New Renewable Hydrothermal Liquefaction (HTL) Biofuel: A Combustion and Emissions Study in an Optical Engine
Abstract
:1. Introduction
2. Materials and Methods
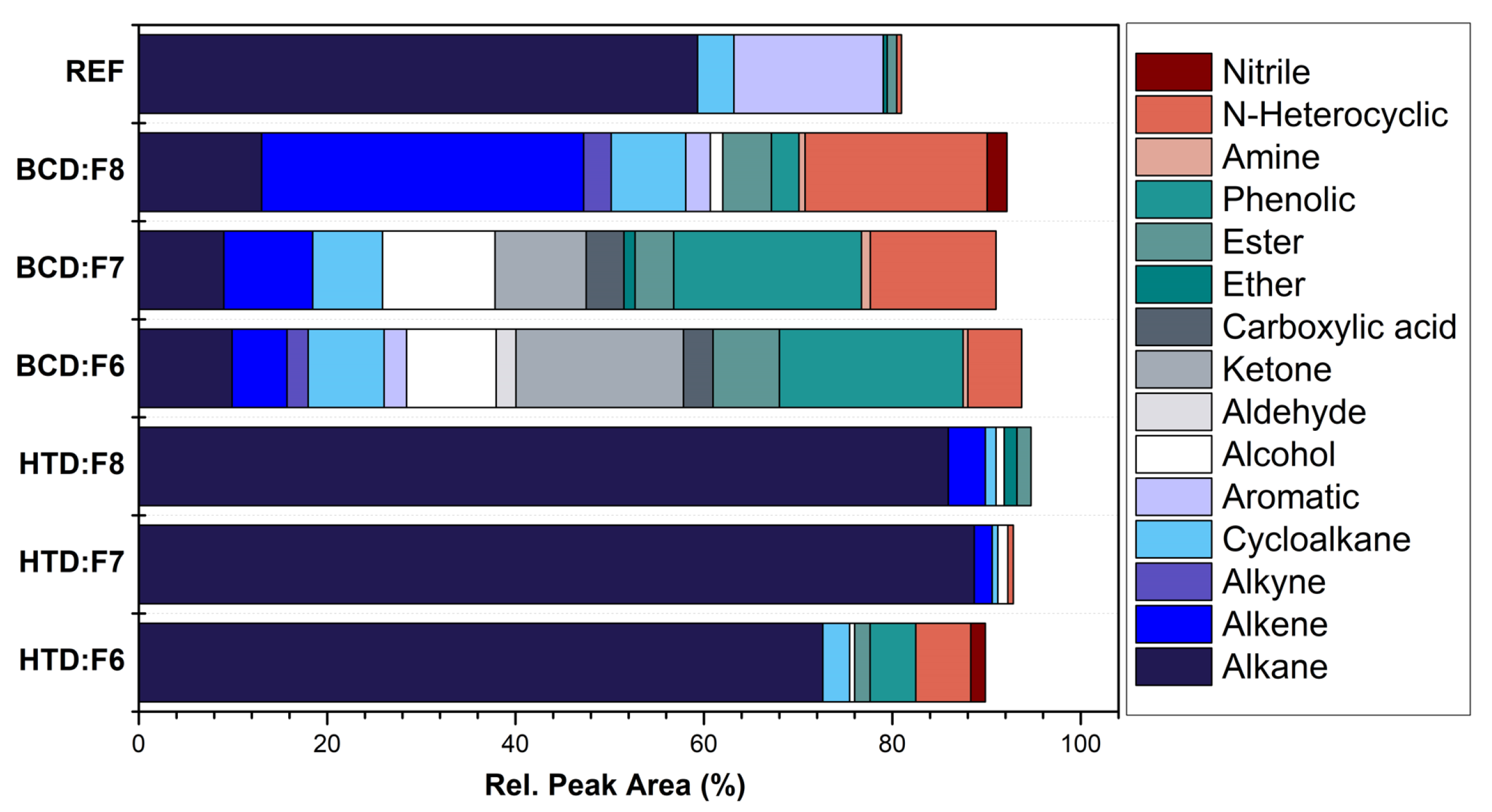
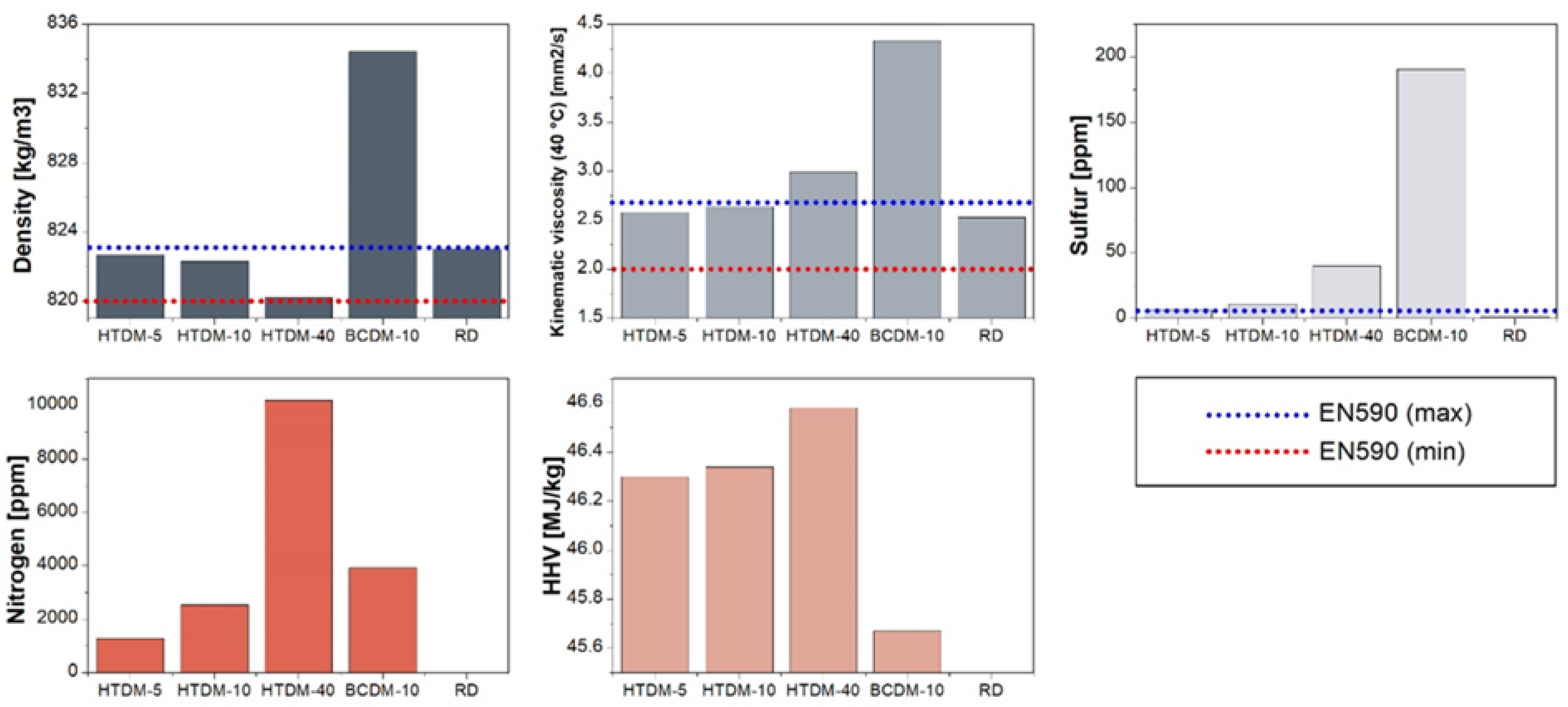
2.1. Fuel Production and Chemical Composition
2.2. Fuel Selection and Parameters
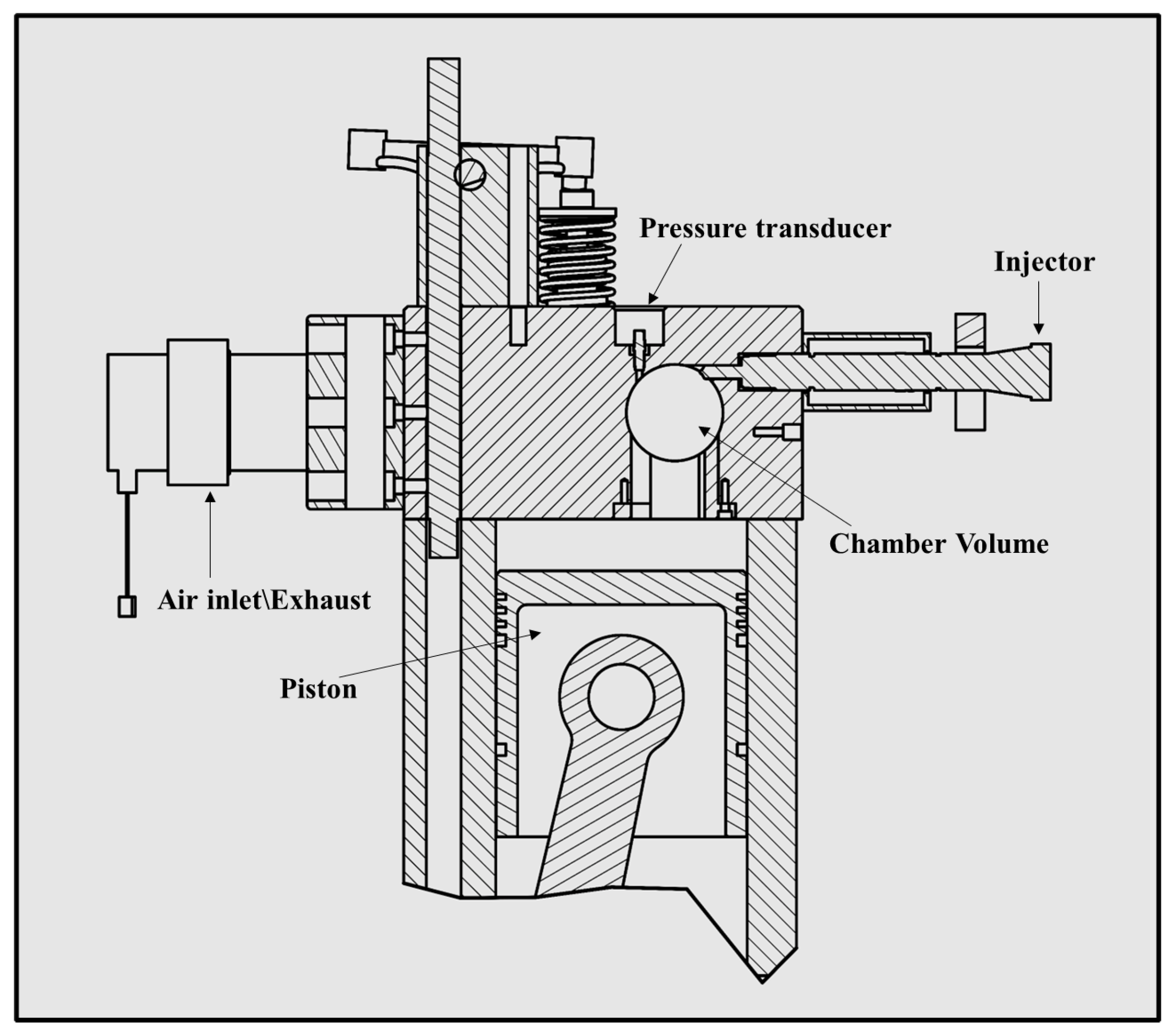
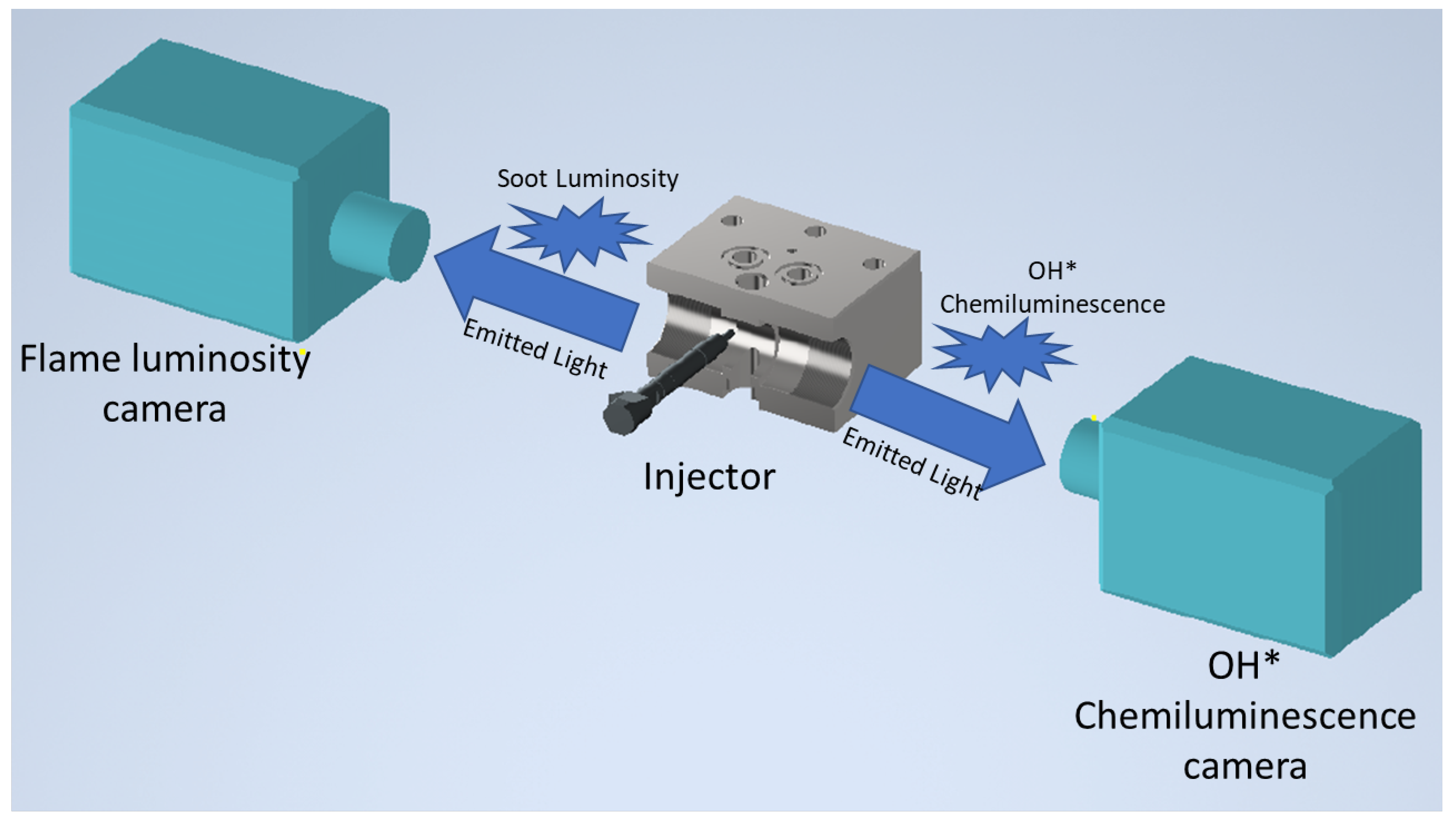
2.3. Experimental Setup
2.3.1. The Optical Accessible Compression Ignition Chamber (OACIC)
2.3.2. Optical Setup
3. Results and Discussion
3.1. Combustion Characteristics
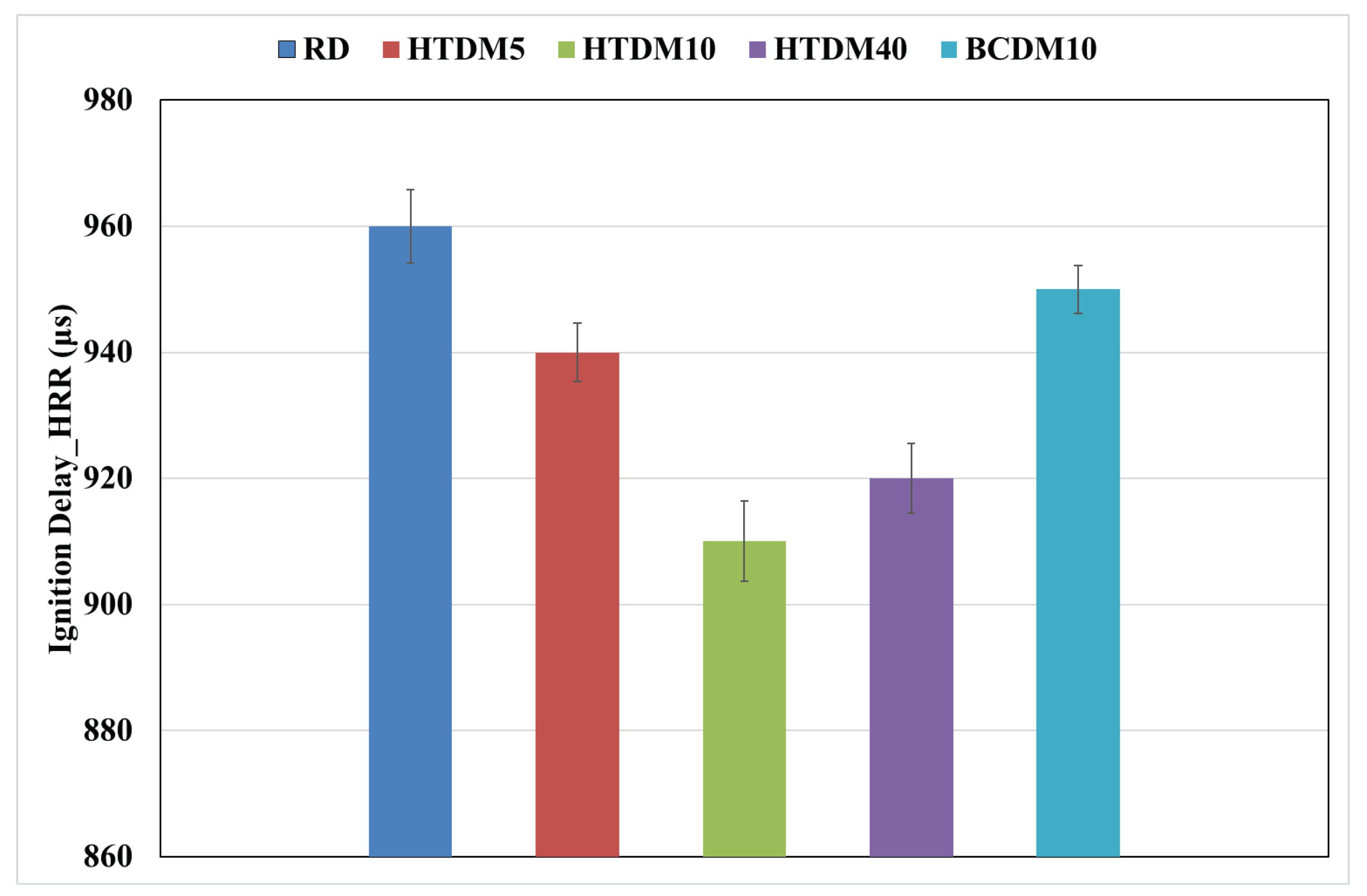
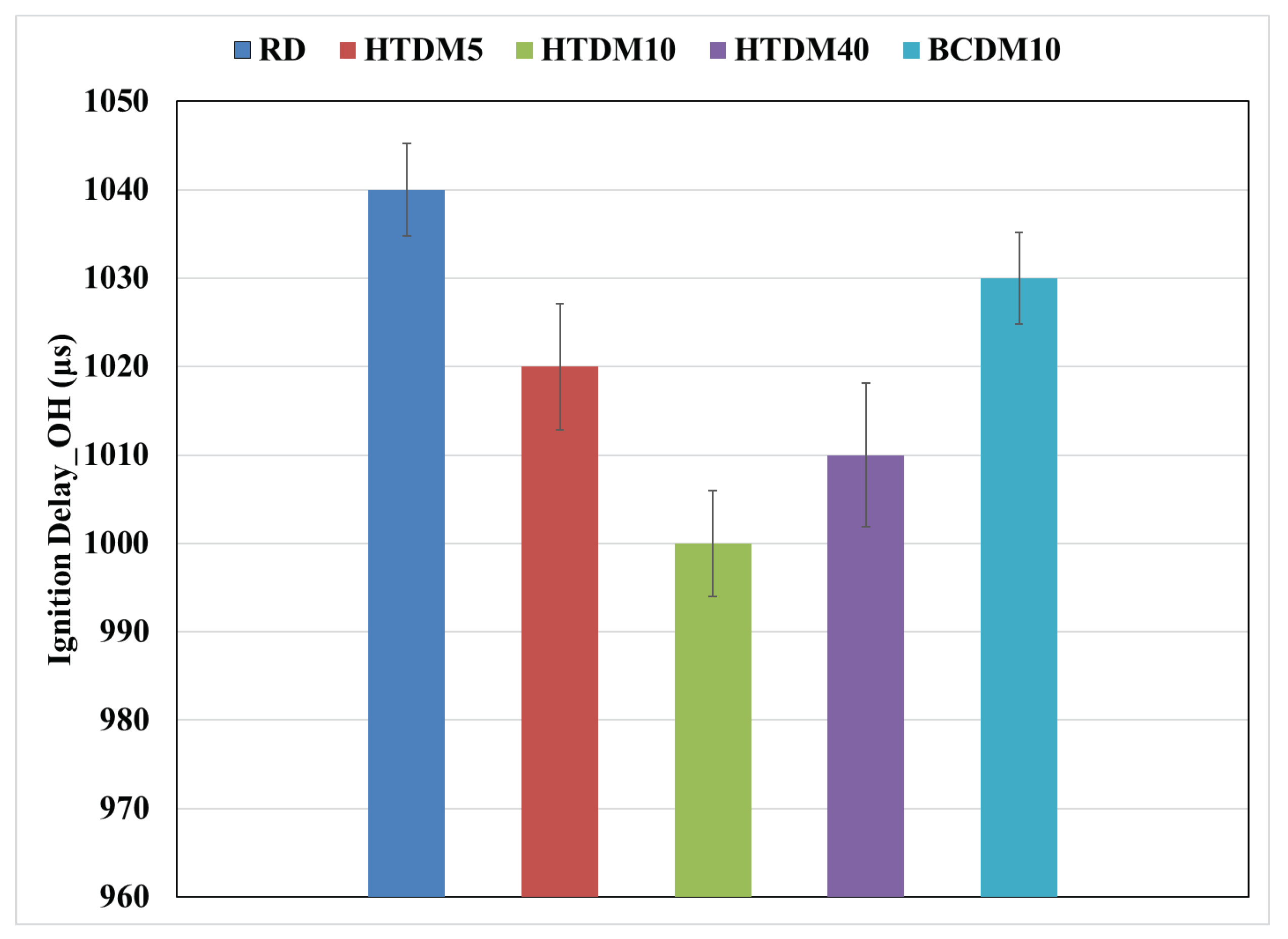
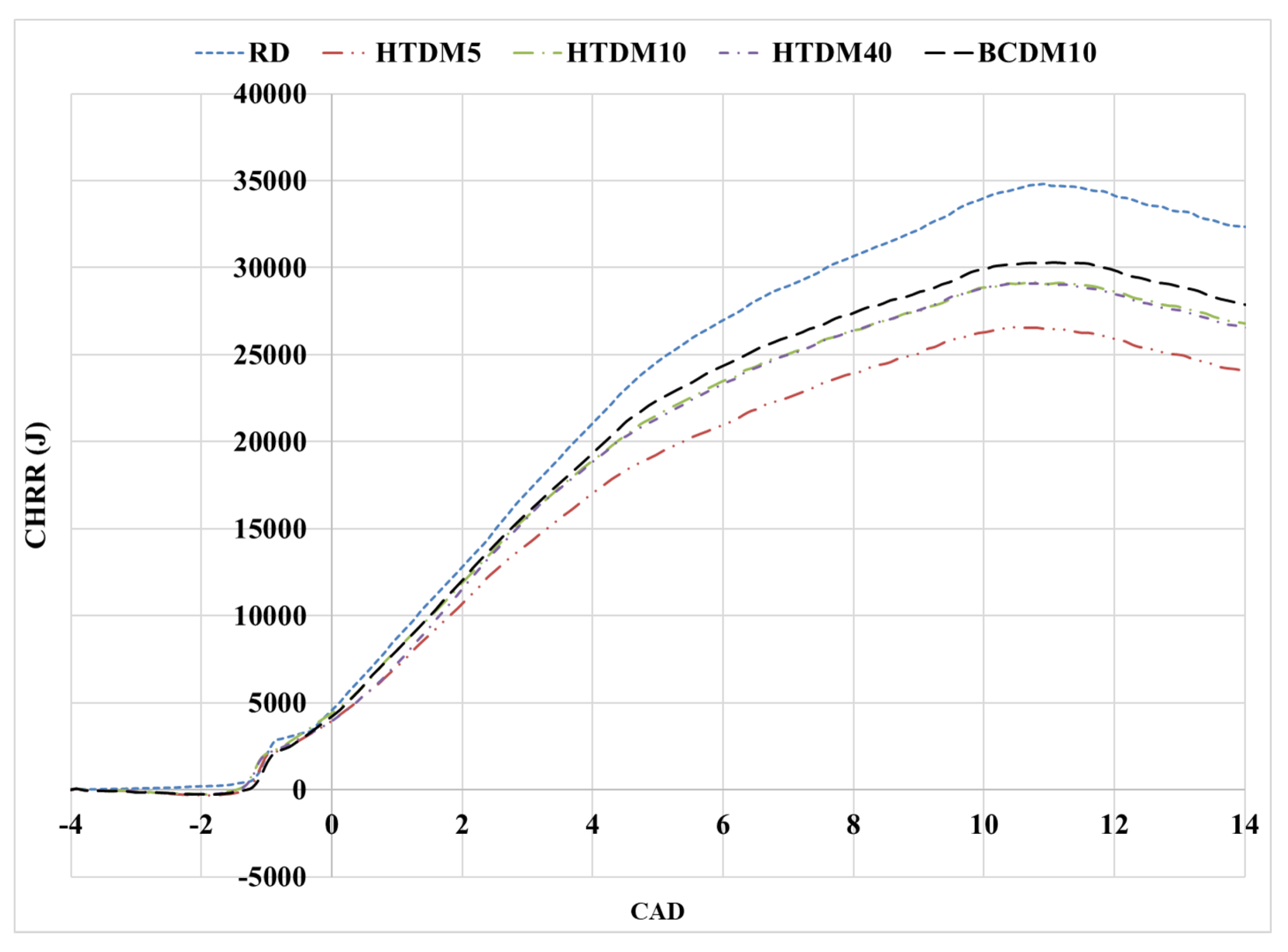
3.1.1. Heat Release Rate (HRR)
3.1.2. In-Cylinder Pressure
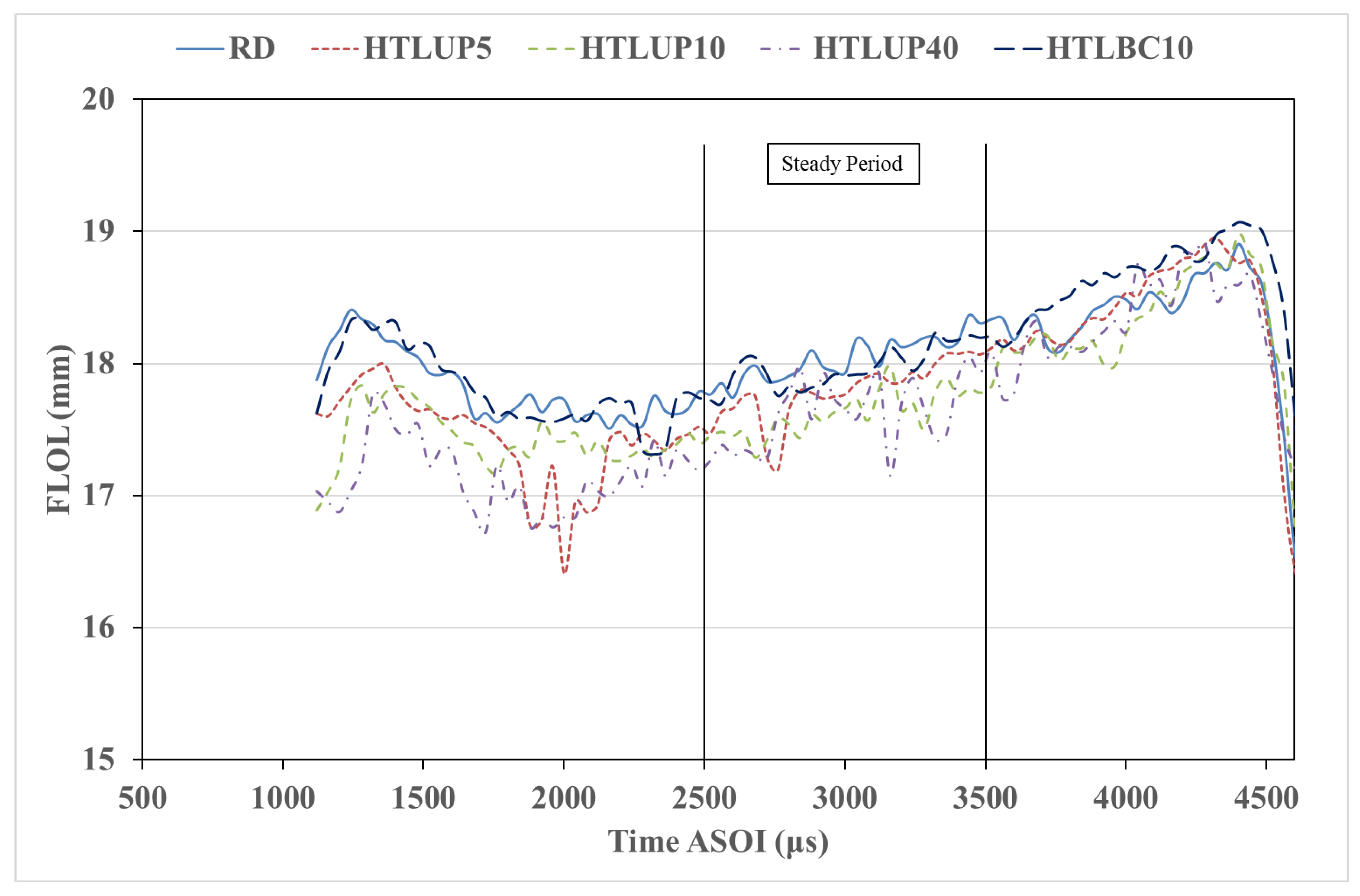
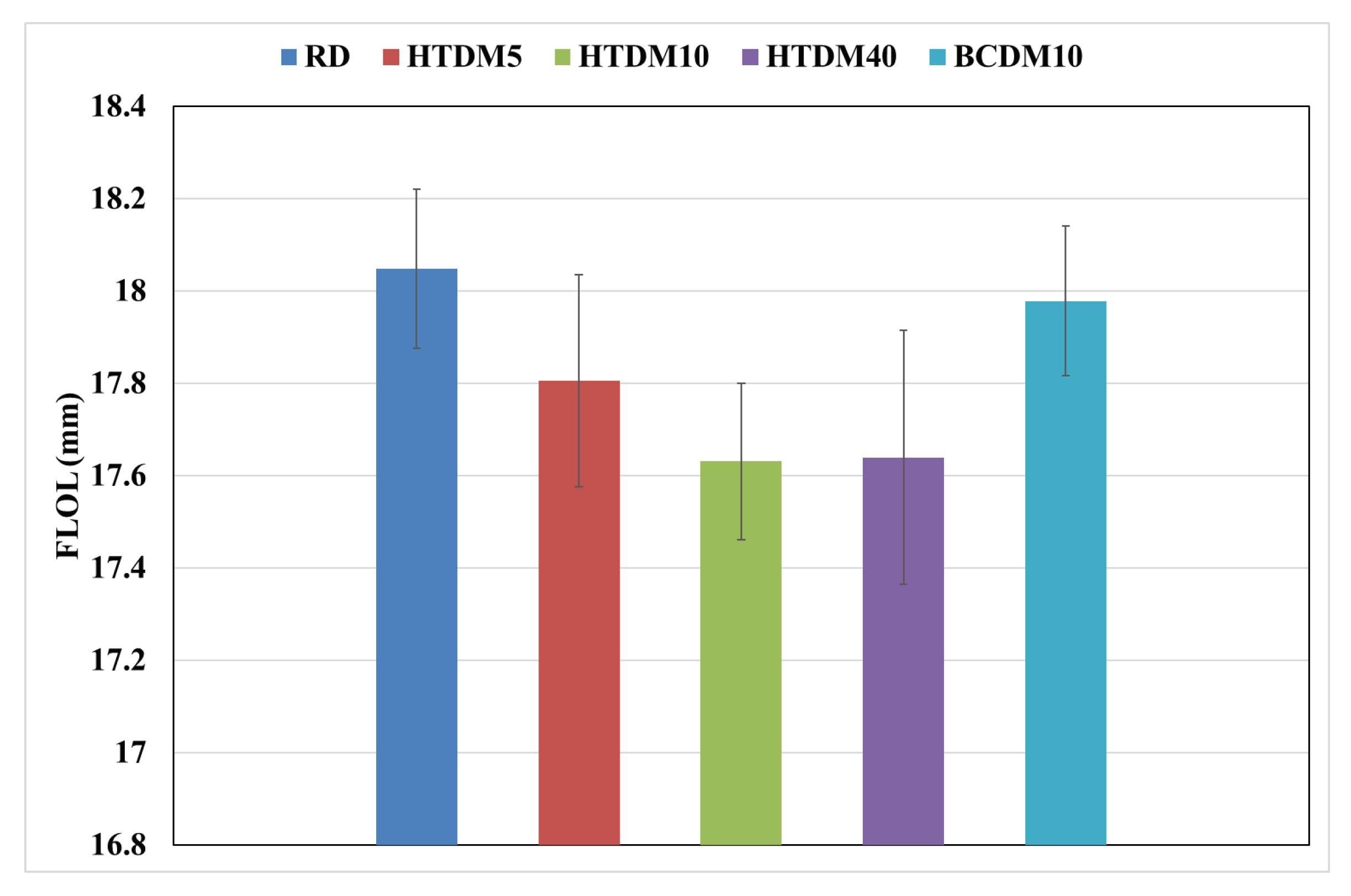
3.1.3. Flame Lift-Off Length (FLOL)
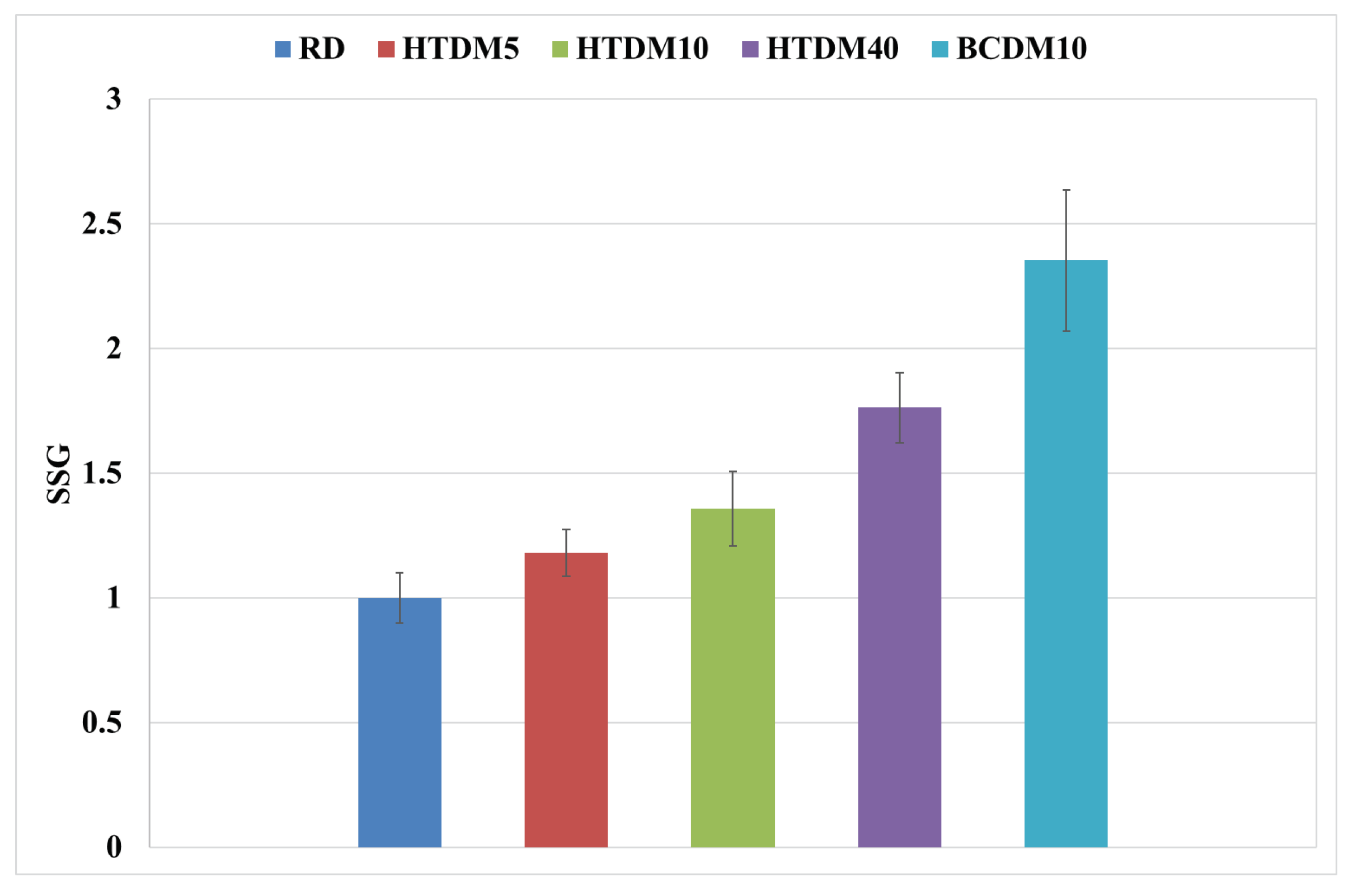
3.1.4. In-Flame Soot
3.2. Exhaust Emissions
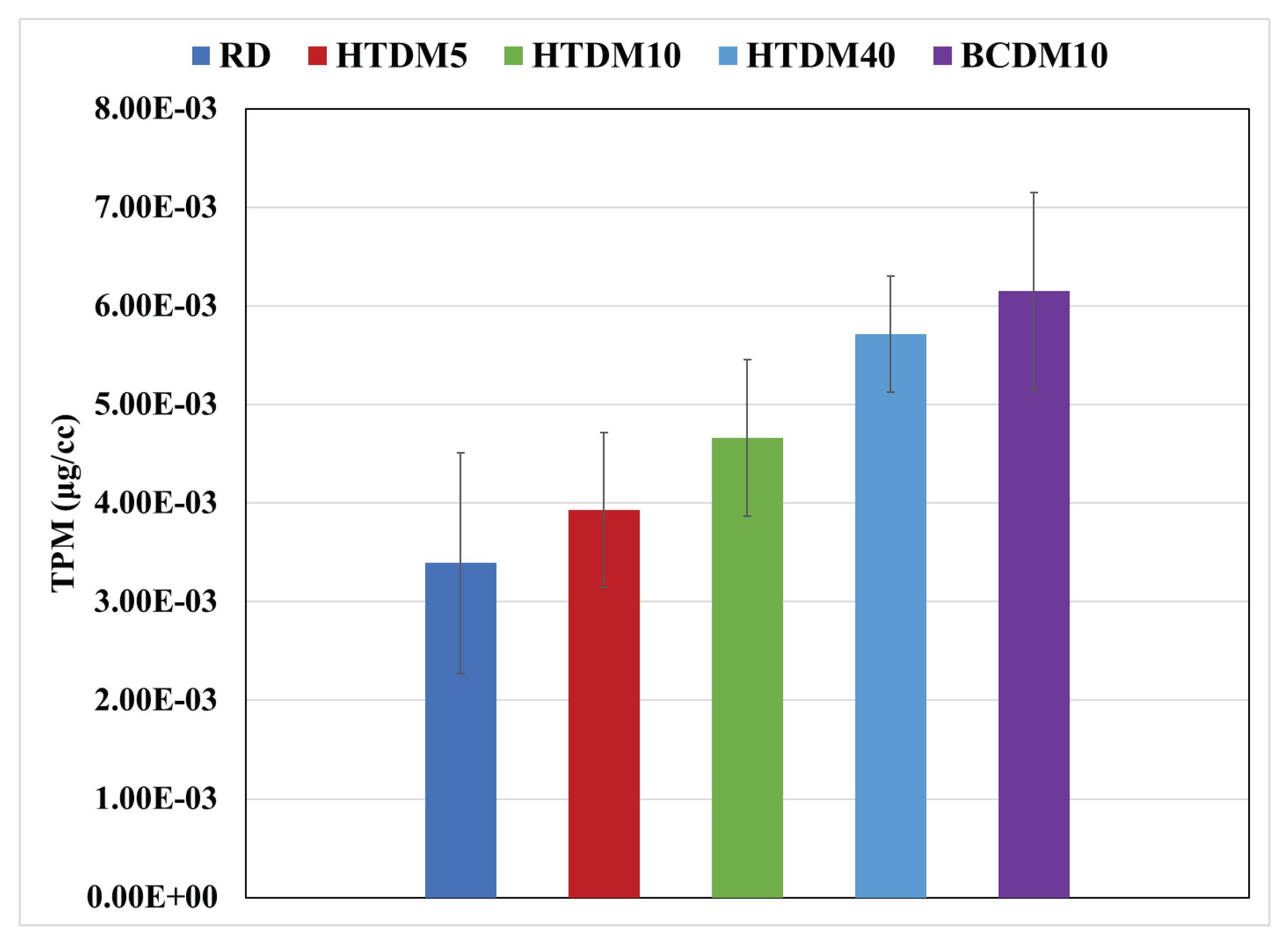
3.2.1. Particulate Matter (PM)
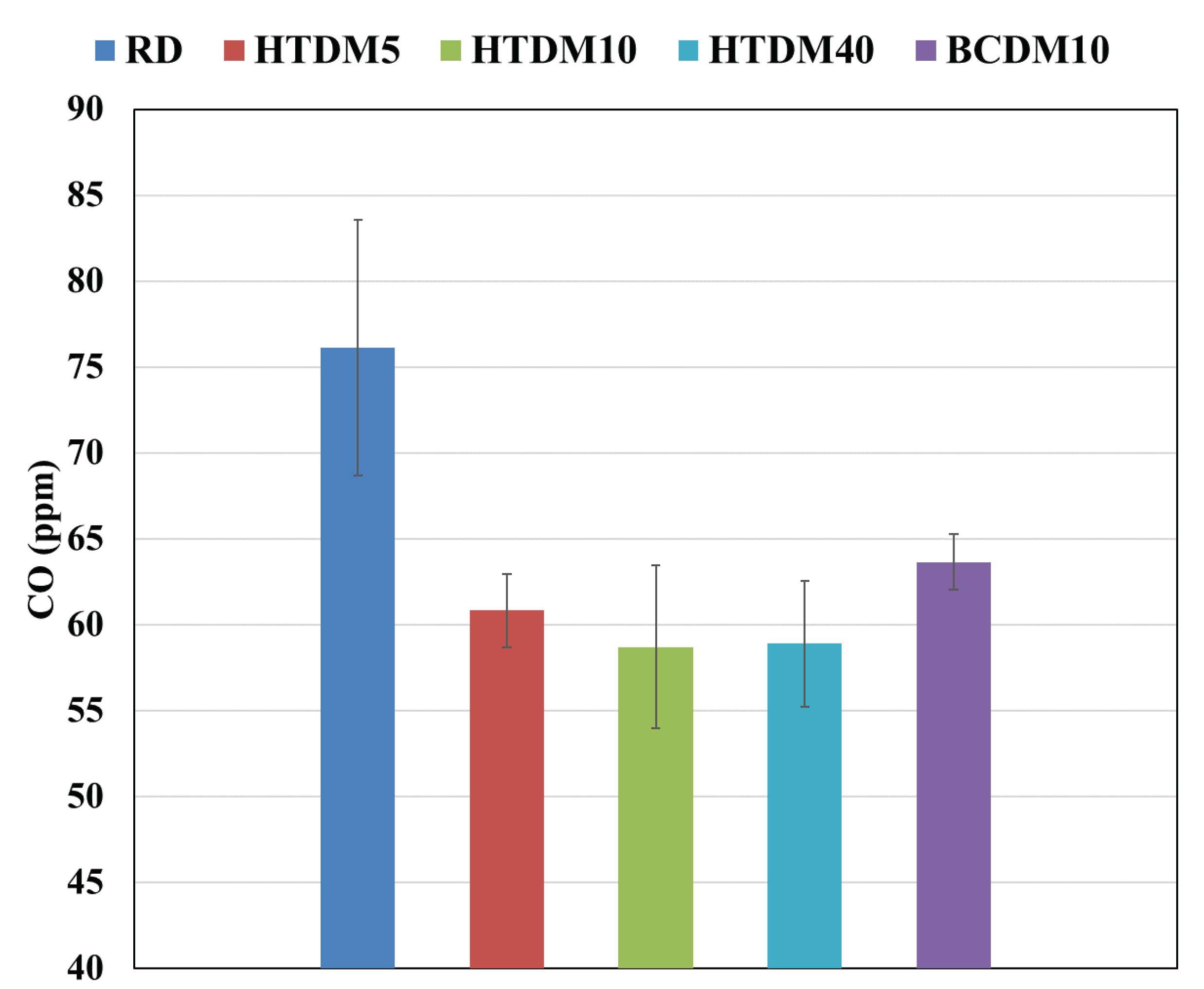
3.2.2. Carbon Monoxide (CO)
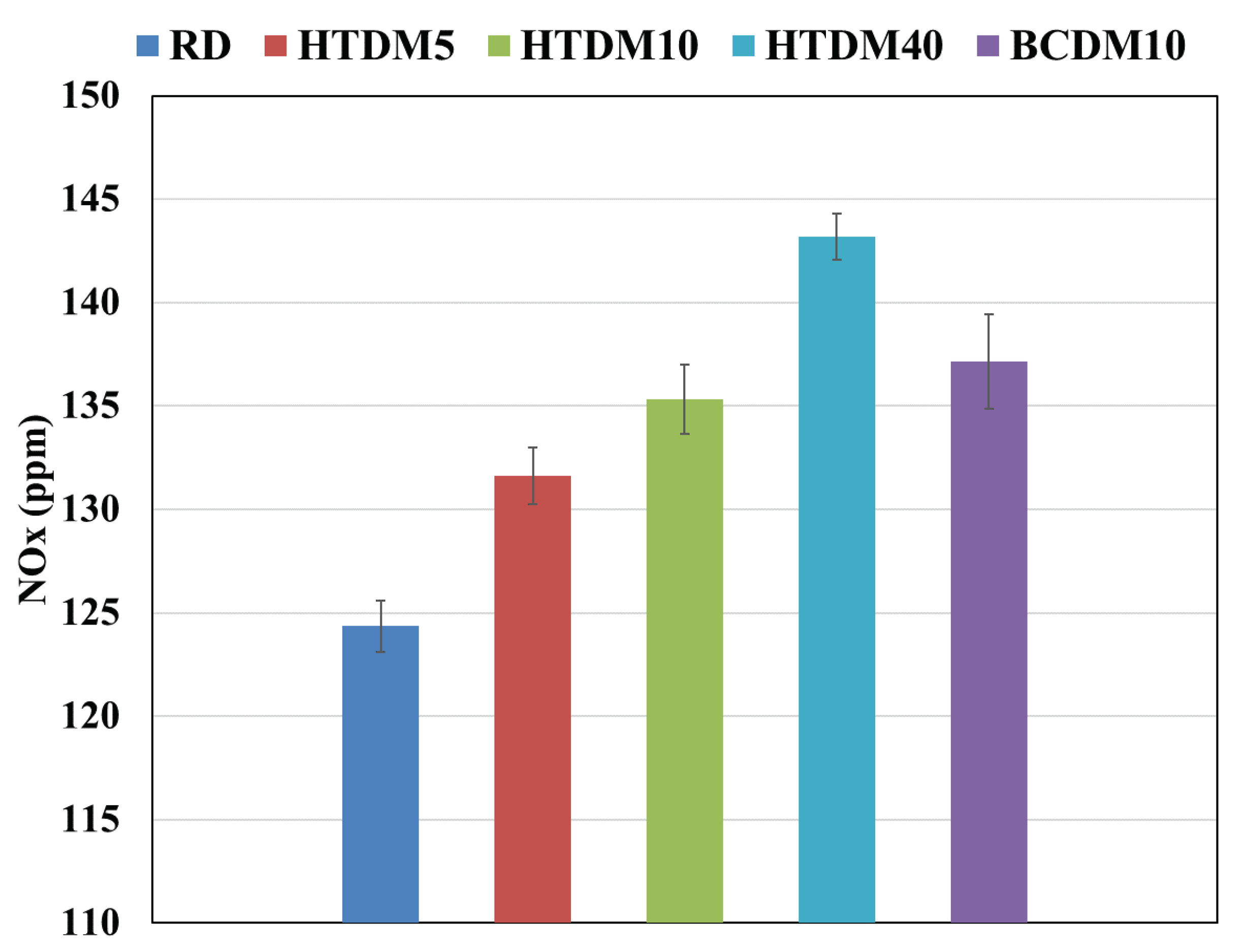
3.2.3. Nitrogen Oxides (NOx)
3.2.4. Carbon Dioxide Emissions (CO2)
4. Conclusions
- The ignition delay of the upgraded blends was shorter compared to the RD and nonupgraded blends but still within the range of engine optimization and operation control.
- The peak in-cylinder pressure is similar for all the tested fuels.
- The flame lift-off length was shorter in the case of the upgraded blends than that of the RD and nonupgraded blends. The nonupgraded blends had the longest flame lift-off length, which can influence combustion efficiency.
- The in-flame soot formation was higher for the upgraded blends compared to the RD and was less relative to the nonupgraded blend.
- The PM emissions in terms of total particulate mass (TPM) were higher in the case of the upgraded blends compared to the RD and were lower relative to the nonupgraded blend.
- The CO emissions were lower in the case of the upgraded blends compared to the RD and nonupgraded blends.
- The NOx emissions were found to be higher for the upgraded blends relative to the RD and lower compared to the nonupgraded blend.
- The CO2 emissions were found to be similar for all the tested fuels.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations Climate Change Conference UCC, Cop26 Glasgow Climate Pact. 2021. No. 28. Available online: https://unfccc.int/documents/310475 (accessed on 20 August 2023).
- Rehfeldt, M.; Worrell, E.; Eichhammer, W.; Fleiter, T. A review of the emission reduction potential of fuel switch towards biomass and electricity in European basic materials industry until 2030. Renew. Sustain. Energy Rev. 2020, 120, 109672. [Google Scholar] [CrossRef]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Ahmed, S.; Warne, T.; Smith, E.; Goemann, H.; Linse, G.; Greenwood, M.; Kedziora, J.; Sapp, M.; Kraner, D.; Roemer, K.; et al. Systematic review on effects of bioenergy from edible versus inedible feedstocks on food security. NPJ Sci. Food 2021, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Lark, T.J.; Hendricks, N.P.; Smith, A.; Pates, N.; Spawn-Lee, S.A.; Bougie, M.; Booth, E.G.; Kucharik, C.J.; Gibbs, H.K. Environmental outcomes of the US Renewable Fuel Standard. Proc. Natl. Acad. Sci. USA 2022, 119, e2101084119. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Su, X.; He, J.; Zhang, P.; Xu, H.; Zhou, C. Investigation on combustion characteristics of cyclopentanol/diesel fuel blends in an optical engine. Renew. Energy 2021, 167, 811–829. [Google Scholar] [CrossRef]
- Bleta, R.; Schiavo, B.; Corsaro, N.; Costa, P.; Giaconia, A.; Interrante, L.; Monflier, E.; Pipitone, G.; Ponchel, A.; Sau, S.; et al. Robust Mesoporous CoMo/γ-Al2O3 Catalysts from Cyclodextrin-Based Supramolecular Assemblies for Hydrothermal Processing of Microalgae: Effect of the Preparation Method. ACS Appl. Mater. Interfaces 2018, 10, 12562–12569. [Google Scholar] [CrossRef]
- Laredo, G.C.; Reza, J.; Meneses Ruiz, E. Hydrothermal liquefaction processes for plastics recycling: A review. Clean. Chem. Eng. 2023, 5, 100094. [Google Scholar] [CrossRef]
- Saengsuriwong, R.; Onsree, T.; Phromphithak, S.; Tippayawong, N. Biocrude oil production via hydrothermal liquefaction of food waste in a simplified high-throughput reactor. Bioresour. Technol. 2021, 341, 125750. [Google Scholar] [CrossRef]
- Kohansal, K.; Toor, S.; Sharma, K.; Chand, R.; Rosendahl, L.; Pedersen, T.H. Hydrothermal liquefaction of pre-treated municipal solid waste (biopulp) with recirculation of concentrated aqueous phase. Biomass Bioenergy 2021, 148, 106032. [Google Scholar] [CrossRef]
- He, P.; Chen, L.; Shao, L.; Zhang, H.; Lü, F. Municipal solid waste (MSW) landfill: A source of microplastics?—Evidence of microplastics in landfill leachate. Water Res. 2019, 159, 38–45. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Brown, R.J.; Rainey, T.J. A review of hydrothermal liquefaction bio-crude properties and prospects for upgrading to transportation fuels. Energies 2015, 8, 6765–6794. [Google Scholar] [CrossRef]
- Sandquist, J.; Tschentscher, R.; del Alamo Serrano, G. Hydrothermal liquefaction of organic resources in biotechnology: How does it work and what can be achieved? Appl. Microbiol. Biotechnol. 2019, 103, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Chen, W.T.; Zhang, Y.; Lee, T.H.; Wu, Z.; Si, B.; Lee, C.F.F.; Lin, A.; Sharma, B.K. Renewable diesel blendstocks produced by hydrothermal liquefaction of wet biowaste. Nat. Sustain. 2018, 1, 702–710. [Google Scholar] [CrossRef]
- Hossain, F.M.; Nabi, M.N.; Rainey, T.J.; Bodisco, T.; Rahman, M.M.; Suara, K.; Rahman, S.M.; Van, T.C.; Ristovski, Z.; Brown, R.J. Investigation of microalgae HTL fuel effects on diesel engine performance and exhaust emissions using surrogate fuels. Energy Convers. Manag. 2017, 152, 186–200. [Google Scholar] [CrossRef]
- Hadhoum, L.; Zohra Aklouche, F.; Loubar, K.; Tazerout, M. Experimental investigation of performance, emission and combustion characteristics of olive mill wastewater biofuel blends fuelled CI engine. Fuel 2021, 291, 120199. [Google Scholar] [CrossRef]
- Nabi, M.N.; Rahman, M.M.; Islam, M.A.; Hossain, F.M.; Brooks, P.; Rowlands, W.N.; Tulloch, J.; Ristovski, Z.D.; Brown, R.J. Fuel characterisation, engine performance, combustion and exhaust emissions with a new renewable Licella biofuel. Energy Convers. Manag. 2015, 96, 588–598. [Google Scholar] [CrossRef]
- Obeid, F.; Van, T.C.; Horchler, E.J.; Guo, Y.; Verma, P.; Miljevic, B.; Brown, R.J.; Ristovski, Z.; Bodisco, T.; Rainey, T. Engine performance and emissions from fuels containing nitrogen and sulphur. Energy Convers. Manag. X 2022, 14, 100179. [Google Scholar] [CrossRef]
- Lee, T.H.; Yang, Z.; Zhang, Y.; Chen, W.T. Investigation of combustion and spray of biowaste based fuel and diesel blends. Fuel 2020, 268, 117382. [Google Scholar] [CrossRef]
- Yang, Z.; Lee, T.H.; Li, Y.; Chen, W.T.; Zhang, Y. Spray and combustion characteristics of pure hydrothermal liquefaction biofuel and mixture blends with diesel. Fuel 2021, 294, 120498. [Google Scholar] [CrossRef]
- Kohansal, K.; Lozano Sanchez, E.; Khare, S.; Oskar Pires Bjørgen, K.; Salman Haider, M.; Castello, D.; Løvås, T.; Aistrup Rosendahl, L.; Helmer Pedersen, T. Automotive sustainable diesel blendstock production through biocrude obtained from hydrothermal liquefaction of municipal solid waste. Fuel 2023, 350, 128770. [Google Scholar] [CrossRef]
- Bjørgen, K.O.P.; Emberson, D.R.; Løvås, T. Optical Measurements of In-Flame Soot in Compression-Ignited Methyl Ester Flames. Energy Fuels 2019, 33, 7886–7900. [Google Scholar] [CrossRef]
- Heywood, J. Internal Combustion Engine Fundamentals, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Tao, C.; Liu, B.; Dou, Y.; Qian, Y.; Zhang, Y.; Meng, S. The experimental study of flame height and lift-off height of propane diffusion flames diluted by carbon dioxide. Fuel 2021, 290, 119958. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, L.; Zhang, M.; Tang, F.; Zhang, X.; Lu, S. Lift-off of jet diffusion flame in sub-atmospheric pressures: An experimental investigation and interpretation based on laminar flame speed. Combust. Flame 2014, 161, 1125–1130. [Google Scholar] [CrossRef]
- Mei, Z.; Mi, J.; Wang, F.; Li, P.; Zhang, J. Chemical flame length of a methane jet into oxidant stream. Flow Turbul. Combust. 2015, 94, 767–794. [Google Scholar] [CrossRef]
- Emberson, D.R.; Wyndorps, J.; Ahmed, A.; Pires Bjørgen, K.O.; Løvås, T. Detailed examination of the combustion of diesel and glycerol emulsions in a compression ignition engine. Fuel 2021, 291, 120147. [Google Scholar] [CrossRef]
- Pham, P.X.; Pham, N.V.; Pham, T.V.; Nguyen, V.H.; Nguyen, K.T. Ignition delays of biodiesel-diesel blends: Investigations into the role of physical and chemical processes. Fuel 2021, 303, 121251. [Google Scholar] [CrossRef]
- Miron, L.; Chiriac, R.; Brabec, M.; Bădescu, V. Ignition delay and its influence on the performance of a Diesel engine operating with different Diesel–biodiesel fuels. Energy Rep. 2021, 7, 5483–5494. [Google Scholar] [CrossRef]
- Mustayen, A.G.; Wang, X.; Rasul, M.G.; Hamilton, J.M.; Negnevitsky, M. Thermodynamic analysis of diesel engine ignition delay under low load conditions. Energy Rep. 2022, 8, 495–501. [Google Scholar] [CrossRef]
- Sardeshmukh, S.; Bedard, M.; Anderson, W. The use of OH* and CH* as heat release markers in combustion dynamics. Int. J. Spray Combust. Dyn. 2017, 9, 409–423. [Google Scholar] [CrossRef]
- He, L.; Guo, Q.; Gong, Y.; Wang, F.; Yu, G. Investigation of OH* chemiluminescence and heat release in laminar methane–oxygen co-flow diffusion flames. Combust. Flame 2019, 201, 12–22. [Google Scholar] [CrossRef]
- Giuliano Albo, P.A.; Lago, S.; Wolf, H.; Pagel, R.; Glen, N.; Clerck, M.; Ballereau, P. Density, viscosity and specific heat capacity of diesel blends with rapeseed and soybean oil methyl ester. Biomass Bioenergy 2017, 96, 87–95. [Google Scholar] [CrossRef]
- Altarazi, Y.S.; Abu Talib, A.R.; Yu, J.; Gires, E.; Abdul Ghafir, M.F.; Lucas, J.; Yusaf, T. Effects of biofuel on engines performance and emission characteristics: A review. Energy 2022, 238, 121910. [Google Scholar] [CrossRef]
- Zhou, C.W.; Farooq, A.; Yang, L.; Mebel, A.M. Combustion chemistry of alkenes and alkadienes. Prog. Energy Combust. Sci. 2022, 90, 100983. [Google Scholar] [CrossRef]
- Yin, Y.; Medwell, P.R.; Gee, A.J.; Foo, K.K.; Dally, B.B. Fundamental insights into the effect of blending hydrogen flames with sooting biofuels. Fuel 2023, 331, 125618. [Google Scholar] [CrossRef]
- Obeid, F.; Van, T.C.; Horchler, E.J.; Guo, Y.; Verma, P.; Miljevic, B.; Brown, R.J.; Ristovski, Z.; Bodisco, T.A.; Rainey, T. Engine performance and emissions of high nitrogen-containing fuels. Fuel 2020, 264, 116805. [Google Scholar] [CrossRef]
- Santhosh, K.; Kumar, G.N. Impact of 1-Hexanol/diesel blends on combustion, performance and emission characteristics of CRDI CI mini truck engine under the influence of EGR. Energy Convers. Manag. 2020, 217, 113003. [Google Scholar] [CrossRef]
- Szybist, J.P.; Kirby, S.R.; Boehman, A.L. NOx emissions of alternative diesel fuels: A comparative analysis of biodiesel and FT diesel. Energy Fuels 2005, 19, 1484–1492. [Google Scholar] [CrossRef]
- Vieira da Silva, M.A.; Lagnier Gil Ferreira, B.; da Costa Marques, L.G.; Lamare Soares Murta, A.; Vasconcelos de Freitas, M.A. Comparative study of NOx emissions of biodiesel-diesel blends from soybean, palm and waste frying oils using methyl and ethyl transesterification routes. Fuel 2017, 194, 144–156. [Google Scholar] [CrossRef]
- Kohl, A.L.; Nielsen, R.B. Control of Nitrogen Oxides. Gas Purif. 1997, 866–945. [Google Scholar] [CrossRef]
- Elkelawy, M.; Alm-Eldin Bastawissi, H.; El Shenawy, E.A.; Taha, M.; Panchal, H.; Sadasivuni, K.K. Study of performance, combustion, and emissions parameters of DI-diesel engine fueled with algae biodiesel/diesel/n-pentane blends. Energy Convers. Manag. X 2021, 10, 100058. [Google Scholar] [CrossRef]





| Engine Type | Four-stroke, single cylinder, indirect DI engine |
| Bore/stroke | 130 mm/140 mm |
| Displaced volume | 1.85 L |
| Compression ratio (CR) | 16.36 |
| Injector | Bosch CR second generation |
| Injector nozzle | Single hole, 62 deg wrt central axis |
| Hole diameter | 0.12 mm |
| Injection pressure | 1000 bar |
| Injection duration | 5 ms |
| Injection timing | 4 CAD before top dead center (BTDC) |
| Speed | 500 rpm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khare, S.; Bjørgen, K.O.P.; Kohansal, K.; Haider, M.S.; Castello, D.; Pedersen, T.H.; Løvås, T.; Emberson, D.R. New Renewable Hydrothermal Liquefaction (HTL) Biofuel: A Combustion and Emissions Study in an Optical Engine. Energies 2023, 16, 6754. https://doi.org/10.3390/en16186754
Khare S, Bjørgen KOP, Kohansal K, Haider MS, Castello D, Pedersen TH, Løvås T, Emberson DR. New Renewable Hydrothermal Liquefaction (HTL) Biofuel: A Combustion and Emissions Study in an Optical Engine. Energies. 2023; 16(18):6754. https://doi.org/10.3390/en16186754
Chicago/Turabian StyleKhare, Shivang, Karl Oskar Pires Bjørgen, Komeil Kohansal, Muhammad Salman Haider, Daniele Castello, Thomas Helmer Pedersen, Terese Løvås, and David Robert Emberson. 2023. "New Renewable Hydrothermal Liquefaction (HTL) Biofuel: A Combustion and Emissions Study in an Optical Engine" Energies 16, no. 18: 6754. https://doi.org/10.3390/en16186754
APA StyleKhare, S., Bjørgen, K. O. P., Kohansal, K., Haider, M. S., Castello, D., Pedersen, T. H., Løvås, T., & Emberson, D. R. (2023). New Renewable Hydrothermal Liquefaction (HTL) Biofuel: A Combustion and Emissions Study in an Optical Engine. Energies, 16(18), 6754. https://doi.org/10.3390/en16186754








