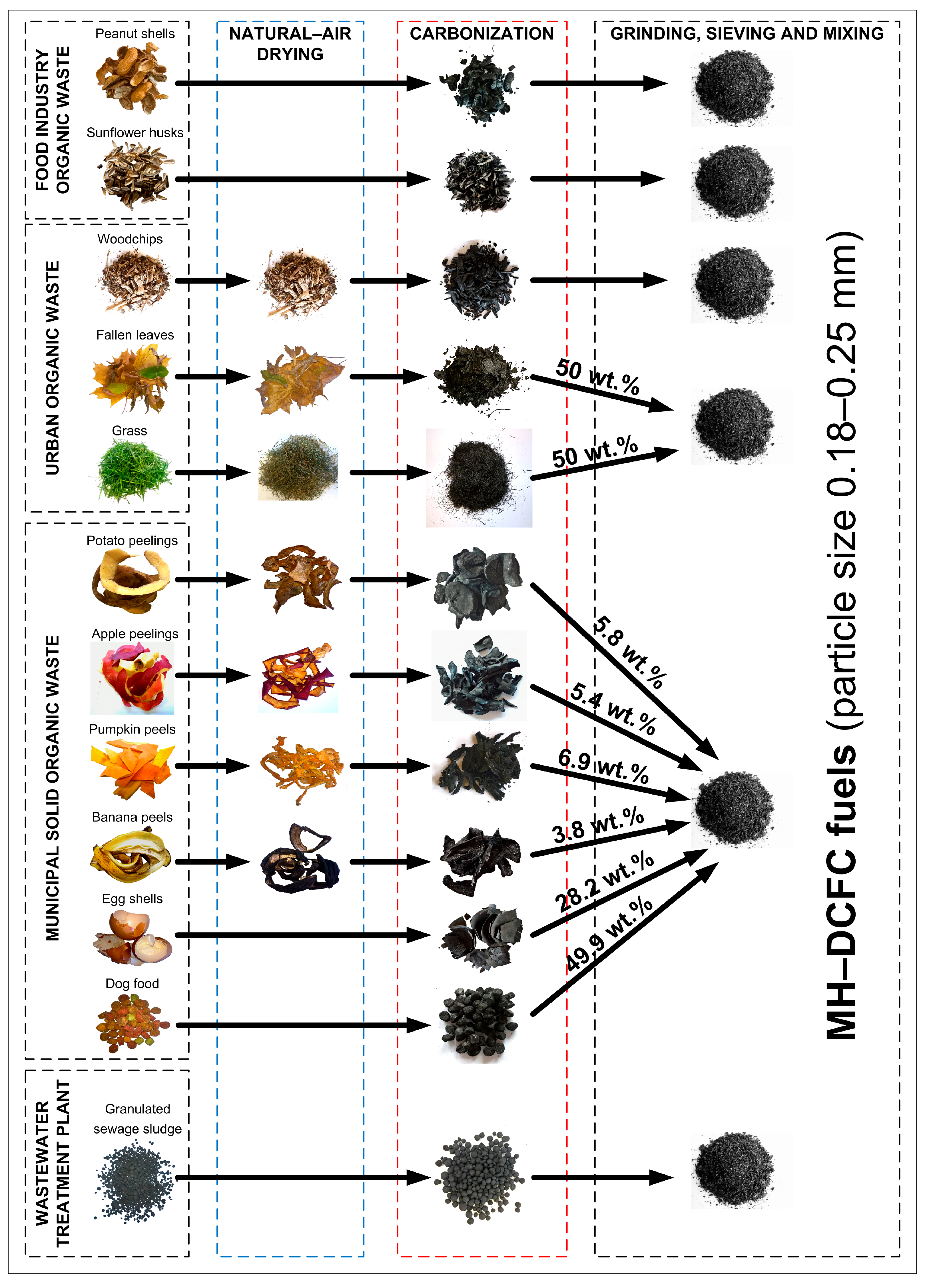

| Weight Proportions [wt.%] (Dry Material—After Carbonization) | |||||
|---|---|---|---|---|---|
| Potato Peelings | Apple Peelings | Pumpkin Peels | Banana Peels | Egg Shells | Dog Food |
| 5.8 | 5.4 | 6.9 | 3.8 | 28.2 | 49.9 |
| Biochar Sample | Proximate Analysis [wt %] | Ultimate Analysis [wt %] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | VM | FC | C | H | N | S | O | |
| Peanuts shells | 0.0003 | 13.49 | 11.50 | 75.01 | 81.02 | 1.38 | 1.24 | 0.25 | 2.62 |
| Sunflower husks | 0.0003 | 9.00 | 16.10 | 74.90 | 84.10 | 2.50 | 2.40 | 0.03 | 1.97 |
| Grass + leaves | 0.0008 | 29.54 | 14.90 | 55.56 | 52.91 | 1.16 | 3.01 | 0.46 | 12.92 |
| Woodchips | 0.0007 | 28.89 | 10.29 | 60.82 | 56.46 | 1.05 | 0.42 | 0.00 | 13.18 |
| Sewage sludge | 0.0003 | 67.41 | 10.05 | 22.54 | 27.58 | 0.71 | 2.06 | 2.221 | 0.02 |
| sMSW | 0.0005 | 44.01 | 13.00 | 42.99 | 44.00 | 0.89 | 2.23 | 0.01 | 8.86 |
| Wavenumber of Absorbance —Peak Centre (cm−1) | Wavenumber of Absorbance —Zone Range (cm−1) | Corresponding Functional Groups (KnowItAll® Database) |
|---|---|---|
| 3400 | 2800–3600 | –OH |
| 1600 | 1550–1680 | C=C |
| 1430 | 1410–1480 | –OH |
| 1350–1460 | C–H | |
| 1430 | Si–C6H5 | |
| 1086 | 1050–1130 | C–O–C or Si–O–Si |
| 875 | 840–880 | N–O |
| 780–980 | C–H | |
| 875 | CaCO3 |
| Fuel Sample | Sunflower Husks | Peanuts Shells | Grass + Leaves | Woodchips | Sewage Sludge | sMSW |
|---|---|---|---|---|---|---|
| OCV [V] | 1.0172 ± 0.0008 | 1.0105 ± 0.006 | 0.9460 ± 0.0024 | 0.9839 ± 0.0011 | 0.9765 ± 0.0010 | 1.0602 ± 0.0007 |
| Fuel Sample | Maximum Power Density | Maximum Current Density | Current Density at 0.7 V |
|---|---|---|---|
| [mW cm−2] | [mA cm−2] | [mA cm−2] | |
| Sunflower husks | 18.35 | 60.63 | 20.92 |
| Peanuts shells | 53.14 | 119.77 | 70.72 |
| Grass + leaves | 27.97 | 73.53 | 34.41 |
| Woodchips | 36.80 | 83.82 | 47.10 |
| Sewage sludge | 5.12 | 16.76 | 6.46 |
| sMSW | 27.25 | 47.06 | 37.07 |
| Organic Waste Type/ Source of Biochar | DCFC Type | Working Temperature | OCV | Maximum Power Density | Maximum Current Density | Ref. |
|---|---|---|---|---|---|---|
| [K] | [V] | [mW cm−2] | [mA cm−2] | |||
| Sunflower husks | MH-DCFC | 723 | 1.02 | 18.35 | 60.63 | This work |
| Peanuts shells | MH-DCFC | 723 | 1.01 | 53.14 | 119.77 | This work |
| Grass+ leaves | MH-DCFC | 723 | 0.95 | 27.97 | 73.53 | This work |
| Woodchips | MH-DCFC | 723 | 0.98 | 36.80 | 83.82 | This work |
| Sewage sludge | MH-DCFC | 723 | 0.98 | 5.12 | 16.76 | This work |
| sMSW | MH-DCFC | 723 | 1.06 | 27.25 | 47.06 | This work |
| Corn cob | H-DCFC 1 | 873 1023 | ≈0.9 1.05 | ≈35 185 | ≈150 ≈500 | [21] |
| Almond shell | H-DCFC | 1023 | 1.07 | 127 | 480 | [22] |
| Olive wood | H-DCFC | 873 973 | ≈1.0 1.02 | ≈32 105 | ≈125 550 | [38] |
| Waste coffee grounds | SO-DCFC | 1023 1173 | ≈0.93 ≈1.03 | ≈55 87.2 | ≈115 ≈260 | [20] |
| Wheat | SO-DCFC | 873 1073 | 0.87 1.18 | 2.8 66.92 | 17.68 138.52 | [30] |
| Spruce | SO-DCFC | 873 1073 | 0.37 1.16 | 1.34 57.4 | 13.26 156.2 | [30] |
| Palm kernel shell | SO-DCFC | 1123 | 0.8 | 3.3 | ≈7.8 | [39] |
| Mesocarp fibre | SO-DCFC | 1123 | 0.89 | 11.8 | 27.1 | [40] |
| Rice husk | SO-DCFC | 1123 | 0.92 | 135 | 140 | [41] |
| Peach pit (activated carbon) | MC-DCFC | 1073 | ≈1.18 | 84 | 124 (at 0.8 V) | [42] |
| Coconut (activated carbon) | MC-DCFC | 1073 | ≈1.22 | 56 | 102 (at 0.8 V) | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacprzak, A.; Włodarczyk, R. Utilization of Organic Waste in a Direct Carbon Fuel Cell for Sustainable Electricity Generation. Energies 2023, 16, 7359. https://doi.org/10.3390/en16217359
Kacprzak A, Włodarczyk R. Utilization of Organic Waste in a Direct Carbon Fuel Cell for Sustainable Electricity Generation. Energies. 2023; 16(21):7359. https://doi.org/10.3390/en16217359
Chicago/Turabian StyleKacprzak, Andrzej, and Renata Włodarczyk. 2023. "Utilization of Organic Waste in a Direct Carbon Fuel Cell for Sustainable Electricity Generation" Energies 16, no. 21: 7359. https://doi.org/10.3390/en16217359
APA StyleKacprzak, A., & Włodarczyk, R. (2023). Utilization of Organic Waste in a Direct Carbon Fuel Cell for Sustainable Electricity Generation. Energies, 16(21), 7359. https://doi.org/10.3390/en16217359







