Development and Characteristics Analysis of Novel Hydrated Salt Composite Adsorbents for Thermochemical Energy Storage
Abstract
:1. Introduction
2. Materials Development and Experimental Methods
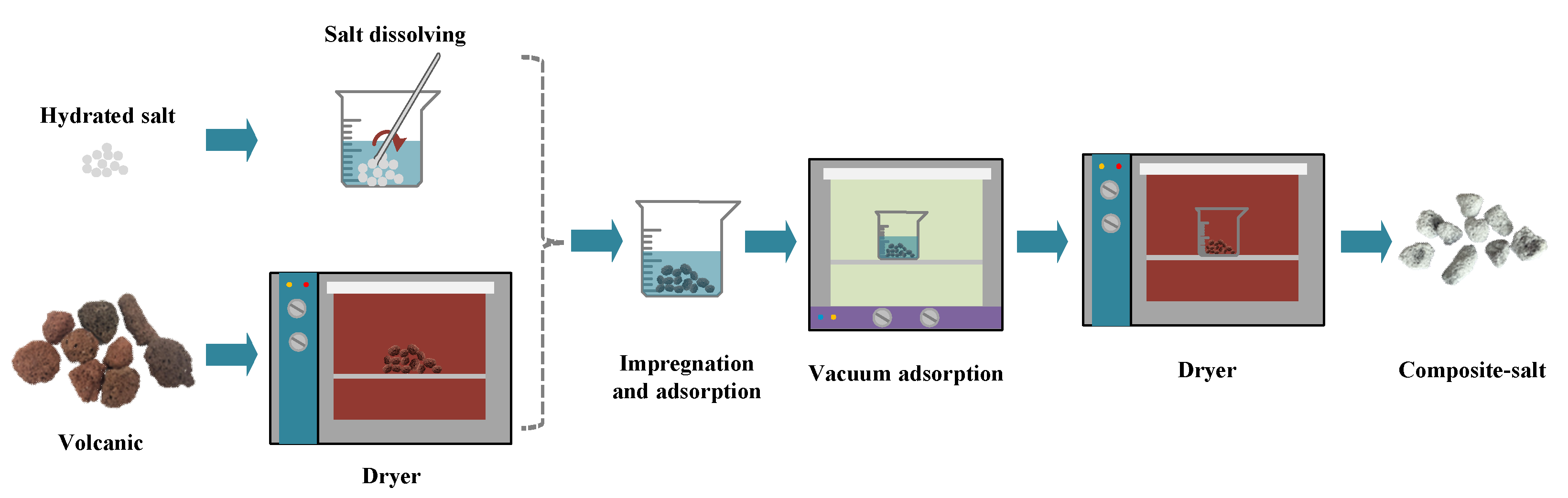
2.1. Development of Composites
2.2. Characterization of Composites
2.3. Thermal Energy Storage Density Measurement
3. Results and Discussion
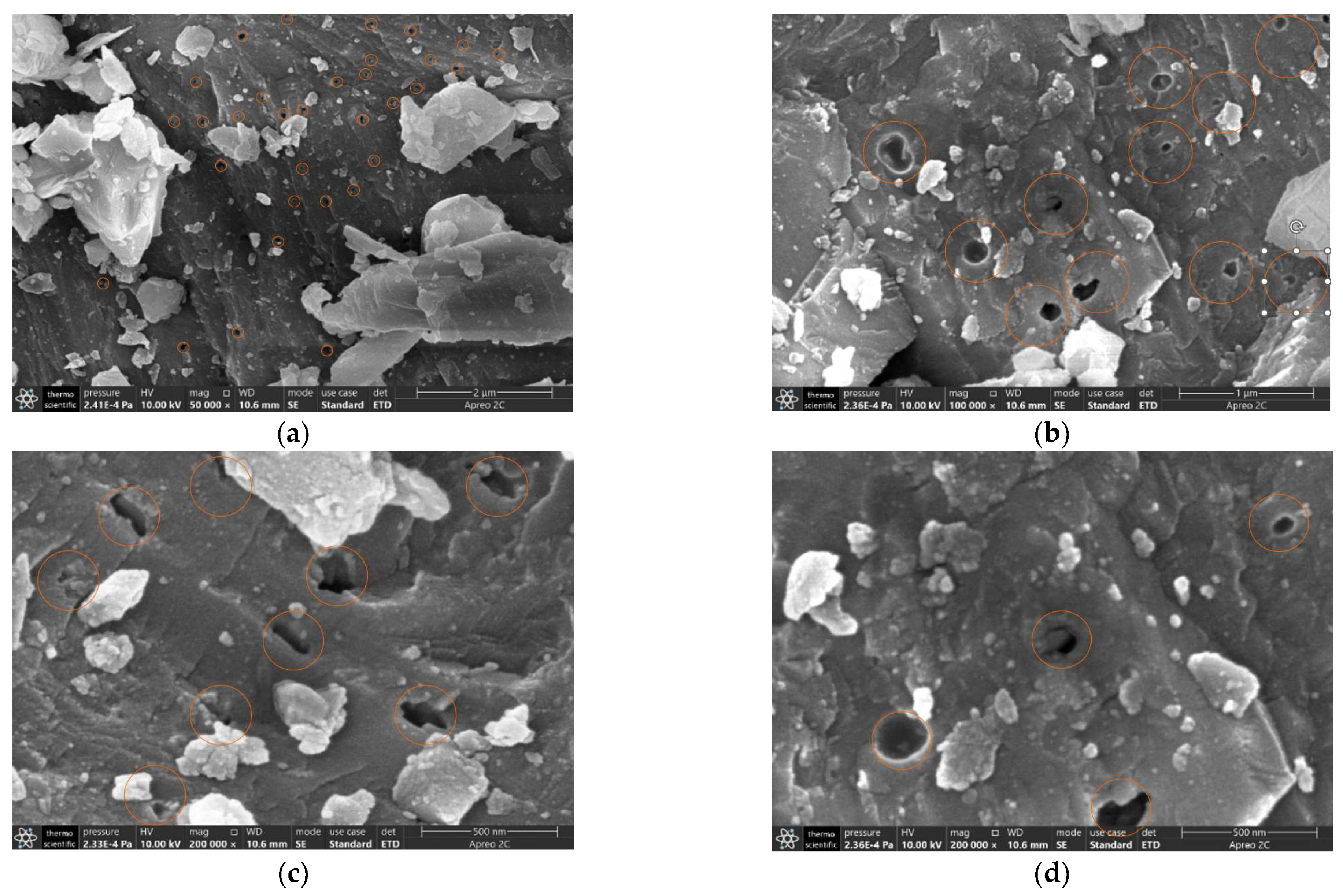
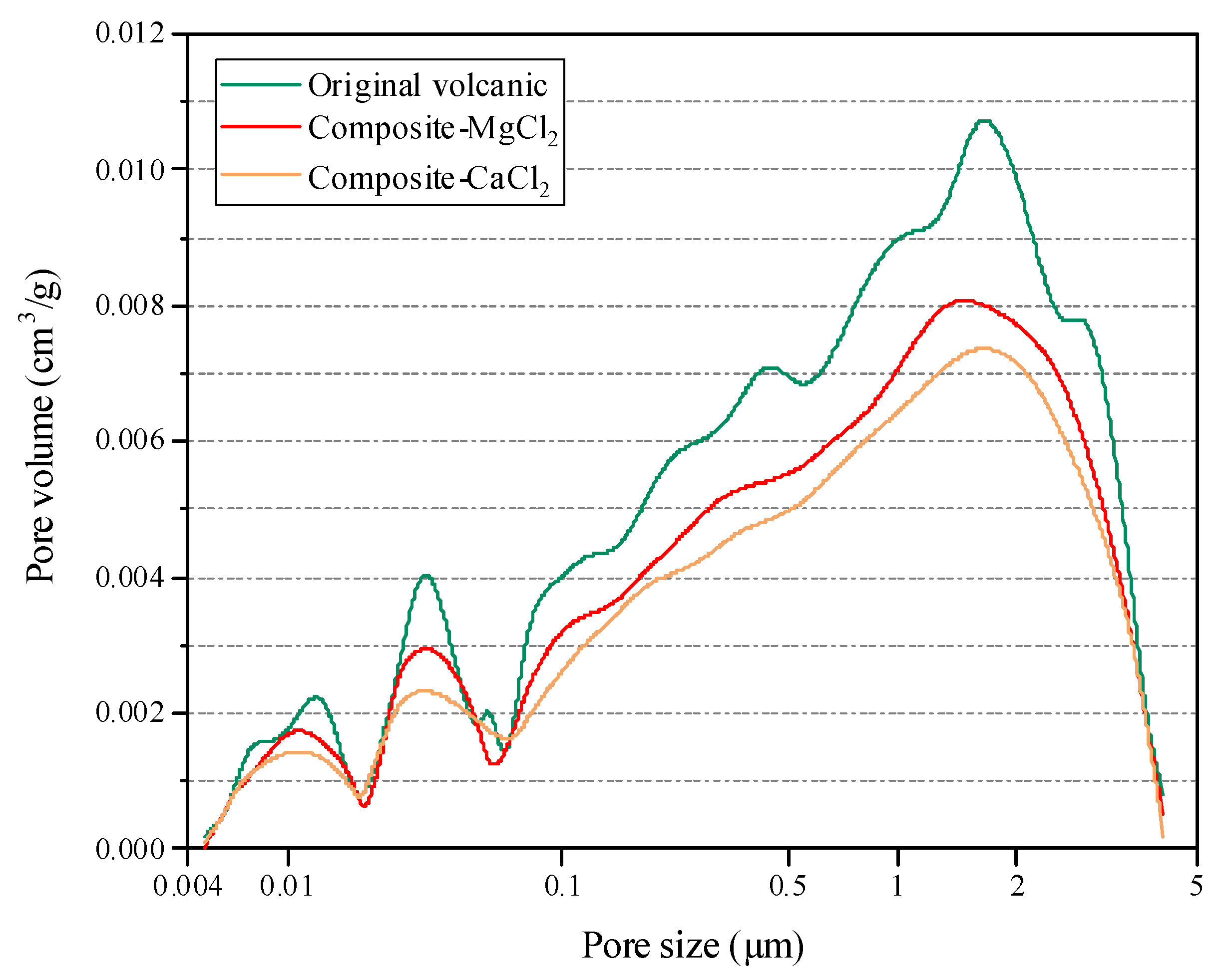
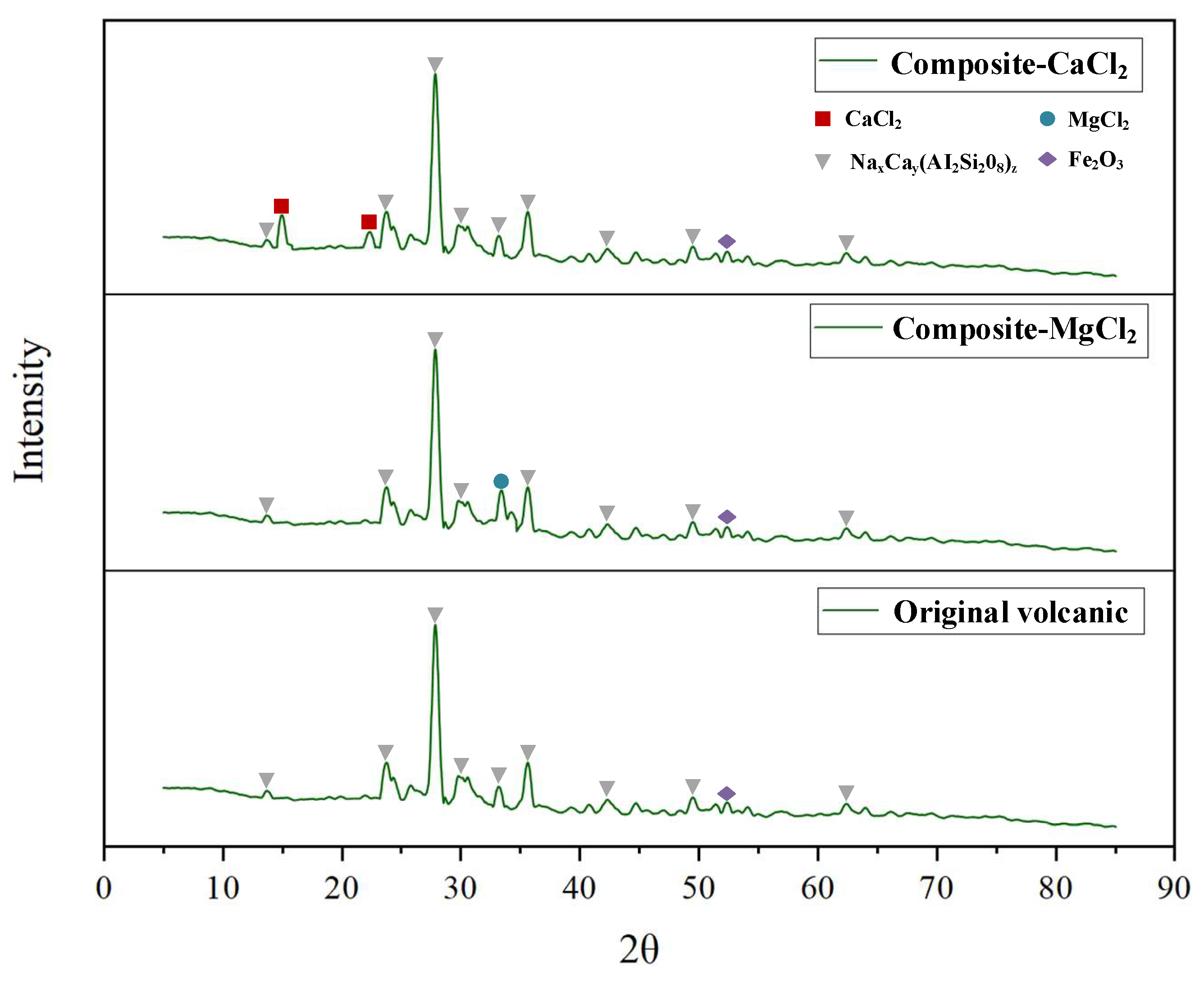
3.1. Pore Morphologies, Properties and Compatibility
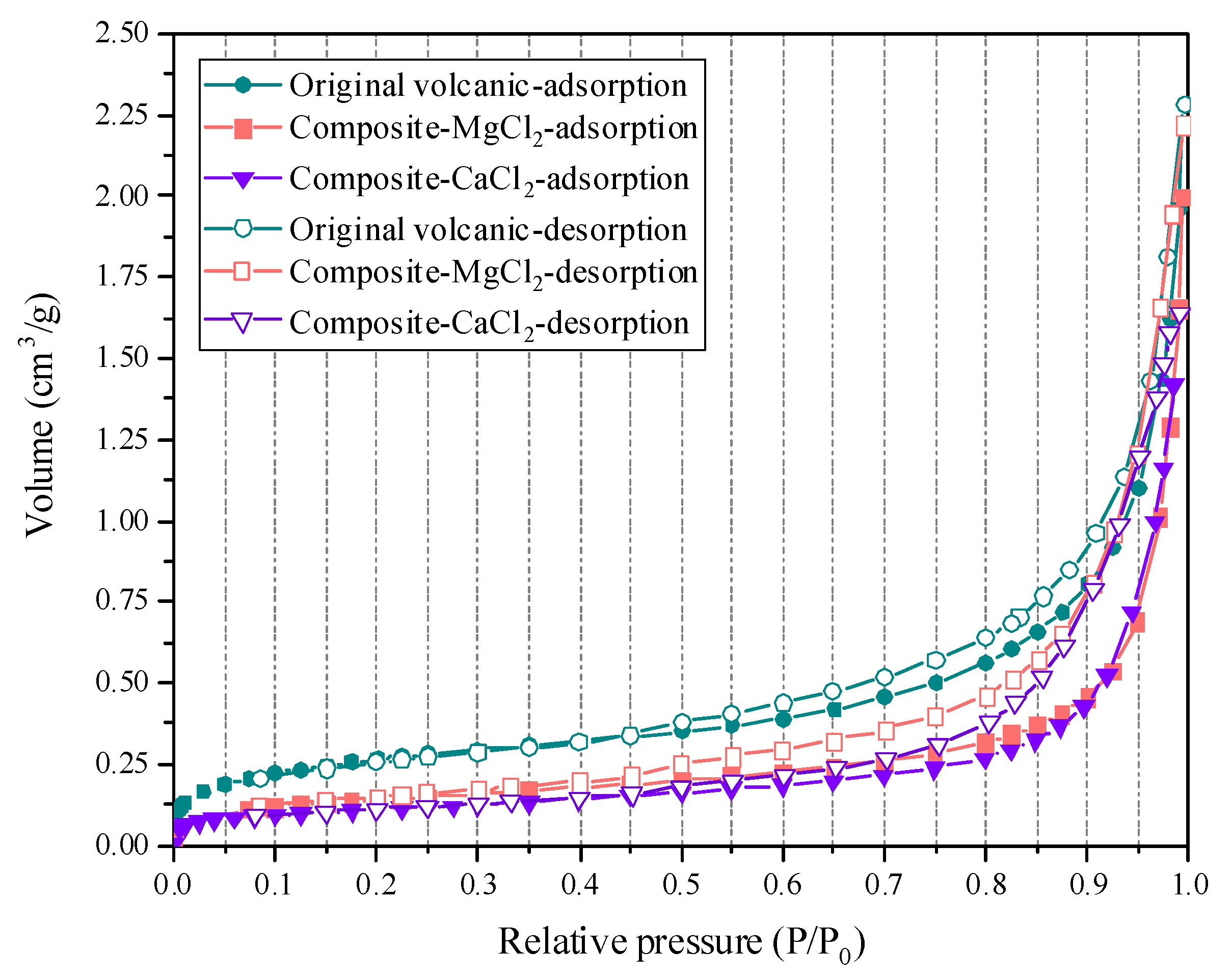
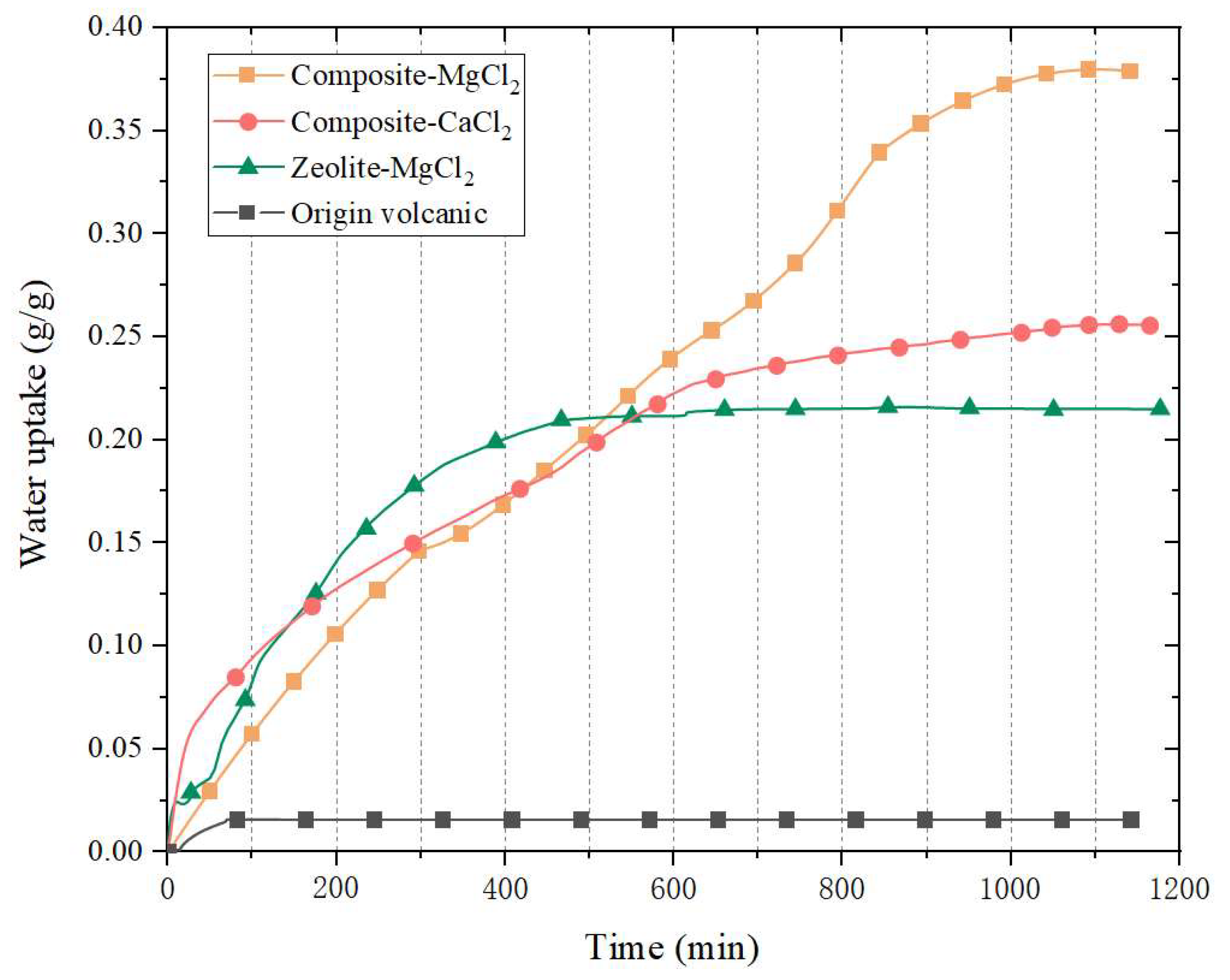
3.2. Adsorption Characteristics
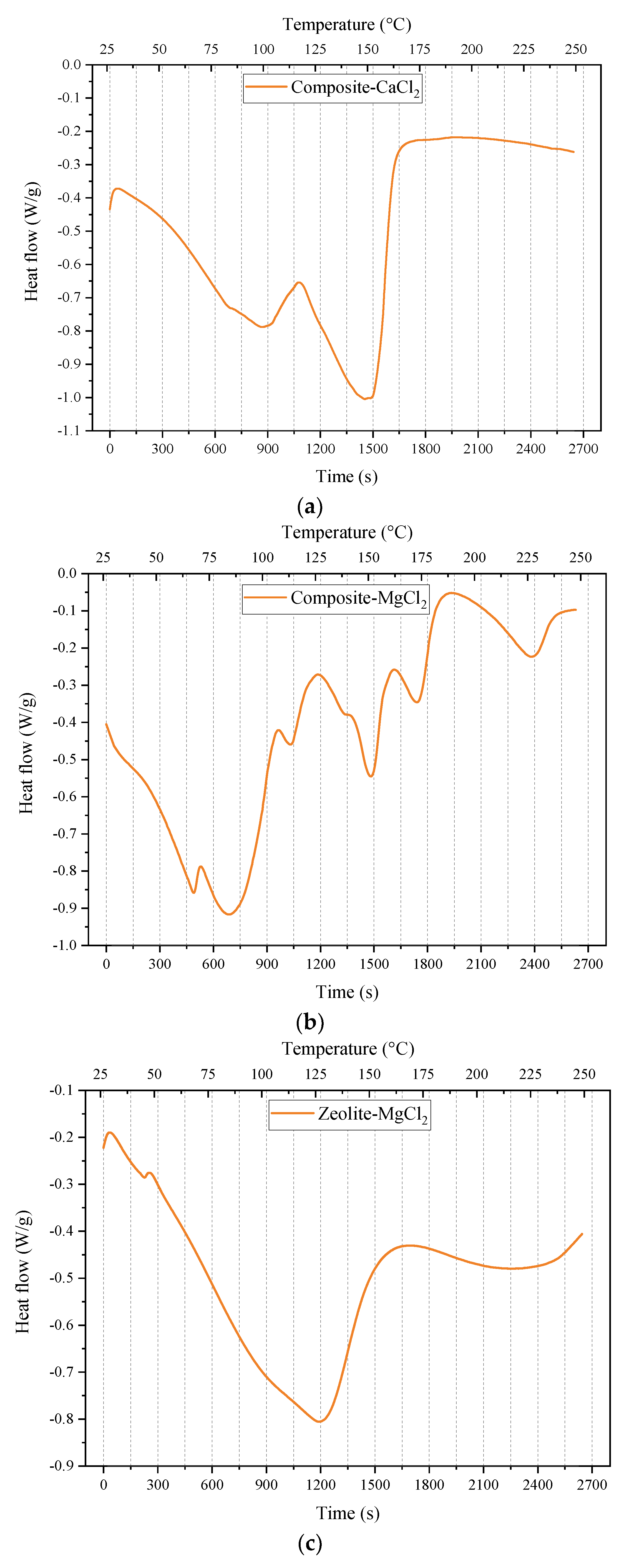
3.3. Thermal Properties Measurement
3.4. Theoretical Evaluation of Thermochemical Energy Storage Density
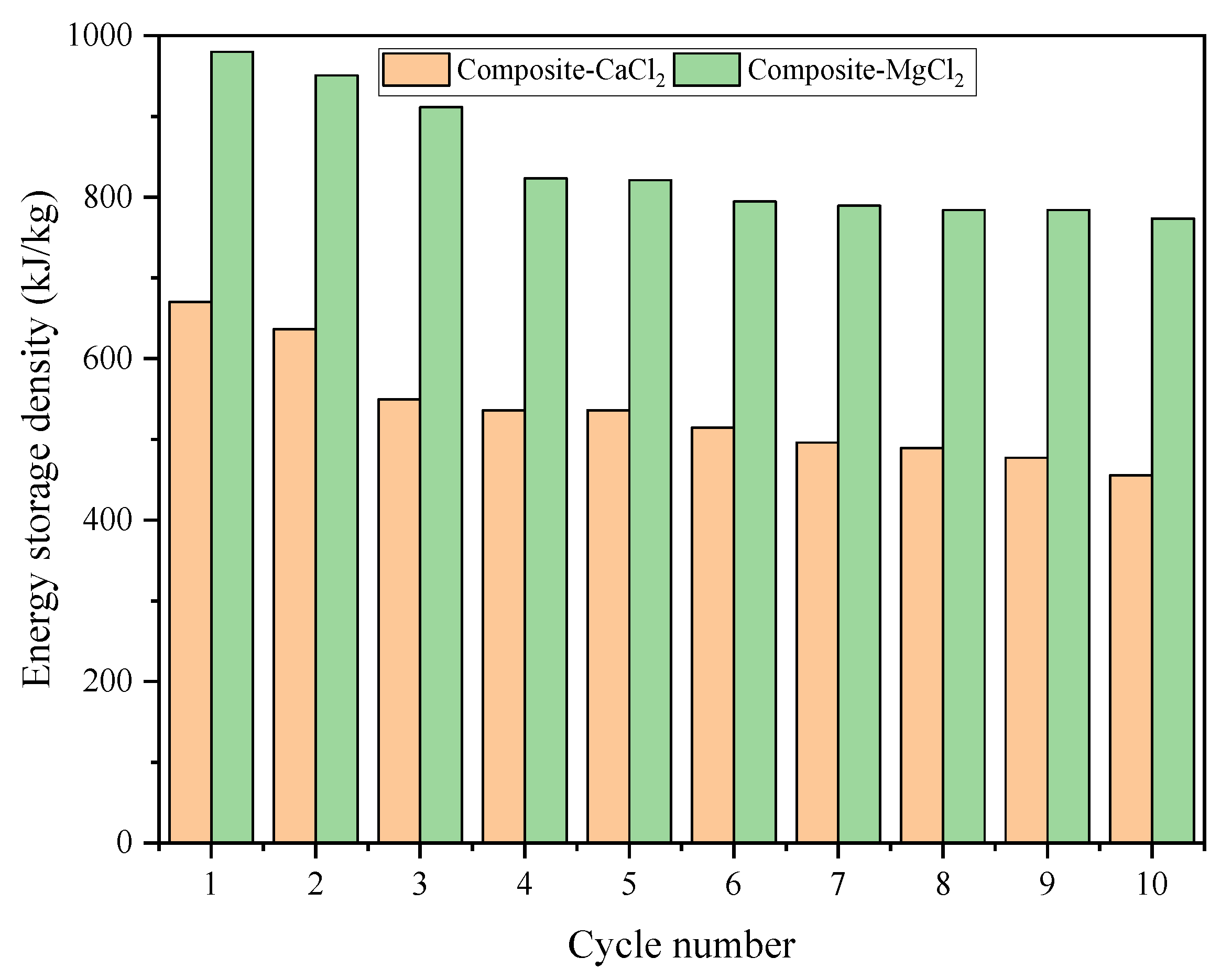
3.5. Cycle Stability
4. Further Discussion
5. Conclusions
- In the composite-CaCl2 and composite-MgCl2, the hydrated salts tend to fill the macropores in the volcanic.
- The water uptake of composite-CaCl2 and composite-MgCl2 e can reach 0.25 g/g and 0.37 g/g, respectively.
- According to TG-DSC measurement, the thermochemical energy storage densities of zeolite-MgCl2, composite-CaCl2 and composite-MgCl2 are 630 kJ/kg, 641 kJ/kg and 983 kJ/kg, respectively. This indicates that the prepared composite-CaCl2 has reached the level of zeolite-MgCl2.
- According to theoretical calculations, the thermochemical energy storage densities of the proposed composites are mainly determined by the hydrated salts. The theoretically calculated energy storage densities of the three materials are not much different from the measured results.
- Compared with other materials, the selected substrate and hydrated salts of the prepared composites have good economy and relatively high thermochemical energy storage performance. Even after many cycles, the energy storage capacity is still high.
- The thermochemical energy storage density of composite-MgCl2 is 1.56 times that of zeolite-MgCl2. The energy storage density of composite-CaCl2 is close to that of zeolite-MgCl2, but the energy storage density cost is only 0.0107 yuan/kJ. Even after many cycles, the energy storage density cost of composite-MgCl2 and composite-CaCl2 is still as low as 0.0218 yuan/kJ and 0.0157 yuan/kJ, respectively.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbols | |
| C | Cost, yuan/kg |
| E | Energy density, kJ/kg |
| h | Dehydration heat, kJ/mol |
| m | Water uptake, g/g |
| M | Relative molecular mass, g/mol |
| P | Pressure, MPa |
| T | Temperature, °C |
| Subscripts | |
| 0 | Saturation |
| d | Density |
| m | Material |
| s | Standard |
| Abbreviations | |
| BET | Brunauer-Emmett-Teller |
| DSC | Differential scanning calorimetry |
| EG | Expanded graphite |
| IEA | International Energy Agency |
| SEM | Scanning electron microscopy |
| TCES | Thermochemical energy storage |
| TES | Thermal energy storage |
| TG | Thermo-gravimetric |
References
- Clark, R.-J.; Mehrabadi, A.; Farid, M. State of the art on salt hydrate thermochemical energy storage systems for use in building applications. J. Energy Storage 2020, 27, 101145. [Google Scholar] [CrossRef]
- Whiting, G.T.; Grondin, D.; Stosic, D.; Bennici, S.; Auroux, A. Zeolite–MgCl2 composites as potential long-term heat storage materials: Influence of zeolite properties on heats of water sorption. Sol. Energy Mater. Sol. Cells 2014, 128, 289–295. [Google Scholar] [CrossRef]
- Mehrabadi, A.; Farid, M. New salt hydrate composite for low-grade thermal energy storage. Energy 2018, 164, 194–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Sorption thermal energy storage: Concept, process, applications and perspectives. Energy Storage Mater. 2020, 27, 352–369. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Recent advances in thermochemical energy storage via solid–gas reversible reactions at high temperature. Energies 2020, 13, 5859. [Google Scholar] [CrossRef]
- Yang, H.; Wang, C.; Tong, L.; Yin, S.; Wang, L.; Ding, Y. Salt Hydrate Adsorption Material-Based Thermochemical Energy Storage for Space Heating Application: A Review. Energies 2023, 16, 2875. [Google Scholar] [CrossRef]
- Li, W.; Klemeš, J.J.; Wang, Q.; Zeng, M. Development and characteristics analysis of salt-hydrate based composite sorbent for low-grade thermochemical energy storage. Renew. Energy 2020, 157, 920–940. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, H.; Liu, S.; Wang, Z.; Deng, S. An experimental study on the binary hydrated salt composite zeolite for improving thermochemical energy storage performance. Renew. Energy 2022, 194, 1163–1173. [Google Scholar] [CrossRef]
- Lefebvre, D.; Tezel, F.H. A review of energy storage technologies with a focus on adsorption thermal energy storage processes for heating applications. Renew. Sustain. Energy Rev. 2017, 67, 116–125. [Google Scholar] [CrossRef]
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Lim, K.; Che, J.; Lee, J. Experimental study on adsorption characteristics of a water and silica-gel based thermal energy storage (TES) system. Appl. Therm. Eng. 2017, 110, 80–88. [Google Scholar] [CrossRef]
- Strong, C.; Carrier, Y.; Tezel, F.H. Experimental optimization of operating conditions for an open bulk-scale silica gel/water vapour adsorption energy storage system. Appl. Energy 2022, 312, 118533. [Google Scholar] [CrossRef]
- Johannes, K.; Kuznik, F.; Hubert, J.-L.; Durier, F.; Obrecht, C. Design and characterisation of a high powered energy dense zeolite thermal energy storage system for buildings. Appl. Energy 2015, 159, 80–86. [Google Scholar] [CrossRef]
- Xu, S.; Lemington; Wang, R.; Wang, L.; Zhu, J. A zeolite 13X/magnesium sulfate–water sorption thermal energy storage device for domestic heating. Energy Convers. Manag. 2018, 171, 98–109. [Google Scholar] [CrossRef]
- Shkatulov, A.; Houben, J.; Fischer, H.; Huinink, H. Stabilization of K2CO3 in vermiculite for thermochemical energy storage. Renew. Energy 2020, 150, 990–1000. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R.; Li, T.; Zhao, Y. Thermochemical characterizations of novel vermiculite-LiCl composite sorbents for low-temperature heat storage. Energies 2016, 9, 854. [Google Scholar] [CrossRef]
- Miao, Q.; Zhang, Y.; Jia, X.; Li, Z.; Tan, L.; Ding, Y. MgSO4-expanded graphite composites for mass and heat transfer enhancement of thermochemical energy storage. Sol. Energy 2021, 220, 432–439. [Google Scholar] [CrossRef]
- Lin, J.; Zhao, Q.; Huang, H.; Mao, H.; Liu, Y.; Xiao, Y. Applications of low-temperature thermochemical energy storage systems for salt hydrates based on material classification: A review. Sol. Energy 2021, 214, 149–178. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, J.; Huang, H.; Wu, Q.; Shen, Y.; Xiao, Y. Optimization of thermochemical energy storage systems based on hydrated salts: A review. Energy Build. 2021, 244, 111035. [Google Scholar] [CrossRef]
- Kumar, E.A.; Jivrakh, K.B.; Babu, K.S. Study of ammonia adsorption/desorption characteristics of CaCl2–Expanded natural graphite composite for thermal energy storage. Therm. Sci. Eng. Prog. 2020, 20, 100752. [Google Scholar] [CrossRef]
- Shere, L.; Trivedi, S.; Roberts, S.; Sciacovelli, A.; Ding, Y. Synthesis and characterization of thermochemical storage material combining porous zeolite and inorganic salts. Heat Transf. Eng. 2018, 40, 1176–1181. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, Y.; Liu, W.; Ma, Z.; Wang, R.; Zhang, X.; Roskilly, A. Thermophysical characterization of magnesium chloride and its application in open sorption thermal energy storage system. Sol. Energy Mater. Sol. Cells 2022, 236, 111528. [Google Scholar] [CrossRef]
- Li, W.; Klemeš, J.J.; Wang, Q.; Zeng, M. Characterisation and sorption behaviour of LiOH-LiCl@ EG composite sorbents for thermochemical energy storage with controllable thermal upgradeability. Chem. Eng. J. 2021, 421, 129586. [Google Scholar] [CrossRef]
- Grekova, A.D.; Gordeeva, L.G.; Aristov, Y.I. Composite “LiCl/vermiculite” as advanced water sorbent for thermal energy storage. Appl. Therm. Eng. 2017, 124, 1401–1408. [Google Scholar] [CrossRef]
- Clark, R.-J.; Farid, M. Experimental investigation into the performance of novel SrCl2-based composite material for thermochemical energy storage. J. Energy Storage 2021, 36, 102390. [Google Scholar] [CrossRef]
- Clark, R.-J.; Gholamibozanjani, G.; Woods, J.; Kaur, S.; Odukomaiya, A.; Al-Hallaj, S.; Farid, M. Experimental screening of salt hydrates for thermochemical energy storage for building heating application. J. Energy Storage 2022, 51, 104415. [Google Scholar] [CrossRef]
- Mastronardo, E.; La Mazza, E.; Palamara, D.; Piperopoulos, E.; Iannazzo, D.; Proverbio, E.; Milone, C. Organic salt hydrate as a novel paradigm for thermal energy storage. Energies 2022, 15, 4339. [Google Scholar] [CrossRef]
- Chao, J.; Xu, J.; Bai, Z.; Wang, P.; Wang, R.; Li, T. Integrated heat and cold storage enabled by high-energy-density sorption thermal battery based on zeolite/MgCl2 composite sorbent. J. Energy Storage 2023, 64, 107155. [Google Scholar] [CrossRef]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. CaCl2-containing composites as thermochemical heat storage materials. Sol. Energy Mater. Sol. Cells 2017, 172, 177–185. [Google Scholar] [CrossRef]
- Xu, S.; Wang, R.; Wang, L.; Zhu, J. Performance characterizations and thermodynamic analysis of magnesium sulfate-impregnated zeolite 13X and activated alumina composite sorbents for thermal energy storage. Energy 2019, 167, 889–901. [Google Scholar] [CrossRef]
- Gaeini, M.; Rouws, A.; Salari, J.; Zondag, H.; Rindt, C. Characterization of microencapsulated and impregnated porous host materials based on calcium chloride for thermochemical energy storage. Appl. Energy 2018, 212, 1165–1177. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, W.; Han, R.; Li, J.; Gao, J.; Zhao, G.; Qin, Y. Scalable Production of EP/CaCl2@ C Multistage Core–Shell Sorbent for Solar-Driven Sorption Heat Storage Application. Energy Fuels 2021, 35, 6845–6857. [Google Scholar] [CrossRef]
- Posern, K.; Kaps, C. Calorimetric studies of thermochemical heat storage materials based on mixtures of MgSO4 and MgCl2. Thermochim. Acta 2010, 502, 73–76. [Google Scholar] [CrossRef]
- Ousaleh, H.A.; Sair, S.; Mansouri, S.; Abboud, Y.; Faik, A.; El Bouari, A. New hybrid graphene/inorganic salt composites for thermochemical energy storage: Synthesis, cyclability investigation and heat exchanger metal corrosion protection performance. Sol. Energy Mater. Sol. Cells 2020, 215, 110601. [Google Scholar] [CrossRef]
- Brancato, V.; Gordeeva, L.G.; Sapienza, A.; Palomba, V.; Vasta, S.; Grekova, A.D.; Frazzica, A.; Aristov, Y.I. Experimental characterization of the LiCl/vermiculite composite for sorption heat storage applications. Int. J. Refrig. 2019, 105, 92–100. [Google Scholar] [CrossRef]
- Liu, H.; Nagano, K.; Togawa, J. A composite material made of mesoporous siliceous shale impregnated with lithium chloride for an open sorption thermal energy storage system. Sol. Energy 2015, 111, 186–200. [Google Scholar] [CrossRef]
- Cammarata, A.; Verda, V.; Sciacovelli, A.; Ding, Y. Hybrid strontium bromide-natural graphite composites for low to medium temperature thermochemical energy storage: Formulation, fabrication and performance investigation. Energy Convers. Manag. 2018, 166, 233–240. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Magnesium sulphate-silicone foam composites for thermochemical energy storage: Assessment of dehydration behaviour and mechanical stability. Sol. Energy Mater. Sol. Cells 2019, 200, 109992. [Google Scholar] [CrossRef]
- d’Ans, P.; Courbon, E.; Permyakova, A.; Nouar, F.; Simonnet-Jegat, C.; Bourdreux, F.; Malet, L.; Serre, C.; Frere, M.; Steunou, N. A new strontium bromide MOF composite with improved performance for solar energy storage application. J. Energy Storage 2019, 25, 100881. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Chao, J.; Yan, T.; Wang, R. High energy-density multi-form thermochemical energy storage based on multi-step sorption processes. Energy 2019, 185, 1131–1142. [Google Scholar] [CrossRef]
- Rammelberg, H.U.; Osterland, T.; Priehs, B.; Opel, O.; Ruck, W.K. Thermochemical heat storage materials–Performance of mixed salt hydrates. Sol. Energy 2016, 136, 571–589. [Google Scholar] [CrossRef]
- Sögütoglu, L.; Donkers, P.; Fischer, H.; Huinink, H.; Adan, O. In-depth investigation of thermochemical performance in a heat battery: Cyclic analysis of K2CO3, MgCl2 and Na2S. Appl. Energy 2018, 215, 159–173. [Google Scholar] [CrossRef]
- Ferchaud, C.J.; Zondag, H.A.; Veldhuis, J.B.J.; De Boer, R. Study of the reversible water vapour sorption process of MgSO4. 7H2O and MgCl2. 6H2O under the conditions of seasonal solar heat storage. J. Phys. Conf. Ser. 2012, 395, 012069. [Google Scholar] [CrossRef]
- Molenda, M.; Stengler, J.; Linder, M.; Wörner, A. Reversible hydration behavior of CaCl2 at high H2O partial pressures for thermochemical energy storage. Thermochim. Acta 2013, 560, 76–81. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D. Effect of graphene oxide aerogel on dehydration temperature of graphene oxide aerogel stabilized MgCl2·6H2O composites. Sol. Energy 2019, 184, 202–208. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, W.; Han, R.; Gao, J.; Zhao, G.; Qin, Y.; Wang, C. Influence of minerals with different porous structures on thermochemical heat storage performance of CaCl2-based composite sorbents. Sol. Energy Mater. Sol. Cells 2022, 243, 111769. [Google Scholar] [CrossRef]
- Gibson, N.; Kuchenbecker, P.; Rasmussen, K.; Hodoroaba, V.-D.; Rauscher, H. Volume-specific surface area by gas adsorption analysis with the BET method. In Characterization of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 265–294. [Google Scholar]
- Singh, S.B.; De, M. Alumina based doped templated carbons: A comparative study with zeolite and silica gel templates. Microporous Mesoporous Mater. 2018, 257, 241–252. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, X.; Shi, L.; Yan, T.; Zhang, J. Enhanced capacitive deionization of graphene/mesoporous carbon composites. Nanoscale 2012, 4, 5440–5446. [Google Scholar] [CrossRef] [PubMed]
- Kishor, R.; Singh, S.B.; Ghoshal, A.K. Role of metal type on mesoporous KIT-6 for hydrogen storage. Int. J. Hydrogen Energy 2018, 43, 10376–10385. [Google Scholar] [CrossRef]
- Kazemifard, S.; Nayebzadeh, H.; Saghatoleslami, N.; Safakish, E. Application of magnetic alumina-ferric oxide nanocatalyst supported by KOH for in-situ transesterification of microalgae cultivated in wastewater medium. Biomass Bioenergy 2019, 129, 105338. [Google Scholar] [CrossRef]
- Iyimen-Schwarz, Z.; Lechner, M.D. Energiespeicherung durch chemische Reaktionen. I. DSC-Messungen zur quantitativen Verfolgung der Enthalpieänderungen von Speicherstoffen für die Hin-und Rückreaktion. Thermochim. Acta 1983, 68, 349–361. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Rammelberg, H.U.; Lele, A.F.; Korhammer, K.; Watts, B.A.; Schmidt, T.; Ruck, W.K. A review on the use of calcium chloride in applied thermal engineering. Appl. Therm. Eng. 2015, 75, 513–531. [Google Scholar] [CrossRef]
- van Alebeek, R.; Scapino, L.; Beving, M.; Gaeini, M.; Rindt, C.; Zondag, H. Investigation of a household-scale open sorption energy storage system based on the zeolite 13X/water reacting pair. Appl. Therm. Eng. 2018, 139, 325–333. [Google Scholar] [CrossRef]
- Alraddadi, S.; Assaedi, H. Physical properties of mesoporous scoria and pumice volcanic rocks. J. Phys. Commun. 2021, 5, 115018. [Google Scholar] [CrossRef]
- Mourhly, A.; Khachani, M.; El Hamidi, A.; Kacimi, M.; Halim, M.; Arsalane, S. The synthesis and characterization of low-cost mesoporous silica SiO2 from local pumice rock. Nanomater. Nanotechnol. 2015, 5, 35. [Google Scholar] [CrossRef]



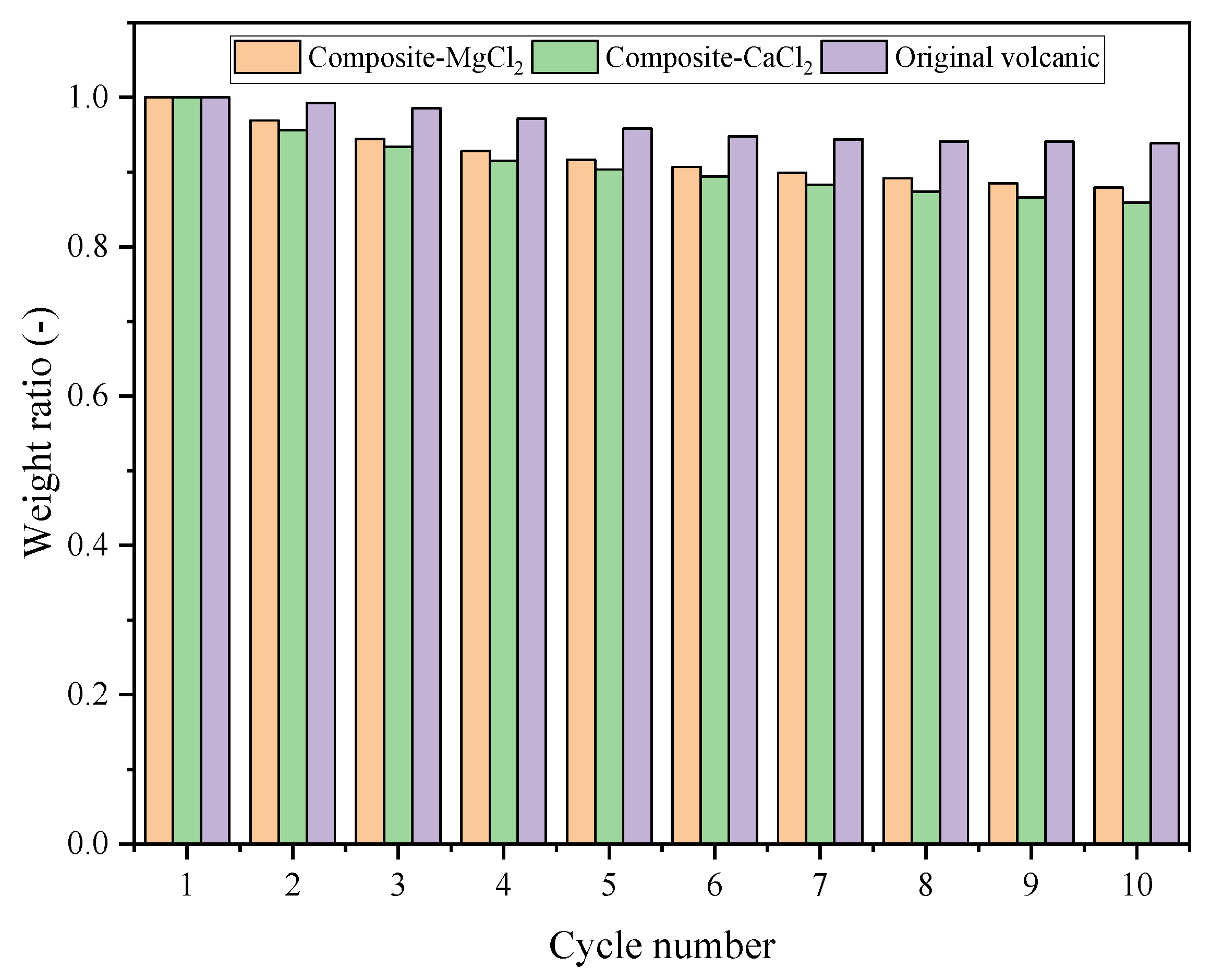
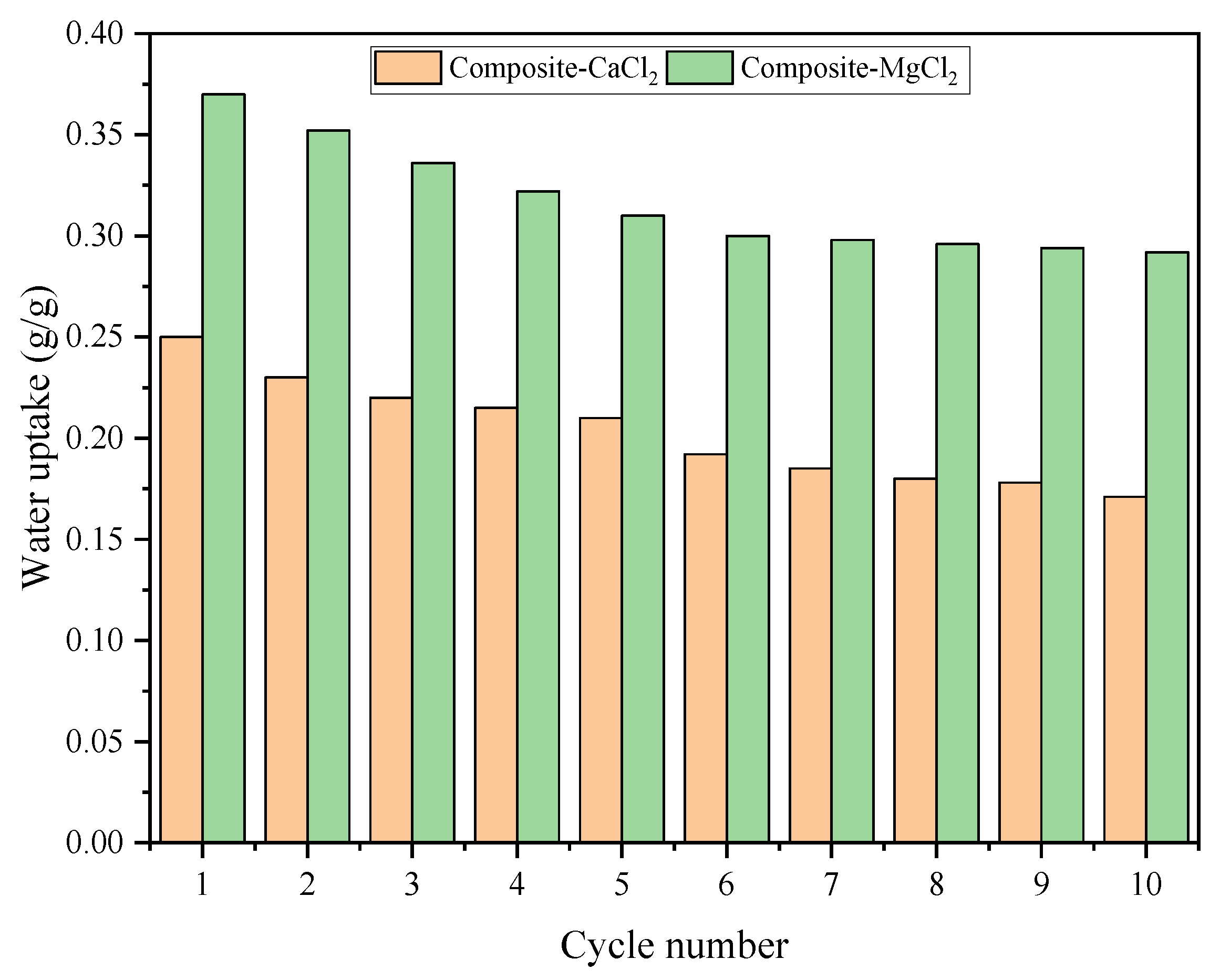

| References | Substrate | Hydrated Salt | TS (°C) | Ed (kJ/kg) | CM (yuan/kg) | CM/Ed (yuan /kJ) |
|---|---|---|---|---|---|---|
| [2] | zeolite (Faujasite Na-X) | MgCl2 | 160 | 1173 | 33.5 | 0.0286 |
| [28] | zeolite 13x | MgCl2 | 175 | 968 | 35 | 0.0362 |
| [29] | silica gel | CaCl2 | 135 | 746 | 29 | 0.0389 |
| [29] | zeolite 13x | MgCl2/CaCl2 | 137 | 722 | 32 | 0.0443 |
| [30] | zeolite 13x | MgSO4 | 148 | 636 | 30 | 0.0472 |
| [31] | vermiculite | CaCl2 | 150 | 1600 | 22 | 0.0138 |
| [32] | expanded perlite | CaCl2 | 140 | 956 | 24 | 0.0251 |
| [33] | attapulgite granulate | MgCl2/MgSO4 | 130 | 1100 | 63 | 0.0573 |
| [34] | graphite oxide | MgCl2 | 153 | 1280 | 99 | 0.0781 |
| [35] | vermiculite | LiCl | 80 | 2150 | 215 | 0.1000 |
| [36] | siliceous shale | LiCl | 60 | 324 | 52 | 0.1600 |
| [7] | expanded graphite | LiOH | 92 | 1400 | 327 | 0.2336 |
| [37] | expanded graphite | SrBr2 | 150 | 600 | 900 | 1.5000 |
| [38] | porous copper foam | MgSO4 | 150 | 960 | 3000 | 3.1250 |
| [39] | MIL-101 (Cr) | SrBr2 | 80 | 1350 | 5000 | 3.7037 |
| Item | Value | |
|---|---|---|
| Zeolite-MgCl2 | Initial zeolite weight (g) | 30.15 |
| MgCl2 solution concentration (%) | 20.00 | |
| After drying (g) | 31.72 | |
| Salt content (wt%) | 4.95 | |
| Composite-CaCl2 | Initial volcanic weight (g) | 42.58 |
| CaCl2 solution concentration (%) | 54.00 | |
| After drying (g) | 60.08 | |
| Salt content (wt%) | 29.13 | |
| Composite-MgCl2 | Initial volcanic weight (g) | 39.21 |
| MgCl2 solution concentration (%) | 36.50 | |
| After drying (g) | 58.47 | |
| Salt content (wt%) | 32.94 | |
| Apparatuses | Type | Accuracy |
|---|---|---|
| SEM | JEOL JSM-7800F Prime | - |
| XRD | BrukerAXS X’Pert MPD | ±0.01° |
| BET | ASAP2460 | - |
| Pore size analyzer | AutoPore IV 9510 | ±2.0% |
| Constant temperature and humidity chamber | Binder KMF115 | ±0.1 °C or ±2.0% |
| Simultaneous thermal analyzer | Netzsch STA 449F5 | 0.1 μg or ±1.0% |
| Item | Original Volcanic | Composite-MgCl2 | Composite-CaCl2 | |
|---|---|---|---|---|
| Mesopore (<100 nm) | Surface area (m2/g) | 0.985 | 0.857 | 0.800 |
| Pore volume (cm3/g) | 0.00353 | 0.00307 | 0.00281 | |
| Average pore size (µm) | 0.0144 | 0.0125 | 0.0119 | |
| Macropore (>100 nm) | Surface area (m2/g) | 7.348 | 4.843 | 3.184 |
| Pore volume (cm3/g) | 0.525 | 0.323 | 0.283 | |
| Average pore size (µm) | 0.286 | 0.223 | 0.198 | |
| Item | Price (yuan/kg) | CM/Ed (yuan/kJ) |
|---|---|---|
| Zeolite | 35 | / |
| Volcanic | 0.3 | / |
| MgCl2 | 25 | / |
| CaCl2 | 10 | / |
| Zeolite-MgCl2 | 43.75 | 0.0516 |
| Composite-CaCl2 | 7.17 | 0.0107 |
| Composite-MgCl2 | 16.86 | 0.0174 |
| Composite-CaCl2 (after 10 cycles) | 7.17 | 0.0157 |
| Composite-MgCl2 (after 10 cycles) | 16.86 | 0.0218 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, Z.; Liu, S.; Wang, Z.; Shen, Y. Development and Characteristics Analysis of Novel Hydrated Salt Composite Adsorbents for Thermochemical Energy Storage. Energies 2023, 16, 6572. https://doi.org/10.3390/en16186572
Wang Y, Zhang Z, Liu S, Wang Z, Shen Y. Development and Characteristics Analysis of Novel Hydrated Salt Composite Adsorbents for Thermochemical Energy Storage. Energies. 2023; 16(18):6572. https://doi.org/10.3390/en16186572
Chicago/Turabian StyleWang, Yihan, Zicheng Zhang, Shuli Liu, Zhihao Wang, and Yongliang Shen. 2023. "Development and Characteristics Analysis of Novel Hydrated Salt Composite Adsorbents for Thermochemical Energy Storage" Energies 16, no. 18: 6572. https://doi.org/10.3390/en16186572
APA StyleWang, Y., Zhang, Z., Liu, S., Wang, Z., & Shen, Y. (2023). Development and Characteristics Analysis of Novel Hydrated Salt Composite Adsorbents for Thermochemical Energy Storage. Energies, 16(18), 6572. https://doi.org/10.3390/en16186572







