Effects of Operating Conditions on the Oxygen Removal Performance of the Deoxo Chamber in the Water Electrolysis System
Abstract
:1. Introduction
2. Numerical Condition and Methods
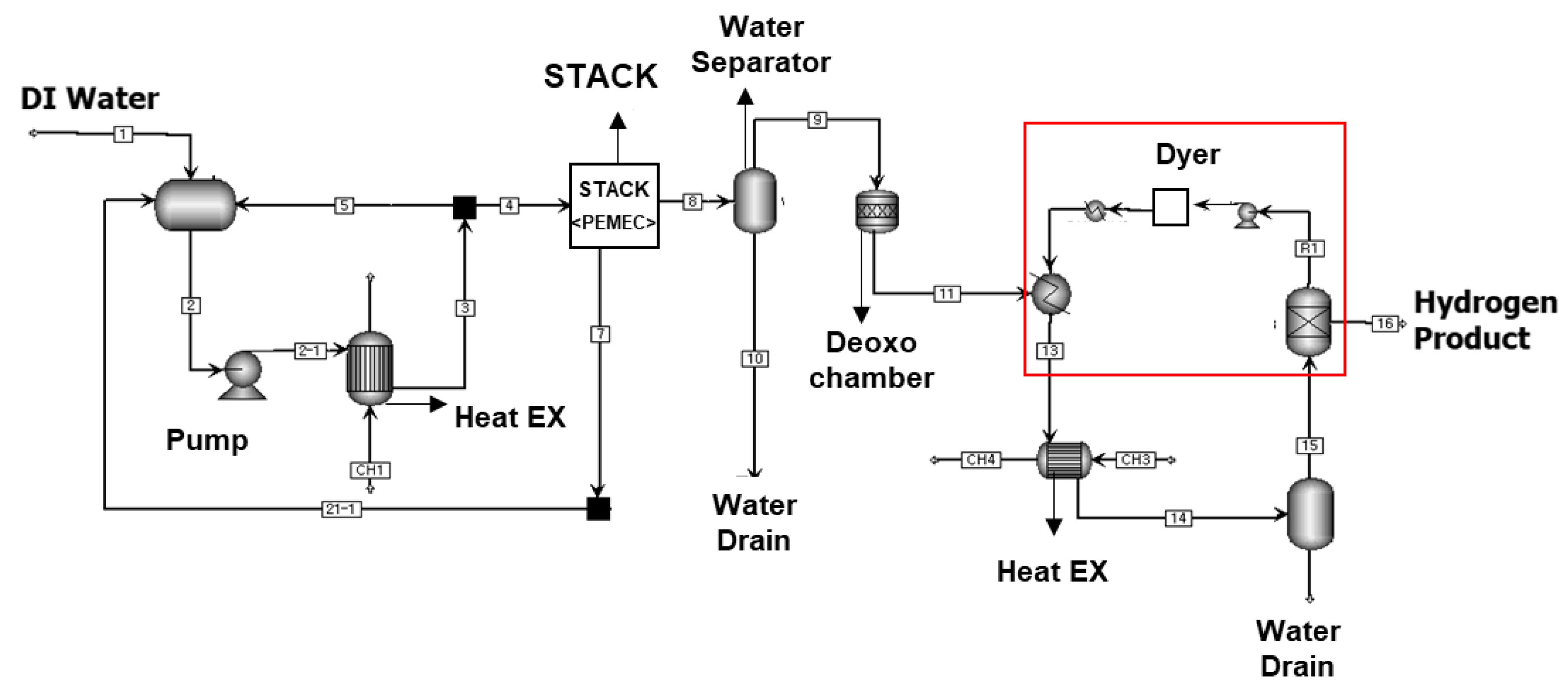
2.1. Process Analysis of the Electrolysis System
2.2. Physical Models and Numerical Methods
2.2.1. Governing Equation
2.2.2. Catalyst Reaction
2.2.3. Reaction Model for Oxygen Removal
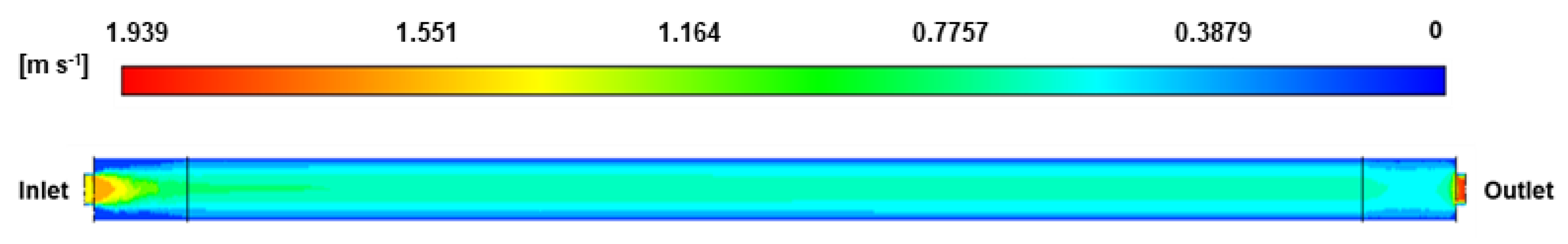
2.2.4. Geometric of the Deoxo Chamber and Boundary Condition
3. Results and Discussion
3.1. Process Analysis Temperature of the Water Electrolysis System
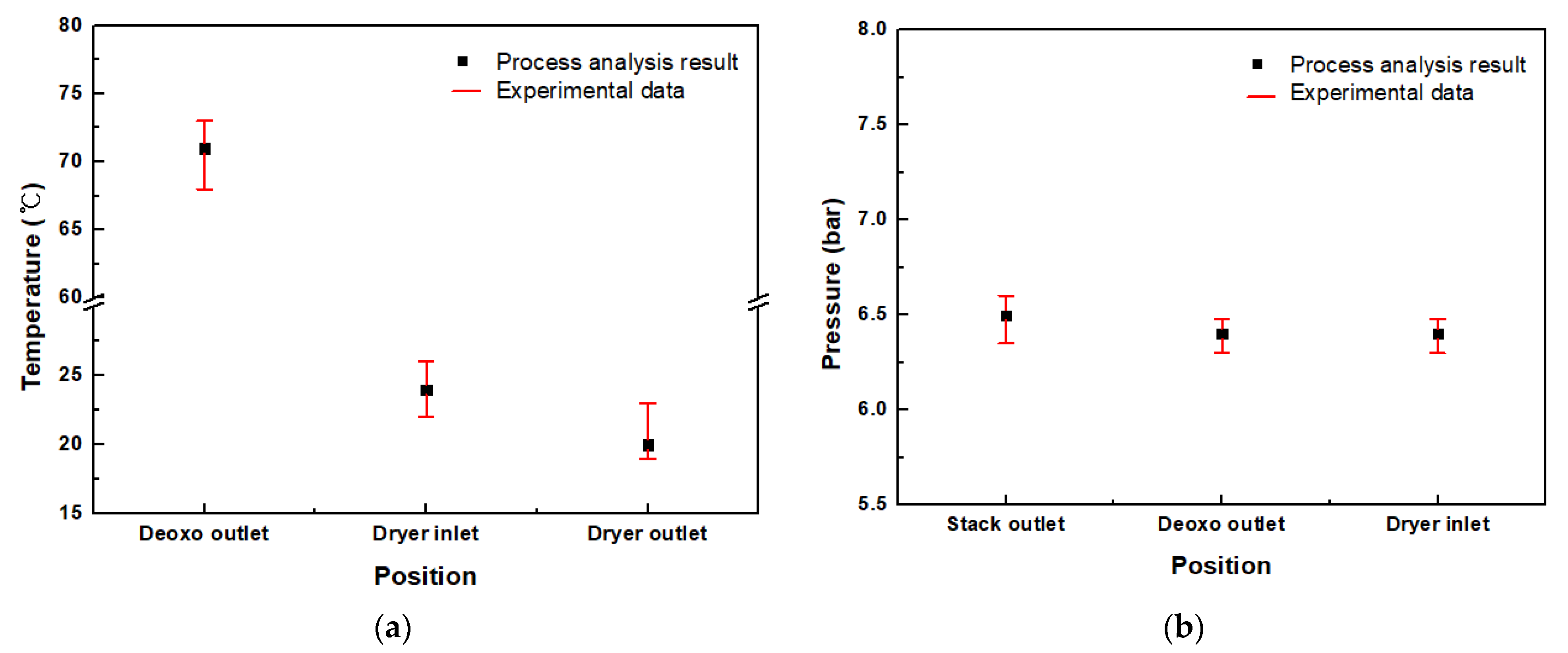
3.1.1. Temperature Comparison of the Test Results and Process Analysis
3.1.2. Pressure Comparison of the Test Results and Process Analysis
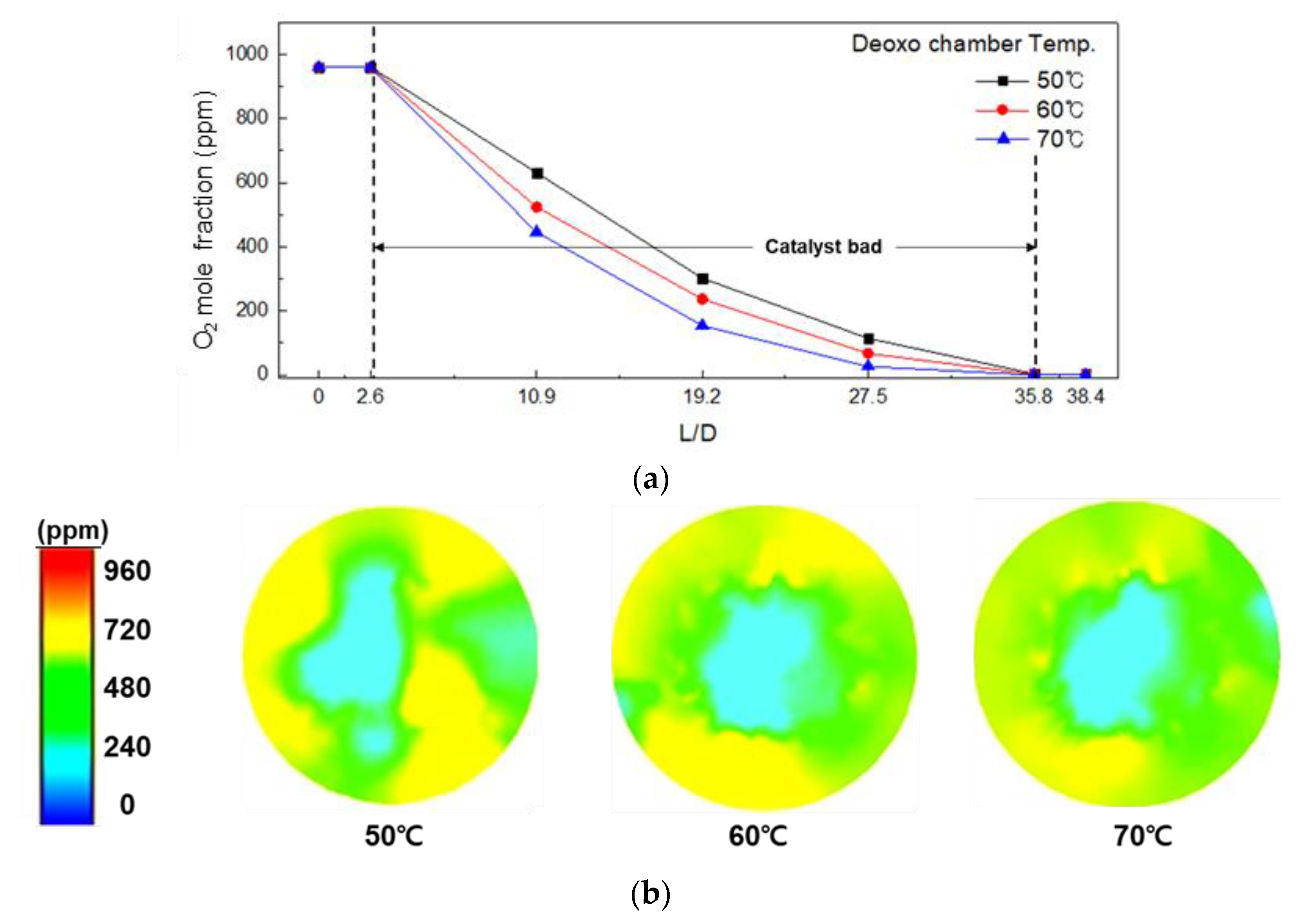
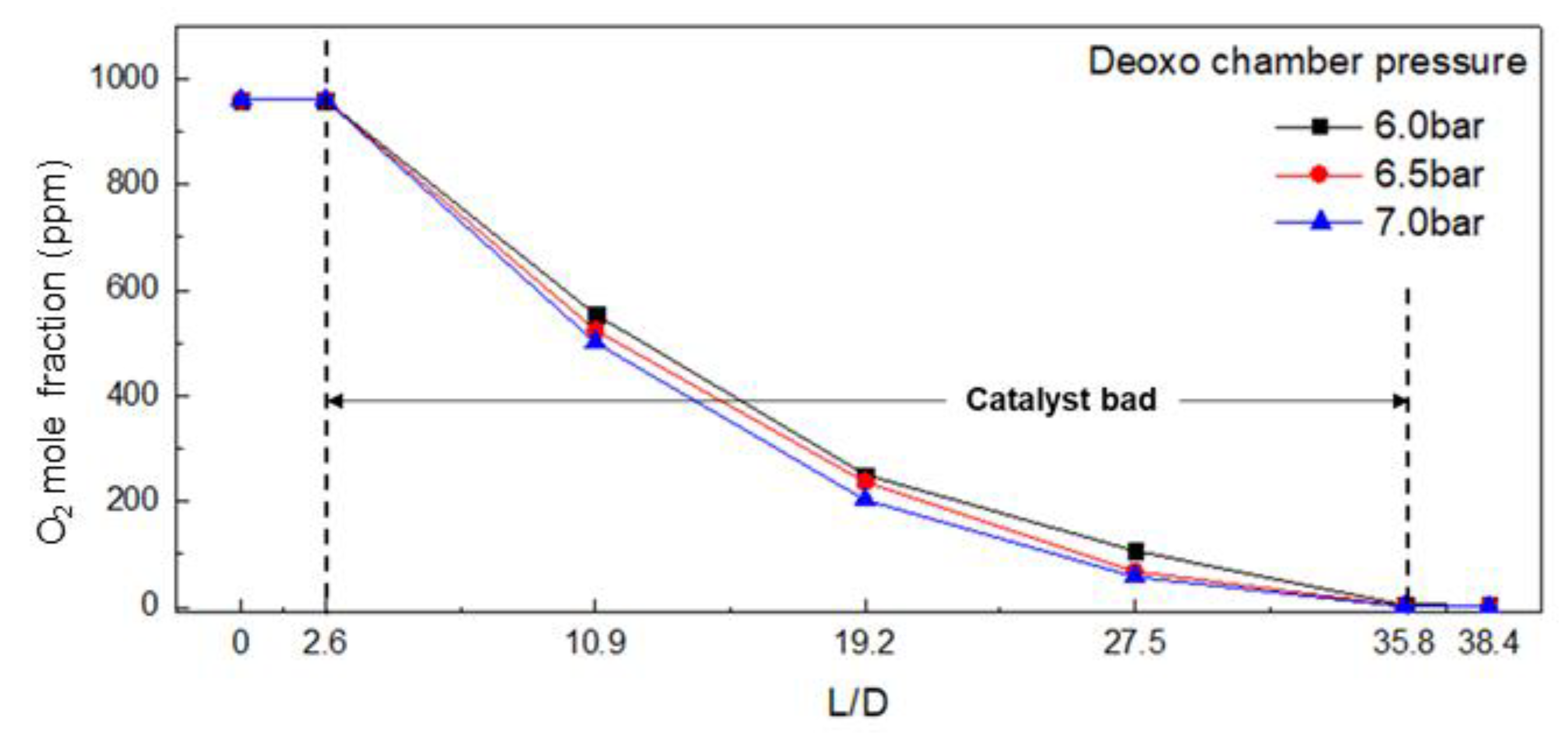
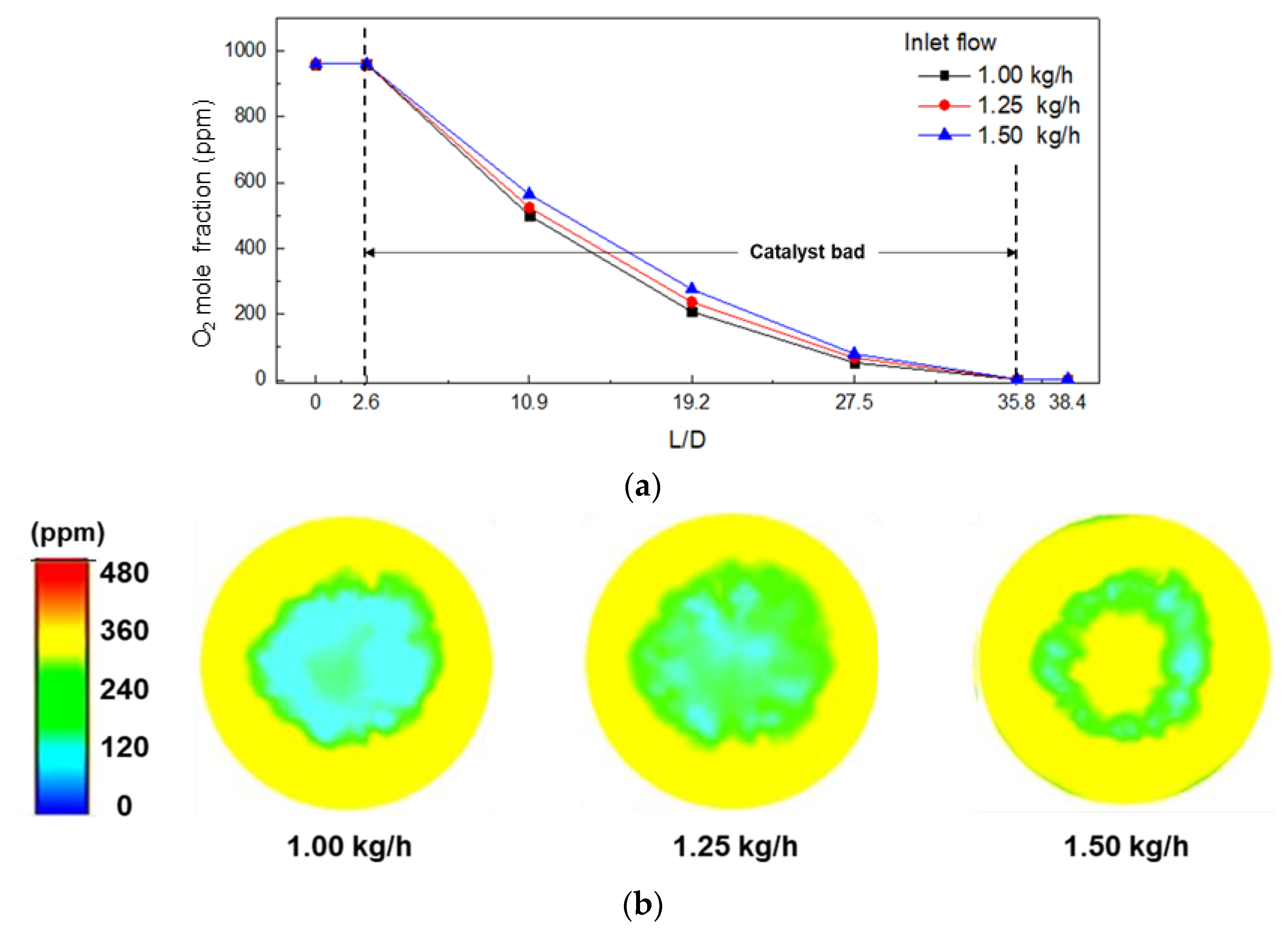
3.2. Effect of Operating Conditions on the O2 Mole Fraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Chen, X.; Wang, L. Increasing permeability of coal seams using the phase energy of liquid carbon dioxide. J. CO2 Util. 2017, 19, 112–119. [Google Scholar] [CrossRef]
- Zhang, X.P.; Cheng, X.M. Energy consumption, carbon emissions, and economic growth in China. Ecol. Econ. 2009, 68, 2706–2712. [Google Scholar] [CrossRef]
- Schlenker, W.; Roberts, M.J. Nonlinear temperature effects indicate severe damages to U.S. crop yields under climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 15594–15598. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Trihamdani, A.R.; Kubota, T.; Iizuka, S.; Phuong, T.T.T. Impacts of land use changes from the Hanoi Master Plan 2030 on urban heat islands: Part 2. Influence of global warming. Sustain. Cities Soc. 2017, 31, 95–108. [Google Scholar] [CrossRef]
- Bale, J.S.; Masters, G.J.; Hodkinson, I.D.; Awmack, C.; Bezemer, T.M.; Brown, V.K. Herbivory in global climate change research: Direct effects of rising temperature on insect herbivores. Glob. Change Biol. 2002, 8, 1–16. [Google Scholar] [CrossRef]
- McCarthy, M.P.; Best, M.J.; Betts, R.A. Climate change in cities due to global warming and urban effects. Geophys. Res. Lett. 2010, 37, 1–5. [Google Scholar] [CrossRef]
- Chiu, Y.B. Carbon dioxide, income and energy: Evidence from a non-linear model. Energy Econ. 2017, 61, 279–288. [Google Scholar] [CrossRef]
- Edelenbosch, O.Y.; Kermeli, K.; Crijns-Graus, W.; Worrell, E.; Bibas, R.; Fais, B. Comparing projections of industrial energy demand and greenhouse gas emissions in long-term energy models. Energy 2017, 122, 701–710. [Google Scholar] [CrossRef]
- Ogunbode, C.A.; Doran, R.; Böhm, G. Exposure to the IPCC special report on 1.5 °C global warming is linked to perceived threat and increased concern about climate change. Clim. Change 2020, 158, 361–375. [Google Scholar] [CrossRef]
- Schröder, V.; Emonts, B.; Janßen, H.; Schulze, H.P. Explosion Limits of Hydrogen/Oxygen Mixtures at Initial Pressures up to 200 bar. Chem. Eng. Technol. 2004, 27, 847–851. [Google Scholar] [CrossRef]
- Schug, C.A. Operational characteristics of high-pressure, high-efficiency water-hydrogen-electrolysis. Int. J. Hydrogen Energy 1998, 23, 1113–1120. [Google Scholar] [CrossRef]
- Ligen, Y.; Vrubel, H.; Girault, H. Energy efficient hydrogen drying and purification for fuel cell vehicles. Int. J. Hydrogen Energy 2020, 45, 10639–10647. [Google Scholar] [CrossRef]
- Pyle, W. Hydrogen Purification; Home Power: Ashland, OR, USA, 1998. [Google Scholar]
- Kim, G.J.; Shin, J.H.; Chang, H.S. Study on the role of Pt and Pd in Pt-Pd/TiO2 bimetallic catalyst for H2 oxidation at room temperature. Int. J. Hydrogen Energy 2020, 45, 17276–17286. [Google Scholar] [CrossRef]
- Lalik, E.; Kosydar, R.; Tokarz-Sobieraj, R.; Witko, M.; Szumełda, T.; Kołodziej, M. Humidity induced deactivation of Al2O3 and SiO2 supported Pd, Pt, Pd-Pt catalysts in H2+O2 recombination reaction: The catalytic, microcalorimetric and DFT studies. Appl. Catal. A-Gen. 2015, 501, 27–40. [Google Scholar] [CrossRef]
- Haug, P.; Koj, M.; Turek, T. Influence of process conditions on gas purity in alkaline water electrolysis. Int. J. Hydrogen Energy 2017, 42, 9406–9418. [Google Scholar] [CrossRef]
- Yasnev, I.M.; Mel’nichenko, A.N.; Gurskii, V.S. A cellular ceramic as a catalyst support for radiolytic gas recombination devices. Russ. J. Appl. Chem. 2020, 93, 927–932. [Google Scholar] [CrossRef]
- Sung, Y.; Choi, M.; Park, T.; Choi, C.; Park, Y.; Choi, G. Synergistic effect of mixer and mixing chamber on flow mixing and NOx reduction in a marine urea-SCR system. Chem. Eng. Process. Process Intensif. 2020, 150, 107888. [Google Scholar] [CrossRef]
- Chapman, D.L.; Gregory, G. The catalysis by palladium of the union of hydrogen and oxygen. Proc. R Soc. Lond A. 1934, 147, 68–75. [Google Scholar]
- Adams, B.D.; Chen, A. The role of palladium in a hydrogen economy. Mater. Today 2011, 14, 282–289. [Google Scholar] [CrossRef]
- Völkening, S.; Bedürftig, K.; Jacobi, K.; Wintterlin, J.; Ertl, G. Dual-Path mechanism for catalytic oxidation of hydrogen on platinum surfaces. Phy.s Rev. Lett. 1999, 83, 2672–2675. [Google Scholar] [CrossRef]
- Kramer, J.F.; Reihani, S.S.; Jackson, G.S. Low-temperature combustion of hydrogen on supported Pd catalysts. Proc. Combust. Inst. 2002, 29, 989–996. [Google Scholar] [CrossRef]
- Kwon, S.; Eom, S.; Yang, J.S.; Choi, G. Development of an In-House Code for Dry Tower of Heat Transfer Analysis in Hydrogen Purification System. Energies 2023, 16, 5090. [Google Scholar] [CrossRef]

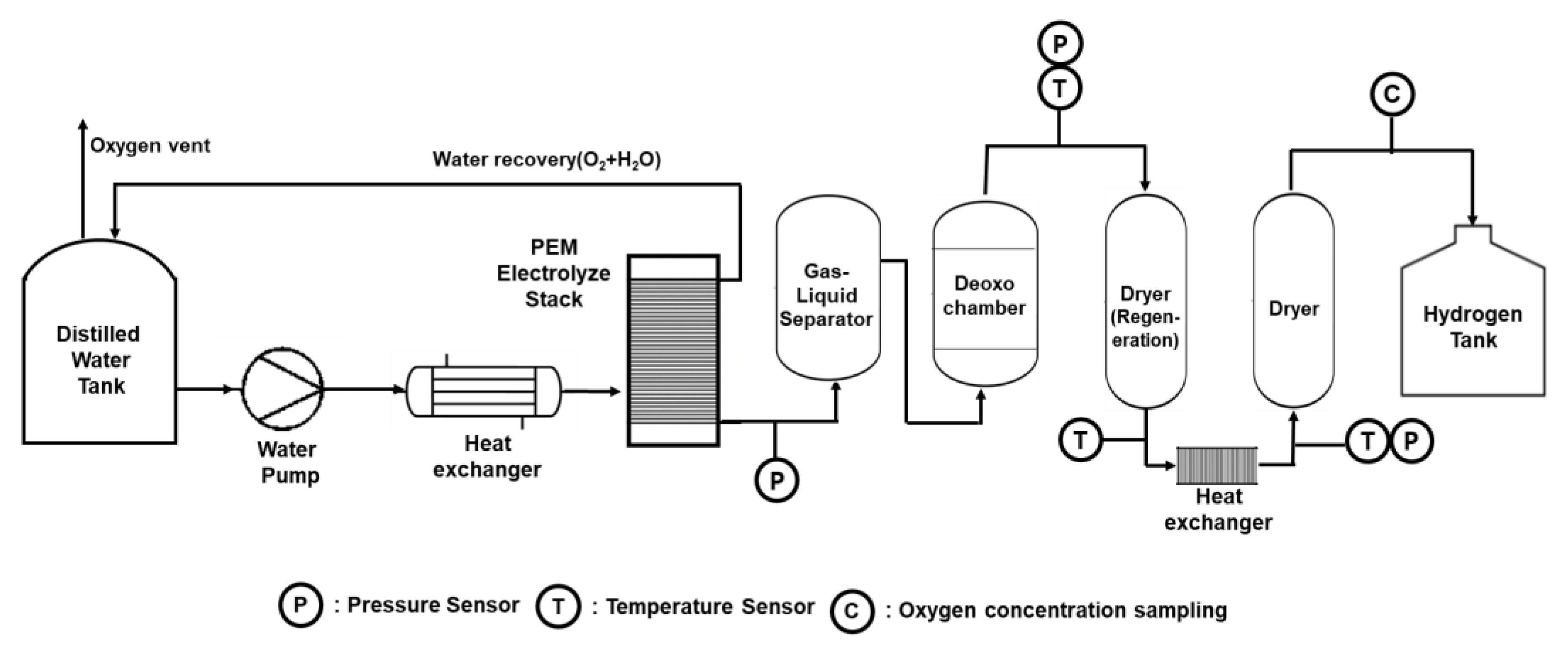

| Deoxo Chamber | ||
|---|---|---|
| Diameter of inlet and outlet | 0.5D | |
| Chamber inner diameter | D | |
| Length | 38.4D | |
| Catalyst | Mean diameter [mm] | 3 |
| Porosity | 0.397–0.476 | |
| Length | 33.2D | |
| Inlet flow [kg/h] | 1.00, 1.25, 1.50 | |
| Inlet temperature [°C] | 50, 60, 70 | |
| Pressure [bar] | 6.0, 6.5, 7.0 | |
| Mole Fractions | H2 | 0.97250 |
| O2 | 0.00096 | |
| H2O | 0.02654 | |
| Stack Inlet | Stack Outlet | Deoxo Inlet | Deoxo Outlet | Dyer Inlet | Dyer Outlet | ||
|---|---|---|---|---|---|---|---|
| Temperature | °C | 50.0 | 60.0 | 60.0 | 71.0 | 24.0 | 20.0 |
| Pressure | bar | 2.0 | 6.5 | 6.5 | 6.4 | 6.4 | 6.4 |
| Mass Flows | kg/h | 2337.135 | 35.090 | 1.247 | 1.247 | 1.247 | 0.988 |
| Volume Flow | m3/h | 2.411 | 1.896 | 1.861 | 1.745 | 1.745 | 1.611 |
| Mole Flows | kmol/h | 129.729 | 2.384 | 0.505 | 0.504 | 0.504 | 0.490 |
| H2O | kmol/h | 129.728 | 1.892 | 0.013 | 0.014 | 0.014 | 0.000 |
| H2 | kmol/h | 0.000 | 0.491 | 0.491 | 0.490 | 0.490 | 0.490 |
| O2 | kmol/h | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mole Fractions | |||||||
| H2O | 0.99999 | 0.79376 | 0.02654 | 0.028484 | 0.028484 | 0.00000 | |
| H2 | 0.00000 | 0.20604 | 0.97250 | 0.971514 | 0.971514 | 0.999998 | |
| O2 | 0.00001 | 0.00020 | 0.00096 | 0.000002 | 0.000002 | 0.000002 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, S.; Eom, S.; Choi, G. Effects of Operating Conditions on the Oxygen Removal Performance of the Deoxo Chamber in the Water Electrolysis System. Energies 2023, 16, 6685. https://doi.org/10.3390/en16186685
Kwon S, Eom S, Choi G. Effects of Operating Conditions on the Oxygen Removal Performance of the Deoxo Chamber in the Water Electrolysis System. Energies. 2023; 16(18):6685. https://doi.org/10.3390/en16186685
Chicago/Turabian StyleKwon, Sooin, Seongyong Eom, and Gyungmin Choi. 2023. "Effects of Operating Conditions on the Oxygen Removal Performance of the Deoxo Chamber in the Water Electrolysis System" Energies 16, no. 18: 6685. https://doi.org/10.3390/en16186685
APA StyleKwon, S., Eom, S., & Choi, G. (2023). Effects of Operating Conditions on the Oxygen Removal Performance of the Deoxo Chamber in the Water Electrolysis System. Energies, 16(18), 6685. https://doi.org/10.3390/en16186685





