Abstract
In recent years there has been a significant increase in the demand for lithium all over the world. Lithium is widely used primarily in the production of batteries for electric vehicles and portable electronic devices, and in many other industries such as production of aluminum, ceramics, glass, polymers, greases, and pharmaceuticals. In order to maintain the balance between supply and demand for lithium on the global market, it is essential to search for alternative sources of this element. Therefore, efforts are being made to obtain lithium from unconventional sources, an example of which is the recovery of lithium from oilfield brines. This article provides an up-to-date review of the literature in this particular field based on data from different sources (scientific literature databases, patent databases, company websites and industrial online newspapers). The current achievements and future perspectives for the lithium recovery from brines generated during oil and gas extraction were critically reviewed. An emphasis was placed on chemistry of lithium-contained oilfield brines, technologies (both pretreatment and direct lithium extraction) suitable for lithium recovery and industrial results obtained from pilot trials.
1. Introduction
Lithium is a silvery-white metal that is currently of great interest to both scientists and industries due to its growing importance in the global economy. Lithium is used in the production of aluminum, ceramics, glass, polymers, lubricants, pharmaceuticals, and lithium-ion batteries for portable electronic devices (e.g., smartphones, laptops, tablets). However, the largest consumer of lithium is the electric vehicle sector, as lithium is a key component of rechargeable batteries [1,2,3,4,5,6]. The demand for lithium is expected to grow rapidly in the coming decades, which can be attributed to lithium’s vital role in sustainable energy transitions [6,7,8].
The primary lithium resources including hard rocks and continental brines are very unevenly distributed around the world. More than 60% of the world’s lithium production comes from the following eight locations: Salar de Atacama 1 and 2 in Chile, Salar del Hombre Muerto and Salar de Olaroz in Argentina, the Clayton Valley in the USA, Lake Zabuye, Dongtai Salt Lake and Xitai Salt Lake in China [9]. The growing demand for lithium raises concerns about security and sustainability of its supplies [10,11]. To prevent shortages, spent lithium-ion batteries [2,3,12] as well as geothermal waters [1,3] and oilfield brines (brines generated during oil and gas extraction, including shale gas, considered as wastewater from oil and gas industry) [13], which are more uniformly distributed and available in most countries, are considered as alternative sources of lithium. Even seawater was considered as a potential lithium source [14], but until today there is no economically efficient technology. In the case of geothermal waters, there are many reviews focusing on this issue (e.g., [1,15,16,17]). Thus, in the following section we only briefly present the most important achievements in this field for comparison with the area of oilfield brine exploitation.
Geothermal systems are especially often studied as the combination of renewable power and heat production with direct lithium extraction allows to lower the environmental footprint of the whole facility [18]. Sanjuan et al. [19] identified six lithium-rich geothermal areas in Europe (Italy, Germany, France and the United-Kingdom) with Li+ concentrations ranging from 125 to 480 mg/L. The first kilograms of battery-grade lithium carbonate from geothermal waters were produced in 2021 under the EuGeLi (European Geothermal Lithium Brine) project located in Alsace, at the French/German border [20]. Encouraged by the success of this project the Eramet and Électricité de Strasbourg signed the memorandum of understanding aimed at developing lithium production from geothermal brines in the municipality of Rittershoffen. The ambitious goal of this cooperation is to achieve lithium production of 10,000 tons per year by the end of 2030 [21]. The German company Vulcan Energy Resources announced the results of its feasibility study for lithium extraction in the Upper Rhine Valley [22]. According to their strategy, drilling to increase brine flow will begin in mid-2023 and lithium production will start by the end of 2025. The full-scale lithium mine in the Albemarle’s Silver Peak site in Nevada’s Clayton Valley, USA produces about 5000 tons of lithium per year [23]. Numerous laboratory studies confirm the suitability of various techniques to recover lithium from geothermal waters [24,25,26] and positive pilot results encourage full commercialization.
As was presented above, there are excellent reviews available in the literature that summarize the current state of knowledge of lithium recovery from geothermal brines, but articles describing lithium extraction from petroleum brines are still lacking. Therefore, to fill this gap, our mini review discusses the possibility of recovering lithium from oilfield brines based on the available literature data. The aim of this review is to show the potential of petroleum brines in the context of lithium production. To date, oilfield brines have only been mentioned as a potential resource, and information regarding their use is scattered. Our ambition is to summarize the state of the art in this area (based on information presented in the scientific literature, patents, patent applications, technical reports and press releases) and encourage various stakeholders (oil & gas operators, public opinion, policymakers, scientists) to increase both research and legislative activity toward better utilization of reservoir waters.
2. Volume, Current Usage, and Composition of Oilfield Brines
Firstly, it should be noted that in the literature oilfield brines are also often described as waters produced from gas and shale gas production [2,13]. Therefore, for simplicity, we use the term oilfield brine as the designation of all kinds of generated waters during oil, gas and shale gas production in this mini review.
The formation of water extracted with hydrocarbons from the reservoir to the surface is a major waste stream generated in the oil industry. The volume of produced water is estimated by some authors at 39.5 Mm3/day globally [27], but this is only a rough approximation because some data are not publicly available and in different countries there are different reporting strategies. The Produced Water Society estimates that the volume of produced water from conventional onshore and offshore fields globally will almost double from 25 billion m3/year in 2020 to 38.6 billion m3/year in 2030 [28].
The most detailed information is provided by Veil et al. [29,30,31] who report every few years on the volumes, compositions, and management of produced brines in the USA. On average, 3 m3 of water is extracted per 1 m3 of oil (water–oil ratio, WOR = 3). The amount of water produced varies over the lifetime of the well [30]. At the beginning of well exploitation water production is negligible for conventional wells, but after a few years the water breakthrough occurs and there is a regular increase in its production. The inflow of reservoir water into unconventional wells is even faster and more difficult to predict [32]. Figure 1a shows a typical profile of oil and water production from conventional and unconventional reservoirs. For conventional reservoirs in the early stage of exploitation, the production of water is negligible (blue line in Figure 1a) while the oil production (green line) is steadily growing. Usually, 2–3 years after the start of production, a particular well reaches a stable peak in oil production, lasting several years. Over time, oil production decreases and water production increases, which continues until the operator decides to decommission the well. For unconventional reservoirs (shales, tight sandstones), due to the low permeability of the rock matrix, hydraulic fracturing is required to start the production, hence high water consumption (and then water production as flowback) occurs at the beginning of the operation. After a short plateau stage, the production of both water and oil decreases. In the case of mature oilfields, the water–oil ratio is as high as 10–50 m3/m3 in the final stage of exploitation. The high water–oil ratio reduces the profitability of exploitation and may lead to the final closure of production. In industry jargon, such a situation is referred to as “water killed the well” [33]. Figure 1b shows the WOR values in different states/areas of the USA. In Texas, which accounts for 40% of the oil production in the USA, the WOR is 7.8. In general, the WORs vary from 0.5 m3/m3 for New York to 57.7 m3/m3 for Arkansas [31]. The calculated values have a high degree of uncertainty but show the global trend well, oilfields actually produce much more water than oil.
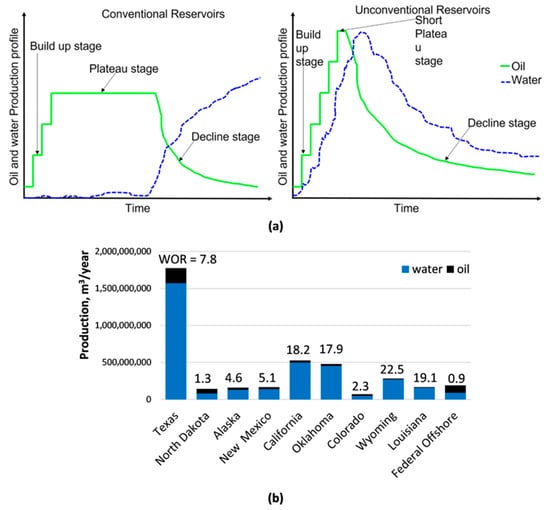
Figure 1.
(a) Typical production profile for conventional and unconventional reservoirs (reproduced from Ref. [32] with permission from the Royal Society of Chemistry), (b) WOR values in selected states in the USA [31].
Exploration of new hydrocarbon reservoirs is very expensive, and the prevailing trend today is to maximize the exploitation of mature fields, despite the significant production of reservoir water. In most cases the produced water is re-injected into production zones to maintain the reservoir pressure and enhance oil recovery. It is estimated that about 40% of this water goes to other, non-hydrocarbon-producing formations solely for disposal [29]. Re-injection of water into disposal wells does not bring any additional profits, it only creates the opportunity to avoid landfill fees.
In order to improve the profitability of oilfield exploitation, it is necessary to maximize the use of brine, hence the increasingly advocated “beneficial reuse”, i.e., the use of brine for tank cleaning, preparation of fracturing fluids or irrigation purposes. The perception of produced water is changing and now it is considered as a valuable resource for the production of potable water and valuable chemical elements [34]. Depending on the volume of brine, geological, reservoir, and technological conditions, as well as local legislation, there are currently several ways to manage this waste stream.
Figure 2 shows produced water management practices in California, USA. As you can see in the case of California, more than 80% of the produced water is re-injected into the formation, either for enhanced oil recovery or for disposal. For oilfield operations, produced water can be used for well stimulation (e.g., hydraulic fracturing), water flooding, and enhanced oil recovery, thereby decreasing the demand for other sources of water. The increased demand for available water resources has driven interest in the reuse of produced water for off-site, non-oilfield applications including irrigation, municipal, livestock watering, and industrial uses.
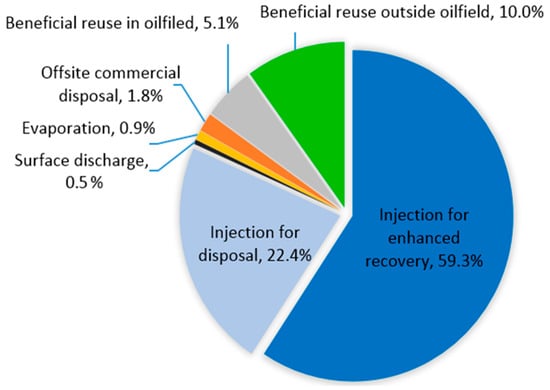
Figure 2.
Produced water management practices in California, USA [31].
Produced water management practices vary from state to state and even from one oilfield to another. The reuse of produced water for irrigation is not a new concept. Approximately 13% of the coal bed methane (CBM) water in Wyoming and 26% of the CBM water in Montana were used for irrigation in 2009 [29]. Kern County in California has been reusing its produced water for agriculture for decades [35]. The treating of the produced water to meet agricultural standards may be technologically and economically feasible when comparing the cost of treatment to the cost of disposal in commercial wells, as shown by McCurdy et al. [36].
The tendency to use the formation waters for water recovery is popular also in other regions in the world. Successful irrigation projects are reported in Australia [37]. The Arabian Gulf region conducts an ambitious water management and agricultural development policies aiming to reinforce their food security through the reuse of formation water [38]. The above-described field experiences confirm the technical and economic feasibility of beneficial reuse of produced water even if only water (for agriculture or industrial purposes) is a final product in some cases.
In addition, a more recent direction is recovery of raw materials from produced waters. Since oilfield brines are usually strongly saline, the minerals present in them can be a valuable chemical resource [34]. The composition of reservoir brines is very diverse and complex, they contain dissolved and dispersed hydrocarbons, dissolved inorganic salts, dissolved gases and radionuclides [39,40]. Among the various elements, lithium is currently receiving the most attention due to the growing demand associated with electromobility.
The best oilfield brines recognized for lithium content are in the USA. Waters from Devonian formations in the Williston Basin (North Dakota) contain from 100 to 288 mg of Li+ per liter, in the Jurassic Smackover Formation in the Gulf of Mexico (Texas and Arkansas) the lithium concentration changes from 50 to 572 mg/L and in Texas Cretaceous reservoirs is in the range of 132–333 mg/L [13,41]. High Li+ values of up to 140 mg/L were found in the Devonian carbonate units (the Leduc and Swan Hills formations) in the Alberta province in Canada [42]. Li et al. [43] reported Li+ concentrations ranging from 110 to 237 mg/L in the Nanyishan and Dafengshan oilfields in the Qaidam Basin, Tibetan Plateau. The recent findings of Yu et al. [44] confirm variable Li+ contents from 7.5 to 150 mg/L in the Qianjiang Formation in central China.
Hitchon et al. [45] defined a “detailed exploration threshold value” for Li+ of 75 mg/L as a minimal acceptable lithium concentration ensuring profitability of its production. Some authors decrease this limit to 50 ppm. According to the Polish Geological Institute—National Research Institute the commercial recovery may be considered from waters containing at least one of the listed elements exceeding the following amounts: lithium—10, iodine—15, boron—100, bromine—200, magnesium—2000, potassium—1000, strontium—500, cesium—0.5 and rubidium—3 mg/L [46].
Taking this into account, many of the reported oilfield brines may be considered as a promising lithium resource. The complex geochemistry of produced water is challenging, but a properly selected pretreatment allows us to overcome this drawback.
The suitability of brine for lithium production is determined by its chemical composition. Numerous research and review papers discuss in detail different groups of compounds present in reservoir waters [39,47,48]. The total dissolved solids (TDS) of oilfield waters vary widely from 5.6 g/L to 313.7 g/L [39] and most of them are of the Na-Cl type. Among the anions, chlorides dominate, with carbonates, bicarbonates, sulfates, sulfides, bromides, and iodides in smaller amounts. Unlike surface water, nitrates and phosphates are rare in reservoir brines. The most common cations are Na+, K+, Mg2+, Ca2+, Fe2+, Mn2+. Heavy metals (Pb2+, Cd2+, Hg2+, Cu2+, Zn2+) appear in trace amounts. For a long time, lithium content was not determined in these waters because it usually occurs in small amounts and does not affect the re-injection process. Table 1 summarizes lithium concentrations in selected oilfield brines. As shown in Table 1, despite the location, oilfield brines are highly mineralized with chloride content varying from 75 to 201 g/L. Some fields are typical sandstones, while others are carbonate reservoirs, as evidenced by high calcium and magnesium contents. Lithium concentration varies from 28 to 505 mg/L and the example of the Smackover Formation shows that it can have different values for different production horizons.

Table 1.
Chemical composition of selected oilfield brines.
In general, there is no relationship between the lithium content and other elements. Therefore, the concentration of lithium cannot be predicted on the basis of simplified analyses of basic ions and must be determined separately for each time. Yu et al. [44] created a unique database containing hydrochemical data from 155 oil wells. The statistical analysis of this dataset confirms that lithium concentration has a weak relationship with the salinity and content of individual ions as shown in Figure 3a–d. The correlation coefficient for data shown in Figure 3 is lower than 0.3, which indicates that there is no linear relationship between the lithium content and other variables.
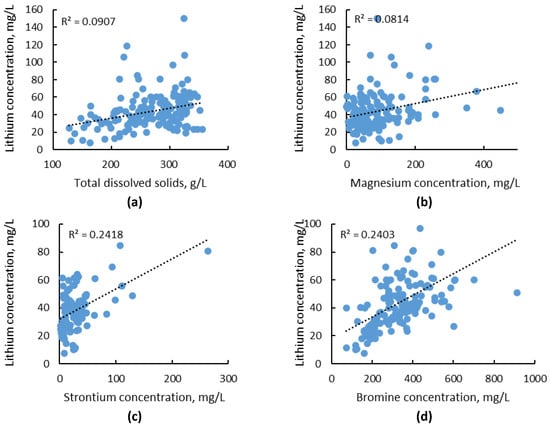
Figure 3.
Lithium concentration vs. (a) total dissolved solids, (b) magnesium concentration, (c) strontium concentration, (d) bromine concentration in oilfield brines from the Qianjiang Formation, China (based on data from Ref. [44]).
It should be mentioned that oilfield brines contain lower lithium concentration than continental brines, for example concentration of main ions in continental brine from Atacama, Chile are equal to 6.5–9.1 wt% for sodium, 1.79–3.13 wt% for potassium, 245–530 mg/kg for calcium, 0.93–1.30 wt% for magnesium, and 1500–2420 mg/kg for lithium [52]. Conventional solar evaporation/crystallization technology can be applied for brines where the concentration of lithium ranges from 2000 mg/L to 6 wt%, so in the case of oilfield brines ([Li+] = 28–505 mg/L) other approaches must be applied, such as direct lithium extraction [1].
3. Impact of Produced Water Chemistry on Lithium Recovery
Many lithium recovery methods are sensitive to the other compounds in the brine [1,8,13]. Therefore, oilfield brines may need to be pre-treated to remove all compounds which can impair lithium extraction. Our previous work [8] shows that the content of other ions may significantly hinder lithium sorption, and only a few materials (Mn- and Ti-based powders) may be applied to fully saturated brines. The selectivity of sorbents is often determined by the gaps in the crystal lattice, and in the case of membranes—by the diameter of their pores. Considering the size of the ion radii [53] the competitive sorption of Li+ and Mg2+ will occur and this is a common and most important problem for oilfield brines. Of course, a large excess of other ions and their accumulation on the surface of the sorbent will hinder the access of lithium ions to the active centers so high salinity is not desirable.
Based on [1,8,53] we propose to categorize physicochemical parameters of water into four classes (from 1 to 4) depending of their impact on lithium recovery:
- Class 1—the low impact parameter—this parameter has a low impact on lithium recovery;
- Class 2—the moderate impact parameter—this parameter has a moderate impact on lithium recovery, its increased or decreased value can affect the process but it can be rather easily adjusted (does not pose a major technological challenge);
- Class 3—the high impact parameter—this parameter strongly affects lithium recovery, its impact can be reduced but it will require additional technological operations, which may result in new problems such as precipitation of sludge, chemical degradation of sorbents, etc.;
- Class 4—the critical parameter—failure to meet the minimum levels of the parameter will result in reduced efficiency and cost-effectiveness of the entire recovery process.
Figure 4 shows the classification of water parameters influencing the lithium recovery, and Table 2 describes the role of each parameter in detail.
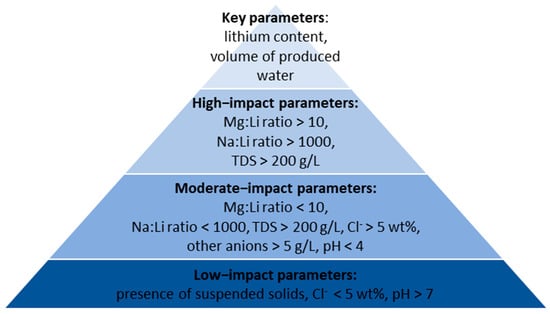
Figure 4.
Classification of water parameters influencing on lithium recovery.

Table 2.
Effect of brine chemistry on lithium recovery.
Figure 4 uses the pyramid concept to visualize the importance of the various factors affecting lithium recovery. At the very top are the two most important parameters, i.e., the lithium content in the brine and the amount of brine extracted. These two parameters determine the amount of lithium available, and more importantly, they cannot be easily influenced by applying certain technical solutions. High salinity and increased concentrations of Na+ and Mg2+ ions were identified as high-impact parameters. As explained in Table 2, these ions compete with lithium sorption, and their presence reduces the quality of the final lithium carbonate. Technical solutions are available to mitigate their impact, but they may cause some operational complications. Some parameters, such as pH and the content of other anions, only slightly affect lithium recovery and may be easily adjusted. There are numerous interdependencies between water parameters, and the correction of one of them may affect the others. Overall, Figure 4 and Table 2 provide only general guidelines and each brine must be considered separately.
4. Pretreatment of Produced Water Prior to Lithium Recovery
Lithium recovery technologies from brines are still in the testing and implementation phase, hence no standards have been set for brine pretreatment. It can be assumed that produced water used in the lithium recovery plant as a feed must meet the certain criteria resulting from the limitations of the methods used in the process. Low suspended solid levels are required if adsorption technology is used, and low organic compound concentrations are required if solvent extraction or membrane methods are used.
Moreover, the resulting brine after lithium recovering will be re-injected into the formation so its composition should not adversely affect the permeability of the near-wellbore zone. Thus, suspended solids, ions which tends to form insoluble precipitates (Ca2+, Mg2+, Fe3+, Mn4+), and organic compounds should be removed from the brine.
To sum up, pretreatment of raw oilfield brine may consist of subsequent operations: filtration, coagulation, flotation, adsorption and ultrafiltration. There are numerous reviews available in the literature that describe the produced water treatment technologies [28,54,55,56] so here they will be only briefly outlined. Figure 5 shows a typical treatment scheme of oil/water mixture in the surface installation.
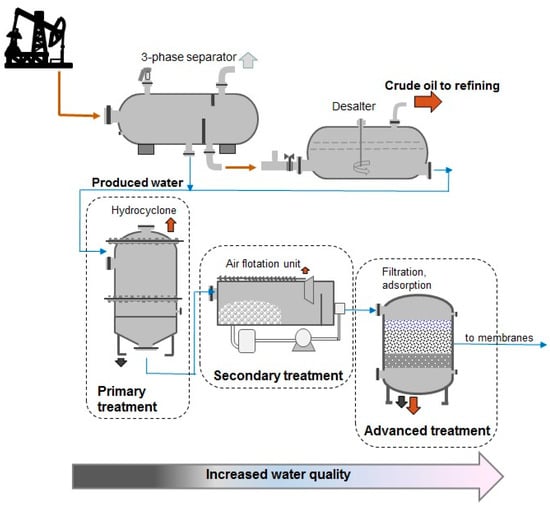
Figure 5.
Schematic of crude oil dehydration and produced water treatment (based on [57]).
According to the literature [57], the mixture of crude oil and water is usually separated in high-pressure three-phase separators. The oil is directed to the desalting and stabilization system while the water goes to the treatment system. The primary treatment includes mechanical methods such as gravity separation or centrifugation. In practice, the gravity method can be used to separate solid particles with a size of 10−1 to 10−3 mm and oil droplets with a diameter of more than 150 μm. In secondary methods some chemicals or gases are applied. Coagulants and flocculants facilitate the separation of smaller particles. Dissolved air flotation (DAF) is also an effective technique to enhance small oil droplets removal. Filter columns are sometimes connected in series—on prefiltration (mechanical) filters solids are separated, while on adsorption beds selected dissolved contaminants (ions, hydrocarbons) are removed. Typical filter beds include gravel, sand, anthracite, diatomite, zeolites, walnut shells, etc. [58]. The advanced treatment includes chemical adsorption methods and membrane desalination. The combination of primary, secondary and advanced treatment methods improves the quality of water, resulting in potable water.
Biological decomposition can also take place in the filter bed. Results of Freedman et al. [59] show that biologically active filtration achieves more than 90% dissolved organic carbon removal. The finely dispersed and dissolved hydrocarbons are efficiently removed using adsorption beds.
The work of Doyle et al. [60] describes industrial trials where adsorption on modified organoclays reduced total petroleum content below 10 ppm. The BTEX fractions can be removed from brine by amine-modified zeolites [61] or hydrophobized biomass [62]. Yang et al. [63] compared the sorption properties of classical walnut shell filters and modified polyethylene fiber filters. The synthetic filters reduced petroleum content below 2.4 mg/L and suspended solids < 2 mg/L. The techniques described above are being combined into specific process sequences to meet water quality requirements.
One of the proposed methods for recovering lithium from brines are membrane techniques. However, such an approach requires much more rigorous water pretreatment than in the case of sorption or solvent extraction. Membrane module suppliers usually specify the required parameters of the input flux and here may be assumed that produced water prior lithium recovery also should meet these criteria. Table 3 shows the recommended limits of the quality parameters of the feed water.

Table 3.
General guideline for acceptable feed water to a membrane system.
Çakmakci et al. [66] proposed a complete system for treating reservoir water prior a reverse osmosis (RO) desalination. For the pretreatment of water before the RO module, the following technological sequence was considered the most effective: gravity separator, DAF unit, 1 μm ceramic or metallic cartridge filter and 0.2 μm ceramic or metallic microfiltration and activated carbon adsorption. In addition to microfiltration, ultrafiltration can also be used to play a shielding role, thereby increasing the lifetime of the membrane. A common problem that arises during the operation of nanomembranes (including nanomembranes predicted for lithium recovery) are scaling and fouling phenomena. This issue can be effectively reduced by the addition of divalent ion complexing agents to the water.
Patrick et al. [67] described an alternative way to treat water before the membrane process (Figure 6). After using classical treatment methods in the form of the dissolved air flotation process and walnut shell bed filtration, they proposed softening the water with lime and the removal of oil in a membrane bioreactor.
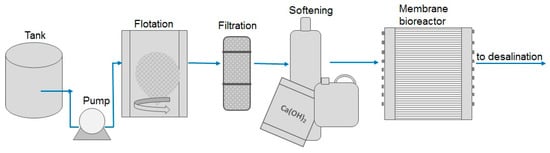
Figure 6.
Schematic diagram of the proposed water pretreatment prior lithium recovery (based on [67]).
The authors assumed an ambitious goal to produce potable water of a quality consistent with the federal requirements (oil and grease < 5 mg/L, TDS < 600 mg/L, boron < 0.2 mg/L). After conventional pretreatment, the TDS was 6200 mg/L, oil and grease—15 mg/L and boron—50 mg/L. Traditional treatment methods work well in case of removing emulsified oil and dispersed solids, but exhibit low efficiency in removing some specific compounds such as boron, silica, ammonia and dissolved organics. Despite these limitations, DAF and filtration are often applied due to their simplicity and low cost of operation. Warm lime softening allows removing carbonate hardness and silica by a simple precipitation way. As a result, the silica content is reduced by 80%, but additional sludge is produced. In the membrane bioreactor, the produced water first enters a bioreactor (biologically active tank) with aerobic bacteria, where sulfides are oxidized to sulfates, and ammonia to nitrates. After this biological treatment, the water is pumped into a tubular membrane microfiltration system. The removal efficiency of oil, grease and solids in such a system is >97%. The authors estimate that the actual cost of treating the produced water will be 0.15 USD per 1 bbl. of water, while the revenue from the sale of reclaimed water will be 0.05–0.1 USD/bbl. This example shows that advanced water treatment may only be beneficial if the production of potable water is combined with the recovery of valuable chemical elements like lithium [67].
In addition to the removal of suspended solids, colloids and organics, some lithium recovery technologies require reduction of concentrations of sodium, potassium, calcium, and magnesium ions. Although sorbents and membranes are designed to provide a high selectivity towards lithium, some alkali and alkaline earth metals are still captured by them. This poses a major complication because the resulting lithium concentrate becomes de facto contaminated with them. On the other hand, it is difficult to avoid this situation when in the raw brine the ratio of Na+:Li+ is of the order of 1000:1 and for Mg2+:Li+ is about 20:1. Lithium for commercial applications in lithium-ion batteries must be practically free of admixtures of other metals.
Separation of lithium from sodium and potassium can be carried out at the brine pretreatment stage or as refining of the resulting lithium concentrate. According to the literature, sodium can be separated from lithium using ion-exchange resins such as Dowex’s AG50W-XS [68], while some zeolites allow an efficient separation of potassium [69]. Removal of calcium and magnesium ions usually involves their precipitation as insoluble salts. Precipitation of magnesium in the form of Mg(OH)2 using lime was described in U.S. Patent 8691169 [70]. Magnesium can also be precipitated as oxalates and then roasted to obtain commercial MgO [68]. Laitala et al. [71] proposed precipitating Ca2+ and Mg2+ with Na2CO3 and Ca(OH)2 in the first step, and subsequent solvent extraction with di-(2-ethylhexyl)phosphoric acid (D2EHPA) to remove residual amounts of these ions, ultimately reducing their concentration below 0.01 g/L. Iron and manganese can be easily removed on commercially available adsorption beds. Christopher et al. [72] investigated the possibility of recovering iron, manganese, zinc and lead from geothermal waters in the Salton Sea, USA, but the process was not cost-effective.
Pretreatment of brine prior lithium recovery can be a technological challenge, but ongoing laboratory studies confirm the effectiveness of conventional methods such as adsorption, ion exchange resins and precipitation techniques. Future work in this area should focus on minimizing chemicals use and reducing solid waste.
5. Methods of Recovering Lithium from Oilfield Brines Reported in the Scientific Literature
The number of scientific publications on the extraction of lithium from various sources is steadily increasing, but data on specific processes or technologies for recovering lithium from oilfield brines are scarce. Several technologies, such as solar evaporation, precipitation, adsorption, ion exchange, solvent extraction and membrane processes can be used to produce lithium from different types of brines [1,2,3,4,5]. However, when it comes to the recovery of lithium from oilfield brines, only some of the listed methods can be applied [13]. Solar evaporation and precipitation with sodium carbonate are recommended primarily for brines containing lithium in concentrations above 500 mg/L, e.g., for salt lake brines. Moreover, evaporation process is geographically limited, dependent on environmental factors and time-consuming (10–24 months), because brine must lose around 90% of their original water content. And in the case of precipitation, the main disadvantage is the generation of a large amount of waste [5,6,13,73].
For oilfield brine, the aluminate precipitation method was proposed [74]. In this method, aluminum chloride and sodium hydroxide are added to the brine, resulting in the formation of amorphous aluminum hydroxide, which precipitates lithium. In the next step, the precipitate containing lithium is separated from the solution, and then lithium is released from the precipitate by applying the appropriate condition (acidic or alkaline) [6,74]. Yang et al. [74] recovered lithium from calcium chloride-type oilfield brine (prepared solution of calcium chloride and lithium chloride and oilfield brine with high calcium content) using aluminum chloride and sodium hydroxide (aluminum hydroxide created by this mixture precipitates Li+). First, the influence of temperature, Al3+:Li+ molar ratio, OH−:Al3+ molar ratio and contact time between Al(OH)3 and brine on the efficiency of lithium recovery was investigated using synthetic solution. Optimized operating parameters (temperature = 35 °C, Al3+:Li+ molar ratio = 4.5, OH−:Al3+ molar ratio = 2.6, contact time = 6 h) for maximum lithium recovery were then used to extract lithium from concentrated oilfield brine (Ca2+ = 100.1 g/L, Mg2+ = 5.75 g/L, Li+ = 1.29 g/L). The lithium recovery efficiency obtained was 75.6%. In addition, the researchers found that the presence of calcium and magnesium ions in the brine had a slight negative effect on lithium recovery efficiency [74].
Some researchers [75,76,77] have investigated the applicability of the solvent extraction method for recovering lithium from simulated shale gas produced water (hypersaline wastewater generated after hydraulic fracturing). Solvent extraction (or liquid-liquid extraction) is a method of separating compounds based on their relative solubility in two different immiscible (or slightly soluble) phases (aqueous and organic) [6]. Typically, an organic solvent is added to the brine (usually diluted) to form organic lithium complexes, which are transferred to the organic phase. Most other metals remain in aqueous solution. The organic phase containing the extracted lithium complexes is then stripped with an acidic solution to remove the lithium [6,75].
Jang et al. [75] proposed a two-stage solvent extraction technique and used synthetic fracturing fluid characterized by the same concentration of components (Ca2+, Li+, Mg2+, Na+, Sr2+, and Ba2+) as the Marcellus shale produced water. As the extractant D2EHPA diluted in kerosene was applied. The first step was the removal of divalent cations using 1.0 M D2EHPA. It was found that eight repetition cycles were the most efficient process (more than 94.4% removal efficiency was achieved, while lithium loss was 25.1%). The second stage was aimed at recovering lithium—the highest lithium extraction efficiency (41.2%) was observed when 1.5 M D2EHPA and 0.3 M tributyl phosphate were used. The main limitations of the proposed method for recovering lithium from shale gas produced water observed by the researchers are the need to use a large volume of organic solvents and to dilute the fracturing fluid due to the very high level of total dissolved solids [75].
Lee and Chung [76] studied the influence of different types of alkanes (n-hexane, n-undecane, and n-hexadecane) and different concentrations of n-hexane on lithium recovery by solvent extraction from simulated shale gas production water. Organic compounds were added to the prepared fracturing fluid. The experimental procedure was the same as described in the study by Jang et al. [75]. The study proved that the presence of organic compounds in the simulated shale gas production water adversely affects the efficiency of lithium recovery in the proposed two-stage liquid extraction, especially in the first stage of the process. In addition, it was observed that the overall lithium recovery efficiency after two extraction steps decreased with an increasing alkane chain length and an increasing n-hexane concentration. To summarize, the removal of organic compounds, especially long chain alkanes, is essential before using solvent extraction to recover lithium from oilfield brines [76].
Zante et al. [77] investigated solvent extraction of lithium from a simulated brine obtained from shale gas production using a bifunctional ionic liquid ([methyl tri(octyl)ammonium chloride (Aliquat-336)][D2EHPA] dissolved in n-dodecane). The researchers, like Jang et al. [75] and Lee and Chung [76], proposed a two-step lithium extraction process. In the first step, divalent metals (Mg2+, Ca2+, Sr2+, and Ba2+) were removed using five successive cycles of extraction with 1 M D2EHPA dissolved in n-dodecane. The efficiency of removing divalent ions was about 80%, and less than 25% of lithium was removed during this step. In the second step, lithium was extracted using one single cycle of extraction with 1 M [Aliquat-336][D2EHPA] ionic liquid. The obtained efficiency of lithium recovery in the second stage was 83% and was higher than that reported by Jang et al. [75]. However, Zante et al. [77] did not analyze the effect of organic compounds on the proposed extraction process with a bifunctional ionic liquid in their study. Meanwhile, real oilfield brines contain organic compounds that can interfere with lithium recovery by solvent extraction.
In summary, solvent extraction may not be the best option for recovering lithium from brine formed as a byproduct of the oil and gas industry, because it requires the removal of divalent cations (some of which have a higher affinity for the solvent than lithium) and organic compounds from the brine. In addition, solvent extraction consumes large volumes of extractants (some of which are quite expensive) and generates large amounts of environmentally harmful waste.
Adsorption with manganese- and titanium-based adsorbents has been reported by several authors [78,79,80,81,82] as an effective method for recovering lithium from petroleum brines. Lithium-ion sieves are considered to be one of the most promising materials for recovering lithium from highly saline brines with low lithium content due to their high adsorption capacity and excellent selectivity towards lithium [78,79,80,81,82].
Titanium-based adsorbent H2TiO3 was used to recover lithium from synthetic shale gas produced water [78,79,80]. H2TiO3 with a layered structure is obtained from Li2TiO3 precursor by replacing H+ with Li+ using an acid solution. The first step, i.e., synthesis of the lithium-titanium oxide precursor can be accomplished by one of the following methods: solid-state reaction, sol-gel technique, and hydrothermal synthesis [83,84,85]. However, the solid reaction method is most often used because of its environmental friendliness, simple operation, low cost, and lack of solvents. In addition, it is recommended as suitable for large-scale production [86,87]. The conventional solid-state process uses lithium salts, such as lithium carbonate and titanium dioxide as raw materials, which are calcinated at around 700 °C for several hours, and then cooled down to room temperature [84,85,86,87]. The second step, i.e., the delithiation process aims to obtain H2TiO3 lithium-ion sieves by pickling Li2TiO3 into hydrochloric acid. During this process, lithium ions are released from the precursor and then replaced with hydrogen ions by ion exchange [84,85,86,87]. The process of lithium adsorption on titanium-based adsorbent is ion exchange in nature (ion exchange between hydrogen cations from the adsorbent and lithium ions from the solution) and is described by Equation (1) [87]:
Jang and Chung [78] studied recovery of lithium from simulated shale gas produced water (similar to Marcellus shale gas produced water) by the precipitation adsorption–desorption method. In the beginning, the researchers prepared H2TiO3 adsorbent using Li2CO3 and TiO2 (Li:Ti molar ratio = 2:1). Raw materials were well mixed and calcinated at 700 °C for 4 h. The obtained Li2TiO3 powder was then stirred in 0.2 M HCl for 24 h at room temperature. After the delithiation process, the adsorbent was washed with deionized water, filtered, and dried at room temperature. Before adsorption, divalent ions were removed from the brine using Na2CO3 as a precipitating agent. The efficiency of the precipitation was above 96%. Adsorption tests were conducted at 30 °C, using 10 mL of simulated shale gas produced water and 0.03 g of adsorbent. Adsorption was studied for 48 h. Desorption involved washing the adsorbent several times with distilled water and then treating it with 0.2 M hydrochloric acid solution (40 mL). The adsorbent with HCl solution was stirred for one day. The achieved lithium desorption capacity was 3.66 mmol/g (lithium recovery was 54.4%). In addition, the researchers proved that the adsorption equilibrium time was about 24 h, and lithium was recovered selectively [78].
In another studies, Jang and Chung [79,80] investigated the effect of different alkanes (n-hexane, n-undecane, and n-hexadecane) and different concentrations of n-hexane on the adsorption of lithium from synthetic shale gas produced water using H2TiO3 adsorbent. In one study [79], the researchers examined the influence of organic compounds on lithium recovery by the precipitation adsorption–desorption method, while in the other [80], the influence of the organic compounds on lithium recovery from the pH buffered synthetic brine solution (KHCO3 was used as a pH buffer, the pH of the buffered shale gas produced water during the adsorption tests was about 6.5). The experimental parameters for both studies [79,80] were similar to those previously reported [78]. Organic compounds were added to the prepared brine solution. The results of the study showed that increasing the concentration of n-hexane decreases the amount of lithium recovered, so if there is a possibility, organic compounds should be removed from the brine before adsorption. Removal of organic compounds will increase the efficiency of lithium recovery and selectivity of the adsorbent towards lithium. However, the authors demonstrated that the H2TiO3 adsorbent showed good selectivity to lithium even in the presence of alkanes, so it can be used to recover lithium from oilfield brine containing large amounts of organic compounds [79,80].
Manganese-based adsorbents were used to recover lithium from fracturing flowback and produced water originating from the Duvernay Formation in Alberta, Canada [81] and shale gas flowback and produced water in the Sichuan Basin, China [82].
Currently, several lithium-selective manganese-based sorbents are known, including λ-MnO2, MnO2⋅0.3H2O, and MnO2⋅0.5H2O derived from spinel-type precursors LiMn2O4, Li1.33Mn1.67O4 (Li4Mn5O12), and Li1.6Mn1.6O4 (Li2Mn2O5), respectively. The synthesis of manganese-based sorbents, like titanium-based sorbents, involves obtaining a precursor, followed by its acid treatment [3,20,87]. Precursors can be synthesized using various methods. For example, solid-phase synthesis (calcination of lithium and manganese compounds mixed in the appropriate Li:Mn molar ratio at >400 °C for several hours), co-precipitation method, hydrothermal method or sol-gel method [20,83,87]. Synthesis of the precursor is an extremely important step because the type of precursor determines the ability of the adsorbent to selectively adsorb lithium [87].
Seip et al. [81] conducted a study on lithium recovery from a real field-collected fracturing flowback and produced water using two manganese-based sorbents (HMO-2 and HMO-3) that were prepared under different conditions. The first stage of the research involved the synthesis of sorbents using the co-precipitation method and delithiation in which 3.0 M LiOH and 0.375 M MnCl2 were mixed at a Li:Mn molar ratio of 2:1 in the case of HMO-2 and 3:1 in the case of HMO-3, then H2O2 was added to oxidize Mn2+ and the mixtures were dried at 90 °C for 16 h; the obtained precursors were ground and calcinated at 450 °C for 4 h, and then pickled into 0.5 M sulfuric acid at 20 °C for 1 h. The resulting HMO-3 was pure Li1.33Mn1.67O4, while HMO-2 was a mixture of Li1.33Mn1.67O4 and Mn2O3. Before using sorbents, the oil layer was removed from fracturing flowback and produced water by centrifugation, and the brine solution was heated to 70 °C. Adsorption tests were carried out at pH 8 for 30 min with the use of a sorbent at a dose of 2 g/L. After adsorption, the sorbent was treated with 0.5 M H2SO4 to desorb lithium ions. It was found that adsorption capacity for both sorbents was comparable (18 mg/g for HMO-2 and 17 mg/g for HMO-3). During the experiments, the researchers observed several problems, e.g., sorbent losses or a decrease in the pH of the brine solution during adsorption due to the release of protons from the ion exchange site, which resulted in a decrease in adsorption efficiency. The limited ability to regenerate the sorbent in an acidic environment was also a big problem, leading to manganese losses in the sorbent. In addition, the researchers pointed out the possibility of a manganese reduction in the sorbent structure during lithium sorption by organic molecules present in the brine, which could lead to increased sorbent loss through reductive dissolution. So, before using manganese-based sorbents, the fracturing flowback and produced water would need to be pre-treated to remove organic compounds [81].
The attempt to remove lithium from raw and pre-treated shale gas flowback and produced water from Gongxian, Sichuan Basin, China was studied by Tian et al. [82] using H1.33Mn1.67O4 as an adsorbent. First, the researchers synthesized a manganese-based adsorbent using a solid phase reaction method (Li2CO3 and MnCO3 at a Li:Mn molar ratio = 1.33:1.67 were calcined at 500 °C for 4 h, and then cooled to room temperature) and pickling with 0.5 mol/L HCl (1 g of precursor was added to 1 L of acid and stirred for 24 h, ten filtered, washed with ultrapure water, and dried at 50 °C for 24 h). In the next step, the researchers evaluated the suitability of the prepared adsorbent for recovering lithium from raw and pre-treated (precipitation with Na2CO3) shale gas flowback and produced water. Adsorption tests involved investigation of the effect of pH on lithium adsorption (pH: 4–11), adsorption kinetics (time: 1–72 h), adsorption isotherm (adsorbent mass: 0.015–0.1 g), adsorption selectivity, and reusability of the adsorbent (regeneration with HCl). The adsorption process was carried out in a shaking incubator (200 rpm) for 48 h at 25 °C using 0.05 g of adsorbent and 50 mL of solution. Tian et al. [82] proved that H1.33Mn1.67O4 adsorbent had high selectivity towards lithium (lithium partition coefficient for raw and pre-treated brine was 731.58 mL/g and 1073.58 mL/g, respectively), precipitation before adsorption improved the adsorbent performance (lithium adsorption capacity in the raw and pre-treated brine was 13.27 mg/g and 16.24 mg/g, respectively), brine pH had a significant impact on lithium adsorption capacity (lithium adsorption capacity increased with increasing pH of solution), and the adsorption capacity of lithium was stable and showed only a slight decrease (5.35% for raw and 3.53% for pre-treated shale gas flowback and produced water) after four adsorption–desorption cycles [82].
Summarizing the research on the recovery of lithium from oil and gas produced waters using titanium- and manganese-based adsorbents, it can be concluded that both types of adsorbents are selective towards lithium, their adsorption capacity to lithium strongly depends on the pH of the brine and increases when the brine is pre-treated to remove divalent ions and organic compounds. For some manganese adsorbents, problems with sorbent loss during regeneration in acidic solution have been pointed out.
In conclusion, there are few scientific articles that discuss specific examples of lithium recovery technology from petroleum brines. Only several research teams are dealing with this issue, with most of the reported studies concerning experiments performed only on a laboratory scale and with the use of synthetic brine solutions. All the previous studies discussed in this review are preliminary and require further experiments to optimize the proposed lithium recovery method. Based on the analysis of the presented literature data, it can be stated that adsorption with titanium and manganese-based adsorbents seems to be the most promising option for recovery of lithium from oilfield brines. The advantages of this method include minimal required pretreatment (some authors indicate that precipitation with Na2CO3 is sufficient), low environmental impact (compared to solvent extraction), and high adsorption efficiency. The main disadvantages of using adsorbents for lithium recovery are relatively slow sorption kinetics, dependence on the pH of the solution, and powder form of the adsorbent which makes some limitation for industrial applications (recovery of the sorbent after the adsorption process may be difficult, therefore the adsorbent should be subjected to granulation).
In our opinion, in the future, great emphasis should be placed on developing a suitable form of sorbents (granules or monoliths) to improve the sorption kinetics and efficiency of the sorption column. In addition, one of the most important problems to be solved before industrial application of this technology is to improve the chemical and mechanical stability of the sorbent during long-term use, including resistance to acidic conditions during desorption and resistance to osmotic shock during the change of ionic strength between sorption and desorption phases. Currently, the scientific literature does not present pilot column trials involving multiple sorption and desorption cycles, in which granular sorbent is used to recover lithium from oilfield brines. It should also be noted that sorbent technology may be integrated with membrane technology, including modification of the membrane material with lithium-selective sieves, as well as electrochemical methods (e.g., electrochemical ion-pumping technology), but such studies have also not yet been reported in the scientific literature.
6. Industrial Technologies for Recovering Lithium from Oilfield Brine—Current Status and Future Perspectives
Current technological progress (especially enormous growth in the lithium-ion battery market) requires the use of many critical materials that are difficult to access, including lithium [7]. Because lithium production is concentrated in only a few locations in the world, lithium supply chains are very fragile [9]. Due to this, many countries are looking for local sources from which lithium can be recovered. Many such sources are proposed in the literature, including seawater, geothermal brines, oil, gas and shale gas field brines, salt lake brines as well as many kinds of solid deposits [1,13,14,20,81,88,89,90,91,92,93]. Also, in the case of brines from oil fields, there are many articles (both scientific and press) indicating such produced waters as potential sources of lithium (e.g., [13,56,94]).
However, looking closer, there are relatively few scientific articles covering this area in detail. Most of the available information are press releases (mostly addressed to potential investors) focusing on new investments in pilot plants, and research projects carried out by start-ups. There are several reports available online on the lithium in reservoir brines which were commissioned and accepted by government agencies such as the U.S. Department of Energy and the Alberta Research Council. Sources of information on the projects in other regions of the world are limited. To the best of our knowledge, only one government-sponsored project is currently underway in Europe (Poland’s CompLithium Project focusing on development of “Complex technology for lithium and potable water recovery from produced waters”) [95] and a single pilot plant research project is being carried out in China. Another source of information are patent applications in this area. On the other hand, predominantly described studies in scientific articles concern the laboratory trials which were mostly performed using simulated brines so drawing the conclusions from such studies is also limited.
An unclear picture of the situation is a typical phenomenon in areas where intensive research work is carried out by competing companies. In such situations, it is difficult to verify the actual achievements. Nevertheless, we will try to summarize the worldwide efforts in the considered area including the pilot scale research and industrial tests.
Kumar et al. [13] reviewed perspectives for lithium recovery from produced water in the USA. The authors concluded that general lithium oilfield deposits in the USA contain more than 750,000 metric tons of lithium equivalent. The authors also compared several lithium recovery technologies from the point of view of usage of oilfield brine as a lithium source. The authors stated that the most promising technologies are metal oxide adsorbent-based technologies as well as membrane technologies. Aluminum-based sorbents were developed by FMC (Tuscon, AZ, USA), Simbol (Pleasanton, CA, USA), and Eramet (Paris, France) companies, titanium oxide-based sorbents were developed by Neometals (West Perth, Australia), manganese oxide-based sorbents were developed by JOGMEC (Tokyo, Japan), and the membrane technology was developed by MGX (Vancouver, BC, Canada). In the article, there is no information about pilot tests for lithium recovery from oilfield brines with the use of the above-mentioned technologies. There is only notice about lithium recovery projects with Smackover brines [13].
Currently, in the area of the Smackover Formation, Standard Lithium Ltd. (Vancouver, BC, Canada) is performing two lithium recovery projects: the Lanxess Project and the Southwest Arkansas Project which are located in Southern Arkansas near the Louisiana border [96].
According to the press release, Standard Lithium Ltd. and Lanxess Corporation (Cologne, Germany) signed the agreement (dated 23 February 2022) “that streamlines and expedites the plan for development of the first commercial lithium project in Arkansas, which is to be constructed at an operational Lanxess facility in El Dorado, Arkansas”. The direct lithium extraction (DLE) process will be used for lithium recovery in this project [97]. At present, pilot plant operates according to the technology developed by Standard Lithium Ltd. from 2020. The company completed the Definitive Feasibility Study in September 2022. Annual production of lithium carbonate was assumed to be 20,900 metric tons. A detailed technical report on the preliminary economic evaluation of the Lanxess Smackover project is available online [98]. Standard Lithium Ltd. also signed a joint development agreement with Koch Technology Solutions “to share data and jointly develop and commercialize integrated lithium brine processing flowsheets for Standard Lithium’s exclusive use in the Smackover Formation” in May 2023 [99]. It should be mentioned that above-described project is a brownfield project, so CAPEX (capital expenditure) is significantly reduced.
The second project in the Smackover region carried out by Standard Lithium Ltd. is the Southwest Arkansas Project. Currently exploited oil and gas mining infrastructure including wells, collection systems, easements, and gas processing facilities will be used in the project. Preliminary economic assessment for the project was completed in November 2022 and the full technical report is available online [100]. A target annual production of 30,000 tons of battery-grade lithium hydroxide was assumed.
According to the patent application [101], technology developed by Standard Lithium Ltd. is based on application of lithium-ion sieves (LISs) to recover lithium from brine. Oxides of titanium and niobium are used as LISs. Metatitanic acid (prepared from lithium titanate) was used in the described example as an adsorbent. The technological scheme presented in the application consists of six reactors. Feed brine is contacted with the regenerated metatitanic acid (slurry in water) in the first reactor which is equipped with stirrer. During the ion exchange process, pH is adjusted with NaOH and maintained at pH 7.8. The resulting suspension is separated by membrane filtration. Lithium-loaded metatitanic acid slurry is pumped into a second reactor. Washing of loaded sorbent by water is carried out in the second reactor. Obtained suspension is separated by membrane filtration. The washed slurry of the loaded sorbent is pumped to the thickener reactor, where solids concentration is increased approximately to 500 g/L. Thickened slurry is directed to the regeneration reactor. Lithium loaded metatitanic acid is contacted with 0.2 M HCl. Lithium ions pass into a liquid phase and metatitanic acid is regenerated. The regeneration reactor is not equipped with a membrane filtration module. LIS slurry and LiCl/HCl solution simply overflow to the next reactor which is the first of two counter-currently operated acid wash reactors. The majority of the HCl/LiCl solution is washed from the first stage. Regenerated metatitanic acid is washed by water in the second stage and directed to the first reactor and contacted with feed brine. A continuous 12 h test was described in the patent application. According to the description, lithium concentration in the brine was reduced from 244 mg/L to 61 mg/L, and a 75% recovery rate was achieved. Lithium eluate which was produced contain 4300 mg/L of lithium ions [101].
The second region where oilfield brine is considered as a source of lithium is Alberta province in Canada. Currently in Alberta several companies are active (i.e., E3 Lithium Ltd., Empire Metals Corp., MGX Minerals/PurLucid, Prism Diversified, Indigo Exploration Inc.).
Indigo Exploration Inc. (Vancouver, Canada) announced its exploration plans for its Alberta-based lithium brine projects (Fox Creek and Leduc projects) for 2023, which includes well sampling and aquifer modelling and resource estimation. Moreover, preliminary economic assessment for each project is planned to be completed in 2023 [102].
E3 Lithium Ltd. formerly E3 Metals Corp. (Calgary, AB, Canada) is implementing the Clearwater Lithium Project.. Preliminary economic assessment for Clearwater Lithium Project was completed in December 2020 and is available online [103]. According to the technical report, existing lithium processing and oilfield technologies will be combined with E3’s direct lithium extraction (DLE) based on lithium selective sorbent technology to create a full lithium extraction process. In 2023, E3 Lithium Ltd. has started the construction of a pilot plant. According to the announcement (dated 13 July 2023), construction of the pilot plant is currently progressing according to schedule [104]. According to the patent applications the company is developing spinel lithium manganese oxides (Li1.3–1.6Mn1.6–1.7O4)-based sorbents. Laboratory trials of batch sorption of lithium ions from complex brine are described as examples. Initial lithium concentration in the brine was 70 mg/L. Recovery rate was in the range from 46% to 69%, stripping efficiency was in the range from 88% to 100% [105,106].
Empire Metals Corp. (Vancouver, BC, Canada) is leading Fox Creek Lithium Project [107].
MGX Minerals (Vancouver, BC, Canada) in cooperation with PurLucid (Calgary, AB, Canada) developed technology for rapid lithium extraction from oilfield brines. Technology is described in detail in patent applications US2020299805 (A1) and US2019276328 (A1) [108,109]. In the first stage, pH of brine is corrected (depending on the silica concentration) and polyaluminum chloride is used for removing of colloidal silica using RSL™ system. Also, magnesium and calcium ions are removed during the pretreatment process using hydroxide precipitation at pH increased up to 10. Resulting permeate is a feed for lithium recovery. Lithium-ion sieves based on titanium oxides are used as adsorbents. The process is carried out in column reactor. Pellets of LISs with additives (zeolites, aerogels) are used as a column filling.
It also should be noted that MGX Minerals announced (dated 9 May 2023) filing of notice of work for drilling at GC Lithium (LCT) Project in British Columbia [110].
Prism Diversified (Calgary, Canada) published a NI 43-101 compliant technical report on the Clear Hills lithium potential in 2018 which is available online [111].
Moreover, Volt Lithium Corp. (Calgary, AB, Canada) announced confirmation of the commencement of the pilot project to test its proprietary DLE process in a simulated commercial environment. Volt Lithium Corp. intends to demonstrate its ability to extract lithium from oilfield brine in scale by processing up to 250,000 liters of brine from its Rainbow Lake project in Northwest Alberta through the second quarter of 2023. Results from this pilot project were expected to be released by the company before the end of June 2023 [112].
In September 2022, it was announced that Prairie Lithium acquired oil wells slated for abandonment to advance their lithium resource research located in Saskatchewan (Canada) [113]. Prairie Lithium developed DLE technology based on lithium-ion sieves and tested it on a pilot scale. In this case, 400 m3 of brine was processed, the maximum throughput was of 17 m3 of brine, or 10 kg LCE per day [114]. Prairie Lithium has been developing its technology since 2019 [115,116].
Another company which operates in the field of advancing and scaling up lithium extraction processes from unconventional brines, such as geothermal and oilfield brines, is Recion Technologies [117]. Technology developed by the company was disclosed in patent application [118]. The first stage of the technology is the contacting of lithium exchange sorbent with feed brine. Lithium concentrate is produced in the course of lithium-loaded sorbent regeneration with an acid solution. The produced lithium concentrate, according to the described example, contained 1572 ppm of lithium (lithium level in feed brine was 161 ppm). The concentrate was polished using an ion exchange process because the concentrate also contained magnesium and calcium ions (95 ppm and 442 ppm, respectively). Amberlite™ IRC747 resin was used for removal of multivalence ions from the solution. The treated lithium concentrate was mixed with 3 M potassium phosphate tribasic at 70 °C and lithium phosphate was precipitated. The precipitate was washed with water several times. The final precipitate was dissolved in sulfuric acid. Concentrations of subsequent ions in obtained solution were equal: 167,140 ppm for Li, 228 ppm for B, 10,140 ppm for Na, 30 ppm for Mg, 2662 ppm for K, 8708 ppm for Ca and 2045 ppm for Sr. Such a solution was used as an anolyte in two-compartment electrolysis. LiOH was produced in the catholyte compartment during electrolysis. The final LiOH solution contained 1445 ppm of Li, 31 ppm of Na, 2 ppm of K, 1 ppm of Ca. Levels of other elements were below the detection limit [118].
Pilot scale projects as well as business interest can also be seen in countries other than the USA and Canada.
According to a press release, PetroChina Southwest Oil & Gasfield Company (Chengdu, China) has produced the first batch of lithium carbonate from gas-field water in a gas reservoir named Longwangmiao Formation, marking the successful commissioning of the first Chinese pilot plant for lithium extraction from gas-field water [119].
In the Ukraine, oilfield brines were also considered as a potential source of lithium, but only preliminary studies (estimation of lithium levels in produced water in several mining facilities) were conducted [120].
Preliminary laboratory tests of lithium recovery from oilfield brine (containing 6.8–7.9 mg/L of lithium ions) collected from the Mangistaumunaygaz facility (Aktau, Kazakhstan) were performed. Aluminum hydroxide-based sorbents were applied [121].
Russian company OOO Ekostar-Nautekh (Novosibirsk, Russia) developed a technology for lithium enrichment for the individual oilfield brine deposits of Na–Ca–Mg chloride type that are widespread on the Siberian platform. The developed method was tested on a large scale [90,122]. According to the technology description, lithium is recovered using aluminum hydroxide-based sorbents. In the next step, lithium ions are eluted from the sorbent bed. Subsequently, calcium and magnesium ions are precipitated from eluate. Purified diluted lithium chloride solution is concentrated in evaporator or using reverse osmosis. Afterward, NaCl and KCl crystals are separated. Filtrate containing 190–200 kg/m3 of total dissolved solids is purified from traces of calcium and magnesium ions. In the final step, lithium carbonate is precipitated [122].
Despite the many scientific papers published recently concerning the development of novel direct lithium recovery, methods in almost all the cases described above, only the lithium-ion sieve adsorption processes are applied for lithium recovery from oilfield brines on a larger (than laboratory) scale. The most commonly used LISs are materials that have been known for more than two decades: manganese oxides, titanium oxides, and layered aluminum hydroxides. Current efforts are directed towards process engineering and comprehensive development of the recovery process until pure battery-grade lithium carbonate is obtained. According to the presented state-of-the-art research, the process of production of battery-grade lithium carbonate from oilfield brine will be a multi-stage process containing at least the following steps:
- Pretreatment of oilfield brine, including removal of organic compounds, suspended solids, colloidal silica, and probably borates;
- Recovery of lithium using lithium ion sieves;
- Regeneration of lithium loaded LISs;
- Polishing of produced lithium concentrate (i.e., removal of calcium, magnesium, strontium, and other impurities);
- Concentration of polished solution of lithium salt using reverse osmosis, membrane processes or evaporation until reaching a concentration at which precipitation of lithium carbonate is possible or precipitation of lithium phosphate and dissolution precipitate in acidic solution, and subsequent operations necessary to obtain a concentrated lithium salt solution;
- Lithium carbonate precipitation as a final step.
Moreover, as part of the technology, methods of recycling or managing side streams must be developed. Taking into account the relatively low lithium concentration in feed oilfield brines as well as a high CAPEX of complex facility for the DLE process, it is likely that production will be profitable only in places with a large volume of brine production. In the case of oil and gas facility, there is a possibility to collect produced water from all wells in one facility which can increase the return on investment. Moreover, often such infrastructure for water collection as well as for the re-injection of brine to the formation, already exists in production places, what is a great advantage for such investments. Potable and technological water recovery from oilfield brine may be considered as another advantage in some regions. It is sometimes believed that recovering lithium can give a depleted gas well a new life. Therefore, gas & oil companies are interested in such technologies.
To summarize, there is great interest in the area of lithium recovery from oilfield brine, not only from scientific but also from the business point of view. Recently, many companies have developed their own direct lithium recovery technologies. Some of them tested such technologies on a pilot scale, but currently there is no full-scale plant for lithium recovery from oilfield brine. In our opinion, the Lanxess Smackover Project seems to be the most advanced.
According to the available technical reports as well as definitive feasibility studies, recovery of lithium (sometimes coupled with recovery of other materials) may be profitable but it depends on many factors specific to a given implementation site. Currently, it can be said that such technologies are not mature, and it is difficult to determine which of the directions of development will be dominant in the future. Recovering lithium from low-quality sources, such as oilfield brines, may be a good and more environmentally friendly alternative compared to extracting lithium from solid deposits and clays, but the spread of this approach will depend on the level of growth in lithium demand and the levels of recovery from spent batteries and other products.
The range of lithium concentrations in oilfield brines is similar to the range of lithium concentrations in geothermal brines, so it is reasonable to briefly compare the advantages and disadvantages of technologies for recovering lithium from both brines. The main disadvantage of recovering lithium from oilfield brines compared to geothermal brines is the high content of organic substances and particulate matter, which must be removed during pretreatment process. Moreover, materials used for lithium recovery (e.g., sorbents) must be resistant to traces of these substances (e.g., in the case of oilfield brines traces of particular matter may clog the pores of the sorbent, organic substances may physically adsorb on the surface of the sorbent and block the sorbent for lithium adsorption). Such problems may increase both operational (lower recovery efficiency, more frequent sorbent replacement) and capital costs (more complex and sophisticated pretreatment plant) in comparison with lithium recovery from a geothermal brine plant.
The major capital cost of greenfield investment in lithium recovery from formation brines (in both cases, geothermal and oilfield brines) is the cost of drilling wells. Preference will therefore be given to investments using already existing infrastructure. To sum up, a lithium recovery plant should be considered as an additional module in a facility in which core activity is something else (e.g., gas and oil production or heat and electricity production from geothermal energy). In such a business environment, the potential of a given industry/technology can be valued on the basis of what assets are currently available for such technology. In the case of lithium recovery from formation brines, there is a possibility of producing formation water. In the case of geothermal technology, for example, in the USA, there are currently about 2000 geothermal wells in operation [1]. In the case of the gas and oil industry, the estimated number of operating wells is ca. 1,000,000 [123]. According to Veil [31], in the USA, the annual volume of produced oilfield brine is estimated at 3.4 billion m3/year, while for geothermal brine, annual production (and reinjection) is estimated at ca. 1.0–1.4 billion m3/year (calculated based on electricity produced (17 billion kWh in 2022 [124]) and the typical production rate of a binary plant, which ranges from 393,000 to 512,000 gallons/day/MW [125]). Therefore, it can be concluded that in the USA, the amount of available raw material (brine) is at least double for oilfield brines compared to geothermal brines. However, it should be noted that in other countries such raw material supply potential may be different. Moreover, since brines from various formations contain different concentrations of lithium, such a simple comparison is not accurate. Only on the basis of a comparison of lithium content it may be determined whether lithium recovery from oilfield brines can compete with lithium recovery from geothermal brines.
The estimated annual potential for lithium production in the USA from geothermal brines, according to Stringfellow and Dobson [1], is in the range of 6800 to 34,236 MT/y of lithium equivalent (assuming 80% recovery efficiency and 90% operation time), while the target annual production of lithium for the two Smackover projects is equal, ca. 12,600 MT/y [98,100]. To summarize, the expected volumetric scales of lithium production from oilfield and geothermal brines are similar for projects in the USA.
Stringfellow and Dobson [1] reported that for lithium recovery processes from geothermal brines tested on a pilot or full scale, the efficiencies obtained were below 60%. Meanwhile, the efficiency of the process developed for Lanxess Smackover oilfield brine project was 90% [98]. Adsorption technology is considered the first choice for both geothermal and oilfield brines. The operational cost of a 20,000 ton/year lithium carbonate plant producing Li2CO3 from geothermal brine (Salton Sea) containing 400 mg/L of lithium was estimated at 3850 USD/MT of lithium carbonate [88], while for Lanxess Smackover oilfield brine project (lithium average concentration equal to 168 mg/L) operational cost was estimated at 4319 USD/MT of lithium carbonate [98]. Used brines, both geothermal and oilfield brines, are typically reinjected into the formation, but they may also be used as a source of potable or industrial water, so the environmental impact of both technologies will be similar.
To sum up, the prospect of recovering lithium from both geothermal and oilfield brines is similar, and it is difficult to answer the question of which raw material is more promising, or which technology is more profitable. In our opinion, there is no general answer to such a question. The profitability of a lithium recovery plant from both geothermal and oilfield brine may be high, but it depends on a number of factors related to the location of the plant (brine composition, volume production capacity, currently existing infrastructure, etc.).
7. Conclusions
This mini review summarizes the actual knowledge concerning the methods of lithium recovery from oilfield brines, including the influence of brine composition on the recovery process as well as a brief summary of pretreatments methods which must be integrated into the lithium recovery process. The current status of the technology from a scientific and business point of view as well as the future perspectives were also presented. Moreover, the volume, composition, and actual usage of oilfield brines were also briefly discussed.
In many countries, oilfield brines are considered a potential source of lithium and other valuable materials. There are many reviews and articles focusing on oilfield brines and discussing the perspective of using brines as feed for lithium recovery processes. However, there are few scientific articles in which experimental studies were carried out. Moreover, predominantly simulated oilfield brines were used as research material, and only laboratory experiences were described.
Most of the information about pilot plant tests and industrial efforts in this area come from press releases and patent literature. According to the presented view, there are many small companies investing in the development of DLE technologies that allow the recovery of lithium from oilfield brines. Furthermore, international corporate giants such as Lanxess are also interested in this field. Lithium recovery from oilfield brines pilot plants have so far been constructed in USA, Canada, China, and Russia.
Author Contributions
Conceptualization, E.K., G.R. and M.M.; methodology, G.R., E.K. and M.M.; investigation, G.R., E.K. and M.M.; writing—original draft preparation, E.K., G.R. and M.M.; writing—review and editing, M.M., G.R. and E.K.; visualization, E.K., G.R. and M.M.; supervision, E.K.; project administration, E.K.; funding acquisition, E.K. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Centre for Research and Development (NCBR) in the frame of Project Contract No. LIDER/34/0174/L-12/20/NCBR/2021 under the LEADER Program.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Stringfellow, W.T.; Dobson, P.F. Technology for the recovery of lithium from geothermal brines. Energies 2021, 14, 6805. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Li, L.; Deshmane, V.G.; Paranthaman, M.P.; Bhave, R.; Moyer, B.A.; Harrison, S. Lithium recovery from aqueous resources and batteries: A brief review. Johns. Matthey Technol. Rev. 2018, 62, 161–176. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Talens Peiró, L.; Villalba Méndez, G.; Ayres, R.U. Lithium: Sources, production, uses, and recovery outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef]
- Murphy, O.; Haji, M.N. A review of technologies for direct lithium extraction from low Li+ concentration aqueous solutions. Front. Chem. Eng. 2022, 4, 1008680. [Google Scholar] [CrossRef]
- Greim, P.; Solomon, A.A.; Breyer, C. Assessment of lithium criticality in the global energy transition and addressing policy gaps in transportation. Nat. Commun. 2020, 11, 4570. [Google Scholar] [CrossRef]
- Knapik, E.; Rotko, G.; Marszałek, M.; Piotrowski, M. Comparative study on lithium recovery with ion-selective adsorbents and extractants: Results of multi-stage screening test with the use of brine simulated solutions with increasing complexity. Energies 2023, 16, 3149. [Google Scholar] [CrossRef]
- Vera, M.L.; Torres, W.R.; Galli, C.I.; Chagnes, A.; Flexer, V. Environmental impact of direct lithium extraction from brines. Nat. Rev. Earth Environ. 2023, 4, 149–165. [Google Scholar] [CrossRef]
- Hu, X.; Wang, C.; Lim, M.K.; Chen, W.-Q.; Teng, L.; Wang, P.; Wang, H.; Zhang, C.; Yao, C.; Ghadimi, P. Critical systemic risk sources in global lithium-ion battery supply networks: Static and dynamic network perspectives. Renew. Sustain. Energy Rev. 2023, 173, 113083. [Google Scholar] [CrossRef]
- Alessia, A.; Alessandro, B.; Maria, V.-G.; Carlos, V.-A.; Francesca, B. Challenges for sustainable lithium supply: A critical review. J. Clean. Prod. 2021, 300, 126954. [Google Scholar] [CrossRef]
- Marcinov, V.; Klimko, J.; Takáčová, Z.; Pirošková, J.; Miškufová, A.; Sommerfeld, M.; Dertmann, C.; Friedrich, B.; Oráč, D. Lithium production and recovery methods: Overview of lithium losses. Metals 2023, 13, 1213. [Google Scholar] [CrossRef]
- Kumar, A.; Fukuda, H.; Hatton, T.A.; Lienhard, J.H. Lithium recovery from oil and gas produced water: A need for a growing energy industry. ACS Energy Lett. 2019, 4, 1471–1474. [Google Scholar] [CrossRef]
- Ooi, K.; Miyai, Y.; Katoh, S. Recovery of lithium from seawater by manganese oxide adsorbent. Sep. Sci. Technol. 1986, 21, 755–766. [Google Scholar] [CrossRef]
- Suud, E.M.; Suryantini; Mubarok, M.Z. Lithium extraction method from geothermal brine to find suitable method for geothermal fields in Indonesia: A Review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1159, 012011. [Google Scholar] [CrossRef]
- Warren, I. Techno-Economic Analysis of Lithium Extraction from Geothermal Brines; National Renewable Energy Laboratory: Golden, CO, USA, 2021. [Google Scholar]
- Ighalo, J.O.; Amaku, J.F.; Olisah, C.; Adeola, A.O.; Iwuozor, K.O.; Akpomie, K.G.; Conradie, J.; Adegoke, K.A.; Oyedotun, K.O. Utilisation of adsorption as a resource recovery technique for lithium in geothermal water. J. Mol. Liq. 2022, 365, 120107. [Google Scholar] [CrossRef]
- Weinand, J.M.; Vandenberg, G.; Risch, S.; Behrens, J.; Pflugradt, N.; Linßen, J.; Stolten, D. Low-carbon lithium extraction makes deep geothermal plants cost-competitive in future energy systems. Adv. Appl. Energy 2023, 11, 100148. [Google Scholar] [CrossRef]
- Sanjuan, B.; Gourcerol, B.; Millot, R.; Rettenmaier, D.; Jeandel, E.; Rombaut, A. Lithium-rich geothermal brines in Europe: An up-date about geochemical characteristics and implications for potential Li resources. Geothermics 2022, 101, 102385. [Google Scholar] [CrossRef]
- EuGeLi: Lithium Extraction from Geothermal Brines in Europe. Available online: https://www.Brgm.Fr/En/Current-Project/Eugeli-Lithium-Extraction-Geothermal-Brines-Europe (accessed on 29 July 2023).
- French Lithium for Electric Vehicle Batteries. Available online: https://www.eramet.com/en/activities/innovate-design/eugeli-project (accessed on 29 July 2023).
- Vulcan Zero Carbon Lithium™ Project Phase One dfs Results and Resources-Reserves Update. Available online: https://v-er.eu/vulcan-zero-carbon-lithium-project-phase-one-dfs-results-and-resources-reserves-update/ (accessed on 29 July 2023).
- Harris, P. Albemarle to Double Silver Peak Lithium Production. Available online: https://www.mining-journal.com/energy-minerals-news/news/1402126/ablemarle-to-double-silver-peak-lithium-production (accessed on 29 July 2023).
- Sutijan, S.; Darma, S.A.; Hananto, C.M.; Sujoto, V.S.H.; Anggara, F.; Jenie, S.N.A.; Astuti, W.; Mufakhir, F.R.; Virdian, S.; Utama, A.P.; et al. Lithium separation from geothermal brine to develop critical energy resources using high-pressure nanofiltration technology: Characterization and optimization. Membranes 2023, 13, 86. [Google Scholar] [CrossRef]
- Chen, S.; Chen, Z.; Wei, Z.; Hu, J.; Guo, Y.; Deng, T. Titanium-based ion sieve with enhanced post-separation ability for high performance lithium recovery from geothermal water. Chem. Eng. J. 2021, 410, 128320. [Google Scholar] [CrossRef]
- Miao, J.; Zhao, K.; Guo, F.; Xu, L.; Xie, Y.; Deng, T. Novel LIS-doped mixed matrix membrane absorbent with high structural stability for sustainable lithium recovery from geothermal water. Desalination 2022, 527, 115570. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, K.T.; Ogolo, N.A.; Angelis-Dimakis, A.; Albert, O. Physicochemical assessment and treatment of produced water: A case study in Niger Delta Nigeria. Pet Res. 2023, 8, 87–95. [Google Scholar] [CrossRef]
- Clark, C.E.; Veil, J.A. Produced Water Volumes and Management Practices in the United States; U.S. Department of Energy—National Energy Technology Laboratory: Houston, TX, USA, 2009. [Google Scholar]
- Veil, J.U.S. Produced Water Volumes and Management Practices in 2012; The Ground Water Protection Council: Oklahoma City, OK, USA, 2015. [Google Scholar]
- Veil, J.U.S. Produced Water Volumes and Management Practices in 2017; Ground Water Research and Education Foundation: Oklahoma City, OK, USA, 2020. [Google Scholar]
- Eyitayo, S.I.; Watson, M.C.; Kolawole, O.; Xu, P.; Lawal, K.A.; Wigwe, M.E.; Giussani, A. Novel systematic approach for produced water volume quantification applicable for beneficial reuse. Environ. Sci. Adv. 2023, 2, 508–528. [Google Scholar] [CrossRef]
- Armenta, M. Mechanisms and Control of Water Inflow to Wells in Gas Reservoirs with Bottom Water Drive. Doctoral Dissertation, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2003. [Google Scholar]
- Miranda, M.A.; Ghosh, A.; Mahmodi, G.; Xie, S.; Shaw, M.; Kim, S.; Krzmarzick, M.J.; Lampert, D.J.; Aichele, C.P. Treatment and recovery of high-value elements from produced water. Water 2022, 14, 880. [Google Scholar] [CrossRef]
- Dolan, F.C.; Cath, T.Y.; Hogue, T.S. Assessing the feasibility of using produced water for irrigation in Colorado. Sci. Total Environ. 2018, 640–641, 619–628. [Google Scholar] [CrossRef]
- McCurdy, R. Underground Injection Wells for Produced Water Disposal. Proceedings of the Technical Workshops for the Hydraulic Fracturing Study: Water Resources Management. 2011. Available online: https://user-content.perma.cc/media/2014/10/19/5/37/3WF-8E9B/ (accessed on 29 July 2023).
- Mallants, D.; Šimůnek, J.; Torkzaban, S. Determining water quality requirements of coal seam gas produced water for sustainable irrigation. Agric. Water Manag. 2017, 189, 52–69. [Google Scholar] [CrossRef]
- Jaffar, S.; Khaliq, A.; Ahmed, M.; Al-Wardy, M.; Al-Busaidi, A.; Choudri, B.S. Wastewater and sludge management and research in Oman: An overview. J. Air Waste Manag. Assoc. 2017, 67, 267–278. [Google Scholar] [CrossRef]
- Alley, B.; Beebe, A.; Rodgers, J., Jr.; Castle, J.W. Chemical and physical characterization of produced waters from conventional and unconventional fossil fuel resources. Chemosphere 2011, 85, 74–82. [Google Scholar] [CrossRef]
- Knapik, E.; Chruszcz-Lipska, K. Chemistry of reservoir fluids in the aspect of CO2 injection for selected oil reservoirs in Poland. Energies 2020, 13, 6456. [Google Scholar] [CrossRef]
- Uliasz-Misiak, B. Wody towarzyszące złożom węglowodorów jako potencjalne źródło jodu, litu i strontu. Miner. Res. Manag. 2016, 32, 31–44. [Google Scholar] [CrossRef]
- Hitchon, B.; Underschultz, J.R.; Bachu, S. Industrial Mineral Potential of Alberta Formation Waters; Alberta Research Council: Edmonton, AB, Canada; Alberta Geological Survey: Edmonton, AB, Canada, 1993. [Google Scholar]
- Li, J.; Chen, F.; Ling, Z.; Li, T. Lithium sources in oilfield waters from the Qaidam Basin, Tibetan Plateau: Geochemical and Li isotopic evidence. Ore Geol. Rev. 2021, 139, 104481. [Google Scholar] [CrossRef]
- Yu, X.; Wang, C.; Huang, H.; Wang, J.; Yan, K. Lithium and brine geochemistry in the Qianjiang Formation of the Jianghan Basin, central China. Sci. Rep. 2023, 13, 4445. [Google Scholar] [CrossRef] [PubMed]
- Hitchon, B.; Bachu, S.; Underschultz, J.R.; Yuan, L.P. Industrial Mineral Potential of Alberta Formation Waters; Alberta Research Council: Edmonton, AB, Canada; Alberta Geological Survey: Edmonton, AB, Canada, 1995. [Google Scholar]
- Bukowski, K.; Czapowski, G. Wody Mineralne Jako Źródło Surowców Chemicznych. Available online: http://surowce-chemiczne.pgi.gov.pl/wody_min.htm (accessed on 29 July 2023).
- Dawoud, H.D.; Saleem, H.; Alnuaimi, N.A.; Zaidi, S.J. Characterization and treatment technologies applied for produced water in Qatar. Water 2021, 13, 3573. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and teuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Collins, A.G. Geochemistry of Liquids, Gases, and Rocks from the Smackover Formation; U.S. Bureau of Mines: Washington, DC, USA, 1974. [Google Scholar]
- Knapik, E.; Chruszcz-Lipska, K.; Łukańko, Ł.; Wysocki, S. Reuse of flowback water from hydraulic fracturing for drilling mud preparation and secondary hydrocarbon recovery. Energies 2021, 14, 5921. [Google Scholar] [CrossRef]
- Engle, M.A.; Reyes, F.R.; Varonka, M.S.; Orem, W.H.; Ma, L.; Ianno, A.J.; Schell, T.M.; Xu, P.; Carroll, K.C. Geochemistry of formation waters from the Wolfcamp and “Cline” Shales: Insights into brine origin, reservoir connectivity, and fluid flow in the Permian Basin, USA. Chem. Geol. 2016, 425, 76–92. [Google Scholar] [CrossRef]
- Garrett, D.E. Handbook of Lithium and Natural Calcium Chloride: Their Deposits, Processing, Uses and Properties, 1st ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Petersková, M.; Valderrama, C.; Gibert, O.; Cortina, J.L. Extraction of valuable metal ions (Cs, Rb, Li, U) from reverse osmosis concentrate using selective sorbents. Desalination 2012, 286, 316–323. [Google Scholar] [CrossRef]
- Yousef, R.; Qiblawey, H.; El-Naas, M.H. Adsorption as a process for produced water treatment: A review. Processes 2020, 8, 1657. [Google Scholar] [CrossRef]
- Zaman, H.G.; Baloo, L.; Pendyala, R.; Singa, P.K.; Ilyas, S.U.; Kutty, S.R.M. Produced water treatment with conventional adsorbents and MOF as an alternative: A review. Materials 2021, 14, 7607. [Google Scholar] [CrossRef]
- Sanchez-Rosario, R.; Hildenbrand, Z.L. Produced water treatment and valorization: A techno-economical review. Energies 2022, 15, 4619. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The formation, stabilization and separation of oil–water emulsions: A review. Processes 2022, 10, 738. [Google Scholar] [CrossRef]
- Purchas, D.; Sutherland, K. (Eds.) Handbook of Filter Media, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; ISBN 9780080507774. [Google Scholar]
- Freedman, D.E.; Riley, S.M.; Jones, Z.L.; Rosenblum, J.S.; Sharp, J.O.; Spear, J.R.; Cath, T.Y. Biologically active filtration for fracturing flowback and produced water treatment. J. Water Process Eng. 2017, 18, 29–40. [Google Scholar] [CrossRef]
- Doyle, D.H.; Brown, A.B. Produced Water Treatment and Hydrocarbon Removal with Organoclay. In Proceedings of the SPE 63100 Presented at the 2000 SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, 1–4 October 2000. [Google Scholar]
- Janks, J.S.; Cadena, F. Investigations into the Use of Modified Zeolites for Removing Benzenes, Toluene and Xylene from Saline Produced Water. In Produced Water: Technological/Environmental Issues and Solutions; Ray, J.P., Engelhardt, F.R., Eds.; Plenum Publishing Corp.: New York, NY, USA, 1992; pp. 473–488. [Google Scholar]
- Knapik, E.; Chruszcz-Lipska, K.; Stopa, J.; Marszałek, M.; Makara, A. Separation of BTX fraction from reservoir brines by sorption onto hydrophobized biomass in a fixed-bed-column system. Energies 2020, 13, 1064. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Wang, Z. Oilfield produced water treatment with surface modified fiber ball media filtration. Water Sci. Technol. 2002, 46, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Water Chemistry and Pretreatment. Fouling Prevention. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/water-solutions/public/documents/en/RO-NF-FilmTec-Guidelines-for-Feedwater-Manual-Exc-45-D01578-en.pdf (accessed on 29 July 2023).
- Pretreatment for Membrane Processes. Available online: https://www.amtaorg.com/wp-content/uploads/12_Pretreatment.pdf (accessed on 29 July 2023).
- Çakmakce, M.; Kayaalp, N.; Koyuncu, I. Desalination of produced water from oil production fields by membrane processes. Desalination 2008, 222, 176–186. [Google Scholar] [CrossRef]
- Patrick, B.; Tsang, P.E.; Christopher, J.; Martin, P.E. Economic evaluation of treating oilfield produced water for potable use. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium and Western Regional Meeting, Bakersfield, CA, USA, 16–18 March 2004. [Google Scholar] [CrossRef]
- Meshram, P.; Pandey, B.D.; Mankhand, T.R. Extraction of lithium from primary and secondary sources by pre-treatment, leaching and separation: A comprehensive review. Hydrometallurgy 2014, 150, 192–208. [Google Scholar] [CrossRef]
- Harrison, S. Technologies for Extracting Valuable Metals and Compounds from Geothermal Fluids; DE-EE0002790; Department of Energy, Geothermal Technologies Program: Washington, DC, USA, 2014. [Google Scholar]
- Perez, W.; Barrientos, H.A.C.; Suarez, C.; Bravo, M. Method for the Production of Battery Grade Lithium Carbonate from Natural and Industrial Brines. U.S. Patent No. 8691169, 8 April 2014. [Google Scholar]
- Laitala, H.; Karonen, J.; Haavanlammi, L. Process and Equipment for Producing Pure Lithium-Containing Solution. U.S. Patent No. 9725787, 8 August 2017. [Google Scholar]
- Christopher, D.H.; Stewart, M.; Rice, J. The Recovery and Separation of Mineral Values from Geothermal Brines; BuMines OFR 81-75; Hazen Research, Inc.: Golden, CO, USA; US Bureau of Mines: Washington, DC, USA, 1975. [Google Scholar]
- Díaz Nieto, C.H.; Mata, M.A.; Palacios, C.J.O.; Palacios, N.A.; Torres, W.R.; Vera, M.L.; Flexer, V. Transmembrane fluxes during electrolysis in high salinity brines: Effects on lithium and other raw materials recovery. Electrochim. Acta 2023, 454, 142401. [Google Scholar] [CrossRef]
- Yang, H.-J.; Li, Q.-H.; Li, B.; Guo, F.-Q.; Meng, Q.-F.; Li, W. Optimization of operation conditions for extracting lithium ions from calcium chloride-type oil field brine. Int. J. Miner. Metall. Mater. 2012, 19, 290–294. [Google Scholar] [CrossRef]
- Jang, E.; Jang, Y.; Chung, E. Lithium recovery from shale gas produced water using solvent extraction. Appl. Geochem. 2017, 78, 343–350. [Google Scholar] [CrossRef]
- Lee, J.; Chung, E. Lithium recovery by solvent extraction from simulated shale gas produced water—Impact of organic compounds. Appl. Geochem. 2020, 116, 104571. [Google Scholar] [CrossRef]
- Zante, G.; Trébouet, D.; Boltoeva, M. Solvent extraction of lithium from simulated shale gas produced water with a bifunctional ionic liquid. Appl. Geochem. 2020, 123, 104783. [Google Scholar] [CrossRef]
- Jang, Y.; Chung, E. Adsorption of lithium from shale gas produced water using titanium based adsorbent. Ind. Eng. Chem. Res. 2018, 57, 8381–8387. [Google Scholar] [CrossRef]
- Jang, Y.; Chung, E. Influence of alkanes on lithium adsorption and desorption of a H2TiO3 ion sieve adsorbent in synthetic shale gas-produced water. Ind. Eng. Chem. Res. 2019, 58, 21897–21903. [Google Scholar] [CrossRef]
- Jang, Y.; Chung, E. Lithium adsorptive properties of H2TiO3 adsorbent from shale gas produced water containing organic compounds. Chemosphere 2019, 221, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Seip, A.; Safari, S.; Pickup, D.M.; Chadwick, A.V.; Ramos, S.; Velasco, C.A.; Cerrato, J.M.; Alessi, D.S. Lithium recovery from hydraulic fracturing flowback and produced water using a selective ion exchange sorbent. Chem. Eng. J. 2021, 426, 130713. [Google Scholar] [CrossRef]
- Tian, L.; Liu, Y.; Tang, P.; Yang, Y.; Wang, X.; Chen, T.; Bai, Y.; Tiraferri, A.; Liu, B. Lithium extraction from shale gas flowback and produced water using H1.33Mn1.67O4 adsorbent. Resour. Conserv. Recycl. 2022, 185, 106476. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Y.; Wan, P.; Gasem, K.; Wang, K.; He, T.; Adidharma, H.; Fan, M. Extraction of lithium with functionalized lithium ion-sieves. Prog. Mater. Sci. 2016, 84, 276–313. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Ma, P. Research progress on new types of H2TiO3 lithium-ion sieves: A review. Metals 2023, 13, 977. [Google Scholar] [CrossRef]
- Yu, H.; Naidu, G.; Zhang, C.; Wang, C.; Razmjou, A.; Han, D.S.; He, T.; Shon, H. Metal-based adsorbents for lithium recovery from aqueous resources. Desalination 2022, 539, 115951. [Google Scholar] [CrossRef]
- Tangkas, I.W.C.W.H.; Sujoto, V.S.H.; Astuti, W.; Jenie, S.N.A.; Anggara, F.; Utama, A.P.; Petrus, H.T.B.M.; Sutijan. Synthesis of titanium ion sieves and its application for lithium recovery from artificial Indonesian geothermal brine. J. Sustain. Metall. 2023, 9, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Orooji, Y.; Nezafat, Z.; Nasrollahzadeh, M.; Shafiei, N.; Afsari, M.; Pakzad, K.; Razmjou, A. Recent advances in nanomaterial development for lithium ion-sieving technologies. Desalination 2022, 529, 115624. [Google Scholar] [CrossRef]
- Ventura, S.; Bhamidi, S.; Hornbostel, M.; Anoop, N. Selective Recovery of Lithium from Geothermal Brines; Publication Number: CEC500-2020-020; California Energy Commission: Sacramento, CA, USA, 2020. [Google Scholar]
- Richter, A. Successful Production of Battery-Grade Geothermal Lithium Carbonate, France. Think GeoEnergy. Available online: https://www.thinkgeoenergy.com/successful-production-of-battery-grade-geothermal-lithium-carbonate-france/ (accessed on 29 July 2023).
- Ryabtsev, A.D.; Kotsupalo, N.P.; Kurakov, A.A.; Nemkov, N.M.; Vakhromeev, A.G. Rational use of multicomponent brines extracted together with oil. Theor. Found. Chem. Eng. 2020, 54, 756–761. [Google Scholar] [CrossRef]
- Jadar Project Website. Available online: https://riotintoserbia.com/ (accessed on 29 July 2023).
- Cinovec Lithium Project Website. Available online: https://britishlithium.co.uk/project/cinovec-lithium-czech-republic/ (accessed on 29 July 2023).
- 5 Ongoing Lithium Projects in Africa. Available online: https://venturesafrica.com/5-ongoing-lithium-projects-in-africa/ (accessed on 29 July 2023).
- Xiao, F. Characterization and treatment of Bakken oilfield produced water as a potential source of value-added elements. Sci. Total Environ. 2021, 770, 145283. [Google Scholar] [CrossRef]
- CompLithium. Technologia Kompleksowego Odzysku Litu i Użytkowej z Odpadowych Wód Złożowych. Available online: https://complithium.agh.edu.pl/ (accessed on 29 July 2023).
- Standard Lithium Ltd. Arkansas Smackover Projects. Available online: https://www.standardlithium.com/projects/arkansas-smackover (accessed on 29 July 2023).
- Standard Lithium and Lanxess Finalize Plan for First Commercial Lithium Project in Arkansas. Available online: https://www.standardlithium.com/investors/news-events/press-releases/detail/113/standard-lithium-and-lanxess-finalize-plan-for-first (accessed on 29 July 2023).
- Standard Lithium Ltd. NI 43—101 Technical Report, Preliminary Economic Assessment of LANXESS Smackover Project. Available online: https://d1io3yog0oux5.cloudfront.net/standardlithium/files/pages/standardlithium/db/369/description/PEA_.pdf (accessed on 29 July 2023).
- Standard Lithium Signs Joint Development Agreement with Koch Technology Solutions. Available online: https://www.standardlithium.com/investors/news-events/press-releases/detail/143/standard-lithium-signs-joint-development-agreement-with (accessed on 29 July 2023).
- Standard Lithium Ltd. Preliminary Economic Assessment of Sw Arkansas Lithium Project, Ni 43—101 Technical Report. Available online: https://d1io3yog0oux5.cloudfront.net/standardlithium/files/pages/standardlithium/db/369/description/Standard_Lithium_SW_Arkansas_Lithium_Project_PEA_-_November_25th.pdf (accessed on 29 July 2023).
- Brown, C.J. Process for Recovery of Lithium from Brine. International. Patent Appl. No. PCT/CA2020/000069, 9 June 2020. [Google Scholar]
- Indigo Exploration Outlines Exploration Plan to Advance its Alberta Lithium Brines Projects. Available online: https://www.newsfilecorp.com/release/162761/Indigo-Exploration-Outlines-Exploration-Plan-to-Advance-its-Alberta-Lithium-Brines-Projects (accessed on 29 July 2023).
- E3 Metals Corp. NI 43-101 Technical Report, Preliminary Economic Assessment, Clearwater Lithium Project, Alberta, Canada. Available online: https://www.e3lithium.ca/_resources/reports/technical/20210917-NI43-101-PEA-Report-Amended-Final.pdf?v=0.511 (accessed on 29 July 2023).
- E3 Lithium Announces Field Pilot Plant on Track for August Start as Construction Continues. Available online: https://www.e3lithium.ca/newsroom/news-releases/e3-lithium-announces-field-pilot-plant-on-track-for-august-start-as-construction-continues (accessed on 29 July 2023).
- Alessi, D.; Safarimohsen-Abad, S. Sorbent Compositions and Methods of Manufacture for Use in Concentrating Lithium from Brines. International Patent Appl. No. PCT/CA2020/051763, 18 December 2020. [Google Scholar]
- Jastrzebska, R.; Sharma, M. Lithium Manganese Oxide Spinel Sorbent Compounds and Methods of Synthesis. International Patent Appl. No. PCT/CA2021/051782, 10 December 2021. [Google Scholar]
- Empire Metals Corp. Fox Creek Project Website. Available online: https://www.empiremetalscorp.com/fox-creek/ (accessed on 29 July 2023).
- Mceachern, P.M.; Wong, N.; Andric, M. Method and Apparatus for the Treatment of Water with the Recovery of Metals. U.S. Patent Appl. No. 16/624744, 24 September 2020. [Google Scholar]
- Lazerson, J.; Marks, A. Method for the Production of Lithium Carbonate from Salt Brines. U.S. Patent Appl. No. 16/348,109, 12 September 2019. [Google Scholar]
- MGX Minerals Announces Filing of Notice of Work for Drilling at GC Lithium (LCT) Project, British Columbia. Available online: https://www.mgxminerals.com/_files/ugd/b0543a_2d053839e7904b00a5e3b000155293f0.pdf (accessed on 29 July 2023).
- NI 43-101 Technical Report, on the Lithium Brines of the Clear Hills Property Alberta, Canada Prepared for Prism Diversified Ltd. Calgary Alberta by Edward Lyons Tekhne Research Inc. Available online: https://www.miningdataonline.com/reports/ClearHills_TechnicalReport_04152018.pdf (accessed on 29 July 2023).
- Volt Lithium Meets Key Milestone with Start-Up of Pilot Project. Available online: https://voltlithium.com/wp-content/uploads/2023/04/23-03-29-Pilot-Announcement-1.pdf (accessed on 29 July 2023).
- Prairie Lithium Acquires Oil Wells Slated for Abandonment to Advance their Lithium Resource Research. Available online: https://www.businesswire.com/news/home/20220920005109/en/Prairie-Lithium-Acquires-Oil-Wells-Slated-for-Abandonment-to-Advance-their-Lithium-Resource-Research (accessed on 29 July 2023).
- Prairie Lithium Direct Lithium Extraction (DLE) Technology—Presentation. Available online: https://www.prairielithium.ca/_files/ugd/74e06a_8611aa698f6a49bdb31ea09134058b3f.pdf (accessed on 29 July 2023).
- Prairie Lithium Website. Available online: https://www.prairielithium.ca/about (accessed on 29 July 2023).
- Ireland, I. Methods and Systems for Recovery of Valuable Target Species from Brine Solutions. International Patent Appl. No. PCT/CA2021/050591, 29 April 2021. [Google Scholar]
- Recion Technologies, Inc. Website. Available online: https://reciontechnologies.com/# (accessed on 29 July 2023).
- Safarimohsen-Abad, S.; Alessi, D. Lithium Extraction Process. International Patent Appl. No. PCT/CA2021/050523, 16 April 2021. [Google Scholar]
- PetroChina Produces First Batch of Lithium Carbonate from Gas-Field Water. Available online: https://www.globaltimes.cn/page/202301/1283176.shtml (accessed on 29 July 2023).
- Reva, M.V.; Chomko, D.F.; Chomko, F. Prospects lithium extraction from produced water in oil and gas fields of Ukrainian. Geoinformatics 2021, 2021, 1–7. [Google Scholar] [CrossRef]
- Karshigina, Z.B.; Abisheva, Z.S.; Bochevskaya, E.G.; Toilanbay, G.A. Recovery of lithium from oil and gas field brines. Tsvetnye Met. 2020, 7, 26–32. [Google Scholar] [CrossRef]
- Ryabtsev, A.D.; Titarenko, V.I.; Kotsupalo, N.P.; Menzheres, L.T.; Mamylova, E.V.; Kurakov, A.A.; Nemkov, N.M.; Kurakov, A.A.; Antonov, S.A.; Gushchina, E.P. Method of obtaining lithium concentrate from lithium-bearing natural brines and processing thereof into lithium chloride or lithium carbonate. U.S. Patent No. 11396452, 26 July 2022. [Google Scholar]
- U.S. Oil and Natural Gas Wells by Production Rate. Available online: https://www.eia.gov/petroleum/wells/ (accessed on 27 August 2023).
- Geothermal Explained. Use of Geothermal Energy. Available online: https://www.eia.gov/energyexplained/geothermal/use-of-geothermal-energy.php (accessed on 27 August 2023).
- Toba, A.-L.; Nguyen, R.T.; Cole, C.; Neupane, G.; Paranthaman, M.P. U.S. lithium resources from geothermal and extraction feasibility. Resour. Conserv. Recycl. 2021, 169, 105514. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).