A Comprehensive Review in Microwave Pyrolysis of Biomass, Syngas Production and Utilisation
Abstract
:1. Introduction
2. Literature Review
2.1. Biofuels
2.2. Gasification vs. Pyrolysis
2.3. Pyrolysis Oil
2.4. Fischer–Tropsch Synthesis
2.5. Comparison to DME
3. Motivation to Produce F-T Fuel
4. Microwave Pyrolysis
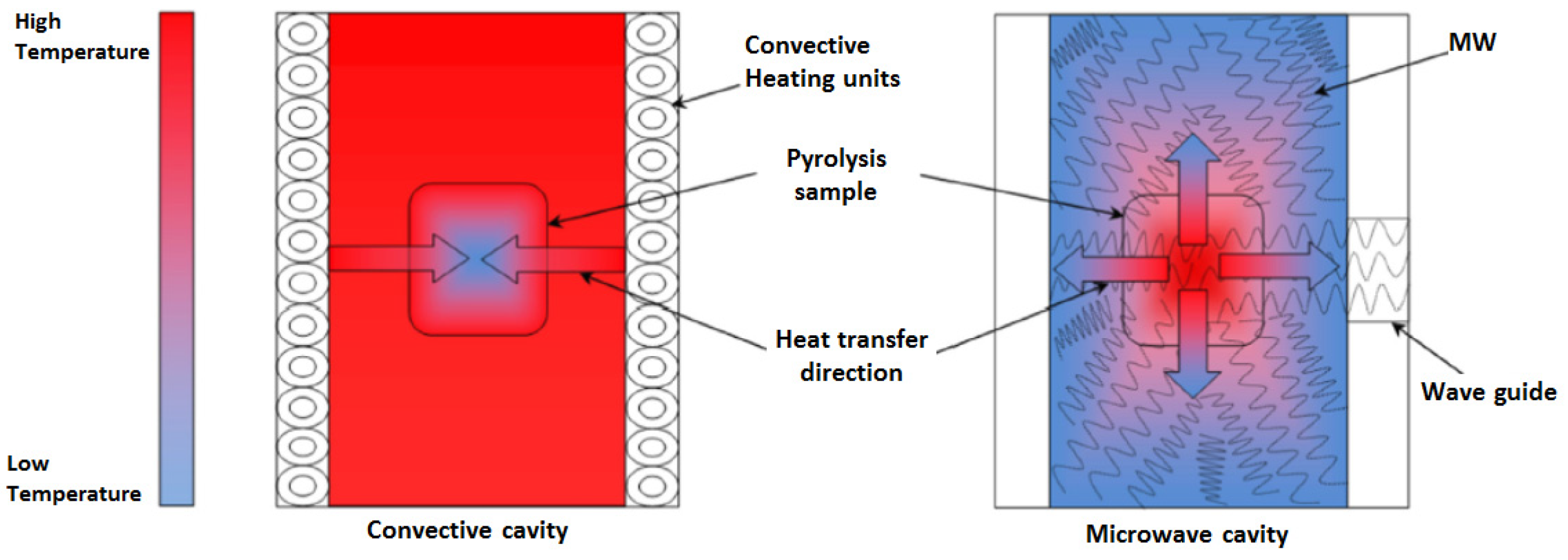
4.1. Heating Mechanism
4.2. Advantages of Microwave Heating
4.2.1. Heating and Energy Transfer
4.2.2. Higher Heating Rate and Efficiency
4.2.3. Material Selective Heating
5. Optimum Microwave Pyrolysis (MWP) Parameters
5.1. Microwave Absorbers
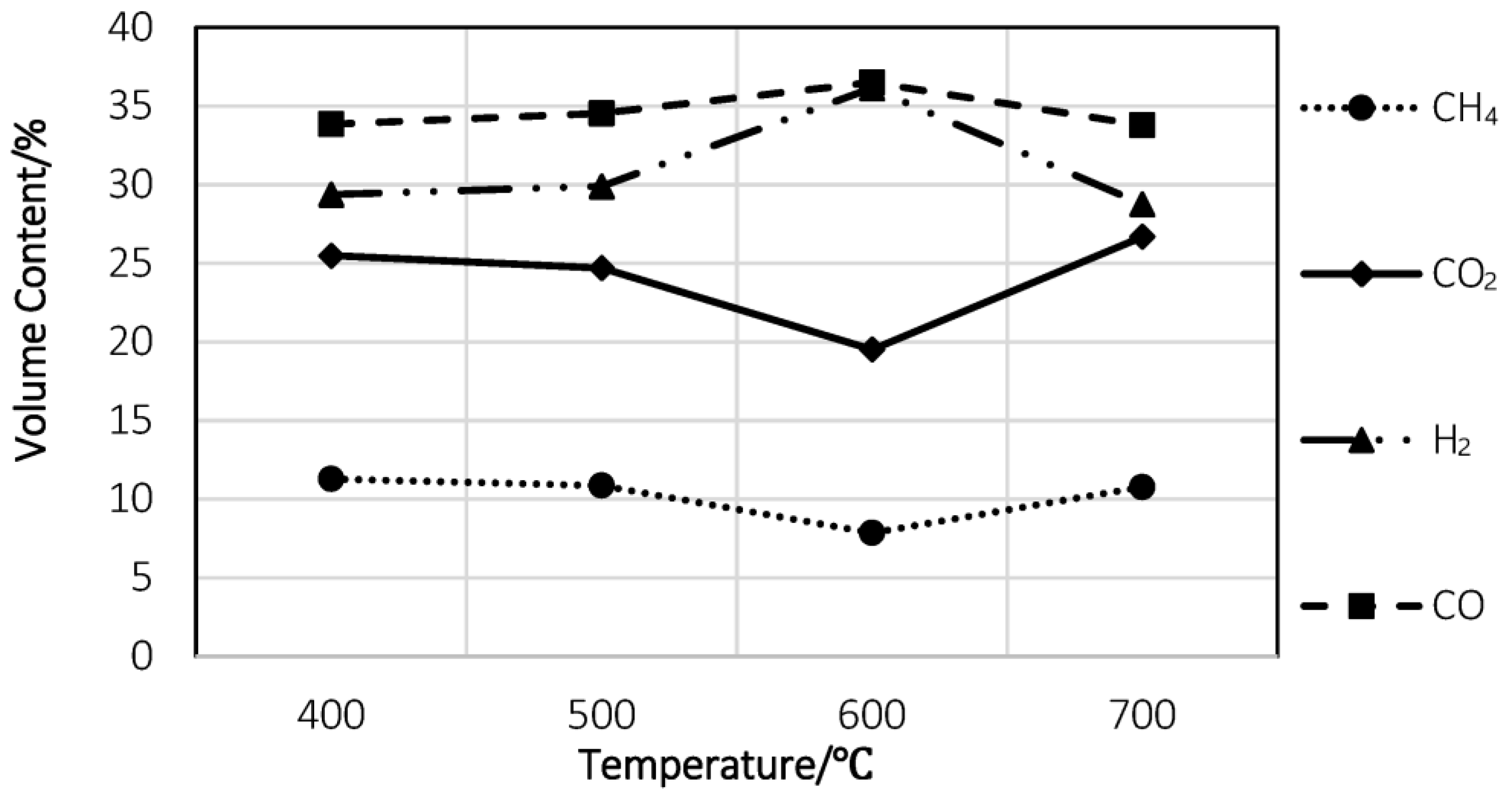
5.2. Feedstock Types and Syngas Production
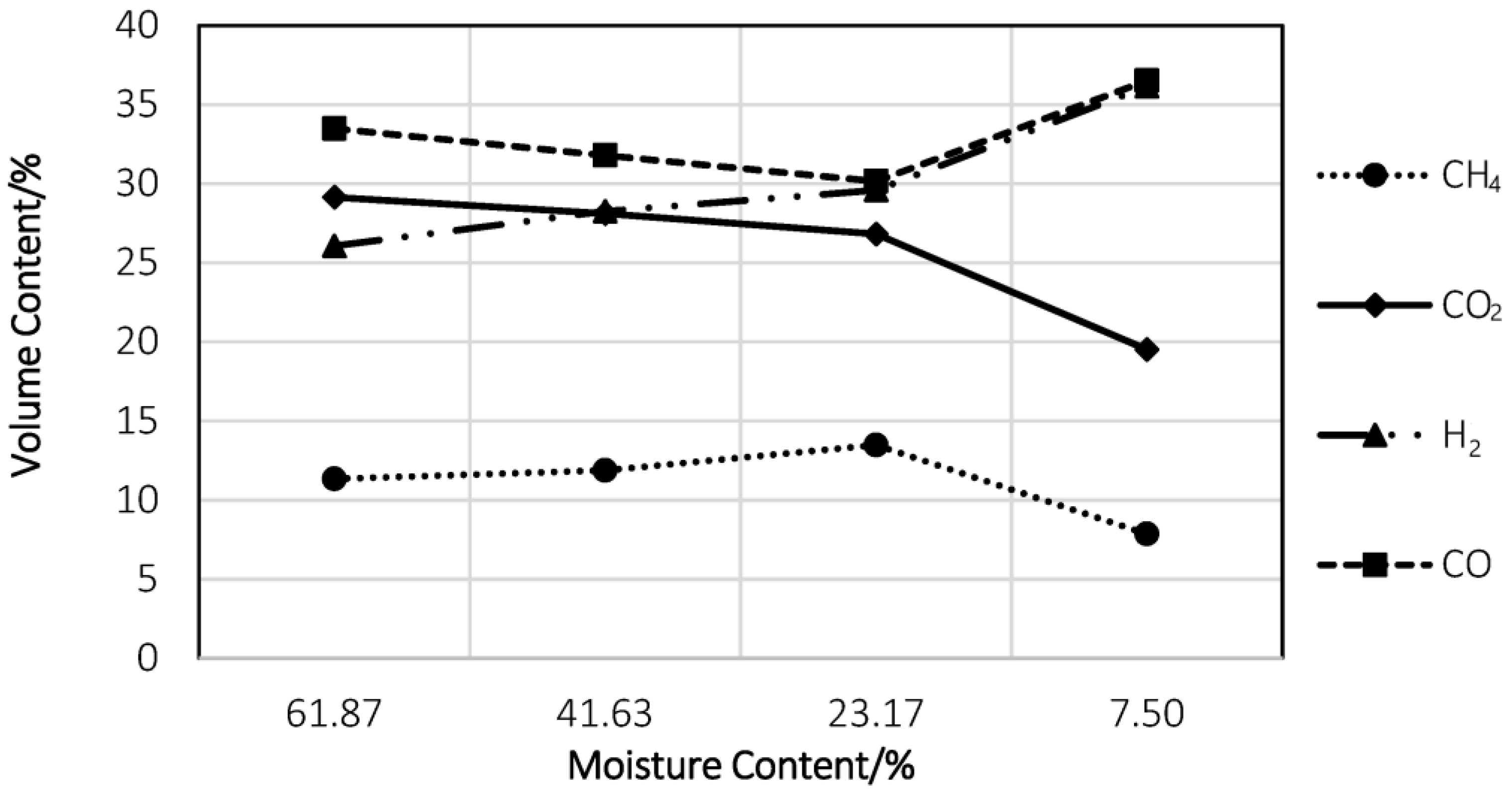
5.3. Moisture Content
6. Comparison of Microwave and Conventional Pyrolysis
6.1. Gas and Syngas Yields
6.2. Hot Spots and Microplasmas
6.3. Rates of Decomposition
7. Improvements for Microwave Pyrolysis Technology
7.1. Improvements on Syngas Yield
7.2. Scalability
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shafiee, S.; Topal, E. When will fossil fuel reserves be diminished? Energy Policy 2009, 37, 181–189. [Google Scholar] [CrossRef]
- Institute Environmental and Energy Study. Biofuels versus Gasoline: The Gap Is Widening; Institute Environmental and Energy Study: Washington, DC, USA, 2016. [Google Scholar]
- Patel, M.; Zhang, X.; Kumar, A. Techno-economic and life cycle assessment on lignocellulosic biomass thermochemical conversion technologies: A review. Renew. Sustain. Energy Rev. 2016, 53 (Suppl. C), 1486–1499. [Google Scholar] [CrossRef]
- Doustdar, O.; Wyszynski, M.L.; Mahmoudi, H.; Tsolakis, A. Enhancing the properties of Fischer-Tropsch fuel produced from syngas over Co/SiO2 catalyst: Lubricity and Calorific Value. In Proceedings of the 2016 IOP Conference Series: Materials Science and Engineering, Bali, Indonesia, 19–20 March 2016; Volume 148, p. 012092. [Google Scholar]
- Mahmoudi, H.; Mahmoudi, M.; Doustdar, O.; Jahangiri, H.; Tsolakis, A.; Gu, S.; LechWyszynski, M. A review of Fischer Tropsch synthesis process, mechanism, surface chemistry and catalyst formulation. Biofuels Eng. 2017, 2, 11–31. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Jahangiri, H.; Doustdar, O.; Akbari, N.; Wood, J.; Tsolakis, A.; Wyszynski, M.L. Maximizing paraffin to olefin ratio employing simulated nitrogen-rich syngas via Fischer-Tropsch process over Co3O4/SiO2 catalysts. Fuel Process. Technol. 2020, 208, 106477. [Google Scholar] [CrossRef]
- Biofuels America’s Advanced. ‘Biodiesel’. 2016. Available online: https://biofuels-news.com/news/us-biodiesel-market-sees-record-growth-in-2016/ (accessed on 1 January 2023).
- Sims, R.; Taylor, M.; Saddler, J.; Mabee, W. From 1st to 2nd Generation Biofuel Technologies; OECD/IEA: Paris, France, 2008; pp. 6–21. [Google Scholar]
- Doustdar, O.; Wyszynski, M.L.; Tsolakis, A.; Mahmoudi, H. Bio-Ketones from lignocellulosic biomass: Experimental investigation on fuel properties, combustion and emission characteristics of cyclopentanone blend with diesel in compression ignition engine. Combust. Engines 2017, 56. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Talluri, G.; Scarlat, N.; Prussi, M. The challenge of forecasting the role of biofuel in EU transport decarbonisation at 2050: A meta-analysis review of published scenarios. Renew. Sustain. Energy Rev. 2021, 139, 110715. [Google Scholar] [CrossRef]
- Shamsul, N.S.; Kamarudin, S.K.; Rahman, N.A.; Kofli, N.T. An overview on the production of bio-methanol as potential renewable energy. Renew. Sustain. Energy Rev. 2014, 33 (Suppl. C), 578–588. [Google Scholar] [CrossRef]
- Nogueira, L.A.H.; Souza, G.M.; Cortez, L.A.B.; de Brito Cruz, C.H. 9—Biofuels for Transport. In Future Energy, 3rd ed.; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 173–197. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Hodgson, E.; Lewys-James, A.; Rao Ravella, S.; Thomas-Jones, S.; Perkins, W.; Gallagher, J. Optimisation of slow-pyrolysis process conditions to maximise char yield and heavy metal adsorption of biochar produced from different feedstocks. Bioresour. Technol. 2016, 214 (Suppl. C), 574–581. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of feedstock type and pyrolysis temperature on potential applications of biochar. J. Anal. Appl. Pyrolysis 2016, 120 (Suppl. C), 200–206. [Google Scholar] [CrossRef]
- Burhenne, L.; Damiani, M.; Aicher, T. Effect of feedstock water content and pyrolysis temperature on the structure and reactivity of spruce wood char produced in fixed bed pyrolysis. Fuel 2013, 107 (Suppl. C), 836–847. [Google Scholar] [CrossRef]
- Li, C.; Aston, J.E.; Lacey, J.A.; Thompson, V.S.; Thompson, D.N. Impact of feedstock quality and variation on biochemical and thermochemical conversion. Renew. Sustain. Energy Rev. 2016, 65 (Suppl. C), 525–536. [Google Scholar] [CrossRef]
- E4Tech. Review of Technologies for Gasification of Biomass and Wastes; NNFCC: York, UK, 2009; Volume 09-008. [Google Scholar]
- Morf, P.; Hasler, P.; Nussbaumer, T. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips. Fuel 2002, 81, 843–853. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass pyrolysis—A review of modelling, process parameters and catalytic studies. Renew. Sustain. Energy Rev. 2015, 50 (Suppl. C), 1081–1096. [Google Scholar] [CrossRef]
- Al-Rahbi, A.S.; Onwudili, J.A.; Williams, P.T. Thermal decomposition and gasification of biomass pyrolysis gases using a hot bed of waste derived pyrolysis char. Bioresour. Technol. 2016, 204 (Suppl. C), 71–79. [Google Scholar] [CrossRef] [PubMed]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38 (Suppl. C), 594–608. [Google Scholar] [CrossRef]
- Lu, Q.; Li, W.-Z.; Zhu, X.-F. Overview of fuel properties of biomass fast pyrolysis oils. Energy Convers. Manag. 2009, 50, 1376–1383. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Fan, L.; Zhou, N.; Min, M.; Cheng, Y.; Peng, P.; Anderson, E.; Wang, Y.; et al. Microwave-Assisted Pyrolysis of Biomass for Bio-Oil Production. Pyrolysis 2017, 129–166. [Google Scholar]
- Gill, S.S.; Tsolakis, A.; Dearn, K.D.; Rodríguez-Fernández, J. Combustion characteristics and emissions of Fischer–Tropsch diesel fuels in IC engines. Prog. Energy Combust. Sci. 2011, 37, 503–523. [Google Scholar] [CrossRef]
- Alleman, T.L.; McCormick, R.L. Fischer-Tropsch Diesel Fuels—Properties and Exhaust Emissions: A Literature Review; SAE International: Warrendale, PA, USA, 2003. [Google Scholar]
- Miyamoto, N.; Ogawa, H.; Shibuya, M.; Arai, K.; Esmilaire, O. Influence of the Molecular Structure of Hydrocarbon Fuels on Diesel Exhaust Emissions; SAE International: Warrendale, PA, USA, 1994. [Google Scholar]
- Andrade Torres, F.; Doustdar, O.; Herreros, J.M.; Li, R.; Poku, R.; Tsolakis, A.; Martins, J.; Vieira de Melo, S.A.B. A Comparative Study of Biofuels and Fischer–Tropsch Diesel Blends on the Engine Combustion Performance for Reducing Exhaust Gaseous and Particulate Emissions. Energies 2021, 14, 1538. [Google Scholar] [CrossRef]
- Torres, F.A.; Doustdar, O.; Herreros, J.M.; Li, R.; Poku, R.; Tsolakis, A.; Martins, J.; Vieira de Melo, S.A.B. Fischer-Tropsch Diesel and Biofuels Exergy and Energy Analysis for Low Emissions Vehicles. Appl. Sci. 2021, 11, 5958. [Google Scholar] [CrossRef]
- Szybist, J.P.; Kirby, S.R.; Boehman, A.L. NOx Emissions of Alternative Diesel Fuels: A Comparative Analysis of Biodiesel and F-T Diesel. Energy Fuels 2005, 19, 1484–1492. [Google Scholar] [CrossRef]
- Kim, Y.-D.; Yang, C.-W.; Kim, B.-J.; Moon, J.-H.; Jeong, J.-Y.; Jeong, S.-H.; Lee, S.-H.; Kim, J.-H.; Seo, M.-W.; Lee, S.-B.; et al. Fischer–tropsch diesel production and evaluation as alternative automotive fuel in pilot-scale integrated biomass-to-liquid process. Appl. Energy 2016, 180 (Suppl. C), 301–312. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for Fischer–Tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef]
- Tayebi, J.; Atashi, H.; Tabrizi, F.F.; Gholizadeh, J. The Effect of Operating Conditions on the Selectivity Products in the Fischer-Tropsch Reaction. Appl. Res. J. 2016, 2, 215–219. [Google Scholar]
- Riyahin, M.; Atashi, H.; Kalhori, D.M. Effect of process conditions on Fischer–Tropsch synthesis product selectivity over an industrial iron-based catalyst in slurry reactor. Pet. Sci. Technol. 2016, 34, 1211–1218. [Google Scholar] [CrossRef]
- Park, W.; Park, S.; Reitz, R.D.; Kurtz, E. The effect of oxygenated fuel properties on diesel spray combustion and soot formation. Combust. Flame 2017, 180, 276–283. [Google Scholar] [CrossRef]
- Himabindu, M.; Ravikrishna, R.V. Potential of bio-DME as a transportation fuel for India. J. Renew. Sustain. Energy 2010, 2. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.S. Combustion performance and emission reduction characteristics of automotive DME engine system. Prog. Energy Combust. Sci. 2013, 39, 147–168. [Google Scholar] [CrossRef]
- Platform European Biofuels Technology. Liquid, Synthetic Hydrocarbons; Platform European Biofuels Technology, 2011. [Google Scholar]
- Platform European Biofuels Technology. Dimethyl Ether (DME) Fact Sheet; Platform European Biofuels Technology, 2016. [Google Scholar]
- Park, S.H.; Lee, C.S. Applicability of dimethyl ether (DME) in a compression ignition engine as an alternative fuel. Energy Convers. Manag. 2014, 86 (Suppl. C), 848–863. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Verbeek, R.; Van der Weide, J. Global Assessment of Dimethyl-Ether: Comparison with Other Fuels; SAE International: Warrendale, PA, USA, 1997. [Google Scholar]
- Zhang, S.; Dong, Q.; Zhang, L.; Xiong, Y. High quality syngas production from microwave pyrolysis of rice husk with char-supported metallic catalysts. Bioresour. Technol. 2015, 191 (Suppl. C), 17–23. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.; Chia, L.H.L. Thermal and non-thermal interaction of microwave radiation with materials. J. Mater. Sci. 1995, 30, 5321–5327. [Google Scholar] [CrossRef]
- Mushtaq, F.; Mat, R.; Ani, F.N. A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew. Sustain. Energy Rev. 2014, 39 (Suppl. C), 555–574. [Google Scholar] [CrossRef]
- Zhang, X.; Hayward, D.O. Applications of microwave dielectric heating in environment-related heterogeneous gas-phase catalytic systems. Inorganica Chim. Acta 2006, 359, 3421–3433. [Google Scholar] [CrossRef]
- Beneroso, D.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Microwave pyrolysis of microalgae for high syngas production. Bioresour. Technol. 2013, 144 (Suppl. C), 240–246. [Google Scholar] [CrossRef]
- Fernández Díez, Y.; Arenillas de la Puente, A.; Menéndez Díaz J, Á. Microwave Heating Applied to Pyrolysis. In Advances in Induction and Microwave Heating of Mineral and Organic Materials; InTech: Houston TX, USA, 2011; Chapter 31; pp. 723–752. [Google Scholar]
- Czarnocka, J. The Use of Microwave Pyrolysis for Biomass Processing. Arch. Automot. Eng. 2015, 67, 11–21. [Google Scholar]
- Thostenson, E.T.; Chou, T.W. Microwave processing: Fundamentals and applications. Compos. Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Mamaeva, A.; Tahmasebi, A.; Tian, L.; Yu, J. Microwave-assisted catalytic pyrolysis of lignocellulosic biomass for production of phenolic-rich bio-oil. Bioresour. Technol. 2016, 211 (Suppl. C), 382–389. [Google Scholar] [CrossRef]
- Pianroj, Y.; Jumrat, S.; Werapun, W.; Karrila, S.; Tongurai, C. Scaled-up reactor for microwave induced pyrolysis of oil palm shell. Chem. Eng. Process. Process Intensif. 2016, 106 (Suppl. C), 42–49. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Effects of lignocellulosic composition and microwave power level on the gaseous product of microwave pyrolysis. Energy 2015, 89 (Suppl. C), 974–981. [Google Scholar] [CrossRef]
- Zhao, X.; Song, Z.; Liu, H.; Li, Z.; Li, L.; Ma, C. Microwave pyrolysis of corn stalk bale: A promising method for direct utilization of large-sized biomass and syngas production. J. Anal. Appl. Pyrolysis 2010, 89, 87–94. [Google Scholar] [CrossRef]
- Mokhtar, N.M.; Omar, R.; Idris, A. Microwave Pyrolysis for Conversion of Materials to Energy: A Brief Review. Energy Sources Recovery Util. Environ. Eff. 2012, 34, 2104–2122. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Liu, L.; Li, K.; Wang, X.; Li, H. Experimental study of microwave-assisted pyrolysis of rice straw for hydrogen production. Int. J. Hydrogen Energy 2016, 41, 2263–2267. [Google Scholar] [CrossRef]
- van der Laan, G.P. Kinetic, Selectivity and Scale Up of the Fischer-Tropsch Synthesis; University of Groningen: Groningen, The Netherlands, 1999. [Google Scholar]
- Hu, J.; Yu, F.; Lu, Y. Application of Fischer–Tropsch Synthesis in Biomass to Liquid Conversion. Catalysts 2012, 2, 303. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Microwave pyrolysis of lignocellulosic biomass: Heating performance and reaction kinetics. Energy 2016, 100 (Suppl. C), 137–144. [Google Scholar] [CrossRef]
- Beneroso, D.; Bermúdez, J.M.; Montes-Morán, M.A.; Arenillas, A.; Menéndez, J.A. Microwave-induced cracking of pyrolytic tars coupled to microwave pyrolysis for syngas production. Bioresour. Technol. 2016, 218 (Suppl. C), 687–691. [Google Scholar] [CrossRef] [PubMed]
- Beneroso, D.; Monti, T.; Kostas, E.T.; Robinson, J. Microwave pyrolysis of biomass for bio-oil production: Scalable processing concepts. Chem. Eng. J. 2017, 316 (Suppl. C), 481–498. [Google Scholar] [CrossRef]
- Aishwarya, K.N.; Sindhu, N. Microwave Assisted Pyrolysis of Plastic Waste. Procedia Technol. 2016, 25 (Suppl. C), 990–997. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Y.; Zhao, X.; Sun, J.; Wang, W.; Mao, Y.; Ma, C. Microwave pyrolysis of tire powders: Evolution of yields and composition of products. J. Anal. Appl. Pyrolysis 2017, 123 (Suppl. C), 152–159. [Google Scholar] [CrossRef]




| Fuel Properties | F-T Diesel | DME | Diesel |
|---|---|---|---|
| Formula | CnH2n+2 | CH3OCH3 | C14H30 |
| Molecular weight (g/mol) | - | 46.07 | 198.4 |
| Density at 20 °C | 0.76 | 0.67 | 0.83 |
| Normal boiling point (°C) | 200–350 | −24.9 | 125–400 |
| LHV (MJ/kg) | 43.247 | 28.882 | 42.791 |
| Viscosity [mm2/s] at 20 °C | 4 | 0.15 | 5 |
| Octane number | >74 | 55–60 | 40–55 |
| Oxygen content (%) | 0 | 34.8 | 0 |
| Sulphur content (ppm) | 0 | 0 | ≈250 |
| Emissions | F-T Diesel | DME (%) |
|---|---|---|
| NOx | −6 | −75 |
| PM | −37 | −100 |
| HC | −42 | −76 |
| CO | −27 | −50 |
| RS | RH | CS | SB | SP | CG | BL | |
|---|---|---|---|---|---|---|---|
| Moisture | 9.32 | 6.34 | 8.58 | 8.61 | 5.30 | 7.97 | 7.14 |
| Calorific value (MJ/kg) | 16.16 | 15.91 | 17.06 | 16.92 | 17.03 | 16.78 | 15.75 |
| Proximate analysis (wt.%) | |||||||
| Volatile matter | 79.22 | 80.45 | 82.58 | 86.02 | 80.4 | 78.69 | 71.59 |
| Fixed Carbon | 12.27 | 8.70 | 12.48 | 9.93 | 15.42 | 14.25 | 16.57 |
| Ash | 8.51 | 10.85 | 4.94 | 4.05 | 4.54 | 7.06 | 11.84 |
| Ultimate analysis (wt.%) | |||||||
| C | 45.76 | 43.98 | 49.38 | 48.88 | 46.47 | 44.89 | 39.98 |
| H | 6.22 | 5.94 | 6.52 | 6.71 | 6.23 | 6.14 | 5.81 |
| N | 0.52 | 0.40 | 0.63 | 0.27 | 0.92 | 0.35 | 1.12 |
| O | 47.50 | 49.68 | 43.47 | 44.15 | 46.38 | 48.62 | 53.09 |
| Lignocellulosic analysis (wt.%) | |||||||
| Extractives | 4.39 | 5.52 | 5.27 | 5.44 | 8.18 | 12.35 | 5.28 |
| Hemicellulose | 31.12 | 28.03 | 28.94 | 27.40 | 26.40 | 30.03 | 25.55 |
| Cellulose | 38.14 | 30.42 | 43.97 | 46.55 | 41.11 | 33.10 | 34.14 |
| Lingin | 26.35 | 36.02 | 21.82 | 20.61 | 24.31 | 24.52 | 35.03 |
| Agricultural Residual | Microwave Power Level (W) | Molecular of Gaseous Component (mmol) | |||
|---|---|---|---|---|---|
| H2 | CH4 | CO | CO2 | ||
| Rice straw | 300 | 7.75 | 2.53 | 26.26 | 7.43 |
| 400 | 13.88 | 4.16 | 35.98 | 9.46 | |
| 500 | 20.13 | 5.46 | 41.46 | 11.42 | |
| Rice husk | 300 | 5.56 | 1.03 | 23.32 | 4.93 |
| 400 | 10.79 | 3.79 | 19.90 | 6.59 | |
| 500 | 17.38 | 4.89 | 35.40 | 8.83 | |
| Corn stover | 300 | 7.52 | 2.91 | 32.72 | 6.06 |
| 400 | 12.79 | 4.56 | 39.40 | 8.53 | |
| 500 | 19.54 | 6.88 | 50.32 | 10.30 | |
| Sugarcane bagasse | 300 | 6.34 | 2.35 | 38.64 | 4.15 |
| 400 | 11.47 | 6.56 | 43.37 | 7.61 | |
| 500 | 18.56 | 7.38 | 50.72 | 9.27 | |
| Sugarcane peel | 300 | 5.06 | 1.76 | 31.85 | 3.42 |
| 400 | 8.81 | 4.72 | 39.79 | 5.80 | |
| 500 | 15.31 | 6.40 | 48.43 | 7.91 | |
| Coffee grounds | 300 | 7.50 | 2.12 | 25.27 | 6.85 |
| 400 | 11.59 | 4.06 | 33.89 | 8.63 | |
| 500 | 19.52 | 5.07 | 42.01 | 10.65 | |
| Bamboo leaves | 300 | 4.40 | 1.50 | 27.29 | 3.88 |
| 400 | 9.20 | 3.45 | 34.56 | 5.15 | |
| 500 | 13.53 | 4.92 | 43.12 | 8.35 | |
| Feedstock | Product Yield and Comparison | Microwave | Conventional Pyrolysis |
|---|---|---|---|
| Coffee hull pellets | Oil yield (wt.%) | 9.80–13.57 | 7.90–9.19 |
| Pine sawdust | H2 vol% | 16–32 | 55–65 |
| CO col% | 41–48 | 0.17–0.36 | |
| CO2 Vol% | 6 - 28 | 0.45–0.65 | |
| Coffee hull | Gas yield (wt.%) | 60–75 | 55–65 |
| Syngas (H2 + CO) L/g of biomass feed | 0.41–0.62 | 0.17–0.36 | |
| CO2 production | 0.20–0.34 | 0.45–0.65 | |
| Wheat straw bales | Syngas (H2 + CO) vol.% | 54% of total gas vol. (37% H2) | <40% of the total gas vol. |
| Corn straw bales | 54% of total gas vol. (35% H2) | <40% of the total gas vol. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Qahtani, A.M. A Comprehensive Review in Microwave Pyrolysis of Biomass, Syngas Production and Utilisation. Energies 2023, 16, 6876. https://doi.org/10.3390/en16196876
Al-Qahtani AM. A Comprehensive Review in Microwave Pyrolysis of Biomass, Syngas Production and Utilisation. Energies. 2023; 16(19):6876. https://doi.org/10.3390/en16196876
Chicago/Turabian StyleAl-Qahtani, Ali Mubarak. 2023. "A Comprehensive Review in Microwave Pyrolysis of Biomass, Syngas Production and Utilisation" Energies 16, no. 19: 6876. https://doi.org/10.3390/en16196876
APA StyleAl-Qahtani, A. M. (2023). A Comprehensive Review in Microwave Pyrolysis of Biomass, Syngas Production and Utilisation. Energies, 16(19), 6876. https://doi.org/10.3390/en16196876






