Abstract
In a world facing increasing environmental and energy challenges, anaerobic digestion of agrifood by-products and food waste could contribute to the production of green energy while reducing greenhouse gas emissions into the atmosphere. Anaerobic digestion is a biological process capable of breaking down and stabilising organic matter in the absence of oxygen and converting it into a renewable source of energy, known as biogas. Biomethane production also enables the generation of electricity and produces digestate, a by-product of the digestion process that can be used as a soil conditioner or fertiliser. This review aims to highlight how substrate pretreatment, together with the optimisation of operating parameters, application of additives, recirculation of digestate and frequent feeding, can increase biogas production. An overview of the basics of the anaerobic digestion of agrifood by-products and food waste is provided, including feedstock characteristics (nutrient content, particle size and inhibitory compounds) and process parameters (process configuration, pH, temperature, total and volatile solids, total Kjeldahl nitrogen, ammonium, chemical oxygen demand, carbon/nitrogen ratio, retention time, organic loading rate, etc.). In addition, recent studies in the field of processes, equipment and pretreatments that can significantly improve the anaerobic digestion process of agricultural and food wastes were classified and discussed. Finally, the challenges and future perspectives of biogas production from the agrifood sector are addressed.
1. Introduction
Significant environmental damage has been caused by the extraction and combustion of fossil fuels. Today, climate scientists have a common opinion on the existence, causes and negative consequences of global warming due to the emission of carbon dioxide (CO2) and methane (CH4) in the atmosphere [1]. Only in recent years has there been a shift in consumption, with a preference for renewable sources to non-renewable ones thanks to current energy policies. As reported by Pramanik et al., (2019) [2], it is estimated that 1/3 of edible food is lost globally through the food supply chain. This includes both food loss, which occurs along the entire supply or production chain up to final consumption (farming techniques, harvesting and transport, transformation), and food waste, which occurs at the final stage of the supply chain due to a mismanagement of supply or poor dietary habits. There is also an embedded greenhouse gas effect from activities related to agriculture and food production. Total global emissions from agriculture and related land use activities reached over 9 billion tonnes of carbon dioxide equivalent (Gt CO2-eq), according to the FAOSTAT Analytical Brief 2018 [3]. On-farm crop and livestock activities accounted for more than half of this total. In 2018, agriculture and related land use emissions accounted for 17 per cent of global greenhouse gas (GHG) emissions from all sectors. This is down from 24 per cent in the 2000s. In this context, anaerobic digestion (AD) of biomass such as agricultural by-products and food waste represents a reliable way to reduce GHG emissions and use waste to be eliminated in by-products for clean energy production. Indeed, residues from food industries, livestock farms and municipal waste still contain a lot of matter that can be converted into biogas and biomethane, therefore allowing for lower consumption of non-renewable sources and lower production of polluting gases. AD is a complex biological process in which organic matter is converted by a bacterial consortium into biogas in the absence of oxygen. The biogas produced consists mainly of 50–75% methane (CH4), 25–50% carbon dioxide (CO2), water vapour (H2O) and traces of oxygen (O2), nitrogen (N2) and hydrogen sulphide (H2S) [4]. Today, biogas is used for combined heat and power (CHP) production or purified and fed into natural gas networks to be used as vehicle fuel, for domestic purposes or in fuel cells [5]. In addition, AD generates a by-product consisting of a stabilised, odourless and nutrient-rich digestate, which can be used as fertiliser or as soil conditioner for agronomic purposes [6], considering its high content of plant macronutrients including nitrogen (N), phosphorus (P), potassium (K) and sulphur (S), as well as various micronutrients and organic matter [7]. Biogas can be produced from different substrates depending on their availability, such as agrifood residues and by-products, livestock manure, organic municipal waste and so on. These substrates have a varying composition, which influences biogas and methane yields. Considering the above, this paper reports the fundamentals concerning the anaerobic digestion of agrifood by-products and food waste regarding the production of biogas and biomethane, focusing on biomass characteristics and process parameters. This review is organised into four parts. Process, physicochemical parameters and operational factors affecting AD performance are discussed in the first part. The second part is focused on pretreatments to which organic substrates have been subjected. The third part is a discussion of the operating parameters of continuous reactors. The last part is focused on batch and continuous anaerobic digestion processes and innovative studies carried out in the field.
2. Anaerobic Digestion (AD) Process
Anaerobic digestion (AD) is a serial multi-stage biologic process in which organic matter is decomposed and stabilised in the absence of oxygen [8]. With the participation of different groups of anaerobic microorganisms, various types of substrates of organic origin can be converted into a renewable energy source known as biogas, a gas mixture containing essentially 50–75% methane (CH4), 30–40% carbon dioxide (CO2) and other minor gases like H2S, H2 and O2 [9,10]. This gas mixture, which is suitably purified, can be used as a substitute for fossil fuels to generate heat or electricity [11,12]. The anaerobic digestion process can be divided into four phases: hydrolysis, acidogenesis, acetogenesis and, the most important for the production of methane, methanogenesis, as reported in Figure 1 [2]. The microorganisms that carry out the degradation responses in each of these stages differ widely in their physiology, nutrient requirements, growth kinetics and sensitivity to the medium. There are different groups of microorganisms, but we can point out two main ones: acid-producing microorganisms and methane-producing microorganisms [13]. Hydrolysis is the first stage of anaerobic digestion; an initial degradation of organic matter, intermediated by enzymes, is observed through the breakdown of macromolecules such as lipids, polysaccharides and proteins into molecules of a lower molecular weight. These molecules serve as an ideal substrate for the growth of the anaerobic bacterial consortium [14]. During acidogenesis, the monomers produced in the hydrolytic phase are processed by the consortium of facultative and obligatory anaerobic bacteria. These molecules are further degraded into short-chain organic acids, such as butyric acids, propanoic acids, acetic acids, alcohols, hydrogen and carbon dioxide. The concentration of hydrogen formed as an intermediate product during this phase can influence the entire digestion process. In general, simple sugars, fatty acids and amino acids are converted into organic acids and alcohols during this phase. The products formed in the acidogenisc phase are consumed as substrates by acetogenic microorganisms. Anaerobic oxidations take place in this phase. Acetogenic bacteria are able to convert volatile fatty acids (VFAs) and alcohols into acetate, hydrogen and carbon dioxide. In the methanogenic phase, methane and carbon dioxide are produced from intermediates by methanogenic bacteria under total anaerobiosis conditions and are subject to various parameters (temperature, pH, alkalinity, etc.) [15].
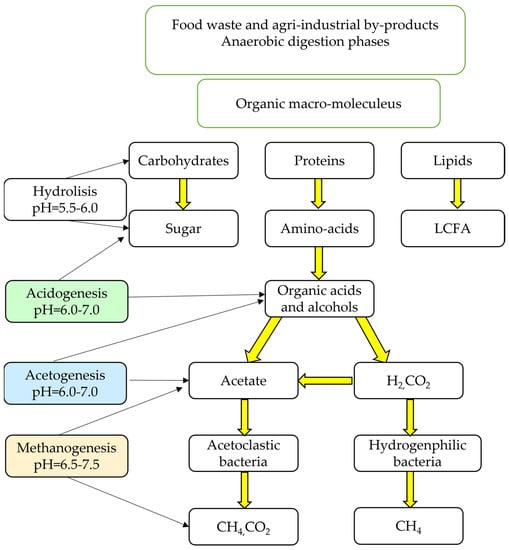
Figure 1.
Flow chart of anaerobic digestion. Information collected from Pramanik et al., 2019 [2].
2.1. Physicochemical Parameters and Operational Factors Affecting AD Performances
Anaerobic digestion is a very sensitive process. In fact, even a slight variation in process parameters can affect the system efficiency. These process parameters include temperature, pH and alkalinity, as well as physicochemical features of the substrates, including total solids (TSs) and total volatile solids (TVSs), ammonium, total Kjeldahl nitrogen (TKN), concentration of volatile fatty acids (VFAs), C/N ratio, chemical oxygen demand (COD) and sample quantity.
2.1.1. Temperature
In general, the microbes and bacterial consortia involved in anaerobic digestion only operate optimally at certain temperatures. For this reason, it is important that the fermentation temperature is kept stable and that there are no sudden drops or rises in temperature during the fermentation process. The operating temperatures of a biogas plant can be different. It is possible to work in mesophilic (37 ± 2 °C) conditions, which is the most common choice due to low operating costs, or in thermophilic (55 ± 2 °C) or psychrophilic (10–25 °C) conditions [16]. If the temperature drops or rises too abruptly during the methanisation process, methane production may stop. In fact, methanogenic microorganisms are very sensitive to changes in temperature. According to Geradi, (2003), [17] daily temperature fluctuations should be kept below 1 °C for thermophilic biogas processes and within 2–3 °C for mesophilic processes. When starting up a biogas plant, the inoculum should already be adapted to the future operating temperature in order to reduce the adaptation time and consequently the duration of the start-up phase [7].
2.1.2. pH
The pH value provides a rough indication of the state of the fermentation process [18]. Fluctuations in pH during the methanisation phase can halt the process. Due to the buffering capacity of biogas plants, which is dependent on dissolved CO2, carbonate and ammonia, a change in pH is not detectable until a significant imbalance in the process has occurred. A low pH value can lead to the formation of potentially harmful agents such as ammonia. During the first three phases, microorganisms function well in a pH range of 4 to 8.5, whereas values between 6.5 and 7.2 are required during the methanogenic phase. Therefore, while it provides important information for process monitoring, pH measurement is not an early indicator of process imbalance. But to ensure that the methanogenesis process takes place, it is recommended a stable pH be maintained using buffer solutions such as NaOH, Na2CO3 and Ca(OH)2 when necessary [19].
2.1.3. Alkalinity
Alkalinity represents the ability of a system to neutralise protons and is generally expressed in terms of calcium carbonate concentration. This is determined analytically on the liquid phase present in the reactor via titration with hydrochloric acid [20]. The alkalinity of an anaerobic digester is determined by the presence of ammonia originating from the degradation of the protein matrix and bicarbonate derived from the dissolution of carbon dioxide (CO2) in the medium, which form a system capable of buffering the decrease in the pH due to the accumulation of volatile fatty acids [21]. Generally, alkalinity values of the order of 3000–5000 mg of CaCO3 per litre are typical for anaerobic digesters operating in stable conditions [20].
2.1.4. Total Solid Content and Volatile Solid Content
Water is a key parameter for initiating the AD process. Water content is important for the solubilisation of nutrients and the bacterial consortium. Depending on the water content, anaerobic digestion can come in three different types: dry, semi-dry and wet. The moisture content in dry anaerobic digestion is around 10%, in semi-dry anaerobic digestion it is approximately 15–20%, and in wet anaerobic digestion, the content is higher at 20%. In dry anaerobic digestion, the filling of the reactor volume, the energy and water consumption needed for handling the mass to be digested and the management/disposal of sludge and wastewater are drastically reduced. Total solids (TSs) and total volatile solids (TVSs) can be determined to determine an adequate reactor content in terms of inoculum and biomass. TVS content provides approximative information about the content of organic fraction suitable to be decomposed and converted into biogas [20,21,22].
2.1.5. Ammonium
Wastes with high concentrations of protein or nitrogen, such as pig manure, dairy products and chicken manure, can produce high levels of free ammonia, which is toxic to methanogenic microorganisms, resulting in low biogas production. Ammonia nitrogen is less inhibitory in its ionic form (NH4⁺) compared to free ammonia (NH3), but the distribution between these forms depends on the temperature and pH. The system can tolerate a total ammonia nitrogen concentration range between 1500 and 7000 mgNL−1 [23]. When the ammonia content is high, methanogenesis is inhibited; this is usually the result of the accumulation of volatile fatty acids (VFAs) to the point where the buffer capacity of the digester is not effective and the pH drops below 6 with a corresponding progressive loss of methane production [24,25]. In the literature, very different inhibitory concentrations of ammonium nitrogen are given. According to Chen et al., 2008 [23], the maximum amount of ammonium should not exceed 14 g NH4⁺-NL−1.
2.1.6. Total Kjeldahl Nitrogen
The nitrogen content of a feedstock can be approximated by determining the total Kjeldahl nitrogen (TKN). In this analysis, organic nitrogen is converted to ammonium nitrogen by boiling the feed samples in the presence of sulphuric acid and a catalyst. Then, similar to NH4⁺-N analysis, a base is added and ammonia is distilled from the alkaline solution into an acidic solution (usually boric acid), wherein ammonia is absorbed quantitatively and measured. Monitoring TKN content in the feedstock can be important because a change from nitrogen-rich feedstock will lead to ammonia accumulation in the digester, which can cause ammonia inhibition [26].
2.1.7. Concentration of Volatile Fatty Acids
Volatile fatty acids are short-chain fatty acids (C-3, C-4, C5), such as acetic, propionic, butyric and valeric acids, or their branched isomers, produced during anaerobic digestion. They are intermediate metabolites in the AD process produced during the acidification step (acidogenesis) and are the precursors of methane [7]. The concentration of VFAs is expressed as the concentration of acetic acid in the volume of material (mg∙L−1) and depends on the quantity and quality of the material loaded into the digester and the balance between acid-forming bacteria and methanogens bacteria [21]. The ratio of acetic acid to propionic acid is an especially good indicator of process stability [27].
2.1.8. Carbon/Nitrogen Ratio
Nitrogen plays an important role in increasing the microbial population. The C/N ratio indicates the total ammonia nitrogen (TAN) released, the accumulation of volatile fatty acids (VFAs) within the digester and the nutrient level of a substrate [11]. In the literature, the ratio of C/N needed to achieve anaerobic digestion is said to be between 20:1 and 30:1, with an optimal fixed ratio of 25:1 enabling suitable growth of the bacterial consortium in an AD system [28].
2.1.9. Chemical Oxygen Demand
According to Method 5135 [29], chemical oxygen demand (COD) is a parameter that indicates the total chemically oxidisable material in a sample and therefore a parameter that indicates the energy content (or organic pollution) of a feedstock. It represents the quantity of oxygen necessary to oxidise, in the presence of a strong oxidising agent, the organic and inorganic substances present in a litre of sample in an acid environment. The chemical oxygen demand is expressed in mg∙L−1 COD, defined as milligrams of O2 consumed per litre of sample. The field of application is determined by the technical specifications of the commercial tests used. Theoretically, 1 g of COD (assuming that only organic carbon compounds are oxidised) is equivalent to a potential of 350 mL of methane [16,17,18,19,20,21,22]. Hence, the analysis of this parameter is important as it consists in a theoretical predictive factor regarding the amount of methane that could be obtained from a substrate in an anaerobic digestion process.
2.1.10. Sample Quantity
According to VDI 4630 [16], during anaerobic digestion, certain parameters must be taken into consideration to prevent inhibition of the process, including the following:
The amount of substrate (S) must not be greater than the amount of seeding sludge or inoculum (I). From this assumption, the ratio between (S/I), as expressed by the following formula, must be ≤0.5.
- ▪
- Substrate gas yields should recover more than 80% of the total gas amount of a sample.
- ▪
- The solid content of the batch must not exceed 10% if adequate mass transfer is to be ensured [15].
2.1.11. Nutrients and Toxic Agents
Macronutrients (e.g., P, N and S) are generally used as buffering agents, while micronutrients (Fe, Cr, Ni, Mo, Zn, Co, W and Se) act as a crucial cofactor in enzymatic reactions [19]. The presence of certain factors can inhibit or limit the growth of the bacterial consortium, particularly the methanogenic. The latter consists of microorganisms that are very sensitive both to variations in nutrition and to the presence of inhibitors. The parameters that can negatively affect the entire anaerobic digestion process are represented by the substrate itself and any inhibiting elements present in the matrices used, such as heavy metals, salts, ammonia nitrogen NH4+, residues of pesticides and antibiotics, detergents and disinfectants, solvents, inhibitors from chemical treatments for food preservation, etc. The substrate itself can constitute an inhibition factor as its concentration can regulate and/or slow down the reaction rate of the subsequent stages, having a negative effect on biogas production. For example, propionate is an important intermediate in anaerobic digesters, but it can be toxic if it exceeds 3 g·L−1 [30]. More generally, it has been reported in the literature that high concentrations of volatile fatty acids (VFAs) can have toxic effects by causing a strong acidification of the substrate. Among the compounds that can inhibit the methanogenesis process, we can cite hydrogen sulphide, ammonia nitrogen, salinity, chloroform and other chlorinated products, disinfectants and antibiotics, as well as various metal species. Methanogenic bacteria can tolerate concentrations of hydrogen sulphide of up to 1000 mg·kgTS−1 even if the actual ability to produce methane is seriously compromised at 200 mg·kgTS−1. In general, the optimal conditions for the growth of methanogenic bacteria are obtained for sulphide concentrations between 8 and 22 mg·kgTS−1 [31]. It has been observed that ammonia nitrogen concentrations between 1500 and 3000 mg·L−1 are inhibitory at a pH below 7.4, while concentrations above 3000 mg·L−1 are toxic at any pH value [21]. The establishment of a high salinity reaction environment can negatively affect the anaerobic digestion process, blocking methanogenesis, and so the tolerance limit can be identified to range from 250 to 500 Mm. The inhibiting action of metal ions mainly concerns the inhibition of the enzymes responsible for the biosynthesis of methane by the bacterial consortium. Studies performed on RU anaerobic digesters [32] indicate that there is a significant reduction in volatile fatty acids when the iron concentration is increased inside the digester (from 4000 to 400 mg·L−1). Similar effects of a reduction in methane yield can also be attributed to other metals, such as zinc (toxicity limit = 160 mg·L−1), copper (toxicity limit = 170 mg·L−1), chromium and cadmium (toxicity limit = 180 mg·L−1) [20].
3. Substrates and Possible Pretreatments
Since waste from food processing and the food industry still has a high content of bioavailable molecules that can be readily metabolised by the bacterial consortium to produce large quantities of biomethane through anaerobic digestion, it is the best raw material as it constitutes an excellent substrate [31]. The origins of these types of substrates can be diverse, though they mainly derive from across the agro-industrial production chain. Furthermore, the entire food processing and sales sector also produces huge amounts of food waste and by-products. The same applies to food products of animal origin, such as meat and meat products, and the entire dairy sector produces huge amounts of waste. In addition, all places where food is prepared and served, such as restaurants, canteens, etc., should be included. Finally, there is a mass of food waste that accumulates daily in consumers’ homes. If these large quantities of waste are not collected and turned into energy or animal feed, they inevitably end up in landfills. But if they are intercepted, they can be used as perfect substrates for anaerobic digestion and produce biomethane. The first step in screening various substrates is essential to remove other types of waste, such as inert materials, plastics and packaging and stones, from the biogas chain. This first screening step is necessary to remove inorganic substances that may enter the reactor. Inorganic material can in fact cause blockages and plant shutdowns, or simply fill the reactor volume with inert material, greatly reducing the useful volume of the reactor. Moreover, this type of initial screening also allows the recovery of other recyclable materials, such as plastic and metal. A recurring problem with food waste, besides its seasonality, is its heterogeneity of origin. The nutrient content of food waste determines the nutrient requirements for the bacterial consortium for anaerobic digestion [33,34]. Furthermore, prior to the AD process, the substrates used may require some pre-treatments which help the consortium of bacteria to degrade the organic component more easily and, consequently, make it more available for bacterial enzymes, enhancing biogas and biomethane production. In this regard, we can distinguish several pretreatments:
- ▪
- Mechanical;
- ▪
- Thermal;
- ▪
- Chemical;
- ▪
- Ultrasound;
- ▪
- Biological.
3.1. Mechanical and Particle Size Reduction
Mechanical pretreatment consists in subjecting waste to high mechanical stress by cutting, squeezing and sieving. The basic process, however, only involves reducing the particle size of the waste. Indeed, in order for substrates to be immediately attacked and transformed by the bacterial consortium, it is necessary to reduce the size of these wastes, as their grain size can compromise biogas production. The larger the substrates, the greater the likelihood of digester clogging. For this reason, it is recommended that the grain size of the waste be reduced. By reducing the waste particle size and increasing the surface area of the waste, the biomolecules are made more readily available to the bacterial consortium, thus reducing the start-up time of the digestion process, greatly improving biogas production and shortening retention times. When the waste particle size is 25 μm, the methane yield is higher, so the particle size of the material is directly related to the difference in the total number of microbes exposed [33,35]. The problem with this type of pretreatment is the wear and tear to which the machinery is subjected, so robust and reliable equipment is advised to prevent both damage to property and objects and forced plant shutdowns [36]. It is performed with mills and makes the substrate pieces smaller or squeezes them to break the cell structure, increasing the specific surface area of the biomass. This offers a greater possibility of enzymatic attack, which is particularly important for lignocellulosic substrates. Reducing particle size not only increases the rate of enzymatic degradation but can also reduce the viscosity in digesters and can reduce the problems resulting from particle settling or foam formation. One of the main disadvantages is that mills can be damaged by inert materials in the substrate, such as stones or pieces of metal, and repairs to the equipment can be very expensive [37]. A test sieve with a mesh size of 10 mm should be used in the reduction in the sample (e.g., crushing and grinding). If fibrous or other material that is difficult to crush is present, it must be cut, crushed or otherwise processed to a particle size of less than 10 mm. In this case, the size reduction method has a decisive influence on the particle size range. If the material is overheated during comminution, volatile components may be lost [16].
3.2. Thermal Pretreatment
In pure thermal pretreatment, the substrate is heated (typically 125 to 190 °C) under pressure and kept at this temperature for up to one hour. It is often carried out in conjunction with chemical or mechanical ones. The maximum temperature varies from one substrate to another and based on batch AD tests, has been found to be 175 °C for sludge (a 52% increase in methane production), 190 °C for crops and 160 °C for brewers’ spent grains. Thermal pretreatment is less effective than thermochemical one, but has the advantage that no chemicals need to be used or taken into account during the subsequent AD phase [36].
3.3. Chemical Pretreatment
Waste can be pretreated with both acidic and alkaline chemicals, involving the use of these substances at different concentrations. For lignin-containing materials, highly corrosive substances are used, which are capable of damaging the lignocellulosic cell walls by dissolving them in blockages for anaerobic digestion. This process allows a significant increase in biogas production [34]. The solution can be prepared with different portions of CaO, NaOH and KOH for alkaline pretreatment and with acids such as HCl, H2SO4, H3PO4 and HNO3 for acidic one [1]. For example Qiao et al., (2022) [38] used potassium ferrate (K2FeO4) to increase sludge hydrolysis and eliminate antibiotics in activated waste sludge (WAS), as their presence can inhibit or kill the bacterial consortium.
3.4. Ultrasound Pretreatment
The term sonication means the use of ultrasonic acoustic waves where there is the need to disintegrate cells, homogenise, emulsify, degas, and disperse products in the biotechnological and chemical sector. This technique, has only been tested in laboratory studies to date, involves the use of ultrasound waves on small quantities of sample. It can be carried out with a power ranging from 0 to 400 w up to 20 to 30 kHz [1]. For example, Zerrouki et al., (2021) [39] used ultrasound as a potential technique for the solubilisation of organic material, using fruit juice effluent in an anaerobic batch reactor in their study. Its effectiveness was evaluated at a low frequency of 20 kHz and at different sonication times (20, 40 and 60 min). Compared to the control, the amount of biogas produced increased by 47.57 and 60% for sonication times of 20, 40 and 60 min, respectively. The methane content of the biogas produced was approximately 59% for the control and 64% for the 60 min sonication. Oleszek et al., 2021 [40] investigated the effects of ultrasound on the biomass of high-protein microalgal species in relation to their suitability for biogas production. The effects of ultrasound were assessed by Fourier transform infrared (FT-IR) spectroscopy of the biomass and analysis of the neutral detergent fibre (NDF) fraction isolated from the biomass. The results indicate a negative effect on the methane fermentation due to the aggregation of the proteins.
3.5. Biological Pretreatment
Biological one is the most promising and the most economical technology for the production of biogas, and is also environmentally friendly [33]. It is a method in which microorganisms are involved, and it is used to break down cross-linked structures in substrates with enzymes. Its main is the degradation of materials in a simple way using microbes, enzymes and fungi. The general advantage of the biological technique over chemical or thermal pretreatment is that it can be carried out at low temperatures without the use of energy and chemicals. A disadvantage is that it can be slower than non-organic methods [1].
4. Biomethane Potential (BMP) Tests and Discontinuous AD of Organic Wastes and Agrifood By-Products
The key parameter for the evaluation of substrates to be used in anaerobic digestion plants is the biogas and biomethane production potential. This indicates the maximum volume of biogas that can be obtained from a given amount of substrate. An accurate evaluation of the biogas potential allows accurate mass balances and process performance analyses for existing or proposed full-scale plants. However, biogas yield, i.e., the amount of gas recovered under technical conditions in a given biogas plant, depends on many factors or variables, such as the kinetics of the degradation process, the rheology and degradation process, the rheology and mixing properties of the digestate, the presence of inhibiting substances and the potential deficiency of nutrients or trace elements. In addition to these factors, pretreatment technologies and technical limitations resulting from disturbing materials or layering the various components can have a significant impact on the specific process performance. Batch testing is a method that helps assess the biogas or methane potential of a given substrate [22]. The batch fermentation test consists in subjecting various substrates to anaerobic digestion in a closed environment and in a discontinuous manner. This type of test can be conducted with all types of substrates and presents the possibility of providing representative information regarding:
- ▪
- Evaluation of the biogas yield and anaerobic biological degradability of a material or a mixture of materials;
- ▪
- Assessment of the anaerobic degradation rate of the substrate under examination;
- ▪
- Evaluation of the possible inhibitory effect of the process due to critical concentrations of certain substances, such as polyphenols and essential oils, which have bactericidal action, therefore compromising bacterial consortium activity and AD performances.
Fermentation tests provide no information regarding the process stability of continuous reactors fed with investigated materials or material mixtures. The practical biogas yield must be gauged considering any potential synergistic effects that may be negative or positive. The capacity for substrate mono-fermentability is assessed during the process conditions, and the ceiling for organic loading rates per unit volume is determined. The outcome of a fermentation test relies mainly on the microbiological activity of the inoculum, which is affected by environmental factors, including temperature, substrate availability and the effectiveness of the biologically active mass. Additionally, the measurement of biogas production must be executed meticulously to ensure accuracy. This means that to achieve comparable results in fermentation tests, it is necessary to define gas data acquisition and evaluation as precisely as possible, in addition creating a fermentation batch [16].
5. Continuous Anaerobic Digestion Process
A continuous process is defined as a continuously or semi-continuously fed system wherein the average residence time of the substrate in the reactor is expressed by the hydraulic residence time (HRT) and that of the bacterial consortium. Depending on the technology adopted, processes can be single-phase or two-phase. In single-phase processes, the biological steps of digestion, hydrolysis/acidogenesis/acetogenesis and methanogenesis take place in the same reactor and simultaneously. In two-phase processes, on the other hand, there are two separate reactors placed in series with each other, each being dedicated to a series of reactions: in the first reactor, hydrolysis/acidogenesis and acetogenesis take place, while in the second reactor, the methanogenic phase develops. This makes it possible to associate the residence time in the reactor with the different kinetics of the microbial strains linked to the two different phases of the digestion process [20].
The reactor management parameters are as follows.
5.1. Average Time of Hydraulic Retention (HRT)
The average hydraulic residence time (HRT) is defined as the ratio of the volume of the reactor considered and the feed rate to the reactor and represents the residence time of each fluid element inside a reactor [26]:
where
- HRT: average time of hydraulic residence (days);
- V: volume of the reactor (m3);
- Q: flow rate to the reactor (m3 per day).
5.2. Average Residence Time of Sludge (SRT)
The average residence time of the sludge inside the reactor is given by the ratio between the total mass of volatile solids present in the reactor and the flow rate of solids extracted from the reactor. If the amount of biomass produced by cell growth is equal to the amount extracted from the reactor, the concentration of active biomass inside remains constant over time, and we will speak of steady-state conditions.
We will then have
where
- SRT: average residence time of the sludge (days);
- V: reactor volume (m3);
- X: concentration of volatile solids inside the reactor (kgVS/m3);
- W: flow rate of volatile substance extracted from the reactor (kgVS/day).
5.3. Volumetric Organic Load (OLR)
The volumetric organic load of substrate applied to the reactor is defined as the amount of substrate entering the reactor, referring to the volume unit of the reactor itself and to time.
Analytically,
where
- OLR: volumetric organic load factor in terms of substrate, referring to the reactor volume (kgsubstrate/m3/day);
- Q: influencing flow (m3/day);
- S: substrate concentration in the influencing flow rate kg/m3;
- V: reactor volume (m3).
This parameter is usually calculated on the basis of the useful volume of the reactor and can be used to refer to different units of measurement to express the biomass concentration (TS, TVS, COD) [41].
5.4. Organic Load Referring to Biomass or Volatile Solids in the Reactor (CF)
This is defined as the amount of substrate entering the reactor and refers to the amount of volatile substance present in the reactor in the unit of time.
That is,
where
- CF: organic load factor in terms of substrate (referring to biomass or volatile solids in the reactor and expressed in kgsubstrate/kgVS/day);
- Q: influencing flow (m3/day);
- S: substrate concentration in the influencing flow (kgVS/m3);
- V: reactor volume (m3);
- X: concentration of volatile solids inside the reactor (kgVS/m3).
5.5. Specific Gas Production (SGP)
This parameter represents the quantity of biogas that is produced per quantity of volatile substance fed to the reactor; it is therefore expressed in terms of m3biogas/kgfeed substrate. This parameter, which is widely used to define the yield of anaerobic digestion processes, is closely related to the biodegradability of the treated substrate rather than to the characteristics of the process used. From an analytical point of view, it is expressed as the ratio
where
- SGP: specific production of biogas (m3biogas/kgfeed substrate);
- Qbiogas: flow rate of biogas produced (m3/day);
- Q: influencing flow (m3/day);
- S: substrate concentration in the influencing flow rate (kgsubstrate/m3).
5.6. Biogas Production Speed (GPR)
This is defined as the flow of biogas produced with respect to the reactor volume and time:
where
- GPR: biogas production rate (m3biogas/m3 reactor/day);
- Qbiogas: flow rate of biogas produced (m3/day);
- V: reactor volume (m3).
5.7. Substrate Removal Efficiency
There are different ways of expressing the substrate removal efficiency during the anaerobic digestion process, which are largely but not exclusively related to the different parameters used to express its concentration (total solid substance, volatile solid substance, COD or BOD). In general, the simplest relationship for the conversion of the substrate into biogas is expressed in percentage terms as follows:
where
- ɳ: percentage of TVSs removed (%);
- Q: influent and effluent flow (m3/day);
- S: TVS concentration in the influencing flow (kg/m3);
- Se: TVS concentration in effluent flow calculated as the difference between the incoming mass and the biogas produced (easier flows quantification) (kg/m3).
In the case of the removal of the volatile substance, referring to the percentage of volatile substance that characterises the influent and effluent of the reactor, the following expression is also suggested [20]:
where
- VSin: percentage of the volatile fraction in the influent (%);
- VSout: percentage of the volatile fraction in the effluent (%).
6. Various Types of Continuous Reactors for Anaerobic Digestion
There are different types of plants that work continuously. Some of the most common types of plants are described below.
6.1. Continuous Stirred Tank Reactor (CSTR)
In this type of reactor, the concentration of the substrate, products and biomass in the effluent is the same as that in the reactor, the content of which is assumed to be homogeneous. This type of process is generally used for the stabilisation of sludge produced in purification plants or for wet or semi-dry processes for the digestion of organic waste.
6.2. Process in a Continuous Reactor with Recirculation
Recirculation is generally used to improve the efficiency of biomass stabilisation processes. The recirculation of part of the effluent after a separation operation allows part of the active biomass extracted with the effluent to be reintroduced into the reactor, thus guaranteeing higher concentrations of the biomass inside the reactor and a residence time of the solids (microorganisms) different from the hydraulic one. This is generally achieved by separating the liquid fraction from the solid one and recirculating the latter inside the reactor. Excess sludge can be purged from the recirculation flow or directly from the reactor [42].
6.3. Continuous Process with Separate Phases
As already reported, the optimal growth conditions for hydrolytic/acidifying bacteria and for methanogenic bacteria are different; therefore, the separation of the phases that lead to methanogenesis appears to be an ideal solution to increase the yields of the two processes. The overall process scheme includes a first phase, hydrolysis and acidification, which takes place in smaller reactors, since retention times can be low (even a few hours), followed by a second phase, in reactors of larger dimensions, in which methanogenesis occurs. This allows the residence time in the reactor to be associated with the different kinetics of the microbial strains connected to the two different phases of the digestion process [16,20].
7. Anaerobic Digestion of Food Waste and Agrifood By-Products
Different matrices can be used in the anaerobic digestion process for methane production, with a more or less abundant yield of biogas. The most commonly used ones come from all solid and liquid manure from livestock activities, such as cattle, pig, sheep and poultry manure [24,43]. They also come from agricultural activities such as silage of various vegetable origin, green manure, pruning residues or from dedicated energy crops. Waste from all these agro-industrial activities is understood as food waste (FW), organic waste from the slaughtering of livestock from meat processing, organic waste from agrifood sector (whey, vegetable waste, yeasts, fats of animal and vegetable origin, wastewater from distilleries), waste from catering activities (restaurants, canteens, etc.) [44] and that produced from household waste and from the organic fraction of municipal solid waste or from material collected under the separate collection of organic waste [24]. Several authors have investigated the potential of using the above-mentioned matrices to produce biogas and biomethane under different conditions, with different yields. Indeed, the physicochemical features of the used matrices, operating parameters and availability of the matrices themselves, which are related to seasonability or consuming habits, strongly affect the progress and the outcomes of the process. An overview of the scientific investigations conducted on the anaerobic digestion of different matrices related to the agrifood sector is reported below.
7.1. Anaerobic Digestion and Co-Digestion of Food Waste
Li et al. 2015 [42] tested the digestion process of vinegar residue using a continuous stirred tank reactor (CSTR). They tested the influence of organic loading rate (OLR) and effluent recirculation on the AD performance of the vinegar residue. Five OLRs were selected, 1.0, 1.5, 2.0, 2.5 and 3.0 gVS L−1 d−1. The highest volumetric methane productivity of 581.88 mLCH4 L−1 was achieved with an OLR of 2.5 gVS L−1 d−1. Palatsi et al., (2011) [45] carried out anaerobic digestion of fresh waste from the slaughter of pigs and cattle, evaluating different ratios between lipids and proteins using a batch test. The resultant methane potentials were high (270–300 LCH4·kgCOD−1). Kafle et al., 2012 [46] used waste from the seafood processing industry for biogas production. The evaluated mixtures were concocted by blending fish remnants with bread remains and brewery barley waste. Their potential to produce methane was assessed. The biogas and methane yield for fish waste silages after 96 days was calculated to be 671–763 mL/gVS and 441–482 mL/gVS, respectively. Meng et al., (2015) [47] tested the effect of different concentrations of FO waste (5, 20, 30, 40 and 50 g/L) on the biomethane produced using batches containing mixtures of floating oil (FO) extracted from food (FW). FO and FO + FW were mono-digested and co-digested. The results showed that FO and FO + FW could be effectively anaerobically converted to biomethane using appropriate loads. For the single digestion of FO, the biomethane yield, TS and VS reduction were 607.7–846.9 mL/g, 69.7–89% and 84.5–92.8% respectively. However, anaerobic digestion appeared to be unstable when the FO concentration was 50 g/L. Maximum FO loads of 40 g/L and 30 g/L were therefore suggested for efficient mono-digestions and co-digestions of FO and FO + FW. Zhang et al., (2014) [48] evaluated the anaerobic co-digestion of food waste and bovine manure in order to define the key parameters that determine the best yield in terms of biogas and methane. The results of both batch and semi-continuous tests indicated that total methane production improved in co-digestion, with an optimal ratio of food waste (FM) to cattle manure (CM) of 2. With this ratio, a total production of methane in the batch corresponding to 388 mL/gVS was obtained. Meanwhile, in the semi-continuous mode, the total production of methane in co-digestion was 317 mL/gVS. Zhang et al., (2012) [49] examined and tested the feasibility of improving biogas production and the stability of the anaerobic single-digestion process for food waste (containing the main meat, rice and vegetables) through co-digestion using fresh leachate from a urban solid waste (USW) incineration plant with the aim of identifying the key factors that regulate the performance and stability of anaerobic digestion. For this purpose, a series of semi-continuous experiments were carried out. During their tests, anaerobic co-digestion with fresh leachate showed much better performance and stability in terms of exhibiting high yields of CH4 (375.9–506.3 mL/gVSadded), no VFA inhibition and a stable pH (7.2–7.8). Yong et al., 2015 [50] tested the BMP using food waste and straw in mesophilic conditions. Laboratory-scale blends were used with different ratios of FW to straw with an OLR of 5 gVS/L. The methane production yield (MPY) reached 0.392 m3/kgVS with an optimal mixing ratio of FW to straw of 5:1. Li et al., (2017) [51] evaluated methane yield based on the assessment of 12 types of food waste, considering a substrate/inoculum ratio of 1:2 on a volatile base. Experimental data and model simulation results suggested that higher methane production (530–548 mL/gVS) and volatile solid removal efficiencies (65.0–67.8%) can be obtained when the percentage of lipids is between 77.8 and 78.2% and that of proteins is between 54.7 and 58.2%. Meanwhile, a shorter digestion retention time could occur if the carbohydrate content is higher than 47.6%, the protein content is less than 24.1% and the lipid content is less than 28.3%. Park et al., (2012) [52] used the biomass residue of algae in co-digestion with fat, oil and fat waste (FOG) rich in lipids to evaluate the effect on methane yield. The co-digestion of the algae biomass residue and FOG produced 0.54 LCH4/gVS/day with a volumetric productivity of the reactor of 1.62 LCH4/day. Lipids contributed significantly to methane production, accounting for 68–83% of the total methane potential. Xu and Li, (2012) [53] investigated the feasibility of solid-state anaerobic digestion (SS-AD) of expired dog food and stewed maize for biogas production. The substrate was tested at three different inoculum-to-substrate ratios (S/I), 2, 4 and 6, using sludge digester effluent as an inoculum. The most favourable methane yield obtained was 304.4 L/kgVSfeed, which was achieved using the substrate consisting of 50% corn stover and 50% dog food. Kazimierowicz et al., 2021 [54] conducted a study on a laboratory scale using food waste products under mesophilic (37 °C) and thermophilic (55 °C) conditions. The maximum biogas yield was obtained in the mesophilic digestion of the substrate mixture containing 50% meat, 40% dairy and 10% fruit and vegetables. It was 740.4 ± 19.9 mLCH4/gVS biogas with 68.6 ± 1.8% methane. The effects of the substrate-to-inoculum ratio (S/I), the alkalinity sources (sodium bicarbonate and oyster shells) and the mixing ratio of inoculum to food waste were studied by Lee et al., (2019) [55]. The digester with an S/I =1, using a mixture of crushed oyster shells and sodium bicarbonate as a buffer, had the highest methane yield (183 mLCH4/gVS). The same authors reported that the addition of waste-activated sludge to food and catering waste mitigated acidification (pH 6.86 ± 0.12) during the start-up period and improved digester stability. Blends with FW/YW/WAS = 0.8:1.7:0.5 had higher methane yields (134 ± 15 mLCH4/gVS) than blends with FW/YW/WAS = 1:1:1. The aim of the study conducted by Rattanapan et al., (2019) [56] was to test biogas production from the co-digestion of food waste (FW) and domestic wastewater under mesophilic (35 ± 1 °C) and thermophilic (55 ± 1 °C) conditions. The highest biomethane potential, 0.78 mL CH4·mgVS−1, was obtained with a food waste to domestic wastewater ratio of 10:90 w/v at mesophilic temperatures. Tixeira et al., (2021) [57] used domestic waste coffee grounds (DSCGs) that came from the infusion of coffee and industrial waste coffee grounds (ISCGs) co-digested with food waste (FW). The reactors were fed with SCGs in the proportions of 0%, 25%, 50%, 75% and 100% dry weight, using a substrate/inoculum ratio of 1. BMP tests were performed for 45 days at mesophilic temperatures (35 ± 2 °C). BMP levels were highest with 25% DSCG (0.345 Nm3CH4/kgVS), 25% ISCG (0.351 Nm3CH4/kgVS) and 75% DSCG (0.301 Nm3CH4/kgVS) samples. On the other hand, the 75% ISCG sample had a low percentage of BMP (0.188 Nm3CH4/kgVS), due to the release of inhibitory compounds as the percentage of added SCGs increased. Megido et al., (2021) [58] tested the anaerobic digestion, in thermophilic conditions (55 °C), of blends of food waste (FW) from supermarket scraps. The tested matrices were bakery products, butchery waste, cooked meats and cheeses, fish waste, fruit and vegetables. In addition to the different mixtures, different types of digesters were tested, including laboratory-scale induced bed reactors (IBRs) and fully stirred tank reactors (CSTRs), at different organic loading rates (OLRs), i.e., 3.0, 3.6 and 4.6 kg of volatile solids (VSs) per m3 of reactor a day. Regardless of the type of reactor used, an OLR of 3.6 kgVS/m3/day was optimal, achieving up to 48.1% more methane production per kg of waste treated compared to the other OLRs tested. Overall, there were no statistically significant differences (p-value < 0.05) between IBR and CSTR performance at the same OLR. However, at the optimal OLR, the IBR achieved an average methane production of 1.5 LCH4/Lreactor/day (426.7 LCH4/kgVS) and the highest VS removal (89.0% in average). This reactor obtained 22.1% more CH4 than the CSTR and the highest biogas methane content (66.9% CH4).
7.2. The Anaerobic Digestion and Co-Digestion of Agrifood By-Products
Beyond food waste, several authors focused on the recovery of agrifood by-products for the production of biogas and biomethane. Indeed, Vitez et al., (2020) [9] investigated the possibility of using waste corn kernels, peas, crushed corn kernels, green beans, mixed vegetables (broccoli, cauliflower, peas and carrots), corn leaves and corn husk as co-substrates for anaerobic management. They conducted digestion tests using batch reactors (5 dm3) for 21 days at 42 °C in thermophilic conditions. During this period, the quantity and quality of the biogas produced were monitored. Biogas production after 21 days of hydraulic retention time ranged from 0.6773 m3/kg of organic dry matter (peas) to 1.1108 m3/kg of organic dry matter (mixed vegetables). All substrates had a final biogas methane concentration between 59.43 and 65.97% vol. The production of biogas from crushed maize grain was greater than that produced from substrates with a similar nutrient composition (maize grain). Lin et al., 2011 [59] investigated the biomethane potential of fruit and vegetable waste (FVW) and food waste (FW). Individual anaerobic digestion tests were conducted at the organic loading rate (OLR) of 3 kgVS/(m3·day) using a laboratory scale CSRT reactor at 35 °C. The optimal mixing ratio was 1:1 for the co-digestion and the methane production yield was 0.49 m3CH4·kgvs and the optimal chemical soluble oxygen demand (sCOD) removal efficiencies of volatile solids were 74.9% and 96.1%, respectively. Shen et al., (2013) [60] carried out a similar mix with varying organic load ratios (OLRs) in single-phase and two-phase systems, respectively. Their results demonstrate that single-phase digestion is more effective than two-phase digestion, with a 4.1% increase in CH4 production at lower OLRs being achieved (<2.0 gVS·L−1·day−1). However, at a higher OLR level (P2.0 gVS·L−1·day−1), two-step digestion achieved a higher CH4 production of 0.351–0.455 L·gVS·L−1·day−1, which was 7.0–15.8% greater than that of the single-phase digestion. Moreover, the two-step digestion demonstrated more stable functioning and a greater OLR processing capacity. Furthermore, bioenergy recovery revealed that the two-phase system presented a higher bioenergy yield overall than the single-phase one. Benalia et al., (2021) [61] evaluated the production of biogas and biomethane from olive mill wastewater by testing blends containing 0% (control), 20% and 30% v/v olive mill wastewater (OMWW) in a reactor under mesophilic conditions. Their research highlighted the production of greater quantities of biogas (80.22± 24.49 NL·kg·VS−1) and methane (47.68 ± 17.55 NL·kg·VS−1) using 30% v/v OMWW.
Zema et al., (2018) [62] evaluated methane production through anaerobic digestion, in mesophilic conditions, of industrial orange peels using a pilot plant (84 L) with semi-continuous feeding at an increasing organic loading rate (OLR) and content of essential oil (EO) until the inhibition process was complete. The highest daily specific methane yield was achieved at an OLR of 1.0 gVS·L−1 and EO of 47.6 mg·L−1·d−1. Beniche et al., (2017) [63] proposed mixing food waste (FW) with leaves and stems of cabbage and cauliflower (CCF) at different carbon/nitrogen (C/N) ratios. Excellent results were obtained during the study with a C/N ratio = 45. The methane yield was 475 mLCH4/gVS, with an organic loading rate (OLR) of 0.06 kg of VS/m3·h for the CCF and FW mixture (CCF + FW).
7.3. Anaerobic Digestion of Food Waste and Agrifood By-Products Undergoing Pretreatment and Novelties in the Experimental Field
In this section, studies on the pretreatment of substrates used in anaerobic digestion and new plant innovations are discussed. Chaurasia et al., (2021) [64] investigated the effect of some pretreatments on fruit, food and vegetable wastes. The effect of alkaline, hydrothermal, thermal and ultrasonic pretreatment of fruit, food and vegetable waste (FFVW) on anaerobic co-digestion (AcoD) was tested for the reduction in total solids (TSs), volatile solids (VSs) and biogas/methane production. The mesophilic anaerobic co-digestion of FFVW pretreated with cattle manure was carried out in a 1 L batch digester on a laboratory scale for 30 days at a temperature of 40 ± 2 °C. A reduction of 16.89% TSs and 19.44% VSs was observed during the ultrasonic pretreatment, while 106.81 mL of biogas/gVS and 29.92 mL of CH4/gVS were generated. In addition, alkaline pretreatment showed a significant improvement in biogas production, but was less economical. El Gnaoui et al., (2019) [65] used food waste (FW) as a substrate for anaerobic digestion by subjecting it to heat pretreatment (HPT) of variable duration. This study investigated the effects of HPT on the physicochemical properties and the improvement in methane yield (MY). As a function of temperature and treatment time, HPT reduced the percentage of VSs compared to the raw FW. In addition, anaerobic digestion (AD) of pretreated FW was tested at 100 °C for 30 min, and the MY was 382.82 mL STPCH4/gVS, 23.68% higher than that of untreated food waste. Sun et al., (2024) [66] examined eight process variables in an agricultural biogas plant, including biomass type, reactor/feeding, volatile solids, pH, organic load rate, hydraulic retention time, temperature and reactor volume; artificial neural networks (ANNs) were used to analyse biogas production rate. Variables were selected using the cuckoo optimisation algorithm (COA), the multiverse optimisation algorithm (MVO), the alloy sampling algorithm (LCA), the evaporation water cycle algorithm (ERWCA), stochastic fractal search (SFS) and learning-based optimisation (TLBO). These models are based on bio-inspired algorithms and demonstrate promising outcomes in forecasting biogas production results. Beltramo et al., (2019) [67] used ANN technology to predict the production rate considering 15 process variables. Concentration of volatile fatty acids, TS, TVS, acid detergent fibre, acid detergent lignin, neutral detergent fibre, ammonium nitrogen, HRT, OLR were measured. They used different algorithms (ant colony optimisation and genetic algorithms) to perform the variable selection. The best results they obtained were those where optimised ANN models with optimised ACO-GA were used. In this case, the prediction error was reduced to 6.24% and the R2 increased to 0.90. To develop and test a system for monitoring and controlling variables in the anaerobic digestion (AD) process, Cruz et al., (2019) [68] conducted batch tests under mesophilic conditions (37 °C) with a substrate/inoculum ratio of 1:2. Variables examined included pH and temperature in the liquid phase and pressure, temperature, methane yield and biogas volume in the gas phase. The variables examined included pH and temperature in the liquid phase and pressure, temperature, methane yield and volume of biogas in the gas phase. The developed system based on Arduino produced a cumulative average biogas concentration of 0.67 Lmethane, with a concentration of 51.46%. Meanwhile, Bernardi et al., (2017) [69] developed a prototype for the anaerobic digestion of olive mill wastewater (OMWW), with pH and temperature as variables. The medium-scale, fully automated prototype was equipped with a thermos-regulable heating cover. The digestion chamber was fed with acid and alkaline through two charging lines. Scarcello et al., (2023) [70] implemented control logic to manage the prototype described earlier. The control logic was implemented to keep the temperature and pH values within a certain range to ensure optimum process parameters. The intelligent automation system consists of three PLC units that manage sensors to collect temperature and pH data for process control and pressure and flow sensors to determine biogas production. A remote control interface was designed for manual or automatic control of the plant. The interface also allows process parameters to be set and process progress to be monitored.
Farhat et al., (2018) [71] examined thermal pretreatment of municipal sewage sludge (MSS) in combination with anaerobic co-digestion of olive processing wastewater (OPW) to improve the disintegration of complex organic matter and its bioconversion into biogas. The authors conducted an anaerobic co-digestion of pretreated municipal solid waste (PMSS) mixed with pressurised organic wastewater (OPW) and examined the effect of increasing the percentage of OPW on biomethane potential (BMP). The best results were obtained with mixtures of PMSS/OPW (80%/20% and 70%/30%), which were also tested in batch reactors. In conclusion, the thermal pretreatment of MSS and the addition of OPW significantly improved the methane yield (50–160%) and the stability of the waste. Farhat et al., (2018) [72] also tested the co-digestion of waste-activated sludge (WAS) combined with olive processing wastewater (OPW). Various WAS/OPW ratios were examined (100% WAS, 90% WAS/10% OPW, 80% WAS/20% OPW, 70% WAS/30% OPW, 60% WAS/40% OPW and 50% WAS/50% OPW) in sequential anaerobic batch reactors (ASBRs). Optimal results were achieved with ratios of (WAS/OPW) 90%/10% and 80%/20%. Zema et al. (2018) [73] conducted experimental batch tests under mesophilic and thermophilic conditions on the anaerobic digestion of olive mill wastewater (OMWW) mixed with other agro-industrial by-products. Tests were conducted to determine the potential biogas production and sensitivity of the process to inhibitory compounds. The mixtures contained varying percentages of OMWW, digested manure and citrus peels. The results presented showed that mixtures containing MSW percentages above 20% (v/v) had low methane yields due to having higher concentrations of polyphenols (PPs) and/or volatile fatty acids (concentrations above 0.8 g kg−1 and 2.4 g L−1, respectively). The addition of other substrates, such as citrus peels, in other tests may have induced synergistic PP and essential oil (EO) inhibition effects on microbial consortium growth. Furthermore, their study revealed that thermophilic processes were more sensitive to these inhibitory compounds than mesophilic ones. Tufaner, (2020) [74] tested the use of primary anaerobic digestion and the Fenton process to treat olive processing wastewater (OPW). The tests were conducted on a laboratory scale using a rising flow anaerobic reactor (UASB) under mesophilic conditions (36.5–37 °C) with an organic loading rate (OLR) of 1 kg COD m3d−1 and an HRT of 10 days. During the experiment, a COD removal of 76.8% was achieved. The effluent from the anaerobic treatment was further treated via the Fenton treatment process, using Fe2+ and H2O2. Fenton treatment achieved a COD and colour removal of 91% and 96%, respectively, under optimised conditions. To remove 1 g of COD from anaerobically treated wastewater, 19 mg of Fe2+ and 250 mg/L H2O2 were required. Thanks to the Fenton process, approximately 98% of the COD of diluted raw sewage could be successfully treated at a ratio of 1:8.
8. Conclusions
It can be said that anaerobic digestion can be considered a highly reliable process for the recovery of huge amounts of wasted food and agrifood by-products along the production and marketing chain for the production of clean and green energy. Indeed, this biological process makes it possible to address current environmental and ecological challenges through the sustainable use of food waste instead of conventional disposal methods. Moreover, it provides high-value-added products, mainly biogas and biomethane, to produce heat and energy needed in food processing, and digestate that could be used as organic fertiliser or soil conditioner for agronomic purposes. Also, if the applied technology is further developed, research and scientific advances could significantly increase the quantities of biogas that can be “extracted” from these substrates, though there are still many challenges to overcome, ranging from defining the best bacterial consortium to sourcing raw materials close to “home”. Pretreatment, for example, can be an excellent opportunity for process optimisation, reducing the residence time in anaerobic reactors and significantly increasing the production of biomethane. However, as Pramanik et al., (2019) [2] report, this technology should be improved for pilot-scale systems, as it is often limited to the laboratory scale. Depending on whether the system used is chemical, thermal, ultrasonic or biological, it also needs to be optimised to reduce the inhibiting compounds and thus the energy consumed, or to increase the hydrolysis rate. Finally, the agrifood sector is strongly encouraged to consider anaerobic digestion of food waste and by-products in the production process to further improve its sustainability in a circular economy context.
Author Contributions
Conceptualisation, A.N., S.B. and B.B.; methodology, A.N., S.B. and B.B.; validation, A.N., S.B., B.B. and G.Z.; investigation, A.N.; resources, B.B. and G.Z.; writing—original draft preparation, A.N. and S.B.; writing—review and editing, A.N., S.B. and B.B.; visualisation, A.N., S.B. and B.B.; supervision, S.B., B.B. and G.Z.; project administration, B.B. and G.Z.; funding acquisition, B.B. and G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of University and Research (MUR) within the framework of the PhD programme in Agricultural, Food and Forestry Sciences (XXXVI cycle) activated at the Mediterranean University of Reggio Calabria and by “MEC—Marketplace Ecosostenibile Calabria” (CUP B31B20000520005), funded under the “Intelligent Factory, Agrifood and Life Sciences” call of the Ministry of Economic Development (MISE).
Data Availability Statement
Not acceptable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- FAOSTAT. Emissions Due to Agriculture. Global, Regional and Country Trends 1990–2018; Analytical Brief 18; FAO: Rome, Italy, 2020; ISSN 2709-0078. [Google Scholar]
- Thompson, E.; Wang, Q.; Li, M. Anaerobic digester systems (ADS) for multiple dairy farms: A GIS analysis for optimal site selection. Energy Policy 2013, 61, 114–124. [Google Scholar] [CrossRef]
- Omar, R.; Harun, R.M.; Ghazi, T.I.M.; Ghazi, W.A.K.; Ab Karim Ghani, W.A.W.; bin Idris, R. Anaerobic treatment of cattle manure for biogas production. In Proceedings of the 2008 AIChE Annual Meeting, Philadelphia, PA, USA, 16–21 November 2008; pp. 1–10. [Google Scholar]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M. Anaerobic digestion of animal waste: Effect of mode of mixing. Water Res. 2005, 39, 3597–3606. [Google Scholar] [CrossRef]
- Drosg, B. Process Monitoring in Biogas Plants; Technical Brochure, no. January; IEA Bioenergy: Paris, France, 2013. [Google Scholar]
- Wu, D.; Li, L.; Zhao, X.; Peng, Y.; Yang, P.; Peng, X. Anaerobic digestion: A review on process monitoring. Renew. Sustain. Energy Rev. 2019, 103, 1–12. [Google Scholar] [CrossRef]
- Vitez, T.; Dokulilova, T.; Vitezova, M.; Elbl, J.; Kintl, A.; Kynicky, J.; Hladky, J.; Brtnicky, M. The Digestion of Waste from Vegetables and Maize Processing. Waste Biomass Valorization 2020, 11, 2467–2473. [Google Scholar] [CrossRef]
- Khanh Nguyen, V.; Kumar Chaudhary, D.; Hari Dahal, R.; Hoang Trinh, N.; Kim, J.; Chang, S.W.; Hong, Y.; Duc La, D.; Nguyen, X.C.; Hao Ngo, H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Pellera, F.-M.; Gidarakos, E. Anaerobic digestion of solid agroindustrial waste in semi-continuous mode: Evaluation of mono-digestion and co-digestion systems. Waste Manag. 2017, 68, 103–119. [Google Scholar] [CrossRef]
- Sun, C.; Cao, W.; Banks, C.J.; Heaven, S.; Liu, R. Biogas production from undiluted chicken manure and maize silage: A study of ammonia inhibition in high solids anaerobic digestion. Bioresour. Technol. 2016, 218, 1215–1223. [Google Scholar] [CrossRef]
- Harris, P.W.; McCabe, B.K. Review of pre-treatments used in anaerobic digestion and their potential application in high-fat cattle slaughterhouse wastewater. Appl. Energy 2015, 155, 560–575. [Google Scholar] [CrossRef]
- Christy, P.M.; Gopinath, L.; Divya, D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew. Sustain. Energy Rev. 2014, 34, 167–173. [Google Scholar] [CrossRef]
- Adekunle, K.F.; Okolie, J.A. A Review of Biochemical Process of Anaerobic Digestion. Adv. Biosci. Biotechnol. 2015, 6, 205–212. [Google Scholar] [CrossRef]
- VDI 4630 INGENIEURE. In Fermentation of Organic Materials: Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests; Verlag des Vereins Deutscher Ingenieure: Düsseldorf, Germany, 2006.
- Gerardi, M.H. Nitrification and Denitrification in the Activated Sludge Process; Wastewater Microbiology SERIES; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Staley, B.F.; Reyes, F.L.d.L.; Barlaz, M.A. Effect of spatial differences in microbial activity, pH, and substrate levels on methanogenesis initiation in refuse. Appl. Environ. Microbiol. 2011, 77, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Dubey, B.K. A critical review on operating parameters and strategies to improve the biogas yield from anaerobic digestion of organic fraction of municipal solid waste. Renew. Energy 2019, 143, 779–797. [Google Scholar] [CrossRef]
- Cecchi, F.; Battistoni, P.; Pavan, P.; Bolzanella, D.; Innocenti, L. Digestione Anaerobica Della Frazione Organica dei Rifiuti Solidi; APAT: Rome, Italy, 2005; p. 178. [Google Scholar]
- Adani, F.; Schievano, A.; D’Imporzano, G. I Fattori che Rendono Ottimale la Razione per il Digestore; L’Informatore Agrario: Verona, Italy, 2008; Volume 40, pp. 19–24. [Google Scholar]
- Weinrich, S.; Schäfer, F.; Bochmann, G.; Liebetrau, J. Value of Batch Tests for Biogas Potential Analysis; IEA Bioenergy: Paris, France, 2018; p. 10. [Google Scholar]
- Chen, Y.; Jay, J.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Abouelenien, F.; Namba, Y.; Kosseva, M.R.; Nishio, N.; Nakashimada, Y. Enhancement of methane production from co-digestion of chicken manure with agricultural wastes. Bioresour. Technol. 2014, 159, 80–87. [Google Scholar] [CrossRef]
- Fricke, K.; Santen, H.; Wallmann, R.; Hüttner, A.; Dichtl, N. Operating problems in anaerobic digestion plants resulting from nitrogen in MSW. Waste Manag. 2007, 27, 30–43. [Google Scholar] [CrossRef]
- Drosg, B.; Fuchs, W.; Al Seadi, T.; Madsen, M.; Linke, B. Nutrient Recovery by Biogas Digestate Processing; Technical Brochure; IEA Bioenergy: Paris, France, 2020. [Google Scholar]
- Marchaim, U.; Krause, C. Propionic to acetic acid ratios in overloaded anaerobic digestion. Bioresour. Technol. 1993, 43, 195–203. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- de Zorzi, P.; Balzamo, S.; Barbizzi, S.; Calabretta, E.; Rosamilia, M.; Potalivo, S. Procedura di Misurazione per la Determinazione Della Richiesta Chimica di Ossigeno (COD) Mediante Test in Cuvetta: Metodo 5135; ISPRA: Rome, Italy, 2014.
- Gourdon, R.; Vermande, P. Effects of Propionic Acid Concentration on Anaerobic Digestion of Pig Manure. Biomass 1987, 13, 1–12. [Google Scholar] [CrossRef]
- Visser, A. The Anaerobic Treatment of Sulfate Containing Wastewater. Ph.D. Thesis, Wageningen Agricultural University, Wageningen, The Netherlands, 1995; p. 157. [Google Scholar]
- Speece, R.E. Anaerobic biotechnology for industrial wastewater treatment a description of several installations. Environ. Sci. Technol. 1983, 17, 416A–427A. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, P.; Ayyadurai, S.; Arunachalam, K.D.; Mishra, G.; Chen, W.-H.; Juan, J.C.; Naqvi, S.R. Current technologies of biochemical conversion of food waste into biogas production: A review. Fuel 2022, 323, 124321. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Mmabyalwa, S.; Edison, M.; Habtom, T. Effect of particle size on anaerobic digestion of different feedstocks. S. Afr. J. Chem. Eng. 2015, 20, 11–26. [Google Scholar]
- Dahunsi, S. Mechanical pretreatment of lignocelluloses for enhanced biogas production: Methane yield prediction from biomass structural components. Bioresour. Technol. 2019, 280, 18–26. [Google Scholar] [CrossRef]
- Montgomery, L.F.R.; Bochmann, G. Pretreatment of Feedstock for Enhanced Biogas Production; Technical Brochure; IEA Bioenergy: Paris, France, 2014. [Google Scholar]
- Qiao, Z.; Xu, S.; Zhang, W.; Shi, S.; Zhang, W.; Liu, H. Potassium ferrate pretreatment promotes short chain fatty acids yield and antibiotics reduction in acidogenic fermentation of sewage sludge. J. Environ. Sci. 2022, 120, 41–52. [Google Scholar] [CrossRef]
- Zerrouki, S.; Rihani, R.; Lekikot, K.; Ramdhane, I. Enhanced biogas production from anaerobic digestion of wastewater from the fruit juice industry by sonolysis: Experiments and modelling. Water Sci. Technol. 2021, 84, 644–655. [Google Scholar] [CrossRef]
- Oleszek, M.; Krzemińska, I. Biogas production from high-protein and rigid cell wall microalgal biomasses: Ultrasonication and FT-IR evaluation of pretreatment effects. Fuel 2021, 296, 120676. [Google Scholar] [CrossRef]
- FNR. Leitfaden Biogas. Available online: https://mediathek.fnr.de/leitfaden-biogas.html (accessed on 18 August 2023).
- Li, L.; Feng, L.; Zhang, R.; He, Y.; Wang, W.; Chen, C.; Liu, G. Anaerobic digestion performance of vinegar residue in continuously stirred tank reactor. Bioresour. Technol. 2015, 186, 338–342. [Google Scholar] [CrossRef]
- Folino, A.; Calabrò, P.S.; Zema, D.A. Effects of ammonia stripping and other physico-chemical pretreatments on anaerobic digestion of swine wastewater. Energies 2020, 13, 3413. [Google Scholar] [CrossRef]
- Jiang, J.; Li, L.; Cui, M.; Zhang, F.; Liu, Y.; Liu, Y.; Long, J.; Guo, Y. Anaerobic digestion of kitchen waste: The effects of source, concentration, and temperature. Biochem. Eng. J. 2018, 135, 91–97. [Google Scholar] [CrossRef]
- Palatsi, J.; Viñas, M.; Guivernau, M.; Fernandez, B.; Flotats, X. Anaerobic digestion of slaughterhouse waste: Main process limitations and microbial community interactions. Bioresour. Technol. 2011, 102, 2219–2227. [Google Scholar] [CrossRef] [PubMed]
- Kafle, G.K.; Kim, S.H.; Sung, K.I. Ensiling of fish industry waste for biogas production: A lab scale evaluation of biochemical methane potential (BMP) and kinetics. Bioresour. Technol. 2013, 127, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Li, S.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Chufo, A.; Jaffar, M.; Li, X. Evaluating biomethane production from anaerobic mono- and co-digestion of food waste and floatable oil (FO) skimmed from food waste. Bioresour. Technol. 2015, 185, 7–13. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Anaerobic co-digestion of food waste with MSW incineration plant fresh leachate: Process performance and synergistic effects. Chem. Eng. J. 2015, 259, 795–805. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Li, H.; Borrion, A.; Yu, Z.; Li, J. Kinetic studies on organic degradation and its impacts on improving methane production during anaerobic digestion of food waste. Appl. Energy 2018, 213, 136–147. [Google Scholar] [CrossRef]
- Park, S.; Li, Y. Evaluation of methane production and macronutrient degradation in the anaerobic co-digestion of algae biomass residue and lipid waste. Bioresour. Technol. 2012, 111, 42–48. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y. Solid-state co-digestion of expired dog food and corn stover for methane production. Bioresour. Technol. 2012, 118, 219–226. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of methane fermentation as a valorisation method for food waste products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Lee, E.; Bittencourt, P.; Casimir, L.; Jimenez, E.; Wang, M.; Zhang, Q.; Ergas, S.J. Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag. 2019, 95, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Rattanapan, C.; Sinchai, L.; Suksaroj, T.T.; Kantachote, D.; Ounsaneha, W. Biogas production by co-digestion of canteen food waste and domestic wastewater under organic loading rate and temperature optimization. Environments 2019, 6, 16. [Google Scholar] [CrossRef]
- Teixeira, R.A.; Bueno, B.A.; Borges, R.M.; Bringhenti, J.R. Biochemical Methane Potential of Spent Coffee Grounds via Co-digestion with Food Waste. BioEnergy Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Megido, L.; Negral, L.; Fernández-Nava, Y.; Suárez-Peña, B.; Ormaechea, P.; Díaz-Caneja, P.; Castrillón, L.; Marañón, E. Impact of organic loading rate and reactor design on thermophilic anaerobic digestion of mixed supermarket waste. Waste Manag. 2021, 123, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zuo, J.; Gan, L.; Li, P.; Liu, F.; Wang, K.; Chen, L.; Gan, H. Effects of mixture ratio on anaerobic co-digestion with fruit and vegetable waste and food waste of China. J. Environ. Sci. 2011, 23, 1403–1408. [Google Scholar] [CrossRef]
- Shen, F.; Yuan, H.; Pang, Y.; Chen, S.; Zhu, B.; Zou, D.; Liu, Y.; Ma, J.; Yu, L.; Li, X. Performances of anaerobic co-digestion of fruit & vegetable waste (FVW) and food waste (FW): Single-phase vs. two-phase. Bioresour. Technol. 2013, 144, 80–85. [Google Scholar] [CrossRef]
- Benalia, S.; Falcone, G.; Stillitano, T.; De Luca, A.I.; Strano, A.; Gulisano, G.; Zimbalatti, G.; Bernardi, B. Increasing the content of olive mill wastewater in biogas reactors for a sustainable recovery: Methane productivity and life cycle analyses of the process. Foods 2021, 10, 1029. [Google Scholar] [CrossRef]
- Zema, D.A.; Zappia, G.; Benalia, S.; Zimbalatti, G.; Perri, E.; Urso, E.; Tamburino, V.; Bernardi, B. Limiting factors for anaerobic digestion of olive mill wastewater blends under mesophilic and thermophilic conditions. J. Agric. Eng. 2018, 49, 130–137. [Google Scholar] [CrossRef]
- Beniche, I.; Hungría, J.; El Bari, H.; Siles, J.A.; Chica, A.F.; Martín, M.A. Effects of C/N ratio on anaerobic co-digestion of cabbage, cauliflower, and restaurant food waste. Biomass Convers. Biorefinery 2021, 11, 2133–2145. [Google Scholar] [CrossRef]
- Chaurasia, A.K.; Siwach, P.; Shankar, R.; Mondal, P. Effect of pre-treatment on mesophilic anaerobic co-digestion of fruit, food and vegetable waste. Clean Technol. Environ. Policy 2021, 25, 603–616. [Google Scholar] [CrossRef]
- El Gnaoui, Y.; Karouach, F.; Bakraoui, M.; Barz, M.; El Bari, H. Mesophilic anaerobic digestion of food waste: Effect of thermal pretreatment on improvement of anaerobic digestion process. Energy Rep. 2020, 6, 417–422. [Google Scholar] [CrossRef]
- Sun, Y.; Dai, H.-L.; Moayedi, H.; Le, B.N.; Adnan, R.M. Predicting steady-state biogas production from waste using advanced machine learning-metaheuristic approaches. Fuel 2023, 355, 129493. [Google Scholar] [CrossRef]
- Beltramo, T.; Klocke, M.; Hitzmann, B. Prediction of the biogas production using GA and ACO input features selection method for ANN model. Sci. Total Environ. 2019, 6, 349–356. [Google Scholar] [CrossRef]
- Cruz, I.A.; de Melo, L.; Leite, A.N.; Sátiro, J.V.M.; Andrade, L.R.S.; Torres, N.H.; Padilla, R.Y.C.; Bharagava, R.N.; Tavares, R.F.; Ferreira, L.F.R. A new approach using an open-source low cost system for monitoring and controlling biogas production from dairy wastewater. J. Clean. Prod. 2019, 241, 118284. [Google Scholar] [CrossRef]
- Bernardi, B.; Benalia, S.; Zema, D.; Tamburino, V.; Zimbalatti, G. An automated medium scale prototype for anaerobic co-digestion of olive mill wastewater. Inf. Process. Agric. 2017, 4, 316–320. [Google Scholar] [CrossRef]
- Scarcello, L.; Benalia, S.; Zimbalatti, G.; Fazari, A.; Bernardi, B. A Smart Automation System for the Management and Control of a Medium Scale Digester Plant. In AIIA 2022: Biosystems Engineering Towards the Green Deal: Improving the Resilience of Agriculture, Forestry and Food Systems in the Post-Covid Era; Springer: Berlin/Heidelberg, Germany, 2023; pp. 917–925. [Google Scholar] [CrossRef]
- Farhat, A.; Asses, N.; Ennouri, H.; Hamdi, M.; Bouallagui, H. Combined effects of thermal pretreat-ment and increasing organic loading by co-substrate addition for enhancing municipal sewage sludge anaerobic digestion and energy production. Process Saf. Environ. Prot. 2018, 119, 14–22. [Google Scholar] [CrossRef]
- Farhat, A.; Manai, I.; Gtari, M.; Bouallagui, H. Effect of enhancing nutrient balance in anaerobic digester feedstock by co-substrate addition on the microbial diversity and energy production from municipal sewage sludge. J. Biosci. Bioeng. 2018, 126, 497–506. [Google Scholar] [CrossRef]
- Zema, D.A.; Fòlino, A.; Zappia, G.; Calabrò, P.S.; Tamburino, V.; Zimbone, S.M. Anaerobic digestion of orange peel in a semi-continuous pilot plant: An environmentally sound way of citrus waste management in agro-ecosystems. Sci. Total Environ. 2018, 630, 401–408. [Google Scholar] [CrossRef]
- Tufaner, F. Evaluation of COD and color removals of effluents from UASB reactor treating olive oil mill wastewater by Fenton process. Sep. Sci. Technol. 2020, 55, 3455–3466. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).