Effect of Interfacial Oxide Layers on Self-Doped PEDOT/Si Hybrid Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
3. Results
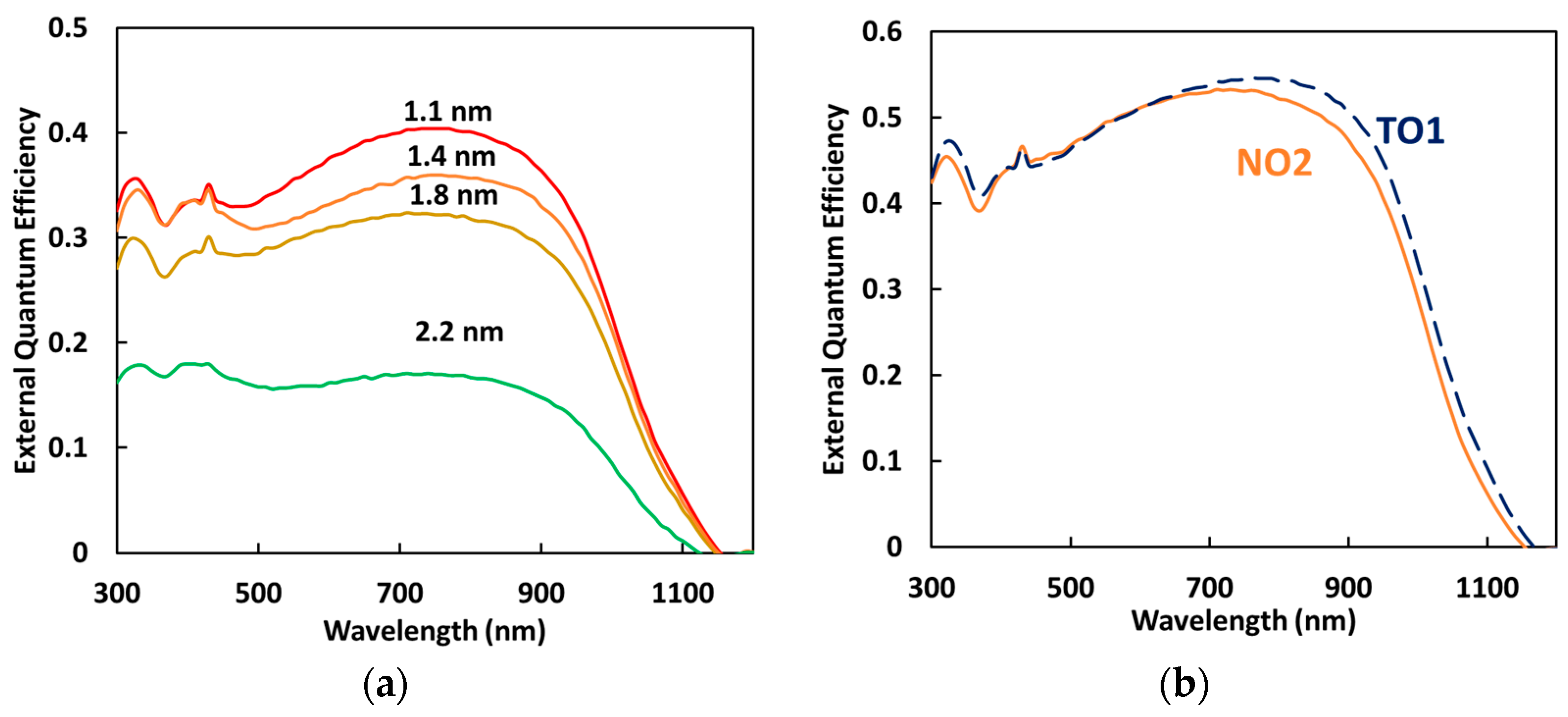
3.1. Optical Properties
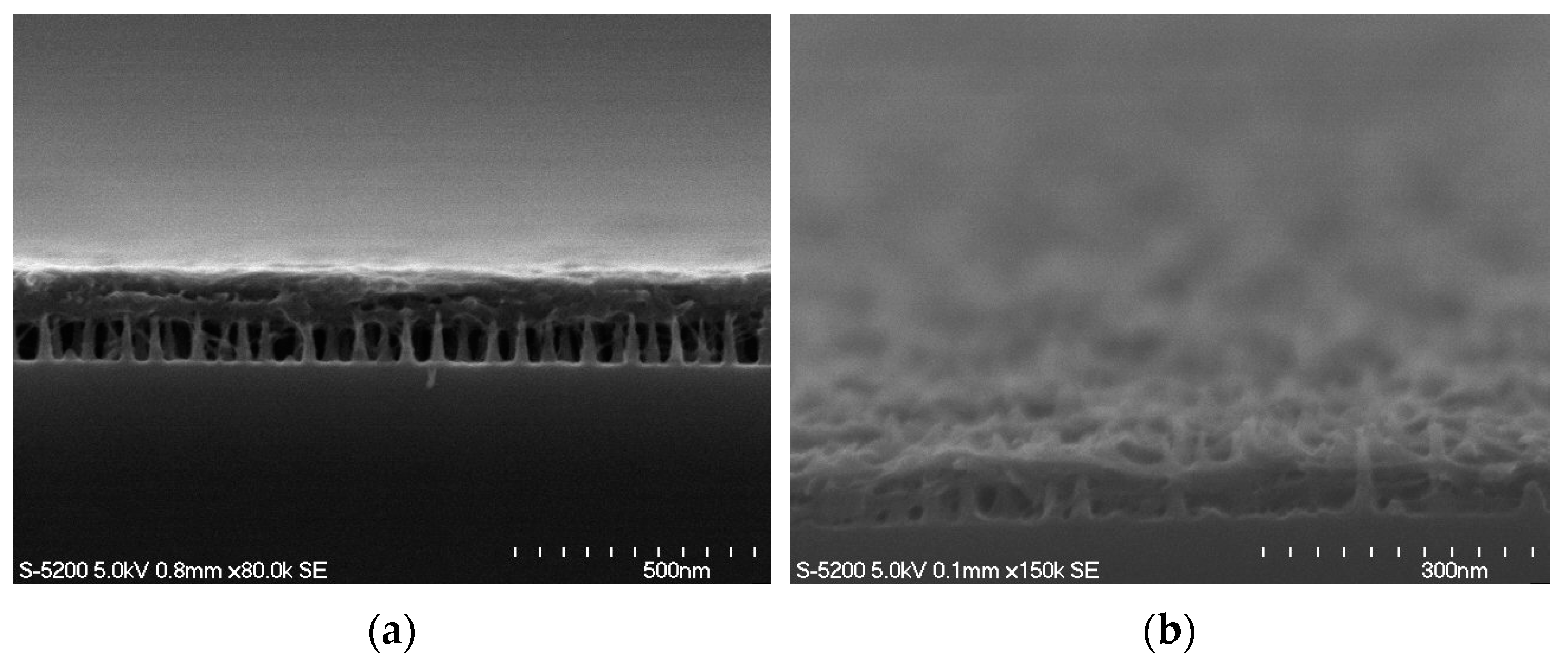
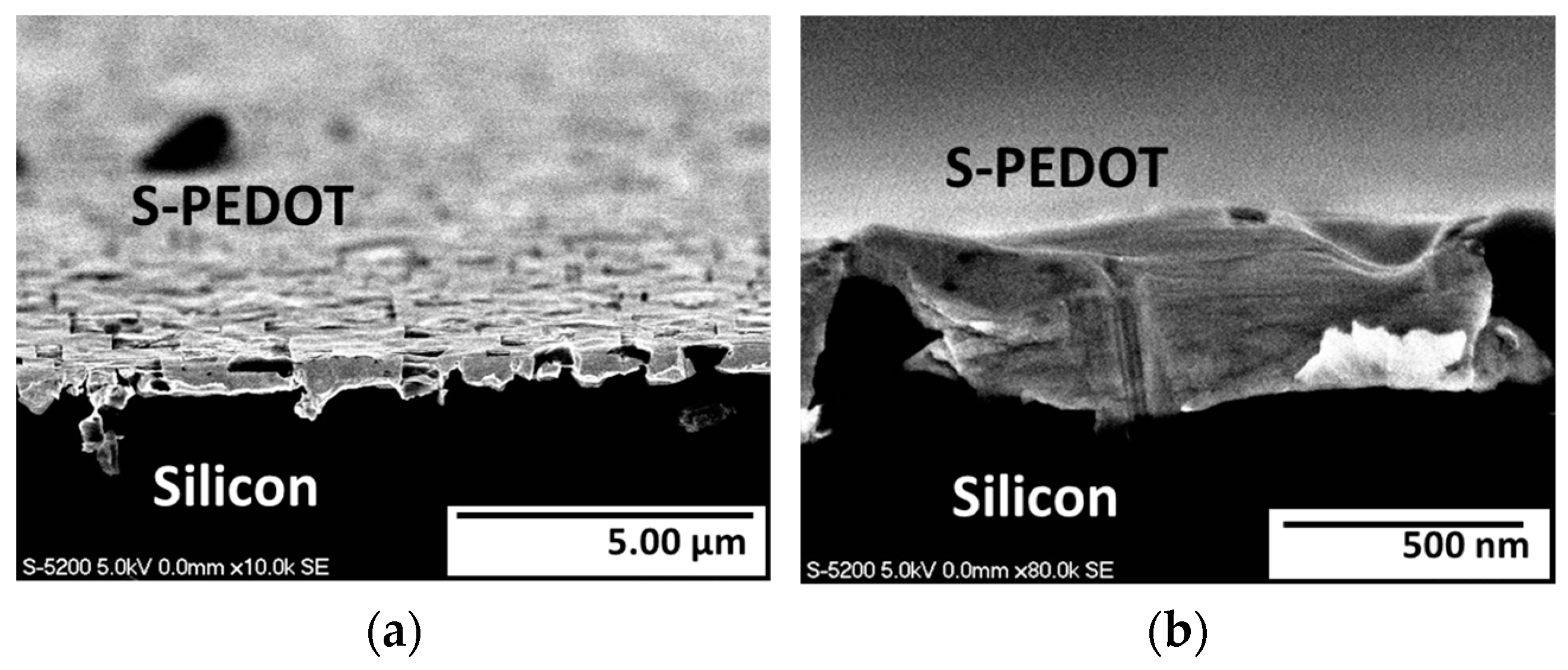
3.2. Surface Morphology
3.3. Characterization of Interfacial Oxides
3.4. Solar Cell Characteristics
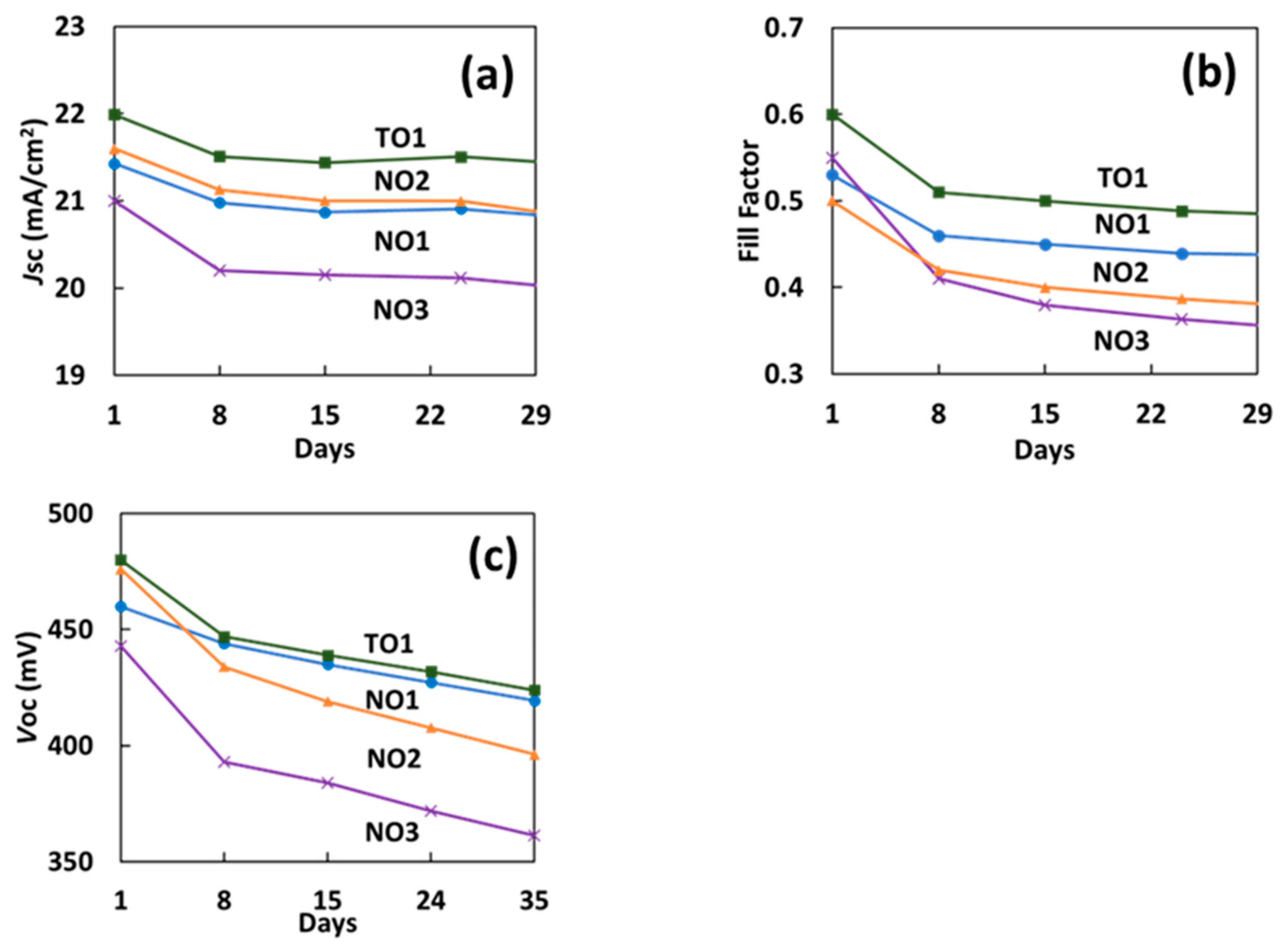
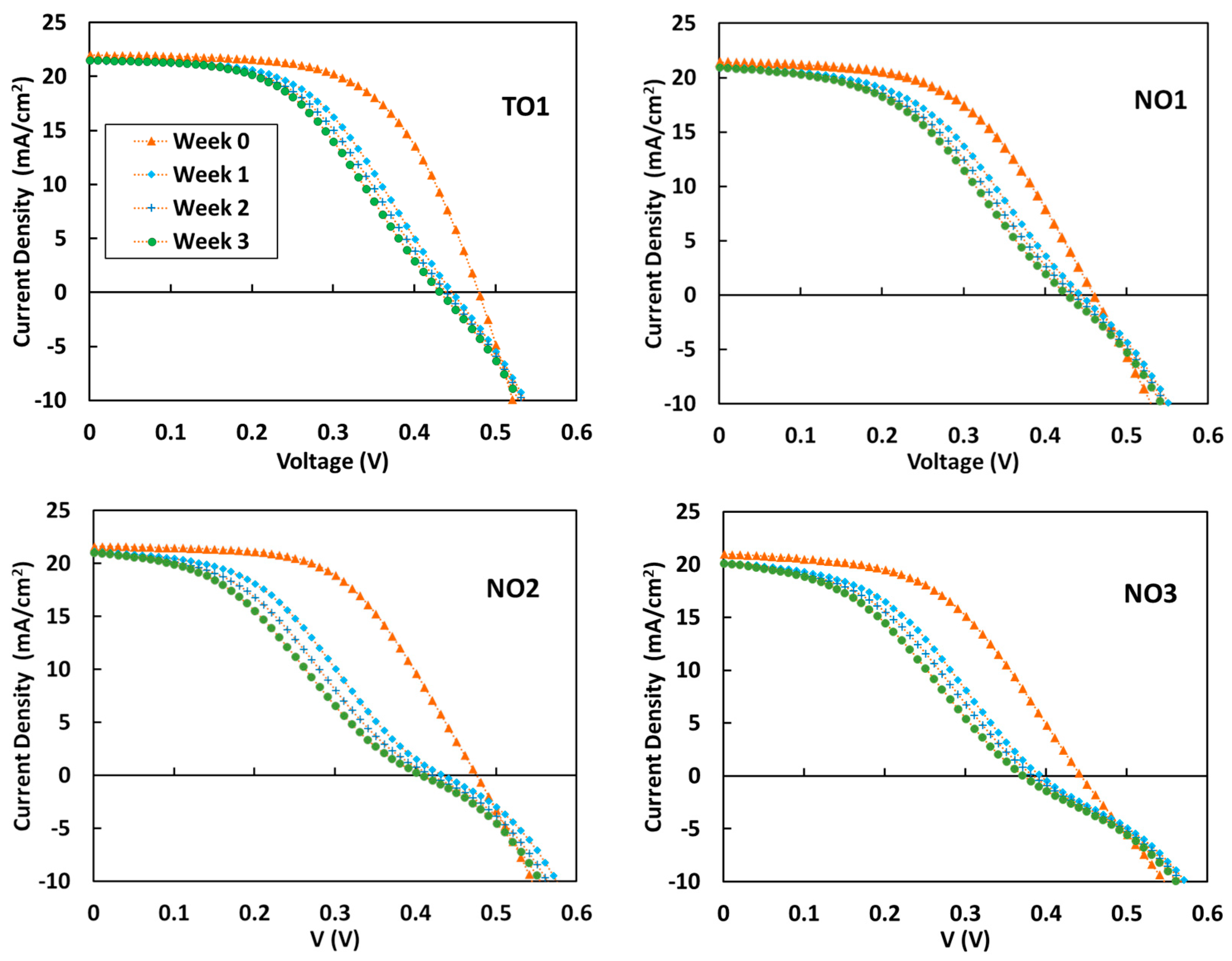
3.5. Durability of S-PEDOT/Si SOHCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arias, P.A.; Bellouin, N.; Jones, R.G.; Naik, V.; Plattner, G.-K.; Rogelj, J.; Sillmann, J.; Storelvmo, T.; Thorne, P.W.; Trewin, B.; et al. Technical Summary. Clim. Chang. 2021, 51, 221–227. [Google Scholar]
- Höller, R.; Gudopp, D.; Leschinsky, T. Solar PV Cost Reduction Potential in Japan. Preprints 2019, 2019040065. [Google Scholar] [CrossRef]
- Kimura, K. Solar Power Generation Costs in Japan. Available online: https://www.renewable-ei.org/en/activities/reports/20211014.php (accessed on 3 May 2023).
- Matsui, T.; Bivour, M.; Hermle, M.; Sai, H. Atomic-Layer-Deposited TiOx Nanolayers Function as Efficient Hole-Selective Passivating Contacts in Silicon Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 49777–49785. [Google Scholar] [CrossRef]
- Poorkazem, K.; Liu, D.; Kelly, T.L. Fatigue Resistance of a Flexible, Efficient, and Metal Oxide-Free Perovskite Solar Cell. J. Mater. Chem. A 2015, 3, 9241–9248. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Liu, Z.; Lee, E.-C. A Transparent Poly(3,4-Ethylenedioxylenethiophene):Poly(Styrene Sulfonate) Cathode for Low Temperature Processed, Metal-Oxide Free Perovskite Solar Cells. J. Mater. Chem. A 2017, 5, 6974–6980. [Google Scholar] [CrossRef]
- Dianetti, M.; Di Giacomo, F.; Polino, G.; Ciceroni, C.; Liscio, A.; D’Epifanio, A.; Licoccia, S.; Brown, T.M.; Di Carlo, A.; Brunetti, F. TCO-Free Flexible Organo Metal Trihalide Perovskite Planar-Heterojunction Solar Cells. Sol. Energy Mater. Sol. Cells 2015, 140, 150–157. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, S.-C.; Wang, Z.; Ma, W.; Wang, J.; Yin, Z.; Tang, C.; Cai, D.; Zheng, Q. Indacenodithiophene-Based Wide Bandgap Copolymers for High Performance Single-Junction and Tandem Polymer Solar Cells. Nano Energy 2017, 33, 313–324. [Google Scholar] [CrossRef]
- Prosa, M.; Tessarolo, M.; Bolognesi, M.; Cramer, T.; Chen, Z.; Facchetti, A.; Fraboni, B.; Seri, M.; Ruani, G.; Muccini, M. Efficient and Versatile Interconnection Layer by Solvent Treatment of PEDOT:PSS Interlayer for Air-Processed Organic Tandem Solar Cells. Adv. Mater. Interfaces 2016, 3, 1600770. [Google Scholar] [CrossRef]
- Okamoto, K.; Kishibe, K.; Sano, N.; Tanabe, K. PEDOT:PSS-Mediated Semiconductor Wafer Bonding for Built-in Middle Subcells in Multijunction Solar Cells. Appl. Phys. Express 2023, 16, 036502. [Google Scholar] [CrossRef]
- Yoon, S.-S.; Khang, D.-Y. High Efficiency (>17%) Si-Organic Hybrid Solar Cells by Simultaneous Structural, Electrical, and Interfacial Engineering via Low-Temperature Processes. Adv. Energy Mater. 2018, 8, 1702655. [Google Scholar] [CrossRef]
- Hu, W.; Igarashi, M.; Lee, M.-Y.; Li, Y.; Samukawa, S. Realistic Quantum Design of Silicon Quantum Dot Intermediate Band Solar Cells. Nanotechnology 2013, 24, 265401. [Google Scholar] [CrossRef]
- Shi, H.; Liu, C.; Jiang, Q.; Xu, J. Effective Approaches to Improve the Electrical Conductivity of PEDOT:PSS: A Review. Adv. Electron. Mater. 2015, 1, 1500017. [Google Scholar] [CrossRef]
- Yamashita, M.; Otani, C.; Shimizu, M.; Okuzaki, H. Effect of Solvent on Carrier Transport in Poly(3,4-Ethylenedioxythiophene)/Poly(4-Styrenesulfonate) Studied by Terahertz and Infrared-Ultraviolet Spectroscopy. Appl. Phys. Lett. 2011, 99, 143307. [Google Scholar] [CrossRef]
- Ouyang, J.; Xu, Q.; Chu, C.-W.; Yang, Y.; Li, G.; Shinar, J. On the Mechanism of Conductivity Enhancement in Poly(3,4-Ethylenedioxythiophene):Poly(Styrene Sulfonate) Film through Solvent Treatment. Polymer 2004, 45, 8443–8450. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jung, J.H.; Lee, D.E.; Joo, J. Enhancement of Electrical Conductivity of Poly(3,4-Ethylenedioxythiophene)/Poly(4-Styrenesulfonate) by a Change of Solvents. Synth. Met. 2002, 126, 311–316. [Google Scholar] [CrossRef]
- Nardes, A.M.; Janssen, R.A.J.; Kemerink, M. A Morphological Model for the Solvent-Enhanced Conductivity of PEDOT:PSS Thin Films. Adv. Funct. Mater. 2008, 18, 865–871. [Google Scholar] [CrossRef]
- Jönsson, S.K.M.; Birgerson, J.; Crispin, X.; Greczynski, G.; Osikowicz, W.; Denier Van Der Gon, A.W.; Salaneck, W.R.; Fahlman, M. The Effects of Solvents on the Morphology and Sheet Resistance in Poly(3,4-Ethylenedioxythiophene)–Polystyrenesulfonic Acid (PEDOT–PSS) Films. Synth. Met. 2003, 139, 1–10. [Google Scholar] [CrossRef]
- Venkatesan, R.; Sheik Kadar Maideen, S.M.T.; Chandhiran, S.; Kushvaha, S.S.; Sagadevan, S.; Venkatachalapathy, V.; Mayandi, J. Fabrication and Characterization of Si/PEDOT: PSS-Based Heterojunction Solar Cells. Electronics 2022, 11, 4145. [Google Scholar] [CrossRef]
- Jäckle, S.; Liebhaber, M.; Niederhausen, J.; Büchele, M.; Félix, R.; Wilks, R.G.; Bär, M.; Lips, K.; Christiansen, S. Unveiling the Hybrid N-Si/PEDOT:PSS Interface. ACS Appl. Mater. Interfaces 2016, 8, 8841–8848. [Google Scholar] [CrossRef]
- He, L.; Jiang, C.; Wang, H.; Lai, D. Rusli High Efficiency Planar Si/Organic Heterojunction Hybrid Solar Cells. Appl. Phys. Lett. 2012, 100, 073503. [Google Scholar] [CrossRef]
- Angel Trujillo, D.A.; Iyer, A.; Hack, J.; Opila, R.L. Role of Oxide at Interface between Organic Layer and Silicon Substrate in Hybrid Solar Cells. J. Mater. Res. 2021, 36, 557–570. [Google Scholar] [CrossRef]
- Yano, H.; Kudo, K.; Marumo, K.; Okuzaki, H. Fully Soluble Self-Doped Poly(3,4-Ethylenedioxythiophene) with an Electrical Conductivity Greater than 1000 S cm−1. Sci. Adv. 2019, 5, eaav9492. [Google Scholar] [CrossRef]
- Muckley, E.S.; Jacobs, C.B.; Vidal, K.; Mahalik, J.P.; Kumar, R.; Sumpter, B.G.; Ivanov, I.N. New Insights on Electro-Optical Response of Poly(3,4-Ethylenedioxythiophene):Poly(Styrenesulfonate) Film to Humidity. ACS Appl. Mater. Interfaces 2017, 9, 15880–15886. [Google Scholar] [CrossRef] [PubMed]
- Dupont, S.R.; Novoa, F.; Voroshazi, E.; Dauskardt, R.H. Decohesion Kinetics of PEDOT:PSS Conducting Polymer Films. Adv. Funct. Mater. 2014, 24, 1325–1332. [Google Scholar] [CrossRef]
- Sheng, J.; Fan, K.; Wang, D.; Han, C.; Fang, J.; Gao, P.; Ye, J. Improvement of the SiOx Passivation Layer for High-Efficiency Si/PEDOT:PSS Heterojunction Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 16027–16034. [Google Scholar] [CrossRef]
- Kumari, P.; Sharma, R.K.; Srivastava, A.; Srivastava, S.K. Effect of ZnO BSF Layer on the Performance of PEDOT:PSS/Si Heterojunction Solar Cell. Mater. Today Proc. 2023, 82, 158–162. [Google Scholar] [CrossRef]
- Thomas, J.P.; Zhao, L.; Abd-Ellah, M.; Heinig, N.F.; Leung, K.T. Interfacial Micropore Defect Formation in PEDOT:PSS-Si Hybrid Solar Cells Probed by TOF-SIMS 3D Chemical Imaging. Anal. Chem. 2013, 85, 6840–6845. [Google Scholar] [CrossRef]
- Oshima, R.; Watanabe, Y.; Yamanaka, M.; Kawanami, H.; Sakamoto, I.; Matsubara, K.; Sakata, I. High-Quality SiGe Films Grown with Compositionally Graded Buffer Layers for Solar Cell Applications. J. Cryst. Growth 2013, 378, 226–229. [Google Scholar] [CrossRef]
- Knotter, D.M. Etching Mechanism of Vitreous Silicon Dioxide in HF-Based Solutions. J. Am. Chem. Soc. 2000, 122, 4345–4351. [Google Scholar] [CrossRef]
- Nielsen, H.; Hackleman, D. Some Illumination on the Mechanism of SiO2 Etching in HF Solutions. J. Electrochem. Soc. 1983, 130, 708–712. [Google Scholar] [CrossRef]
- Biesinger, M.C. Accessing the Robustness of Adventitious Carbon for Charge Referencing (Correction) Purposes in XPS Analysis: Insights from a Multi-User Facility Data Review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Niimi, H.; Yang, H.; Lucovsky, G.; Keister, J.W.; Rowe, J.E. Barrier Layer Model Determined by XPS Data for Tunneling Current Reductions at Monolayer Nitrided Si–SiO2 Interfaces. Appl. Surf. Sci. 2000, 166, 485–491. [Google Scholar] [CrossRef]
- Khalilov, U.; Pourtois, G.; Huygh, S.; Van Duin, A.C.T.; Neyts, E.C.; Bogaerts, A. New Mechanism for Oxidation of Native Silicon Oxide. J. Phys. Chem. C 2013, 117, 9819–9825. [Google Scholar] [CrossRef]
- Sato, R.; Wasai, Y.; Izumi, Y.; Ueno, K.; Shirai, H. Influence of Effective Mass on Carrier Concentration for PEDOT:PSS and S-PEDOT Thin Films Studied by Ellipsometry and Hall Measurement. J. Phys. Chem. C 2023, 127, 13196–13206. [Google Scholar] [CrossRef]
- Schmidt, J.; Titova, V.; Zielke, D. Organic-Silicon Heterojunction Solar Cells: Open-Circuit Voltage Potential and Stability. Appl. Phys. Lett. 2013, 103, 183901. [Google Scholar] [CrossRef]
- Yameen, M.; Srivastava, S.K.; Singh, P.; Prathap, P.; Vandana; Rauthan, C.M.S.; Singh, P.K. Stability Study of PEDOT:PSS/Micro-Textured Silicon Hetero-Junction Solar Cells. Adv. Sci. Lett. 2014, 20, 1540–1544. [Google Scholar] [CrossRef]
- Hossain, J.; Mondal, B.K.; Mostaque, S.K.; Ahmed, S.R.A.; Shirai, H. Optimization of Multilayer Anti-Reflection Coatings for Efficient Light Management of PEDOT:PSS/c-Si Heterojunction Solar Cells. Mater. Res. Express 2020, 7, 015502. [Google Scholar] [CrossRef]
- Koch, N.; Vollmer, A.; Elschner, A. Influence of Water on the Work Function of Conducting Poly(3,4-Ethylenedioxythiophene)/Poly(Styrenesulfonate). Appl. Phys. Lett. 2007, 90, 043512. [Google Scholar] [CrossRef]
- Huang, J.; Miller, P.F.; Wilson, J.S.; De Mello, A.J.; De Mello, J.C.; Bradley, D.D.C. Investigation of the Effects of Doping and Post-Deposition Treatments on the Conductivity, Morphology, and Work Function of Poly(3,4-Ethylenedioxythiophene)/Poly(Styrene Sulfonate) Films. Adv. Funct. Mater. 2005, 15, 290–296. [Google Scholar] [CrossRef]
- Yun, D.-J.; Ra, H.; Kim, J.; Hwang, I.; Lee, J.; Rhee, S.-W.; Chung, J. Characterizing Annealing Effect of Poly (3,4-Ethylenedioxythiophene) Polymerized with Poly (4-Styrenesulfonate) Conjugated Film on the Molecular Arrangement and Work Function by Core-Level and Valence-Level Band Spectra. ECS J. Solid State Sci. Technol. 2012, 1, M10–M14. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; De Kok, M.M.; Vinken, E.; Maturova, K.; Janssen, R.A.J. Conductivity, Work Function, and Environmental Stability of PEDOT:PSS Thin Films Treated with Sorbitol. Org. Electron. 2008, 9, 727–734. [Google Scholar] [CrossRef]
- Bailo, D.; Generosi, A.; Albertini, V.R.; Caminiti, R.; De Bettignies, R.; Paci, B. Time-Resolved Morphological Study of ‘PEDOT:PSS’ Hole Transporting Layer for Polymer Solar Cells. Synth. Met. 2012, 162, 808–812. [Google Scholar] [CrossRef]
- Gutierrez-Fernandez, E.; Ezquerra, T.A.; García-Gutiérrez, M.-C. Additive Effect on the Structure of PEDOT:PSS Dispersions and Its Correlation with the Structure and Morphology of Thin Films. Polymers 2021, 14, 141. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Rezaie, S.S.; Facchini-Rakovich, M.; Gudi, D.; Montemagno, C.; Gupta, M. Tuning PEDOT:PSS Conductivity to Obtain Complementary Organic Electrochemical Transistor. Org. Electron. 2019, 66, 148–155. [Google Scholar] [CrossRef]





| Device Structure | VOC (mV) | JSC (mA/cm2) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|
| Ag/S-PEDOT/SiOx/Si/Au-Sb/Au | 482 | 22.0 | 61.0 | 6.35 | This work |
| Au/PEDOT:PSS/SiOx/Si/In:Ga | 548 | 30.5 | 73.0 | 12.2 | [20] |
| Ag/PEDOT:PSS/SiOx/Si/Ti/Pd/Ag | 580 | 25.8 | 62.4 | 9.4 | [21] |
| Ag/PEDOT:PSS/SiOx/Si/Ti-Ag | 490 | 33.2 | 64.5 | 10.48 | [26] |
| Ag/PEDOT:PSS/SiOx/Si/n-ZnO/In:Ga | 508 | 22.2 | 51.7 | 5.84 | [27] |
| Ag/PEDOT:PSS/SiOx/Si/Al | 510 | 24.3 | 41.2 | 5.08 | [28] |
| Sample | Preparation | Thickness (nm) | Oxygen Saturation (%) |
|---|---|---|---|
| NBO | NBO for 400 s | 4.62 | 95 |
| NO1 | 30 s HF etch after NBO | 1.37 | 83 |
| NO2 | 40 s HF etch after NBO | 1.03 | 43 |
| NO3 | 50 s HF etch after NBO | 0.52 | 55 |
| TO1 | 150 °C for 15 min | 1.07 | 54 |
| SOHC | VOC (mV) | JSC (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| NO1 | 453.25 ± 6.75 | 21.41 ± 0.28 | 50.25 ± 3 | 4.92 ± 0.33 |
| (Best) | (460.00) | (21.69) | (53.00) | (5.25) |
| NO2 | 474.25 ± 8.75 | 21.73 ± 0.45 | 54 ± 2 | 5.59 ± 0.10 |
| (Best) | (483.00) | (22.18) | (56.00) | (5.69) |
| NO3 | 419.5 ± 13.50 | 21.25 ± 0.70 | 49.5 ± 0.5 | 4.43 ± 0.2 |
| (Best) | (433.00) | (21.95) | (50.00) | (4.63) |
| TO1 | 460 ± 22.00 | 21.54 ± 0.45 | 55 ± 6 | 5.53 ± 0.82 |
| (Best) | (482.00) | (21.99) | (61.00) | (6.35) |
| TO1 (PEDOT:PSS) | 270 ± 127.00 | 25.02 ± 0.78 | 38.40 ± 3.86 | 3.25 ± 1.08 |
| (Best) | (397.00) | (25.82) | (42.26) | (4.33) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, A.; Oshima, R.; Ohori, D.; Sasaki, T.; Yano, H.; Okuzaki, H.; Tokumasu, T.; Endo, K.; Samukawa, S. Effect of Interfacial Oxide Layers on Self-Doped PEDOT/Si Hybrid Solar Cells. Energies 2023, 16, 6900. https://doi.org/10.3390/en16196900
Saha A, Oshima R, Ohori D, Sasaki T, Yano H, Okuzaki H, Tokumasu T, Endo K, Samukawa S. Effect of Interfacial Oxide Layers on Self-Doped PEDOT/Si Hybrid Solar Cells. Energies. 2023; 16(19):6900. https://doi.org/10.3390/en16196900
Chicago/Turabian StyleSaha, Aditya, Ryuji Oshima, Daisuke Ohori, Takahiko Sasaki, Hirokazu Yano, Hidenori Okuzaki, Takashi Tokumasu, Kazuhiko Endo, and Seiji Samukawa. 2023. "Effect of Interfacial Oxide Layers on Self-Doped PEDOT/Si Hybrid Solar Cells" Energies 16, no. 19: 6900. https://doi.org/10.3390/en16196900
APA StyleSaha, A., Oshima, R., Ohori, D., Sasaki, T., Yano, H., Okuzaki, H., Tokumasu, T., Endo, K., & Samukawa, S. (2023). Effect of Interfacial Oxide Layers on Self-Doped PEDOT/Si Hybrid Solar Cells. Energies, 16(19), 6900. https://doi.org/10.3390/en16196900








