Abstract
The maritime sector is among the most polluting industrial sectors in the world. To oppose this and following the global trend towards carbon neutrality, the International Maritime Organization (IMO) introduced the objective to reduce the CO2 emission of vessels by the year 2030 of 40% and at the same time the European Union will introduce the maritime sector into the ETS system. Therefore, there is a need to reduce the emissions of the working vessels, and this can be accomplished through the Carbon Capture and Storage (CCS). There are many possible CCS technologies that can be applied to vessels: the one that has already been studied the most is the ammine scrubbing of the exhaust gasses. In parallel, other technologies have been proposed to reduce volume and energy needs, which are the Molten Carbonate Fuel Cells (MCFCs), membrane technologies, fixed bed absorption processes and limestone. The review shows how, depending on the used vessel type, the technology to be used may vary, and proposes some preferential options for different applications. The obtained results can be of relevant importance in the present context of energy transition promoting immediate retrofitting to respond to the urgent request for intervention.
1. Introduction
CO2 has a major role in global emissions and climate change [1] as it is produced globally in almost all industrial activities. This makes the reduction of this kind of emission very important to mitigate the effects its increase can have on the ecosystem [2].
Although it seems like the CO2 emissions could be slowing down their rate of growth, when looking at emission data after the post-pandemic industrial boom [3], the trend shows a steadily increase in recent years [4]. It also has to be noted that yearly data still have a high degree of uncertainty [5], and therefore trusting the long-term trend is far more appropriate.
The introduction of new and improved technologies that have the objective to reduce the effects of CO2 production are being developed at a fast rate, some of the most promising ones being the carbon capture and storage technologies, which are receiving a huge boost in terms of the amount of research performed and interest from the industry even from an economical point of view [6,7,8].
The reduction of CO2 in the maritime sector is receiving a lot of attention lately following the release of the International Maritime Organisation (IMO) plan of action for the CO2 reduction that points towards carbon neutrality in the year 2050 and a 40% reduction that must be reached in the maritime sector until 2030 compared to 2008 [9,10,11]. As can be seen in Table 1, the maritime transportation sector is responsible for around 2% of the total amount of CO2 produced [3] globally yearly, and containers, oil tankers and bulk carriers accounted for about 50% of CO2 emission from international shipping in 2018 [12]. The IMO strategy time plan is divided in three periods [13]:

Table 1.
Reassuming table of the CO2 emissions globally and in the maritime sector [3].
- Short-term, which includes the initial study of the possible CO2 reduction solution;
- Mid-term, where the best measures are selected;
- Long-term, where selected measures are developed in order to achieve the reduction target.
Short-term measurements can be divided into two categories: operative and technical approaches. Different technical and operative criteria are developed to achieve carbon neutrality on board, such as, respectively, Energy Efficiency Existing Ship Index (EEXI) for existing ships and Energy Efficiency Design Index (EEDI) for the new ships. EEXI limits the CO2 emitted per unit of transport supply, modifying the speed to maintain the highest possible efficiency, and the amount depends on the type and capacity of the ship [14]. A younger ship has higher environmental efficiency than the existing one, so its design is improved through EEDI [12].
Furthermore, Europe introduced stricter rules about control of the emission values [15], meaning that a strong push to an emission-neutral future is inevitable to keep the maritime sector competitive both in terms of emissions and economically, seeing their possible introduction into the Emission Trading Scheme (ETS) system in Europe in 2022 [16]. As of today, this measure is provisional, but if this agreement will be adopted, in 2024 Europe will become the first jurisdiction to put an explicit carbon price on the maritime sector [17]. The ETS system is a carbon market proposed by the EU to reduce emissions, incentivizing those industry actors who invest into emission reduction with the possibility to sell their shares of CO2 emissions, while the ones who decide to not invest can still operate but must buy these shares, making the operating costs higher [18].
There are different ways to reduce the emission of CO2 in vessels including the implementation of more efficient and innovative propulsion systems [19,20], the use of carbon-free fuels [21,22,23,24,25], the application of more efficient heat recovery units [26,27], or the CCS [28], which will be addressed in this review.
The field of CCS is one of the possible options that can be used to retrofit vessels without the need to change the whole internal combustion unit for the onboard energy demand [29], therefore being able to reduce the cost connected to the implementation of the IMO regulations, as older vessels can still be used without the need for heavy investments. The idea of applying CCS on the flue gasses of internal combustion engine vehicles is very appealing [30], yet the space connected with such a system might not always be available, i.e., inside cars. The problem of space is present in vessels as well, but the few spaces that can be found still allow for some CCS systems on board. In this sector, the established technology, amine solvent scrubbing, occupies, in terms of scrubbing and CO2 storage, about 1–2% of the total volume available in the ship [31].
Today, ship-based carbon capture is still promoted by a few projects, such as EverLoNG [32], aimed to increase the Technology Readiness Level (TRL) of prototypes to be applied on ships fuelled with Liquified Natural Gas (LNG), and few scientific articles are available as maritime applications are still quite rare and just under evaluation. For example, Figure 1 compares the number of publications concerning carbon capture in the last ten years focused, respectively, on the maritime sector and on the industrial sector more generally. However, it can also be observed that the topic of maritime carbon capture has gained increasing interest in recent years.
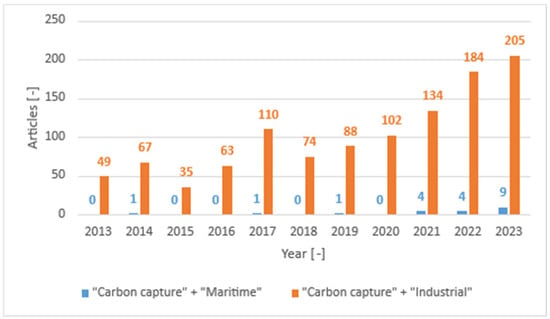
Figure 1.
Comparison between the results of a bibliographic search on ScienceDirect using the keywords “Carbon capture” + “Maritime” (blue) and “Carbon capture” + “Industrial” (orange).
Moreover, both on a European level but also locally in Italy, projects that are centred around the downstream storage or utilization of captured CO2 are being funded and are seeing success [33,34,35]. This means that while this review is focused on the application of CCS on vessels, CO2 can be used or stored in further steps, something that makes this even more interesting on an industrial level.
Aim of the Review
The first aim is to evaluate the major CCS techniques that can be applied onboard, looking at the main strong and weak points that each technology has to offer and taking a look at how the captured CO2 can be stored. The removal efficiency was looked at in order to see whether the technologies are viable to stay inside the new limits imposed by 2030.
To accomplish this, literature research was coupled with opinions from industrial players in the field of on-board CCS, trying to interest both the academic and industrial worlds, bringing the attention of scientific research to this issue and helping shipowners to direct their choices.
Reassuming, the main aims of this work are:
- An overview of the CCS technologies that could be applied for on board usage;
- A brief description of the systems that might be used in these cases;
- A collection of the available literature data;
- A comparison of vantages and disadvantages of the more promising solutions with respect to different types of vessels.
2. Different Carbon Capture Techniques
The capture is the first part of the Carbon Capture and Storage (CCS) technology that needs to be analysed. The capture step determines the reduction of the CO2 and therefore the reduction of Global Warming Potential (GWP) connected with the use of a technology. The choice of the technology must be made considering many parameters:
- GWP reduction capacity: The IMO regulations mentioned before, having the imposition to reduce by 40% the output of CO2 by 2030, is the first limit that needs to be respected. Looking over this limited goal, the real objective being 2050 zero emissions, means that the more the technology can capture, less likely it is that a new change will have to be made soon on the vessel, making it more future proof.
- Volume: What sets the maritime application aside from other CCS applications that can be applied in other industrial fields, i.e., in the energy production [35], are the reduced spaces that are available for the implementation of the technology, as previously mentioned. Therefore, these technologies must be very space efficient and able to be applied in an already existing system.
- CO2 purity: The output concentration of the captured CO2 is also interesting when looking at possible applications that it might find later in its lifecycle, as there are obviously different ideas on how to handle the captured quantities of CO2 [36]. The one way could be to store the CO2 in depleted natural gas reservoirs [37,38], but on the other hand it could also be interesting to reuse the captured CO2, i.e., to favour synthetic fuel production [39,40]. On-board applications are the best-case solution and will be discussed later.
- Energy needs: CCS systems themselves need energy to work properly, causing the need to look at how much energy the system needs and how this affects the energy that can already be generated on board with the auxiliary motors that are being used [41]. A consideration must be made, looking at the fact that if a technology is too energy hungry, the growth in terms of energy might be compensated by burning more fuel and in producing more emissions, therefore if the system needs more energy the effective reduction in terms of CO2 can be found as
The existent energy generation on board, in different cases, is not able to supply this need, so it is necessary to introduce new generation systems, but it is not always feasible or it is too expensive.
The main capture technologies, such as Solvent Scrubbing, Molten Carbonate Fuel Cells, Calcium Hydroxide to limestone reaction, Membrane, Fixed Bed adsorption and desorption, and Ionic Liquid, will be described in the following chapters as they differ from land-based processes [42].
The main issues, when comparing to the same system used in land-based processes, are common for all technologies. First of all, as already mentioned, in maritime applications the space is an issue that is of primary importance, also because these systems are often added on to already existing vessels and therefore need to be worked in close to the engine bay, where space utilization is already exasperated. This problem concerns not only the technologies as such, but also the storage of the degraded materials and captured CO2. Generally, this issue is not so critical in land-based processes, where the storage facilities can be expanded at will. Also, the discharging of these materials is more critical with respect to land cases, where transportation is easier thanks to piping or vehicles, while still not neglectable. In addition to this, many of the CCS technologies use chemicals to work correctly, which need to be produced or regenerated, typically using a plant separate to the CCS system. The coupling of these systems in the land-based industry can be accomplished, as far as the space allows it, while this cannot always be carried out for marine applications, therefore another weak link in the CCS application on board can be the need for the loading of raw chemicals. All these aspects entail both a physical issue, as the unloading and loading processes need to be implemented, and a logistical one, as not all harbours are or will be able to provide such services and the routes will need to be adapted to accommodate this issue [43].
Another important aspect that has to be considered is the safety concerns, still regarding both storage and utilization of the chemical intermediates needed for the single processes. This issue is of a different relevancy when looking at the different technologies. The absorption-based processes use solvents and need special recipients for these, but the storage itself does not cause concern, as do the solid chemical-based reactions, were the real issue stems from the space. Much more problematic is the application of the MCFC as it uses hydrogen, typically coming from reforming or cracking processes (e.g., methane or ammonia). This means that there is the necessity for fuel storage and treatment on board and that during the operation hydrogen is used in molecular form. In land-based applications, the safety conditions are easier to respect, while on board a ship, the monitoring of the system will need to be refined to compensate for an evident impossibility of redundant physical safety measures.
The stability of the system is also a criticality when it comes to the difference in application in land- and maritime-based CCS applications. In land applications, the system integration grants the system inherent stability as it is anchored to the ground. In maritime applications, the CCS system it anchored to the vessel, which itself is in motion thanks to the waves and varying atmospheric conditions, this can be of concern for the different system, as it can change the fluid dynamics of the used liquid chemicals. For example, in absorption processes the motion can cause preferential pathways to be formed inside the columns making the system less efficient, while in MCFC systems can affect molten carbonate distribution, even if component capillarity should mitigate the effects. This means that in maritime application stabilizing systems might need to be put in place to compensate this issue.
Finally, operating conditions could also be affected by constraints of opportunity, like reduce temperature or pressure to reduce energy consumption on board as well as minimize risks.
On the basis of the above considerations, the study of carbon capture systems in land-based applications can help to understand the functioning principles and evaluate the maritime applicability, but the importance of furthering research in the specific field of on-board use cannot be understated, as the issues at hand need to be taken into account.
2.1. Solvent Scrubbing
Scrubbing the post-combustion gasses with solvents can be used, as it allows for a high efficiency separation with a successive purification step, which allows for the recovery of the solvent that can then be reused [44]. This technique is already well proven in absorption from flue gasses on land [45], where it still presents some limitation connected to the high energy requirements given by the regeneration process of the solvent [46]. In maritime applications, there are also other limitations connected with the usage of this technique being the height of the column, which is limited by the volumes that can be used on board. Considering that for on-shore applications a height of around 25 m is given for the absorbing bed [47], and the head and bottom of the column are still to be added, the height can exceed 50 m [48].
The scrubbing process works like a normal scrubber does, feeding the flue gasses from below and using liquid form solvents to extract the CO2 from the current through an adsorption process, allowing the CO2-free current to exit on top, and separating the CO2-rich solvent current, which will be sent into a separation unit, where the CO2 will be released from the solvent, which can then be reused [49]. The released CO2 can be further elaborated depending on the desired storage or usage. The mechanism can be seen visually in Figure 2.
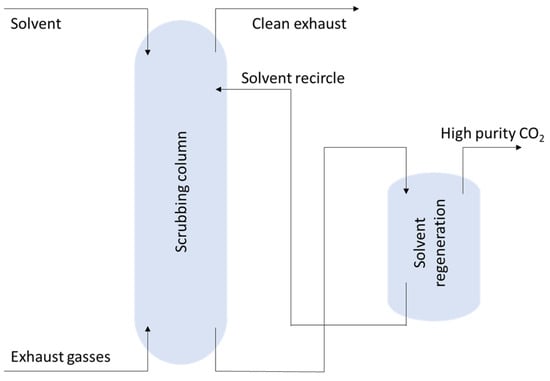
Figure 2.
Schematics of a possible scrubbing and solvent regeneration unit.
The reaction that occurs depends on the solvent choice. In maritime applications, the main criteria to select the solvent are kinetic, degradation, corrosion, toxicity and energy penalty [50]. There are different possible solvents; the most common ones are MonoEthanolAmina (MEA), NH3 and MethylDiEthanolAmine (MDEA), while in research studies DiIsoPropanolAmine (DIPA), which has less energy to regenerate and is less corrosive [51], and MDEA with piperazine (PZ) as promoter, this addition allows lower degradation and higher kinetic rates [52], were proposed. To achieve better results in terms of regeneration, different studies propose a pressure reduction (1 bar to 0.2 bar), which leads to an acceleration of the desorption kinetics; in particular it boosts desorption of about 16% at 363 K to about 70% at 323 K [53].
MEA-based technologies, being the most established ones on an industrial scale [54], different reaction mechanisms have been proposed [55,56,57,58,59] that describe how the CO2 is captured by the solvent, but in the literature are also present a reaction mechanism for DiEthanolAmine (DEA) and TriEthanolAmine (TEA) [60]. Reassuming the results described in the afore mentioned articles, the main mechanism for primary and secondary amine is as can be seen in the following reactions:
where R represents a substituent attached to the amino nitrogen and Equation (1) represents the formation of zwitterion, which in reaction (2) transfers a proton to a non-ionized amine, forming the corresponding carbamate. The mayor problem connected with MEA is its high energy cost for the regeneration, as it is a water-based solvent [60]. The first step (1) determines the rate, while the second one (2) takes place instantaneously.
Regarding tertiary amines, reaction (3) shows their mechanism by the formation of a protonated amine and a bicarbonate anion.
The mechanisms shown are typically happening at room temperature, while the reverse reactions being the desorption and regeneration of the solvent are made at 110 °C and at atmospheric pressure [58].
A possible other solution to optimize the regeneration process with a reducing cost and energy requirements is to work with a vacuum process. The reduction of the pressure of the system favours the release of the dissolved component, and therefore less energy is needed, about 9–15% less than the conventional MEA regeneration process, which requires high temperatures to work. Using this technology, the regeneration process was performed at 75 °C and 20 kPa [61].
As far as ammonia goes (NH3), different possible mechanism have been observed [62], where the most promising one seems to be the one using aqueous ammonia, with the reaction happening as follows:
As with the case before, the working temperature for the adsorption is room temperature, while for regeneration of the solvent the operating temperature is 80 °C [63].
To install this system, some external systems are also required, those being the reboiler for the solvent regeneration and heat exchangers between the CO2-rich and the CO2-poor streams coming from the two towers. The regeneration process is very energy hungry, typically the objective is to minimize the energy needed by pushing for very high temperatures for short times, as to reduce the solvent thermal destruction to a minimum [64].
As can be seen in Figure 2, in the first column, the exhaust is already being released, yet the second column is necessary to allow for the regeneration of the solvent inside the system, meaning that the system always requires two columns, otherwise the used solvent has to be discharged at the port and a fresh load has to be loaded on, which can be a problem, seeing that this can cause the vessel to need to stop in ports that have the capabilities to accomplish this.
Depending on the choice of solvent that is being used, the efficiency for the process can be very different, as can be seen in Table 2, where several simulated systems are analysed. It is important to remember that depending on the fuel purity that is being used the efficiency also changes.

Table 2.
Removal efficiency for different types of solvents as a CCS technique (the values presented are derived from simulations).
Using Key Performance Indicator (KPI), it is possible to compare different solvents, which can be used in this technology, as illustrated in Table 3.

Table 3.
KPI for different solvents in solvent scrubbing technology (5 = good performance, 1 = bad performance) [69].
Where maturity indicates the technology level; energy penalty is a parameter that evaluates the energy loss that the integration of the CCS system on board gives to the system as a whole and it also takes into account the penalty given by increasing the capture rate; CO2 loading is a measure of the concentration of CO2 required in the inlet stream for amines to work correctly; OPEX, as known, indicates the operational expenditure.
2.2. Molten Carbonate Fuel Cells
While molten carbonate fuel cells (MCFCs) are already studied in an established manner for CCS purposes in different fields like the steel industry [70] or energy generation from natural gas [71] or coal [72], with systems being applied in development phase or during retrofitting [72], the application in the maritime field is under study and still in development [73]. The advantage stemming from this kind of system is that the MCFCs can have an energy co-generation and CO2 separation starting from burnt gases with low CO2 concentration, like motor flue gas [74]. These characteristics make it a competitive system compared with other technologies, which have low efficiency, with flue gas having a low CO2 concentration and, in addition, allows it to have a positive energy output [75].
Molten carbonate fuel cells work at high temperatures (around 650 °C) using typically nickel-based electrodes and molten carbonates (of Li, Na and/or K) as electrolytes inside a lithium aluminate matrix [76]. The high temperatures allow the electrolytes to stay fluid and move through the matrix as cations CO32−. Furthermore, the passage of CO32−, which becomes oxidized to CO2, allows the CO2 to be selectively separated from flue gas [77]. Operating pressure is in the range 1–8 atm [78], but, even if higher pressure increases performance, for maritime applications the atmospheric pressure is preferable to simplify the balance of the plant and avoid safety problems. The working principle can be seen in Figure 3, even though the details of the mechanism are still being researched [79,80,81].
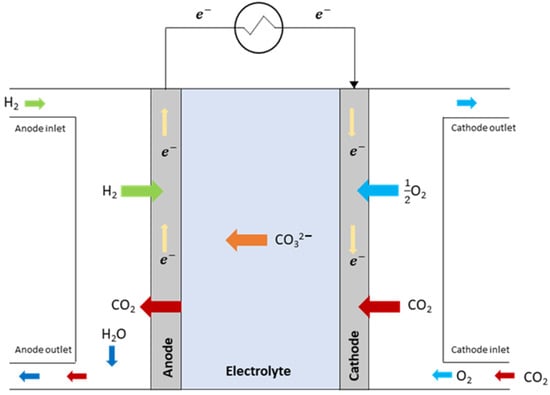
Figure 3.
MCFC working principle. On the left side the anode, on the right side the cathode, both typically nickel based. In the centre part (blue in the figure), the carbonate-based electrolyte, which is supported by a lithium aluminate matrix, allows the ions to pass through one electrode side to the other. The outside circuit allows the flow of the electrons to close the system.
The main reactions that occur in this system are different according to the side of the cell.
At the anode side, the oxidation of hydrogen results in the following reaction [82]:
while at the cathode side, the oxygen is reduced:
Making the complete reaction:
In addition, secondary reactions, based on the migration of hydroxide ions from cathode (9) to anode (8), are involved in this system due to the presence of water on the cathode side [83]:
Overall reaction:
Experimental tests demonstrate that these parallel reactions produce electricity, thanks to the ion transportation, but it reduces the CO2 capture efficiency because the ions that are transported are not only CO32−, but also OH− [84].
There may also be other reactions that can cause problems for the process, for example the Boudouard reaction [85] that can be activated by the presence of CO formed by the water shift reaction. By adding enough vapour, it is possible to inhibit such Boudouard reactions, reducing the amount of carbon depositing itself on the electrode, and therefore favouring long-term durability [86].
As far as maritime application goes, as mentioned before, the case studies are still very limited [87], and the found data are resumed in Table 4. It has also to be mentioned that the MCFC needs to stay at high temperatures to work correctly, meaning that this must be considered when looking at its application because it is necessary, for example, to keep the cells warm also when the vessel stops in the port [73]. This issue is easily overcoming for ship having high size because, when propulsion generators are closed, the auxiliar be kept opened for ship-service, so MCFC is still powered.

Table 4.
Reassuming table of the main applications of MCFC and their reduction efficiency (the values presented are derived from simulations).
Furthermore, the application of such a system on board would require some attention when looking at the implementation of it. Firstly, as is evident when looking at the above seen Figure 3, this system uses hydrogen, which must be either stored or produced on board the vessel itself. The first option, being the storage on board, can be problematic because of different reasons, mainly connected with safety and the volumes required to keep the storage at the right temperature and pressures [88]. This is the reason why the second choice of an on-board production can be much more appealing. The production can usually be performed through a reforming process of either methanol [89] or methane [90]. It is also important to look at the purity of the entering exhaust from pollutant like SOx, which can influence the cell itself depending on its concentration and the operating conditions [91].
MCFC cells have also been proposed in the past for maritime applications on board with objectives other than CCS, some of them being the substitution of the propulsion unit with an MCFC system, like in the Viking Lady ship. This makes it one of the most environmentally friendly vessels being used today [92]. Other theoretical studies applied this power substitution on tanker ships in order to analyse the possibility to reduce the emissions using an MCFC system fed directly by natural gas and water internally reformed [93].
2.3. Calcium Hydroxide to Limestone Reaction
The objective of this technology is to use the reaction of calcium hydroxide with CO2 that produces calcium carbonate, which is also called limestone and is stable in water.
This reaction is exothermic and therefore favoured at low temperatures [94]. The main factors that influence the carbonation capacity are space velocity, grain size, presence of moisture and chemical composition of the CO2 stream [95].
The stable solid product can then be discharged into the sea without causing any harm [96,97]; in fact, in theory, it is even possible to reduce the effect of sea acidification by using the obtained limestones [98]. This might still require some further regulations, as even though limestone is stable, there are not yet any regulations around this topic, showcasing the speed at which the CCS technologies are evolving relatively to the regulatory environment.
Another chemical process, starting from sodium hydroxide, allows CO2 adsorption and consequently limestone production [99]:
Both reactions are exothermic.
Starting from industrial test, by reducing the flow rate of the exhaust gas, fed in the scrubber inlet, and some design parameters, CO2 capture increases from about 20–30% to 60% [100].
There are several advantages from this technology, which should not be underestimated: no requirements for the storage tank compared to CO2 storing in gaseous or liquid form; the possibility to sell the product, whose price usually fluctuates from 20 to 50 $/ton, as “blue-” ; the possibility to discharge the product into the sea with environmental benefits; easier technology and process.
However, there are some issues connected with this technology, firstly being the space required to stock the reactants (3.19 kgCaCO3/kgCO2 considering the single Calcium Hydroxide system, while by the addition of the overall system efficiency, with limestone calcination being an energy-intensive process, the carbon penalty is about 44.9%, [101]) so that the cargo ships already dedicated to the transport of materials could be the preferable type of vessel for this CCS solution.
A second issue with this technology is that usually the calcium hydroxide is obtained through calcium oxide, which in turn is obtained through the heating of limestone that causes the opposite reaction of the CCS technique to happen [102,103]:
As can be seen from (15), the production of calcium hydroxide itself produces carbon dioxide, therefore, if the CO2 is not abated at the production site, the CCS net CO2 abatement is negative, as some CO2 is also produced for heating the system [101].
2.4. Membrane
This technique is proposed for LNG-fuelled vessels, as the outcoming flue gasses have the right amounts of CO2 and O2 for this system to work correctly (in terms of mole fractions: CO2 ≈ 0.03 and O2 ≈ 0.16, [104]). It operates by selectively permeating CO2, while letting the rest of the gas through. This has the huge advantage connected to the reduced dimensions and the cost, when comparing it to the ammine systems, which are in essence the most established Onboard Carbon Capture and Storage [104].
This technology could be developed in two different ways: Membrane Gas Separator or Membrane Contactor. The first system is constituted by a dense membrane and the selectivity is determined by the membrane material, while the second one has a porous membrane and a solvent, used to absorb CO2, which characterised the selectivity. The feed composition is the key to comparing these two systems: in the first case, a high CO2 concentration (up to 40 mol%) is required to have a good efficiency, while in the second case, this parameter does not influence the overall efficiency [105]. For this reason, in maritime CO2 capture, MC application is preferred to MSG application. Figure 4 shows the schematic representation of the membrane-separation MC process.
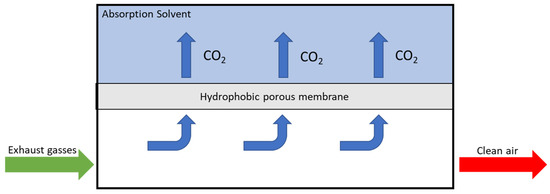
Figure 4.
Schematic representation of the membrane separation MC process.
Obliviously, in MC, the membrane materials should be hydrophobic in order to avoid the wetting of the membrane. MC should have high chemical and thermal stability and high porosity. In addition, the liquid concentration affects the efficiency of CO2 absorption [106].
The most common microporous membrane from polymeric materials are PolyTetraFluoroEthylene (PTFE), PolyPropilene (PP), and PolyVinilDeFluoride (PVDF). The first one has better performance in terms of hydrophobicity than the other materials, but it is more expensive. PP is the cheapest material, but it is less hydrophobic than the other polymers, which contain fluorine; for this reason, the middle way between affordability and material characteristics is PVDF [105].
During the last few years, ceramic materials have also been studied for this application. The main issue with the latter material is that they must be processed to achieve the hydrophobicity and the right specific surface area, so they have a lower TRL in comparison with the polymeric category [107].
2.5. Fixed Bed Adsorption and Desorption
The use of potassium carbonate in fixed beds seems to be a good alternative, as it can chemically bind the CO2 into potassium bicarbonate [108]. This unit should be placed after the SO2 scrubbing that is necessary and already widely applied and can work at low temperatures (around 50 °C) as the absorption process is exothermic. The reaction is generally written as [109]
The bed is then regenerated at around 150–200 °C releasing almost pure CO2 that can then be stored as desired [110]. Figure 5 represents both steps of the process.
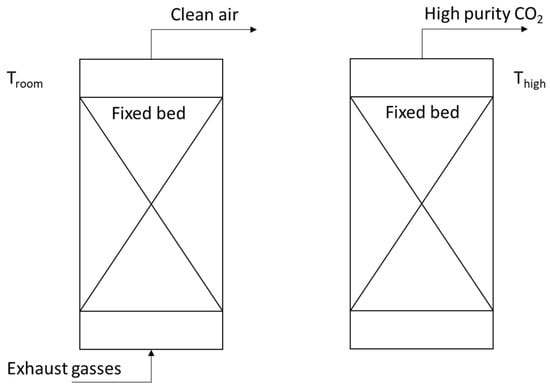
Figure 5.
(Left) First step of the process, the CO2 exhaust gasses flow over the fixed bed and the CO2 is absorbed. (Right) Second step, the temperature is set higher, and the CO2 is released.
Alternatively, a fixed bed based on hollow fibres based on alumina supported CaO can be used, where the reversible reaction of calcium carbonate is used to capture CO2 [111] on the fixed bed and then released again [112]. The reaction is the opposite of the one seen in (14) and (16).
Both techniques use a reversible chemical reaction to separate the hot stream of flue gas from CO2, and they then regenerate the bed to be able to reuse it. While very simple, this technique can suffer from typical adsorbing bed problems, being poisoned by pollutants and aging, which are already studied processes [113].
2.6. Ionic Liquids
If the previous technologies have been already taken into consideration for maritime applications, at least in theoretical feasibility analyses, instead there are no similar studies on Ionic Liquids (ILs) to the authors’ knowledge. These solvents, organic or inorganic and constituted by anions and cations, could be a further option.
ILs are liquid at temperatures below 100 °C, have high thermal stability and, compared with other solvents, have higher CO2 selectively, because the molecules that possess the electric quadruple moment have higher solubilities than the other [114]. On the other hand, they have high viscosity, which implies an increasing of operative costs, in terms of energy requirements, like pumps and mass transfer rates [115].
There are different ILs, which have several methods to capture CO2. In order of prominent techniques: imidazolium carboxylate, amino acids and aprotic heterocyclic. The first category has higher CO2 selectivity, but shows high viscosity [116]. The amino acid ILs have a good CO2 capture, but the high viscosity causes the reduction of the reaction rate [117]. In order to overcome this issue and to improve the CO2 absorption, a possible solution is the functionalized Ionic Liquids, called Task Specific ILs, which are improved by the addition of suitable moieties into conventional ILs. Usually, these liquids have lower volatility, so their regeneration is accomplished by heating at 80–100 °C, for several hours under vacuum [118]. In addition, TSILs, on one hand, are considered environmentally friendly, due to their low vapor pressure, non-flammability and high stability, on the other hand, the addition of moieties has a key role in terms of toxicity [119]. The most common groups used to functionalize ILs could be cationic or anionic, imidazolium, pyridinium, pyrolidinium, phosphonium, ammonium or tetrafluoroborate, lactate, hexafluorophosphate, respectively [120]. The last category, aprotic heterocyclic ILs, has great CO2 adsorption, with high reaction rate and high decomposition temperature, but presents high heats of absorption [107].
In order to reduce the cost-effective and the regeneration, encapsulated ILs in hydrophobic polymeric materials are developed. This configuration shows 3.05 kgCO2/kgILs at working conditions of 25 bar as pressure and room temperature, using 1-hexyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide as ILs [121].
It is interesting to note that the system solution is very similar to the one seen for amine absorption as they both use a similar principle; however, it must be noted that the TRL for the two technologies is significantly different (for the amine-based system TRL is 9, while for ILs it is 2–3 [122]).
3. Discussion of the Results
The main key performance indicators are summarised in Table 5 referring to the discussed technologies.

Table 5.
Different capture techniques with the fuel exhaust gasses they can treat, efficiency and specific duty required to operate them. (The values presented are derived from simulations) (* For the MCFC system, the duty is negative as the system is able to provide energy instead of needing it, the control volume).
It is important to remember that LNG is a cleaner fuel when it comes to pollutants than its diesel counterpart [125], but in economic terms it still makes sense to evaluate the diesel, because the jump to LNG costs cargo space and diesel is still considered the most cost-efficient fuel [25].
After having reviewed several technologies, it becomes clear that each one of those has its own peculiarity that can show advantages or disadvantages depending on the application. An in-depth analysis has been carried out on the three most developed technologies to better understand the level of applicability of each one in the maritime sector. The analysis has been performed on 6 type of ships that differs one from the other for several aspects such as typical route, space availability for the onboard installation of new technologies and so on. The ships analysed in this study are: cruise, ferry, LNG carrier, bulk carrier, tanker and container.
In order to analyse these aspects, an applicability score has been considered as a sum of eight factors, each of those with a different weight depending on the specific technology considered, targeting 100 as the maximum achievable score, as illustrated in the Table 6.

Table 6.
Weight of the parameters that were analysed given in order to be able to elaborate a fair comparison between them.
Each factor is then affected by a multiplier (from 0 to 1) that, being ship-type specific, characterizes how the specific factor is impacting on the specific ship-type.
The factors that have been considered are the following:
- Route length: The longer the route is, the more complex the technology implementation is, in terms of reagents or CO2 to be stored, driving the dimension of the equipment.
- Route-planning: Having a fixed trading scheme facilitates the logistics for the reagents or CO2 supply and handling while unplanned voyages make it more complex, especially if trading is carried out in remote locations as typically happens for merchant vessels.
- Space availability: Space is one of the main challenges, especially talking about retrofit. Optimize the performances is always one of the main topics during the development of a new technology, but space constraints are indubitably impacting on the possibility of installing a system on board a ship. Even if for certain ships the available space could be considered more or easier to be used, the loss of cargo capacity must be considered and the trade-off carefully evaluated.
- Maintenance: The impact that maintenance has cannot be considered as much as others but is still worth evaluating. Maintenance is to be considered not merely in terms of cost for it but also in terms of personnel capability and availability and passenger ships are typically better structured then others in this aspect.
- Reagent/fuel transportation capability: Similarly to space availability, this factor can have a huge impact depending on the ship type. However, the weight this factor has varies among the different technologies depending on the actual necessity of carrying reagents or fuel in big quantities.
- Public opinion: It is worth considering the public opinion on the evaluation, even if with minimal impact, being an important driver for shipowners, especially for passenger ships. Some technologies can be seen more environmentally friendly or more advanced than others, resulting in being more appealing for public opinion.
- Technology cost: Seen mainly as the capex for the technology implementation, this can be a driver for certain maritime segments (cost of the technology implementation compared with the ship value).
- Levelized capturing cost: This is indubitably one of the main drivers for the technology implementation. This parameter considers capex, opex, amortization, additional income or expenditures from CO2 handling, carbon taxes or credits over the remaining ship-lifetime period and allows us to better compare the different technologies with the most objective analysis.
The choice of indexes that were used is illustrated in Table 7, while the applications for different vessels are available in Appendix A.

Table 7.
Applicability factor for several CCS technologies.
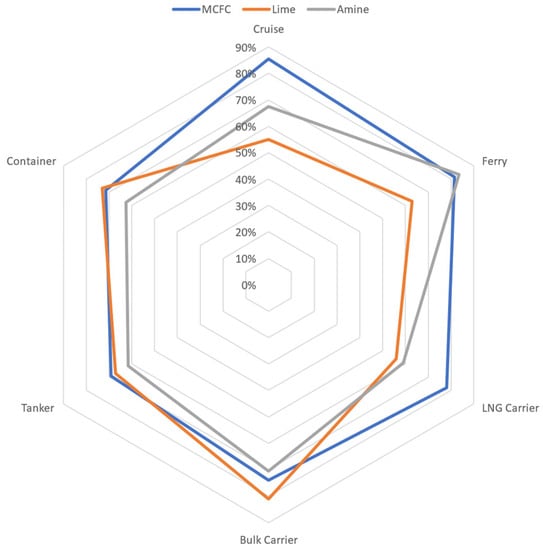
Figure 6.
Graphical representation of the results obtained through the weighted method mentioned above.
A detailed economic analysis would be useful to refine the results, but it would require an industrial experience still lacking in the maritime sector which, for example, presents peculiar engineering issues for technology implementation on board, so that just general economic assessments not based on real cases are available in the literature so far [126]. Experience on industrial sites on land suggests costs for abatement of CO2 ranging from 0.5 up to 3 EUR per kg of removed CO2 to even negative values in certain solutions that imply the sale of CO2 combined with the production of additional energy, depending on the application contexts [127,128,129]. In any case, CCS can allow very low costs in comparison to alternative solutions. For example, the IEA (International Energy Agency) estimated that the exclusion of CCS as a carbon mitigation tool for the power sector would increase the costs of emissions mitigation by around 3.5 USD by 2050, a 70% increase in mitigation costs if only alternative solutions, including renewables, were instead employed over that time [130,131].
Finally, storage technology can be as varied as the capture technologies themselves, depending on many factors. Some of these parameters are, for example, the intended use of the captured CO2 and the available spaces. There are three main options when it comes to the storage: liquefied, compressed, and as solid compounds. The most energy efficient technique is to liquefy the CO2 [132], but this still this does not mean that it does not have its problems when looking at the energy efficiency of the system and the cargo loss by installing a liquefaction system [133]. Liquefaction is very useful as it allows for a huge reduction in volumes (about 1/550 times [127]) and therefore requires less storage space [134], which is already very limited in vessels. In particular, cruise ships have very tight spaces, while cargo ships have more space at their disposal, even though using the space for the CO2 storage would take away from possible space for cargo itself. The real problem here resides in the temperature drop that is needed for the liquefaction, connected with a discrete pressure increase, from the pressure of CCS, usually 1.2–3.5 bar, to the pressure of liquefaction, which for this application is 15 bar [135]. This can be a burden and add to the energy needs of the CCS system. This choice is usually implemented when the obtained CO2 is very pure, as residues of air can cause the liquefaction process to be less energy efficient [136]. Different ways to liquefy the CO2 on-board were found, and the results can be seen in the Table 8.

Table 8.
Reassuming table of the main liquefaction techniques.
When looking at the specific case of LNG powered Internal Combustion Energy (ICE), it is possible to reduce the necessary volume needed for the storage by inserting the separated CO2 inside the LNG tanks, as the two materials do not mix. The CO2 is pressurised into a dense phase (15 bar), which is usually less energy costly then a complete liquefaction, and in these conditions the cost is almost 7% lower than the 7 bar pressure liquefaction case [138]. Dense phase CO2 has the behaviour of a fluid and a gas at the same time and is the most used phase when looking at land-based applications like piping [140,141]. The CO2 that will be present inside the tank will not cause problems to the LNG itself, as there will be no mixture between the two phases because the CO2 is much lighter than LNG. By doing this, another storage unit can be avoided, even though some studies showed that the use of this tank might not cover the entire volume needed to collect the amount of CO2 that is being collected [65].
Some of the technologies that were presented above already include the storage in the capture step [99], like the limestone formation through carbon hydroxide. This has some advantages, as there is no need to work with the CO2 after the capture step, yet the problem is the storage, because storing the solids is much more difficult, in terms of volume. In the Table 9, the main advantages and disadvantage of each storage technique were reassumed.

Table 9.
Main advantages and disadvantages of the different CO2 storage techniques.
However, even though the CO2 will be collected with the objective of storing or reusing it in on-land applications, this does not mean that it is not possible to find applications for the CO2 even on board. While there are no studies still showing that CO2 specifically collected with CCS can be used for these reasons, CO2 can be used as a flooding agent to suffocate fire in the vital parts of the ship, for example, the engine bay [142]. There are many other utilisation technologies, and most of them are still being researched and have yet to find their footing in the industry, but it cannot be excluded that one day they can be used as on-board applications [143,144,145].
4. Conclusions
The On-board Carbon Capture and Storage (OCCS) technologies are very promising when it comes to reaching the IMO regulations, as using the CCS systems it is possible to retrofit even vessels using still very heavy fossil fuels like diesel with integrated cleaning systems obtaining good results. It should also be mentioned that the CCS technologies have a fundamental rule in order to save the Carbon Credits (currently, the price, according to European Union Allowance, is about 100 USD/tonCO2 [146]).
The most established technology is the use of ammine scrubbers, but the other technologies presented show a fast growth in research interest and technological advancements.
Therefore, it is difficult to evaluate which CCS technique is the best one without looking at the whole system that must be treated, starting from the incoming fuel and the space that can be used, and the desired outputs. In the maritime field, moreover, specific variables come into play when evaluating the most suitable CCS system, such as the vessel’s route in terms of both length and the level of industrialization and CO2 supply of the port areas where it docks. These variables are directly related to the type of vessel.
From a first analysis on the three main capture technologies analysed in this article (ammine adsorption, calcium hydroxide and MCFC), the ammines show an interesting utilization for ferries, calcium hydroxide lime technology has potential good results with bulk carriers and the MCFC technology has advantages for cruise and LNG carriers.
These assessments may undergo variations soon depending on the developments of the laws around carbon transportation and storage work on an international scale [147]. When looking at the main question of this review as to how these technologies might be able to provide an answer to the IMO regulations, this can be clearly answered positively as all the above-mentioned technologies are able to reduce the emission enough to respect the legislation. Yet, the main issues are not with respecting these limits, but with the intrinsic limitation that comes from a maritime application, like the volumes needed both for the capture and the storage system, which still require the technologies to be optimised to reach the right compromise between space and abatement efficiencies. Furthermore, the need for unloading stations of the captured CO2 is another challenge. Still, seeing as the industry is pushing in these regards, as seen in the projects mentioned in the introduction, and the papers that are being published, the prospective of the CCS technologies as an answer to the new limitations is surely viable.
Reassuming, the main results of the work are
- -
- Research in this field is still without real applications, but is attracting increasing attention;
- -
- There are numerous possible CCS technologies that have already been tested on land and appear promising on board;
- -
- There are some intrinsic limitations to the on-board environment for system installations, mainly related to space and safety;
- -
- The choice of the appropriate CCS systems on board depends on the type of vessel;
- -
- Molten Carbonate Fuel Cells could be applied satisfactorily on all ships, but amines would be preferable for ferries and lime for cargoes.
5. Future Work
As highlighted by the results of the review, CCS technologies have a great potential to aid in the future direction of a decarbonised maritime sector. Both already established technologies (solvent scrubbing, molten carbon fuel cells, calcium hydroxide to limestone reaction and fixed bed adsorption and desorption), but also newly ones (membranes, ionic liquids), are receiving more and more attention, as highlighted by the increase in works in these years, even if still low in absolute numbers. Authors are working on the technological development of some of these possible solutions and hope to be able to make a significant contribution soon.
Although decarbonising is the most immediate way of staying inside the newly imposed limitation to the emissions provided by the legislators, in the long run, to reach the objective of net zero emissions by 2050, there will be a need to couple CCS with both efficient and economical renewable energy storage and production systems [148,149,150].
It is important to remember that, while single technology solutions might appear viable, a broader approach to the issue at hand can be beneficial to reach the final objective of net zero emissions.
Author Contributions
Conceptualization, M.A., F.L., B.B. and D.B.; Methodology, F.L. and D.B.; Writing—original draft, R.R. and L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
Acronym reassuming table:
| CCS | Carbon Capture and Storage |
| DIPA | DiIsoPropanolAmine |
| DEA | DiEthanolAmine |
| EEDI | Energy Efficiency Design Index |
| EEXI | Energy Efficiency Existing Ship Index |
| ETS | Emission Trading Scheme |
| GWP | Global Warming Potential |
| ICE | Internal Combustion Engines |
| IMO | International Maritime Organisation |
| LNG | Liquefied Natural Gas |
| KPI | Key Performance Indicator |
| MC | Membrane Contactor |
| MDEA | Methyl DiEthanolAmine |
| MEA | MonoEthanolAmine |
| MGS | Membrane Gas Separator |
| OCCS | On-board Carbon Capture and Storage |
| TEA | TriEthanolAmine |
| TRL | Technology Readiness Level |
| TSILs | Task Specific Ionic Liquids |
Appendix A

| Vessel | Route-Length | Route-Planning | Space Availability | Available Personal | Reagent/Fuel Transportation Capability | Public Opinion | Technology Cost | Levelized Capturing Cost | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–2 Days | 1 Week | 2 Weeks or More | Fixed Routes | Planned in Advance | Not Planned | Space Available w/o Cargo Loss | Space Available w/ Cargo Loss | Little Space Available | Crew Available | Mid Crew Available | Little Crew Available | Space Available w/o Cargo Loss | Space Available w/ Cargo Loss | Little Space Available | Good | Neutral | Bad | Expensive Ship | Mid-Level Ship | Cheap Ship | High | Mid | Low | ||
| Cruise | MCFC | X | X | X | X | X | X | X | X | ||||||||||||||||
| Limestone | X | X | X | X | X | X | X | X | |||||||||||||||||
| Amine | X | X | X | X | X | X | X | X | |||||||||||||||||
| Ferry | MCFC | X | X | X | X | X | X | X | X | ||||||||||||||||
| Limestone | X | X | X | X | X | X | X | X | |||||||||||||||||
| Amine | X | X | X | X | X | X | X | X | |||||||||||||||||
| LNG Carrier | MCFC | X | X | X | X | X | X | X | X | ||||||||||||||||
| Limestone | X | X | X | X | X | X | X | X | |||||||||||||||||
| Amine | X | X | X | X | X | X | X | X | |||||||||||||||||
| Bulk Carrier | MCFC | X | X | X | X | X | X | X | X | ||||||||||||||||
| Limestone | X | X | X | X | X | X | X | X | |||||||||||||||||
| Amine | X | X | X | X | X | X | X | X | |||||||||||||||||
| Tanker | MCFC | X | X | X | X | X | X | X | X | ||||||||||||||||
| Limestone | X | X | X | X | X | X | X | X | |||||||||||||||||
| Amine | X | X | X | X | X | X | X | X | |||||||||||||||||
| Container | MCFC | X | X | X | X | X | X | X | X | ||||||||||||||||
| Limestone | X | X | X | X | X | X | X | X | |||||||||||||||||
| Amine | X | X | X | X | X | X | X | X | |||||||||||||||||
References
- Scheffer, M.; Brovkin, V.; Cox, M. Positive feedback between global warming and atmospheric CO2 concentration inferred from past climate change. Geophys. Res. Lett. 2006, 33, 1–4. [Google Scholar] [CrossRef]
- Arnell, N.W.; Cannell, M.G.; Hulme, M.; Kovats, R.S.; Mitchell, J.F.; Nicholls, R.J.; Parry, M.L.; Livermore, M.T. The Consequences of CO2 Stabilisation for the Impacts of Climate Change. Clim. Change 2002, 53, 413–446. [Google Scholar] [CrossRef]
- Crippa, M.; Guizzardi, D.; Banja, M.; Solazzo, E.; Muntean, M.; Schaaf, E.; Pagani, F.; Monforti-Ferrario, F.; Olivier, J.G.; Quadrelli, R. CO2 Emissions of All World Countries. JRC Science of Policy Report; European Commission: Brussels, Belgium, 2022. [Google Scholar] [CrossRef]
- Pathak, M.; Slade, R.; Shukla, P.R.; Skea, J.; Pichs-Madruga, R.; Urge-Vorsat, D. WG III Contribution to the Sixth Assessment Report; IPCC: Geneva, Switzerland, 2022. [Google Scholar]
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J. Global Carbon Budget. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Longa, F.D.; Detz, R.; van der Zwaan, B. Integrated assessment projections for the impact of innovation on CCS deployment in Europe. Int. J. Greenh. Gas Control 2020, 103, 103–133. [Google Scholar]
- Fiorini, A.; Pasimeni, F.; Georgakaki, A.; Tzimas, E. Analysis of the European CCS Research and Innovation Landscape. Energy Procedia 2017, 114, 7651–7658. [Google Scholar] [CrossRef]
- Størset, S.Ø.; Tangen, G.; Berstad, D.; Eliasson, P.; Hoff, K.A.; Langørgen, Ø.; Munkejord, S.T.; Roussanaly, S.; Torsæter, M. Profiting from CCS innovations: A study to measure potential value creation from CCS research and development. Int. J. Greenh. Gas Control 2019, 83, 208–215. [Google Scholar] [CrossRef]
- IMO. IMO Strategy on Reduction of GHG Emission from Ships. Available online: http://www.imo.org (accessed on 3 January 2023).
- Comer, B.; Chen, C.; Rutherford, D. Relating Short-Term Measures to IMO’s Minimum 2050 Emissions Reduction Target; Emissions Reduction Target; International Council on Clean Transportation. 2018. Available online: https://theicct.org/publication/relating-short-term-measures-to-imos-minimum-2050-emissions-reduction-target/ (accessed on 13 May 2023).
- Joung, T.H.; Kang, S.G.; Lee, J.K.; Ahn, J. The IMO initial strategy for reducing Greenhouse Gas (GHG) emissions, and its follow-up actions towards 2050. J. Int. Marit. Saf. Environ. Aff. Shipp. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Rutherford, D.; Mao, X.; Comer, B. Potential CO2 reductions under the Energy Efficiency Existing Ship Index. Int. Counc. Clean. Transp. 2020, 222, 1–12. Available online: https://theicct.org/publication/potential-co2-reductions-under-the-energy-efficiency-existing-ship-index/ (accessed on 23 April 2023).
- United Nation Climate Change. Emissions from Fuel Used for International Aviation and Maritime Transport. Available online: https://unfccc.int/ (accessed on 4 January 2023).
- Chuah, L.F.; Mokhtar, K.; Ruslan, S.M.; Bakar, A.A.; Abdullah, M.A.; Osman, N.H.; Bokhari, A.; Mubashir, M.; Show, P.L. Implementation of the energy efficiency existing ship index and carbon intensity indicator on domestic ship for marine environmental protection. Environ. Res. 2023, 222, 115–348. [Google Scholar] [CrossRef] [PubMed]
- Fedi, L. The monitoring, reporting and verification of ships’ carbon dioxide emissions: A European substantial policy measure towards accurate and transparent carbon dioxide quantification. Ocean Yearbook. Online 2017, 31, 381–417. [Google Scholar] [CrossRef]
- Cariou, P.; Lindstad, E.; Jia, H. The impact of an EU maritime emissions trading system on oil trades. Transp. Res. Part D Transp. Environ. 2021, 99, 102992. [Google Scholar] [CrossRef]
- Ordinary Legislative Procedure. Interinstitutional Negotiations-ETS. Available online: https://www.europarl.europa.eu/ (accessed on 4 January 2023).
- EU ETS. Available online: https://climate.ec.europa.eu/eu-action/eu-emissions-trading-system-eu-ets_en (accessed on 2 January 2023).
- Levander, O. New concepts in ferry propulsion. Wärtsilä Technol. J. 2007, pp. 45–50. Available online: http://www.123seminarsonly.com/Seminar-Reports/020/44752818-Ferry-Propulsion-New-Concept.pdf (accessed on 2 March 2023).
- Ling-Chin, J.; Roskilly, A. Investigating the implications of a new-build hybrid power system for Roll-on/Roll-off cargo ships from a sustainability perspective—A life cycle assessment case study. Appl. Energy 2016, 181, 416–434. [Google Scholar] [CrossRef]
- Bengtsson, S.; Andersson, K.; Fridell, E. A comparative life cycle assessment of marine fuels: Liquefied natural gas and three other fossil fuels. J. Eng. Marit. Environ. 2011, 225, 97–110. [Google Scholar] [CrossRef]
- Chiong, M.C.; Kang, H.S.; Shaharuddin, N.M.; Mat, S.; Quen, L.K.; Ten, K.H.; Ong, M.C. Challenges and opportunities of marine propulsion with alternative fuels. Renew. Sustain. Energy Rev. 2021, 149, 111–397. [Google Scholar] [CrossRef]
- Gabiña, G.; Martin, L.; Basurko, O.C.; Clemente, M.; Aldekoa, S.; Uriondo, Z. Waste oil-based alternative fuels for marine diesel engines. Fuel Process. Technol. 2016, 153, 28–36. [Google Scholar] [CrossRef]
- Paulauskiene, T.; Bucas, M.; Laukinaite, A. Alternative fuels for marine applications: Biomethanol-biodiesel-diesel blends. Fuel 2019, 248, 161–167. [Google Scholar] [CrossRef]
- Law, L.C.; Mastorakos, E.; Evans, S. Estimates of the Decarbonization Potential of Alternative Fuels for Shipping as a Function of Vessel Type, Cargo, and Voyage. Energies 2022, 15, 7468. [Google Scholar] [CrossRef]
- Shu, G.; Liang, Y.; Wei, H.; Tian, H.; Zhao, J.; Liu, L. A review of waste heat recovery on two-stroke IC engine aboard ships. Renew. Sustain. Energy Rev. 2013, 19, 385–401. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, K.; Deng, K. A review of waste heat recovery from the marine engine with highly efficient bottoming power cycles. Renew. Sustain. Energy Rev. 2020, 120, 109–611. [Google Scholar] [CrossRef]
- IEA. CO2 Capture and Storage: A Key Carbon Abatement Option; IEA: Paris, France, 2008. [Google Scholar]
- NETL. Carbon Sequestration Technology Roadmap and Program Plan 2007, Ensuring the Future of Fossil Energy Systems through the Successful Deployment of Carbon Capture and Storage Technologies; NETL: Albany, OR, USA, 2007; Available online: http://cepac.cheme.cmu.edu/pasi2008/slides/siirola/library/reading/2007Roadmap.pdf (accessed on 31 August 2023).
- García-Mariaca, A.; Llera-Sastresa, E. Review on carbon capture in ice driven transport. Energies 2021, 14, 6865. [Google Scholar] [CrossRef]
- Maelum, M.; Mathisen, A.; Jayarathna, C.; Skagestad, R.; Belgaroui, J.; Dijkhuizen, C. Ship-Based CO2 Capture–Port Integration. In Proceedings of the 16th International Conference on Greenhouse Gas Control Technologies, Lyon, France, 23–27 October 2022. [Google Scholar]
- EverLong. Available online: https://everlongccus.eu/about-the-project (accessed on 21 August 2023).
- Carbon Capture, Storage and Utilization. Available online: https://energy.ec.europa.eu/topics/oil-gas-and-coal/carbon-capture-storage-and-utilisation_en (accessed on 21 July 2023).
- ENI. Cattura, Stoccaggio e Riutilizzo della CO2 (CCUS). Available online: https://www.eni.com/it-IT/attivita/gestione-anidride-carbonica.html (accessed on 25 August 2023).
- Carbon Capture and Storage (CCS) in Italy. Available online: https://ocre-geoscience.com/carbon-capture-and-storage-ccs-in-italy/ (accessed on 6 June 2023).
- Su, D.; Herraiz, L.; Lucquiaud, M.; Thomson, C.; Chalmers, H. Thermal integration of waste to energy plants with Post-combustion CO2 capture. Fuel 2023, 332, 126004. [Google Scholar] [CrossRef]
- Al Baroudi, H.; Awoyomi, A.; Patchigolla, K.; Jonnalagadda, K.; Anthony, E.J. A review of large-scale CO2 shipping and marine emissions management for carbon capture, utilization and storage. Appl. Energy 2021, 287, 116510. [Google Scholar] [CrossRef]
- Global CCS Institute. Knowledge Sharing Report. CO2 Liquid Logistics Shipping Concept (LLSC): Overall Supply Chain Optimization; Global CCS Institute: Melbourne, Australia, 2011; Available online: https://www.globalccsinstitute.com/resources/publications-reports-research/knowledge-sharing-report-co2-liquid-logistics-shipping-concept-llsc-overall-supply-chain-optimization/ (accessed on 7 July 2023).
- Ren, B.; Xu, Y.; Huang, Y.W.; She, C.; Sun, B. Methanol production from natural gas reforming and CO2 capturing process, simulation, design, and technical-economic analysis. Energy 2023, 263, 125–879. [Google Scholar] [CrossRef]
- Hidalgo, D.; Martín-Marroquín, J.M. Power-to-methane, coupling CO2 capture with fuel production: An overview. Renew. Sustain. Energy Rev. 2020, 132, 110057. [Google Scholar] [CrossRef]
- Fang, S.; Xu, Y.; Li, Z.; Ding, Z.; Liu, L.; Wang, H. Optimal Sizing of Shipboard Carbon Capture System for Maritime Greenhouse Emission Control. IEEE Trans. Ind. Appl. Inst. Electr. Electron. Eng. Inc. 2019, 55, 5543–5553. [Google Scholar] [CrossRef]
- NETL. Carbon Dioxide Capture Handbook; NETL: Albany, OR, USA, 2015. Available online: https://netl.doe.gov/sites/default/files/netl-file/Carbon-Dioxide-Capture-Handbook-2015.pdf (accessed on 4 June 2023).
- EverLong. Available online: https://everlongccus.eu/work-packages/work-package-2 (accessed on 17 August 2023).
- Emis. Scrubbing Definition. Available online: https://emis.vito.be/en/bat/tools-overview/sheets/gas-scrubbing-general#:~:text=A%20scrubber%20is%20a%20waste,technique%20for%20many%20gaseous%20emissions (accessed on 21 December 2022).
- Haszeldine, R.S. Carbon capture and storage: How green can black be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.; Miller, G.; Aandi, I.; Gadsden, R.; Davison, J. Performance and Costs of CO2 Capture at Gas Fired Power Plants. Energy Procedia 2013, 37, 2443–2452. [Google Scholar] [CrossRef][Green Version]
- Agbonghae, E.O.; Hughes, K.J.; Ingham, D.B.; Ma, L.; Pourkashanian, M. Optimal process design of commercial-scale amine-based CO2 capture plants. Ind. Eng. Chem. Res. 2014, 53, 14815–14829. [Google Scholar] [CrossRef]
- Luo, X.; Wang, M. Study of solvent-based carbon capture for cargo ships through process modeling and simulation. Appl. Energy 2017, 195, 402–413. [Google Scholar] [CrossRef]
- Lin, Y.J.; Rochelle, G.T. Approaching a reversible stripping process for CO2 capture. Chem. Eng. J. 2016, 283, 1033–1043. [Google Scholar] [CrossRef]
- Liang, Z.H.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K.; et al. Recent progress and new developments in post-combustion carbon-capture technology with amine-based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar] [CrossRef]
- Haghtalab, A.; Eghbali, H.; Shojaeian, A. Experiment and modeling solubility of CO2 in aqueous solutions of Diisopropanolamine + 2-amino-2-methyl-1-propanol + Piperazine at high pressures. J. Chem. Thermodyn. 2014, 71, 71–83. [Google Scholar] [CrossRef]
- Ji, C.; Yuan, S.; Huffman, M.; El-Halwagi, M.M.; Wang, Q. Post-combustion carbon capture for tank to propeller via process modeling and simulation. J. CO2 Util. 2021, 51, 101655. [Google Scholar] [CrossRef]
- Wehrung, Q.; Destefanis, E.; Caviglia, C.; Bernasconi, D.; Pastero, L.; Bruno, M.; Bernasconi, A.; Magnetti Vernai, A.; Di Rienzo, A.; Pavese, A. Experimental Modeling of CO2 Sorption/Desorption Cycle with MDEA/PZ Blend: Kinetics and Regeneration Temperature; Università di Torino: Turin, Italy, 2023; status (submitted). [Google Scholar]
- Jung, J.; Jeong, Y.S.; Lim, Y.; Lee, C.S.; Han, C. Advanced CO2 capture process using MEA scrubbing: Configuration of a split flow and phase separation heat exchanger. Energy Procedia 2013, 37, 1778–1784. [Google Scholar] [CrossRef]
- Arstad, B.; Blom, R.; Swang, O. CO2 absorption in aqueous solutions of alkanolamines: Mechanistic insight from quantum chemical calculations. J. Phys. Chem. A 2007, 111, 1222–1228. [Google Scholar] [CrossRef]
- Da Silva, E.F.; Svendsen, H.F. Ab initio study of the reaction of carbamate formation from CO2 and alkanolamines. Ind. Eng. Chem. Res. 2004, 43, 3413–3418. [Google Scholar] [CrossRef]
- Xie, H.B.; Zhou, Y.; Zhang, Y.; Johnson, J.K. Reaction mechanism of monoethanolamine with CO2 in aqueous solution from molecular modelling. J. Phys. Chem. A 2010, 114, 11844–11852. [Google Scholar] [CrossRef] [PubMed]
- Danckwerts, P.V. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 1979, 34, 443–446. [Google Scholar] [CrossRef]
- Lv, B.; Guo, B.; Zhou, Z.; Jing, G. Mechanisms of CO2 Capture into Monoethanolamine Solution with Different CO2 Loading during the Absorption/Desorption Processes. Environ. Sci. Technol. 2015, 49, 10728–10735. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fang, M.; Pan, Y.; Yan, S.; Luo, Z. Amine-based absorbents selection for CO2 membrane vacuum regeneration technology by combined absorption-desorption analysis. Chem. Eng. Sci. 2013, 93, 238–249. [Google Scholar] [CrossRef]
- Oh, S.Y.; Binns, M.; Cho, H.; Kim, J.K. Energy minimization of MEA-based CO2 capture process. Appl. Energy 2016, 169, 353–362. [Google Scholar] [CrossRef]
- Liu, J.; Wang, S.; Zhao, B.; Tong, H.; Chen, C. Absorption of carbon dioxide in aqueous ammonia. Energy Procedia 2009, 1, 933–940. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, Y. Analysis on Regeneration Energy of NH3-based CO2 Capture with Equilibrium-based Simulation Method. Energy Procedia 2017, 114, 1480–1487. [Google Scholar] [CrossRef]
- Chung, W.; Roh, K.; Lee, J.H. Design and evaluation of CO2 capture plants for the steelmaking industry by means of amine scrubbing and membrane separation. Int. J. Greenh. Gas Control 2018, 74, 259–270. [Google Scholar] [CrossRef]
- Van den Akker, J.T. Carbon Capture Onboard LNG-Fueled Vessels: A Feasibility Study. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2 October 2017. Available online: https://repository.tudelft.nl/islandora/object/uuid:a94741f3-c7cb-4970-80d1-bceebff4e423?collection=education (accessed on 31 August 2023).
- Awoyomi, A.; Patchigolla, K.; Anthony, E.J. CO2/SO2 emission reduction in CO2 shipping infrastructure. Int. J. Greenh. Gas Control 2019, 88, 57–70. [Google Scholar] [CrossRef]
- Awoyomi, A.; Patchigolla, K.; Anthony, E.J. Process and economic evaluation of an onboard capture system for LNG-fueled CO2 carriers. Ind. Eng. Chem. Res. 2020, 59, 6951–6960. [Google Scholar] [CrossRef]
- Lee, S.; Yoo, S.; Park, H.; Ahn, J.; Chang, D. Novel methodology for EEDI calculation considering onboard carbon capture and storage system. Int. J. Greenh. Gas Control 2021, 105, 103241. [Google Scholar] [CrossRef]
- Asimakopoulou, A. MemCCSea: Carbon Capture at Sea Project Concept & Key Objective; CATO: Rotterdam, The Netherlands, 2022; Available online: https://www.co2-cato.org/cato-download/5823/20220623_160651_MemCCSea.pdf (accessed on 19 June 2023).
- Mastropasqua, L.; Pierangelo, L.; Spinelli, M.; Romano, M.C.; Campanari, S.; Consonni, S. Molten carbonate fuel cells retrofits for CO2 capture and enhanced energy production in the steel industry. Int. J. Greenh. Gas Control 2019, 88, 195–208. [Google Scholar] [CrossRef]
- Campanari, S.; Chiesa, P.; Manzolini, G.; Giannotti, A.; Federici, F.; Bedont, P.; Parodi, F. Application of MCFCs for active CO2 capture within natural gas combined cycles. Energy Procedia 2011, 4, 1235–1242. [Google Scholar] [CrossRef]
- Carapellucci, R.; di Battista, D.; Cipollone, R. The retrofitting of a coal-fired subcritical steam power plant for carbon dioxide capture: A comparison between MCFC-based active systems and conventional MEA. Energy Convers. Manag. 2019, 194, 124–139. [Google Scholar] [CrossRef]
- Archetti, M.; Audasso, E.; Bosio, B.; Bove, D. High temperature fuel cells to reduce CO2 emission in the maritime sector. E3S Web Conf. 2022, 334, 1–8. [Google Scholar] [CrossRef]
- Barckholtz, T.A.; Taylor, K.M.; Narayanan, S.; Jolly, S.; Ghezel-Ayagh, H. Molten carbonate fuel cells for simultaneous CO2 capture, power generation, and H2 generation. Appl. Energy 2022, 313, 118553. [Google Scholar] [CrossRef]
- Campari, S.; Manzolini, G.; Chiesa, P. Using MCFC for high efficiency CO2 capture from natural gas combined cycles: Comparison of internal and external reforming. Appl. Energy 2013, 112, 772–783. [Google Scholar] [CrossRef]
- Audasso, E.; Kim, K.; Accardo, G.; Sung, K.H.; Yoon, P.S. Investigation of molten carbonate electrolysis cells performance for H2 production and CO2 capture. J. Power Sources 2022, 523, 231039. [Google Scholar] [CrossRef]
- Rexed, I. Applications for Molten Carbonate Fuel Cells; KTH Royal Institute of Technology, School of Chemical Science and Engineering: Stockholm, Sweden, 2014. [Google Scholar]
- Perez-Trujillo, J.P.; Elizalde-Blancas, F.; Della Pietra, M.; McPhail, S.J. A numerical and experimental comparison of a single reversible molten carbonate cell operating in fuel cell mode and electrolysis mode. Appl. Energy 2018, 226, 1037–1055. [Google Scholar] [CrossRef]
- Audasso, E.; Bosio, B.; Bove, D.; Arato, E.; Barckholtz, T.; Kiss, G.; Rosen, J.; Elsen, H.; Gutierrez, R.B.; Han, L. New, Dual-Anion Mechanism for Molten Carbonate Fuel Cells Working as Carbon Capture Devices. J. Electrochem. Soc. 2020, 167, 8. [Google Scholar] [CrossRef]
- Audasso, E.; Bosio, B.; Bove, D.; Arato, E.; Barckholtz, T.; Kiss, G.; Rosen, J.; Elsen, H.; Gutierrez, R.B.; Han, L. The Effects of Gas Diffusion in Molten Carbonate Fuel Cells Working as Carbon Capture Devices. J. Electrochem. Soc. 2020, 167, 11. [Google Scholar] [CrossRef]
- Morita, H.; Kawase, M.; Mugikura, Y. AsaDegradation mechanism of molten carbonate fuel cell based on long-term performance: Long-term operation by using bench-scale cell and post-test analysis of the cell. J. Power Sources 2010, 195, 6988–6996. [Google Scholar] [CrossRef]
- Barckholtz, T.; Elsen, H.; Kalamaras; Kiss, G.; Rosen, J.; Bove, D.; Audasso, E.; Bosio, B. Experimental and Modeling Investigation of CO3−−/OH− Equilibrium Effects on Molten Carbonate Fuel Cell Performance in Carbon Capture Applications. Frontiers 2021, 9, 669761. [Google Scholar] [CrossRef]
- Bove, D.; Audasso, E.; Barckholtz, T.; Kiss, G.; Rosen, J.; Bosio, B. Process analysis of molten carbonate fuel cells in carbon capture applications. Int. J. Hydrog. Energy 2021, 46, 15032–15045. [Google Scholar] [CrossRef]
- Rosen, J.; Geary, T.; Hilmi, A.; Blanco-Gutierrez, R.; Yuh, C.Y.; Pereira, C.S.; Han, L.; Johnson, R.A.; Willman, C.A.; Ghezel-Ayagh, H. Molten Carbonate Fuel Cell Performance for CO2 Capture from Natural Gas Combined Cycle Flue Gas. J. Electrochem. Soc. 2020, 167, 6. [Google Scholar] [CrossRef]
- Boudouard Equilibrium. Available online: https://www.giessereilexikon.com/en/foundry-lexicon/Encyclopedia/show/boudouard-equilibrium-4705/?cHash=e172a61ebbc38ae84221e9903d5ec7a4 (accessed on 21 December 2022).
- Randstro, S. New Materials for the Molten Carbonate Fuel Cell. Ph.D. Thesis, KTH, School of Chemical Science and Engineering (CHE), Chemical Engineering and Technology, Applied Electrochemistry, Stockholm, Sweden, 2008. Available online: https://www.diva-portal.org/smash/get/diva2:13180/FULLTEXT01.pdf (accessed on 16 May 2023).
- Bosio, B.; Archetti, M.; Audasso, E.; Bove, D. Process analysis of a molten carbonate fuel cell on-board application to reduce vessel CO2 emissions. Chem. Eng. Process. Process Intensif. 2023, 190, 109415. [Google Scholar] [CrossRef]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of hydrogen safety during storage, transmission, and applications processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Takahashi, T.; Kawabata, M.; Yoshida, A.; Kai, T.; Kimura, H.; Inoue, A. Methanol Steam Reforming over Copper Catalyst Prepared from Amorphous Cu-Zr Alloy with Minute Amounts of Noble Metals. Stud. Surf. Sci. Catal. 2007, 172, 293–296. [Google Scholar]
- Office of Energy Efficiency & Renewable Energy. Methane Reforming. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming (accessed on 2 January 2023).
- Della Pietra, M.; Discepoli, G.; Bosio, B.; McPhail, S.J.; Barelli, L.; Bidini, G.; Ribes-Greus, A. Experimental investigation of SO2 poisoning in a Molten Carbonate Fuel Cell operating in CCS configuration. Int. J. Hydrog. Energy 2016, 41, 18822–18836. [Google Scholar] [CrossRef]
- Ship Technology. Viking Lady. Available online: https://www.ship-technology.com/projects/viking-lady/ (accessed on 11 January 2023).
- Inal, O.B.; Deniz, C. Emission Analysis of LNG Fuelled Molten Carbonate Fuel Cell System for a Chemical Tanker Ship: A Case Study. Mar. Sci. Technol. Bull. 2020, 10, 118–133. [Google Scholar] [CrossRef]
- Han, S.J.; Yoo, M.; Kim, D.W.; Wee, J.H. Carbon dioxide capture using calcium hydroxide aqueous solution as the absorbent. Energy Fuels 2011, 25, 3825–3834. [Google Scholar] [CrossRef]
- Costagliola, M.A.; Prati, M.V.; Perretta, G. Post combustion CO2 capture with calcium and lithium hydroxide. Sci. Rep. 2022, 12, 10518. [Google Scholar] [CrossRef]
- Caserini, S.; Pagano, D.; Campo, F.; Abbà, A.; De Marco, S.; Righi, D.; Renforth, P.; Grosso, M. Potential of Maritime Transport for Ocean Liming and Atmospheric CO2 Removal. Front. Clim. 2021, 3, 575900. [Google Scholar] [CrossRef]
- Archetti, M.; Bosio, B. Road to maritime sector decarbonization. In Technology and Science for the Ship of the Future; Rizzuto, E., Ruggiero, V., Eds.; IOS Press: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Harvey, L.D.D. Mitigating the atmospheric CO2 increase and ocean acidification by adding limestone powder to upwelling regions. J. Geophys. Res. Ocean. 2008, 113, 4. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, H. Carbon capture and storage-Solidification and storage of carbon dioxide captured on ships. Ocean Eng. 2014, 91, 172–180. [Google Scholar] [CrossRef]
- Ecospray Technologies S.R.L. Internal Technical Report, Carbon Capture with Calcium Hydroxide; Ecospray Technologies S.R.L.: Alzano Scrivia, Italy, 2023. [Google Scholar]
- Foteinis, S.; Andresen, J.; Campo, F.; Caserini, S.; Renforth, P. Life cycle assessment of ocean liming for carbon dioxide removal from the atmosphere. J. Clean. Prod. 2022, 370, 133309. [Google Scholar] [CrossRef]
- Limestone to Calcium Hydroxide. Available online: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Block/1_s-Block_Elements/Group__2_Elements%3A_The_Alkaline_Earth_Metals/Z020_Chemistry_of_Calcium_(Z20)/Calcium_Hydroxide (accessed on 25 June 2023).
- Limestone and Calcium Oxide. Available online: https://www.essentialchemicalindustry.org/chemicals/calcium-carbonate.html (accessed on 3 July 2023).
- Oh, J.; Anantharaman, R.; Zahid, U.; Lee, P.S.; Lim, Y. Process design of onboard membrane carbon capture and liquefaction systems for LNG-fueled ships. Sep. Purif. Technol. 2022, 282, 120052. [Google Scholar] [CrossRef]
- Siagian, U.W.R.; Raksajati, A.; Himma, N.F.; Khoiruddin, K.; Wenten, I.G. Membrane-based carbon capture technologies: Membrane gas separation vs. membrane contactor. J. Nat. Gas. Sci. Eng. 2019, 67, 172–195. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Z.; Zhang, L.; Zhang, Z. Decarburization characteristics of coalbed methane by membrane separation technology. Fuel 2019, 242, 470–478. [Google Scholar] [CrossRef]
- Damartzis, T.; Asimakopoulou, A.; Koutsonikolas, D.; Skevis, G.; Georgopoulou, C.; Dimopoulos, G.; Nikolopoulos, L.; Bougiouris, K.; Richter, H.; Lubenau, U. Solvents for Membrane-Based Post-Combustion CO2 Capture for Potential Application in the Marine Environment. Appl. Sci. 2022, 12, 6100. [Google Scholar] [CrossRef]
- Nasiman, T.; Kanoh, H. CO2 Capture by a K2CO3-Carbon Composite under Moist Conditions. Ind. Eng. Chem. Res. 2020, 59, 3405–3412. [Google Scholar] [CrossRef]
- Borhani, T.N.G.; Azarpour, A.; Akbari, V.; Alwi, S.R.W.; Manan, Z.A. CO2 capture with potassium carbonate solutions: A state-of-the-art review. Int. J. Greenh. Gas. Control 2015, 41, 142–162. [Google Scholar] [CrossRef]
- Balsamo, M.; Erto, A.; Lancia, A.; di Natale, F. Carbon dioxide capture from model marine diesel engine exhaust by means of K2CO3-based sorbents. Chem. Eng. Trans. 2016, 52, 415–420. [Google Scholar]
- Krödel, M.; Landuyt, A.; Abdala, P.M.; Müller, C.R. Mechanistic Understanding of CaO-Based Sorbents for High-Temperature CO2 Capture: Advanced Characterization and Prospects. ChemSusChem 2020, 13, 6259–6272. [Google Scholar] [CrossRef]
- Larkin, C.; Morrison, J.; Hemmings, M.; Guanhong, L.; Zhang, G.; Oliva, F.; García–García, F.R. Hollow Fibre Adsorption Unit for On-board Carbon Capture: The Key to Reducing Transport Emissions. Carbon. Capture Sci. Technol. 2022, 2, 100034. [Google Scholar] [CrossRef]
- Olson, J. Rates of Poisoning in Fixed-Bed Reactors. Ind. Eng. Chem. Fundam. 1968, 7, 185–188. [Google Scholar] [CrossRef]
- Ramdin, M. CO2 Capture with Ionic Liquids: Experiments and Molecular Simulations. Ph.D. Thesis, Mechanical, Maritime and Materials Engineering, Department of Process and Energy, Delft University of Technology, Delft, The Netherlands, 1 December 2015. Available online: https://repository.tudelft.nl/islandora/object/uuid%3Ab6ed1913-75b8-44c4-8e6e-4d8744917b84 (accessed on 28 May 2023).
- Aghaie, M.; Rezaei, N.; Zendehboudi, S. A systematic review on CO2 capture with ionic liquids: Current status and future prospects. Renew. Sustain. Energy Rev. 2018, 96, 502–525. [Google Scholar] [CrossRef]
- Stevanovic, S.; Podgorsek, A.; Moura, L.; Santini, C.C.; Padua, A.A.H.; Gomes, M.F.C. Absorption of carbon dioxide by ionic liquids with carboxylate anions. Int. J. Greenh. Gas Control 2013, 17, 78–88. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 absorption studies in amino acid-anion based ionic liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Dong, H.; Zhao, Z.; Zhang, S.; Huang, Y. Carbon capture with ionic liquids: Overview and progress. Energy Environ. Sci. 2012, 5, 6668–6681. [Google Scholar] [CrossRef]
- Ramdin, M.; De Loos, T.W.; Vlugt, T.J.H. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. Carbon dioxide absorption studies using amine-functionalized ionic liquids. J. Ind. Eng. Chem. 2014, 20, 2497–2509. [Google Scholar] [CrossRef]
- Kaviani, S.; Kolahchyan, S.; Hickenbottom, K.L.; Lopez, A.M.; Nejati, S. Enhanced solubility of carbon dioxide for encapsulated ionic liquids in polymeric materials. Chem. Eng. J. 2018, 354, 753–757. [Google Scholar] [CrossRef]
- Kearns, D.; Liu, H.; Consoli, C. Technology Readiness and Costs of CCS; Global CCS Institute: Melbourne, Australia, 2021; Available online: https://www.globalccsinstitute.com/wp-content/uploads/2021/03/Technology-Readiness-and-Costs-for-CCS-2021-1.pdf (accessed on 6 May 2023).
- Ramezani, R.; Di Felice, L.; Gallucci, F. A Review on Hollow Fiber Membrane Contactors for Carbon Capture: Recent Advances and Future Challenges. Processes 2022, 10, 2103. [Google Scholar] [CrossRef]
- Zhai, H.; Rubin, E.S. Systems analysis of ionic liquids for post-combustion CO2 capture at coal-fired power plants. Energy Procedia 2014, 63, 1321–1328. [Google Scholar] [CrossRef]
- Al-Enazi, A.; Okonkwo, E.C.; Bicer, Y.; Al-Ansari, T. A review of cleaner alternative fuels for maritime transportation. Energy Rep. 2021, 7, 1962–1985. [Google Scholar] [CrossRef]
- Bulk, S.; Is carbon capture on ship feasible? Oil Gas. Clim. Initiat. 2021. Available online: https://www.ogci.com/wp-content/uploads/2023/04/OGCI_STENA_MCC_November_2021.pdf (accessed on 21 May 2023).
- Panja, P.; McPherson, B.; Deo, M. Techno-Economic Analysis of Amine-based CO2 Capture Technology: Hunter Plant Case Study. Carbon Capture Sci. Technol. 2022, 3, 100041. [Google Scholar] [CrossRef]
- Roussanaly, S.; Anantharaman, R. Cost-optimal CO2 capture ratio for membrane-based capture from different CO2 sources. Chem. Eng. J. 2017, 327, 618–628. [Google Scholar] [CrossRef]
- Wood and Department for Business, Energy and Industrial Strategy. Assessing the Cost Reduction Potential and Competitiveness of Novel (Next Generation) UK Carbon Capture Technology. 13333-8820-RP-001 Benchmarking State-of-the-Art and Next Generation Technologies. 2018. Available online: https://www.woodplc.com (accessed on 15 June 2023).
- International CCS Knowledge Centre. The Shand CCS Feasibility Study. Public Report. 2018. Available online: https://ccsknowledge.com/ (accessed on 26 June 2023).
- CCS Report. Available online: https://www.iea.org/fuels-and-technologies/carbon-capture-utilisation-and-storage (accessed on 20 May 2023).
- Benson, S.M.; Orr, F.M. Carbon dioxide capture and storage. MRS Bull. 2008, 33, 303–305. [Google Scholar] [CrossRef]
- Mc-Kinney, M.; Møller Center for Zero Carbon Shipping. A Case Study of the Largest Shipping Segments, Main Carbon-Based Fuels, and Full and Partial Application as Part of a Newbuild or Retrofit. 2022, pp. 1–15. Available online: https://cms.zerocarbonshipping.com/media/uploads/publications/The-role-of-onboard-carbon-capture-in-martime-decarbonization.pdf (accessed on 7 August 2023).
- Race, J.; Aghajani, H.; Wetenhall, B.; Benson, S.D.; Chalmers, H.; Ferrari, M.C.; Li, J. Impact of CO2 impurity on CO2 compression, liquefaction and transportation. Energy Procedia 2014, 63, 2764–2778. [Google Scholar] [CrossRef]
- Chen, F.; Morosuk, T. Exergetic and economic evaluation of CO2 liquefaction processes. Energies 2021, 14, 7174. [Google Scholar] [CrossRef]
- Wilkes, M.D.; Mukherjee, S.; Brown, S. Linking CO2 capture and pipeline transportation: Sensitivity analysis and dynamic study of the compression train. Int. J. Greenh. Gas Control 2021, 11, 103449. [Google Scholar] [CrossRef]
- Øi, L.E.; Eldrup, N.; Adhikari, U.; Bentsen, M.H.; Badalge, J.L.; Yang, S. Simulation and cost comparison of CO2 liquefaction. Energy Procedia 2016, 86, 500–510. [Google Scholar] [CrossRef]
- Witkowski, A.; Majkut, M.; Rulik, S. Analysis of pipeline transportation systems for carbon dioxide sequestration. Arch. Thermodyn. 2014, 35, 117–140. [Google Scholar] [CrossRef]
- Deng, H.; Roussanaly, S.; Skaugen, G. Techno-economic analyses of CO2 liquefaction: Impact of product pressure and impurities. Int. J. Refrig. 2019, 103, 301–315. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.D.; Adu, E.; Yang, J.P.; Shen, Q.W.; Tian, L.; Wu, L. Influence of dense phase CO2 pipeline transportation parameters. Int. J. Heat. Technol. 2016, 34, 479–484. [Google Scholar] [CrossRef]
- Gough, C.; O’keefe, L.; Mander, S. COOLTRANS (Dense Phase Carbon Dioxide Pipeline Transportation) WP5.2.1 Social Impacts of the Installation of Pipeline Network; Tyndall Manchester Climate Change Research: Manchester, UK, 2012; Available online: https://documents.manchester.ac.uk/display.aspx?DocID=42714 (accessed on 9 August 2023).
- Maritime Engineering Study Materials. Available online: https://marineengineeringonline.com/co2-flooding-system-on-ships/ (accessed on 14 February 2023).
- Basini, L.E.; Furesi, F.; Baumgärtl, M.; Mondelli, N.; Pauletto, G. CO2 capture and utilization (CCU) by integrating water electrolysis, electrified reverse water gas shift (E-RWGS) and methanol synthesis. J. Clean. Prod. 2022, 377, 134280. [Google Scholar] [CrossRef]
- Parekh, A.; Chaturvedi, G.; Dutta, A. Sustainability analyses of CO2 sequestration and CO2 utilization as competing options for mitigating CO2 emissions. Sustain. Energy Technol. Assess. 2023, 55, 102942. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research progress on CO2 capture and utilization technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- EU Carbon Credits Trading. Available online: https://tradingeconomics.com/commodity/carbon (accessed on 30 March 2023).
- Weber, V. Are we ready for the ship transport of CO2 for CCS? Crude solutions from international and European law. Rev. Eur. Comp. Int. Environ. Law 2021, 30, 387–395. [Google Scholar] [CrossRef]
- Giannelos, S.; Konstantelos, I.; Strbac, G. A new class of planning models for option valuation of storage technologies under decision-dependent innovation uncertainty. In Proceedings of the 2017 IEEE Manchester PowerTech, Manchester, UK, 18–22 June 2017. [Google Scholar] [CrossRef]
- Giannelos, S.; Jain, A.; Borozan, S.; Falugi, P.; Moreira, A.; Bhakar, R.; Mathur, J.; Strbac, G. Long-Term Expansion Planning of the Transmission Network in India under Multi-Dimensional Uncertainty. Energies 2021, 14, 7813. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, W.; Huang, Y.; Li, P.; Dong, L. Simultaneous optimization of renewable energy and energy storage capacity with the hierarchical control. CSEE J. Power Energy Syst. 2022, 8, 95–104. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).