The Use of Dolomite to Produce a Magnesium Potassium Phosphate Matrix for Radioactive Waste Conditioning
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of Used Dolomite Powder
2.2. Synthesis of the MPP Matrix Samples
2.3. Research Methods for Experimental Samples
3. Results and Discussion
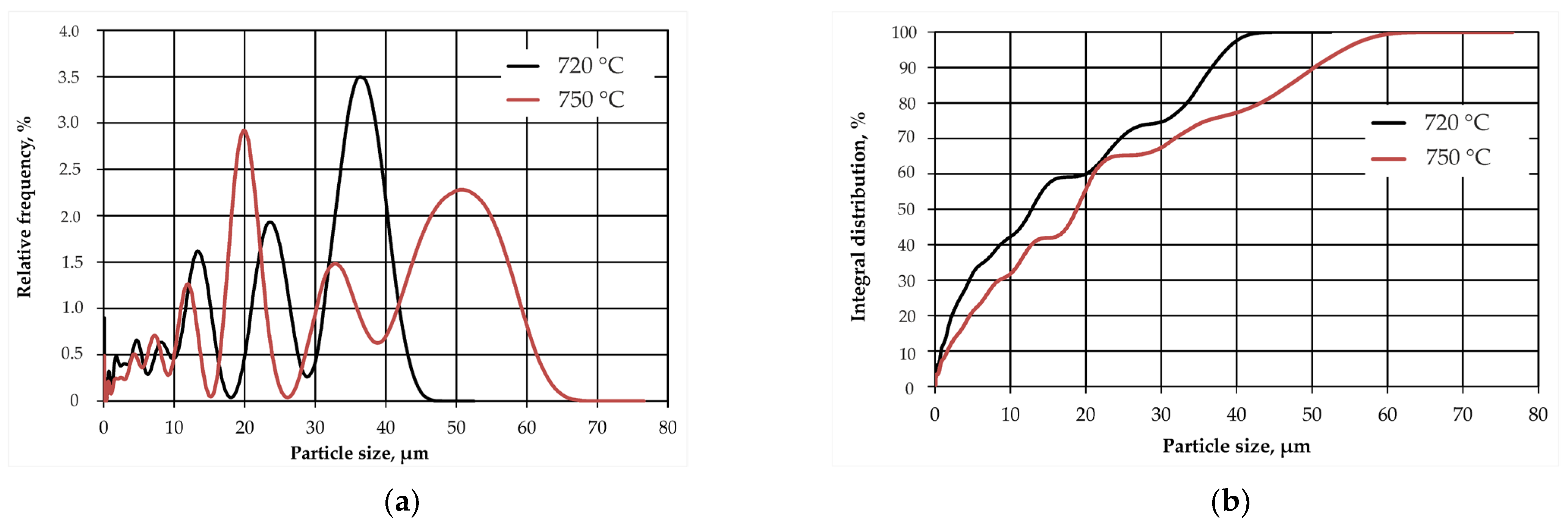
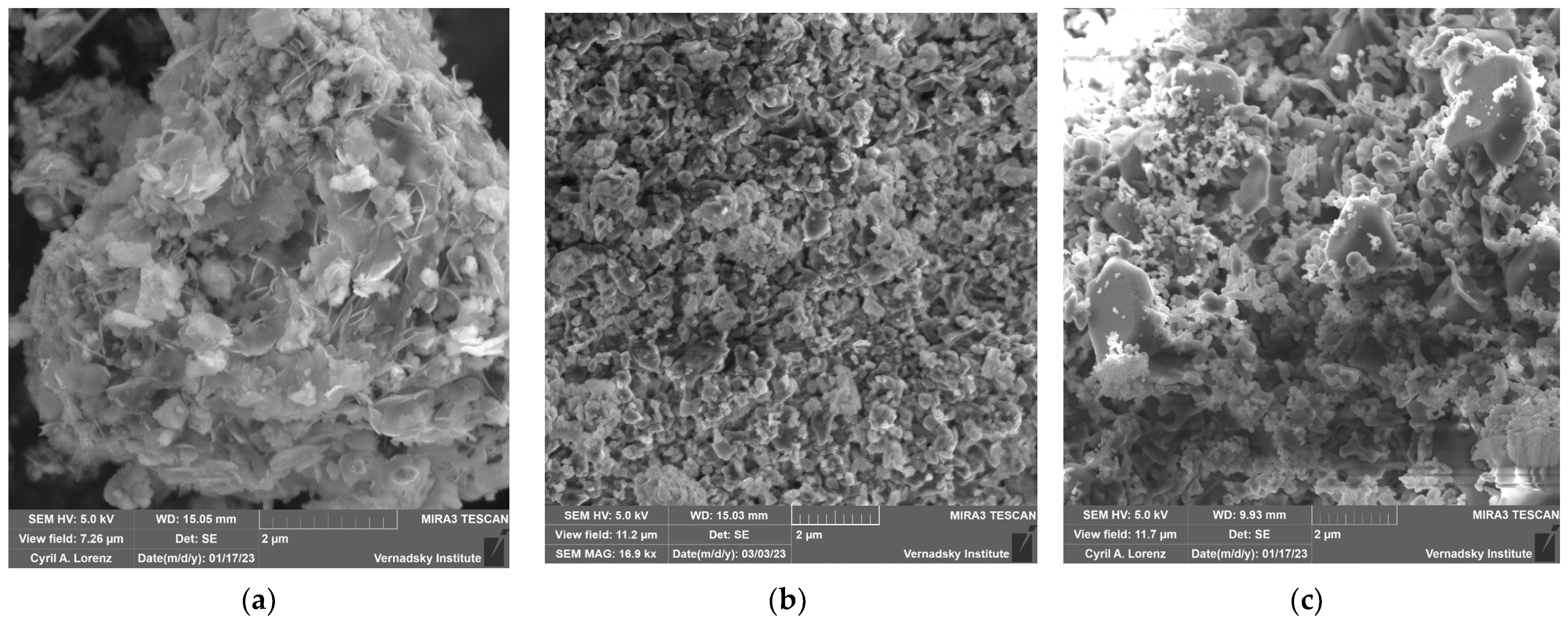
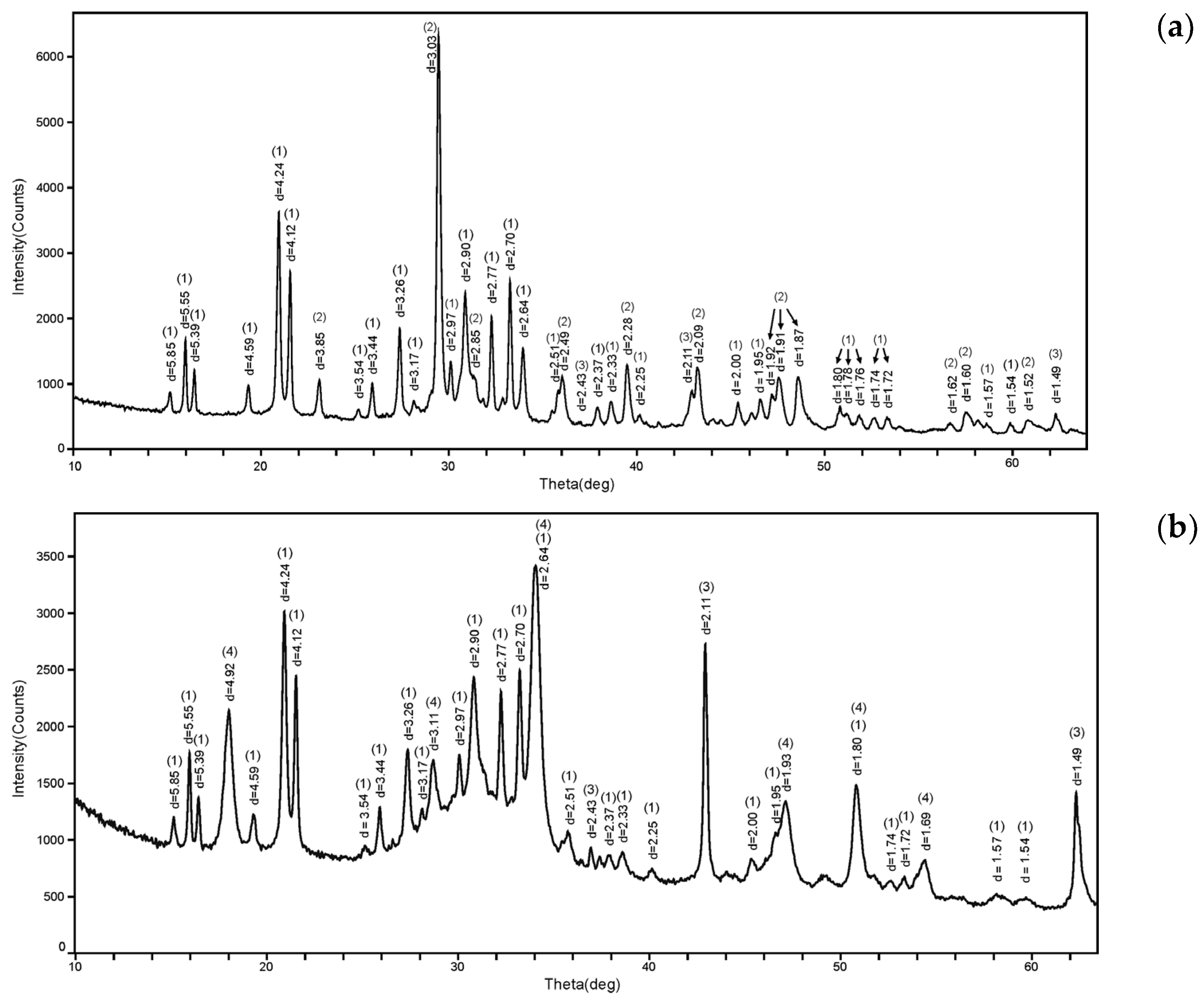
3.1. Effect of Calcination of Dolomite
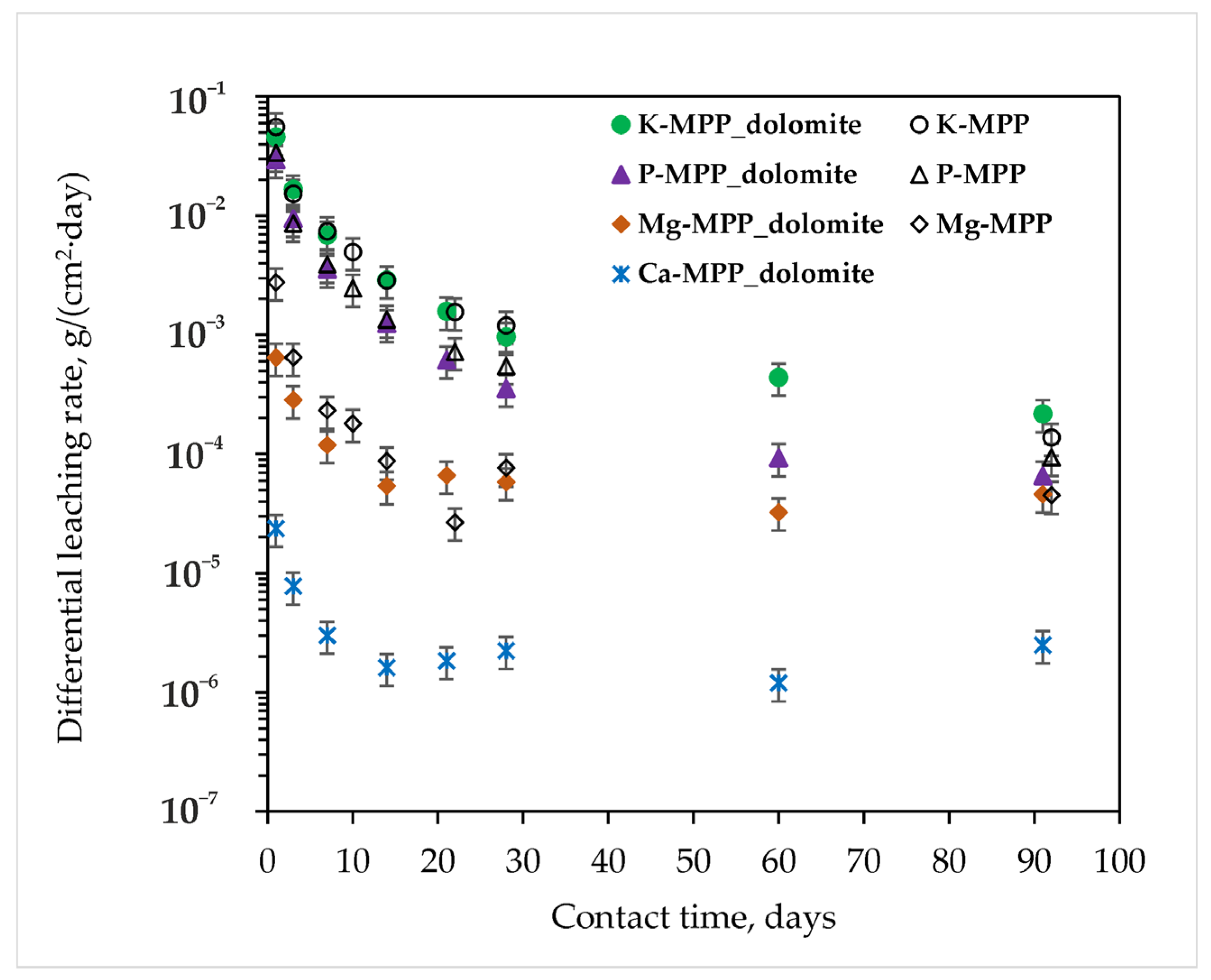
3.2. Study of the MPP Matrix Samples
4. Conclusions
- -
- Calcination of dolomite at 720 °C for 1.5 h results in the production of MgO, CaO, and CaCO3 in the ratios of 1.00, 0.42, and 1.44, respectively. Increasing the calcination temperature of dolomite up to 750 °C and the calcination time up to 24 h results in the decomposition of CaCO3 and the production of CaO; the resulting MgO/CaO ratio was 1/1.18.
- -
- The target crystalline phase of the matrix, MgKPO4 × 6H2O, was confirmed in all the studied MPP compounds obtained from calcined dolomite samples. At the same time, differences in calcination conditions resulted in compounds with different properties. It was shown that the quality indicators of compound obtained from dolomite after calcination at 720 °C exceed those of compound obtained from dolomite after calcination at 750 °C to solve the problem of RW conditioning. Thus, the compound under milder calcination conditions (720 °C, 1.5 h) has a higher density (1.88 g/cm3 vs. 1.67 g/cm3) and strength (25.8 MPa vs. 7.5 MPa) with reduced porosity (PV and PA are 4 and 2 times less, respectively).
- -
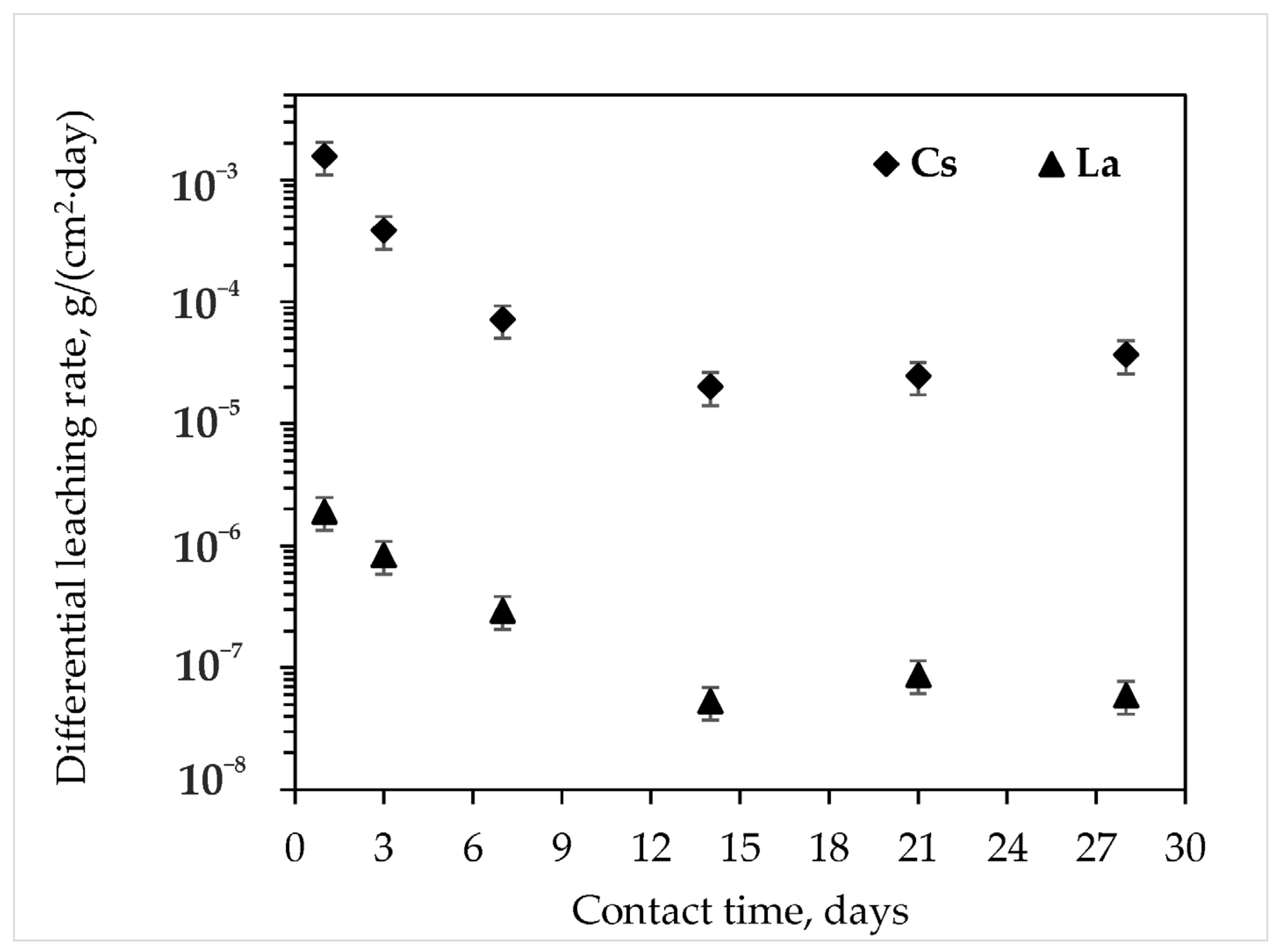
- It should be especially emphasized that the leaching rate of structure-forming elements from the MPP compound obtained from dolomite after calcination at 720 °C is similar to their leaching rate from the blank MPP matrix obtained by using expensive magnesium oxide as a hardener. Thus, the differential leaching rates of K, P, and M from the compound are 2.2 × 10−4, 6.6 × 10−5, 4.6 × 10−5 g/(cm2∙day), respectively. Most importantly, the leaching rates of RW components from this compound meet the normative requirements for industrial cement matrices. The differential leaching rate of cesium from the compound was 3.7 × 10−5 g/(cm2∙day), which corresponds to the normative requirements in Russia: no more than 10−3 g/(cm2∙day).
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Atomic Energy Agency. Available online: https://www.iaea.org/topics/radioactive-waste-and-spent-fuel-management (accessed on 28 April 2023).
- Ojovan, M.I.; Steinmetz, H.J. Approaches to Disposal of Nuclear Waste. Energies 2022, 15, 7804. [Google Scholar] [CrossRef]
- Drace, Z.; Ojovan, M.I.; Samanta, S.K. Challenges in Planning of Integrated Nuclear Waste Management. Sustainability 2022, 14, 14204. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Danilov, S.S.; Matveenko, A.V.; Frolova, A.V.; Belova, K.Y.; Petrov, V.G.; Vinokurov, S.E.; Myasoedov, B.F. Perspective Compounds for Immobilization of Spent Electrolyte from Pyrochemical Processing of Spent Nuclear Fuel. Appl. Sci. 2021, 11, 11180. [Google Scholar] [CrossRef]
- Corkhill, C.; Hyatt, N. Nuclear Waste Management; IOP Publishing Ltd.: Bristol, UK, 2018; pp. 1–30. [Google Scholar]
- Rakhimova, N. Recent Advances in Alternative Cementitious Materials for Nuclear Waste Immobilization: A Review. Sustainability 2023, 15, 689. [Google Scholar] [CrossRef]
- Covill, A.; Hyatt, N.C.; Hill, J.; Collier, N.C. Development of magnesium phosphate cements for encapsulation of radioactive waste. Adv. Appl. Ceram. 2011, 110, 151–156. [Google Scholar] [CrossRef]
- Cau Dit Coumes, C.; Lambertin, D.; Lahalle, H.; Antonucci, P.; Cannes, C.; Delpech, S. Selection of a mineral binder with potentialities for the stabilization/solidification of aluminum metal. J. Nucl. Mater. 2014, 453, 31–40. [Google Scholar] [CrossRef]
- Gardner, L.J.; Walling, S.A.; Lawson, S.M.; Sun, S.; Bernal, S.A.; Corkhill, C.L.; Provis, J.L.; Apperley, D.C.; Iuga, D.; Hanna, J.V.; et al. Characterization of and Structural Insight into Struvite-K, MgKPO4·6H2O, an Analogue of Struvite. Inorg. Chem. 2021, 60, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Holdsworth, A.F.; Eccles, H.; Halman, A.M.; Mao, R.; Bond, G. Low-Temperature Continuous Flow Synthesis of Metal Ammonium Phosphates. Sci. Rep. 2018, 8, 13547. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: Twenty-First Century Materials with Diverse Applications, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–422. ISBN 978-0-08-100380-0. [Google Scholar]
- Vinokurov, S.E.; Kulikova, S.A.; Frolova, A.V.; Danilov, S.S.; Belova, K.Y.; Rodionova, A.A.; Myasoedov, B.F. New Methods and Materials in Nuclear Fuel Fabrication and Spent Nuclear Fuel and Radioactive Waste Management. In Advances in Geochemistry, Analytical Chemistry, and Planetary Sciences; Kolotov, V.P., Bezaeva, N.S., Eds.; Springer: Cham, Switzerland, 2023; pp. 579–594. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Belova, K.Y.; Tyupina, E.A.; Vinokurov, S.E. Conditioning of Spent Electrolyte Surrogate LiCl-KCl-CsCl Using Magnesium Potassium Phosphate Compound. Energies 2020, 13, 1963. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.-P.; Berlepsch, P.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F. Struvite-(K), KMgPO4∙6H2O, the potassium equivalent of struvite—A new mineral. Eur. J. Miner. 2008, 20, 629–633. [Google Scholar] [CrossRef]
- Milestone, N.B. Reactions in cement encapsulated nuclear wastes: Need for toolbox of different cement types. Adv. Appl. Ceram. 2006, 105, 13–20. [Google Scholar] [CrossRef]
- Wagh, A.S.; Strain, R.; Jeong, S.Y.; Reed, D.; Krause, T.; Singh, D. Stabilization of Rocky Flats Pu-contaminated ash within chemically bonded phosphate ceramics. J. Nucl. Mater. 1999, 265, 295–307. [Google Scholar] [CrossRef]
- Lai, Z.; Lai, X.; Shi, J.; Lu, Z. Effect of Zn2+ on the early hydration behavior of potassium phosphate based magnesium phosphate cement. Constr. Build. Mater. 2016, 129, 70–78. [Google Scholar] [CrossRef]
- Banks, E.; Chianelli, R.; Korenstein, R. Crystal Chemistry of Struvite Analogs of the Type MgMPO4∙6H2O (M+ = K+, Rb+, Cs+, T1+, NH4+). Inorg. Chem. 1975, 14, 1634–1639. [Google Scholar] [CrossRef]
- Wagh, A.; Sayenko, S.; Shkuropatenko, V.; Tarasov, R.; Dykiy, M.P.; Svitlychniy, Y.O.; Virych, V.; Ulybkina, E.A. Experimental study on cesium immobilization in struvite structures. J. Hazard. Mater. 2016, 302, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Walling, S.A.; Provis, J.L. Magnesia-based cements: A journey of 150 years, and cements for the future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Chartier, D.; Sanchez-Canet, J.; Antonucci, P.; Esnouf, S.; Renault, J.-P.; Farcy, O.; Lambertin, D.; Parraud, S.; Lamotte, H.; Cau Dit Coumes, C. Behaviour of magnesium phosphate cement-based materials under gamma and alpha irradiation. J. Nucl. Mater. 2020, 541, 152411. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Tyupina, E.A. Effect of characteristics of magnesium oxide powder on composition and strength of magnesium potassium phosphate compound for solidifying radioactive waste. Russ. J. Appl. Chem. 2019, 92, 490–497. [Google Scholar] [CrossRef]
- Formosa, J.; Lacasta, A.M.; Navarro, A.; del Valle-Zermeño, R.; Niubó, M.; Rosell, J.R.; Chimenos, J.M. Magnesium Phosphate Cements formulated with a low-grade MgO by-product: Physico-mechanical and durability aspects. Constr. Build. Mater. 2015, 91, 150–157. [Google Scholar] [CrossRef]
- Kulikova, S.A.; Vinokurov, S.E.; Khamizov, R.K.; Vlasovskikh, N.S.; Belova, K.Y.; Dzhenloda, R.K.; Konov, M.A.; Myasoedov, B.F. The Use of MgO Obtained from Serpentinite in the Synthesis of a Magnesium Potassium Phosphate Matrix for Radioactive Waste Immobilization. Appl. Sci. 2021, 11, 220. [Google Scholar] [CrossRef]
- Yu, J.; Qian, J.; Wang, F.; Li, Z.; Jia, X. Preparation and properties of a magnesium phosphate cement with dolomite. Cem. Concr. Res. 2020, 138, 106235. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, S.; Wu, X. Deicer-scaling resistance of phosphate cement-based binder for rapid repair of concrete. Cem. Concr. Res. 2002, 32, 165–168. [Google Scholar] [CrossRef]
- Yu, J.; Qian, J.; Wang, F.; Qin, J.; Dai, X.; You, C.; Jia, X. Study of using dolomite ores as raw materials to produce magnesium phosphate cement. Constr. Build. Mater. 2020, 253, 119147. [Google Scholar] [CrossRef]
- Baghriche, M.; Achour, S.; Baghriche, O. Combined effect of cement kiln dust and calcined dolomite raw on the properties of performance magnesium phosphate cement. Case Stud. Constr. Mater. 2020, 13, e00386. [Google Scholar] [CrossRef]
- Kristóf-Makó, É.; Juhász, A.Z. The effect of mechanical treatment on the crystal structure and thermal decomposition of dolomite. Thermochim. Acta 1999, 342, 105–114. [Google Scholar] [CrossRef]
- Abdullah, S.F.A.; Saleh, S.S.M.; Mohammad, N.F.; Idris, M.S.; Saliu, H.R. Effect of Thermal Treatment on Natural Dolomite. J. Phys. Conf. Ser. 2021, 2080, 012009. [Google Scholar] [CrossRef]
- Dmitrieva, A.V.; Kalenova, M.Y.; Kulikova, S.A.; Kuznetsov, I.V.; Koshcheev, A.M.; Vinokurov, S.E. Magnesium-Potassium Phosphate Matrix for Immobilization of 14C. Russ. J. Appl. Chem. 2018, 91, 641–646. [Google Scholar] [CrossRef]
- GOST R 52126-2003; Radioactive Waste. Long Time Leach Testing of Solidified Radioactive Waste Forms. Gosstandart 305: Moscow, Russia, 2003; pp. 1–8.
- Mandrino, D.; Paulin, I.; Kržmanc, M.M.; Škapin, S.D. Physical and chemical treatments influence on the thermal decomposition of a dolomite used as a foaming agent. J. Therm. Anal. Calorim. 2018, 31, 1125–1134. [Google Scholar] [CrossRef]
- Vinokurov, S.E.; Kulikova, S.A.; Krupskaya, V.V.; Myasoedov, B.F. Magnesium potassium phosphate compound for radioactive waste immobilization: Phase composition, structure, and physicochemical and hydrolytic durability. Radiochemistry 2018, 60, 70–78. [Google Scholar] [CrossRef]
- Ropp, R.C. Group 16 (O, S, Se, Te) Alkaline Earth Compounds. In Encyclopedia of the Alkaline Earth Compounds; Elsevier: Oxford, UK, 2013; Chapter 3; pp. 105–197. [Google Scholar] [CrossRef]
- Benjamin, M.M. Water Chemistry, 2nd ed.; McGraw-Hill: New York, NY, USA, 2002; ISBN 978-0-07-238390-4. [Google Scholar]
- Rumble, J.R. Handbook of Chemistry and Physics, 99th ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1138561632. [Google Scholar]
- NP-019-15; Federal Norms and Rules in the Field of Atomic Energy Use “Collection, Processing, Storage and Conditioning of Liquid Radioactive Waste. Safety Requirements”; With Changes (Order No. 299 Dated 13 September 2021). Rostekhnadzor: Moscow, Russia, 2015; pp. 1–22.
- Vinokurov, S.E.; Kulikova, S.A.; Myasoedov, B.F. Magnesium Potassium Phosphate Compound for Immobilization of Radioactive Waste Containing Actinide and Rare Earth Elements. Materials 2018, 11, 976. [Google Scholar] [CrossRef]
- Ojovan, M.I.; Lee, W.E. Glassy Wasteforms for Nuclear Waste Immobilization. Metall. Mater. Trans. A 2011, 42A, 837–851. [Google Scholar] [CrossRef]



| Chemical Compound | Composition (wt%) |
|---|---|
| MgO | 26.68 |
| CaO | 36.30 |
| SiO2 | 0.44 |
| Al2O3 | 0.28 |
| TiO2 | 0.014 |
| Fe2O3 | 0.14 |
| K2O | 0.01 |
| P2O5 | 0.10 |
| Cr | 0.0015 |
| Cu | 0.0027 |
| Zn | 0.0040 |
| Rb | 0.0008 |
| Sr | 0.0102 |
| Y | 0.0006 |
| Zr | 0.0016 |
| Nb | 0.0008 |
| Pb | 0.0101 |
| LOI * | 36.17 |
| Phase | Component Phase (wt%) | |
|---|---|---|
| 720 °C | 750 °C | |
| CaCO3 | 50.3 | - |
| MgO | 34.9 | 45.9 |
| CaO | 14.8 | 54.1 |
| Parameter | Sample | |
|---|---|---|
| MPP_dolomite_720 | MPP_dolomite_750 | |
| Density (ρ), g/cm3 | 1.88 | 1.67 |
| Volumetric porosity (PV), % | 2.1 | 8.3 |
| Apparent porosity (PA), % | 9.3 | 19.0 |
| Compressive strength, MPa | 25.8 | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulikova, S.A.; Belova, K.Y.; Frolova, A.V.; Vinokurov, S.E. The Use of Dolomite to Produce a Magnesium Potassium Phosphate Matrix for Radioactive Waste Conditioning. Energies 2023, 16, 5513. https://doi.org/10.3390/en16145513
Kulikova SA, Belova KY, Frolova AV, Vinokurov SE. The Use of Dolomite to Produce a Magnesium Potassium Phosphate Matrix for Radioactive Waste Conditioning. Energies. 2023; 16(14):5513. https://doi.org/10.3390/en16145513
Chicago/Turabian StyleKulikova, Svetlana A., Kseniya Y. Belova, Anna V. Frolova, and Sergey E. Vinokurov. 2023. "The Use of Dolomite to Produce a Magnesium Potassium Phosphate Matrix for Radioactive Waste Conditioning" Energies 16, no. 14: 5513. https://doi.org/10.3390/en16145513
APA StyleKulikova, S. A., Belova, K. Y., Frolova, A. V., & Vinokurov, S. E. (2023). The Use of Dolomite to Produce a Magnesium Potassium Phosphate Matrix for Radioactive Waste Conditioning. Energies, 16(14), 5513. https://doi.org/10.3390/en16145513







