A Review of Sensing Technologies for New, Low Global Warming Potential (GWP), Flammable Refrigerants †
Abstract
:1. Introduction
- capable of operating safely with any 2 L refrigerant without becoming an ignition source themselves,
- very high reliability during the lifetime of a typical system (25-year lifetime desired/5-year lifetime minimum),
- ready to operate with smart control and fault detection and diagnostics systems employed in these applications,
- wireless capabilities (Internet of Things connectivity) to simplify distribution in typical supermarket stores,
- capable of measuring refrigerant concentration with sufficient accuracy, and of providing adequate response time according to the appropriate safety standards,
- acceptable cost for mass-production equipment such as refrigeration systems.
| Refrigerant Number | Type | Chemical Name/Composition Mass | Chemical Formula/Composition Tolerance | OEL, ppm v/v | RCL | GWP (AR4) | ||
|---|---|---|---|---|---|---|---|---|
| ppm v/v | lb/Mcf | g/ | ||||||
| Methane series | ||||||||
| 32 | HFC | difluoromethane (methylene fluoride) | 1000 | 36,000 | 4.8 | 77 | 675 | |
| Ethane series | ||||||||
| 143a | HFC | 1,1,1-trifluoroethane | 1000 | 21,000 | 4.5 | 70 | 4470 | |
| Unsaturated Organic Compounds | ||||||||
| 1234yf | HFO | 2,3,3,3-tetrafluoro-1-propene | 500 | 16,000 | 4.7 | 75 | 4 | |
| 1234ze(E) | HFO | Trans-1,3,3,3-tetrafluoro-1-propene | 800 | 16,000 | 4.7 | 75 | 6 | |
| Zeotropes | ||||||||
| 444A | HFC blend | R-32/152a/1234ze(E) 12.0/5.0/83.0 | ±1/±1/±2 | 850 | 21,000 | 5.1 | 81 | 87 |
| 444B | HFC blend | R-32/152a/1234ze(E) 41.5/10.0/48.5 | ±1/±1/±1 | 890 | 23,000 | 4.3 | 69 | 293 |
| 445A | HFC blend | R-744/134a/1234ze(E) 6.0/9.0/85.0 | ±1/±1/±2 | 930 | 16,000 | 4.2 | 67 | 129 |
| 446A | HFC blend | R-32/1234ze(E)/600 68.0/29.0/3.0 | // | 960 | 16,000 | 2.5 | 39 | 459 |
| 447A | HFC blend | R-32/125/1234ze(E) 68.0/3.5/28.5 | // | 900 | 16,000 | 2.6 | 42 | 582 |
| 447B | HFC blend | R-32/125/1234ze(E) 68.0/8.0/24.0 | // | 970 | 30,000 | 23 | 360 | 739 |
| 451A | HFC blend | R-1234yf/134a 89.8/10.2 | ±0.2/±0.2 | 520 | 18,000 | 5.3 | 81 | 146 |
| 451B | HFC blend | R-1234yf/134a 88.8/11.2 | ±0.2/±0.2 | 530 | 18,000 | 5.3 | 81 | 160 |
| 452B | HFC blend | R-32/125/1234yf 67.0/7.0/26.0 | ±2.0/±1.5/±2.0 | 870 | 30,000 | 23 | 360 | 696 |
| 454A | HFC blend | R-32/1234yf 35.0/65.0 | +2.0/−2.0, +2.0/−2.0 | 690 | 16,000 | 28 | 450 | 236 |
| 454B | HFC blend | R-32/1234yf 68.9/31.1 | +1.0/−1.0, +1.0/−1.0 | 850 | 19,000 | 22 | 360 | 465 |
| 454C | HFC blend | R-32/1234yf 21.5/78.5 | ±0.2/±0.2 | 620 | 19,000 | 29 | 460 | 145 |
| 455A | HFC blend | R-744/32/1234yf 3.0/21.5/75.5 | //±2.0 | 650 | 30,000 | 23 | 380 | 145 |
| 457A | HFC blend | R-32/1234yf/152a 18.0/70.0/12.0 | // | 650 | 15,000 | 25 | 400 | 136 |
| 459A | R-32/1234yf/1234ze(E) 68.0/26.0/6.0 | /±/ | 870 | 27,000 | 23 | 360 | ||
| 459B | R-32/1234yf/1234ze(E) 21.0/69.0/10.0 | /±/ | 640 | 16,000 | 30 | 470 | ||
| Azeotropes | ||||||||
| 516A | R-1234yf/134a/152a 77.5/8.5/14.0 | ±// | 590 | 27,000 | 7.0 | 110 | ||
2. Gas Sensors
2.1. Recognition and Transduction Mechanisms
- Thermometric transduction is based on the registration of the thermal effect of a catalytic reaction of the analyte near the surface of a sensing element. It is suitable for catalytic processes that generate a lot of heat such as the combustion of flammable gases.
- Resistive/capacitive transduction is based on measuring the change of the resistance/capacitance of the sensing material due to interaction with a gas.
- Electrochemical transduction is based on measuring the change in electrical potential or current due to ion/electron transfer reactions at the surface of a solid sensing element.
- Optical transduction is achieved by detecting modulation of some properties of electromagnetic radiation in ultraviolet–visible–infrared domains during its interaction with a sensing element that is commonly the gas itself.
- Acoustic transduction entails the measurement of the characteristics of acoustic waves produced within the sensing element as a result of the recognition process.
2.2. Performance Characteristics
- Selectivity is the extent to which a sensor can determine a particular analyte without interfering with other components.
- Operating limits, detection and quantification capabilities:
- sensitivity is the change in a sensor’s response to the unit change in concentration,
- limit of detection (LOD) is the lowest concentration level that can be distinguished from the absence of a substance (blank value, ) within a stated confidence interval,
- response range is the range between LOD and the concentration at which the sensor response starts to significantly deviate from the calibration function,
- resolution is the smallest detectable change in concentration. It is the ratio of the smallest detectable change in response to the sensitivity of a sensor.
- Environmental characteristics (operating temperature, humidity, etc.) within which the sensor maintains its accuracy.
- Dynamic characteristics:
- warm-up time is the time delay between the excitation signal and the moment when the sensor can operate within its specified accuracy,
- response time is the time required for a sensor to attain a stationary response after adding the analyte.
- Reliability of the measurement, defined by the
- accuracy is the discrepancy between the measured and true concentrations,
- precision is the discrepancy between independent measurements under similar conditions.
- Lifetime, stability, reversibility: drift or aging is related to irreversible degradation of sensor materials.
- Response to harsh conditions as defined in UL and MIL-STD-883 standards.
3. Electrically Transduced Sensors
3.1. Semiconductor Sensors
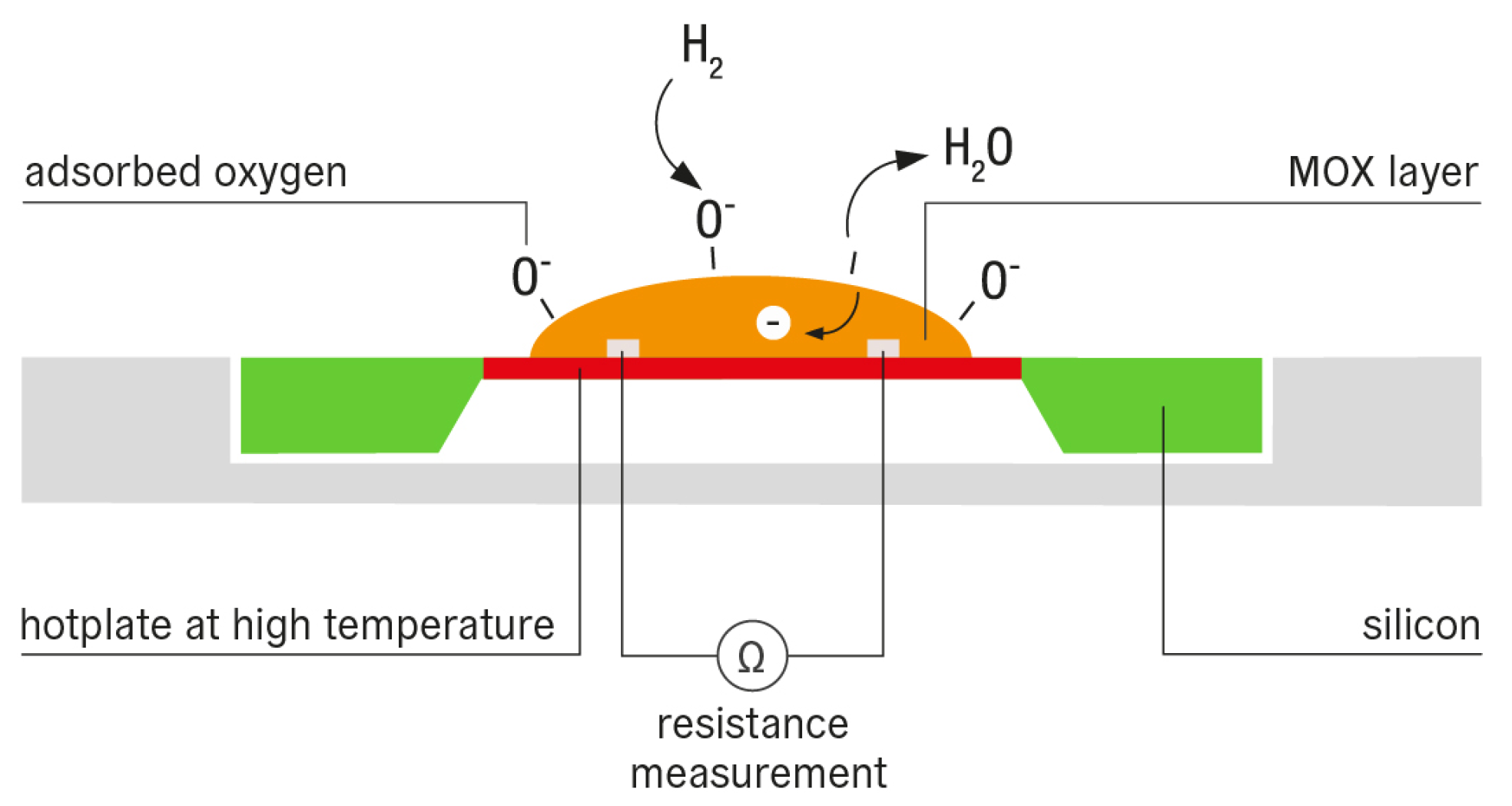
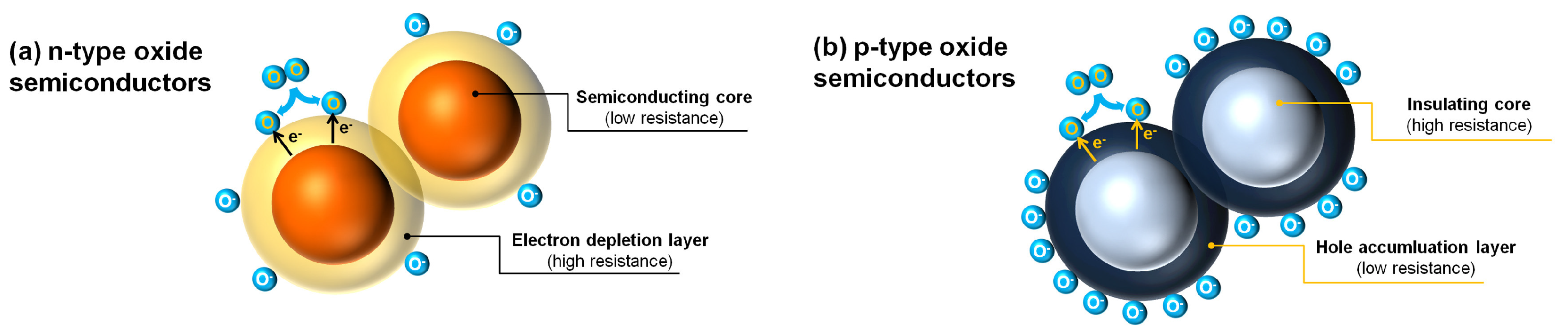
3.1.1. Metal Oxide Semiconductors (MOS)
Sensing Mechanism
Advantages and Limitations
Strategies for Improvement
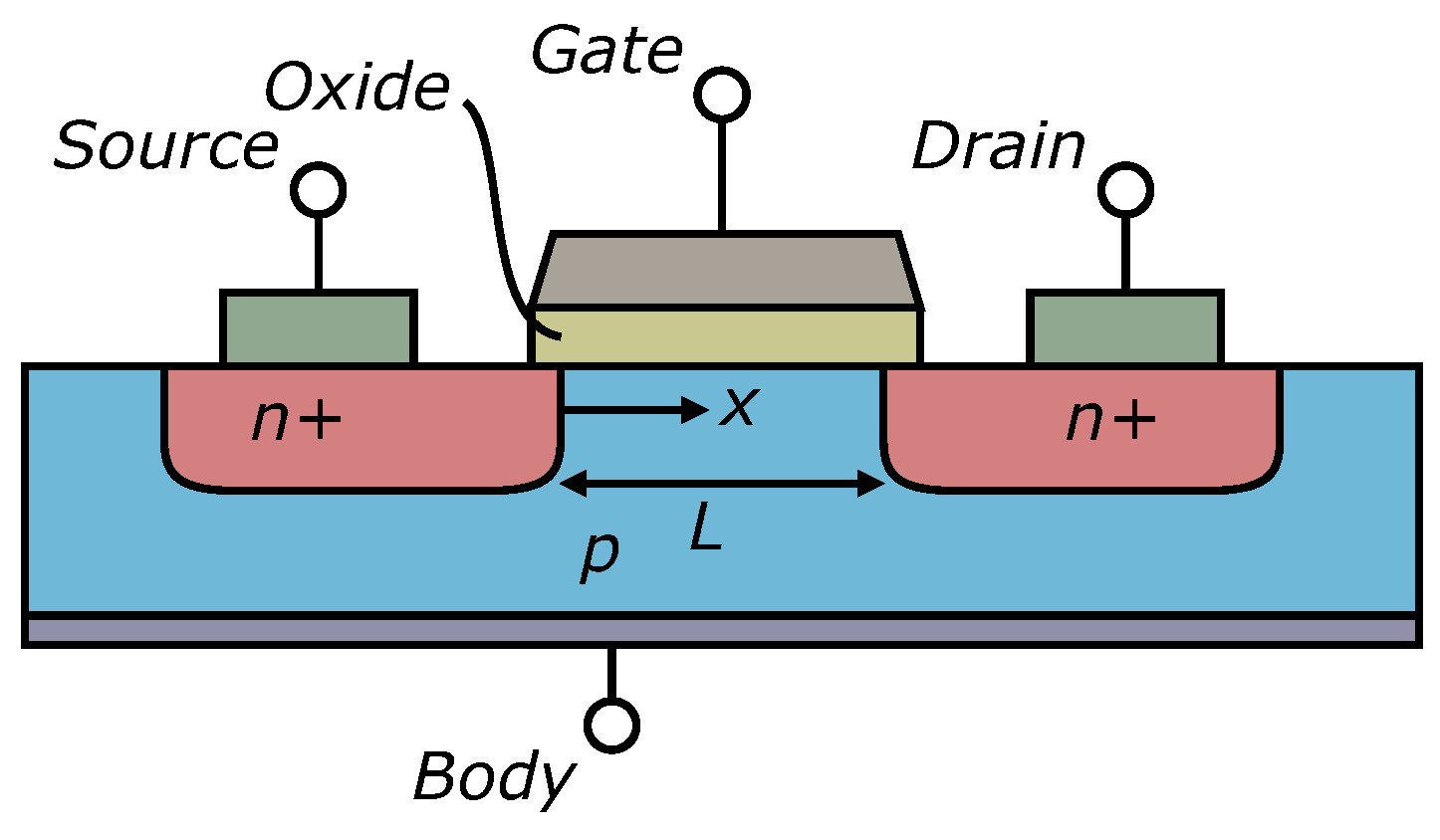
3.1.2. Field Effect Transistors (FET)
Sensing Mechanism
Advantages and Limitations
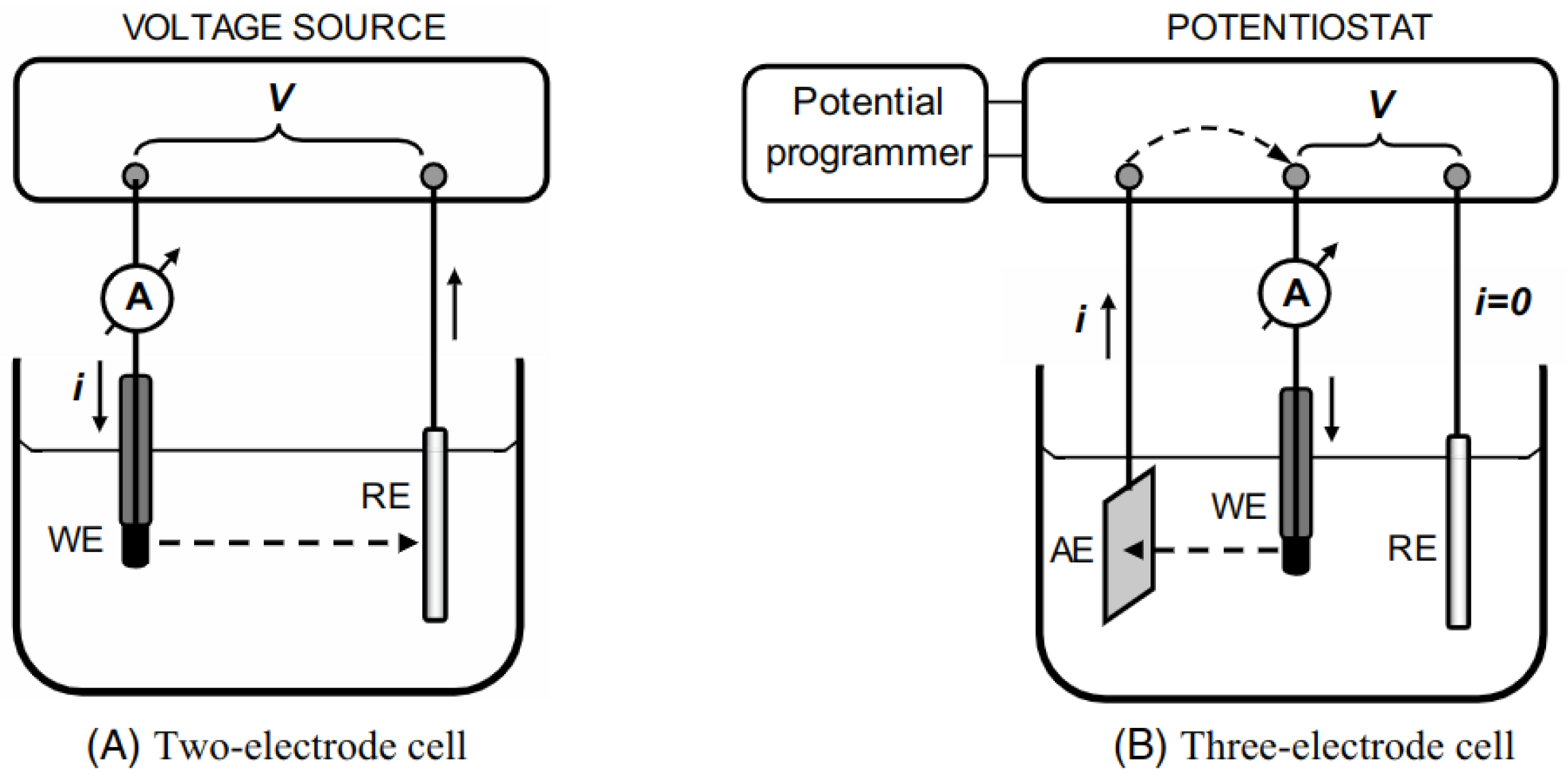
3.2. Electrochemical Sensors
3.2.1. Sensing Mechanism
3.2.2. Advantages and Limitations
3.2.3. Strategies for Improvement
4. Spectrochemical Sensors
4.1. Absorption Spectroscopy
4.1.1. Sensing Mechanism
4.1.2. Advantages and Limitations
4.1.3. Strategies for Improvement
4.2. Vibrational Spectroscopy
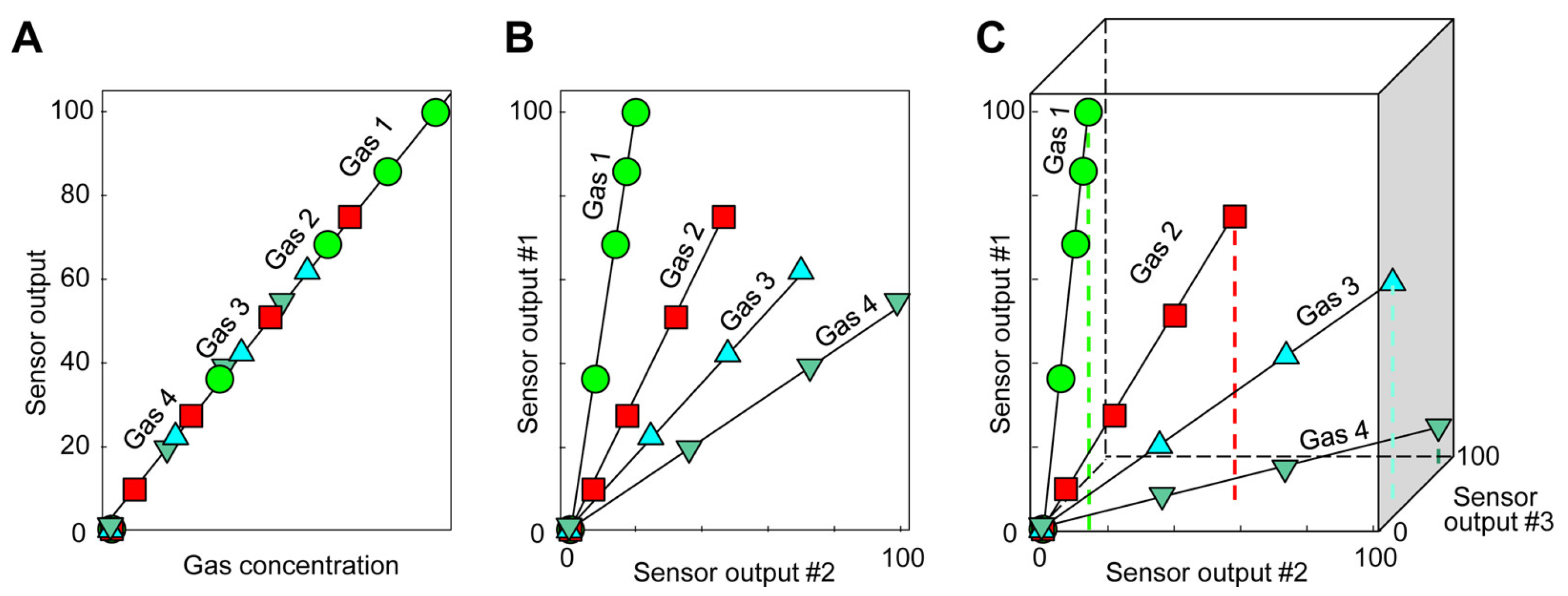
5. Sensor Arrays, Multivariable Sensors, and Data Processing
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, V.; Fricke, B.; Bansal, P. Comparative analysis of various CO2 configurations in supermarket refrigeration systems. Int. J. Refrig. 2014, 46, 86–99. [Google Scholar] [CrossRef]
- ANSI/ASHRAE 34-2019; Designation and Safety Classification of Refrigerants. ASHRAE: Atlanta, GA, USA, 2019.
- ANSI/ASHRAE 15-2019; Safety Standard for Refrigeration Systems. ASHRAE: Atlanta, GA, USA, 2019.
- 60335-2-89; Standard for Household And Similar Electrical Appliances—Safety—Part 2-40: Particular Requirements for Electrical Heat Pumps, Air-Conditioners and Dehumidifiers. 3rd ed. UL Enterprise: Northbrook, IL, USA, 2019.
- 60335-2-40; Household and Similar Electrical Appliances–Safety–Part 2-89: Particular Requirements for Commercial Refrigerating Appliances and Ice-Makers with an Incorporated or Remote Refrigerant Unit or Motor-Compressor. 2nd ed. UL Enterprise: Northbrook, IL, USA, 2021.
- Wagner, M.; Ferenchiak, R. Leak Detection of A2L Refrigerants in HVACR Equipment; Technical Report 9009; AHRTI: Washington, DC, USA, 2017. [Google Scholar]
- Refrigerant Leak Detection: IR vs. Semiconductor Sensors. Available online: https://www.nenvitech.com/news/refrigerant-leak-detection-ir-vs-semiconductor-sensors/ (accessed on 30 September 2022).
- Eckstein, M.; Lee, K. Market Trends in Refrigerant Leakage Detection. Available online: https://sensirion.com/products/product-insights/specialist-articles/market-trends-in-refrigerant-leakage-detection/ (accessed on 25 September 2022).
- Zheng, J.; Zang, F.; Yu, C.N.; Elbel, S. Refrigerant Detector Characteristics for Use in HVACR Equipment; Technical Report 9014; AHRTI: Washington, DC, USA, 2021. [Google Scholar]
- Summary of Findings from 2020 AHRTI Report, “Refrigerant Detector Characteristics for Use in HVACR Equipment—Phase I”. Available online: https://nevadanano.com/wp-content/uploads/2020/05/SM-AN-0012-04-Leak-Detection-Technologies-for-A2L-Refrigerants-in-HVACR-Equipment-AHRTI-2020-Phase-I-Report-Summary.pdf (accessed on 30 September 2022).
- Banica, F.G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Fraden, J. Handbook of Modern Sensors: Physics, Designs, and Applications; Springer International Publishing: Cham, Switzerland, 1998. [Google Scholar]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Raju, P.; Li, Q. Semiconductor materials and devices for gas sensors. J. Electrochem. Soc. 2022, 169, 057518. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Hübner, M.; Simion, C.; Tomescu-Stănoiu, A.; Pokhrel, S.; Bârsan, N.; Weimar, U. Influence of humidity on CO sensing with p-type CuO thick film gas sensors. Sens. Actuators B Chem. 2011, 153, 347–353. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, L.; Ma, J.; Ren, Y.; Cheng, X.; Zhu, Y.; Zhao, D.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y. Ordered mesoporous tin oxide semiconductors with large pores and crystallized walls for high-performance gas sensing. ACS Appl. Mater. Interfaces 2018, 10, 1871–1880. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Tian, F.; Liang, H.; Wang, K.; Zhao, X.; Lu, Z.; Jiang, K.; Yang, L.; Lou, X. From the surface reaction control to gas-diffusion control: The synthesis of hierarchical porous SnO2 microspheres and their gas-sensing mechanism. J. Phys. Chem. C 2015, 119, 15963–15976. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Shi, L.; Li, G.D.; Sun, L.; Zou, X. Revealing the relationship between energy level and gas sensing performance in heteroatom-doped semiconducting nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 29795–29804. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, A.; Komem, Y. The effect of grain size on the sensitivity of nanocrystalline metal-oxide gas sensors. J. Appl. Phys. 2004, 95, 6374–6380. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Cho, B.K.; Brinzari, V. Grain Size Effects in Sensor Response of Nanostructured SnO2− and In2O3− Based Conductometric Thin Film Gas Sensor. Crit. Rev. Solid State Mater. Sci. 2009, 34, 1–17. [Google Scholar] [CrossRef]
- Ma, J. (Ed.) Gas Sensors; IOP Publishing: Bristol, UK, 2021; pp. 2053–2563. [Google Scholar]
- Liang, S.; Li, J.; Wang, F.; Qin, J.; Lai, X.; Jiang, X. Highly sensitive acetone gas sensor based on ultrafine α-Fe2O3 nanoparticles. Sens. Actuators B Chem. 2017, 238, 923–927. [Google Scholar] [CrossRef]
- Zhou, S.; Chen, M.; Lu, Q.; Zhang, Y.; Zhang, J.; Li, B.; Wei, H.; Hu, J.; Wang, H.; Liu, Q. Ag nanoparticles sensitized In2O3 nanograin for the ultrasensitive HCHO detection at room temperature. Nanoscale Res. Lett. 2019, 14, 365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jia, C.; Kong, Q.; Fan, N.; Chen, G.; Guan, H.; Dong, C. ZnO-decorated In/Ga oxide nanotubes derived from bimetallic In/Ga MOFs for fast acetone detection with high sensitivity and selectivity. ACS Appl. Mater. Interfaces 2020, 12, 26161–26169. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Chen, H.; Li, G.D.; Shi, Z.; Zou, H.; Zou, X. Ultrathin In2O3 nanosheets with uniform mesopores for highly sensitive nitric oxide detection. ACS Appl. Mater. Interfaces 2017, 9, 16335–16342. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Guo, Z.; Su, Y.; Jin, X.B.; Tang, X.H.; Huang, J.R.; Huang, X.J.; Li, M.Q.; Liu, J.H. Hierarchical morphology-dependent gas-sensing performances of three-dimensional SnO2 nanostructures. ACS Sens. 2017, 2, 102–110. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Güell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuators B Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Han, Y.; Liu, Y.; Su, C.; Chen, X.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; Yang, Z. Sonochemical synthesis of hierarchical WO3 flower-like spheres for highly efficient triethylamine detection. Sens. Actuators B Chem. 2020, 306, 127536. [Google Scholar] [CrossRef]
- Ma, J.; Ren, Y.; Zhou, X.; Liu, L.; Zhu, Y.; Cheng, X.; Xu, P.; Li, X.; Deng, Y.; Zhao, D. Pt Nanoparticles Sensitized Ordered Mesoporous WO3 Semiconductor: Gas Sensing Performance and Mechanism Study. Adv. Funct. Mater. 2018, 28, 1705268. [Google Scholar] [CrossRef]
- Shao, F.; Hoffmann, M.; Prades, J.; Zamani, R.; Arbiol, J.; Morante, J.; Varechkina, E.; Rumyantseva, M.; Gaskov, A.; Giebelhaus, I.; et al. Heterostructured p-CuO (nanoparticle)/n-SnO2 (nanowire) devices for selective H2S detection. Sens. Actuators B Chem. 2013, 181, 130–135. [Google Scholar] [CrossRef]
- Xu, F.; HO, H.P. Light-Activated Metal Oxide Gas Sensors: A Review. Micromachines 2017, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuators B Chem. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Berger, F.; Sanchez, J.B.; Heintz, O. Detection of hydrogen fluoride using SnO2-based gas sensors: Understanding of the reactional mechanism. Sens. Actuators B Chem. 2009, 143, 152–157. [Google Scholar] [CrossRef]
- Kwon, C.H.; Yun, D.H.; Hong, H.K.; Kim, S.R.; Lee, K.; Lim, H.Y.; Yoon, K.H. Multi-layered thick-film gas sensor array for selective sensing by catalytic filtering technology. Sens. Actuators B Chem. 2000, 65, 327–330. [Google Scholar] [CrossRef]
- Wong, K.; Tang, Z.; Sin, J.; Chan, P.; Cheung, P.; Hiraoka, H. Study on selectivity enhancement of tin dioxide gas sensor using non-conducting polymer membrane. In Proceedings of the 1995 IEEE Hong Kong Electron Devices Meeting, Hong Kong, China, 1 July 1995; pp. 42–45. [Google Scholar] [CrossRef]
- Ng, K.T.; Boussaid, F.; Bermak, A. A CMOS Single-Chip Gas Recognition Circuit for Metal Oxide Gas Sensor Arrays. IEEE Trans. Circuits Syst. I Regul. Pap. 2011, 58, 1569–1580. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; He, T.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Mickelson, W.; Carraro, C.; Maboudian, R. In situ localized growth of porous tin oxide films on low power microheater platform for low temperature CO detection. ACS Sens. 2016, 1, 339–343. [Google Scholar] [CrossRef]
- Hong, S.; Wu, M.; Hong, Y.; Jeong, Y.; Jung, G.; Shin, W.; Park, J.; Kim, D.; Jang, D.; Lee, J.H. FET-type gas sensors: A review. Sens. Actuators B Chem. 2021, 330, 129240. [Google Scholar] [CrossRef]
- Field-Effect Transistor. Available online: https://en.wikipedia.org/wiki/Field-effect_transistor (accessed on 30 September 2022).
- Yenn, T.S. Electrochemical vs. Semiconductor Gas Detection—A Critical Choice. Industrial Automation Asia, August 2019; 42–43. [Google Scholar]
- Dirac, P.A.M.; Bohr, N.H.D. The quantum theory of the emission and absorption of radiation. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1927, 114, 243–265. [Google Scholar] [CrossRef]
- Grynberg, G.; Aspect, A.; Fabre, C.; Cohen-Tannoudji, C. Introduction to Quantum Optics: From the Semi-Classical Approach to Quantized Light; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar] [CrossRef]
- Palzer, S. Photoacoustic-Based Gas Sensing: A Review. Sensors 2020, 20, 2745. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.K. Non-Dispersive Infrared Gas Sensing Technology: A Review. IEEE Sens. J. 2022, 22, 6–15. [Google Scholar] [CrossRef]
- Jia, X.; Roels, J.; Baets, R.; Roelkens, G. On-Chip Non-Dispersive Infrared CO2 Sensor Based on an Integrating Cylinder. Sensors 2019, 19, 4260. [Google Scholar] [CrossRef]
- Haas, J.; Mizaikoff, B. Advances in Mid-Infrared Spectroscopy for Chemical Analysis. Annu. Rev. Anal. Chem. 2016, 9, 45–68. [Google Scholar] [CrossRef]
- Questions and Answers about Tunable Diode Laser Gas Spectroscopy (TDLS). Available online: https://www.axetris.com/en/lgd/frequently-asked-questions-tdls-technology (accessed on 30 September 2022).
- Chen, Y.; Wang, Z.; Li, Z.; Zheng, H.; Dai, J. Development of an Online Detection Setup for Dissolved Gas in Transformer Insulating Oil. Appl. Sci. 2021, 11, 12149. [Google Scholar] [CrossRef]
- Scholz, L.; Palzer, S. Photoacoustic-based detector for infrared laser spectroscopy. Appl. Phys. Lett. 2016, 109, 041102. [Google Scholar] [CrossRef]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Dudzik, G.; Krzempek, K.; Abramski, K.; Wysocki, G. Solid-state laser intra-cavity photothermal gas sensor. Sens. Actuators B Chem. 2021, 328, 129072. [Google Scholar] [CrossRef]
- Koppens, F.; Mueller, T.; Avouris, P.; Ferrari, A.; Vitiello, M.; Polini, M. Photodetectors based on graphene, other two-dimensional materials and hybrid systems. Nat. Nanotechnol. 2014, 9, 780–793. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.; Lin, H.; Deckoff-Jones, S.; Hempel, M.; Lu, A.Y.; Richardson, K.A.; Palacios, T.; Kong, J.; Hu, J.; Englund, D. Waveguide-integrated mid-infrared photodetection using graphene on a scalable chalcogenide glass platform. Nat. Commun. 2022, 13, 3915. [Google Scholar] [CrossRef]
- Rohman, A.; Windarsih, A.; Lukitaningsih, E.; Rafi, M.; Betania, K.; Fadzillah, N. A The use of FTIR and Raman spectroscopy in combination with chemometrics for analysis of biomolecules in biomedical fluids: A review. Biomed. Spectrosc. Imaging 2019, 8, 55–71. [Google Scholar] [CrossRef]
- Janata, J. Principles of Chemical Sensors; Springer: Boston, MA, USA, 2009. [Google Scholar]
- Hannon, A.; Lu, Y.; Li, J.; Meyyappan, M. A Sensor Array for the Detection and Discrimination of Methane and Other Environmental Pollutant Gases. Sensors 2016, 16, 1163. [Google Scholar] [CrossRef]
- Rubio, R.; Santander, J.; Fonseca, L.; Sabaté, N.; Gràcia, I.; Cané, C.; Udina, S.; Marco, S. Non-selective NDIR array for gas detection. Sens. Actuators B Chem. 2007, 127, 69–73. [Google Scholar] [CrossRef]
- Gupta, R.K.; Alqahtani, F.H.; Dawood, O.M.; Carini, M.; Criado, A.; Prato, M.; Garlapati, S.K.; Jones, G.; Sexton, J.; Persaud, K.C.; et al. Suspended graphene arrays for gas sensing applications. 2D Mater. 2020, 8, 025006. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Song, Z.; Ye, W.; Fan, Z. Smart gas sensor arrays powered by artificial intelligence. J. Semicond. 2019, 40, 111601. [Google Scholar] [CrossRef]
- Yaqoob, U.; Younis, M.I. Chemical Gas Sensors: Recent Developments, Challenges, and the Potential of Machine Learning—A Review. Sensors 2021, 21, 2877. [Google Scholar] [CrossRef] [PubMed]
- Michelucci, U.; Baumgartner, M.; Venturini, F. Optical Oxygen Sensing with Artificial Intelligence. Sensors 2019, 19, 777. [Google Scholar] [CrossRef]
- Kang, M.; Cho, I.; Park, J.; Jeong, J.; Lee, K.; Lee, B.; Del Orbe Henriquez, D.; Yoon, K.; Park, I. High accuracy real-time multi-gas identification by a batch-uniform gas sensor array and deep learning algorithm. ACS Sens. 2022, 7, 430–440. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Multivariable sensors for ubiquitous monitoring of gases in the era of internet of things and industrial internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Meier, D.C.; Raman, B.; Semancik, S. Detecting Chemical Hazards with Temperature-Programmed Microsensors: Overcoming Complex Analytical Problems with Multidimensional Databases. Annu. Rev. Anal. Chem. 2009, 2, 463–484. [Google Scholar] [CrossRef] [PubMed]

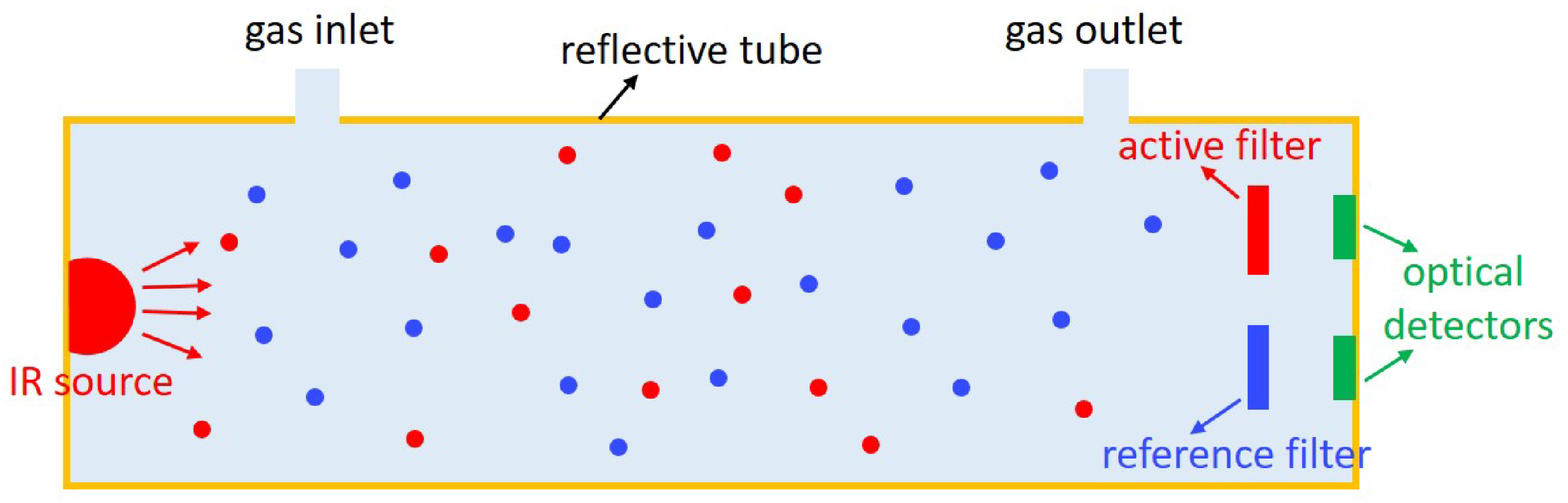
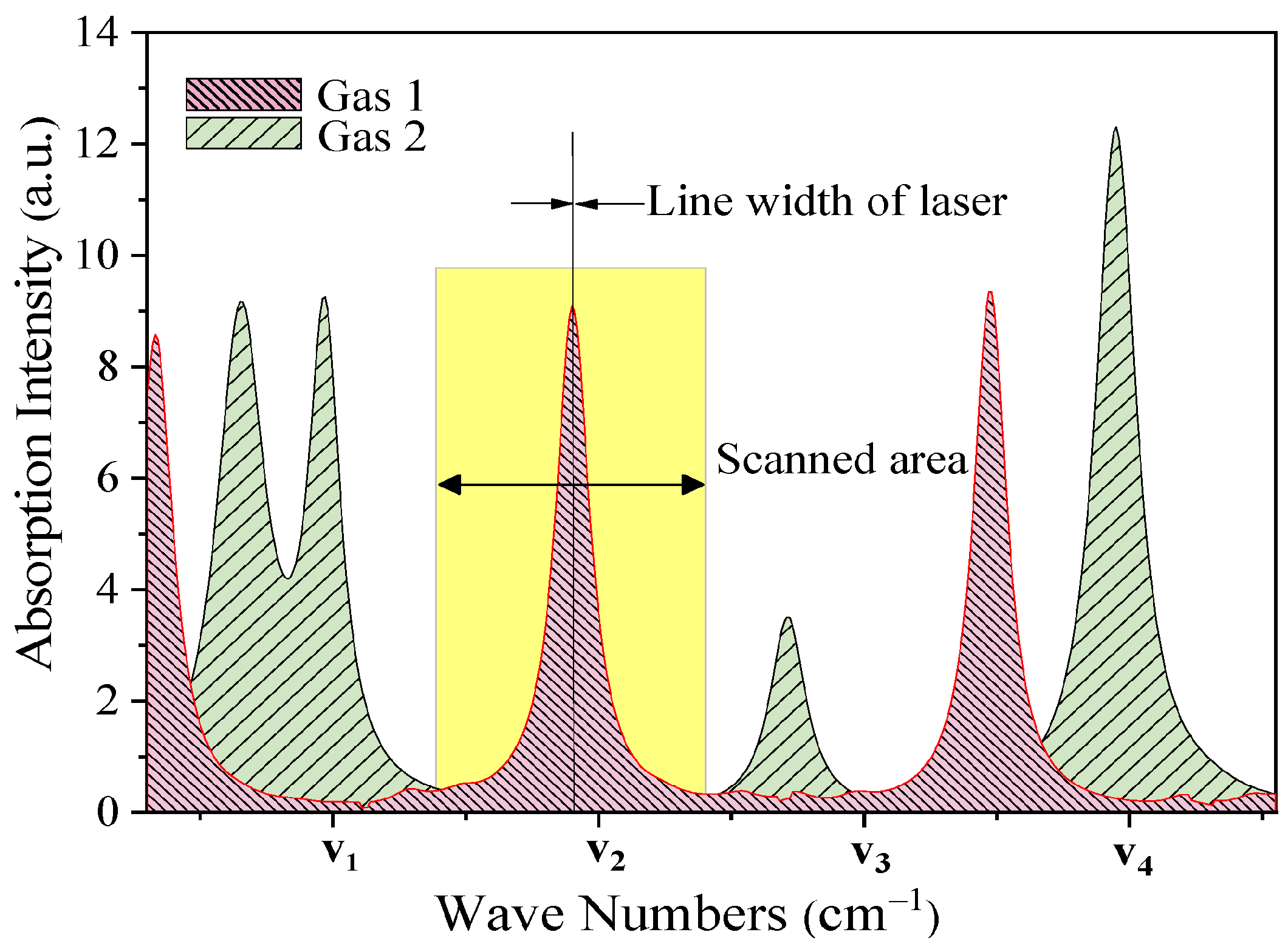
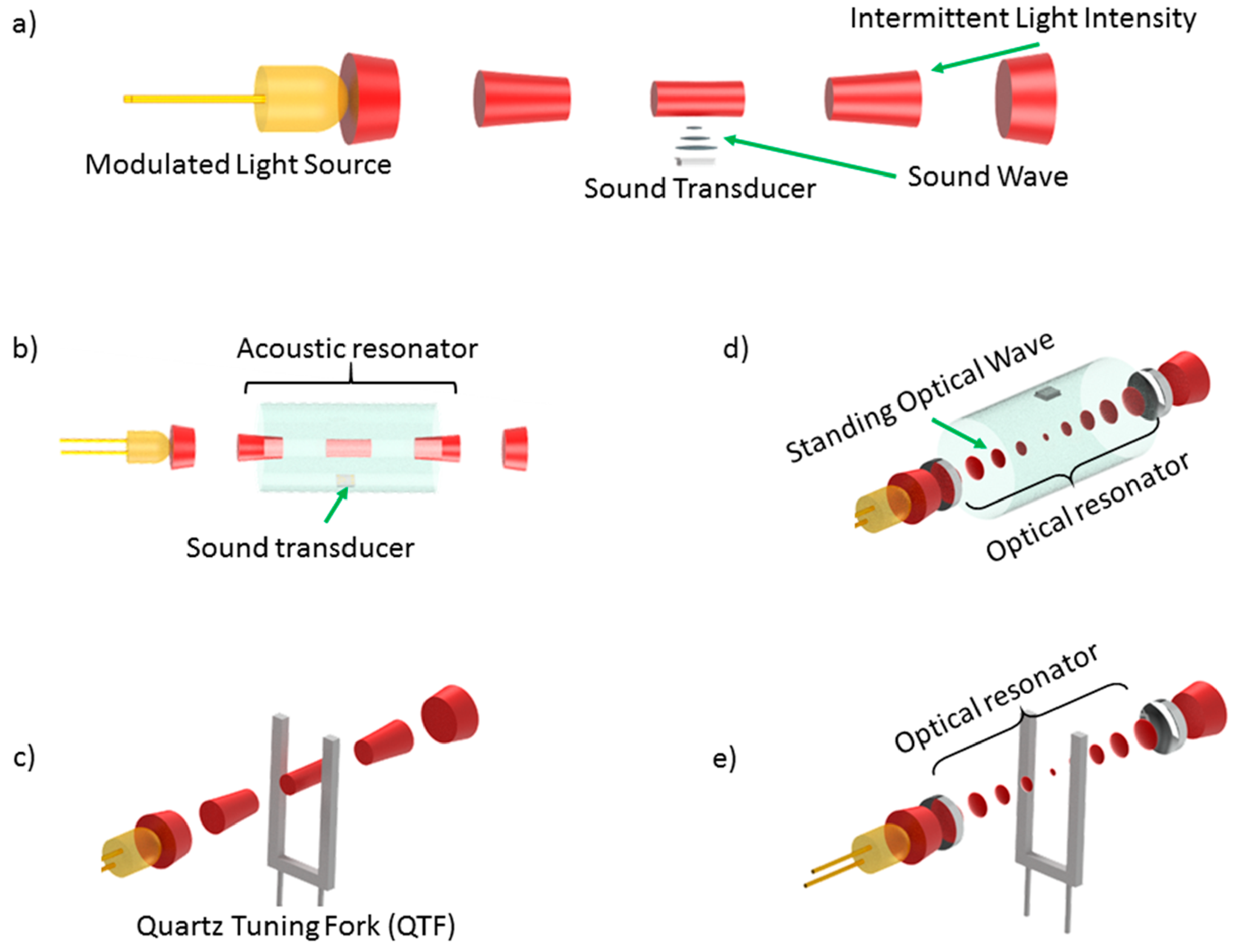
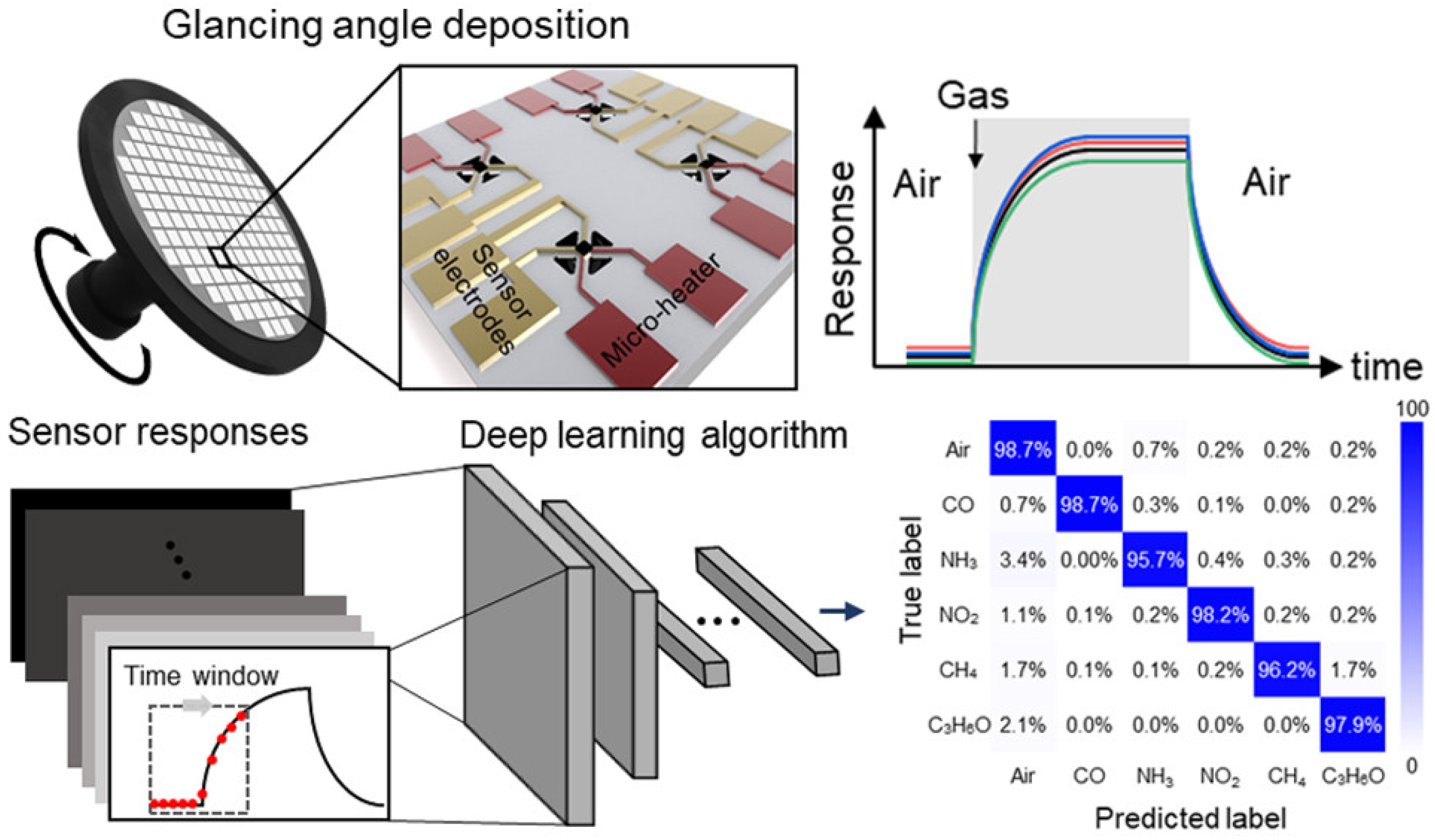
| Infrared | EC | MOS | Catalytic | Heated Diode | ||
|---|---|---|---|---|---|---|
| Features | ||||||
| Cost | Handheld | USD 300–400 | USD 100–500 | |||
| Stationary | USD 1000–12,000 | USD 250–1600 | USD 500–1300 | USD 700–1500 | ||
| Sensing element | USD 100–200 | USD 3–100 | USD 50–100 | |||
| Size | 1–20 lbs. | 0.5–4 lbs. | 1 × 1 × 1 in | 2–3.5 lbs. | Handheld system | |
| Power requirements | 13–30 VDC 4–5 W | 12–30 VDC 4–10 W | 12–24 VDC 1–5 W | 12–24 VDC 1 –10 W | Battery-operated | |
| Refrigerant types | HFCs, HFOs, HCs, CFCs, HCFCs | CFC, HFCs, HCFCs, HFOs | HCs, , other flammable gases | HFCs, HFOs, and blends | ||
| Calibration | PIR: Required every 6 months NDIR: Calibration not required. Re-zeroing is required every C internal temp. change or every year | Required every 12 months | Recommended every 6 months | Required every 3–6 months depending on environment where used | Automatic or manual zeroing | |
| Limitations | ||||||
| Measurement range | 0–10,000 ppm | 0–1000 ppm | 20–10,000 ppm | 0–1000 ppm 0–100% LEL | 6.6- oz/yr high/low sensitivity | |
| Response time | Single-zone: 5–30 s Multi-zone: 5–300 s | T90: <90 s | T90: 15–90 s | T50: 5–10 s T90: 20–30 s | 0.5–1 s warm-up: 30 s recovery: ∼9 s | |
| Operating temperature | −40–167 F −40–75 C | −4–122 F −20–50 C | −30–158 F −34–170 C | −40–300 F −40–150 C | −4–122 F −20–50 C | |
| Humidity | 0–10% some sensors require non-condensing environment | 15–90% | 0–95% | 0–95% | unknown | |
| Vibration | Sensor can be placed inside a protective structure | Sensor can be placed inside a protective structure | Should not be affected by normal workplace vibrations | Typically, not impactful | n/a | |
| False-triggering chemicals | None | Organic solvents (e.g., alcohols, acetone), cross-sensitivity with other gases | Gasoline, diesel, and propane exhaust, fumes from solvents, paints, and cleansers | None | Moisture, oils, sensors are not selective | |
| Interfering chemicals | Acetylene, overexposure of refrigerant gas | None | Ethanol, silicones, highly corrosive gases, alkaline metals, overexposure to refrigerant, condensation | Silicone or sulfur, heavy metals, halogenated hydrocarbons, overexposure to refrigerant, poisoning | Moisture, oils, overexposure to refrigerant | |
| Reliability | ||||||
| Lifetime | Handheld: 5 years Stationary: 10–15 years | 1–3 years (based on exposure to gas) | 3–5 years | 2–5 years | 2–3 years, up to 5 years | |
| Repairable | Replace air filters every year | EC cell can be replaced | Sensing element can be replaced | Sensing element can be replaced | Sensing element and filters can be replaced | |
| Self-testing abilities | Certain monitoring devices incorporate active diagnostics that continuously monitor the system for proper operation | None observed | Compensator element acts as a constant control mechanism | n/a | ||
| Letter Code | Sensor Type | % of Requirements Passed | Average Time Delay, [s] | Average Time Constant, [s] | [s] |
|---|---|---|---|---|---|
| A | MMM | 100% | 4.5 | 0.25 | 4.75 |
| B | NDIR | 96% | 1.6 | 15.8 | 17.4 |
| C | TC | 86% | 0.0 | 0.1 | 0.1 |
| D | NDIR | 79% | 0.1 | 13.7 | 13.8 |
| E | MOS | 75% | Cannot be determined | ||
| F | MOS | 64% | Cannot be determined | ||
| Attribute | Range | Test method |
|---|---|---|
| Concentration/accuracy |
| Signal response vs. concentration |
| Time constant/response time |
| Signal response time after leak initiation |
| Sensitivity and selectivity |
| Presence of humidity, temperature, , other refrigerants, poison species: co-injection |
| Repeatability |
| Simultaneous evaluation |
| Reliability |
| Exposure to different test conditions |
| Location |
| Impact of density |
| Robustness (vibration/shock) | – | Vibration sensitivity, shock sensitivity |
| Flexibility |
| Cross compatibility feasibility |
| Structure | Description | Typical Morphology |
|---|---|---|
| 0-dimensional | All dimensions are nanometric | Quantum dot, nanoparticles, clusters |
| 1-dimensional | Two dimensions are nanometric | Nanowires, nanorods, nanotubes, nanofibers |
| 2-dimensional | One dimension is nanometric | Thin film, nanosheets, nanobelts, nanoplates |
| 3-dimensional | Composed of lower-dimensional structures | Nanoflowers, nanospheres |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reshniak, V.; Cheekatamarla, P.; Sharma, V.; Yana Motta, S. A Review of Sensing Technologies for New, Low Global Warming Potential (GWP), Flammable Refrigerants. Energies 2023, 16, 6499. https://doi.org/10.3390/en16186499
Reshniak V, Cheekatamarla P, Sharma V, Yana Motta S. A Review of Sensing Technologies for New, Low Global Warming Potential (GWP), Flammable Refrigerants. Energies. 2023; 16(18):6499. https://doi.org/10.3390/en16186499
Chicago/Turabian StyleReshniak, Viktor, Praveen Cheekatamarla, Vishaldeep Sharma, and Samuel Yana Motta. 2023. "A Review of Sensing Technologies for New, Low Global Warming Potential (GWP), Flammable Refrigerants" Energies 16, no. 18: 6499. https://doi.org/10.3390/en16186499
APA StyleReshniak, V., Cheekatamarla, P., Sharma, V., & Yana Motta, S. (2023). A Review of Sensing Technologies for New, Low Global Warming Potential (GWP), Flammable Refrigerants. Energies, 16(18), 6499. https://doi.org/10.3390/en16186499







