Abstract
Bioethanol is the most widely used alternative transportation fuel to petrol. Bioethanol is considered a clean, renewable, and environmentally friendly fuel that can contribute to climate change mitigation, decreased environmental pollution, and enhanced energy security. Commercial bioethanol production is based on traditional agricultural crops such as corn, sugarcane, and sugarbeet, primarily used as food and feed. In order to meet the growing demand for this fuel and decrease competition in the food and biofuel sectors for the same feedstock, other raw materials and process technologies have been intensively studied. Lignocellulosic biomass is one of the most abundant renewable resources, with it being rich in compounds that could be processed into energy, transportation fuels, various chemical compounds, and diverse materials. Bioethanol production from lignocellulosic biomass has received substantial attention in recent decades. This review gives an overview of bioethanol production steps from lignocellulosic biomass and challenges in the production process. The following aspects of bioethanol production are covered here, including pretreatment methods, process strategies, strain development, ethanol isolation and purification, and technical hurdles.
1. Introduction
Increasing public concern over the sustainability of natural resources, the climate, and environmental issues have encouraged the development of environmentally friendly processes and new sustainable natural bio-based products relying on recent technological advances in biotechnology. Energy plays a decisive role in human and social improvements as well as economic development. The growing demand for energy and depleting petroleum sources encourage extensive research for renewable and environmentally acceptable alternative fuels and the development of their production [1]. Today, bioethanol is the most common liquid biofuel, predominately produced from sugar- and starch-based feedstocks which are also used as food and animal feed. Second-generation bioethanol is produced from non-edible lignocellulose-rich biomass such as energy crops, agricultural and forestry residues, and industrial and municipal waste [2]. Such lignocellulosic feedstocks are not directly associated with food security for energy conversion and are available at a reasonable price as a renewable source [3]. In this context, forestry and agro-industrial residues, such as sugarcane bagasse and straw, wheat straw, rice straw, rice husk, corn stover, cotton stalks, and wood chips and sawdust, have been investigated as raw materials in process development for biofuel production and use.
Lignocellulosic biomass, widely available at relatively low cost in different forms, is a suitable raw feedstock for bioethanol production due to its relatively high carbohydrate content. It consists mainly of cellulose (30–50%), hemicelluloses (20–40%), and lignin (20–30%). Carbohydrate composition differs regarding biomass origin [4]. Cellulose and hemicelluloses can be converted into fermentable sugars, chemicals, and materials. Lignin is a source for producing high-value-added products, such as phenolic resins, epoxy resins, adhesives, and polyolefins.
The biochemical conversion of lignocellulosic biomass into bioethanol by microbial fermentation is considered attractive due to the mild operating conditions. Due to its complex and recalcitrant structure, lignocellulosic biomass cannot be directly used as a carbon source for microorganism growth and bioethanol production. Therefore, lignocellulosic biomass is subjected to pretreatment before the saccharification step and fermentation [5,6]. Pretreatment changes the physical, chemical, and rheological properties of lignocellulosic biomass, increasing the sugar yield through the hydrolysis of structural carbohydrates and thus improving bioethanol titer, yield, and productivity. The additional step in bioethanol production from lignocellulosic biomass significantly increases production, making it economically uncompetitive with conventional fossil fuels or first-generation bioethanol produced from sugar or starch-based feedstocks [7]. After pretreatment, lignocellulosic biomass is subjected to cellulase hydrolysis and fermentation by the yeast Saccharomyces cerevisiae [8].
2. Recalcitrant Structure of Lignocellulosic Biomass
Lignocellulosic biomass mainly comprises cellulose, hemicelluloses, lignin (Figure 1), and minor amounts of proteins, ash, and pectin. The composition of lignocellulosic biomass and plant parts varies from one species to another, but it also depends on the growth condition and plant age. Cellulose is most abundant in hardwoods (40–55%, wt) and softwoods (45–50%, wt), while hemicellulose is dominant in grasses (35–50%, wt) and wheat straw (50%, wt). Softwoods and hardwoods are rich in lignin (25–35%, wt and 18–25%, wt respectively) compared to grasses (10–30%, wt). All three major polymers (Figure 1) are mutually connected, forming a heteropolymeric structure in plant cell walls resistant to microbial and chemical degradation [9].
Cellulose is a linear polymer of hundreds to over tens of thousands of glucose units linked via β-1,4 glycosidic bonds. Parallel cellulose chains (20–300) are linked together by hydrogen and weaker van der Waals bonds forming microfibrils, which are insoluble in water and most organic solvents. Microfibrils often associate and form bundles. Cellulose microfibers have a highly ordered (crystalline) and disordered (amorphous) structure, which is more susceptible to enzyme and acid hydrolysis. Cellulose microfibrils are embedded with the matrix of amorphous hemicellulose. A matrix of lignin surrounds the whole, providing mechanical support and strength to the plant [10].
Hemicellulose is an amorphous branched heteropolymer consisting of hexose (glucose and mannose), pentose sugars (xylose and arabinose), and uronic acids. It usually contains a linear chain of xylan and considerable variation in the branch backbone chains, including glucuronic acid, mannan, galactomannan, and glucomannan [11]. Due to complex branching and acetylation patterns, hemicelluloses can be more easily enzymatically degraded than cellulose, except for particular recalcitrant oligomeric structures [12]. Also, they are relatively sensitive to operating conditions. Therefore, the temperature and retention time must be controlled to avoid forming unwanted products, such as furfurals and hydroxymethyl furfurals, which later inhibit fermentation [9].
Lignin is a complex, amorphous, relatively hydrophobic, and highly branched heteropolymer made of alcohols (monolignols) with an aromatic ring. It has been described in many studies as being able to severely enhance the recalcitrance of lignocellulose deconstruction. Lignin is responsible for the wall’s structural rigidity and brittleness. It fills the space between hemicelluloses and wraps the cellulose skeleton, making a lignocellulose matrix. Predominant structural components are p-coumaryl alcohol, sinapyl alcohol, and coniferyl alcohol, connected via carbon–carbon and ether bonds.
The recalcitrant structure of lignocellulosic biomass affects the subsequent steps in its conversion to different products [13]. Factors affecting the recalcitrance of lignocellulosic biomass are strongly connected and are complicated to separate. They are mainly related to the cellulose-specific surface area, crystallinity, degree of polymerization (DP), pore size and volume, chemical factors related to lignin composition and content, and the content of hemicelluloses and acetyl groups [14].
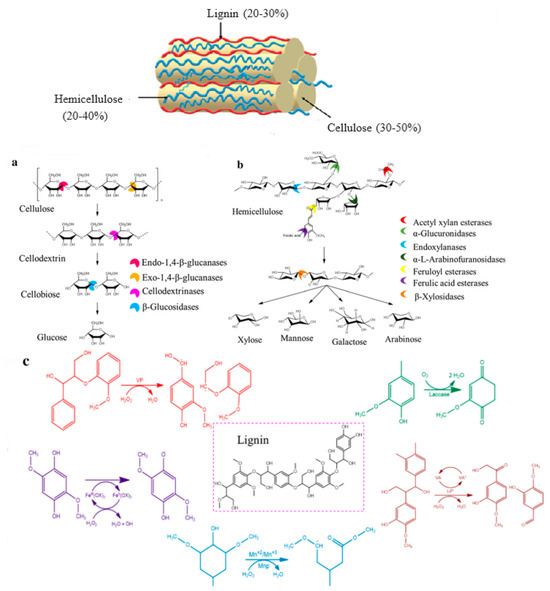
Figure 1.
Schematic representation of the structural composition of lignocellulosic biomass along with the action of the major lignocellulolytic enzyme involved in lignocellulosic biomass degradation: (a) Cellulase action mechanism; (b) hemicellulase/xylanase action mechanism; and (c) ligninase/Laccase action mechanism. Modified from Rajeswari et al. [15].
3. Pretreatment of Lignocellulosic Biomass
The bioenergy industry depends on specific biomass feedstock, whether producing biofuels, biopower, or other bioproducts. Biomass cannot be fed into conversion infeed systems until it undergoes some form of pretreatment level, such as size reduction. From an economic point of view, pretreatment is one of the most critical steps in lignocellulosic biomass conversion to bioproducts, and the efficiency of pretreatment is a crucial issue in obtaining a higher product yield. The different natures and complex hierarchical structures of lignocellulosic biomass have presented pretreatment as the most critical step during biomass conversion to biofuels [5]. The most important objectives of the pretreatment step are increasing the biomass surface area, dissolving hemicellulose and/or lignin, and reducing biomass particle sizes. During pretreatment, chemical and/or physical modification of the lignocellulosic structure improves cellulose hydrolysis by cellulases due to the increased accessibility of the substrate surface to enzyme/s [6]. However, the difference in the chemical composition and physical and rheological properties of lignocellulosic biomass of various origins directly affects bio-refinery capacity, increases pretreatment and conversion costs, and reduces yield [7].
Various pretreatment methods serve to fractionate, solubilize, and hydrolyze the biomass and separate cellulose, hemicellulose, and lignin from lignocellulosic biomass. Pretreatment methods are classified into four main types, physical, chemical, physico-chemical, and biological. Physical pretreatment mainly increases the accessible surface area for hydrolysis through biomass size reduction, while some methods also reduce the crystallinity of the lignocellulosic biomass. Chemical methods loosen the holocellulose and lignin structure network and/or dislocate lignin and hemicellulose in the presence of chemicals (e.g., alkalies, acids, organic solvents, etc.). Physico-chemical methods combine harsh conditions such as high temperatures, pressure, and/or the presence of chemicals. In biological pretreatments, natural microorganisms (fungi, bacteria, and microbial consortia) are used to break the cell wall of lignocellulosic material. Biological pretreatment methods are eco-friendly, involve no chemicals, and cause the delignification of lignocellulosic biomass [6].
Therefore, ideal pretreatment procedures must meet specific criteria: (1) increase the surface area accessibility; (2) decrease cellulose crystallinity; (3) increase the hemicellulose and lignin solubility; (4) alter the lignin structure; (5) improve enzymatic hydrolysis to fermentable sugars; (6) diminish the loss of sugars; (7) minimize the formation of by-products that inhibit subsequent hydrolysis and fermentation processes; and (8) be economically profitable [16].
3.1. Physical Pretreatments
Physical pretreatments can open the structure of lignocellulosic biomass by disrupting its surface structure and reducing the particle size using shear or compression forces [17]. The biomass characteristics and final particle size are the most decisive influencing factors for physical pretreatment. However, the high energy consumption and relatively low efficiency are the main drawbacks of this technology [18]. Table 1 shows a review and the mode of action of different types of physical pretreatments as well as the advantages and disadvantages of each.

Table 1.
Mechanisms, advantages, and disadvantages of different physical pretreatment methods.
3.1.1. Extrusion
Extrusion is the most conventional mechanical method of biomass pretreatment where the lignocellulosic biomass is subjected to high temperatures (>300 °C) under shear mixing. The crystalline and amorphous regions of biomass residues are disrupted due to the combined effects of high temperatures in the vessel and the shearing force caused by rotating screw blades. Screw speed and barrel temperature are two factors responsible for the disruption of lignocellulosic biomass and cause fibrillation, defibrillation, and fiber shortening. Depending on the varied extrusion conditions, the method increases glucose from 44% to 66% [17]. The process is cost-effective because it requires significant energy and is difficult to scale up for industrial purposes [18].
3.1.2. Mechanical Pretreatment
Mechanical pretreatment is usually the first step in bioethanol production. This can be carried out by milling, chipping, or grinding. Particle size reduction is needed to simplify the handling material and enhance the specific surface area. The desired particle size is dependent on the subsequent steps that will be followed during the entire process. The raw material size is usually between 10 to 30 mm when chipping and 0.2 to 2 mm in milling or grinding [19]. Processes like vibratory milling, colloid milling, hammer milling, and two-roll milling are used to enhance lignocellulosic biomass digestibility compared to traditional ordinary ball milling [20]. The parameters to be considered for effective milling operation include the biomass feeding rate, initial biomass size, technology parameters, time, and biomass moisture content. Factors like capital and operating costs, possibilities of scale-up, and equipment depreciation are crucial for this process. However, a requirement of high energy input makes it economically non-feasible [21].
3.1.3. Microwave
Microwave-assisted pretreatment of lignocellulosic biomass is a robust and very efficient process in pretreating switchgrass and Miscanthus, resulting in high lignin removal and increased reducing sugar yields, ranging from 40 to 60%. A large microwave irradiator is required for large-scale pretreatment, which is expensive, energy-consuming, and has limited use in industrial processes. The second major disadvantage is the non-uniform heating of biomass and high-temperature generation, which leads to the formation of inhibitors, which lower expected yields and increase operational costs. For higher efficiency and cost-effective results, the microwave method is usually used in combination with chemical pretreatment [21].
3.1.4. Ultrasound
Ultrasound pretreatment is another method used to enhance the digestibility of lignocellulosic biomass through delignification and surface erosion. The processing time is short, and pretreatment is conducted under lower operating temperatures with minimal chemical usage. The results show that when the cellulosic suspension is treated with energy by irradiation, enzymatic hydrolysis is enhanced by approximately 200%, though the mode of action is still unknown. Therefore, it is assumed that the hydrogen bond of the cellulose crystalline structure breaks upon supplying adequate energy. The ultrasound method efficiency could vary depending on the ultrasonic frequency, the solvent used, and the reactor design. This method is often combined with other pretreatment methods, similar to microwave-assisted pretreatment [22].
3.2. Chemical Pretreatments
Chemical pretreatment is the most applied method on a commercial scale. Compared with the other pretreatment methods, this method is considered very promising since it can effectively degrade more complex structured substrates [23]. Chemical pretreatment can be carried out using organic or inorganic compounds, which leads to the disruption of the lignocellulosic structure through interaction with the intra or intermolecular bonds of cellulose, hemicellulose, and lignin [24]. Chemicals, including acids, alkalies, organic solvents, ionic liquids (ILs), deep eutectic solvents (DESs), oxidizing agents, and ozone, are some of the standard chemical pretreatment methods used in biorefinery. The mode of action, advantages, and disadvantages of different chemical pretreatment methods are summarized in Table 2.

Table 2.
Mechanisms, advantages, and disadvantages of different chemical pretreatment methods.
3.2.1. Acids
Acid pretreatment is one of the most widely used pretreatment methods. The technology relies upon certain factors, including the type of acids (weak or strong), their concentrations (diluted to concentrated), liquid-to-solid ratios, and temperature (30 °C to 210 °C). It causes lignin and hemicellulose solubilization and an improvement in cellulose accessibility. The method is suitable for low lignin content biomass since the lignin is not degraded from the raw material [25]. This process has significant limitations in forming inhibitory compounds like furfural, 5-hydroxymethyl furfural, phenolics, etc. [26]. There are two types of acid pretreatments:
- Weak acid hydrolysis or dilute acid hydrolysis is one of the most commonly applied techniques for lignocellulosic biomass. There are two ways to approach this process: first, a continuous flow process at a high temperature is used mainly for low solids loading when T > 160 °C and the substrate concentration is 5–10% wt. The second way is a batch process at low temperature, mainly for high solids loading when T ≤ 160 °C and the substrate concentration is 10–40% wt. Organic acids, like maleic acid and fumaric acid, can be used for this pretreatment method instead of inorganic acids [27]. This method has shown good performance in recovering hemicellulosic sugars, but these sugars might be further converted to furan compounds, furfural, and 5-hydroxymethyl furfural, potent inhibitors of microbial fermentation. Also, acids are corrosive. This method is most suitable for lignocellulosic biomass with low lignin content, as lignin is not removed in this process.
- Strong acid hydrolysis: Sulfuric acid and hydrochloric acid have been used widely to treat lignocellulosic biomass as they are potent reagents for cellulose hydrolysis [28]. Enzymes are not needed after concentrated acid hydrolysis for saccharification. Advantages include feedstock flexibility, high monomeric sugar yield, and mild temperature requirements. The drawbacks are the corrosive nature of the acids, and acid recycling is needed for the economy. Several industries are in the process of the commercialization of the strong acid hydrolysis treatment of lignocellulosic biomass for bioethanol production. Concentrated acid requires corrosion-resistant equipment as it is corrosive and toxic. On an industrial scale, dilute acid treatments are more feasible [28].
Acid pretreatment can also be categorized using two different approaches. The first is high temperatures and short reaction times; the second is low process temperatures and longer reaction times (30–90 min). The main drawbacks of acid hydrolysis are (1) the high energy input requirement as a high temperature is needed, (2) a corrosion-resistant specific reaction vessel being required because of the corrosive nature of acids, and (3) the generation of inhibitory compounds [29].
3.2.2. Alkalies
Similar to acid pretreatment, alkaline pretreatment is one of the major chemical pretreatment technologies. It has shown great promise for the solubilization of lignocellulose biomass using various bases, including NaOH, KOH, Ca(OH)2 (lime), aqueous ammonia, NH4OH, and NaOH in combination with H2O2 or others [23]. Pretreatment with alkalies causes biomass swelling, which increases the internal surface area of the biomass and decreases both the degree of polymerization and cellulose crystallinity. One significant effect of alkali pretreatment is its selectivity to remove mainly the lignin portion of lignocellulosic biomass without degrading carbohydrates. However, it increases the content of the reactive holocellulosic part (total polysaccharide fraction, cellulose, and hemicellulose), which is suitable for enzymatic hydrolysis. They saponify intermolecular ester bonds, which crosslink xylan hemicelluloses and lignin [30]. During treatment, alkalies react with hemicellulose by removing the acetyl groups and various uronic acid substitutions, improving the accessibility of enzymes to hemicellulose and cellulose [31].
Since salts are formed using these compounds, which can be incorporated into the substrate, they need to be recycled or removed [32].
The process conditions are relatively mild, and the reaction time can be very long. Mild conditions prevent lignin condensation, resulting in high lignin solubility, especially for biomass containing low lignin content (softwood, grasses, etc.). Mild conditions also prevent sugars’ degradation to furan compounds and organic acids. Oxygen or air addition to the reaction mixture significantly improves the delignification process [33]. The effectiveness of alkaline pretreatment varies, depending on the substrate and treatment conditions. In general, alkaline pretreatment is more effective on hardwood, herbaceous crops, and agricultural residues with low lignin content than on softwood with high lignin content [34].
3.2.3. Organic Solvents
This method combines organic or aqueous organic solvent mixtures with or without inorganic acid catalysts for delignification. Various organic solvent mixtures have been used, like triethylene glycol, ethylene glycol, ethanol, methanol, tetrahydrofurfuryl alcohol, and acetone under specific temperatures and pressures and with specific catalysts [29].
Organosolv pretreatment has been used extensively for high-quality lignin extraction (value-added product). Due to efficient lignin removal, around 90% of the sugar was recovered after enzymatic hydrolysis of the treated biomass. The main drawback of the organosolv process is solvent and catalyst costs. Solvent removal and recovery can reduce operational costs considerably [28]. As organic solvents are flammable, the safety measures should be impeccable. Uncontrolled use can be the cause of fires and explosions. This additional cost makes the process non-economical. Organic solvents have shown an inhibitory effect on enzymatic hydrolysis; therefore, their removal is required before hydrolysis, which increases operation costs [35].
3.2.4. Ionic Liquids (ILs)
Another pretreatment solvent is ionic liquids (ILs), which represent salts of organic origin with melting points below 100 °C [36].
The use of ionic liquids (ILs) in the pretreatment of lignocellulosic biomass is attracting increasing attention due to the high solubility of biomass in ionic liquids, which results in increased sugar and carbohydrate yields [37].
This method uses biomass with ILs in a ratio of 1:10 w/w, while the temperature ranges from 100 °C to 150 °C. Ionic liquids behave like salt, combining small inorganic anions and large organic cations. They exist as a liquid at relatively low temperatures (room temperature). At high temperatures, room-temperature ionic liquids can form hydrogen bonds with cellulose as anions like formate, acetate, chloride, or alkyl phosphonate are present. It has enormous potential for substrate production, achieving more than 90% cellulose digestibility [38]. However, some significant limitations exist for ILs used for bioethanol production from lignocellulosic biomass. ILs or room temperature ionic liquids (RTIL) that remain in the biomass can interfere with hydrolytic enzyme activity and downstream fermentation processes, which affects sugar and ethanol yields [39]. Also, the disadvantages are the high energy requirement to recycle pure ILs, increased waste generation with difficult recovery, and the high cost of the process. Despite these limitations, the development of this pretreatment could offer significant potential for biorefinery processes in the future.
3.2.5. Deep Eutectic Solvents (DESs)
The utilization of deep eutectic solvents (DESs) as a class of the chemical pretreatment process started in 2015 [40]. DESs are considered as a green solvent like ILs, but comparatively, DESs are cheaper and easier to prepare than ILs [41]. They are used for the delignification of lignocellulosic materials without condensation reactions [42]. The results are the efficient dissolution of biomass components and increased carbohydrate recovery yield, allowing the breakage of the strong intermolecular hydrogen bond [43]. One of the novel classes of DESs, hydrogen bonding DESs (formic and acetic acids), showed successful a delignification rate and cellulose digestibility on rice straw as substrate [44]. However, to fully understand the mechanism of DESs, additional research is needed to improve the efficiency of the pretreatment process.
3.2.6. Oxidizing Agents
The most commonly used one is hydrogen peroxide, which helps the delignification process during treatment. At 25–30 °C, 1–2% H2O2 is effective in the recovery of most hemicelluloses; 50% of lignin can be dissolved using hydrogen peroxide. The results are fivefold higher than with NaOH [45]. During this process, several reactions occur, like electrolytic substitution, alkyl or aryl ether linkage breakage, side chain displacement, or the oxidative breakdown of aromatic nuclei. The result is the formation of carboxylic acid, which acts as an inhibitor to microbial growth and has to be removed by washing [46]. This method is effective for the pretreatment of rice hulls, with it showing 96% sugar conversion after hydrolysis [47].
3.2.7. Ozonolysis
Ozonolysis is a very efficient oxidative pretreatment method. In this method, lignocellulosic biomass is pretreated with ozone, which does not affect the holocellulose portion; it degrades only lignin by attacking its aromatic ring structure. Various types of lignocellulosic biomasses have been treated using ozone, such as wheat straw, bagasse, pine, and peanut [48]. This process usually occurs at room temperature (around 30 °C) and under normal pressure. Inhibitory compounds are not generated during this process; therefore, the saccharification and fermentation are unaffected, and the pretreatment process is sustainable [49]. Two major factors influence this process: the type of lignocellulosic biomass and the moisture content. Ozonolysis is a successful method due to lignin polymer degradation and minimal effects on cellulose and hemicellulose content. Still, the requirement of a large amount of ozone makes the process expensive and non-feasible at an industrial scale [50].
3.3. Physicochemical Pretreatments
Several promising, non-classical methods, including the use of steam explosion (SE) and liquid hot water (LHW) pretreatment, CO2 explosion, wet oxidation (WO), and ammonia fiber expansion (AFEX), have been proposed as commercial solutions for lignocellulosic biomass pretreatments. Table 3 highlights the mode of action and the advantages or disadvantages of the physicochemical pretreatments discussed in this work.

Table 3.
Mechanisms, advantages, and disadvantages of different physicochemical pretreatment methods.
3.3.1. Steam Explosion (SE)
Steam explosion (SE) is also called autohydrolysis and is one of the most promising used lignocellulosic biomass pretreatment methods (catalyzed or uncatalyzed) [51]. Boiling water is maintained as saturated steam (160–250 °C) in a pressured container for a short time (30 s to 20 min); then, the pressure is dropped suddenly to disrupt the recalcitrant nature of the biomass. Hemicellulose is usually broken down with the additional significant transformation of lignin and the conversion of the acetyl branches of hemicellulose to organic acids. The reaction catalyzes favorably and contributes to easier digestion of the lignocellulosic biomass [52]. This method is generally considered a cost-effective and greener pretreatment method compared to chemical processes as no toxic waste is produced from chemicals or catalysts [53].
The SE pretreatment process is carried out in two steps: (1) a steam boiling phase and (2) an explosion phase. The temperatures in this first stage are around 160 °C–260 °C to provoke the hydrolytic breakdown of the lignocellulosic matrix. The process’s second stage corresponds to converting thermal energy into mechanical energy. The pressure suddenly drops down, leading to vapor expansion inside the fibers and disrupting the fibrous structure [54]. Various factors influencing this process are the residence time, reaction temperature, moisture content in the biomass, and raw material particle size. To improve the results, acids or alkalies have been tested. SE’s advantages include the high recovery of sugars, lower capital investment and environmental impact, more energy-efficient strategy, the possibility of using larger particle sizes, the addition of an acid or alkali catalyst, and the feasibility at an industrial scale [55].
3.3.2. Liquid Hot Water (LHW)
Liquid hot water is a hydrothermal pretreatment method requiring no catalyst, chemical addition, or rapid decompression. High pressure is used at high temperatures to maintain the liquid state of water. Hydrothermal pretreatment, hydrothermolysis, aqueous fractionation, solvolysis, or aquasolv terms are also used. The temperature ranges from 170 °C–230 °C, and pressure (>5 MPa) is usually used from seconds to hours during the process [56]. The liquid hot water pretreatment removes hemicelluloses from lignocellulosic biomass, increasing cellulose accessibility. The slurry obtained after pretreatment contains liquid and solid fractions. The liquid fraction contains hemicellulose-derived sugars, and the solid fraction contains cellulose. Better pH control (4–7) minimizes the non-specific degradation of sugars and avoids toxic compound formation [57]. Liquid hot water can primarily result in a high hemicellulosic sugar fraction in the form of oligomers and reduce by-product formation. The residence time and reaction temperature are the most significant factors in this process. Three methods have been developed to promote effective contact between the liquid hot water and biomass, i.e., co-current, counter-current, and flow-through.
In the co-current method, biomass slurry and water are heated to the desired temperature and held for an optimized residence time, after which they are cooled down. In the counter-current method, the slurry biomass and liquid hot water move opposite each other. In the flow-through process, the biomass is kept stationary, and the liquid hot water is passed through it [58]. As no catalyst or corrosion-resistant material is required in this process, it is considered economical. The main advantages are high pentose recovery and lower toxic compound generation. Still, increased water demand and high energy requirements make it non-feasible at an industrial scale [59].
3.3.3. CO2 Explosion
Supercritical carbon dioxide (scCO2) explosion has attracted much attention as a viable and greener alternative than other biomass pretreatment techniques [60]. Supercritical fluids refer to any substance above its critical temperature and pressure and exhibit unique physicochemical properties such as liquid-like solvating power and gas-like mass transfer. Typically used as an extraction solvent in many applications, CO2 has become important as a solvent for the pretreatment of different varieties of biomass. When combined with water, carbonic acid forms, which favors polymer hydrolysis. Once the biomass is pretreated, the explosive release of CO2 disrupts the cellulose and hemicellulose structure, thus increasing the accessible surface area for enzyme hydrolysis. The lower temperatures used in the process aid in the stability of the sugars and prevent the degradation observed in other pretreatments [61].
CO2 is an abundant, non-toxic, non-flammable, and recyclable gas, revealing a low critical temperature and pressure (31.1 °C and 73.8 bar, respectively) [62]. In addition, CO2 is emitted from various industrial production processes, including brewing, ethanol fermentation, and cement production; therefore, it can be supplied in large quantities and at a relatively low cost [63]. Compared to ammonia-based pretreatment methods, this method is cost-effective, and toxic compound generation is much lower than in steam explosion [64].
3.3.4. Wet Oxidation (WO)
Wet oxidation (WO), a pretreatment method of treating lignocellulose with water, air, or oxygen for 30 min at temperatures above 120 °C, has been effective for different biomass resources. Wet oxidation is well-suited as a pretreatment method for producing ethanol and biogas from agricultural residues. This method is suitable for biomass with high lignin content. The process becomes exothermic when the temperature reaches more than 170 °C, making it self-sufficient with heat [65]. This approach for pretreatment catalyzes acid formation from oxidative and hydrolytic reactions. The entire portion of lignocellulosic biomass (cellulose, hemicelluloses, and lignin) is affected during the process. Hemicellulose effectively breaks down into very low molecular weight sugars soluble in water. It also facilitates the de-esterification of hemicellulose acetyl groups to produce organic acids. Cellulose degrades partially, and lignin undergoes cleavage. Due to degradation, the surface area of the cellulose becomes highly accessible for hydrolysis. The addition of alkaline agents (e.g., Na2CO3) enhances hemicellulose solubilization [66].
3.3.5. Ammonia Fiber Expansion (AFEX)
AFEX is another type of pretreatment process in which liquid ammonia is used to treat biomass at a relatively mild temperature (90 °C–100 °C) for a limited time (30–60 min), followed by rapid pressure release [67]. The method involves contacting raw material with gaseous or liquid ammonia at a relatively high concentration (0.3 to 3.0 g ammonia/g dry biomass) within a pressurized vessel. The moisture from the biomass reacts with ammonia to produce ammonium hydroxide. The result is physical disruption of lignocellulosic biomass fibers and the decrystallization of cellulose to some extent. Due to swelling caused by the rapid expansion of liquid ammonia, this process can modify or reduce the effective crystallinity of the cellulosic fiber and lignin matrix [68]. It increases the digestibility of biomass through the deacetylation process. Toxic compounds are not produced using it; washing with water is not compulsory. AFEX is less effective in the pretreatment of woody biomass than agricultural residues and herbaceous biomass [69].
3.4. Biological Pretreatments
The biological pretreatment of lignocellulose biomass is considered a cheap, environmentally friendly, and efficient alternative to conventional pretreatment technologies. The enzyme extracts or the ligninolytic potential of certain microorganisms and macrofungi are used to reduce the raw material’s insubordination and enhance its digestibility by hydrolytic enzymes. However, the enzymes are generally considered costly [70].
Pretreatment can be performed directly by growing the microorganism or macrofungi on the lignocellulosic biomass or indirectly using the enzyme extracts. The typical candidates are filamentous fungi (molds), filamentous macroscopic fungi, and microbial consortia [71].
Based on the composition of the specific lignocellulosic biomass, a suitable microbial consortium or lignocellulosic enzyme mix is used to remove lignin and hemicellulose effectively. The optimum incubation temperature, pH, and incubation time depend on the microbial consortium.
Biological pretreatments are eco-friendly and inexpensive compared to organic solvents or AFEX, where the cost of chemicals and infrastructure is high [72]. However, to carry out biological pretreatment on a large scale, a capacious sterile area is required, and it is necessary to maintain aseptic conditions during the pretreatment process, which may be expensive. The second disadvantage of this technology is that the employed microorganisms consume a significant portion of carbohydrates (cellulose or hemicellulose). This results in lower outputs and increases the treatment cost [70]. The mode of action and the advantages and disadvantages of biological pretreatments are presented in Table 4. Despite environmental benefits, biological pretreatment is still not a viable alternative to conventional methods for large-scale applications, mainly owing to the slow process of the biodegradation of lignocellulosic biomass. Expanded knowledge about existing ligninolytic and cellulolytic systems includes discovering new enzymes with the potential for effective pretreatment and full utilization of lignocellulosic biomass in biorefineries.

Table 4.
Mechanisms, advantages, and disadvantages of different biological pretreatment methods.
3.4.1. Fungal
Over the past few decades, the fungal pretreatment of lignocellulosic biomass has been widely investigated due to the ability of fungi to depolymerize and mineralize lignin. In addition to cellulase-degrading enzymes, endoglucanases, exoglucanases, and β-glucosidases, fungi secrete ligninolytic enzymes, including laccases, lignin peroxidases, and manganese peroxidases [73]. They are generally classified into brown-rot, soft-rot, and white-rot fungi due to their specific ability to utilize the different components of lignocellulosic biomass and the morphological characteristics and the type of decay. White and brown-rot fungi belong to Basidiomycota, while soft-rot are primarily members of the Ascomycota phylum [74].
White-rot fungi such as Phanerochaete chrysosporium, Ceriporiopsis subvermispora, Echinodontium taxodii, Physisporinus vitreus, Pleurotus, and Bjerkandera species possess an oxidative ligninolytic system which degrades lignin and opens the phenyl rings. This system of lignin-modifying enzymes, LMEs, comprises a phenol oxidase, laccase (Lcc, EC 1.10.3.2), and three peroxidases, lignin peroxidase (LiP, EC 1.11.1.14), manganese peroxidase (MnP, EC 1.11.1.13), and versatile peroxidase (VP, 1.11.1.16) [75]. Laccase was first described 130 years ago and is one of the oldest known enzymes. LiP and MnP were first discovered in P. chrysosporium. Versatile peroxidase (VP) is a new addition to the group of LMEs and was found in a strain of Pleurotus eryngii [76].
Brown-rot fungi (Tyromyces balsemeus, Poria placenta, and Lentinus lepidius) can degrade cellulose and hemicellulose by slightly modifying the lignin structure. Endo-cellulases, exo-cellulases, cellobiohydrolase, and β-glucosidases cause cellulose degradation. In contrast, the action of other enzymes such as endo-xylanases, endo-α-L-arabinase, β-galactosidase, and β-glucosidases induces hemicellulose degradation. These microorganisms can completely degrade lignin to CO2 and H2O due to the action of lignin-degrading enzymes such as Lcc, LiP, and MnP regulated by carbon and nitrogen sources [77].
Soft-rot fungi were not classified as a decay type until the 1950s, although their damage to wood was first observed in the 1860s [78]. Most soft-rot fungi belong to the Ascomycota species. There are two types of soft-rot attacks. Type 1 involves the formation of diamond-shaped cavities aligned with the cellulose microfibril angle within the S-2 cell wall layer. Type 2 is a more generalized erosion of the S-2 cell wall layer from the lumen outward [79]. Type 2 attacks can be noticed more often, but some species can produce both types of damage depending on the timber and environmental conditions. Soft-rot fungi are often found in extreme and wetter conditions less suitable for white- and brown-rot fungi. Soft-rot damage presents an exciting mixture of white and brown rot characteristics. Several soft-rot fungi are known to produce laccase, also involved in white-rot fungi lignin degradation. However, they can degrade lignin, as evidenced by the cavities and erosion they cause [79].
3.4.2. Bacterial
Different species of bacteria, such as Bacillus, Clostridium, Streptomyces, Cellulomonas, Microbispora, and Nocardia, can produce cellulases [28,80]. Also, they are known for their characteristics in alteration, solubilization, and lignin degradation. They adapt relatively quickly to new conditions and substrates and multiply after a longer or shorter lag phase. Unlike fungi, bacterial strains degrade lignocellulose by tunneling into the interior cell walls or making erosions in the cellulose microfibrils and have extensive interactions for lignin degradation, which shows the potential to process lignocellulosic waste biomass. Bacterial ligninolytic enzymes are actively involved in degrading phenols, aromatic amines, and other xenobiotic molecules [81].
Aerobic bacteria have a mechanism for breaking down lignocellulosic biomass through the free enzyme system. In contrast, anaerobic bacteria use an alternative lignocellulolytic system that uses complex protein structures such as cellulosomes and xylanosomes as supporting enzymes for hydrolysis lignocellulosic biomass [82]. Bacteria also possess many characteristics for producing hydrolytic enzymes, which are vital for the degradation of lignocellulosic biomass. Several studies have shown the bacterial degradation of lignocellulosic biomass with some identified bacteria such as Acetovibrio, Bacillus, Bacteroides, Cellulomanas, Clostridium, Erwinia, Microbispora, Ruminococcus, Streptomyces, and Thermomonospora [83].
3.4.3. Microbial Consortia
This type of biological pretreatment involves using enzyme-producing microorganisms that can co-exist naturally or through adaptation in pretreatment activities. The enzymes produced are purified and used for pretreatment, or the microbe growth is allowed to yield the enzyme directly into the medium [71]. Single strains or microbial consortia can secrete hydrolytic enzymes during their metabolism, disintegrating the lignocellulosic biomass to convert the biopolymers into smaller fragments [84]. Studies have shown that multi-species consortia produce more active lignocellulosic enzyme complexes than single-strain cultures because they demonstrate higher stability to environmental conditions (temperature, pH, inhibitors, and others) and higher redundancy.
Although biological pretreatment, including single or mixed strain cultivation, has been applied in the hydrolysis of lignocellulosic materials, these strategies are not in accord with the degradation characteristics of lignocelluloses in nature. Lignocellulosic biomass is degraded with the cooperation of many microorganisms that produce various cellulolytic and hemicellulolytic enzymes under aerobic and anaerobic conditions. Microbial consortia are ideal for converting lignocellulose biomass substrates by providing the complex metabolic functions necessary for efficient polymerization. The degradation of cellulosic materials utilizing microbial consortia or complex communities has been proposed as a highly efficient approach for biotechnological application since it avoids the problems of feedback regulation and metabolite repression posed by single isolated strains. Bacteria members of the specialized communities were isolated and identified, like Raoultella/Klebsiella, Enterobacter amnigenus, Arthrobacter intermedia, Citrobacter, and Pseudomonas putida and the fungi species Penicillium citrinum and Coniochaeta ligniaria [85]. Several researchers have successfully constructed a mixed microbial consortium and obtained good results in applying the hydrolysis of lignocellulosic materials [71]. However, although their metabolic degradation capacity was tested, the main reasons for the selection have not been fully clarified.
3.4.4. Enzymatic
Biological pretreatment can also be carried out by adding enzymes directly to the lignocellulosic substrate, thereby eliminating the requirement for microbial growth. Enzymatic pretreatment involves purified, semi-purified, or crude enzymes (oxidative and hydrolytic) principally produced by bacteria and fungi [53]. It is gaining attention due to its relatively short reaction periods and more accessible control. Compared to microbial cells, most cellulolytic enzymes, as free catalysts, are less sensitive to lignocellulose-derived inhibitors [86].
Because of its complexity, the hydrolysis of lignocellulose requires numerous enzymes with different specificities to work synergistically. Those enzyme cocktails performed better than the single enzyme [87]. Also, it was found that the soluble chemical oxygen demand released by the mixed enzyme treatment was more than twice that of the single enzyme [53]. The variation in structure between substrates from different sources and the effect of different pretreatments increases the complexity of developing a standard method. In a generic sense, microorganisms produce two types of enzyme systems for lignocellulose degradation: (1) freely released enzyme systems, which are produced mainly by many aerobic bacteria and fungi, and (2) multi-enzyme complexes named cellulosomes, which are primarily found in anaerobic bacteria such as Clostridium [88].
Three major groups of enzymes are required to work synergistically to degrade cellulose: endoglucanases, exoglucanases (cellobiohydrolases), and β-glucosidases. Endoglucanases (endo-β-(1,4)-glucan hydrolases) are characterized by their hydrolysis of internal β-(1,4)-glucosidic linkages. These enzymes attack the low-crystallinity regions of the cellulose fibers. Exoglucanases (exo-β-(1–4)-glucanases) remove the cellobiose units from the free chain ends. They have a preference for attacking longer chain substrates than β-glucosidases. In addition, β-glucosidases hydrolyze cellobioses and other short-chain β-1,4-oligosaccharides, releasing glucose monomers. Most β-glucosidases are active on a range of β-dimers of glucose [89].
Due to its more complicated composition compared to cellulose, hemicellulose requires a more significant number of different enzymes to be hydrolyzed effectively. Enzymes involved in the degradation of hemicellulose can be divided into depolymerizing enzymes (cleaving the backbone) and those that remove substituents [90].
Two main classes of enzymes catalyze lignin degradation: peroxidases (lignin and manganese) and laccases. These enzymes, working together, lead to the complete degradation of lignin. Lignin peroxidase is a heme-containing glycoprotein that degrades non-phenolic lignin units and requires hydrogen peroxide as the oxidant. Manganese peroxidases act on phenolic and non-phenolic lignin units through lipid peroxidation reactions. They oxidize Mn2+ to Mn3+, which then oxidizes phenol rings to phenoxy radicals, leading to the decomposition of the compound. On the other hand, laccases catalyze the oxidation of phenolic units, phenolic compounds, and aromatic amines to radicals [91]. Industries are significantly expected to implement ligninolytic enzymes for delignification and bleaching systems. Still, no fundamental concept of these discoveries can fulfill the efficiency of its application. Auspicious results have been obtained using ligninolytic enzymes for delignification. However, it still shows a high cost of application or even restriction in performance or technical feasibility, which depends on the enzymatic system.
There are indications that many other enzymes contribute to lignocellulose degradation in poorly understood ways. These so-called accessory enzymes act on less abundant linkages found in lignocellulosic biomass and include carbon-binding modules (CBMs), arabinases, lyases, pectinases, galactanases, and several types of esterases and polysaccharide monooxygenases (PMOs). Among these auxiliary enzymes, the most relevant groups are CBMs, PMOs, and the glucuronyl esterase family-15 class (CE15) [92].
Enzyme catalysis has the characteristic of high efficiency, but the degradation ability of a single enzyme is limited due to the complex and diverse structure of lignin. The overall performance of the enzymatic pretreatment of lignocellulosic biomass is yet to be evaluated thoroughly. The major drawback is the high cost of enzymes, which sometimes may not be commensurate with the yield of products. A techno-economic analysis should be performed to compare it to physicochemical procedures for possible industrial implementation [93].
4. Bioethanol
The production of biofuels is of rapidly growing interest for several reasons, including energy independence, reducing reliance on fossil fuels, the development of rural regions, and a reduction in greenhouse gas emissions [94]. The annual production of biofuels globally increased from 139.4 in 2016 to 174.9 billion liters in 2022, with an incline of 8% due to the COVID-19 pandemic in 2020. The leading producers of biofuels are still the USA, with 57.5 and 14.5 billion liters of bioethanol and biodiesel, respectively, followed by Brazil, with 35.6 billion liters of bioethanol. Still, most of the ethanol is produced from sugar-based feedstocks, sugarcane in Brazil, and corn in the United States of America (USA), while the production of lignocellulosic bioethanol is negligible (<0.2 of total annual production) [95]. Bioethanol is a widely used biofuel for transportation and is available in different blends with gasoline for use in conventional and flexible fuel vehicles. Nowadays, most vehicles use E10 and E15 blends with 10 and 15% ethanol, respectively. In comparison, flexible fuel vehicles run on the blend E85, which contains from 51% to 83% ethanol, depending on geography and the season, and pure hydrated ethanol (93% ethanol; 7% water) [94,96]. Nowadays, bioethanol is commonly produced in bio-refineries, which integrate several processes, generating multiple products, including energy, various chemicals, and biomaterials derived from feedstock/s to enhance the sustainability and economic competitiveness of production and reduce environmental concerns.
4.1. Bio-Refineries
A bio-refinery is a facility for sustainable biomass processing into food/feed ingredients, chemicals, materials, and bioenergy in an environmentally sound, socially acceptable, and cost-competitive manner (Figure 2). The most commonly used biomass is classified into four groups based on origin:
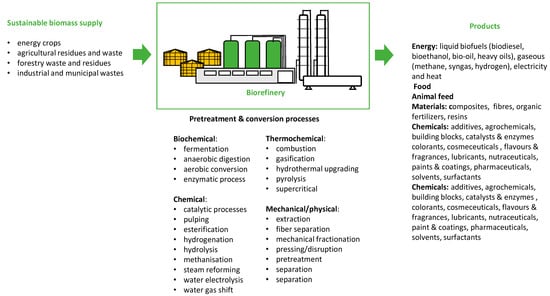
Figure 2.
Bio-refinery concept [97,98].
- (1)
- Energy crops: herbaceous energy crops (e.g., switchgrass, miscanthus, bamboo, AND sweet sorghum), woody energy crops (e.g., hybrid poplar, hybrid willow, silver maple, AND eastern cottonwood), agricultural crops (oil crops, e.g., jatropha, oilseed rape, sunflower, castor oil, palm, and coconut; cereals, e.g., barley, wheat, oats, maize, and rye; and sugar and starchy crops, e.g., sweet sorghum, potato, and sugarcane), and aquatic crops (e.g., giant kelp, other seaweed, and microalga);
- (2)
- Agricultural residues and waste: agricultural residues (e.g., sugar cane bagasse, corn stover, cobs, stalks and leaves, wheat straw, rice straw, rice hulls, nut hulls, and barley straw) and by-products and waste (wood processing by-products, e.g., sawdust, bark, branches, and leaves/needles; animal manure);
- (3)
- Forestry waste and residues harvested for non-commercial purposes (e.g., renewal pruning and forest restoration);
- (4)
- Industrial and municipal waste: e.g., municipal solid waste (e.g., wastepaper, cardboard, wood waste, and yard waste), sewage sludge, and industrial waste. [2].
A similar concept used in bio-refineries has been applied for decades in the food processing industry to produce starch from potatoes, wheat, and corn, crystalline sugar, wine, beer, and vegetable oil, as well as in pulp and paper production. A range of marketable products has been developed from intermediate or final products as well as waste streams such as feed, materials, biomaterials, energy, chemicals, etc. The production of fossil fuels from crude oil in convectional petroleum refineries is based on a comparable concept. In addition to fuels for transport, petroleum refineries generate electricity and various high-value chemicals using optimized and mature technology, creating additional income [97].
Several classification systems of biorefineries are described in the literature based on the source of feedstock, technological implementation status and biomass conversion route used, and product(s) targeted [97]. The commonly used classification is based on the feedstock. First-generation biorefineries utilize cereal (e.g., wheat, corn, rice, and barley), edible oilseed crops (e.g., rapeseed, sunflower, and soybeans), and sugar crops (e.g., sugar beet and sugarcane). Most of the currently produced biofuels are classified as first generation. The production of biofuels in second-generation bio-refineries relies on non-edible feedstocks such as lignocellulosic biomass (agriculture and forest residues such as corn stover and cobs and wheat straw), food waste, and energy crops like Jerusalem artichoke, Miscanthus x giganteus, millet, and jatropha. In third-generation biorefineries, phototrophically grown algal and microalgal biomass is processed into biofuels and other value-added products [98,99].
Feedstock for the production of biofuels greatly affects the economic and environmental sustainability of the process. The production costs for biofuels produced from edible crops (first-generation biofuels) are significantly lower compared to biofuels produced from lignocellulosic biomass and waste (second-generation biofuels), photographically grown algae (third-generation biofuel), or genetically modified microorganisms and crops (fourth-generation biofuel). In 2018, the price of bioethanol produced from sugarcane was USD 0.56/L, while the price of bioethanol produced from sugarcane bagasse was more than two times higher (USD 1.33/L) [100]. The production of advanced biofuels still strongly depends on subsidies and other market interventions to compete economically with fossil fuels. Despite limitations related to the cost-effectiveness in scaling to the commercial level, second- to fourth-generation biofuels have greater potential to reduce greenhouse gas emissions than first-generation biofuels. The environmental and economic feasibility of first-generation biofuel production is questionable since it depends on edible crops that compete with food production for the same feedstock and requires arable land, fertilizers, and water for plant growth. Moreover, increased feedstock demand leads to land use change and deforestation, accelerating climate change and biodiversity loss [98].
4.2. Strategies for Bioethanol Production
Two major routes for converting lignocellulosic biomass to biofuels are (1) biochemical and (2) thermochemical (Figure 3). Gasification, pyrolysis, and hydrothermal liquefaction are extensively used thermochemical methods for the conversion of lignocellulosic biomass. The main products of thermochemical conversion are gases (gasification) and liquids (pyrolysis and hydrothermal liquefaction), which are processed into biofuels and other valuable chemicals. Gasification includes the thermal decomposition of biomass at high temperatures (>800 °C) followed by a partial oxidation process to produce a mixture of gases consisting mainly of carbon monoxide and hydrogen, called synthetic gas or syngas, which are further used for the production of mixed alcohols or Fischer–Tropsch hydrocarbons via catalytic reaction [101,102]. Pyrolysis also involves the thermal decomposition of lignocellulosic biomass, which is conducted at lower temperatures (400–600 °C) in the absence of oxygen. Lignocellulosic biomass is converted into liquid bio-oil, solid biochar, and non-condensable gases rich in CO, CH4, and H2. After purification, bio-oil is used as feedstock for the production of different biofuels [103]. Hydrothermal liquefaction, also known as hydrous pyrolysis, involves the slow thermal decomposition of biomass under pressure from 4 to 22 MPa and temperatures lower than other thermochemical conversion processes (temperature: 250–400 °C). Hydrothermal liquefaction is less energy-intensive and more economically feasible due to lower operating temperatures and the omission of the drying step needed in other thermochemical conversion processes [104].
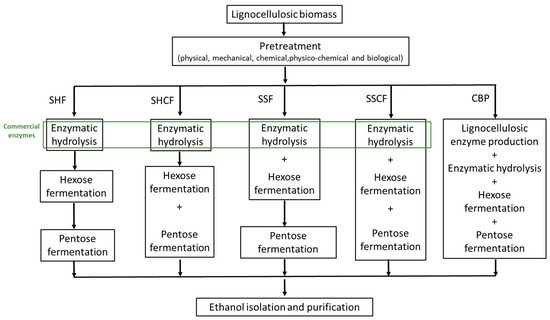
Figure 3.
Process configuration of bioethanol production from lignocellulosic biomass: SHF, separate hydrolysis and fermentation; SHCF, separate hydrolysis and co-fermentation; SSF, simultaneous saccharification and fermentation; SSCF, simultaneous saccharification and co-fermentation; and CBP, consolidated bioprocess.
The biochemical route of bioethanol production involves three main steps: pretreatment, enzymatic hydrolysis, and fermentation. The pretreatment step alters the recalcitrant structure of lignocellulosic biomass, making the structural carbohydrates more accessible to hydrolytic enzymes in the subsequent step. Pretreated biomass is further hydrolyzed to simple sugars by cellulases and hemicellulases. Besides enzymes involved in the hydrolysis of carbohydrates, commercial enzymatic mixtures may also contain lignin-degrading auxiliary enzymes and lignin-modifying enzymes that improve the rate of hydrolysis and yield of simple sugars [105]. The reducing sugar-rich hydrolyzate obtained by enzymatic saccharification is further used as a carbon source for bioethanol production by Saccharomyces cerevisiae.
Lignocellulosic hydrolysate is mostly composed of hexoses (glucose) and pentoses (xylose and arabinose), which theoretically yield 0.51 g ethanol per 1 g of sugar. In most of the current pilot, demonstration, and commercial plants, hydrolysis of lignocellulosic biomass and fermentation of sugars are performed separately or simultaneously. Separate hydrolysis and fermentation (SHF) is a process in which hydrolysis and lignocellulosic biomass are performed in separate tanks under optimal temperature for cellulase and xylanase hydrolysis (45–50 °C) and microorganism growth (30–37 °C; Figure 3). Nevertheless, this process also has several drawbacks that outweigh these advantages, including the higher capital cost for separate tanks, cellulase inhibition by end-products (glucose and cellobiose), and microorganism inhibition by the high osmotic pressure in lignocellulosic slurry. Simultaneous saccharification and fermentation (SSF) lowers the capital costs by performing hydrolysis and fermentation in one tank, consequently decreasing the energy cost, risk of contamination, cellulase and microorganism inhibition, and process time. Furthermore, potential sugar loss is avoided by omitting the filtration step after enzymatic hydrolysis. The major drawback of this process is the conditions (i.e., pH and temperature) under which fermentation and enzyme hydrolysis are conducted. The temperature of ~37 °C at which SSFs are usually running is suboptimal for microbial growth (optimal T = 30–32 °C for yeasts) and cellulase hydrolysis (optimal T = 45–50 °C). The ethanol yield in SSF is generally higher than in SHF [106,107]. Table 5 presents several SSF and SHF processes using different lignocellulosic biomass. In order to further enhance the ethanol productivity and sugar yield by SSF, thermotolerant yeasts that are more resistant to a higher temperature range of 40–45 °C have been applied in production. Several strains, such as Kluyveromyces marxianus, Candida brassicae, and Saccharomyces uvarum have shown relatively high ethanol yields [108,109].
Lignocellulosic biomass may contain 5%–20% or more of the carbohydrates composed of pentose sugars such as xylose and arabinose [110]. Several techno-economic studies have shown that using pentoses for bioethanol production directly contributes to economic ethanol production [111]. Instead of S. cerevisiae which is unable to ferment xylose, bacterial and non-conventional yeast strains that metabolize a wide range of sugars, including xylose, can be used in bioethanol production, e.g., Candida intermedia, Scheffersomyces stipites, Candida shehatae, Kluyveromyces marxianus [108,112,113,114], and Bacillus macerans [115,116]. Despite wide substrate acceptance, the ethanol yield of these microorganisms is significantly lower than S. cerevisiae.
In order to improve the conversion yields of xylose-to-ethanol and ethanol concentration, specific traits of S. cerevisiae, non-conventional yeasts, and Escherichia coli have been modified by genetic engineering. However, the conversion of xylose to ethanol and ethanol productivity is rather low [117,118,119] (Table 5).
In SSH and SSF processes, cellulose- and hemicellulose-containing streams produced by pretreatment are separately fermented. Using wild-type or recombinant microorganisms capable of the simultaneous conversion of pentose and hexose to bioethanol reduces the number of steps in SSH and SSF processes regardless of whether fermentation and hydrolysis are conducted separately or simultaneously (Figure 3). Thus, bioethanol can be produced through the simultaneous scarification and co-fermentation (SSCF) of xylose and glucose or by co-fermentation of these sugars after the hydrolysis step by separate hydrolysis and co-fermentation (SHCF) [106,107].
The conversion of lignocellulosic biomass to bioethanol includes several steps depending on the process configuration, substrate composition, and microorganism characteristics, which inevitably increase the product price. Conversion of the substrate to bioethanol in a single step, used in the production of first-generation bioethanol, would reduce capital and production costs and make the process of second-generation bioethanol more economically competitive. According to techno-economical studies, the most economically feasible strategy for bioethanol production is consolidated bioprocessing (CBP) (Figure 3) [106,120]. This process combines cellulase/hemicellulase production, saccharification, and fermentation. The integrated process relies on microorganisms which, in addition to high specific growth rates, high ethanol yield, and productivity, produce enzymes for lignocellulosic biomass hydrolysis and metabolize pentose and hexose simultaneously. Wild-type microorganisms and genetically modified bacterial, yeast, and fungi strains were applied in CBP, obtaining low productivity and product yields [106,121]. An efficient and robust strain with preferred properties for industrial application has not yet been reported in the literature (Table 5). Davison et al. reported on S. cerevisiae strain coexpressing genes from Trichoderma reesei endoglucanase and Saccharomycopsis fibuligera β-glucosidase and producing 4.05 g/L ethanol with a conversion yield of 83.7% [122]. Another approach includes co-culturing wild-type microorganisms that produce enzymes needed for the hydrolysis of lignocellulosic biomass and the fermentation of a glucose–xylose mixture in a single step. Co-cultivation of Aspergillus oryzae and S. cerevisiae NCYC479 on brewer’s spent grain resulted in a high ethanol yield of 37 g/L [123]. The critical challenge in the further progress of the CBP strategy is still developing efficient microorganisms using advanced methods of genetic engineering, mutagenesis, and adaptive evolution. Alternatively, screening for microbial diversity, in particular, microbial communities living in extreme environments, might reveal novel microorganisms with a combination of favorable properties.
The low ethanol concentration in culture broth produced by batch cultivation on lignocellulosic hydrolysate negatively affects the economy of the fermentation process as well as product isolation and purification. The increased volume of the bioreactors and tanks for hydrolysis enlarges the capital costs for bioethanol production. Therefore, increasing the bioethanol concentration should be one of the goals for improving the cost-effectiveness of the process. Consequently, the bioprocess efficiency would increase, while the capital, labor, and energy costs as well as the water demand would decrease [124].
Thermochemical pretreatment is commonly used as a pretreatment step since it efficiently reduces the recalcitrance of lignocellulosic biomass by degrading the lignin and decreasing cellulose crystallinity. However, pretreatment generates several degradation by-products, such as furans, phenols, and organic acids, negatively affecting microorganism growth, product yield, and cellulase/hemicellulase activity. The inhibition effect is most pronounced in fermentations at high substrate loadings due to the increased inhibitor concentration [58,106,125,126]. The inhibitory effect could be decreased or avoided by applying several approaches. First, implementing SSF instead of SHF enables the microorganism to adapt to the increasing concentration of inhibitors and improve the product yield. Also, different chemical, biological, and physical methods can be applied to detoxify lignocellulosic slurries. High inoculum loading can help to overcome the inhibition effect, or instead of commonly used producing strains, one can adopt microbial species and strains exhibiting resistance to inhibitors. Finally, the adaptation of the producing strain through mutagenesis or improvement through genetic engineering can also be applied [126].
A critical factor in developing a cost-effective biorefinery includes utilizing low-valued feedstocks such as lignocellulosic biomass and efficient conversion into biofuels and different bio-based products that generate income and contribute to the economic feasibility of production. Regardless of the pretreatment method and fermentation configuration employed in bioethanol production, lignin-rich waste streams in the form of black liquor after the pretreatment step and solid (together with cell biomass) obtained by centrifugation of the culture broth after fermentation are obtained. Depending on the lignocellulosic biomass composition and production process, these streams can contain more than 20% (w/w) of lignin calculated based on the total lignocellulosic biomass weight used in the process. Lignin can be used as an energy source for power generation in biorefineries and feedstock for the production of biofuels and valuable chemicals (such as asphalts, bioplastics, biopolymers, and resins). Furthermore, the sugar streams generated in the process can also be used to develop several sugar-based coproducts (Figure 3, [127]).

Table 5.
The efficiency of bioethanol production related to the pretreatment method, used microorganisms, and process configuration.
Table 5.
The efficiency of bioethanol production related to the pretreatment method, used microorganisms, and process configuration.
| Lignocellulosic Biomass | Pretreatment | Enzyme Hydrolysis/Substrate and Conditions | Microorganism | Process Config. | Major Findings | Ref. |
|---|---|---|---|---|---|---|
| Oil palm empty fruit bunches | Two-step pretreatment: (1) 0.2 M H2SO4 at 121 °C for 53 min, and (2) 0.2 M H2SO4 at 121 °C for 53 min; biomass loading: 12.50% (w/v) | Enzyme: 20 FPU cellulase/gLCB a and 4 IU ß-glucosidase/gLCB b substrate loading 10% (w/v) hydrolysis at 37.5 °C for 72 h; | K. marxinus | SHF, batch | Ethanol yield: 0.258 g/gLCB c Ethanol concentration 25.80 g/L | [128] |
| As above | As above | Enzyme: 20 FPU cellulase/gLCB a and 4 IU ß-glucosidase/gLCB b | As above | SSF, batch | Ethanol yield 0.281 g/gLCB c Ethanol concentration 28.10 g/L Substrate loading: 10% (w/v) | [128] |
| g Woody and herbaceous biomass | h Steam explosion conditions: 190–210 °C, 2–8 min depending on lignocellulosic biomass | Enzyme: 15 FPU/gLCB a | K. marxianus CECT10875 | SSF, batch | Ethanol concentration 16.2–19.0 g/L Ethanol yield 60.9–71.2% of theoretical yield Fermentation temperature: 42 °C | [129] |
| Corncob residue | KOH pretreatment | Enzyme: 22 FPU cellulase/gLCB a substrate loading 7.5% (w/v) | S. cerevisiae TC-5 | SSF, fed-batch | Ethanol concentration 31.96 g/L Ethanol productivity 0.222 g/L h Fermentation temperature: 40 °C | [130] |
| Wheat straw | Steam explosion: 220 °C and 2.5 min | Enzyme: 15 FPU cellulase/gC e and 15 IU ß-glucosidase/gC f | Kluyveromyces marxianus CECT 10875 | SSF, fed-batch | Ethanol concentration 36.2 g/L Ethanol yield: 0.33 g/gG d Substrate loading: initial 10 (w/v) + 4% (w/v) addition after 12 h; Fermentation temperature: 42 °C | [131] |
| Sugarcane bagasse | Steam pretreatment with 0.5% (w/v) H2SO4 at 121 °C for 30 min. | 15 FPU/ gLCB a | Saccharomyces cerevisiae | SSF, fed-batch | Ethanol concentration 65.43 g/L Cumulative substrate concentration ~20% (w/w) | |
| Corn cobs | 2% NaOH at 120 °C for 15 min; solid-to-liquid ratio of 1:5 (w/v) | - | S. cerevisiae YI13 co-producing BGLI and EGII | CBP, batch | Ethanol concentration 4.05 g/L Conversion yield (83.7%) after 168 h | [122] |
| Brewers spent grains | Dried and ground | - | Co-culture Aspergillus oryzae and S. cerevisiae NCYC479 | CBP, batch | Ethanol concentration 37 g/L after 10-day incubation at 15 °C | [123] |
a Cellulase loading, FPU per g of lignocellulosic biomass (FPU/gLCB); b ß-glucosidase loading, IU per g of lignocellulosic biomass (U/gLCB); c ethanol yield, g of ethanol per g of lignocellulosic biomass (g/gLCB); d ethanol yield, g of ethanol per g of total glucose in the pretreated lignocellulosic biomass (g/gG); e cellulase loading, FPU per g of cellulose in lignocellulosic biomass (FPU/gC); f ß-glucodidase loading, IU per g of cellulose in lignocellulosic biomass (U/gC); g lignocellulosic woody (poplar and eucalyptus) and herbaceous (Sorghum sp. bagasse, wheat straw and Brassica carinata residue) biomass; hpoplar and eucalyptus biomass, 210 °C, 4 min; wheat straw, 190 °C, 8 min; sweet sorghum bagasse, 210 °C, 2 min and B. carinata residue, 210 °C, 8 min.
4.3. Bioethanol Recovery and Purification
The final step of bioethanol production is the recovery of the product from the culture broth, commonly achieved by conventional distillation. Dehydration methods are further applied to reduce the water content below 0.2% (v/v) or 1.0% (v/v) according to two well-established fuel standards, EN 15376 (Europe) and ASTM D 4806 (USA), respectively [132,133]. Distillation and dehydration are the most energy-intensive steps significantly contributing to greenhouse gas emissions (e.g., CO2). A low bioethanol concentration in the culture broth adds significant capital and operating costs and negatively affects the energy balance of the process. The concentration of bioethanol produced by different bioprocesses using sugar-based substrates is typically between 5–12% (vol /vol), while the concentration of bioethanol produced from lignocellulosic biomass is lower, below 40 g/L (Table 5). According to several studies, the distillation of culture broth with an ethanol concentration below 40 g/L is economically unfeasible [124,134]. Distillation is the most common unit operation based on the relative volatility or boiling temperature difference of the components in the mixture. Distillation of the water–ethanol mixture results in an azeotrope binary mixture containing 95.6% (wt) ethanol at 78.15 °C, and further enrichment of the vaporous phase with ethanol is not possible. Therefore, the process of ethanol recovery and purification on a large scale is conducted in two steps. Diluted ethanol solution, i.e., the culture broth is first concentrated by ordinary distillation at atmospheric pressure to 92.4% (wt) ethanol, followed by dehydration. Several separation methods have been developed for recovering anhydrous ethanol (>99.5%, wt) which include various distillation techniques and hybrid processes that combine distillation with other unit operations for breaking the azeotrope, like adsorption (distillation + molecular sieves) or pervaporation (distillation + membranes) [135,136].
4.3.1. Distillation-Based Processes
In order to separate ethanol from the azeotropic mixture by distillation, the condition and configuration of the distillation process have been modified. Several distillation techniques used for breaking the azeotropic mixture have been reported in the literature: simple vacuum distillation, azeotropic distillation, extractive distillation, and pressure-swing distillation. However, these distillation techniques are tremendously energy-demanding and require high capital costs. Anhydrous ethanol can be obtained by simple distillation under low pressure (0.11 atm), i.e., vacuum distillation. However, due to high operational costs, vacuum distillation is not applied in industrial ethanol separation [135].
In azeotropic distillation, the third component, the entrainer, is added to the azeotropic ethanol–water mixture in order to modify the equilibrium and obtain pure products. The entrainer forms a ternary azeotropic mixture, changing the relative volatilities of the other two components. Various entrainers for the separation of ethanol–water mixtures have been studied: benzene [136,137,138], toluene [139], cyclohexane [136], gasoline additives (tert-amyl methyl ether, TAME) [140], etc. Azeotropic distillation is conducted using two columns: first, a dehydration column for the generation of pure ethanol (>99%), and second, an entrainer column for entrainer recovery. The water–ethanol mixture is introduced into the dehydration column (first column), and the entrainer is fed above the feed tray. The entrainer is recovered from the overhead area of the column, while bioethanol is collected at the bottom. The entrainer can form a homogenous (homogenous azeotropic distillation) or heterogeneous mixture (heterogeneous azeotropic distillation) with ethanol–water solution along the distillation column. If a heterogeneous mixture is formed, the overhead vapor is condensed and fed to decenter to separate the ethanol–entrainer and water–entrainer streams. The ethanol–entrained phase is refluxed back to the dehydration column (first column), while the water–entrainer phase is further processed in the second column for the recovery of the entrainer which is fed to the dehydration column [135].
Like azeotropic distillation, extractive distillation also requires an entrainer to separate ethanol from an aqueous solution. For a long time, it was considered as a special type of azeotropic distillation. However, these two types of distillations have different process configurations and obey different feasibility rules [141]. Various types of entrainers have been studied in the literature: liquid solvents (e.g., ethylene glycol [142], glycerol [143], dissolved salts (e.g., lithium chloride, calcium chloride, sodium chloride, and potassium chloride [144]), ionic liquids (e.g., 1-butyl-3-methylimidazolium chloride, 1-ethyl-3-methylimidazolium tetrafluoroborate) [145], 1-ethyl-3-methylpyridinium ethylsulfate [146], hyperbranched polymers (polyesters and polyesteramides and polyethylene glycol) [147], and complex solvents [148]. Extractive distillation configuration depends on the entrainer used for ethanol purification. Conventionally, liquid solvent with a high-boiling point used as an entrainer is introduced in the extractive distillation column above the ethanol–water feed. The extractive column is divided into three sections: the rectifying section (above the entrainer feed), the extractive section (between two feeds), and the stripping section (stages below the ethanol–water mixture feed). The addition of an entrainer increases the relative volatility of the ethanol without forming the new azeotrope. Ethanol goes to the top of the column while water collects at the bottom of the distillation column with added solvent. The solvent is recovered by distillation and returned to the extractive section of the extractive column [149]. The main disadvantage of this type of extractive distillation is the high solvent-to-feed (ethanol–water) mass ratio, which exceeds 5:1, entailing large energy consumption for costs involved in reboiler and condenser duty (extractive distillation) and entrainer recovery (second distillation column). A substantial energy saving could be achieved using soluble salts instead of liquid solvents. Dissolving salt in an ethanol–water mixture enhances the relative volatility of ethanol due to the salting-out effect on ethanol. The salt is directly introduced at the top or near the top trey of the extractive distillation column. Salt dissolves in the reflux stream, flows downward along the column concurrent with the water stream, and collects as the bottom product. It is further recycled by evaporation or drying. Ethanol (extracted component) evaporates due to higher relative volatility compared to water and collects as a top distillate product free of the entrainer. When potassium acetate is used as an entrainer instead of ethylene glycol, the ratio of entrainer to feed is reduced by 50 times (0.06 mol/mol). Due to the low entrainer-to-feed ratio, extraction distillation with dissolved salt is much less energy intensive and has a higher production capacity than extraction distillation with liquid solvents. Furthermore, salts are less toxic and not volatile, so they do not contaminate the overhead product. Ionic liquids (ILs) have also been investigated as alternative entrainers that could replace conventional volatile organic solvents in extractive distillation due to unique properties such as non-volatility, thermal stability, environmental benignity, and easy recovery after extraction. The addition of ILs has a similar effect on the separation of components of an azeotropic mixture such as dissolved salt. ILs can be easily recovered using simple flash distillation or gas stripping [146,150].
Unlike the other distillation methods, pressure-swing distillation does not depend on an entrainer to separate the ethanol–water azeotrope. It relies on the change in azeotrope composition with applied pressure. Two distillation columns are used, the first operating at high pressure and the second operating at low pressure. In the case of an azeotrope with minimum boiling, such as water–ethanol, high-purity product streams are collected on the bottom of columns while the distillate stream from the high-pressure column is recycled back to the low-pressure column. Iqbal and Akhlaq produced nearly pure ethanol (99.7%, mol/mol) obtained at the bottom of the high-pressure column (10 atm), while water (99.5%, mol/mol) was collected at the bottom of the first column (1 atm) [151].
4.3.2. Adsorption
Adsorption is a cost-effective and environmentally friendly alternative to extractive and azeotrope distillation and is used on a large scale to purify ethanol. Many traditional porous adsorbents have been applied to capture either water or ethanol from distillates and culture broths, such as zeolites [152,153,154,155], silicate [156], activated carbons [157], composite adsorbents (e.g., polyvinyl alcohol/zeolite/carbon composites, and composite silica–divinylbenzene) [158,159,160], polymeric resins, activated carbons metal−organic frameworks [160,161], etc. Biomass has also been investigated as an alternative adsorbent since it is an abundant, eco-friendly, inexpensive, and easily regenerated material. Various starch and lignocellulose-based biomaterials have been applied for that purpose, including canola meal [162], oat hull, flax shives [163], cassava starch [164], rice straw, and paddy husk [165].
Depending on the configuration of the ethanol production and purification process, the adsorbent is applied to the ethanol–water mixture in the vapor or liquid phase. A vaporous stream leaving the top of the distillation column after the first purification step by conventional distillation can be directly introduced to the adsorption column, avoiding additional vaporization steps and reducing production costs. Regeneration of the adsorbent for next cycle application is usually carried out by heating (for zeolites: 200–250 °C), lowering the pressure, and reducing the pressure in combination with purging the adsorbent bed with an inert gas [152].
The similar physical–chemical properties of water and ethanol make the search for suitable adsorbents challenging. Both molecules are highly polar and bind strongly on common adsorbents. The molecular size and polarity of water and ethanol are 2.8 and 4.4 A and 0.36 and 0.65, respectively [135,152,153]. The separation efficiency depends on the pore dimensions of the microporous structure of the adsorbent with respect to the molecular sizes or interactions between the adsorbent and components of the mixture. The adsorbent can also be applied to the liquid phase containing ethanol–water binary mixtures or complex mixtures such as fermentation broth. The 3A and 4A zeolite molecular sieves (with microspores smaller than 3 and 4 Angstroms, respectively) have been used to dry ethanol from liquid and vapor ethanol–water mixtures. Unlike ethanol, water molecules easily penetrate into the microporous channels of molecular sieves and adsorb [152,153].
Although bio-based adsorbents have lower separation capacity than adsorbents applied to large-scale ethanol purification (e.g., zeolites), the main advantage of using adsorbents is the possibility of omitting the regeneration step. The water-saturated adsorbent can be directly used as feedstock (substrate) for bioethanol production and fresh biomass for the ethanol dehydration step. Many conventional adsorbents have been studied for ethanol separation, but most of them exhibit stronger interactions with water than ethanol. Since water is the main component in the cultivation broth, it is essential to capture the component present in relatively low concentrations, i.e., ethanol. Therefore, designing new adsorbents that selectively capture ethanol from dilute ethanol mixtures is crucial for the feasibility of bioethanol production. Another advantage of in-situ ethanol removal is an increase in process productivity. Applying zeolite NaZSM-5 for in situ ethanol recovery during fermentation in bioreactor growth inhibition by-products was avoided, improving the process efficiency [166].
4.3.3. Membrane Separation
Over the last few decades, membrane separation processes and materials have been extensively studied and evaluated. The membrane process emerged as a sustainable and energy-efficient alternative to conventional azeotropic distillation. Membrane systems are built in a modular form that is adaptive to any particular demand. The main characteristics are easy operation, low maintenance, and low space requirements. Different membrane processes have been employed for bioethanol isolation and dehydration, including membrane distillation [167], pervaporation [168,169,170], vapor permeation [171], nanofiltration [172], reverse osmosis [173], and forward osmosis [174].
Pervaporation is one of the most studied processes for ethanol dehydration (ethanol–water mixture with high ethanol concentration) and isolation and purification from diluted mixtures, i.e., culture broth. The membranes comprise a selective layer that accomplishes separation and a porous support layer to provide mechanical strength. The ethanol-containing stream is brought into contact with the selective side of the membrane. The membrane acts as a semipermeable barrier that allows specific component(s) to permeate the membrane to the permeate side and retain the other components of a mixture (retenate). The three steps in pervaporation separation are the sorption of the specific component on the feed side of the membrane, permeation through the membrane, and desorption by evaporation on the permeate side where the permeate vapor is condensed at atmospheric pressure (condenser). The driving force for molecules’ permeation through the pervaporation membrane is a difference in chemical potential on both sides of the membrane. The chemical potential difference is maintained by purging with inert gas (sweep gas pervaporation) or applying a vacuum (vacuum pervaporation) on the permeate side of the membrane or by keeping the temperature difference (thermopervaporation) on both sides of the membrane [135,175].
Pervaporation membranes are symmetric (dense) and asymmetric (dense/porous) based on their morphology. Due to limited permeate flux through relatively thick layers, dense membranes are unsuitable on an industrial scale. Asymmetric membranes composed of a thin separation layer on the surface of microporous support has a higher total flux of permeate compared to dense membranes. Pervaporation membranes are manufactured in three types of geometries: flat-sheet, tubular, and hollow-fiber membranes [168]. Membranes used for the separation of binary ethanol–water mixtures are ethanol-selective (hydrophobic) or water–selective (hydrophilic). Pervaporation with ethanol-selective hydrophobic membranes is considered the most promising alternative to the conventional processes currently used for ethanol recovery on a large scale since the concentration of ethanol in the culture broth is rather low. Based on the materials used for manufacturing membranes, they are polymeric, inorganic, and mixed-matrix membranes made of polymeric and inorganic materials [135,175,176].
Inorganic membranes are produced from silica, alumina (Al2O3), or zeolite. Due to their narrow pore size distribution and intrinsic surface properties, these membranes exhibit excellent separation performance, including having a high separation factor and permeation flux. Furthermore, inorganic membranes have high solvent resistance, temperature stability, and mechanical strength. Unlike polymeric membranes, they do not suffer from swelling problems [177]. Zeolites are hydrated aluminosilicates characterized by pores and cavities of molecular dimensions. Zeolite membranes consist of a polycrystalline zeolite layer on top of a porous inorganic layer which provides mechanical strength with low mass-transfer resistance. These membranes can be water- or ethanol-selective. The most studied hydrophobic ethanol selective zeolite membranes are silicate-1 (pure silica) and ZSM-5 (some Si atoms substituted by Al). The silicate-1 membrane is more hydrophobic than ZSM-5 since it does not contain aluminium atoms in its structure. Incorporating tri and tetravalent elements, such as aluminium, boron, germanium, and iron zirconium, into a zeolite structure change the pore size and hydrophobicity of the membrane, affecting the sorption and diffusion properties of the zeolite material and changing the membrane flux and selectivity (e.g., Al-ZSM-5, B-ZSM-5, etc.) [168]. The best-performing ethanol selective membrane, Ti-silicate-1, with a separation factor of 127 and permeate flux of 770 g m−2 h−1 has substituted titanium atoms into the silicate-1 structure [Chen et al., 2008]. For the dehydration of ethanol solutions containing 5–10% of water the following inorganic materials are used: hybrid silicas and three types of zeolite, Linde Type A (NaA), Chabazite (CHA) and T-type, and are reported in the literature. The Linde Type A (LTA) zeolite has incorporated sodium counter ions in the 1:1 Si:Al aluminosilicate lattice, also known as NaA or 4A zeolite [169,178]. The stability of NaA membranes in acidic and high-water activity environments has been improved using more stable zeolite materials recently developed and commercialized. Lower Al content in T-type membranes compared to NaA zeolite decreases their hydrophobicity and improves acid stability [169].
Mixed-matrix membranes (MMM) combine the favorable characteristics of inorganic and polymeric membranes, easy processability and low costs of polymeric materials, and the superior selectivity and high stability of inorganic materials. They consist of inorganic moieties in the form of micro or nano-particles (discrete phase) incorporated into a polymer matrix (continuous phase) [168,170]. Most of the ethanol-selective mixed-matrix membranes are based on polydimethylsiloxane (PDMS), although other materials have been studied, such as poly(1-trimethylsilyl-1-propyne); PTMSP [179,180], poly(ether block amide) [181], poly(methylphenylsiloxane) [182], etc. The most common inorganic materials used as filler are zeolites (silicalite-1 and ZSM-5) [183], fumed silica [184], metal–organic frameworks [185], zeolitic imidazolate frameworks (ZIFs) [186], and carbon nanotubes [187], etc.
Diverse polymeric materials have been extensively investigated as potential materials for pervaporation membrane manufacturing. Hydrophobic polymeric membranes made of polydimethylsiloxane (PDMS) and poly(1-trimethylsilyl1-propyne) (PTMSP) have been widely applied in ethanol removal from diluted ethanol solution. The separation factor and permeation fluxes for PDSM membrane are generally below 10 and 1000 g m−2 h−1, respectively. PTMSP membranes exhibit better separation characteristics than PDMS membranes (so-called silicone rubber), with separation factors ranging from 9 to 26. Despite good performance characteristics, PTMSP membranes have not been applied on a large scale due to membrane deterioration caused by the chemical and physical degradation of polymer structure. In order to improve the separation characteristics and stability, PDMS and PTMSP membranes have been modified. Various modifications of these membranes have been reported in the literature, including blending, blocking, or grafting with different polymers by incorporating fillers forming the mixed-matrix membrane (MMM) [168]. Furthermore, asymmetric composite membranes consisting of thin selective layers of polymer on highly porous support material exhibited improved separation performance. Li et al. synthesized a composite membrane consisting of a PDMS ultra-thin selective layer (thickness from 0.5 to 8 μm) on porous polytetrafluoroethylene (PTFE) support. The membrane exhibits a high flux of 2016 g m−2 h−1 with a separation factor of 12 for the separation of ethanol from a 5 wt% solution with stable operation over 200 h [188]. Furthermore, several other polymers have been proposed for the recovery of alcohol diluted aqueous solutions by pervaporation, including homopolymers and copolymers of siloxane (e.g., polymethylethoxysiloxane (PMES), polymethylphenylsiloxane (PMPS), polyphenylmethylsiloxane (PPMS), polydimethylsiloxane-imide (PDSI), polysiloxaneimides (PSI), polyoctylmethylsiloxane (POMS), etc.), poly(vinyltriethoxysilane) (PVTES) and PVTES copolymerized with other substances (e.g., dimethyldiethoxysilane and PDMS), poly(ether block amide) (PEBA), polytetrafluoroethylene (PTFE), perfluoroprpane (PFP), etc. [168]. Compared to inorganic membranes, polymeric membranes have high processability, lower separation performance (separation factor, permeate flux, and stability), and low cost [189].
4.3.4. Advanced Hybrid Processes
The high operational costs of the distillation-based process encourage the development of new innovative separation processes. Integrating bioethanol separation with the fermentation process enables maintaining bioethanol at low concentrations by continuous removal. Avoiding yeast inhibition increases ethanol yield and process efficiency, shortens the fermentation time, and decreases the production costs. Several advanced hybrid processes for in situ bioethanol removal have been described in the literature, which combine fermentation with vacuum fermentation [190,191], flash fermentation [113,191,192], gas stripping [193,194], adsorption [195,196,197], solvent extraction [198,199,200,201,202], and membrane pervaporation [168,203,204].
In vacuum fermentation, a bioreactor is maintained under vacuum using a vacuum pump, allowing ethanol to boil off from the culture broth at the fermentation temperature. Ethanol is recovered by condensation in the condenser. Also, carbon dioxide, a known inhibitor of yeast metabolism, is also removed, improving the fermentation rate. Due to continuous product and by-product removal, high productivity is obtained, especially when substrate feeding is applied (fed-batch cultivation). Huang et al. studied bioethanol production by vacuum fermentation using food waste as substrate at high solid loading (35%, w/w) and obtained complete sugar conversion and increased the product yield (358 g of ethanol /kg substrate by vacuum fermentation versus 327 g of ethanol /kg substrate in conventional fermentation) [190]. However, several difficulties are connected with applying a vacuum during fermentation. Carbon dioxide generated by yeast metabolism must be compressed from the bioreactor pressure up to atmospheric pressure, increasing production costs [205]. Furthermore, yeast cells have to be supplied with oxygen to ensure growth and product accumulation. Instead of air, culture broth has to be aerated by pure oxygen due to the low solubility of oxygen under reduced pressure [191].
These difficulties are overcome in flash fermentation, where the fermentation process is separated from ethanol recovery. The low ethanol concentration is maintained by the periodical circulation of culture broth to a vacuum chamber while the bioreactor remains at atmospheric pressure. Since the fermentation is conducted at atmospheric pressure, dissolved oxygen levels can be maintained by adding air instead of pure oxygen. Furthermore, this process configuration enables a reduction in compression costs since carbon dioxide is not compressed [191,192].
Gas stripping is a simple, low-cost method for the in situ removal of ethanol. Stripping gas, air, CO2, or N2 is sparged in the culture broth, causing the evaporation of volatile compounds, including bioethanol, which is further recovered in a condenser. The main benefits of in situ ethanol removal are the improvement of productivity and the reduction in process water and energy use, especially in fermentation conducted at higher substrate loadings. Nevertheless, applying high substrate loadings in batch and fed-batch fermentations accumulates toxic non-volatile compounds in culture broth that can originate from yeast metabolism or pretreated lignocellulosic biomass [194]. Wang et al. continuously removed produced bioethanol during the simultaneous saccharification and solid-state fermentation of rice straw using nitrogen as the stripping gas. In situ product removal resulted in low concentrations of bioethanol and high substrate consumption [193].
Bioethanol and inhibitors from pretreated lignocellulosic biomass can be removed by liquid–liquid extraction by mixing culture broth with organic solvent during fermentation. Desirable solvent characteristics are high capacity for ethanol (described by equilibrium distribution coefficient, KD), high selectivity for ethanol over water, and biocompatibility (nontoxicity) with producing microorganisms. The extracted bioethanol can be recovered by distillation or back extraction. Numerous solvents were tested as extractants for ethanol recovery from culture broth, including alcohols, esters, alkanes, hydrocarbons, synthetic aliphatic hydrocarbon solvents, ketones, amines, acids, chlorinated hydrocarbons, aromatics, polymers, ionic liquids, etc. [198,199,200,201]. Despite high selectivity, alcohols and esters show toxicity to ethanol-producing microorganisms [198]. In order to avoid cell death, extraction can be conducted in separate containers with cell-free culture broth instead of in a bioreactor. The test against the producing microorganism showed that toxicity of alcohol decreases with carbon chain length. Thus, toxicity experiments showed growth inhibition of Zymomonas mobilis by n-amyl alcohol. However, using n-amyl alcohol as a solvent in extractive fermentation with Zymomonas mobilis improved ethanol yield and productivity compared to conventional fermentation [202].
Adsorption by a molecular sieve is a commonly used method for removing the remaining water from bioethanol–water mixtures in the final step of the commercial production of fuel-grade bioethanol. The adsorption method has also been applied in several studies for the in situ removal of bioethanol from culture broth during fermentation to maintain the low bioethanol concentration and avoid inhibition. The main disadvantages of the direct application of adsorbent in fermentation broth are a decrease in adsorbent capacity due to the unspecific binding of microbial cells and fermentation broth components (nutrients and cell metabolites), the toxicity of adsorbents to microbial cells, a decrease in adsorbent lifetime due to irreversible adsorption, etc. The toxicity of the adsorbents to cells could be overcome by using immobilized microorganisms instead of free cells or by conducting the adsorption in separate steps (e.g., fluidized bed or fixed-bed column) with cell-free culture broth after cell separation [191,195,196,197]. Jones et al. used F-600 activated carbon in bioethanol fermentation using E. coli KO11 (ATCC 55124). The direct addition of an optimized quantity of adsorbent to the fermentation broth improved ethanol yield and resulted in the complete depletion of the carbon source [195]. Hashi et al. studied in situ bioethanol removal by combining carbon dioxide stripping and the adsorption of bioethanol onto activated carbon WV-B 1500. A mathematical model was developed to predict the performance of the adsorption column [196]. A similar approach was applied by Seo et al. in recovering pure bioethanol. Bioethanol was first recovered from fermentation broth in three steps: gas stripping of bioethanol from the culture broth, preconcentration from the gaseous phase by adsorption onto molecular-sieving carbon (5A), and selective dehydration driven by the molecular-sieving effect during the desorption phase [197].
Pervaporation is an effective and energy-efficient separation method for the dehydration of alcohols from water–alcohol mixtures like ethanol, isopropanol, butanol, etc. Nowadays, pervaporation is commercially used for the dehydration of the azeotropic ethanol-water mixture at a large scale in the production of fuel-grade bioethanol. Pervaporation is also a promising method for in situ bioethanol recovery from diluted complex mixtures such as fermentation broth since the pervaporation membranes are nontoxic to microorganisms and conditions under which the pervaporation process is conducted, which do not influence cell growth [206]. Polydimethylsiloxane (PDMS) is mostly studied for the separation of ethanol from aqueous solution on a lab scale due to its excellent stability and satisfactory separation performance [203]. The dramatic decline in pervaporation membrane performance (bioethanol permeability and selectivity) is observed upon membrane exposure to fermentation broths. Deterioration of membrane performance is attributed to the adhesion of components in culture broth such as sugars, inorganic salts, extracellular polymers like lipids, polysaccharides and peptides, cell metabolites, and biofouling of the membrane surface through the adhesion of microorganisms and growth in the form of biofilm [168,203]. Therefore, the design of a hydrophobic pervaporation membrane with anti-fouling properties is critical for the application of pervaporation in the recovery of bioethanol from fermentation broth. Lowering the membrane surface hydrophobicity not only decreases the adhesion of culture broth components and cells to a membrane but also decreases separation performance. Biofouling of the membrane could be decreased by using immobilized cells in a fluidized bed or fixed-bed column instead of free cells (stirred tank bioreactor) or cell-free culture broth (after cell harvesting by centrifugation or microfiltration), which can be used as a feed stream in the pervaporation module [204]. Vapor permeation, currently used in the dehydration of organic solvents from aqueous mixtures at an industrial scale, is a more suitable method for the separation of bioethanol from fermentation broth since direct contact between the membrane with the culture broth is avoided [207,208].
5. Conclusions
The current global energy crisis and environmental issues are the major problems facing our society today. Biofuels produced from renewable and sustainable feedstocks through environmentally friendly routes enhance energy security, mitigate climate change, and contribute to rural development and economic growth. Second-generation bioethanol production in a biorefinery manner relies on using abundant and low-cost biomass such as plant biomass, agriculture, forestry, and industrial and municipal waste rich in lignocellulose. The production costs of lignocellulosic bioethanol significantly exceed the cost of bioethanol production from sugar- and starch-based feedstocks due to the complexity of production and high energy consumption. The main challenges in production include lignocellulosic recalcitrance, high enzyme costs, the inhibition of cellulases, hemicellulases and microorganisms by simple sugars and lignocellulose-derived inhibitors, low bioethanol yield, and the high energy demand for bioethanol recovery and dehydration. The pretreatment step is one of the costliest and most energy-intensive steps in second-generation biorefineries, which reduces the recalcitrance structure of lignocellulosic biomass and improves the sugar yield by hydrolysis. The major disadvantage of the physico-chemical pretreatments, which have been recognized as the most cost-effective methods for large-scale production, is the generation of diverse inhibitors that decrease the enzyme cellulase and hemicellulase activity and microorganism growth rate. Therefore, the improvement of established and the design of new sustainable pretreatment methods with lower energy and/or chemical consumption, reduced water use, minimized inhibitor generation, an improved delignification rate, and carbohydrate recovery is needed. Moreover, the design of new genetically modified microorganisms with preferable traits, including cellulolytic and hemicellulolytic activity, simultaneous assimilation of hexose and pentose sugars, and high resistance to inhibitors would improve the production yields, simplify the production process, and reduce the production costs.
Despite the intensive research on developing new and improving conventional bioethanol separation methods in the last few decades, distillation-based methods are still used in recovering bioethanol from fermentation broth. In recent years membrane separation techniques have emerged as a sustainable and energy-efficient alternative to conventional methods. The application of hybrid processes consisting of azeotropic distillation and membrane pervaporation for the dehydration of azeotropic ethanol–water mixtures have decreased the energy and operating costs of large-scale bioethanol separation. New advanced hybrid methods for in situ bioethanol recovery integrate fermentation with bioethanol separation. These methods can potentially reduce the energy and operating costs of bioethanol recovery, but further research is needed for their optimization before transfer to large-scale production.
Author Contributions
S.B., conceptualization and writing—original draft preparation; K.M., writing—review and editing; B.Š., editing and project administration; M.I.Š., conceptualization, writing—review and editing, and supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Croatian Science Foundation under the project “Sustainable production of biochemicals from waste lignocellulose containing feed-stocks” (Croatian Science Foundation no. 9717) and the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Contract No. 451-03-47/2023-01/200135 and 451-03-47/2023-01/200287).
Data Availability Statement
Not applicable.
Acknowledgments
This work was supported by the Croatian Science Foundation under the project “Sustainable production of biochemicals from waste lignocellulose containing feed-stocks” (Croatian Science Foundation no. 9717), the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (Contract No. 451-03-47/2023-01/200135 and 451-03-47/2023-01/200287), and the Bilateral Project Serbia/Croatia (337-00-205/2019-09/35) (2019–2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2023, 10, 29. [Google Scholar] [CrossRef]
- Maity, S.K. Opportunities, recent trends and challenges of integrated biorefinery: Part I. Renew. Sustain. Energy Rev. 2015, 43, 1427–1445. [Google Scholar] [CrossRef]
- Deora, P.S.; Verma, Y.; Muhal, R.A.; Goswami, C.; Singh, T. Biofuels: An alternative to conventional fuel and energy source. Mater. Today Proc. 2022, 48, 1178–1184. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess 2017, 4, 7. [Google Scholar] [CrossRef]
- Kumar, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of second generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Ambat, I.; Srivastava, V.; Sillanpää, M. Recent advancement in biodiesel production methodologies using various feedstock: A review. Renew. Sustain. Energy Rev. 2018, 90, 356–369. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hagerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Amoah, J.; Ogura, K.; Schmetz, Q.; Kondo, A.; Ogino, C. Co-fermentation of xylose and glucose from ionic liquid pretreated sugar cane bagasse for bioethanol production using engineered xylose assimilating yeast. Biomass Bioenergy 2019, 128, 105283. [Google Scholar] [CrossRef]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.-S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Tišma, M.; Busić-Kojić, A.; Planinić, M. Bio-based products from lignocellulosic waste biomass; a state of art. Chem. Biochem. Eng. Q. 2021, 35, 139–156. [Google Scholar] [CrossRef]
- McCann, M.C.; Carpita, N.C. Biomass recalcitrance: A multi-scale, multi-factor, and conversion-specific property. J. Exp. Bot. 2015, 66, 4109–4118. [Google Scholar] [CrossRef] [PubMed]
- Weidener, D.; Dama, M.; Dietrich, S.K.; Ohrem, B.; Pauly, M.; Leitner, W.; Dominguez de Maria, P.; Grande, P.M.; Klose, H. Multiscale analysis of lignocellulose recalcitrance towards OrganoCat pretreatment and fractionation. Biotechnol. Biofuels 2020, 13, 155. [Google Scholar] [CrossRef]
- Rajeswari, G.; Jacob, S.; Kumar Chandel, A.; Kumar, V. Unlocking the potential of insect and ruminant host symbionts for recycling of lignocellulosic carbon with biorefinery approach: A review. Microb. Cell Fact. 2021, 20, 107. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar]
- Tsapekos, P.; Kougias, P.G.; Egelund, H.; Larsen, U.; Pedersen, J.; Trenel, P.; Angelidaki, I. Mechanical pretreatment at harvesting increases the bioenergy output from marginal land grasses. Renew. Energy 2017, 111, 914–921. [Google Scholar] [CrossRef]
- Perez-Rodríguez, N.; García-Bernet, D.; Domínguez, J. Faster methane production after sequential extrusion and enzymatic hydrolysis of vine trimming shoots. Environ. Chem. Lett. 2018, 16, 295–299. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef]
- Jedrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and chemical pretreatment of lignocellulosic biomass. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Elsevier: Amsterdam, Netherlands, 2019; pp. 143–196. [Google Scholar] [CrossRef]
- Bussemaker, M.J.; Zhang, D. Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind. Eng. Chem. Res. 2013, 52, 3563–3580. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Hernandez-Beltran, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Gupta, A.; Verma, J.P. Sustainable Bio-Ethanol Production from Agro-Residues: A Review. Renew. Sustain. Energy Rev. 2015, 41, 550–567. [Google Scholar] [CrossRef]
- Liu, Z.; Fels, M.; Dragone, G.; Mussatto, S.I. Effects of inhibitory compounds derived from lignocellulosic biomass on the growth of the wild-type and evolved oleaginous yeast Rhodosporidium toruloides. Ind. Crops Prod. 2021, 170, 113799. [Google Scholar] [CrossRef]
- Ilanidis, D.; Wu, G.; Stagge, S.; Martín, C.; Jönsson, L.J. Effects of redox environment on hydrothermal pretreatment of lignocellulosic biomass under acidic conditions. Bioresour. Technol. 2021, 319, 124211. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Cho, J.; Talaiekhozani, A.; Sabbagh, F.; Hashemi, B.; Rupani, P.F.; Mohammadi, A.A. Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy 2020, 199, 117457. [Google Scholar] [CrossRef]
- Kim, S.-J.; Brandizzi, F. The plant secretory pathway for the trafficking of cell wall polysaccharides and glycoproteins. Glycobiology 2016, 26, 940–949. [Google Scholar] [CrossRef]
- Chang, V.S.; Holtzapple, M.T. Fundamental factors affecting biomass enzymatic reactivity. In Twenty-First Symposium on Biotechnology for Fuels and Chemicals, Proceedings of the Twenty-First Symposium on Biotechnology for Fuels and Chemicals, Fort Collins, CO, USA, 2–6 May 1999; Human Press: Totowa, NJ, USA, 2000; Volume 84–86, pp. 5–37. [Google Scholar] [CrossRef]
- Morais de Carvalho, D.; Queiroz, J.H.; Colodette, J.L. Assessment of alkaline pretreatment for the production of bioethanol from eucalyptus, sugarcane bagasse and sugarcane straw. Ind. Crops Prod. 2016, 94, 256. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S. Comparison of Various Alkaline Pretreatment Methods of Lignocellulosic Biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, D.; Singh, S.; Cheng, G. Recent developments in ionic liquid pretreatment of lignocellulosic biomass for enhanced bioconversion. Sustain. Energy Fuels 2021, 6, 1655–1667. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Goto, M. Ionic liquid pretreatment of lignocellulosic biomass for enhanced enzymatic delignification. Adv. Biochem. Eng. Biotechnol. 2018, 168, 61–77. [Google Scholar] [CrossRef]
- Asim, A.M.; Uroos, M.; Naz, S.; Sultan, M.; Griffin, G.; Muhammad, N.; Khan, A.S. Acidic ionic liquids: Promising and cost-effective solvents for processing of lignocellulosic biomass. J. Mol. Liq. 2019, 287, 110943. [Google Scholar] [CrossRef]
- Khan, A.S.; Man, Z.; Bustam, M.A.; Nasrullah, A.; Ullah, Z.; Sarwono, A.; Shah, F.U.; Muhammad, N. Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids. Carbohydr. Polym. 2018, 181, 208–214. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Mutrakulcharoen, P.; Chuetor, S.; Cheenkachorn, K.; Tantayotai, P.; Panakkal, E.J.; Sriariyanun, M. Recent situation and progress in biorefining process of lignocellulosic biomass: Toward green economy. Appl. Sci. Eng. Prog. 2020, 13, 299–311. [Google Scholar] [CrossRef]
- Chen, Z.; Bai, X.; Lusi, A.; Zhang, H.; Wan, C. Insights into structural changes of lignin toward tailored properties during deep eutectic solvent pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 9783–9793. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.M.; Hayyan, M.; Hayyan, A.; Basirun, W.J.; Salleh, H.M.; Mirghani, M.E.S. A grand avenue to integrate deepeutectic solvents into biomass processing. Biomass Bioenergy 2020, 137, 105550. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Application of deep eutectic solvents in biomass pretreatment and conversion. Green Energy Environ. 2019, 4, 95–115. [Google Scholar] [CrossRef]
- Li, C.; Huang, C.; Zhao, Y.; Zheng, C.; Su, H.; Zhang, L.; Luo, W.; Zhao, H.; Wang, S.; Huang, L.-J. Effect of choline-based deep eutectic solvent pretreatment on the structure of cellulose and lignin in bagasse. Processes 2021, 9, 384. [Google Scholar] [CrossRef]
- Bensah, E.C.; Mensah, M. Chemical pretreatment methods for the production of cellulolytic ethanol: Technologies and Innovations. Int. J. Chem. Eng. 2013, 2013, 719607. [Google Scholar] [CrossRef]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Jose, D.; Kitiborwornkul, N.; Sriariyanun, M. A review on chemical pretreatment methods of lignocellulosic biomass: Recent advances and progress. Appl. Sci. Eng. Prog. 2022, 15, 6210. [Google Scholar] [CrossRef]
- Travaini, R.; Martín-Juárez, J.; Lorenzo-Hernando, A.; Bolado-Rodríguez, S. Ozonolysis: An advantageous pretreatment for lignocellulosic biomass revisited. Bioresour. Technol. 2016, 199, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Peng, L.; Chen, K.; Zhu, Z. Enhanced enzymatic saccharification of sugarcane bagasse pretreated by combining O2 and NaOH. Bioresour. Technol. 2016, 214, 692–699. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Chen, Z.; Li, Y.; Wang, R.; Luo, X.; Cai, G.; Li, Y.; Yu, Q.; Lu, J. Ozonolysis pretreatment of maize stover: The interactive effect of sample particle size and moisture on ozonolysis process. Bioresour. Technol. 2015, 183, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front. Chem. 2021, 9, 705358. [Google Scholar] [CrossRef]
- Lee, J.; Hong, J.; Jeong, S.; Chandran, K.; Park, K.Y. Interactions between substrate characteristics and microbial communities on biogas production yield and rate. Bioresour. Technol. 2020, 303, 122934. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.U.; Usman, M.; Ashraf, M.A.; Dutta, N.; Luo, G.; Zhang, S. A review of recent advancements in pretreatment techniques of lignocellulosic materials for biogas production: Opportunities and limitations. Chem. Eng. J. Adv. 2022, 10, 100263. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, X.P.; Duong, D.Q.; Ağbulut, U.; Len, C.; Nguyen, P.Q.P.; Kchaou, M.; Chen, W.-S. Steam explosion as sustainable biomass pretreatment technique for biofuel production: Characteristics and challenges. Biores. Technol. 2023, 385, 129398. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the Structural and Chemical Changes of Plant Biomass Following Steam Explosion Pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef]
- Sanchez, O.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Biores. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Gutierrez, J.M.; Verlinden, R.A.J.; van der Meer, P.C.; van der Wielen, L.A.M.; Straathof, A.J.J. Liquid Hot Water Pretreatment of Lignocellulosic Biomass at Lab and Pilot Scale. Processes 2021, 9, 1518. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing theireffects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Li, M.; Cao, S.; Meng, X.; Studer, M.; Wyman, C.E.; Ragauskas, A.J.; Pu, Y. The effect of liquid hot water pretreatment on the chemical–structural alteration and the reduced recalcitrance in poplar. Biotechnol. Biofuels 2017, 10, 237. [Google Scholar] [CrossRef]
- Toscan, A.; Morais, A.R.C.; Paixão, S.M.; Alves, L.; Andreaus, J.; Camassola, M.; Dillon, A.J.P.; Lukasik, R.M. High-pressure carbon dioxide/water pretreatment of sugarcane bagasse and elephant grass: Assessment of the effect of biomass composition on process efficiency. Bioresour. Technol. 2017, 224, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 787532. [Google Scholar] [CrossRef]
- Narayanaswamy, N.; Faik, A.; Goetz, D.J.; Gu, T. Supercritical carbon dioxide pretreatment of corn stover and switchgrass for lignocellulosic ethanol production. Bioresour. Technol. 2011, 102, 6995–7000. [Google Scholar] [CrossRef]
- Cormos, A.-M.; Dragan, S.; Petrescu, L.; Sandu, V.; Cormos, C.-C. Techno-economic and environmental evaluations of decarbonized fossil-intensive industrial processes by reactive absorption & adsorption CO2 capture systems. Energies 2020, 13, 1268. [Google Scholar] [CrossRef]
- Sohni, S.; Hashim, R.; Nidaullah, H.; Sulaiman, O.; Leh, C.P.; Lamaming, J.; Arai, T.; Kosugi, A. Enhancing the enzymatic digestibility of oil palm biomass using supercritical carbon dioxide-based pretreatment towards biorefinery application. Ind. Crops Prod. 2020, 157, 112923. [Google Scholar] [CrossRef]
- Martín, C.; Dixit, P.; Momayez, F.; Jönsson, L.J. Hydrothermal Pretreatment of Lignocellulosic Feedstocks to Facilitate Biochemical Conversion. Front. Bioeng. Biotechnol. 2022, 10, 846592. [Google Scholar] [CrossRef] [PubMed]
- Ahring, B.K.; Biswas, R.; Ahamed, A.; Teller, P.J.; Uellendahl, H. Making lignin accessible for anaerobic digestion by wet-explosion pretreatment. Bioresour. Technol. 2015, 175, 182–188. [Google Scholar] [CrossRef]
- Karki, B.K.; Muthukumarappan, Y.; Wang, B.; Dale, V.; Balan, W.; Gibbons, R.; Karunanity, C. Physical characteristics of AFEX-pretreated and densified switchgrass, prairie cordgrass, and corn stover. Biomass Bioenergy 2015, 78, 164–174. [Google Scholar] [CrossRef]
- Campbell, T.J.F.; Teymouri, B.; Bals, J.; Glassbrook, C.D.; Nielson, T.; Videto, J. A packed bed ammonia fiber expansion reactor system for pretreatment of agricultural residues at regional depots. Biofuels 2013, 4, 23–34. [Google Scholar] [CrossRef]
- Chundawat, S.P.S.; Bals, B.; Campbell, T.; Sousa, L.; Gao, D.; Jin, M. Primer on ammonia fiber expansion pretreatment. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; Wyman, C., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 169–200. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Naqvi, S.R.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Chi, N.T.L. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Olajuyigbe, F.M.; Adetuyi, O.Y.; Fatokun, C.O. Characterization of free and immobilized laccase from Cyberlindnera fabianii and application in degradation of bisphenol A. Int. J. Biol. Macromol. 2019, 125, 856–864. [Google Scholar] [CrossRef]
- Usman, M.; Ren, S.; Ji, M.; Sompong, O.; Qian, Y.; Luo, G.; Zhang, S. Characterization and biogas production potentials of aqueous phase produced from hydrothermal carbonization of biomass–Major components and their binary mixtures. Chem. Eng. J. 2020, 388, 124201. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.L.; Tonnis, B.; Habteselassie, M.; Liao, X.; Huang, Q. Fungal pretreatment of switchgrass for improved saccharification and simultaneous enzyme production. Bioresour. Technol. 2013, 135, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 111, 1797. [Google Scholar] [CrossRef]
- Ma, F.; Huang, X.; Ke, M.; Shi, Q.; Chen, Q.; Shi, C.; Zhang, J.; Zhang, X.; Yu, H. Role of selective fungal delignification in overcoming the saccharification recalcitrance of bamboo culms. ACS Sustain. Chem. Eng. 2017, 5, 8884–8894. [Google Scholar] [CrossRef]
- Castoldi, R.; Bracht, A.; de Morais, G.R.; Baesso, M.L.; Correa, R.C.G.; Peralta Muniz, R.T.; Polizeli, M.L.; Marquez de Souza, C.G.; Peralta, R.M. Biological pretreatment of Eucaliptus grandis sawdust with white-rot fungi: Study of degradation patterns and saccharification kinetics. Chem. Eng. J. 2014, 258, 240–246. [Google Scholar] [CrossRef]
- Shi, J.; Chinn, M.S.; Sharma-Shivappa, R.R. Microbial pretreatment of cotton stalks by solid state cultivation of Phanerochaete chrysosporium. Bioresour. Technol. 2008, 99, 6556–6564. [Google Scholar] [CrossRef] [PubMed]
- Savory, J.G. Brreakdown of timber by Actinomycetes and Fungi imperfecti. Ann. Appl. Biol. 1954, 41, 3336–3347. [Google Scholar] [CrossRef]
- Goodell, B.; Winandy, J.E.; Morrell, J.J. Fungal Degradation of Wood: Emerging Data, New Insights and Changing Perceptions. Coatings 2020, 10, 1210. [Google Scholar] [CrossRef]
- Sari, S.L.A.; Pangastuti, A.; Susilowati, A.; Purwoko, T.; Mahajoeno, E.; Hidayat, W.; Mardhena, I.; Pununtun, D.F.; Kurniawati, D.; Anitasari, R. Cellulolytic and hemicellulolytic bacteria from the gut of Oryctes rhinoceros larvae. Biodiversitas J. Biol. Divers. 2016, 17, 78–83. [Google Scholar] [CrossRef]
- Yan, X.; Wang, Z.; Zhang, K.; Si, M.; Liu, M.; Chai, L.; Liu, X.; Shi, Y. Bacteria-enhanced dilute acid pretreatment of lignocellulosic biomass. Bioresour. Technol. 2017, 245, 419–425. [Google Scholar] [CrossRef]
- Malgas, S.; Thoresen, M.; van Dyk, J.S.; Pletschke, B.I. Time dependence of enzyme synergism during the degradation of model and natural lignocellulosic substrates. Enzym. Microb. Technol. 2017, 103, 1–11. [Google Scholar] [CrossRef]
- Lee, S.; Kang, M.; Bae, J.-H.; Sohn, J.-H.; Sung, B.H. Bacterial Valorization of Lignin: Strains, Enzymes, Conversion Pathways, Biosensors, and Perspectives. Front. Bioeng. Biotechnol. 2019, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Ferdeș, M.; Dincă, M.N.; Moiceanu, G.; Zăbavă, B.Ș.; Paraschiv, G. Microorganisms and enzymes used in the biological pretreatment of the substrate to enhance biogas production: A Review. Sustainability 2020, 12, 7205. [Google Scholar] [CrossRef]
- Jimenez, D.J.; Korenblum, E.; van Elsas, J.D. Novel multispecies microbial consortia involved in lignocellulose and 5-hydroxymethylfurfural bioconversion. Appl. Microbiol. Biotechnol. 2013. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- Bhushan, S.; Rana, M.S.; Bhandari, M.; Sharma, A.K.; Simsek, H.; Prajapati, S.K. Enzymatic pretreatment of algal biomass has different optimal conditions for biogas and bioethanol routes. Chemosphere 2021, 284, 131264. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Shoham, Y.; Lamed, R. Lignocellulose-decomposing bacteria and their enzyme systems. In The Prokaryotes; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 215–265. [Google Scholar]
- Koupaie, E.H.; Dahadka, S.; Lakeh, A.A.B.; Azizi, A.; Elbeshishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production—A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef]
- Van Dyk, J.; Pletschke, B. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.S.; Gama, R.; Morrison, D.; Swart, S.; Pletschke, B.I. Food processing waste: Problems, current management and prospects for utilisation of the lignocellulose component through enzyme synergistic degradation. Renew. Sustain. Energy Rev. 2013, 26, 521–531. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Khuong, L.S.; Masjuki, H.H.; Zulkifli, N.W.M.; Mohamad, E.N.; Kalam, M.A.; Alabdulkarem, A.; Jamshaid, M. Effect of gasoline–bioethanol blends on the properties and lubrication characteristics of commercial engine oil. RSC Adv. 2017, 7, 15005–15019. [Google Scholar] [CrossRef]
- IEA. Biofuel Production by Country/Region and Fuel Type, 2016–2022; IEA: Paris, France, 2021; Available online: https://www.iea.org/data-and-statistics/charts/biofuel-production-by-country-region-and-fuel-type-2016-2022 (accessed on 9 January 2023).
- U.S. Department of Energy. Energy Efficiency and Renewable Energy. Available online: https://afdc.energy.gov/fuels/ethanol_blends.html (accessed on 9 January 2023).
- de Jong, E.; Jungmeier, G. Biorefinery concepts in comparison to petrochemical refineries. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–33. [Google Scholar] [CrossRef]
- Mat Aron, N.S.; Khoo, K.S.; Chew, K.W.; Show, P.L.; Chen, W.H.; Nguyen, T.H.P. Sustainability of the four generations of biofuels–a review. Int. J. Energy Res. 2020, 44, 9266–9282. [Google Scholar] [CrossRef]
- Grubišić, M.; Šantek, B.; Zorić, Z.; Čošić, Z.; Vrana, I.; Gašparović, B.; Čož-Rakovac, R.; Šantek, M.I. Bioprospecting of Microalgae Isolated from the Adriatic Sea: Characterisation of Biomass, Pigment, Lipid, Fatty Acid Composition, Antioxidant and Antimicrobial Activity. Molecules 2022, 27, 1248. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuel. Bioprod. Biorefin. 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- Hoekman, S.K. Biofuels in the US–challenges and opportunities. Renew. Energ. 2009, 34, 14–22. [Google Scholar] [CrossRef]
- Akbarian, A.; Andooz, A.; Kowsari, E.; Ramakrishna, S.; Asgari, S.; Cheshmeh, Z.A. Challenges and opportunities of lignocellulosic biomass gasification in the path of circular bioeconomy. Bioresour. Technol. 2022, 362, 127774. [Google Scholar] [CrossRef]
- Pecha, M.B.; Arbelaez, J.I.M.; Garcia-Perez, M.; Chejne, F.; Ciesielski, P.N. Progress in understanding the four dominant intra-particle phenomena of lignocellulose pyrolysis: Chemical reactions, heat transfer, mass transfer, and phase change. Green Chem. 2019, 21, 2868–2898. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Raj, T.; Chen, C.W.; Ponnusamy, V.K.; Tahir, N.; Kim, S.H.; Dong, C.D. Lignin valorisation via enzymes: A sustainable approach. Fuel 2022, 311, 122608. [Google Scholar] [CrossRef]
- Salehi Jouzani, G.; Taherzadeh, M.J. Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: A comprehensive review. Biofuel Res. J. 2015, 2, 152–195. [Google Scholar] [CrossRef]
- Santek, M.I.; Beluhan, S.; Santek, B. Production of microbial lipids from lignocellulosic biomass. In Advances in Biofuels and Bioenergy; Nageswara-Rao, M., Soneji, J.R., Eds.; IntechOpen: London, UK, 2018; pp. 137–164. [Google Scholar] [CrossRef]
- Leonel, L.V.; Arruda, P.V.; Chandel, A.K.; Felipe, M.G.A.; Sene, L. Kluyveromyces marxianus: A potential biocatalyst of renewable chemicals and lignocellulosic ethanol production. Crit. Rev. Biotechnol. 2021, 41, 1131–1152. [Google Scholar] [CrossRef] [PubMed]
- Spindler, D.D.; Wyman, C.E.; Mohagheghi, A.; Grohmann, K. Thermotolerant yeast for simultaneous saccharification and fermentation of cellulose to ethanol. Appl. Biochem. Biotechnol. 1988, 17, 279–293. [Google Scholar] [CrossRef]
- Hahn-Hägerdal, B.; Galbe, M.; Gorwa-Grauslund, M.F.; Lidén, G.; Zacchi, G. Bio-ethanol–the fuel of tomorrow from the residues of today. Trends Biotechnol. 2006, 24, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Carpio, R.R.; Secchi, S.G.; Barros, R.O.; Oliveira, R.A.; Queiroz, S.; Teixeira, R.S.S.; Secchi, A.R. Techno-economic evaluation of second-generation ethanol from sugarcane bagasse: Commercial versus on-site produced enzymes and use of the xylose liquor. J. Clean. Prod. 2022, 369, 133340. [Google Scholar] [CrossRef]
- Moreno, A.D.; Tomás-Pejó, E.; Olsson, L.; Geijer, C. Candida intermedia CBS 141442: A novel glucose/xylose co-fermenting isolate for lignocellulosic bioethanol production. Energies 2020, 13, 5363. [Google Scholar] [CrossRef]
- Farias, D.; Atala, D.I.P.; Maugeri, F. Improving bioethanol production by Scheffersomyces stipitis using retentostat extractive fermentation at high xylose concentration. Biochem. Eng. J. 2017, 121, 171–180. [Google Scholar] [CrossRef]
- Guan, D.; Li, Y.; Shiroma, R.; Ike, M.; Tokuyasu, K. Sequential incubation of Candida shehatae and ethanol-tolerant yeast cells for efficient ethanol production from a mixture of glucose, xylose and cellobiose. Bioresour. Technol. 2013, 132, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Schepers, H.-J.; Bringer-Meyer, S.; and Sahm, H. Fermentation of D-xylose to ethanol by Bacillus macerans. Z. Für Naturforschung 1987, 42, 401–407. [Google Scholar] [CrossRef]
- Dogaris, I.; Mamma, D.; Kekos, D. Biotechnological production of ethanol from renewable resources by Neurospora crassa: An alternative to conventional yeast fermentations? Appl. Microbiol. Biotechnol. 2013, 97, 1457–1473. [Google Scholar] [CrossRef]
- He, B.; Hao, B.; Yu, H.; Tu, F.; Wei, X.; Xiong, K.; Xia, T. Double integrating XYL2 into engineered Saccharomyces cerevisiae strains for consistently enhanced bioethanol production by effective xylose and hexose co-consumption of steam-exploded lignocellulose in bioenergy crops. Renew. Energ. 2022, 186, 341–349. [Google Scholar] [CrossRef]
- Valdehuesa, K.N.G.; Ramos, K.R.M.; Nisola, G.M.; Bañares, A.B.; Cabulong, R.B.; Lee, W.-K.; Liu, H.; Chung, W.-J. Everyone loves an underdog: Metabolic engineering of the xylose oxidative pathway in recombinant microorganisms. Appl. Microbiol. Biotechnol. 2018, 102, 7703–7716. [Google Scholar] [CrossRef]
- Chacón, A.; Martinez, A.; Poggi-Varaldo, H.M.; Villa-Tanaca, L.; Ramos-Valdivia, A.C.; Ponce-Noyola, T. Xylose Metabolism in Bioethanol Production: Saccharomyces cerevisiae vs. Non-Saccharomyces Yeasts. BioEnergy Res. 2021, 15, 905–923. [Google Scholar] [CrossRef]
- Aui, A.; Wang, Y.; Mba-Wright, M. Evaluating the economic feasibility of cellulosic ethanol: A meta-analysis of techno-economic analysis studies. Renew. Sustain. Energy Rev. 2021, 145, 111098. [Google Scholar] [CrossRef]
- Schuster, B.G.; Chinn, M.S. Consolidated bioprocessing of lignocellulosic feedstocks for ethanol fuel production. BioEnergy Res. 2013, 6, 416–435. [Google Scholar] [CrossRef]
- Davison, S.A.; Keller, N.T.; van Zyl, W.H.; den Haan, R. Improved cellulase expression in diploid yeast strains enhanced consolidated bioprocessing of pretreated corn residues. Enzym. Microb. Technol. 2019, 131, 109382. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.; Smart, K.A.; James, S.; Cook, D.J. Bioethanol production from brewers spent grains using a fungal consolidated bioprocessing (CBP) approach. Bioenergy Res. 2017, 10, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Koppram, R.; Tomás-Pejó, E.; Xiros, C.; Olsson, L. Lignocellulosic ethanol production at high-gravity: Challenges and perspectives. Trends Biotechnol. 2014, 32, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Šantek, M.I.; Grubišić, M.; Perečinec, M.G.; Beluhan, S.; Šantek, B. Lipid production by Mortierella isabellina from pretreated corn cobs and effect of lignocellulose derived inhibitors on growth and lipid synthesis. Process Biochem. 2021, 109, 46–58. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef]
- Saini, R.; Kaur, A.; Saini, J.K.; Patel, A.K.; Varjani, S.; Chen, C.W.; Singhania, R.R.; Dong, C.D. Trends in Lignin Biotransformations for Bio-Based Products and Energy Applications. Bioenergy Res. 2022, 16, 88–104. [Google Scholar] [CrossRef]
- Sukhang, S.; Choojit, S.; Reungpeerakul, T.; Sangwichien, C. Bioethanol production from oil palm empty fruit bunch with SSF and SHF processes using Kluyveromyces marxianus yeast. Cellulose 2020, 27, 301–314. [Google Scholar] [CrossRef]
- Ballesteros, M.; Oliva, J.M.; Negro, M.J.; Manzanares, P.; Ballesteros, I. Ethanol from lignocellulosic materials by a simulataneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochem. 2004, 39, 1843–1848. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Chaiyaso, T. Bioethanol production from cellulose-rich corncob residue by the thermotolerant Saccharomyces cerevisiae TC-5. J. Fungi 2021, 7, 547. [Google Scholar] [CrossRef]
- Tomás-Pejó, E.; Oliva, J.M.; González, A.; Ballesteros, I.; Ballesteros, M. Bioethanol production from wheat straw by the thermotolerant yeast Kluyveromyces marxianus CECT 10875 in a simultaneous saccharification and fermentation fed-batch process. Fuel 2009, 88, 2142–2147. [Google Scholar] [CrossRef]
- ASTM. Standard Specification for Denatured Fuel Ethanol for Blending with Gasolines for Use as Automotive Spark-Ignition Fuel. In Annual Book of ASTM Standards; ASTM Standard D4806-11; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- EN 15376; Automotive Fuels—Ethanol as a Blending Component for Petrol—Requirements and Test Methods. European Committee for Standarization: Brussels, Belgium, 2007.
- Zhao, X.Q.; Zi, L.H.; Bai, F.W.; Lin, H.L.; Hao, X.M.; Yue, G.J.; Ho, N.W.Y. Bioethanol from lignocellulosic biomass. Adv. Biochem. Eng. Biotechnol. 2012, 128, 25–51. [Google Scholar] [CrossRef]
- Huang, H.J.; Ramaswamy, S.; Tschirner, U.W.; Ramarao, B.V. A review of separation technologies in current and future biorefineries. Sep. Purif. Technol. 2008, 62, 1–21. [Google Scholar] [CrossRef]
- Kunnakorn, D.; Rirksomboon, T.; Siemanond, K.; Aungkavattana, P.; Kuanchertchoo, N.; Chuntanalerg, P.; Wongkasemjit, S. Techno-economic comparison of energy usage between azeotropic distillation and hybrid system for water-ethanol separation. Renew. Energy 2013, 51, 310–316. [Google Scholar] [CrossRef]
- Luyben, W.L. Distillation Economic Optimization. Distillation Design and Control Using Aspen TM Simulation; Wiley-Blackwell: Hoboken, NJ, USA, 2006; pp. 85–97. [Google Scholar]
- Luyben, W.L. Control of a multiunit heterogeneous azeotropic distillation process. AIChE J. 2006, 52, 623–637. [Google Scholar] [CrossRef]
- Zhao, L.; Lyu, X.; Wang, W.; Shan, J.; Qiu, T. Comparison of heterogeneous azeotropic distillation and extractive distillation methods for ternary azeotrope ethanol/toluene/water separation. Comput. Chem. Eng. 2017, 100, 27–37. [Google Scholar] [CrossRef]
- Popescu, A.E.P.; Pellin, J.L.; Bonet, J.; Llorens, J. Bioethanol dehydration and mixing by heterogeneous azeotropic distillation. J. Clean. Prod. 2021, 320, 128810. [Google Scholar] [CrossRef]
- Gerbaud, V.; Rodriguez-Donis, I.; Hegely, L.; Lang, P.; Denes, F.; You, X. Review of extractive distillation. Process design, operation, optimization and control. Chem. Eng. Res. Des. 2019, 141, 229–271. [Google Scholar] [CrossRef]
- Meirelles, A.; Weiss, S.; Herfurth, H. Ethanol dehydration by extractive distillation. J. Chem. Technol. Biotechnol. 1992, 53, 181–188. [Google Scholar] [CrossRef]
- García-Herreros, P.; Gómez, J.M.; Gil, I.D.; Rodríguez, G. Optimization of the design and operation of an extractive distillation system for the production of fuel grade ethanol using glycerol as entrainer. Ind. Eng. Chem. Res. 2011, 50, 3977–3985. [Google Scholar] [CrossRef]
- de Jesús Hernández-Hernández, E.; Cabrera-Ruiz, J.; Hernández-Escoto, H.; Gutiérrez-Antonio, C.; Hernández, S. Simulation study of the production of high purity ethanol using extractive distillation: Revisiting the use of inorganic salts. Chem. Eng. Process. Process Intensif. 2022, 170, 108670. [Google Scholar] [CrossRef]
- Martínez-Galmiche, I.F.; Ramírez-Corona, N.; Conde-Mejía, C.; Sánchez-Sánchez, K.B.; Gani, R.; Jiménez-Gutiérrez, A. Design of energy-efficient ionic liquid-based extractive distillation systems for ethanol dehydration including alternatives for ionic liquid recovery. Chem. Eng. Res. Des. 2022, 188, 238–248. [Google Scholar] [CrossRef]
- Fadia, G.; Hassiba, B.; Weifeng, S. Separation of ethanol–water mixture by extractive distillation using pyridinium-based ionic liquid 1-ethyl-3-methylpyridinium ethylsulfate. Chem. Eng. Process. Process Intensif. 2022, 173, 108815. [Google Scholar] [CrossRef]
- Seiler, M.; Köhler, D.; Arlt, W. Hyperbranched polymers: New selective solvents for extractive distillation and solvent extraction. Sep. Purif. Technol. 2003, 30, 179–197. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Yu, G.; Dai, C.; Lei, Z. Extractive distillation using ionic liquids-based mixed solvents combined with dividing wall column. Sep. Purif. Technol. 2021, 269, 118713. [Google Scholar] [CrossRef]
- Karimi, S.; Karri, R.R.; Tavakkoli Yaraki, M.; Koduru, J.R. Processes and separation technologies for the production of fuel-grade bioethanol: A review. Environ. Chem. Lett. 2021, 19, 2873–2890. [Google Scholar] [CrossRef]
- Lei, Z.; Xi, X.; Dai, C.; Zhu, J.; Chen, B. Extractive distillation with the mixture of ionic liquid and solid inorganic salt as entrainers. AIChE J. 2014, 60, 2994–3004. [Google Scholar] [CrossRef]
- Iqbal, A.; Ahmad, S.A. Pressure swing distillation of azeotropic mixture–A simulation study. Perspect. Sci. 2016, 8, 4–6. [Google Scholar] [CrossRef][Green Version]
- Sowerby, B.; Crittenden, B.D. An experimental comparison of type A molecular sieves for drying the ethanol-water azeotrope. Gas Sep. Purif. 1988, 2, 77–83. [Google Scholar] [CrossRef]
- Wang, Y. Measurements and modeling of water adsorption isotherms of zeolite linde-type A crystals. Ind. Eng. Chem. Res. 2020, 59, 8304–8314. [Google Scholar] [CrossRef]
- Adnađević, B.; Mojović, Z.; Abu Rabi, A. The kinetics of ethanol adsorption from the aqueous phase onto zeolite NaZSM-5. Adsorption 2008, 14, 123–131. [Google Scholar] [CrossRef]
- Chaibi, A.; Boucheffa, Y.; Bendjaballah-Lalaoui, N. TGA investigation of water and ethanol adsorption over LTA zeolites. Microporous Mesoporous Mater. 2021, 324, 111285. [Google Scholar] [CrossRef]
- Hajilari, M.; Shariati, A.; Khosravi-Nikou, M. Equilibrium adsorption of bioethanol from aqueous solution by synthesized silicalite adsorbents: Experimental and modeling. Adsorption 2019, 25, 13–31. [Google Scholar] [CrossRef]
- Hajilari, M.; Shariati, A.; Khosravi-Nikou, M. Mass transfer determination of ethanol adsorption on activated carbon: Kinetic adsorption modeling. Heat Mass Transf. 2019, 55, 2165–2171. [Google Scholar] [CrossRef]
- Laksmono, J.A.; Sudibandriyo, M.; Saputra, A.H.; Haryono, A. Structured polyvinyl alcohol/zeolite/carbon composites prepared using supercritical fluid extraction techniques as adsorbent for bioethanol dehydration. Int. J. Chem. Eng. 2019, 2019, 6036479. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Divinagracia, M.F.; Choi, A.E.S.; Ong, D.C.; Chung, T.W. Applicability of composite silica–divinylbenzene in bioethanol dehydration: Equilibrium, kinetic, thermodynamic, and regeneration analysis. Energy Fuels 2019, 33, 7347–7356. [Google Scholar] [CrossRef]
- Tang, Y.; Tanase, S. Water-alcohol adsorptive separations using metal-organic frameworks and their composites as adsorbents. Microporous Mesoporous Mater. 2020, 295, 109946. [Google Scholar] [CrossRef]
- Claessens, B.; De Staercke, M.; Verstraete, E.; Baron, G.V.; Cousin-Saint-Remi, J.; Denayer, J.F. Identifying selective adsorbents for the recovery of renewable isobutanol. ACS Sustain. Chem. Eng. 2020, 8, 9115–9124. [Google Scholar] [CrossRef]
- Ranjbar, Z.; Tajallipour, M.; Niu, C.H.; Dalai, A.K. Water removal from ethanol vapor by adsorption on canola meal after protein extraction. Ind. Eng. Chem. Res. 2013, 52, 14429–14440. [Google Scholar] [CrossRef]
- Ghanbari, S.; Niu, C.H. Characterization of a high-performance biosorbent for natural gas dehydration. Energy Fuels 2018, 32, 11979–11990. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Gao, X.; Li, X. Preparation and characterization of cassava starch-based adsorbents for separating of azeotropic ethanol-water in biofuels ethanol production. J. Chem. Technol. Biotechnol. 2016, 91, 977–984. [Google Scholar] [CrossRef]
- Kularathne, I.W.; Gunathilake, C.; Yatipanthalawa, B.S.; Kalpage, C.S.; Rathneweera, A.C.; Rajapakse, S. Production of green energy–ethanol dehydration using rice straw, rice husk and castor oil. Biomass Convers. Biorefinery 2021, 11, 1597–1610. [Google Scholar] [CrossRef]
- Einicke, W.D.; Gläser, B.; Schöoullner, R. In-situ recovery of ethanol from fermentation broth by hydrophobic adsorbents. Acta Biotechnol. 1991, 11, 353–358. [Google Scholar] [CrossRef]
- Shirazi, M.M.A.; Kargari, A.; Tabatabaei, M. Sweeping gas membrane distillation (SGMD) as an alternative for integration of bioethanol processing: Study on a commercial membrane and operating parameters. Chem. Eng. Commun. 2015, 202, 457–466. [Google Scholar] [CrossRef]
- Peng, P.; Lan, Y.; Liang, L.; Jia, K. Membranes for bioethanol production by pervaporation. Biotechnol. Biofuels 2021, 14, 10. [Google Scholar] [CrossRef]
- Vane, L.M. Review: Membrane materials for the removal of water from industrial solvents by pervaporation and vapor permeation. J. Chem. Technol. Biotechnol. 2019, 94, 343–365. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Rao, L.; Mitra, S. Graphene Oxide-Carbon Nanotube (GO-CNT) Hy-brid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol. Membranes 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Hietaharju, J.; Kangas, J.; Tanskanen, J. Analysis of the permeation behavior of ethanol/water mixtures through a polydimethylsiloxane (PDMS) membrane in pervaporation and vapor permeation conditions. Sep. Purif. Technol. 2019, 227, 115738. [Google Scholar] [CrossRef]
- Saha, K.; Maharana, A.; Sikder, J.; Chakraborty, S.; Curcio, S.; Drioli, E. Continuous production of bioethanol from sugarcane bagasse and downstream purification using membrane integrated bioreactor. Catal. Today 2019, 331, 68–77. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Mai, Z.; Hung, W.S.; Matsuyama, H. Osmotically assisted solvent reverse osmosis membrane for dewatering of aqueous ethanol solution. J. Membr. Sci. 2023, 672, 121434. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, Z.; Wang, D.K.; Diniz da Costa, J.C. A novel ethanol dehydration process by forward osmosis. Chem. Eng. J. 2013, 232, 397–404. [Google Scholar] [CrossRef]
- Wei, P.; Cheng, L.H.; Zhang, L.; Xu, X.H.; Chen, H.L.; Gao, C.J. A review of membrane technology for bioethanol production. Renew. Sustain. Energy Rev. 2014, 30, 388–400. [Google Scholar] [CrossRef]
- Peng, P.; Shi, B.; Lan, Y. A review of membrane materials for ethanol recovery by pervaporation. Sep. Sci. Technol. 2010, 46, 234–246. [Google Scholar] [CrossRef]
- Wee, S.L.; Tye, C.T.; Bhatia, S. Membrane separation process—Pervaporation through zeolite membrane. Sep. Purif. Technol. 2008, 63, 500–516. [Google Scholar] [CrossRef]
- Lin, Y.S. Microporous and dense inorganic membranes: Current status and prospective. Sep. Purif. Technol. 2001, 25, 39–55. [Google Scholar] [CrossRef]
- Claes, R.; Vandezande, P.; Mullens, S.; Sitter, K.D.; Peeters, R.; Van Bael, M.K. Preparation and benchmarking of thin film supported PTMSP-silica pervaporation membranes. J. Membr. Sci. 2012, 389, 265–271. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Konstas, K.; Doherty, C.M.; Wood, C.D.; Mulet, X.; Xie, Z.; Ng, D.; Hill, M.R.; Lau, C.H.; Shao, L. Organic microporous nanofllers with unique alcohol afnity for superior ethanol recovery toward sustainable biofuels. ChemSusChem 2017, 10, 1887–1891. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Li, Q.; Liu, G.; Liu, G.; Jin, W. Mixed-matrix hollow fiber composite membranes comprising of PEBA and MOF for pervaporation separation of ethanol/water mixtures. Sep. Purif. Technol. 2019, 214, 2–10. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zhu, G.; Ban, Y.; Xu, L.; Yang, W. An organophilic pervaporation membrane derived from metal–organic framework nanoparticles for efficient recovery of bio-alcohols. Angew. Chem. Int. Ed. 2011, 50, 10636–10639. [Google Scholar] [CrossRef]
- Liu, X.; Hu, D.; Li, M.; Zhang, J.; Zhu, Z.; Zeng, G.; Zhang, Y.; Sun, Y. Preparation and characterization of Silicalite-1/PDMS surface sieving pervaporation membrane for separation of ethanol/water mixture. J. Appl. Polym. Sci. 2015, 132, 42460. [Google Scholar] [CrossRef]
- Tang, X.; Wang, R.; Xiao, Z.; Shi, E.; Yang, J. Preparation and pervaporation performances of fumed-silica-filled polydimethylsiloxane–polyamide (PA) composite membranes. J. Appl. Polym. Sci. 2007, 105, 3132–3137. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Wang, J.; Gascon, J.; Li, J.; van der Bruggen, B. Metal–organic frameworks based membranes for liquid separation. Chem. Soc. Rev. 2017, 46, 7124–7144. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Yu, X. Preparation of Zeolitic Imidazolate Framework-91 and its modeling for pervaporation separation of water/ethanol mixtures. Sep. Purif. Technol. 2020, 237, 116330. [Google Scholar] [CrossRef]
- Shafiei Amrei, S.; Asghari, M.; Esfahanian, M.; Zahraei, Z. Highly selective carbon nanotube-coupled graphene oxide-incorporated polydimethylsiloxane membrane for pervaporative membrane bioreactor ethanol production. J. Chem. Technol. Biotechnol. 2020, 95, 1604–1613. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Cai, P.; Cao, T.; Zhang, N.; Wang, N.; An, Q.F. Liquid–liquid interface induced PDMS-PTFE composite membrane for ethanol perm-selective pervaporation. AIChE J. 2020, 68, e17694. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.J.; Lee, K.; Foster, R.I. A short review on hydrophobic pervaporative inorganic membranes for ethanol/water separation applications. Korean J. Chem. Eng. 2022, 39, 2263–2274. [Google Scholar] [CrossRef]
- Huang, H.; Qureshi, N.; Chen, M.H.; Liu, W.; Singh, V. Ethanol production from food waste at high solids content with vacuum recovery technology. J. Agric. Food Chem. 2015, 63, 2760–2766. [Google Scholar] [CrossRef]
- Roffler, S.R.; Blanch, H.W.; Wilke, C.R. In situ recovery of fermentation products. Trends Biotechnol. 1984, 2, 129–136. [Google Scholar] [CrossRef]
- Rivera, E.C.; Atala, D.I.P.; Filho, F.M.; Carvalho da Costa, A.; Filho, R.M. Development of real-time state estimators for reaction–separation processes: A continuous flash fermentation as a study case. Chem. Eng. Process. Process Intensif. 2010, 49, 402–409. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Liao, Q.; Lv, F.L.; Zhu, X.; Ran, Y.; Hou, C.J. Solid simultaneous saccharification and fermentation of rice straw for bioethanol production using nitrogen gas stripping. RSC Adv. 2015, 5, 55328–55335. [Google Scholar] [CrossRef]
- Jin, M.; Sarks, C.; Bals, B.D.; Posawatz, N.; Gunawan, C.; Dale, B.E.; Balan, V. Toward high solids loading process for lignocellulosic biofuel production at a low cost. Biotechnol. Bioeng. 2017, 114, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.A.; Gandier, J.A.; Thibault, J.; Tezel, F.H. Enhanced ethanol production through selective adsorption in bacterial fermentation. Biotechnol. Bioprocess Eng. 2011, 16, 531–541. [Google Scholar] [CrossRef]
- Hashi, M.; Thibault, J.; Tezel, F.H. Recovery of Ethanol from Carbon Dioxide Stripped Vapor Mixture: Adsorption Prediction and Modeling. Ind. Eng. Chem. Res. 2010, 49, 8733–8740. [Google Scholar] [CrossRef]
- Seo, D.J.; Takenaka, A.; Fujita, H.; Mochidzuki, K.; Sakoda, A. Practical considerations for a simple ethanol concentration from a fermentation broth via a single adsorptive process using molecular-sieving carbon. Renew. Energy 2018, 118, 257–264. [Google Scholar] [CrossRef]
- Matsumura, M.; Märkl, H. Application of solvent extraction to ethanol fermentation. Appl. Microbiol. Biotechnol. 1984, 20, 371–377. [Google Scholar] [CrossRef]
- Job, C.; Schertler, C.; Staudenbauer, W.L.; Blass, E. Selection of organic solvents for in situ extraction of fermentation products from Clostridium thermohydrosulfuricum cultures. Biotechnol. Tech. 1989, 3, 315–320. [Google Scholar] [CrossRef]
- Munson, C.L.; King, C.J. Factors influencing solvent selection for extraction of ethanol from aqueous solutions. Ind. Eng. Chem. Process Des. Dev. 1984, 23, 109–115. [Google Scholar] [CrossRef]
- Kollerup, F.; Daugulis, A.J. Ethanol production by extractive fermentation-solvent identification and prototype development. Can. J. Chem. Eng. 1986, 64, 598–606. [Google Scholar] [CrossRef]
- Widjaja, T.; Altway, A.; Permanasari, A.R.; Gunawan, S. Production of Ethanol as A Renewable Energy by Extractive Fermentation. Appl. Mech. Mater. 2014, 493, 300–305. [Google Scholar] [CrossRef]
- Fan, S.; Liu, J.; Tang, X.; Wang, W.; Xiao, Z.; Qiu, B.; Wang, Y.; Jian, S.; Qin, Y.; Wang, Y. Process operation performance of PDMS membrane pervaporation coupled with fermentation for efficient bioethanol production. Chin. J. Chem. Eng. 2019, 27, 1339–1347. [Google Scholar] [CrossRef]
- Serna-Vázquez, J.; Zamidi Ahmad, M.; Castro-Muñoz, R. Simultaneous production and extraction of bio-chemicals produced from fermentations via pervaporation. Sep. Purif. Technol. 2021, 279, 119653. [Google Scholar] [CrossRef]
- Maiorella, B.; Blanch, H.W.; Wilke, C.R. Rapid Ethanol Production. In Proceedings of the AIChE 72nd National Meeting, San Francisco, CA, USA, 25 November 1979; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1979. [Google Scholar]
- Zentou, H.; Abidin, Z.Z.; Yunus, R.; Biak, D.R.A.; Korelskiy, D. Overview of Alternative Ethanol Removal Techniques for Enhancing Bioethanol Recovery from Fermentation Broth. Processes 2019, 7, 458. [Google Scholar] [CrossRef]
- Olukman, M.; Şanlı, O.; Solak, E.K. Synthesis of magnetite in poly(vinyl alcohol) matrix and its use in separation of acetone/water mixtures via pervaporation, vapor permeation with and without temperature difference methods. Vacuum 2015, 120, 107–115. [Google Scholar] [CrossRef]
- Vane, L.M.; Alvarez, F.R. Membrane-assisted vapor stripping: Energy efficient hybrid distillation-vapor permeation process for alcohol-water separation. J. Chem. Technol. Biotechnol. 2008, 83, 1275–1287. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).