Pollutant Emissions from Municipal Biowaste Composting: Comparative Analysis and Contribution of N-Containing Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates for Composting
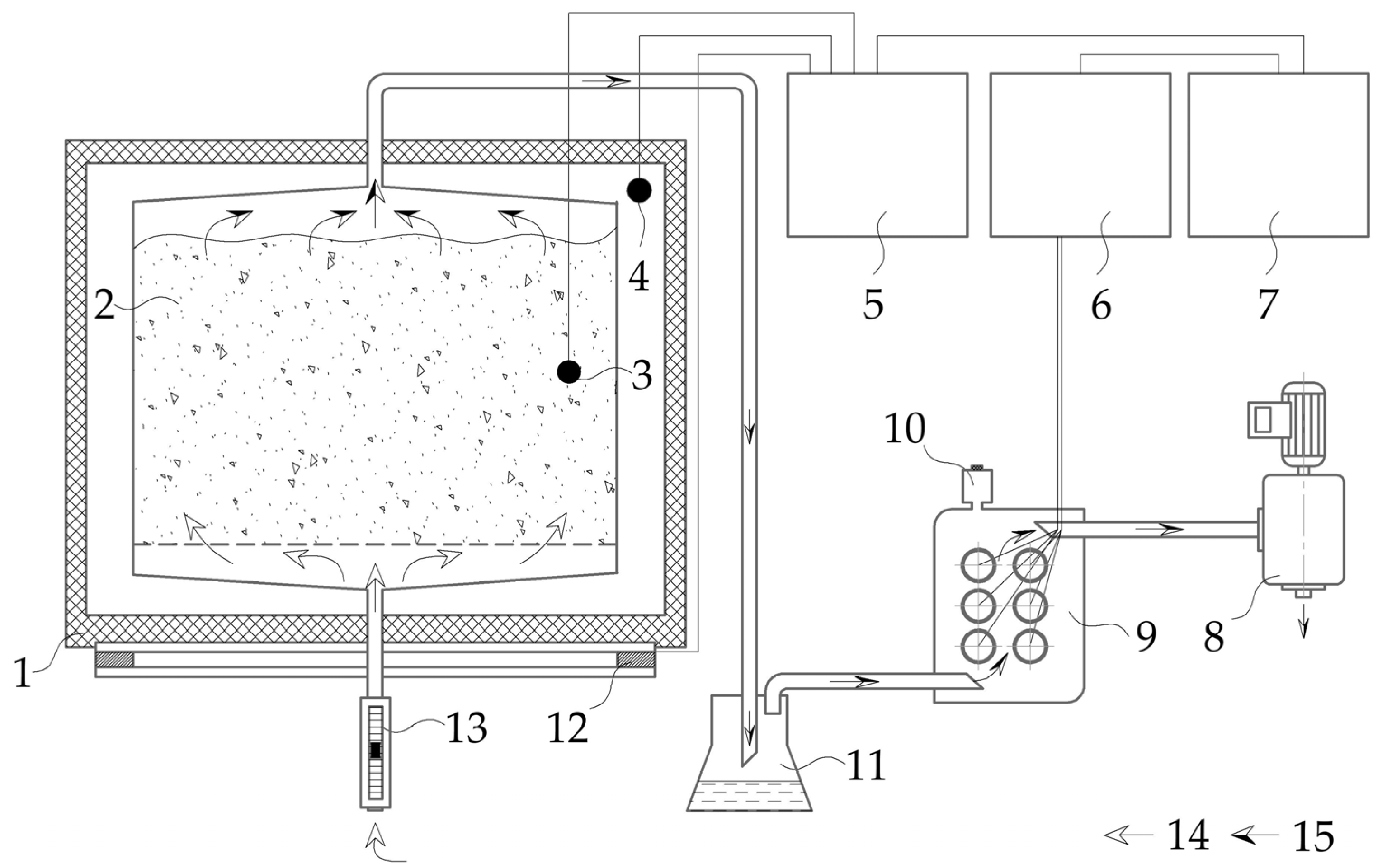
2.2. Experimental Composting Setup
2.3. Measurements of Substrates Parameters
2.4. VOC Sampling and Analysis
2.5. Statistical Data Analysis
3. Results
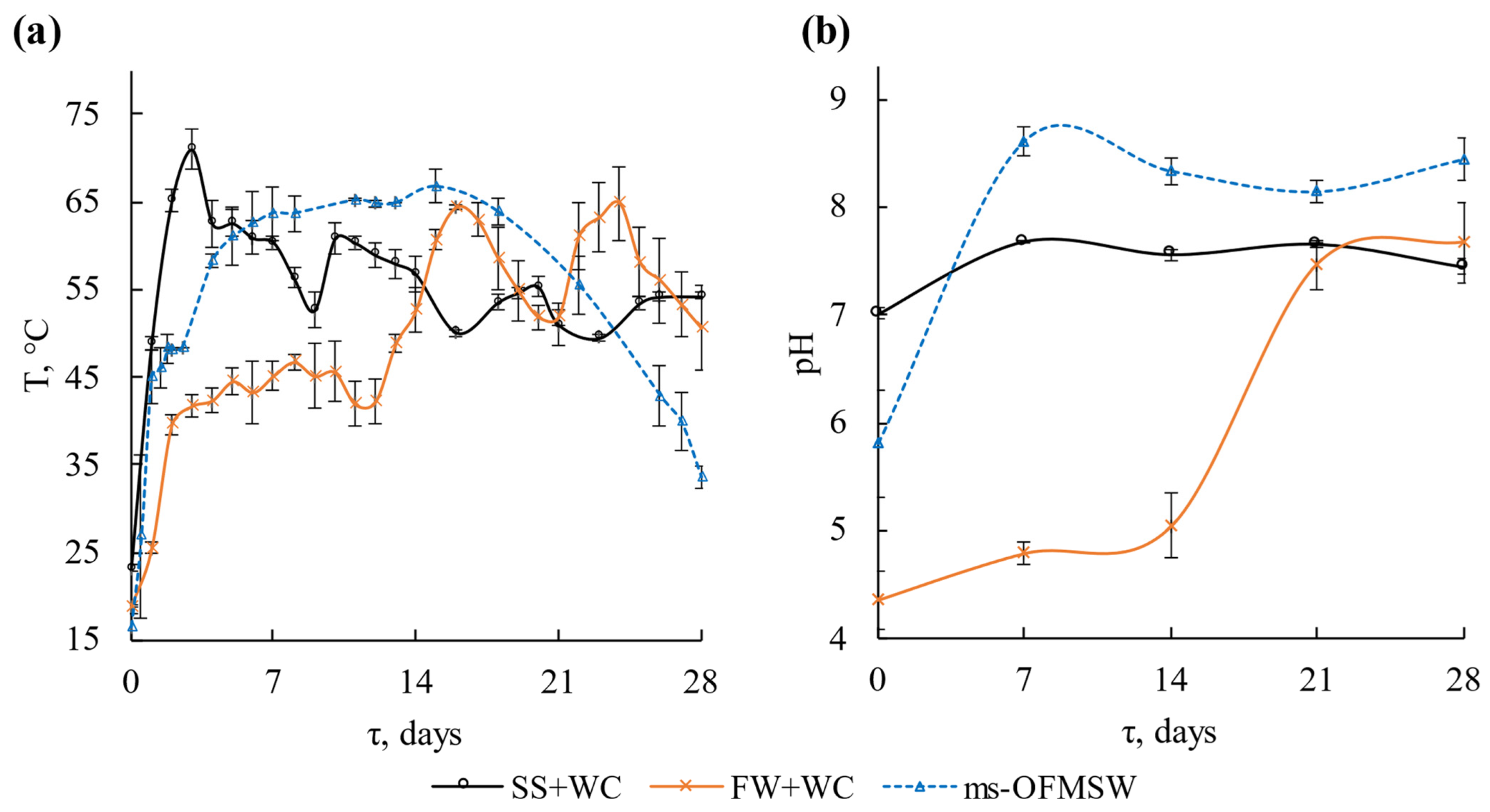
3.1. Dynamics of Physicochemical Parameters
3.2. VC Emission Dynamics
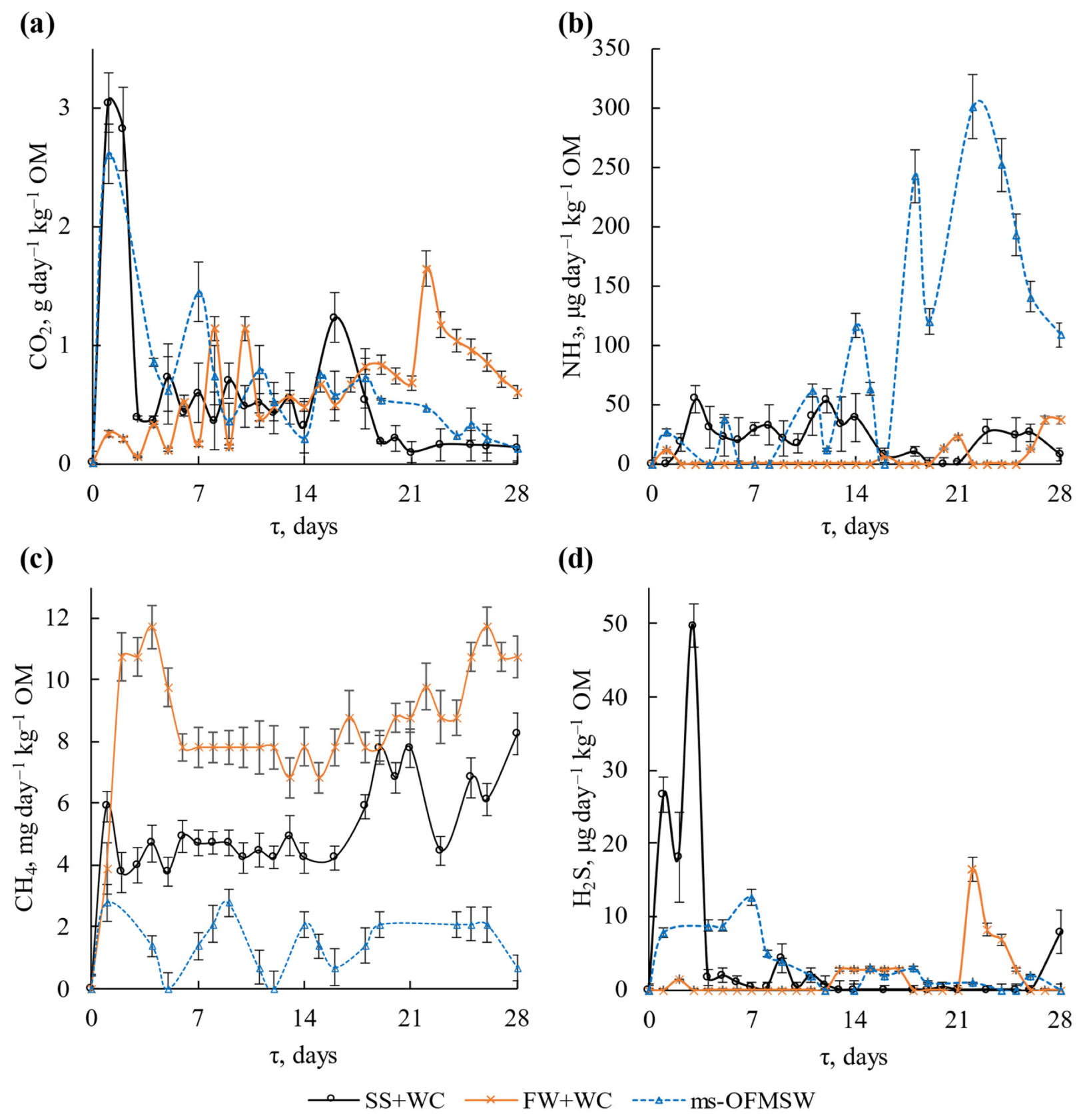
3.2.1. Emission of Carbon Dioxide, Ammonia, Methane and Hydrogen Sulphide
3.2.2. VOC Emission
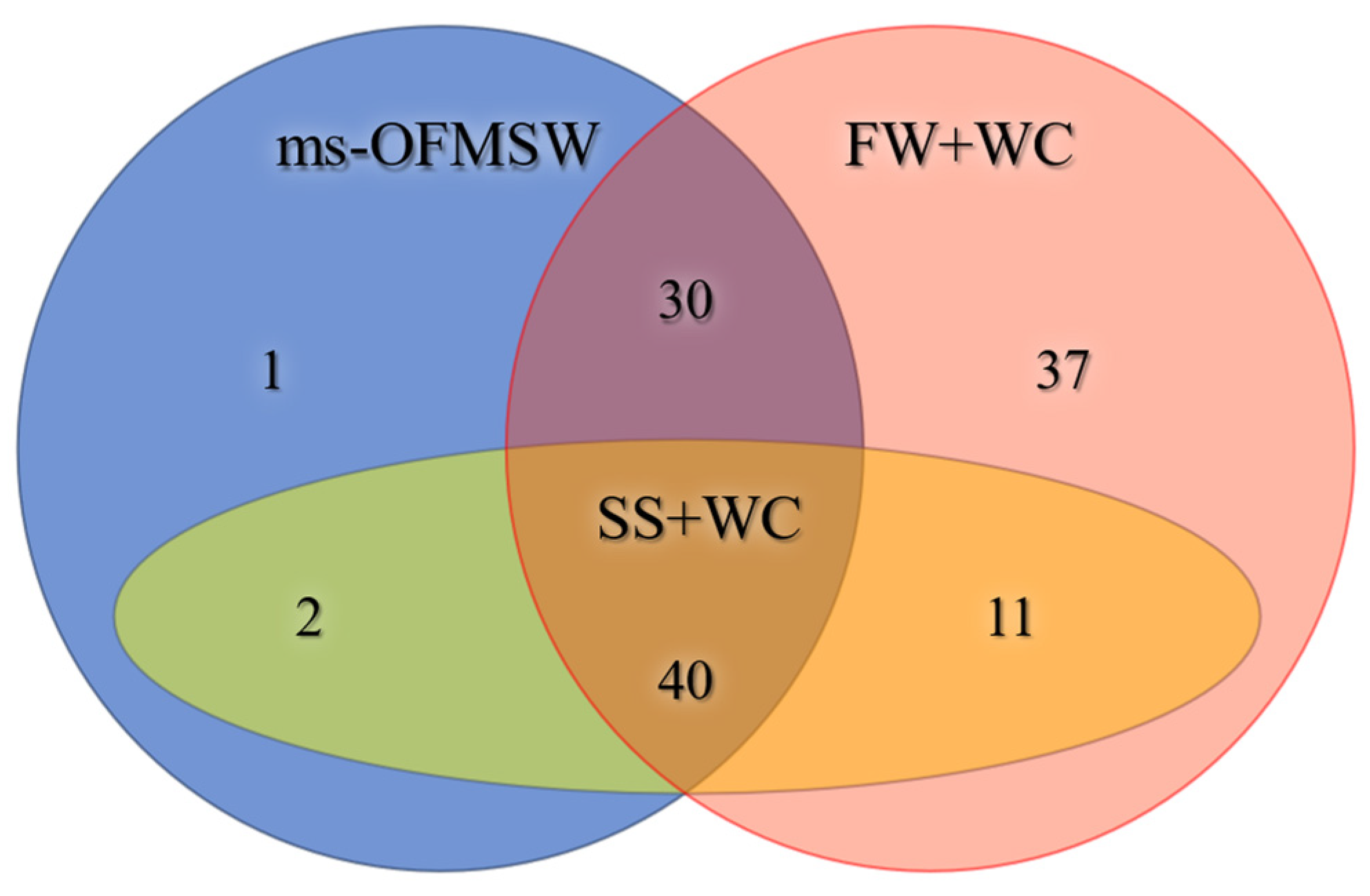
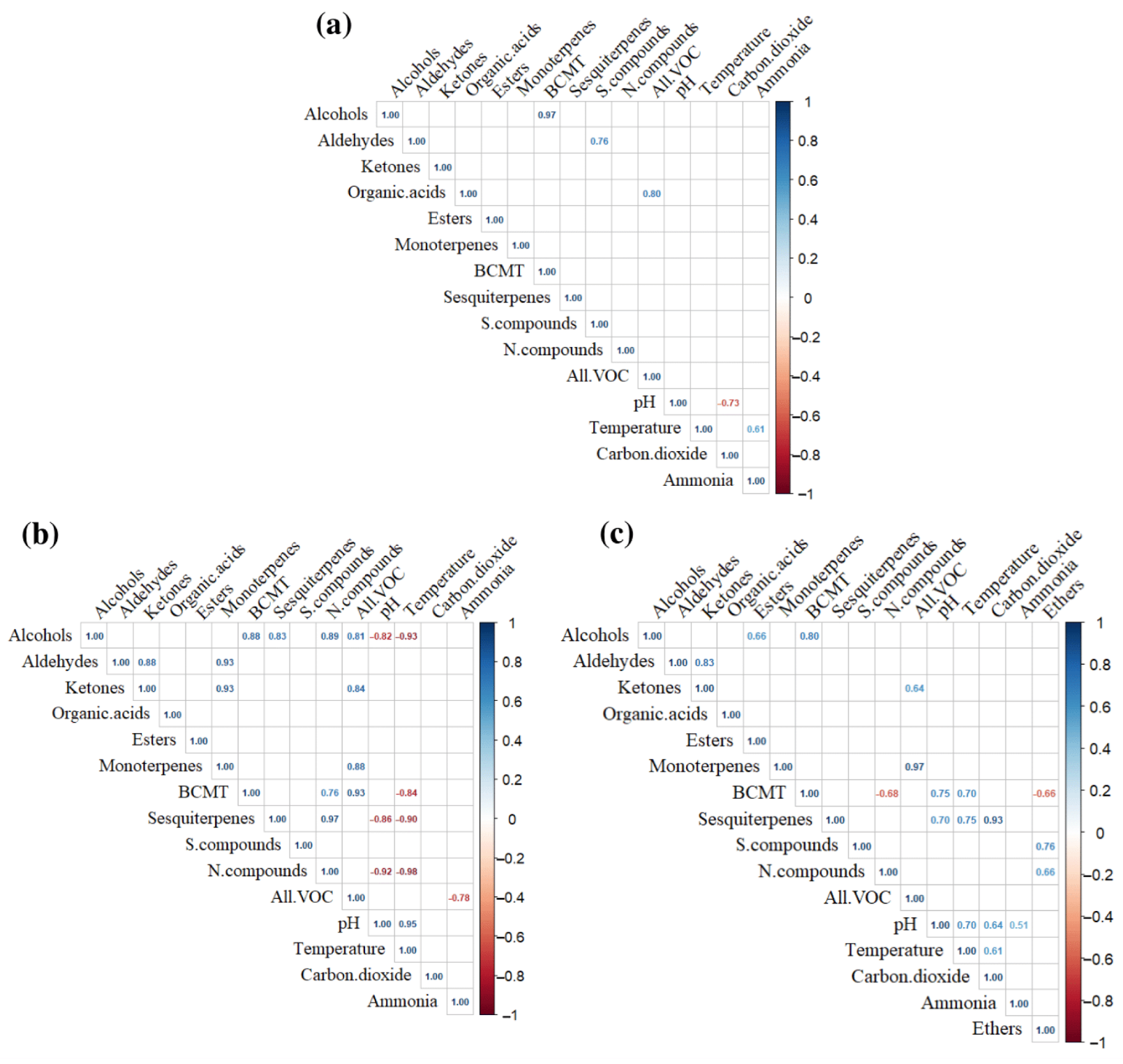
3.3. Chemical and Correlational Analysis of VOC Composition
3.3.1. SS+WC
3.3.2. ms-OFMSW
3.3.3. FW+WC
3.4. N-Containing Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Towprayoon, S.; Shmarin, S.; Gao, Q.; Osama Abdel-Aziz, A.; Farkaš, J.; Zanzottera, N.M.; Ijaz, M.; Sharma, C. Waste Generation, Composition and Management Data. In 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; IPCC Task Force on National Greenhouse Gas Inventories: Bangkok, Thailand, 2019; Volume 5. [Google Scholar]
- Nordahl, S.L.; Preble, C.V.; Kirchstetter, T.W.; Scown, C.D. Greenhouse Gas and Air Pollutant Emissions from Composting. Environ. Sci. Technol. 2023, 57, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Teklehaimanot, G.Z.; Kamika, I.; Coetzee, M.A.A.; Momba, M.N.B. Population Growth and Its Impact on the Design Capacity and Performance of the Wastewater Treatment Plants in Sedibeng and Soshanguve, South Africa. Environ. Manag. 2015, 56, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.M.-C.; Bodirsky, B.L.; Krueger, T.; Mishra, A.; Popp, A. The World’s Growing Municipal Solid Waste: Trends and Impacts. Environ. Res. Lett. 2020, 15, 074021. [Google Scholar] [CrossRef]
- Rincón, C.A.; De Guardia, A.; Couvert, A.; Le Roux, S.; Soutrel, I.; Daumoin, M.; Benoist, J.C. Chemical and Odor Characterization of Gas Emissions Released during Composting of Solid Wastes and Digestates. J. Environ. Manag. 2019, 233, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, M.F.; Liu, Y.; Duan, Z.; Guo, H.; Xu, S.; Wang, H.; Lu, W. Volatile Compounds Emission and Health Risk Assessment during Composting of Organic Fraction of Municipal Solid Waste. J. Hazard. Mater. 2017, 327, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Defoer, N.; De Bo, I.; Van Langenhove, H.; Dewulf, J.; Van Elst, T. Gas Chromatography—Mass Spectrometry as a Tool for Estimating Odour Concentrations of Biofilter Effluents at Aerobic Composting and Rendering Plants. J. Chromatogr. A 2002, 970, 259–273. [Google Scholar] [CrossRef]
- Kume, K.; Ohura, T.; Amagai, T.; Fusaya, M. Field Monitoring of Volatile Organic Compounds Using Passive Air Samplers in an Industrial City in Japan. Environ. Pollut. 2008, 153, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Rovira, J.; Vilavert, L.; Nadal, M.; Figueras, M.J.; Schuhmacher, M. Health Risks for the Population Living in the Vicinity of an Integrated Waste Management Facility: Screening Environmental Pollutants. Sci. Total Environ. 2015, 518–519, 363–370. [Google Scholar] [CrossRef]
- Allievi, M.J.; Silveira, D.D.; Cantão, M.E.; Filho, P.B. Bacterial Community Diversity in a Full Scale Biofilter Treating Wastewater Odor. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2018, 77, 2014–2022. [Google Scholar] [CrossRef]
- Pachaiappan, R.; Cornejo-Ponce, L.; Rajendran, R.; Manavalan, K.; Femilaa Rajan, V.; Awad, F. A Review on Biofiltration Techniques: Recent Advancements in the Removal of Volatile Organic Compounds and Heavy Metals in the Treatment of Polluted Water. Bioengineered 2022, 13, 8432–8477. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, G.; Gao, D.; Chen, T.; Wu, F.; Niu, M.; Zou, K. Odor Composition Analysis and Odor Indicator Selection during Sewage Sludge Composting. J. Air Waste Manag. Assoc. 2016, 66, 930–940. [Google Scholar] [CrossRef] [PubMed]
- González, D.; Guerra, N.; Colón, J.; Gabriel, D.; Ponsá, S.; Sánchez, A. Filling in Sewage Sludge Biodrying Gaps: Greenhouse Gases, Volatile Organic Compounds and Odour Emissions. Bioresour. Technol. 2019, 291, 121857. [Google Scholar] [CrossRef] [PubMed]
- Raclavská, H.; Růžičková, J.; Raclavský, K.; Juchelková, D.; Kucbel, M.; Švédová, B.; Slamová, K.; Kacprzak, M. Effect of Biochar Addition on the Improvement of the Quality Parameters of Compost Used for Land Reclamation. Environ. Sci. Pollut. Res. 2023, 30, 8563–8581. [Google Scholar] [CrossRef]
- Maulini-Duran, C.; Artola, A.; Font, X.; Sánchez, A. A Systematic Study of the Gaseous Emissions from Biosolids Composting: Raw Sludge versus Anaerobically Digested Sludge. Bioresour. Technol. 2013, 147, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, J.; Shi, D.; Liu, G.; Zhao, Y.; Shimaoka, T. Environmental Challenges Impeding the Composting of Biodegradable Municipal Solid Waste: A Critical Review. Resour. Conserv. Recycl. 2017, 122, 51–65. [Google Scholar] [CrossRef]
- Schiavon, M.; Martini, L.M.; Corrà, C.; Scapinello, M.; Coller, G.; Tosi, P.; Ragazzi, M. Characterisation of Volatile Organic Compounds (VOCs) Released by the Composting of Different Waste Matrices. Environ. Pollut. 2017, 231, 845–853. [Google Scholar] [CrossRef]
- Gutiérrez, M.C.; Hernández-Ceballos, M.A.; Márquez, P.; Chica, A.F.; Martín, M.A. Identification and Simulation of Atmospheric Dispersion Patterns of Odour and VOCs Generated by a Waste Treatment Plant. Atmos. Pollut. Res. 2023, 14, 101636. [Google Scholar] [CrossRef]
- Agapiou, A.; Vasileiou, A.; Stylianou, M.; Mikedi, K.; Zorpas, A.A. Waste Aroma Profile in the Framework of Food Waste Man-agement through Household Composting. J. Clean. Prod. 2020, 257, 120340. [Google Scholar] [CrossRef]
- Agapiou, A.; Vamvakari, J.P.; Andrianopoulos, A.; Pappa, A. Volatile Emissions during Storing of Green Food Waste under Different Aeration Conditions. Environ. Sci. Pollut. Res. 2016, 23, 8890–8901. [Google Scholar] [CrossRef]
- Smet, E.; Van Langenhove, H.; De Bo, I. The Emission of Volatile Compounds during the Aerobic and the Combined Anaerobic/Aerobic Composting of Biowaste. Atmos. Environ. 1999, 33, 1295–1303. [Google Scholar] [CrossRef]
- Nagata, Y. Measurement of Odor Threshold Value Review of “Measurement of Threshold Value by Tiriangle Odor Bag Method Bull. Jap. Env. Sanit. Cent. No. 17. 1990” Paper. J. Jpn. Assoc. Odor Environ. 2012, 43, 401–407. [Google Scholar] [CrossRef]
- Martínez-Valdez, F.J.; Martínez-Ramírez, C.; Martínez-Montiel, L.; Favela-Torres, E.; Soto-Cruz, N.O.; Ramírez-Vives, F.; Saucedo-Castañeda, G. Rapid Mineralisation of the Organic Fraction of Municipal Solid Waste. Bioresour. Technol. 2015, 180, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, P.; Garau, G.; Deiana, P.; Melis, P. Evolution of Carbon Compounds during Municipal Solid Waste Composting: Suitability of Chemical and Biochemical Parameters in Defining the Stability and Maturity of the End Product. Dyn. Soil Dyn. Plant 2009, 3, 17–31. [Google Scholar]
- Palaniveloo, K.; Amran, M.A.; Norhashim, N.A.; Mohamad-Fauzi, N.; Peng-Hui, F.; Hui-Wen, L.; Kai-Lin, Y.; Jiale, L.; Chian-Yee, M.G.; Jing-Yi, L.; et al. Food Waste Composting and Microbial Community Structure Profiling. Processes 2020, 8, 723. [Google Scholar] [CrossRef]
- Schwalb, M.; Rosevear, C.; Chin, R.; Barrington, S. Food Waste Treatment in a Community Center. Waste Manag. 2011, 31, 1570–1575. [Google Scholar] [CrossRef]
- Mironov, V.; Vanteeva, A.; Merkel, A. Microbiological Activity during Co-Composting of Food and Agricultural Waste for Soil Amendment. Agronomy 2021, 11, 928. [Google Scholar] [CrossRef]
- Mironov, V.; Vanteeva, A.; Sokolova, D.; Merkel, A.; Nikolaev, Y. Microbiota Dynamics of Mechanically Separated Organic Fraction of Municipal Solid Waste during Composting. Microorganisms 2021, 9, 1877. [Google Scholar] [CrossRef]
- Ivanov, Y.; Mironov, V. Test Results In-Vessel Composting System at the Cattle Farm Located in the Central Part of Russia. AMA Agric. Mech. Asia Africa Lat. Am. 2018, 49, 86–90. [Google Scholar]
- Navarro, A.F.; Cegarra, J.; Roig, A.; Garcia, D. Relationships between Organic Matter and Carbon Contents of Organic Wastes. Bioresour. Technol. 1993, 44, 203–207. [Google Scholar] [CrossRef]
- Arrigoni, J.P.; Paladino, G.; Garibaldi, L.A.; Laos, F. Inside the Small-Scale Composting of Kitchen and Garden Wastes: Thermal Performance and Stratification Effect in Vertical Compost Bins. Waste Manag. 2018, 76, 284–293. [Google Scholar] [CrossRef]
- Cesaro, A.; Belgiorno, V.; Guida, M. Compost from Organic Solid Waste: Quality Assessment and European Regulations for Its Sustainable Use. Resour. Conserv. Recycl. 2015, 94, 72–79. [Google Scholar] [CrossRef]
- Laor, Y.; Parker, D.; Pagé, T. Measurement, Prediction, and Monitoring of Odors in the Environment: A Critical Review. Rev. Chem. Eng. 2014, 30, 139–166. [Google Scholar] [CrossRef]
- Font, X.; Artola, A.; Sánchez, A. Detection, Composition and Treatment of Volatile Organic Compounds from Waste Treatment Plants. Sensors 2011, 11, 4043–4059. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Zhang, H.; Jiang, T. Correlation Coefficients for a Study with Repeated Measures. Comput. Math. Methods Med. 2020, 2020, 7398324. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.V.; Bochkova, E.A.; Gannesen, A.V.; Vanteeva, A.V.; Russkova, Y.I.; Nozhevnikova, A.N. Dynamics of Biological Processes during Composting of Anaerobically Digested Wastewater Sludge. Microbiology 2020, 89, 470–482. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of Aeration Rate, C/N Ratio and Moisture Content on the Stability and Maturity of Compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef]
- Mironov, V.; Moldon, I.; Shchelushkina, A.; Zhukov, V.; Zagustina, N. Bio-Drying of Municipal Wastewater Sludge: Effects of High Temperature, Low Moisture Content and Volatile Compounds on the Microbial Community. Fermentation 2023, 9, 570. [Google Scholar] [CrossRef]
- Pagans, E.; Barrena, R.; Font, X.; Sánchez, A. Ammonia Emissions from the Composting of Different Organic Wastes. Dependency on Process Temperature. Chemosphere 2006, 62, 1534–1542. [Google Scholar] [CrossRef]
- Mao, I.-F.; Tsai, C.-J.; Shen, S.-H.; Lin, T.-F.; Chen, W.-K.; Chen, M.-L. Critical Components of Odors in Evaluating the Performance of Food Waste Composting Plants. Sci. Total Environ. 2006, 370, 323–329. [Google Scholar] [CrossRef]
- Jokela, J.P.Y.; Kettunen, R.H.; Rintala, J.A. Methane and Leachate Pollutant Emission Potential from Various Fractions of Municipal Solid Waste (MSW): Effects of Source Separation and Aerobic Treatment. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2002, 20, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Zhang, X.; Yan, Z.; Cheng, Y.; Li, H.; Xu, G. Monitoring Volatile Organic Compounds in Different Pear Cultivars during Storage Using HS-SPME with GC-MS. Foods 2022, 11, 3778. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.I.; Arnáiz, N.; Font, R.; Carratalá, A. Chemical Characterization of Emissions from a Municipal Solid Waste Treatment Plant. Waste Manag. 2014, 34, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- He, Z.; Chen, K.; Huang, C.; Xin, X.; Tan, H.; Jiang, J.; Wu, X.; Zhai, J. Microbial Metabolism and Health Risk Assessment of Kitchen Waste Odor VOCs. Environ. Sci. Pollut. Res. 2023, 30. [Google Scholar] [CrossRef] [PubMed]
- Dewan, A.; Cichy, A.; Zhang, J.; Miguel, K.; Feinstein, P.; Rinberg, D.; Bozza, T. Single Olfactory Receptors Set Odor Detection Thresholds. Nat. Commun. 2018, 9, 2887. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Rappert, S. Pyrazines: Occurrence, Formation and Biodegradation. Appl. Microbiol. Biotechnol. 2010, 85, 1315–1320. [Google Scholar] [CrossRef]
- Rosenfeld, P.E.; Clark, J.J.J.; Hensley, A.R.; Suffet, I.H. The Use of an Odour Wheel Classification for the Evaluation of Human Health Risk Criteria for Compost Facilities. Water Sci. Technol. 2007, 55, 345–357. [Google Scholar] [CrossRef]
- Jang, J.-K. Amines as Occupational Hazards for Visual Disturbance. Ind. Health 2016, 54, 101–115. [Google Scholar] [CrossRef]
- Wójcik, W.; Łukasiewicz, M.; Puppel, K. Biogenic Amines: Formation, Action and Toxicity—A Review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef]
- Feng, Z.; Zhang, J.; Chen, G.; Ge, Y.; Zhang, X.; Zhu, H. Extracellular Expression of L-Aspartate-α-Decarboxylase from Bacillus Tequilensis and Its Application in the Biosynthesis of β-Alanine. Appl. Biochem. Biotechnol. 2019, 189, 273–283. [Google Scholar] [CrossRef]
- Deng, W.; Ogita, S.; Ashihara, H. Ethylamine Content and Theanine Biosynthesis in Different Organs of Camellia Sinensis Seedlings. Z. Naturforsch. C 2014, 64, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Jameson, E.; Quareshy, M.; Chen, Y. Methodological Considerations for the Identification of Choline and Carnitine-Degrading Bacteria in the Gut. Methods 2018, 149, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; DaCosta, K.A.; Fox, J.G. Endogenous Formation of Dimethylamine. Biochem. J. 1985, 232, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, G.J.; Proctor, N.H. Proctor and Hughes’ Chemical Hazards of the Workplace; John Wiley & Sons: Hoboken, NJ, USA, 2014; ISBN 0471268836. [Google Scholar]


| Parameters | Substrate Type | ||
|---|---|---|---|
| SS+WC | ms-OFMSW | FW+WC | |
| pH | 6.72 ± 0.05 | 5.14 ± 0.08 | 4.06 ± 0.22 |
| Electrical conductivity (EC, µS cm−1) | 1024 ± 0.03 | 1310 ± 12 | 857 ± 24 |
| Mass fraction of water (%) | 58.90 ± 4.71 | 44.87 ± 2.24 | 69.8 ± 2.2 |
| Kjeldahl total nitrogen (N, %) | 2.3 ± 0.3 | 1.38 ± 0.30 | 1.21 ± 0.18 |
| Ash content (%) | 38.12 ± 0.71 | 40.55 ± 6.93 | 12.84 ± 1.04 |
| Organic matter (OM, %) | 65.45 ± 1.50 | 59.45 ± 6.93 | 87.16 ± 1.04 |
| Total content of organic carbon (C, %) | 38.50 ± 0.60 | 29.73 ± 3.47 | 43.58 ± 0.58 |
| C/N ratio | 14.95 ± 1.20 | 21.54 ± 3.77 | 36.02 ± 0.76 |
| Germination index (GI, %) | 102.6 ± 24.7 | 34.0 ± 4.0 | 73.69 ± 12.40 |
| Classification [35] | Composting Substrates | ||||||
|---|---|---|---|---|---|---|---|
| Chemical Groups | FW+WC | ms-OFMSW | SS+WC | ||||
| Subgroup | Compound | TAP *, CU | % ** | TAP *, CU | % ** | TAP *, CU | % ** |
| ALCOHOLS | 8944 ± 1073 | 3.0% | 3155 ± 347 | 5.4% | 470 ± 52 | 19.4% | |
| Monohydric | Ethanol | 1970 ± 217 | 0.7% | 218 ± 24 | 0.4% | 74 ± 8 | 3.1% |
| Dimethyloctanol | 347 ± 38 | 0.1% | 284 ± 31 | 0.5% | 83 ± 9 | 3.4% | |
| Monoterpenoids | Terpinen-4-ol | 681 ± 75 | 0.2% | 381 ± 42 | 0.7% | 4 ± 2 | 0.2% |
| β-Fenchol | 208 ± 23 | 0.1% | 379 ± 42 | 0.7% | n.d. | n.d. | |
| Sesquiterpenoids | cis-Sesquisabinene hydrate | n.d. | n.d. | 384 ± 42 | 0.7% | 83 ± 9 | 3.4% |
| ETHERS | 2598 ± 270 | 0.9% | n.d. | n.d. | n.d. | n.d. | |
| Diisopropyl ether | 1531 ± 153 | 0.5% | n.d. | n.d. | n.d. | n.d. | |
| Ethylhexyl ether | 546 ± 55 | 0.2% | n.d. | n.d. | n.d. | n.d. | |
| ALDEHYDES | 5675 ± 567 | 1.9% | 365 ± 36 | 0.6% | 71 ± 7 | 3.0% | |
| Acetaldehyde | 680 ± 68 | 0.2% | n.d. | n.d. | 65 ± 6 | 2.7% | |
| Propanal | 2012 ± 201 | 0.7% | n.d. | n.d. | n.d. | n.d. | |
| Nonanal | 24 ± 2 | <0.1% | 136 ± 14 | 0.2% | n.d. | n.d. | |
| KETONES | 25,657 ± 2822 | 8.6% | 1106 ± 122 | 1.9% | 75 ± 8 | 3.1% | |
| Acetone | 22,173 ± 2439 | 7.4% | n.d. | n.d. | 5 ± 2 | 0.2% | |
| 2-Methyl vinyl ketone | 1182 ± 130 | 0.4% | n.d. | n.d. | n.d. | n.d. | |
| 1-Heptene-3-one | 205 ± 23 | 0.1% | 404 ± 44 | 0.7% | n.d. | n.d. | |
| Terpenoid-ketones | Fenchone | 205 ± 23 | 0.1% | 229 ± 25 | 0.4% | 22 ± 2 | 0.9% |
| α-Pinocarvone | 487 ± 54 | 0.2% | 139 ± 15 | 0.2% | 36 ± 4 | 1.5% | |
| ORGANIC ACIDS (Org-acids) | 3418 ± 410 | 1.1% | 944 ± 113 | 1.6% | 331 ± 33 | 13.7% | |
| Acetic acid | 1156 ± 139 | 0.4% | 61 ± 7 | 0.1% | 126 ± 13 | 5.2% | |
| Propionic acid | 508 ± 61 | 0.2% | 349 ± 42 | 0.6% | 26 ± 3 | 1.1% | |
| Butyric acid | 1202 ± 144 | 0.4% | 444 ± 53 | 0.8% | 103 ± 10 | 4.3% | |
| ESTERS | 2341 ± 281 | 0.8% | 691 ± 83 | 1.2% | 33 ± 4 | 1.4% | |
| Methyl acetate | 610 ± 73 | 0.2% | 54 ± 7 | 0.1% | n.d. | n.d. | |
| Phenyl propyl carbonate | 766 ± 92 | 0.3% | 206 ± 25 | 0.4% | 21 ± 2 | 0.9% | |
| MONOTERPENES (MT) | 225,929 ± 18,074 | 75.4% | 41,938 ± 3355 | 72.2% | 96 ± 7 | 4.0% | |
| α- & β-Terpinene | 2499 ± 200 | 0.8% | 977 ± 78 | 1.7% | n.d. | n.d. | |
| δ-Terpinene | 541 ± 43 | 0.2% | 113 ± 9 | 0.2% | 29 ± 2 | 1.2% | |
| D-Limonene | 214,289 ± 17,143 | 71.5% | 40,179 ± 3214 | 69.2% | n.d. | n.d. | |
| Acyclic MT | β-Myrcene | 6423 ± 514 | 2.1% | n.d. | n.d. | 48 ± 4 | 2.0% |
| BICYCLIC MONOTERPENES (BCMT) | 3268 ± 294 | 1.1% | 2952 ± 266 | 5.1% | 40 ± 3 | 1.7% | |
| δ- & β-Pinene | 759 ± 77 | 0.3% | 2952 ± 266 | 5.1% | 36 ± 4 | 1.4% | |
| α-Pinene | 1377 ± 124 | 0.5% | n.d. | n.d. | 5 ± 2 | 0.2% | |
| Camphene | 798 ± 72 | 0.3% | n.d. | n.d. | n.d. | n.d. | |
| SESQUITERPENES (ST) | 2620 ± 236 | 0.9% | 4500 ± 405 | 7.7% | 762 ± 61 | 31.4% | |
| α-Ylangene | 45 ± 4 | <0.1% | 2765 ± 249 | 4.8% | 37 ± 3 | 1.5% | |
| β-Elemene | 83 ± 7 | <0.1% | 192 ± 17 | 0.3% | 131 ± 10 | 5.4% | |
| α-, β- & γ-Calacorene | 357 ± 32 | 0.2% | 512 ± 33 | 0.9% | 164 ± 14 | 6.8% | |
| α- & β-Selinene | 416 ± 37 | 0.2% | 107 ± 10 | 0.2% | 27 ± 2 | 1.1% | |
| Classification [35] | Composting Substrates | ||||||
|---|---|---|---|---|---|---|---|
| Chemical Groups | FW+WC | ms-OFMSW | SS+WC | ||||
| Subgroup | Compound | TAP *, CU | % ** | TAP *, CU | % ** | TAP *, CU | % ** |
| S-COMPOUNDS | 1911 ± 191 | 0.6% | 601 ± 60 | 1.0% | 46 ± 4 | 1.9% | |
| Dimethyl sulphide | 509 ± 51 | 0.2% | 16 ± 2 | <0.1% | n.d. | n.d. | |
| Dimethyl disulphide | 257 ± 26 | 0.1% | 138 ± 14 | 0.2% | n.d. | n.d. | |
| Dimethyl trisulphide | 1145 ± 115 | 0.4% | 447 ± 45 | 0.8% | 46 ± 4 | 1.9% | |
| N-COMPOUNDS | 17,165 ± 1888 | 5.7% | 1842 ± 203 | 3.2% | 495 ± 45 | 20.4% | |
| Amines | Trimethylamine | 24 ± 3 | <0.1% | 44 ± 5 | 0.1% | 56 ± 5 | 2.3% |
| Dimethylamine | 553 ± 61 | 0.2% | 45 ± 5 | 0.1% | 200 ± 18 | 8.2% | |
| Ethylamine | 8531 ± 938 | 2.8% | 755 ± 83 | 1.3% | 110 ± 10 | 4.5% | |
| Ethylmethylamine | 659 ± 72 | 0.2% | n.d. | n.d. | 117 ± 11 | 4.8% | |
| Diethylamine | 813 ± 89 | 0.3% | 12 ± 1 | <0.1% | 4 ± 1 | 0.2% | |
| tert-Butylamine | 3708 ± 408 | 1.2% | n.d. | n.d. | n.d. | n.d. | |
| Heterocyclic | 1-Methylpiperazine | 506 ± 56 | 0.2% | 426 ± 47 | 0.7% | n.d. | n.d. |
| 2,3-Dimethylpirazine | 310 ± 34 | 0.1% | 422 ± 46 | 0.7% | n.d. | n.d. | |
| 2,5-Dimethylpirazine | 308 ± 34 | 0.1% | n.d. | n.d. | n.d. | n.d. | |
| 3,5-Dimethylpyrazole | 287 ± 32 | 0.1% | 53 ± 6 | 0.1% | n.d. | n.d. | |
| Alkanolamine | Ethanolamine | 1281 ± 141 | 0.4% | 85 ± 9 | 0.1% | 9 ± 1 | 0.4% |
| Ketoximes | Ethyl acetohydroxamate | 186 ± 20 | 0.1% | n.d. | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mironov, V.; Zhukov, V.; Moldon, I.; Zagustina, N.; Shchelushkina, A.; Ostrikova, V.; Kotova, I.B.; Zhang, S. Pollutant Emissions from Municipal Biowaste Composting: Comparative Analysis and Contribution of N-Containing Organic Compounds. Energies 2023, 16, 7271. https://doi.org/10.3390/en16217271
Mironov V, Zhukov V, Moldon I, Zagustina N, Shchelushkina A, Ostrikova V, Kotova IB, Zhang S. Pollutant Emissions from Municipal Biowaste Composting: Comparative Analysis and Contribution of N-Containing Organic Compounds. Energies. 2023; 16(21):7271. https://doi.org/10.3390/en16217271
Chicago/Turabian StyleMironov, Vladimir, Vitaly Zhukov, Ivan Moldon, Nataliya Zagustina, Anna Shchelushkina, Valeria Ostrikova, Irina B. Kotova, and Shenghua Zhang. 2023. "Pollutant Emissions from Municipal Biowaste Composting: Comparative Analysis and Contribution of N-Containing Organic Compounds" Energies 16, no. 21: 7271. https://doi.org/10.3390/en16217271
APA StyleMironov, V., Zhukov, V., Moldon, I., Zagustina, N., Shchelushkina, A., Ostrikova, V., Kotova, I. B., & Zhang, S. (2023). Pollutant Emissions from Municipal Biowaste Composting: Comparative Analysis and Contribution of N-Containing Organic Compounds. Energies, 16(21), 7271. https://doi.org/10.3390/en16217271






