Hydrothermal but Not Mechanical Pretreatment of Wastewater Algae Enhanced Anaerobic Digestion Energy Balance due to Improved Biomass Disintegration and Methane Production Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Rate Raceway Ponds for Algae Cultivation in Wastewater
2.2. Algae Biomass Pretreatment, Experimental and Theoretical Methane Potential
2.2.1. Mechanical and Hydrothermal Pretreatments
2.2.2. Solubilization of Organic Matter
2.2.3. Particle-Size Distribution
2.2.4. Experimental Biomethane Potential
2.2.5. Theoretical Biomethane Potential
2.3. Modelling Gas Production Kinetics and Analyzing Statistics
2.4. Simulation of the Impact of Pretreatment on the Energy Balance Parameters of a Scaled Anaerobic Digestion (AD) System
2.5. Analytical Techniques
3. Results and Discussion
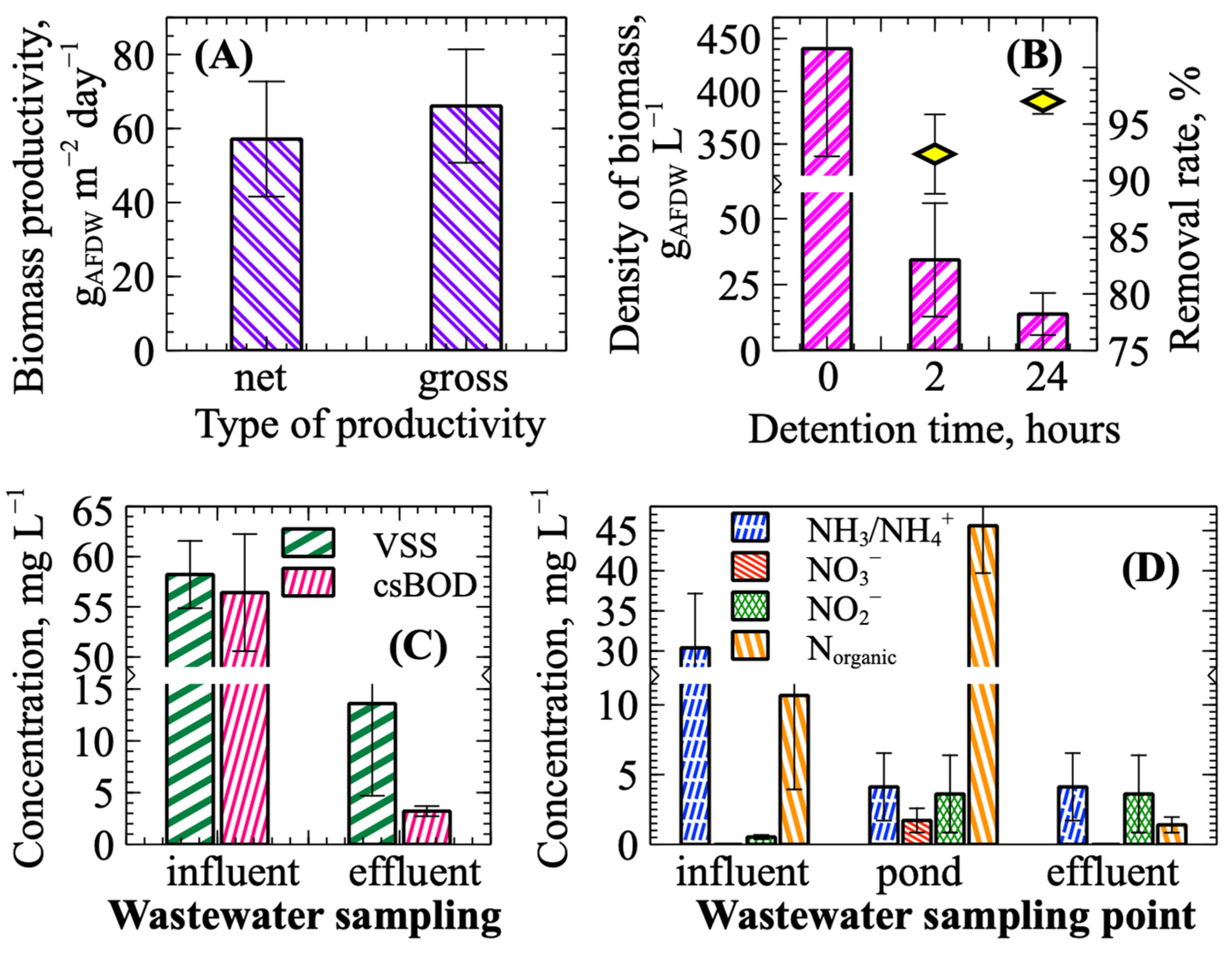
3.1. Algae Cultivation in Wastewater Ponds
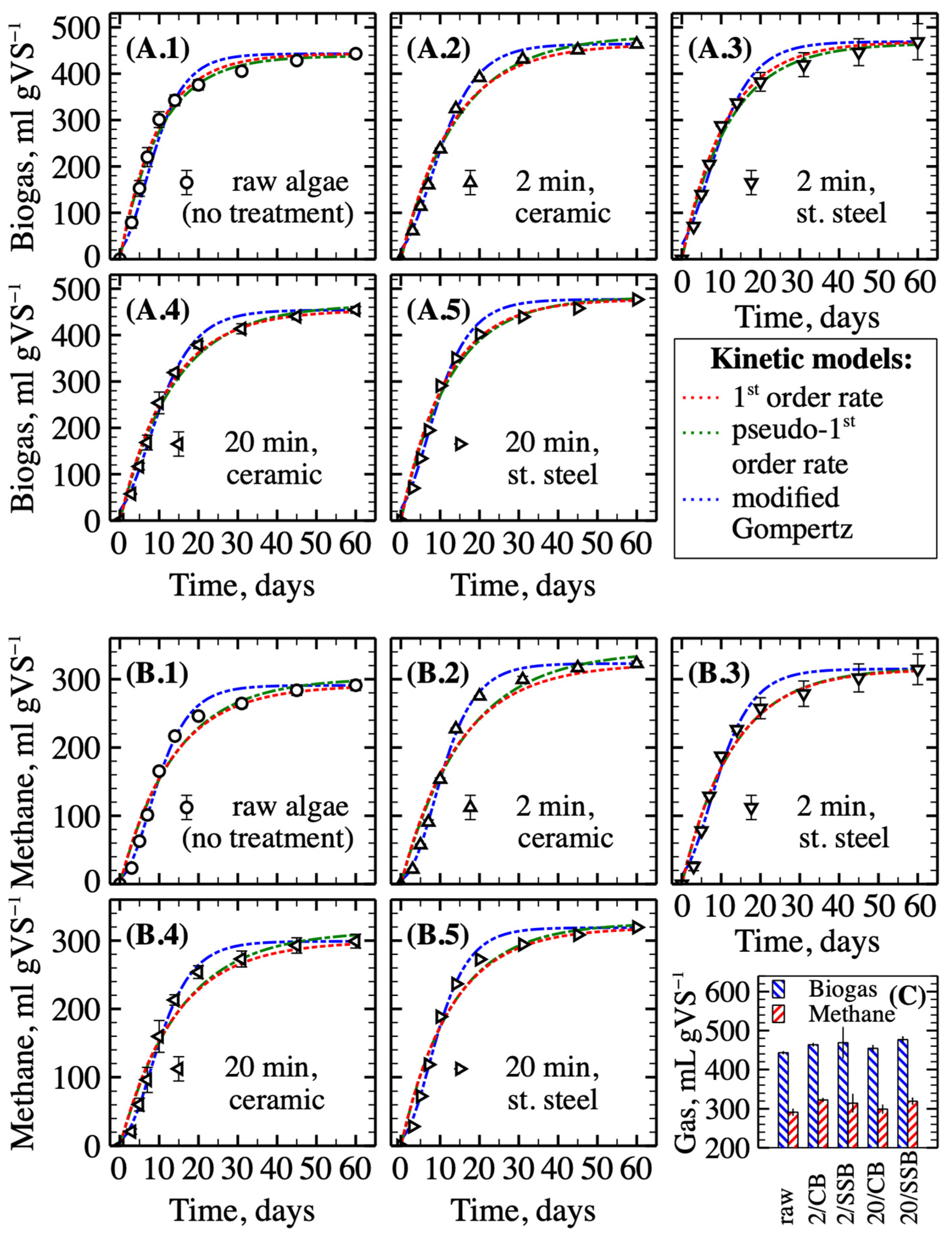
3.2. Enhancing of Biogas and Methane Yields through Algal Biomass Disintegration by Mechanical Pretreatment
3.3. Enhancing Biogas and Methane Yields through Algal Biomass Disintegration by Hydrothermal Pretreatment
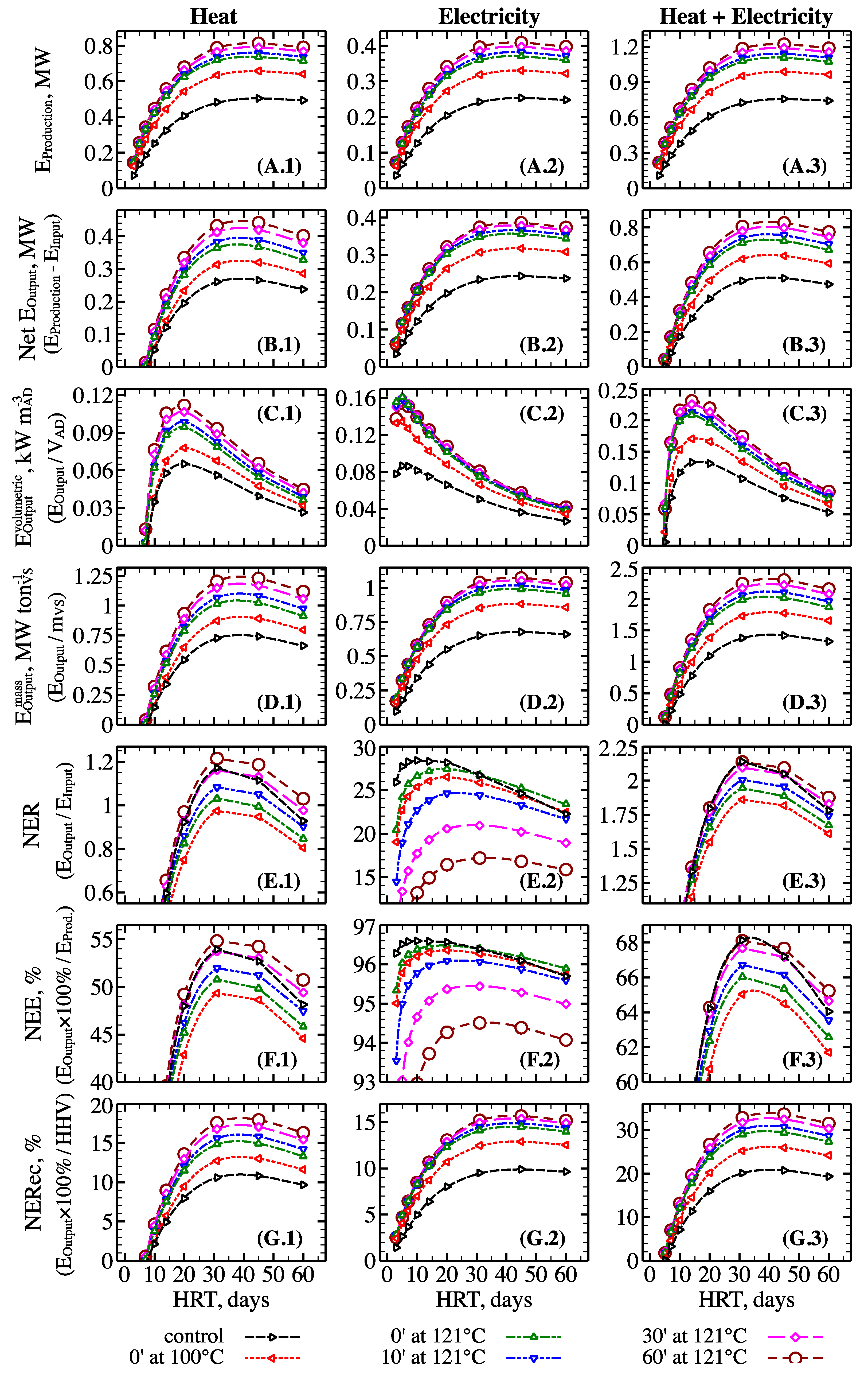
3.4. Assessment of Energy Balance for Anaerobic Digestion of Raw and Hydrothermally Pretreated Biomass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.W.T.; Benemann, J.R. A Realistic Technology and Engineering Assessment of Algae Biofuel Production; UC: Berkeley, CA, USA, 2010; p. 178. [Google Scholar]
- Sydney, E.B.; da Silva, T.E.; Tokarski, A.; Novak, A.C.; de Carvalho, J.C.; Woiciecohwski, A.L.; Larroche, C.; Soccol, C.R. Screening of microalgae with potential for biodiesel production and nutrient removal from treated domestic sewage. Appl. Energy 2011, 88, 3291–3294. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Min, M.; Hu, B.; Chen, P.; Ruan, R. Local bioprospecting for high-lipid producing microalgal strains to be grown on concentrated municipal wastewater for biofuel production. Bioresour. Technol. 2011, 102, 6909–6919. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, A.E.M.; Leite, G.B.; Belhaj, M.A.; Hallenbeck, P.C. Screening microalgae native to Quebec for wastewater treatment and biodiesel production. Bioresour. Technol. 2014, 157, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Liu, K.; Nasr, L.K.; Byers, N.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Bouwer, E.J. Bioprospecting of microalgae for integrated biomass production and phytoremediation of unsterilized wastewater and anaerobic digestion centrate. Appl. Microbiol. Biotechnol. 2015, 99, 6139–6154. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Kligerman, D.C.; Byers, N.; Nasr, L.K.; Cua, C.; Chow, S.; Su, C.; Tang, Y.; Betenbaugh, M.J.; Bouwer, E.J. Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res. 2016, 19, 278–290. [Google Scholar] [CrossRef]
- Massimi, R.; Kirkwood, A.E. Screening microalgae isolated from urban storm- and wastewater systems as feedstock for biofuel. PeerJ 2016, 4, e2396. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Kucek, L.A.; Hill, E.; Pinchuk, G.E.; Mundree, S.G.; Beliaev, A.S. Conversion of Stranded Waste-Stream Carbon and Nutrients into Value-Added Products via Metabolically Coupled Binary Heterotroph-Photoautotroph System. Bioresour. Technol. 2018, 26, 68–75. [Google Scholar] [CrossRef]
- Guo, R.; Chen, J. Application of alga-activated sludge combined system (AASCS) as a novel treatment to remove cephalosporins. Chem. Eng. J. 2015, 260, 550–556. [Google Scholar] [CrossRef]
- Xie, B.; Gong, W.; Tian, Y.; Qu, F.; Luo, Y.; Du, X.; Tang, X.; Xu, D.; Lin, D.; Li, G.; et al. Biodiesel production with the simultaneous removal of nitrogen, phosphorus and COD in microalgal-bacterial communities for the treatment of anaerobic digestion effluent in photobioreactors. Chem. Eng. J. 2018, 350, 1092–1102. [Google Scholar] [CrossRef]
- Bohutskyi, P.; McClure, R.S.; Hill, E.A.; Nelson, W.C.; Chrisler, W.B.; Nuñez, J.R.; Renslow, R.S.; Charania, M.A.; Lindemann, S.R.; Beliaev, A.S. Metabolic effects of vitamin B12 on physiology, stress resistance, growth rate and biomass productivity of Cyanobacterium stanieri planktonic and biofilm cultures. Algal Res. 2019, 42, 101580. [Google Scholar] [CrossRef]
- Xie, B.; Bishop, S.; Stessman, D.; Wright, D.; Spalding, M.H.; Halverson, L.J. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J. 2013, 7, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Chow, S.; Ketter, B.; Fung Shek, C.; Yacar, D.; Tang, Y.; Zivojnovich, M.; Betenbaugh, M.J.; Bouwer, E.J. Phytoremediation of agriculture runoff by filamentous algae poly-culture for biomethane production, and nutrient recovery for secondary cultivation of lipid generating microalgae. Bioresour. Technol. 2016, 222, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Spierling, R.E.; Phan, D.; Kopachevsky, A.M.; Tang, Y.; Betenbaugh, M.J.; Bouwer, E.J.; Lundquist, T.J. Bioenergy from wastewater resources: Nutrient removal, productivity and settleability of indigenous algal-bacteria polyculture, and effect of biomass composition variability on methane production kinetics and anaerobic digestion energy balance. Algal Res. 2018, 36, 217–228. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Phan, D.; Spierling, R.E.; Kopachevsky, A.M.; Bouwer, E.J.; Lundquist, T.J.; Betenbaugh, M.J. Production of lipid-containing algal-bacterial polyculture in wastewater and biomethanation of lipid extracted residues: Enhancing methane yield through hydrothermal pretreatment and relieving solvent toxicity through co-digestion. Sci. Total Environ. 2019, 653, 1377–1394. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; García, J.; Ferrer, I. Impact of low temperature pretreatment on the anaerobic digestion of microalgal biomass. Bioresour. Technol. 2013, 138, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Solé, M.; García, J.; Ferrer, I. Biogas production from microalgae grown in wastewater: Effect of microwave pretreatment. Appl. Energy 2013, 108, 168–175. [Google Scholar] [CrossRef]
- Passos, F.; Hernández-Mariné, M.; García, J.; Ferrer, I. Long-term anaerobic digestion of microalgae grown in HRAP for wastewater treatment. Effect of microwave pretreatment. Water Res. 2014, 49, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Passos, F.; Felix, L.; Rocha, H.; Pereira, J.d.O.; de Aquino, S. Reuse of microalgae grown in full-scale wastewater treatment ponds: Thermochemical pretreatment and biogas production. Bioresour. Technol. 2016, 209, 305–312. [Google Scholar] [CrossRef]
- Nallathambi Gunaseelan, V. Anaerobic digestion of biomass for methane production: A review. Biomass Bioenergy 1997, 13, 83–114. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Climent, M.; Ferrer, I.; Baeza, M.d.M.; Artola, A.; Vázquez, F.; Font, X. Effects of thermal and mechanical pretreatments of secondary sludge on biogas production under thermophilic conditions. Chem. Eng. J. 2007, 133, 335–342. [Google Scholar] [CrossRef]
- Bayr, S.; Kaparaju, P.; Rintala, J. Screening pretreatment methods to enhance thermophilic anaerobic digestion of pulp and paper mill wastewater treatment secondary sludge. Chem. Eng. J. 2013, 223, 479–486. [Google Scholar] [CrossRef]
- Costa, A.G.; Pinheiro, G.C.; Pinheiro, F.G.C.; Dos Santos, A.B.; Santaella, S.T.; Leitão, R.C. Pretreatment strategies to improve anaerobic biodegradability and methane production potential of the palm oil mesocarp fibre. Chem. Eng. J. 2013, 230, 158–165. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Nilsen, P.J.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biomethane potential of wheat straw: Influence of particle size, water impregnation and thermal hydrolysis. Chem. Eng. J. 2014, 242, 254–259. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Zhang, Z.; Ma, D.; Wang, L.; Xu, G. Hydrothermal pretreatment for biogas production from anaerobic digestion of antibiotic mycelial residue. Chem. Eng. J. 2015, 279, 530–537. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Lang, S.; Xian, P.; Xie, T. Kinetics of combined thermal pretreatment and anaerobic digestion of waste activated sludge from sugar and pulp industry. Chem. Eng. J. 2016, 295, 131–138. [Google Scholar] [CrossRef]
- Koch, F.; Sañudo-Wilhelmy, S.A.; Fisher, N.S.; Gobler, C.J. Effect of vitamins B1and B12on bloom dynamics of the harmful brown tide alga, Aureococcus anophagefferens(Pelagophyceae). Limnol. Oceanogr. 2013, 58, 1761–1774. [Google Scholar] [CrossRef]
- Schwede, S.; Rehman, Z.-U.; Gerber, M.; Theiss, C.; Span, R. Effects of thermal pretreatment on anaerobic digestion of Nannochloropsis salina biomass. Bioresour. Technol. 2013, 143, 505–511. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Thermal pretreatment to improve methane production of Scenedesmus biomass. Biomass Bioenergy 2012, 40, 105–111. [Google Scholar] [CrossRef]
- Cho, S.; Park, S.; Seon, J.; Yu, J.; Lee, T. Evaluation of thermal, ultrasonic and alkali pretreatments on mixed-microalgal biomass to enhance anaerobic methane production. Bioresour. Technol. 2013, 143, 330–336. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Betenbaugh, M.J.; Bouwer, E.J. The effects of alternative pretreatment strategies on anaerobic digestion and methane production from different algal strains. Bioresour. Technol. 2014, 155, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Mendez, L.; Mahdy, A.; Demuez, M.; Ballesteros, M.; González-Fernández, C. Effect of high pressure thermal pretreatment on Chlorella vulgaris biomass: Organic matter solubilisation and biochemical methane potential. Fuel 2014, 117, 674–679. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Methane production of thermally pretreated Chlorella vulgaris and Scenedesmus sp. biomass at increasing biomass loads. Appl. Energy 2014, 129, 238–242. [Google Scholar] [CrossRef]
- Keymer, P.; Ruffell, I.; Pratt, S.; Lant, P. High pressure thermal hydrolysis as pre-treatment to increase the methane yield during anaerobic digestion of microalgae. Bioresour. Technol. 2013, 131, 128–133. [Google Scholar] [CrossRef]
- Marsolek, M.D.; Kendall, E.; Thompson, P.L.; Shuman, T.R. Thermal pretreatment of algae for anaerobic digestion. Bioresour. Technol. 2014, 151, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Ferrer, I. Microalgae Conversion to Biogas: Thermal Pretreatment Contribution on Net Energy Production. Environ. Sci. Technol. 2014, 48, 7171–7178. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, J.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. The IWA Anaerobic Digestion Model No 1 (ADM1). Water Sci. Technol. 2002, 45, 65–73. [Google Scholar] [CrossRef]
- Batstone, D.J. Modelling and control in anaerobic digestion: Achievements and challenges. In Proceedings of the 13th World Congress on Anaerobic Digestion (AD 13), Santiago de Compostela, Spain, 25–28 June 2012. [Google Scholar]
- Wu, B. Integration of mixing, heat transfer, and biochemical reaction kinetics in anaerobic methane fermentation. Biotechnol. Bioeng. 2012, 109, 2864–2874. [Google Scholar] [CrossRef]
- Sadino-Riquelme, C.; Hayes, R.E.; Jeison, D.; Donoso-Bravo, A. Computational fluid dynamic (CFD) modelling in anaerobic digestion: General application and recent advances. Crit. Rev. Environ. Sci. Technol. 2018, 48, 39–76. [Google Scholar] [CrossRef]
- Flores-Alsina, X.; Solon, K.; Kazadi Mbamba, C.; Tait, S.; Gernaey, K.V.; Jeppsson, U.; Batstone, D.J. Modelling phosphorus (P), sulfur (S) and iron (Fe) interactions for dynamic simulations of anaerobic digestion processes. Water Res. 2016, 95, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yang, Y.; Chen, S.; Zhao, M.; Zhu, Z.; Yang, S.; Qu, Y.; Ma, Q.; He, Z.; Zhou, J.; et al. Long-term successional dynamics of microbial association networks in anaerobic digestion processes. Water Res. 2016, 104, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fitamo, T.; Treu, L.; Boldrin, A.; Sartori, C.; Angelidaki, I.; Scheutz, C. Microbial population dynamics in urban organic waste anaerobic co-digestion with mixed sludge during a change in feedstock composition and different hydraulic retention times. Water Res. 2017, 118, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Phan, D.; Kopachevsky, A.M.; Chow, S.; Bouwer, E.J.; Betenbaugh, M.J. Synergistic Co-Digestion of Wastewater Grown Algae-Bacteria Polyculture Biomass and Cellulose to Optimize Carbon-to-Nitrogen Ratio and Application of Kinetic Models to Predict Anaerobic Digestion Energy Balance. Bioresour. Technol. 2018, 269, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [PubMed]
- López, I.; Passeggi, M.; Borzacconi, L. Validation of a simple kinetic modelling approach for agro-industrial waste anaerobic digesters. Chem. Eng. J. 2015, 262, 509–516. [Google Scholar] [CrossRef]
- Brunn, L.; Dornack, C.; Bilitewski, B. Application of laboratory scale experiments to industrial scale in case of anaerobic waste treatment. Fresenius Environ. Bull. 2009, 18, 196–203. [Google Scholar]
- Sell, S.T.; Burns, R.T.; Moody, L.B.; Raman, D.R. Comparison of Methane Production from Bench- and Sub Pilot-Scale Anaerobic Digesters. Appl. Eng. Agric. 2011, 27, 821–825. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Schwede, S.; Gerber, M.; Span, R. Scale up of laboratory scale to industrial scale biogas plants. In Proceedings of World Renewable Energy Congress-Sweden; 8–13 May 2011; Linköping University Electronic Press: Linköping, Sweden, 2011; pp. 48–55. [Google Scholar]
- Connelly, S.; Shin, S.G.; Dillon, R.J.; Ijaz, U.Z.; Quince, C.; Sloan, W.T.; Collins, G. Bioreactor Scalability: Laboratory-Scale Bioreactor Design Influences Performance, Ecology, and Community Physiology in Expanded Granular Sludge Bed Bioreactors. Front. Microbiol. 2017, 8, 664. [Google Scholar] [CrossRef] [PubMed]
- Camoying, M.G.; Yñiguez, A.T. FlowCAM optimization: Attaining good quality images for higher taxonomic classification resolution of natural phytoplankton samples. Limnol. Oceanogr. Methods 2016, 14, 305–314. [Google Scholar] [CrossRef]
- Buswell, A.M.; Mueller, H.F. Mechanism of Methane Fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Fogler, H.S. Predicting Conversion Directly from the Residence Time Distribution. In Elements of Chemical Reaction Engineering, 5th ed.; Prentice Hall: Hoboken, NJ, USA, 2016; pp. 807–844. [Google Scholar]
- Lue-Hing, C. Municipal Sewage Sludge Management: A Reference Text on Processing, Utilization and Disposal, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1998; Volume 4, p. 790. [Google Scholar]
- Naegele, H.-J.; Lemmer, A.; Oechsner, H.; Jungbluth, T. Electric energy consumption of the full scale research biogas plant “Unterer Lindenhof”: Results of longterm and full detail measurements. Energies 2012, 5, 5198–5214. [Google Scholar] [CrossRef]
- Eaton, A.D.; Franson, M.A.H. Standard Methods for the Examination of Water & Wastewater, 21st ed.; American Public Health Association, American Water Works Association, and the Water Environment Federation: New York, NY, USA, 2005. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.L.; Mat Yasin, N.H.; Derek, C.J.C.; Lim, J.K. Optimization of microalgae coagulation process using chitosan. Chem. Eng. J. 2011, 173, 879–882. [Google Scholar] [CrossRef]
- Rwehumbiza, V.M.; Harrison, R.; Thomsen, L. Alum-induced flocculation of preconcentrated Nannochloropsis salina: Residual aluminium in the biomass, FAMEs and its effects on microalgae growth upon media recycling. Chem. Eng. J. 2012, 200–202, 168–175. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Influence of hydrothermal pretreatment on microalgal biomass anaerobic digestion and bioenergy production. Water Res. 2015, 68, 364–373. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Pérez-Elvira, S.; Aymerich, E.; Fdz-Polanco, F. Assessment of the influence of thermal pre-treatment time on the macromolecular composition and anaerobic biodegradability of sewage sludge. Bioresour. Technol. 2011, 102, 660–666. [Google Scholar] [CrossRef]
- Bougrier, C.; Delgenès, J.P.; Carrère, H. Effects of thermal treatments on five different waste activated sludge samples solubilisation, physical properties and anaerobic digestion. Chem. Eng. J. 2008, 139, 236–244. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, H.; Chen, S.; Dichtl, N.; Dai, X.; Li, N. Effects of thermal hydrolysis on organic matter solubilization and anaerobic digestion of high solid sludge. Chem. Eng. J. 2015, 264, 174–180. [Google Scholar] [CrossRef]
- Wilson, C.A.; Novak, J.T. Hydrolysis of macromolecular components of primary and secondary wastewater sludge by thermal hydrolytic pretreatment. Water Res. 2009, 43, 4489–4498. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lee, E.; Dilbeck, M.P.; Liebelt, M.; Zhang, Q.; Ergas, S.J. Thermal pretreatment of microalgae for biomethane production: Experimental studies, kinetics and energy analysis. J. Chem. Technol. Biotechnol. 2017, 92, 399–407. [Google Scholar] [CrossRef]
- Alzate, M.E.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biochemical methane potential of microalgae: Influence of substrate to inoculum ratio, biomass concentration and pretreatment. Bioresour. Technol. 2012, 123, 488–494. [Google Scholar] [CrossRef]
- Cui, B.; Liu, C.; Rong, H.; Luo, S.; Guo, D.; Ji, B. CO2 favors the lipid and biodiesel production of microalgal-bacterial granular sludge. Results Eng. 2023, 17, 100980. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Bouwer, E. Biogas Production from Algae and Cyanobacteria Through Anaerobic Digestion: A Review, Analysis, and Research Needs. In Advanced Biofuels and Bioproducts; Springer: New York, NY, USA, 2013; pp. 873–975. [Google Scholar] [CrossRef]
- Ngo, P.L.; Udugama, I.A.; Gernaey, K.V.; Young, B.R.; Baroutian, S. Mechanisms, status, and challenges of thermal hydrolysis and advanced thermal hydrolysis processes in sewage sludge treatment. Chemosphere 2021, 281, 130890. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.K.; Mitra, I.; Kleiven, H.; Holte, H.R.; Svensson, K. Cambi Thermal Hydrolysis Process (CambiTHP) for sewage sludge treatment. In Clean Energy and Resource Recovery; Elsevier: Amsterdam, The Netherlands, 2022; pp. 405–422. [Google Scholar] [CrossRef]
- Deleris, S.; Bausseron, A.; Cretenot, D.; Chauzy, J. Anaerobic digestion enhanced by thermal hydrolysis: First reference BIOTHELYS® at Saumur, France. Water Pract. Technol. 2008, 3, wpt2008004. [Google Scholar] [CrossRef]
- Pereboom, J.; Luning, L.; Hol, A.; van Dijk, L.; de Man, A.W.A. Full scale experiences with TurboTec® continuous thermal hydrolysis at WWTP Venlo (NL) and Apeldoorn (NL). In Proceedings of the Aqua-Enviro 19th European Biosolids and Organic Residuals Conference and Exhibition, Manchester, UK, 17–19 November 2014. [Google Scholar]
- Gurieff, N.; Bruus, J.; Hoejsgaard, S.; Boyd, J.; Kline, M. Maximizing Energy Efficiency and Biogas Production: EXELYS™—Continuous Thermal Hydrolysis. Proc. Water Environ. Fed. 2011, 2011, 642–656. [Google Scholar] [CrossRef]
- Geraats, B. Lysotherm® Sludge Hydrolysis Five Year Experience with a Novel Approach for Operational Savings. In Proceedings of In Proceedings of the 19th European Biosolids & Organic Resources Conference & Exhibition, Manchester, UK, 17–19 November 2014. [Google Scholar]


| AD Parameter | Equation | # |
|---|---|---|
| Estimation of Biogas and Methane Production by Scaled AD (Equations (9)–(11)) | ||
| Gas production | (9) | |
| Biomass residence time distribution fxn | (10) | |
| Numerical solution for gas production | (11) | |
| —biomass SRT, d. | ||
| Estimation of Energy Input for Scaled AD (Equations (12)–(14)) | ||
| Total energy input | (12) | |
| heat input | (13) | |
| electricity input | (14) | |
| —wet mass; —specific heat, 4.19 kJ kg−1 °C−1; & —ambient (10 °C) and digestion (35 °C) T; —heat transfer coeff., W m−2 °C−1; —digester surface area, m2; —mixing energy as 3.8 W per digester m3 [58]; —CHP unit operation, 74 W per methane m3 [59] | ||
| Estimation of Energy Output from Scaled AD (Equations (15)–(19)) | ||
| Total energy production | (15) | |
| heat production | (16) | |
| heat from boiler | (17) | |
| heat from CHP | (18) | |
| electricity production | (19) | |
| where: and corresponding to utilization of 5% of biogas in boiler and 90% in CHP (remaining 5% is flared); —gas yieled, m−3; —methane LHV of, 36.6 MJ m−3; , and —energy conversion efficiencies 85%, 55% and 30%, respectively. | ||
| Scaled AD System Evaluation Metrics (Equations (20)–(25)) | ||
| Net Energy Output | (20) | |
| Volume-specific Net Energy Output | (21) | |
| Mass-specific Net Energy Output | (22) | |
| Net Energy Ratio | (23) | |
| Net Energy Efficiency | (24) | |
| Net Energy Recovery | (25) | |
| —digested biomass in ton of ash-free dry weight (organic matter); —volume of scaled AD, —algae biomass high heating value. | ||
| Parameter | Relative Content (% dw A) |
|---|---|
| Ash | 19.9 ± 0.7 |
| Volatile solids | 80.1 ± 0.5 |
| Crude protein B | 51.6 |
| Total lipids | 23 ± 0.4 |
| Fatty acid methyl esters | 9.9 ± 0.6 |
| Carbon | 46.9 |
| Nitrogen | 8.26 |
| Hydrogen | 7.06 |
| Biomass formula | C6.62H12.0O1.93N |
| Theoretical biogas yield, Lbiogas gVS−1 | 1.09 |
| Theoretical methane yield, LCH4 gVS−1 | 0.65 |
| Biogas methane content, % | 60 |
| Sample | 1st-Order Equation Model | Pseudo-1st-Order Equation Model | Modified Gompertz Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k, d−1 | RMSE | R2 | k1, d−1 | k2, d−1 | Pbiodeg | RMSE | R2 | k, mL gVS−1 d−1 | λ, d | RMSE | R2 | |
| Biogas production | ||||||||||||
| Raw algae | 0.104 | 18.3 | 0.990 | 0.099 | 0.000 | 0.403 | 15.9 | 0.989 | 29.6 | 0.00 | 20.8 | 0.985 |
| 2 min CB | 0.076 | 21.7 | 0.989 | 0.069 | 0.000 | 0.444 | 20.1 | 0.987 | 26.1 | 0.94 | 26.1 | 0.997 |
| 2 min SSB | 0.090 | 18.6 | 0.991 | 0.084 | 0.000 | 0.428 | 15.9 | 0.991 | 29.3 | 0.75 | 29.3 | 0.984 |
| 20 min CB | 0.080 | 19.5 | 0.990 | 0.073 | 0.000 | 0.428 | 18.2 | 0.989 | 25.8 | 0.70 | 25.8 | 0.993 |
| 20 min SSB | 0.088 | 20.3 | 0.990 | 0.082 | 0.000 | 0.443 | 18.7 | 0.989 | 28.9 | 0.51 | 28.9 | 0.991 |
| Methane production | ||||||||||||
| Raw algae | 0.076 | 19.1 | 0.976 | 0.071 | 0.000 | 0.465 | 18.6 | 0.974 | 19.1 | 1.79 | 10.2 | 0.993 |
| 2 min CB | 0.069 | 24.9 | 0.974 | 0.062 | 0.000 | 0.526 | 23.4 | 0.971 | 20.3 | 2.49 | 6.7 | 0.998 |
| 2 min SSB | 0.078 | 17.0 | 0.980 | 0.077 | 0.000 | 0.488 | 16.9 | 0.980 | 19.1 | 0.97 | 15.9 | 0.984 |
| 20 min CB | 0.072 | 20.3 | 0.978 | 0.066 | 0.000 | 0.484 | 19.4 | 0.975 | 18.9 | 1.98 | 8.9 | 0.995 |
| 20 min SSB | 0.079 | 19.8 | 0.978 | 0.076 | 0.000 | 0.502 | 19.5 | 0.977 | 21.1 | 1.60 | 11.0 | 0.993 |
| Sample | 1st-Order Equation Model | Pseudo-1st-Order Equation Model | Modified Gompertz Model | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| k, d−1 | RMSE | R2 | k1, d−1 | k2, d−1 | Pbiodeg | RMSE | R2 | k, mL gVS−1 d−1 | λ, d | RMSE | R2 | |
| Biogas production | ||||||||||||
| Raw algae | 0.104 | 18.3 | 0.990 | 0.099 | 0.000 | 0.403 | 15.9 | 0.989 | 29.6 | 0.09 | 20.8 | 0.985 |
| 0 min at 100 °C | 0.096 | 18.0 | 0.994 | 0.099 | 0.001 | 0.454 | 14.1 | 0.994 | 32.6 | 0.00 | 29.4 | 0.978 |
| 0 min at 121 °C | 0.114 | 25.6 | 0.988 | 0.107 | 0.000 | 0.547 | 24.1 | 0.987 | 46.5 | 0.51 | 21.3 | 0.992 |
| 10 min at 121 °C | 0.108 | 27.2 | 0.988 | 0.101 | 0.000 | 0.565 | 25.0 | 0.987 | 44.9 | 0.43 | 25.5 | 0.989 |
| 30 min at 121 °C | 0.106 | 27.2 | 0.989 | 0.099 | 0.000 | 0.581 | 25.2 | 0.988 | 45.1 | 0.42 | 25.6 | 0.989 |
| 60 min at 121 °C | 0.104 | 30.9 | 0.987 | 0.096 | 0.000 | 0.620 | 29.1 | 0.986 | 48.6 | 0.66 | 23.8 | 0.992 |
| Methane production | ||||||||||||
| Raw algae | 0.076 | 19.1 | 0.976 | 0.071 | 0.000 | 0.465 | 18.6 | 0.974 | 19.1 | 1.8 | 10.2 | 0.993 |
| 0 min at 100 °C | 0.088 | 13.6 | 0.989 | 0.091 | 0.000 | 0.564 | 13.5 | 0.989 | 22.6 | 0.0 | 19.9 | 0.979 |
| 0 min at 121 °C | 0.106 | 21.7 | 0.980 | 0.106 | 0.000 | 0.623 | 21.7 | 0.979 | 33.9 | 1.0 | 14.4 | 0.992 |
| 10 min at 121 °C | 0.099 | 21.7 | 0.981 | 0.098 | 0.000 | 0.651 | 21.6 | 0.981 | 32.4 | 0.9 | 17.1 | 0.990 |
| 30 min at 121 °C | 0.098 | 22.8 | 0.981 | 0.096 | 0.000 | 0.681 | 22.7 | 0.981 | 33.5 | 0.9 | 17.5 | 0.990 |
| 60 min at 121 °C | 0.096 | 26.2 | 0.979 | 0.092 | 0.000 | 0.712 | 25.8 | 0.978 | 35.3 | 1.3 | 14.7 | 0.994 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohutskyi, P.; Phan, D.; Spierling, R.E.; Lundquist, T.J. Hydrothermal but Not Mechanical Pretreatment of Wastewater Algae Enhanced Anaerobic Digestion Energy Balance due to Improved Biomass Disintegration and Methane Production Kinetics. Energies 2023, 16, 7146. https://doi.org/10.3390/en16207146
Bohutskyi P, Phan D, Spierling RE, Lundquist TJ. Hydrothermal but Not Mechanical Pretreatment of Wastewater Algae Enhanced Anaerobic Digestion Energy Balance due to Improved Biomass Disintegration and Methane Production Kinetics. Energies. 2023; 16(20):7146. https://doi.org/10.3390/en16207146
Chicago/Turabian StyleBohutskyi, Pavlo, Duc Phan, Ruth E. Spierling, and Trygve J. Lundquist. 2023. "Hydrothermal but Not Mechanical Pretreatment of Wastewater Algae Enhanced Anaerobic Digestion Energy Balance due to Improved Biomass Disintegration and Methane Production Kinetics" Energies 16, no. 20: 7146. https://doi.org/10.3390/en16207146
APA StyleBohutskyi, P., Phan, D., Spierling, R. E., & Lundquist, T. J. (2023). Hydrothermal but Not Mechanical Pretreatment of Wastewater Algae Enhanced Anaerobic Digestion Energy Balance due to Improved Biomass Disintegration and Methane Production Kinetics. Energies, 16(20), 7146. https://doi.org/10.3390/en16207146






