HKUST-1 as a Positive Electrode Material for Supercapattery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the HKUST-1
2.3. Synthesis of N-Doped Graphene
2.4. Characterization
2.5. Electrochemical Analyses
2.6. Supercapattery Assembly and Electrochemical Measurement
3. Results and Discussion
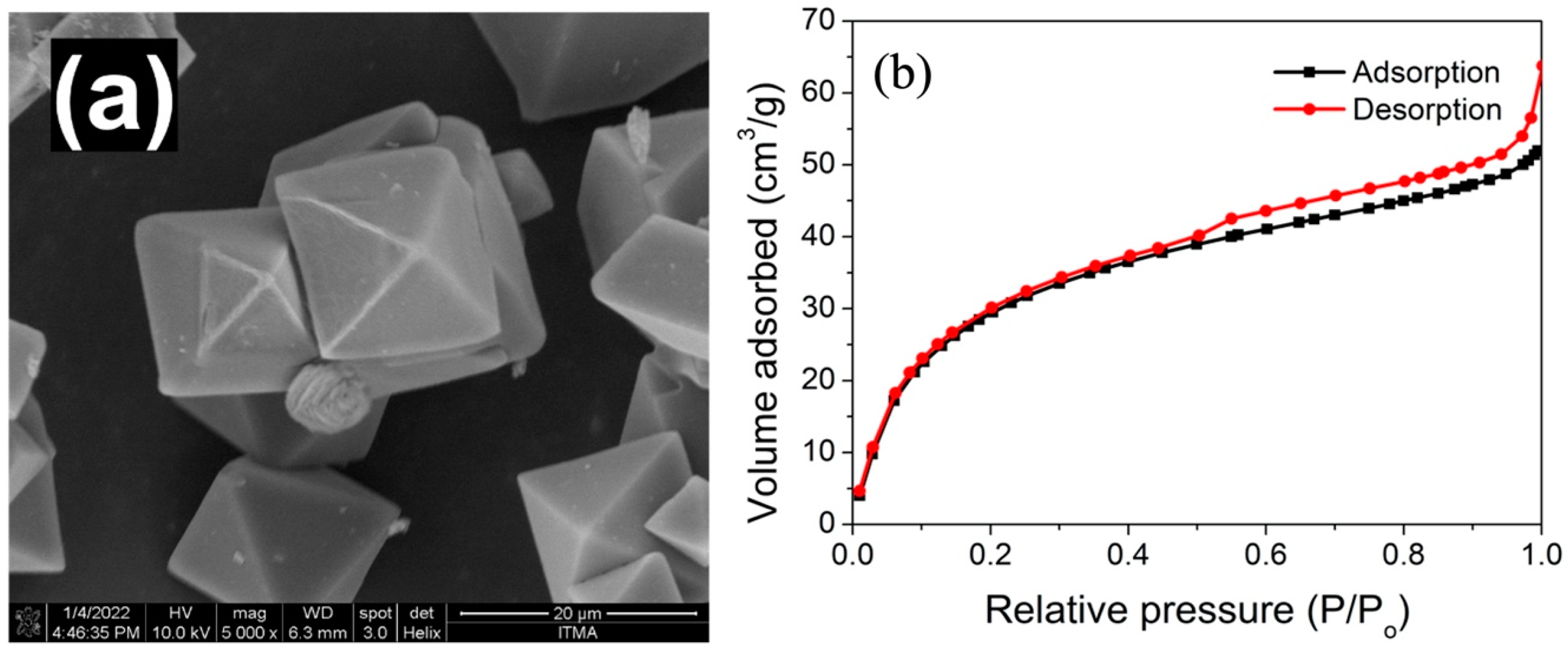
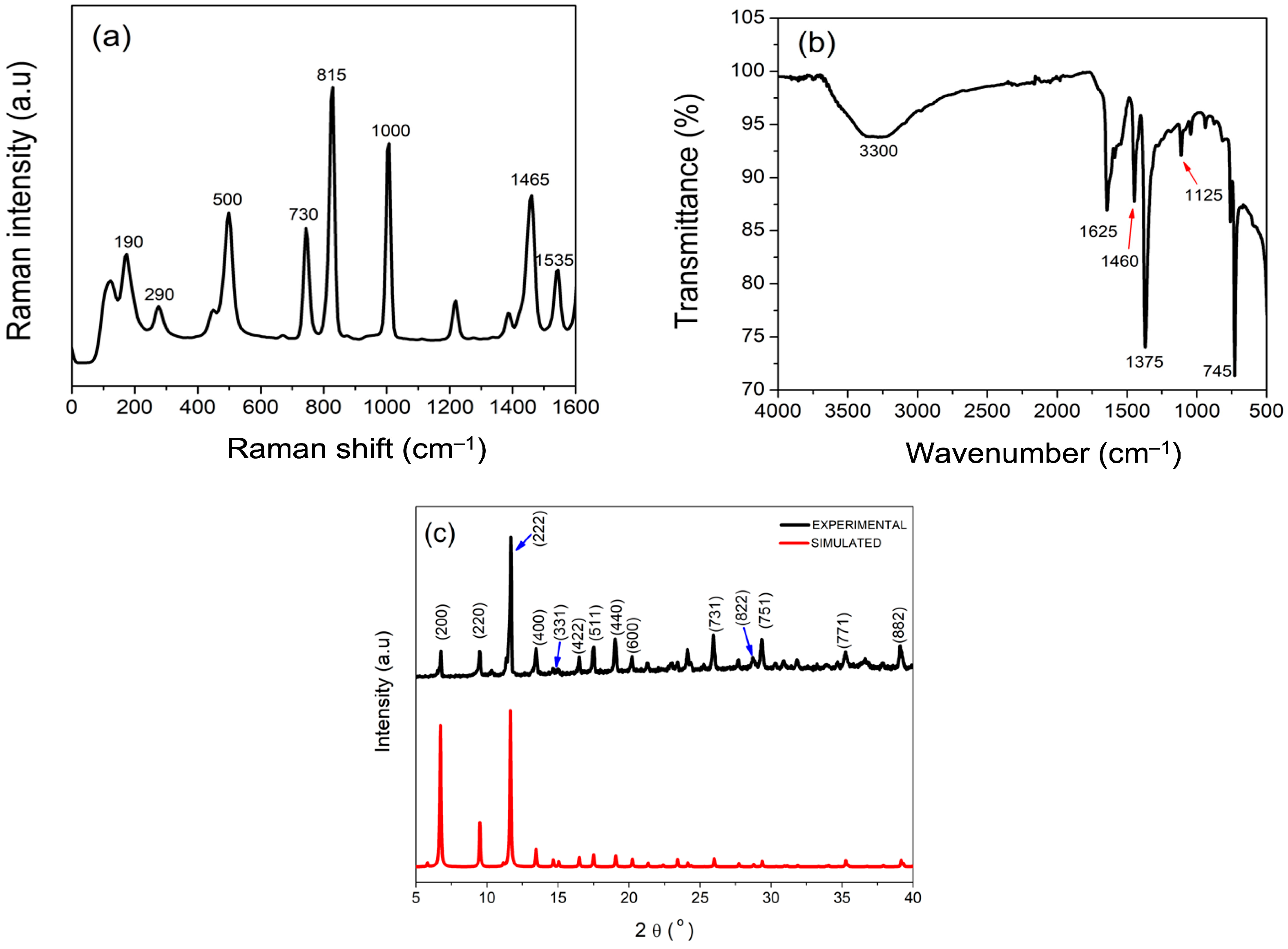
3.1. Physicochemical Characterizations
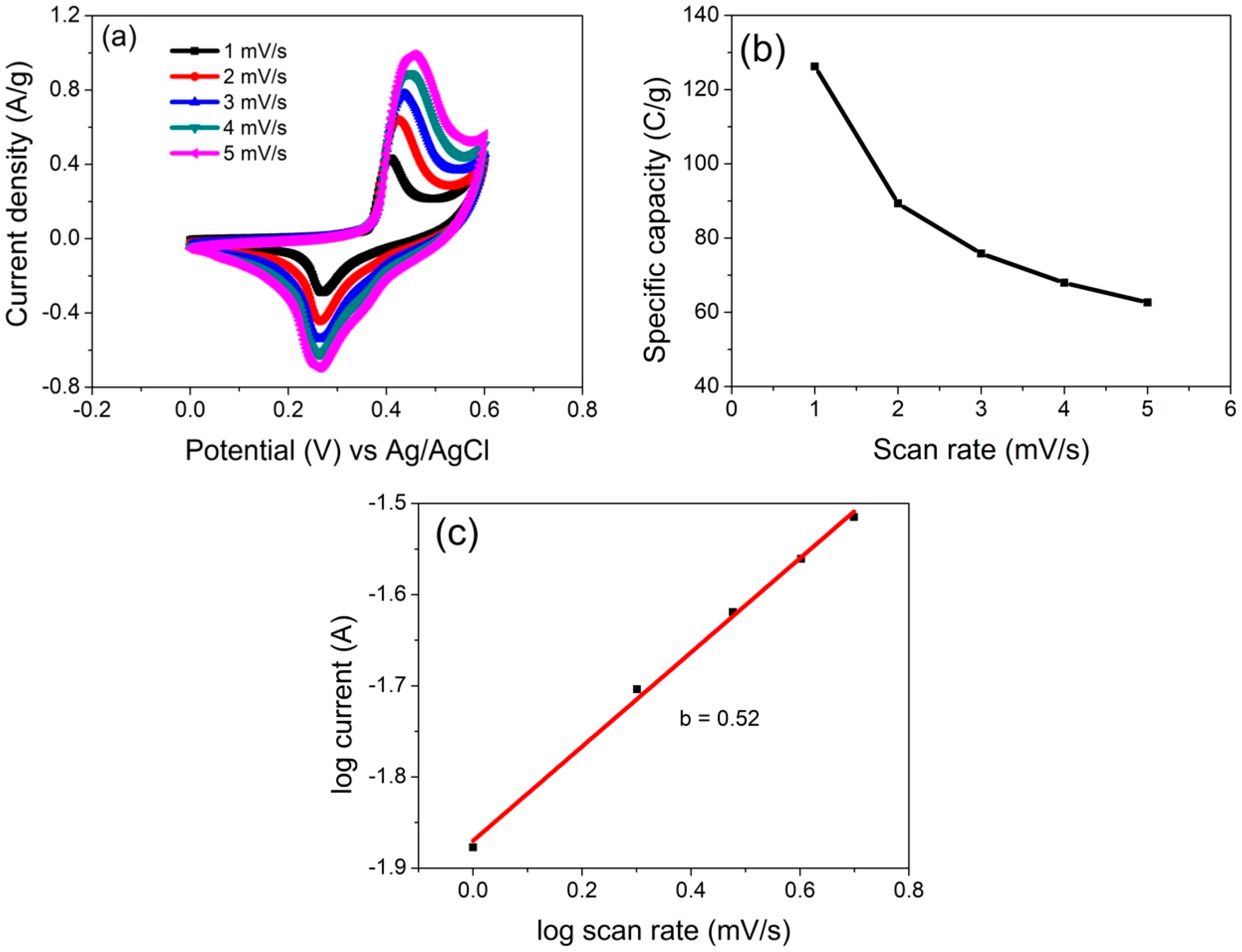
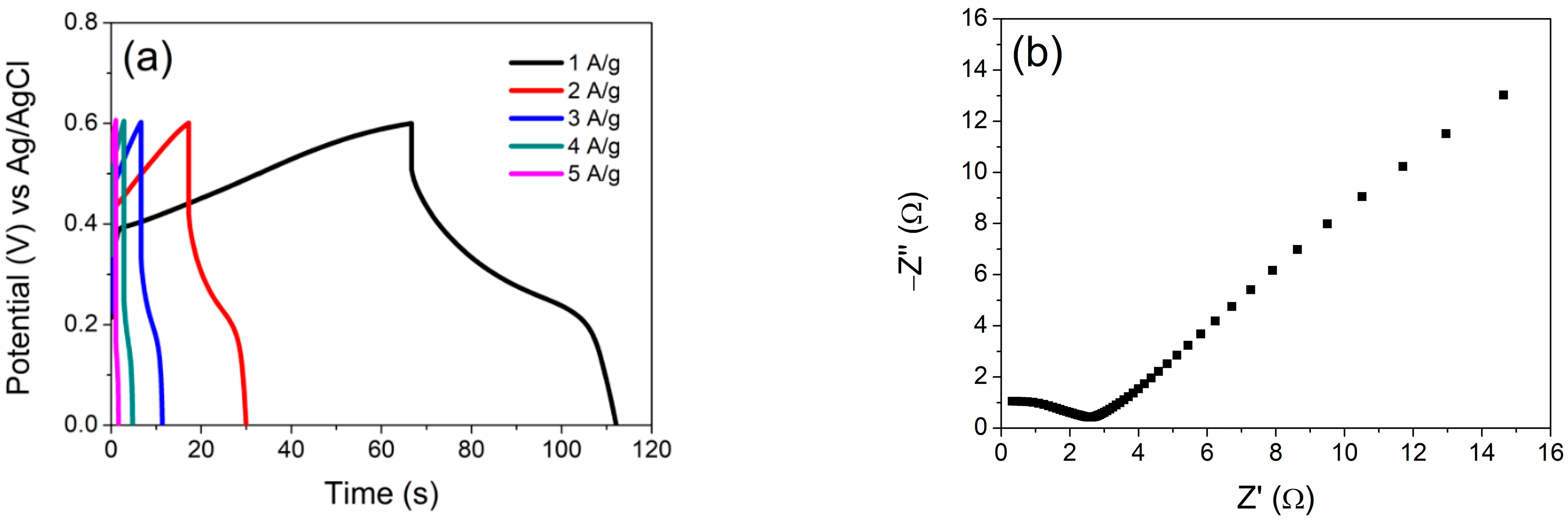
3.2. Electrochemical Characterization
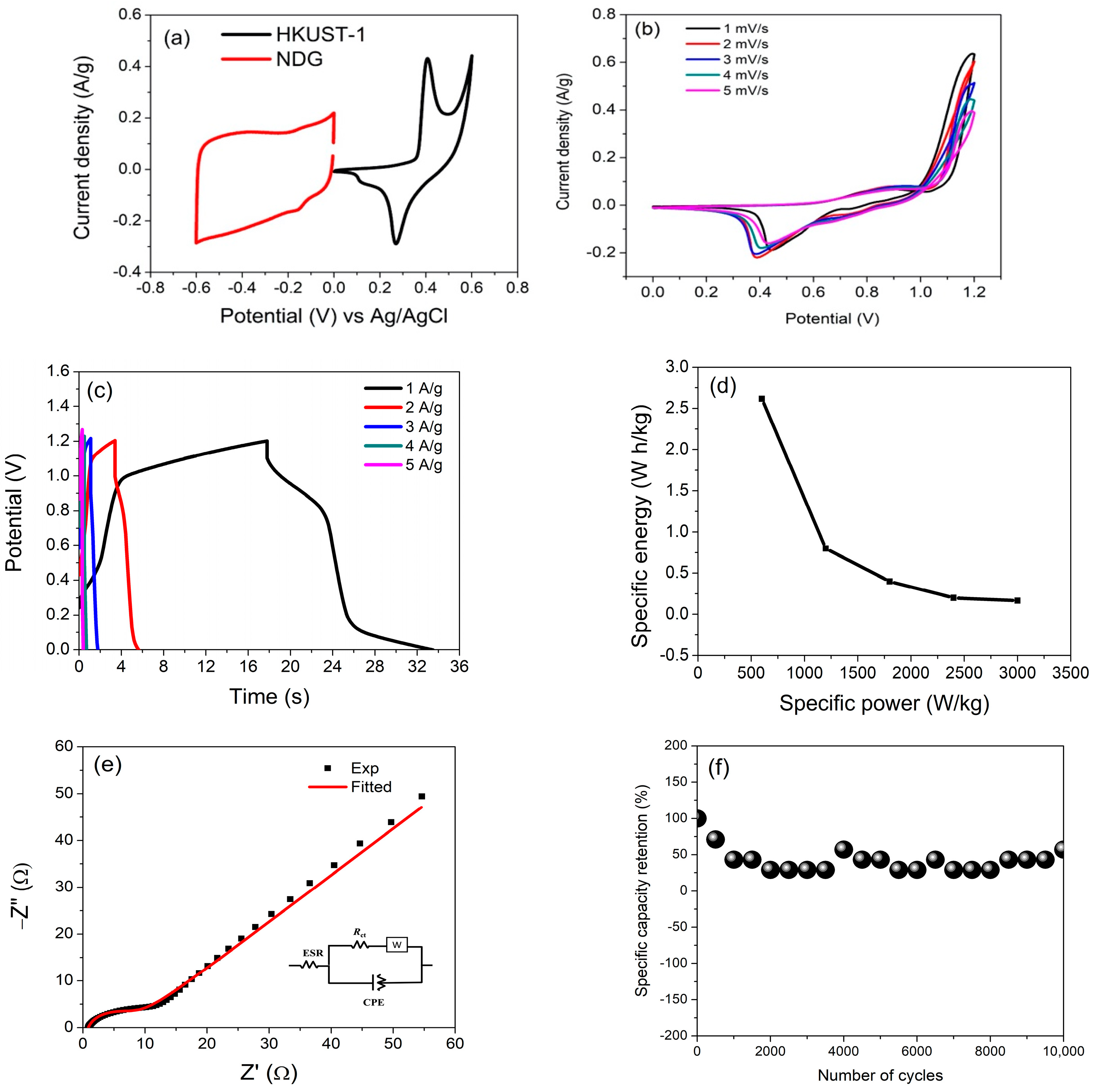
3.3. Electrochemical Performance of Assembled Supercapattery
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Faisal, M.M.; Ali, S.R.; Sanal, K.C.; Iqbal, M.W.; Iqbal, M.Z.; Saeed, A. Highly porous terpolymer-MOF composite electrode material for high performance supercapattery devices. J. Electroanal. Chem. 2021, 893, 115321. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Ali, S.R.; Alzaid, M. A facile approach to investigate the charge storage mechanism of MOF/PANI based supercapattery devices. Solid State Ion. 2020, 354, 115411. [Google Scholar] [CrossRef]
- Raihana, M.K.; Padmanathan, N.; Eswaramoorthi, V.; McNulty, D.; Sahadevan, J.; Mohanapriya, P.; Muthu, S.E. Reduced graphene oxide/VSB-5 composite micro/nanorod electrode for high energy density supercapattery. Electrochim. Acta 2021, 391, 138903. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Omar, F.S.; Ramesh, K.; Ramesh, S.; Shajan, X.S. Three-dimensional hierarchical nanostructured porous TiO2 aerogel/Cobalt based metal-organic framework (MOF) composite as an electrode material for supercapattery. J. Energy Storage 2020, 32, 101750. [Google Scholar] [CrossRef]
- Raman, V.; Gándara, F.; Azman, N.H.N.; Mohamed Tahir, M.I.; Abdul Rahman, M.B.; Sulaiman, Y. Facile synthesis and characterisations of novel Mn-1,2,4-triazole framework (UPMOF-4) as a promising electrode material for supercapattery device. J. Energy Storage 2023, 62, 106867. [Google Scholar] [CrossRef]
- Raman, V.; Gándara, F.; Mohamed Tahir, M.I.; Abdul Rahman, M.B.; Sulaiman, Y. 1,2,4-Triazole (Htrz) functionalised 2D-manganese-organic framework (UPMOF-5) as a battery-type electrode for supercapattery. J. Electroanal. Chem. 2023, 929, 117122. [Google Scholar] [CrossRef]
- Iqbal, J.; Numan, A.; Ansari, M.O.; Jagadish, P.R.; Jafer, R.; Bashir, S.; Mohamad, S.; Ramesh, K.; Ramesh, S. Facile synthesis of ternary nanocomposite of polypyrrole incorporated with cobalt oxide and silver nanoparticles for high performance supercapattery. Electrochim. Acta 2020, 348, 136313. [Google Scholar] [CrossRef]
- Iqbal, J.; Numan, A.; Jafer, R.; Bashir, S.; Jilani, A.; Mohammad, S.; Khalid, M.; Ramesh, K.; Ramesh, S. Ternary nanocomposite of cobalt oxide nanograins and silver nanoparticles grown on reduced graphene oxide conducting platform for high-performance supercapattery electrode material. J. Alloys Compd. 2020, 821, 153452. [Google Scholar] [CrossRef]
- Ediati, R.; Laharto, P.B.F.; Safitri, R.; Mahfudhah, H.; Oktavia Sulistiono, D.; Denisa Syukrie, T.; Nadjib, M. Synthesis of HKUST-1 with addition of Al-MCM-41 as adsorbent for removal of methylene blue from aqueous solution. Mater. Today Proc. 2021, 46, 1799–1806. [Google Scholar] [CrossRef]
- Amrollahi Bioki, H.; Moshaii, A.; Borhani Zarandi, M. Performance improvement of ambient-condition fabricated perovskite solar cells using an interfacial HKUST-1 MOF on electron transfer layer. Surf. Interfaces 2021, 27, 101579. [Google Scholar] [CrossRef]
- Ai, Y.; Gao, N.; Wang, Q.; Gao, F.; Hibbert, D.B.; Zhao, C. Electrosynthesis of HKUST-1 on a carbon-nanotube-modified electrode and its application for detection of dihydroxybenzene isomers. J. Electroanal. Chem. 2020, 872, 114161. [Google Scholar] [CrossRef]
- Zhao, H.; Lei, M.; Liu, T.; Huang, T.; Zhang, M. Synthesis of composite material HKUST-1/LiCl with high water uptake for water extraction from atmospheric air. Inorg. Chim. Acta 2020, 511, 119842. [Google Scholar] [CrossRef]
- Dong, J.; Li, P.; Guan, H.; Ge, C.; Bai, Y.; Zhao, Y.; Zhang, X. The synthesis of HKUST-1/SiO2 composite material based on 3D printing. Inorg. Chem. Commun. 2020, 117, 107975. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Khan, J.; Gul, A.; Siddique, S.; Alzaid, M.; Saleem, M.; Iqbal, M.J. Copper doped cobalt-manganese phosphate ternary composites for high-performance supercapattery devices. J. Energy Storage 2021, 35, 102307. [Google Scholar] [CrossRef]
- Kim, B.C.; Manikandan, R.; Yu, K.H.; Park, M.-S.; Kim, D.-W.; Park, S.Y.; Justin Raj, C. Efficient supercapattery behavior of mesoporous hydrous and anhydrous cobalt molybdate nanostructures. J. Alloys Compd. 2019, 789, 256–265. [Google Scholar] [CrossRef]
- Shahabuddin, S.; Numan, A.; Shahid, M.M.; Khanam, R.; Saidur, R.; Pandey, A.K.; Ramesh, S. Polyaniline-SrTiO3 nanocube based binary nanocomposite as highly stable electrode material for high performance supercapaterry. Ceram. Int. 2019, 45, 11428–11437. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Alam, S.; Afzal, A.M.; Iqbal, M.J.; Yaqoob, K.; Kamran, M.A.; Karim, M.R.A.; Alherbi, T. Binary composites of strontium oxide/polyaniline for high performance supercapattery devices. Solid State Ion. 2020, 347, 115276. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Faisal, M.M.; Sulman, M.; Ali, S.R.; Alzaid, M. Facile synthesis of strontium oxide/polyaniline/graphene composite for the high-performance supercapattery devices. J. Electroanal. Chem. 2020, 879, 114812. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Khan, J. Optimization of cobalt-manganese binary sulfide for high performance supercapattery devices. Electrochim. Acta 2021, 368, 137529. [Google Scholar] [CrossRef]
- Shinde, N.M.; Xia, Q.X.; Yun, J.M.; Shinde, P.V.; Shaikh, S.M.; Sahoo, R.K.; Mathur, S.; Mane, R.S.; Kim, K.H. Ultra-rapid chemical synthesis of mesoporous Bi2O3 micro-sponge-balls for supercapattery applications. Electrochim. Acta 2019, 296, 308–316. [Google Scholar] [CrossRef]
- Haider, S.S.; Iqbal, M.Z.; Zakar, S.; Afzal, A.M.; Yaqoob, K.; Aftab, S. Superior performance of electrodeposited CoMnS as novel electrode material for supercapattery devices. J. Energy Storage 2021, 39, 102608. [Google Scholar] [CrossRef]
- Oyedotun, K.O.; Madito, M.J.; Momodu, D.Y.; Mirghni, A.A.; Masikhwa, T.M.; Manyala, N. Synthesis of ternary NiCo-MnO2 nanocomposite and its application as a novel high energy supercapattery device. Chem. Eng. J. 2018, 335, 416–433. [Google Scholar] [CrossRef]
- Ramulu, B.; Chandra Sekhar, S.; Nagaraju, G.; Yu, J.S. Rational design and construction of nickel molybdate nanohybrid composite for high-performance supercapattery. Appl. Surf. Sci. 2020, 515, 146023. [Google Scholar] [CrossRef]
- Gurusamy, L.; Karuppasamy, L.; Anandan, S.; Liu, N.; Lee, G.-J.; Liu, C.-H.; Wu, J.J. Enhanced performance of charge storage supercapattery by dominant oxygen deficiency in crystal defects of 2-D MoO3−x nanoplates. Appl. Surf. Sci. 2021, 541, 148676. [Google Scholar] [CrossRef]
- Alam, S.; Iqbal, M.Z. Nickel-manganese phosphate: An efficient battery-grade electrode for supercapattery devices. Ceram. Int. 2021, 47, 11220–11230. [Google Scholar] [CrossRef]
- Tarimo, D.J.; Oyedotun, K.O.; Mirghni, A.A.; Mutuma, B.; Sylla, N.F.; Murovhi, P.; Manyala, N. Enhanced electrochemical performance of supercapattery derived from sulphur-reduced graphene oxide/cobalt oxide composite and activated carbon from peanut shells. Int. J. Hydrogen Energy 2020, 45, 33059–33075. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azman, N.H.N.; Alias, M.M.; Sulaiman, Y. HKUST-1 as a Positive Electrode Material for Supercapattery. Energies 2023, 16, 7072. https://doi.org/10.3390/en16207072
Azman NHN, Alias MM, Sulaiman Y. HKUST-1 as a Positive Electrode Material for Supercapattery. Energies. 2023; 16(20):7072. https://doi.org/10.3390/en16207072
Chicago/Turabian StyleAzman, Nur Hawa Nabilah, Muhammad Mustaqhim Alias, and Yusran Sulaiman. 2023. "HKUST-1 as a Positive Electrode Material for Supercapattery" Energies 16, no. 20: 7072. https://doi.org/10.3390/en16207072
APA StyleAzman, N. H. N., Alias, M. M., & Sulaiman, Y. (2023). HKUST-1 as a Positive Electrode Material for Supercapattery. Energies, 16(20), 7072. https://doi.org/10.3390/en16207072




