Benchmarking Electrolytes for the Solid Oxide Electrolyzer Using a Finite Element Model
Abstract
:1. Introduction
- To develop a theoretical model to assess polarization and impedance for three different SOECs based on the following criteria: the model shall evaluate the performances of three electrolyte materials, namely yttrium-stabilized zirconia (YSZ), gadolinium-doped ceria (GDC), and scandium-, cerium-, and gadolinium-doped zirconia (SCGZ).
- To ensure that the model reveals how operating conditions such as temperature and pressure affect the performance of cell.
2. Materials and Methods
2.1. Multiphysics Modeling
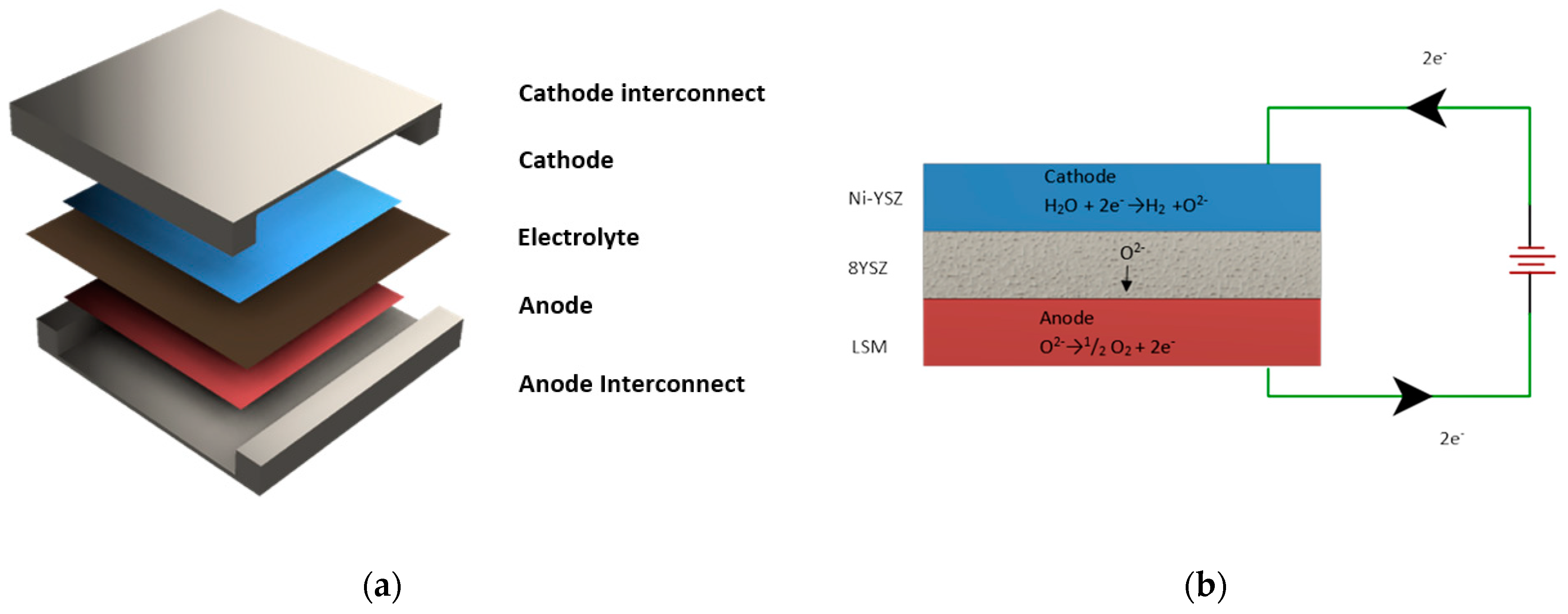
2.1.1. Model Description
- (i)
- Continuity equation.
- (ii)
- Momentum-conservation equation (Brinkman equation—modified Navier–Stokes).
- (iii)
- Species conservation (mass balance).
- (iv)
- Charge balance and electrochemical kinetics (Butler–Volmer equation and Nernst equation).
- (1)
- The kinetics and the mass transfer occurring the cell are in steady state.
- (2)
- Model is operated only in low- and intermediate-current regions and, thus, the mass-transport loss is assumed be negligible and ignored.
- (3)
- The gas species involved in the reaction follows ideal gas law.
- (4)
- The species are assumed to follow Newton’s law of viscosity.
- (5)
- All flows are assumed to be laminar due to the low velocity of gas species.
2.1.2. Governing Equations
- Transport in Gas Channel
- Transport in Electrode
- Electrochemical Equations and Charge Balance
- Impedance Equations
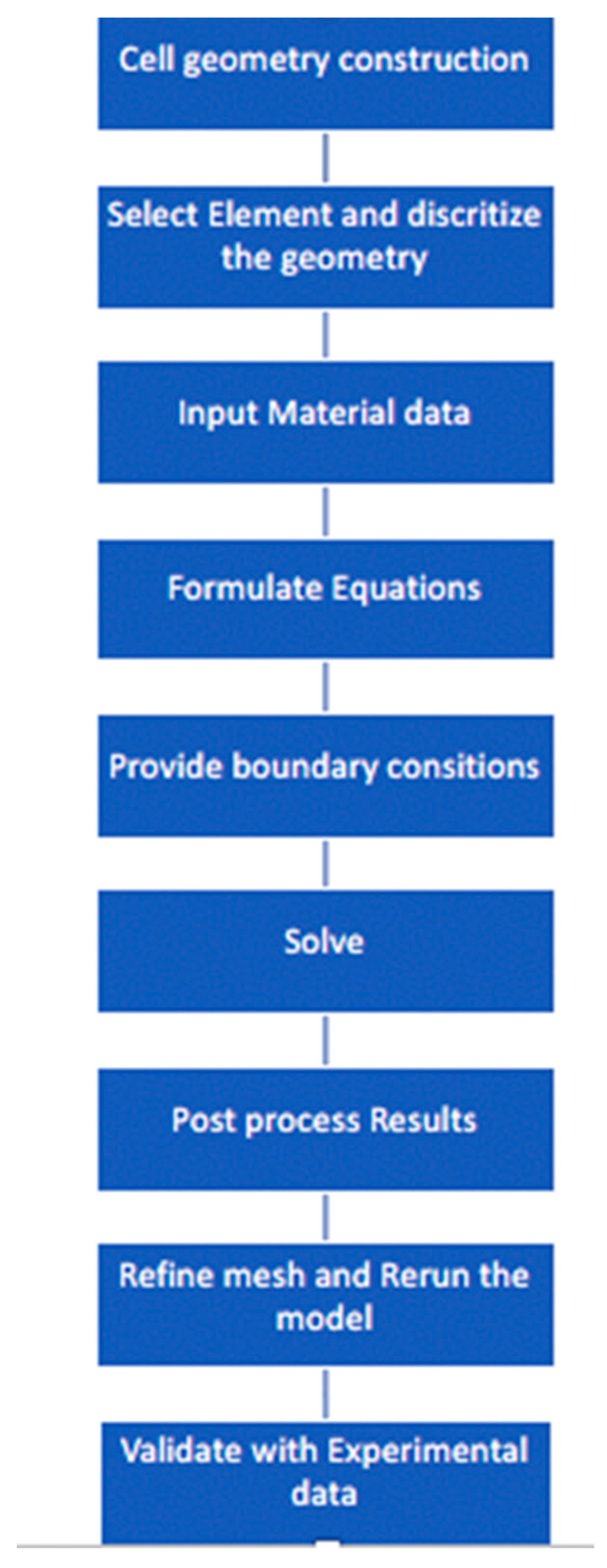
2.1.3. Numerical Details
2.1.4. Validation Criteria
3. Results and Discussion
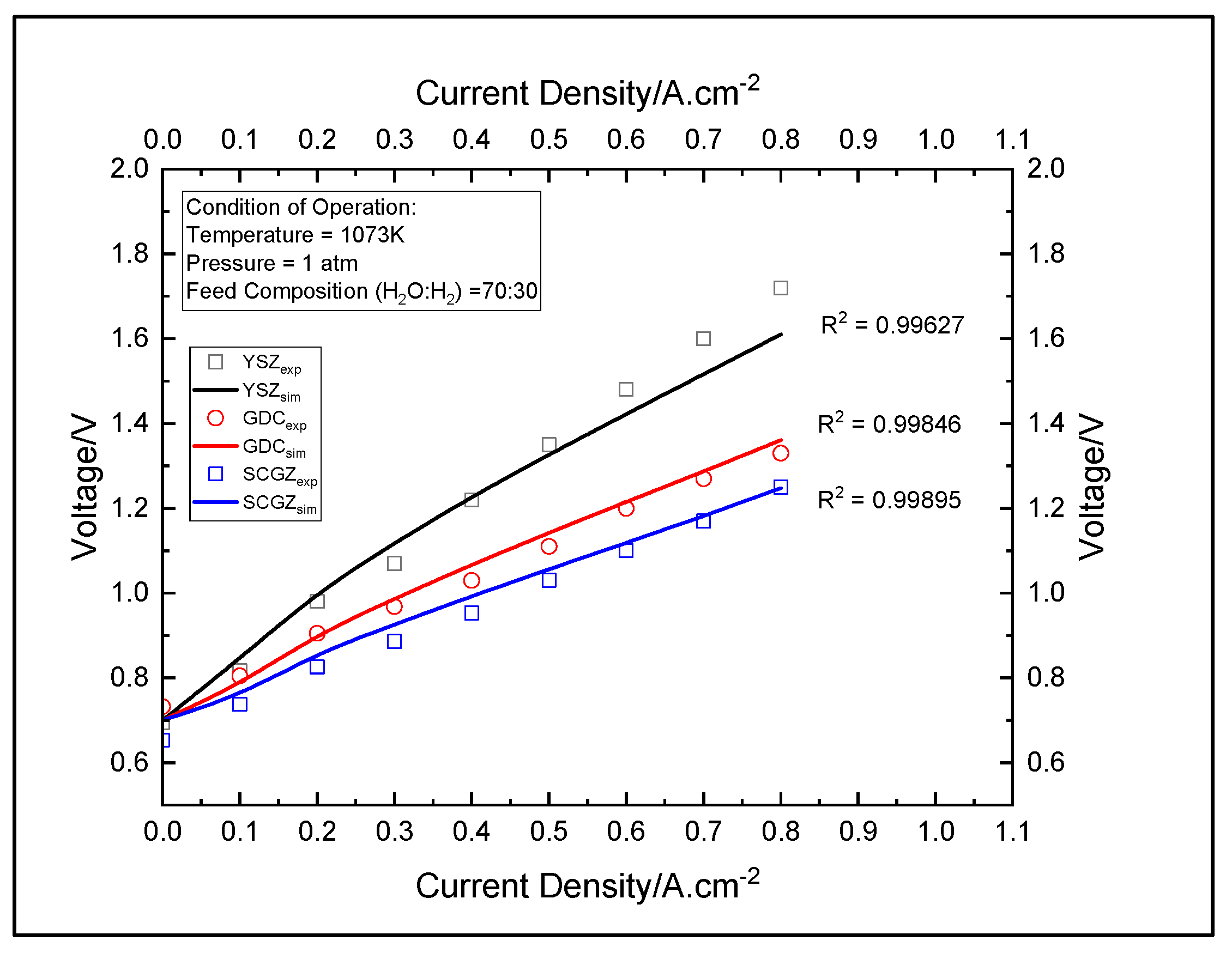
3.1. Validity of the Model
3.2. Comparison of the Electrolytes
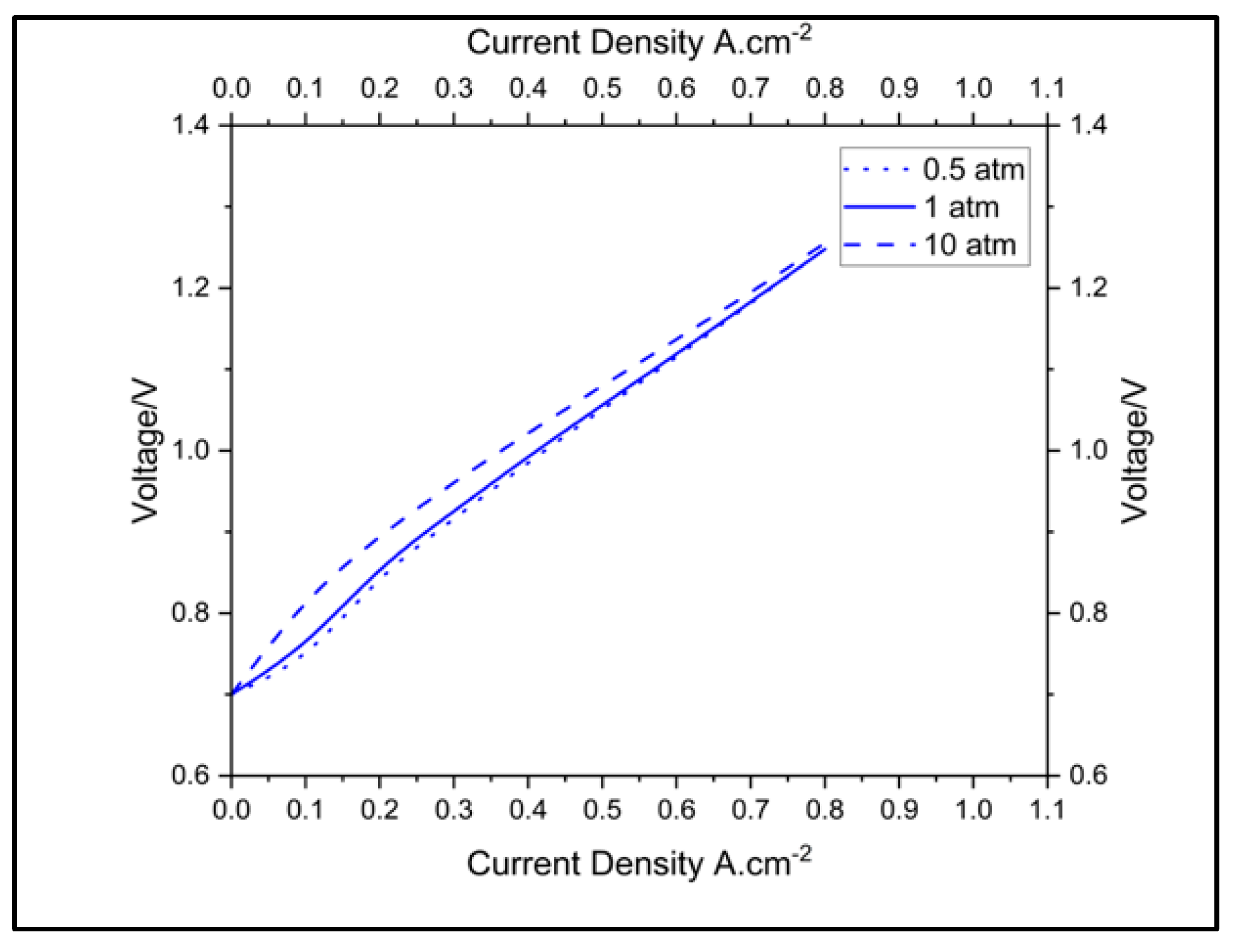
3.3. Effects of Operating Conditions on Cell Performance
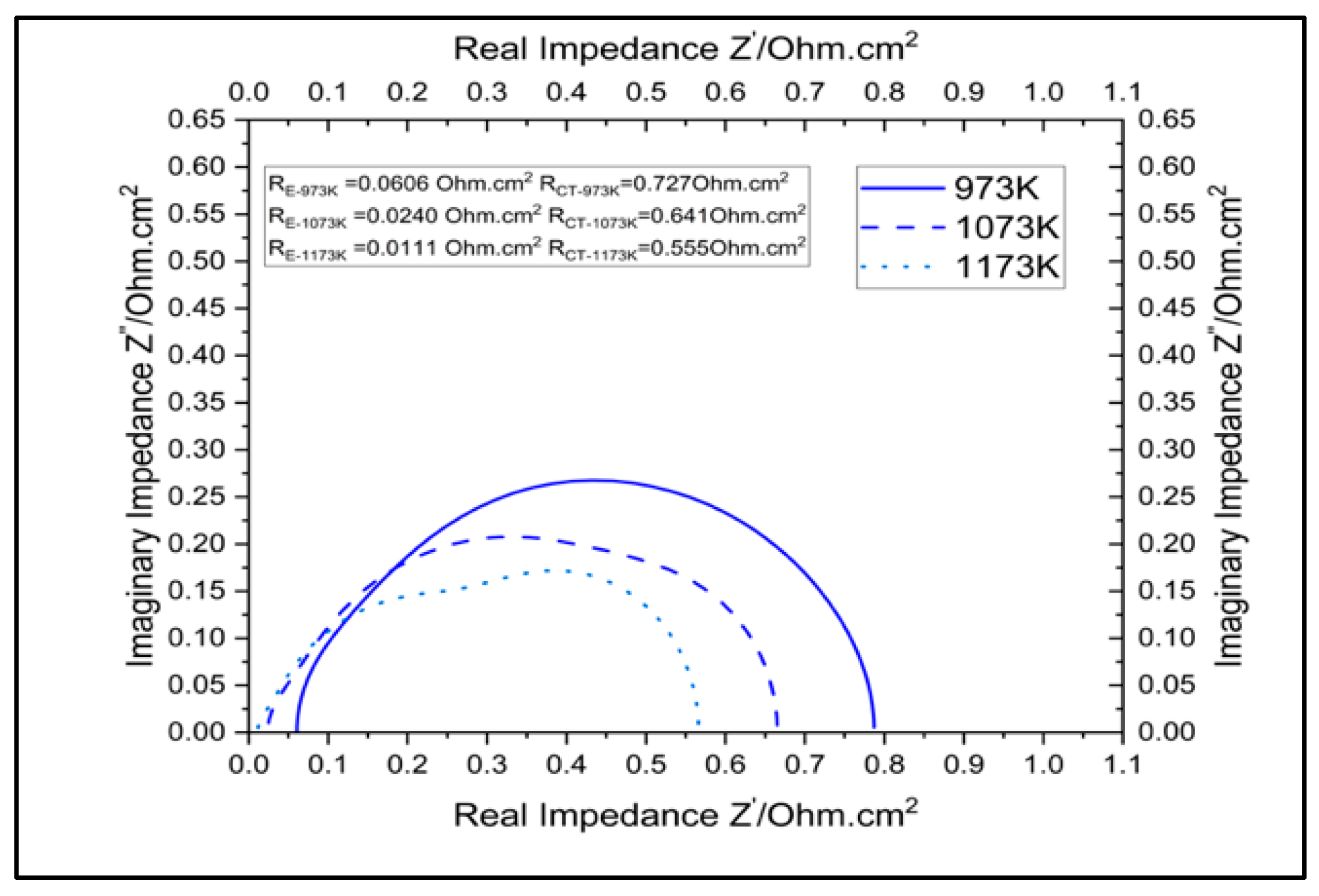
3.3.1. Impact of Temperature on Cell Performance
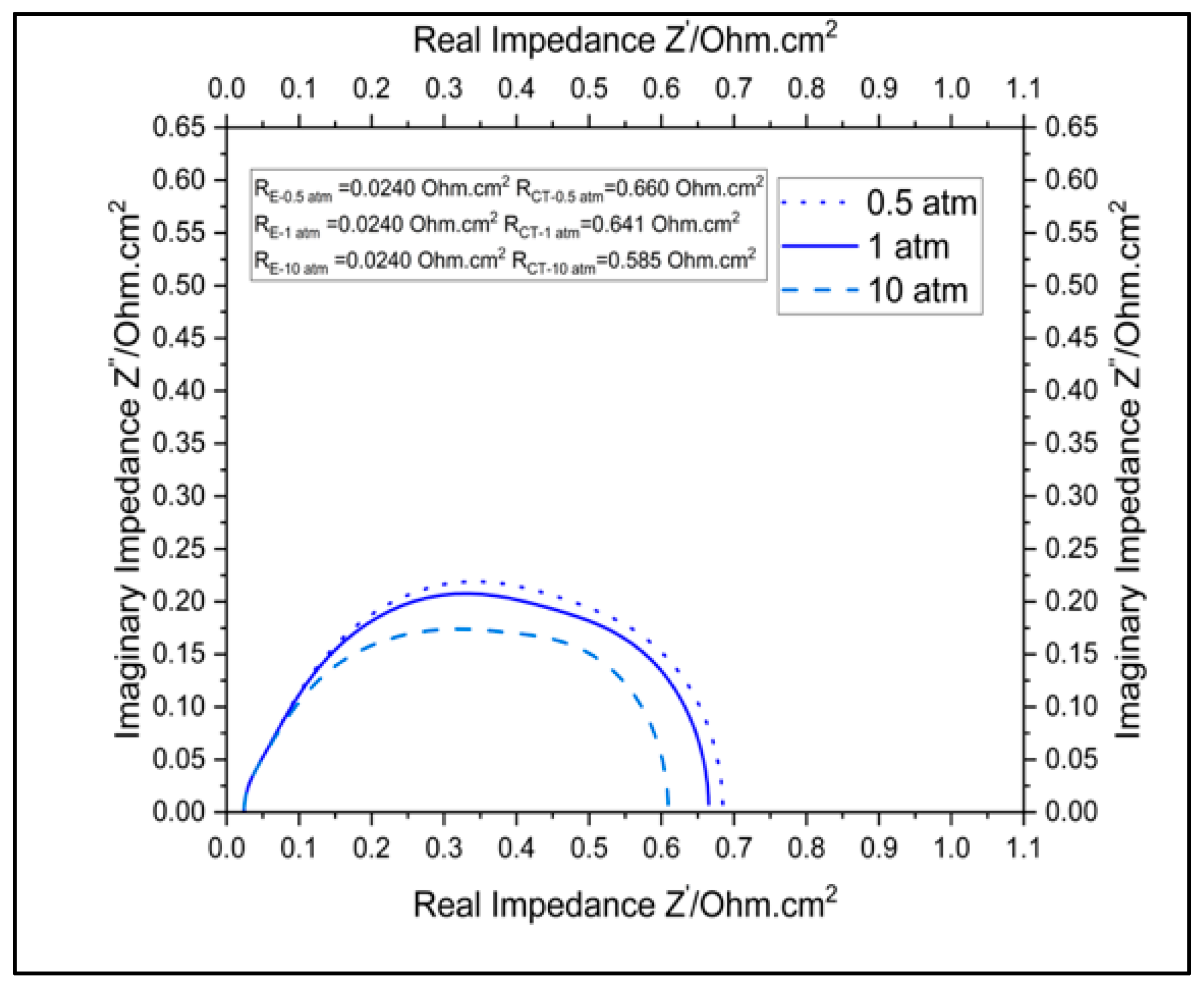
3.3.2. Impact of Pressure on Cell Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| SOEC | Solid oxide electrolysis cell |
| EECD | Electrochemical energy-conversion device |
| SCGZ | Scandium-doped zirconia (SCGZ) |
| YSZ | Yttrium-stabilized zirconia |
| GDC | Gadolinium-doped ceria |
| CeO2 | Cerium oxide |
| CO2 | Carbondioxide |
| TPB | Triple-phase boundary |
| ASR | Area-specific resistance |
| FEM | Finite-element method |
| SOFC | Solid oxide fuel cell |
References
- IEA. World Energy Outlook 2022; License: CC BY 4.0; IEA: Paris, France, 2022. [Google Scholar]
- IEA. Global Hydrogen Review 2022; License: CC BY 4.0; IEA: Paris, France, 2022. [Google Scholar]
- Hawkes, G.L.; O’Brien, J.E.; Stoots, C.M.; Herring, J.S.; Shahnam, M. Computational fluid dynamics model of a planar solid-oxide electrolysis cell for hydrogen production from nuclear energy. Nucl. Technol. 2004, 158, 132–144. [Google Scholar] [CrossRef]
- Grondin, D.; Deseure, J.; Brisse, A.; Zahid, M.; Ozil, P. Simulation of a high temperature electrolyzer. J. Appl. Electrochem. 2010, 40, 933–941. [Google Scholar] [CrossRef]
- Yildiz, B.; Smith, J.; Sofu, T. Thermal-Fluid and Electrochemical Modeling and Performance Study of a Planar Solid Oxide Electrolysis Cell: Analysis on SOEC Resistances, Size, and Inlet Flow Conditions; No. ANL-06/52; Argonne National Lab. (ANL): Argonne, IL, USA, 2008. [Google Scholar]
- Xu, Z.; Zhang, X.; Li, G.; Xiao, G.; Wang, J.Q. Comparative performance investigation of different gas flow configurations for a planar solid oxide electrolyzer cell. Int. J. Hydrogen Energy 2017, 42, 10785–10801. [Google Scholar] [CrossRef]
- Navasa, M.; Yuan, J.; Sundén, B. Computational fluid dynamics approach for performance evaluation of a solid oxide electrolysis cell for hydrogen production. Appl. Energy 2015, 137, 867–876. [Google Scholar] [CrossRef]
- Jin, X.; Xue, X. Computational fluid dynamics analysis of solid oxide electrolysis cells with delaminations. Int. J. Hydrogen Energy 2010, 35, 7321–7328. [Google Scholar] [CrossRef]
- Jensen, S.H.; Hauch, A.; Knibbe, R.; Jacobsen, T.; Mogensen, M. Modeling degradation in SOEC impedance spectra. J. Electrochem. Soc. 2013, 160, F244. [Google Scholar] [CrossRef]
- Shi, Y.; Cai, N.; Li, C.; Bao, C.; Croiset, E.; Qian, J.; Hu, Q.; Wang, S. Simulation of electrochemical impedance spectra of solid oxide fuel cells using transient physical models. J. Electrochem. Soc. 2008, 155, B270. [Google Scholar] [CrossRef]
- Weber, A.Z.; Borup, R.L.; Darling, R.M.; Das, P.K.; Dursch, T.J.; Gu, W.; Harvey, D.; Kusoglu, A.; Litster, S.; Mench, M.M.; et al. A critical review of modeling transport phenomena in polymer-electrolyte fuel cells. J. Electrochem. Soc. 2014, 161, F1254. [Google Scholar] [CrossRef]
- Bianchi, F.R.; Baldinelli, A.; Barelli, L.; Cinti, G.; Audasso, E.; Bosio, B. Multiscale modeling for reversible solid oxide cell operation. Energies 2020, 13, 5058. [Google Scholar] [CrossRef]
- Ni, M. Modeling of a solid oxide electrolysis cell for carbon dioxide electrolysis. Chem. Eng. J. 2010, 164, 246–254. [Google Scholar] [CrossRef]
- Ni, M. 2D thermal modeling of a solid oxide electrolyzer cell (SOEC) for syngas production by H2O/CO2 co-electrolysis. Int. J. Hydrogen Energy 2012, 37, 6389–6399. [Google Scholar] [CrossRef]
- COMSOL Multiphysics® v. 5.6. Fuel cell and Electrolyser Module User’s Guide; COMSOL: Burlington, MA, USA, 2020; pp. 75–84.
- COMSOL Multiphysics® v. 5.6. Solid Oxide Electrolyzer Using Thermodynamics; Application ID: 74001; COMSOL: Burlington, MA, USA, 2020.
- Xu, H.; Ni, M. High-temperature electrolysis and co-electrolysis. In Power to Fuel; Academic Press: Cambridge, MA, USA, 2021; pp. 51–73. [Google Scholar]
- Temluxame, P.; Puengjinda, P.; Peng-Ont, S.; Ngampuengpis, W.; Sirimungkalakul, N.; Jiwanuruk, T.; Sornchamni, T.; Kim-Lohsoontorn, P. Comparison of ceria and zirconia based electrolytes for solid oxide electrolysis cells. Int. J. Hydrogen Energy 2020, 46, 24568–24580. [Google Scholar] [CrossRef]
- Kendall, K.; Kendall, M. High-Temperature Solid Oxide Fuel Cells for the 21st Century: Fundamentals, Design and Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Yashima, M.; Masato, K.; Masahiro, Y. Metastable-stable phase diagrams in the zirconia-containing systems utilized in solid-oxide fuel cell application. Solid State Ion. 1996, 86, 1131–1149. [Google Scholar] [CrossRef]
- Ruh, R.; Garrett, H.J.; Domagala, R.F.; Patel, V.A. The system zirconia scandia. J. Am. Ceram. Soc. 1977, 60, 399–403. [Google Scholar] [CrossRef]
- Barbir, F. PEM Fuel Cells: Theory and Practice; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Kulikovsky, A.A.; Berg, P. Positioning of a reference electrode in a PEM fuel cell. J. Electrochem. Soc. 2015, 162, F843. [Google Scholar] [CrossRef]
- Finklea, H.; Chen, X.; Gerdes, K.; Pakalapati, S.; Celik, I. Analysis of SOFCs using reference electrodes. J. Electrochem. Soc. 2013, 160, F1055. [Google Scholar] [CrossRef]
- Cimenti, M.; Co, A.C.; Birss, V.I.; Hill, J.M. Distortions in Electrochemical Impedance Spectroscopy Measurements Using 3 Electrode Methods in SOFC. I. Effect of Cell Geometry. Fuel Cells 2007, 7, 364–376. [Google Scholar] [CrossRef]
- Cimenti, M.; Co, A.C.; Birss, V.I.; Hill, J.M. Distortions in Electrochemical Impedance Spectroscopy Measurements Using 3 Electrode Methods in SOFC. II. Effect of Electrode Activity and Relaxation Times. Fuel Cells 2007, 7, 377–391. [Google Scholar]
- Tran, A.-T.; Huet, F.; Ngo, K.; Rousseau, P. Artefacts in electrochemical impedance measurement in electrolytic solutions due to the reference electrode. Electrochim. Acta 2011, 56, 8034–8039. [Google Scholar] [CrossRef]



| Sl No | Cathode (Material–Thickness–Porosity) | Anode (Material–Thickness–Porosity) | Electrolyte (Material–Thickness) | Steam-Feed Rate | Feed Composition | Temperature K | Parameters Studied | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | Ni-YSZ-25 μm-0.37 | LSM-25 μm-0.37 | YSZ-140 μm | 4.33 × 10−6 kgs−1. | N2:H2:H2O-0.51:0.0074:0.483 | 1073 | Temperature, Nernst potential, current density, hydrogen-concentration distribution across electrolyte | [2] |
| 2 | Ni-YSZ-240 μm-0.3 | LSM-20 μm-0.3 | YSZ-7 μm | 1.25 ms−1 | N2: H2O-0.3:0.7 (molar) | 1073 | Overpotential across cell, steam distribution across hydrogen electrode, temperature profile across flow channels | [3] |
| 3 | Ni-YSZ-25 μm-0.37 | LSM-25 μm-0.37 | YSZ-140 μm SSZ-140 μm | 4 × 10−6 kgs−1. | N2:H2:H2O-0.5:0.006098:0.493902 | 1073 | Effect of cell active area, ASR, effect of flow rates | [4] |
| 4 | Ni-YSZ-10 μm-0.4 | LSM-20 μm-0.4 | YSZ-10 μm | 0.217 Lmin−1 | H2: H2O-0.1:0.9 | 1073 | Multi-channel cell-flow configuration, distribution of hydrogen concentration, water concentration, temperature profile, activation overpotential across electrodes | [5] |
| 5 | Ni-YSZ-510 μm-0.5 | LSM-60 μm-0.5 | YSZ-100 μm | 1.61 × 10−5 kgs−1. | H2: H2O-0:1 | 1073 | Distribution of current and concentrations across TPB layer for various condition | [6] |
| 6 | Ni-YSZ-500 μm-0.5 | LSM-30 μm-0.5 | YSZ-15 μm | 0.040 Lmin−1. | H2: H2O-0.1:0.9 | 1073 | Modeling of delamination in SOEC and effect of delamination size and location | [7] |
| Dimensions of the Cell | ||
| Cell length | 1.25 | cm |
| Cell width | 0.6 | cm |
| Cathode thickness | 80 | μm |
| Anode thickness | 400 | μm |
| Electrolyte thickness | 20 | μm |
| Number of ribs in gas channel | 3 | |
| Length of rib | 1 | cm |
| Width of rib | 0.15 | cm |
| Thickness of rib | 0.05 | cm |
| Operating Condition | ||
| Temperature | 800 | °C |
| Pressure | 1 | atm |
| Feed composition (H2O:H2) | 0.7:0.3 | |
| Feed-flow rate | 8.5 × 10−8 | kgs−1 |
| Material Properties | ||
| Electrolyte porosity | 0.225 | |
| Electrode porosity | 0.4 | |
| Electrolyte conductivity | Sm−1 | |
| Electrochemical Parameters | ||
| Cathode reference exchange-current density | 1500 | A.m−2 |
| Cathode charge-transfer coefficient / | 0.5/0.5 | |
| Cathode pressure dependence | 1/1 | |
| Anode reference exchange-current density | 2000 | A.m−2 |
| Anode charge-transfer coefficient / | 0.5/0.5 | |
| Anode pressure dependence | 0.25 | |
| Parameter | RE Ohm.cm2 | RCT Ohm.cm2 | |
|---|---|---|---|
| Temperature | 973 K | 0.0606 | 0.727 |
| 1073 K | 0.024 | 0.641 | |
| 1173 K | 0.0111 | 0.555 | |
| Pressure | 0.5 atm | 0.02395 | 0.660 |
| 1 atm | 0.02395 | 0.641 | |
| 10 atm | 0.02395 | 0.58535 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivas, S.; Dhanushkodi, S.R.; Chidambaram, R.K.; Skrzyniowska, D.; Korzen, A.; Taler, J. Benchmarking Electrolytes for the Solid Oxide Electrolyzer Using a Finite Element Model. Energies 2023, 16, 6419. https://doi.org/10.3390/en16186419
Srinivas S, Dhanushkodi SR, Chidambaram RK, Skrzyniowska D, Korzen A, Taler J. Benchmarking Electrolytes for the Solid Oxide Electrolyzer Using a Finite Element Model. Energies. 2023; 16(18):6419. https://doi.org/10.3390/en16186419
Chicago/Turabian StyleSrinivas, Sriram, Shankar Raman Dhanushkodi, Ramesh Kumar Chidambaram, Dorota Skrzyniowska, Anna Korzen, and Jan Taler. 2023. "Benchmarking Electrolytes for the Solid Oxide Electrolyzer Using a Finite Element Model" Energies 16, no. 18: 6419. https://doi.org/10.3390/en16186419
APA StyleSrinivas, S., Dhanushkodi, S. R., Chidambaram, R. K., Skrzyniowska, D., Korzen, A., & Taler, J. (2023). Benchmarking Electrolytes for the Solid Oxide Electrolyzer Using a Finite Element Model. Energies, 16(18), 6419. https://doi.org/10.3390/en16186419








