Abstract
The use of zero-carbon and carbon-neutral fuels reduces emissions of conventional pollutants, but their emissions can be toxic and have various adverse effects on human health. This article reviews the possible combustion products of zero-carbon and carbon-neutral fuels, as well as their cytotoxic effects and potential health risks. At the same time, the review outlines biological models and toxicity detection methods commonly used in pollutant toxicity studies. Metals, nitrogen oxides (NOX), and ammonia (NH3) emitted from the combustion of metal fuels, hydrogen fuels, and ammonia fuels in zero-carbon fuels are harmful to human health. Exhaust emissions from carbon-neutral fuels, particularly biodiesel, and their blends with gasoline/diesel are cytotoxic, leading to severe cellular damage, such as oxidative damage, inflammatory responses, DNA damage, cell death, or apoptosis. Moreover, the normal function of the human body’s respiratory, cardiovascular, immune, digestive, urinary, and nervous systems may also be impacted by these fuel emissions according to cytotoxic research. Cytotoxicity of fuel combustion products is usually related to the fuel type, time, dose, and cell line used in the experiment. This review provides some ideas for the exhaust emission management of zero-carbon and carbon-neutral fuels and human health assessment. It also presents a theoretical and experimental basis for further research, including in vivo experiments.
1. Introduction
Since the beginning of the 21st century, energy demand and consumption around the world have increased with the rapid growth of the global population and the development of industry and transportation [1]. According to the Energy Institute (EI), fossil fuels (oil, coal, and gas) remained a major source of primary energy in 2022, accounting for about 82% of the world’s primary energy consumption [2]. Due to the non-renewable nature of fossil fuels and the growing depletion of fossil fuel reserves, there is a global energy crisis [3]. According to a 2021 report by the International Energy Agency (IEA), fossil fuel combustion produces CO, CO2, NOx, SOx, HC, various trace metal elements, volatile organic compounds (VOCs), and particulate matter (PM) [4]. High greenhouse gas emissions have caused global warming, melting glaciers, and rising sea levels [5]. Additionally, these harmful pollutants also contribute to serious air pollution, which unavoidably puts human health at risk by increasing the incidence of diseases like asthma, pneumonia, lung cancer, stroke, and heart disease [6]. World Health Organization (WHO) data show that almost all of the global population (99%) breathe air that exceeds WHO guideline limits [7]. Air pollution has become a major problem that needs to be urgently addressed.
From the perspective of sustainable development and environmental protection, many countries have introduced strict policies and emission standards to reduce the consumption of fossil fuels and the emission of motor vehicle exhaust pollutants [8,9,10,11,12]. Researchers are actively looking for ways to reduce pollutant emissions from engines, including engine modifications, vehicle exhaust after-treatment, and improvements in combustion technologies [13]. In addition, countries are developing clean, renewable alternative energy sources, such as solar, wind, hydro, ocean, geothermal, and biomass, to reduce fossil fuel consumption [14]. However, they are confronted with challenges such as intermittent production or geographical dependence [15,16]. Therefore, they need to be processed and converted into secondary energy use, which means that their energy is stored in a secondary energy carrier.
Solar fuel, electric fuel, and biomass fuel are secondary energy carriers that have attracted much attention in recent years. Solar fuels are produced directly (photocatalytic) or indirectly (photoelectrochemical, solar thermochemical) by solar energy [17,18,19,20]. Electric fuels are gas/liquid fuels or synthetic fuels produced with renewable electricity as energy and carbon dioxide as the main carbon source [21,22]. Biomass fuel is a renewable fuel produced by photosynthetic organisms (microalgae, macroalgae, bacteria, plants, etc.) or fungi through biological processes [23,24,25,26]. Solar fuel uses renewable solar energy, and the electricity used for electric fuel production also comes from renewable energy [27]. Both, in turn, can re-reduce the CO2 produced by combustion back into fuel [28]. In this sense, solar fuel and electric fuel belong to the category of zero-carbon fuel and carbon-neutral fuel. The CO2 released into the atmosphere when biomass fuel is burned can be reabsorbed by photosynthetic organisms, which means that the biomass fuel consumes the CO2 produced by combustion during its production [29]. In this way, biomass fuels do not have any net impact on the carbon concentration of the biosphere, achieving carbon neutrality in the whole life-cycle process. Therefore, biomass fuel can also be called a carbon-neutral fuel.
Recently, the goal of “carbon neutrality” has been proposed, which is the pursuit of “net-zero emissions” of carbon dioxide in energy and transportation systems. To achieve this, it is crucial to increase the use of zero-carbon fuels and carbon-neutral fuels [30]. Zero-carbon fuels are fuels that do not produce carbon emissions when burned. Carbon-neutral fuel refers to the fuel produced by using renewable energy to re-reduce the emitted CO2 through electrocatalysis, photocatalysis, thermal catalysis, and other technologies [31]. Researchers are trying to explore and apply binary or multi-component blends of zero-carbon fuels/carbon-neutral fuels with gasoline/diesel [32,33,34,35]. Conventional pollutants emitted by engines are significantly reduced by using these alternative fuels [32,33,34,35].
When evaluating their toxicity to humans, zero-carbon fuels, carbon-neutral fuels, and their blends’ combustion chemistry need to be studied in depth. It is necessary to take into account the content of various pollutants created by combustion, new changes in the physical and chemical characteristics of pollutants, and the production of potentially harmful pollutants. Therefore, this paper first briefly reviews the categories of gas-phase and particulate-phase pollutants emitted by zero-carbon and carbon-neutral fuel combustion.
Currently, appropriate chemical analysis and bioassays have been performed to understand the toxicity of fuel exhaust [36,37,38]. However, in vivo toxicity studies in humans and animals with zero-carbon/carbon-neutral fuels are limited due to ethical and safety concerns. The exhaust toxicity of these new fuels is currently concentrated in in vitro studies, especially in vitro cytotoxicity studies. The damage and death caused by pollutants can eventually manifest as changes at the cellular level [39]. Pathological changes at the cellular level may alter or inhibit the functioning of various systems in the body, which may eventually lead to disease [40]. Therefore, this article focuses on the in vitro cytotoxic effects of carbon-neutral fuel exhaust pollutants and their effects on human health. These toxic effects involve cytotoxicity, oxidative stress or oxidative damage, inflammatory response, and genotoxicity (carcinogenicity and mutagenicity), ultimately affecting the normal functioning of the respiratory, circulatory, immune, and nervous systems. These cytotoxicity studies can predict acute and chronic toxicity in vivo, preliminarily assess the harm of fuel combustion products to human health, provide prerequisites for animal testing, and offer a theoretical basis for subsequent human-exposure experiments. Finally, toxicity detection methods and major biological exposure models are briefly introduced. In summary, understanding the strength of the toxic effects of zero-carbon and carbon-neutral fuels can help us better assess the impact of combustion products on human health, improve fuel composition, design effective engine and after-treatment systems, develop new emissions regulations, and implement effective prevention strategies to protect public health.
2. Zero-Carbon and Carbon-Neutral Fuel Combustion Products
2.1. Zero-Carbon Fuel Combustion Products
2.1.1. Metal Fuels
Metal fuels (e.g., aluminum, magnesium, iron, beryllium, and lithium) have been proposed as recyclable carriers for a large number of green renewable energy sources [27,41]. Ideally, the complete combustion products of metals are metal oxides without producing other waste products, including CO2 [42]. As shown in Figure 1, a zero-carbon electrolysis process powered by primary renewable energy re-reduces metal oxides recovered or captured from combustion or reaction products to an unlimited amount of metal fuels [27]. In addition, metal combustion can lead to NOx emissions [43,44].
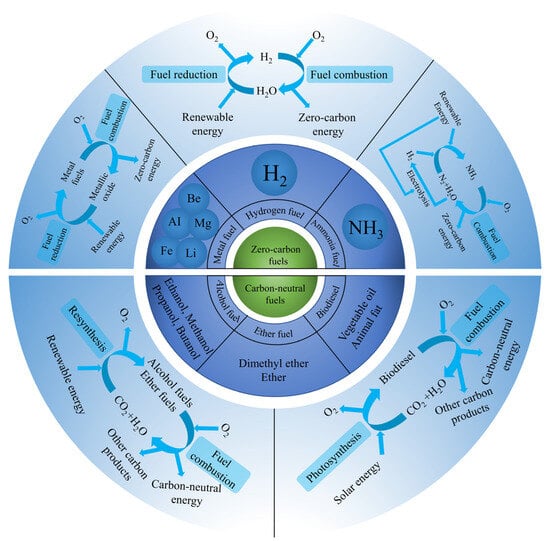
Figure 1.
Illustration of the zero-carbon fuel cycle and carbon-neutral fuel cycle.
2.1.2. Hydrogen
Hydrogen (H2) is an efficient, environmentally friendly, and sustainable energy source, often considered an ideal electric fuel or solar fuel [27]. Green hydrogen, produced using renewable energy, is expected to be at the heart of future zero-carbon energy carriers (Figure 1) [45,46,47,48]. Hydrogen does not contain carbon, and its products of complete combustion in the air are mainly water, producing no hydrocarbons and carbon oxides [49]. However, hydrogen combustion produces NOx due to the high combustion temperature [50].
2.1.3. Ammonia
Ammonia (NH3) is representative of zero-carbon fuels (Figure 1). Green ammonia is carbonless ammonia produced by electrolysis using renewable energy or biomass gasification [51,52,53]. NH3 only produces N2 and H2O when completely burned in pure oxygen [54]. However, there are also significant NOx and unburned NH3 emissions when NH3 is burned in an engine [54,55,56]. Dual-fuel combustion of standard hydrocarbon fuels and NH3 also generates carbon emissions and higher NH3 and NOx emissions [57].
2.2. Carbon-Neutral Fuel Combustion Products
2.2.1. Alcohol Fuels
Alcohol fuels refer to liquid fuels containing alcohol, mainly methanol, ethanol, propanol, butanol, and so on. As shown in Figure 1, alcohol fuels have the potential to be used in a near-carbon-neutral manner when they are produced from captured carbon dioxide and renewable energy sources [58,59,60]. Not only can they be burned in engines as fuel separately, but they are also often used as additives to be blended with gasoline or diesel to make a blended fuel [61]. Table 1 lists the combustion products of alcohol fuels. The constituent elements of alcohol are C, H, and O, so the main combustion products in engines are CO, CO2, and H2O. Moreover, there are other conventional contaminants: NOx and HC, such as methane (CH4), ethane (C2H4), and acetylene (C2H2) [62]. The unconventional pollutants produced by the combustion of alcohol fuels mainly include aldehydes, ketones, acids, unburned alcohols, enols, and olefins [63,64,65,66,67,68,69,70,71,72,73,74]. Alcohols do not contain unsaturated bonds such as C=C, so their combustion does not produce soot [75]. Furthermore, alcohols also do not contain sulfur and there are no sulfur oxides in the combustion products [75].

Table 1.
Combustion products of alcohol fuels, ether fuels, and biodiesel.
2.2.2. Ether Fuels
Ethers are expected to become renewable fuels and additives for future advanced internal combustion engines. Ethers can be produced from a variety of feedstocks, such as fossil fuels, residual oil, scrap, biomass, and renewable energy [76,77]. As shown in Figure 1, like alcohol fuels, ether fuels may also achieve the goal of carbon neutrality when they are produced from captured carbon dioxide and renewable energy sources. Methyl tert-butyl ether (MTBE) and dimethyl ether (DME) are currently the two most commonly used ether fuels [78]. Table 1 lists the combustion products of ethers. The main combustion products are CO2 and H2O. The incomplete combustion products also include CO, NOx, HC, PM, and incompletely burned ethers [79,80]. Ethers do not contain unsaturated bonds and sulfur, so they do not produce soot and sulfur oxides [79]. However, as an oxygenated fuel, ether also has some unconventional emissions: carbonyl products (aldehydes, ketones, acids, esters), peroxides such as hydrogen peroxide (H2O2) and hydroperoxymethyl formate (HPMF), olefins, alkynes, alcohols, enols, benzene, and other pollutants [81,82,83,84,85,86,87,88,89,90].
2.2.3. Biodiesel
Biodiesel is a typical carbon-neutral biomass fuel (Figure 1). Biodiesel is often referred to as a methyl or ethyl-ester-fuel-containing vegetable fat, animal fat, or other triacylglycerol-containing substances [91]. Biodiesel can be used directly or mixed with other fuels [92]. Biodiesel is environmentally friendly and produces fewer harmful pollutants when burned. Table 1 shows the types of pollutants produced by biodiesel combustion. Biodiesel is mainly composed of three elements, namely C, H, and O, so the main combustion products are CO2 and H2O. The composition of biodiesel is complicated due to the residue of alcohols, alkalis, acids, and other impurities in the preparation process of biodiesel. Therefore, biodiesel often produces CO, NOx, HC (methane, ethane, ethylene, 1-butene, 1,3-butadiene, acetylene, propyne, etc.), PM, and other incomplete combustion products [93,94]. Biodiesel combustion also produces some unconventional pollutants: carbonyl substances (aldehydes, ketones, carboxylic acids, esters), monocyclic aromatic hydrocarbons (MAHs), polycyclic aromatic hydrocarbons (PAHs) [94,95,96,97,98,99]. Unconventional pollutants are more harmful to the human body although they account for a small proportion of all products. Biodiesel has low or no sulfur content and does not contain aromatic hydrocarbons, so its combustion produces low levels of sulfides and aromatic hydrocarbons [100].
2.2.4. Blended Fuels
Since pure fuel has its shortcomings in physical and chemical properties, the direct use of pure fuel may reduce the performance of the engine, and the effect often does not meet expectations. Therefore, depending on the advantages and disadvantages of each fuel characteristic, binary or multivariate blends are prepared to improve fuel characteristics and optimize engine performance at an acceptable cost. Currently, commonly mixed fuels are gasoline/diesel–biodiesel, gasoline/diesel–alcohol/ether, biodiesel–alcohol/ether, and gasoline/diesel–biodiesel–ester/alcohol. Table 2 shows the trends in regulated pollutant emissions after the use of some experimental fuel blends. The emission trends in CO, CO2, NOx, HC, and PM measured by different experiments are inconsistent. This may be due to different types, volume ratios, and test conditions of alcohol/ether and biodiesel added to gasoline or diesel fuel.

Table 2.
Trends in pollutant emissions from the combustion of common fuel blends.
3. Toxicity of Zero-Carbon Fuels
Currently, there are few in vitro cytotoxicity studies on exhaust from engines fueled with metal, hydrogen, and ammonia. The health risk potential of these fuels can be inferred from the toxicity of the single pollution produced by the fuels. The combustion of all three fuels produces NOx. Common NOx with greater health effects are nitric oxide (NO), nitrogen dioxide (NO2), and nitrous oxide (N2O) [151]. Exposure to NOx, even for short periods, can lead to a variety of respiratory diseases: inflammatory reactions in the lungs and bronchi, respiratory symptoms (cough, wheezing, dyspnea), asthma, and severe acute respiratory syndrome [152]. Furthermore, NOx plays a role in the formation of ozone (O3), which can cause severe respiratory diseases and reduce lung function in exposed populations [153]. Unburned NH3 is emitted in large quantities during the use of ammonia fuel. Exposure to NH3 for some time can have serious effects on human health, with signs of exposure manifesting as eye irritation, skin inflammation, severe cough, and burning of the nose, throat, and respiratory tract [154,155]. Severe exposure to high concentrations of NH3 can lead to lung disease, permanent blindness, and even death [154,155].
4. Toxicity of Biodiesel and Biodiesel Blends
4.1. Types of Cytotoxic Effects
The toxic effects of emissions from biodiesel and its fuel blends involve cytotoxicity, oxidative stress, inflammatory response, and genotoxicity (carcinogenicity and mutagenicity) (Figure 2). In terms of cytotoxicity, cell morphological changes, the release of cytoplasmic components (e.g., LDH and AK), mitochondrial activity (e.g., MTT, MTS, XTT, and WST-1 assays), lysosomal integrity (neutral red uptake), adenosine triphosphate (ATP) levels, and the number of apoptosis and necrotic cells (trypan blue, propidium iodide and annexin staining) are often used as indicators of cytotoxicity or cell viability [156,157,158]. Biodiesel treatment increased the content of LDH and decreased cell viability [159]. A PM concentration-dependent increase in the number of necrotic cells was observed after exposure to PM of biodiesel blends [160,161].
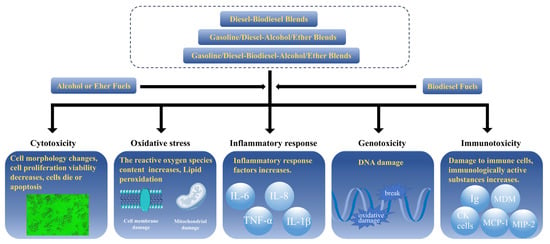
Figure 2.
Cytotoxic effects of carbon-neutral fuel combustion products.
Oxidative stress was assessed by measuring ROS production, depletion of free radical scavengers or antioxidant enzymes, and proteins that characterize cellular oxidative stress [162,163]. The inflammatory response was measured by measuring inflammatory markers, such as cyclooxygenase-2 (COX-2), interleukins (IL-1, IL-4, IL-6, IL-8, IL-10, IL-13), tumor necrosis factor (TNF-α, TNF-β), monocytes chemotactic protein-1 (MCP-1/CCL2), macrophage inflammatory protein-2 (MIP-2), interferon-γ (INF-γ), and exogenous metabolic enzyme (CYP1A1, CYP1B1) [163,164,165]. Cell culture studies have shown an increase in intracellular ROS production following exposure to PMs from the combustion of hydrogenated vegetable oil (HVO), rapeseed methyl ester (RME), fatty acid methyl ester (FAME), bean-based biodiesel, and other biodiesel fuels [161,163,166,167,168,169]. It has been suggested that engine type, rather than biodiesel type, is an important factor in ROS production [169]. Cells exposed to biodiesel increased the expression of SOD1 in the superoxide dismutase gene [170]. One study observed an immediate increase in transcripts coding for heme oxygenase-1 (HO-1/HMOX1) proteins associated with oxidative stress in cells after exposure to biodiesel [159]. Two other studies have also demonstrated that biodiesel fuel increased HMOX1 gene expression in cells [171,172]. The GSH/GSSG ratio fell after 48 h of cell culture in mediums containing biodiesel exhaust particles [173]. Exposure to different types of biodiesel exhaust induced the release of pro-inflammatory factors (e.g., IL-6, IL-8) and expression of the CYP1A1 gene [160,170,171,174].
In terms of genotoxicity, DNA damage is mainly detected by comet assay (CA)/single-cell gel electrophoresis (SCGE), micronucleus (MN) assay, agarose gel electrophoresis, cytoplasmic division blocking micronucleus (CBMN) assay, products formed after DNA damage (γ-H2AX, 8-OhdG), and Ames test [175]. After exposure to biodiesel, comet assays revealed increased levels of DNA strand break (SB) and formamidopyrimidine DNA glycosylase (FPG)-sensitive sites, causing cellular DNA damage [169]. Other findings support this conclusion [176,177].
4.2. Exposure of Specific Cell Lines in Different Biological Systems
4.2.1. Exposure of Respiratory System Cell Lines
The effects of biodiesel exhaust on the respiratory system are usually analyzed using various types of airway epithelial cells and alveolar cells [178]. The airway epithelium acts as the first line of defense against inhalation injury (viral, bacterial, air pollutants, and other environmental pollutants) [179]. After Mullins et al. exposed human epithelial cell line (NuLi-1 and 10KT) cultures to diluted exhaust from the combustion of 100% FAME and 20% FAME mixtures, significant apoptosis and loss of cell viability occurred, and IL-6 and IL-8 production increased [180]. Exposure to exhaust gases from 100% soy biodiesel or 20% soy biodiesel–diesel blends induced cell deaths and the release of immune mediators from human-respiratory-tract epithelial cells compared to air control and ultra-low-sulfur diesel (ULSD) engine exhaust [181]. Aqueous solutions of extractable organic matter (EOM) from PM of soy methyl ester (SME) or soy ethyl ester (SEE) can increase the release of pro-inflammatory cytokines IL-8 and IL-6 by respiratory epithelial cells [182]. Similarly, Traviss et al. found that B20 exhaust gases from waste yellow oils and used cooking oils increased IL-6 and IL-8 gene expression in human bronchial epithelial cells (BEAS-2B) [183]. Studies have indicated that after 6 h of exposure to soy biodiesel, BEAS-2B cells did not exhibit any change in the expression of cytokine genes (IL-6, IL-8, TNF, MCP-1, IL-1b) [181]. However, MCP-1 and IL-8 gene expression was upregulated in human alveolar basal epithelial cells (A549) [181].
Exposure to combustion particles from FAME biodiesel blends can cause DNA damage and chromosomal damage in A549 cells and BEAS-2B cells [177]. Cells exhibit increased levels of single-strand breaks (SSBs), increased MN frequency, or dysregulation of gene expression associated with DNA damage signaling pathways, but do not induce double-strand breaks and oxidative DNA damage (FPG) in cells [177]. This is supported by Cervena and Barraud, whose findings found that REM biodiesel exhaust induced DNA damage in BEAS-2B cells and A549 cells [184,185]. Furthermore, the DNA of epithelial cell lines (HepG2) was severely damaged by PM organics from diesel +10% biodiesel (D+BIO) and diesel +10% HVO (D+HVO), according to Valentina et al. [186]. BEAS-2B cells exhibit cytotoxicity and DTT activity (oxidative potential) and develop oxidative stress (HMOX-1 gene expression upregulation) after exposure to pure HVO and HVO–biodiesel blend PM emissions [187]. Another study also observed that BEAS-2B cells exhibited mild cytotoxicity associated with alterations in ATP/ADP ratios, increased expression of oxidative stress marker genes (NQO1 and HO-1), inflammatory marker genes (IL-8 and IL-6), and xenobiotic metabolic marker genes (CYP1A1 and CYP1B1) after 3 h of exposure to second-generation biofuel (B2G) extracted from lignocellulosic biomass [188]. Betha et al. also noted that biodiesel (B100) from waste cooking oil (WCO) caused higher cytotoxicity and oxidative stress in A549 cells than ULSD [173].
Steiner et al. performed experiments on a complex three-dimensional cell model of the human airway epithelium and reported severe oxidative stress and pro-inflammatory responses in cells after exposure to 100% RME engine exhaust [172]. Landwehr et al. exposed primary airway epithelial cells to diluted exhaust gases from engines fueled with biodiesel made from soybean oil, rapeseed oil, waste cooking oil, tallow oil, palm oil, and cottonseed oil. Shea butter biodiesel was the most toxic, with exposure leading to a significant decrease in cell viability and increased release of several immune mediators, including IL-6 and IL-8 [189]. In one study, PAHs in biodiesel exhaust were cytotoxic in human alveolar epithelial cells (HPAEpiC), manifested by increased oxidative stress (SOD, GSH, and ROS), decreased cell viability, increased extracellular LDH, and apoptosis promotion [190]. During the FTP-75 test cycle, RME’s PM extract was slightly more toxic to L929 cells (mouse lung fibroblasts) than biodiesel [191].
After exposing human bronchial epithelial cells cultured at the air–liquid interface (ALI) to the emissions of coconut oil and diesel fuel mixture (D90C10, D85C15, D80C20), compared with filtered air exposure, the metabolic viability of the cells was reduced, IL-8 and IL-6 secretion increased, and CYP1A1 gene expression significantly increased [170]. D80C20 decreased the percentage of viable cells, but there was no significant effect on the expression of the apoptotic and anti-apoptotic biomarkers genes (CASP3, BCL2) [170]. The 24 h exposure of primary human airway epithelial cells grown on the ALI to diluted exhaust gases from 100% canola biodiesel and 100% or 20% shea butter biodiesel blends increased protein release, indicating damage to epithelial cells [192]. Exposure to shea butter biodiesel exhaust induced increased permeability of ALI cultures [192].
This evidence suggests that biodiesel exhaust exposure leads to loss of airway epithelial cell viability, cell death, or apoptosis, ultimately affecting the normal functioning of the respiratory system. Biodiesel may increase the permeability of the epithelial barrier, leading to the loss of the defensive function of the epithelial barrier. The resulting damage can invade the underlying lung tissue, resulting in irreversible damage to the lung parenchymal tissue, such as a reduced gas-exchange area and impaired lung function, accelerating the development of respiratory diseases, such as asthma, pneumonia, emphysema, and chronic obstructive pulmonary disease [193]. In addition, biodiesel exhaust exposure also causes oxidative stress and inflammatory reactions in the respiratory tract and lung cells, which may cause bronchitis, pneumonia, and even systemic inflammation. Notably, biodiesel exhaust causes DNA damage and reduces the DNA repair capacity of respiratory cells, resulting in mutations. The mutations may lead to irregular cell proliferation and promote tumor development, increasing the risk of morbidity and death associated with lung cancer.
4.2.2. Exposure of Cardiovascular System Cell Lines
In the cardiovascular system, intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are mechanistic intermediates of cancer and vascular disease. PM from the combustion of conventional diesel significantly raised the expression of ICAM-1 and VCAM-1 protein in human-umbilical-cord endothelial cells (HUVECs), but no such increase was seen in the PM from the combustion of 20% biodiesel [169]. Exposure to biodiesel exhaust PM triggers the loss of primary human-vascular-smooth-muscle cells (hVSMCs), which is strongly associated with the apoptosis of vascular wall cells [194]. It is speculated that biodiesel exhaust may cause damage to cardiovascular cells in the circulatory system, promote the occurrence of cardiovascular disease, and accelerate the development of atherosclerotic plaque. The underlying mechanism for this damage may be the ability of ultrafine particles emitted by biodiesel to bypass the respiratory barrier and the cerebral barrier and directly enter the bloodstream, directly causing inflammation of the brain and/or systemic inflammation of the respiratory and cardiovascular systems [195,196].
4.2.3. Exposure of Immune System Cell Lines
In terms of the immune system, an innate immune response barrier consists of neutrophils, monocytes/macrophages, and natural killer (NK) cells [197]. These cells are essential in maintaining immune homeostasis and tolerance [198]. They are the first line of defense against pathogens, contaminants, or inhaled particulate matter and are considered sentinel cells in immune response, responsible for initiating and resolving the immune response to the invading substance [199]. They mediate non-specific immune responses that are strong and quick enough to prevent infection without inducing damaging inflammation [200]. Therefore, due to the ubiquitous presence of macrophages throughout the human body, including the lungs, the toxic effects of fuel combustion emissions on macrophages or monocyte-derived macrophages (MDMs) are often considered.
Jalava et al. found that PM from RME and HVO caused dose-dependent fragmentation of chromosomal DNA strands in mouse macrophages (RAW264.7), with the highest concentrations (150 and 300 mg/mL) of PM causing cytotoxicity and apoptosis [161,166]. Another study also reported the toxicity of exposure to water-soluble components extracted from exhaust PMs of diesel and biodiesel blends (BD30) on RAW 264.7 cells [201]. ROS levels increased and cell viability was significantly reduced (15–70%) after exposure to 100 μL PM for 24 h compared to untreated cell controls [201]. Furthermore, exposure of rat alveolar macrophages (AMs) to soybean biodiesel blend (B20) exhaust particles induced a dose-dependent increase in inflammatory signaling [202]. B20 exposure resulted in elevated prostaglandin E2 (PGE2) release at lower particle concentrations compared to low-sulfur petroleum diesel (PDEP) [202]. Previous studies also reported a significant increase in TNF-α levels and dose-dependent increases in chemokine MIP-2 concentrations after exposure of the RAW264.7 cell line to RME and HVO biofuels compared with the control group [161]. ROS production increases when human monocytes (THP-1) are exposed to PM from 20% animal fat or RME blends, while CCL2 and IL-8 gene expressions remain unaffected [169]. Another study of THP-1 cells showed that exposure to PM from 20% waste oil biodiesel–80% petroleum diesel blends for 24 h can trigger an inflammatory response comparable to petroleum diesel particles [183]. Human U937-derived macrophages exposed to biodiesel emission samples produced with heavy-duty diesel vehicles elevated inflammatory markers (COX-2 and IL-8) and upregulated CYP1A1 gene expression [203]. Tooker et al. found that exposure to PAHs during the differentiation of monocytes (U937) into macrophages (MDMs) did not change the number of MDMs, but exposure to a unique mixture of PAHs resulted in altered immune markers [204]. The percentage of DNA migration and the degree of cell damage increased compared to controls after human lymphocytes were exposed to EOM from PM of biodiesel blends (72% soybean oil and 28% palm oil) [205]. Metals associated with the organic composition of biodiesel may play an important role in molecular mechanisms involved in cell proliferation and immune response [206].
It can be seen that biodiesel exposure can cause damage to immune cells, triggering oxidative stress, inflammatory responses, and DNA damage. These injuries lead to functional deficiencies, such as phagocytosis, which may provoke allergies, autoimmune diseases, and various bacterial infections.
4.2.4. Exposure of Skin and Other System Cell Lines
Biodiesel exhaust can enter the body through skin contact [207]. The biofuels produced by esterifying fried vegetable oils showed more cytotoxic activity against A431 skin cells when compared to those produced by esterifying waste animal fats [208]. Biodiesel may be associated with kidney and liver diseases. PM from 20% biodiesel–mineral diesel blends is mutagenic and cytotoxic to human embryonic kidney 293T cells (HEK 293T) [209]. Cavalcante et al. reported that the exposure of the ZFL zebrafish liver cell line to a soluble portion of biodiesel exhaust particles increased ROS production, glutathione S-transferase activity, and DNA damage, resulting in cytotoxic, biochemical, and genotoxic changes in ZFL cells [210].
4.3. Degree of Cytotoxic of Biodiesel
4.3.1. Lower Toxicity Than Diesel
Some evidence suggests that biodiesel engine exhaust has low or no cytotoxic effects. BEAS-2B cells did not show any toxic effects after exposure to extracts from biodiesel exhaust particles, and levels of IL-6 and IL-8 protein secretion did not change [206]. Similarly, HepG2 cell lines did not exhibit cytotoxic after exposure to soluble organics extracted from exhaust particles of three fuels (ULSD, ULSD+13% HVO, ULSD+20% HVO) [211]. Some studies have found that biodiesel is less toxic than diesel. Biodiesel engine exhaust PM is safer for human lung cells than its mineral diesel counterpart [172]. Under real conditions, cytotoxicity was 8.5 times higher after exposure of the BEAS-2B cell line to PM from petroleum diesel for 24 h than that of PM from a 20% waste oil biodiesel blend [212].
In terms of mutagenicity, exhaust from RME is less mutagenic than that from diesel [191]. This may be due to a lower concentration of highly polar mutagens (PAHs, aromatic amines, nitroaromatic hydrocarbons, and oxygenated PAHs) emitted by biodiesel–mineral diesel blends compared to the benchmark mineral diesel, resulting in a lower mutagenic potential for exhaust emissions [213]. In terms of oxidative stress and inflammatory response, PM from a 20% animal fat or RME blend was less or equal in cell ROS production, DNA damage, and CCL2 and IL-8 gene expression compared to PM from conventional diesel fuel (D100) combustion [169]. Novotna et al. observed high levels of DNA strand breaking and oxidative damage DNA after 4 h exposure of A549 cells to DCM organics extracted from petroleum diesel and pure biodiesel [214]. While these results indicate the potential health benefits of biodiesel, the toxic effects of each biodiesel exhaust gas may vary depending on the chemical composition and organ systems examined.
4.3.2. Higher Toxicity Than Diesel
A few studies have come to the opposite conclusion, finding that biodiesel is more toxic than diesel [215]. Miriam et al. reported that PM from B50 (50% RME+50% diesel) resulted in increased cytotoxicity and IL-6 release from BEAS-2B cells compared to PM from diesel [160]. PM organics from biodiesel blends (diesel+10% HVO) exhibit higher genotoxicity compared to conventional ULSD [186]. The particles emitted by biodiesel engines are more harmful than those emitted by diesel engines, possibly because they contain more intermediate combustion products, NPs, toxic trace metals, and harmful PAHs [209].
4.3.3. Causes of the Toxicity Contradiction
The reasons for the contradiction of biodiesel toxicity may be related to factors such as biodiesel feedstock type, biodiesel content, gas and particle-phase properties, exposure time, engine conditions, and other factors. Even though biodiesel is not a major component of fuel, the amount of biodiesel in the fuel blend can significantly affect the cytotoxicity of exhaust gases [192]. The particulate-phase extract of 10% palm oil biodiesel induced cell death at lower doses and was more cytotoxic than the particulate-phase extract of 20% palm oil biodiesel [216]. Vaughan et al. reported that a low proportion of coconut oil–diesel blends (10% and 15%) reduced inflammation and increased antioxidant expression compared with conventional diesel, while a high proportion of coconut oil blends (20%) reduced cell viability and increased inflammation [170]. There is a difference in the toxicity of biodiesel gas-phase emissions and particulate-phase emissions to cells. Palm oil biodiesel gas-phase extracts showed higher cytotoxicity and genotoxicity in A549 cells but lower in the particulate phase [216]. The toxicity of biodiesel semi-volatile particles is higher than that of solid particles [217].
The toxicity of biodiesel has a time effect, and different exposure times activate different regulatory pathways, resulting in different toxic effects [218]. Water-soluble fractions from biodiesel and its blends with diesel are cytotoxic to human cell lines. Leme et al. reported a dose-dependent response to toxic effects in human T-cell leukemia (Jurkat) and human liver cancer cells (HepG2) in water systems contaminated with pure biodiesel and its diesel blend (B5) [219]. The presence or absence of after-treatment systems (particulate traps and oxidation catalysts) is critical to the health effects of exhaust gas exposure. The results of cytotoxicity experiments found that the cytotoxicity, oxidative, and pro-inflammatory properties of biodiesel vehicle exhaust equipped with a diesel particulate filter (DPF) were reduced, which may be due to the reduction in PM mass and particle number due to the application of DPF [160]. Different driving conditions also have some influence on the toxicity of biodiesel. Particles collected under rural driving conditions had more cytotoxic and inflammatory responses but lower oxidative potential than those collected under urban driving conditions [160].
5. Toxicity of Alcohol and Alcohol Blends
The physicochemical properties and toxicity of PM from oxygenated fuel blend change because the thermochemistry properties of oxygenated fuels alter the combustion chemistry. The addition of oxygenated fuels (alcohols/ethers) to gasoline/diesel can reduce PM emissions [142]. Determining changes in PM properties caused by different types of oxygenated fuel mixtures can help assess PM toxicity.
5.1. Exposure of Respiratory System Cell Lines
Several experiments have investigated the toxic effects of gasoline–alcohol fuels on respiratory cell lines. One study used MN assay and CA of A549 cells to compare the genotoxicity of gasoline and methanol exhaust. The results showed that methanol vehicle exhaust did not cause DNA damage and chromosomal damage to A549 cells, while gasoline vehicle exhaust had strong cytotoxicity and genotoxicity [220]. Liang et al. also found that gasoline exhaust is more genotoxic than methanol exhaust, and methanol exhaust is more cytotoxic than gasoline exhaust within a certain dose range [221]. A 72% and 83% reduction in genotoxicity was seen from ethanol–gasoline blends (E10, E85) compared to gasoline [222]. Some dysregulated genes and processes were identified, such as oxidative stress, lipid and steroid metabolism, PPARα signaling, and immune response after 4 h exposure of BEAS-2B cells to subtoxic doses of PM extract from E15 (85% gasoline + 15% ethanol) [223]. On the other hand, after 24 h exposure, genes and pathways associated with the metabolism of PAHs and aromatic hydrocarbon receptors (AhR) were activated, and multiple genes and pathways associated with cancer promotion and progression were dysregulated [223].
Bisig et al. found that multicellular human lung models consisting of human bronchial cells (16HBE14o-), supplemented with human-monocyte-derived dendritic cells (MDDCs) and monocyte-derived macrophages (MDMs), did not cause observable cytotoxicity, morphological changes, oxidative stress, and DNA damage after 6 h exposure to aerosols excreted from ethanol–gasoline mixture (E10, E85) [224]. However, an increase in cell death, oxidative stress, and pro-inflammatory cytokines was observed in cells exposed to diesel exhaust [224]. Rossner et al. exposed the BEAS-2B cell line to complete emissions of PM produced by the gasoline–20% ethanol mixture (E20) and found that lipid oxidation was reduced, but concentrations of AA metabolites and chemokines with anti-inflammatory effects increased [225]. Sima et al. investigated the toxic effects of OME from PM produced by gasoline blended with different ethanol contents and found that 5-day exposure induced significant cytotoxicity in BEAS-2B cells and low cytotoxicity in 3D cell models of human airways (MucilAir™) [226]. Compared to the control group, ROS production decreased, CYP1A1 gene expression was upregulated, and histone phosphorylation was not significantly increased in two cell cultures, i.e., double-stranded DNA damage was not evident [226]. Arias et al. reported that soluble organic fractions (SOFs) extracted from PM produced by two fuels (ULSD, ULSD+13% butanol) had no cytotoxic activity against HepG2 cells and produced genotoxic reactions at the same level [211].
These studies have shown low levels of oxidative stress, cytotoxic effects, and genotoxic effects caused by alcohols and their fuel mixtures on respiratory system cell lines. However, these fuel emissions can cause an inflammatory response in cells. In summary, the addition of ethanol to gasoline has advantages in reducing the risk of respiratory diseases from engine exhaust.
5.2. Exposure of Immune System Cell Lines
One study reported that the exposure of RAW264.7 cells to PM from ethanol–gasoline blends (E10, E8) decreased cell viability and metabolic viability and raised the level of the inflammatory response (TNF-α and MIP-2) but did not increase oxidative stress levels compared to control cells [227]. Another study found that rat alveolar macrophages (NR8383) produced cytotoxic responses similar to environmental aerosols but secreted more TNF-α after being exposed to PM from three ethanol–gasoline blends (E10, E30, and E78) [228]. The E78 mixture showed minimal oxidation potential, while E30 showed the greatest oxidation potential [228]. Natural killer (NK) cells eliminate pathogen infection through secretory pathways and death-receptor-mediated pathways, as well as by secreting immunomodulatory cytokines, such as interferon (IFN)-γ and granzyme B [229]. In addition, NK cells, which can directly or indirectly attack and kill tumor cells, have been described as the main effector cells against cancer in innate immunity [230]. Co-culture models consisting of bronchial epithelial cells (ECs) and NK cells did not detect toxic effects after being exposed to an 85% ethanol–gasoline mixture (E85) compared to air controls for the short term [231]. This evidence suggests that alcohol fuel emissions have damaging effects on macrophages and may lead to systemic inflammation and immune system disease. However, alcohol fuel emissions are not significantly toxic to NK cells, indicating that this fuel may have an advantage in reducing cancer incidence.
5.3. Exposure of Other System Cell Lines
Agarwal et al. reported that the cytotoxicity, ROS production potential, and mutagenicity of PM from 10% ethanol–gasoline blends on human embryonic kidney (HEK) 293 cells were lower than those of gasoline [142]. This suggests that alcohol fuel may cause less damage to the kidney organs than gasoline.
6. Toxicity of Gasoline/Diesel—Biodiesel—Alcohol/Ether Blends
The characteristics of particulate matter can change when biodiesel and ethanol are added to diesel, and ethanol has a higher influence on these changes than biodiesel [232]. PM from DB10 (90% diesel + 10% biodiesel) vehicles reduced RAW 264.7 macrophage death (−60.8%) compared to PM from pure diesel (D100) vehicles, while PM from DE10 (90% diesel + 9% ethanol + 1% biodiesel) vehicles increased cell death (84.1%) [232]. This suggests that alcohol blends may be more immunotoxic than biodiesel blends. Ternary fuel blends can reduce the genotoxicity associated with PM. Yang et al. showed that PM10 produced by fuel blends composed of waste cooking oil/butanol/diesel (B10W20, B10W40) significantly reduced diesel-induced genotoxicity of A549 cells and diesel-induced mutagenicity of CHO-K1 cells [233]. Diluted engine exhaust from grapeseed, bran, or coconut biodiesel blended with 10% (v/v) diethylene glycol dimethyl ether (DGDME) resulted in reduced permeability, increased cell damage, and increased IL-6 and IL-8 release of primary human airway epithelial cells growing at the gas–liquid interface [234]. Collectively, these results suggest that these fuel blends are cytotoxic. Blends of biodiesel with oxygenated fuels may be a potential alternative fuel for diesel engines as they have less cytotoxic effects than diesel.
7. Biological Models and Methods for Toxicity Assessment
7.1. Biological Models
Air pollution caused by vehicle emissions has posed a huge threat to the ecological environment and biological health. Vehicle emissions affect a broad spectrum, from biogeochemical cycles to microbial communities, and from plant development to animal and human health. Under such circumstances, there is an increasing need to develop and validate microbial, plant, animal, and human-based toxicity testing models and methods. This multifaceted assessment can help better predict the actual impact of vehicle exhaust emissions on ecosystems and understand their effects from the cellular level to the organismal level [235].
As shown in Figure 3, bacteria, yeast, filamentous fungi, and protozoa are commonly used microbial models [236]. Model plants that have been used for air pollutant assessment include Arabidopsis, tobacco, wheat, rice, soybean, maize, and sorghum [237,238,239]. The plants evaluated for toxicity of aquatic organisms are mainly algae, such as chlorella and microalgae [240,241]. The selection of plant species is related to geographical factors, and researchers usually choose common plant species in the area to be studied. Model animals currently commonly used for toxicity studies are small rodents (e.g., mice and rats), Drosophila, and nematodes (e.g., C. elegans) [242,243,244,245]. The model animals commonly used in aquatic environment toxicity assessments mainly include zebrafish, Daphnia magna, and Artemia [246,247,248]. Animal model experiments have made many important contributions to our understanding of the relationship of entire organisms to pollutants, the human disease process caused by pollutants, and the prediction of the impact of pollutants on human health [249]. However, toxic effects in humans may differ significantly from those in animals due to distinct species characteristics [250]. Animal models ignore the detailed changes that occur at the level of individual cells.
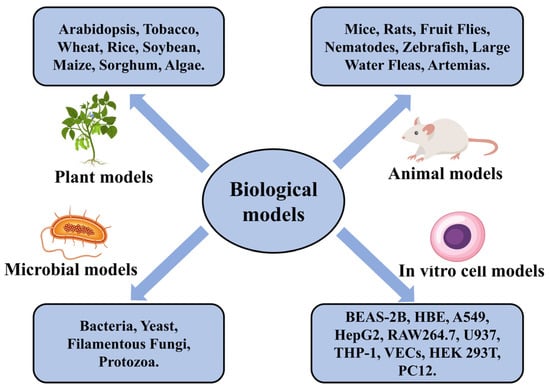
Figure 3.
Biological model for toxicological studies.
In vitro, cell models have evolved from simple cell-free biochemical assays to monoculture systems under submerged conditions to the latest and advanced multicellular cultures grown at air–liquid interfaces. Monoculture cell lines commonly used in respiratory toxicity studies include human nasal epithelial cells (HNECs), human bronchial epithelial cell lines (HBECs) (e.g., BEAS-2B, HBEC3KT, 16-HBE, NL-20), human alveolar basal epithelial cells (e.g., A549, NCI-H441, HPAEpiC), human lung adenocarcinoma cell line (CALU-3), and mouse lung fibroblast cell line (L929) [191,251,252,253,254,255]. Cell models commonly used in other human toxicity studies are the human epidermal carcinoma cell line (A431), normal human keratinocytes (NHKs), the human hepatocellular carcinoma cell line (HepG2), mouse macrophages (RAW264.7), the human lymphoblastoid cell line (MCL-5), the human monocytic cell line (U937, THP-1) and monocyte-derived macrophages (MDM), vascular endothelial cells (VECs), human pulmonary artery endothelial cells (HPAECs), human embryonic kidney cells (HEK 293T), and the pheochromocytoma line (PC12) [208,211,254,256,257,258,259,260,261]. Although the monoculture system under immersion conditions is easy to operate and low in cost, it cannot reflect the actual in vivo cell–cell interaction, cannot truly simulate the exposure process, and is difficult to expose cells to gaseous or vaporized exhaust pollutants [262,263]. In recent years, the air–liquid interface (ALI) in vitro cell-exposure technology has been a way to expose cells to all or part of the components of engine exhaust particulate matter and gas-phase substances [264]. The system more realistically simulates in vivo inhalation exposure conditions [265]. Additionally, cell culture systems used to study air-pollution-mediated respiratory toxicity also include microfluidic organ chips, organoids, and human precision-cut tissue slices [266,267,268,269].
7.2. Toxicity Detection Methods
Toxicity testing on plants involves all aspects of plant growth, development, and reproduction, for example, plant growth indicators (seed germination rate, plant height, root length, plant phenotype, biomass, leaf quality), physiological and biochemical indicators (chlorophyll content, carotenoid content, proline content, sugar content, relative water content of leaves, photosynthesis parameters), oxidative stress indicators, and genotoxicity indicators (DNA damage) [238,270,271,272,273,274].
In vivo toxicity assessments in mammals and humans are often evaluated using animal experiments, epidemiological studies, and human clinical studies [275,276]. By exposing laboratory animal models to an artificially defined environment, the effects of pollutant exposure on animal behavior, the pathological changes in tissues and organs, the impact of pollutant exposure on the morbidity and mortality of certain human diseases, and the carcinogenicity of pollutants can be evaluated [277,278,279,280,281].
In vitro toxicity assessment in mammals and humans typically includes cytotoxicity, oxidative stress, inflammatory biomarkers, genotoxicity, neurotoxicity, and reproductive toxicity. Cytotoxicity can be mainly divided into the following aspects of detection [282,283,284,285,286,287,288,289]: (i) Cell morphological damage observations commonly use instruments, such as microscopes, including optical microscope (OM), electron microscope (EM), scanning tunneling microscope (STM), and atomic force microscope (AFM); (ii) Cell death identification commonly uses trypan blue staining; (iii) Apoptosis and cell cycle are often measured using fluorescent dye probes and flow cytometry, and the expression levels of proteins closely associated with apoptosis are detected using Western blot and RT-PCR; (iv) Detection of metabolic or proliferative activity (WST-1 assay, XTT assay, MTT assay, CCK-8 assay, Alamar Blue assay, AB detection) and detection of DNA synthesis ([3H]-TdR method, BrdU immunoassay, EdU labeling method) usually use cell tracing, which can be performed by various techniques such as in vivo imaging, microplate or flow cytometry; (v) The detection methods of cell membrane damage usually include the following: changes in cell membrane permeability based on lactate dehydrogenase (LDH) detection, comparison of membrane surface structure changes before and after contamination exposure by transmission electron microscope (TEM) and atomic force microscope, evaluation of cell membrane fluidity based on lipid diffusion coefficient and fluorescence recovery rate, and rapid detection of cell membrane potential changes based on fluorescent dyes and fluorescence microscopy; (vi) The effects of contaminants on cell morphology and cell and organelle movement were studied by (a) Coomassie blue staining, (b) immunofluorescence staining, and (c) RT-PCR and Western blot with microscopy and flow cytometry; (vii) Organelle damage mainly focuses on mitochondrial damage of mitochondrial morphology and mitochondrial membrane potential; (viii) Real-time cell analyzer (RTCA) is a new technology for detecting cytotoxicity. It is a transient cell-inductance continuous recording system based on impedance. The resistance is closely related to the cells’ adhesion, quantity, volume size, and morphological changes. The cytotoxic response is dynamically monitored in real time by detecting the resistance value.
Detection of oxidative damage typically includes several aspects [287,290,291,292,293,294,295,296]: (i) Intracellular radicals can be determined directly by electron spin resonance (ESR) and chemiluminescence; (ii) Lipid peroxidative damage is usually reflected by the content of malondialdehyde (MDA), a by-product determined by thiobarbituric acid reactive substances (TBARS); (iii) Protein oxidative damage is often determined by traditional detection techniques, such as colorimetry, spectrophotometry, or enzyme-linked immunosorbent assay (ELISA), to determine hydrazone generated by the reaction of 2,4-dinitrophenylhydrazine with protein carbonyls; (iv) DNA oxidative damage is most commonly detected by the biomarker 8-hydroxydeoxyguanosine (8-OHdG); (v) Changes in key protein expression in multiple signaling pathways involved in oxidative damage are detected by Western blotting. The method for measuring the inflammatory response is described in Section 4.1. At present, there are some mature and systematic experimental methods for in vitro genotoxicity studies [297,298,299,300]: the Ames test for bacterial mutagenicity, the comet assay/single-cell gel electrophoresis, DNA double-strand break analysis, micronucleus test, chromosomal aberration test, gene mutation test, giant DNA adduct formation or oxidative DNA damage detection, genome-wide gene expression analysis, the occurrence of apoptosis and subdiploid DNA content analyzed by annexin V/PI staining, and the detection of relevant gene expression changes by RT-PCR.
8. Conclusions and Prospect
The metal particles produced by the combustion of metal fuels and the NH3 produced by the combustion of ammonia fuels are toxic and can negatively affect human health. Hydrogen fuel generally does not adversely affect the environment and human health. It is worth noting that the combustion of these three types of zero-carbon fuels leads to the production of NOx, which can cause respiratory diseases in humans. Future research on metal fuel will require a detailed assessment of toxic metal and NOx emission levels, as well as consideration of advanced combustion strategies or after-treatment technologies to mitigate emissions. Further use of ammonia fuel also requires controlling NH3 and NOx emissions, developing new NH3 combustion systems, and establishing more complete after-treatment systems.
Biodiesel has negative effects on various cell lines, including those in the immunological, circulatory, and respiratory systems, according to in vitro cytotoxicity studies. However, there are conflicting results in the literature regarding biodiesel-induced cytotoxicity, which may be related to differences in fuel type, fuel content, exposure time, engine configuration, and PM emissions sampling. These results cannot be directly compared due to different experimental conditions. It is necessary to establish more effective assessment methods for pollutant emissions assessment methods to compare the toxicity of different types of biodiesels. Most studies have shown that biodiesel and its fuel blends cause lower cytotoxicity than diesel, suggesting potential health benefits for biodiesel when used as an alternative to diesel. The use of alcohols and their fuel mixtures can reduce the cytotoxicity of diesel or gasoline engine exhaust gases and does not even cause adverse cellular responses. From a human health point of view, alcohol fuels can be considered carbon-neutral alternatives. When biodiesel and alcohol are added to gasoline or diesel as additives, the toxic effects vary due to the changing proportions. However, in comparison, the fuel blends with these additives are less toxic than pure gasoline or pure diesel. Unfortunately, the cytotoxicity of ethers and their fuel blends has been less studied. Further research is needed to determine whether their toxicity has the same effect as alcohol fuels.
It is recommended that more detailed studies be conducted in the future on toxic components in carbon-neutral fuel emissions to reduce toxic component emissions. In addition, the toxicity of respiratory system cell lines and immune system cell lines have been well studied. Toxicity studies of various cell lines in other systems should be given greater focus to better understand the diseases caused in each system by biodiesel. After sufficient in vitro toxicity assessment data are available, in vivo toxicity assessments should be considered in the future to more accurately assess human health risks. It is worth mentioning that the current toxicity studies of alternative fuels focus on the toxicity of PM or PM extracts. The overall toxicity of all biodiesel emissions (gas phase and particle phase) need further research. In addition, more effective and realistic biological models need to be developed and used to better simulate the adverse effects of alternative fuels on the environment and human health. Simple, rapid, and cost-effective cytotoxicity assay systems should also be further developed to assess cytotoxicity in multiple ways.
Author Contributions
Conceptualization, C.J.; Data curation, X.L.; Writing—original draft, X.L.; Methodology, T.X.; Validation, J.D.; Formal analysis, Z.G.; Software, J.L.; Supervision, C.D.; Investigation, J.H.; Syntax check, A.E.A.; Writing—review and editing, A.E.A.; Funding acquisition, Q.Z.; Project administration, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the financial support to the research provided by the National Natural Science Foundation of China through the Project of 52176125. Also, the project was supported by Open Fund of Shanghai Key Laboratory of Plant Functional Genomics and Resources (PFGR202302). This research is also supported by the Open Research Fund of State Environmental Protection Key Laboratory of Vehicle Emission Control and Simulation, Chinese Research Academy of Environmental Sciences (VECS2022K06) and the Fundamental Research Funds for the Central Public-interest Scientific Institution (2022YSKY-05).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| NOx | Nitrogen Oxides |
| HC | Hydrocarbon |
| CO | Carbon Monoxide |
| PM | Particulate Matter |
| CO2 | Carbon Dioxide |
| VOCs | Volatile Organic Compounds |
| NO | Nitric Oxide |
| NO2 | Nitrogen Dioxide |
| N2O | Nitrous Oxide |
| MAHs | Monocyclic aromatic hydrocarbons |
| PAHs | Polycyclic aromatic hydrocarbons |
| LDH | Lactate Dehydrogenase |
| SOF | Soluble organic compounds |
| EOM | Extractable organic matter |
| AK | Adenylate Kinase |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| COX-2 | Cyclooxygenase-2 |
| CYP1A1 | Cytochrome P450 1A1 |
| CYP1B1 | Cytochrome P450 1B1 |
| 8-OhdG | 8-hydroxydeoxyguanosine |
| TUNEL | TdT-mediated dUTP nick end labeling |
| HVO | Hydrogenated vegetable oil |
| SSBs | Single-strand breaks |
| MN | Micronucleus |
| NQO1 | NAD (P)H: Quinone oxidoreductase 1 |
| PPAR | Peroxisome proliferator-activated receptor |
| [3H]-TdR | [3H] thymidine deoxyribose |
| BrdU | 5-bromo-2-deoxyuridine |
| EdU | 5-ethynyl-2′-deoxyuridine |
References
- Ramos, J.L.; Pakuts, B.; Godoy, P.; Garcia-Franco, A.; Duque, E. Addressing the energy crisis: Using microbes to make biofuels. Microb. Biotechnol. 2022, 15, 1026–1030. [Google Scholar] [CrossRef]
- EI. Statistical Review of World Energy. 2023. Available online: https://www.energyinst.org/statistical-review (accessed on 2 August 2023).
- Escobar, J.C.; Lora, E.S.; Venturini, O.J.; Yáñez, E.E.; Castillo, E.F.; Almazan, O. Biofuels: Environment, technology and food security. Renew. Sustain. Energy Rev. 2009, 13, 1275–1287. [Google Scholar] [CrossRef]
- IEA. Global Energy Review. 2021. Available online: https://www.iea.org/reports/global-energy-review-2021 (accessed on 16 May 2023).
- Sharma, S.; Agarwal, S.; Jain, A. Significance of Hydrogen as Economic and Environmentally Friendly Fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Xue, W.; Zhan, Q.; Zhang, Q.; Wu, Z. Spatiotemporal Variations of Particulate and Gaseous Pollutants and Their Relations to Meteorological Parameters: The Case of Xiangyang, China. Int. J. Environ. Res. Public Health 2020, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, H. Impact of ambient air pollution on physical activity and sedentary behavior in children. BMC Public Health 2023, 23, 357. [Google Scholar] [CrossRef]
- Mendoza-Villafuerte, P.; Suarez-Bertoa, R.; Giechaskiel, B.; Riccobono, F.; Bulgheroni, C.; Astorga, C.; Perujo, A. NOx, NH3, N2O and PN real driving emissions from a Euro VI heavy-duty vehicle. Impact of regulatory on-road test conditions on emissions. Sci. Total Environ. 2017, 609, 546–555. [Google Scholar] [CrossRef]
- Wallington, T.J.; Anderson, J.E.; Dolan, R.H.; Winkler, S.L. Vehicle Emissions and Urban Air Quality: 60 Years of Progress. Atmosphere 2022, 13, 650. [Google Scholar] [CrossRef]
- Park, J.; Shin, M.; Lee, J.; Lee, J. Estimating the effectiveness of vehicle emission regulations for reducing NOx from light-duty vehicles in Korea using on-road measurements. Sci. Total Environ. 2021, 767, 144250. [Google Scholar] [CrossRef]
- Selleri, T.; Melas, A.D.; Joshi, A.; Manara, D.; Perujo, A.; Suarez-Bertoa, R. An Overview of Lean Exhaust deNOx Aftertreatment Technologies and NOx Emission Regulations in the European Union. Catalysts 2021, 11, 404. [Google Scholar] [CrossRef]
- Warkins, J.; Tao, T.; Shen, M.; Lyu, S. Application of Low-Mass Corning® FLORA® Substrates for Cold-Start Emissions Reduction to Meet Upcoming LEV III SULEV30 Regulation Requirement; SAE Technical Paper, SAE International: Warrendale, PA, USA, 2020. [Google Scholar] [CrossRef]
- Cheng, Y.; Chow, J.C.; Watson, J.G.; Zhou, J.; Liu, S.; Cao, J. Decreasing concentrations of carbonaceous aerosols in China from 2003 to 2013. Sci. Rep. 2021, 11, 5352. [Google Scholar] [CrossRef] [PubMed]
- Pushpaanjali, G.; Arivarasu, L.; Rani, L.S. Global Renewable Energy. J. Complement. Med. Res. 2020, 11, 95–101. [Google Scholar] [CrossRef]
- Sergio, M.; Antonio, D.A.; Paolo, R. A dynamic decision model for energy-efficient scheduling of manufacturing system with renewable energy supply. J. Clean. Prod. 2020, 270, 122028. [Google Scholar] [CrossRef]
- Notton, G.; Nivet, M.-L.; Voyant, C.; Paoli, C.; Darras, C.; Motte, F.; Fouilloy, A. Intermittent and stochastic character of renewable energy sources: Consequences, cost of intermittence and benefit of forecasting. Renew. Sustain. Energy Rev. 2018, 87, 96–105. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Y.; Chen, S.; Zhang, L. Slow photons for solar fuels. Chin. J. Catal. 2018, 39, 379–389. [Google Scholar] [CrossRef]
- Kalamaras, E.; Maroto-Valer, M.M.; Shao, M.; Xuan, J.; Wang, H. Solar carbon fuel via photoelectrochemistry. Catal. Today 2018, 317, 56–75. [Google Scholar] [CrossRef]
- Yadav, D.; Banerjee, R. A review of solar thermochemical processes. Renew. Sustain. Energy Rev. 2016, 54, 497–532. [Google Scholar] [CrossRef]
- Qu, W.; Hong, H.; Jin, H. A spectral splitting solar concentrator for cascading solar energy utilization by integrating photovoltaics and solar thermal fuel. Appl. Energy 2019, 248, 162–173. [Google Scholar] [CrossRef]
- Hani, A.; Hameed, B.H. Electrofuels as emerging new green alternative fuel: A review of recent literature. Energy Convers. Manag. 2022, 254, 115213. [Google Scholar] [CrossRef]
- Brynolf, S.; Taljegard, M.; Grahn, M.; Hansson, J. Electrofuels for the transport sector: A review of production costs. Renew. Sustain. Energy Rev. 2018, 81, 1887–1905. [Google Scholar] [CrossRef]
- Keasling, J.; GarciaMartin, H.; Lee, T.S.; Mukhopadhyay, A.W.; Singer, S.; Sundstrom, E. Microbial production of advanced biofuels. Nat. Rev. Microbiol. 2021, 19, 701–715. [Google Scholar] [CrossRef]
- Tobío-Pérez, I.; Alfonso-Cardero, A.; Díaz-Domínguez, Y.; Pohl, S.; Piloto-Rodríguez, R.; Lapuerta, M. Thermochemical Conversion of Sargassum for Energy Production: A Comprehensive Review. BioEnergy Res. 2022, 15, 1872–1893. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Poudyal, R.S.; Tiwari, I.; Voloshin, R.A.; Zharmukhamedov, S.K.; Nam, H.G.; Zayadan, B.K.; Bruce, B.D.; Hou, H.J.M.; Allakhverdiev, S.I. Biofuel production: Challenges and opportunities. Int. J. Hydrogen Energy 2017, 42, 8450–8461. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Guillemin, G.J.; Gupta, V.K.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. A comprehensive review on anaerobic fungi applications in biofuels production. Sci. Total Environ. 2022, 829, 154521. [Google Scholar] [CrossRef]
- Bergthorson, J.M. Recyclable metal fuels for clean and compact zero-carbon power. Prog. Energy Combust. Sci. 2018, 68, 169–196. [Google Scholar] [CrossRef]
- Marzi, E.; Morini, M.; Gambarotta, A. Analysis of the Status of Research and Innovation Actions on Electrofuels under Horizon 2020. Energies 2022, 15, 618. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Ho, S.-H. Microalgae as a solution of third world energy crisis for biofuels production from wastewater toward carbon neutrality: An updated review. Chemosphere 2022, 291, 132863. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Costa, M.; Sun, Z.; He, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/air, NH3/H2/air, NH3/CO/air and NH3/CH4/air premixed flames. Combust. Flame 2019, 206, 214–226. [Google Scholar] [CrossRef]
- Cheng, Y.; Hou, P.; Wang, X.; Kang, P. CO2 Electrolysis System under Industrially Relevant Conditions. Acc. Chem. Res. 2022, 55, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, B.S.; Singh, R.K.; Cho, H.M.; Lim, H.C. Practice of diesel fuel blends using alternative fuels: A review. Renew. Sustain. Energy Rev. 2016, 59, 1358–1368. [Google Scholar] [CrossRef]
- Verger, T.; Azimov, U.; Adeniyi, O. Biomass-based fuel blends as an alternative for the future heavy-duty transport: A review. Renew. Sustain. Energy Rev. 2022, 161, 112391. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Tsolakis, A.; Alagumalai, A.; Mahian, O.; Lam, S.S.; Pan, J.; Peng, W.; Tabatabaei, M.; Aghbashlo, M. Use of hydrogen in dual-fuel diesel engines. Prog. Energy Combust. Sci. 2023, 98, 101100. [Google Scholar] [CrossRef]
- Chai, W.S.; Bao, Y.; Jin, P.; Tang, G.; Zhou, L. A review on ammonia, ammonia-hydrogen and ammonia-methane fuels. Renew. Sustain. Energy Rev. 2021, 147, 111254. [Google Scholar] [CrossRef]
- Strandell, M.; Zakrisson, S.; Alsberg, T.; Westerholm, R.; Winquist, L.; Rannug, U. Chemical analysis and biological testing of a polar fraction of ambient air, diesel engine, and gasoline engine particulate extracts. Environ. Health Perspect. 1994, 102, 85–92. [Google Scholar] [CrossRef]
- Pereira, S.A.; Araújo, V.Q.; Reboucas, M.V.; Vieira, F.S.V.; de Almeida, M.V.A.; Chinalia, F.A.; Nascimento, I.A. Toxicity of biodiesel, diesel and biodiesel/diesel blends: Comparative sub-lethal effects of water-soluble fractions to microalgae species. Bull. Environ. Contam. Toxicol. 2011, 88, 234–238. [Google Scholar] [CrossRef]
- Oh, S.-M.; Chung, K.-H. Identification of mammalian cell genotoxins in respirable diesel exhaust particles by bioassay-directed chemical analysis. Toxicol. Lett. 2006, 161, 226–235. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Cambre, M.; Lee, H.-J. The Toxicity of Nanoparticles Depends on Multiple Molecular and Physicochemical Mechanisms. Int. J. Mol. Sci. 2017, 18, 2702. [Google Scholar] [CrossRef]
- Zahedi, A.; Phandthong, R.; Chaili, A.; Leung, S.; Omaiye, E.; Talbot, P. Mitochondrial Stress Response in Neural Stem Cells Exposed to Electronic Cigarettes. iScience 2019, 16, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Julien, P.; Bergthorson, J.M. Enabling the metal fuel economy: Green recycling of metal fuels. Sustain. Energy Fuels 2017, 1, 615–625. [Google Scholar] [CrossRef]
- Bergthorson, J.M.; Goroshin, S.; Soo, M.J.; Julien, P.; Palecka, J.; Frost, D.L.; Jarvis, D.J. Direct combustion of recyclable metal fuels for zero-carbon heat and power (vol 160, pg 368, 2015). Appl. Energy 2017, 202, 784. [Google Scholar] [CrossRef]
- Laraqui, D.; Allgaier, O.; Schönnenbeck, C.; Leyssens, G.; Brilhac, J.-F.; Lomba, R.; Dumand, C.; Guézet, O. Experimental study of a confined premixed metal combustor: Metal flame stabilization dynamics and nitrogen oxides production. Proc. Combust. Inst. 2019, 37, 3175–3184. [Google Scholar] [CrossRef]
- Garra, P.; Leyssens, G.; Allgaier, O.; Schonnenbeck, C.; Tschamber, V.; Brilhac, J.F.; Tahtouh, T.; Guezet, O.; Allano, S. Magnesium/air combustion at pilot scale and subsequent PM and NOx emissions. Appl. Energy 2017, 189, 578–587. [Google Scholar] [CrossRef]
- Li, S.; Tan, E.C.D.; Dutta, A.; Snowden-Swan, L.J.; Thorson, M.R.; Ramasamy, K.K.; Bartling, A.W.; Brasington, R.; Kass, M.D.; Zaimes, G.G.; et al. Techno-economic Analysis of Sustainable Biofuels for Marine Transportation. Environ. Sci. Technol. 2022, 56, 17206–17214. [Google Scholar] [CrossRef]
- Dong, Z.Y.; Yang, J.; Yu, L.; Daiyan, R.; Amal, R. A green hydrogen credit framework for international green hydrogen trading towards a carbon neutral future. Int. J. Hydrogen Energy 2022, 47, 728–734. [Google Scholar] [CrossRef]
- Dimitriou, P.; Tsujimura, T. A review of hydrogen as a compression ignition engine fuel. Int. J. Hydrogen Energy 2017, 42, 24470–24486. [Google Scholar] [CrossRef]
- Staffell, I.; Scamman, D.; Abad, A.V.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Barreiros, R.C.S.; da Silva, M.F.; Casazza, A.A.; Converti, A.; Sarubbo, L.A. Use of Hydrogen as Fuel: A Trend of the 21st Century. Energies 2022, 15, 311. [Google Scholar] [CrossRef]
- Stępień, Z. A Comprehensive Overview of Hydrogen-Fueled Internal Combustion Engines: Achievements and Future Challenges. Energies 2021, 14, 6504. [Google Scholar] [CrossRef]
- Hanfei, Z.; Ligang, W.; Jan Van, H.; François, M.; Umberto, D. Techno-economic comparison of green ammonia production processes. Appl. Energy 2019, 259, 114135. [Google Scholar] [CrossRef]
- Jianyun, Z.; Li, J.; Yanhong, L.; San Ping, J.; Shuangyin, W. Green Synthesis of Nitrogen-to-Ammonia Fixation: Past, Present, and Future. Energy Environ. Mater. 2021, 5, 452–457. [Google Scholar] [CrossRef]
- Hanchu, W.; Prodromos, D.; Qi, Z. Harnessing the Wind Power of the Ocean with Green Offshore Ammonia. ACS Sustain. Chem. Eng. 2021, 9, 14605–14617. [Google Scholar] [CrossRef]
- Jun, L.; Shini, L.; Danan, C.; Rongjun, W.; Noriyuki, K.; Lisheng, D.; Hongyu, H. A Review on Combustion Characteristics of Ammonia as a Carbon-Free Fuel. Front. Energy Res. 2021, 9, 760356. [Google Scholar] [CrossRef]
- Hideaki, K.; Akihiro, H.; Somarathne, K.D.; Kunkuma, A.; Ekenechukwu, C.O. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Chehade, G.; Dincer, I. Progress in green ammonia production as potential carbon-free fuel. Fuel 2021, 299, 120845. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Shenbagamuthuraman, V.; Patel, A.; Khanna, S.; Banerjee, E.; Parekh, S.; Karthick, C.; Ashok, B.; Velvizhi, G.; Nanthagopal, K.; Ong, H.C. State of art of valorising of diverse potential feedstocks for the production of alcohols and ethers: Current changes and perspectives. Chemosphere 2022, 286, 131587. [Google Scholar] [CrossRef]
- Sarp, S.; Gonzalez Hernandez, S.; Chen, C.; Sheehan, S.W. Alcohol Production from Carbon Dioxide: Methanol as a Fuel and Chemical Feedstock. Joule 2021, 5, 59–76. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ali, O.M.; Sidik, N.A.C.; Yusaf, T.; Kadirgama, K.; Kettner, M. Alcohol and ether as alternative fuels in spark ignition engine: A review. Renew. Sustain. Energy Rev. 2018, 82, 2586–2605. [Google Scholar] [CrossRef]
- Bharath, B.K.; Arul Mozhi Selvan, V. Influence of Higher Alcohol Additives in Methanol–Gasoline Blends on the Performance and Emissions of an Unmodified Automotive SI Engine: A Review. Arab. J. Sci. Eng. 2021, 46, 7057–7085. [Google Scholar] [CrossRef]
- Yusri, I.M.; Mamat, R.; Najafi, G.; Razman, A.; Awad, O.I.; Azmi, W.H.; Ishak, W.F.W.; Shaiful, A.I.M. Alcohol based automotive fuels from first four alcohol family in compression and spark ignition engine: A review on engine performance and exhaust emissions. Renew. Sustain. Energy Rev. 2017, 77, 169–181. [Google Scholar] [CrossRef]
- Andreis, G.S.L.; Vaz, F.A.; De Bortoli, A.L. Bioethanol combustion based on a reduced kinetic mechanism. J. Math. Chem. 2013, 51, 1584–1598. [Google Scholar] [CrossRef]
- Guimaraes, C.; Custodio, D.; de Oliveira, R.; Varandas, L.; Arbilla, G. Comparative study of automotive, aircraft and biogenic emissions of aldehydes and aromatic compounds. Bull. Environ. Contam. Toxicol. 2010, 84, 180–184. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Oßwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol combustion chemistry. Prog. Energy Combust. Sci. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- Feng, B.; Zhai, Y.; Zhang, L. Toward the Mechanism Study of Pd/γ-Al2O3-Assisted Bioalcohol Combustion in a Flow Reactor. Energy Fuels 2021, 35, 14954–14962. [Google Scholar] [CrossRef]
- Su, S.; Ge, Y.; Wang, X.; Zhang, M.; Hao, L.; Tan, J.; Shi, F.; Guo, D.; Yang, Z. Evaluating the In-Service Emissions of High-Mileage Dedicated Methanol-Fueled Passenger Cars: Regulated and Unregulated Emissions. Energies 2020, 13, 2680. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Mei, B.; Li, Y.; Cao, C.; Zou, J.; Yang, J.; Cheng, Z. Experimental and kinetic modeling study of n-propanol and i-propanol combustion: Flow reactor pyrolysis and laminar flame propagation. Combust. Flame 2019, 207, 171–185. [Google Scholar] [CrossRef]
- Frassoldati, A.; Cuoci, A.; Faravelli, T.; Niemann, U.; Ranzi, E.; Seiser, R.; Seshadri, K. An experimental and kinetic modeling study of n-propanol and iso-propanol combustion. Combust. Flame 2010, 157, 2–16. [Google Scholar] [CrossRef]
- Welz, O.; Savee, J.D.; Eskola, A.J.; Sheps, L.; Osborn, D.L.; Taatjes, C.A. Low-temperature combustion chemistry of biofuels: Pathways in the low-temperature (550–700 K) oxidation chemistry of isobutanol and tert-butanol. Proc. Combust. Inst. 2013, 34, 493–500. [Google Scholar] [CrossRef]
- Grana, R.; Frassoldati, A.; Faravelli, T.; Niemann, U.; Ranzi, E.; Seiser, R.; Cattolica, R.; Seshadri, K. An experimental and kinetic modeling study of combustion of isomers of butanol. Combust. Flame 2010, 157, 2137–2154. [Google Scholar] [CrossRef]
- Bai, F.-Y.; Chen, M.-Y.; Liu, X.-H.; Ni, S.; Tang, Y.-Z.; Pan, X.-M.; Zhao, Z. Kinetics and mechanism of OH-mediated degradation of three pentanols in the atmosphere. New J. Chem. 2021, 45, 16543–16556. [Google Scholar] [CrossRef]
- Wang, G.; Yuan, W.; Li, Y.; Zhao, L.; Qi, F. Experimental and kinetic modeling study of n-pentanol pyrolysis and combustion. Combust. Flame 2015, 162, 3277–3287. [Google Scholar] [CrossRef]
- Hashemi, H.; Christensen, J.M.; Glarborg, P. High-pressure pyrolysis and oxidation of ethanol. Fuel 2018, 218, 247–257. [Google Scholar] [CrossRef]
- Geng, P.; Cao, E.; Tan, Q.; Wei, L. Effects of alternative fuels on the combustion characteristics and emission products from diesel engines: A review. Renew. Sustain. Energy Rev. 2017, 71, 523–534. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Bell, A.T.; Toste, F.D. Synthesis of Biomass-Derived Ethers for Use as Fuels and Lubricants. ChemSusChem 2019, 12, 2835–2858. [Google Scholar] [CrossRef]
- Styring, P.; Dowson, G.R.M.; Tozer, I.O. Synthetic Fuels Based on Dimethyl Ether as a Future Non-Fossil Fuel for Road Transport From Sustainable Feedstocks. Front. Energy Res. 2021, 9, 663331. [Google Scholar] [CrossRef]
- Awad, O.I.; Mamat, R.; Ibrahim, T.K.; Hammid, A.T.; Yusri, I.M.; Hamidi, M.A.; Humada, A.M.; Yusop, A.F. Overview of the oxygenated fuels in spark ignition engine: Environmental and performance. Renew. Sustain. Energy Rev. 2018, 91, 394–408. [Google Scholar] [CrossRef]
- Putrasari, Y.; Lim, O. Dimethyl Ether as the Next Generation Fuel to Control Nitrogen Oxides and Particulate Matter Emissions from Internal Combustion Engines: A Review. ACS Omega 2022, 7, 32–37. [Google Scholar] [CrossRef]
- Lin, Q.; Tay, K.L.; Yu, W.; Zong, Y.; Yang, W.; Rivellini, L.-H.; Ma, M.; Lee, A.K.Y. Polyoxymethylene dimethyl ether 3 (PODE3) as an alternative fuel to reduce aerosol pollution. J. Clean. Prod. 2021, 285, 124857. [Google Scholar] [CrossRef]
- Han, D.; Yin, H.; Qian, E.; Ye, L.; Liu, D. Pyrolysis and catalysis of dimethyl ether in a flow reactor. Fuel 2020, 263, 116700. [Google Scholar] [CrossRef]
- Vin, N.; Herbinet, O.; Battin-Leclerc, F. Diethyl ether pyrolysis study in a jet-stirred reactor. J. Anal. Appl. Pyrolysis 2016, 121, 173–176. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, H.; Zuo, Q.; Zheng, Z.; Wang, J.; Yin, W.; Yao, M. Experimental and kinetic modeling studies of polyoxymethylene dimethyl ether (PODE) pyrolysis in jet stirred reactor. J. Anal. Appl. Pyrolysis 2021, 159, 105332. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, H.; Yin, W.; Wang, J.; Li, W.; Wang, Z.; Xing, L.; Gao, X.; Mei, B.; Zhang, Y.; et al. Experimental and kinetic modeling study of di-n-propyl ether and diisopropyl ether combustion: Pyrolysis and laminar flame propagation velocity. Combust. Flame 2022, 237, 111809. [Google Scholar] [CrossRef]
- Serinyel, Z.; Dayma, G.; Glasziou, V.; Lailliau, M.; Dagaut, P. A pyrolysis study on C4–C8 symmetric ethers. Proc. Combust. Inst. 2021, 38, 329–336. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Q.; Ito, S.; Wada, Y. Oxidation characteristics and products of five ethers at low temperature. Fuel 2016, 165, 513–525. [Google Scholar] [CrossRef]
- Guo, H.; Sun, W.; Haas, F.M.; Farouk, T.; Dryer, F.L.; Ju, Y. Measurements of H2O2 in low temperature dimethyl ether oxidation. Proc. Combust. Inst. 2013, 34, 573–581. [Google Scholar] [CrossRef]
- Li, R.; Herreros, J.M.; Tsolakis, A.; Yang, W. Chemical kinetic study on ignition and flame characteristic of polyoxymethylene dimethyl ether 3 (PODE3). Fuel 2020, 279, 118423. [Google Scholar] [CrossRef]
- Andersen, A.; Carter, E.A. Insight into Selected Reactions in Low-Temperature Dimethyl Ether Combustion from Born−Oppenheimer Molecular Dynamics. J. Phys. Chem. A 2006, 110, 1393–1407. [Google Scholar] [CrossRef]
- Zhou, S.; Barnes, I.; Zhu, T.; Klotz, B.; Albu, M.; Bejan, I.; Benter, T. Product Study of the OH, NO3, and O3 Initiated Atmospheric Photooxidation of Propyl Vinyl Ether. Environ. Sci. Technol. 2006, 40, 5415–5421. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, K.; Luis, F.R. Biodiesel fuels. Prog. Energy Combust. Sci. 2016, 58, 36–59. [Google Scholar] [CrossRef]
- Gaur, A.; Dwivedi, G.; Baredar, P.; Jain, S. Influence of blending additives in biodiesel on physiochemical properties, engine performance, and emission characteristics. Fuel 2022, 321, 124072. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, Y.L.; Egolfopoulos, F.N.; Tsotsis, T.T. Fundamental Study of the Oxidation Characteristics and Pollutant Emissions of Model Biodiesel Fuels. Ind. Eng. Chem. Res. 2010, 49, 10392–10398. [Google Scholar] [CrossRef]
- Wallington, T.J.; Hurley, M.D.; Maurer, T.; Barnes, I.; Becker, K.H.; Tyndall, G.S.; Orlando, J.J.; Pimentel, A.S.; Bilde, M. Atmospheric Oxidation Mechanism of Methyl Formate. J. Phys. Chem. A 2001, 105, 5146–5154. [Google Scholar] [CrossRef]
- Li, R.; Wang, Z.; Xu, G. Study on Carbonyl Emissions of Diesel Engine Fueled with Biodiesel. Int. J. Chem. Eng. 2017, 2017, 1409495. [Google Scholar] [CrossRef]
- McCormick, R.L. The impact of biodiesel on pollutant emissions and public health. Inhal. Toxicol. 2007, 19, 1033–1039. [Google Scholar] [CrossRef]
- He, C.; Ge, Y.; Tan, J.; You, K.; Han, X.; Wang, J. Characteristics of polycyclic aromatic hydrocarbons emissions of diesel engine fueled with biodiesel and diesel. Fuel 2010, 89, 2040–2046. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y.; Guan, C.; Cheung, C.S.; Huang, Z. Effect of biodiesel on PAH, OPAH, and NPAH emissions from a direct injection diesel engine. Environ. Sci. Pollut. Res. 2018, 25, 34131–34138. [Google Scholar] [CrossRef] [PubMed]
- Karavalakis, G.; Deves, G.; Fontaras, G.; Stournas, S.; Samaras, Z.; Bakeas, E. The impact of soy-based biodiesel on PAH, nitro-PAH and oxy-PAH emissions from a passenger car operated over regulated and nonregulated driving cycles. Fuel 2010, 89, 3876–3883. [Google Scholar] [CrossRef]
- Das, A.K.; Sahu, S.K.; Panda, A.K. Current status and prospects of alternate liquid transportation fuels in compression ignition engines: A critical review. Renew. Sustain. Energy Rev. 2022, 161, 112358. [Google Scholar] [CrossRef]
- Canakci, M.; Ozsezen, A.N.; Alptekin, E.; Eyidogan, M. Impact of alcohol–gasoline fuel blends on the exhaust emission of an SI engine. Renew. Energy 2013, 52, 111–117. [Google Scholar] [CrossRef]
- Edwin Geo, V.; Jesu Godwin, D.; Thiyagarajan, S.; Saravanan, C.G.; Aloui, F. Effect of higher and lower order alcohol blending with gasoline on performance, emission and combustion characteristics of SI engine. Fuel 2019, 256, 115806. [Google Scholar] [CrossRef]
- Ratcliff, M.A.; Luecke, J.; Williams, A.; Christensen, E.; Yanowitz, J.; Reek, A.; McCormick, R.L. Impact of Higher Alcohols Blended in Gasoline on Light-Duty Vehicle Exhaust Emissions. Environ. Sci. Technol. 2013, 47, 13865–13872. [Google Scholar] [CrossRef]
- Varol, Y.; Öner, C.; Öztop, H.; Altun, Ş. Comparison of methanol, ethanol, or n-butanol blending with unleaded gasoline on exhaust emissions of an SI engine. Energy Sources Part A Recovery Util. Environ. Eff. 2014, 36, 938–948. [Google Scholar] [CrossRef]
- Stansfield, P.A.; Bisordi, A.; OudeNijeweme, D.; Williams, J.; Gold, M.; Ali, R. The performance of a modern vehicle on a variety of alcohol-gasoline fuel blends. SAE Int. J. Fuels Lubr. 2012, 5, 813–822. [Google Scholar] [CrossRef][Green Version]
- Yesilyurt, M.K. A detailed investigation on the performance, combustion, and exhaust emission characteristics of a diesel engine running on the blend of diesel fuel, biodiesel and 1-heptanol (C7 alcohol) as a next-generation higher alcohol. Fuel 2020, 275, 117893. [Google Scholar] [CrossRef]
- Wu, S.; Bao, J.; Wang, Z.; Zhang, H.; Xiao, R. The regulated emissions and PAH emissions of bio-based long-chain ethers in a diesel engine. Fuel Process. Technol. 2021, 214, 106724. [Google Scholar] [CrossRef]
- Gao, W.; Liu, J.; Sun, P.; Yang, C.; Fang, J. Gaseous Emissions and Particle Microstructure Characteristics of PODE/Diesel Blend Fuel. Int. J. Automot. Technol. 2019, 20, 607–617. [Google Scholar] [CrossRef]
- Venugopal, T.; Ramesh, A. Performance, combustion and emission characteristics of a spark-ignition engine with simultaneous injection of n-butanol and gasoline in comparison to blended butanol and gasoline. Int. J. Energy Res. 2013, 38, 1060–1074. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, P.; Liu, Y. Investigation on combustion and emission performance of a common rail diesel engine fueled with diesel-ethylene glycol dual fuel. Appl. Therm. Eng. 2018, 142, 43–55. [Google Scholar] [CrossRef]
- Nour, M.; Attia, A.M.A.; Nada, S.A. Combustion, performance and emission analysis of diesel engine fuelled by higher alcohols (butanol, octanol and heptanol)/diesel blends. Energy Convers. Manag. 2019, 185, 313–329. [Google Scholar] [CrossRef]
- Nayak, S.K.; Behera, G.R.; Mishra, P.C.; Sahu, S.K. Biodiesel vs diesel: A race for the future. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 1453–1460. [Google Scholar] [CrossRef]
- Imdadul, H.K.; Zulkifli, N.W.M.; Masjuki, H.H.; Kalam, M.A.; Kamruzzaman, M.; Rashed, M.M.; Rashedul, H.K.; Alwi, A. Experimental assessment of non-edible candlenut biodiesel and its blend characteristics as diesel engine fuel. Environ. Sci. Pollut. Res. 2017, 24, 2350–2363. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Wang, J.; Tan, D.; Zhang, Z.; Yang, D. Effects of Different Biodiesel-Diesel Blend Fuel on Combustion and Emission Characteristics of a Diesel Engine. Processes 2021, 9, 1984. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, X.; Zhong, X.; Hu, B.; Liu, H.; Yao, M. Experimental study on the combustion and emissions fueling biodiesel/n-butanol, biodiesel/ethanol and biodiesel/2,5-dimethylfuran on a diesel engine. Energy 2016, 115, 539–549. [Google Scholar] [CrossRef]
- Pérez, A.; Mateos, D.; García, C.; Caraveo, C.; Montero, G.; Coronado, M.; Valdez, B. Quantitative Evaluation of the Emissions of a Transport Engine Operating with Diesel-Biodiesel. Energies 2020, 13, 3594. [Google Scholar] [CrossRef]
- Alptekin, E. Emission, injection and combustion characteristics of biodiesel and oxygenated fuel blends in a common rail diesel engine. Energy 2017, 119, 44–52. [Google Scholar] [CrossRef]
- Devasani, S.K.R.; Vodnala, S.; Singarapu, D.; Nair, J.N. A comprehensive review on performance, combustion and emissions of ternary and quaternary biodiesel/diesel blends. Environ. Sci. Pollut. Res. 2021, 29, 51083–51094. [Google Scholar] [CrossRef] [PubMed]
- Bari, S. Performance, combustion and emission tests of a metro-bus running on biodiesel-ULSD blended (B20) fuel. Appl. Energy 2014, 124, 35–43. [Google Scholar] [CrossRef]
- Di, Y.; Cheung, C.S.; Huang, Z. Experimental investigation on regulated and unregulated emissions of a diesel engine fueled with ultra-low sulfur diesel fuel blended with biodiesel from waste cooking oil. Sci. Total Environ. 2008, 407, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Mariappan, M.; Panithasan, M.S.; Venkadesan, G. Pyrolysis plastic oil production and optimisation followed by maximum possible replacement of diesel with bio-oil/methanol blends in a CRDI engine. J. Clean. Prod. 2021, 312, 127687. [Google Scholar] [CrossRef]
- Mangesh, V.L.; Padmanabhan, S.; Tamizhdurai, P.; Ramesh, A. Experimental investigation to identify the type of waste plastic pyrolysis oil suitable for conversion to diesel engine fuel. J. Clean. Prod. 2020, 246, 119066. [Google Scholar] [CrossRef]
- Prakash, S.; Abraham Baby, S.J.; Manikandan, K. Emission study of alcohol–biodiesel blends propelled diesel engine. Int. J. Ambient Energy 2021, 42, 292–296. [Google Scholar] [CrossRef]
- Devarajan, Y.; Munuswamy, D.B.; Nagappan, B.; Pandian, A.K. Performance, combustion and emission analysis of mustard oil biodiesel and octanol blends in diesel engine. Heat Mass Transf. 2018, 54, 1803–1811. [Google Scholar] [CrossRef]
- Roy, M.M.; Calder, J.; Wang, W.; Mangad, A.; Diniz, F.C.M. Cold start idle emissions from a modern Tier-4 turbo-charged diesel engine fueled with diesel-biodiesel, diesel-biodiesel-ethanol, and diesel-biodiesel-diethyl ether blends. Appl. Energy 2016, 180, 52–65. [Google Scholar] [CrossRef]
- Jia, D.; Deng, X.; Lei, J. Analysis on the impact of biodiesel–ethanol–diesel fuel on the performance and emissions of a diesel engine. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 3013–3025. [Google Scholar] [CrossRef]
- Nagpure, A.G.; Chavan, S.B.; Rathod, W.S. Effects of diesel-Calophyllum Inophyllum biodiesel-propyl alcohol blends on performance, emissions and combustion of diesel engine. Environ. Prog. Sustain. Energy 2022, 41, e13834. [Google Scholar] [CrossRef]
- Yesilyurt, M.K.; Eryilmaz, T.; Arslan, M. A comparative analysis of the engine performance, exhaust emissions and combustion behaviors of a compression ignition engine fuelled with biodiesel/diesel/1-butanol (C4 alcohol) and biodiesel/diesel/n-pentanol (C5 alcohol) fuel blends. Energy 2018, 165, 1332–1351. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, Q.; Liang, J.; Yang, C. The performance and emissions characteristics of diesel/biodiesel/alcohol blends in a diesel engine. Energy Rep. 2021, 7, 1016–1024. [Google Scholar] [CrossRef]
- Seela, C.R.; Alagumalai, A.; Pugazhendhi, A. Evaluating the feasibility of diethyl ether and isobutanol added Jatropha Curcas biodiesel as environmentally friendly fuel blends. Sustain. Chem. Pharm. 2020, 18, 100340. [Google Scholar] [CrossRef]
- Galloni, E.; Fontana, G.; Staccone, S.; Scala, F. Performance analyses of a spark-ignition engine firing with gasoline–butanol blends at partial load operation. Energy Convers. Manag. 2016, 110, 319–326. [Google Scholar] [CrossRef]
- Noorollahi, Y.; Azadbakht, M.; Ghobadian, B. The effect of different diesterol (diesel–biodiesel–ethanol) blends on small air-cooled diesel engine performance and its exhaust gases. Energy 2018, 142, 196–200. [Google Scholar] [CrossRef]
- Kumar, S.; Prakash, R.; Murugan, S.; Singh, R.K. Performance and emission analysis of blends of waste plastic oil obtained by catalytic pyrolysis of waste HDPE with diesel in a CI engine. Energy Convers. Manag. 2013, 74, 323–331. [Google Scholar] [CrossRef]
- Datta, A.; Mandal, B.K. Numerical investigation of the performance and emission parameters of a diesel engine fuelled with diesel-biodiesel-methanol blends. J. Mech. Sci. Technol. 2016, 30, 1923–1929. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Tao, H.; Bagaber, S.A.; Ali, O.M.; Kamil, M.; Ma, X.; Awad, O.I. Prediction of emissions and performance of a gasoline engine running with fusel oil–gasoline blends using response surface methodology. Fuel 2019, 253, 1–14. [Google Scholar] [CrossRef]
- Li, Y.; Gong, J.; Deng, Y.; Yuan, W.; Fu, J.; Zhang, B. Experimental comparative study on combustion, performance and emissions characteristics of methanol, ethanol and butanol in a spark ignition engine. Appl. Therm. Eng. 2017, 115, 53–63. [Google Scholar] [CrossRef]
- Ozsezen, A.N.; Canakci, M. Performance and combustion characteristics of alcohol–gasoline blends at wide-open throttle. Energy 2011, 36, 2747–2752. [Google Scholar] [CrossRef]
- Balan, K.; Yashvanth, U.; Booma Devi, P.; Arvind, T.; Nelson, H.; Devarajan, Y. Investigation on emission characteristics of alcohol biodiesel blended diesel engine. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1879–1889. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Prashumn; Chandra, K. Di-ethyl ether-diesel blends fuelled off-road tractor engine: Part-I: Technical feasibility. Fuel 2022, 308, 121972. [Google Scholar] [CrossRef]
- Yilmaz, N.; Ileri, E.; Atmanli, A. Performance of biodiesel/higher alcohols blends in a diesel engine. Int. J. Energy Res. 2016, 40, 1134–1143. [Google Scholar] [CrossRef]
- Razak, N.H.; Hashim, H.; Yunus, N.A.; Klemeš, J.J. Reducing diesel exhaust emissions by optimisation of alcohol oxygenates blend with diesel/biodiesel. J. Clean. Prod. 2021, 316, 128090. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Singh, A.P.; Gupta, T.; Agarwal, R.A.; Sharma, N.; Pandey, S.K.; Ateeq, B. Toxicity of exhaust particulates and gaseous emissions from gasohol (ethanol blended gasoline)-fuelled spark ignition engines. Environ. Sci. Process. Impacts 2020, 22, 1540–1553. [Google Scholar] [CrossRef]
- Nabi, M.N.; Zare, A.; Hossain, F.M.; Ristovski, Z.D.; Brown, R.J. Reductions in diesel emissions including PM and PN emissions with diesel-biodiesel blends. J. Clean. Prod. 2017, 166, 860–868. [Google Scholar] [CrossRef]
- Tsai, J.H.; Chen, S.J.; Huang, K.L.; Lin, W.Y.; Lee, W.J.; Lin, C.C.; Hsieh, L.T.; Chiu, J.Y.; Kuo, W.C. Emissions from a generator fueled by blends of diesel, biodiesel, acetone, and isopropyl alcohol: Analyses of emitted PM, particulate carbon, and PAHs. Sci. Total Environ. 2013, 466, 195–202. [Google Scholar] [CrossRef]
- Tüccar, G.; Ozgür, T.; Aydın, K. Effect of diesel–microalgae biodiesel–butanol blends on performance and emissions of diesel engine. Fuel 2014, 132, 47–52. [Google Scholar] [CrossRef]
- Zhu, L.; Xiao, Y.; Cheung, C.S.; Guan, C.; Huang, Z. Combustion, gaseous and particulate emission of a diesel engine fueled with n-pentanol (C5 alcohol) blended with waste cooking oil biodiesel. Appl. Therm. Eng. 2016, 102, 73–79. [Google Scholar] [CrossRef]
- Zhang, Z.; Balasubramanian, R. Investigation of particulate emission characteristics of a diesel engine fueled with higher alcohols/biodiesel blends. Appl. Energy 2016, 163, 71–80. [Google Scholar] [CrossRef]
- Liu, H.; Ma, X.; Li, B.; Chen, L.; Wang, Z.; Wang, J. Combustion and emission characteristics of a direct injection diesel engine fueled with biodiesel and PODE/biodiesel fuel blends. Fuel 2017, 209, 62–68. [Google Scholar] [CrossRef]
- Seeniappan, K.; Venkatesan, B.; Krishnan, N.N.; Kandhasamy, T.; Arunachalam, S.; Seeta, R.K.; Depoures, M.V. A comparative assessment of performance and emission characteristics of a DI diesel engine fuelled with ternary blends of two higher alcohols with lemongrass oil biodiesel and diesel fuel. Energy Environ. 2021, 33, 1134–1159. [Google Scholar] [CrossRef]
- Emiroğlu, A.O.; Şen, M. Combustion, performance and exhaust emission characterizations of a diesel engine operating with a ternary blend (alcohol-biodiesel-diesel fuel). Appl. Therm. Eng. 2018, 133, 371–380. [Google Scholar] [CrossRef]
- Mancebo, S.E.; Wang, S.Q. Recognizing the impact of ambient air pollution on skin health. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2326–2332. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Skrzypek, M.; Kowalski, M.; Cyrys, J. Effect of NOx and NO2 Concentration Increase in Ambient Air to Daily Bronchitis and Asthma Exacerbation, Silesian Voivodeship in Poland. Int. J. Environ. Res. Public Health 2020, 17, 754. [Google Scholar] [CrossRef]
- Grout, L.; Baker, M.G.; French, N.; Hales, S. A Review of Potential Public Health Impacts Associated With the Global Dairy Sector. GeoHealth 2020, 4, e2019GH000213. [Google Scholar] [CrossRef] [PubMed]
- Hossain, R.; Hassan, K.; Sahajwalla, V. Utilising problematic waste to detect toxic gas release in the environment: Fabricating a NiO doped CuO nanoflake based ammonia sensor from e-waste. Nanoscale Adv. 2022, 4, 4066–4079. [Google Scholar] [CrossRef]
- Abdelfattah, I.; El-Saied, F.A.; Almedolab, A.A.; El-Shamy, A.M. Biosorption as a Perfect Technique for Purification of Wastewater Contaminated with Ammonia. Appl. Biochem. Biotechnol. 2022, 194, 4105–4134. [Google Scholar] [CrossRef]
- Hong, S.-H.; Szili, E.J.; Fenech, M.; Gaur, N.; Short, R.D. Genotoxicity and cytotoxicity of the plasma jet-treated medium on lymphoblastoid WIL2-NS cell line using the cytokinesis block micronucleus cytome assay. Sci. Rep. 2017, 7, 3854. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, N.; Soeteman-Hernández, L.G.; Carrière, M.; Boyles, M.; McLean, P.; Catalán, J.; Katsumiti, A.; Cabellos, J.; Delpivo, C.; Sánchez Jiménez, A.; et al. The State of the Art and Challenges of In Vitro Methods for Human Hazard Assessment of Nanomaterials in the Context of Safe-by-Design. Nanomaterials 2023, 13, 472. [Google Scholar] [CrossRef]
- Xia, M.; Huang, R.; Witt, K.L.; Southall, N.; Fostel, J.; Cho, M.-H.; Jadhav, A.; Smith, C.S.; Inglese, J.; Portier, C.J.; et al. Compound cytotoxicity profiling using quantitative high-throughput screening. Environ. Health Perspect. 2008, 116, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Hawley, B.; L’Orange, C.; Olsen, D.B.; Marchese, A.J.; Volckens, J. Oxidative stress and aromatic hydrocarbon response of human bronchial epithelial cells exposed to petro- or biodiesel exhaust treated with a diesel particulate filter. Toxicol. Sci. 2014, 141, 505–514. [Google Scholar] [CrossRef]
- Gerlofs-Nijland, M.E.; Totlandsdal, A.I.; Tzamkiozis, T.; Leseman, D.L.A.C.; Samaras, Z.; Låg, M.; Schwarze, P.; Ntziachristos, L.; Cassee, F.R. Cell toxicity and oxidative potential of engine exhaust particles: Impact of using particulate filter or biodiesel fuel blend. Environ. Sci. Technol. 2013, 41, 5931–5938. [Google Scholar] [CrossRef]
- Jalava, P.I.; Aakko-Saksa, P.; Murtonen, T.; Happo, M.S.; Markkanen, A.; Yli-Pirilä, P.; Hakulinen, P.; Hillamo, R.; Mäki-Paakkanen, J.; Salonen, R.O.; et al. Toxicological properties of emission particles from heavy duty engines powered by conventional and bio-based diesel fuels and compressed natural gas. Part. Fibre Toxicol. 2012, 9, 37. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Lankoff, A.; Brzoska, K.; Czarnocka, J.; Kowalska, M.; Lisowska, H.; Mruk, R.; Øvrevik, J.; Wegierek-Ciuk, A.; Zuberek, M.; Kruszewski, M. A comparative analysis of in vitro toxicity of diesel exhaust particles from combustion of 1st- and 2nd-generation biodiesel fuels in relation to their physicochemical properties-the FuelHealth project. Environ. Sci. Pollut. Res. 2017, 24, 19357–19374. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.-F.; Wu, T.; Wang, B.; Wei, Y.-Y.; Kong, Q.-X.; Ye, Y.; Yin, H.-F.; Li, J.-B. The Regulation of Seventeen Inflammatory Mediators are Associated with Patient Outcomes in Severe Fever with Thrombocytopenia Syndrome. Sci. Rep. 2018, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.-K.; Saad Eldin, W.F.; El-Ghareeb, W.R.; Elhelaly, A.E.; Khedr, M.H.E.; Li, X.; Huang, X.-C. Effects of Pyrene on Human Liver HepG2 Cells: Cytotoxicity, Oxidative Stress, and Transcriptomic Changes in Xenobiotic Metabolizing Enzymes and Inflammatory Markers with Protection Trial Using Lycopene. BioMed Res. Int. 2019, 2019, 7604851. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Tapanainen, M.; Kuuspalo, K.; Markkanen, A.; Hakulinen, P.; Happo, M.S.; Pennanen, A.S.; Ihalainen, M.; Yli-Pirilä, P.; Makkonen, U. Toxicological effects of emission particles from fossil-and biodiesel-fueled diesel engine with and without DOC/POC catalytic converter. Inhal. Toxicol. 2010, 22, 48–58. [Google Scholar] [CrossRef]
- Fukagawa, N.K.; Li, M.; Poynter, M.E.; Palmer, B.C.; Parker, E.; Kasumba, J.; Holmén, B.A. Soy biodiesel and petrodiesel emissions differ in size, chemical composition and stimulation of inflammatory responses in cells and animals. Environ. Sci. Technol. 2013, 47, 12496–12504. [Google Scholar] [CrossRef]
- Martin, N.R.; Kelley, P.; Klaski, R.; Bosco, A.; Moore, B.; Traviss, N. Characterization and comparison of oxidative potential of real-world biodiesel and petroleum diesel particulate matter emitted from a nonroad heavy duty diesel engine. Sci. Total Environ. 2019, 655, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Hemmingsen, J.G.; Møller, P.; Nøjgaard, J.K.; Roursgaard, M.; Loft, S. Oxidative stress, genotoxicity, and vascular cell adhesion molecule expression in cells exposed to particulate matter from combustion of conventional diesel and methyl ester biodiesel blends. Environ. Sci. Technol. 2011, 45, 8545–8551. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.; Stevanovic, S.; Banks, A.P.; Zare, A.; Rahman, M.M.; Bowman, R.V.; Fong, K.M.; Ristovski, Z.D.; Yang, I.A. The cytotoxic, inflammatory and oxidative potential of coconut oil-substituted diesel emissions on bronchial epithelial cells at an air-liquid interface. Environ. Sci. Pollut. Res. 2019, 26, 27783–27791. [Google Scholar] [CrossRef]
- Skuland, T.S.; Refsnes, M.; Magnusson, P.; Oczkowski, M.; Gromadzka-Ostrowska, J.; Kruszewski, M.; Mruk, R.; Myhre, O.; Lankoff, A.; Øvrevik, J. Proinflammatory effects of diesel exhaust particles from moderate blend concentrations of 1st and 2nd generation biodiesel in BEAS-2B bronchial epithelial cells—The FuelHealth project. Environ. Toxicol. Pharmacol. 2017, 52, 138–142. [Google Scholar] [CrossRef]
- Steiner, S.; Czerwinski, J.; Comte, P.; Popovicheva, O.; Kireeva, E.; Müller, L.; Heeb, N.; Mayer, A.; Fink, A.; Rothen-Rutishauser, B. Comparison of the toxicity of diesel exhaust produced by bio-and fossil diesel combustion in human lung cells in vitro. Atmos. Environ. 2013, 81, 380–388. [Google Scholar] [CrossRef]
- Betha, R.; Pavagadhi, S.; Sethu, S.; Hande, M.P.; Balasubramanian, R. Comparative in vitro cytotoxicity assessment of airborne particulate matter emitted from stationary engine fuelled with diesel and waste cooking oil-derived biodiesel. Atmos. Environ. 2012, 61, 23–29. [Google Scholar] [CrossRef]
- Vaughan, A.; Stevanovic, S.; Jafari, M.; Bowman, R.V.; Fong, K.M.; Ristovski, Z.D.; Yang, I.A. Primary human bronchial epithelial cell responses to diesel and biodiesel emissions at an air-liquid interface. Toxicol. Vitr. 2019, 57, 67–75. [Google Scholar] [CrossRef]
- Barabadi, H.; Najafi, M.; Samadian, H.; Azarnezhad, A.; Vahidi, H.; Mahjoub, M.A.; Koohiyan, M.; Ahmadi, A. A Systematic Review of the Genotoxicity and Antigenotoxicity of Biologically Synthesized Metallic Nanomaterials: Are Green Nanoparticles Safe Enough for Clinical Marketing? Medicina 2019, 55, 439. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.A.; Nelson, G.B.; Mutlu, E.; Warren, S.H.; Gilmour, M.I.; DeMarini, D.M. DNA adducts induced by in vitro activation of extracts of diesel and biodiesel exhaust particles. Inhal. Toxicol. 2015, 31, 167. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Wegierek-Ciuk, A.; Brzoska, K.; Wojewodzka, M.; Meczynska-Wielgosz, S.; Gromadzka-Ostrowska, J.; Mruk, R.; Øvrevik, J.; Kruszewski, M.; Lankoff, A. Genotoxic potential of diesel exhaust particles from the combustion of first-and second-generation biodiesel fuels—The FuelHealth project. Environ. Sci. Pollut. Res. 2017, 24, 24223–24234. [Google Scholar] [CrossRef]
- Wronski, S.; Dannenmaier, J.; Schild, S.; Macke, O.; Müller, L.; Burmeister, Y.; Seilheimer, B.; Müller, M. Engystol reduces onset of experimental respiratory syncytial virus-induced respiratory inflammation in mice by modulating macrophage phagocytic capacity. PLoS ONE 2018, 13, e0195822. [Google Scholar] [CrossRef]
- Hirota, J.A.; Marchant, D.J.; Singhera, G.K.; Moheimani, F.; Dorscheid, D.R.; Carlsten, C.; Sin, D.; Knight, D. Urban particulate matter increases human airway epithelial cell IL-1β secretion following scratch wounding and H1N1 influenza A exposure in vitro. Exp. Lung Res. 2015, 41, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Mullins, B.J.; Kicic, A.; Ling, K.-M.; Mead-Hunter, R.; Larcombe, A.N. Biodiesel exhaust–induced cytotoxicity and proinflammatory mediator production in human airway epithelial cells. Environ. Toxicol. 2016, 31, 44–57. [Google Scholar] [CrossRef]
- Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; O’Leary, R.A.; Kicic, A.; Mullins, B.J.; Larcombe, A.N. Soy Biodiesel Exhaust is More Toxic than Mineral Diesel Exhaust in Primary Human Airway Epithelial Cells. Environ. Sci. Technol. 2019, 53, 11437–11446. [Google Scholar] [CrossRef]
- Swanson, K.J. Release of the pro-inflammatory markers IL-8 & IL-6 by BEAS-2B cells following in vitro exposure to biodiesel extracts. Open Toxicol. J. 2006, 3, 8–15. [Google Scholar] [CrossRef]
- Traviss, N.; Li, M.; Lombard, M.; Thelen, B.A.; Palmer, B.C.; Poynter, M.E.; Mossman, B.T.; Holmén, B.A.; Fukagawa, N.K. Petrodiesel and waste grease biodiesel (B20) emission particles at a rural recycling center: Characterization and effects on lung epithelial cells and macrophages. Air Qual. Atmos. Health 2014, 7, 59–70. [Google Scholar] [CrossRef]
- Cervena, T.; Rossnerova, A.; Sikorova, J.; Beranek, V.; Vojtisek-Lom, M.; Ciganek, M.; Topinka, J.; Rossner, P., Jr. DNA damage potential of engine emissions measured in vitro by micronucleus test in human bronchial epithelial cells. Basic Clin. Pharmacol. Toxicol. 2017, 121, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Barraud, C.; Corbière, C.; Pottier, I.; Estace, E.; Blanchard, K.; Logie, C.; Lagadu, S.; Kéravec, V.; Pottier, D.; Dionnet, F. Impact of after-treatment devices and biofuels on diesel exhausts genotoxicity in A549 cells exposed at air-liquid interface. Toxicol. Vitr. 2017, 45, 426–433. [Google Scholar] [CrossRef]
- Nieto Marin, V.; Echavarria Mazo, L.V.; Londono Berrio, M.; Orozco Jimenez, L.Y.; Estrada Velez, V.; Pablo Isaza, J.; Cristina Ortiz-Trujillo, I. Genotoxicity of organic material extracted from particulate matter of alternative fuels. Environ. Sci. Pollut. Res. 2021, 28, 17844–17852. [Google Scholar] [CrossRef]
- McCaffery, C.; Zhu, H.W.; Ahmed, C.M.S.; Canchola, A.; Chen, J.Y.; Li, C.G.; Johnson, K.C.; Durbin, T.D.; Lin, Y.H.; Karavalakis, G. Effects of hydrogenated vegetable oil (HVO) and HVO/biodiesel blends on the physicochemical and toxicological properties of emissions from an off-road heavy-duty diesel engine. Fuel 2022, 323, 124283. [Google Scholar] [CrossRef]
- Juárez-Facio, A.T.; Rogez-Florent, T.; Méausoone, C.; Castilla, C.; Mignot, M.; Devouge-Boyer, C.; Lavanant, H.; Afonso, C.; Morin, C.; Merlet-Machour, N.; et al. Ultrafine Particles Issued from Gasoline-Fuels and Biofuel Surrogates Combustion: A Comparative Study of the Physicochemical and In Vitro Toxicological Effects. Toxics 2022, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; Brooks, P.; King, A.; O’Leary, R.A.; Kicic, A.; Mullins, B.J.; Larcombe, A.N. Fuel feedstock determines biodiesel exhaust toxicity in a human airway epithelial cell exposure model. J. Hazard. Mater. 2021, 420, 126637. [Google Scholar] [CrossRef] [PubMed]
- Ke, S.; Liu, Q.; Yao, Y.; Zhang, X.; Sui, G. An in vitro cytotoxicities comparison of 16 priority polycyclic aromatic hydrocarbons in human pulmonary alveolar epithelial cells HPAEpiC. Toxicol. Lett. 2018, 290, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Bünger, J.; Krahl, J.; Franke, H.-U.; Munack, A.; Hallier, E. Mutagenic and cytotoxic effects of exhaust particulate matter of biodiesel compared to fossil diesel fuel. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1998, 415, 13–23. [Google Scholar] [CrossRef]
- Landwehr, K.R.; Hillas, J.; Mead-Hunter, R.; King, A.; O’Leary, R.A.; Kicic, A.; Mullins, B.J.; Larcombe, A.N. Toxicity of different biodiesel exhausts in primary human airway epithelial cells grown at air-liquid interface. Sci. Total Environ. 2022, 832, 155016. [Google Scholar] [CrossRef]
- Faber, S.C.; McNabb, N.A.; Ariel, P.; Aungst, E.R.; McCullough, S.D. Exposure Effects Beyond the Epithelial Barrier: Transepithelial Induction of Oxidative Stress by Diesel Exhaust Particulates in Lung Fibroblasts in an Organotypic Human Airway Model. Toxicol. Sci. 2020, 177, 140–155. [Google Scholar] [CrossRef]
- Posma, J.J.; Kilinç, E.; Borissoff, J.I.; Haegens, A.; Jedynska, A.D.; Ten Cate, H.; Kooter, I.M.; Spronk, H.M. Abstract 599: Exposure to Biodiesel Exhaust Triggers Atherosclerotic Plaque Destabilisation through Increased Apoptosis of Vascular Smooth Muscle Cells in the Arterial Vessel Wall. Arterioscler. Thromb. Vasc. Biol. 2018, 38, A599. [Google Scholar] [CrossRef]
- Martens, D.S.; Cox, B.; Janssen, B.G.; Clemente, D.B.; Nawrot, T.S. Prenatal Air Pollution and Newborns’ Predisposition to Accelerated Biological Aging EDITORIAL COMMENT. Obstet. Gynecol. Surv. 2018, 73, 259–260. [Google Scholar] [CrossRef]
- Sears, C.G.; Sears, L.; Zierold, K.M. Sex differences in the association between exposure to indoor particulate matter and cognitive control among children (age 6–14 years) living near coal-fired power plants. Neurotoxicol. Teratol. 2020, 78, 106855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zink, F.; Hezel, F.; Vogt, J.; Wachter, U.; Wepler, M.; Loconte, M.; Kranz, C.; Hellmann, A.; Mizaikoff, B.; et al. Metabolic substrate utilization in stress-induced immune cells. Intensive Care Med. Exp. 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sarangi, P.P. Inflammation driven metabolic regulation and adaptation in macrophages. Clin. Immunol. 2022, 246, 109216. [Google Scholar] [CrossRef] [PubMed]
- Sangani, R.G.; Deepak, V.; Anwar, J.; Patel, Z.; Ghio, A.J. Cigarette Smoking, and Blood Monocyte Count Correlate with Chronic Lung Injuries and Mortality. Int. J. Chronic Obstr. Pulm. Dis. 2023, 18, 431–446. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, E.; Wasserman, G.A.; Morgan, M.; O’Carroll, D.; Ramirez, N.-G.P.; Gummuluru, S.; Rah, J.Y.; Gower, A.C.; Ieong, M.; Quinton, L.J.; et al. The RNA uridyltransferase Zcchc6 is expressed in macrophages and impacts innate immune responses. PLoS ONE 2017, 12, e0179797. [Google Scholar] [CrossRef]
- Gali, N.K.; Yang, F.; Cheung, C.S.; Ning, Z. A comparative analysis of chemical components and cell toxicity properties of solid and semi-volatile PM from diesel and biodiesel blend. J. Aerosol Sci. 2017, 111, 51–64. [Google Scholar] [CrossRef]
- Bhavaraju, L.; Shannahan, J.; William, A.; McCormick, R.; McGee, J.; Kodavanti, U.; Madden, M. Diesel and biodiesel exhaust particle effects on rat alveolar macrophages with in vitro exposure. Chemosphere 2013, 104, 126–133. [Google Scholar] [CrossRef]
- Vogel, C.F.A.; Kado, S.Y.; Kobayashi, R.; Liu, X.; Wong, P.; Na, K.; Durbin, T.; Okamoto, R.A.; Kado, N.Y. Inflammatory marker and aryl hydrocarbon receptor-dependent responses in human macrophages exposed to emissions from biodiesel fuels. Chemosphere 2018, 220, 993–1002. [Google Scholar] [CrossRef]
- Tooker, B.C.; Quinn, K.; Armstrong, M.; Bauer, A.K.; Reisdorph, N. Comparing the effects of an exposure to a polycyclic aromatic hydrocarbon mixture versus individual polycyclic aromatic hydrocarbons during monocyte to macrophage differentiation: Mixture exposure results in altered immune metrics. J. Appl. Toxicol. 2021, 41, 1568–1583. [Google Scholar] [CrossRef]
- Soriano, J.A.; García-Contreras, R.; de la Fuente, J.; Armas, O.; Orozco-Jiménez, L.Y.; Agudelo, J.R. Genotoxicity and mutagenicity of particulate matter emitted from diesel, gas to liquid, biodiesel, and farnesane fuels: A toxicological risk assessment. Fuel 2020, 282, 118763. [Google Scholar] [CrossRef]
- Gioda, A.; Rodríguez-Cotto, R.I.; Amaral, B.S.; Encarnación-Medina, J.; Ortiz-Martínez, M.G.; Jiménez-Vélez, B.D. Biodiesel from soybean promotes cell proliferation in vitro. Toxicol. Vitr. 2016, 34, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Nakai, K.; Tsuruta, D. What Are Reactive Oxygen Species, Free Radicals, and Oxidative Stress in Skin Diseases? Int. J. Mol. Sci. 2021, 22, 10799. [Google Scholar] [CrossRef] [PubMed]
- Skowroń, J.; Miranowicz-Dzierzawska, K.; Zapor, L. Cytotoxicity of biofuels produced by esterification of waste materials: Vegetable oils or animal fats on A431 skin cells. Toxicol. Lett. 2015, 238, S345. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Singh, A.P.; Gupta, T.; Agarwal, R.A.; Sharma, N.; Rajput, P.; Pandey, S.K.; Ateeq, B. Mutagenicity and Cytotoxicity of Particulate Matter Emitted from Biodiesel-Fueled Engines. Environ. Sci. Technol. 2018, 52, 14496–14507. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, D.G.S.M.; da Silva, N.D.G.; Marcarini, J.C.; Mantovani, M.S.; Marin-Morales, M.A.; Martinez, C.B.R. Cytotoxic, biochemical and genotoxic effects of biodiesel produced by different routes on ZFL cell line. Toxicol. Vitr. 2014, 28, 1117–1125. [Google Scholar] [CrossRef]
- Arias, S.; Estrada, V.; Ortiz, I.C.; Molina, F.J.; Agudelo, J.R. Biological toxicity risk assessment of two potential neutral carbon diesel fuel substitutes. Environ. Pollut. 2022, 308, 119677. [Google Scholar] [CrossRef]
- Martin, N.; Lombard, M.; Jensen, K.R.; Kelley, P.; Pratt, T.; Traviss, N. Effect of biodiesel fuel on “real-world”, nonroad heavy duty diesel engine particulate matter emissions, composition and cytotoxicity. Sci. Total Environ. 2017, 586, 409–418. [Google Scholar] [CrossRef]
- Mutlu, E.; Warren, S.H.; Matthews, P.P.; Schmid, J.E.; Kooter, I.M.; Linak, W.P.; Ian Gilmour, M.; DeMarini, D.M. Health effects of soy-biodiesel emissions: Bioassay-directed fractionation for mutagenicity. Inhal. Toxicol. 2015, 27, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Novotná, B.; Sikorová, J.; Milcová, A.; Pechout, M.; Dittrich, L.; Vojtíšek-Lom, M.; Rossner Jr, P.; Brzicová, T.; Topinka, J. The genotoxicity of organic extracts from particulate truck emissions produced at various engine operating modes using diesel or biodiesel (B100) fuel: A pilot study. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2019, 845, 403034. [Google Scholar] [CrossRef] [PubMed]
- Brito, J.M.; Belotti, L.; Toledo, A.C.; Antonangelo, L.; Silva, F.S.; Alvim, D.S.; Andre, P.A.; Saldiva, P.H.N.; Rivero, D.H.R.F. Acute cardiovascular and inflammatory toxicity induced by inhalation of diesel and biodiesel exhaust particles. Toxicol. Sci. 2010, 116, 67–78. [Google Scholar] [CrossRef]
- Botero, M.L.; Mendoza, C.; Arias, S.; Hincapié, O.D.; Agudelo, J.R.; Ortiz, I.C. In vitro evaluation of the cytotoxicity, mutagenicity and DNA damage induced by particle matter and gaseous emissions from a medium-duty diesel vehicle under real driving conditions using palm oil biodiesel blends. Environ. Pollut. 2020, 265, 115034. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Lin, T.-C.; Wang, Y.-J.; Ho, W.-L. Biological toxicities of emissions from an unmodified engine fueled with diesel and biodiesel blend. J. Environ. Sci. Health Part A 2008, 43, 1735–1743. [Google Scholar] [CrossRef]
- Libalova, H.; Vrbova, K.; Brzicova, T.; Sikorova, J.; Milcova, A.; Vojtisek Lom, M.; Beranek, V.; Klema, J.; Neca, J.; Ciganek, M.; et al. Comparative analysis of the toxic responses of organic extracts from diesel/biodiesel engine emissions in human lung BEAS-2B cells. Toxicol. Lett. 2016, 258, S281–S282. [Google Scholar] [CrossRef]
- Leme, D.M.; Grummt, T.; Heinze, R.; Sehr, A.; Skerswetat, M.; de Marchi, M.R.R.; Machado, M.C.; de Oliveira, D.P.; Marin-Morales, M.A. Cytotoxicity of water-soluble fraction from biodiesel and its diesel blends to human cell lines. Ecotoxicol. Environ. Saf. 2011, 74, 2148–2155. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Zhang, Q.; Liang, Y.; Li, C.-Q.; Lai, C.-C.; Huang, W.-B.; Wu, D.-S. Comparative study on the genotoxicity of gasoline-fueled vehicle exhaust and methanol-fueled vehicle exhaust. J. Sichuan Univ. Med. Sci. Ed. 2005, 36, 249–252. [Google Scholar]
- Liang, Y.; Zhan, L.; Zhang, Z.; Zhang, H.; Zeng, X.; Gou, X.; Lin, C.; Cai, C.; Shao, X.; Shao, G.; et al. Genotoxicity comparison between gasoline- and methanol-fueled exhaust by TK gene mutation assay. J. Biomed. Eng. 2005, 22, 347–350. [Google Scholar]
- Muñoz, M.; Heeb, N.V.; Haag, R.; Honegger, P.; Zeyer, K.; Mohn, J.; Comte, P.; Czerwinski, J. Bioethanol Blending Reduces Nanoparticle, PAH, and Alkyl- and Nitro-PAH Emissions and the Genotoxic Potential of Exhaust from a Gasoline Direct Injection Flex-Fuel Vehicle. Environ. Sci. Technol. 2016, 50, 11853–11861. [Google Scholar] [CrossRef] [PubMed]
- Líbalová, H.; Závodná, T.; Vrbová, K.; Sikorová, J.; Vojtíšek-Lom, M.; Beránek, V.; Pechout, M.; Kléma, J.; Ciganek, M.; Machala, M.; et al. Transcription profiles in BEAS-2B cells exposed to organic extracts from particulate emissions produced by a port-fuel injection vehicle, fueled with conventional fossil gasoline and gasoline-ethanol blend. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2021, 872, 503414. [Google Scholar] [CrossRef] [PubMed]
- Bisig, C.; Roth, M.; Müller, L.; Comte, P.; Heeb, N.; Mayer, A.; Czerwinski, J.; Petri-Fink, A.; Rothen-Rutishauser, B. Hazard identification of exhausts from gasoline-ethanol fuel blends using a multi-cellular human lung model. Environ. Res. 2016, 151, 789–796. [Google Scholar] [CrossRef]
- Rossner, P.; Cervena, T.; Vojtisek-Lom, M.; Neca, J.; Ciganek, M.; Vrbova, K.; Ambroz, A.; Novakova, Z.; Elzeinova, F.; Sima, M.; et al. Markers of lipid oxidation and inflammation in bronchial cells exposed to complete gasoline emissions and their organic extracts. Chemosphere 2021, 281, 130833. [Google Scholar] [CrossRef]
- Sima, M.; Cervena, T.; Elzeinova, F.; Ambroz, A.; Beranek, V.; Vojtisek-Lom, M.; Klema, J.; Ciganek, M.; Rossner, P. The impact of extractable organic matter from gasoline and alternative fuel emissions on bronchial cell models (BEAS-2B, MucilAir™). Toxicol. Vitr. 2022, 80, 105316. [Google Scholar] [CrossRef]
- Hakkarainen, H.; Aakko-Saksa, P.; Sainio, M.; Ihantola, T.; Rönkkö, T.J.; Koponen, P.; Rönkkö, T.; Jalava, P.I. Toxicological evaluation of exhaust emissions from light-duty vehicles using different fuel alternatives in sub-freezing conditions. Part. Fibre Toxicol. 2020, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Roth, P.; Durbin, T.D.; Shafer, M.M.; Hemming, J.; Antkiewicz, D.S.; Asa-Awuku, A.; Karavalakis, G. Emissions from a flex fuel GDI vehicle operating on ethanol fuels show marked contrasts in chemical, physical and toxicological characteristics as a function of ethanol content. Sci. Total Environ. 2019, 683, 749–761. [Google Scholar] [CrossRef]
- Le, C.T.T.; Ahn, S.Y.; Kang, S.-M.; Ko, E.-J. Functional NK Cell Activation by Ovalbumin Immunization with a Monophosphoryl Lipid A and Poly I:C Combination Adjuvant Promoted Dendritic Cell Maturation. Vaccines 2021, 9, 1061. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Fu, T.; Jiang, Y.-Z.; Shao, Z.-M. Natural killer cells in cancer biology and therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef]
- Roth, M.; Usemann, J.; Bisig, C.; Comte, P.; Czerwinski, J.; Mayer, A.C.R.; Beier, K.; Rothen-Rutishauser, B.; Latzin, P.; Müller, L. Effects of gasoline and ethanol-gasoline exhaust exposure on human bronchial epithelial and natural killer cells in vitro. Toxicol. Vitr. 2017, 45, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.K.; Ghadikolaei, M.A.; Chen, S.H.; Fadairo, A.A.; Ng, K.W.; Lee, S.M.Y.; Xu, J.C.; Lian, Z.D.; Li, S.; Wong, H.C.; et al. Physicochemical and cell toxicity properties of particulate matter (PM) from a diesel vehicle fueled with diesel, spent coffee ground biodiesel, and ethanol. Sci. Total Environ. 2022, 842, 153873. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-M.; Wang, C.-C.; Lin, Y.-C.; Jhang, S.-R.; Lin, L.-J.; Lin, Y.-C. Development of novel alternative biodiesel fuels for reducing PM emissions and PM-related genotoxicity. Environ. Res. 2017, 156, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, K.R.; Nabi, M.N.; Rasul, M.G.; Kicic, A.; Mullins, B.J. Biodiesel Exhaust Toxicity with and without Diethylene Glycol Dimethyl Ether Fuel Additive in Primary Airway Epithelial Cells Grown at the Air–Liquid Interface. Environ. Sci. Technol. 2022, 56, 14640–14648. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.; Santos, S.M.A.; Fernandes, M.A.S.; Deus, C.M.; Martins, J.D.; Pedroso de Lima, M.C.; Vicente, J.A.F.; Videira, R.A.; Jurado, A.S. Improving pollutants environmental risk assessment using a multi model toxicity determination with in vitro, bacterial, animal and plant model systems: The case of the herbicide Alachlor. Environ. Pollut. 2021, 286, 117239. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.A. Microbial bioassays in environmental toxicity testing. Adv. Appl. Microbiol. 2021, 115, 115–158. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-F.; Wang, S.-L.; Lee, D.-C.; Hsiao, S.S.-Y.; Hashimoto, Y.; Yeh, K.-C. Assessment of indium toxicity to the model plant Arabidopsis. J. Hazard. Mater. 2019, 387, 121983. [Google Scholar] [CrossRef]
- Bahl, A.; Loitsch, S.M.; Kahl, G. Transcriptional activation of plant defence genes by short-term air pollutant stress. Environ. Pollut. 1995, 89, 221–227. [Google Scholar] [CrossRef]
- Anand, P.; Mina, U.; Khare, M.; Kumar, P.; Kota, S.H. Air pollution and plant health response-current status and future directions. Atmos. Pollut. Res. 2022, 13, 101508. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.; An, S.-A.; Khim, J.S. Methodological advances and future directions of microalgal bioassays for evaluation of potential toxicity in environmental samples: A review. Environ. Int. 2023, 173, 107869. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Ji, Z.; Sun, J.; Liu, X. Distinct responses of Chlorella vulgaris upon combined exposure to microplastics and bivalent zinc. J. Hazard. Mater. 2022, 442, 130137. [Google Scholar] [CrossRef]
- Bendtsen, K.M.; Gren, L.; Malmborg, V.B.; Shukla, P.C.; Tunér, M.; Essig, Y.J.; Krais, A.M.; Clausen, P.A.; Berthing, T.; Loeschner, K.; et al. Particle characterization and toxicity in C57BL/6 mice following instillation of five different diesel exhaust particles designed to differ in physicochemical properties. Part. Fibre Toxicol. 2020, 17, 38. [Google Scholar] [CrossRef]
- Noël, A.; Ashbrook, D.G.; Xu, F.; Cormier, S.A.; Lu, L.; O’Callaghan, J.P.; Menon, S.K.; Zhao, W.; Penn, A.L.; Jones, B.C. Genomic Basis for Individual Differences in Susceptibility to the Neurotoxic Effects of Diesel Exhaust. Int. J. Mol. Sci. 2022, 23, 12461. [Google Scholar] [CrossRef]
- Posgai, R.; Ahamed, M.; Hussain, S.M.; Rowe, J.J.; Nielsen, M.G. Inhalation method for delivery of nanoparticles to the Drosophila respiratory system for toxicity testing. Sci. Total Environ. 2009, 408, 439–443. [Google Scholar] [CrossRef]
- Yan, C.; Wu, X.; Cao, X.; Li, M.; Zhou, L.; Xiu, G.; Zeng, J. In vitro and in vitro toxicity study of diesel exhaust particles using BEAS-2B cell line and the nematode Caenorhabditis elegans as biological models. Environ. Sci. Pollut. Res. 2021, 28, 60704–60716. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Huang, Z.; Zhang, W.; Ren, Y. Investigating the neurotoxicity of environmental pollutants using zebrafish as a model organism: A review and recommendations for future work. NeuroToxicology 2022, 94, 235–244. [Google Scholar] [CrossRef]
- Diego Stéfani, T.M.; Laura-Jayne, A.E.; Gabriela, H.D.S.; Romana, P.; Aline, M.Z.M.; Hossein Hayat, D.; Anastasios, G.P.; Adalberto, F.; Antreas, A.; Georgia, M.; et al. Daphnia magna and mixture toxicity with nanomaterials—Current status and perspectives in data-driven risk prediction. Nano Today 2022, 43, 101430. [Google Scholar] [CrossRef]
- Pikula, K.; Tretyakova, M.; Zakharenko, A.; Johari, S.A.; Ugay, S.; Chernyshev, V.; Chaika, V.; Kalenik, T.; Golokhvast, K. Environmental Risk Assessment of Vehicle Exhaust Particles on Aquatic Organisms of Different Trophic Levels. Toxics 2021, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.B.; Krieger, K.; Khan, F.M.; Huffman, W.; Chang, M.; Naik, A.; Yongle, R.; Hameed, I.; Krieger, K.; Girardi, L.N.; et al. The current state of animal models in research: A review. Int. J. Surg. 2019, 72, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Yoon, J.-Y. Organ-on-a-chip for assessing environmental toxicants. Curr. Opin. Biotechnol. 2017, 45, 34–42. [Google Scholar] [CrossRef]
- Castro-Gálvez, Z.; Garrido-Armas, M.; Palacios-Arreola, M.I.; Torres-Flores, U.; Rivera-Torruco, G.; Valle-Rios, R.; Amador-Muñoz, O.; Hernández-Hernández, A.; Arenas-Huertero, F. Cytotoxic and genotoxic effects of Benzo[ghi]perylene on the human bronchial cell line NL-20. Toxicol. Vitr. 2019, 61, 104645. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Gamage, A.M.; Liu, J. Human Nasal Epithelial Cells (hNECs) Generated by Air-Liquid Interface (ALI) Culture as a Model System for Studying the Pathogenesis of SARS-CoV-2. Methods Mol. Biol. 2022, 2452, 213–224. [Google Scholar] [CrossRef]
- Cidem, A.; Bradbury, P.; Traini, D.; Ong, H.X. Modifying and Integrating in vitro and ex vivo Respiratory Models for Inhalation Drug Screening. Front. Bioeng. Biotechnol. 2020, 8, 581995. [Google Scholar] [CrossRef]
- Vegard Sæ ter, G.; Elisabeth, K.; Tonje, S.; Magne, R.; Johan, Ø.; Mats, G.; Marit, L. 76 Combined Exposure to road wear Particles and Diesel Exhaust Particles Induces Enhanced pro-Inflammatory Responses in Cells of the Human Airways. Ann. Work Expo. Health 2023, 67, i94. [Google Scholar] [CrossRef]
- Ke, S.; Liu, Q.; Zhang, X.; Yao, Y.; Yang, X.; Sui, G. Cytotoxicity analysis of biomass combustion particles in human pulmonary alveolar epithelial cells on an air–liquid interface/dynamic culture platform. Part. Fibre Toxicol. 2021, 18, 31. [Google Scholar] [CrossRef]
- Lee, E.; Kim, H.-J.; Lee, M.; Jin, S.H.; Hong, S.H.; Ahn, S.; Kim, S.O.; Shin, D.W.; Lee, S.-T.; Noh, M. Cystathionine metabolic enzymes play a role in the inflammation resolution of human keratinocytes in response to sub-cytotoxic formaldehyde exposure. Toxicol. Appl. Pharmacol. 2016, 310, 185–194. [Google Scholar] [CrossRef]
- Shah, U.-K.; Seager, A.L.; Fowler, P.; Doak, S.H.; Johnson, G.E.; Scott, S.J.; Scott, A.D.; Jenkins, G.J.S. A comparison of the genotoxicity of benzo[a]pyrene in four cell lines with differing metabolic capacity. Mutation research. Genet. Toxicol. Environ. Mutagen. 2016, 808, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, C.; Zhang, X.; Kang, X.; Li, Y.; Zhang, W.; Chen, Y.; Liu, Y.; Wang, W.; Ge, M.; et al. Exposure to PM2.5 aggravates Parkinson’s disease via inhibition of autophagy and mitophagy pathway. Toxicology 2021, 456, 152770. [Google Scholar] [CrossRef]
- Sim, H.; Noh, Y.; Choo, S.; Kim, N.; Lee, T.; Bae, J.-S. Suppressive Activities of Fisetin on Particulate Matter-induced Oxidative Stress. Biotechnol. Bioprocess Eng. 2021, 26, 568–574. [Google Scholar] [CrossRef]
- Xie, W.; You, J.; Zhi, C.; Li, L. The toxicity of ambient fine particulate matter (PM2.5) to vascular endothelial cells. J. Appl. Toxicol. 2021, 41, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Guastadisegni, C.; Kelly, F.J.; Cassee, F.R.; Gerlofs-Nijland, M.E.; Janssen, N.A.H.; Pozzi, R.; Brunekreef, B.; Sandström, T.; Mudway, I. Determinants of the proinflammatory action of ambient particulate matter in immortalized murine macrophages. Environ. Health Perspect. 2010, 118, 1728–1734. [Google Scholar] [CrossRef]
- Upadhyay, S.; Palmberg, L. Air-liquid interface: Relevant in vitro models for investigating air pollutant-induced pulmonary toxicity. Toxicol. Sci. 2018, 164, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Lenz, A.G.; Karg, E.; Brendel, E.; Hinze-Heyn, H.; Maier, K.L.; Eickelberg, O.; Stoeger, T.; Schmid, O. Inflammatory and oxidative stress responses of an alveolar epithelial cell line to airborne zinc oxide nanoparticles at the air-liquid interface: A comparison with conventional, submerged cell-culture conditions. BioMed Res. Int. 2013, 2013, 652632. [Google Scholar] [CrossRef]
- Zarcone, M.C.; Duistermaat, E.; Alblas, M.J.; van Schadewijk, A.; Ninaber, D.K.; Clarijs, V.; Moerman, M.M.; Vaessen, D.; Hiemstra, P.S.; Kooter, I.M. Effect of diesel exhaust generated by a city bus engine on stress responses and innate immunity in primary bronchial epithelial cell cultures. Toxicol. Vitr. 2018, 48, 221–231. [Google Scholar] [CrossRef]
- Guénette, J.; Breznan, D.; Thomson, E.M. Establishing an air-liquid interface exposure system for exposure of lung cells to gases. Inhal. Toxicol. 2022, 34, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, P.; Qin, J. Microfluidic Organs-on-a-Chip for Modeling Human Infectious Diseases. Acc. Chem. Res. 2021, 54, 3550–3562. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yin, F.; Li, Z.; Su, W.; Li, D. Advances of microfluidic lung chips for assessing atmospheric pollutants exposure. Environ. Int. 2023, 172, 107801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Stegmayr, J.; Wagner, D.E. The dawn of the omics era in human precision-cut lung slices. Eur. Respir. J. 2021, 58, 2100203. [Google Scholar] [CrossRef]
- Yang, Z.; Xiao, Y.; Jiao, T.; Zhang, Y.; Chen, J.; Gao, Y. Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza sativa L.) Seedlings and the Relevant Physiological Responses. Int. J. Environ. Res. Public Health 2020, 17, 1260. [Google Scholar] [CrossRef]
- Delias, D.S.; Da-Silva, C.J.; Martins, A.C.; de Oliveira, D.S.C.; do Amarante, L. Iron toxicity increases oxidative stress and impairs mineral accumulation and leaf gas exchange in soybean plants during hypoxia. Environ. Sci. Pollut. Res. 2021, 29, 22427–22438. [Google Scholar] [CrossRef]
- Song, U.; Kim, J. Assessment of the potential risk of 1,2-hexanediol using phytotoxicity and cytotoxicity testing. Ecotoxicol. Environ. Saf. 2020, 201, 110796. [Google Scholar] [CrossRef]
- Shahbaz, M.; Akram, A.; Mehak, A.; Haq, E.U.; Fatima, N.; Wareen, G.; Fitriatin, B.N.; Sayyed, R.Z.; Ilyas, N.; Sabullah, M.K. Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress. Plants 2023, 12, 761. [Google Scholar] [CrossRef]
- Villarini, M.; Fatigoni, C.; Dominici, L.; Maestri, S.; Ederli, L.; Pasqualini, S.; Monarca, S.; Moretti, M. Assessing the genotoxicity of urban air pollutants using two in situ plant bioassays. Environ. Pollut. 2009, 157, 3354–3356. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Song, B.; Xiao, R.; Zeng, G.; Gong, J.; Chen, M.; Xu, P.; Zhang, P.; Shen, M.; Yi, H. Assessing the human health risks of perfluorooctane sulfonate by in vivo and in vitro studies. Environ. Int. 2019, 126, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Zavala, J.; Freedman, A.N.; Szilagyi, J.T.; Jaspers, I.; Wambaugh, J.F.; Higuchi, M.; Rager, J.E. New Approach Methods to Evaluate Health Risks of Air Pollutants: Critical Design Considerations for In Vitro Exposure Testing. Int. J. Environ. Res. Public Health 2020, 17, 2124. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Wei, L.; Hou, Z.; Li, J.; Zhang, C.; Lin, D. Microplastics altered contaminant behavior and toxicity in natural waters. J. Hazard. Mater. 2021, 425, 127908. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, T.; Li, X.; Sun, H.; Xia, X.; Ji, X.; Zhang, J.; Liu, M.; Lin, Y.; Zhang, R.; et al. Inhaled tire-wear microplastic particles induced pulmonary fibrotic injury via epithelial cytoskeleton rearrangement. Environ. Int. 2022, 164, 107257. [Google Scholar] [CrossRef]
- Lundbäck, M.; Mills, N.L.; Lucking, A.; Barath, S.; Donaldson, K.; Newby, D.E.; Sandström, T.; Blomberg, A. Experimental exposure to diesel exhaust increases arterial stiffness in man. Part. Fibre Toxicol. 2009, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Naughton, S.X.; Pasinetti, G.M. Role of diesel exhaust exposure in promoting Alzheimer’s disease susceptibility by impairing glymphatic drainage of amyloid beta (aβ) and other toxic metabolites from the brain. Alzheimer’s Dement. 2021, 17, e056165. [Google Scholar] [CrossRef]
- Hesterberg, T.W.; Bunn, W.B.; McClellan, R.O.; Hart, G.A.; Lapin, C.A. Carcinogenicity studies of diesel engine exhausts in laboratory animals: A review of past studies and a discussion of future research needs. Crit. Rev. Toxicol. 2005, 35, 379–411. [Google Scholar] [CrossRef]
- Asma, S.T.; Imre, K.; Morar, A.; Herman, V.; Acaroz, U.; Mukhtar, H.; Arslan-Acaroz, D.; Shah, S.R.A.; Gerlach, R. An Overview of Biofilm Formation–Combating Strategies and Mechanisms of Action of Antibiofilm Agents. Life 2022, 12, 1110. [Google Scholar] [CrossRef]
- Gupta, G.; Cappellini, F.; Farcal, L.; Gornati, R.; Bernardini, G.; Fadeel, B. Copper oxide nanoparticles trigger macrophage cell death with misfolding of Cu/Zn superoxide dismutase 1 (SOD1). Part. Fibre Toxicol. 2022, 19, 33. [Google Scholar] [CrossRef] [PubMed]
- Mandavilli, B.S.; Yan, M.; Clarke, S. Cell-Based High Content Analysis of Cell Proliferation and Apoptosis. Methods Mol. Biol. 2017, 1683, 47–57. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xu, Y.; Wang, M.; Zhu, Q.; Li, W.; Wang, X.; Liu, J.; Ma, B.; Wu, K. Neurotransmitter noradrenaline downregulate cytoskeletal protein expression of VSMCs. Exp. Mol. Pathol. 2012, 94, 79–83. [Google Scholar] [CrossRef]
- Barcellini-Couget, S.; de Sousa, G.; Rahmani, R. The use of Real-Time Cell Analyzer (RTCA) in in vitro toxicology. Toxicol. Lett. 2013, 221, S163. [Google Scholar] [CrossRef]
- Xiong, P.; Huang, X.; Ye, N.; Lu, Q.; Zhang, G.; Peng, S.; Wang, H.; Liu, Y. Cytotoxicity of Metal-Based Nanoparticles: From Mechanisms and Methods of Evaluation to Pathological Manifestations. Adv. Sci. 2022, 9, 2106049. [Google Scholar] [CrossRef]
- Oshima, A.; Nakashima, H.; Sumitomo, K. Evaluation of Lateral Diffusion of Lipids in Continuous Membranes between Freestanding and Supported Areas by Fluorescence Recovery after Photobleaching. Langmuir 2019, 35, 11725–11734. [Google Scholar] [CrossRef]
- Liu, X.; Xia, J.; Tang, J.; Zhou, M.; Chen, D.; Liu, Z. Ginsenoside Rh_2 induces apoptosis and autophagy of K562 cells by activating p38. China J. Chin. Mater. Med. 2017, 42, 146–151. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Zhang, L.; Wang, H.; Ho, C.-T.; Li, S.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M. 8-oxoguanine and 8-oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef] [PubMed]
- Buss, H.; Chan, T.P.; Sluis, K.B.; Domigan, N.M.; Winterbourn, C.C. Protein carbonyl measurement by a sensitive ELISA method. Free Radic. Biol. Med. 1997, 24, 1352. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Issakidis-Bourguet, E. A Simplified Method to Assay Protein Carbonylation by Spectrophotometry. Methods Mol. Biol. 2022, 2526, 135–141. [Google Scholar] [CrossRef]
- Pesez, M. 2:4-Dinitrophenylhydrazine, a suitable reagent for the colorimetric determination of carbonyl compounds. J. Pharm. Pharmacol. 1959, 11, 475–476. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ma, J.; Cai, K.; Chen, H.; Xie, K.; Xu, B.; Quan, D.; Du, J. Isoflavones from Semen Sojae Preparatum Improve Atherosclerosis and Oxidative Stress by Modulating Nrf2 Signaling Pathway through Estrogen-Like Effects. Evid. Based Complement. Altern. Med. 2022, 2022, 4242099. [Google Scholar] [CrossRef] [PubMed]
- Favier, A. Oxidative stress: Value of its demonstration in medical biology and problems posed by the choice of a marker. Ann. Biol. Clin. 1997, 55, 9–16. [Google Scholar]
- Cordelli, E.; Bignami, M.; Pacchierotti, F. Comet assay: A versatile but complex tool in genotoxicity testing. Toxicol. Res. 2021, 10, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Libalova, H.; Rossner, P.; Vrbova, K.; Brzicova, T.; Sikorova, J.; Vojtisek-Lom, M.; Beranek, V.; Klema, J.; Ciganek, M.; Neca, J.; et al. Comparative Analysis of Toxic Responses of Organic Extracts from Diesel and Selected Alternative Fuels Engine Emissions in Human Lung BEAS-2B Cells. Int. J. Mol. Sci. 2016, 17, 1833. [Google Scholar] [CrossRef] [PubMed]
- Tutty, M.A.; Vella, G.; Vennemann, A.; Wiemann, M.; Prina-Mello, A. Evaluating nanobiomaterial-induced DNA strand breaks using the alkaline comet assay. Drug Deliv. Transl. Res. 2022, 12, 2243–2258. [Google Scholar] [CrossRef]
- Hamouchene, H.; Arlt, V.M.; Giddings, I.; Phillips, D.H. Influence of cell cycle on responses of MCF-7 cells to benzo[a]pyrene. BMC Genom. 2011, 12, 333. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).