Insights into the Reaction Kinetics of Hydrazine-Based Fuels: A Comprehensive Review of Theoretical and Experimental Methods
Abstract
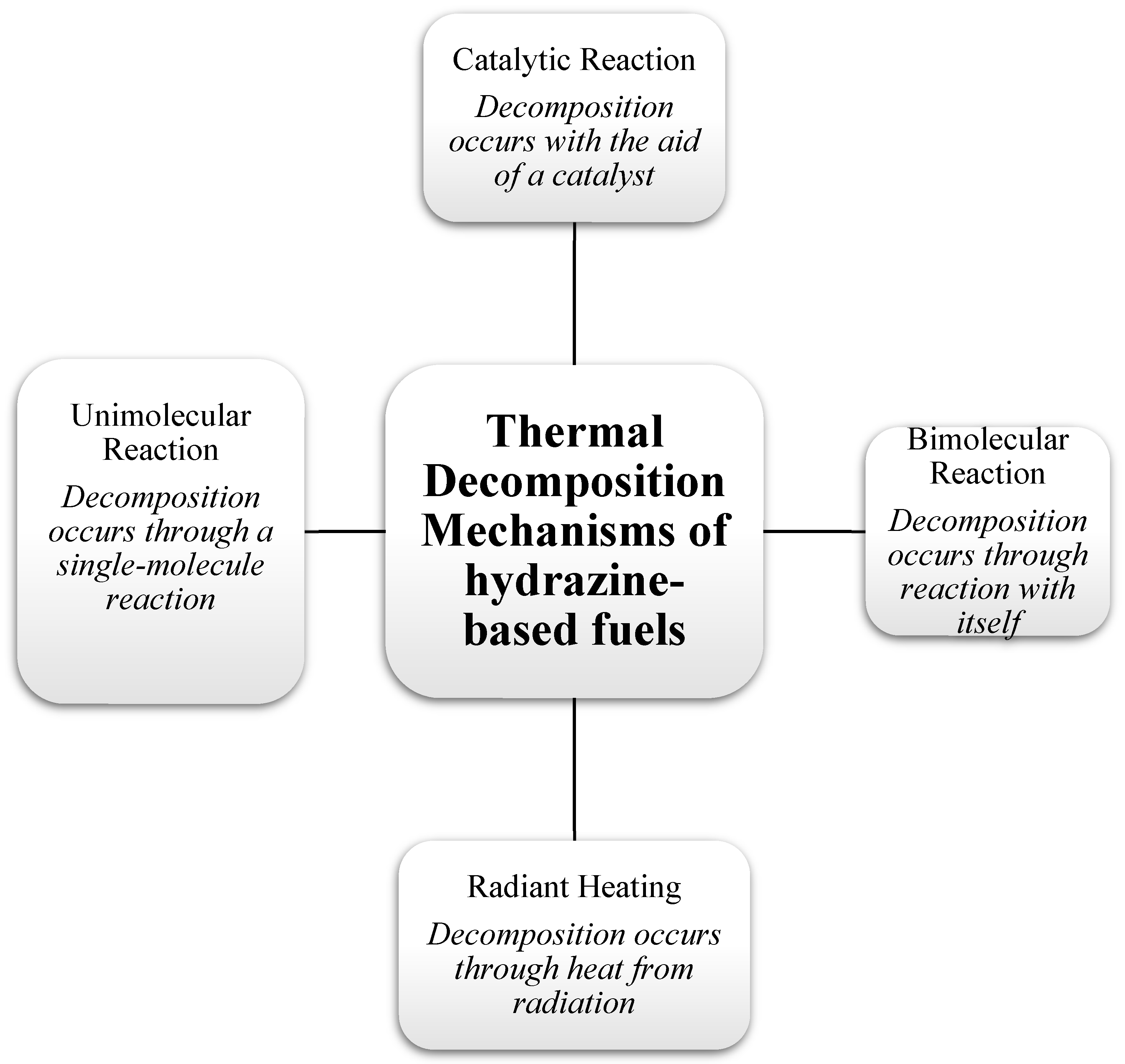
:1. Introduction
2. Research Status on Anhydrous Hydrazine Fuel
2.1. Hydrazine Combinations with Other Species
2.2. Critical Assessment of the Research on Hydrazine
2.3. Research Status on MMH Fuel
Critical Assessment of Existing Literature
2.4. Research Status on UDMH Fuel
Critical Assessment of UMDH Literature
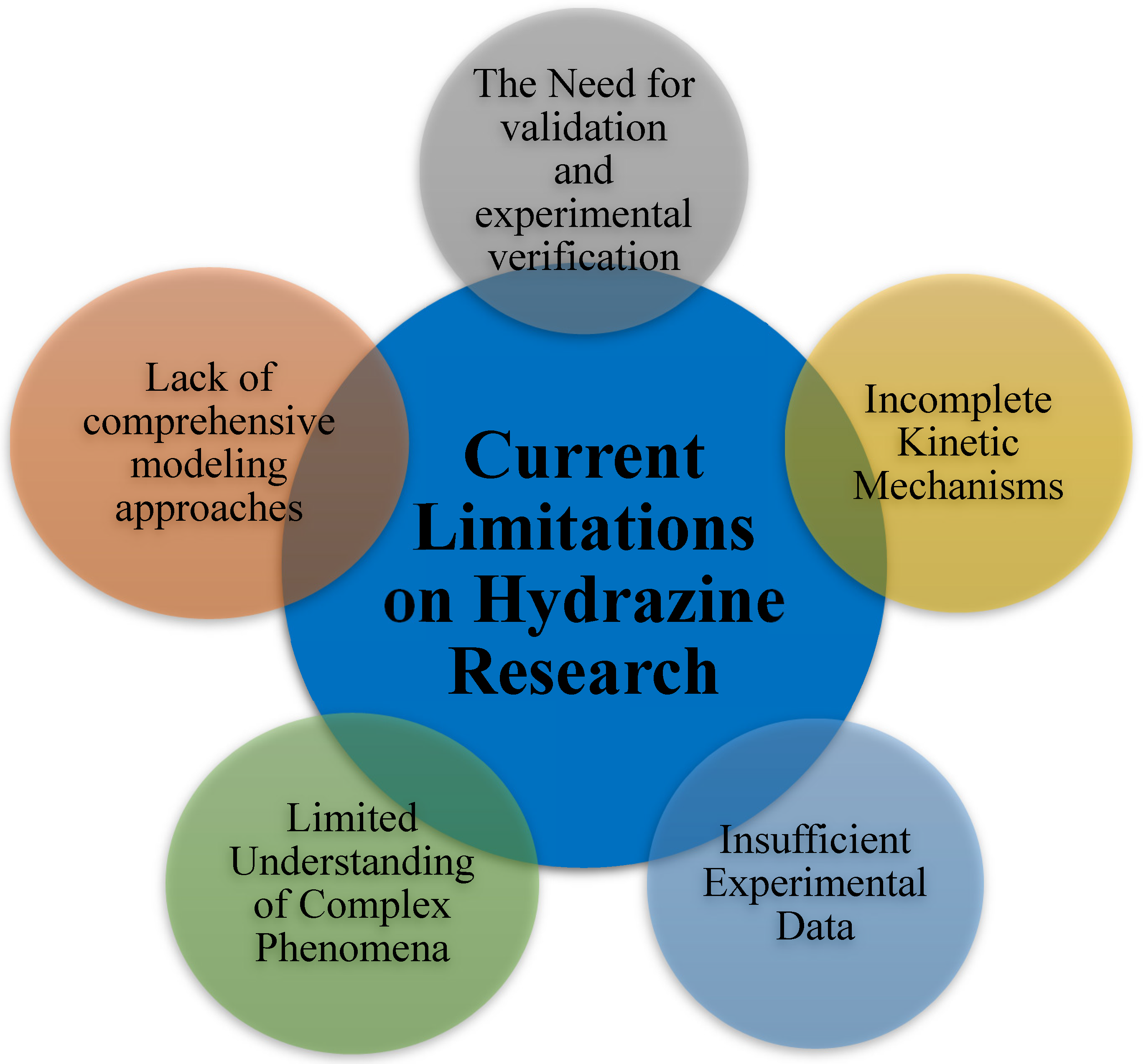
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.S.; Zhang, X. Direct Dynamics Study on the Hydrogen Abstraction Reactions N2H4 + R → N2H3 + RH (R = NH2, CH3). J. Chem. Phys. 2006, 125, 064304. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Zhang, X.; Zhang, S.W. Direct Dynamics Study on the Hydrogen Abstraction Reaction N2H4 + H → N2H3 + H2. J. Phys. Chem. A 2003, 107, 6055–6061. [Google Scholar] [CrossRef]
- Gray, P.; Lee, J.C.; Spencer, M. Combustion, Ammonia Flame and Explosion of Hydrazine and Ammonia I-The Spontaneous Ignition of Pure Gaseous Hydrazine. Combust. Flame 1963, 7, 315–321. [Google Scholar] [CrossRef]
- Kaufman, F.; Gerri, N.J. Experimental Studies of Thermal Explosions and of Moderately Fast Reactions. In Symposium (International) on Combustion; Elsevier: Amsterdam, The Netherlands, 1961; Volume 8, pp. 619–627. [Google Scholar] [CrossRef]
- Auzanneau, M.; Roux, M. Self-Detonation Range in Inert Atmosphere of Ternary Systems Using Hydrogen Peroxide and Hydrazine or Hydrazine Derivatives. Combust. Sci. Technol. 1990, 73, 505–520. [Google Scholar] [CrossRef]
- Schliebs, R. Book Review: Hydrazine and Its Derivatives. Preparation, Properties, Applications. By E. W. Schmidt. Angew. Chem. Int. Ed. Engl. 1985, 24, 1006–1007. [Google Scholar] [CrossRef]
- Durgapal, U.C.; Dutta, P.K.; Pant, G.C.; Ingalgaonkar, M.B.; Oka, V.Y.; Umap, B.B. Studies on Hypergolicity of Several Liquid Fuels with Fuming Nitric Acids as Oxidizers. Propellants Explos. Pyrotech. 1987, 12, 149–153. [Google Scholar] [CrossRef]
- Kubal, T.D.; Dambach, E.M.; Son, S.F.; Anderson, W.E.; Pourpoint, T.L. Aspects of monomethylhydrazine and red fuming nitric acid ignition. In Proceedings of the 46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Nashville, TN, USA, 25–28 July 2010. [Google Scholar] [CrossRef]
- Osmont, A.; Catoire, L.; Klapötke, T.M.; Vaghjiani, G.L.; Swihart, M.T. Thermochemistry of Species Potentially Formed during NTO/MMH Hypergolic Ignition. Propellants Explos. Pyrotech. 2008, 33, 209–212. [Google Scholar] [CrossRef]
- Palaszewski, B.; Ianovski, L.S.; Carrick, P. Propellant Technologies: Far-Reaching Benefits for Aeronautical and Space-Vehicle Propulsion. J. Propuls. Power 1998, 14, 641–648. [Google Scholar] [CrossRef]
- Anderson, W.R.; Fontijn, A. Gas-Phase Kinetics for Propellant Combustion Modeling: Requirements and Experiments. Overv. Recent Res. Energetic Mater. 2005, 16, 191–239. [Google Scholar] [CrossRef]
- Catoire, L.; Dupré, G.; Paillard, C.E. Study of the Reactivity Between Monomethylhydrazine and “Nitrogen Peroxide” or Oxygen in the Gaseous Phase. Int. Conf. Spacecr. Propuls. 1994, 1, hal-01665660. [Google Scholar]
- Li, X.; Wu, J.; Hou, T.; Li, H.; Wang, L.; Ding, J.; Wan, H.; Guan, G. Synthesis and Characterization of Hypergolic Salts Based on Bis(1H-1,2,3-Triazole-1-Yl) Dihydroborate Anion. J. Mol. Struct 2022, 1261, 132850. [Google Scholar] [CrossRef]
- Eremeeva, A.M.; Kondrasheva, N.K.; Khasanov, A.F.; Oleynik, I.L. Environmentally Friendly Diesel Fuel Obtained from Vegetable Raw Materials and Hydrocarbon Crude. Energies 2023, 16, 2121. [Google Scholar] [CrossRef]
- Kondrasheva, N.; Eremeeva, A. Production of Biodiesel Fuel from Vegetable Raw Materials. J. Min. Inst. 2023, 260, 248–256. [Google Scholar] [CrossRef]
- Mays, L.O.; Farmer, M.J.; Smith, J.E. A Laser Diagnostic Technique to Measure Chemical Delay Time in Hypergolic Combustion. Combust. Sci. Technol. 1998, 134, 127–138. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Gao, J. CARNOT: A Fragment-Based Direct Molecular Dynamics and Virtual-Reality Simulation Package for Reactive Systems. J. Chem. Theory Comput. 2022, 18, 1297–1313. [Google Scholar] [CrossRef]
- Moldoveanu, S.C. Pyrolysis of Organic Molecules: Applications to Health and Environmental Issues; Techniques and Instrumentation in Analytical Chemistry; Moldoveanu, S.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; Volume 28, ISBN 9780444531131. [Google Scholar]
- Sun, H.; Vaghjiani, G.L.; Law, C.K. Ab Initio Kinetics of Methylamine Radical Thermal Decomposition and H-Abstraction from Monomethylhydrazine by H-Atom. J. Phys. Chem. A 2020, 124, 3747–3753. [Google Scholar] [CrossRef]
- Cohen, N. The O + NH3 Reaction: A Review. Int. J. Chem. Kinet. 1987, 19, 319–362. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowmanaf, C.T. Mechanism and Modeling of Nitrogen Chemistry in Combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Tsang, W.; Herron, J.T. Chemical Kinetic Data Base for Propellant Combustion I. Reactions Involving NO, NO2, HNO, HNO2, HCN and N2O. J. Phys. Chem. Ref. Data 1991, 20, 609–663. [Google Scholar] [CrossRef]
- Allen, M.T.; Yeiter, R.A.; Dryer, F.L. High Pressure Studies of Moist Carbon Monoxide/Nitrous Oxide Kinetics. Combust. Flame 1997, 109, 449–470. [Google Scholar] [CrossRef]
- Adams, G.K.; Stocks, G.W. The combustion of hydrazine. In Proceedings of the Symposium (International) on Combustion, Cambridge, MA, USA, 1–5 September 1952; Massachusetts Institute of Technology: Cambridge, MA, USA, 1953; Volume 4, pp. 239–248. [Google Scholar]
- Eberstein, I.J.; Glassman, I. The Gas-Phase Decomposition of Hydrazine and Its Methyl Derivatives. In Proceedings of the Tenth Symposium (International) on Combustion, Cambridge, UK, 17–21 August 1965; Volume 10, pp. 365–374. [Google Scholar]
- Gilbert, M. The Hydrazine Flame. Combust Flame 1958, 2, 137–148. [Google Scholar] [CrossRef]
- Gray, P.; Holland, S. The Effect of Isotopic Substitution on the Decomposition Flame of Hydrazine. Combust Flame 1970, 14, 203–216. [Google Scholar] [CrossRef]
- Gray, P.; Lee, J.C.; Leach, H.A.; Taylor, D.C. The Propagation and Stability of The Decomposition Flame of Hydrazine. Symp. (Int.) Combust. 1957, 6, 255–263. [Google Scholar] [CrossRef]
- Gray, P.; Spencer, M. Studies Of The Combustion of Dimethyl Hydrazine and Related Compounds. Symp. (Int.) Combust. 1963, 9, 148–157. [Google Scholar] [CrossRef]
- Mchale, E.T.; Knox, B.E.; Palmer, H.B. Determination of the decomposition kinetics of hydrazine using a single-pulse shock tube. In Proceedings of the Ten Symposium (International) on Combustion, Cambridge, UK, 17–21 August 1965; The Combustion Institute: Pittsburgh, PA, USA, 1965; pp. 341–351. [Google Scholar]
- Moberbly, W.H. Shock Tube Study of Hydrazine Decomposition. J. Phys. Chem. 1962, 66, 366–368. [Google Scholar] [CrossRef]
- Diesen, R.W. Mass Spectral Studies of Kinetics behind Shock Waves. II. Thermal Decomposition of Hydrazine. J. Chem. Phys. 1963, 39, 2121–2128. [Google Scholar] [CrossRef]
- Kerr, J.A.; Sekhar, R.C.; Trotman-Dickenson, A.F. The Pyrolyses of Hydrazines and Benzylamines. C-C and N-N Bond Dissociation Energies. J. Chem. Soc. 1963, 3217–3225. [Google Scholar] [CrossRef]
- Meyer, E.; Olschewski, H.A.; Troe, J.; Wagner, H.G. Investigation of N2H4 and H2O2 decomposition in low and high pressure shock waves. In Proceedings of the Symposium (International) on Combustion, University of Poitiers, Poitiers, France, 14–20 July 1968; Volume 12, pp. 345–355. [Google Scholar]
- Michel, K.W.; Wagner, H.G. The Pyrolysis and oxidation of hydrazine behind shock waves. In Proceedings of the Tenth Symposium (International) on Combustion, University of Cambridge, Cambridge, UK, 17–21 August 1964; pp. 353–364. [Google Scholar]
- Konnov, A.A.; De Ruyck, J. Kinetic Modeling of the Thermal Decomposition of Ammonia. Combust. Sci. Technol. 2000, 152, 23–37. [Google Scholar] [CrossRef]
- Bowman, C.T.; Birkeland, J. Alternative hydrocarbon fuels: Combustion and chemical kinetics. In Alternative Hydrocarbon Fuels: Combustion and Chemical Kinetics; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 1978; Volume 62, pp. 1–480. ISBN 0-915928-25-6. [Google Scholar]
- Konnov, A.A.; De Ruyck, J. Kinetic Modeling of the Decomposition and Flames of Hydrazine. Combust Flame 2001, 124, 106–126. [Google Scholar] [CrossRef]
- Catoire, L.; Luche, J.; Dupré, G.; Paillard, C. Critical Reactions for the Hydrazine Vapor Detonations. Shock Waves 2001, 11, 97–103. [Google Scholar] [CrossRef]
- Sawyer, R.F.; Glassman, I. Gas-Phase Reactions Of Hydrazine With Nitrogen Dioxide, Nitric Oxide, And Oxygen. Symp. (Int.) Combust. 1967, 11, 861–869. [Google Scholar] [CrossRef]
- Izato, Y.I.; Shiota, K.; Miyake, A. A Detailed Mechanism for the Initial Hypergolic Reaction in Liquid Hydrazine/Nitrogen Tetroxide Mixtures Based on Quantum Chemistry Calculations. Combust. Flame 2021, 229, 111389. [Google Scholar] [CrossRef]
- Wasko, R.A. Reaction of Hydrazine and Nitrogen Tetroxide in a Lowpressure Environment. AIAA J. 1963, 1, 1919–1920. [Google Scholar] [CrossRef]
- Chuan, R.L.; Wilber, P.C. Ignition of Hypergolic Propellants in a Simulated Space Environment. J. Spacecr. Rocket. 1967, 4, 282–284. [Google Scholar] [CrossRef]
- Tanaka, M.; Daimon, W.; Itsuro, K. Explosion Phenomenon from Contact of Hypergolic Liquids. J. Propuls. Power 1985, 1, 314–316. [Google Scholar] [CrossRef]
- Daimon, W.; Gotoh, Y.; Kimura, I. Mechanism of Explosion Induced by Contact of Hypergolic Liquids. J. Propuls. Power 1991, 7, 946–952. [Google Scholar] [CrossRef]
- Miyajima, H.; Sakamoto, H. Gas Phase Ignition of Hydrazine with Nitrogen Dioxide. Combust. Sci. Technol. 1973, 8, 199–200. [Google Scholar] [CrossRef]
- Daimon, W.; Tanaka, M.; Kimura, I. The mechanisms of explosions induced by contact of hypergolic liquid propellants, hydrazine and nitrogen tetroxide. In Proceedings of the Twentieth Symposium (International) on Combustion, The University of Michigan, Ann Arbor, MI, USA, 12–17 August 1984; Volume 206, pp. 2065–2071. [Google Scholar]
- Ohminami, K.; Sawai, S.; Uesugi, K.T.; Yamanish, N.; Koshi, M. Hydrazine and di-nitrogen tetroxide combustion model inside film-cooled bipropellant thruster. In Proceedings of the 45th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Denver, CO, USA, 2–5 August 2009. [Google Scholar]
- He, B.; Nie, W.; Feng, S.; Su, L.; Zhuang, F. Effects of NTO Oxidizer Temperature and Pressure on Hypergolic Ignition Delay and Life Time of UDMH Organic Gel Droplet. Propellants Explos. Pyrotech. 2013, 38, 665–684. [Google Scholar] [CrossRef]
- Daimon, Y.; Terashima, H.; Koshi, M. Origin of Hypergolic Ignition of N2H4/NO2 Mixtures. Sci. Technol. Energ. Mater. 2013, 74, 17–22. [Google Scholar]
- Vaghjiani, G.L.; Sun, H.; Chambreau, S.D. Experimental and Theoretical Investigations of the Radical−radical Reaction: N2H3 + NO2. J. Phys. Chem. A 2020, 124, 10434–10446. [Google Scholar] [CrossRef]
- Daimon, Y.; Terashima, H.; Koshi, M. Chemical Kinetics of Hypergolic Ignition in N2H4/N2O4-NO2 Gas Mixtures. J. Propuls. Power 2014, 30, 707–716. [Google Scholar] [CrossRef]
- Lai, K.Y.; Zhu, R.; Lin, M.C. Why Mixtures of Hydrazine and Dinitrogen Tetroxide Are Hypergolic? Chem. Phys. Lett. 2012, 537, 33–37. [Google Scholar] [CrossRef]
- Le Huyen, T.; Raghunath, P.; Lin, M.C. Ab Initio Chemical Kinetics for Hypergolic Reactions of Nitrogen Tetroxide with Hydrazine and Methyl Hydrazine. Comput. Chem. 2019, 1163, 112505. [Google Scholar] [CrossRef]
- Asatryan, R.; Bozzelli, J.W.; Da Silva, G.; Swinnen, S.; Tho Nguyen, M. Formation and Decomposition of Chemically Activated and Stabilized Hydrazine. J. Phys. Chem. A 2010, 114, 6235–6249. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.M.; Bozzelli, J.W. Combustion chemistry of nitrogen. In Gas Phase Combustion Chemistry; Gardiner, W.C., Jr., Ed.; Springer Science: Berlin/Heidelberg, Germany, 2000; pp. 125–341. [Google Scholar]
- Zhou, C.W.; Li, Y.; O’Connor, E.; Somers, K.P.; Thion, S.; Keesee, C.; Mathieu, O.; Petersen, E.L.; DeVerter, T.A.; Oehlschlaeger, M.A.; et al. A Comprehensive Experimental and Modeling Study of Isobutene Oxidation. Combust Flame 2016, 167, 353–379. [Google Scholar] [CrossRef]
- Just, T. Experimental Studies on the Reaction Kinetics of 1,1-Dimethylhydrazine and Oxygen; Technical Memorandum, TM; NASA-TM-77947, NAS 1.15:77947, Accession Number: 86N21630; NASA Headquarters: Washington, DC, USA, 1985. [Google Scholar]
- Golden, D.M.; Solly, R.K.; Gac, N.A.; Benson, S.W. Very Low-pressure Pyrolysis. VII. The Decomposition of Methylhydrazine, 1,1-dimethylhydrazine, 1,2-dimethylhydrazine, and Tetramethylhydrazine. Concerted Deamination and Dehydrogenation of Methylhydrazine. Int. J. Chem. Kinet. 1972, 4, 433–448. [Google Scholar] [CrossRef]
- Li, S.; Davidson, D.F.; Hanson, R.K. Shock Tube Study of the Pressure Dependence of Monomethylhydrazine Pyrolysis. Combust. Flame 2014, 161, 16–22. [Google Scholar] [CrossRef]
- Catoire, L.; Bassin, X.; Dupre, G.; Paillard, C. Experimental Study and Kinetic Modeling of the Thermal Decomposition of Gaseous Monomethylhydrazine. Application to Detonation Sensitivity. Shock Waves 1996, 6, 139–146. [Google Scholar] [CrossRef]
- Sun, H.; Law, C.K. Thermochemical and Kinetic Analysis of the Thermal Decomposition of Monomethylhydrazine: An Elementary Reaction Mechanism. J. Phys. Chem. A 2007, 111, 3748–3760. [Google Scholar] [CrossRef]
- Sun, H.; Catoire, L.; Law, C.K. Thermal Decomposition of Monomethylhydrazine: Shock Tube Experiments and Kinetic Modeling. Int. J. Chem. Kinet. 2009, 41, 176–186. [Google Scholar] [CrossRef]
- Cook, R.D.; Pyun, S.H.; Cho, J.; Davidson, D.F.; Hanson, R.K. Shock Tube Measurements of Species Time-Histories in Monomethyl Hydrazine Pyrolysis. Combust Flame 2011, 158, 790–795. [Google Scholar] [CrossRef]
- Zhang, P.; Klippenstein, S.J.; Sun, H.; Law, C.K. Ab Initio Kinetics for the Decomposition of Monomethylhydrazine (CH3NHNH2). Proc. Combust. Inst. 2011, 33, 425–432. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Law, C.K. Ab Initio Kinetics for Thermal Decomposition of CH3N•NH2, Cis-CH3NHN•H, Trans-CH3NHN•H, and C•H2NNH2 Radicals. J. Phys. Chem. A 2012, 116, 8419–8430. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, M.J.; Ishikawa, Y. H-Atom Abstraction from CH3NHNH2 by NO2: CCSD(T)/6-311++G(3df,2p)//MPWB1K/6-31+G(d,p) and CCSD(T)/6-311+G(2df,p)//CCSD/6-31+G(d,p) Calculations. J. Phys. Chem. A 2006, 110, 6129–6138. [Google Scholar] [CrossRef] [PubMed]
- Kanno, N.; Tani, H.; Daimon, Y.; Terashima, H.; Yoshikawa, N.; Koshi, M. Computational Study of the Rate Coefficients for the Reactions of NO2 with CH3NHNH, CH3NNH2, and CH2NHNH2. J. Phys. Chem. A 2015, 119, 7659–7667. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.G.; Wang, S.; Dasgupta, S.; Thynell, S.T.; Goddard, W.A.; Zybin, S.; Yetter, R.A. Experimental and Quantum Mechanics Investigations of Early Reactions of Monomethylhydrazine with Mixtures of NO2 and N2O4. Combust. Flame 2013, 160, 2557–2559. [Google Scholar] [CrossRef]
- Harris, G.W.; Atkinson, R.; Pitts, J. Kinetics of The Reactions of The Hydroxyl Radical with Hydrazine And Methylhydrazine. J. Phys. Chem. 1979, 83, 2557–2559. [Google Scholar] [CrossRef]
- Vaghjiani, G.L. Gas Phase Reaction Kinetics of O Atoms with (CH3)2NNH2, CH3NHNH2, and N2H4, and Branching Ratios of the OH Product. J. Phys. Chem. A 2001, 105, 4682–4690. [Google Scholar] [CrossRef]
- Liu, H.X.; Ying, W.; Lei, Y.; Liu, J.Y.; Hong, G.; Li, Z.S.; Sun, C.C. CH3NHNH2 + OH Reaction: Mechanism and Dynamics Studies. J. Comput. Chem. 2009, 30, 2194–2204. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, P.; Law, C.K. Gas-Phase Kinetics Study of Reaction of OH Radical with CH3NHNH2 by Second-Order Multireference Perturbation Theory. J. Phys. Chem. A 2012, 116, 5045–5056. [Google Scholar] [CrossRef]
- Vaghjiani, G.L. UV Absorption Cross Sections, Laser Photodissociation Product Quantum Yields, and Reactions of H Atoms with Methylhydrazines at 298 K. J. Phys. Chem. A 1997, 101, 4167–4171. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Wen, J.; Zhang, J. Mechanisms and Kinetics of Hydrogen Abstraction of Methylhydrazine and Deuterated Methylhydrazine with H/D Atoms. Chem. Acc. 2013, 132, 1321. [Google Scholar] [CrossRef]
- Diévart, P.; Catoire, L. Contributions of Experimental Data Obtained in Concentrated Mixtures to Kinetic Studies: Application to Monomethylhydrazine Pyrolysis. J. Phys. Chem. A 2020, 124, 6214–6236. [Google Scholar] [CrossRef] [PubMed]
- Ilincic, N.; Anderson, W.R.; Seshadri, K.; Meagher, N.E. Simplified chemical-kinetic mechanisms for characterizing the structure of the dark zones of double base and nitramine propellants. In Proceedings of the Twenty-Sixth Symposium (International) on Combustion, Campi Phlegraci, Italy, 28 July–2 August 1996; pp. 1997–2006. [Google Scholar]
- Catoire, L.; Swihart, M.T. Thermochemistry of Species Produced from Monomethylhydrazine in Propulsion and Space-Related Applications. J. Propuls. Power 2002, 18, 1242–1253. [Google Scholar] [CrossRef]
- Catoire, L.; Ludwig, T.; Bassin, X.; Dupré, G.; Paillard, C. Kinetic modeling of the ignition delays in monomethylhydrazine/oxygen/argon mixtures. In Proceedings of the Twenty-Seventh Symposium (International) on Combustion, Boulder, CO, USA, 2–7 August 1998; pp. 2359–2365. [Google Scholar]
- Catoire, L.; Chaumeix, N.; Paillard, C. Chemical Kinetic Model for Monomethylhydrazine/Nitrogen Tetroxide Gas-Phase Combustion and Hypergolic Ignition. J. Propuls. Power 2004, 20, 87–92. [Google Scholar] [CrossRef]
- Glarborg, P.; Bendtsen, A.B.; Miller, J.A. Nitromethane Dissociation: Implications for the CH3 + NO2 Reaction. Int. J. Chem. Kinet. 1999, 31, 591–602. [Google Scholar] [CrossRef]
- Anderson, W.R.; Mcquaid, M.J.; Nusca, M.J.; Kotlar, A.J. A Detailed, Finite-Rate, Chemical Kinetics Mechanism for Monomethylhydrazine-Red Fuming Nitric Acid Systems. 2010. Available online: http://www.arl.army.mil/arlreports/2010/ARL-TR-5088.pdf (accessed on 2 July 2023).
- Catoire, L.; Chaumeix, N.; Pichon, S.; Paillard, C. Visualizations of Gas-Phase NTO/MMH Reactivity. J. Propuls. Power 2006, 22, 120–126. [Google Scholar] [CrossRef]
- Hou, L.; Fu, P.; Ba, Y. Chemical Mechanism of MMH/NTO and Simulation in a Small Liquid Rocket Engine. Combust. Sci. Technol. 2019, 191, 2208–2225. [Google Scholar] [CrossRef]
- Huang, D.; Liu, X.X.; Wang, X.J.; Yang, Y.X.; Mu, X.G. Mechanism of Forming Nitrosodimethylamine by Oxidation of Unsym-Dimethylhydrazine in the Atmosphere. Hanneng Cailiao/Chin. J. Energ. Mater. 2019, 27, 35–40. [Google Scholar] [CrossRef]
- Tuazon, E.C.; Carter, W.P.L.; Winer, A.M.; Pitts, J.N. Reactions of Hydrazines with Ozone under Simulated Atmospheric Conditions. Env. Sci. Technol. 1981, 15, 823–828. [Google Scholar] [CrossRef]
- Hong-Bo, H.; Hong-Yu, C.; Yu, Y.; Feng, Z.; Ji-Hui, Y.; Dong, Z. Investigation of Chemical Kinetic Model for Hypergolic Propellant of Monomethylhydrazine and Nitrogen Tetroxide. J. Energy Resour. Technol. 2021, 143, 4048593. [Google Scholar] [CrossRef]
- Liu, Y.D.; Zhong, R. Comparison of N-Nitrosodimethylamine Formation Mechanisms from Dimethylamine during Chloramination and Ozonation: A Computational Study. J. Hazard. Mater. 2017, 321, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, G.; Chen, J.; Wang, B.; Huang, J.; Deng, S. Unveiling Formation Mechanism of Carcinogenic N-Nitrosodimethylamine in Ozonation of Dimethylamine: A Density Functional Theoretical Investigation. J. Hazard. Mater. 2014, 279, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.L. GC-MS Analysis of Unsymmetrical Dimethylhydrazine and Its Initial Oxidation Products. Chin. J. Energ. Mater. 2004, 12, 89–92. [Google Scholar]
- Zhang, L.; Liu, X.; Wang, X. Oxidation Products and Reaction Mechanism of O2 and Gas-Liquid Two Phase UDMH. Chin. J. Explos. Propellants 2017, 40, 88–92. [Google Scholar]
- Bellerby, J.M. The Chemical Effects of Storing Hydrazine Containing Carbon Dioxide Impurity in Stainless Steel Systems. J. Hazard. Mater. 1983, 7, 187–197. [Google Scholar] [CrossRef]
- Fu, Y.; Luo, S.; Zhang, M.; Liu, D.; Sun, B.; Liu, Z.; Wang, D.; Wang, X.; Rong, M. Chemical Kinetics of Unsymmetrical Dimethylhydrazine (UDMH) Degradation in Wastewater by ·OH Radical. Plasma Chem. Plasma Process. 2022, 42, 363–375. [Google Scholar] [CrossRef]
- Bakaikina, N.V.; Kenessov, B.; Ul’yanovskii, N.V.; Kosyakov, D.S. Quantification of Transformation Products of Rocket Fuel Unsymmetrical Dimethylhydrazine in Soils Using SPME and GC-MS. Talanta 2018, 184, 332–337. [Google Scholar] [CrossRef]
- Bukenov, B.; Baimatova, N.; Kenessov, B. Quantification of Transformation Products of Rocket Fuel Unsymmetrical Dimethylhydrazine in Air Using Solid-Phase Microextraction. J. Sep. Sci. 2022, 45, 614–622. [Google Scholar] [CrossRef]
- Huang, D.; Liu, X.; Wang, X.; Huang, Z.; Xie, Z.; Wang, H. Investigation on the Compositionsofunsymmetrical Dimethylhydrazine Treatment with Different Oxidants Using Solid-Phase Micro-Extraction-Gas Chromatography-Mass Spectrometer. R. Soc. Open Sci. 2019, 6, 190263. [Google Scholar] [CrossRef]
- Ruomeng, H.; Ying, J.; Yuanzheng, H.; Keke, S.; Huixin, Z. TiO2-Reduced Graphene Oxide for the Removal of Gas-Phase Unsymmetrical Dimethylhydrazine. New J. Chem. 2021, 45, 394–402. [Google Scholar] [CrossRef]
- Yi, L.; Guo, L.; Jin, H.; Kou, J.; Zhang, D.; Wang, R. Gasification of Unsymmetrical Dimethylhydrazine in Supercritical Water: Reaction Pathway and Kinetics. Int. J. Hydrogen Energy 2018, 43, 8644–8654. [Google Scholar] [CrossRef]
- McNary, C.P.; Demireva, M.; Martens, J.; Berden, G.; Oomens, J.; Hamlow, L.A.; Rodgers, M.T.; Armentrout, P.B. Infrared Multiple Photon Dissociation Action Spectroscopy of Protonated Unsymmetrical Dimethylhydrazine and Proton-Bound Dimers of Hydrazine and Unsymmetrical Dimethylhydrazine. Phys. Chem. Chem. Phys. 2021, 23, 25877–25885. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Mu, X.; Wang, X. Review on the leak detection technology of unsymmetrical dimethylhydrazine. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Kuala Lumpur, Malaysia, 12–14 April 2019; Volume 295. [Google Scholar]
- Swami, U.; Senapathi, K.; Srinivasulu, K.M.; Desingu, J.; Chowdhury, A. Ignition Delays of Mixtures of the Non-Hypergolic Energetic Ionic Liquid Hydroxyethylhydrazinium Nitrate Blended with Unsymmetrical Dimethylhydrazine. Propellants Explos. Pyrotech. 2019, 44, 1139–1146. [Google Scholar] [CrossRef]
- Rodin, I.A.; Anan’Eva, I.A.; Smolenkov, A.D.; Shpigun, O.A. Determination of the Products of the Oxidative Transformation of Unsymmetrical Dimethylhydrazine in Soils by Liquid Chromatography/Mass Spectrometry. J. Anal. Chem. 2010, 65, 1405–1410. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, Y.; Zhou, Y.; Liu, Z.; Feng, X.S. Unsymmetrical Dimethylhydrazine and Related Compounds in the Environment: Recent Updates on Pretreatment, Analysis, and Removal Techniques. J. Hazard. Mater. 2022, 432, 128708. [Google Scholar] [CrossRef]
- Smirnov, A.V.; Panteleyev, M.A.; Krivetskiy, V.V.; Gaskov, A.M. Catalytic Oxidation of Unsymmetrical Dimethylhydrazine on Pt/SiO2. Russ. J. Appl. Chem. 2016, 89, 1109–1118. [Google Scholar] [CrossRef]
- Yi, L.; Wang, L.; Guo, L.; Jin, H.; Cao, W.; Xu, J.; Wei, W. Molecular Dynamic Study on Hydrogen Production from Unsymmetrical Dimethylhydrazine in Supercritical Water. J. Mol. Liq. 2021, 339, 117051. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, X.; He, Z.; Wu, J. Experimental Study on the Combustion and Microexplosion of Freely Falling Gelled Unsymmetrical Dimethylhydrazine (UDMH) Fuel Droplets. Energies 2012, 5, 3126–3136. [Google Scholar] [CrossRef]
- Feng, S.; He, B.; He, H.; Su, L.; Hou, Z.; Nie, W.; Guo, X. Experimental Studies the Burning Process of Gelled Unsymmetrical Dimethylhydrazine Droplets under Oxidant Convective Conditions. Fuel 2013, 111, 367–373. [Google Scholar] [CrossRef]
- Keshavarz, M.H.; Ramadan, A.; Mousaviazar, A.; Zali, A.; Esmaeilpour, K.; Atabaki, F.; Shokrolahi, A. Reducing Dangerous Effects of Unsymmetrical Dimethylhydrazine as a Liquid Propellant by Addition of Hydroxyethylhydrazine—Part I: Physical Properties. J. Energ. Mater. 2011, 29, 46–60. [Google Scholar] [CrossRef]
- Sholokhova, A.Y.; Grinevich, O.I.; Matyushin, D.D.; Buryak, A.K. Machine Learning-Assisted Non-Target Analysis of a Highly Complex Mixture of Possible Toxic Unsymmetrical Dimethylhydrazine Transformation Products with Chromatography-Mass Spectrometry. Chemosphere 2022, 307, 135764. [Google Scholar] [CrossRef] [PubMed]
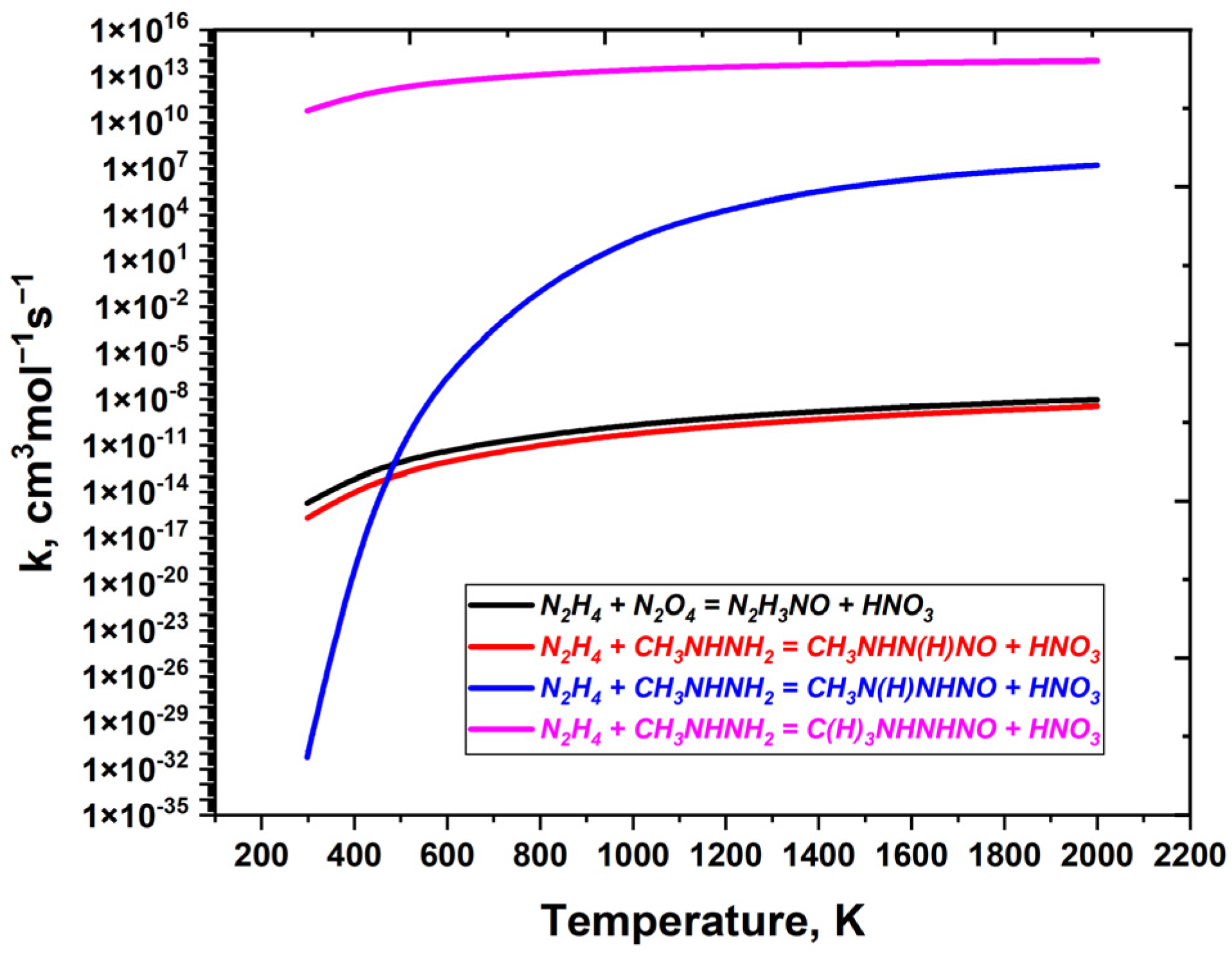
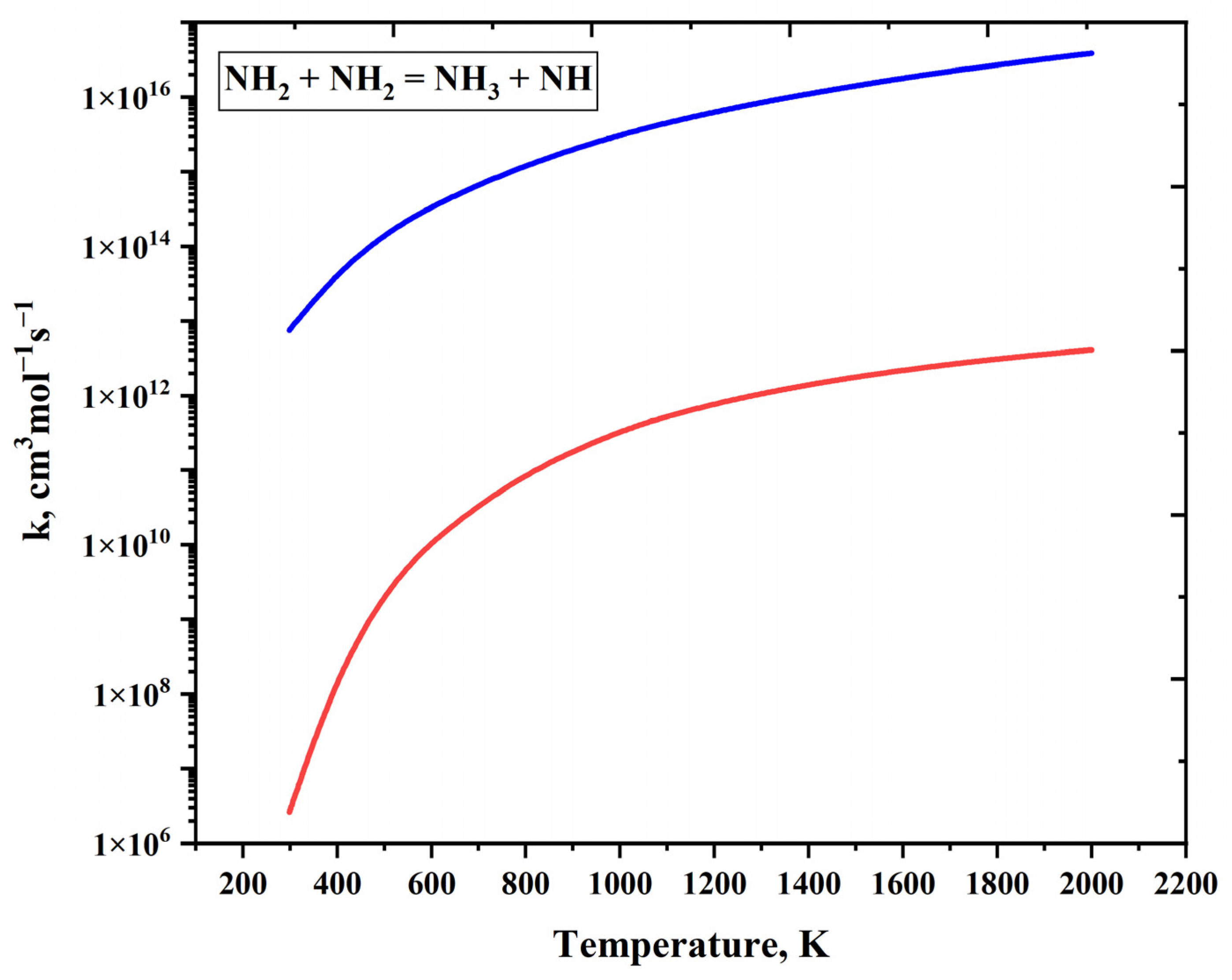

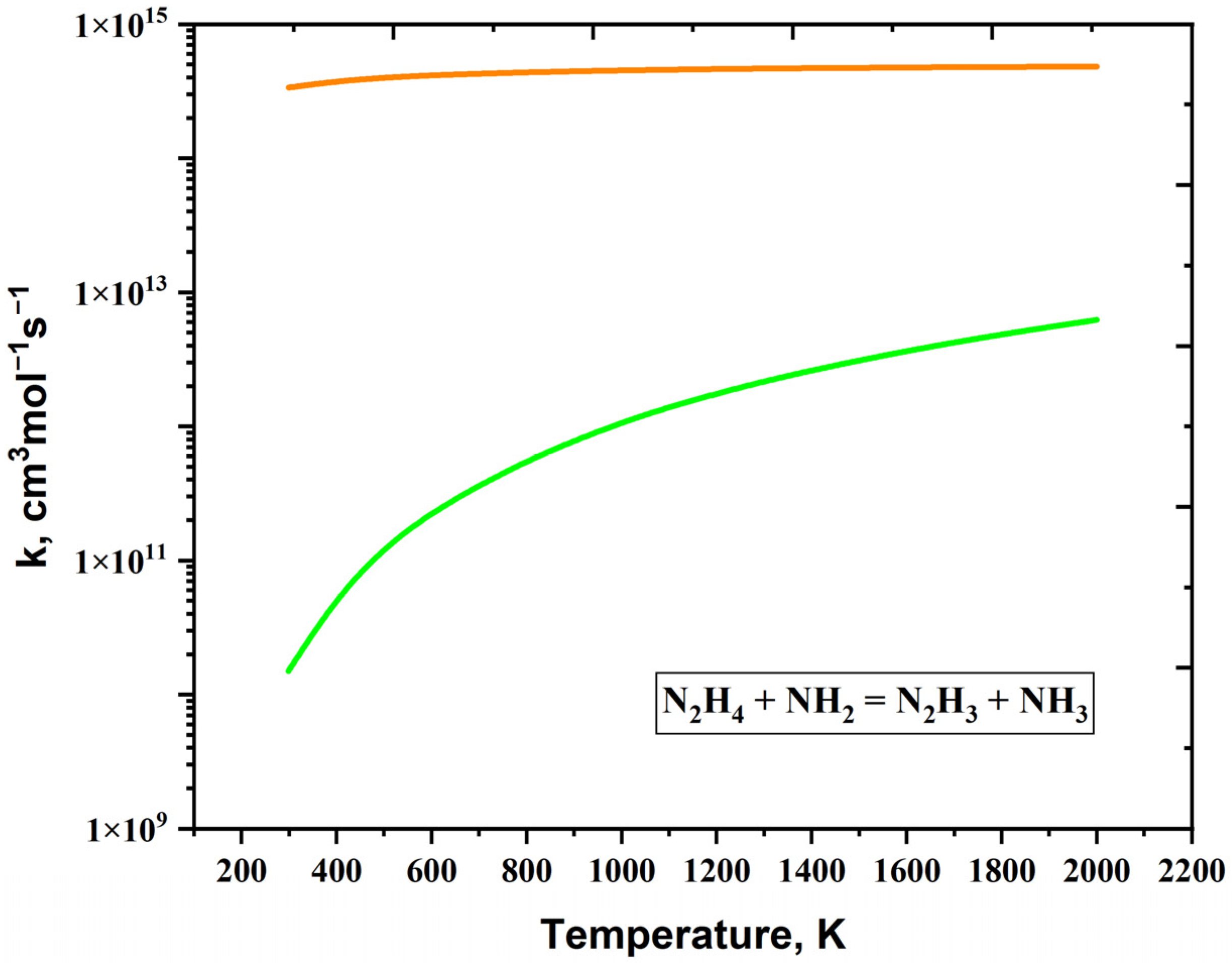
| Reaction | A (s−1 or cm3mol−1s−1) | n | Ea (cal/mol) | References |
|---|---|---|---|---|
| NH2 + NH2 = N2H4 | 3.12 × 1013 | 0 | 0 | [55] |
| NH2 + NH2 = N2H4 | 5.14 × 1010 | 0 | 0 | [56] |
| N2H4 (+M) = NH2 + NH2 (+M) | 5.00 × 1014 | 0 | 251.21 | [38] |
| NH2 + NH2 = NH3 + NH | 3.37 × 107 | 2.82 | 2.22 × 103 | [55] |
| NH2 + NH2 = NH3 + NH | 5.00 × 1013 | 0 | 9.94 × 103 | [39] |
| N2H4 + H = N2H3 + H2 | 9.60 × 108 | 1.5 | 4.83834 × 103 | [39,56] |
| N2H4 + H = N2H3 + H2 | 7.00 × 1012 | 0 | 10.47 | [38] |
| N2H4 + NH2 = N2H3 + NH3 | 3.70 × 106 | 1.94 | 1.62934 × 103 | [39,56] |
| N2H4 + NH2 = N2H3 + NH3 | 1.80 × 106 | 1.71 | −5.78 | [38] |
| Researchers | Details |
|---|---|
| Avery et al. [24] | Constructed a model for hydrazine decomposition |
| Just [58] | Conducted a series of studies on the N2H4/NTO combination fuel |
| Sawyer and Glassman et al. [40] | Studied the gas-phase reactions of N2H4 with O2, NO, and NO2 |
| Konnov et al. [36] | Adjusted the reaction rate constant for the reaction N2H4 + NH2 → N2H3 + NH3 based on the decomposition of N2H4 and flame experimental data |
| Konnov et al. [38] | Developed a kinetic reaction mechanism for the decomposition of hydrazine at high temperatures, containing 51 reactions and 11 substances |
| Ohminami et al. [48] | Developed a gas-phase chemical reaction mechanism of N2H4/NTO, containing 64 and 22 chemical reactions, and performed a series of CFD simulations |
| Daimon et al. [50] | Investigated the nature and mechanism of contact explosions of spontaneous liquid propellants |
| Izato et al. [41] | Presented a chemical mechanism for the early stage of hypergolic ignition in liquid hydrazine/nitrogen tetroxide mixtures |
| Golden et al. [59] | Studied the decomposition of hydrazine fuels at pressures of 0.1–10 mTorr by the ultra-low-pressure pyrolysis technique (VLPP). They measured the reaction rate constants for the decomposition of Hydrazine single molecules due to collisions |
| Study | Description and Findings |
|---|---|
| Kerr et al. [33] | Investigated primary reaction rate constants for uniform bond breaking of N-N bonds in N2H4 and MMH |
| Eberstein and Glassman [25] | Studied decomposition reactions of N2H4, MMH, and UDMH in adiabatic flowers at atmospheric pressure 750–1000 K |
| Catoire et al. [9] | Conducted a pyrolysis study of 1–3% MMH/argon mixtures in 38.4 mm excitation tubes in the temperature range of 1040 to 1370 K and pressure range of 1.4 to 4.5 atm. MMH can decompose exothermally at 70–80 mol% argon dilution. Pure gaseous MMH is much less sensitive to explosive bombardment than hydrazine |
| Sun and Law [62] | Investigated the kinetics of the thermal decomposition reaction of MMH using quantum Rice–Ramsperger–Kassel (QRRK) theory and pressure drop master equation analysis. N-N and C-N bond-breaking reactions are the main reaction pathways simulating the homogeneous decomposition of MMH under atmospheric pressure conditions |
| Sun et al. [65] | Using laser absorption techniques, measured the time histories of NH2 and NH3 during MMH pyrolysis after reflecting excitation waves in an excitation tube. N-N bond splitting is the most sensitive reaction pathway to simulate the homogeneous decomposition of MMH at high pressures. Overall MMH decomposition rate is consistent with first-order kinetics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Bruce, F.N.O.; Bai, X.; Ren, X.; Li, Y. Insights into the Reaction Kinetics of Hydrazine-Based Fuels: A Comprehensive Review of Theoretical and Experimental Methods. Energies 2023, 16, 6006. https://doi.org/10.3390/en16166006
Wu J, Bruce FNO, Bai X, Ren X, Li Y. Insights into the Reaction Kinetics of Hydrazine-Based Fuels: A Comprehensive Review of Theoretical and Experimental Methods. Energies. 2023; 16(16):6006. https://doi.org/10.3390/en16166006
Chicago/Turabian StyleWu, Jin, Frederick Nii Ofei Bruce, Xin Bai, Xuan Ren, and Yang Li. 2023. "Insights into the Reaction Kinetics of Hydrazine-Based Fuels: A Comprehensive Review of Theoretical and Experimental Methods" Energies 16, no. 16: 6006. https://doi.org/10.3390/en16166006
APA StyleWu, J., Bruce, F. N. O., Bai, X., Ren, X., & Li, Y. (2023). Insights into the Reaction Kinetics of Hydrazine-Based Fuels: A Comprehensive Review of Theoretical and Experimental Methods. Energies, 16(16), 6006. https://doi.org/10.3390/en16166006







