1. Introduction
Carbonate oil and gas reserves comprise approximately 40% of the world’s total oil and gas reserves, with their production constituting around 60% of global oil and gas output. This makes them an essential sector in oil and gas development [
1]. A prime example is the Daniudi gas field located in the northeastern part of the Ordos Basin. The deep Ordovician Majiagou Formation, a critical gas-producing layer in the Daniudi gas field, is primarily composed of carbonate rocks and evaporite strata. From bottom to top, it can be categorized into six sections, named from Ma Yi to Ma Liu. Furthermore, the Ma Wu section is divided into 10 sub-sections, sequentially labelled from Ma Wu 1 to Ma Wu 10 [
2].
Before 2011, vertical well development was the adopted strategy in the Daniudi gas field. Although multiple horizons of the Majiagou Formation yielded an industrial gas flow, the average test output of 0.62 × 10
4 m
3/d was insufficient for the field’s natural gas economic development needs. Consequently, in 2012, horizontal well sublevel acid fracturing technology was introduced, increasing the average test output to 2.72 × 10
4 m
3/d, thus satisfying economic development demands. This is a significant breakthrough in developing tight and low-permeability carbonate reservoirs [
3].
During the development of Upper Paleozoic clastic rocks, potassium ammonium based drilling fluid, formulated to protect the reservoir, was employed as the drilling and completion fluid. The primary treatment agents used were polyacrylonitrile potassium salt and polyacrylonitrile ammonium salt. These agents function by inhibiting shale water absorption and expansion via lattice fixation and ion exchange of potassium and ammonium ions. This approach effectively addresses the issue of block falling triggered by uneven expansion pressure, subsequently achieving the goal of inhibiting shale hydration, dispersion, and stabilizing borehole walls. By concurrently reducing the density and filtration loss of the drilling fluid, the ancient clastic gas reservoir is well-protected [
4].
The development strategy for Lower Paleozoic carbonate rocks, specifically the Ma Wu 5 member in the Daniudi gas field, mirrored that of the Upper Paleozoic clastic rocks. However, Ma Wu 5 member experienced significant weathering, erosion, and chemical dissolution due to the uplift of Paleozoic Caledonian movement at the end of sedimentation, forming natural fractured reservoirs of various origins with pressures ranging from 120 to 150 MPa [
5]. As a result, the use of potassium ammonium based drilling fluid with low density and low filtration as a reservoir protection method faced severe challenges.
This issue arose primarily because the potassium ammonium based drilling fluid, composed of elements such as bentonite, graphite lubricant, and ultrafine calcium carbonate, allows 6 to 10% of the system’s solid phase to penetrate deep into the carbonate rock due to pressure differences. The deeper it penetrates, the greater the fracture closing pressure and the narrower the fracture width of the carbonate rock becomes [
6]. When the drilling fluid circulation ceases, the equivalent liquid column pressure decreases, leading to fracture closure, compaction of the solids that have entered the formation, and plastic deformation or fragmentation. Such changes prevent the fracture from returning to its original state, causing the solid phase to block the seepage channels and inducing a permanent reduction in reservoir permeability [
7,
8,
9,
10,
11,
12]. Additionally, the solid phase in potassium ammonium based drilling fluid consists mainly of non-acid soluble solids, limiting the effectiveness of acid fracturing fluid in dissolving its solid phase, thereby hindering the extraction of natural gas from Lower Paleozoic carbonate rocks.
To mitigate reservoir damage caused by the solid phase, solid-free drilling fluids with polymers and salts as primary treatment agents have been internationally used since the 1980s. Although the density of these drilling fluids ranges from 1.00 to 1.75 g/cm
3, their high-temperature resistance is limited, allowing them to operate only at temperatures below 100 °C [
13]. However, post 1990s, the enhanced temperature resistance of polymer treatment agents led to the development of various solid-free drilling fluids.
For instance, solid-free brine polymer drilling fluids use soluble salts such as sodium chloride, potassium chloride, or calcium chloride as weighting agents and inhibitors, exhibiting good rheological properties [
14,
15]. Their main disadvantage is that they cause significant corrosion to drilling tools and equipment [
16].
Solid-free formate drilling fluids, which utilize sodium formate, potassium formate, and cesium formate as weighting agents and inhibitors, supplemented by polymer tackifiers and fluid loss reducers, have several advantages. These include a broad adjustable density range (1.05 to 2.30 g/cm
3), good high-temperature stability, strong inhibition, low corrosion, and environmental friendliness. Their primary downside is the associated high cost [
17].
Alkyl glycoside solid-free drilling fluid is a type of drilling and completion fluid that incorporates methyl glycoside into a polymer drilling fluid to provide reservoir protection. These fluids have a low water activity and easily form an isolation film, effectively inhibiting clay hydration and expansion and reducing reservoir water-locking effects [
18]. The main disadvantage is that a significant amount of methyl glucoside is needed, increasing the cost.
Solid-free weak gel drilling fluids create a hexagonal space grid structure via intermolecular winding, possessing good sand suspension carrying capacity, lubrication, and inhibition. They are resistant to temperatures up to 140 °C. The disadvantages are the weak gel strength of the polymer, long macromolecular chains, large volume, and high viscosity. When these enter the formation, they are prone to adsorption and retention in the pores, causing oil and gas flow obstruction and reducing oil and gas well productivity. Thus, after drilling the oil and gas reservoir, it is necessary to break the gel and reverse discharge the mud cake and its residue formed by the solid-free weak gel drilling fluid on the wellbore [
19].
In summary, the current development strategy for solid-free drilling fluids prioritizes meeting drilling engineering requirements first. Then, specific acidic gel breakers, oxidation gel breakers, or biodegradable agents are selected based on the characteristics of the drilling fluid to degrade the polymer and return it to the formation. Consequently, the field construction process requires an added gel-breaking step to remove blockages, which is relatively complex [
20]. If potassium ammonium based drilling fluid is used for completion in the Daniudi gas field, and there is no specific gel breaker or gel breaker technology, reservoir damage is inevitable.
To address this issue, we propose a new approach based on the characteristics of acidizing fracturing stimulation in carbonate rock completion. We suggest using completion acid fluid as the gel breaker for solid-free drilling fluid, and screening out viscosity enhancers and fluid loss reducers that are easy to dissolve and break the gel. Considering the characteristics of carbonate rock clay content and low formation pressure in the Daniudi gas field, we formulate a set of low-density solid-free drilling fluid that is easy to dissolve and break the gel. Based on the reservoir characteristics, the target improvements for the drilling fluid are as follows: lower the drilling and completion fluid density to less than 1.05 g/cm
3 to reduce the invasion depth of the solid phase and polymer caused by liquid column pressure [
21]; lower the solid phase to less than 2% to alleviate the damage of the solid phase to the reservoir [
22]; and select a drilling and completion fluid with acid solubility greater than 95% to resolve the damage of polymer to solid-phase invasion within the production radius of the reservoir [
23]. With these changes, the reservoir permeability recovery value should exceed 95%, thus effectively assisting the efficient exploration and beneficial development of carbonate rock reservoirs.
2. Materials and Methods
The Daniudi gas field is classified as a three low oil and gas reservoir. The pore pressure coefficient of the Ma 5 gas reservoir ranges from 0.98 to 1.02, while the porosity falls between 0.7% and 2.0%. The matrix permeability is less than 0.1 mD, whereas the fracture permeability varies from 0.64 to 38.10 mD, characterizing it as a typical fractured formation [
24]. The productivity of this reservoir primarily depends on the number and conductivity of fractures. Hence, the preservation of fracture conductivity directly influences subsequent development output.
2.1. Optimization of Acid-Soluble Treatment Agent
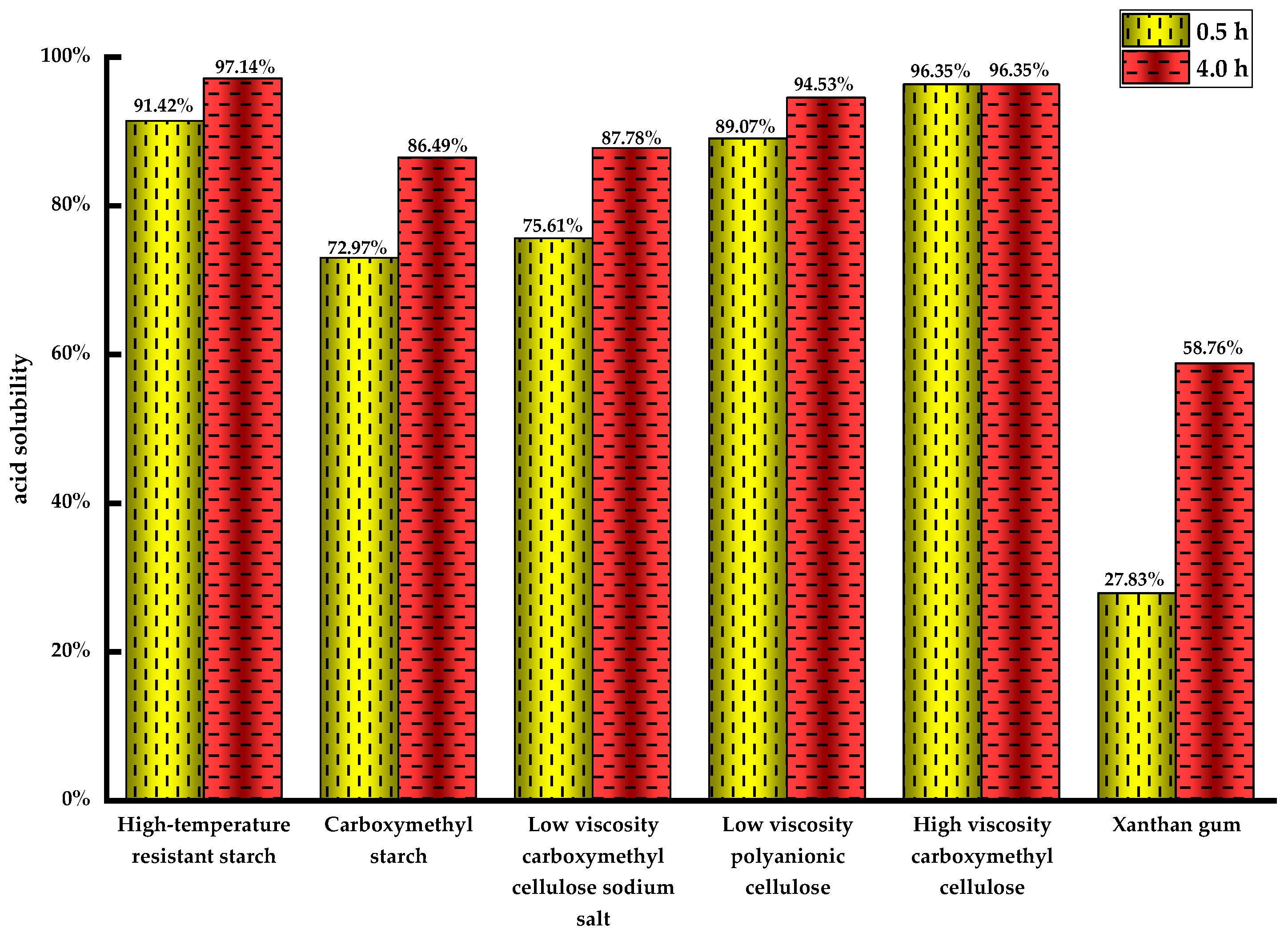
Generally, acid solutions can effectively degrade glycoside polymers such as xanthan gum, starch, and cellulose derivatives. Therefore, acid solubility tests were conducted for 12 groups of six commonly used natural additives in solid-free drilling fluids, including heat-resistant starch, sodium carboxymethyl cellulose, and Xanthan gum.
In the experiment, a specified amount of drilling fluid additive was weighed in proportion, dissolved in 400 mL of water using a variable-frequency high-speed stirrer, and then stirred at high speed for 15 min after being sealed and cured for 16 h. The apparent viscosity before acid addition was measured using a digital automatic rheometer. Subsequently, 2% of 1:1 hydrochloric acid was weighed and added to a stirring cup containing 400 mL of drilling fluid. This mixture was stirred at a high speed of 10,000 rpm for 10 min, transferred into a beaker, and heated in a water bath at 90 °C for 0.5 and 4 h. After removal, it was stirred for an additional 5 min. The apparent viscosity after acid addition was measured using a digital automatic rheometer. Acid solubility is the difference between the apparent viscosity before and after adding acid, divided by the rate of change in the apparent viscosity before adding acid [
25]. Shorter acid dissolution times are preferable for subsequent construction and for facilitating the backflow of the drilling and completion fluid [
26]. The experimental results are illustrated in
Figure 1.
Figure 1 illustrates that the acid dissolution rate of the six treatments increases with longer dissolution time. High-temperature-resistant starch, low-viscosity carboxymethyl cellulose, and high-viscosity carboxymethyl cellulose demonstrate superior acid dissolution rates after 4 h, whereas the dissolution rate of Xanthan gum is only 58.76%, rendering it unsuitable for facile acid dissolution formulas. Therefore, the selected fluid loss reducers and viscosity enhancers of drilling fluid are high-temperature-resistant starch and both low- and high-viscosity carboxymethyl cellulose.
2.2. Optimization of Starch Fungicide
Bacteria often cause degradation of starch additives in drilling fluids, resulting in additive failure [
27]. This increases the water loss of drilling fluid, complicating the restoration of drilling fluid performance in a short timeframe and raising the risk of wellbore instability [
28]. Conventional fungicides pose varying degrees of toxicity to humans, animals, and aquatic organisms, and an accumulation of these fungicides in the environment can pose ecological risks [
29]. Therefore, environmentally friendly fungicides such as bis-quaternary amine, quaternary ammonium salt, and organic base were selected for evaluation using the bacterial bottle method.
In the experiment, 1600 mL of field slurry water was used, with 2% high-temperature-resistant starch added. After uniform stirring, the mixture turned red and was divided evenly into four parts. No. 1 part is puree, and the other three parts had bis-quaternary amine, quaternary ammonium salt, and organic alkali fungicides added at 0.1% dosage, respectively, and labeled as Nos. 2 to 4. After uniform stirring, 1 mL of drilling fluid from each part was extracted using a sterile syringe and injected into separate bacterial bottles. These bottles were placed in a constant temperature box and cured for 7 days at 30 °C. The efficacy of the fungicides was determined by observing color changes in the bacterial bottles [
30].
The results show that No. 1, the puree test bottle, changed from red to yellow, indicating that the high-temperature-resistant starch would cultivate sulfuric acid-reducing bacteria in the drilling fluid. Nos. 2 and 3 also transitioned from red to yellow, demonstrating insufficient bactericidal capabilities of bis-quaternary amine and quaternary ammonium salt fungicides, with bacterial growth still present. No. 4, with the organic base fungicide, remained red, indicating its efficacy. Consequently, the organic alkali fungicide was chosen for the solid-free acid-soluble drilling fluid.
2.3. Lubricant Preference
Horizontal wells necessitate superior lubricity in drilling fluids. While solid-free drilling fluid possesses good lubricity, it struggles to meet the requirements of friction reduction during drilling and completion [
31]. As both fungicides and grease lubricants contain surfactants, their compatibility significantly impacts the lubricating performance of the combined treatment.
During the experiment, two 400 mL samples of clear water were taken, with 0.1% organic alkali fungicide added to each. Subsequently, 2% oil-based lubricant II and vegetable oil lubricant were, respectively, added to the samples. After stirring at 10,000 rpm for 20 min, the extreme pressure lubrication coefficient was measured. Then, both drilling fluids were transferred into aging tanks and heated in a high-temperature roller furnace at 120 °C for 16 h. After this, the extreme pressure lubrication coefficient was tested again. Compatibility was evaluated based on the lubrication coefficient before and after the hot rolling process [
32].
The results show that the lubrication coefficient of both oil-based lubricant II and vegetable oil lubricant was almost the same at room temperature, specifically 0.08 and 0.06, respectively. After hot rolling for 16 h, the lubrication coefficients were 0.17 and 0.09, respectively. The lubrication coefficient of oil-based lubricant II increased by 112%, suggesting its incompatibility with the organic base fungicide. On the other hand, the extreme pressure lubrication coefficient of vegetable oil lubricant increased by only 50%, remaining under 0.10, indicating better compatibility.
2.4. Temperature Resistance Test
Indoor preparations of solid-free and acid-soluble drilling fluids, along with potassium ammonium based drilling fluids, were made. After curing at room temperature for 16 h, they were heated in a high-temperature roller furnace at 120 °C for 16 h. Subsequent measurements of comprehensive properties of the two sets of drilling fluids were undertaken.
The composition of the solid-free acid-soluble drilling fluid was as follows: clear water, 0.3 to 0.8% low-viscosity sodium carboxymethyl cellulose, which is cellulose reacting with sodium hydroxide to form alkali cellulose, then it reacts with chloroacetic acid to form CMC-Na, 1.0 to 2.0% high-temperature-resistant starch, 0.1 to 0.3% high-viscosity sodium carboxymethyl cellulose, 2.0 to 3.0% vegetable oil lubricant, mainly is rapeseed oil, 0.05 to 0.10% organic base fungicide is glutaraldehyde, and 0.05 to 0.10% caustic soda is sodium hydroxide.
The composition of the potassium ammonium based drilling fluid included: clear water, 3.0 to 4.0% bentonite, 0.1 to 0.2% soda ash, 0.1 to 0.2% caustic soda, 0.2 to 0.5% low-viscosity polyanionic cellulose, 0.3 to 0.5% polyacrylonitrile potassium salt, 0.5 to 2.0% polyacrylonitrile ammonium salt, 2.0 to 3.0% emulsified asphalt, 2.0 to 3.0% lignite resin, 3.0 to 5.0% superfine calcium carbonate, 0.3 to 0.5% partially hydrolyzed polyacrylonitrile potassium salt, and 1.0 to 2.0% graphite lubricant.
Following 16 h of heating at 120 °C in a high-temperature roller furnace, the performance of the aged first set of drilling fluid was tested. Its properties were as follows: density 1.02 g/cm3, medium-pressure filtration loss 6.8 mL, plastic viscosity 19 mPa·s, yield point 6 Pa, initial gel strength 0.5 Pa, terminal gel strength 1.0 Pa, viscosity coefficient of mud cake 0.06, extreme pressure lubrication coefficient 0.09, and solid content 0%.
Similarly, the performance of the aged second set of drilling fluid was tested. Its properties were as follows: density 1.08 g/cm3, low-temperature filtration rate 5.2 mL/30 min, plastic viscosity 17.0 mPa·s, yield point 8.0 Pa, initial gel strength 1.5 Pa, terminal gel strength 2.0 Pa, viscosity coefficient of mud cake 0.08, extreme pressure lubrication coefficient 0.10, and solid content 6.5%.
The experimental results reveal that the temperature resistance of the first solid-free acid-soluble drilling fluid reached 120 °C, higher than the formation temperature of the Lower Paleozoic reservoir in the Daniudi gas field. When compared with the performance of the potassium ammonium based drilling fluid, the density of the first solid-free acid-soluble drilling fluid was lower by 0.06 g/cm3, and the solid content of the first solid-free acid-soluble drilling fluid was lower by 6.5%. This reduction could potentially decrease the bottom hole liquid column pressure and solid phase.
2.5. Acid Solubility Test
The acid solubility of the two sets of drilling fluids was assessed based on the previously mentioned acid solubility experimental method and drilling fluid formula.
Before acid was added, the apparent viscosity of the drilling fluid was 20.0 mPa·s. After acid dissolution, the viscosity decreased to 18.3 mPa·s, an acid dissolution rate of 8.50%, and the acid dissolution rate of the mud cake was 35%. In contrast, the apparent viscosity of the solid-free acid-soluble drilling fluid decreased from 22.0 mPa·s before adding acid to only 1.0 mPa·s after acid dissolution, yielding an acid dissolution rate of 95.45%. The solid-free mud cake did not require acid dissolution.
2.6. Permeability Recovery Value Test
Fractured carbonate cores with a permeability above 1.0 mD from the Ma 5 stratum in the Daniudi gas field were chosen. The pH of the drilling fluid was adjusted to neutral following acid dissolution. The reservoir protection abilities of the two sets of drilling fluids were assessed using the permeability recovery value before and after damage (
Table 1).
Prior to contamination, the permeability of the Ma 5 fractured carbonate cores in the A well was 3.23 mD. After contamination with the potassium ammonium based drilling fluid, the permeability dropped to 1.66 mD, resulting in a permeability recovery value of 51.40%. The permeability of the Ma 5 fractured carbonate cores from the B well before contamination was 1.70 mD. Contamination by the solid-phase-free acid-soluble drilling fluid reduced the permeability to 1.63 mD, yielding a permeability recovery value of 95.88%.
The experimental results demonstrate that the permeability recovery value of the solid-free acid-soluble drilling fluid was 44.38% higher than that of the potassium ammonium based drilling fluid.
2.7. Field Test
The Daniudi gas field, situated at the junction of Yulin in Shanxi Province and Ordos City in Inner Mongolia, lies in the northeast of the Ordos Basin, spanning an area of approximately 2000 km
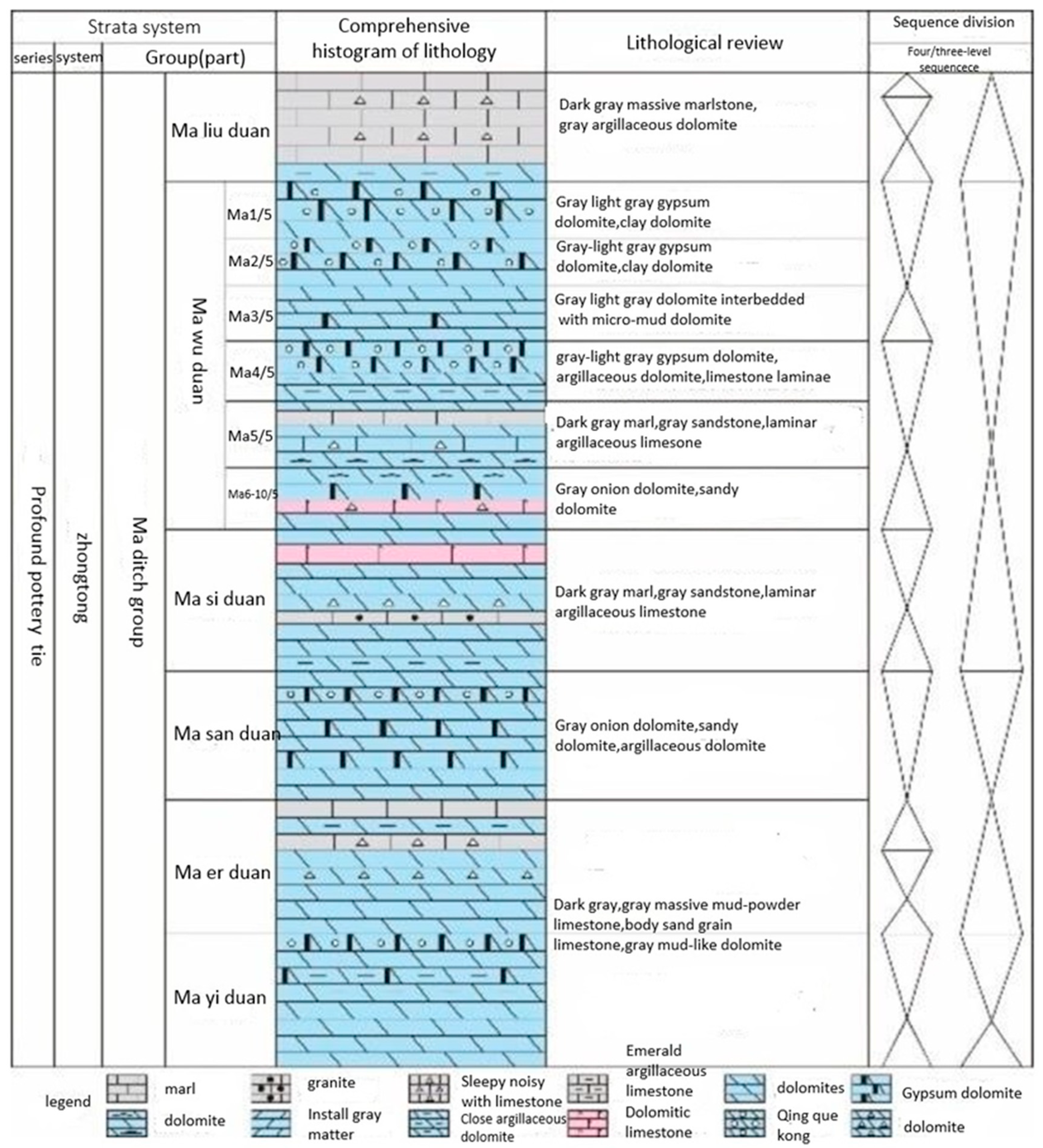
2. In the structural segmentation of the Ordos Basin, the Daniudi gas field is attributed to the northern slope of northern Shanxi. The Majiagou Formation of the Ordovician in the Ordos Basin is predominantly an evaporite deposit composed of carbonate rocks within an extensive inland region. The formation experienced multiple transgressions and regressions during its sedimentary period, with the first, third, and fifth members of the Majiagou Formation being regressive deposits and the second, fourth, and sixth members being transgressive deposits. Notably, the fourth member of the Majiagou Formation exhibits the widest transgression range. The lithofacies of the Majiagou Formation are primarily composed of micrite dolomite, mud-silt dolomite, silt dolomite, and gypsum dolomite, with a minor distribution of granular dolomite and oolitic limestone. The Majiagou formation are illustrated in
Figure 2.
The construction methodology of solid-free acid-soluble drilling fluid in the Daniudi gas field has been continually refined, with successful completion in more than ten wells. This paper presents an example of the first well, DK-A, which has been commissioned, detailing the application process of the solid-free acid-soluble drilling fluid.
The target stratum of the well is the Ma Wu 5 member of the Majiagou Formation from the Ordovician, possessing a vertical depth of 2976.20 m and a planned horizontal section length of 1200 m. After a hydration period of 24 h, the drilling fluid’s performance was evaluated, yielding a Marsh funnel viscosity of 67 s, density of 1.02 g/cm3, solid content of 0.5%, medium-pressure filtration of 8.0 mL, plastic viscosity of 17.0 mPa·s, yield point of 8.0 Pa, dynamic plastic ratio of 0.48, initial gel strength of 2.0 Pa, terminal gel strength of 3.0 Pa, bentonite content of 0%, and mud cake viscosity coefficient of 0.08.
The drilling fluid, post-shearing through the bit water hole, exhibited reduced viscosity and shear force, although it remained adequate for construction requirements. The drilling fluid’s performance was maintained and adjusted in response to changes in performance parameters during drilling. The drilling fluid performance remained essentially stable during the drilling process. Marsh funnel viscosity ranged between 35 and 43 s, density varied from 1.02 to 1.05 g/cm3, solid concentration from 0.5% to 2.0%, low-temperature and low-pressure filtration fluctuated between 5.0 and 6.0 mL/30 min, plastic viscosity shifted from 7.0 to 10.0 mPa·s, yield point ranged from 3.5 to 6.0 Pa, dynamic plastic ratio varied from 0.50 to 0.60 Pa/mPa·s, initial gel strength from 1.0 to 1.5 Pa, and terminal gel strength from 1.0 to 2.0 Pa. The bentonite content remained at 0, and the mud cake viscosity coefficient ranged from 0.03 to 0.08. Drilling in the horizontal section was completed in 9.42 days, drilling 1300 m, with an electric logging diameter expansion rate of 3.21%. The drilling process was smooth, with no recorded incidents or complexities. The estimated cost of the drilling fluid in the horizontal section was CNY 312,000, but the actual cost was CNY 131,400, signifying a cost reduction of 57.88%.
3. Results and Discussion
3.1. Mitigating Solid-Phase Invasion and Damage to the Reservoir
Laboratory tests reveal that the thermal resistance of solid-free acid-soluble drilling fluid can reach 120 °C, which is higher than the formation temperature of the Lower Paleozoic reservoir in the Daniudi gas field. In comparison to the performance of potassium ammonium based drilling fluid, the density of this drilling fluid decreased by 0.06 g/cm3, and the solid content of this drilling fluid decreased by 6.5%, resulting in a substantial reduction in bottom hole liquid column pressure and solid phase.
During the drilling process of DK-A well, the vibrating screen is used with a 200 mesh sieve cloth, and the integrated machine and centrifuge are used continuously for 24 h to enhance the ability to remove inferior solid phases. During the drilling of the horizontal section, the density increased from 1.02 g/cm
3 of the freshly mixed slurry to 1.05 g/cm
3. In the third opening of the DK-B well, diluted potassium ammonium based drilling fluid (density 1.20~1.25 g/cm
3) was used. The drilling density ranged from 1.08 to 1.10 g/cm
3, while the final density was 1.12 g/cm
3, 1.5~2.0 MPa higher than the bottom hole pressure differential of the solid-free, low-density, acid-soluble drilling fluid.
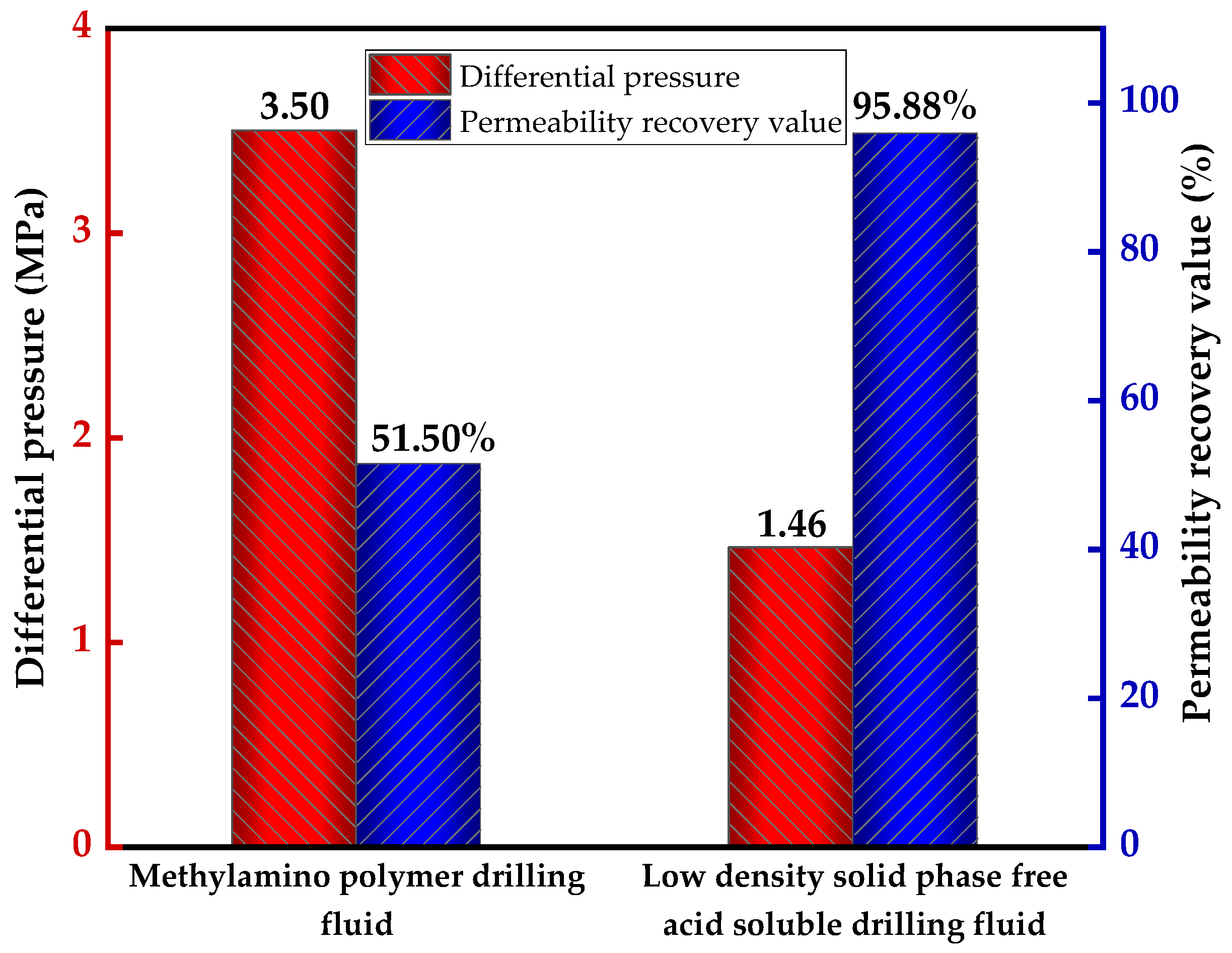
Figure 3 illustrates the comparison of bottom hole pressure differential and core permeability recovery value for the DK-A and DK-B wells.
Figure 3 demonstrates that the pressure differential of the solid-free, acid-soluble drilling fluid during the third borehole drilling was 1.46 MPa, which is 2.04 MPa lower than the 3.50 MPa of the potassium ammonium based drilling fluid. Furthermore, the permeability recovery value was 95.88%, considerably higher than the 51.50% of the potassium ammonium based drilling fluid.
These conclusions, derived from both laboratory testing and field applications, are essentially consistent. In terms of the action mechanism, the filtration amount per unit percolation area (Vf/A) according to the static filtration equation of the drilling fluid, is directly proportional to parameters such as the square root of mud cake permeability (K), solid content (Ce/cm−1), filtration pressure differential (P), and filtrate time (T); inversely, it is proportional to the square root of filtrate viscosity (μ).
Comparing the DK-A and DK-B wells, assuming that other factors remained constant, the use of methylamine drilling fluid led to a larger pressure differential, which in turn resulted in greater fluid filtration. Consequently, the solid phase in the drilling fluid invaded the reservoir deeper, causing more significant reservoir damage. The damage made flow back after acid fracturing more difficult, resulting in lower gas production from the DK-B well compared to the DK-A well.
3.2. Enhancing Permeability Recovery and Gas Production
The solid-free, acid-soluble drilling fluid in the laboratory can achieve over 90% acid solubility after contact with acid for 0.5 h, peaking at 95.45% after 4 h. This allows the recovery value of formation permeability to be restored to 95.88% in a short time. Conversely, the acid solubility of the potassium ammonium group is only 8.5%, making it difficult to remove plugging with an acid solution, and yielding a permeability recovery value of just 51.50%.
In field applications, 16 sections and 34 clusters underwent acid fracturing in the DK-A well, with section intervals ranging from 65 to 100 m, and averaging 80 m. The amount of fluid entering the ground was 23,000 cubic meters (containing 0.17 × 10
4 m
3 of acid liquid), the volume of added sand was 0.11 × 10
4 m
3, and the formation fracture pressure ranged from 31.8 to 87.1 MPa, with a pump stop pressure from 29.3 to 60.8 MPa. After 15 days, the oil pressure was 5.2 MPa, the casing pressure was 10.8 MPa, the flow-back rate was 36.10%, and the average daily gas production was 6.16 × 10
4 m
3, which is 105% higher than the 3 × 10
4 m
3/d from scheme.
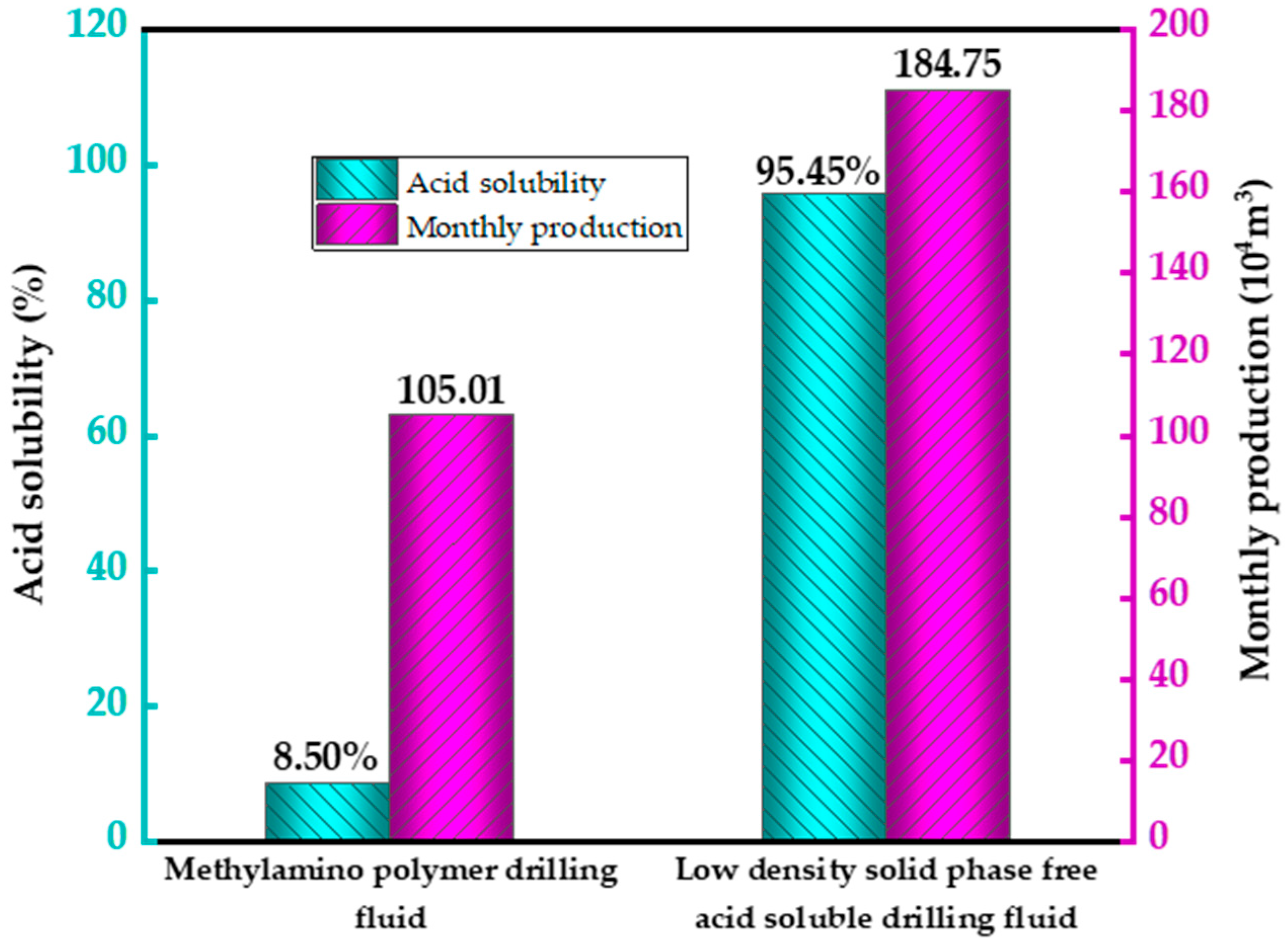
Figure 4 is a comparison chart of acid solubility rate and daily output between potassium ammonium based drilling fluid and solid-free, low-density, acid-soluble drilling fluid.
Figure 4 shows that when compared to the DK-B well, which has a wellhead distance of 5 km, the cumulative gas production within a few months was 105.01 × 10
4 m
3; the DK-A well, which uses solid-free, acid-soluble drilling fluid, yielded a cumulative gas production of 184.75 × 10
4 m
3 in the same month, reflecting a monthly production increase of 75.94%.
In the tight carbonate gas reservoir of the Daniudi gas field, the use of solid-free, acid-soluble drilling fluid results in a small bottom hole pressure difference that aligns well with the lower formation pore pressure. This leads to fewer solid particles and treatment agents being squeezed into the micro-fractures of rock strata, thereby creating favorable conditions for maintaining maximum reservoir channel unobstructedness during later acid fracturing flowback.
In terms of the action mechanism, acid fracturing is used for reservoir reconstruction. After the drilling fluid remaining in the micro-fractures of rock strata contacts acid fluid, it reacts with polymer treatment agents such as sodium carboxymethyl cellulose and high-temperature-resistant starch in the drilling fluid. These macromolecules then transform into smaller molecules, resulting in glucose, carboxymethyl cellulose, and other substances that do not seriously obstruct the reservoir channel. Consequently, it can be concluded that the solid-free, acid-soluble drilling fluid causes minimal damage to the reservoir. The related chemical reactions are as follows:
The reservoir of this gas field primarily comprises carbonate minerals, and micro-fractures in the reservoir are developed, causing the acid liquid to mainly advance along the fracture channel. The chemical reaction between carbonate rocks and acid liquid produces CO
2, H
2O, and other substances, which can severely corrode the rock stratum. After acidification, acid-etched channels or fracture channels with ultra-high conductivity are formed in the formation, and the seepage area increases, thereby providing the primary reason for high permeability recovery. The related chemical reactions are:
In conclusion, the laboratory test results and field application collectively suggest that, given the reservoir characteristics of the Daniudi gas field, the use of solid-free, acid-soluble drilling fluid causes minimal damage to the carbonate reservoir, exhibits low plugging removal pressure, and yields a high permeability recovery value. This leads to superior acid fracturing stimulation effects and can meet the benefit development needs of oil and gas field companies.