Numerical Study on the Combustion Properties of Ammonia/DME and Ammonia/DMM Mixtures
Abstract
:1. Introduction
2. Kinetic Modeling
2.1. Numerical Approach
2.2. Mechanism Optimization
3. Results and Discussion
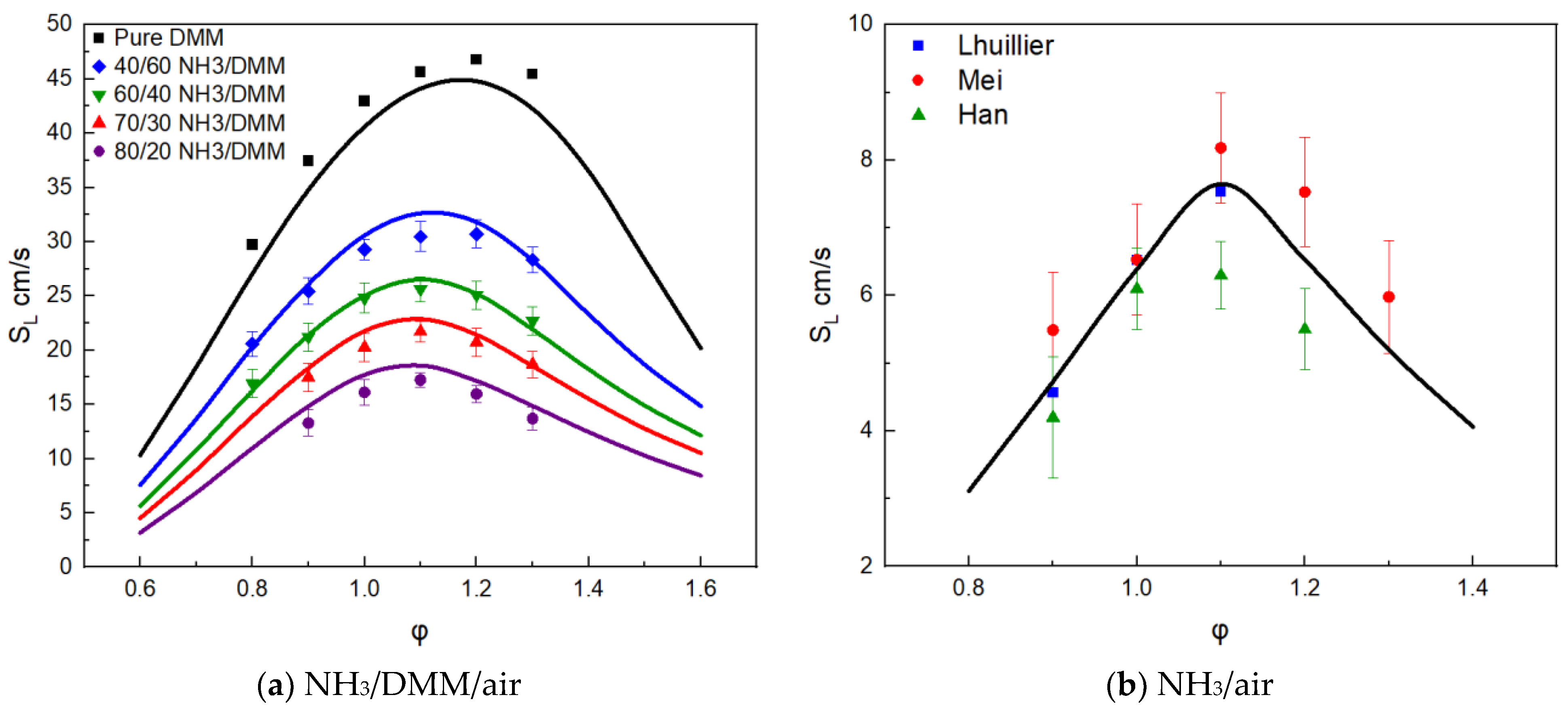
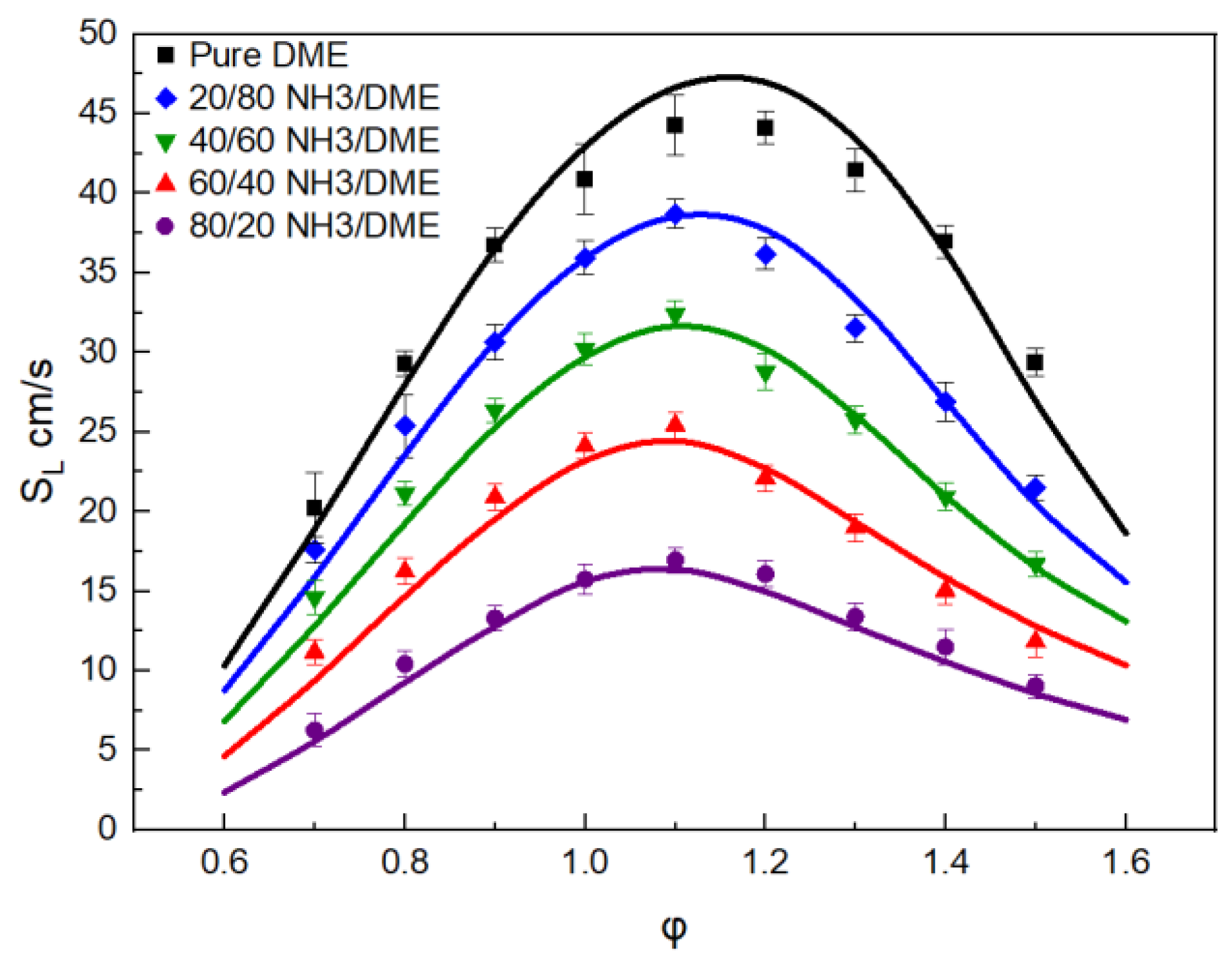
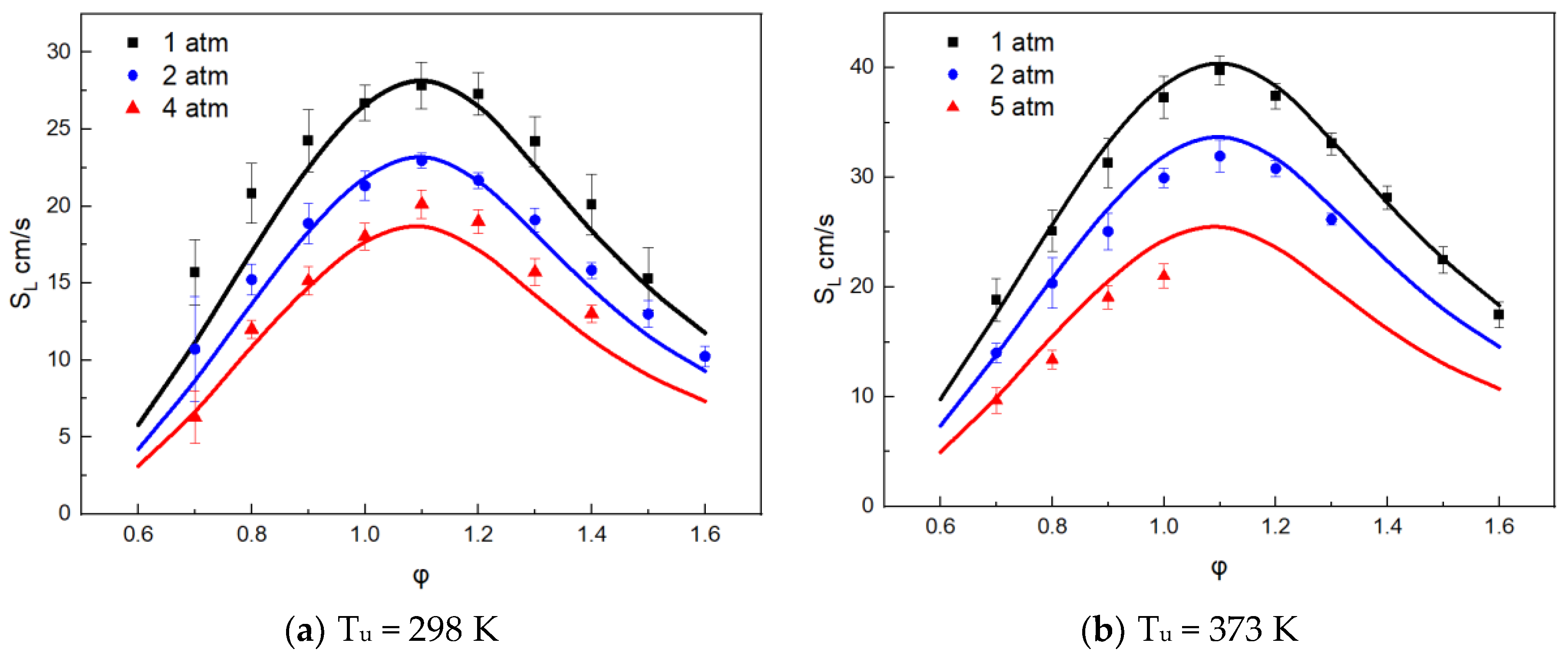
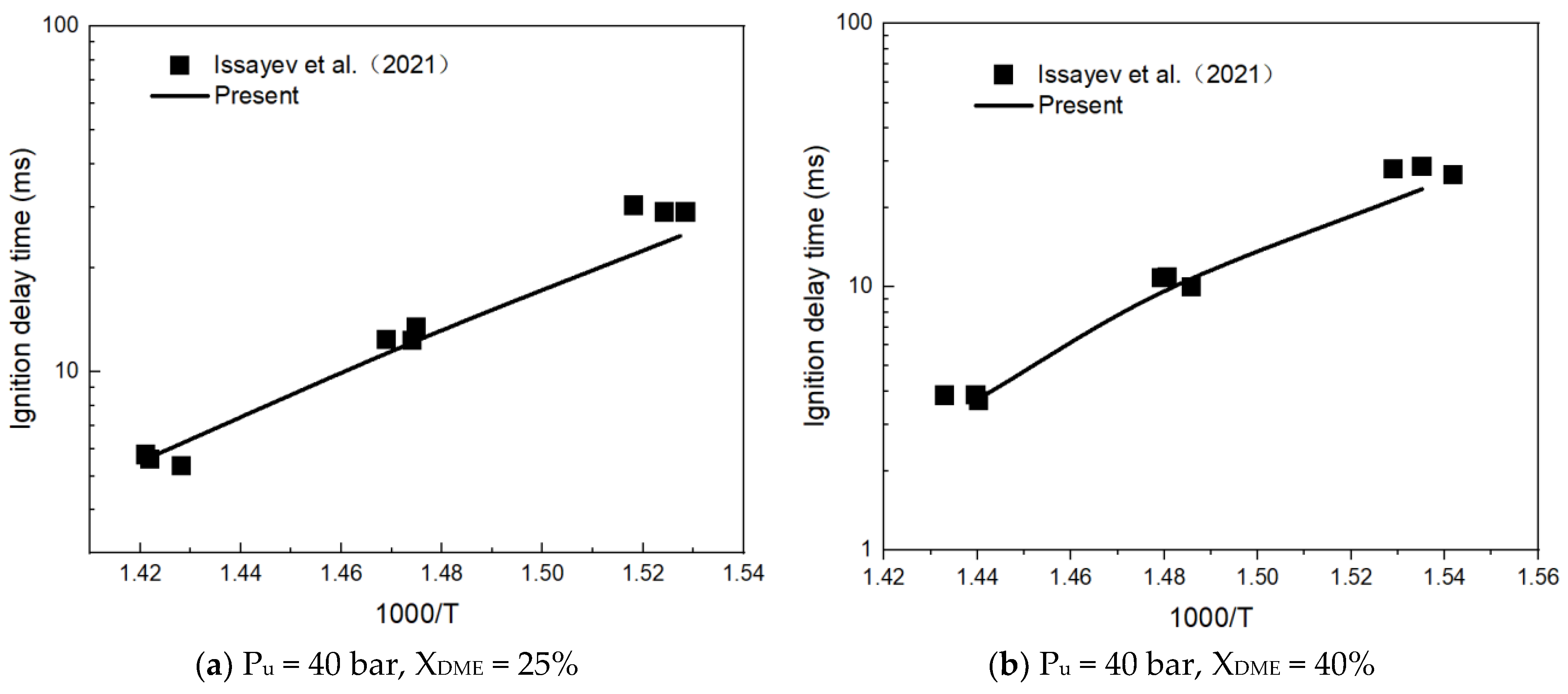
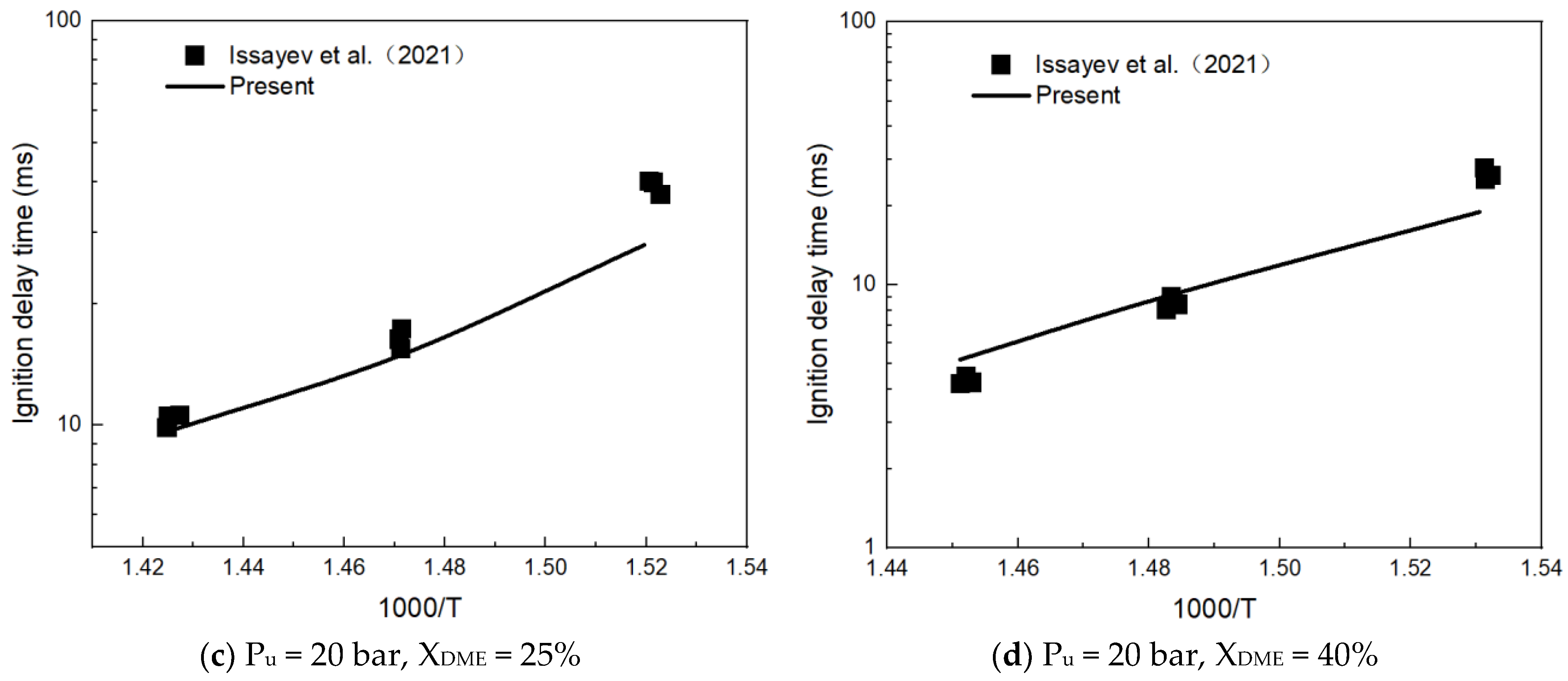
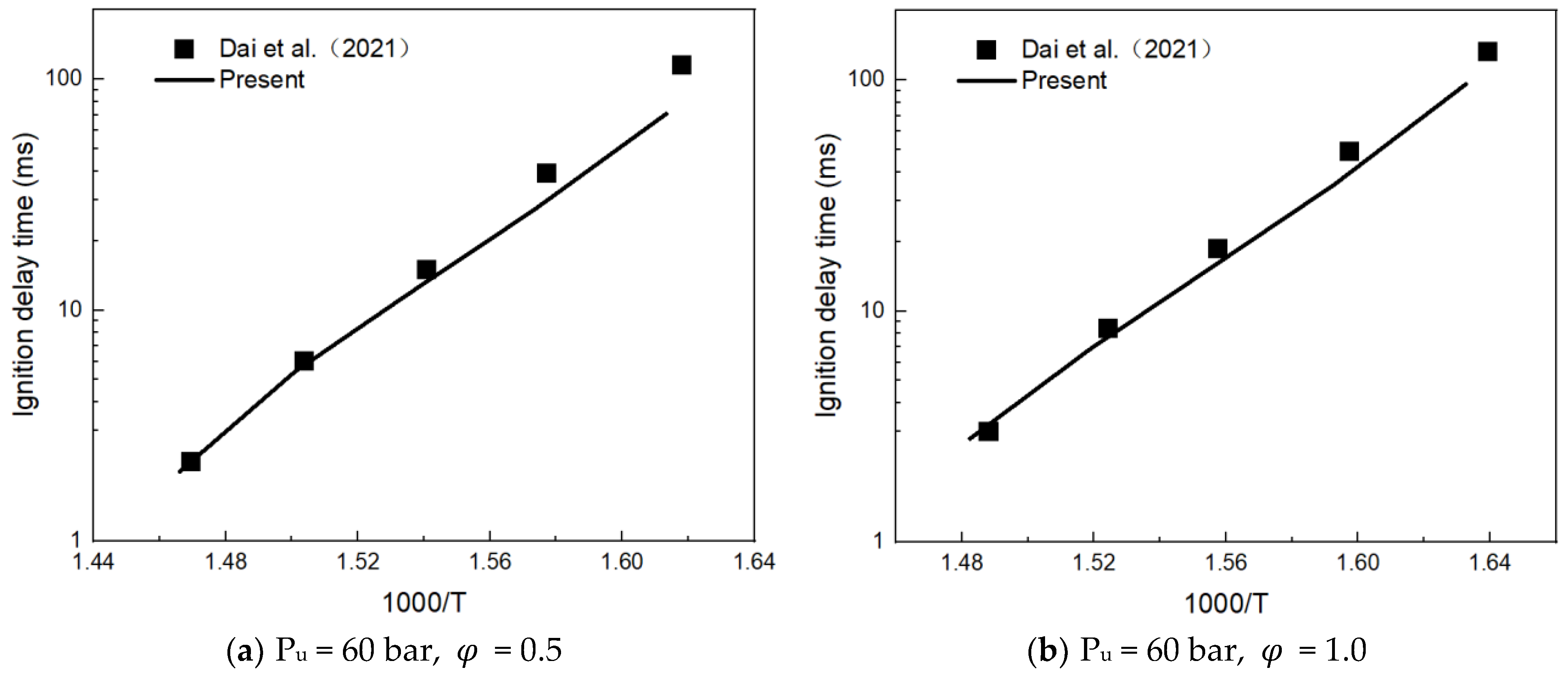
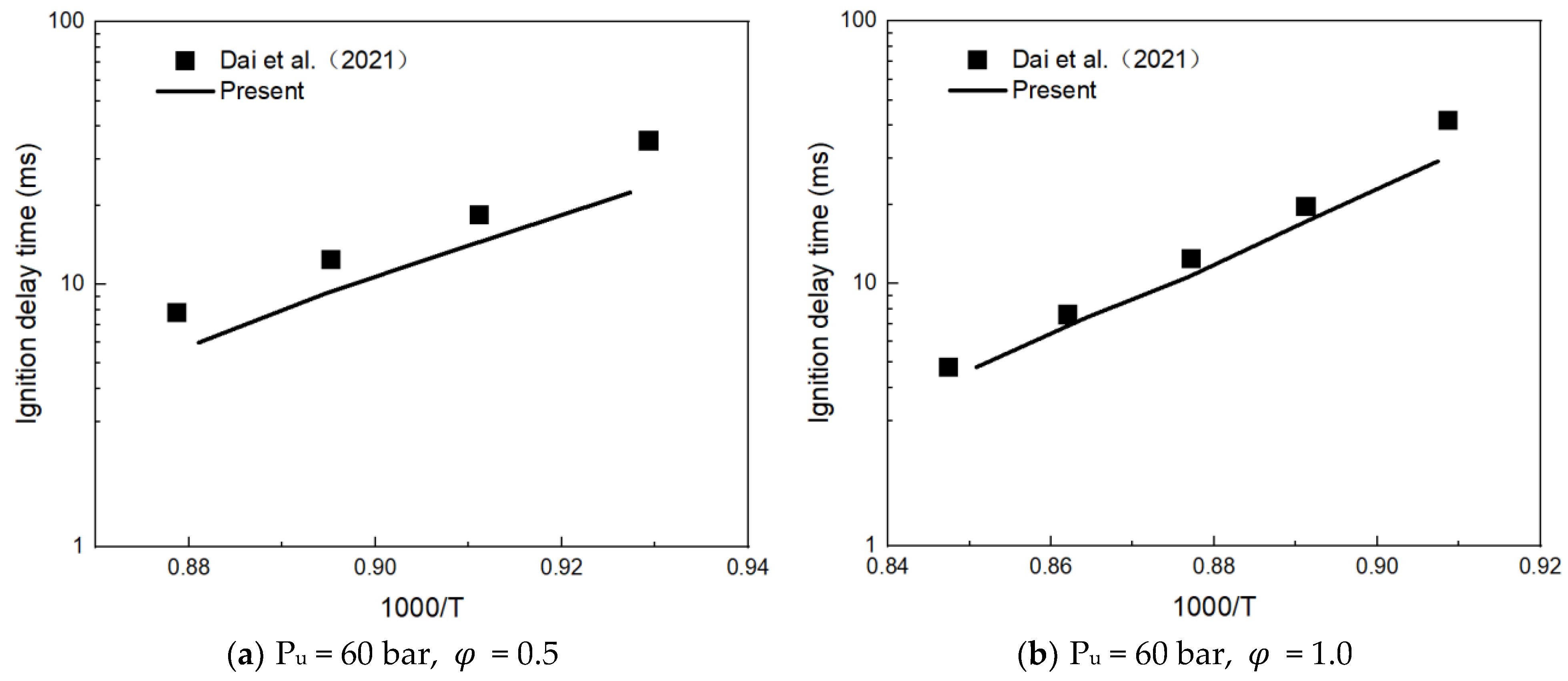
3.1. Mechanism Validation
3.2. Kinetic Analysis
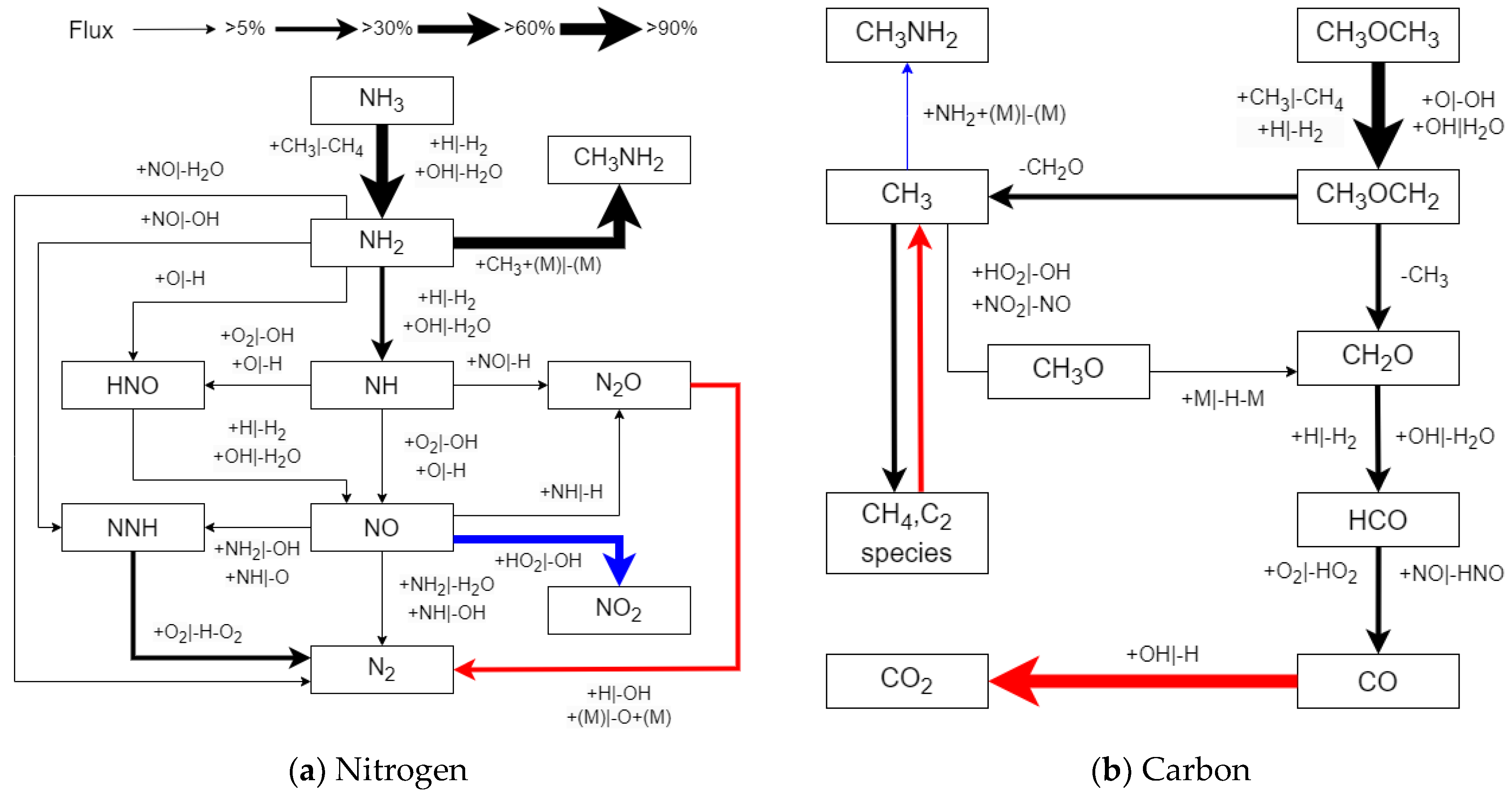
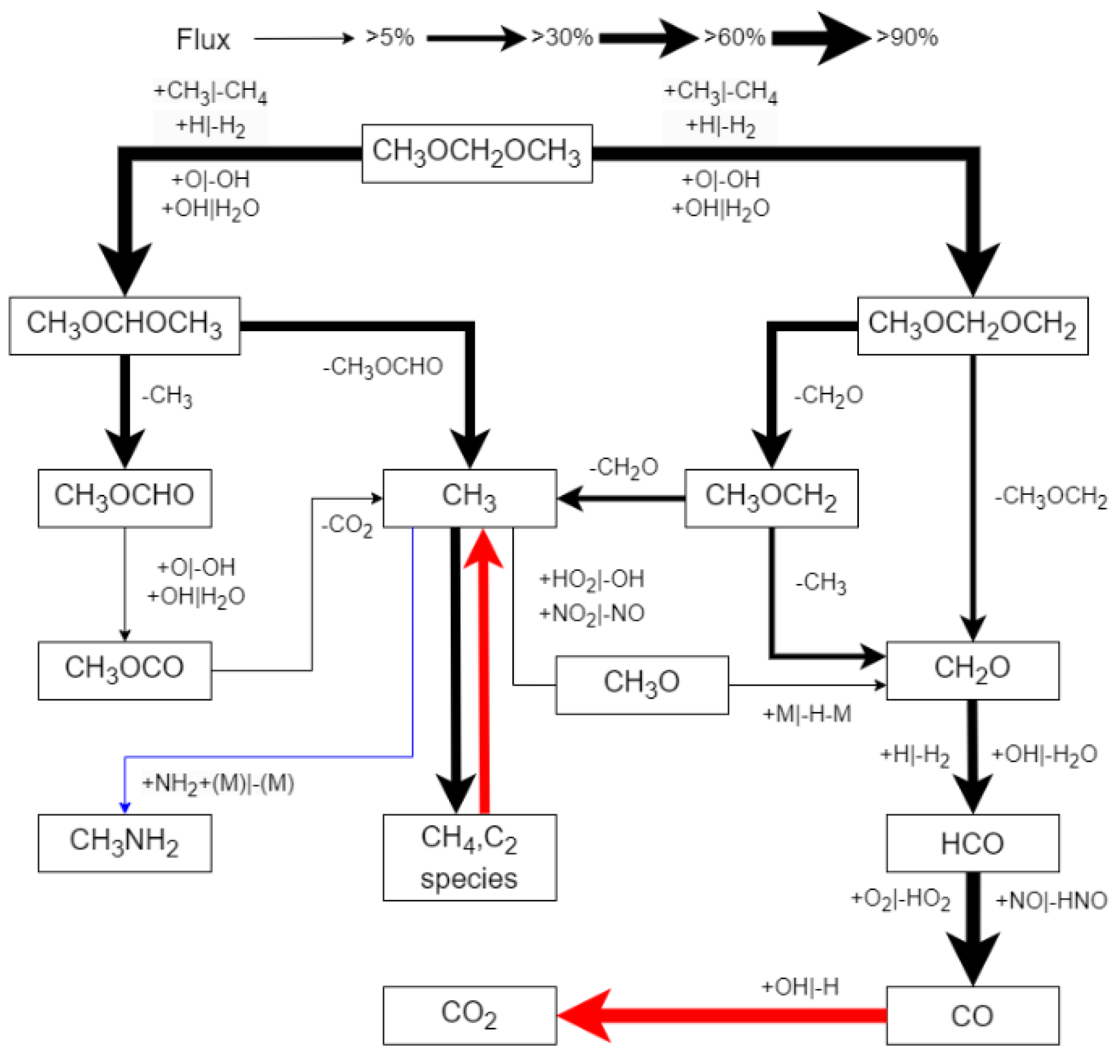
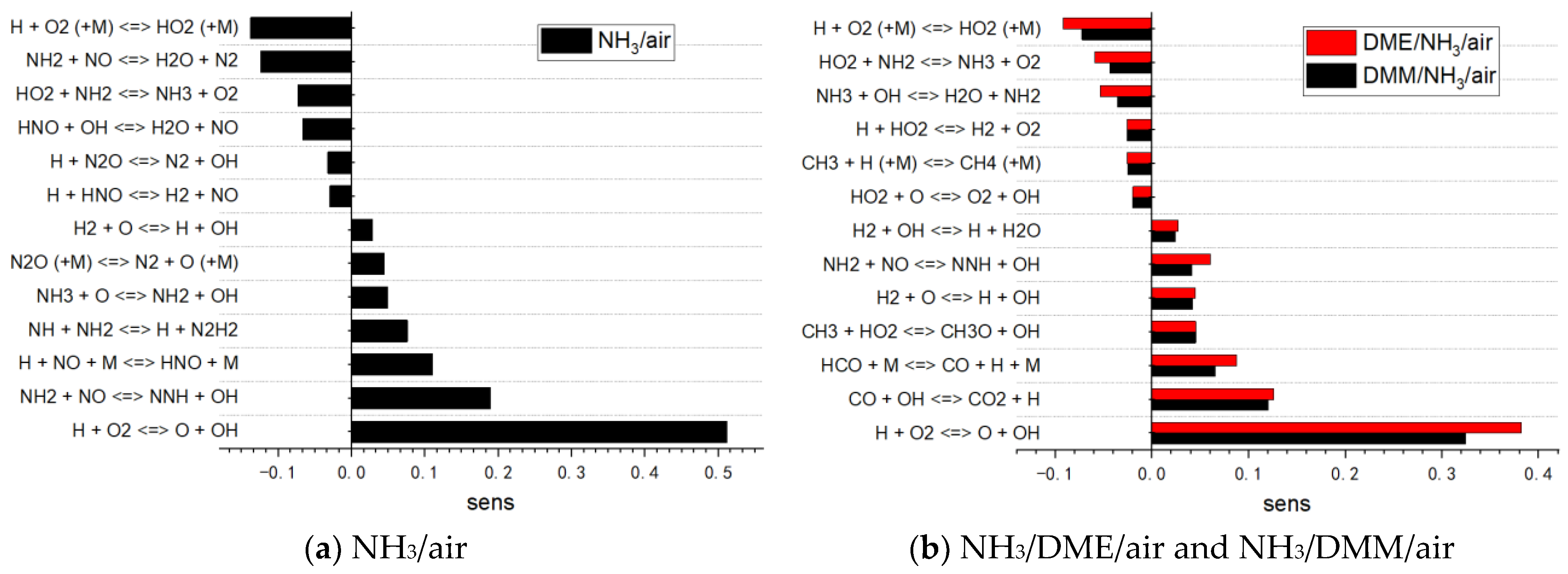
3.2.1. Reaction Pathway and Sensitivity Analysis
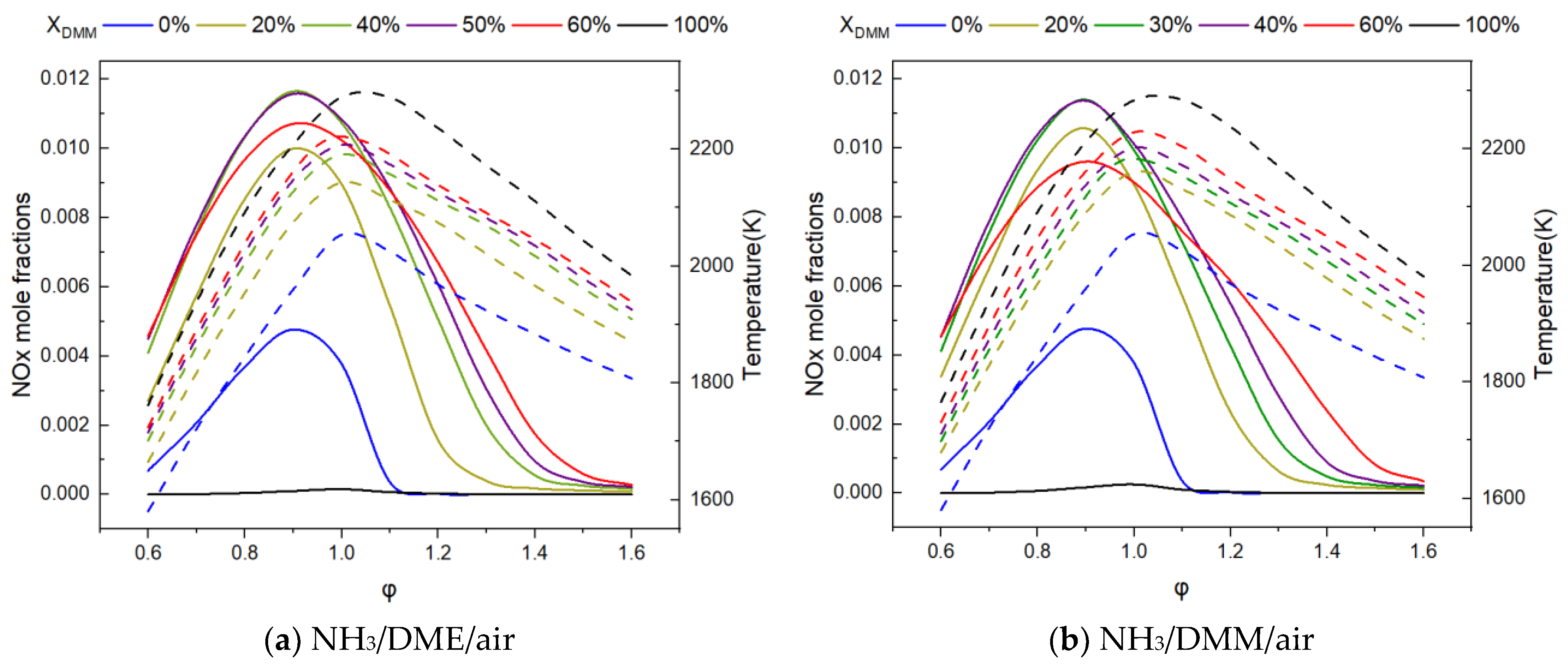
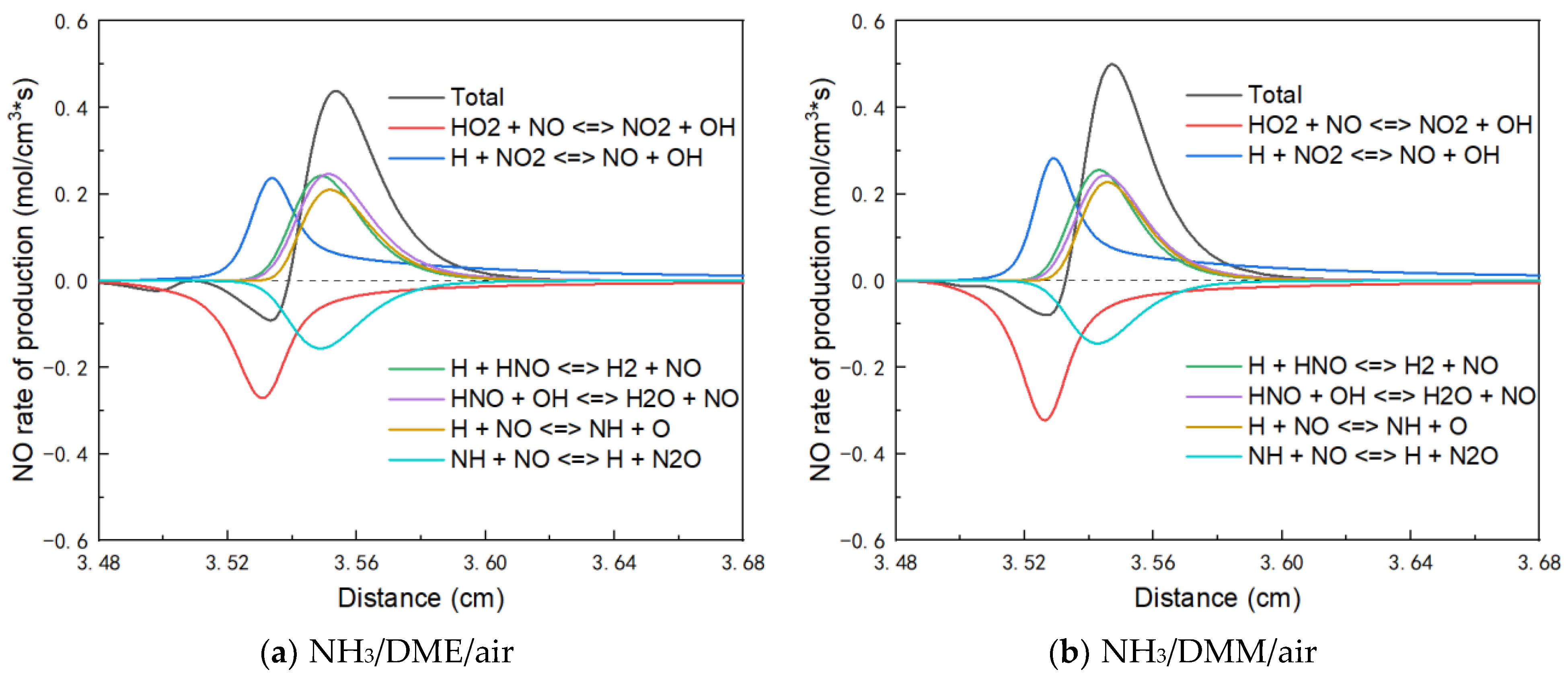
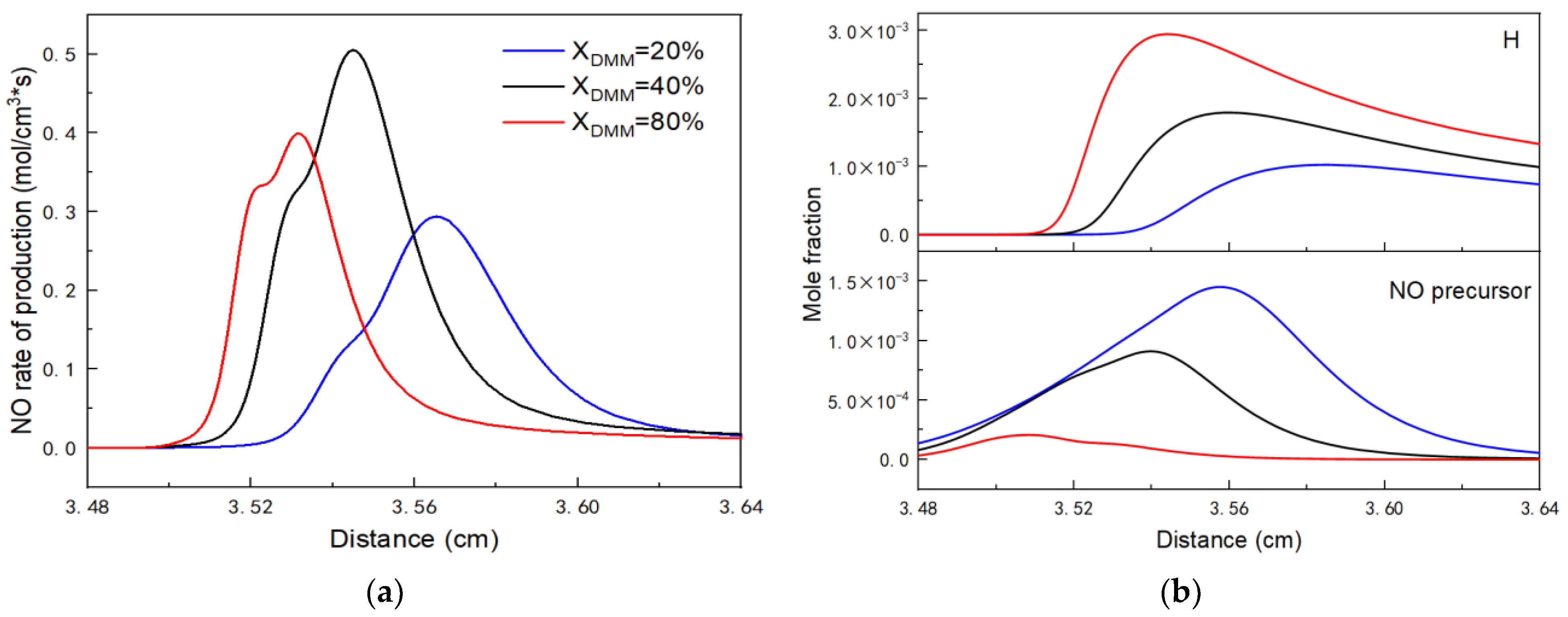
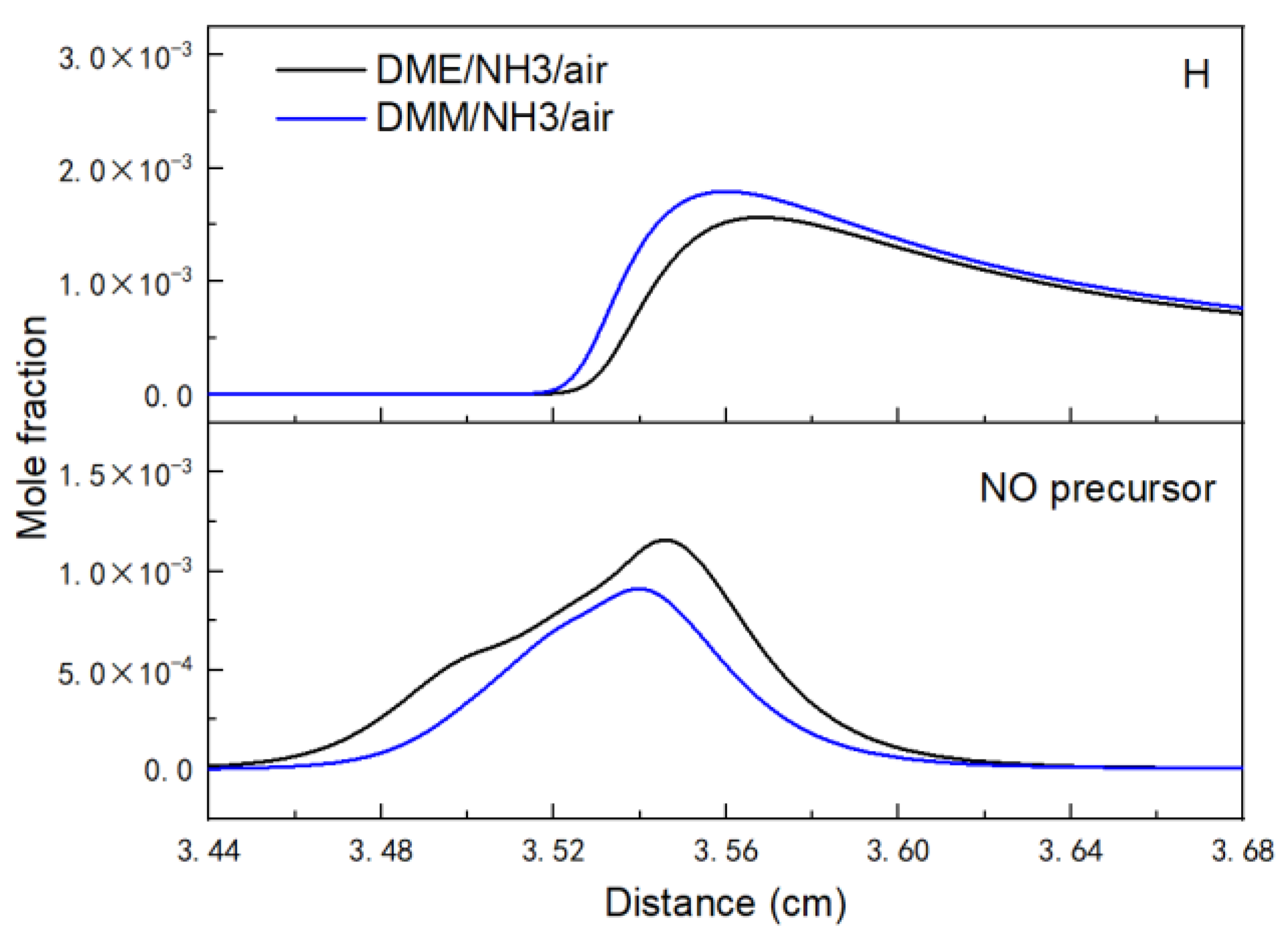
3.2.2. NOx Emission Analysis
4. Summary and Conclusions
- Updates of some key reactions using the latest dataset, e.g., NH, NNH, and H-relevant reactions and the interactions between DMM and NH2/NOx are crucial to increase the accuracy of the present mechanism.
- Reaction path analysis revealed that early C–N interaction reactions play an important role in the oxidation pathway of NH3. The dehydrogenation of NH3 leads to the formation of NH2, which then combines with a significant amount of CH3 produced by the oxidation of DMM through collisions with other radicals, forming CH3NH2.
- The analysis of NOx emission shows that fuel NOx coming from NH3 dominates the NOx emissions and NO turns out to be the main component of NOx emissions.
- The calculated NOx emissions initially increase and then decrease with higher DME or DMM fraction, reaching a peak around a fraction of 40%. This phenomenon can be attributed to the ‘trade-off’ relationship between the high-activity radicals (e.g., H, OH, and O) and NO precursors promoted by the addition of DME or DMM.
- The difference in NOx mole fraction between NH3/DMM and NH3/DME flames does not exceed 830 ppm according to the calculations.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| LBV | laminar burning velocity |
| IDT | ignition delay time |
| ROP | rate of production |
| NH3 | ammonia |
| NOx | nitrogen oxides |
| DME | dimethyl ether |
| DMM | dimethoxymethane |
| PODEn | polyoxymethylene dimethyl ethers |
| C2 | compounds containing two carbon atoms |
| C–N | carbon and nitrogen |
| Tu, | temperature |
| Pu, | pressure |
| reactor volume | |
| heat capacity at constant volume | |
| heat capacity at constant pressure | |
| density | |
| gas constant | |
| XDME | DME fraction |
| XDMM | DMM fraction |
| mass flow rates of reactants entering the reactor. | |
| mass flow rates of products leaving the reactor. | |
| heat sources or losses | |
| axial velocity | |
| pressure eigenvalue | |
| dynamic viscosity | |
| thermal conductivity | |
| mass flow rates of each species | |
| equivalence ratio | |
| the rate constant of the i-th reaction | |
| laminar burning velocity | |
| normalized sensitivity coefficients |
References
- Chiong, M.C.; Chong, C.T.; Ng, J.H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of Combustion Technologies in the Ammonia-Fuelled Engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Kang, L.; Pan, W.; Zhang, J.; Wang, W.; Tang, C. A Review on Ammonia Blends Combustion for Industrial Applications. Fuel 2023, 332, 126150. [Google Scholar] [CrossRef]
- Verkamp, F.J.; Hardin, M.C.; Williams, J.R. Ammonia Combustion Properties and Performance in Gas-Turbine Burners. Symp. Combust. 1967, 11, 985–992. [Google Scholar] [CrossRef]
- Starkman, E.S.; Samuelsen, G.S. Flame-Propagation Rates in Ammonia-Air Combustion at High Pressure. Symp. Combust. 1967, 11, 1037–1045. [Google Scholar] [CrossRef]
- Wang, N.; Huang, S.; Zhang, Z.; Li, T.; Yi, P.; Wu, D.; Chen, G. Laminar Burning Characteristics of Ammonia/Hydrogen/Air Mixtures with Laser Ignition. Int. J. Hydrogen Energy 2021, 46, 31879–31893. [Google Scholar] [CrossRef]
- Dai, L.; Gersen, S.; Glarborg, P.; Levinsky, H.; Mokhov, A. Experimental and Numerical Analysis of the Autoignition Behavior of NH3 and NH3/H2 Mixtures at High Pressure. Combust. Flame 2020, 215, 134–144. [Google Scholar] [CrossRef]
- Tian, Z.; Li, Y.; Zhang, L.; Glarborg, P.; Qi, F. An Experimental and Kinetic Modeling Study of Premixed NH3/CH4/O2/Ar Flames at Low Pressure. Combust. Flame 2009, 156, 1413–1426. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Z.; Zhang, T.; Zhai, Y.; Hou, R.; Tian, Z.Y.; Ji, C. A Comparative Study on the Laminar C1–C4 n-Alkane/NH3 Premixed Flame. Fuel 2022, 324, 124732. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; He, Y.; Zhu, R.; Zhu, Y.; Zhou, Z.; Cen, K. Experimental and Kinetic Study on the Laminar Burning Velocities of NH3 Mixing with CH3OH and C2H5OH in Premixed Flames. Combust. Flame 2021, 229, 111392. [Google Scholar] [CrossRef]
- Xu, H.; Wang, J.; Zhang, C.; Dai, L.; He, Z.; Wang, Q. Numerical Study on Laminar Burning Velocity of Ammonia Flame with Methanol Addition. Int. J. Hydrogen Energy 2022, 47, 28152–28164. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Measurement and Modelling of the Laminar Burning Velocity of Methane-Ammonia-Air Flames at High Pressures Using a Reduced Reaction Mechanism. Combust. Flame 2019, 204, 162–175. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, X.; Ma, S.; Cui, M.; Guo, H.; Cao, Z.; Li, Y. Experimental and Kinetic Modeling Investigation on the Laminar Flame Propagation of Ammonia under Oxygen Enrichment and Elevated Pressure Conditions. Combust. Flame 2019, 210, 236–246. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Lhuillier, C.; Barbosa, A.A.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C.; Seidel, L.; Mauss, F. An Experimental and Modeling Study of Ammonia with Enriched Oxygen Content and Ammonia/Hydrogen Laminar Flame Speed at Elevated Pressure and Temperature. Proc. Combust. Inst. 2021, 38, 2163–2174. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Harding, L.B.; Glarborg, P.; Miller, J.A. The Role of NNH in NO Formation and Control. Combust. Flame 2011, 158, 774–789. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and Modeling Study on the High-Temperature Oxidation of Ammonia and Related NOx Chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Seidel, L.; Zeuch, T.; Mauss, F.; Chemie, P.; Göttingen, G.; Gmbh, L.D.; Chaussee, B. Combustion A Detailed Kinetic Mechanism for the Oxidation of Ammonia Including the Formation and Reduction of Nitrogen Oxides. Energy Fuels 2018, 32, 10202–10217. [Google Scholar] [CrossRef]
- Wang, B.; Dong, S.; Jiang, Z.; Gao, W.; Wang, Z.; Li, J.; Yang, C.; Wang, Z.; Cheng, X. Development of a Reduced Chemical Mechanism for Ammonia/n-Heptane Blends. Fuel 2023, 338, 127358. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Wang, P.; Niu, B.; Liu, J. Construction and Derivation of a Series of Skeletal Chemical Mechanisms for N-Alkanes with Uniform and Decoupling Structure Based on Reaction Rate Rules. Combust. Flame 2022, 236, 111785. [Google Scholar] [CrossRef]
- Dong, S.; Wang, B.; Jiang, Z.; Li, Y.; Gao, W.; Wang, Z.; Cheng, X.; Curran, H.J. An Experimental and Kinetic Modeling Study of Ammonia/n -Heptane Blends. Combust. Flame 2022, 246, 112428. [Google Scholar] [CrossRef]
- Pellegrini, L.; Marchionna, M.; Patrini, R.; Beatrice, C.; Del Giacomo, N.; Guido, C. Combustion Behaviour and Emission Performance of Neat and Blended Polyoxymethylene Dimethyl Ethers in a Light-Duty Diesel Engine. SAE Tech. Pap. 2012, 01, 1053. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Liu, H.; Liu, F.; Wang, Z.; Wang, J. Combustion and Emission Characteristics of Diesel Engine Fueled with Biodiesel/PODE Blends. Appl. Energy 2017, 206, 425–431. [Google Scholar] [CrossRef]
- Yin, G.; Li, J.; Zhou, M.; Li, J.; Wang, C.; Hu, E.; Huang, Z. Experimental and Kinetic Study on Laminar Flame Speeds of Ammonia/Dimethyl Ether/Air under High Temperature and Elevated Pressure. Combust. Flame 2022, 238, 111915. [Google Scholar] [CrossRef]
- Xiao, H.; Li, H. Experimental and Kinetic Modeling Study of the Laminar Burning Velocity of NH3/DME/Air Premixed Flames. Combust. Flame 2022, 245, 16–19. [Google Scholar] [CrossRef]
- Dai, L.; Hashemi, H.; Glarborg, P.; Gersen, S.; Marshall, P.; Mokhov, A.; Levinsky, H. Ignition Delay Times of NH3/DME Blends at High Pressure and Low DME Fraction: RCM Experiments and Simulations. Combust. Flame 2021, 227, 120–134. [Google Scholar] [CrossRef]
- Issayev, G.; Giri, B.R.; Elbaz, A.M.; Shrestha, K.P.; Mauss, F.; Roberts, W.L.; Farooq, A. Ignition Delay Time and Laminar Flame Speed Measurements of Ammonia Blended with Dimethyl Ether: A Promising Low Carbon Fuel Blend. Renew. Energy 2022, 181, 1353–1370. [Google Scholar] [CrossRef]
- Zhao, Z.; Chaso, M.; Kazakov, A. Thermal Decomposition Reaction and a Comprehensive Kinetic Model of Dimethyl Ether. Int. J. Chem. Kinet. 2007, 43, 154–160. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; He, Y.; Zhu, Y.; Cen, K. Experimental and Kinetic Modeling Study of Laminar Burning Velocities of NH3/Syngas/Air Premixed Flames. Combust. Flame 2020, 213, 1–13. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Eckart, S.; Elbaz, A.M.; Giri, B.R.; Fritsche, C.; Seidel, L.; Roberts, W.L.; Krause, H.; Mauss, F. A Comprehensive Kinetic Model for Dimethyl Ether and Dimethoxymethane Oxidation and NOx Interaction Utilizing Experimental Laminar Flame Speed Measurements at Elevated Pressure and Temperature. Combust. Flame 2020, 218, 57–74. [Google Scholar] [CrossRef]
- Capriolo, G.; Brackmann, C.; Lubrano Lavadera, M.; Methling, T.; Konnov, A.A. An Experimental and Kinetic Modeling Study on Nitric Oxide Formation in Premixed C3alcohols Flames. Proc. Combust. Inst. 2021, 38, 805–812. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, M.; Zhao, C.; Tian, H.; Tian, J.; Long, W.; Bi, M. Study of Combustion and NO Chemical Reaction Mechanism in Ammonia Blended with DME. Fuel 2022, 319, 123832. [Google Scholar] [CrossRef]
- Gross, C.W.; Kong, S.C. Performance Characteristics of a Compression-Ignition Engine Using Direct-Injection Ammonia-DME Mixtures. Fuel 2013, 103, 1069–1079. [Google Scholar] [CrossRef]
- Elbaz, A.M.; Giri, B.R.; Issayev, G.; Shrestha, K.P.; Mauss, F.; Farooq, A.; Roberts, W.L. Experimental and Kinetic Modeling Study of Laminar Flame Speed of Dimethoxymethane and Ammonia Blends. Energy Fuels 2020, 34, 14726–14740. [Google Scholar] [CrossRef]
- Shrestha, K.P.; Vin, N.; Herbinet, O.; Seidel, L.; Battin-Leclerc, F.; Zeuch, T.; Mauss, F. Insights into Nitromethane Combustion from Detailed Kinetic Modeling—Pyrolysis Experiments in Jet-Stirred and Flow Reactors. Fuel 2020, 261, 116349. [Google Scholar] [CrossRef]
- Sun, W.; Wang, G.; Li, S.; Zhang, R.; Yang, B.; Yang, J.; Li, Y.; Westbrook, C.K.; Law, C.K. Speciation and the Laminar Burning Velocities of Poly(Oxymethylene) Dimethyl Ether 3 (POMDME3) Flames: An Experimental and Modeling Study. Proc. Combust. Inst. 2017, 36, 1269–1278. [Google Scholar] [CrossRef]
- Li, N.; Sun, W.; Liu, S.; Qin, X.; Zhao, Y.; Wei, Y.; Zhang, Y. A Comprehensive Experimental and Kinetic Modeling Study of Dimethoxymethane Combustion. Combust. Flame 2021, 233, 111583. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Moffat, H.K.; Schoegl, I.; Speth, R.L.; Weber, B.W. Cantera: An object-oriented software toolkit for chemical kinetics, thermodynamics, and transport processes (Version 2.4.0). Available online: http://www.cantera.org (accessed on 16 October 2022).
- Felden, A. Cantera Tutorials—A Series of Tutorials to Get Started with the Python Interface of Cantera, 1st ed.; Cerfacs: Toulouse, France, 2015; pp. 37–87. [Google Scholar]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A Comprehensive Modeling Study of n-Heptane Oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, C.; Wang, D.; Zhang, T.; Zhai, Y.; Wang, S. Experimental and Numerical Study on Laminar Burning Velocity and Premixed Combustion Characteristics of NH3/C3H8/Air Mixtures. Fuel 2023, 331, 125936. [Google Scholar] [CrossRef]
- Li, Y.; Sarathy, S.M. Probing Hydrogen–Nitrogen Chemistry: A Theoretical Study of Important Reactions in NxHy, HCN and HNCO Oxidation. Int. J. Hydrogen Energy 2020, 45, 23624–23637. [Google Scholar] [CrossRef]
- Nakamura, H.; Hasegawa, S.; Tezuka, T. Kinetic Modeling of Ammonia/Air Weak Flames in a Micro Flow Reactor with a Controlled Temperature Profile. Combust. Flame 2017, 185, 16–27. [Google Scholar] [CrossRef]
- Fernandes, R.X.; Luther, K.; Troe, J.; Ushakov, V.G. Experimental and Modelling Study of the Recombination Reaction H + O2 (+M) → HO2 (+M) between 300 and 900 K, 1.5 and 950 Bar, and in the Bath Gases M = He, Ar, and N2. Phys. Chem. Chem. Phys. 2008, 10, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- GRI-Mech 3.0—University of California, Berkeley. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 12 March 2023).
- Sutherland, J.W.; Patterson, P.M.; Klemm, R.B. Rate Constants for the Reaction, O(3P)+H2O=OH+OH, over the Temperature Range 1053 K to 2033 K Using Two Direct Techniques. Symp. Combust. 1990, 23, 51–57. [Google Scholar] [CrossRef]
- Burke, M.P.; Goldsmith, C.F.; Klippenstein, S.J.; Welz, O.; Huang, H.; Antonov, I.O.; Savee, J.D.; Osborn, D.L.; Zádor, J.; Taatjes, C.A.; et al. Multiscale Informatics for Low-Temperature Propane Oxidation: Further Complexities in Studies of Complex Reactions. J. Phys. Chem. A 2015, 119, 7095–7115. [Google Scholar] [CrossRef]
- Baulch, H.M.; Schindler, D.W.; Turner, M.A.; Findlay, D.L.; Paterson, M.J.; Vinebrooke, R.D. Effects of Warming on Benthic Communities in a Boreal Lake: Implications of Climate Change. Limnol. Oceanogr. 2005, 50, 1377–1392. [Google Scholar] [CrossRef]
- Joshi, A.V.; Wang, H. Master Equation Modeling of Wide Range Temperature and Pressure Dependence of CO + OH → Products. Int. J. Chem. Kinet. 2006, 38, 57–73. [Google Scholar] [CrossRef]
- Vermeire, F.H.; Carstensen, H.H.; Herbinet, O.; Battin-Leclerc, F.; Marin, G.B.; Van Geem, K.M. Experimental and Modeling Study of the Pyrolysis and Combustion of Dimethoxymethane. Combust. Flame 2018, 190, 270–283. [Google Scholar] [CrossRef]
- Jacobs, S.; Döntgen, M.; Alquaity, A.B.S.; Kopp, W.A.; Kröger, L.C.; Burke, U.; Pitsch, H.; Leonhard, K.; Curran, H.J.; Heufer, K.A. Detailed Kinetic Modeling of Dimethoxymethane. Part II: Experimental and Theoretical Study of the Kinetics and Reaction Mechanism. Combust. Flame 2019, 205, 522–533. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental Investigation on Laminar Burning Velocities of Ammonia/Hydrogen/Air Mixtures at Elevated Temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Costa, M.; Sun, Z.; He, Y.; Cen, K. Experimental and Kinetic Modeling Study of Laminar Burning Velocities of NH3/Air, NH3/H2/Air, NH3/CO/Air and NH3/CH4/Air Premixed Flames. Combust. Flame 2019, 206, 214–226. [Google Scholar] [CrossRef]
- Li, R.; Konnov, A.A.; He, G.; Qin, F.; Zhang, D. Chemical Mechanism Development and Reduction for Combustion of NH3/H2/CH4 Mixtures. Fuel 2019, 257, 116059. [Google Scholar] [CrossRef]
| No. | Reaction | A | n | Ea | Ref. |
|---|---|---|---|---|---|
| 1 | NH + NO = N2O + H | 2.7 × 1015 | −0.78 | 20.0 | [40] |
| 2 | NH2 + OH = NH + H2O | 2.04 × 104 | 2.52 | −616.032 | [41] |
| 3 | NH3 + OH = NH2 + H2O | 3.25 × 1012 | 0.0 | 2120.0 | [42] |
| 4 | NNH + O = NH + NO | 5.2 × 1011 | 0.388 | −409.0 | [15] |
| 5 | NH2 + NO = H2O + N2 | 9.5 × 1016 | −1.44 | 268.0 | [25] |
| 6 | NH2 + NO2 = H2NO + NO | 2.0 × 1019 | −2.369 | 870.0 | [14] |
| 7 | HNO + O2 = NO + HO2 | 2.0 × 1013 | 0.0 | 14896.0 | [25] |
| 8 | H2NO + NO2 = HONO + HNO | 6.0 × 1012 | 0.0 | 2000.0 | [25] |
| 9 | N2H3 + HO2 = N2H4 + O2 | 9.2 × 105 | 1.94 | 2126.1 | [25] |
| 10 | H + O2 + M = HO2 + M | 4.65 × 1012 | 0.44 | 0.0 | [43] |
| 11 | H + OH + M = H2O + M | 3.5 × 1022 | −2.0 | 0.0 | [44] |
| 12 | O + H2O = OH + OH | 6.7 × 107 | 1.704 | 14986.8 | [45] |
| 13 | HO2 + OH = H2O + O2 | 1.93 × 1020 | −2.49 | 584 | [46] |
| 14 | HO2 + O = O2 + OH | 1.0 × 1013 | 0.0 | −4452 | [47] |
| 15 | CH2 + O2 = HCO + OH | 1.06 × 1013 | 0.0 | 1500.0 | [44] |
| 16 | HCO + O2 = CO + HO2 | 13.45 × 1012 | 0.0 | 400.0 | [44] |
| 17 | CO + OH = CO2 + H | 8.7 × 104 | 2.053 | −355.7 | [48] |
| 18 | CH3OCH2OCH3 + H = CH3OCH2OCH2 + H2 | 5.04 × 106 | 2.3 | 6453.155 | [49] |
| 19 | CH3OCH2OCH3 + H = CH3OCHOCH3 + H2 | 2.18 × 1010 | 1.155 | 6548.757 | [49] |
| 20 | CH3OCH2OCH3 + M = CH3OCH2O + CH3 + M | 2.33 × 1019 | −0.66 | 84139.5 | [50] |
| 21 | CH3OCH2OCH3 + NH2 = CH3OCH2OCH2 + NH3 | 1.8 × 100 | 3.61 | 4353.0 | est DME |
| 22 | CH3OCH2OCH3 + NH2 = CH3OCHOCH3 + NH3 | 3.79 × 103 | 2.426 | 4475.0 | est DME |
| 23 | CH3OCH2OCH3 + NO2 = CH3OCH2OCH2 + HONO | 5.8 × 101 | 3.5 | 23755.0 | est DME |
| 24 | CH3OCH2OCH3 + NO2 = CH3OCHOCH3 + HONO | 9.93 × 102 | 3.112 | 22010.0 | est DME |
| 25 | CH3OCH2OCH3 + NO2 = CH3OCH2OCH2 + HNO2 | 6.5 × 102 | 3.0 | 23176.0 | est DME |
| 26 | CH3OCH2OCH3 + NO2 = CH3OCHOCH3 + HNO2 | 1.11 × 104 | 2.667 | 21473.0 | est DME |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Q.; Dai, L.; Zhang, M.; Yu, C. Numerical Study on the Combustion Properties of Ammonia/DME and Ammonia/DMM Mixtures. Energies 2023, 16, 6929. https://doi.org/10.3390/en16196929
Zhang Y, Wang Q, Dai L, Zhang M, Yu C. Numerical Study on the Combustion Properties of Ammonia/DME and Ammonia/DMM Mixtures. Energies. 2023; 16(19):6929. https://doi.org/10.3390/en16196929
Chicago/Turabian StyleZhang, Yuanpu, Qian Wang, Liming Dai, Ming Zhang, and Chunkan Yu. 2023. "Numerical Study on the Combustion Properties of Ammonia/DME and Ammonia/DMM Mixtures" Energies 16, no. 19: 6929. https://doi.org/10.3390/en16196929
APA StyleZhang, Y., Wang, Q., Dai, L., Zhang, M., & Yu, C. (2023). Numerical Study on the Combustion Properties of Ammonia/DME and Ammonia/DMM Mixtures. Energies, 16(19), 6929. https://doi.org/10.3390/en16196929









