New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas
Abstract
:1. Introduction
2. Steam Reforming
3. Dry Reforming
4. Partial Oxidation
5. Autothermal Dual/Tri-Reforming
6. Thermo-Catalytic Decomposition
7. Discussion
8. Novel Perspectives
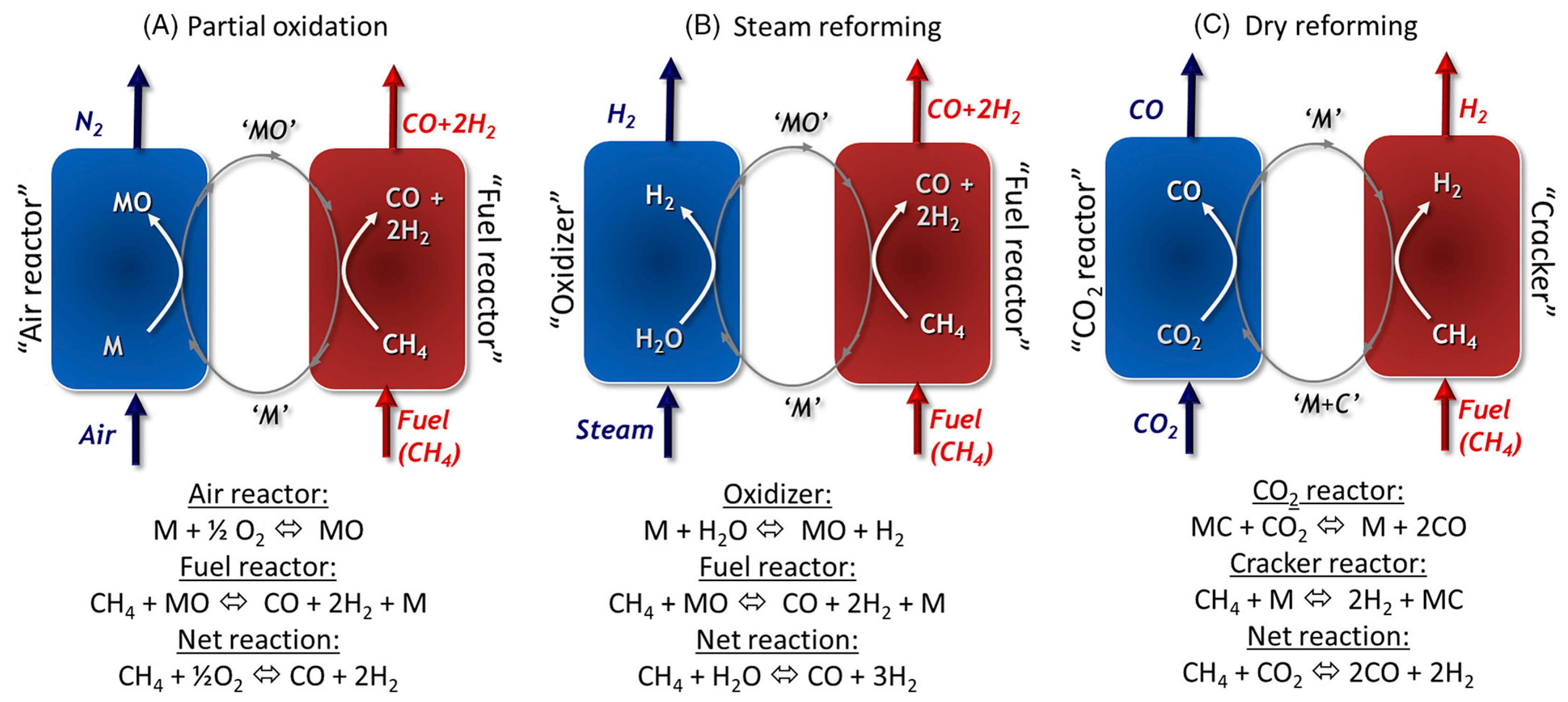
8.1. Chemical Looping
8.2. Electrically Asssited Reforming
8.3. Multistage Processes
8.4. Hydrogen Purification by Membranes
8.5. Catalyst Patterning
9. Conclusions
Funding
Conflicts of Interest
Abbreviations
| CL | chemical looping |
| CL-DRM | Chemical-cooping dry reforming of methane |
| CRM | critical raw material |
| DR | dry reforming |
| ATSR | autothermal steam reforming |
| ATDR | autothermal dry reforming |
| 2-R | dual reforming |
| TR | tri-reforming |
| CCS | carbon capture and storage |
| DFT | density functional theory |
| FB | fluidized bed |
| MPEC | mixed-conducting ceramic–ceramic composite |
| MW | microwave |
| NG | natural gas |
| OC | oxygen carrier |
| PO | partial oxidation |
| PSA | pressure swing adsorption |
| SR | steam reforming |
| TCD | thermo-catalytic decomposition |
| TGA | thermo-gravimetric analysis |
| TPR | temperature-programmed reduction |
| WGS | water gas shift |
References
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an Energy Vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). The Future of Hydrogen; IEA: Paris, France, 2019.
- Riera, J.A.; Lima, R.M.; Knio, O.M. A Review of Hydrogen Production and Supply Chain Modeling and Optimization. Int. J. Hydrogen Energy 2023, 48, 13731–13755. [Google Scholar] [CrossRef]
- Rasul, M.G.; Hazrat, M.A.; Sattar, M.A.; Jahirul, M.I.; Shearer, M.J. The Future of Hydrogen: Challenges on Production, Storage and Applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A Review on Hydrogen Production and Utilization: Challenges and Opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Okolie, J.A.; Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Futuristic Applications of Hydrogen in Energy, Biorefining, Aerospace, Pharmaceuticals and Metallurgy. Int. J. Hydrogen Energy 2021, 46, 8885–8905. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the Role of Hydrogen in the 21st Century Energy Transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
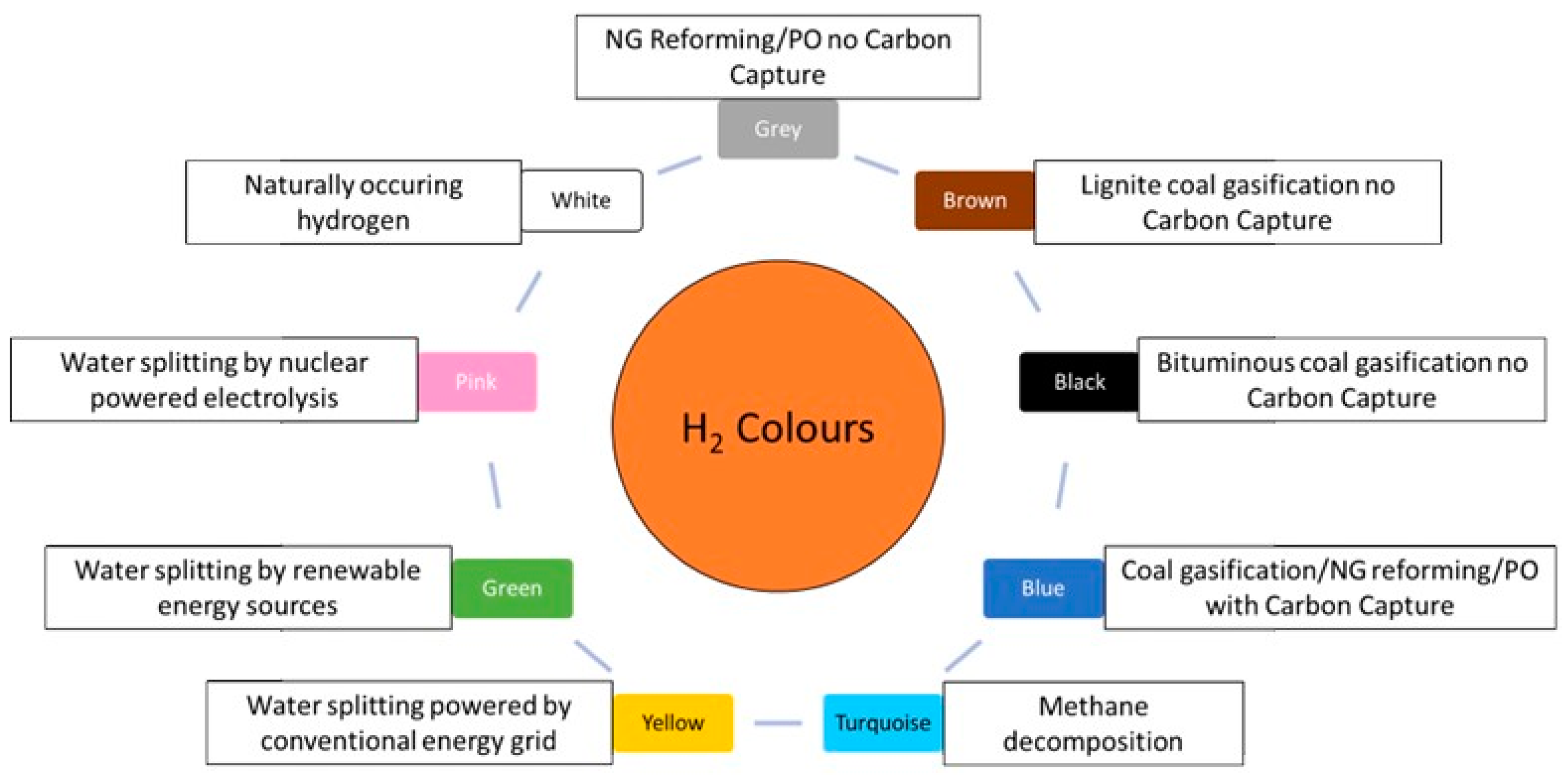
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Monteiro, E.; Brito, P.S.D. Hydrogen Supply Chain: Current Status and Prospects. Energy Storage 2023, e466. [Google Scholar] [CrossRef]
- Bridgwater, A. V Renewable Fuels and Chemicals by Thermal Processing of Biomass. Chem. Eng. J. 2003, 91, 87–102. [Google Scholar] [CrossRef]
- Incer-Valverde, J.; Korayem, A.; Tsatsaronis, G.; Morosuk, T. “Colors” of Hydrogen: Definitions and Carbon Intensity. Energy Convers. Manag. 2023, 291, 117294. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Vo, D.V.N.; Chelliapan, S.; Joo, S.W.; Vasseghian, Y. Latest Eco-Friendly Avenues on Hydrogen Production towards a Circular Bioeconomy: Currents Challenges, Innovative Insights, and Future Perspectives. Renew. Sustain. Energy Rev. 2022, 168, 112916. [Google Scholar] [CrossRef]
- Borowski, P.F.; Karlikowska, B. Clean Hydrogen Is a Challenge for Enterprises in the Era of Low-Emission and Zero-Emission Economy. Energies 2023, 16, 1171. [Google Scholar] [CrossRef]
- AlHumaidan, F.S.; Absi Halabi, M.; Rana, M.S.; Vinoba, M. Blue Hydrogen: Current Status and Future Technologies. Energy Convers. Manag. 2023, 283, 116840. [Google Scholar] [CrossRef]
- Angeli, S.D.; Monteleone, G.; Giaconia, A.; Lemonidou, A.A. State-of-the-Art Catalysts for CH4 Steam Reforming at Low Temperature. Int. J. Hydrogen Energy 2014, 39, 1979–1997. [Google Scholar] [CrossRef]
- Summa, P.; Samojeden, B.; Motak, M. Dry and Steam Reforming of Methane. Comparison and Analysis of Recently Investigated Catalytic Materials. A Short Review. Pol. J. Chem. Technol. 2019, 21, 31–37. [Google Scholar] [CrossRef]
- Fan, Z.; Weng, W.; Zhou, J.; Gu, D.; Xiao, W. Catalytic Decomposition of Methane to Produce Hydrogen: A Review. J. Energy Chem. 2021, 58, 415–430. [Google Scholar] [CrossRef]
- Chung, W.T.; Mekhemer, I.M.A.; Mohamed, M.G.; Elewa, A.M.; EL-Mahdy, A.F.M.; Chou, H.H.; Kuo, S.W.; Wu, K.C.W. Recent Advances in Metal/Covalent Organic Frameworks Based Materials: Their Synthesis, Structure Design and Potential Applications for Hydrogen Production. Coord. Chem. Rev. 2023, 483, 215066. [Google Scholar] [CrossRef]
- Saeidi, S.; Sápi, A.; Khoja, A.H.; Najari, S.; Ayesha, M.; Kónya, Z.; Asare-Bediako, B.B.; Tatarczuk, A.; Hessel, V.; Keil, F.J.; et al. Evolution Paths from Gray to Turquoise Hydrogen via Catalytic Steam Methane Reforming: Current Challenges and Future Developments. Renew. Sustain. Energy Rev. 2023, 183, 113392. [Google Scholar] [CrossRef]
- Ganguli, A.; Bhatt, V. Hydrogen Production Using Advanced Reactors by Steam Methane Reforming: A Review. Front. Therm. Eng. 2023, 3, 1143987. [Google Scholar] [CrossRef]
- Yang, W.W.; Ma, X.; Tang, X.Y.; Dou, P.Y.; Yang, Y.J.; He, Y.L. Review on Developments of Catalytic System for Methanol Steam Reforming from the Perspective of Energy-Mass Conversion. Fuel 2023, 345, 128234. [Google Scholar] [CrossRef]
- Qin, T.; Yuan, S. Research Progress of Catalysts for Catalytic Steam Reforming of High Temperature Tar: A Review. Fuel 2023, 331, 125790. [Google Scholar] [CrossRef]
- Deng, Y.; Li, S.; Appels, L.; Zhang, H.; Sweygers, N.; Baeyens, J.; Dewil, R. Steam Reforming of Ethanol by Non-Noble Metal Catalysts. Renew. Sustain. Energy Rev. 2023, 175, 113184. [Google Scholar] [CrossRef]
- Yadav, A.K.; Vaidya, P.D. A Review on Butanol Steam Reforming for Renewable Hydrogen Production. J. Indian Chem. Soc. 2023, 100, 101050. [Google Scholar] [CrossRef]
- Amjad, U.E.S.; Quintero, C.W.M.; Ercolino, G.; Italiano, C.; Vita, A.; Specchia, S. Methane Steam Reforming on the Pt/CeO2 Catalyst: Effect of Daily Start-Up and Shut-Down on Long-Term Stability of the Catalyst. Ind. Eng. Chem. Res. 2019, 58, 16395–16406. [Google Scholar] [CrossRef]
- Androulakis, A.; Yentekakis, I.V.; Panagiotopoulou, P. Dry Reforming of Methane over Supported Rh and Ru Catalysts: Effect of the Support (Al2O3, TiO2, ZrO2, YSZ) on the Activity and Reaction Pathway. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Geng, H.; Zhao, H.; Yu, S.; Li, D.; Lei, H.; Zhang, Y. High Performance of Conversion and Selectivity of Methane to CO via Pt-Pd Core-Shell Structural Catalyst. Appl. Catal. B 2023, 324, 122189. [Google Scholar] [CrossRef]
- Singh, A.K.; Pal, P.; Rathore, S.S.; Sahoo, U.K.; Sarangi, P.K.; Prus, P.; Dziekański, P. Sustainable Utilization of Biowaste Resources for Biogas Production to Meet Rural Bioenergy Requirements. Energies 2023, 16, 5409. [Google Scholar] [CrossRef]
- Yin, W.; Guilhaume, N.; Schuurman, Y. Model Biogas Reforming over Ni-Rh/MgAl2O4 Catalyst. Effect of Gas Impurities. Chem. Eng. J. 2020, 398, 125534. [Google Scholar] [CrossRef]
- Chaghouri, M.; Hany, S.; Cazier, F.; Tidahy, H.L.; Gennequin, C.; Abi-Aad, E. Impact of Impurities on Biogas Valorization through Dry Reforming of Methane Reaction. Int. J. Hydrogen Energy 2022, 47, 40415–40429. [Google Scholar] [CrossRef]
- Postels, S.; Abánades, A.; von der Assen, N.; Rathnam, R.K.; Stückrad, S.; Bardow, A. Life Cycle Assessment of Hydrogen Production by Thermal Cracking of Methane Based on Liquid-Metal Technology. Int. J. Hydrogen Energy 2016, 41, 23204–23212. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen Production, Storage, Utilisation and Environmental Impacts: A Review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Gholkar, P.; Shastri, Y.; Tanksale, A. Renewable Hydrogen and Methane Production from Microalgae: A Techno-Economic and Life Cycle Assessment Study. J. Clean. Prod. 2021, 279, 123726. [Google Scholar] [CrossRef]
- Ji, M.; Wang, J. Review and Comparison of Various Hydrogen Production Methods Based on Costs and Life Cycle Impact Assessment Indicators. Int. J. Hydrogen Energy 2021, 46, 38612–38635. [Google Scholar] [CrossRef]
- Megia, P.J.; Vizcaino, A.J.; Calles, J.A.; Carrero, A. Hydrogen Production Technologies: From Fossil Fuels toward Renewable Sources. A Mini Review. Energy Fuels 2021, 35, 16403–16415. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.; Eduok, U. A Critical Review on the Current Technologies for the Generation, Storage, and Transportation of Hydrogen. Int. J. Hydrogen Energy 2022, 47, 13771–13802. [Google Scholar] [CrossRef]
- Chen, L.; Qi, Z.; Zhang, S.; Su, J.; Somorjai, G.A. Catalytic Hydrogen Production from Methane: A Review on Recent Progress and Prospect. Catalysts 2020, 10, 858. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Advances in Hydrogen Production from Natural Gas Reforming. Adv. Energy Sustain. Res. 2021, 2, 2100097. [Google Scholar] [CrossRef]
- Suleman, F.; Dincer, I.; Agelin-Chaab, M. Environmental Impact Assessment and Comparison of Some Hydrogen Production Options. Int. J. Hydrogen Energy 2015, 40, 6976–6987. [Google Scholar] [CrossRef]
- IRENA. Hydrogen (IRENA). Available online: https://www.irena.org/Energy-Transition/Technology/Hydrogen#:~:text=As%20at%20the%20end%20of,around%204%25%20comes%20from%20electrolysis (accessed on 24 August 2023).
- Wang, J.; Liu, Z.; Ji, C.; Liu, L. Heat Transfer and Reaction Characteristics of Steam Methane Reforming in a Novel Composite Packed Bed Microreactor for Distributed Hydrogen Production. Energies 2023, 16, 4347. [Google Scholar] [CrossRef]
- Levalley, T.L.; Richard, A.R.; Fan, M. The Progress in Water Gas Shift and Steam Reforming Hydrogen Production Technologies—A Review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Kaiwen, L.; Bin, Y.; Tao, Z. Economic Analysis of Hydrogen Production from Steam Reforming Process: A Literature Review. Energy Sources Part B Econ. Plan. Policy 2018, 13, 109–115. [Google Scholar] [CrossRef]
- Ozbilen, A.; Dincer, I.; Rosen, M.A. Comparative Environmental Impact and Efficiency Assessment of Selected Hydrogen Production Methods. Environ. Impact Assess. Rev. 2013, 42, 1–9. [Google Scholar] [CrossRef]
- Argyris, P.A.; Wright, A.; Taheri Qazvini, O.; Spallina, V. Dynamic Behaviour of Integrated Chemical Looping Process with Pressure Swing Adsorption in Small Scale On-Site H2 and Pure CO2 Production. Chem. Eng. J. 2022, 428, 132606. [Google Scholar] [CrossRef]
- Ochoa Bique, A.; Maia, L.K.K.; La Mantia, F.; Manca, D.; Zondervan, E. Balancing Costs, Safety and CO2 Emissions in the Design of Hydrogen Supply Chains. Comput. Chem. Eng. 2019, 129, 106493. [Google Scholar] [CrossRef]
- Sehested, J. Sintering of Nickel Steam-Reforming Catalysts. J. Catal. 2003, 217, 417–426. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in Reforming and Partial Oxidation of Hydrocarbons for Hydrogen Production and Fuel Cell Applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Salcedo, A.; Lustemberg, P.G.; Rui, N.; Palomino, R.M.; Liu, Z.; Nemsak, S.; Senanayake, S.D.; Rodriguez, J.A.; Ganduglia-Pirovano, M.V.; Irigoyen, B. Reaction Pathway for Coke-Free Methane Steam Reforming on a Ni/CeO2 Catalyst: Active Sites and the Role of Metal-Support Interactions. ACS Catal. 2021, 11, 8327–8337. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wang, F.; Zhang, L.; Wang, Y.; Fan, W.; Xu, L.; Yu, H.; Li, Z. Syngas Production from Methane Steam Reforming and Dry Reforming Reactions over Sintering-Resistant Ni@SiO2 Catalyst. Res. Chem. Intermed. 2020, 46, 1735–1748. [Google Scholar] [CrossRef]
- Jeon, K.W.; Kim, J.K.; Kim, B.J.; Jang, W.J.; Kang, Y.C.; Roh, H.S. Ultra-Stable Porous Yolk-Shell Ni Catalysts for the Steam Reforming of Methane with Alkali Poisoning. Chem. Eng. J. 2023, 454, 140060. [Google Scholar] [CrossRef]
- Wang, S.; Nabavi, S.A.; Clough, P.T. A Review on Bi/Polymetallic Catalysts for Steam Methane Reforming. Int. J. Hydrogen Energy 2023, 48, 15879–15893. [Google Scholar] [CrossRef]
- Djaidja, A.; Messaoudi, H.; Kaddeche, D.; Barama, A. Study of Ni-M/MgO and Ni-M-Mg/Al (M=Fe or Cu) Catalysts in The CH4-CO2 and CH4-H2O Reforming. Int. J. Hydrogen Energy 2015, 40, 4989–4995. [Google Scholar] [CrossRef]
- Lu, H.; Shi, X.; Costa, M.; Huang, C. Carcinogenic Effect of Nickel Compounds. Mol. Cell Biochem. 2005, 279, 45–67. [Google Scholar] [CrossRef]
- Al-Mamoori, A.; Krishnamurthy, A.; Rownaghi, A.A.; Rezaei, F. Carbon Capture and Utilization Update. Energy Technol. 2017, 5, 834–849. [Google Scholar] [CrossRef]
- Joos, L.; Huck, J.M.; Van Speybroeck, V.; Smit, B. Cutting the Cost of Carbon Capture: A Case for Carbon Capture and Utilization. Faraday Discuss. 2016, 192, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Rackley, S.A. Overview of Carbon Capture and Storage. In Carbon Capture and Storage; Butterworth-Heinemann: Oxford, UK, 2017; pp. 23–36. ISBN 9780128120415. [Google Scholar]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: An Update Review. Energies 2010, 3, 1529–1575. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A Review of Recent Developments in Hydrogen Production via Biogas Dry Reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Jung, S.; Lee, J.; Moon, D.H.; Kim, K.H.; Kwon, E.E. Upgrading Biogas into Syngas through Dry Reforming. Renew. Sustain. Energy Rev. 2021, 143, 110949. [Google Scholar] [CrossRef]
- Minh, D.P.; Siang, T.J.; Vo, D.V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Hydrogen Production from Biogas Reforming: An Overview of Steam Reforming, Dry Reforming, Dual Reforming, and Tri-Reforming of Methane. In Hydrogen Supply Chain: Design, Deployment and Operation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–166. ISBN 9780128111970. [Google Scholar]
- Aramouni, N.A.K.; Touma, J.G.; Tarboush, B.A.; Zeaiter, J.; Ahmad, M.N. Catalyst Design for Dry Reforming of Methane: Analysis Review. Renew. Sustain. Energy Rev. 2018, 82, 2570–2585. [Google Scholar] [CrossRef]
- Yan, W.; Chuan, T.; Ching, J.; Lee, D.; Chang, J. Bioresource Technology Biorefineries of Carbon Dioxide: From Carbon Capture and Storage (CCS) to Bioenergies Production. Bioresour. Technol. 2016, 215, 346–356. [Google Scholar] [CrossRef]
- Aramouni, N.A.K. Carbon Mitigation in the Dry Reforming of Methane. Ph.D. Thesis, University of Limerick, Limerick, Ireland, 2020. [Google Scholar]
- González-Castaño, M.; Dorneanu, B.; Arellano-García, H. The Reverse Water Gas Shift Reaction: A Process Systems Engineering Perspective. React. Chem. Eng. 2021, 6, 954–976. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Phan, T.S.; Pham Minh, D. New Performing Hydroxyapatite-Based Catalysts in Dry-Reforming of Methane. Int. J. Hydrogen Energy 2023, 48, 30770–30790. [Google Scholar] [CrossRef]
- Nakajima, E.A.; Oliveira, L.G.; Gasparrini, L.J.; de Queiros Souza, G.E.; Ignacio, A.A.; Alves, H.J.; Borba, C.E. Kinetics of Dry Reforming of Methane Catalyzed by Ni/Si-MCM-41. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G.; Liu, J.; Li, T.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, Y. Dry Reforming of Methane over Ni/SiO2 Catalysts: Role of Support Structure Properties. Fuel 2023, 340, 127490. [Google Scholar] [CrossRef]
- Song, Q.; Ran, R.; Wu, X.; Si, Z.; Weng, D. Dry Reforming of Methane over Ni Catalysts Supported on Micro- and Mesoporous Silica. J. CO2 Util. 2023, 68, 102387. [Google Scholar] [CrossRef]
- Li, S.; Fu, Y.; Kong, W.; Wang, J.; Yuan, C.; Pan, B.; Zhu, H.; Chen, X.; Zhang, Y.; Zhang, J.; et al. Tuning Strong Metal-Support Interactions to Boost Activity and Stability of Aluminium Nitride Supported Nickel Catalysts for Dry Reforming of Methane. Fuel 2023, 343, 127918. [Google Scholar] [CrossRef]
- Haug, L.; Thurner, C.; Bekheet, M.F.; Ploner, K.; Bischoff, B.; Gurlo, A.; Kunz, M.; Sartory, B.; Penner, S.; Klötzer, B. Pivotal Role of Ni/ZrO2 Phase Boundaries for Coke-Resistant Methane Dry Reforming Catalysts. Catalysts 2023, 13, 804. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, Y.; Liu, Y.; Zheng, J.; Deng, J.; Yan, T.; Cheng, D.; Zhang, D. Unraveling the Unique Promotion Effects of a Triple Interface in Ni Catalysts for Methane Dry Reforming. Ind. Eng. Chem. Res. 2023, 62, 4965–4975. [Google Scholar] [CrossRef]
- Georgiadis, A.G.; Siakavelas, G.I.; Tsiotsias, A.I.; Charisiou, N.D.; Ehrhardt, B.; Wang, W.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Mascotto, S.; et al. Biogas Dry Reforming over Ni/LnOx-Type Catalysts (Ln = La, Ce, Sm or Pr). Int. J. Hydrogen Energy 2023, 48, 19953–19971. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Zhang, G.; Liu, J.; Wang, Y.; Zhao, Y.; Li, G.; Lv, Y. Effects of Promoter and Calcination Temperatures on the Catalytic Performance of Y Promoted Co/WC-AC for Dry Reforming of Methane. Chem. Asian J. 2023, 18, e202300319. [Google Scholar] [CrossRef] [PubMed]
- Ewbank, J.L.; Kovarik, L.; Kenvin, C.C.; Sievers, C. Effect of Preparation Methods on the Performance of Co/Al2O3 Catalysts for Dry Reforming of Methane. Green Chem. 2014, 16, 885–896. [Google Scholar] [CrossRef]
- Mohd Jailani, M.S.A.; Miskan, S.N.; Bahari, M.B.; Setiabudi, H.D. Optimization of Dry Reforming of Methane over Yttrium Oxide-Cobalt/Mesoporous Alumina Using Response Surface Methodology. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Owgi, A.H.K.; Jalil, A.A.; Aziz, M.A.A.; Nabgan, W.; Alhassan, M.; Sofi, M.H.M.; Hassan, N.S.; Saravanan, R.; Bahari, M.B. Methane Dry Reforming on Fibrous Silica-Alumina Employing Nanocrystals of Nickel and Cobalt to Recognize the Most Efficient Metal. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Zafarnak, S.; Rahimpour, M.R. Co-Ni Bimetallic Supported on Mullite as a Promising Catalyst for Biogas Dry Reforming toward Hydrogen Production. Mol. Catal. 2023, 534, 112803. [Google Scholar] [CrossRef]
- Liang, D.; Wang, Y.; Chen, M.; Xie, X.; Li, C.; Wang, J.; Yuan, L. Dry Reforming of Methane for Syngas Production over Attapulgite-Derived MFI Zeolite Encapsulated Bimetallic Ni-Co Catalysts. Appl. Catal. B 2023, 322, 122088. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced Carbon-Resistant Dry Reforming Fe-Ni Catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.; Cao, M.; Li, S.; Song, Z.; Qiu, L.; Yu, F.; Li, R.; Yan, X. Structural Evolution of Robust Ni3Fe1 Alloy on Al2O3 in Dry Reforming of Methane: Effect of Iron-Surplus Strategy from Ni1Fe1 to Ni3Fe1. Appl. Catal. B 2023, 331, 122669. [Google Scholar] [CrossRef]
- Chatla, A.; Ghouri, M.M.; El Hassan, O.W.; Mohamed, N.; Prakash, A.V.; Elbashir, N.O. An Experimental and First Principles DFT Investigation on the Effect of Cu Addition to Ni/Al2O3 Catalyst for the Dry Reforming of Methane. Appl. Catal. A Gen. 2020, 602, 117699. [Google Scholar] [CrossRef]
- Han, K.; Wang, S.; Liu, Q.; Wang, F. Optimizing the Ni/Cu Ratio in Ni-Cu Nanoparticle Catalysts for Methane Dry Reforming. ACS Appl. Nano Mater. 2021, 4, 5340–5348. [Google Scholar] [CrossRef]
- Khan, M.A.; Challiwala, M.S.; Prakash, A.V.; Elbashir, N.O. Conceptual Modeling of a Reactor Bed of a Nickel-Copper Bi-Metallic Catalyst for Dry Reforming of Methane. Chem. Eng. Sci. 2023, 267, 118315. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Kandoi, S.; Greeley, J.P.; Mavrikakis, M.; Dumesic, J.A. Molecular-Level Descriptions of Surface Chemistry in Kinetic Models Using Density Functional Theory. Chem. Eng. Sci. 2004, 59, 4679–4691. [Google Scholar] [CrossRef]
- Zhang, D.; Xie, H.; Chen, S.; Zeng, J.; Zhou, G. High-Performance CoCe Catalyst Constructed by the Glucose-Assisted In-Situ Reduction for CH4/CO2 Dry Reforming. Fuel 2023, 344, 128083. [Google Scholar] [CrossRef]
- Loktev, A.S.; Arkhipova, V.A.; Bykov, M.A.; Sadovnikov, A.A.; Dedov, A.G. Novel Samarium Cobaltate/Silicon Carbide Composite Catalyst for Dry Reforming of Methane into Synthesis Gas. Pet. Chem. 2023. [Google Scholar] [CrossRef]
- Loktev, A.S.; Arkhipova, V.A.; Bykov, M.A.; Sadovnikov, A.A.; Dedov, A.G. Cobalt–Samarium Oxide Composite as a Novel High-Performance Catalyst for Partial Oxidation and Dry Reforming of Methane into Synthesis Gas. Pet. Chem. 2023, 63, 317–326. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry Reforming of Methane by Stable Ni-Mo Nanocatalysts on Single-Crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yan, B.; Yao, S.; Xie, Z.; Wu, Q.; Ran, R.; Weng, D.; Zhang, C.; Chen, J.G. LaFe0.9Ni0.1O3 Perovskite Catalyst with Enhanced Activity and Coke-Resistance for Dry Reforming of Ethane. J. Catal. 2018, 358, 168–178. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 Dry-Reforming of Methane over La0.8Sr0.2Ni0.8M0.2O3 Perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of Lattice Oxygen on C-H Activation and Carbon Suppression. Int. J. Hydrogen Energy 2012, 37, 11195–11207. [Google Scholar] [CrossRef]
- Das, S.; Bhattar, S.; Liu, L.; Wang, Z.; Xi, S.; Spivey, J.J.; Kawi, S. Effect of Partial Fe Substitution in La0.9Sr0.1NiO3 Perovskite-Derived Catalysts on the Reaction Mechanism of Methane Dry Reforming. ACS Catal. 2020, 10, 12466–12486. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effects of Ce Substitution at the A-Site of LaNi0.5Fe0.5O3 Perovskite on the Enhanced Catalytic Activity for Dry Reforming of Methane. Appl. Catal. B 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Yao, X.; Cheng, Q.; Attada, Y.; Ould-Chikh, S.; Ramírez, A.; Bai, X.; Mohamed, H.O.; Li, G.; Shterk, G.; Zheng, L.; et al. Atypical Stability of Exsolved Ni-Fe Alloy Nanoparticles on Double Layered Perovskite for CO2 Dry Reforming of Methane. Appl. Catal. B 2023, 328, 122479. [Google Scholar] [CrossRef]
- York, A.P.E.; Xiao, T.; Green, M.L.H. Brief Overview of the Partial Oxidation of Methane to Synthesis Gas. Top. Catal. 2003, 22, 345–358. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Salgansky, E.A.; Arutyunov, V.S.; Sedov, I.V. Non-Catalytic Partial Oxidation of Hydrocarbon Gases to Syngas and Hydrogen: A Systematic Review. Energies 2023, 16, 2916. [Google Scholar] [CrossRef]
- Kalamaras, C.M.; Efstathiou, A.M. Hydrogen Production Technologies: Current State and Future Developments. Conf. Pap. Energy 2013, 2013, 690627. [Google Scholar] [CrossRef]
- Osman, A.I. Catalytic Hydrogen Production from Methane Partial Oxidation: Mechanism and Kinetic Study. Chem. Eng. Technol. 2020, 43, 641–648. [Google Scholar] [CrossRef]
- Elbadawi, A.H.; Ge, L.; Li, Z.; Liu, S.; Wang, S.; Zhu, Z. Catalytic Partial Oxidation of Methane to Syngas: Review of Perovskite Catalysts and Membrane Reactors. Catal. Rev. Sci. Eng. 2021, 63, 1–67. [Google Scholar] [CrossRef]
- Liu, H.; He, D. Recent Progress on Ni-Based Catalysts in Partial Oxidation of Methane to Syngas. Catal. Surv. Asia 2012, 16, 53–61. [Google Scholar] [CrossRef]
- Barbero, J.; Penã, M.A.; Campos-Martin, J.M.; Fierro, J.L.G.; Arias, P.L. Support Effect in Supported Ni Catalysts on Their Performance for Methane Partial Oxidation. Catal. Lett. 2003, 87, 211–218. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Yigit, N.; Wicht, T.; Stöger-Pollach, M.; Lucrédio, A.F.; Marcos, F.C.F.; Assaf, E.M.; Rupprechter, G. Partial Oxidation of Bio-Methane over Nickel Supported on MgO–ZrO2 Solid Solutions. Top. Catal. 2023. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Zhao, Z.; Hu, X.; Ye, Z.; Yao, J.; Buckley, C.E.; Dong, D. Comparison of Fibrous Catalysts and Monolithic Catalysts for Catalytic Methane Partial Oxidation. Renew. Energy 2019, 138, 1010–1017. [Google Scholar] [CrossRef]
- Özdemir, H.; Faruk Öksüzömer, M.A. Synthesis of Al2O3, MgO and MgAl2O4 by Solution Combustion Method and Investigation of Performances in Partial Oxidation of Methane. Powder Technol. 2020, 359, 107–117. [Google Scholar] [CrossRef]
- Khaleel, A.; Pillantakath, A.R.; Adamson, A. Significant Role of Well-Dispersed Fe2+ Ions in the Support of Ni Catalysts in Enhancing Coking Resistance during Partial Oxidation of Methane. Int. J. Hydrogen Energy, 2023; in press. [Google Scholar] [CrossRef]
- Moral, A.; Reyero, I.; Llorca, J.; Bimbela, F.; Gandía, L.M. Partial Oxidation of Methane to Syngas Using Co/Mg and Co/Mg-Al Oxide Supported Catalysts. Catal. Today 2019, 33, 259–267. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidation of Lean Methane over Cobalt Catalysts Supported on Ceria/Alumina. Appl. Catal. A Gen. 2020, 591, 117381. [Google Scholar] [CrossRef]
- Mosayebi, A.; Abedini, R. Effect of Synthesis Solution PH of Co/γ-Al2O3 Catalyst on Its Catalytic Properties for Methane Conversion to Syngas. J. Fuel Chem. Technol. 2018, 46, 311–318. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Arafat, Y.; Ibrahim, A.A.; Shaikh, H.; Atia, H.; Abasaeed, A.E.; Armbruster, U.; Al-Fatesh, A.S. Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni-Co)/ZrO2-Al2O3 Catalysts: Influence of Calcination Temperature. Processes 2019, 7, 141. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Ding, C.; Ma, L.; Xue, Y.; Guo, J.; Wang, S.; Meng, Y.; Zhang, K.; Liu, P. Effect of Cobalt Addition on the Structure and Properties of Ni-MCM-41 for the Partial Oxidation of Methane to Syngas. RSC Adv. 2019, 9, 25508–25517. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.H.; Shahzad, N.; Butt, F.A.; Khan, M.A.; Naeem, N.; Liaquat, R.; Khoja, A.H. Synthesis of Bimetallic Co-Ni/ZnO Nanoprisms (ZnO-NPr) for Hydrogen-Rich Syngas Production via Partial Oxidation of Methane. J. Environ. Chem. Eng. 2021, 9, 106887. [Google Scholar] [CrossRef]
- Shooli, Z.S.; Izadbakhsh, A.; Sanati, A.M. Effect of Copper and Cerium on the Performance of Ni-SBA-16 in the Partial Oxidation of Methane. Reac Kinet. Mech. Cat. 2018, 124, 873–889. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Ferraria, A.M.; do Rego, A.M.B.; Gonçalves, A.P.; Girão, A.V.; Correia, R.; Gasche, T.A.; Branco, J.B. Partial Oxidation of Methane over Bimetallic Copper-Cerium Oxide Catalysts. J. Mol. Catal. A Chem. 2010, 320, 47–55. [Google Scholar] [CrossRef]
- Emamdoust, A.; La Parola, V.; Pantaleo, G.; Testa, M.L.; Farjami Shayesteh, S.; Venezia, A.M. Partial Oxidation of Methane over SiO2 Supported Ni and NiCe Catalysts. J. Energy Chem. 2020, 47, 1–9. [Google Scholar] [CrossRef]
- Loktev, A.S.; Mukhin, I.E.; Bykov, M.A.; Sadovnikov, A.A.; Osipov, A.K.; Dedov, A.G. Novel High-Performance Catalysts for Partial Oxidation and Dry Reforming of Methane to Synthesis Gas. Pet. Chem. 2022, 62, 526–543. [Google Scholar] [CrossRef]
- Gavrikov, A.V.; Loktev, A.S.; Ilyukhin, A.B.; Mukhin, I.E.; Bykov, M.A.; Vorobei, A.M.; Parenago, O.O.; Cherednichenko, K.A.; Sadovnikov, A.A.; Dedov, A.G. Partial Oxidation of Methane to Syngas over SmCoO3-Derived Catalysts: The Effect of the Supercritical Fluid Assisted Modification of the Perovskite Precursor. Int. J. Hydrogen Energy 2023, 48, 2998–3012. [Google Scholar] [CrossRef]
- Wang, H.; Dai, H. Isovalent Substituted La-Gd-Cr Perovskite for the Cleaner Hydrogen Production during Partial Oxidation Methane in Catalytic Packed Bed. Fuel 2023, 340, 127457. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Q.; Yao, Z.; Dou, B.; Shi, Y.; Sun, Y. A Comparative Study of Molybdenum Phosphide Catalyst for Partial Oxidation and Dry Reforming of Methane. Int. J. Hydrogen Energy 2019, 44, 11441–11447. [Google Scholar] [CrossRef]
- Levikhin, A.A.; Boryaev, A.A. High-Temperature Reactor for Hydrogen Production by Partial Oxidation of Hydrocarbons. Int. J. Hydrogen Energy 2023, 48, 28187–28204. [Google Scholar] [CrossRef]
- Khan, S.N.; Yang, Z.; Dong, W.; Zhao, M. Cost and Technology Readiness Level Assessment of Emerging Technologies, New Perspectives, and Future Research Directions in H2 Production. Sustain. Energy Fuels 2022, 6, 4357–4374. [Google Scholar] [CrossRef]
- Basini, L.E.; Guarinoni, A. Short Contact Time Catalytic Partial Oxidation (SCT-CPO) for Synthesis Gas Processes and Olefins Production. Ind. Eng. Chem. Res. 2013, 52, 17023–17037. [Google Scholar] [CrossRef]
- Kale, G.R.; Doke, S.; Anjikar, A. Process Thermoneutral Point in Dry Autothermal Reforming for CO2 Utilization. J. CO2 Util. 2017, 18, 318–325. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Ding, F.; Guo, X.; Wang, G. Syngas Production via Steam-CO2 Dual Reforming of Methane over LA-Ni/ZrO2 Catalyst Prepared by l -Arginine Ligand-Assisted Strategy: Enhanced Activity and Stability. ACS Sustain. Chem. Eng. 2015, 3, 3461–3476. [Google Scholar] [CrossRef]
- Izquierdo, U.; Barrio, V.L.; Requies, J.; Cambra, J.F.; Güemez, M.B.; Arias, P.L. Tri-Reforming: A New Biogas Process for Synthesis Gas and Hydrogen Production. Int. J. Hydrogen Energy 2013, 38, 7623–7631. [Google Scholar] [CrossRef]
- Chein, R.Y.; Hsu, W.H. Analysis of Syngas Production from Biogas via the Tri-Reforming Process. Energies 2018, 11, 1075. [Google Scholar] [CrossRef]
- Mosinska, M.; Szynkowska, M.I.; Mierczynski, P. Oxy-Steam Reforming of Natural Gas on Ni Catalysts-A Minireview. Catalysts 2020, 10, 896. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial Decarbonization via Hydrogen: A Critical and Systematic Review of Developments, Socio-Technical Systems and Policy Options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Garbarino, G.; Busca, G. Facile Synthesis of a Mesoporous Alumina and Its Application as a Support of Ni-Based Autothermal Reforming Catalysts. Int. J. Hydrogen Energy 2016, 41, 3456–3464. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Garbarino, G.; Busca, G. Preparation and Characterization of Mesoporous Nanocrystalline La-, Ce-, Zr-, Sr-Containing Ni-Al2O3 Methane Autothermal Reforming Catalysts. Int. J. Hydrogen Energy 2016, 41, 8855–8862. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M. Ce Promoting Effect on the Activity and Coke Formation of Ni Catalysts Supported on Mesoporous Nanocrystalline γ-Al2O3 in Autothermal Reforming of Methane. Int. J. Hydrogen Energy 2017, 42, 11130–11138. [Google Scholar] [CrossRef]
- Matus, E.V.; Ismagilov, I.Z.; Yashnik, S.A.; Ushakov, V.A.; Prosvirin, I.P.; Kerzhentsev, M.A.; Ismagilov, Z.R. Hydrogen Production through Autothermal Reforming of CH4: Efficiency and Action Mode of Noble (M = Pt, Pd) and Non-Noble (M = Re, Mo, Sn) Metal Additives in the Composition of Ni-M/Ce0.5Zr0.5O2/Al2O3 Catalysts. Int. J. Hydrogen Energy 2020, 45, 33352–33369. [Google Scholar] [CrossRef]
- Araújo, P.M.; da Costa, K.M.; Passos, F.B. Hydrogen Production from Methane Autothermal Reforming over CaTiO3, BaTiO3 and SrTiO3 Supported Nickel Catalysts. Int. J. Hydrogen Energy 2021, 46, 24107–24116. [Google Scholar] [CrossRef]
- Tariq, R.; Maqbool, F.; Abbas, S.Z. Small-Scale Production of Hydrogen via Auto-Thermal Reforming in an Adiabatic Packed Bed Reactor: Parametric Study and Reactor’s Optimization through Response Surface Methodology. Comput. Chem. Eng. 2021, 145, 107192. [Google Scholar] [CrossRef]
- Murmura, M.A.; Diana, M.; Spera, R.; Annesini, M.C. Modeling of Autothermal Methane Steam Reforming: Comparison of Reactor Configurations. Chem. Eng. Process. Process. Intensif. 2016, 109, 125–135. [Google Scholar] [CrossRef]
- Chen, B.; Wang, F. Numerical Simulation of Heat-Pipe and Folded Reformers for Efficient Hydrogen Production through Methane Autothermal Reforming. Int. J. Energy Res. 2020, 44, 10430–10441. [Google Scholar] [CrossRef]
- Shahhosseini, H.R.; Saeidi, S.; Najari, S.; Gallucci, F. Comparison of Conventional and Spherical Reactor for the Industrial Auto-Thermal Reforming of Methane to Maximize Synthesis Gas and Minimize CO2. Int. J. Hydrogen Energy 2017, 42, 19798–19809. [Google Scholar] [CrossRef]
- Gul, H.; Arshad, M.Y.; Tahir, M.W. Production of H2 via Sorption Enhanced Auto-Thermal Reforming for Small Scale Applications-A Process Modeling and Machine Learning Study. Int. J. Hydrogen Energy 2023, 48, 12622–12635. [Google Scholar] [CrossRef]
- Rau, F.; Herrmann, A.; Krause, H.; Fino, D.; Trimis, D. Production of Hydrogen by Autothermal Reforming of Biogas. Energy Procedia 2017, 120, 294–301. [Google Scholar] [CrossRef]
- Rau, F.; Herrmann, A.; Krause, H.; Fino, D.; Trimis, D. Efficiency of a Pilot-Plant for the Autothermal Reforming of Biogas. Int. J. Hydrogen Energy 2019, 44, 19135–19140. [Google Scholar] [CrossRef]
- Montenegro Camacho, Y.S.; Bensaid, S.; Piras, G.; Antonini, M.; Fino, D. Techno-Economic Analysis of Green Hydrogen Production from Biogas Autothermal Reforming. Clean Technol. Environ. Policy 2017, 19, 1437–1447. [Google Scholar] [CrossRef]
- Kelling, R.; Eigenberger, G.; Nieken, U. Ceramic Counterflow Reactor for Autothermal Dry Reforming at High Temperatures. Catal. Today 2016, 273, 196–204. [Google Scholar] [CrossRef]
- Akri, M.; Achak, O.; Granger, P.; Wang, S.; Batiot-Dupeyrat, C.; Chafik, T. Autothermal Reforming of Model Purified Biogas Using an Extruded Honeycomb Monolith: A New Catalyst Based on Nickel Incorporated Illite Clay Promoted with MgO. J. Clean. Prod. 2018, 171, 377–389. [Google Scholar] [CrossRef]
- Dega, F.B.; Chamoumi, M.; Braidy, N.; Abatzoglou, N. Autothermal Dry Reforming of Methane with a Nickel Spinellized Catalyst Prepared from a Negative Value Metallurgical Residue. Renew. Energy 2019, 138, 1239–1249. [Google Scholar] [CrossRef]
- Dega, F.B.; Abatzoglou, N. H 2 S Poisoning and Regeneration of a Nickel Spinellized Catalyst Prepared from Waste Metallurgical Residues, During Dry Autothermal Methane Reforming. Catal. Lett. 2019, 149, 1730–1742. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Hydrogen Enrichment of Biogas via Dry and Autothermal-Dry Reforming with Pure Nickel (Ni) Nanoparticle. Energy 2019, 172, 733–739. [Google Scholar] [CrossRef]
- Khalighi, R.; Bahadoran, F.; Panjeshahi, M.H.; Zamaniyan, A.; Tahouni, N. High Catalytic Activity and Stability of X/CoAl2O4 (X = Ni, Co, Rh, Ru) Catalysts with No Observable Coke Formation Applied in the Autothermal Dry Reforming of Methane Lined on Cordierite Monolith Reactors. Microporous Mesoporous Mater. 2020, 305, 110371. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, G.; Zhu, X.; Guo, Q.; Liao, X.; Chen, X.; Li, K. Optimized Ni-Based Catalysts for Methane Reforming with O2-Containing CO2. Appl. Catal. B 2021, 289, 120033. [Google Scholar] [CrossRef]
- Babakouhi, R.; Alavi, S.M.; Rezaei, M.; Akbari, E.; Varbar, M. Combined CO2 Reforming and Partial Oxidation of Methane over Mesoporous Nanostructured Ni/M-Al2O3 Catalyst: Effect of Various Support Promoters and Nickel Loadings. J. CO2 Util. 2023, 70, 102427. [Google Scholar] [CrossRef]
- Fan, D.; Gao, Y.; Liu, F.; Wei, T.; Ye, Z.; Ling, Y.; Chen, B.; Zhang, Y.; Ni, M.; Dong, D. Autothermal Reforming of Methane over an Integrated Solid Oxide Fuel Cell Reactor for Power and Syngas Co-Generation. J. Power Sources 2021, 513, 230536. [Google Scholar] [CrossRef]
- Kumar, N.; Shojaee, M.; Spivey, J.J. Catalytic Bi-Reforming of Methane: From Greenhouse Gases to Syngas. Curr. Opin. Chem. Eng. 2015, 9, 8–15. [Google Scholar] [CrossRef]
- Mohanty, U.S.; Ali, M.; Azhar, M.R.; Al-Yaseri, A.; Keshavarz, A.; Iglauer, S. Current Advances in Syngas (CO + H2) Production through Bi-Reforming of Methane Using Various Catalysts: A Review. Int. J. Hydrogen Energy 2021, 46, 32809–32845. [Google Scholar] [CrossRef]
- Syed Muhammad Wajahat, U.H.; Ahmad, S.F.; Bamidele, V.A.; Bawadi, A. Syngas Production via Combined Dry and Steam Reforming Methane over Ni-Based Catalyst: A Review. Mater. Res. Proc. 2023, 29, 17–27. [Google Scholar] [CrossRef]
- Jin, B.; Wang, K.; Yu, H.; He, X.; Liang, X. Engineering Oxygen Vacancy-Rich CeOx Overcoating onto Ni/Al2O3 by Atomic Layer Deposition for Bi-Reforming of Methane. Chem. Eng. J. 2023, 459, 141611. [Google Scholar] [CrossRef]
- Panda, S.; Joshi, V.; Shrivastaw, V.; Das, S.; Poddar, M.K.; Bal, R.; Bordoloi, A. Enhanced Coke-Resistant Co-Modified Ni/Modified Alumina Catalyst for Bi-Reforming of Methane. Catal. Sci. Technol. 2023, 13, 4506–4516. [Google Scholar] [CrossRef]
- Von Storch, H.; Becker-Hardt, S.; Sattler, C. (Solar) Mixed Reforming of Methane: Potential and Limits in Utilizing CO2 as Feedstock for Syngas Production—A Thermodynamic Analysis. Energies 2018, 11, 2537. [Google Scholar] [CrossRef]
- Matus, E.V.; Sukhova, O.B.; Ismagilov, I.Z.; Kerzhentsev, M.A.; Li, L.; Ismagilov, Z.R. Bi-Reforming of Methane: Thermodynamic Equilibrium Analysis and Selection of Preferable Reaction Conditions. J. Phys. Conf. Ser. 2021, 1749, 012023. [Google Scholar] [CrossRef]
- Pham, X.H.; Ashik, U.P.M.; Hayashi, J.I.; Pérez Alonso, A.; Pla, D.; Gómez, M.; Pham Minh, D. Review on the Catalytic Tri-Reforming of Methane—Part II: Catalyst Development. Appl. Catal. A Gen. 2021, 623, 118286. [Google Scholar] [CrossRef]
- Alli, R.D.; de Souza, P.A.L.; Mohamedali, M.; Virla, L.D.; Mahinpey, N. Tri-Reforming of Methane for Syngas Production Using Ni Catalysts: Current Status and Future Outlook. Catal. Today 2023, 407, 107–124. [Google Scholar] [CrossRef]
- Soleimani, S.; Lehner, M. Tri-Reforming of Methane: Thermodynamics, Operating Conditions, Reactor Technology and Efficiency Evaluation—A Review. Energies 2022, 15, 7159. [Google Scholar] [CrossRef]
- Kozonoe, C.E.; de Abreu, T.F.; de Brito Alves, R.M.; Schmal, M. Influence of the Material as Support for Nickel on the Product Selectivity of the Tri-Reforming of Methane. Mater. Today Commun. 2023, 35, 105732. [Google Scholar] [CrossRef]
- Nascimento, J.P.; Bezerra, R.D.C.F.; Assaf, E.M.; Lucredio, A.F.; Araujo, R.S.; Saraiva, G.D.; Rodríguez-Castellón, E.; Rodríguez-Aguado, E.; Pinheiro, G.S.; Oliveira, A.C. Investigation of the Deactivation Behavior of MeMo/La2O3-Al2O3- and MeMo/Nb2O5 Supported Catalysts (Me = Pt, Ni, and Co) in Tri-Reforming of Methane. Energy Fuels 2023, 37, 3836–3853. [Google Scholar] [CrossRef]
- Thyssen, V.V.; Lino, A.V.P.; Assaf, J.M.; Assaf, E.M. Syngas Production via Methane Tri-Reforming on Ni/La2O3–AAl2O3 Catalysts. Braz. J. Chem. Eng. 2023. [Google Scholar] [CrossRef]
- Bertoldi, J.; de Campos Roseno, K.T.; Schmal, M.; Lage, V.D.; Lenzi, G.G.; Brackmann, R. La1−x(Ce, Sr)XNiO3 Perovskite-Type Oxides as Catalyst Precursors to Syngas Production through Tri-Reforming of Methane. Int. J. Hydrogen Energy 2022, 47, 31279–31294. [Google Scholar] [CrossRef]
- Jang, J.; Han, M. Tri-Reformer with O2 Side-Stream Distribution for Syngas Production. Int. J. Hydrogen Energy 2022, 47, 9139–9155. [Google Scholar] [CrossRef]
- Arab Aboosadi, Z.; Dehghanfard, E.; Abdosheikhi, M. Modeling of Methane Tri-Reforming Slurry Bubble Column Reactor via Differential Evolution Optimization Method to Produce Syngas. Int. J. Chem. React. Eng. 2022, 20, 911–928. [Google Scholar] [CrossRef]
- Osat, M.; Shojaati, F.; Hafizi, A. A Multi-Objective Optimization of Three Conflicting Criteria in a Methane Tri-Reforming Reactor. Int. J. Hydrogen Energy 2023, 48, 6275–6287. [Google Scholar] [CrossRef]
- Soltanimehr, S.; Rahimpour, M.R.; Shariati, A. Development of a Green Process Based on a Novel Tri-Reforming Reactor to Produce Syngas for Methanol Synthesis: Process Design, Modeling and Multi Objective Optimization. J. Clean. Prod. 2023, 418, 138167. [Google Scholar] [CrossRef]
- McConnachie, M.; Konarova, M.; Smart, S. Literature Review of the Catalytic Pyrolysis of Methane for Hydrogen and Carbon Production. Int. J. Hydrogen Energy 2023, 48, 25660–25682. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroǧlu, T.N. From Hydrocarbon to Hydrogen-Carbon to Hydrogen Economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Msheik, M.; Rodat, S.; Abanades, S. Methane Cracking for Hydrogen Production: A Review of Catalytic and Molten Media Pyrolysis. Energies 2021, 14, 3107. [Google Scholar] [CrossRef]
- Korányi, T.I.; Németh, M.; Beck, A.; Horváth, A. Recent Advances in Methane Pyrolysis: Turquoise Hydrogen with Solid Carbon Production. Energies 2022, 15, 6342. [Google Scholar] [CrossRef]
- Kang, D.; Rahimi, N.; Gordon, M.J.; Metiu, H.; McFarland, E.W. Catalytic Methane Pyrolysis in Molten MnCl2-KCl. Appl. Catal. B 2019, 254, 659–666. [Google Scholar] [CrossRef]
- Hu, X.; Hu, Y.; Xu, Q.; Wang, X.; Li, G.; Cheng, H.; Zou, X.; Lu, X. Molten Salt-Promoted Ni–Fe/Al2O3 Catalyst for Methane Decomposition. Int. J. Hydrogen Energy 2020, 45, 4244–4253. [Google Scholar] [CrossRef]
- Boo, J.; Ko, E.H.; Park, N.K.; Ryu, C.; Kim, Y.H.; Park, J.; Kang, D. Methane Pyrolysis in Molten Potassium Chloride: An Experimental and Economic Analysis. Energies 2021, 14, 8182. [Google Scholar] [CrossRef]
- Kazemi, S.; Alavi, S.M.; Rezaei, M.; Akbari, E. Fabrication and Evaluation of the Mn-Promoted Ni/FeAl2O4 Catalysts in the Thermocatalytic Decomposition of Methane: Impact of Various Promoters. Fuel 2023, 342, 127797. [Google Scholar] [CrossRef]
- Miccio, F. On the Integration between Fluidized Bed and Stirling Engine for Micro-Generation. Appl. Therm. Eng. 2013, 52, 46–53. [Google Scholar] [CrossRef]
- Reshetenko, T.V.; Avdeeva, L.B.; Ismagilov, Z.R.; Chuvilin, A.L.; Ushakov, V.A. Carbon Capacious Ni-Cu-Al2O3 Catalysts for High-Temperature Methane Decomposition. Appl. Catal. A Gen. 2003, 247, 51–63. [Google Scholar] [CrossRef]
- Ammendola, P.; Chirone, R.; Lisi, L.; Ruoppolo, G.; Russo, G. Copper Catalysts for H2 Production via CH4 Decomposition. J. Mol. Catal. A Chem. 2007, 266, 31–39. [Google Scholar] [CrossRef]
- Kunii, D. Levenspiel Octave Fluidization Engineering-Chapter 3: Fluidization and Mapping of Regimes; Butterworth-Heinemann: Newton, MA, USA, 1991; ISBN 978-0-08-050664-7. [Google Scholar]
- Ammendola, P.; Chirone, R.; Ruoppolo, G.; Russo, G. Regeneration Strategies of Deactivated Catalysts for Thermo-Catalytic Decomposition Process in a Fluidized Bed Reactor. Combust. Sci. Technol. 2008, 180, 869–882. [Google Scholar] [CrossRef]
- European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs. European Commission Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020; European Commission, Directorate-General for Internal Market, Industry, Entrepreneurship and SMEs: Brussels, Belgium, 2023. [Google Scholar]
- Bahman, N.; Al-Khalifa, M.; Al Baharna, S.; Abdulmohsen, Z.; Khan, E. Review of Carbon Capture and Storage Technologies in Selected Industries: Potentials and Challenges. Rev. Environ. Sci. Biotechnol. 2023, 22, 451–470. [Google Scholar] [CrossRef]
- Steinberg, M. Fossil Fuel Decarbonization Technology for Mitigating Global Warming. Int. J. Hydrogen Energy 1999, 24, 771–777. [Google Scholar] [CrossRef]
- Shamsi, M.; Moghaddas, S.; Naeiji, E.; Farokhi, S. Techno-Economic, Energy, Exergy, and Environmental Comparison of Hydrogen Production from Natural Gas, Biogas, and Their Combination as Feedstock. Arab. J. Sci. Eng. 2023, 48, 8971–8987. [Google Scholar] [CrossRef]
- Szima, S.; Cormos, C.C. Techno—Economic Assessment of Flexible Decarbonized Hydrogen and Power Co-Production Based on Natural Gas Dry Reforming. Int. J. Hydrogen Energy 2019, 44, 31712–31723. [Google Scholar] [CrossRef]
- Lee, B.; Kim, H.; Lee, H.; Byun, M.; Won, W.; Lim, H. Technical and Economic Feasibility under Uncertainty for Methane Dry Reforming of Coke Oven Gas as Simultaneous H2 Production and CO2 Utilization. Renew. Sustain. Energy Rev. 2020, 133, 110056. [Google Scholar] [CrossRef]
- Farkad Alkhani, A. Catalytic Dry Reforming of Methane: Paving the Road to a Carbon Neutral Industrial Scale Blue Hydrogen Production Process Technology via Monolithic Catalyst-Based Reformer Bolstered by a Techno-Economic Assessment; Columbia University: New York, NY, USA, 2022. [Google Scholar]
- Kumar, R.; Kumar, A.; Pal, A. An Overview of Conventional and Non-Conventional Hydrogen Production Methods. Mater. Today Proc. 2020, 46, 5353–5359. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for CO2-Free H2 Production: A Green Process to Overcome Renewable Energies Unsteadiness. Chem. Ing. Tech. 2020, 92, 1596–1609. [Google Scholar] [CrossRef]
- Cheon, S.; Byun, M.; Lim, D.; Lee, H.; Lim, H. Parametric Study for Thermal and Catalytic Methane Pyrolysis for Hydrogen Production: Techno-Economic and Scenario Analysis. Energies 2021, 14, 6102. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Gu, Z.; Zhu, X.; Qing, S.; Li, K. Chemical-Looping Conversion of Methane: A Review. Energy Technol. 2020, 8, 1900925. [Google Scholar] [CrossRef]
- Mantripragada, H.C.; Veser, G. Hydrogen Production via Chemical Looping Dry Reforming of Methane: Process Modeling and Systems Analysis. AIChE J. 2022, 68, e17612. [Google Scholar] [CrossRef]
- Ugwu, A.; Zaabout, A.; Amini, S. An Advancement in CO2 Utilization through Novel Gas Switching Dry Reforming. Int. J. Greenh. Gas Control 2019, 90, 102791. [Google Scholar] [CrossRef]
- Hu, J.; Galvita, V.V.; Poelman, H.; Marin, G.B. Advanced Chemical Looping Materials for CO2 Utilization: A Review. Materials 2018, 11, 1187. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-Based Oxygen Carriers in Chemical Looping Conversions: A Review. Carbon. Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Stepwise Solar Methane Reforming and Water-Splitting via Lattice Oxygen Transfer in Iron and Cerium Oxides. Energy Technol. 2020, 8, 1900415. [Google Scholar] [CrossRef]
- Tang, M.; Xu, L.; Fan, M. Progress in Oxygen Carrier Development of Methane-Based Chemical-Looping Reforming: A Review. Appl. Energy 2015, 151, 143–156. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Chen, D.; Zhao, K.; Wei, G.; Zheng, A.; Zhao, Z.; Li, H. Investigation on Reactivity of Iron Nickel Oxides in Chemical Looping Dry Reforming. Energy 2016, 116, 53–63. [Google Scholar] [CrossRef]
- Sastre, D.; Galván, C.Á.; Pizarro, P.; Coronado, J.M. Enhanced Performance of CH4 Dry Reforming over La0.9Sr0.1FeO3/YSZ under Chemical Looping Conditions. Fuel 2022, 309, 122122. [Google Scholar] [CrossRef]
- Forutan, H.R.; Karimi, E.; Hafizi, A.; Rahimpour, M.R.; Keshavarz, P. Expert Representation Chemical Looping Reforming: A Comparative Study of Fe, Mn, Co and Cu as Oxygen Carriers Supported on Al2O3. J. Ind. Eng. Chem. 2015, 21, 900–911. [Google Scholar] [CrossRef]
- Löfberg, A.; Guerrero-Caballero, J.; Kane, T.; Rubbens, A.; Jalowiecki-Duhamel, L. Ni/CeO2 Based Catalysts as Oxygen Vectors for the Chemical Looping Dry Reforming of Methane for Syngas Production. Appl. Catal. B 2017, 212, 159–174. [Google Scholar] [CrossRef]
- Guerrero-Caballero, J.; Kane, T.; Haidar, N.; Jalowiecki-Duhamel, L.; Löfberg, A. Ni, Co, Fe Supported on Ceria and Zr Doped Ceria as Oxygen Carriers for Chemical Looping Dry Reforming of Methane. Catal. Today 2019, 333, 251–258. [Google Scholar] [CrossRef]
- Noh, Y.G.; Lee, Y.J.; Kim, J.; Kim, Y.K.; Ha, J.S.; Kalanur, S.S.; Seo, H. Enhanced Efficiency in CO2-Free Hydrogen Production from Methane in a Molten Liquid Alloy Bubble Column Reactor with Zirconia Beads. Chem. Eng. J. 2021, 428, 131095. [Google Scholar] [CrossRef]
- Miccio, F.; Papa, E.; Murri, A.N.; Landi, E.; Medri, V.; Vaccari, A. Fluidized Bed Gasification of Biomass Char by Chemical Looping. Chem. Eng. Trans. 2021, 86, 769–774. [Google Scholar] [CrossRef]
- Kang, D.; Lee, M.; Lim, H.S.; Lee, J.W. Chemical Looping Partial Oxidation of Methane with CO2 Utilization on the Ceria-Enhanced Mesoporous Fe2O3 Oxygen Carrier. Fuel 2018, 215, 787–798. [Google Scholar] [CrossRef]
- García-García, F.R.; Metcalfe, I.S. Chemical Looping Dry Reforming of Methane Using Mixed Oxides of Iron and Cerium: Operation Window. Catal. Commun. 2021, 160, 106356. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, X.; Li, K.; Wei, Y.; He, F.; Wang, H. Moderate-Temperature Chemical Looping Splitting of CO2 and H2O for Syngas Generation. Chem. Eng. J. 2020, 397, 125393. [Google Scholar] [CrossRef]
- Haxel, G.B.; Hedrick, J.B.; Orris, G.J. Rare Earth Elements—Critical Resources for High Technology. Available online: https://pubs.usgs.gov/fs/2002/fs087-02/ (accessed on 25 May 2022).
- Otsuka, K.; Ushiyama, T.; Yamanaka, I. Partial Oxidation of Methane Using the Redox of Cerium Oxide. Chem. Lett. 1993, 22, 1517–1520. [Google Scholar] [CrossRef]
- Schmitt, R.; Nenning, A.; Kraynis, O.; Korobko, R.; Frenkel, A.I.; Lubomirsky, I.; Haile, S.M.; Rupp, J.L.M. A Review of Defect Structure and Chemistry in Ceria and Its Solid Solutions. Chem. Soc. Rev. 2020, 49, 554–592. [Google Scholar] [CrossRef]
- Li, P.; Chen, X.; Li, Y.; Schwank, J.W. A Review on Oxygen Storage Capacity of CeO2-Based Materials: Influence Factors, Measurement Techniques, and Applications in Reactions Related to Catalytic Automotive Emissions Control. Catal. Today 2019, 327, 90–115. [Google Scholar] [CrossRef]
- Nair, M.M.; Abanades, S. Tailoring Hybrid Nonstoichiometric Ceria Redox Cycle for Combined Solar Methane Reforming and Thermochemical Conversion of H2O/CO2. Energy Fuels 2016, 30, 6050–6058. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Rotaru, C.G.; Postole, G.; Florea, M.; Matei-Rutkovska, F.; Pârvulescu, V.I.; Gelin, P. Dry Reforming of Methane on Ceria Prepared by Modified Precipitation Route. Appl. Catal. A Gen. 2015, 494, 29–40. [Google Scholar] [CrossRef]
- Miccio, F.; Landi, E.; Murri, A.N.; Minelli, M.; Doghieri, F.; Storione, A. Fluidized Bed Reforming of Methane by Chemical Looping with Cerium Oxide Oxygen Carriers. Chem. Eng. Res. Des. 2023, 191, 568–577. [Google Scholar] [CrossRef]
- Ackermann, S.; Scheffe, J.R.; Steinfeld, A. Diffusion of Oxygen in Ceria at Elevated Temperatures and Its Application to H2O/CO2 Splitting Thermochemical Redox Cycles. J. Phys. Chem. C 2014, 118, 5216–5225. [Google Scholar] [CrossRef]
- Teh, L.P.; Setiabudi, H.D.; Timmiati, S.N.; Aziz, M.A.A.; Annuar, N.H.R.; Ruslan, N.N. Recent Progress in Ceria-Based Catalysts for the Dry Reforming of Methane: A Review. Chem. Eng. Sci. 2021, 242, 116606. [Google Scholar] [CrossRef]
- Ramírez-Cabrera, E.; Atkinson, A.; Chadwick, D. Reactivity of Ceria, Gd- and Nb-Doped Ceria to Methane. Appl. Catal. B 2002, 36, 193–206. [Google Scholar] [CrossRef]
- Jeong, H.H.; Kwak, J.H.; Han, G.Y.; Yoon, K.J. Stepwise Production of Syngas and Hydrogen through Methane Reforming and Water Splitting by Using a Cerium Oxide Redox System. Int. J. Hydrogen Energy 2011, 36, 15221–15230. [Google Scholar] [CrossRef]
- Matei-Rutkovska, F.; Postole, G.; Rotaru, C.G.; Florea, M.; Pârvulescu, V.I.; Gelin, P. Synthesis of Ceria Nanopowders by Microwave-Assisted Hydrothermal Method for Dry Reforming of Methane. Int. J. Hydrogen Energy 2016, 41, 2512–2525. [Google Scholar] [CrossRef]
- Warren, K.J.; Carrillo, R.J.; Greek, B.; Hill, C.M.; Scheffe, J.R. Solar Reactor Demonstration of Efficient and Selective Syngas Production via Chemical-Looping Dry Reforming of Methane over Ceria. Energy Technol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Chuayboon, S.; Abanades, S.; Rodat, S. Solar Chemical Looping Reforming of Methane Combined with Isothermal H2O/CO2 Splitting Using Ceria Oxygen Carrier for Syngas Production. J. Energy Chem. 2020, 41, 60–72. [Google Scholar] [CrossRef]
- Krenzke, P.T.; Davidson, J.H. Thermodynamic Analysis of Syngas Production via the Solar Thermochemical Cerium Oxide Redox Cycle with Methane-Driven Reduction. Energy Fuels 2014, 28, 4088–4095. [Google Scholar] [CrossRef]
- Storione, A.; Minelli, M.; Doghieri, F.; Landi, E.; Miccio, F. Thermodynamic Study on the Feasibility of a New Combined Chemical Looping Process for Syngas Production. Chem. Eng. Trans. 2021, 86, 1267–1272. [Google Scholar] [CrossRef]
- Warren, K.J.; Scheffe, J.R. Role of Surface Oxygen Vacancy Concentration on the Dissociation of Methane over Nonstoichiometric Ceria. J. Phys. Chem. C 2019, 123, 13208–13218. [Google Scholar] [CrossRef]
- Ackermann, S.; Sauvin, L.; Castiglioni, R.; Rupp, J.L.M.; Scheffe, J.R.; Steinfeld, A. Kinetics of CO2 Reduction over Nonstoichiometric Ceria. J. Phys. Chem. C 2015, 119, 16452–16461. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.; Warren, K.; Scheffe, J.R.; Steinfeld, A. Combined Ceria Reduction and Methane Reforming in a Solar-Driven Particle-Transport Reactor. Ind. Eng. Chem. Res. 2017, 56, 10300–10308. [Google Scholar] [CrossRef]
- Diao, Y.; Wang, H.; Chen, B.; Zhang, X.; Shi, C. Modulating Morphology and Textural Properties of Al2O3 for Supported Ni Catalysts toward Plasma-Assisted Dry Reforming of Methane. Appl. Catal. B 2023, 330, 122573. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, Y.; Mao, Y.; Wang, W.; Sun, J.; Song, Z.; Sun, J.; Zhao, X. Enhanced Dry Reforming of Methane by Microwave-Mediated Confined Catalysis over Ni-La/AC Catalyst. Chem. Eng. J. 2023, 451, 138616. [Google Scholar] [CrossRef]
- Mei, D.; Sun, M.; Liu, S.; Zhang, P.; Fang, Z.; Tu, X. Plasma-Enabled Catalytic Dry Reforming of CH4 into Syngas, Hydrocarbons and Oxygenates: Insight into the Active Metals of γ-Al2O3 Supported Catalysts. J. CO2 Util. 2023, 67, 102307. [Google Scholar] [CrossRef]
- Kim, H.J.; Chun, Y.N. Conversion of Biogas to Renewable Energy by Microwave Reforming. Energies 2020, 13, 4093. [Google Scholar] [CrossRef]
- Gray, J.T.; Che, F.; McEwen, J.S.; Ha, S. Field-Assisted Suppression of Coke in the Methane Steam Reforming Reaction. Appl. Catal. B 2020, 260, 118132. [Google Scholar] [CrossRef]
- Zehetner, E.; Schöny, G.; Fuchs, J.; Pröll, T.; Hofbauer, H. Fluid-Dynamic Study on a Multistage Fluidized Bed Column for Continuous CO2 Capture via Temperature Swing Adsorption. Powder Technol. 2017, 316, 528–534. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, Y.; Yang, Y.; Cai, D.; Song, W.; Wang, N.; Qian, W. A Multi-Stage Fluidized Bed Strategy for the Enhanced Conversion of Methanol into Aromatics. Chem. Eng. Sci. 2019, 204, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Yan, L.; Zhao, F.; Lu, H.; Sun, L.; Zhang, Q. Numerical Simulation of Hydrogen Production via Chemical Looping Reforming in Interconnected Fluidized Bed Reactor. Ind. Eng. Chem. Res. 2014, 53, 4182–4191. [Google Scholar] [CrossRef]
- Durán, P.; Sanz-Martínez, A.; Soler, J.; Menéndez, M.; Herguido, J. Pure Hydrogen from Biogas: Intensified Methane Dry Reforming in a Two-Zone Fluidized Bed Reactor Using Permselective Membranes. Chem. Eng. J. 2019, 370, 772–781. [Google Scholar] [CrossRef]
- Zambrano, D.; Soler, J.; Herguido, J.; Menéndez, M. Conventional and Improved Fluidized Bed Reactors for Dry Reforming of Methane: Mathematical Models. Chem. Eng. J. 2020, 393, 124775. [Google Scholar] [CrossRef]
- Driessen, R.T.; Bos, M.J.; Brilman, D.W.F. A Multistage Fluidized Bed for the Deep Removal of Sour Gases: Proof of Concept and Tray Efficiencies. Ind. Eng. Chem. Res. 2018, 57, 3866–3875. [Google Scholar] [CrossRef]
- Ko, K.J.; Kim, H.; Cho, Y.H.; Lee, H.; Kim, K.M.; Lee, C.H. Overview of Carbon Monoxide Adsorption Performance of Pristine and Modified Adsorbents. J. Chem. Eng. Data 2022, 67, 1599–1616. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas Separation Membrane Materials: A Perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-Efficient Polymeric Gas Separation Membranes for a Sustainable Future: A Review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef]
- Bernardo, G.; Araújo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent Advances in Membrane Technologies for Hydrogen Purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Teplyakov, V. Polymeric Membranes for Hydrogen Separation/Purification. In Current Trends and Future Developments on (Bio-) Membranes: New Perspectives on Hydrogen Production, Separation, and Utilization; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–304. ISBN 9780128173848. [Google Scholar]
- Amosova, O.L.; Malykh, O.V.; Teplyakov, V.V. Integrated Membrane/PSA Systems for Hydrogen Recovery from Gas Mixtures. Desalination Water Treat. 2010, 14, 119–126. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Wang, D.; Flanagan, T.B.; Shanahan, K.L. Permeation of Hydrogen through Pre-Oxidized Pd Membranes in the Presence and Absence of CO. J. Alloys Compd. 2004, 372, 158–164. [Google Scholar] [CrossRef]
- Catalano, J.; Giacinti Baschetti, M.; Sarti, G.C. Hydrogen Permeation in Palladium-Based Membranes in the Presence of Carbon Monoxide. J. Memb. Sci. 2010, 362, 221–233. [Google Scholar] [CrossRef]
- Koutsonikolas, D.E.; Pantoleontos, G.; Karagiannakis, G.; Konstandopoulos, A.G. Development of H2 Selective Silica Membranes: Performance Evaluation through Single Gas Permeation and Gas Separation Tests. Sep. Purif. Technol. 2021, 264, 118432. [Google Scholar] [CrossRef]
- Mercadelli, E.; Gondolini, A.; Ardit, M.; Cruciani, G.; Melandri, C.; Escolástico, S.; Serra, J.M.; Sanson, A. Chemical and Mechanical Stability of BCZY-GDC Membranes for Hydrogen Separation. Sep. Purif. Technol. 2022, 289, 120795. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of Carbon Dioxide for Post-Combustion Capture: A Review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
- Iarikov, D.D.; Ted Oyama, S. Chapter 5—Review of CO2/CH4 Separation Membranes. In Membrane Science and Technology; Oyama, S.T., Stagg-Williams, S.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; Volume 14, pp. 91–115. ISBN 9780444537287. [Google Scholar]
- Minelli, M.; Doghieri, F.; Miccio, F.; Landi, E.; Medri, V. New Hybrid Unit Operation for Gas Separation Membranes Application. Chem. Eng. Trans. 2019, 74, 925–930. [Google Scholar] [CrossRef]
- Li, B.; Kado, S.; Mukainakano, Y.; Nurunnabi, M.; Miyao, T.; Naito, S.; Kunimori, K.; Tomishige, K. Temperature Profile of Catalyst Bed during Oxidative Steam Reforming of Methane over Pt-Ni Bimetallic Catalysts. Appl. Catal. A Gen. 2006, 304, 62–71. [Google Scholar] [CrossRef]
- Karim, A.; Bravo, J.; Datye, A. Nonisothermality in Packed Bed Reactors for Steam Reforming of Methanol. Appl. Catal. A Gen. 2005, 282, 101–109. [Google Scholar] [CrossRef]
- Wesenberg, M.H.; Svendsen, H.F. Mass and Heat Transfer Limitations in a Heterogeneous Model of a Gas-Heated Steam Reformer. Ind. Eng. Chem. Res. 2007, 46, 667–676. [Google Scholar] [CrossRef]
- Al-Otaibi, F.; Xiao, H.; Berrouk, A.S.; Polychronopoulou, K. Numerical Study of Dry Reforming of Methane in Packed and Fluidized Beds: Effects of Key Operating Parameters. ChemEngineering 2023, 7, 57. [Google Scholar] [CrossRef]
- Han, Z.; Shao, Y.; Zhong, W. Experimental and Numerical Study on Characteristics and Mechanism of Particles Attrition in Fluidized Bed. Powder Technol. 2023, 427, 118444. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Zhu, J. Development of Gas-Solid Fluidization: Particulate and Aggregative. Powder Technol. 2023, 421, 118420. [Google Scholar] [CrossRef]
- Côté, A.S.; Delgass, W.N.; Ramkrishna, D. Spatially Patterned Catalytic Reactors. Feasibility Issues. Chem. Eng. Sci. 2001, 56, 1011–1019. [Google Scholar] [CrossRef]
- Mcbride, K.; Turek, T.; Güttel, R. Direct Dimethyl Ether Synthesis by Spatial Patterned Catalyst Arrangement: A Modeling and Simulation Study. AIChE J. 2012, 58, 3468–3473. [Google Scholar] [CrossRef]
- Pajak, M.; Mozdzierz, M.; Chalusiak, M.; Kimijima, S.; Szmyd, J.S.; Brus, G. A Numerical Analysis of Heat and Mass Transfer Processes in a Macro-Patterned Methane/Steam Reforming Reactor. Int. J. Hydrogen Energy 2018, 43, 20474–20487. [Google Scholar] [CrossRef]
- Cherif, A.; Nebbali, R.; Lee, C.J. Numerical Analysis of Steam Methane Reforming over a Novel Multi-Concentric Rings Ni/Al2O3 Catalyst Pattern. Int. J. Energy Res. 2021, 45, 18722–18734. [Google Scholar] [CrossRef]
- Pajak, M.; Buchaniec, S.; Kimijima, S.; Szmyd, J.S.; Brus, G. A Multiobjective Optimization of a Catalyst Distribution in a Methane/Steam Reforming Reactor Using a Genetic Algorithm. Int. J. Hydrogen Energy 2021, 46, 20183–20197. [Google Scholar] [CrossRef]
- Pajak, M.; Brus, G.; Kimijima, S.; Szmyd, J.S. Enhancing Hydrogen Production from Biogas through Catalyst Rearrangements. Energies 2023, 16, 4058. [Google Scholar] [CrossRef]
- Lee, J.; Kim, B.; Han, M. Spatially Patterned Catalytic Reactor for Steam-CO2 Reforming of Methane. Ind. Eng. Chem. Res. 2019, 58, 18731–18741. [Google Scholar] [CrossRef]
- Wu, Z.; Guo, Z.; Yang, J.; Wang, Q. Effect of Diameter Distribution of Particles on Methane Steam Reforming in Multi-Channel Grille-Sphere Composite Packed Bed. Energy Convers. Manag. 2022, 265, 115764. [Google Scholar] [CrossRef]
- Karpilov, I.; Pashchenko, D. Steam Methane Reforming over a Preheated Packed Bed: Heat and Mass Transfer in a Transient Process. Therm. Sci. Eng. Prog. 2023, 42, 101868. [Google Scholar] [CrossRef]
- Cherif, A.; Nebbali, R.; Sheffield, J.W.; Doner, N.; Sen, F. Numerical Investigation of Hydrogen Production via Autothermal Reforming of Steam and Methane over Ni/Al2O3 and Pt/Al2O3 Patterned Catalytic Layers. Int. J. Hydrogen Energy 2021, 46, 37521–37532. [Google Scholar] [CrossRef]
- Cherif, A.; Nebbali, R.; Sen, F.; Sheffield, J.W.; Doner, N.; Nasseri, L. Modeling and Simulation of Steam Methane Reforming and Methane Combustion over Continuous and Segmented Catalyst Beds in Autothermal Reactor. Int. J. Hydrogen Energy 2022, 47, 9127–9138. [Google Scholar] [CrossRef]
- Cherif, A.; Lee, J.S.; Nebbali, R.; Lee, C.J. Novel Design and Multi-Objective Optimization of Autothermal Steam Methane Reformer to Enhance Hydrogen Production and Thermal Matching. Appl. Therm. Eng. 2022, 217, 119140. [Google Scholar] [CrossRef]
- Mundhwa, M.; Thurgood, C.P. Improved Performance of a Catalytic Plate Reactor Coated with Distributed Layers of Reforming and Combustion Catalysts for Hydrogen Production. React. Chem. Eng. 2018, 3, 487–514. [Google Scholar] [CrossRef]
- Lee, S.; Lim, H. Utilization of CO2 Arising from Methane Steam Reforming Reaction: Use of CO2 Membrane and Heterotic Reactors. J. Ind. Eng. Chem. 2020, 91, 201–212. [Google Scholar] [CrossRef]
- Cherif, A.; Nebbali, R.; Lee, C.J. Design and Multiobjective Optimization of Membrane Steam Methane Reformer: A Computational Fluid Dynamic Analysis. Int. J. Energy Res. 2022, 46, 8700–8715. [Google Scholar] [CrossRef]
| Process | Technology Readiness | Catalysts | CRM | Harmfulness | Refs. |
|---|---|---|---|---|---|
| Steam reforming | Commercial | Ni | N | Y | [39,40,41] |
| Ni-Fe | N | Y | [53] | ||
| Dry reforming | Lab scale | Ni | N | Y | [58,59,60,61,62,63,64] |
| Co | Y | Y | [65,66,67,68] | ||
| Ni-Co | Y | Y | [69,70] | ||
| Ni-Cu | N | Y | [73,74,75] | ||
| Ni-Fe | N | Y | [71,72] | ||
| Co-Ce | Y | Y | [88] | ||
| Co-Sm | Y | Y | [89,90] | ||
| Ni-Mo | Y | Y | [91] | ||
| Perovskite * | Y | Y-N | [80,81,82,83,84] | ||
| Partial oxidation | Pilot–Commercial Lab scale | Ni | N | Y | [91,92,93,94,95] |
| Co | Y | Y | [96,97,98] | ||
| Ni-Co | Y | Y | [99,113] | ||
| Ni-Cu | Y | Y | [102,103] | ||
| Ni-Ce | Y | Y | [102,104] | ||
| Cu-Ce | Y | N | [115] | ||
| Perovskite * | Y | Y-N | [105,106,107,108,109,110,111,112,113,114,115,116,117,118,119] | ||
| MoP | Y | N | [120] | ||
| Thermo-catalytic decomposition | Lab scale | Ni-Mn | Y | Y | [177] |
| Ni-Cu-Al | Y | Y | [179] | ||
| Cu | Y | N | [180] |
| Process | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| SR | High H2/CO ratio (=3) Mature technology | Excess high-pressure steam needed (steam/C: 2–5; 3) Endothermic reaction High CO2 emission High capital costs | [49,50,51,52,53] |
| DR | CO2 utilization Applicable to biogas without previous separation | Low H2/CO ratio (=1) RWGS parasitic reaction can decrease the syngas ratio below 1 Strong tendency for carbon deposition Endothermic reaction High reaction temperature needed (risk of catalyst sintering) | [68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,88,89,90,91,92,93,94,95,96] |
| PO | Exothermic process More compact reactors, thanks to fast kinetics Moderate syngas H2/CO ratio (=2) Non-catalytic operation is possible | Pure O2 is needed (expensive ASU unit) Difficult temperature and selectivity control Risk of explosion (CH4-O2 mixture) Coking can deactivate catalyst | [103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120] |
| ATSR | Mature technology Compact reactors Increased energy efficiency Reduced capital costs compared to SR Lower steam and oxygen requirements compared to SR and PO Lower operating cost compared to SR | Pure O2 needed Risk of explosion (CH4-O2 mixture) Coking and sintering can deactivate catalyst Low H2 yield compared to SR | [130,131,132,133,134,135] |
| ATDR | CO2 utilization Applicable to biogas without prior separation Autothermal process Lower oxygen and no steam requirement | Pure O2 needed Risk of explosion (CH4-O2 mixture) Coking and sintering can deactivate catalyst | [144,145,146,147,148,149,150] |
| 2-R | CO2 utilization Applicable to biogas without prior separation Tunable H2/CO ratio Lower carbon deposition compared to pure SR and DR | Strongly endothermic process Coking and sintering can deactivate catalyst | [152,153,154,155,156] |
| TR | CO2 utilization Applicable to biogas without prior separation Tunable H2/CO ratio Presence of oxygen lowers endothermicity Lower carbon deposition compared to pure SR, DR and PO | Pure O2 needed Risk of explosion (CH4-O2 mixture) Coking and sintering can deactivate catalyst Difficult management of competitive oxidation–reforming reaction | [159,160,162,163,165] |
| TCD | Zero or near-zero carbon dioxide emission (no CO2 formation) Highest yield of hydrogen (no CO formation) Less complex separation of produced hydrogen (easy separation from solid carbon) Carbon can be produced in value-added form (nanotubes, nanosheets, etc.) No oxygen or steam requirement | Endothermic reaction Coke formation leads to difficult continuous operation due to catalyst deactivation and reactor clogging Harsh reaction conditions cause problems for durability of reactor materials | [177,179,180] |
| Process | Efficiency (%) | T (°C) | P (Bar) | H2 Cost |
|---|---|---|---|---|
| SR | 70–85 (no CCS) [5,9,14,35,124] 60 (with CCS) [185] | 650–1100 [44] | 3–25 [44] | 0.9–1.8 USD/Kg [14] 2.08 USD/Kg (no CCS)–2.27 USD/Kg (with CCS) [34] 1.83–2.35 USD/Kg [35] 1.54–2.30 USD/Kg [9] |
| DR | 76 (estimated for biogas reforming) [186] 59 (estimated for power and H2 cogeneration) [187] | 600–1000 [53] | N.A. | 0.15 EUR/Nm3 (power and H2 co-generation) [187] 2.38–3.27 USD/Kg (coke-oven-gas reforming) [188] 1.07–1.32 USD/Kg (no CCS)—1.91 USD/kg (with CCS) [189] |
| PO | N.A.* | 800–900 [100] | N.A. * | N.A. |
| ATSR | 90 [14] 60–75 [190] | 800–1200 [131] | 1–30 [131] | 1.48 USD/Kg (with CCS) [34] |
| ATDR | N.A. | N.A. | N.A. | N.A. |
| 2-R | 82 (natural and biogas co-reforming) [188] | N.A. | N.A. | N.A. |
| TR | N.A. | N.A. | N.A. | N.A. |
| TCD | 58 [187,191] | 500–1000 [162,163] | 1 [162] | 2 USD/Kg [8] 2.55–5 USD/Kg [12] 3.53–3.82 USD/kg [192] 1.72 USD/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscherini, M.; Storione, A.; Minelli, M.; Miccio, F.; Doghieri, F. New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas. Energies 2023, 16, 6375. https://doi.org/10.3390/en16176375
Boscherini M, Storione A, Minelli M, Miccio F, Doghieri F. New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas. Energies. 2023; 16(17):6375. https://doi.org/10.3390/en16176375
Chicago/Turabian StyleBoscherini, Mattia, Alba Storione, Matteo Minelli, Francesco Miccio, and Ferruccio Doghieri. 2023. "New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas" Energies 16, no. 17: 6375. https://doi.org/10.3390/en16176375
APA StyleBoscherini, M., Storione, A., Minelli, M., Miccio, F., & Doghieri, F. (2023). New Perspectives on Catalytic Hydrogen Production by the Reforming, Partial Oxidation and Decomposition of Methane and Biogas. Energies, 16(17), 6375. https://doi.org/10.3390/en16176375









