Production of Microbial Lipids by Saitozyma podzolica Zwy2-3 Using Corn Straw Hydrolysate, the Analysis of Lipid Composition, and the Prediction of Biodiesel Properties
Abstract
:1. Introduction
2. Materials and Methods
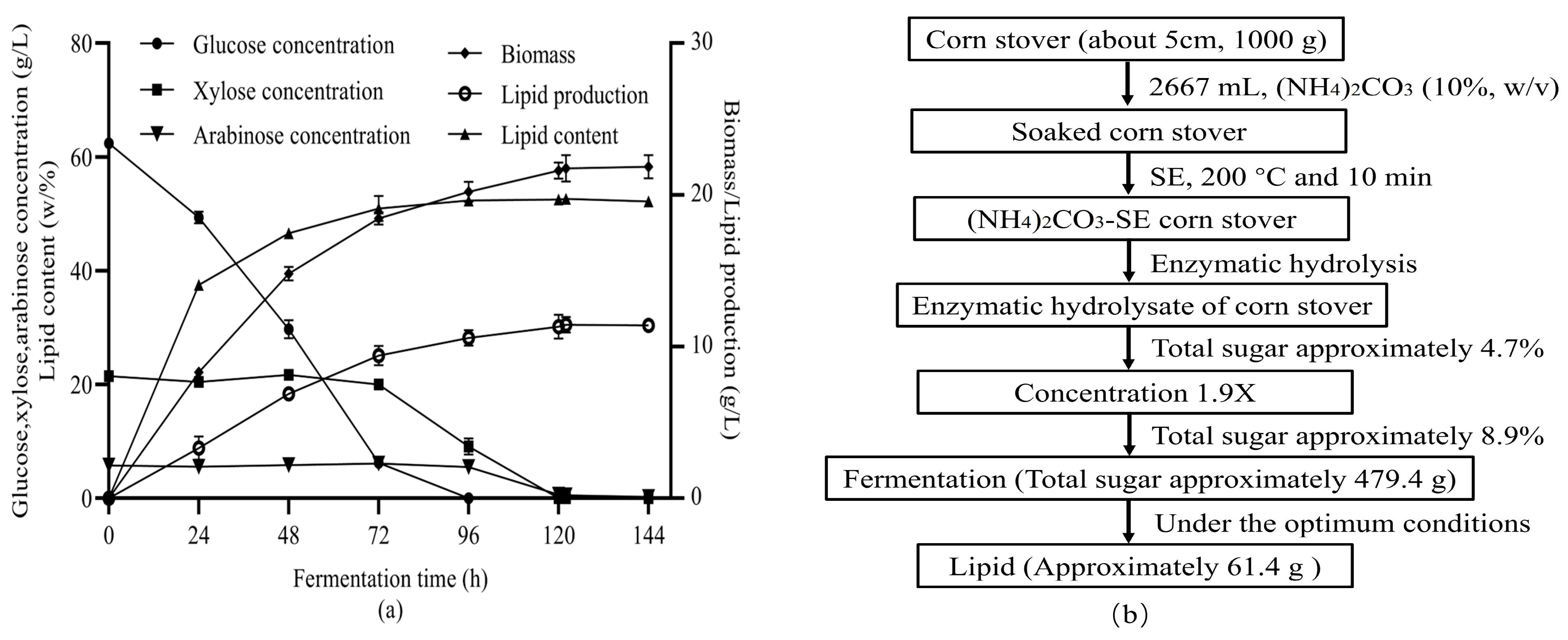
2.1. Processing of Corn Stalks
2.2. Strain, Mediums, and Culture Conditions
2.3. Effect of Major Fermentation Parameters on Biomass and Lipid Production
2.4. Experimental Design for Optimization of Lipid Production Using RSM
2.5. Determination of Cell Dry Weight and Lipid Extraction
2.6. Lipid Composition Analysis by Fatty Acid Methyl Ester (FAME)
2.7. Prediction of Biodiesel Properties Based on the Lipid Profile
2.8. Statistical Analysis
3. Results and Discussion
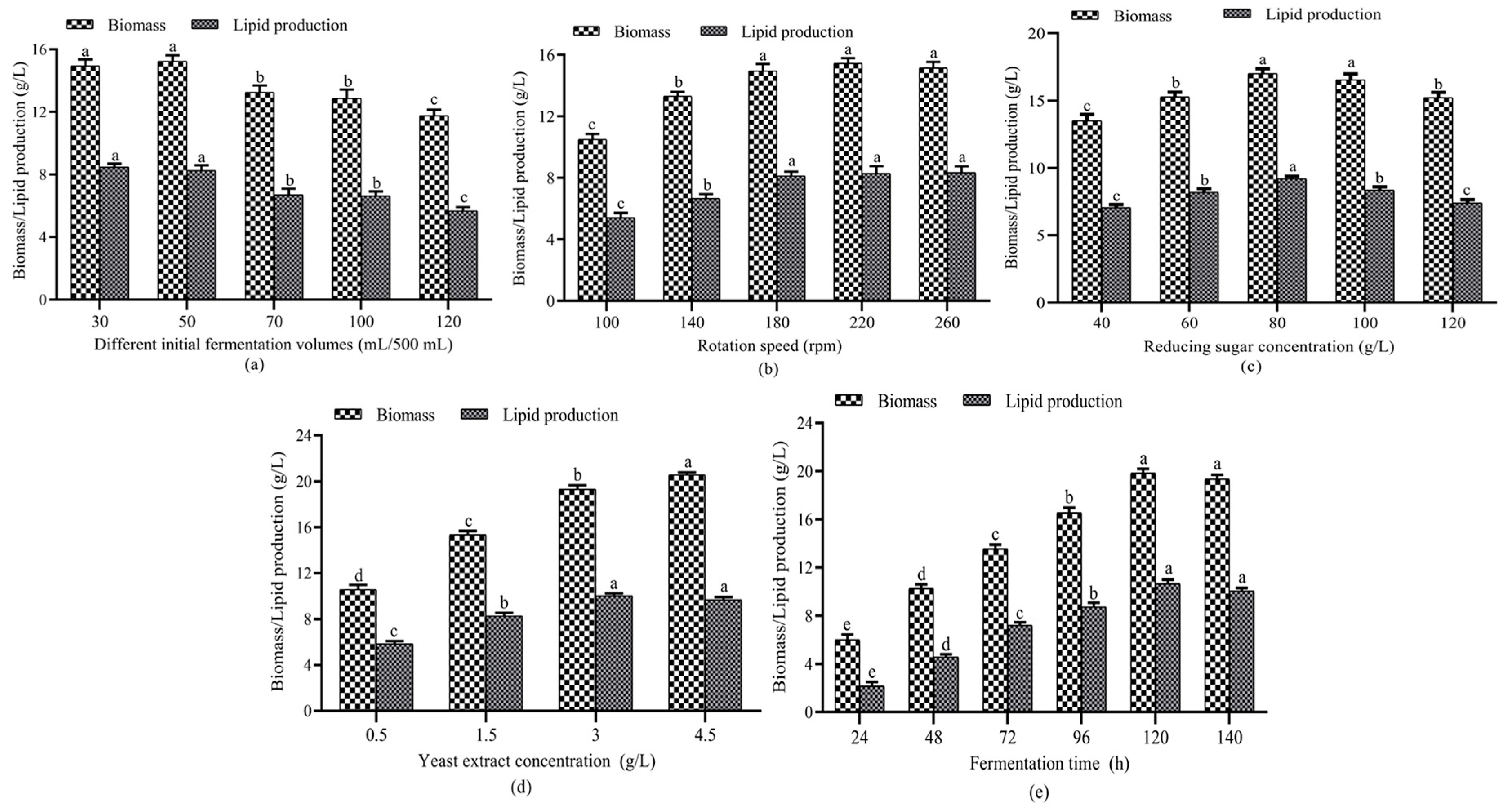
3.1. Optimization of Single-Factor Experiments
3.1.1. Effect of Fermentation Volume and Shaker Speed on Lipid Accumulation
3.1.2. Effect of Reducing Sugar Concentration on Lipid Accumulation
3.1.3. Effect of Yeast Extract Concentration on Lipid Accumulation
3.1.4. Effect of Fermentation Time on Lipid Accumulation
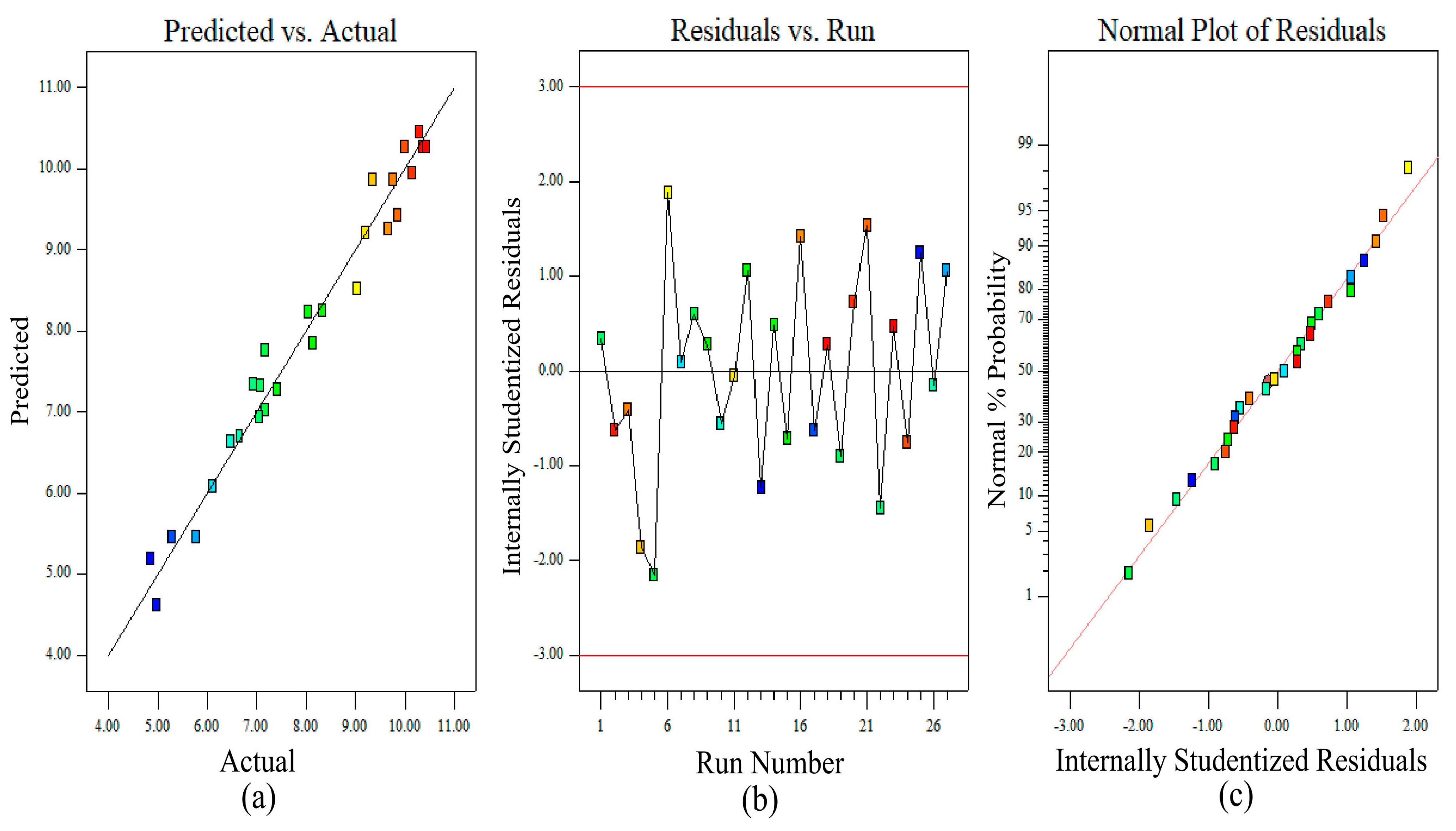
3.2. Assessment of the Regression Model and Adequacy Check for Lipid Production
0.81 × A × B + 0.045 × A × C
+ 0.16 × A × D + 0.65 × B × C + 0.41 × B × D − 0.53 × C × D − 1.37 × A2 − 1.27 × B2 − 1.70 × C2 −
0.81 × D2
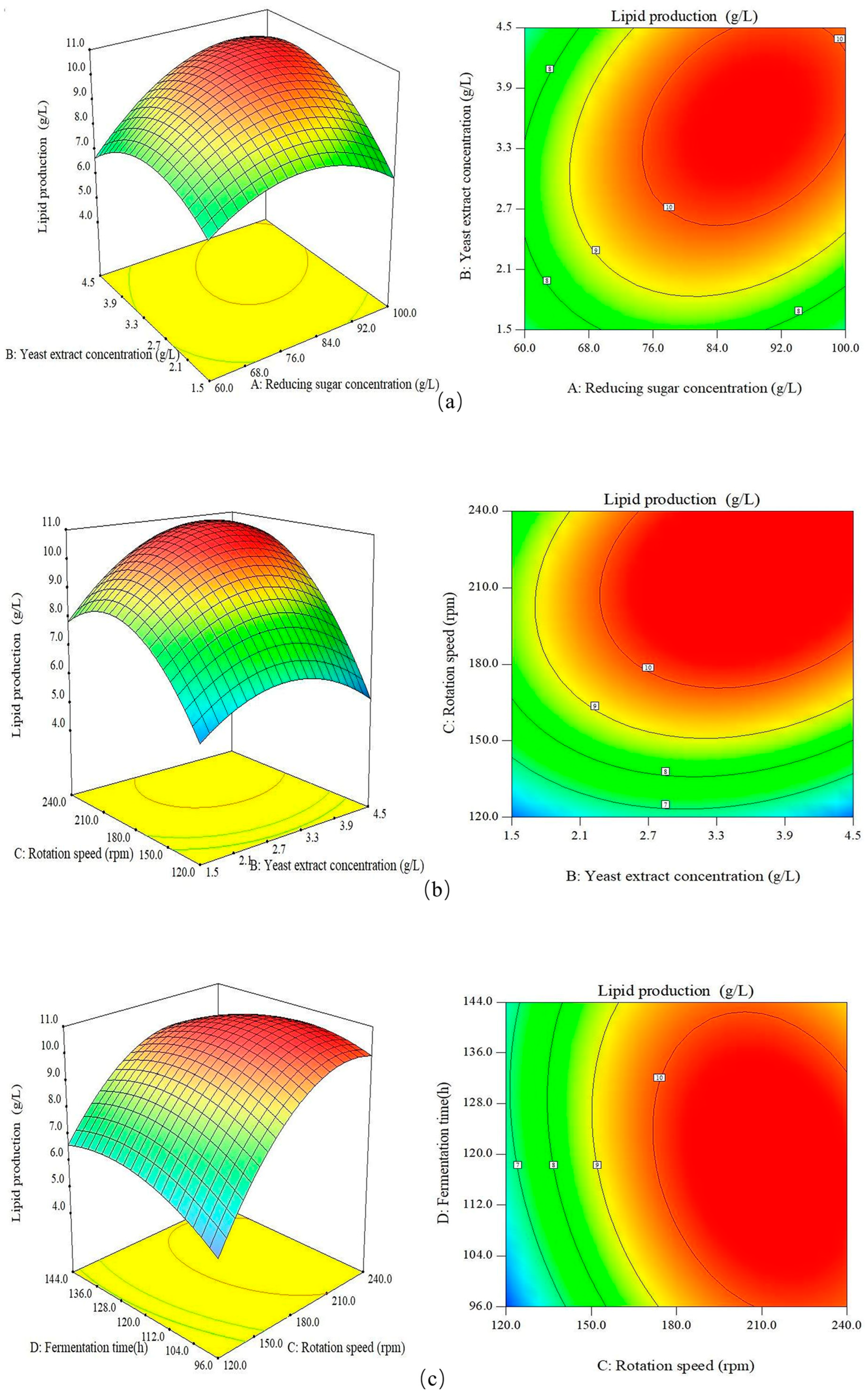
3.3. Response Surface Plots Analysis
3.4. Lipid Accumulation Ability of S. podzolica Zwy2-3 under Optimized Conditions
3.5. Comparative Analysis of Fatty Acid Composition and Proportions under Various Culture Conditions
3.6. Prediction of Biodiesel Properties Based on the Lipid Profile
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leong, W.-H.; Lim, J.-W.; Lam, M.-K.; Uemura, Y.; Ho, Y.-C. Third generation biofuels: A nutritional perspective in enhancing microbial lipid production. Renew. Sustain. Energy Rev. 2018, 91, 950–961. [Google Scholar]
- Kumar, S.; Singhal, M.K.; Sharma, M.P. Predictability of biodiesel fuel properties from the fatty acid composition of the feedstock oils. Arab. J. Sci. Eng. 2022, 47, 5671–5691. [Google Scholar]
- Leung, D.Y.C.; Wu, X.; Leung, M.K.H. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar]
- Oktavian, R.; Poerwadi, B.; Pahleva, M.R.; Wahyu, M.; Muharyanto, S. Synthesis and performance assessment of coconut fiber solid adsorbent for waste cooking oil purification as biodiesel feedstock. Malays. J. Fundam. Appl. Sci. 2020, 16, 374–377. [Google Scholar] [CrossRef]
- Sharma, P.; Usman, M.; Salama, E.-S.; Redina, M.; Thakur, N.; Li, X. Evaluation of various waste cooking oils for biodiesel production: A comprehensive analysis of feedstock. Waste Manag. (Oxford) 2021, 136, 219–229. [Google Scholar] [CrossRef]
- Cho, H.U.; Park, J.M. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef]
- Ochsenreither, K.; Glück, C.; Stressler, T.; Fischer, L.; Syldatk, C. Production strategies and applications of microbial single cell oils. Front. Microbiol. 2016, 7, 1539. [Google Scholar]
- Jin, M.; Slininger, P.J.; Dien, B.S.; Waghmode, S.; Balan, V. Microbial lipid-based lignocellulosic biorefinery: Feasibility and challenges. Trends Biotechnol. 2015, 33, 43–54. [Google Scholar]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhao, M.; Mofijur, M.; Awasthi, M.K.; Kalam, M.A.; Ragauskas, A.; Qi, X. Towards the sustainable conversion of corn stover into bioenergy and bioproducts through biochemical route: Technical, economic and strategic perspectives. J. Clean. Prod. 2023, 400, 136699. [Google Scholar]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Agbulut, U.; Len, C.; Nguyen, P.Q.P.; Kchaou, M.; Chen, W.H. Steam explosion as sustainable biomass pretreatment technique for biofuel production: Characteristics and challenges. Bioresour. Technol. 2023, 385, 129398. [Google Scholar]
- Baral, N.R.; Shah, A. Microbial inhibitors: Formation and effects on acetone-butanol-ethanol fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 9151–9172. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, Z.; Chen, S.; Jin, M. Microbial lipid production from dilute acid and dilute alkali pretreated corn stover via Trichosporon dermatis. Bioresour. Technol. 2020, 295, 122253. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Y.; Liu, W.; Bao, J. Biological removal of inhibitors leads to the improved lipid production in the lipid fermentation of corn stover hydrolysate by Trichosporon cutaneum. Bioresour. Technol. 2011, 102, 9705–9709. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, X.; Hu, X.; Zhou, N. Lignin monomer in steam explosion assist chemical treated cotton stalk affects sugar release. Bioresour. Technol. 2019, 276, 343–348. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, N.; Yao, H.Y.; Qin, H.; Zeng, J.; Ran, Y.L.; Yang, Y.B.; Qiao, D.R.; Cao, Y. Lipid production from corn stover by a cost-efficient system featuring ammonium carbonate-steam explosion and recirculating enzymatic hydrolysis. Biomass Bioenergy 2019, 120, 387–395. [Google Scholar] [CrossRef]
- Pereira, A.S.; Miranda, S.M.; Lopes, M.; Belo, I. Factors affecting microbial lipids production by Yarrowia lipolytica strains from volatile fatty acids: Effect of co-substrates, operation mode and oxygen. J. Biotechnol. 2021, 331, 37–47. [Google Scholar] [CrossRef]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Lopes, M.; Gomes, A.S.; Silva, C.M.; Belo, I. Microbial lipids and added value metabolites production by Yarrowia lipolytica from pork lard. J. Biotechnol. 2018, 265, 76–85. [Google Scholar] [CrossRef]
- Lopes, M.; Miranda, S.M.; Alves, J.M.; Pereira, A.S.; Belo, I. Waste cooking oils as feedstock for lipase and lipid-rich biomass production. Eur. J. Lipid Sci. Technol. 2019, 121, 1800188. [Google Scholar] [CrossRef]
- Huang, X.; Yun, D.; Liao, X.; Bo, P.; He, Y.; Ma, C. Microbial lipid production from enzymatic hydrolysate of corn stover pretreated by combining with biological pretreatment and alkalic salt soaking. Ind. Crops Prod. 2018, 124, 487–494. [Google Scholar]
- Huang, C.; Yang, Y.; Qin, H.; Feng, S.; Yi, C. An oleaginous yeast strain: Screening, identification and optimization of fermentation conditions. Chin. J. Appl. Environ. Biol. 2014, 20, 609–614. [Google Scholar]
- Ran, Y.; Yang, Q.; Zeng, J.; Li, F.; Cao, Y.; Xu, Q.; Qiao, D.; Xu, H.; Cao, Y. Potential xylose transporters regulated by CreA improved lipid yield and furfural tolerance in oleaginous yeast Saitozyma podzolica zwy-2-3. Bioresour. Technol. 2023, 386, 129413. [Google Scholar]
- Gorte, O.; Kugel, M.; Ochsenreither, K. Optimization of carbon source efficiency for lipid production with the oleaginous yeast Saitozyma podzolica DSM 27192 applying automated continuous feeding. Biotechnol. Biofuels 2020, 13, 181. [Google Scholar]
- Qian, X.; Gorte, O.; Chen, L.; Zhang, W.; Dong, W.; Ma, J.; Xin, F.; Jiang, M.; Ochsenreither, K. Continuous self-provided fermentation for microbial lipids production from acetate by using oleaginous yeasts Cryptococcus podzolicus and Trichosporon porosum. Renew. Energy 2020, 146, 737–743. [Google Scholar]
- Qian, X.J.; Gorte, O.; Chen, L.; Zhang, W.M.; Dong, W.L.; Ma, J.F.; Jiang, M.; Xin, F.X.; Ochsenreither, K. Co-production of single cell oil and gluconic acid using oleaginous Cryptococcus podzolicus DSM 27192. Biotechnol. Biofuels 2019, 12, 127. [Google Scholar] [PubMed]
- Veza, I.; Spraggon, M.; Fattah, I.M.R.; Idris, M. Response surface methodology (RSM) for optimizing engine performance and emissions fueled with biofuel: Review of RSM for sustainability energy transition. Results Eng. 2023, 18, 101213. [Google Scholar]
- Guerfali, M.; Ayadi, I.; Sassi, H.E.; Belhassen, A.; Gargouri, A.; Belghith, H. Biodiesel-derived crude glycerol as alternative feedstock for single cell oil production by the oleaginous yeast Candida viswanathii Y-E4. Ind. Crops Prod. 2020, 145, 112103. [Google Scholar]
- Kujawska, N.; Talbierz, S.; Debowski, M.; Kazimierowicz, J.; Zielinski, M. Optimizing docosahexaenoic acid (DHA) production by Schizochytrium sp. grown on waste glycerol. Energies 2021, 14, 1685. [Google Scholar]
- Ratledge, C.; Wynn, J.P. The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv. Appl. Microbiol. 2002, 51, 1–51. [Google Scholar]
- Bhatia, S.K.; Bhatia, R.K.; Yang, Y.-H. An overview of microdiesel—A sustainable future source of renewable energy. Renew. Sustain. Energy Rev. 2017, 79, 1078–1090. [Google Scholar]
- Santek, M.I.; Beluhan, S.; Santek, B. Production of microbial lipids from lignocellulosic biomass. In Advances in Biofuels and Bioenergy; Madhugiri, N.-R., Jaya, R.S., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 137–161. [Google Scholar]
- ASTM D6751-02; Standard Specification for Biodiesel Fuel (B100) Blend Stock for Distillate Fuels. American Society for Testing and Materials: West Conshohocken, PA, USA, 2002.
- EN 14214; Automotive Fuels-Fatty Acid Methyl Esters (FAME) for Diesel Engines-Requirements and Test Methods. European Committee for Standardization: Brussels, Belgium, 2008.
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar]
- Talebi, A.F.; Tabatabaei, M.; Chisti, Y. BiodieselAnalyzer: A user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res. J. 2014, 2, 55–57. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Folch, J. A simple methods for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Ran, Y.L.; Xu, H.; Yang, Q.Z.; Xu, Y.; Yang, H.H.; Qiao, D.R.; Cao, Y. GATA-type transcriptional factor SpGAT1 interacts with SpMIG1 and promotes lipid accumulation in the oleaginous yeast Saitozyma podzolica zwy-2-3. Biotechnol. Biofuels Bioprod. 2022, 15, 103. [Google Scholar]
- Liu, C.H.; Huang, C.C.; Wang, Y.W.; Lee, D.J.; Chang, J.S. Biodiesel production by enzymatic transesterification catalyzed by Burkholderia lipase immobilized on hydrophobic magnetic particles. Appl. Energy 2012, 100, 41–46. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Suwannarat, W.; Niyomdecha, R. Mixed culture of oleaginous yeast Rhodotorula glutinis and microalga Chlorella vulgaris for lipid production from industrial wastes and its use as biodiesel feedstock. New Biotechnol. 2011, 28, 362–368. [Google Scholar] [CrossRef]
- Magdouli, S.; Brar, S.K.; Blais, J.F. Morphology and rheological behaviour of Yarrowia lipolytica: Impact of dissolved oxygen level on cell growth and lipid composition. Process Biochem. 2017, 65, 1–10. [Google Scholar]
- Morão, A.; Maia, C.I.; Fonseca, M.M.R.; Vasconcelos, J.M.T.; Alves, S.S. Effect of antifoam addition on gas-liquid mass transfer in stirred fermenters. Bioprocess. Eng. 1999, 20, 165–172. [Google Scholar] [CrossRef]
- Wang, L.; Chen, H. Increased fermentability of enzymatically hydrolyzed steam-exploded corn stover for butanol production by removal of fermentation inhibitors. Process Biochem. 2011, 46, 604–607. [Google Scholar]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Robles-Iglesias, R.; Naveira-Pazos, C.; Fernández-Blanco, C.; Veiga, M.C.; Kennes, C. Factors affecting the optimisation and scale-up of lipid accumulation in oleaginous yeasts for sustainable biofuels production. Renew. Sustain. Energy Rev. 2023, 171, 113043. [Google Scholar]
- Wang, Z.; He, X.; Yan, L.; Wang, J.; Hu, X.; Sun, Q.; Zhang, H. Enhancing enzymatic hydrolysis of corn stover by twin-screw extrusion pretreatment. Ind. Crops Prod. 2020, 143, 111960. [Google Scholar]
- Noppawan, P.; Lanctôt, A.G.; Magro, M.; Navarro, P.G.; Supanchaiyamat, N.; Attard, T.M.; Hunt, A.J. High pressure systems as sustainable extraction and pre-treatment technologies for a holistic corn stover biorefinery. BMC Chem. 2021, 15, 37. [Google Scholar]
- Tkacova, J.; Caplova, J.; Klempova, T.; Certik, M. Correlation between lipid and carotenoid synthesis in torularhodin-producing Rhodotorula glutinis. Ann. Microbiol. 2017, 67, 541–551. [Google Scholar] [CrossRef]
- Javed, U.; Farooq, R.; Shehzad, F.; Khan, Z. Optimization of HNO3 leaching of copper from old AMD Athlon processors using response surface methodology. J. Environ. Manag. 2018, 211, 22–27. [Google Scholar] [CrossRef]
- Alvarez-Guzman, C.L.; Balderas-Hernandez, V.E.; Gonzalez-Garcia, R.; Ornelas-Salas, J.T.; Vidal-Limon, A.M.; Cisneros-de la Cueva, S.; De Leon-Rodriguez, A. Optimization of hydrogen production by the psychrophilic strain G088. Int. J. Hydrogen Energy. 2017, 42, 3630–3640. [Google Scholar] [CrossRef]
- Muralidhar, R.V.; Chirumamila, R.R.; Marchant, R.; Nigam, P. A response surface approach for the comparison of lipase production by Candida cylindracea using two different carbon sources. Biochem. Eng. J. 2001, 9, 17–23. [Google Scholar] [CrossRef]
- Zhang, G.; French, W.T.; Hernandez, R.; Alley, E.; Paraschivescu, M. Effects of furfural and acetic acid on growth and lipid production from glucose and xylose by Rhodotorula glutinis. Biomass Bioenergy 2011, 35, 734–740. [Google Scholar]
- Gong, Z.; Zhou, W.; Shen, H.; Zhao, Z.K.; Yang, Z.; Yan, J.; Zhao, M. Co-utilization of corn stover hydrolysates and biodiesel-derived glycerol by Cryptococcus curvatus for lipid production. Bioresour. Technol. 2016, 219, 552–558. [Google Scholar]
- Hu, C.; Wu, S.; Qian, W.; Jin, G.; Zhao, Z.K. Simultaneous utilization of glucose and xylose for lipid production by Trichosporon cutaneum. Biotechnol. Biofuels 2011, 4, 25. [Google Scholar]
- Miao, Z.G.; Tian, X.M.; Liang, W.X.; He, Y.W.; Wang, G.Y. Bioconversion of corncob hydrolysate into microbial lipid by an oleaginous yeast Rhodotorula taiwanensis AM2352 for biodiesel production. Renew. Energy 2020, 161, 91–97. [Google Scholar]
- Wang, X.; Xu, Q.; Cheng, J.; Hu, G.; Xie, H. Bio-refining corn stover into microbial lipid and advanced energy material using ionic liquid-based organic electrolyte. Ind. Crops Prod. 2020, 145, 112137. [Google Scholar]
- Gong, Z.W.; Shen, H.W.; Wang, Q.; Yang, X.B.; Xie, H.B.; Zhao, Z.B.K. Efficient conversion of biomass into lipids by using the simultaneous saccharification and enhanced lipid production process. Biotechnol. Biofuels 2013, 6, 36. [Google Scholar] [CrossRef]
- Galafassi, S.; Cucchetti, D.; Pizza, F.; Franzosi, G.; Bianchi, D.; Compagno, C. Lipid production for second generation biodiesel by the oleaginous yeast Rhodotorula graminis. Bioresour. Technol. 2012, 111, 398–403. [Google Scholar]
- Sitepu, I.R.; Ignatia, L.; Franz, A.K.; Wong, D.M.; Faulina, S.A.; Tsui, M.; Kanti, A.; Boundy-Mills, K. An improved high-throughput nile red fluorescence assay for estimating intracellular lipids in a variety of yeast species. J. Microbiol. Methods 2012, 91, 321–328. [Google Scholar] [CrossRef]
- Amaretti, A.; Raimondi, S.; Sala, M.; Roncaglia, L.; De Lucia, M.; Leonardi, A.; Rossi, M. Single cell oils of the cold-adapted oleaginous yeast Rhodotorula glacialis DBVPG 4785. Microb. Cell Fact. 2010, 9, 73. [Google Scholar]
- Giannakis, N.; Carmona-Cabello, M.; Makri, A.; Leiva-Candia, D.; Filippi, K.; Argeiti, C.; Pateraki, C.; Dorado, M.P.; Koutinas, A.; Stylianou, E. Spent coffee grounds and orange peel residues based biorefinery for microbial oil and biodiesel conversion estimation. Renew. Energy 2023, 209, 382–392. [Google Scholar]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol. Biofuels 2015, 8, 104. [Google Scholar] [CrossRef]
- Villegas-Méndez, M.Á.; Montañez, J.; Contreras-Esquivel, J.C.; Salmerón, I.; Koutinas, A.A.; Morales-Oyervides, L. Scale-up and fed-batch cultivation strategy for the enhanced co-production of microbial lipids and carotenoids using renewable waste feedstock. J. Environ. Manag. 2023, 339, 117866. [Google Scholar]
- Lu, H.; Chen, H.; Tang, X.; Yang, Q.; Zhang, H.; Chen, Y.Q.; Chen, W. Metabolomics analysis reveals the role of oxygen control in the nitrogen limitation induced lipid accumulation in Mortierella alpina. J. Biotechnol. 2021, 325, 325–333. [Google Scholar]
- Chang, G.; Wu, J.; Jiang, C.; Tian, G.; Wu, Q.; Chang, M.; Wang, X. The relationship of oxygen uptake rate and kLa with rheological properties in high cell density cultivation of docosahexaenoic acid by Schizochytrium sp. S31. Bioresour. Technol. 2014, 152, 234–240. [Google Scholar]
- Silva, R.M.; Castro, A.R.; Machado, R.; Pereira, M.A. Dissolved oxygen concentration as a strategy to select type and composition of bacterial storage lipids produced during oilfield produced water treatment. Environ. Technol. Innov. 2021, 23, 101693. [Google Scholar]
- Saini, R.; Hegde, K.; Brar, S.K.; Vezina, P. Advanced biofuel production and road to commercialization: An insight into bioconversion potential of Rhodosporidium sp. Biomass Bioenergy 2020, 132, 105439. [Google Scholar]
- Bajpai, D.; Tyagi, V.K. Biodiesel: Source, Production, Composition, Properties and Its Benefits. J. Oleo Sci. 2006, 55, 487–502. [Google Scholar]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar]
- Ibrahim, S.M.; Mustafa, A. Synthesis and characterization of new bifunctional SnZrSi oxide catalysts for biodiesel production. J. Mol. Liq. 2022, 354, 118811. [Google Scholar]
- Mishra, S.; Anand, K.; Mehta, P.S. Predicting cetane number of biodiesel fuels from their fatty acid methyl ester composition. Energy Fuels 2016, 30, 10425–10434. [Google Scholar]
- Mumtaz, M.W.; Adnan, A.; Anwar, F.; Mukhtar, H.; Raza, M.A.; Ahmad, F.; Rashid, U. Response surface methodology: An emphatic tool for optimized biodiesel production using rice bran and sunflower oils. Energies 2012, 5, 3307–3328. [Google Scholar]
- Knothe, G.; Matheaus, A.C.; Ryan, T.W. Cetane numbers of branched and straight-chain fatty esters determined in an ignition quality tester. Fuel 2003, 82, 971–975. [Google Scholar] [CrossRef]
- Yaar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 116817. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Gurav, R.; Choi, Y.-K.; Lee, H.-J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Lee, S.H.; Choi, K.-Y.; et al. Rhodococcus sp. YHY01 a microbial cell factory for the valorization of waste cooking oil into lipids a feedstock for biodiesel production. Fuel 2021, 301, 121070. [Google Scholar] [CrossRef]
- Neff, W.E.; Selke, E.; Mounts, T.L.; Rinsch, W.; Frankel, E.N.; Zeitoun, M.A.M. Effect of triacylglycerol composition and structures on oxidative stability of oils from selected soybean germplasm. J. Am. Oil Chem. Soc. 1992, 69, 111–118. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recenttrends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Kaya, C.; Hamamci, C.; Baysal, A.; Akba, O.; Erdogan, S.; Saydut, A. Methyl ester of peanut (Arachis hypogea L.) seed oil as a potential feedstock for biodiesel production. Renew. Energy 2009, 34, 1257–1260. [Google Scholar] [CrossRef]


| Factors | Independent Variables | Range and Levels | ||
|---|---|---|---|---|
| −1 | 0 | +1 | ||
| A | Reducing sugar concentration (g/L) | 60.00 | 80.00 | 100.00 |
| B | Yeast extract concentration (g/L) | 1.50 | 3.00 | 4.50 |
| C | Rotation speed (rpm) | 120 | 180 | 240 |
| D | Fermentation time (h) | 96 | 120 | 144 |
| Variables | Response | |||||
|---|---|---|---|---|---|---|
| Run | Reducing Sugar Concentration (g/L) | Yeast Extract Concentration (g/L) | Rotation Speed (rpm) | Fermentation Time (h) | Lipid Production (g/L) | |
| Factor A | Factor B | Factor C | Factor D | Actual | Predicted | |
| 1 | 60.00 | 1.50 | 180 | 120 | 7.18 | 7.02 |
| 2 | 100.00 | 1.50 | 180 | 120 | 7.04 | 6.95 |
| 3 | 60.00 | 4.50 | 180 | 120 | 6.65 | 6.69 |
| 4 | 100.00 | 4.50 | 180 | 120 | 9.75 | 9.86 |
| 5 | 80.00 | 3.00 | 120 | 96 | 4.85 | 5.19 |
| 6 | 80.00 | 3.00 | 240 | 96 | 10.15 | 9.95 |
| 7 | 80.00 | 3.00 | 120 | 144 | 6.48 | 6.63 |
| 8 | 80.00 | 3.00 | 240 | 144 | 9.66 | 9.27 |
| 9 | 60.00 | 3.00 | 180 | 96 | 7.41 | 7.28 |
| 10 | 100.00 | 3.00 | 180 | 96 | 9.04 | 8.52 |
| 11 | 60.00 | 3.00 | 180 | 144 | 6.94 | 7.34 |
| 12 | 100.00 | 3.00 | 180 | 144 | 9.19 | 9.20 |
| 13 | 80.00 | 1.50 | 120 | 120 | 5.76 | 5.46 |
| 14 | 80.00 | 4.50 | 120 | 120 | 5.28 | 5.45 |
| 15 | 80.00 | 1.50 | 240 | 120 | 8.14 | 7.85 |
| 16 | 80.00 | 4.50 | 240 | 120 | 10.28 | 10.46 |
| 17 | 60.00 | 3.00 | 120 | 120 | 4.97 | 4.62 |
| 18 | 100.00 | 3.00 | 120 | 120 | 6.11 | 6.08 |
| 19 | 60.00 | 3.00 | 240 | 120 | 8.03 | 8.23 |
| 20 | 100.00 | 3.00 | 240 | 120 | 9.35 | 9.87 |
| 21 | 80.00 | 1.50 | 180 | 96 | 7.16 | 7.76 |
| 22 | 80.00 | 4.50 | 180 | 96 | 8.32 | 8.24 |
| 23 | 80.00 | 1.50 | 180 | 144 | 7.07 | 7.32 |
| 24 | 80.00 | 4.50 | 180 | 144 | 9.86 | 9.43 |
| 25 | 80.00 | 3.00 | 180 | 120 | 10.37 | 10.27 |
| 26 | 80.00 | 3.00 | 180 | 120 | 10.01 | 10.27 |
| 27 | 80.00 | 3.00 | 180 | 120 | 10.44 | 10.27 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 80.78 | 14 | 5.77 | 31.18 | <0.0001 | significant |
| A | 7.21 | 1 | 7.21 | 38.95 | <0.0001 | ** |
| B | 5.06 | 1 | 5.06 | 27.33 | 0.0002 | ** |
| C | 40.92 | 1 | 40.92 | 221.14 | <0.0001 | ** |
| D | 0.43 | 1 | 0.43 | 2.32 | 0.1536 | |
| AB | 2.62 | 1 | 2.62 | 14.18 | 0.0027 | ** |
| AC | 8.100 × 10−3 | 1 | 8.100 × 10−3 | 0.044 | 0.8378 | |
| AD | 0.096 | 1 | 0.096 | 0.52 | 0.4849 | |
| BC | 1.72 | 1 | 1.72 | 9.27 | 0.0102 | * |
| BD | 0.66 | 1 | 0.66 | 3.59 | 0.0825 | |
| CD | 1.12 | 1 | 1.12 | 6.07 | 0.0298 | * |
| A2 | 10.07 | 1 | 10.07 | 54.39 | <0.0001 | ** |
| B2 | 8.60 | 1 | 8.60 | 46.49 | <0.0001 | ** |
| C2 | 15.39 | 1 | 15.39 | 83.17 | <0.0001 | ** |
| D2 | 3.54 | 1 | 3.54 | 19.14 | 0.0009 | ** |
| Residual | 2.22 | 12 | 0.19 | |||
| Lack of Fit | 2.11 | 10 | 0.21 | 3.97 | 0.2178 | not significant |
| Pure Error | 0.11 | 2 | 0.053 | |||
| Cor Total | 83.00 | 26 | ||||
| R2 = 0.9732 | Pred R2 = 0.8504 | |||||
| Adj R2 = 0.9420 | Adeq Precision = 18.187 | |||||
| Lipid Source | Fatty Acid Relative Percentage (%) | |||||
|---|---|---|---|---|---|---|
| C14:0 | C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | |
| Corn | ND c | 7–13 | 2.5–3 | 30.5–43 | 39–52 | 1 |
| Soybean | ND c | 2.3–11 | 2.4–6 | 22–30.8 | 49–53 | ND c |
| Safflower (high oleic) | ND c | 4–8 | 2.3–8 | 73.6–79 | 11–19 | 2–10.5 |
| Palm | 0.62.4 | 32–46.3 | 4–6.3 | 37–53 | 6–12 | ND c |
| Sunflower | ND c | 3.5–6.5 | 1.3–5.6 | 14–43 | 44–68.7 | ND c |
| Olive | 1.3 | 7–18.3 | 1.4–3.3 | 55.5–84.5 | 4–19 | ND c |
| S. Podzolica Zwy2-3 | ND c | 21.53 | 13.05 | 61.84 | 3.58 | ND c |
| Properties | Unit | Zwy2-3 Biodiesel | DSM 27192 Biodiesel | Sunflower Biodiesel | EN 14214 | ASTM D6751-02 |
|---|---|---|---|---|---|---|
| IV | - | 62.09 | 76.53 | 120.4 | ≤120 | ≤120 |
| CN | - | 59.29 | 56.09 | 50.54 | ≥51 | ≥47 |
| υ | mm2/s | 4.10 | 4.02 | 4.68 | 3.5–5.0 | 1.9–6.0 |
| SV | mg/g | 202.46 | 202.11 | 191.1 | - | - |
| OS | h | 35.53 | 14.50 | 2.00 | >8 | >3 |
| ρ | g/cm3 | 0.87 | 0.88 | 0.84 | 0.86–0.9 | 0.86–0.9 |
| HHV | MJ/kg | 39.56 | 39.58 | 43.9 | - | - |
| PP | °C | 0.054 | −0.82 | −3.74 | - | −15 to 10 |
| CP | °C | 6.33 | 5.53 | 4.57 | - | −3 to 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Guo, Y.; Ran, Y.; Yang, Q.; Cao, X.; Yang, H.; Cao, Y.; Xu, Q.; Qiao, D.; Xu, H.; et al. Production of Microbial Lipids by Saitozyma podzolica Zwy2-3 Using Corn Straw Hydrolysate, the Analysis of Lipid Composition, and the Prediction of Biodiesel Properties. Energies 2023, 16, 6630. https://doi.org/10.3390/en16186630
Feng S, Guo Y, Ran Y, Yang Q, Cao X, Yang H, Cao Y, Xu Q, Qiao D, Xu H, et al. Production of Microbial Lipids by Saitozyma podzolica Zwy2-3 Using Corn Straw Hydrolysate, the Analysis of Lipid Composition, and the Prediction of Biodiesel Properties. Energies. 2023; 16(18):6630. https://doi.org/10.3390/en16186630
Chicago/Turabian StyleFeng, Shunli, Yihan Guo, Yulu Ran, Qingzhuoma Yang, Xiyue Cao, Huahao Yang, Yu Cao, Qingrui Xu, Dairong Qiao, Hui Xu, and et al. 2023. "Production of Microbial Lipids by Saitozyma podzolica Zwy2-3 Using Corn Straw Hydrolysate, the Analysis of Lipid Composition, and the Prediction of Biodiesel Properties" Energies 16, no. 18: 6630. https://doi.org/10.3390/en16186630
APA StyleFeng, S., Guo, Y., Ran, Y., Yang, Q., Cao, X., Yang, H., Cao, Y., Xu, Q., Qiao, D., Xu, H., & Cao, Y. (2023). Production of Microbial Lipids by Saitozyma podzolica Zwy2-3 Using Corn Straw Hydrolysate, the Analysis of Lipid Composition, and the Prediction of Biodiesel Properties. Energies, 16(18), 6630. https://doi.org/10.3390/en16186630





