The State of the Art on the Flow Characteristic of an Encapsulated Phase-Change Material Slurry
Abstract
:1. Introduction
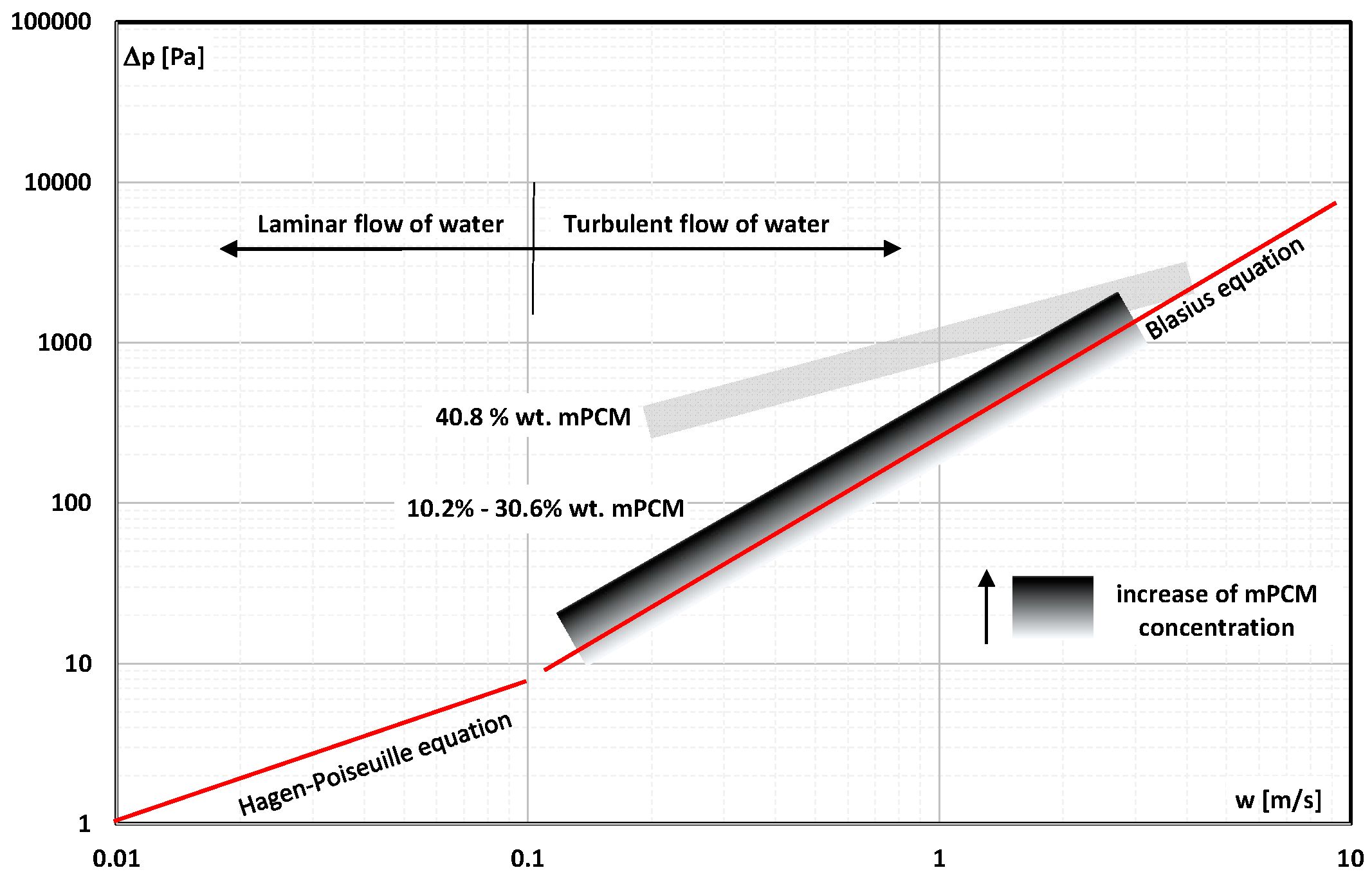
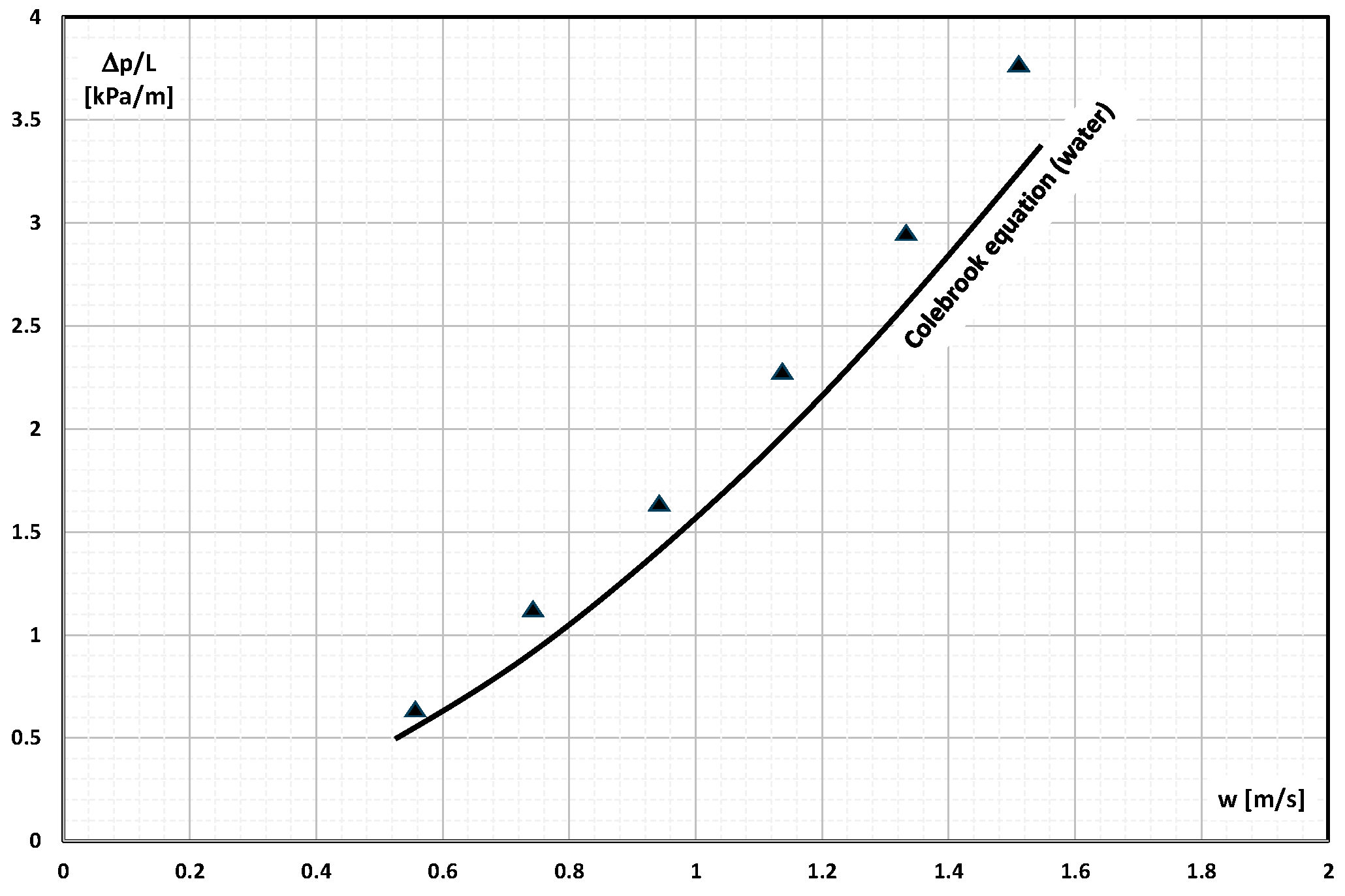
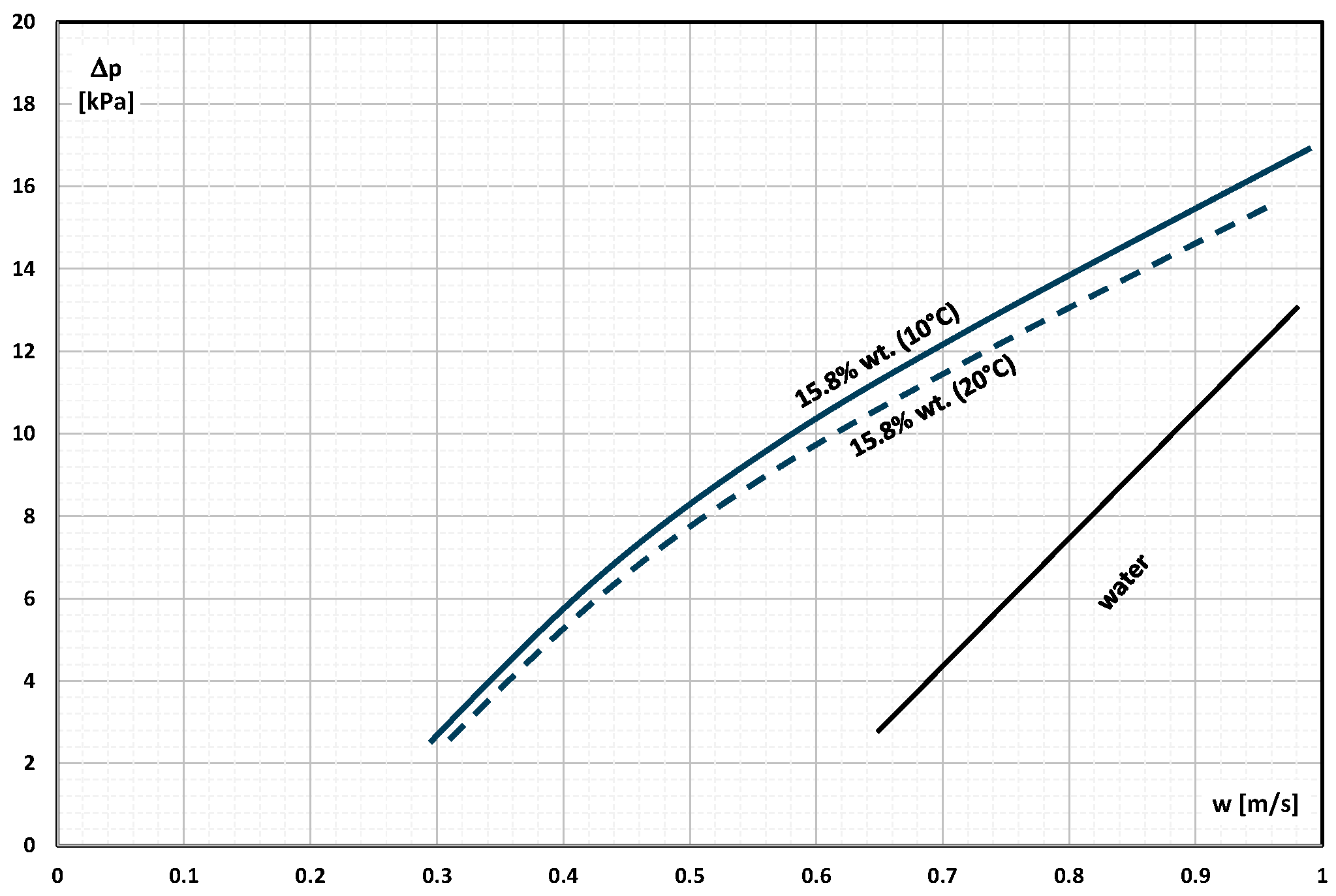
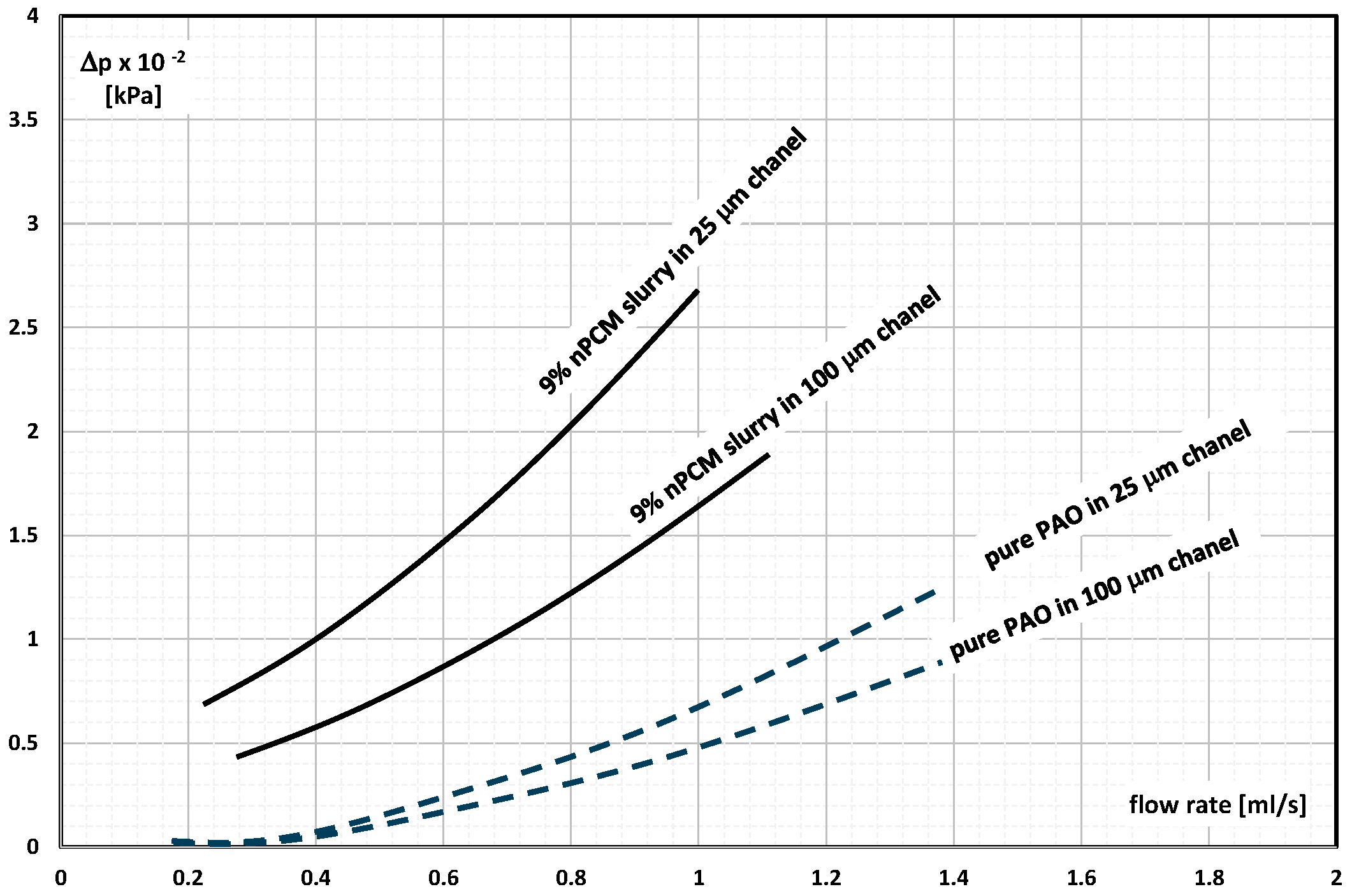
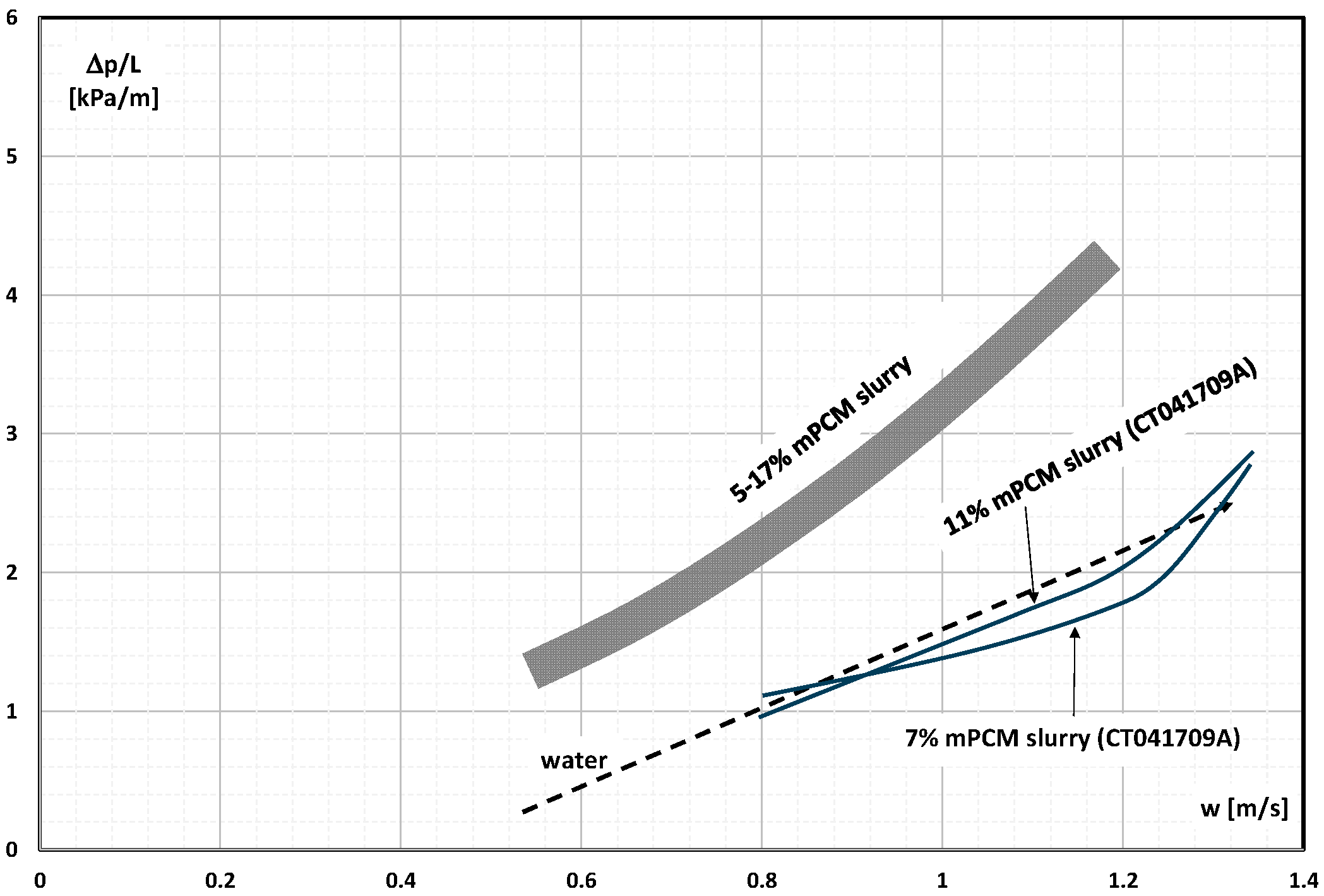
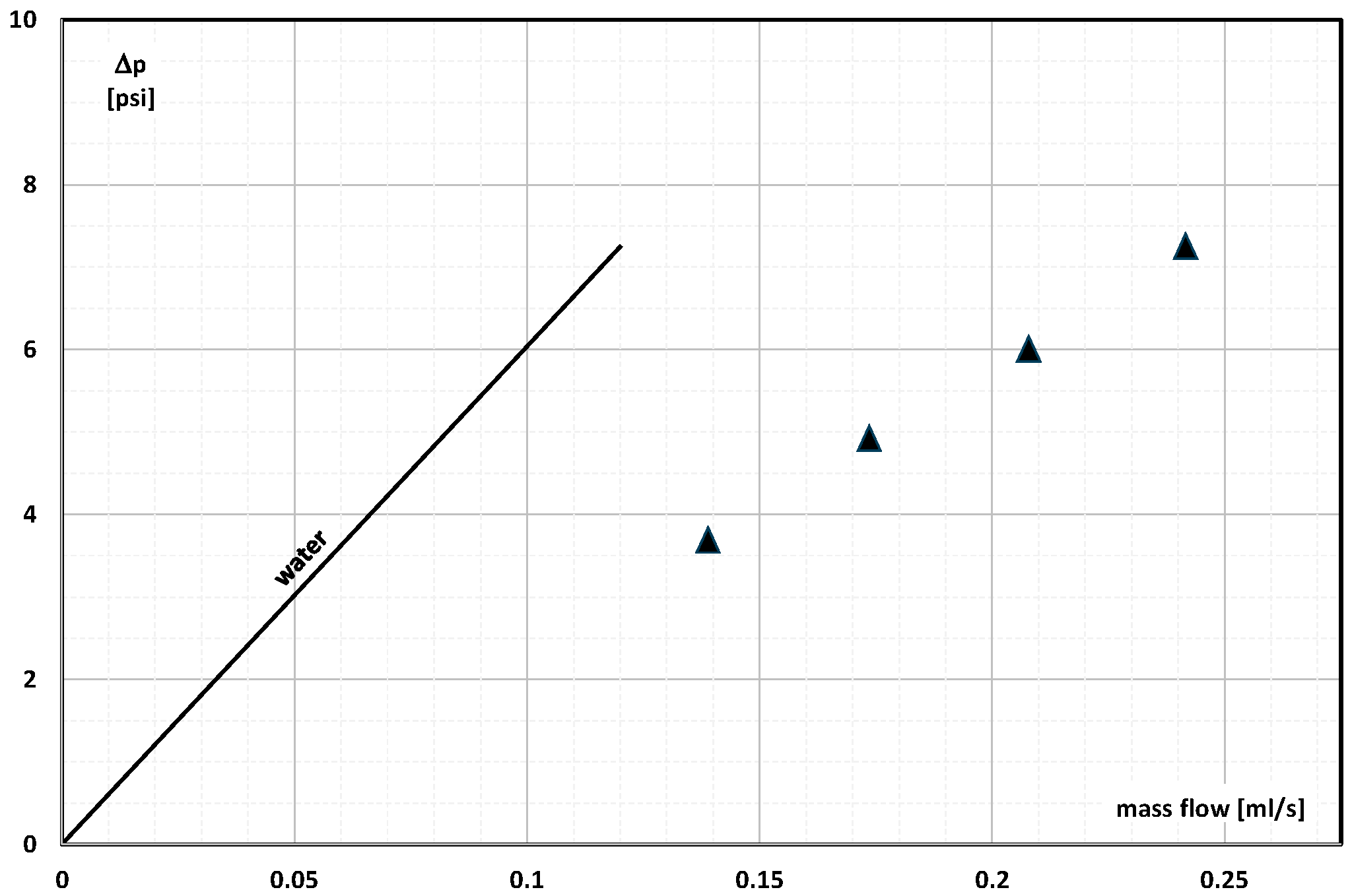
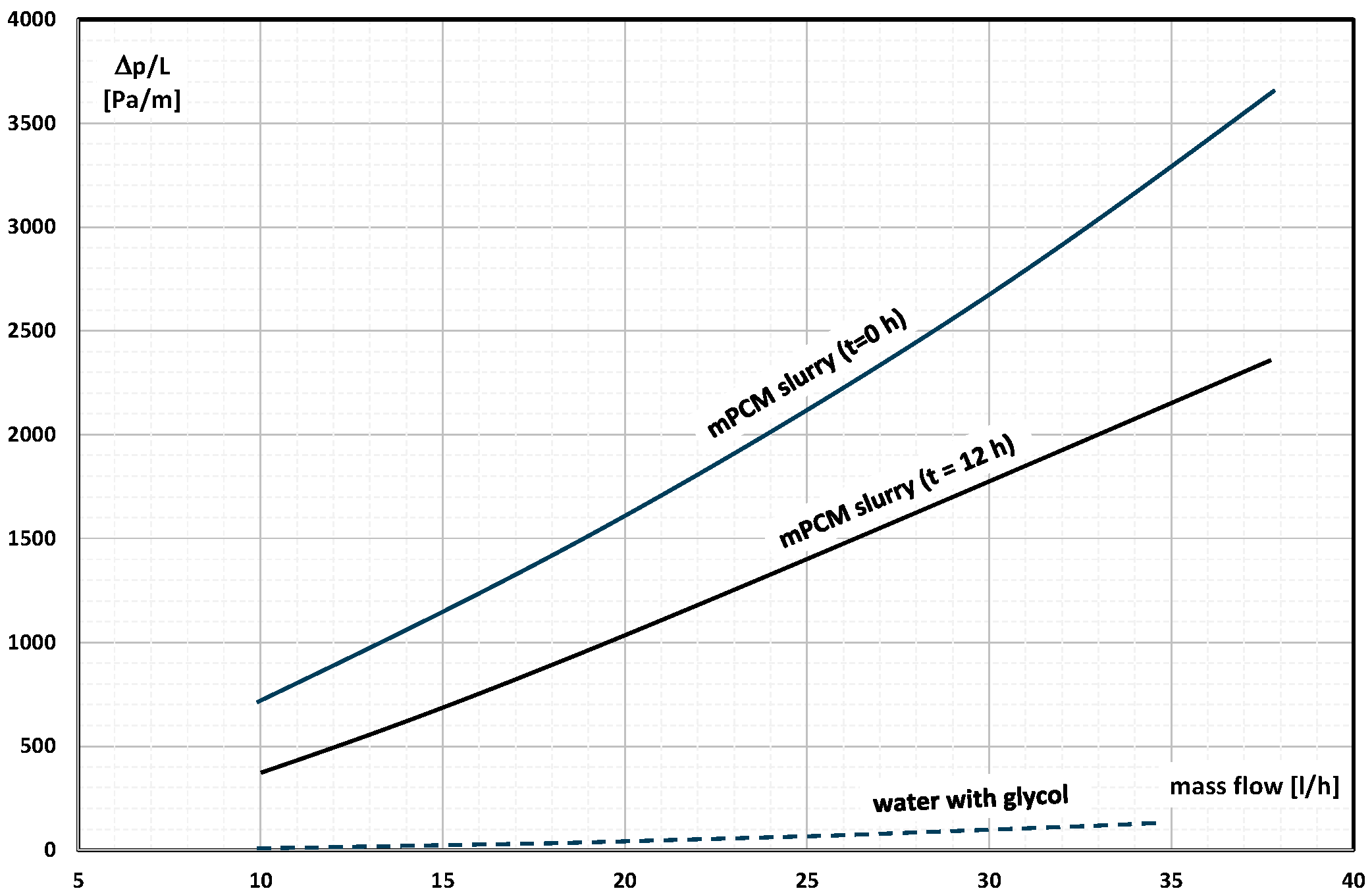
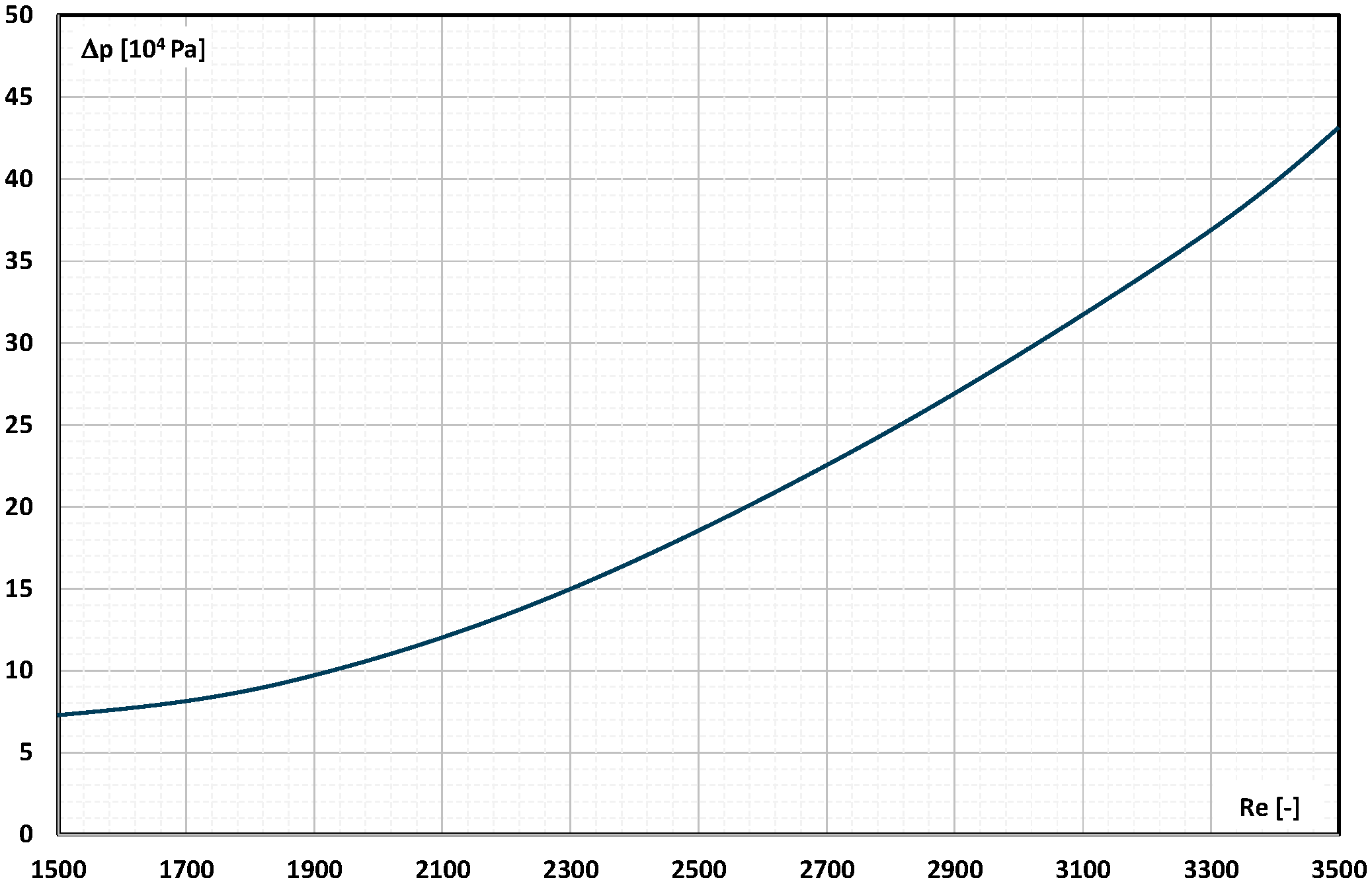
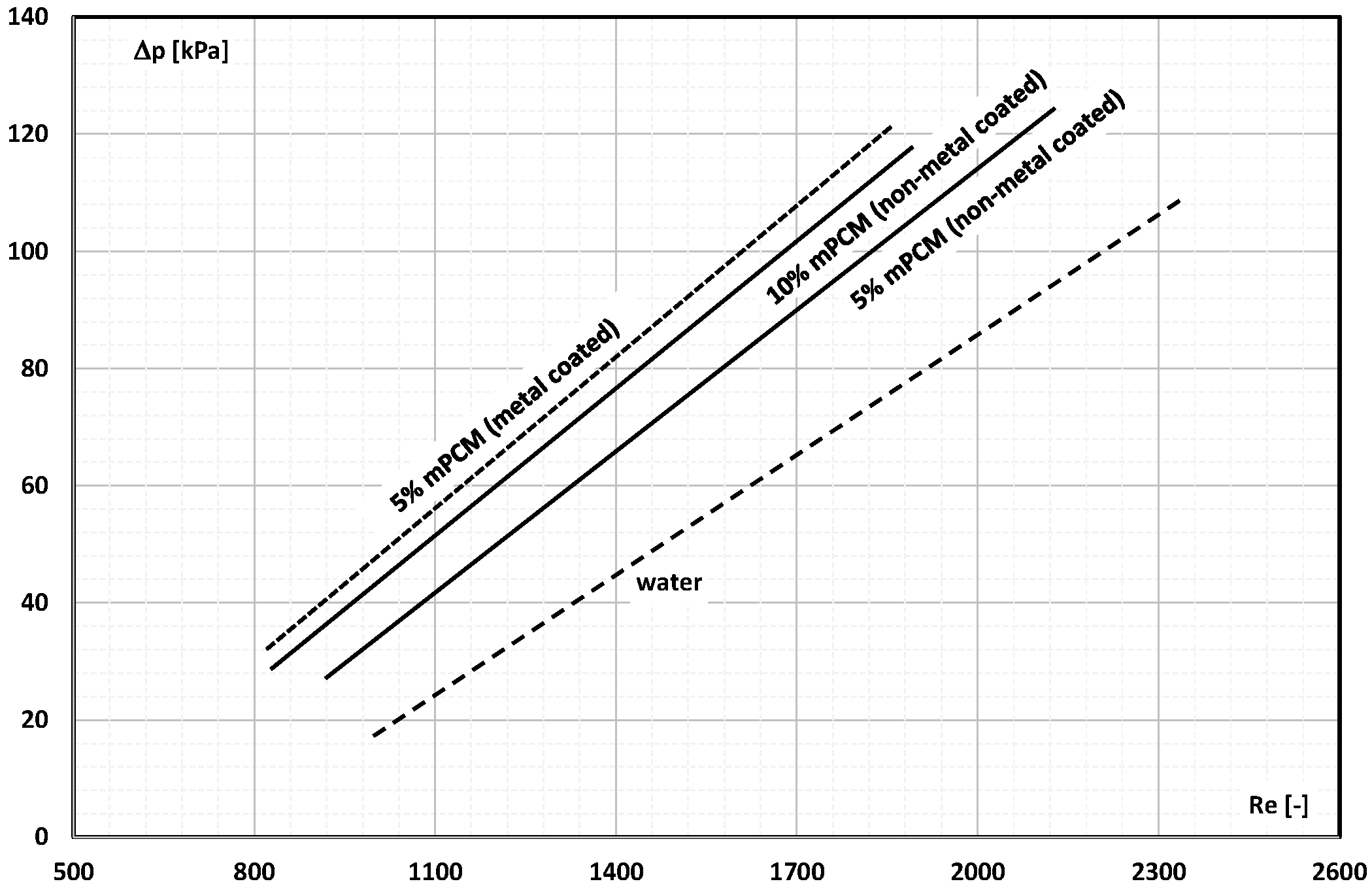
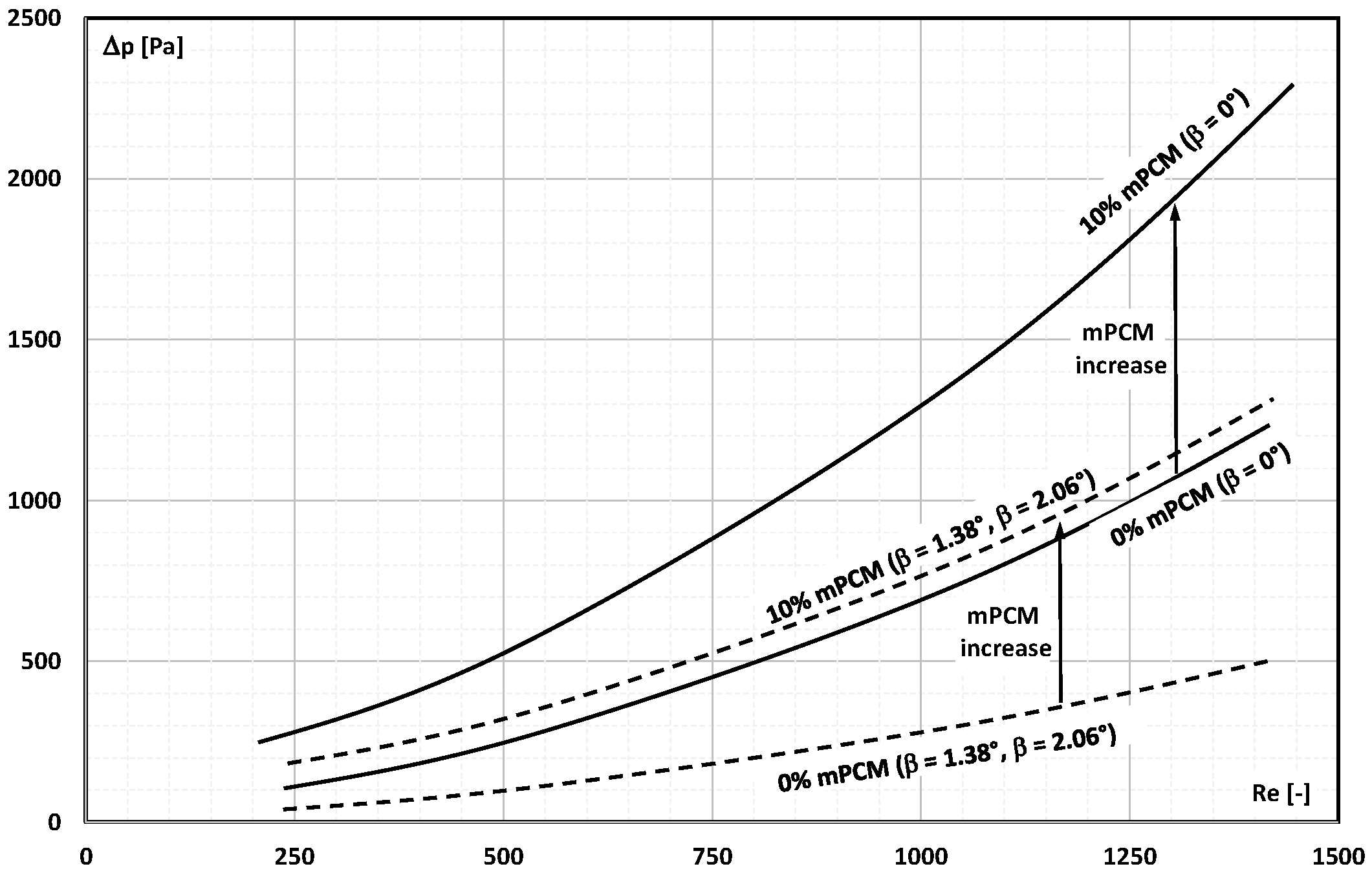
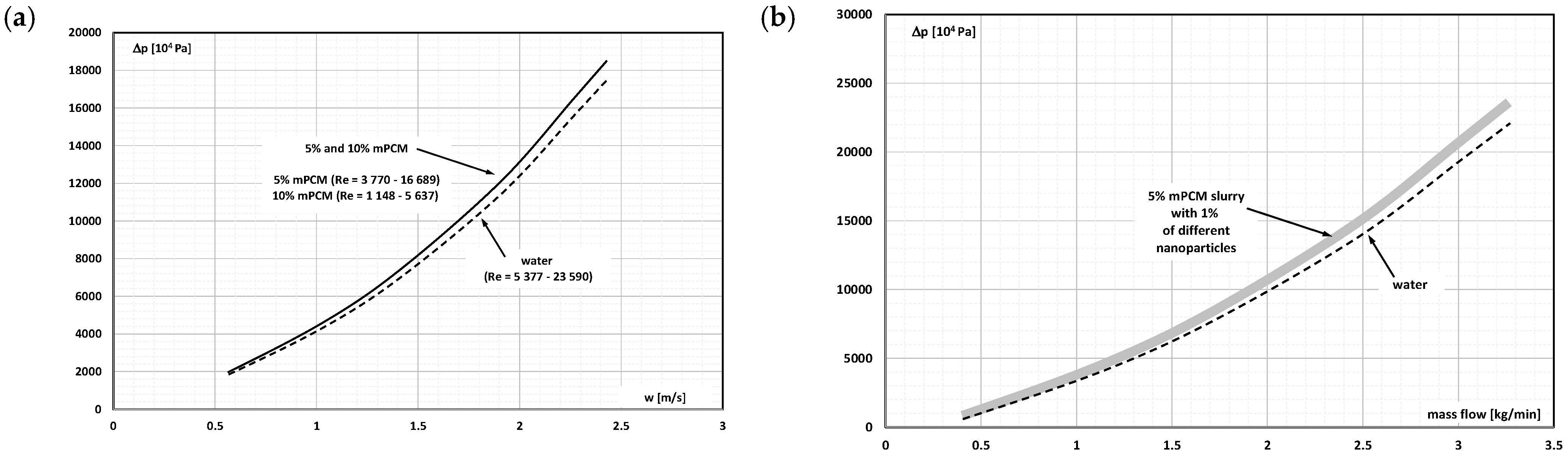
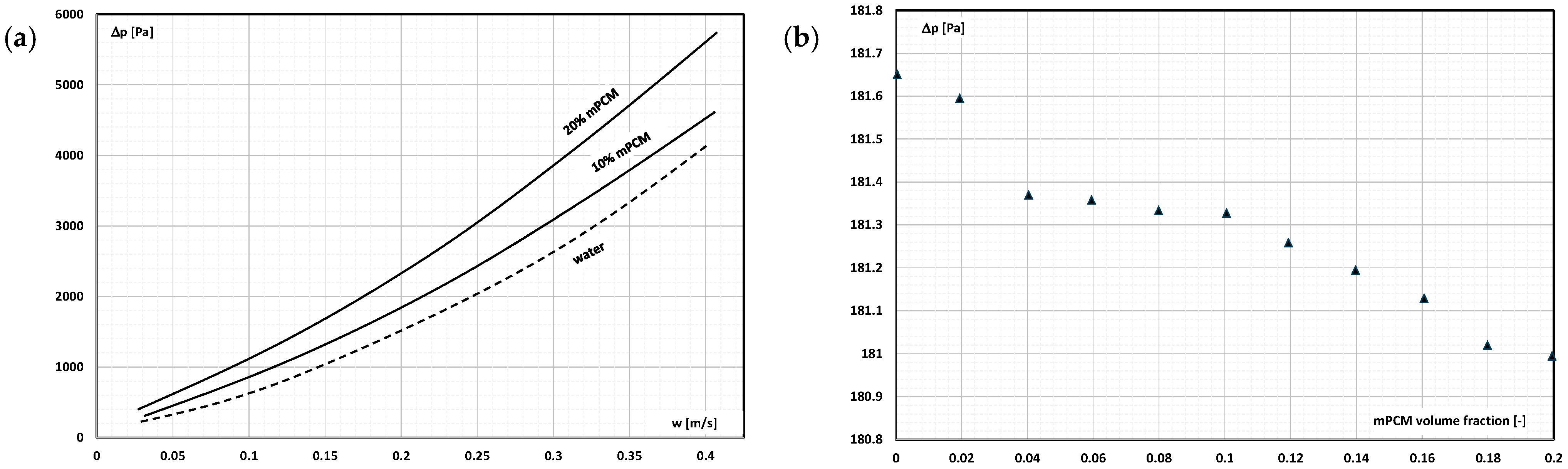
2. The Pressure Drop
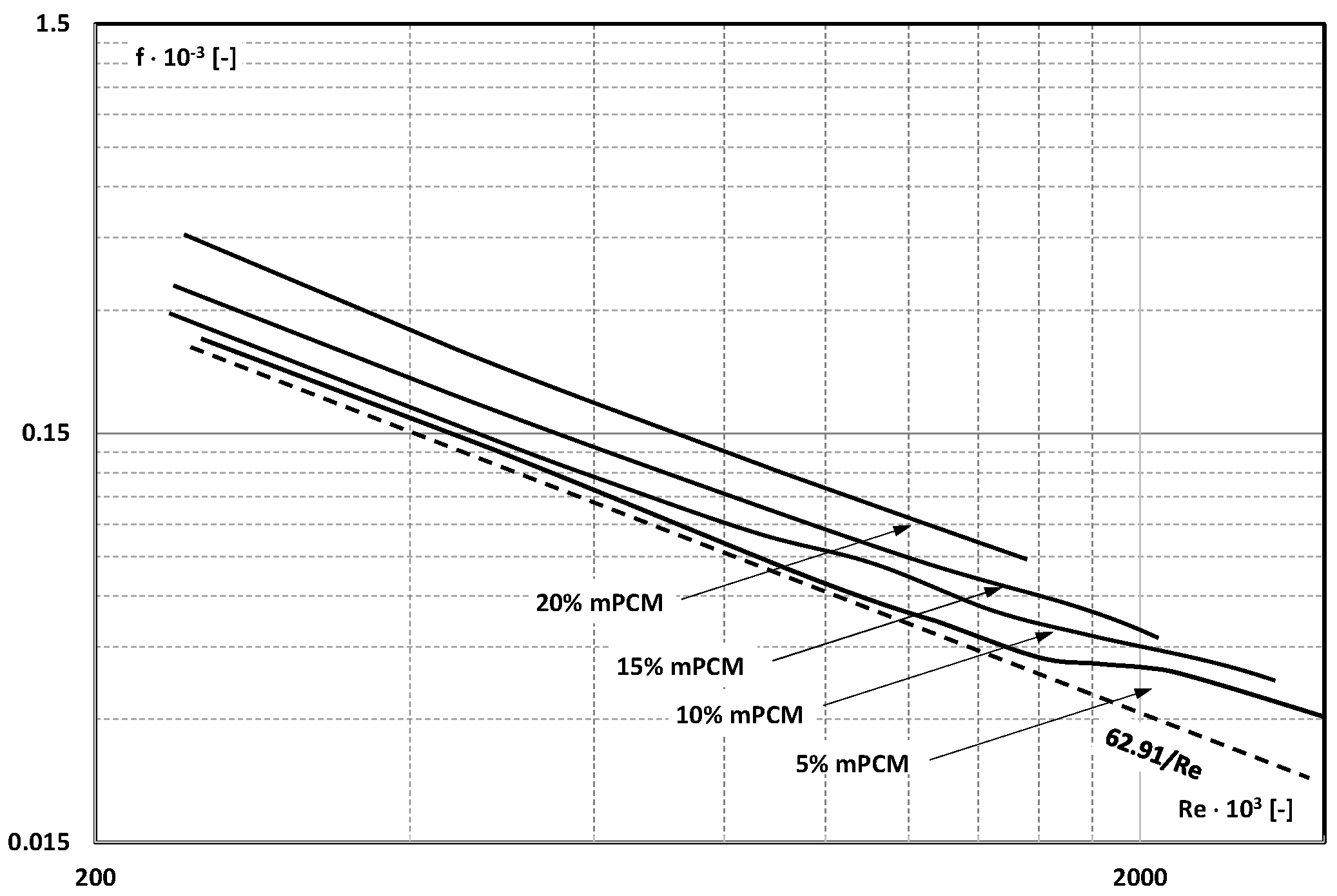
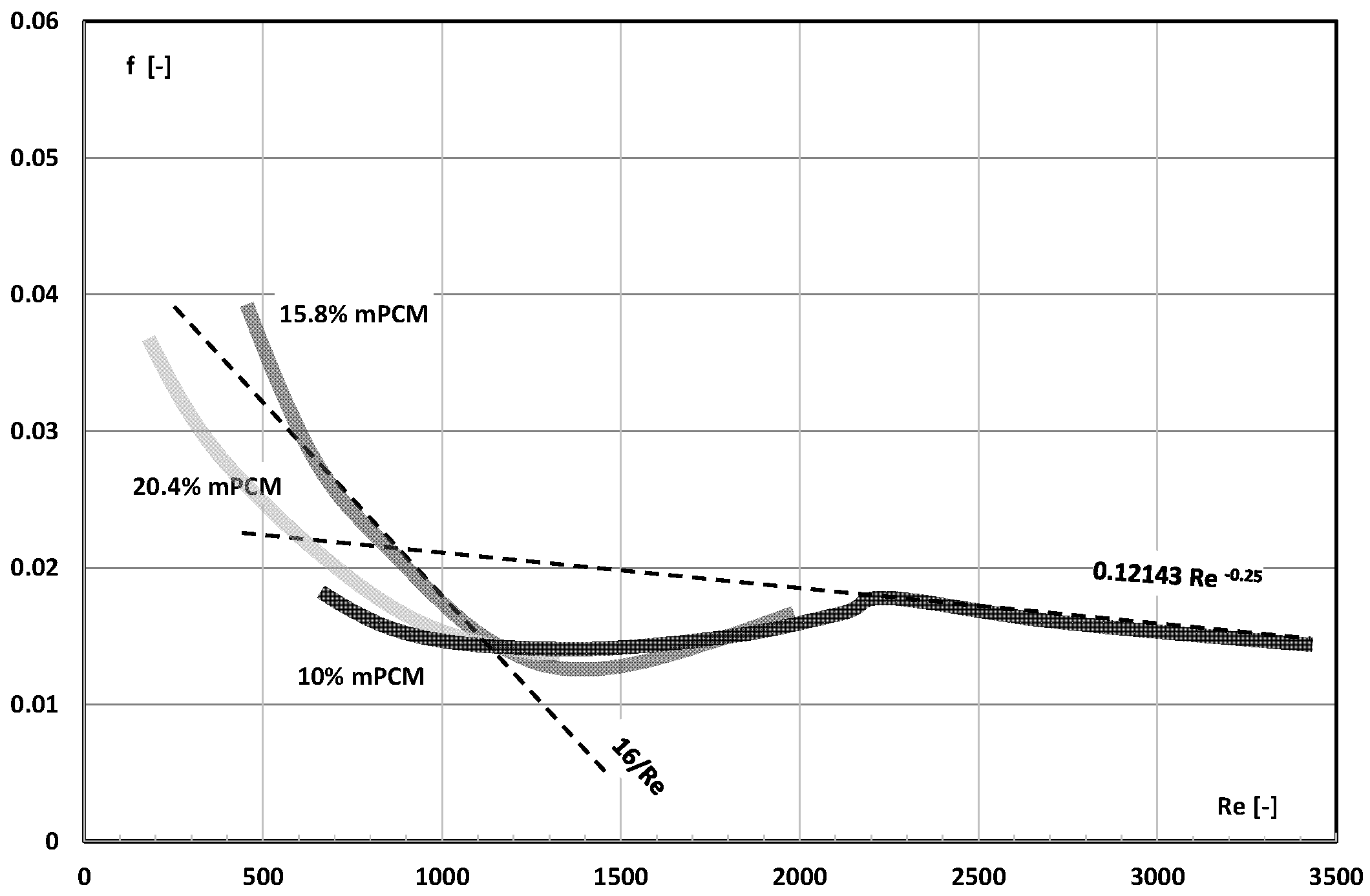
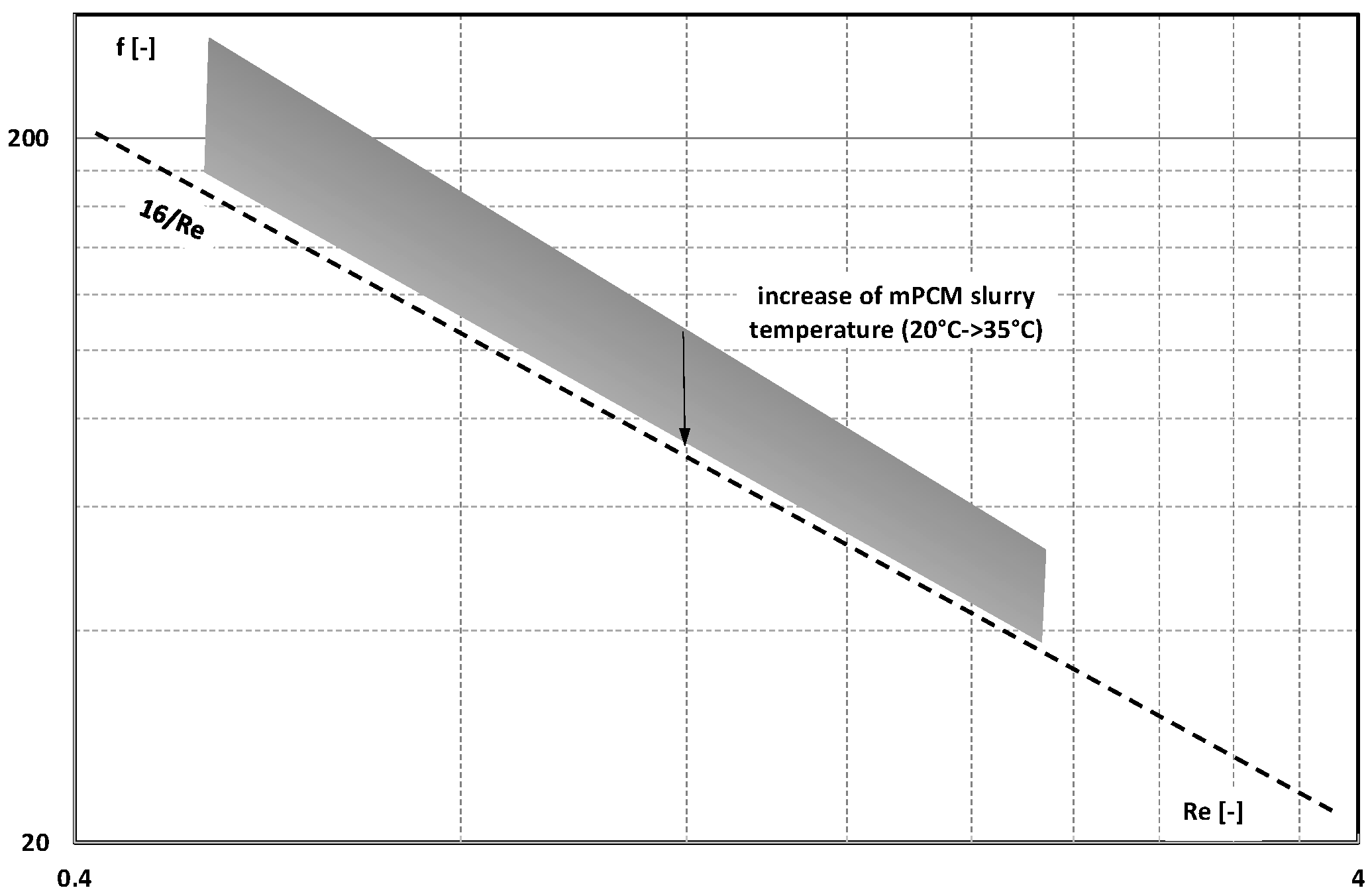
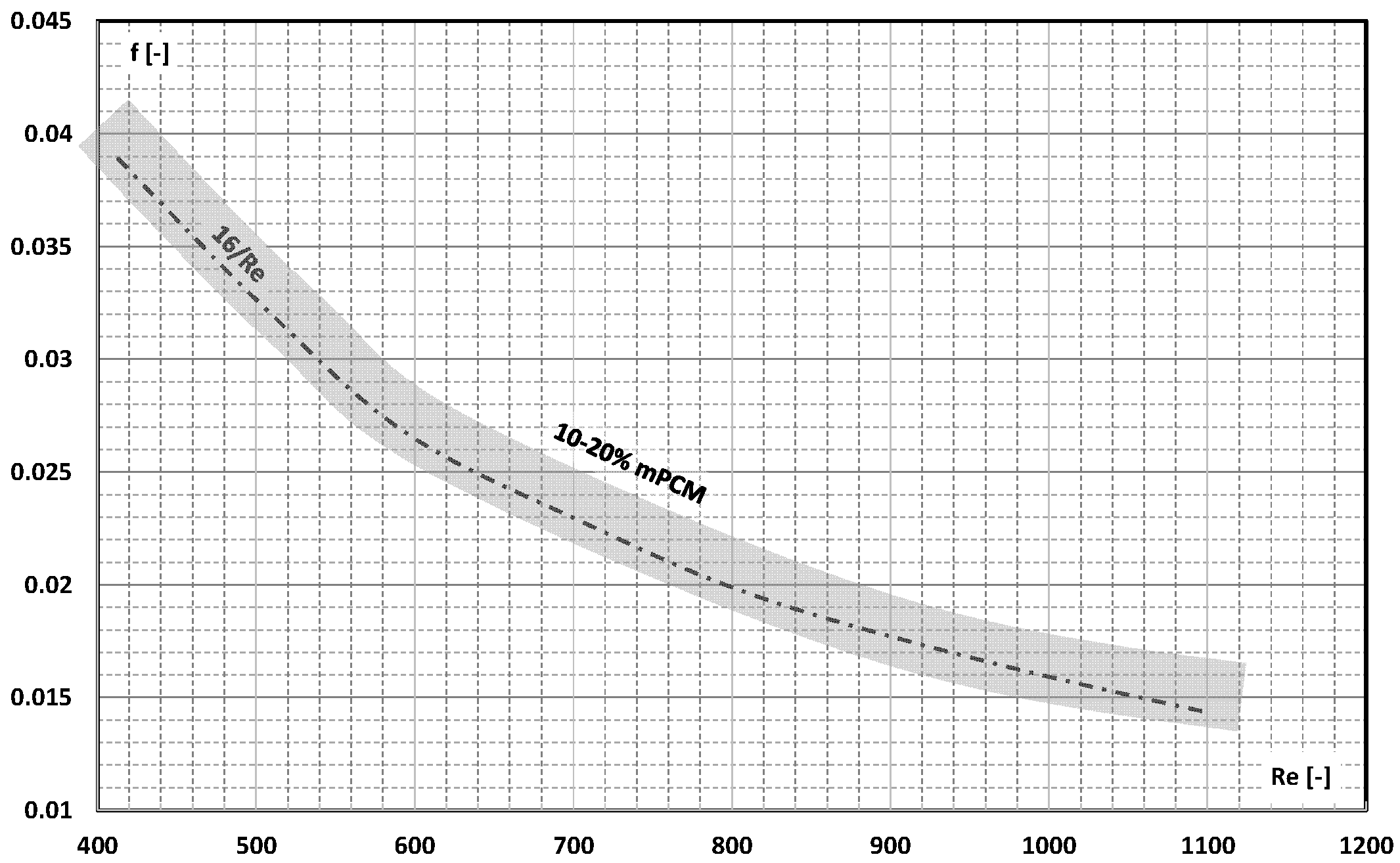
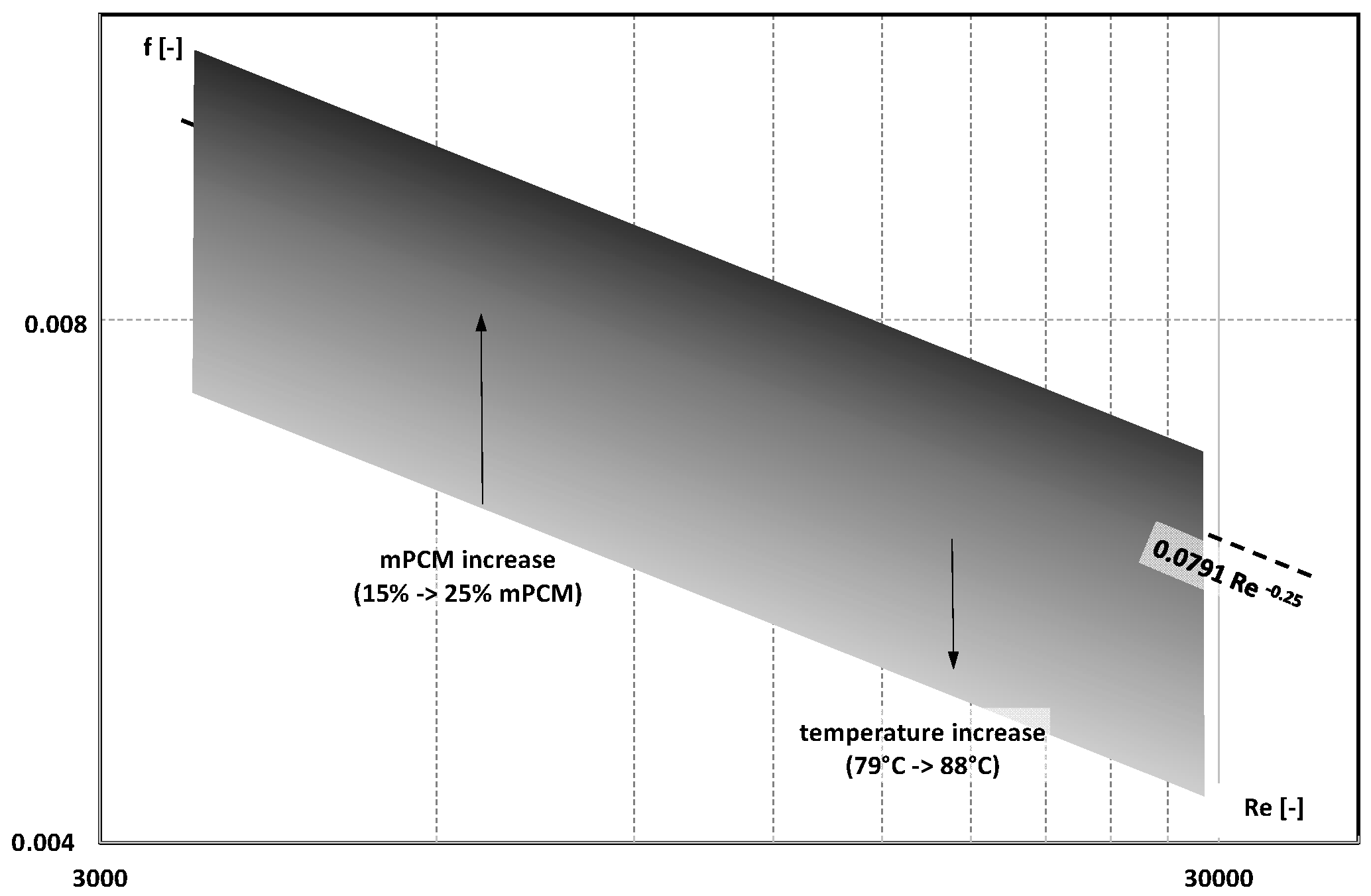
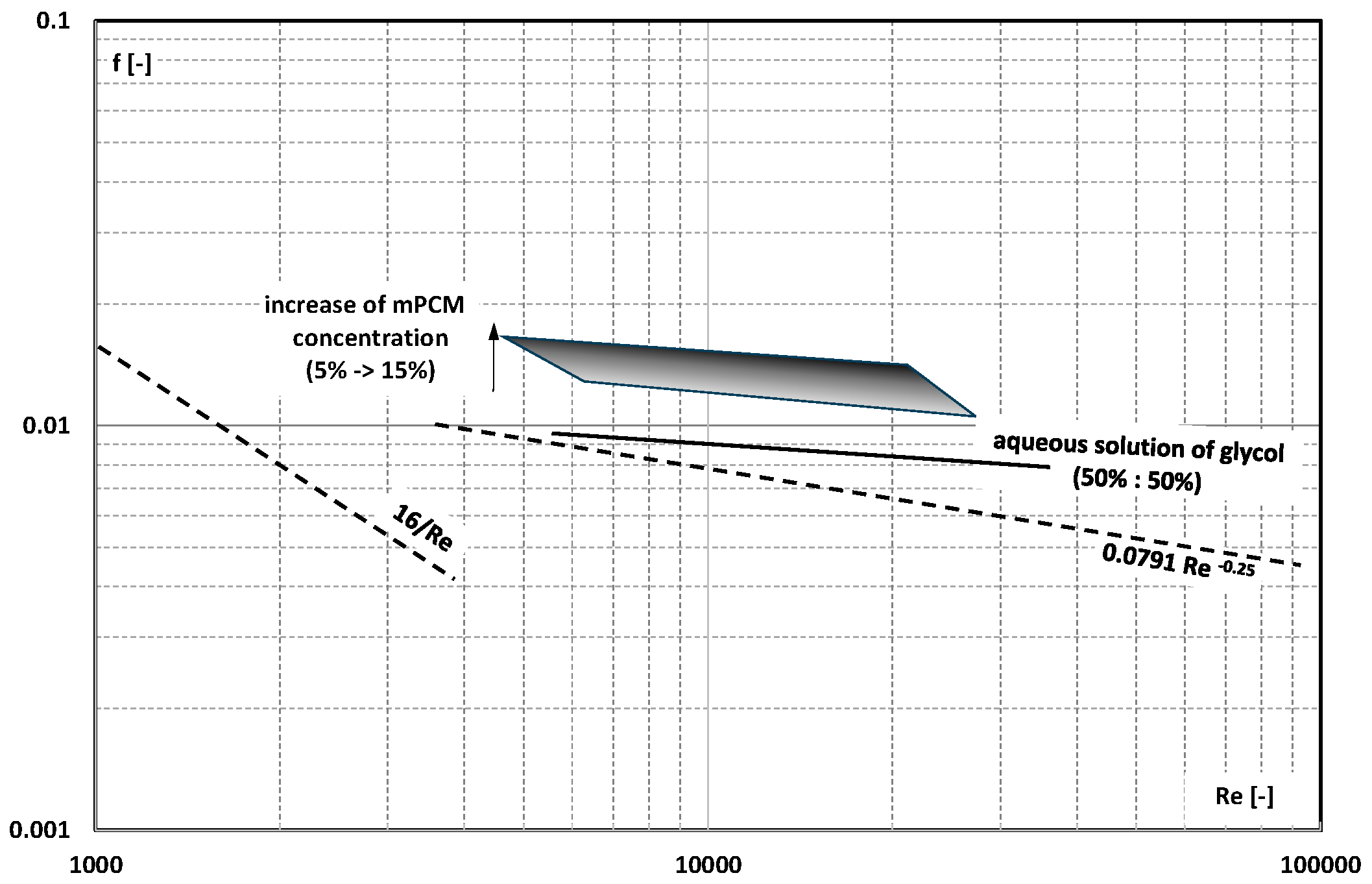
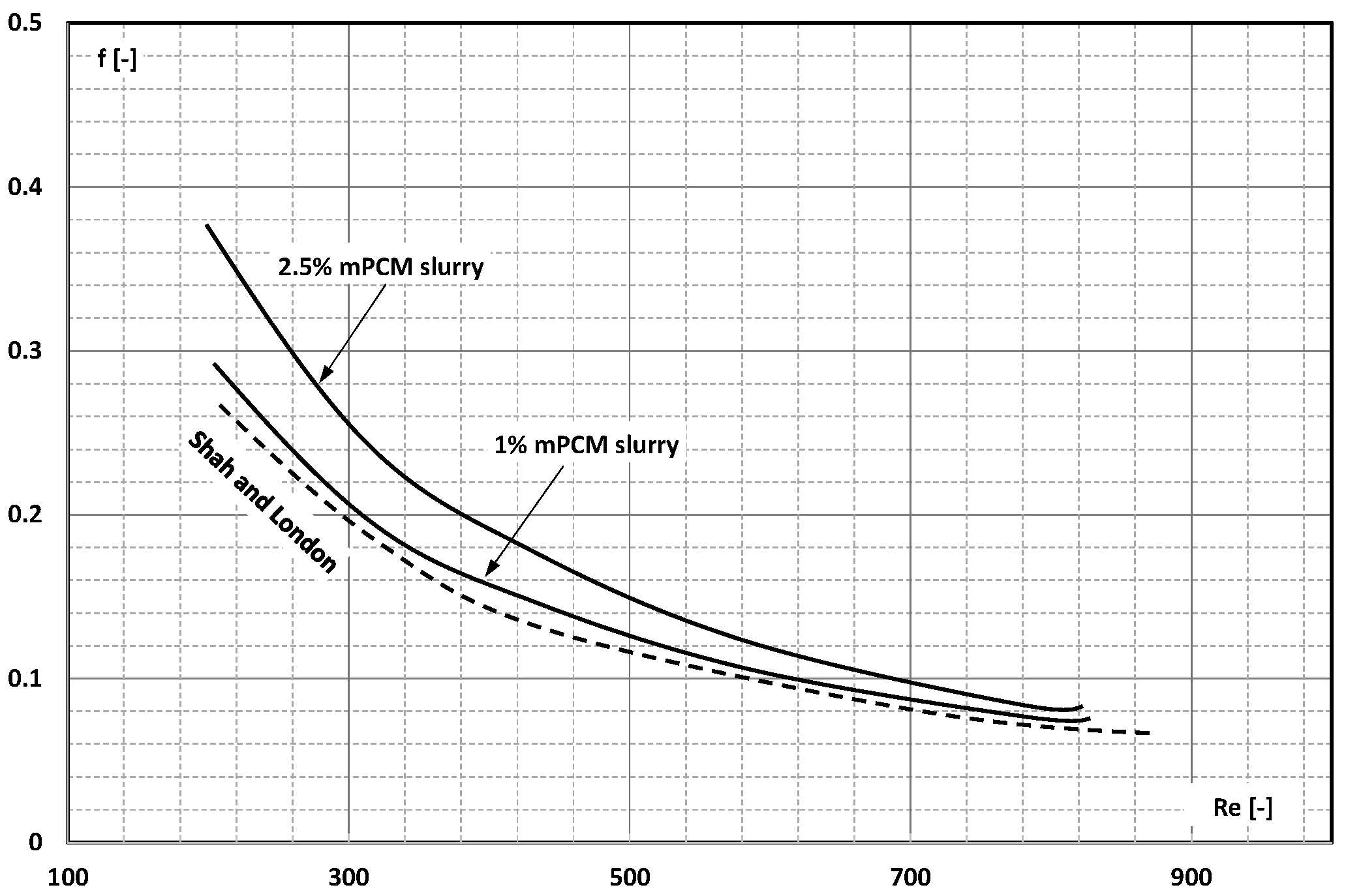
3. Friction Factor
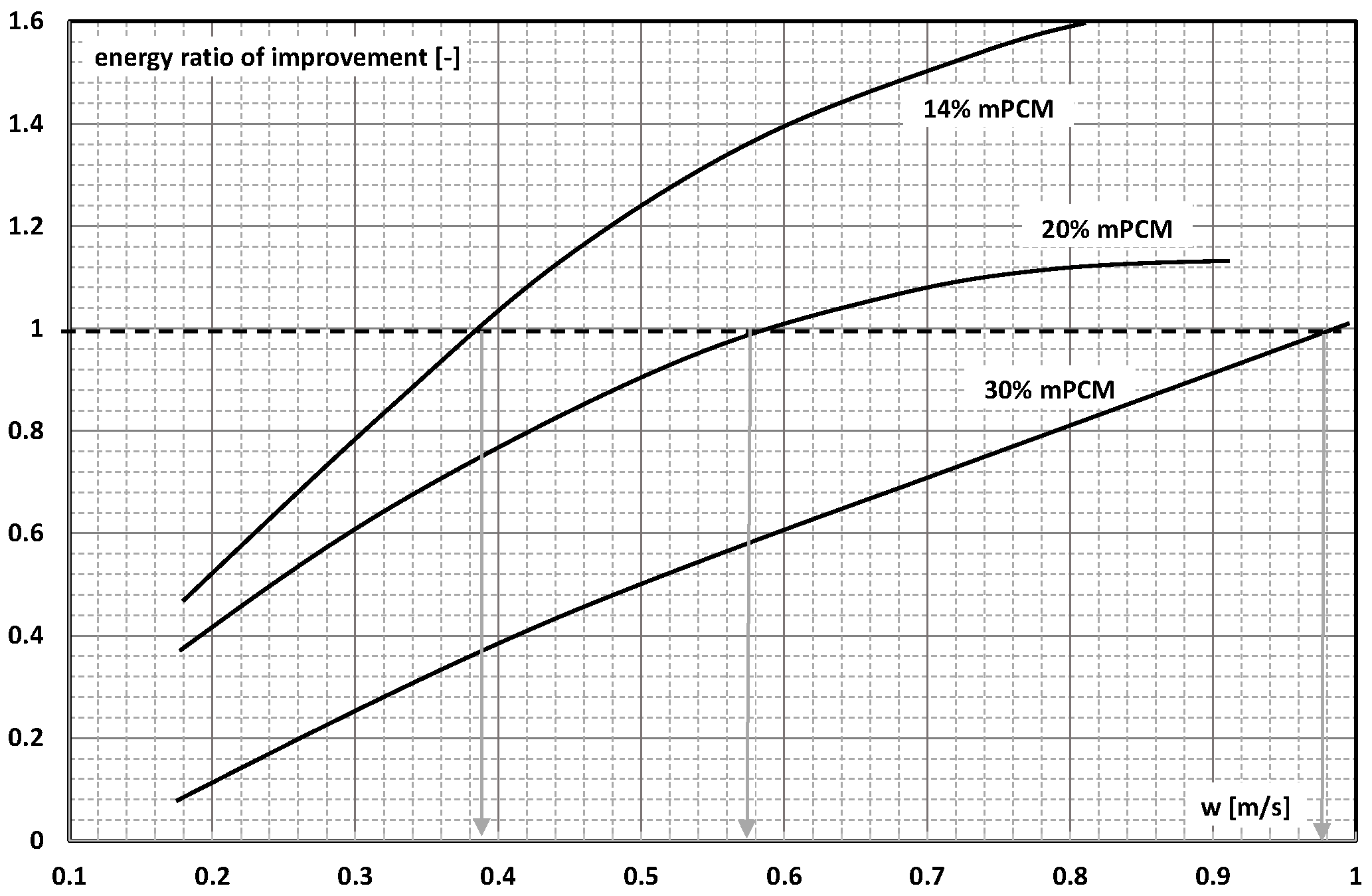
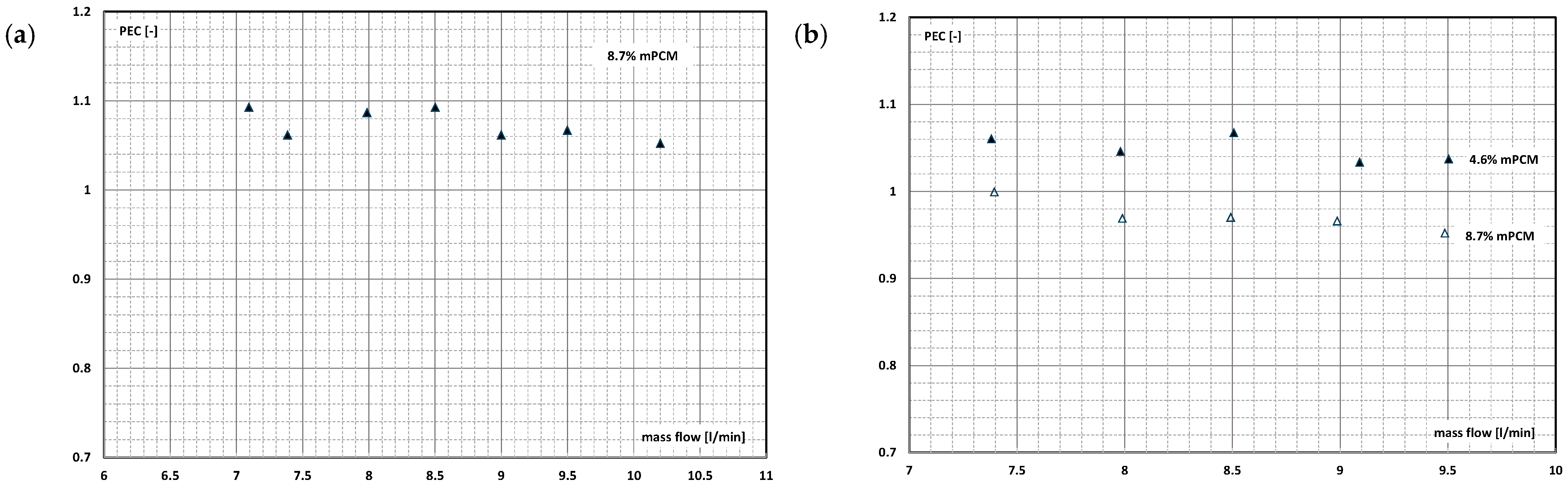
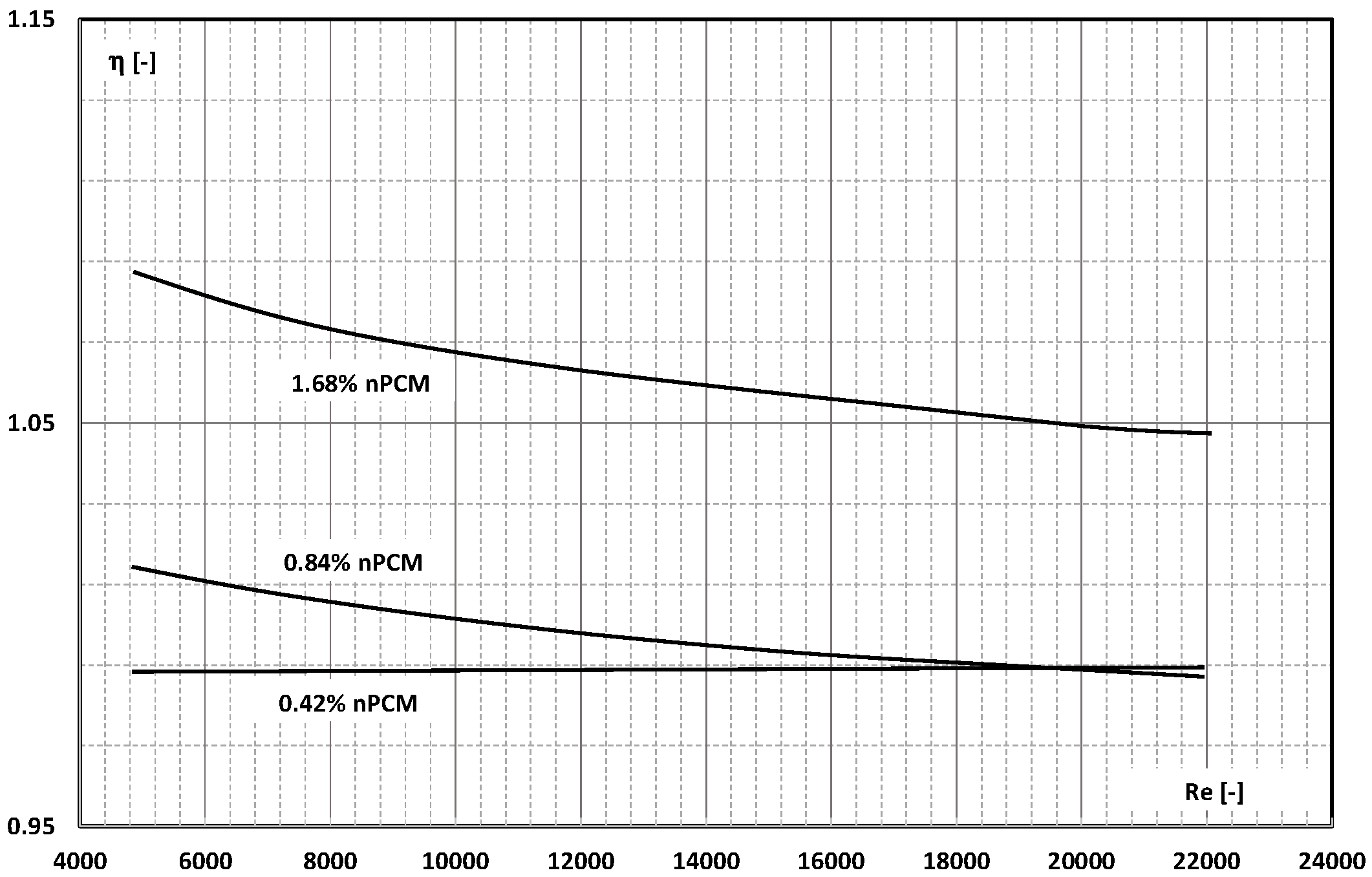
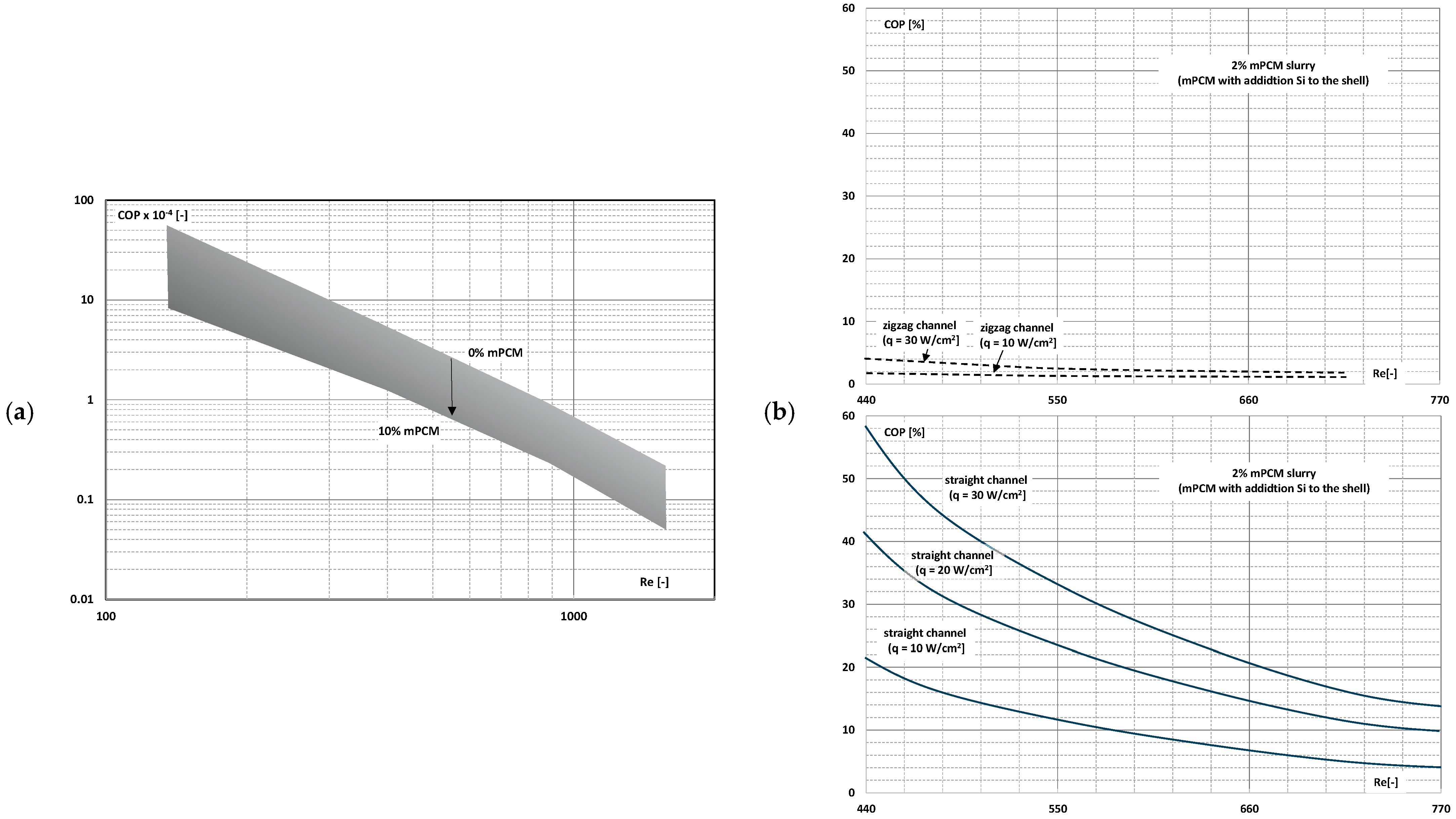
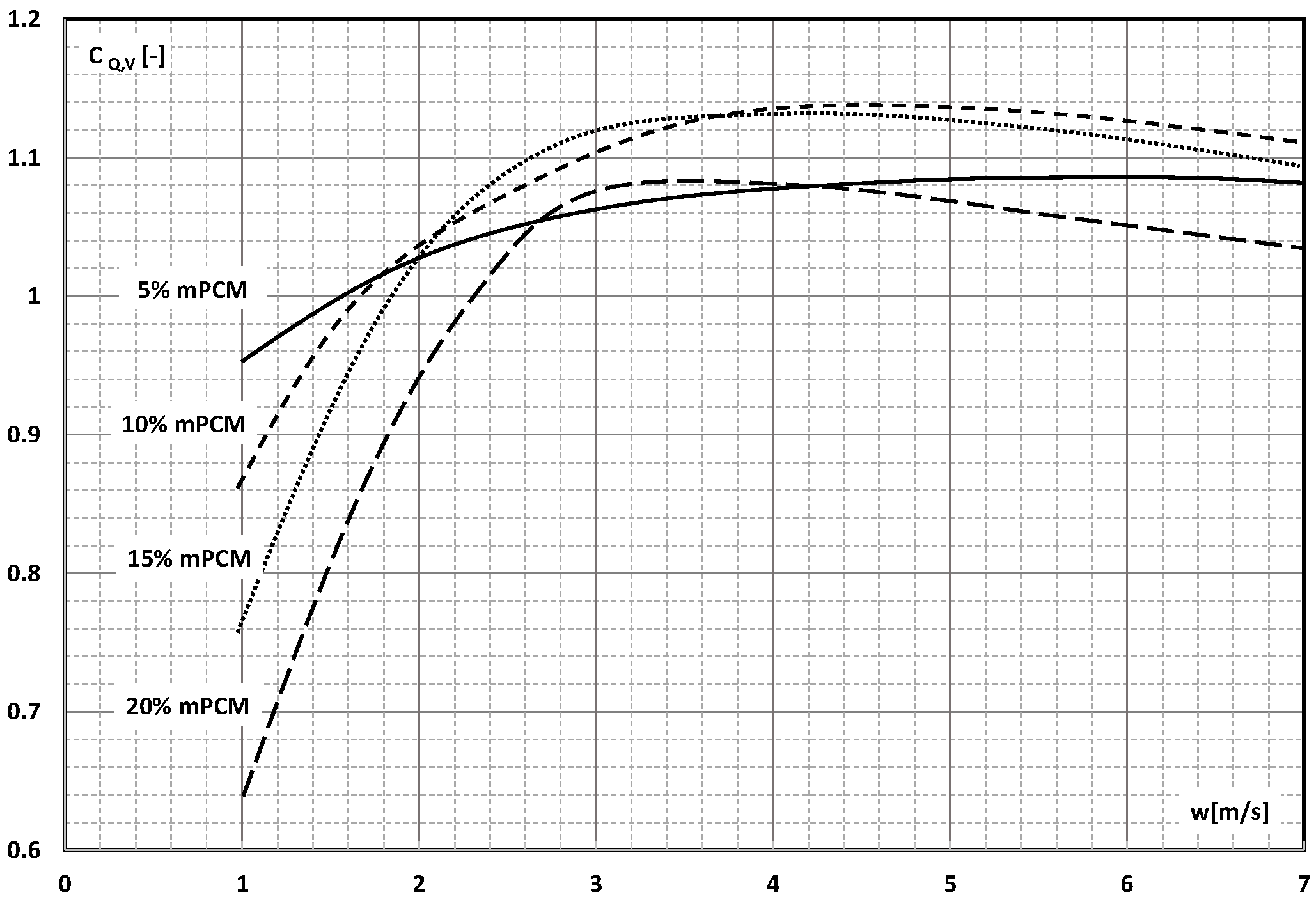
4. System Efficiency Improvement Factor/Energy Ratio of Improvement
5. Conclusions and Outlook
- The number of papers devoted to flow studies of liquids with the addition of mPCM (nPCM) is incomparably smaller than the number of papers devoted to the rheology and heat transfer of these slurries;
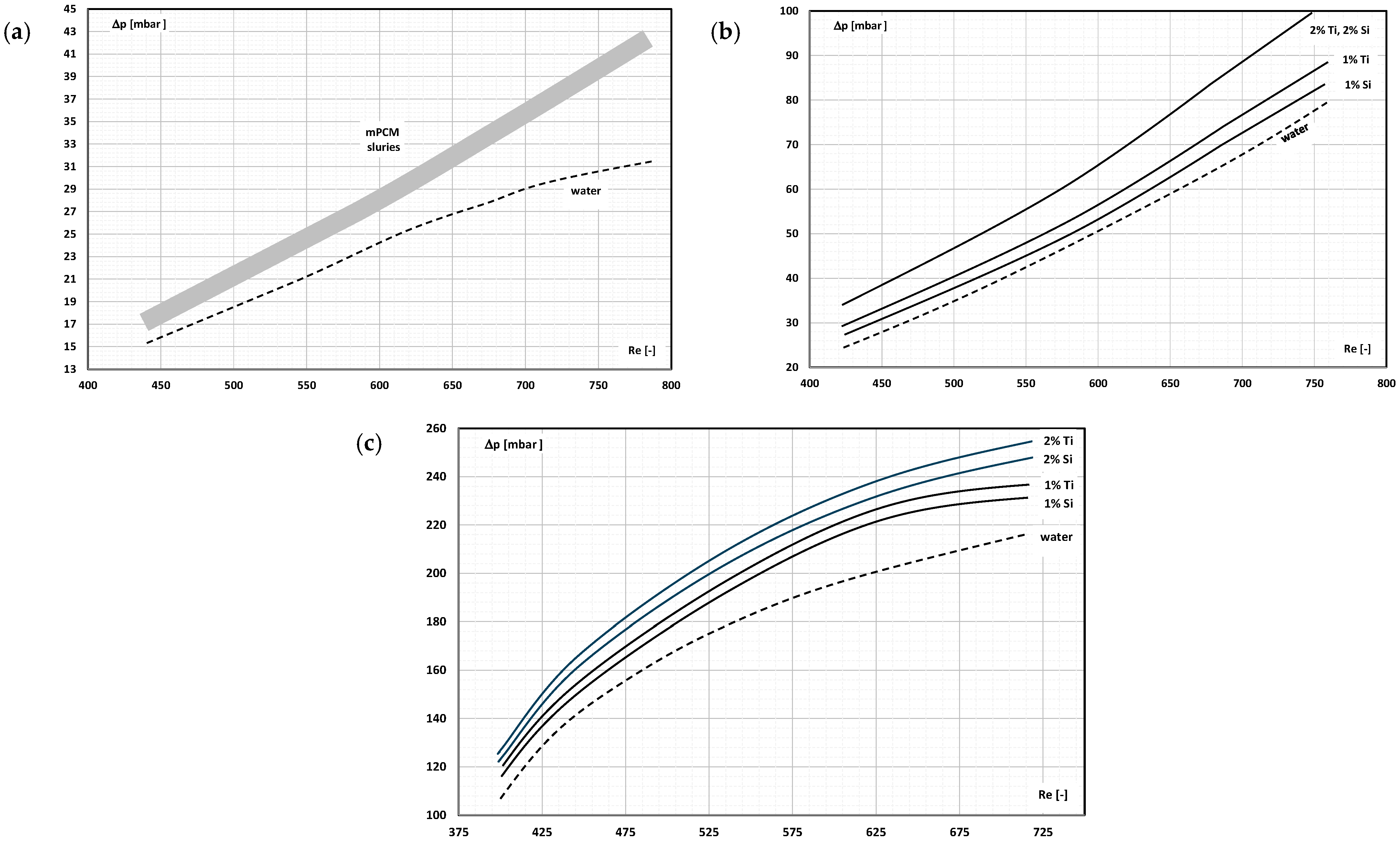
- Occasional experimental studies show the results of measuring the pressure drop during the flow of the mPCM (nPCM) slurry in heat-transfer systems and show that with the increase in the flow velocity of the slurry, the measured pressure drop also increased and was greater the higher the concentration of mPCM (nPCM) in the slurry;
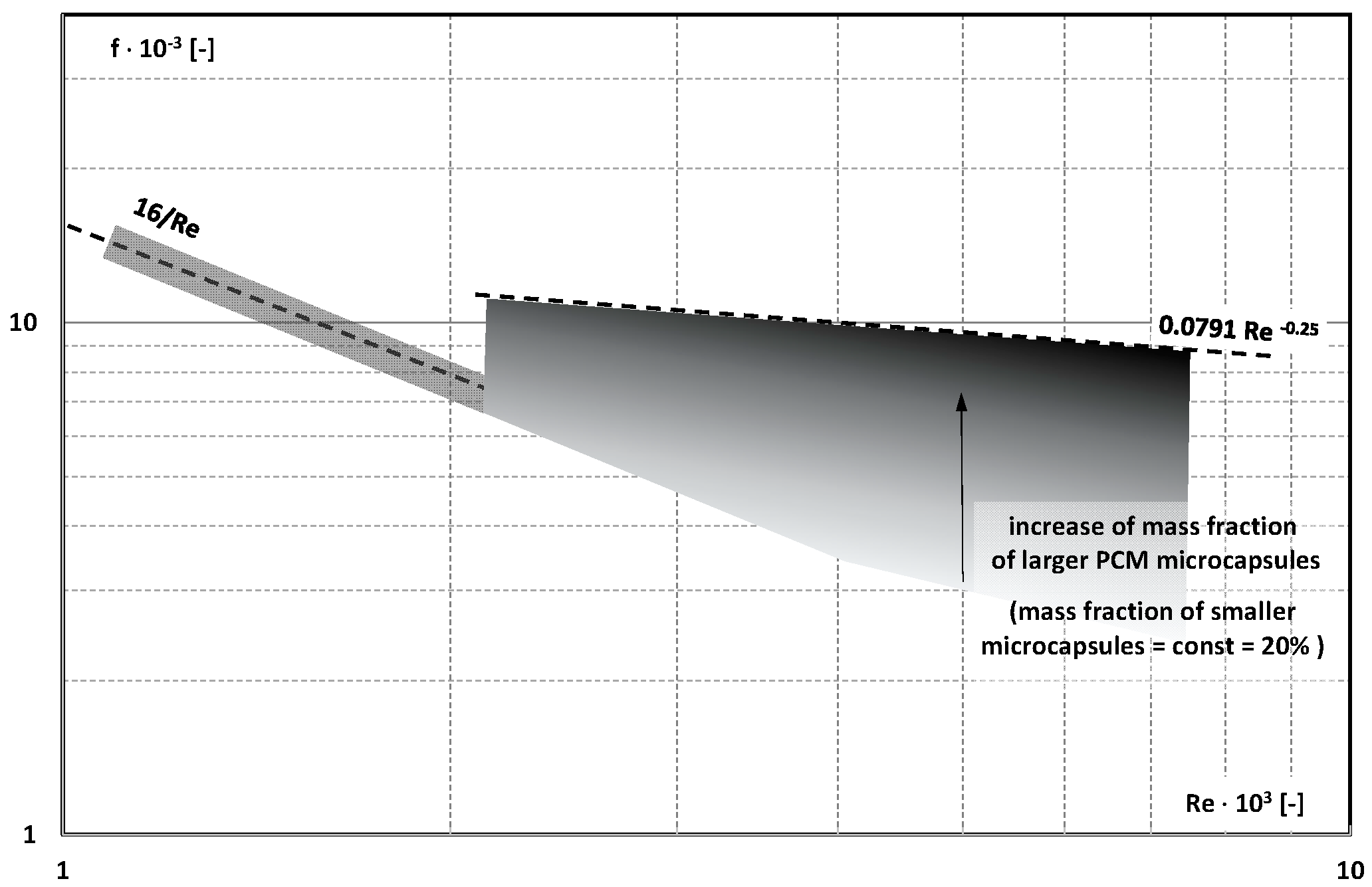
- There is insufficient information in the literature to determine whether and how the type of PCM, its state of matter, the type and size of the PCM, etc., affect the pressure drop of the flowing slurry;
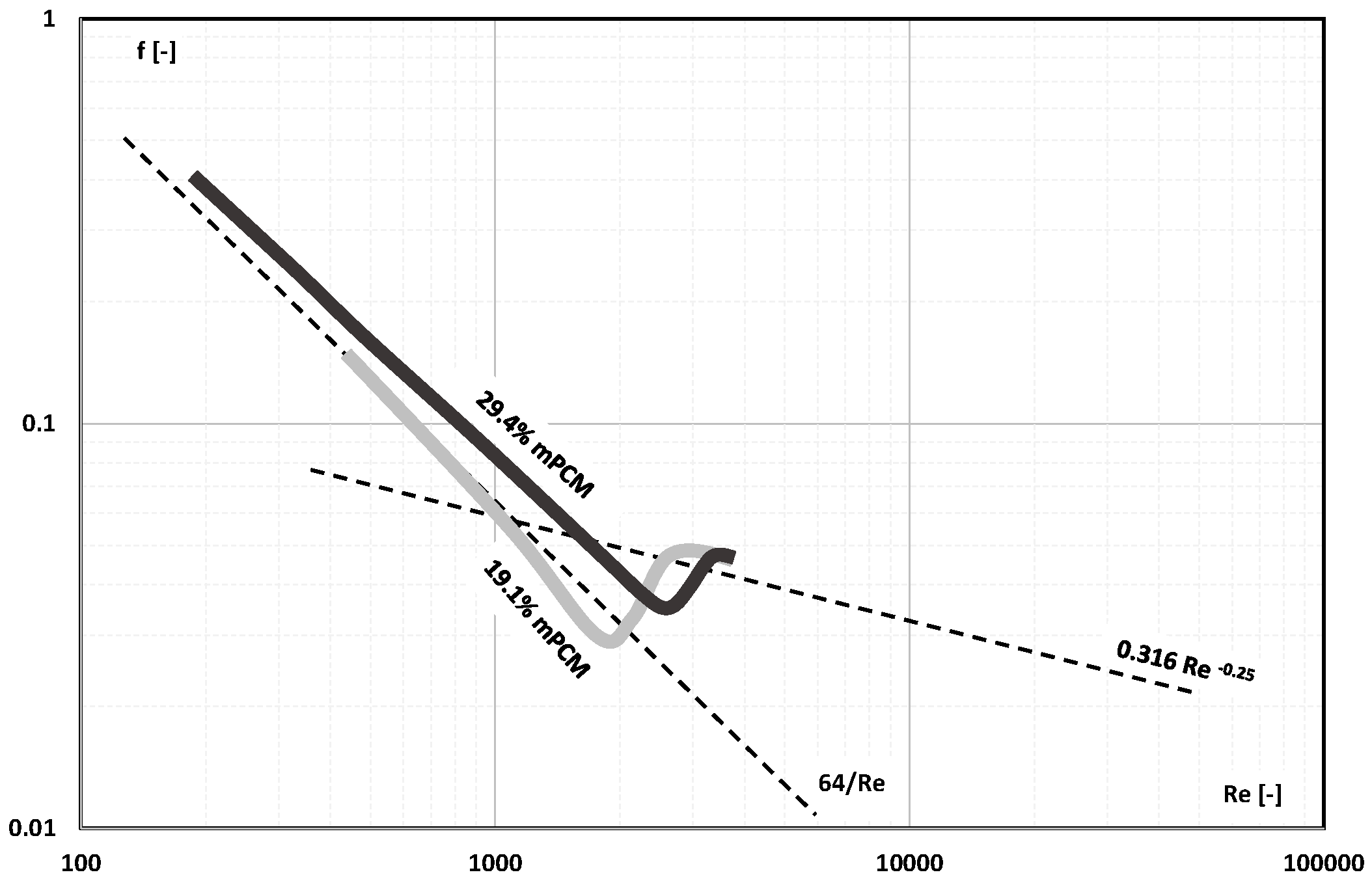
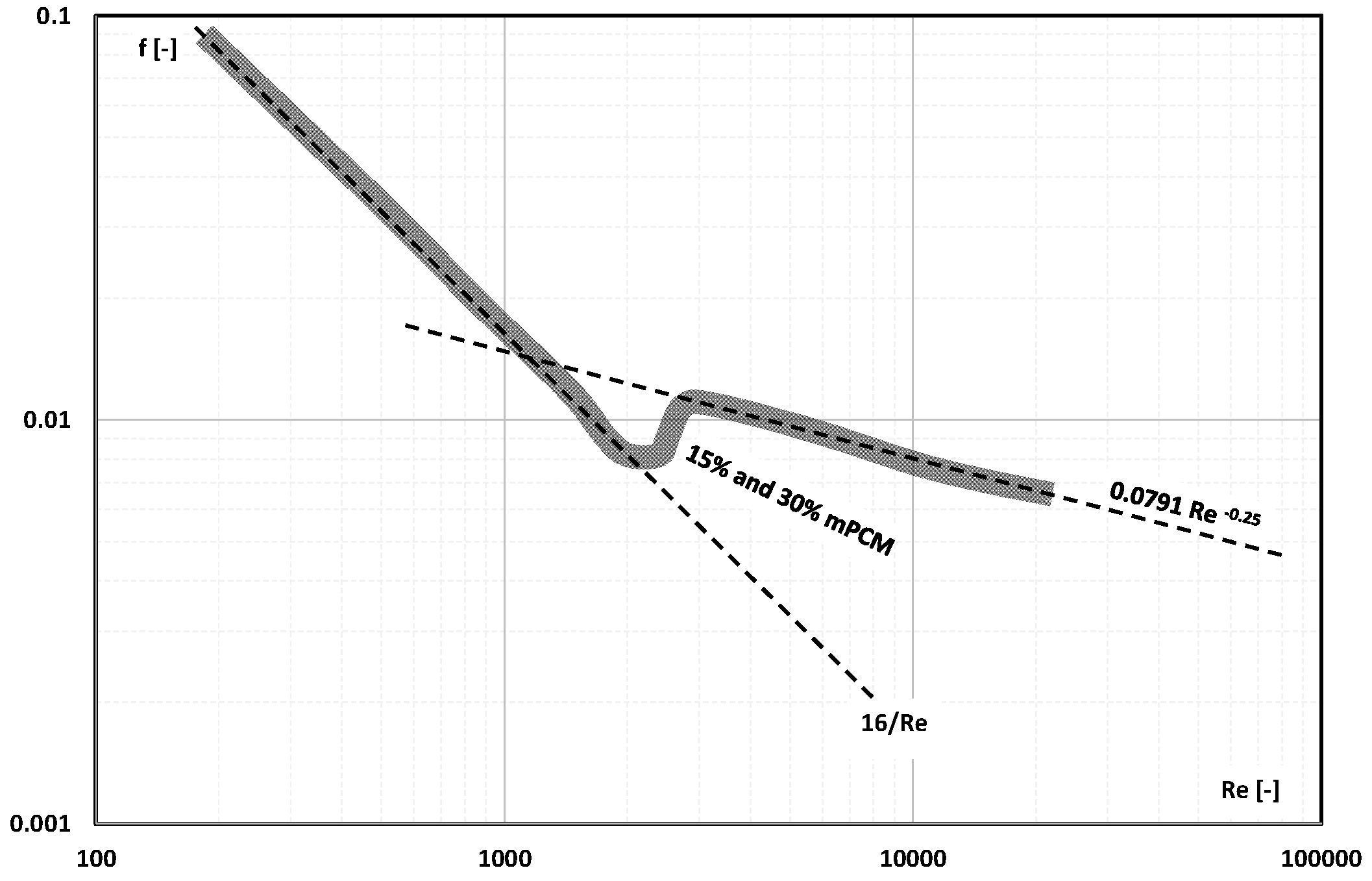
- The information contained in previous publications on the friction factor indicates that in the range of laminar and turbulent motion, it may take values lower, equal to, or higher than the value of this coefficient for the base liquid (usually water);
- There are not enough publications to assess whether and how an increase in the mass fraction of mPCM (nPCM) in the slurry or the matter state of PCM in the capsule affects the value of the friction factor. Current data indicate that it may decrease, increase, or have no effect;
- The use of mPCM (nPCM) slurry in flow heat-transfer systems is a valid concept because despite the increase in pressure drop, the increase in the heat-transfer coefficient is large enough, provided that the slurry flows fast enough;
- There are studies that indicate that the higher the slurry flow, after exceeding the limit value, the further the increase in the flow velocity, resulting in another improvement in the efficiency of the exchanger. It stabilizes asymptotically or reaches a certain maximum value, after which it begins to decrease.
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Channel cross-sectional area (m2) |
| cp | Specific heat (J·kg−1·K−1) |
| CQ,V | Comprehensive coefficient of performance (-) |
| d | Diameter (m) |
| f | Friction factor (Fanning factor) (-) |
| L | Length (m) |
| Nu | Nusselt number (-) |
| p | Pressure (Pa) |
| PEC | Performance evaluation criterion (-) |
| Q | Thermal power (W) |
| Re | Reynolds number (-) |
| T | Temperature (°C) |
| w | Velocity (m·s−1) |
| W | Pumping power (W) |
| Greek letters | |
| η | Energy ratio of improvement (-) |
| Abbreviations | |
| mPCM | Micro-encapsulated PCM |
| nPCM | Nano-encapsulated PCM |
| PCM | Phase-change material |
References
- Ali, H.M. Phase change materials based thermal energy storage for solar energy systems. J. Build. Eng. 2022, 56, 104731. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Agresti, F.; Fedele, L.; Barison, S.; Hermida-Merino, C.; Losada-Barreiro, S.; Bobbo, S.; Piñeiro, M.M. Review on phase change material emulsions for advanced thermal management: Design, characterization and thermal performance. Renew. Sustain. Energy Rev. 2022, 159, 112238. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127, 838–856. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Encapsulation techniques for organic phase change materials as thermal energy storage medium: A review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Junaid, M.F.; ur Rehman, Z.; Čekon, M.; Čurpek, J.; Farooq, R.; Cui, H.; Khan, I. Inorganic phase change materials in thermal energy storage: A review on perspectives and technological advances in building applications. Energy Build. 2021, 252, 111443. [Google Scholar] [CrossRef]
- Mishra, R.K.; Verma, K.; Mishra, V.; Chaudhary, B. A review on carbon-based phase change materials for thermal energy storage. J. Energy Storage 2022, 50, 104166. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, C.; Fang, G. Encapsulation of inorganic phase change thermal storage materials and its effect on thermophysical properties: A review. Sol. Energy Mater. Sol. Cells 2022, 241, 111747. [Google Scholar] [CrossRef]
- Li, M.; Mu, B. Effect of different dimensional carbon materials on the properties and application of phase change materials: A review. Appl. Energy 2019, 242, 695–715. [Google Scholar] [CrossRef]
- Abdeali, G.; Bahramian, A.R. A comprehensive review on rheological behavior of phase change materials fluids (slurry and emulsion): The way toward energy efficiency. J. Energy Storage 2022, 55, 105549. [Google Scholar] [CrossRef]
- Albdour, S.A.; Haddad, Z.; Sharaf, O.Z.; Alazzam, A.; Abu-Nada, E. Micro/nano-encapsulated phase-change materials (ePCMs) for solar photothermal absorption and storage: Fundamentals, recent advances, and future directions. Prog. Energy Combust. Sci. 2022, 93, 101037. [Google Scholar] [CrossRef]
- Chai, L.; Shaukat, R.; Wang, L.; Wang, H.S. A review on heat transfer and hydrodynamic characteristics of nano/microencapsulated phase change slurry (N/MPCS) in mini/microchannel heat sinks. Appl. Therm. Eng. 2018, 135, 334–349. [Google Scholar] [CrossRef]
- Chen, L.; Wang, T.; Zhao, Y.; Zhang, X.R. Characterization of thermal and hydrodynamic properties for microencapsulated phase change slurry (MPCS). Energy Convers. Manag. 2014, 79, 317–333. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental research of viscosity of microencapsulated PCM slurry at the phase change temperature. Int. J. Heat Mass Transf. 2019, 134, 1209–1217. [Google Scholar] [CrossRef]
- Dutkowski, K.; Fiuk, J.J. Experimental investigation of the effects of mass fraction and temperature on the viscosity of microencapsulated PCM slurry. Int. J. Heat Mass Transf. 2018, 126, 390–399. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Zalba, B. Review on phase change material emulsions and microencapsulated phase change material slurries: Materials, heat transfer studies and applications. Renew. Sustain. Energy Rev. 2012, 16, 253–273. [Google Scholar] [CrossRef]
- Dutkowski, K.; Kruzel, M.; Zajączkowski, B.; Białko, B. The experimental investigation of mPCM slurries density at phase change temperature. Int. J. Heat Mass Transf. 2020, 159, 120083. [Google Scholar] [CrossRef]
- Ghoghaei, M.S.; Mahmoudian, A.; Mohammadi, O.; Shafii, M.B.; Jafari Mosleh, H.; Zandieh, M.; Ahmadi, M.H. A review on the applications of micro-/nano-encapsulated phase change material slurry in heat transfer and thermal storage systems. J. Therm. Anal. Calorim. 2021, 145, 245–268. [Google Scholar] [CrossRef]
- Jurkowska, M.; Szczygieł, I. Review on properties of microencapsulated phase change materials slurries (mPCMS). Appl. Therm. Eng. 2016, 98, 365–373. [Google Scholar] [CrossRef]
- Liu, L.; Alva, G.; Huang, X.; Fang, G. Preparation, heat transfer and flow properties of microencapsulated phase change materials for thermal energy storage. Renew. Sustain. Energy Rev. 2016, 66, 399–414. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, X.; Li, P.; Zhao, X.; Wright, A. Micro-encapsulated phase change material (MPCM) slurries: Characterization and building applications. Renew. Sustain. Energy Rev. 2017, 77, 246–262. [Google Scholar] [CrossRef]
- Ran, F.; Chen, Y.; Cong, R.; Fang, G. Flow and heat transfer characteristics of microencapsulated phase change slurry in thermal energy systems: A review. Renew. Sustain. Energy Rev. 2020, 134, 110101. [Google Scholar] [CrossRef]
- Inaba, H. New challenge in advanced thermal energy transportation using functionally thermal fluids. Int. J. Therm. Sci. 2000, 39, 991–1003. [Google Scholar] [CrossRef]
- Alvarado, J.L.; Marsh, C.; Sohn, C.; Phetteplace, G.; Newell, T. Thermal performance of microencapsulated phase change material slurry in turbulent flow under constant heat flux. Int. J. Heat Mass Transf. 2007, 50, 1938–1952. [Google Scholar] [CrossRef]
- Chen, B.; Wang, X.; Zeng, R.; Zhang, Y.; Wang, X.; Niu, J.; Li, Y.; Di, H. An experimental study of convective heat transfer with microencapsulated phase change material suspension: Laminar flow in a circular tube under constant heat flux. Exp. Therm. Fluid Sci. 2008, 32, 1638–1646. [Google Scholar] [CrossRef]
- Dammel, F.; Stephan, P. Heat transfer to suspensions of microencapsulated phase change material flowing through minichannels. J. Heat Transf. 2012, 134, 020907. [Google Scholar] [CrossRef]
- Wu, W.; Bostanci, H.; Chow, L.C.; Hong, Y.; Wang, C.M.; Su, M.; Kizito, J.P. Heat transfer enhancement of PAO in microchannel heat exchanger using nano-encapsulated phase change indium particles. Int. J. Heat Mass Transf. 2013, 58, 348–355. [Google Scholar] [CrossRef]
- Taherian, H.; Alvarado, J.L.; Tumuluri, K.; Thies, C.; Park, C.H. Fluid flow and heat transfer characteristics of microencapsulated phase change material slurry in turbulent flow. J. Heat Transf. 2014, 136, 061704. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Sinha-Ray, S.; Sriram, H.; Yarin, A.L. Flow of suspensions of carbon nanotubes carrying phase change materials through microchannels and heat transfer enhancement. Lab Chip 2014, 14, 494–508. [Google Scholar] [CrossRef]
- Serale, G.; Fabrizio, E.; Perino, M. Design of a low-temperature solar heating system based on a slurry Phase Change Material (PCS). Energy Build. 2015, 106, 44–58. [Google Scholar] [CrossRef]
- Qiu, Z.; Ma, X.; Zhao, X.; Li, P.; Ali, S. Experimental investigation of the energy performance of a novel Micro-encapsulated Phase Change Material (MPCM) slurry based PV/T system. Appl. Energy 2016, 165, 260–271. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Ling, X. An experimental study of the latent functionally thermal fluid with micro-encapsulated phase change material particles flowing in microchannels. Appl. Therm. Eng. 2016, 105, 209–216. [Google Scholar] [CrossRef]
- Roberts, N.S.; Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.A.; Farid, M.M. Efficacy of using slurry of metal-coated microencapsulated PCM for cooling in a micro-channel heat exchanger. Appl. Therm. Eng. 2017, 122, 11–18. [Google Scholar] [CrossRef]
- Ho, C.J.; Chang, P.C.; Yan, W.M.; Amani, M. Comparative study on thermal performance of MEPCM suspensions in parallel and divergent minichannel heat sinks. Int. Commun. Heat Mass Transf. 2018, 94, 96–105. [Google Scholar] [CrossRef]
- Ho, C.J.; Chang, P.C.; Yan, W.M.; Amani, P. Efficacy of divergent minichannels on cooling performance of heat sinks with water-based MEPCM suspensions. Int. J. Therm. Sci. 2018, 130, 333–346. [Google Scholar] [CrossRef]
- Ho, C.J.; Chen, W.C.; Yan, W.M.; Amani, P. Contribution of hybrid Al2O3-water nanofluid and PCM suspension to augment thermal performance of coolant in a minichannel heat sink. Int. J. Heat Mass Transf. 2018, 122, 651–659. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, G.; Dou, B.; Wang, Z.; Goula, M.A. An experimental investigation of forced convection heat transfer with novel microencapsulated phase change material slurries in a circular tube under constant heat flux. Energy Convers. Manag. 2018, 171, 699–709. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, B.; Guo, Y.; Cui, G.; Dou, B.; Wang, Z.; Yan, X. Effect of metal oxide particles on the flow and forced convective heat transfer behaviour of microencapsulated PCM slurry. Sol. Energy 2022, 238, 280–290. [Google Scholar] [CrossRef]
- Pakrouh, R.; Hosseini, M.J.; Bahrampoury, R.; Ranjbar, A.A.; Borhani, S.M. Cylindrical battery thermal management based on microencapsulated phase change slurry. J. Energy Storage 2021, 40, 102602. [Google Scholar] [CrossRef]
- Bai, F.; Chen, M.; Song, W.; Yu, Q.; Li, Y.; Feng, Z.; Ding, Y. Investigation of thermal management for lithium-ion pouch battery module based on phase change slurry and mini channel cooling plate. Energy 2019, 167, 561–574. [Google Scholar] [CrossRef]
- Shaukat, R.; Anwar, Z.; Imran, S.; Noor, F.; Qamar, A. Numerical Study of Heat Transfer Characteristics of mPCM Slurry During Freezing. Arab. J. Sci. Eng. 2021, 46, 7977–7988. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, S.; Zhang, L. Multi-scale modeling and investigation of thermo-fluidic performance of microencapsulated phase-change material slurry. J. Energy Storage 2021, 37, 102502. [Google Scholar] [CrossRef]
- Ashagre, T.B.; Rakshit, D. A study on energy transport performance of Microencapsulated Phase Change Materials (MPCM) slurry. Int. Commun. Heat Mass Transf. 2022, 138, 106321. [Google Scholar] [CrossRef]
- Lian, C.; Wang, Y.; Li, Q.; Li, H.; He, X. Numerical investigation on the performance of microencapsulated phase change material suspension applied to liquid cold plates. Numer. Heat Transf. Part A Appl. 2019, 75, 342–358. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhao, X.; Li, P.; Zhang, X.; Ali, S.; Tan, J. Theoretical investigation of the energy performance of a novel MPCM (Microencapsulated Phase Change Material) slurry based PV/T module. Energy 2015, 87, 686–698. [Google Scholar] [CrossRef]
- Balasubramanian, K.R.; John Peter, R.; B.S, J. Experimental investigation on paraffin encapsulated with Silica and Titanium shell in the straight and re-entrant microchannel heat sinks. Heat Mass Transf. 2023, 59, 1005–1018. [Google Scholar] [CrossRef]
- John Peter, R.; Balasubramanian, K.R.; Ravi Kumar, K. Comparative study on the thermal performance of microencapsulated phase change material slurry in tortuous geometry microchannel heat sink. Appl. Therm. Eng. 2023, 218, 119328. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Sugeno, T.; Ishige, T. An evolution of microencapsulated PCM for use in cold energy transportation medium. In Proceedings of the 31st Intersociety Energy Conversion Engineering Conference, Washington, DC, USA, 11–16 August 1996; p. 96082. [Google Scholar]
- Yamagishi, Y.; Takeuchi, H.; Pyatenko, A.T.; Kayukawa, N. Characteristics of microencapsulated PCM slurry as a heat-transfer fluid. AIChE J. 1999, 45, 696–707. [Google Scholar] [CrossRef]
- Inaba, H.; Kim, M.K.; Horibe, A. Melting heat transfer characteristics of microencapsulated phase change material slurries with plural microcapsules having different diameters. J. Heat Transf. 2004, 126, 558–565. [Google Scholar] [CrossRef]
- Rao, Y.; Dammel, F.; Stephan, P.; Lin, G. Flow frictional characteristics of microencapsulated phase change material suspensions flowing through rectangular minichannels. Sci. China Ser. E Technol. Sci. 2006, 49, 445–456. [Google Scholar] [CrossRef]
- Wang, X.; Niu, J.; Li, Y.; Wang, X.; Chen, B.; Zeng, R.; Song, Q.; Zhang, Y. Flow and heat transfer behaviors of phase change material slurries in a horizontal circular tube. Int. J. Heat Mass Transf. 2007, 50, 2480–2491. [Google Scholar] [CrossRef]
- Ho, C.J.; Chen, W.C.; Yan, W.M. Experimental study on cooling performance of minichannel heat sink using water-based MEPCM particles. Int. Commun. Heat Mass Transf. 2013, 48, 67–72. [Google Scholar] [CrossRef]
- Ho, C.J.; Chen, W.C.; Yan, W.M. Experiment on thermal performance of water-based suspensions of Al 2O3 nanoparticles and MEPCM particles in a minichannel heat sink. Int. J. Heat Mass Transf. 2014, 69, 276–284. [Google Scholar] [CrossRef]
- Cao, F.; Kalinowski, P.; Lawler, J.; Lee, H.S.; Yang, B. Synthesis and heat transfer performance of phase change microcapsule enhanced thermal fluids. J. Heat Transf. 2015, 137, 091018. [Google Scholar] [CrossRef]
- Song, S.; Shen, W.; Wang, J.; Wang, S.; Xu, J. Experimental study on laminar convective heat transfer of microencapsulated phase change material slurry using liquid metal with low melting point as carrying fluid. Int. J. Heat Mass Transf. 2014, 73, 21–28. [Google Scholar] [CrossRef]
- Li, L.Y.; Zou, D.; Ma, X.F.; Liu, X.S.; Hu, Z.G.; Guo, J.R.; Zhu, Y.Y. Preparation and flow resistance characteristics of novel microcapsule slurries for engine cooling system. Energy Convers. Manag. 2017, 135, 170–177. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kurazono, K.; Yamauchi, T. Thermal–hydraulic characteristics of ethylene glycol aqueous solutions containing microencapsulated paraffin. Exp. Therm. Fluid Sci. 2018, 99, 297–303. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Liu, Z. Experimental study on micro-encapsulated phase change material slurry flowing in straight and wavy microchannels. Appl. Therm. Eng. 2021, 190, 116841. [Google Scholar] [CrossRef]
- Jadal, M.; Soto, J.; Boyard, N.; Delaunay, D. Experimental determination of crystallization kinetic model of CENG-PCM composite material. Validation at macro and meso scales. Therm. Sci. Eng. Prog. 2022, 33, 101336. [Google Scholar] [CrossRef]
- Ye, J.; Mo, S.; Jia, L.; Chen, Y. Experimental performance of a LED thermal management system with suspended microencapsulated phase change material. Appl. Therm. Eng. 2022, 207, 118155. [Google Scholar] [CrossRef]
- Yuan, Z.; Liang, K.; Xue, Y.; Yamada, Y.; Isobe, K.; Horibe, A. Experimental study of evaluation of dynamical utilization of a microencapsulated phase change material slurry based on temperature range matching analysis. Int. Commun. Heat Mass Transf. 2022, 130, 105788. [Google Scholar] [CrossRef]
- Zheng, H.; Tian, G.; Zhao, Y.; Xin, X.; Yang, C.; Cao, L.; Ma, Y. Experimental study on the preparation and cool storage performance of a phase change micro-capsule cold storage material. Energy Build. 2022, 262, 111999. [Google Scholar] [CrossRef]
- Fischer, L.; Maranda, S.; Stamatiou, A.; von Arx, S.; Worlitschek, J. Experimental investigation on heat transfer with a Phase Change Dispersion. Appl. Therm. Eng. 2019, 147, 61–73. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, H.; Xuan, Y. Experimental investigation of thermal properties and moisture buffering performance of composite interior finishing materials under different airflow conditions. Build. Environ. 2019, 160, 106175. [Google Scholar] [CrossRef]
- Al-Abidi, A.A.; Bin Mat, S.; Sopian, K.; Sulaiman, M.Y.; Mohammed, A.T. CFD applications for latent heat thermal energy storage: A review. Renew. Sustain. Energy Rev. 2013, 20, 353–363. [Google Scholar] [CrossRef]
- Wan, H.; He, G.Q.; Xue, Z.R.; Li, W.Q. Numerical study and experimental verification on spray cooling with nanoencapsulated phase-change material slurry (NPCMS). Int. Commun. Heat Mass Transf. 2021, 123, 105187. [Google Scholar] [CrossRef]
- Chananipoor, A.; Azizi, Z.; Raei, B.; Tahmasebi, N. Optimization of the thermal performance of nano-encapsulated phase change material slurry in double pipe heat exchanger: Design of experiments using response surface methodology (RSM). J. Build. Eng. 2021, 34, 101929. [Google Scholar] [CrossRef]
- Ghasemi, K.; Mahmud, S.; Tasnim, S. Optimization of microencapsulated phase change material slurry-based porous heat exchanger. J. Energy Storage 2022, 55, 105797. [Google Scholar] [CrossRef]
- Wang, D.; Niu, X.; Yan, Y.; Gao, P.; Duan, D. Research on falling film dehumidification performance of microencapsulated phase change materials slurry. Energy Build. 2021, 235, 110750. [Google Scholar] [CrossRef]
- Mourad, A.; Aissa, A.; Said, Z.; Younis, O.; Iqbal, M.; Alazzam, A. Recent advances on the applications of phase change materials for solar collectors, practical limitations, and challenges: A critical review. J. Energy Storage 2022, 49, 104186. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, R.; Li, J. High latent heat phase change materials (PCMs) with low melting temperature for thermal management and storage of electronic devices and power batteries: Critical review. Renew. Sustain. Energy Rev. 2022, 168, 112783. [Google Scholar] [CrossRef]
- Sharma, M.K. Alternative designs and technological advancements of phase change material integrated photovoltaics: A state-of-the-art review. J. Energy Storage 2022, 48, 104020. [Google Scholar] [CrossRef]
- Yu, K.; Liu, Y.; Yang, Y. Review on form-stable inorganic hydrated salt phase change materials: Preparation, characterization and effect on the thermophysical properties. Appl. Energy 2021, 292, 116845. [Google Scholar] [CrossRef]
- Jiménez-Vázquez, M.; Ramos, F.J.; Garrido, I.; López-Pedrajas, D.; Rodríguez, J.F.; Carmona, M. Production of thermoregulating slurries constituted by nanocapsules from melamine-formaldehyde containing n-octadecane. J. Energy Storage 2022, 51, 104465. [Google Scholar] [CrossRef]
- Ashagre, T.B.; Rakshit, D. Study on flow and heat transfer characteristics of Encapsulated Phase Change Material (EPCM) slurry in Double-Pipe Heat Exchanger. J. Energy Storage 2022, 46, 103931. [Google Scholar] [CrossRef]
- Xu, L.; Pu, L.; Angelo, Z.; Zhang, D.; Dai, M.; Zhang, S. An experimental investigation on performance of microencapsulated phase change material slurry in ground heat exchanger. Renew. Energy 2022, 198, 296–305. [Google Scholar] [CrossRef]
- Shin, D.H.; Kim, S.; Karng, S.W.; Shin, Y. Development of a new type of PCM thermal capsule transport system. Int. J. Heat Mass Transf. 2022, 183, 122034. [Google Scholar] [CrossRef]
- Niu, X.; Xia, R.; Dong, H.; Wang, D.; Duan, D.; Gao, P.; Kosonen, R. Dispersion stability and thermophysical properties of microencapsulated phase change material slurry for liquid desiccant dehumidification. Energy Build. 2021, 240, 110870. [Google Scholar] [CrossRef]
- Alehosseini, E.; Jafari, S.M. Nanoencapsulation of phase change materials (PCMs) and their applications in various fields for energy storage and management. Adv. Colloid Interface Sci. 2020, 283, 102226. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Li, L. Experimental and numerical investigation of laminar heat transfer of microencapsulated phase change material slurry (MPCMS) in a circular tube with constant heat flux. Sustain. Cities Soc. 2020, 52, 101786. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, C.; Fang, G. Numerical evaluation on the flow and heat transfer characteristics of microencapsulated phase change slurry flowing in a circular tube. Appl. Therm. Eng. 2018, 144, 845–853. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, Z.W.; Bai, Z.Y.; Ye, J. Rheological and energy transport characteristics of a phase change material slurry. Energy 2016, 106, 63–72. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Zheng, H. A comprehensive review of hydrodynamic mechanisms and heat transfer characteristics for microencapsulated phase change slurry (MPCS) in circular tube. Renew. Sustain. Energy Rev. 2019, 114, 109312. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, P. Preparation and characterization of nano-sized phase change emulsions as thermal energy storage and transport media. Appl. Energy 2017, 190, 868–879. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Mazo, J.; Marín, J.M.; Zalba, B. Experimental analysis of a microencapsulated PCM slurry as thermal storage system and as heat transfer fluid in laminar flow. Appl. Therm. Eng. 2012, 36, 370–377. [Google Scholar] [CrossRef]
- Delgado, M.; Lázaro, A.; Peñalosa, C.; Zalba, B. Experimental analysis of the influence of microcapsule mass fraction on the thermal and rheological behavior of a PCM slurry. Appl. Therm. Eng. 2014, 63, 11–22. [Google Scholar] [CrossRef]
- Kong, M.; Alvarado, J.L.; Thies, C.; Morefield, S.; Marsh, C.P. Field evaluation of microencapsulated phase change material slurry in ground source heat pump systems. Energy 2017, 122, 691–700. [Google Scholar] [CrossRef]
- Kong, M.S.; Yu, K.; Alvarado, J.L.; Terrell, W. Thermal performance of microencapsulated phase change material slurry in a coil heat exchanger. J. Heat Transf. 2015, 137, 071801. [Google Scholar] [CrossRef]
- Doruk, S.; Şara, O.N.; Karaipekli, A.; Yapıcı, S. Heat transfer performance of water and Nanoencapsulated n-nonadecane based Nanofluids in a double pipe heat exchanger. Heat Mass Transf. Und Stoffuebertragung 2017, 53, 3399–3408. [Google Scholar] [CrossRef]
- Ho, C.J.; Chen, W.C.; Yan, W.M.; Amani, M. Cooling performance of MEPCM suspensions for heat dissipation intensification in a minichannel heat sink. Int. J. Heat Mass Transf. 2017, 115, 43–49. [Google Scholar] [CrossRef]
- Dai, H.; Chen, W. Numerical investigation of heat transfer in the double-layered minichannel with microencapsulated phase change suspension. Int. Commun. Heat Mass Transf. 2020, 119, 104918. [Google Scholar] [CrossRef]
- Dai, H.; Chen, W.; Cheng, Q.; Liu, Y.; Dong, X. Analysis of thermo-hydraulic characteristics in the porous-wall microchannel with microencapsulated phase change slurry. Int. J. Heat Mass Transf. 2021, 165, 120634. [Google Scholar] [CrossRef]
- Dai, H.; Chen, W.; Dong, X.; Liu, Y.; Cheng, Q. Thermohydraulic performance analysis of graded porous media microchannel with microencapsulated phase change material suspension. Int. J. Heat Mass Transf. 2021, 176, 121459. [Google Scholar] [CrossRef]
- Dai, H.; Zhu, C.; Liu, Y. Thermal performance of double-layer porous-microchannel with phase change slurry. Appl. Therm. Eng. 2022, 211, 118457. [Google Scholar] [CrossRef]
- Dai, H.; Liu, Y. Entropy generation analysis on thermo-hydraulic characteristics of microencapsulated phase change slurry in wavy microchannel with porous fins. Appl. Therm. Eng. 2022, 219, 119440. [Google Scholar] [CrossRef]
- Liu, P.; Chen, W. Thermal-hydraulic characteristics analysis in porous-wall corrugated microchannel with microencapsulated phase change slurry. Int. Commun. Heat Mass Transf. 2022, 138, 106316. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutkowski, K.; Kruzel, M. The State of the Art on the Flow Characteristic of an Encapsulated Phase-Change Material Slurry. Energies 2023, 16, 6931. https://doi.org/10.3390/en16196931
Dutkowski K, Kruzel M. The State of the Art on the Flow Characteristic of an Encapsulated Phase-Change Material Slurry. Energies. 2023; 16(19):6931. https://doi.org/10.3390/en16196931
Chicago/Turabian StyleDutkowski, Krzysztof, and Marcin Kruzel. 2023. "The State of the Art on the Flow Characteristic of an Encapsulated Phase-Change Material Slurry" Energies 16, no. 19: 6931. https://doi.org/10.3390/en16196931
APA StyleDutkowski, K., & Kruzel, M. (2023). The State of the Art on the Flow Characteristic of an Encapsulated Phase-Change Material Slurry. Energies, 16(19), 6931. https://doi.org/10.3390/en16196931







