Combustion Behaviors and Unregular Emission Characteristics in an Ammonia–Diesel Engine
Abstract
:1. Introduction
2. Experimental Setup
3. Numerical Methods
4. Results and Discussions
4.1. Combustion Characteristics
4.2. Emission Characteristics
5. Conclusions
- (1)
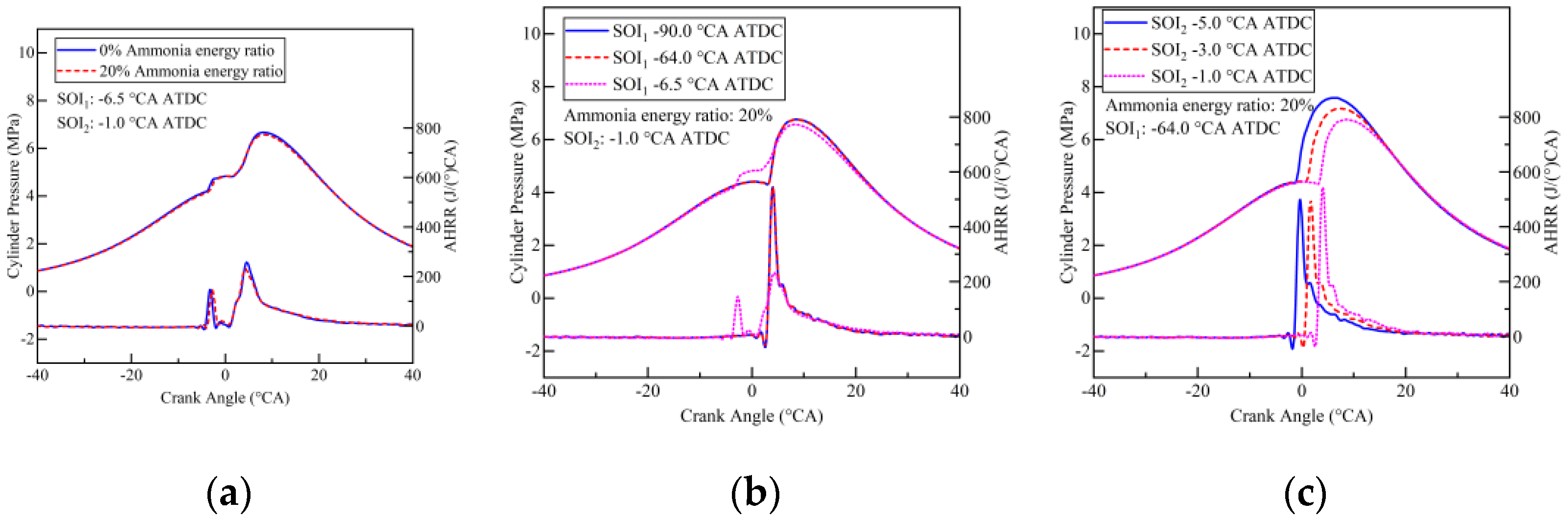
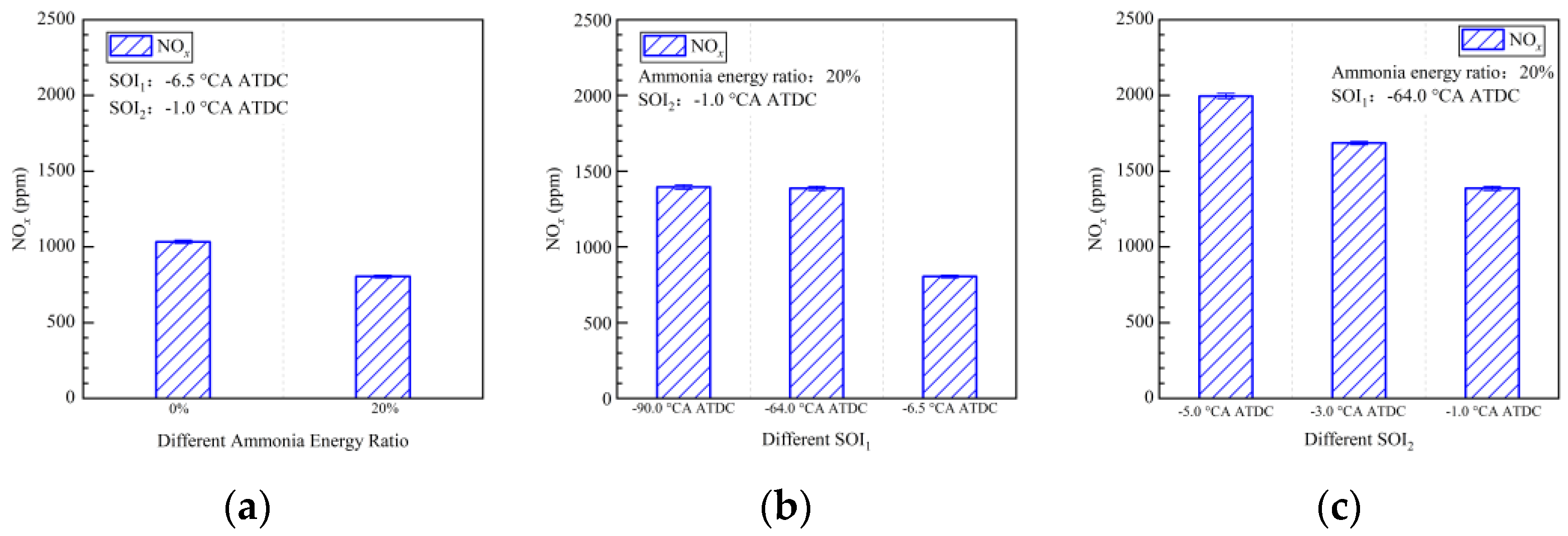
- The introduction of ammonia resulted in delayed combustion and extended combustion duration. Advancing the pilot injection timing (SOI1) led to missing the first stage combustion.
- (2)
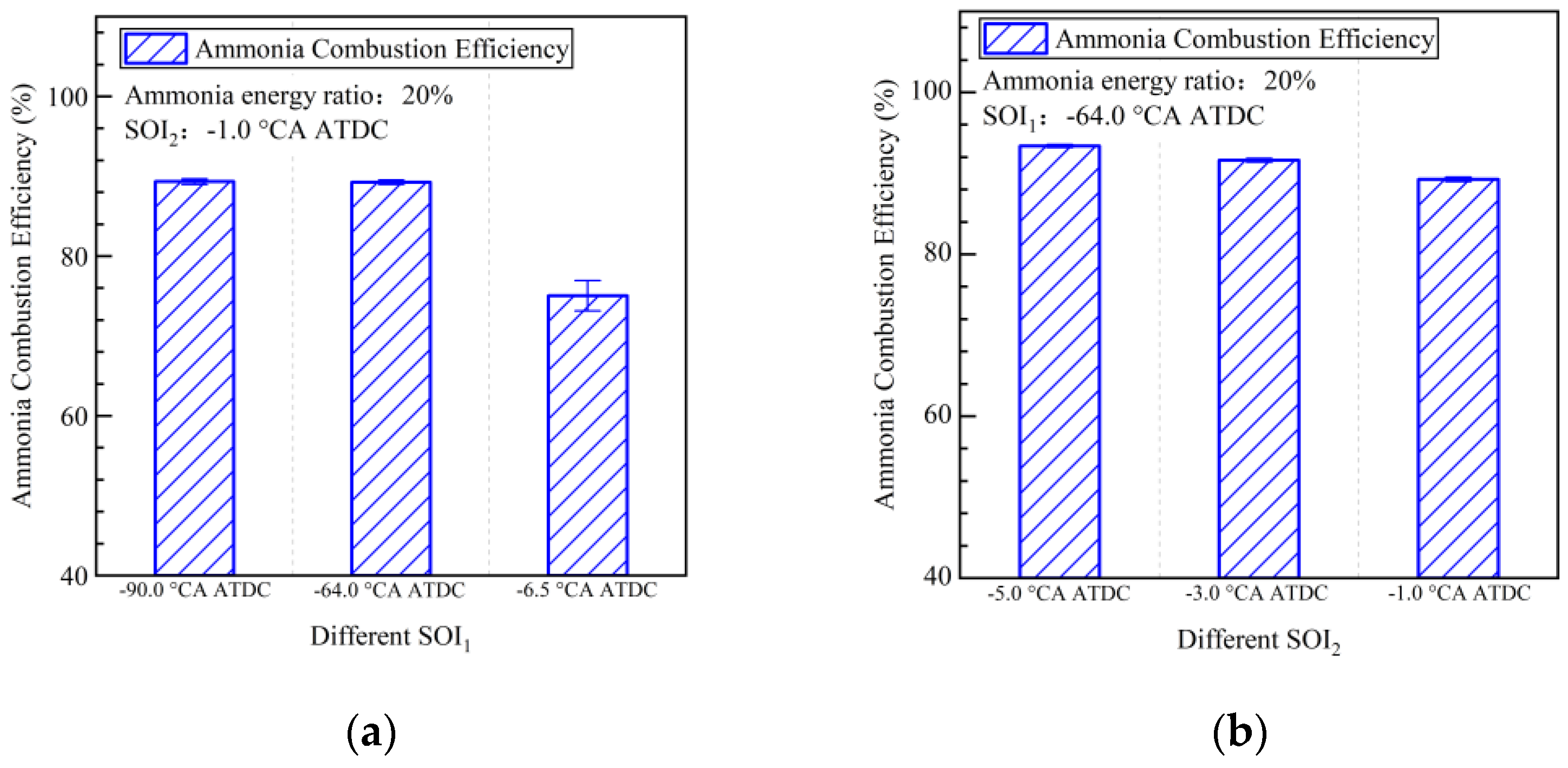
- Strategies involving early pilot injection timing (SOI1) improved ammonia combustion efficiency from 74% to 89%. Diesel injected during pilot injection facilitated ammonia decomposition. The maximum ammonia combustion efficiency achieved in this study was 93%, attained with SOI1 set at −64.0 °CA ATDC and SOI2 at −5.0 °CA ATDC.
- (3)
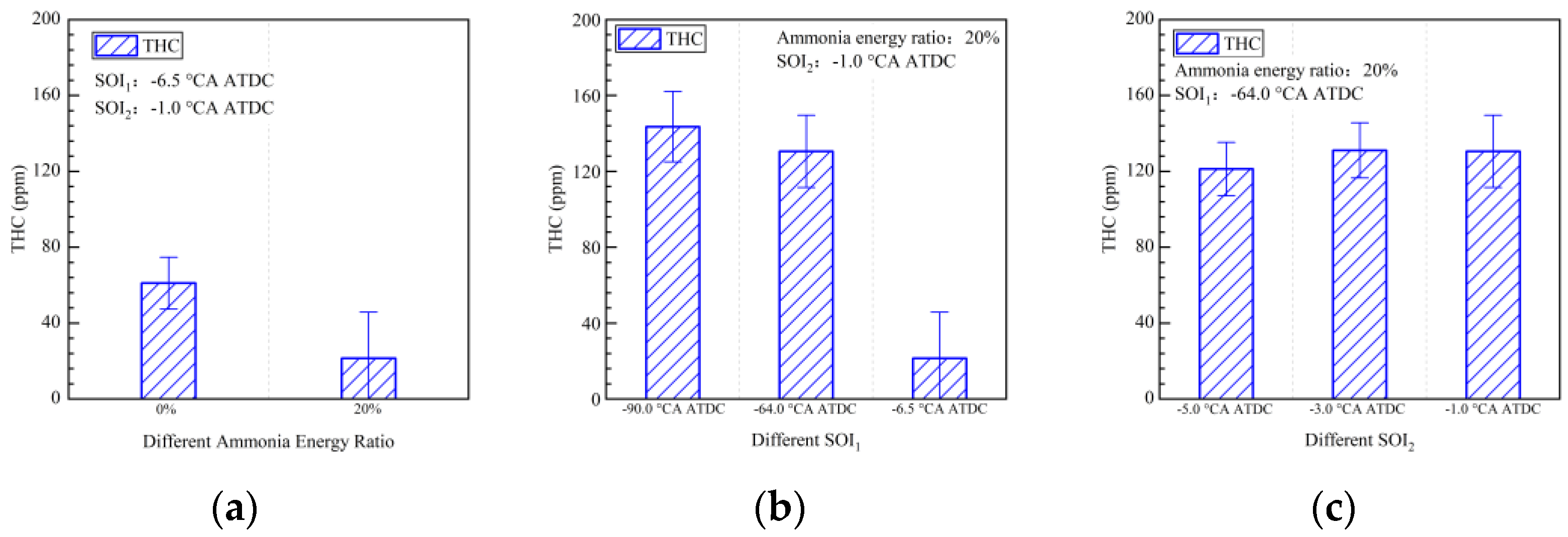
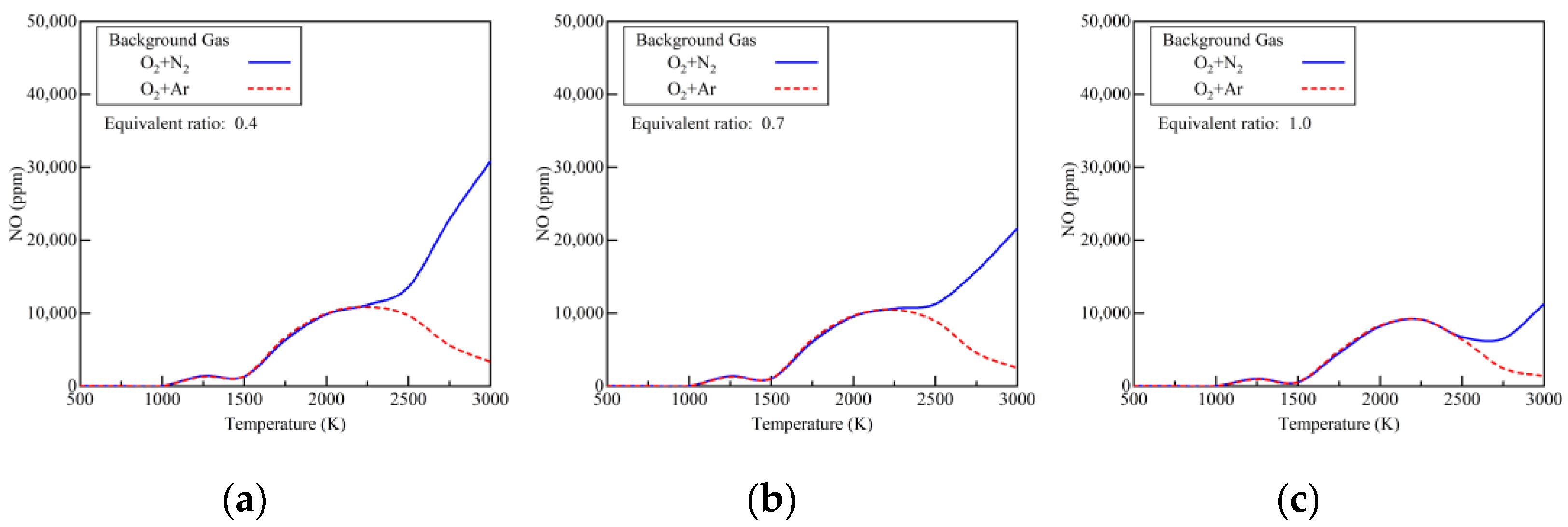
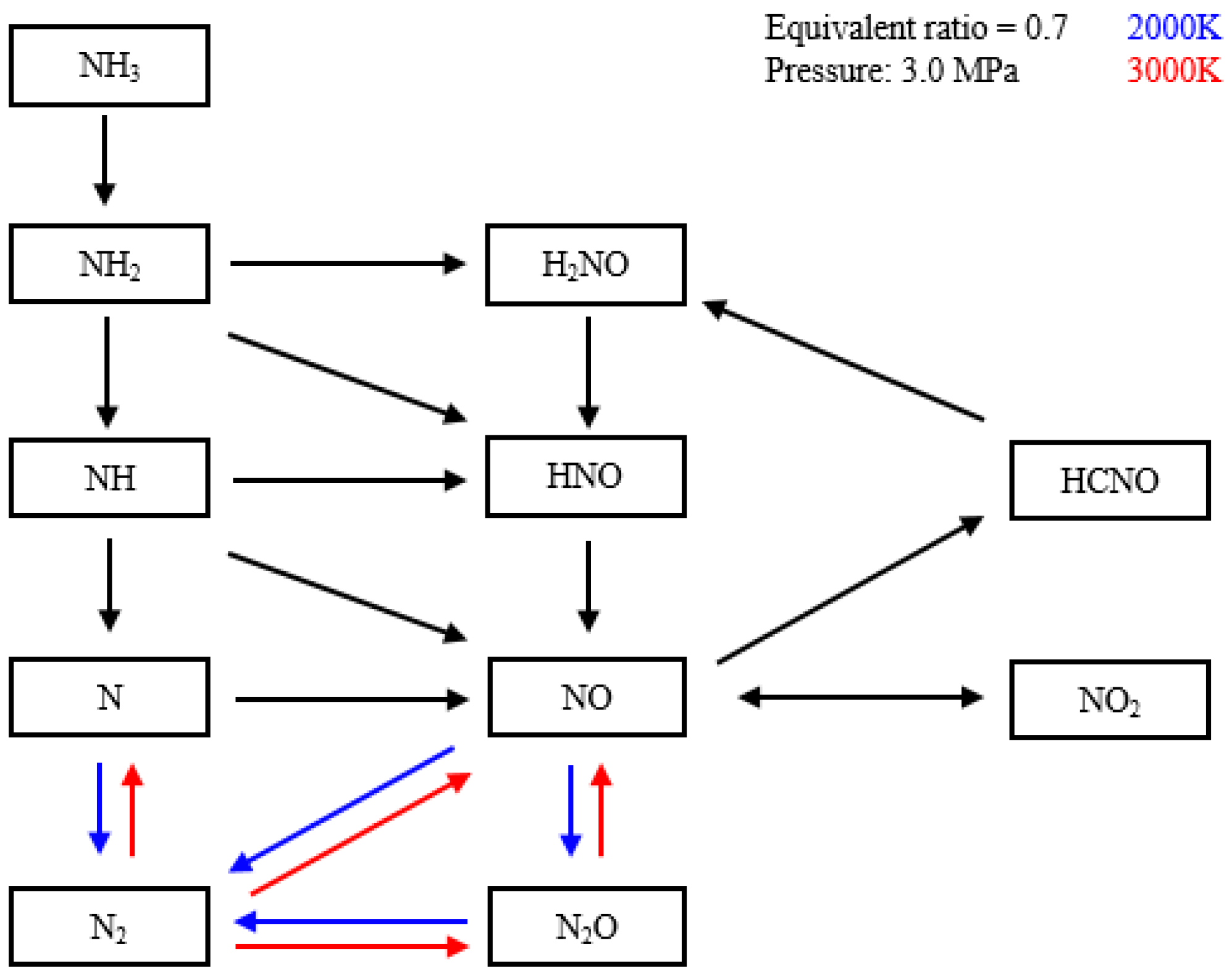
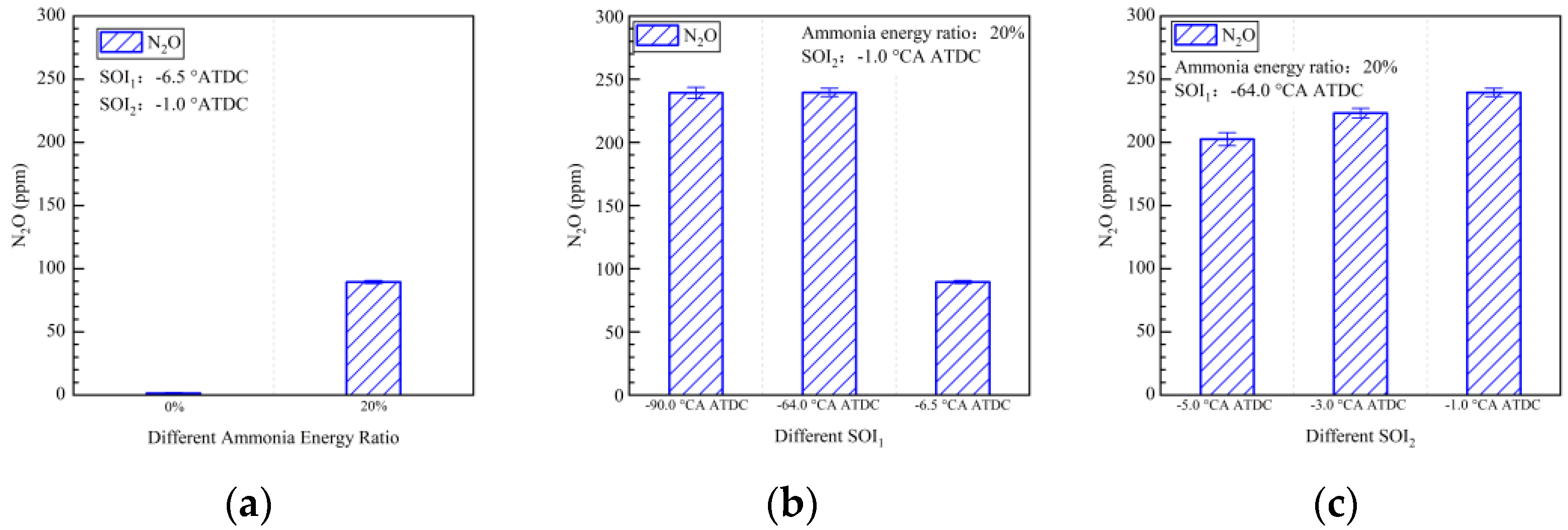
- The early pilot injection strategy yielded poorer emission performance (CO, THC, NOx, N2O, and HCN). Advancing SOI2 accelerated the oxidation process of CO, THC, N2O, and HCN due to higher temperatures. However, thermal NOx emissions worsened.
- (4)
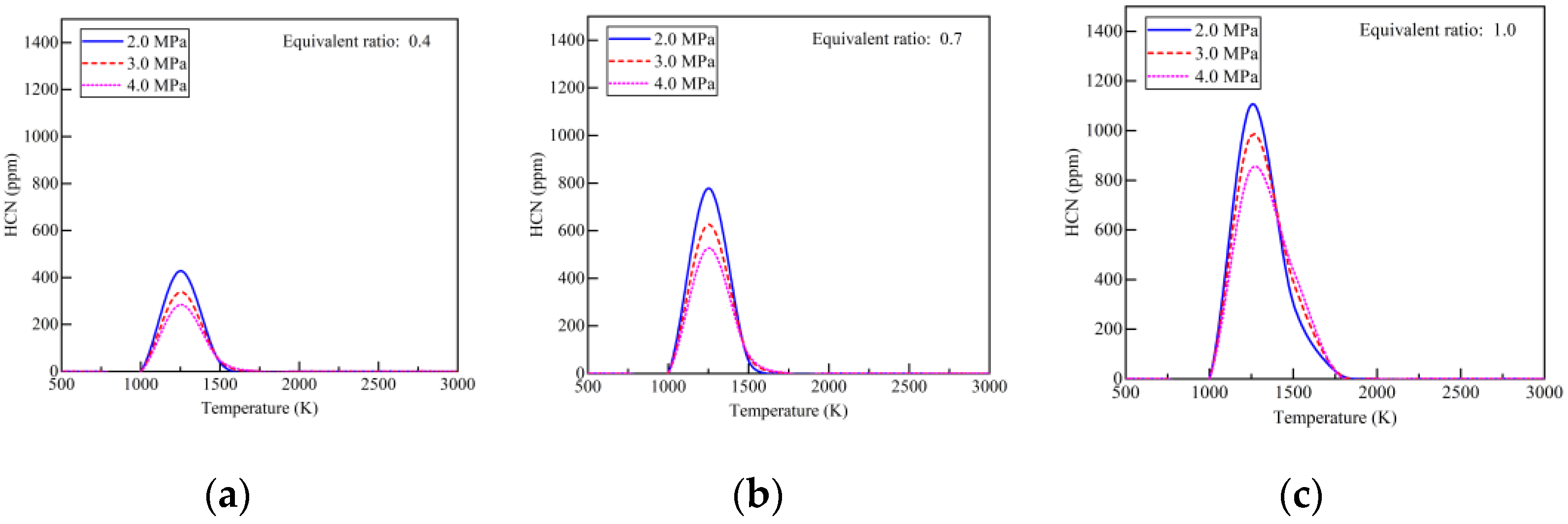
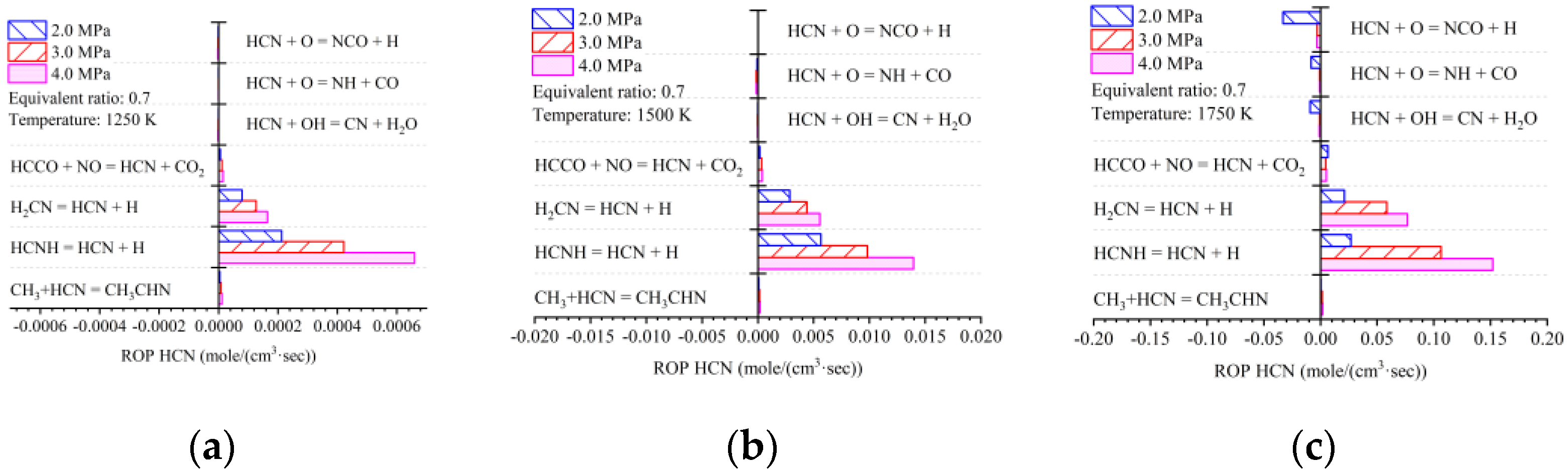
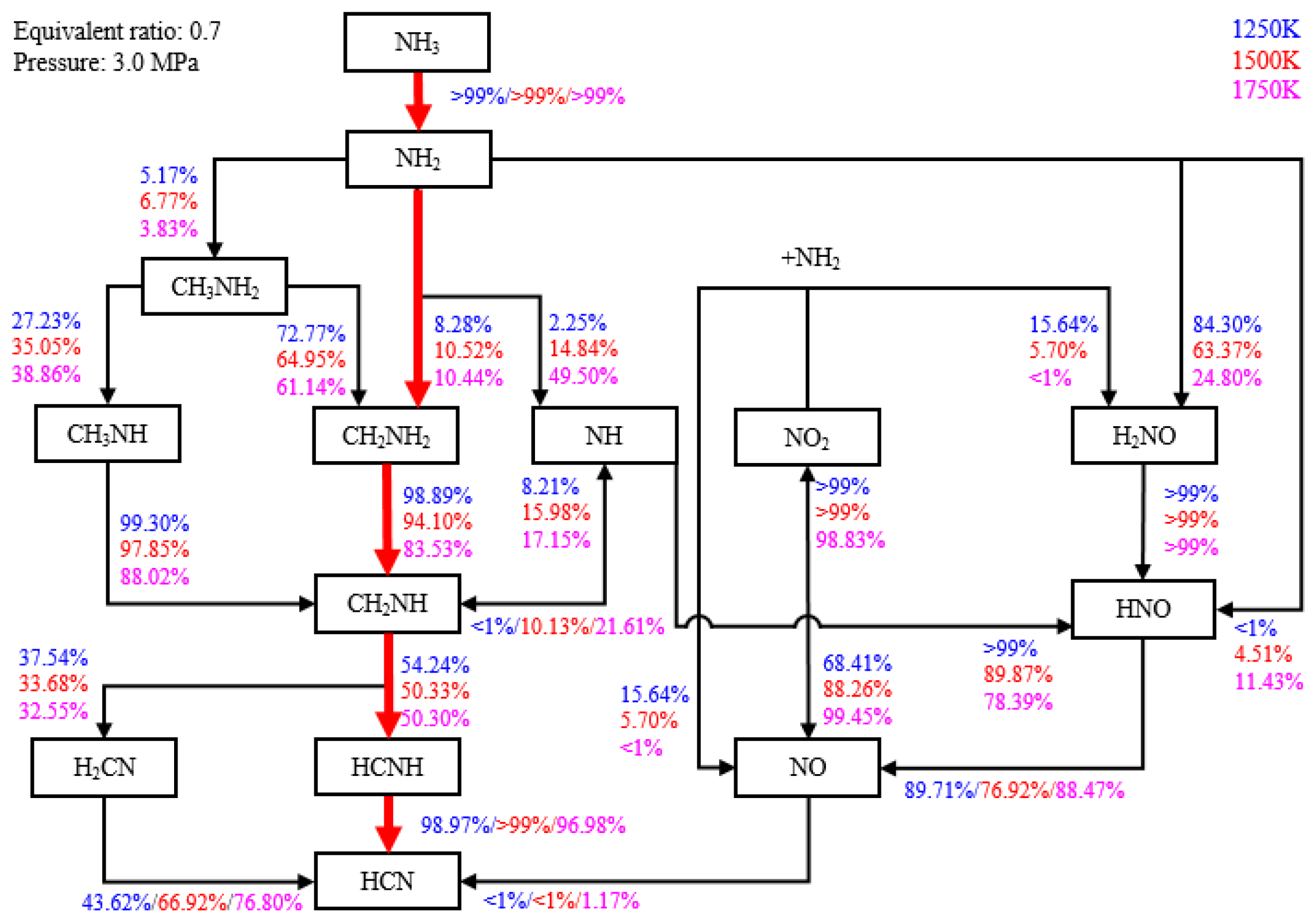
- With the addition of ammonia, HCN emissions increased to 1.7 ppm, more than five times higher than those in diesel-only mode. The early pilot injection strategy stimulated HCN formation. The highest recorded HCN emission was 9.2 ppm. Simulation results indicate that HCN was produced when local temperatures ranged from 1000 K to 1750 K under fuel-lean conditions. Strategies for mitigating HCN emissions could involve increasing pilot injection fuel quantities, heating intake air, or advancing combustion phases closer to TDC.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency, Beyond 20/20 WDS—Reports (iea.org). Available online: https://wds.iea.org/wds/ReportFolders/report-Folders.aspx (accessed on 30 August 2023).
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Berwal, P.; Kumar, S.; Khandelwal, B. A comprehensive review on synthesis, chemical kinetics, and practical application of ammonia as future fuel for combustion. J. Energy Inst. 2021, 99, 273–298. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Rosenthal, A.B. Energy depot—A concept for reducing the military supply burden. In SAE Technical Paper 1965; SAE: Warrendale, PA, USA, 1965; p. 650050. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Wang, S.; Guiberti, T.F.; Roberts, W.L. Review on the recent advances on ammonia combustion from the fundamentals to the applications. Fuel Commun. 2022, 10, 100053. [Google Scholar] [CrossRef]
- Cornelius, W.; Huellmantel, W.L.; Mitchell, H.R. Ammonia as an engine fuel. In SAE Technical Paper 1965; SAE: Warrendale, PA, USA, 1965; p. 650052. [Google Scholar] [CrossRef]
- Gray, J.T.; Dimitroff, E.; Meckel, N.T.; Quillian, R.D. Ammonia fuel—Engine compatibility and combustion. In SAE Technical Paper 1966; SAE: Warrendale, PA, USA, 1966; p. 660156. [Google Scholar] [CrossRef]
- Blarigan, P.V. Advanced internal combustion engine research. In Proceedings of the 2000 DOE Hydrogen Program Review NREL/CP-570-28890 2000, San Ramon, CA, USA, 5 September–5 November 2000; pp. 1–19. [Google Scholar]
- Pearsall, T.J.; Garabedian, C.G. Combustion of anhydrous ammonia in diesel engines. In SAE Technical Paper 1967; SAE: Warrendale, PA, USA, 1967; p. 670947. [Google Scholar] [CrossRef]
- Bro, K.; Pedersen, P.S. Alternative diesel engine fuels: An experimental investigation of methanol, ethanol, methane and ammonia in a D.I. diesel engine with pilot injection. In SAE Technical Paper 1977; SAE: Warrendale, PA, USA, 1977; p. 770794. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.-C. Demonstration of compression-ignition engine combustion using ammonia in reducing greenhouse gas emissions. Energy Fuels 2008, 22, 2963–2971. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.-C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Gill, S.S.; Chatha, G.S.; Tsolakis, A.; Golunski, S.E.; York, A.P.E. Assessing the effects of partially decarbonising a diesel engine by co-fuelling with dissociated ammonia. Int. J. Hydrogen Energy 2012, 37, 6074–6083. [Google Scholar] [CrossRef]
- U.S Evironmental Protection Agency. Understanding Global Warming Potentials. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 30 August 2023).
- Niki, Y.; Yoo, D.-H.; Hirata, K.; Sekiguchi, H. Effects of ammonia gas mixed into intake air on combustion and emissions characteristics in diesel engine. In Proceedings of the ASME 2016 Internal Combustion Engine Fall Technical Conference, Greenville, SC, USA, 9–12 October 2016. [Google Scholar] [CrossRef]
- Niki, Y.; Nitta, Y.; Sekiguchi, H.; Hirata, K. Emission and combustion characteristics of diesel engine fumigated with ammonia. In Proceedings of the ASME 2018 Internal Combustion Engine Division Fall Technical Conference. Volume 1: Large Bore Engines; Fuels; Advanced Combustion, San Diego, CA, USA, 4–7 November 2018. [Google Scholar] [CrossRef]
- Niki, Y.; Nitta, Y.; Sekiguchi, H.; Hirata, K. Diesel fuel multiple injection effects on emission characteristics of diesel engine mixed ammonia gas into intake air. J. Eng. Gas Turbines Power 2019, 141, 061020. [Google Scholar] [CrossRef]
- Niki, Y. Reductions in unburned ammonia and nitrous oxide emissions from an ammonia-assisted diesel engine with early timing diesel pilot injection. J. Eng. Gas Turbines Power 2021, 143, 091014. [Google Scholar] [CrossRef]
- Yousefi, A.; Guo, H.; Dev, S.; Liko, B.; Lafrance, S. Effects of ammonia energy fraction and diesel injection timing on combustion and emissions of an ammonia/diesel dual-fuel engine. Fuel 2021, 314, 122723. [Google Scholar] [CrossRef]
- Yousefi, A.; Guo, H.; Dev, S.; Lafrance, S.; Liko, B. A study on split diesel injection on thermal efficiency and emissions of an ammonia/diesel dual-fuel engine. Fuel 2022, 316, 123412. [Google Scholar] [CrossRef]
- Mi, S.; Wu, H.; Pei, X.; Liu, C.; Zheng, L.; Zhao, W.; Qian, Y.; Lu, X. Potential of ammonia energy fraction and diesel pilot-injection strategy on improving combustion and emission performance in an ammonia-diesel dual fuel engine. Fuel 2023, 343, 127889. [Google Scholar] [CrossRef]
- Jin, S.; Wu, B.; Zi, Z.; Yang, P.; Shi, T.; Zhang, J. Effects of fuel injection strategy and ammonia energy ratio on combustion and emissions of ammonia-diesel dual-fuel engine. Fuel 2023, 314, 127668. [Google Scholar] [CrossRef]
- Pei, Y.Q.; Wang, D.C.; Jin, S.Y.; Gu, Y.C.; Wu, C.L.; Wu, B.Y. A quantitative study on the combustion and emission characteristics of an Ammonia-Diesel Dual-fuel (ADDF) engine. Fuel Process. Technol. 2023, 250, 107906. [Google Scholar] [CrossRef]
- Sun, W.C.; Zeng, W.P.; Guo, L.; Zhang, H.; Yan, Y.Y.; Lin, S.D.; Zhu, G.N.; Jiang, M.Q.; Yu, C.Y.; Wu, F. An optical study of the combustion and flame development of ammonia-diesel dual-fuel engine based on flame chemiluminescence. Fuel 2023, 349, 128507. [Google Scholar] [CrossRef]
- Hiraoka, K.; Matsunaga, D.; Kamino, T.; Honda, Y.; Toshinaga, K.; Murakami, Y.; Nakamura, H. Experimental and numerical analysis on combustion characteristics of ammonia and diesel dual fuel engine. In SAE Technical Paper 2023; 2023-32-0102; SAE: Warrendale, PA, USA, 2023. [Google Scholar]
- Imamori, Y.; Takahashi, T.; Ueda, H.; Yamada, S.; Tanaka, T.; Kogure, R. Experimental and Numerical Investigations of Emission Characteristics from Diesel-Ammonia-Fueled Industry Engines. In SAE Technical Paper 2023; 2023-32-0064; SAE: Warrendale, PA, USA, 2023. [Google Scholar]
- Siegień, I.; Bogatek, R. Cyanide action in plants—From toxic to regulatory. Acta Physiol. Plant. 2006, 28, 483–497. [Google Scholar] [CrossRef]
- Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.; Kellens, R.; Reddy, J.; Steier, N.; Hasenpusch, W. Cyano Compounds, Inorganic. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2012; pp. 673–678. [Google Scholar]
- Klüssmann, J.N.; Ekknud, L.R.; Ivarsson, A.; Schramm, J. Ammonia Application in IC Engines. In Special Report, a Report from the Advanced Motor Fuels Technology Collaboration Programme; IEA: Paris, France, 2020. [Google Scholar]
- Zengel, D.; Koch, P.; Torkashvand, B.; Grunwaldt, J.-D.; Casapu, M.; Deutschmann, O. Emission of toxic HCN during NOx removal by ammonia SCR in the exhaust of lean-burn natural gas engines. Angew. Chem. Int. Ed. 2020, 59, 14423–14428. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B.; Jiang, Z.; Li, Y.; Gao, W.; Wang, Z. An experimental and kinetic modeling study of ammonia/n-heptane blends. Combust. Flame 2022, 246, 112428. [Google Scholar] [CrossRef]
- Mørch, C.S.; Bjerre, A.; Gøttrup, M.P.; Sorenson, S.C.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Shim, E.; Park, H.; Bae, C. Intake air strategy for low HC and CO emissions in dual-fuel (CNG-diesel) premixed charge compression ignition engine. Appl. Energy 2018, 225, 1068–1077. [Google Scholar] [CrossRef]
- Dagaut, P.; Glarborg, P.; Alzueta, M.U. The oxidation of hydrogen cyanide and related chemistry. Prog. Energy Combust. Sci. 2008, 34, 1–46. [Google Scholar] [CrossRef]
- Hong, D.; Guo, X.; Wang, C. A reactive molecular dynamics study of HCN oxidation during pressurized oxy-fuel combustion. Fuel Process. Technol. 2021, 224, 107020. [Google Scholar] [CrossRef]
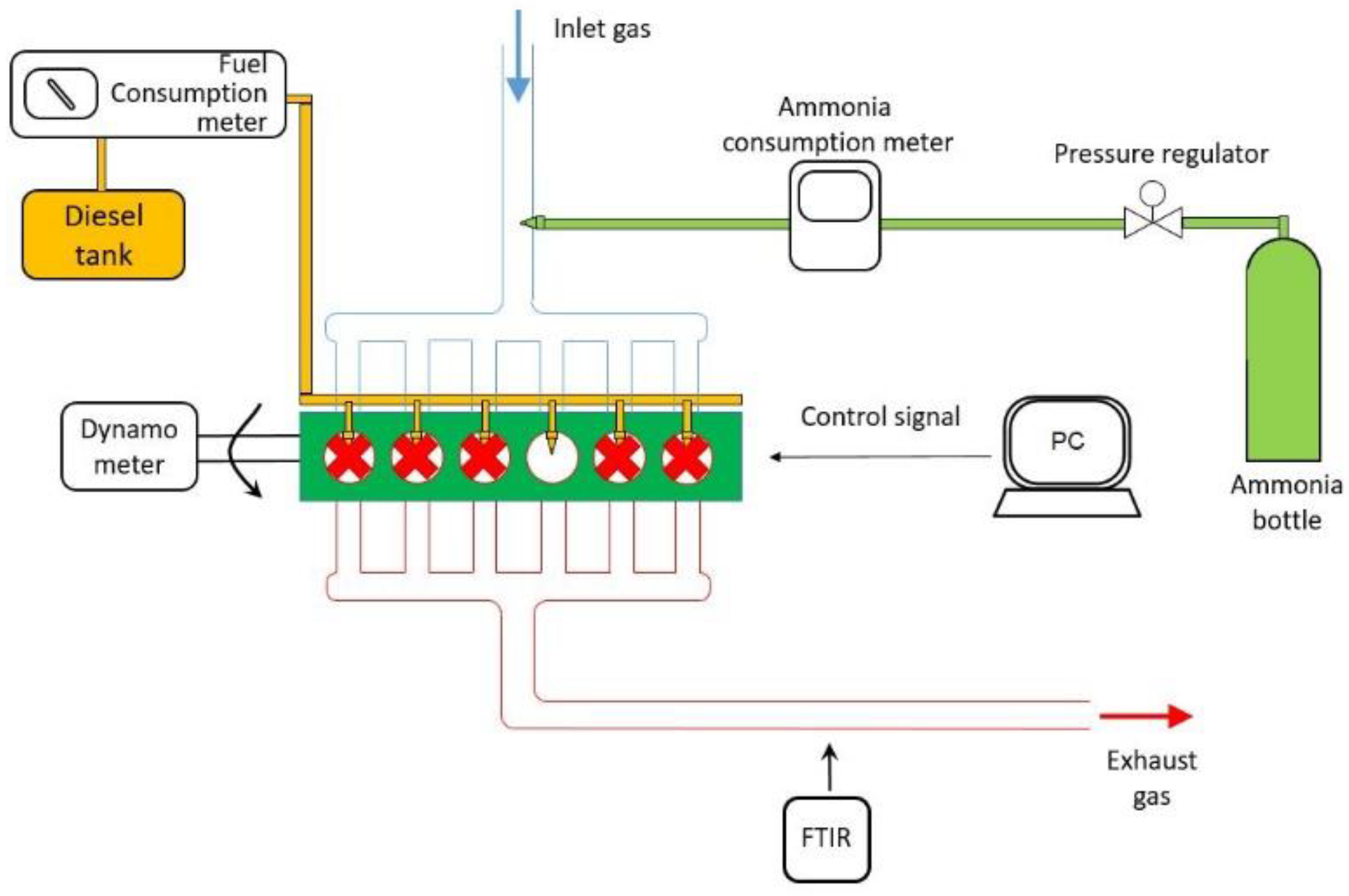



| Description (Units) | Value |
|---|---|
| Bore (mm) | 131 |
| Stroke (mm) | 160 |
| Connecting rod (mm) | 257 |
| Geometric compression ratio (-) | 18.0 |
| Combustion chamber geometry (-) | ω, re-entrant |
| DI nozzle number (-) | 7 |
| Description (Units) | Diesel | Ammonia |
|---|---|---|
| CN (-) | 56.5 | -- |
| Octane number (-) | -- | 130 |
| Carbon contents (%(m/m)) | 86.45 | 0.00 |
| Hydrogen contents (%(m/m)) | 13.49 | 17.65 |
| Oxygen contents (%(m/m)) | 0.05 | 0.00 |
| LHV (MJ/kg) | 42.68 | 18.8 |
| Condition | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Engine speed (r/min) | 800 | 800 | 800 | 800 | 800 | 800 |
| IMEP (MPa) | 0.556 | 0.559 | 0.554 | 0.557 | 0.557 | 0.557 |
| Ammonia injection pressure (MPa) | -- | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Ammonia flow rate (kg/h) | -- | 0.646 | 0.630 | 0.634 | 0.632 | 0.624 |
| DI pilot injection timing (°CA ATDC) | −6.5 | −6.5 | −64.0 | −90.0 | −64.0 | −64.0 |
| DI pilot injection rate (mg/cycle) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| DI main injection timing (°CA ATDC) | −1.0 | −1.0 | −1.0 | −1.0 | −3.0 | −5.0 |
| DI main injection rate (mg/cycle) | Varying | Varying | Varying | Varying | Varying | Varying |
| Common rail pressure (MPa) | 110.0 | 110.0 | 110.0 | 110.0 | 110.0 | 110.0 |
| Condition | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Fuel equivalent ratio (-) | 0.4 | 0.7 | 1.0 | 0.4–1.0 |
| Mole fraction of ammonia (%) | 1.68 | 1.67 | 1.66 | 1.68–1.66 |
| Mole fraction of n-heptane (%) | 0.63 | 1.18 | 1.73 | 0.63–1.73 |
| Mole fraction of O2 (%) | 20.5 | 20.4 | 20.3 | 20.5–20.3 |
| Mole fraction of N2 (%) | 77.2 | 76.7 | 76.3 | 0 |
| Mole fraction of Ar (%) | 0 | 0 | 0 | 77.2–76.3 |
| Temperature (K) | 500–3000 | 500–3000 | 500–3000 | 500–3000 |
| Pressure (MPa) | 2.0–4.0 | 2.0–4.0 | 2.0–4.0 | 3.0 |
| End time (ms) | 1.0 | 1.0 | 1.0 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, K.; Liu, Y.; Chen, Q.; Qi, Y.; Li, L.; Wang, Z. Combustion Behaviors and Unregular Emission Characteristics in an Ammonia–Diesel Engine. Energies 2023, 16, 7004. https://doi.org/10.3390/en16197004
Cai K, Liu Y, Chen Q, Qi Y, Li L, Wang Z. Combustion Behaviors and Unregular Emission Characteristics in an Ammonia–Diesel Engine. Energies. 2023; 16(19):7004. https://doi.org/10.3390/en16197004
Chicago/Turabian StyleCai, Kaiyuan, Yi Liu, Qingchu Chen, Yunliang Qi, Li Li, and Zhi Wang. 2023. "Combustion Behaviors and Unregular Emission Characteristics in an Ammonia–Diesel Engine" Energies 16, no. 19: 7004. https://doi.org/10.3390/en16197004
APA StyleCai, K., Liu, Y., Chen, Q., Qi, Y., Li, L., & Wang, Z. (2023). Combustion Behaviors and Unregular Emission Characteristics in an Ammonia–Diesel Engine. Energies, 16(19), 7004. https://doi.org/10.3390/en16197004






