Numerical Simulation on Effect of Separator Thickness on Coupling Phenomena in Single Cell of PEFC under Higher Temperature Operation Condition at 363 K and 373 K
Abstract
1. Introduction
2. Numerical Simulation
Governing Equations
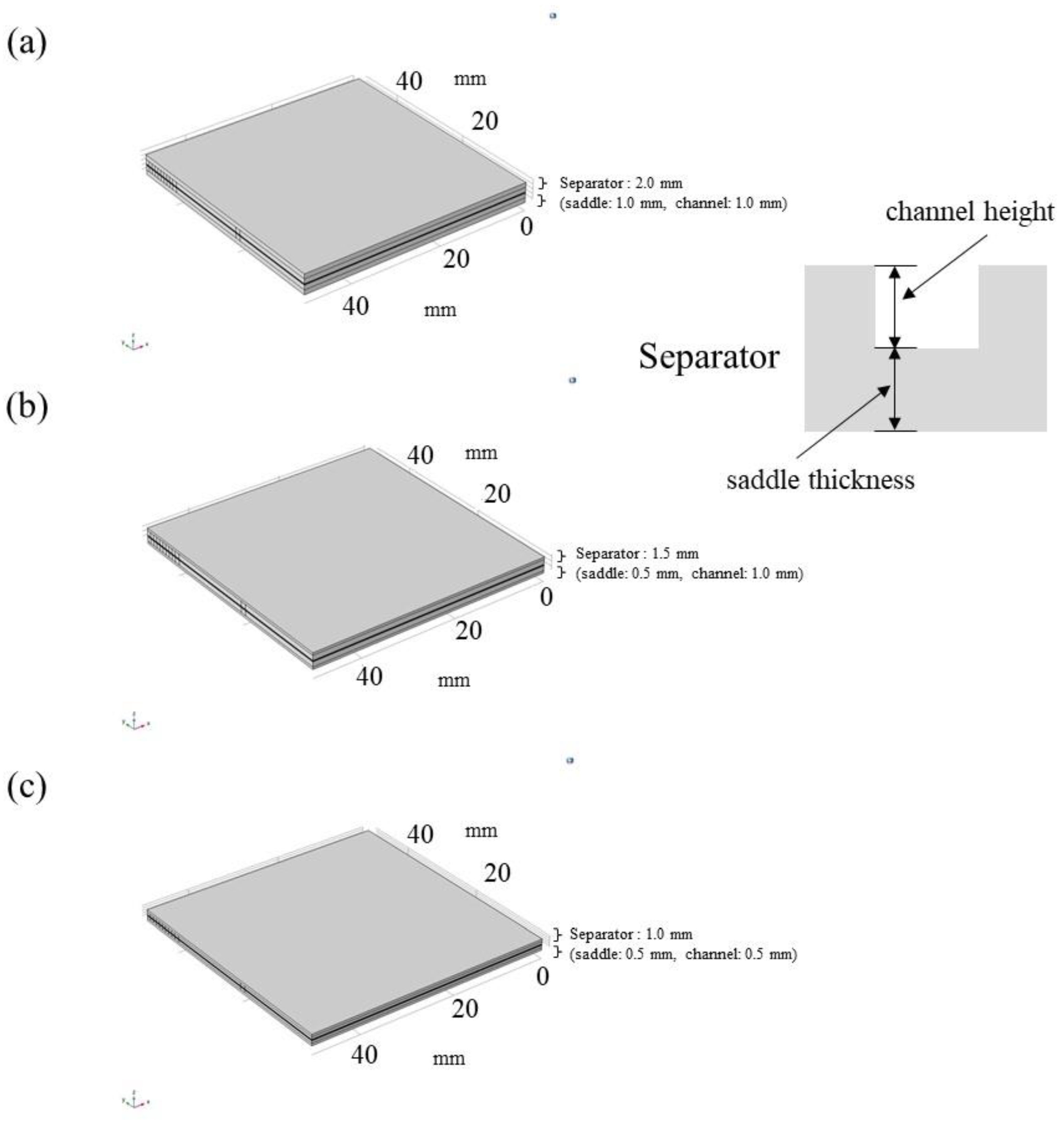
| Cell Components | Dimension | Information |
|---|---|---|
| PEM | Width: 50.0 mm, Length: 50.0 mm, Depth: 0.025 mm | Nafion NRE-211 (manufactured by Du Pont Corp.) |
| Catalyst layer | Width: 50.0 mm, Length: 50.0 mm, Depth: 0.01 mm | Pt/C (Pt: 20 wt%) |
| MPL | Width: 50.0 mm, Length: 50.0 mm, Depth: 0.003 mm | PTFE + carbon black |
| GDL | Width: 50.0 mm, Length: 50.0 mm, Depth: 0.11 mm | TGP-H-030 (manufactured by Toray Corp.) |
| Separator | Width: 75.4 mm, Length: 75.4 mm, Depth: 2.0 mm (sd.t.: 1.0 mm, c.h.: 1.0 mm), 1.5 mm (sd.t.: 0.5 mm, c.h.: 1.0 mm), 1.0 mm (sd.t.: 0.5 mm, c.h.: 0.5 mm); Width: 50.0 mm, Length: 50.0 mm (gas supply area) | Carbon graphite, serpentine flow |
| Physical Parameters | Values |
|---|---|
| Density of H2 [kg/m3] | 7.10×10−2 (at 353 K), 6.89×10−2 (at 363 K), 6.69×10−2 (at 373 K) [29] |
| Density of O2 [kg/m3] | 1.11 (at 353 K), 1.08 (at 363 K), 1.05 (at 373 K) [29] |
| Density of H2O [kg/m3] | 2.95×10−1 (at 353 K), 4.26×10−1 (at 363 K), 6.01×10−1 (at 373 K) [29] |
| Pressure of supply gas at inlet (absolute) (MPa) | 0.4 [18] |
| Viscosity of H2 [Pa·s] | 9.96×10−6 (at 353 K), 1.02×10−5 (at 363 K), 1.03×10−5 (at 373 K) [29] |
| Viscosity of O2 [Pa·s] | 2.35×10−5 (at 353 K), 2.40×10−5 (at 363 K), 2.45×10−5 (at 373 K) [29] |
| Viscosity of H2O [Pa·s] | 1.16×10−5 (at 353 K), 1.19×10−5 (at 363 K), 1.23×10−5 (at 373 K) [29] |
| Binary diffusion constant between H2 and H2O [m2/s] | 9.27×10−5 [30] |
| Binary diffusion constant between O2 and H2O [m2/s] | 3.57×10−5 [30] |
| Porosity of catalyst layer [-] | 0.78 [13,31,32,33,34] |
| Permeability of catalyst layer [m2] | 8.69×10−12 [13,31,32,33,34] |
| Porosity of MPL [-] | 0.60 [13,31,32,33,34] |
| Permeability of MPL [m2] | 1.00×10−13 [13,31,32,33,34] |
| Porosity of GDL [-] | 0.78 [13,31,32,33,34] |
| Permeability of GDL [m2] | 8.69×10−12 [13,31,32,33,34] |
| Porosity of separator [-] | 0.15 [35] |
| Permeability of separator [m2] | 1.50×10−5 [35] |
| Conductivity of PEM [S/m] | 10 [36] |
| Conductivity of catalyst layer [S/m] | 53 [37] |
| Conductivity of MPL [S/m] | 1000 [38] |
| Conductivity of GDL [S/m] | 1250 [39] |
| Conductivity of separator [S/m] | 83,000 [35] |
| Anode reference equilibrium voltage [V] | 0 |
| Cathode reference equilibrium voltage [V] | 1.229 |
| Anode reference exchange current density [A/m2] | 1000 [40] |
| Cathode reference exchange current density [A/m2] | 1 [40] |
| Anode charge transfer constant [-] | 0.5 [41] |
| Cathode charge transfer constant [-] | 0.5 [42] |
| Operation Conditions | Values | |
|---|---|---|
| The initial temperature of cell (Tini) (K) | 353, 363, 373 | |
| Cell voltage (V) | Experimental data are used [18,27,28] | |
| Supply gas condition | Anode | Cathode |
| Gas type | H2 | O2 |
| Temperature of supply gas at inlet (K) | 353, 363, 373 | 353, 363, 373 |
| RH of supply gas (%RH) | 40, 80 | 40, 80 |
| Pressure of supply gas at inlet (absolute) (MPa) | 0.4 | 0.4 |
| Flow rate of supply gas at inlet (NL/min) (Stoichiometric ratio (-)) | 0.210 (1.5) | 0.105 (1.5) |
3. Results and Discussion
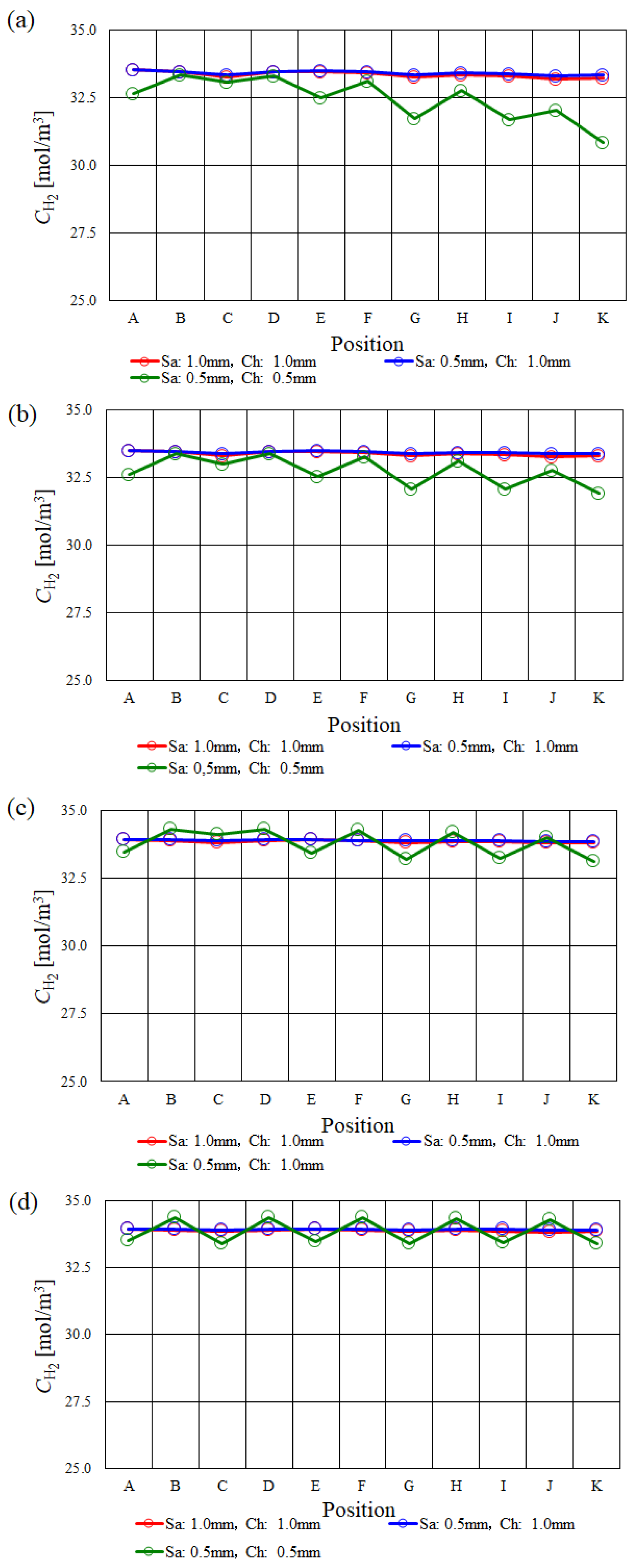
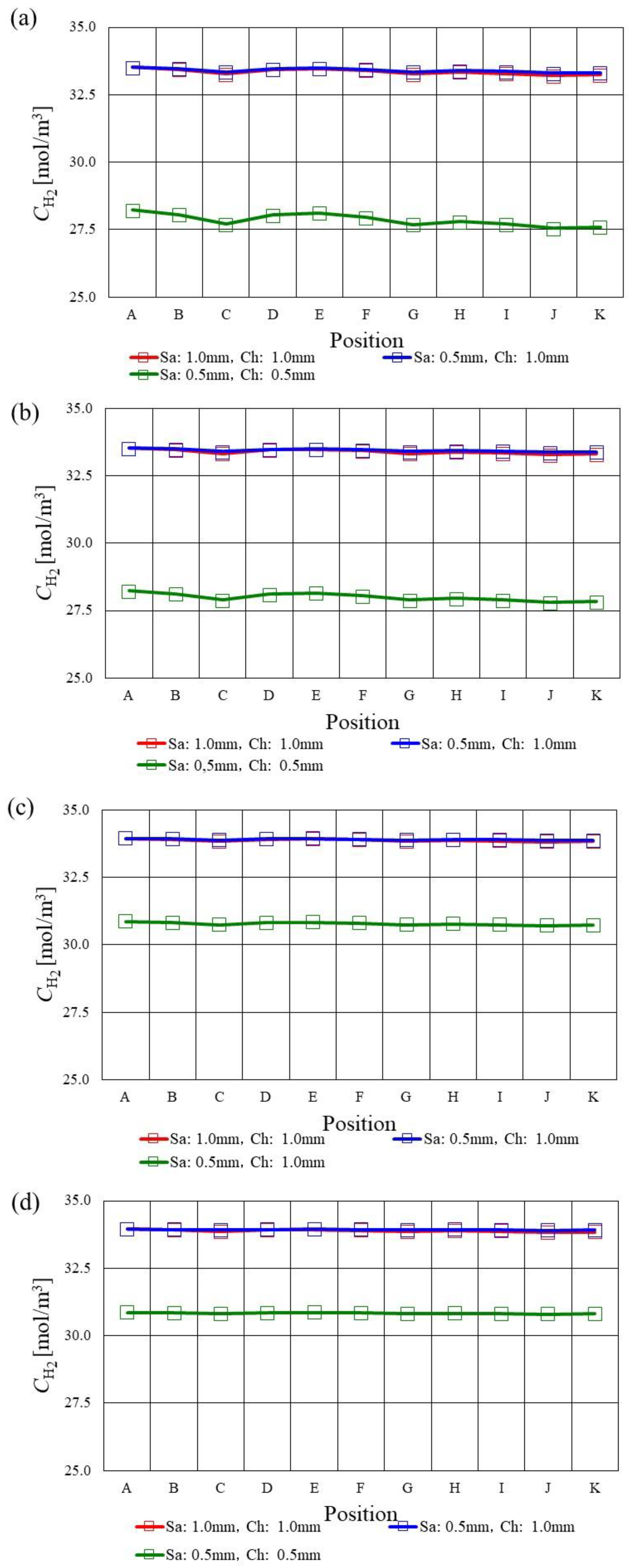
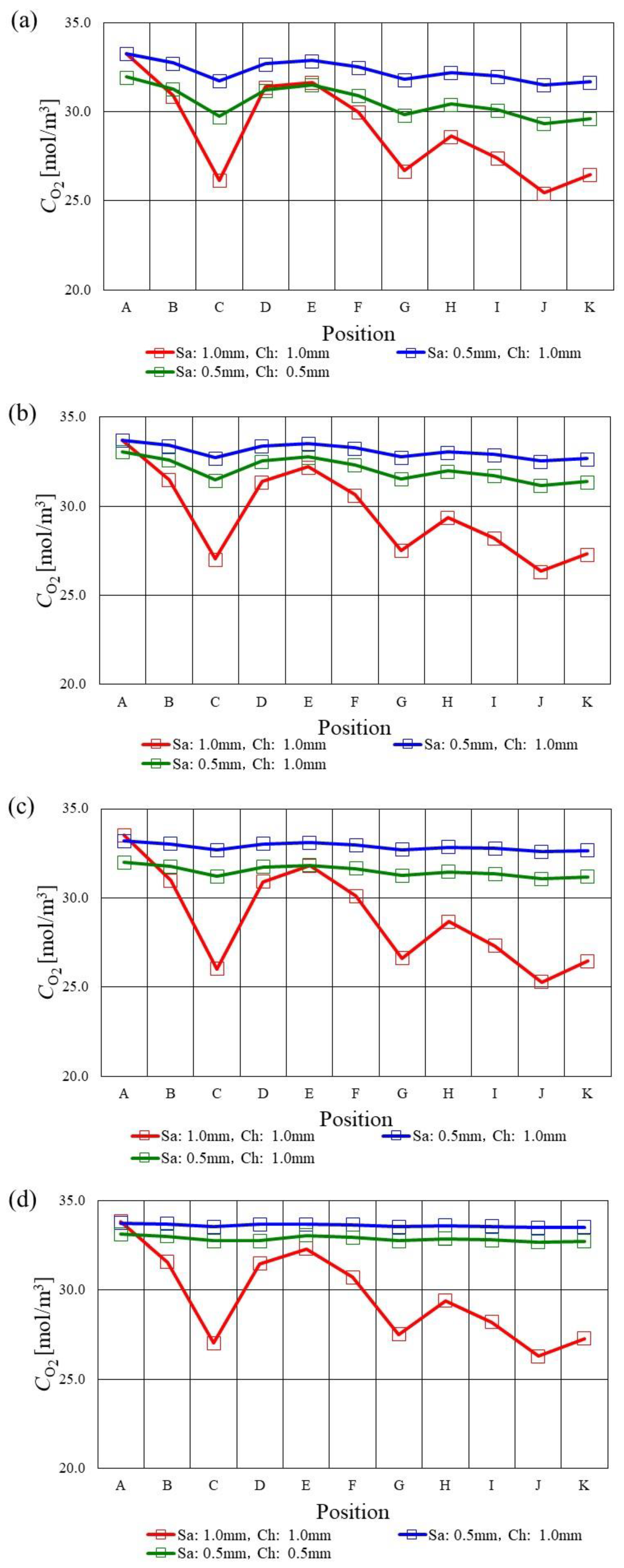
3.1. In-Plane Distribution of Molar Concentration of H2 on the Interface between PEM and Catalyst Layer at the Anode among Various s.t.
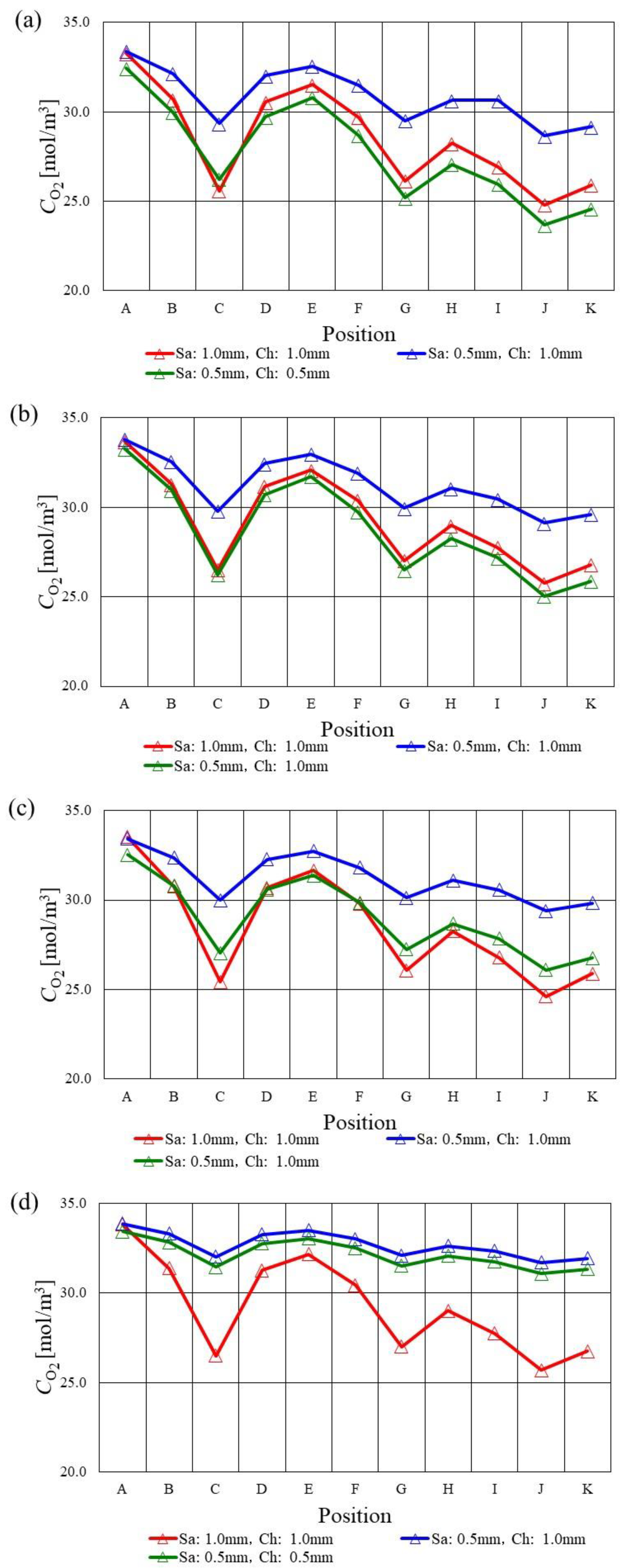
3.2. In-Plane Distribution of Molar Concentration of O2 on the Interface between PEM and Catalyst Layer at the Cathode among Various s.t.
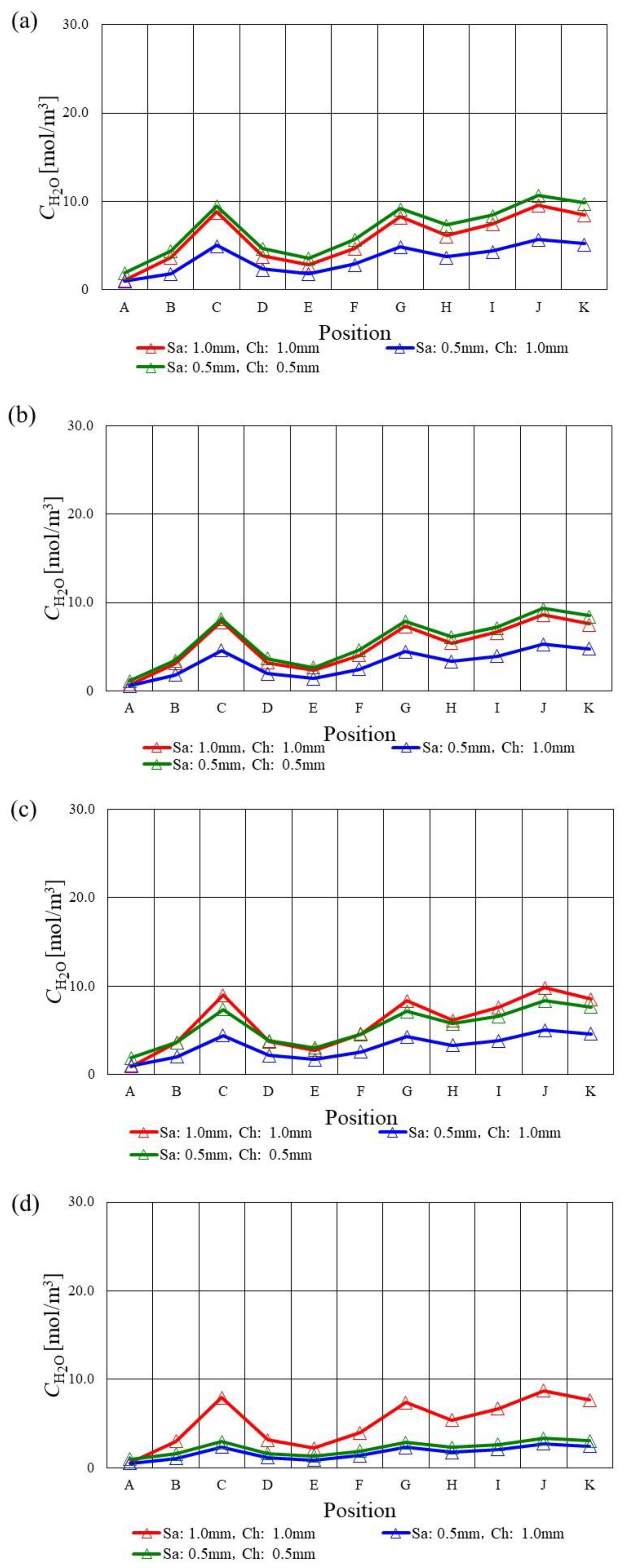
3.3. In-Plane Distribution of Molar Concentration of H2O on the Interface between PEM and Catalyst Layer at the Cathode among Various s.t.
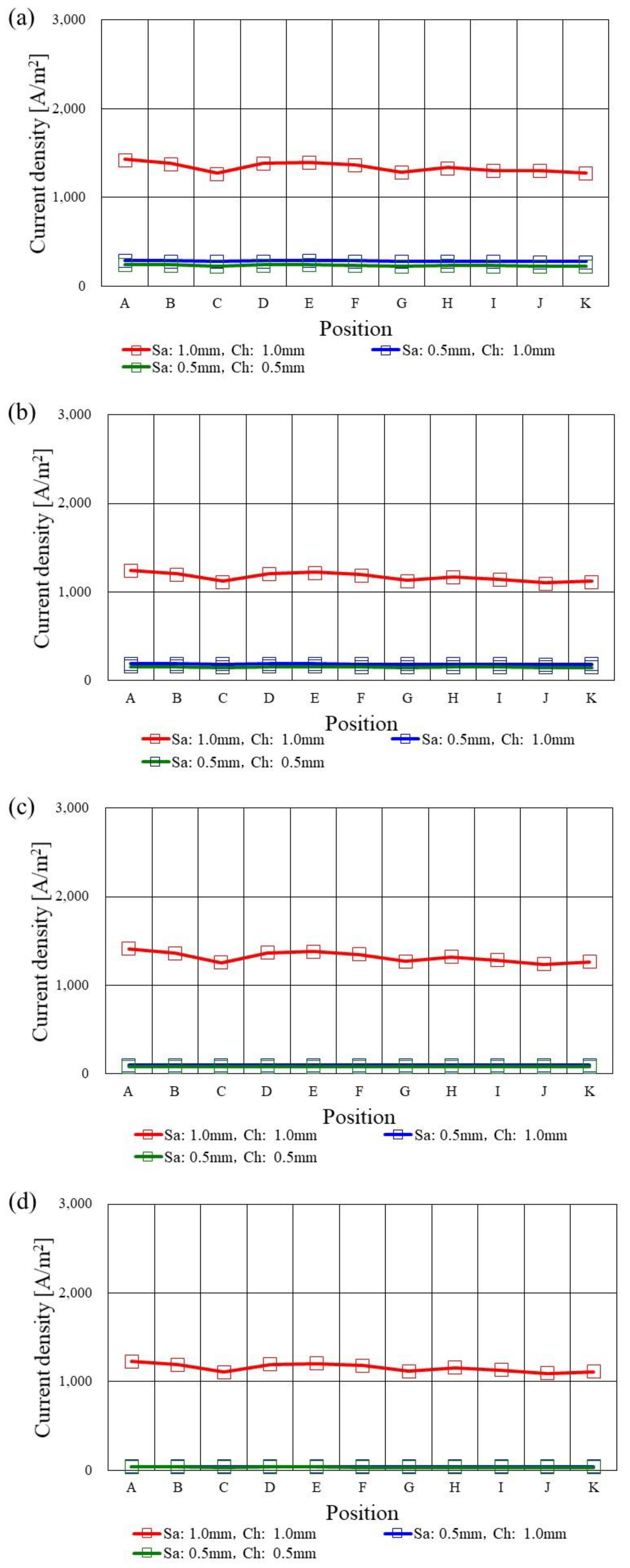
3.4. In-Plane Distribution of Current Density on the Interface between PEM and Catalyst Layer at the Cathode among Various s.t.
3.5. Comparison with the Other Studies and Future Work
4. Conclusions
- (i)
- The molar concentration of H2 keeps approximately the same value along with the gas flow except for in the case of using s.t. of 1.0 mm.
- (ii)
- Regarding s.t. of 1.0 mm, the molar concentration of H2 drops at the points of C, E, G, I and K since the velocity and concentration of H2 in the gas channel is high due to the smaller cross-sectional area of the gas channel. The molar concentration of H2 is smaller compared with the other s.t. cases at Tini = 363 K and 373 K.
- (iii)
- The molar concentration of O2 decreases along with the gas channel at Tini = 353 K and 363 K, while the molar concentration of O2 decreases at the analysis points of C and G especially due to accumulating H2O there.
- (iv)
- The molar concentration of O2 in the case of using s.t. of 2.0 mm is smaller compared with the thinner separator cases at Tini = 373 K, which can also be observed for A40%RH/C40%RH regardless of Tini. Since the catalyst layer is relatively humidified in the case of using s.t. of 2.0 mm because of the large heat capacity, the O2 reduction reaction in the catalyst layer at the cathode progressed well compared with the other s.t.
- (v)
- The molar concentration of H2O decreases at the points of C, G, J and K since H2O accumulates there.
- (vi)
- The molar concentration of H2O keeps a low value along with the gas channel at Tini = 373 K in the case of using s.t. of 1.5 mm and 1.0 mm due to the decrease in the performance of O2 reduction reaction.
- (vii)
- The current density decreases along with the gas channel since the concentration of H2 and O2, which is a driving force to diffuse toward the catalyst layer, decreases along with the gas channel.
- (viii)
- The current density in case of using s.t. of 2.0 mm is the highest among the various s.t. irrespective of Tini, which is the most remarkable for A40%RH/C40%RH. It can be thought that the dehydration of PEM and electrode would be smaller compared with the other s.t. cases.
- (ix)
- At Tini = 373 K, the current densities in case of using s.t. of 1.5 mm and 1.0 mm are very low since the dehydration of PEM and catalyst layer causes the reduction in the performance of H2 oxidization as well as O2 reduction, providing large ohmic and activation over-potential.
- (x)
- From the viewpoint of PEFC manufacturing, this study has revealed that the thickness of the separator is optimized based on the thermal properties of the separator. In addition, this study has revealed that the optimization procedure of thickness is different among PEM, GDL and separator.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- NEDO (New Energy and Industry Technology Development Organization). Available online: https://www.nedo.go.jp/content/100871973 (accessed on 7 December 2022). (In Japanese)
- Zhang, G.; Kandlikar, S.G.A. Critical Review of Cooling Technique in Proton Exchange Membrane Fuel Cell Stacks. Int. J. Hydrogen Energy 2012, 37, 2412–2429. [Google Scholar] [CrossRef]
- Agbossou, K.; Kolhe, M.; Hamelin, J.; Bose, T.K. Performance of a Stand-Alone Renewable Energy System Based on Energy Storage as Hydrogen. IEEE Trans. Energy Convers. 2004, 19, 633–640. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, C.; Hao, D.; Ni, M.; Huang, S.; Liu, D.; Zheng, Y. 3D Non-isothermal Dynamic Simulation of High Temperature Proton Exchange Membrane Fuel Cell in Start-up Process. Int. J. Hydrogen Energy 2021, 46, 2577–2593. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and Recent Development Polymer Electrolyte Membrane for Fuel Cells Operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef]
- Lee, C.Y.; Wng, F.; Kuo, Y.W.; Tsai, C.H.; Cheng, Y.T.; Cheng, C.K.; Lin, J.T. In-situ Measurement of High-temperature Proton Exchange Membrane Fuel Cell Stack Using Flexible Five-in-one Micro Sensor. Sensors 2016, 16, 1731. [Google Scholar] [CrossRef]
- Lee, C.Y.; Weng, F.B.; Kuo, Y.W.; Cheng, Y.T.; Cheng, C.K.; Tsai, C.H.; Lee, T.J. Persistent Effect Test for High Temperature Resistant Integrated Microsensor Embedded in High Temperature Proton Exchange Membrane Fuel Cell Stack. Sens. Actuators A Phys. 2016, A250, 202–209. [Google Scholar] [CrossRef]
- Ryu, S.K.; Vinothkannan, M.; Kim, A.R.; Yoo, D.J. Effect of Type and Stoichiometry of Fuels on Performance of Polybenzimidazole-based Proton Exchange Membrane Fuel Cells Operating at the Temperature Range of 120–160 °C. Energy 2022, 238, 121791. [Google Scholar] [CrossRef]
- Budak, Y.; Devrim, Y. Micro-cogeneration Application of a High-temperature PEM Fuel Cell Stack Operated with Polybenzimidazole Based Membranes. Int. J. Hydrogen Energy 2022, 45, 35198–35207. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Li, W.; Zhang, J.; Lu, D.; Yan, W.; Xiang, Y.; Lu, S. Effect of Catalyst Layer Microstructure on Performance and Stability for High Temperature Polymer Electrolyte Membrane Fuel Cells. J. Power Source 2021, 505, 230059. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, H.; Park, H.; Oh, S.; Ahn, S.H.; Kim, H.J.; Kim, S.K. Performance enhancement of high-temperature polymer electrolyte membrane fuel cells using Pt pulse electrodeposition. J. Power Source 2019, 438, 227022. [Google Scholar] [CrossRef]
- Kanchan, B.K.; Randive, P.; Pati, S. Implications of Non-uniform Porosity Distribution in Gas Diffusion Layer on the Performance of a High Temperature PEM Fuel Cell. Int. J. Hydrogen Energy 2021, 46, 18571–18588. [Google Scholar] [CrossRef]
- Xia, L.; Ni, M.; He, Q.; Xu, Q.; Cheng, C. Optimization of Gas Diffusion Layer in High Temperature PEMFC with the Focuses on Thickness and Porosity. Appl. Energy 2021, 300, 117357. [Google Scholar] [CrossRef]
- Agarwal, H.; Thosar, A.U.; Bhat, S.D.; Lele, A.K. Interdigitated Flow Field Impact on Mass Transport and Electrochemical Reaction in High-temperature Polymer Electrolyte Fuel Cell. J. Power Source 2022, 532, 231319. [Google Scholar] [CrossRef]
- Xia, L.; Xu, Q.; He, Q.; Ni, M.; Seng, M. Numerical Study of High Temperature Proton Exchange Membrane Fuel Cell (HT-PEMFC) with a Focus on Rib Design. Int. J. Hydrogen Energy 2021, 46, 21098–21111. [Google Scholar] [CrossRef]
- Huang, T.; Wang, W.; Yuan, Y.; Huang, J.; Chen, X.; Zhang, J.; Kong, X.; Zhang, Y.; Wan, Z. Optimization of High-temperature Proton Exchange Membrane Fuel Cell Flow Channel Based on Genetic Algorithm. Energy Rep. 2021, 7, 1374–1384. [Google Scholar] [CrossRef]
- Hoppe, E.; Janssen, H.; Muller, M.; Lehnert, W. The Impact of Flow Field Plate Misalignment on the Gas Diffusion Layer Intrusion and Performance of a High-temperature Polymer Electrolyte Fuel. J. Power Source 2021, 501, 230036. [Google Scholar] [CrossRef]
- Nishimura, A.; Kojima, Y.; Ito, S.; Hu, E. Impacts of Separator Thickness on Temperature Distribution and Power Generation Characteristics of a Single PEMFC Operated at Higher Temperature of 363 and 373 K. Energies 2022, 15, 1558. [Google Scholar] [CrossRef]
- Nishimura, A.; Kono, N.; Toyoda, K.; Mishima, D.; Kolhe, M.L. Impact of Separator Thickness on Temperature Distribution in Single Cell of Polymer Electrolyte Fuel Cell Operated at Higher Temperature of 90 °C and 100 °C. Energies 2022, 15, 4203. [Google Scholar] [CrossRef]
- Nanadegani, F.S.; Lay, E.N.; Sunden, B. Computational Analysis of the Impact of a Micro Porous Layer (MPL) on the Characteristics of a High Temperature PEMFC. Elecrochim. Acta 2020, 333, 135552. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, L.; Qin, Z.; Wu, J.; Xi, F.; Mou, G.; Wang, Y.; Jiao, K. A Comprehensive Three-dimensional Model Coupling Channel Multi-phase Flow and Electrochemical Reactions in Proton Exchange Membrane Fuel Cell. Adv. Appl. Energy 2021, 2, 100033. [Google Scholar] [CrossRef]
- Zhang, T.; Li, J.; Li, Q.; Yu, M.; Sun, H. Combination Effects of Flow Field Structure and Assembly Force on Performance of High Temperature Proton Exchange Membrane Fuel Cells. Int. J. Energy Res. 2021, 45, 7903–7917. [Google Scholar] [CrossRef]
- Das, S.K.; Gibson, H.A. These Dimensional Multi-Physics Modeling and Simulation for Assessment of Mass Transport Impact on the Performance of a High Temperature Polymer Electrolyte Membrane Fuel Cell. J. Power Source 2021, 499, 161–188. [Google Scholar] [CrossRef]
- Panesi, A.R.Q.; Silva, R.P.; Cunha, E.F.; Korkischko, I.; Santiago, E.I. Three-dimensional CFD Modeling of H2/O2 HT-PEMFC Based on H3PO4-doped PBI Membranes. Ionics 2021, 27, 3461–3475. [Google Scholar] [CrossRef]
- Penga, Z.; Tolj, I.; Barbir, F. Computational Fluid Dynamics Study of PEM Fuel Cell Performance. Int. J. Hydrogen Energy 2016, 41, 17585–17594. [Google Scholar] [CrossRef]
- Nishimura, A.; Toyoda, K.; Kojima, Y.; Ito, S.; Hu, E. Numerical Simulation on Impacts of Thickness of Nafion Series Membranes and Relative Humidity on PEMFC Operated at 363 K and 373 K. Energies 2021, 14, 8256. [Google Scholar] [CrossRef]
- Nishimura, A.; Kamiya, S.; Okado, T.; Sato, Y.; Hirota, M.; Kolhe, M.L. Heat and Mass Transfer Analysis in Single Cell of PEFC Using Different PEM and GDL at Higher Temperature. Int. J. Hydrogen Energy 2019, 44, 29631–29640. [Google Scholar] [CrossRef]
- Nishimura, A.; Okado, T.; Kojima, Y.; Hirota, M.; Hu, E. Impact of MPL on Temperature Distribution in Single Polymer Electrolyte Fuel Cell with Various Thickness of Polymer Electrolyte Membrane. Energies 2020, 13, 2499. [Google Scholar] [CrossRef]
- Copper, N.J.; Santamaria, A.D.; Becton, M.K.; Park, J.W. Neutron Radiography Measurements of In-situ PEMFC Liquid Water Saturation in 2D & 3D Morphology Gas Diffusion Layers. Int. J. Hydrogen Energy 2017, 42, 16269–16278. [Google Scholar]
- The Japan Society of Mechanical Engineers. JSME Heat Transfer Handbook, 1st ed.; The Japan Society of Mechanical Engineers; Maruzen: Tokyo, Japan, 1993; p. 387. [Google Scholar]
- Nishimura, A.; Yamamoto, K.; Okado, T.; Kojima, Y.; Hirota, M.; Kolhe, M.L. Impact Analysis of MPL and PEM Thickness on Temperature Distribution within PEFC Operating at Relatively Higher Temperature. Energy 2020, 205, 117875. [Google Scholar] [CrossRef]
- Freunberger, S.A.; Reum, M.; Evertz, J.; Wokuan, A.; Buchi, F.M. Measuring the Current Distribution in PEFCs with Sub-Millimeter Resolution. J. Electrochem. Soc. 2006, 153, A2158–A2165. [Google Scholar] [CrossRef]
- Nishimura, A.; Sato, A.; Yoshimura, M.; Kamiya, S.; Hirota, M. Impact of Thickness of Polymer Electrolyte Membrane on Temperature Distribution in Single Cell of Polymer Electrolyte Fuel Cell Operated at High Temperature. J. Energy Power Eng. 2018, 12, 80–92. [Google Scholar] [CrossRef][Green Version]
- Nishimura, A.; Shibuya, K.; Morimoto, A.; Tanaka, S.; Hirota, M.; Nakamura, M.; Kojima, Y.; Narita, M.; Hu, E. Dominant Factor and Mechanism of Coupling Phenomena in Single Cell of Polymer Electrolyte Fuel Cell. Appl. Energy 2012, 1, 73–79. [Google Scholar] [CrossRef]
- Bit Tech. Product Catalog; Bit Tech.: Gosyogawara, Japan, 2008; p. 1. [Google Scholar]
- Reid, R.C.; Prausnitz, J.M.; Poling, B.E. The Properties of Gases and Liquids, 1st ed.; McGraw-Hill: New York, NY, USA, 1987; p. 591. [Google Scholar]
- Merck. Available online: http://www.sigmaaldrich.com/japan/materialscience/alternative/nafion.html (accessed on 5 November 2021).
- Senn, S.M.; Poulikakos, D. Polymer Electrolyte Fuel Cells with Porous Materials as Fluid Distributors and Comparisons with Traditional Channeled Systems. Trans. ASME 2004, 126, 410–418. [Google Scholar] [CrossRef]
- Kang, K.; Ju, H. Numerical Modeling and Analysis of Micro-porous Layer Effects in Polymer Electrolyte Fuel Cells. J. Power Source 2006, 194, 763–773. [Google Scholar] [CrossRef]
- TORAY. Available online: http://www.torayca.com/en/lineup/composites/com_009_01.html (accessed on 5 November 2021).
- Takayama, T. Numerical Simulation of Transient Internal States of PEFC Cell and Stack Considering Control of Anode System. Res. Rep. Mizuho Res. Technol. 2018, 9, 1–14. [Google Scholar]
- Rostami, L.; Nejad, P.M.G.; Vatani, A. A Numerical Investigation of Serpentine Flow Channel with Different Bend Sizes in Polymer Electrolyte Membrane Fuel Cells. Energy 2016, 97, 400–410. [Google Scholar] [CrossRef]
- Nishimura, A.; Toyoda, K.; Mishima, D.; Ito, S.; Hu, E. Numerical Analysis on Impact of Thickness of PEM and GDL with and without MPL on Coupling Phenomena in PEFC Operated at Higher Temperature Such as 363 K and 373 K. Energies 2022, 15, 5936. [Google Scholar] [CrossRef]
- Xing, L.; Das, P.K.; Song, X.; Mamlouk, M.; Scott, K. Numerical Analysis of the Optimum Membrane/Ionomer Water Content of PEMFCs: The Interface of Nafion Ionomer Content and Cathode Relative Humidity. Appl. Energy 2015, 138, 242–257. [Google Scholar] [CrossRef]
- Quan, P.; Lai, M.C. Numerical study of water management in the air flow channel of a PEM fuel cell cathode. J. Power Source 2007, 164, 222–237. [Google Scholar] [CrossRef]
- Jiao, K.; Park, J.; Li, X. Experimental investigation on liquid water removal from the gas diffusion layer by reaction flow in PEM fuel cell. Appl. Energy 2010, 87, 2770–2777. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Ramya, K.; Khalid, M.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Walvekar, R.; Kadhum, A.A.H. Additives in proton exchange membranes for low- and high-temperature fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Salomov, U.R.; Chiavazzo, E.; Fasano, M.; Asinari, P. Pore- and macro-scale simulations of high temperature proton exchange fuel cells—HTPEMFC—And possible strategies for enhancing durability. Int. J. Hydrogen Energy 2017, 42, 26730–26743. [Google Scholar] [CrossRef]
- Nishimura, A.; Yoshimura, M.; Kamiya, S.; Hirota, M.; Hu, E. Impact of relative humidity of supply gas on temperature distributions in single cell of polymer electrolyte fuel cell when operated at high temperature. J. Energy Power Eng. 2017, 11, 706–718. [Google Scholar] [CrossRef][Green Version]
- Akimoto, F.; Sasabe, T.; Yoshida, T.; Naito, H.; Kawamura, K.; Hirai, S. Investigation of Effects of High Temperature and Pressure on a Polymer Electrolyte Fuel Cell with Polarization Analysis and X-ray Imaging of Liquid Water. J. Power Source 2019, 431, 205–209. [Google Scholar]
- Jia, T.; Shen, S.; Zhao, J.; Jin, J.; Pan, B.; Duan, X.; Meng, C.; Che, Q. Ultrathin Membranes Formation via the Layer by Layer Self-assembly of Carbon Natotubes-based Inorganics as High Temperature Proton Exchange Membranes. Int. J. Hydrogen Energy 2020, 45, 14517–14527. [Google Scholar] [CrossRef]
- He, G.; Yamazaki, Y.; Abudula, A. A Three-dimensional Analysis of the Effect of Anisotropic Gas Diffusion Layer (GDL) Thermal Conductivity on the Heat Transfer and Two-phase Behavior in a Proton Exchange Membrane Fuel Cell (PEMFC). J. Power Source 2010, 195, 1551–1560. [Google Scholar] [CrossRef]






| Position | Temperature of Separator Back Surface [K] | |||||
|---|---|---|---|---|---|---|
| Sa: 1.0 mm, Ch: 1.0 mm | Sa: 0.5 mm, Ch: 1.0 mm | Sa: 0.5 mm, Ch: 0.5 mm | ||||
| Anode | Cathode | Anode | Cathode | Anode | Cathode | |
| A | 359.55 | 361.45 | 362.15 | 361.35 | 361.25 | 362.35 |
| B | 359.55 | 360.85 | 362.25 | 361.05 | 361.25 | 361.85 |
| C | 359.35 | 360.55 | 362.35 | 361.05 | 361.45 | 361.75 |
| D | 359.15 | 360.15 | 362.25 | 361.05 | 361.15 | 361.55 |
| E | 359.35 | 360.55 | 362.65 | 361.35 | 361.65 | 362.05 |
| F | 359.55 | 360.95 | 362.65 | 361.15 | 361.95 | 362.05 |
| G | 359.85 | 361.05 | 362.45 | 360.95 | 361.85 | 362.15 |
| H | 359.95 | 361.25 | 362.55 | 361.15 | 361.85 | 362.15 |
| I | 360.45 | 361.25 | 362.85 | 361.45 | 362.25 | 362.15 |
| J | 360.35 | 361.35 | 362.75 | 361.15 | 362.45 | 362.35 |
| K | 359.95 | 361.25 | 362.95 | 361.45 | 362.55 | 362.35 |
| L | 359.65 | 360.85 | 362.75 | 361.45 | 362.25 | 362.35 |
| M | 359.95 | 361.15 | 363.05 | 361.65 | 362.65 | 362.55 |
| N | 360.15 | 361.35 | 363.25 | 361.75 | 363.05 | 362.65 |
| O | 360.55 | 361.45 | 363.25 | 361.55 | 362.95 | 362.55 |
| P | 360.55 | 361.25 | 363.35 | 361.65 | 362.75 | 362.45 |
| Q | 360.85 | 361.65 | 364.05 | 362.25 | 363.05 | 362.65 |
| R | 360.65 | 361.65 | 363.85 | 362.25 | 363.05 | 362.95 |
| S | 360.25 | 361.85 | 363.65 | 362.45 | 363.05 | 363.15 |
| T | 359.95 | 361.35 | 363.55 | 361.95 | 362.75 | 362.65 |
| s.t. = 2.0 mm | ||||
|---|---|---|---|---|
| A80%RH/C80%RH | A80%RH/C40%RH | A40%RH/C80%RH | A40%RH/C40%RH | |
| 353 K | 3.48 mΩ | 4.09 mΩ | 3.69 mΩ | 5.42 mΩ |
| 363 K | 3.38 mΩ | 3.64 mΩ | 3.58 mΩ | 5.19 mΩ |
| 373 K | 3.17 mΩ | 3.54 mΩ | 3.94 mΩ | 3.49 mΩ |
| s.t. = 1.5 mm | ||||
| A80%RH/C80%RH | A80%RH/C40%RH | A40%RH/C80%RH | A40%RH/C40%RH | |
| 353 K | 4.51 mΩ | 4.62 mΩ | 4.56 mΩ | 5.30 mΩ |
| 363 K | 4.36 mΩ | 4.82 mΩ | 4.84 mΩ | 5.50 mΩ |
| 373 K | 4.63 mΩ | 5.06 mΩ | 9.74 mΩ | 5.90 mΩ |
| s.t. = 1.0 mm | ||||
| A80%RH/C80%RH | A80%RH/C40%RH | A40%RH/C80%RH | A40%RH/C40%RH | |
| 353 K | 3.87 mΩ | 4.20 mΩ | 4.17 mΩ | 4.61 mΩ |
| 363 K | 3.93 mΩ | 3.92 mΩ | 3.94 mΩ | 4.99 mΩ |
| 373 K | 4.62 mΩ | 5.00 mΩ | 4.66 mΩ | 5.44 mΩ |
| s.t. = 2.0 mm | ||||
|---|---|---|---|---|
| A80%RH/C80%RH | A80%RH/C40%RH | A40%RH/C80%RH | A40%RH/C40%RH | |
| 353 K | 4.85 mΩ | 5.41 mΩ | 5.10 mΩ | 5.36 mΩ |
| 363 K | 4.55 mΩ | 5.76 mΩ | 5.23 mΩ | 5.19 mΩ |
| 373 K | 4.64 mΩ | 5.38 mΩ | 4.71 mΩ | 4.19 mΩ |
| s.t. = 1.5 mm | ||||
| A80%RH/C80%RH | A80%RH/C40%RH | A40%RH/C80%RH | A40%RH/C40%RH | |
| 353 K | 5.91 mΩ | 6.24 mΩ | 8.53 mΩ | 9.87 mΩ |
| 363 K | 6.65 mΩ | 7.61 mΩ | 7.65 mΩ | 9.22 mΩ |
| 373 K | 10.05 mΩ | 9.12 mΩ | 3.31 mΩ | 12.31 mΩ |
| s.t. = 1.0 mm | ||||
| A80%RH/C80%RH | A80%RH/C40%RH | A40%RH/C80%RH | A40%RH/C40%RH | |
| 353 K | 5.13 mΩ | 6.55 mΩ | 6.00 mΩ | 9.42 mΩ |
| 363 K | 5.99 mΩ | 7.92 mΩ | 7.55 mΩ | 8.58 mΩ |
| 373 K | 8.07 mΩ | 10.89 mΩ | 5.94 mΩ | 20.55 mΩ |
| 353 K | ||||||||
|---|---|---|---|---|---|---|---|---|
| A80%RH, C80%RH | A80%RH, C40%RH | A40%RH, C80%RH | A40%RH, C40%RH | |||||
| Total Voltage [V] | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment |
| Sa: 1.0 mm, Ch: 1.0 mm | 0.56 | 0.56 | 0.54 | 0.54 | 0.54 | 0.54 | 0.51 | 0.51 |
| Sa: 0.5 mm, Ch: 1.0 mm | 0.52 | 0.52 | 0.49 | 0.49 | 0.49 | 0.49 | 0.40 | 0.40 |
| Sa: 0.5 mm, Ch: 0.5 mm | 0.55 | 0.55 | 0.51 | 0.51 | 0.52 | 0.52 | 0.39 | 0.39 |
| A80%RH, C80%RH | A80%RH, C40%RH | A40%RH, C80%RH | A40%RH, C40%RH | |||||
| Total current [A] | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment |
| Sa: 1.0 mm, Ch: 1.0 mm | 4.7 | 20 | 3.9 | 20 | 4.0 | 20 | 3.5 | 20 |
| Sa: 0.5 mm, Ch: 1.0 mm | 3.5 | 20 | 2.5 | 20 | 2.7 | 20 | 1.4 | 20 |
| Sa: 0.5 mm, Ch: 0.5 mm | 3.4 | 20 | 2.8 | 20 | 2.7 | 20 | 1.0 | 20 |
| 363 K | ||||||||
|---|---|---|---|---|---|---|---|---|
| A80%RH, C80%RH | A80%RH, C40%RH | A40%RH, C80%RH | A40%RH, C40%RH | |||||
| Total Voltage [V] | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment |
| Sa: 1.0 mm, Ch: 1.0 mm | 0.55 | 0.55 | 0.52 | 0.52 | 0.53 | 0.53 | 0.49 | 0.49 |
| Sa: 0.5 mm, Ch: 1.0 mm | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.37 | 0.37 |
| Sa: 0.5 mm, Ch: 0.5 mm | 0.49 | 0.49 | 0.47 | 0.47 | 0.45 | 0.45 | 0.36 | 0.36 |
| A80%RH, C80%RH | A80%RH, C40%RH | A40%RH, C80%RH | A40%RH, C40%RH | |||||
| Total current [A] | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment |
| Sa: 1.0 mm, Ch: 1.0 mm | 3.7 | 20 | 3.2 | 20 | 3.6 | 20 | 3.2 | 20 |
| Sa: 0.5 mm, Ch: 1.0 mm | 2.0 | 20 | 1.9 | 20 | 1.7 | 20 | 0.8 | 20 |
| Sa: 0.5 mm, Ch: 0.5 mm | 2.1 | 20 | 1.8 | 20 | 1.4 | 20 | 0.5 | 20 |
| 373 K | ||||||||
|---|---|---|---|---|---|---|---|---|
| A80%RH, C80%RH | A80%RH, C40%RH | A40%RH, C80%RH | A40%RH, C40%RH | |||||
| Total Voltage [V] | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment |
| Sa: 1.0 mm, Ch: 1.0 mm | 0.46 | 0.46 | 0.47 | 0.47 | 0.41 | 0.41 | 0.41 | 0.41 |
| Sa: 0.5 mm, Ch: 1.0 mm | 0.35 | 0.35 | 0.35 | 0.35 | 0.33 | 0.33 | 0.29 | 0.29 |
| Sa: 0.5 mm, Ch: 0.5 mm | 0.38 | 0.38 | 0.33 | 0.33 | 0.28 | 0.28 | 0.23 | 0.23 |
| A80%RH, C80%RH | A80%RH, C40%RH | A40%RH, C80%RH | A40%RH, C40%RH | |||||
| Total current [A] | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment | Simulation | Experiment |
| Sa: 1.0 mm, Ch: 1.0 mm | 3.4 | 20 | 2.9 | 20 | 3.3 | 20 | 2.9 | 20 |
| Sa: 0.5 mm, Ch: 1.0 mm | 0.7 | 20 | 0.5 | 20 | 0.2 | 20 | 0.1 | 20 |
| Sa: 0.5 mm, Ch: 0.5 mm | 0.6 | 20 | 0.4 | 20 | 0.2 | 20 | 0.1 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimura, A.; Mishima, D.; Toyoda, K.; Ito, S.; Kolhe, M.L. Numerical Simulation on Effect of Separator Thickness on Coupling Phenomena in Single Cell of PEFC under Higher Temperature Operation Condition at 363 K and 373 K. Energies 2023, 16, 606. https://doi.org/10.3390/en16020606
Nishimura A, Mishima D, Toyoda K, Ito S, Kolhe ML. Numerical Simulation on Effect of Separator Thickness on Coupling Phenomena in Single Cell of PEFC under Higher Temperature Operation Condition at 363 K and 373 K. Energies. 2023; 16(2):606. https://doi.org/10.3390/en16020606
Chicago/Turabian StyleNishimura, Akira, Daiki Mishima, Kyohei Toyoda, Syogo Ito, and Mohan Lal Kolhe. 2023. "Numerical Simulation on Effect of Separator Thickness on Coupling Phenomena in Single Cell of PEFC under Higher Temperature Operation Condition at 363 K and 373 K" Energies 16, no. 2: 606. https://doi.org/10.3390/en16020606
APA StyleNishimura, A., Mishima, D., Toyoda, K., Ito, S., & Kolhe, M. L. (2023). Numerical Simulation on Effect of Separator Thickness on Coupling Phenomena in Single Cell of PEFC under Higher Temperature Operation Condition at 363 K and 373 K. Energies, 16(2), 606. https://doi.org/10.3390/en16020606









