The Effect of Carbon Nanofibers on the Hydrocracking of Vacuum Residue in the Presence of Formic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents Used
2.2. FA Decomposition Reaction
2.3. Experimental Setup for the Hydrocracking Process
2.4. Analysis of Vacuum Residue and the Products Formed from It
2.5. Chromatographic Analysis of Gaseous Reaction Products
- (1)
- The first part was introduced into a flame ionization detector via an St®-PLOT/Si capillary column (25 m × 0.32 mm × 3 μm) with a SiO2 stationary phase. Ar was used as the carrier gas.
- (2)
- The second part of the sample was introduced into a thermal conductivity detector via a column filled with P1387 coal. Ar was used as the carrier gas.
2.6. Electron Microscopy
3. Results and Discussion
3.1. Formic Acid Decomposition
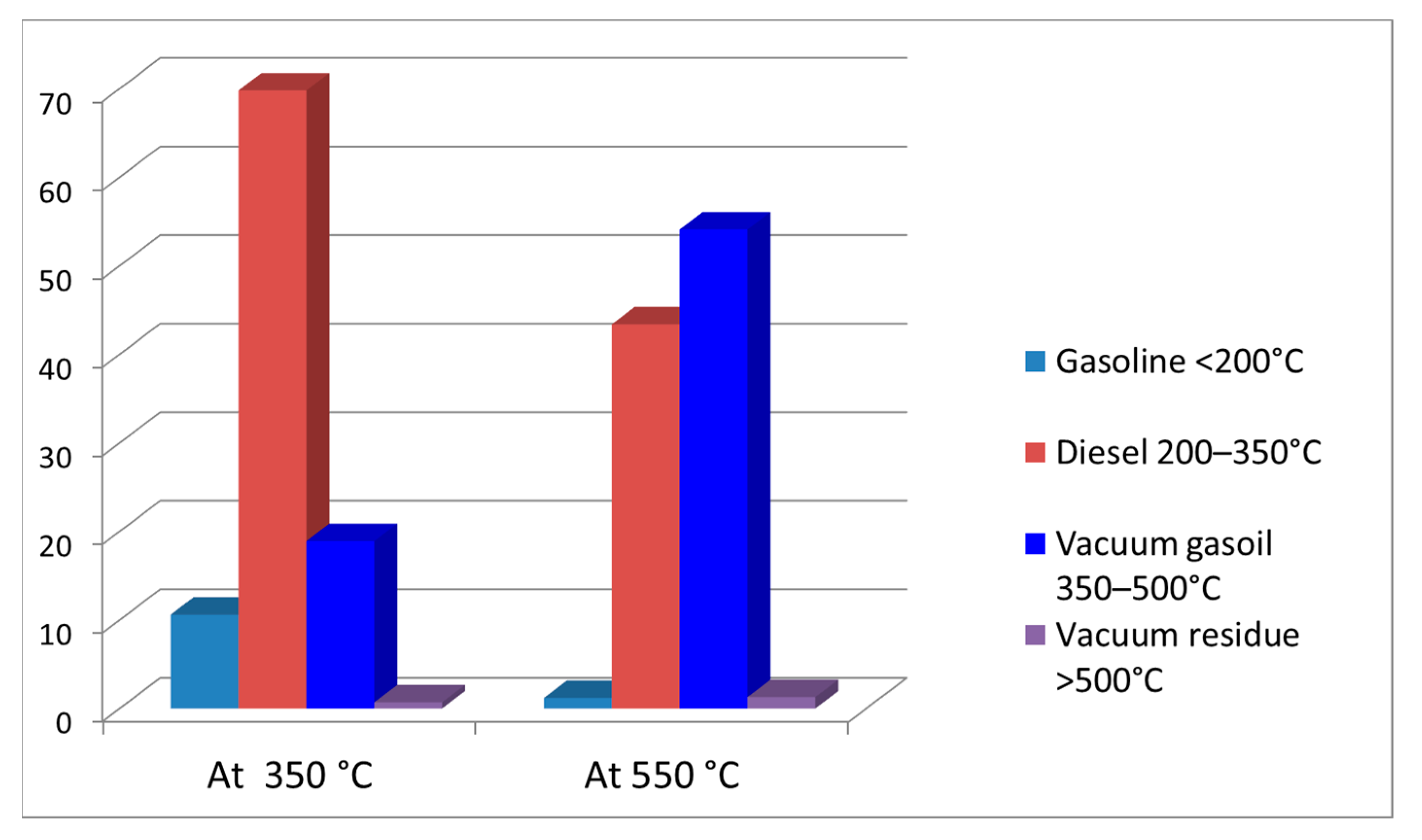
3.2. Thermal Cracking of Vacuum Residue
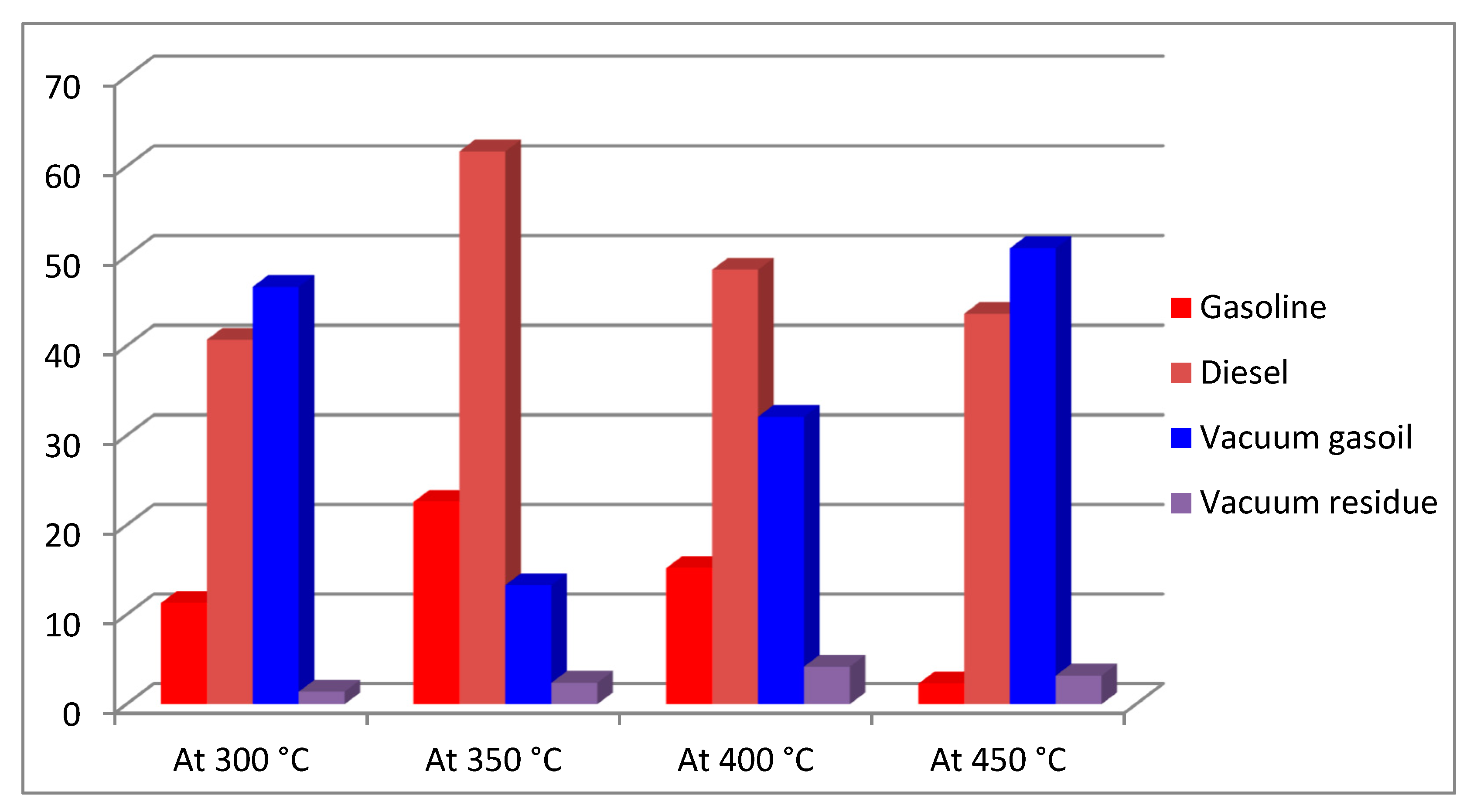
3.3. Hydrocracking of Vacuum Residue in the Presence of FA
3.4. Effect of CNFs Treated with NaOH on the Hydrocracking of Vacuum Residue in the Presence of FA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| CNF | carbon nanofiber |
| FA | formic acid |
| TEM | transmission electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| BET | Brunauer–Emmett–Teller |
| XRD | X-ray diffraction |
| WGS | water–gas shift reaction |
References
- Hosseinpour, M.; Fatemi, S.; Ahmadi, S.J.; Morimoto, M.; Akizuki, M.; Oshima, Y.; Fumoto, E. The synergistic effect between supercritical water and redox properties of iron oxide nanoparticles during in-situ catalytic upgrading of heavy oil with formic acid. Isotopic study. Appl. Catal. B Environ. 2018, 230, 91–101. [Google Scholar] [CrossRef]
- Castañeda, L.C.; Muñoz, J.A.D.; Ancheyta, J. Current situation of emerging technologies for upgrading of heavy oils. Catal. Today 2014, 220–222, 248–273. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-situ heavy and extra-heavy oil recovery: A review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Ancheyta, J. Modeling of Processes and Reactors for Upgrading of Heavy Petroleum; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- ExxonMobil: Diesel Will Surpass Gasoline as the Number One Global Transportation Fuel by 2020, Green Car Congress, 2013. Available online: https://www.greencarcongress.com/2013/03/xom-20130309.html (accessed on 13 March 2013).
- Yan, T.; Xu, J.; Wang, L.; Liu, Y.; Yang, C.; Fang, T. A review of upgrading heavy oils with supercritical fluids. RSC Adv. 2015, 5, 75129–75140. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Nikitin, A.V.; Arutyunov, V.S. Current trends in the production of hydrogen from hydrocarbon feedstock. Sci. J. Russ. Gas Soc. 2020, 1, 50–68. (In Russian) [Google Scholar]
- Hart, A.; Lewis, C.; White, T.; Greaves, M.; Wood, J. Effect of cyclohexane as hydrogen-donor in ultradispersed catalytic upgrading of heavy oil. Fuel Process. Technol. 2015, 138, 724–733. [Google Scholar] [CrossRef]
- Zachariah, A.; Wang, L.; Yang, S.; Prasad, V.; de Klerk, A. Suppression of Coke Formation during Bitumen Pyrolysis. Energy Fuels 2013, 27, 3061–3070. [Google Scholar] [CrossRef]
- Habib, F.K.; Diner, C.; Stryker, J.M.; Semagina, N.; Gray, M.R. Suppression of Addition Reactions during Thermal Cracking Using Hydrogen and Sulfided Iron Catalyst. Energy Fuels 2013, 27, 6637–6645. [Google Scholar] [CrossRef]
- Alshareef, A.H.; Scherer, A.; Tan, X.; Azyat, K.; Stryker, J.M.; Tykwinski, R.R.; Gray, M.R. Formation of Archipelago Structures during Thermal Cracking Implicates a Chemical Mechanism for the Formation of Petroleum Asphaltenes. Energy Fuels 2011, 25, 2130–2136. [Google Scholar] [CrossRef]
- Ovalles, C.; Vallejos, C.; Vásquez, T.; Martinis, J.; Perez-Perez, A.; Cotte, E.; Castellanos, L.; Rodríguez, H. Extra-Heavy Crude Oil Downhole Upgrading Process Using Hydrogen Donors under Steam Injection Conditions. In Proceedings of the SPE International Thermal Operations and Heavy Oil Symposium, Porlamar, Venezuela, 12–14 March 2001. Paper Number: SPE-69692-MS. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, P.; Fan, H.; Meng, Q.; Duan, A.; Chen, Z.; Xu, C. Insights into the effect of solvent on dibenzothiophene hydrodesulfurization. Fuel 2021, 287, 119459. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Soltani, M.; Noofeli, A.; Nathwani, J. An optimization study on heavy oil upgrading in supercritical water through the response surface methodology (RSM). Fuel 2020, 271, 117618. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Dik, P.P.; Chichkan, A.S. Formic Acid as a Hydrogen Donor for Catalytic Transformations of Tar. Energies 2020, 13, 4515. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Dik, P.P.; Nikityonok, A.V.; Chichkan, A.S.; Parmon, V.N. Comparative Analysis of the Effects of Hydrogen and Formic Acid on the Vacuum Residue Hydrocracking. Chem. Eng. J. 2022, 449, 137839. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Bielawski, C.W. Carbocatalysis: Heterogeneous carbons finding utility in synthetic chemistry. Chem. Sci. 2011, 2, 1233–1240. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by graphene-based materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef]
- Ding, Y.; Greiner, M.; Schlögl, R.; Heumann, S. A Metal-Free Electrode: From Biomass-Derived Carbon to Hydrogen. ChemSusChem 2020, 13, 4064–4068. [Google Scholar] [CrossRef]
- Gu, Q.; Ding, Y.; Liu, Z.; Lin, Y.; Schlögl, R.; Heumann, S.; Su, D. Probing the intrinsic catalytic activity of carbon nanotubes for the metal-free oxidation of aromatic thiophene compounds in ionic liquids. J. Energy Chem. 2019, 32, 131–137. [Google Scholar] [CrossRef]
- Wu, S.; Wen, G.; Schlögl, R.; Su, D.S. Carbon nanotubes oxidized by a green method as efficient metal-free catalysts for nitroarene reduction. Phys. Chem. Chem. Phys. 2015, 17, 1567–1571. [Google Scholar] [CrossRef]
- Boehm, H.P. Free radicals and graphite. Carbon 2012, 50, 3154–3157. [Google Scholar] [CrossRef]
- Khavryuchenko, O.V.; Frank, B.; Trunschke, A.; Hermann, K.; Schlögl, R. Quantum-Chemical Investigation of Hydrocarbon Oxidative Dehydrogenation over Spin-Active Carbon Catalyst Clusters. J. Phys. Chem. 2013, 117, 6225–6234. [Google Scholar] [CrossRef]
- Avgul, N.N.; Kiselev, A.V.; Poshkus, D.P. Adsorption of Gases and Vapors on Homogeneous Surfaces; Chemistry: Moscow, Russia, 1975; p. 384. [Google Scholar]
- Abrahamson, J. The Surface Energies of Graphite. Carbon 1973, 11, 337–362. [Google Scholar] [CrossRef]
- Chesnokov, V.V.; Chichkan, A.S. Production of Hydrogen by Methane Catalytic Decomposition over Ni–Cu–Fe/Al2O3 Catalyst. Int. J. Hydrogen Energy 2009, 34, 2979–2985. [Google Scholar] [CrossRef]
- Jia, L.; Bulushev, D.A.; Ross, J.R.H. Formic acid decomposition over palladium based catalysts doped by potassium carbonate. Catal. Today 2016, 259, 453–459. [Google Scholar] [CrossRef]
- Jia, L.; Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. Hydrogen production from formic acid vapour over a Pd/C catalyst promoted by potassium salts: Evidence for participation of buffer-like solution in the pores of the catalyst. Appl. Catal. B Environ. 2014, 160–161, 35–43. [Google Scholar] [CrossRef]
- ASTM D7169; Standard Test Method for Boiling Point Distribution of Samples with Residues Such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D4294; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2021.
- Speight, J.G. The Chemistry and Technology of Petroleum; Marcel Dekker: New York, NY, USA, 1980. [Google Scholar]
- Dickakian, G. Process for Deasphaltenating Cat Cracker Bottoms and for Production of Anisotropic Pitch. U.S. Patent US4427531A, 24 January 1984. [Google Scholar]
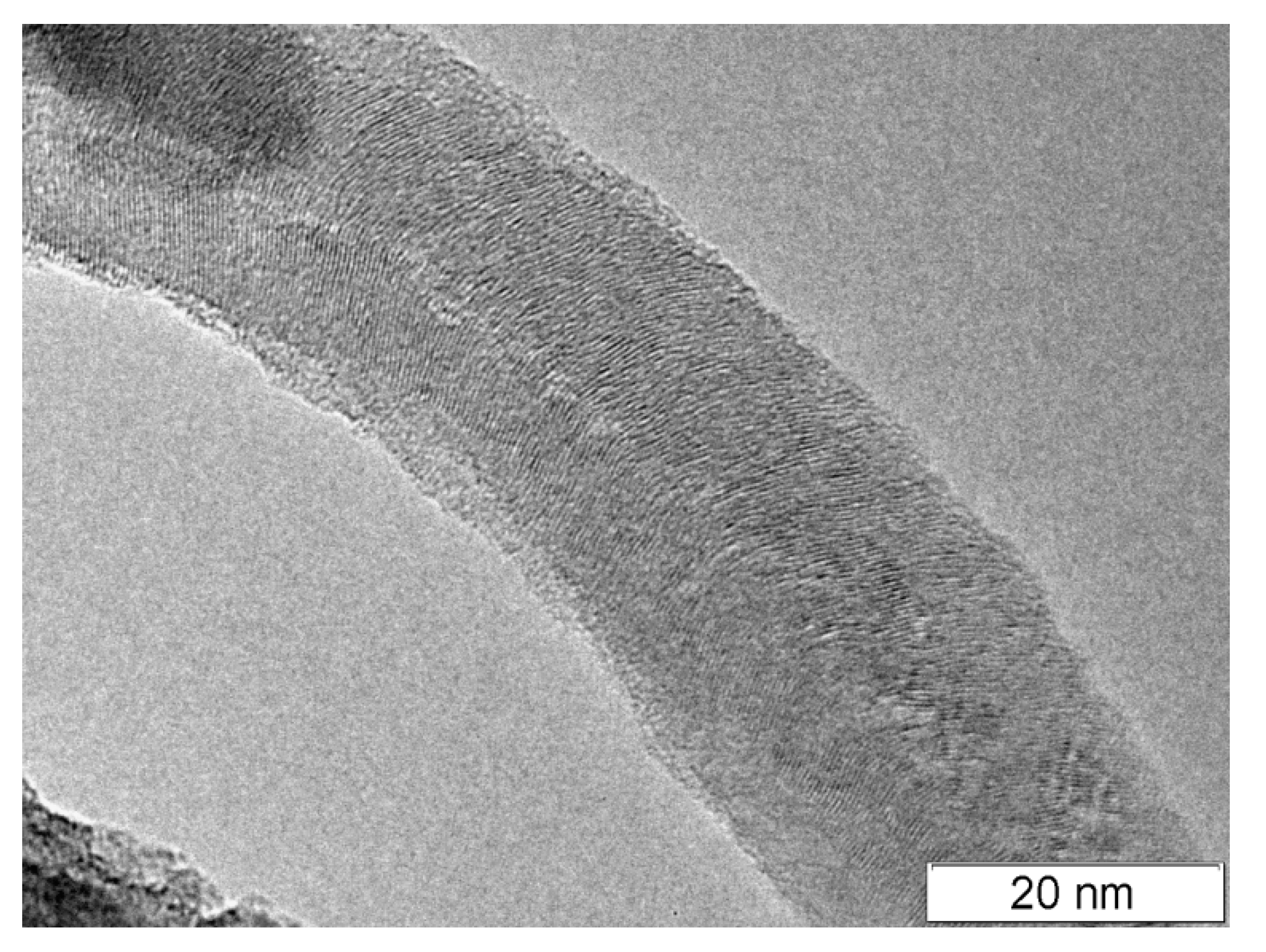
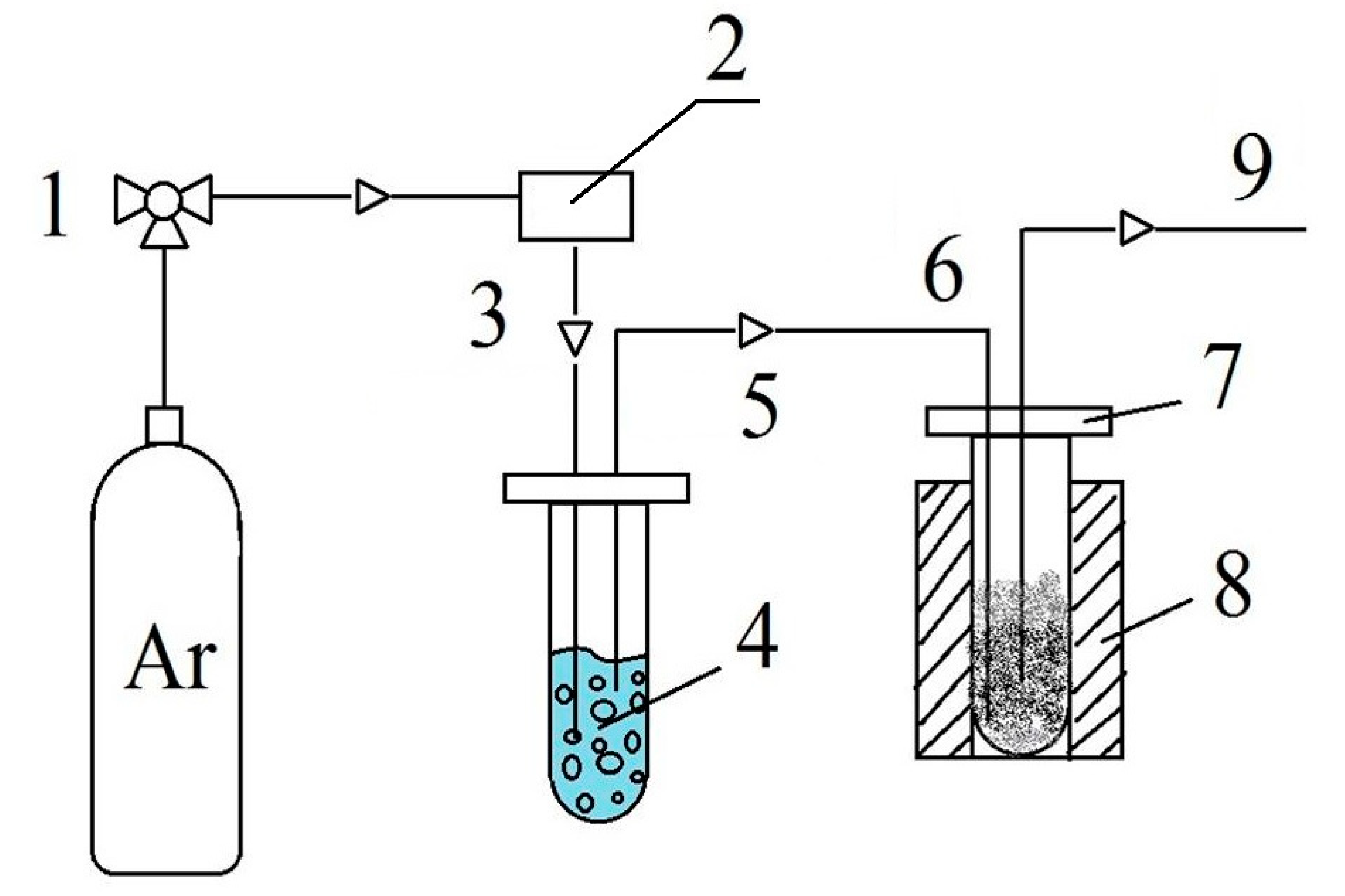



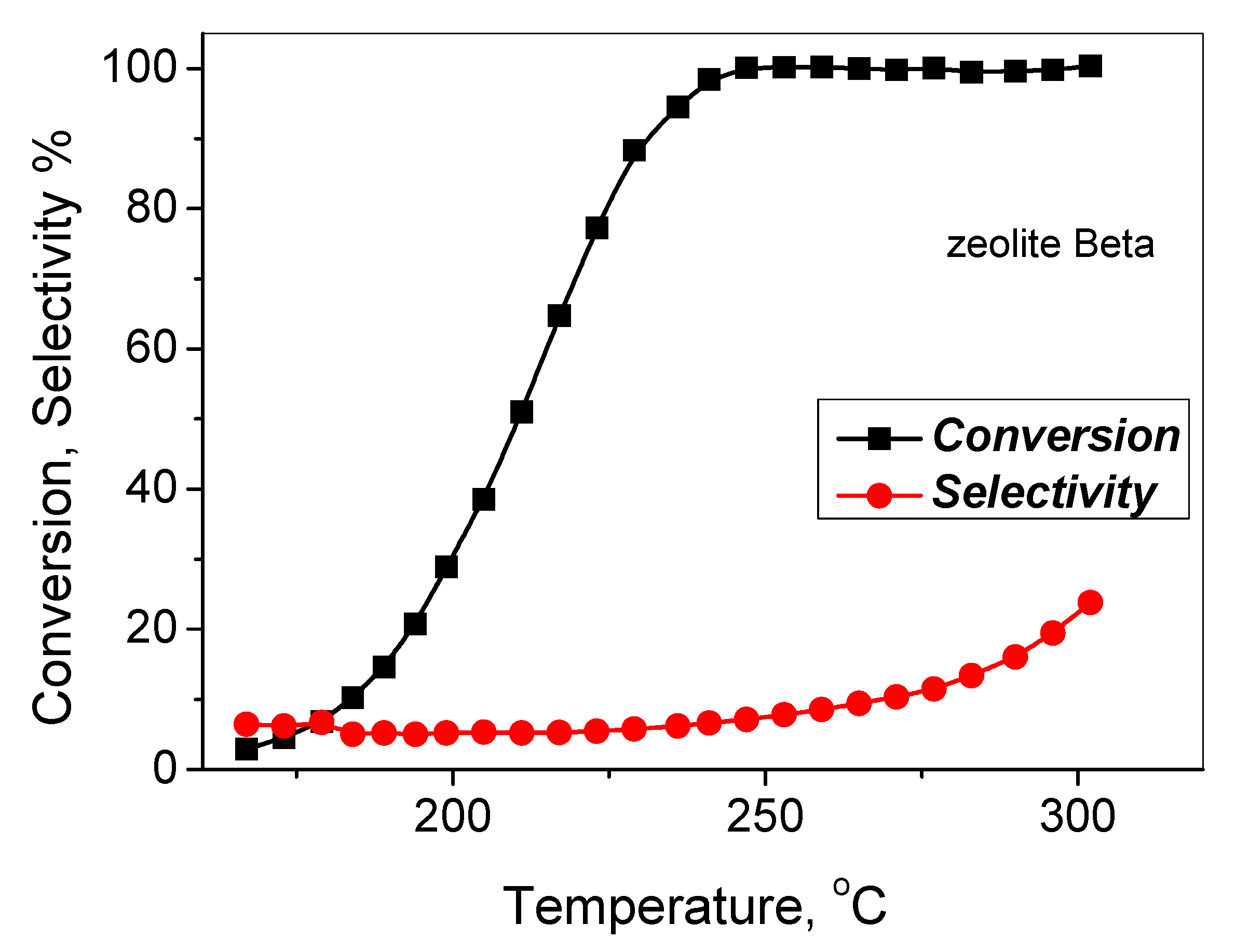
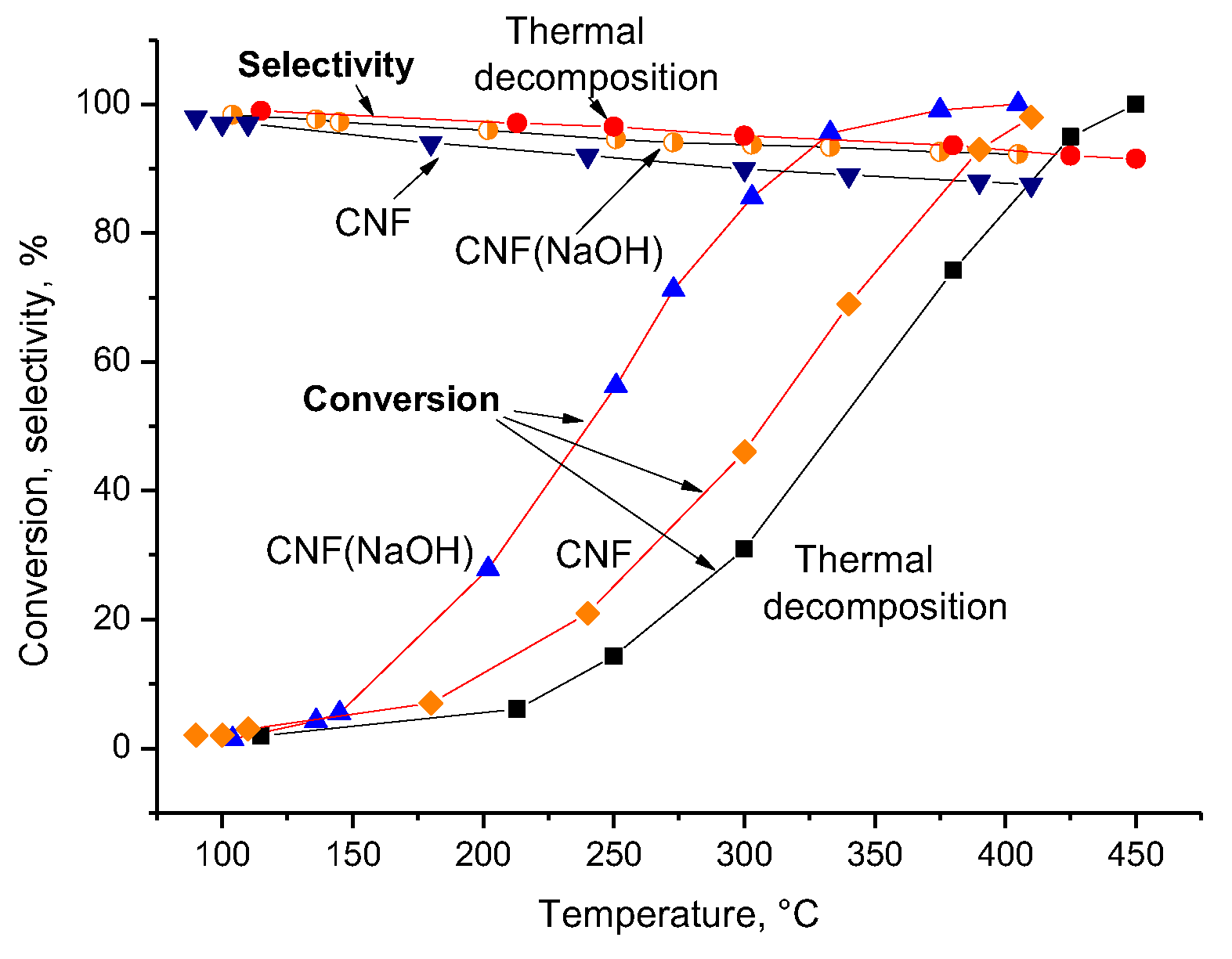



| Zeolite | Vmicro, cm3/g | Vmeso, cm3/g | Vtotal, cm3/g | SBET, m2/g | Average Size of the Crystals, nm |
|---|---|---|---|---|---|
| BEA | 0.22 | 0.15 | 0.37 | 660 | 160 |
| Characteristic | Value |
|---|---|
| Average external diameter, nm | 25–100 |
| Specific surface area, m2/g | 120 |
| Apparent density, g/cm3 | 0.45 |
| Temperature, °C | Conversion of the Vacuum Residue | Selectivity, % | ||
|---|---|---|---|---|
| Gas * | Liquid Hydrocarbons | Coke ** | ||
| 300 | 10 | 5 | 5 | 0 |
| 350 | 52 | 20 | 20 | 12 |
| 400 | 70 | 32 | 23 | 15 |
| 450 | 87 | 48 | 22 | 17 |
| 500 | 91 | 53 | 20 | 18 |
| 550 | 95 | 59 | 17 | 19 |
| 600 | 97 | 67 | 10 | 20 |
| Temperature, °C | Conversion of the Vacuum Residue | Selectivity, % | ||
|---|---|---|---|---|
| Gas * | Liquid Hydrocarbons | Coke ** | ||
| 250 | 13 | 6 | 7 | 0 |
| 300 | 45 | 12 | 28 | 5 |
| 350 | 69 | 28 | 27 | 14 |
| 400 | 85 | 43 | 25 | 17 |
| 450 | 93 | 54 | 21 | 18 |
| 500 | 97 | 61 | 17 | 19 |
| 550 | 100 | 69 | 11 | 20 |
| Temperature, °C | Conversion of the Vacuum Residue | Selectivity, % | |||
|---|---|---|---|---|---|
| Gas * | Liquid Hydrocarbons | Coke ** | |||
| Sulfur Concentration | |||||
| 250 | 23 | 8 | 15 | 1.05 | 0 |
| 300 | 54 | 15 | 34 | 1.00 | 5 |
| 350 | 76 | 32 | 29 | 0.82 | 15 |
| 400 | 89 | 46 | 24 | 0.97 | 19 |
| 450 | 96 | 57 | 19 | 1.33 | 20 |
| 500 | 99 | 65 | 13 | 1.5 | 21 |
| Vacuum Residue | Vacuum Residue + FA | Vacuum Residue + FA + CNF (NaOH) | |
|---|---|---|---|
| Sulfur concentration in liquid products, % | 1.0 | 0.98 | 0.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chesnokov, V.V.; Dik, P.P. The Effect of Carbon Nanofibers on the Hydrocracking of Vacuum Residue in the Presence of Formic Acid. Energies 2023, 16, 6477. https://doi.org/10.3390/en16186477
Chesnokov VV, Dik PP. The Effect of Carbon Nanofibers on the Hydrocracking of Vacuum Residue in the Presence of Formic Acid. Energies. 2023; 16(18):6477. https://doi.org/10.3390/en16186477
Chicago/Turabian StyleChesnokov, Vladimir V., and Pavel P. Dik. 2023. "The Effect of Carbon Nanofibers on the Hydrocracking of Vacuum Residue in the Presence of Formic Acid" Energies 16, no. 18: 6477. https://doi.org/10.3390/en16186477
APA StyleChesnokov, V. V., & Dik, P. P. (2023). The Effect of Carbon Nanofibers on the Hydrocracking of Vacuum Residue in the Presence of Formic Acid. Energies, 16(18), 6477. https://doi.org/10.3390/en16186477






