Study on SI Engine Operation Stability at Lean Condition—The Effect of a Small Amount of Hydrogen Addition
Abstract
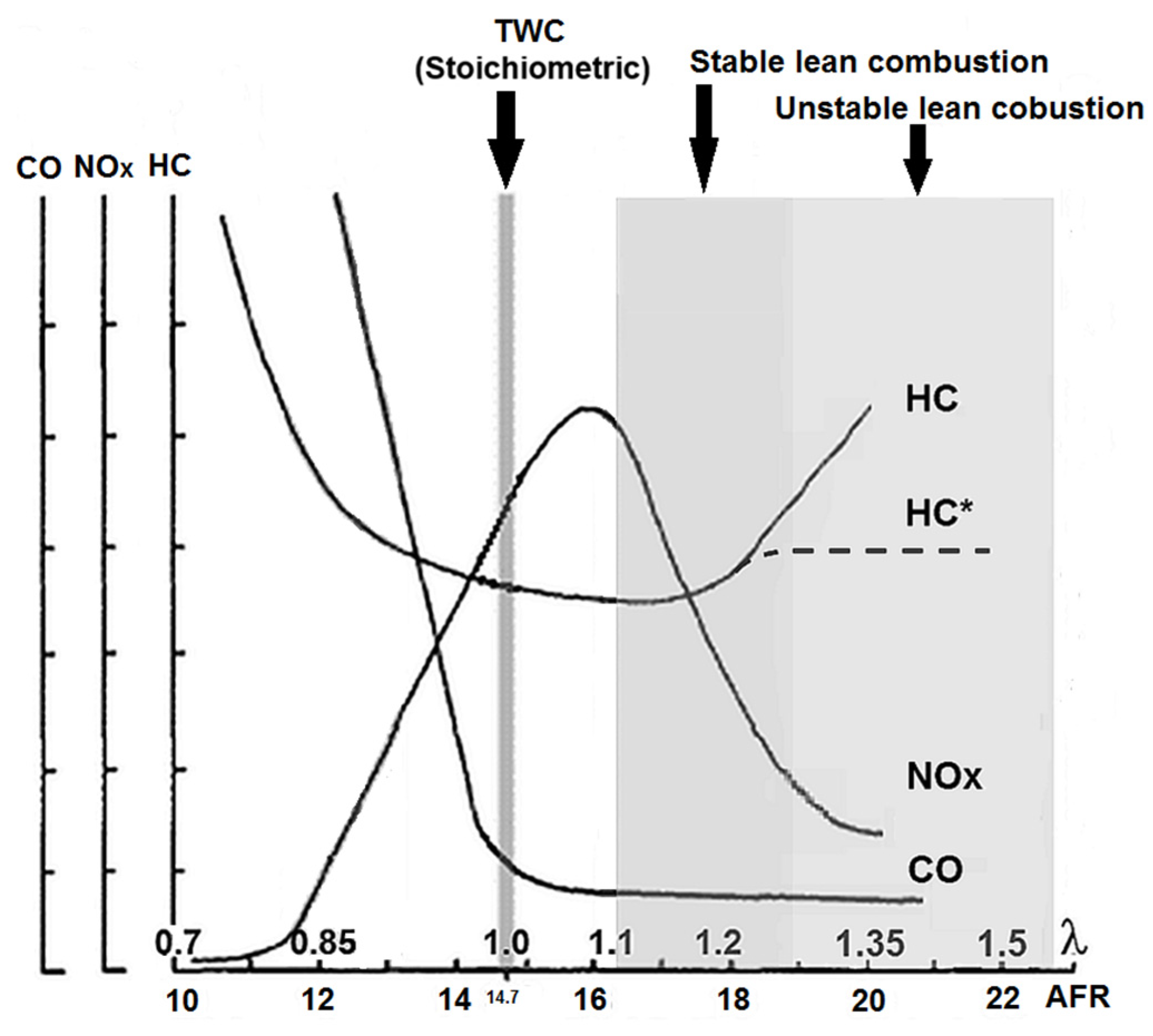
:1. Introduction
2. Experimental Setup and Procedures
2.1. Engine
2.2. Hydrogen Rich Gas Generator Development
2.3. Mesaurement System with HHO Gas Installation
3. Results and Discussion
4. Conclusions
- The results of the laboratory tests show that the HHO gas fuel enhancement enables the improvement of the lean mode combustion stability.
- It should be noted that the addition of pure hydrogen in an amount of at least 1% of the mass fraction is necessary to affect the in-cylinder pressure, but even such a small amount as 0.15–0.3% improves the stability of the lean-burn process. It should be emphasized that, usually, it is not possible to run the modern gasoline engine in unstratified lean-burn mode because of combustion instability and exhaust aftertreatment problems.
- The results of the in-cylinder pressure analysis show that hydrogen enhancement of gasoline in an amount of less than 0.5% of the mass fraction at lean condition has a minor effect on the value of the in-cylinder pressure. However, the impact on the value of the maximum of the pressure derivative due to the shortened flame development and propagation durations is evident.
- The highest impact of a small hydrogen addition on CS is observed at the lowest loads when the instability in combustion at the pure-lean condition is the highest. At a higher load and higher speed when the engine operates more stably, the effect of the enrichment becomes lower.
- A hydrogen enrichment of less than 1% supplied from a small 10–15 dm3 gas cylinder placed onboard a medium or small passenger vehicle may solve the problem of instability at lean conditions in MPI engines at idle or low-load operation. This can be the subject of future research.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFR | Air Fuel Ratio; |
| BMEP | brake mean effective pressure; |
| CAD/°CA | crank angle degree; |
| COV/COVi/COVIMEP | coefficient of variation/COV for the ith cylinder/COV of IMEP; |
| CS | combustion stability coefficient; |
| DC | Direct current; |
| ECU | electronic control unit; |
| EGR | Exhaust Gas Recirculation; |
| FC | fuel cell; |
| FSO | Full scale output; |
| HC | Hydrocarbons; |
| HHO | Hybrid Hydrogen Oxygen; |
| HHV | higher heating value; |
| ICell | Current per generator cell; |
| ICE | Internal combustion engine; |
| IMEP/IMEPi | indicated mean effective pressure/IMEP for the ith cylinder; |
| KOH | potassium hydroxide; |
| LHV | lower heating value; |
| LPM | Liters per Minute indicator; |
| MMW | Milliliters per Minute per Watt indicator; |
| MON | Motor Octane Number; |
| MPI | Multi Point Injection; |
| PWM | Pulse width modulation; |
| RON | Research Octane Number; |
| SI | Spark ignition; |
| TDC | Top dead center; |
| TWC | Three-way catalyst; |
| UCell | Voltage per generator cell; |
| Urev | Reversible voltage; |
| US | Voltage supply; |
| Utn | Thermoneutral voltage; |
| WOT | Wide open throttle; |
| k | Number of neutral electrodes of generator; |
| pH2 | Hydrogen pressure; |
| λ | Excess air coefficient; |
| σi | Standard deviation for the ith cylinder. |
References
- Hwang, H.T.; Varma, A. Hydrogen storage for fuel cell vehicles. Curr. Opin. Chem. Eng. 2014, 42, 42–48. [Google Scholar] [CrossRef]
- Wang, S.; Ji, C.; Zhang, B.; Liu, X. Lean burn performance of a hydrogen-blended gasoline engine at the wide open throttle condition. Appl. Energy 2004, 136, 43–50. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals, 1st ed.; Mcgraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Johansson, B. Cycle to Cycle Variations in S.I. Engines—The Effects of Fluid Flow and Gas Composition in the Vicinity of the Spark Plug on Early Combustion. J. Engines 1996, 105, 2281–2296. [Google Scholar]
- Reyes, M.; Tinaut, F.V.; Giménez, B.; Pérez, A. Characterization of cycle-to-cycle variations in a natural gas spark ignition engine. Fuel 2015, 140, 752–761. [Google Scholar] [CrossRef]
- Davies, T.; Cracknell, R.; Lovett, G.; Cruff, L.; Fowler, J. Fuel Effects in a Boosted DISI Engine; SAE Technical Paper No. 2011-01-1985; SAE: Warrendale, PA, USA, 2011. [Google Scholar]
- Kalghatgi, G. Spark Ignition, Early Flame Development and Cyclic Variation in I.C. Engines; SAE Technical Paper 870163; SAE: Warrendale, PA, USA, 1987. [Google Scholar]
- Sun, Y.; Yu, X.; Dong, W.; Tang, Y. Effects of hydrogen direct injection on engine stability and optimization of control parameters for a combined injection engine. Int. J. Hydrogen Energy 2018, 43, 6723–6733. [Google Scholar] [CrossRef]
- Sherif, S.A.; Yogi Goswami, D.; Stefanakos, E.K.; Steinfeld, A. Handbook of Hydrogen Energy, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Pulkrabek, W.W. Engineering Fundamentals of the Internal Combustion Engine, 1st ed.; Prentnice Hall: Hoboken, NJ, USA, 1997. [Google Scholar]
- Ogden, J.M. Hydrogen: The fuel of the future? Phys. Today 2002, 69, 69–75. [Google Scholar] [CrossRef]
- Yaws, C.L. Gas Data Book, 7th ed.; McGraw-Hill Professional: New York, NY, USA, 2001. [Google Scholar]
- Ji, C.; Wang, S.; Zhang, B. Effect of spark timing on the performance of a hybrid hydrogen-gasoline engine at lean conditions. Int. J. Hydrogen Energy 2010, 35, 2203–2212. [Google Scholar] [CrossRef]
- Fennel, D.; Herreros, J.; Tsolakis, A. Improving gasoline direct injection (GDI) engine efficiency and emissions with hydrogen from exhaust gas fuel reforming. Int. J. Hydrogen Energy 2015, 40, 8607–8619. [Google Scholar] [CrossRef]
- Karagöz, Y.; Sandalci, T.; Dalkihç, A.S. Effects of hydrogen and oxygen enrichment on performance and emissions of an SI engine under idle operating condition. Int. J. Hydrogen Energy 2015, 40, 8607–8619. [Google Scholar] [CrossRef]
- Quetz de Almeida, L.; Monteiro Sales, L.C.; Sodré, J.R. Fuel consumption and emissions from a vehicle operating with ethanol, gasoline and hydrogen produced on-board. Int. J. Hydrogen Energy 2015, 40, 6988–6994. [Google Scholar] [CrossRef]
- Ji, C.; Wang, S. Effect of Hydrogen Addition on Combustion and Emissions Performance of a Spark-ignition Gasoline Engine at 800 rpm and Lean Conditions. In Proceedings of the 18th World Hydrogen Energy Conference—WHEC 2010, Essen, Germany, 16–21 May 2010. [Google Scholar]
- Mori, M.; Mržljak, T.; Drobnič, B.; Sekavčnik, M. Integral Characteristics of Hydrogen Production in Alkaline Electrolysers. Stroj. Vestn.-J. Mech. Eng. 2013, 59, 585–594. [Google Scholar] [CrossRef]
- T-Raissi, A.; Block, D.L. Hydrogen: Automotive fuel of the future. IEEE Power Energy Mag. 2004, 2, 40–55. [Google Scholar] [CrossRef]
- Zoulias, E.; Varkaraki, E.; Lymberopoulos, N. A Review on Water Electrolysis. Cyprus J. Sci. Technol. 2004, 4, 41–71. [Google Scholar]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- De Falco, M. Ethanol membrane reformer and PEMFC system for automotive application. Fuel 2011, 90, 739–747. [Google Scholar] [CrossRef]
- Comotti, M.; Frigo, S. Hydrogen generation system for ammonia-hydrogen fueled internal combustion engines. Int. J. Hydrogen Energy 2015, 40, 10673–10686. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, Z.; Coutteneye, R.A.; Willis, W.S.; Suib, S.L.; Fanson, P.T.; Hirata, H.; Ibe, M. Processing of hydrocarbons in an AC discharge nonthermal plasma reactor: An approach to generate reducing agents for on board automotive exhaust gas cleaning. J. Catal. 2008, 253, 28–36. [Google Scholar] [CrossRef]
- Li, X.; Sun, B.; Zhang, D.; Wang, X.; Bao, L.; Luo, Q. Experimental study on the cycle variation characteristics of direct injection hydrogen engine. Energy Convers. Manag. X 2022, 15, 100260. [Google Scholar] [CrossRef]









| Property | Hydrogen | Gasoline (Unleaded) | Ref. | |
|---|---|---|---|---|
| Lower heating value (LHV) | MJ/kg | 142–120 | 43–44 | [10,11] |
| Higher heating value (HHV) | MJ/kg | 141.8 | 47.3 | [10,11] |
| Air–fuel ratio (AFR) | kg/kg | 34.5 | 14.7 | [10] |
| (2.82 m3/m3) | ||||
| Heating value of the air–fuel mixture (port injection) | MJ/m3 | ~3.0 | ~3.5 | |
| Flammability limits in air at 101.3 kPa | %Vol | 74–4 | 7.1 (7.6)–1.2 (1.0) | [12,11] |
| AFR | kg/kg | 5.0–344.4 | 3.5 (3.3)–22.3 (26.8) | |
| λ | - | 0.14–9.98 | 0.24 (0.22)–1.52 (1.8) | |
| Minimum ignition energy in air: | ||||
| At stoichiometric mixture (λ = 1) | mJ | 0.02 | 0.24 | [11] |
| At lower flammability limit | mJ | 10 | n.a. | [11] |
| Maximum laminar burning velocity | m/s | 2.65–3.25 | 0.37–0.43 | [11] |
| Octane number (RON) | - | >130 | 95–98 | [9,10] |
| Hydrogen | ||||
|---|---|---|---|---|
| pH2 (Ambient) | pH2 (30 MPa) | pH2 (70 MPa) | ||
| % | kg | dm3 | dm3 | dm3 |
| 5 | 2.220–2.280 | 27,300–28,037 | 90.7–93.1 | 38.9–40.0 |
| 4 | 1.776–1.824 | 21,840–22,430 | 72.6–74.5 | 31.2–32.0 |
| 3 | 1.332–1.368 | 16,380–16,822 | 54.4–55.9 | 23.4–24.0 |
| 2 | 0.888–0.912 | 10,920–11,215 | 36.3–37.3 | 15.6–16.0 |
| 1 | 0.444–0.456 | 5460–5607 | 18.1–18.6 | 7.8–8.0 |
| 0.5 | 0.222–0.228 | 2730–2804 | 9.1–9.3 | 3.9–4.0 |
| Compression ratio | 11:1 |
| Bore × Stroke | 75 × 90.5 mm |
| Rated power | 60 kW at 5750 rpm |
| Rated torque | 118 N*m at 2750 rpm |
| Valve train | 4 valves per cylinder Variable intake and exhaust timing |
| Device | Measurement Range | Accuracy |
|---|---|---|
| Gas flowmeter | 0–10 L/min | <0.5% FSO |
| In-cylinder pressure transducer | 0–20 MPa | <±0.5% FSO |
| Piezo amplifier | 144–14,400 pC | ±0.3% |
| Crankshaft angle encoder | 20–20,000 rpm | ±0.1 °CA |
| Lambda meter | 0.645–15.999 | 0.001 |
| Wide band oxygen sensor | 0.650– | ±1.5% |
| Case | 1000 rpm/BMEP = 2 Bar | 3000 rpm/BMEP = 5 Bar | |||||||
|---|---|---|---|---|---|---|---|---|---|
| λ | - | 1.0 | 1.4 | 1.4 | 1.4 | 1.0 | 1.4 | 1.4 | 1.4 |
| H2 | (%) | 0.0 | 0.30 | 0.15 | 0.0 | 0.0 | 0.30 | 0.15 | 0.0 |
| (bar) | 1.587 | 3.384 | 3.154 | 3.202 | 6.452 | 6.934 | 6.717 | 6.440 | |
| σ1 | (bar) | 0.042 | 0.138 | 0.324 | 0.200 | 0.063 | 0.067 | 0.067 | 0.085 |
| CS | (%) | 2.35 | 3.34 | 10.32 | 10.87 | 0.84 | 1.08 | 0.98 | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyko, J.; Słobiński, K.; Jaworski, J.; Mitukiewicz, G.; Bou Nader, W.; Batory, D. Study on SI Engine Operation Stability at Lean Condition—The Effect of a Small Amount of Hydrogen Addition. Energies 2023, 16, 6659. https://doi.org/10.3390/en16186659
Leyko J, Słobiński K, Jaworski J, Mitukiewicz G, Bou Nader W, Batory D. Study on SI Engine Operation Stability at Lean Condition—The Effect of a Small Amount of Hydrogen Addition. Energies. 2023; 16(18):6659. https://doi.org/10.3390/en16186659
Chicago/Turabian StyleLeyko, Jacek, Kamil Słobiński, Jarosław Jaworski, Grzegorz Mitukiewicz, Wissam Bou Nader, and Damian Batory. 2023. "Study on SI Engine Operation Stability at Lean Condition—The Effect of a Small Amount of Hydrogen Addition" Energies 16, no. 18: 6659. https://doi.org/10.3390/en16186659
APA StyleLeyko, J., Słobiński, K., Jaworski, J., Mitukiewicz, G., Bou Nader, W., & Batory, D. (2023). Study on SI Engine Operation Stability at Lean Condition—The Effect of a Small Amount of Hydrogen Addition. Energies, 16(18), 6659. https://doi.org/10.3390/en16186659








