Experimental and Numerical Study of the Ice Storage Process and Material Properties of Ice Storage Coils
Abstract
:1. Introduction
2. Model Formulation and Boundary Conditions
2.1. Model Description
2.2. Physical Formulation
2.2.1. Model Assumptions
2.2.2. Model Equation
2.3. Boundary Conditions
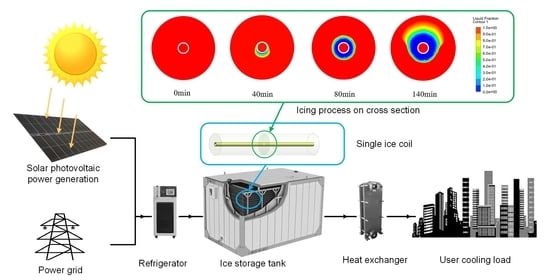
3. Experimental Tests
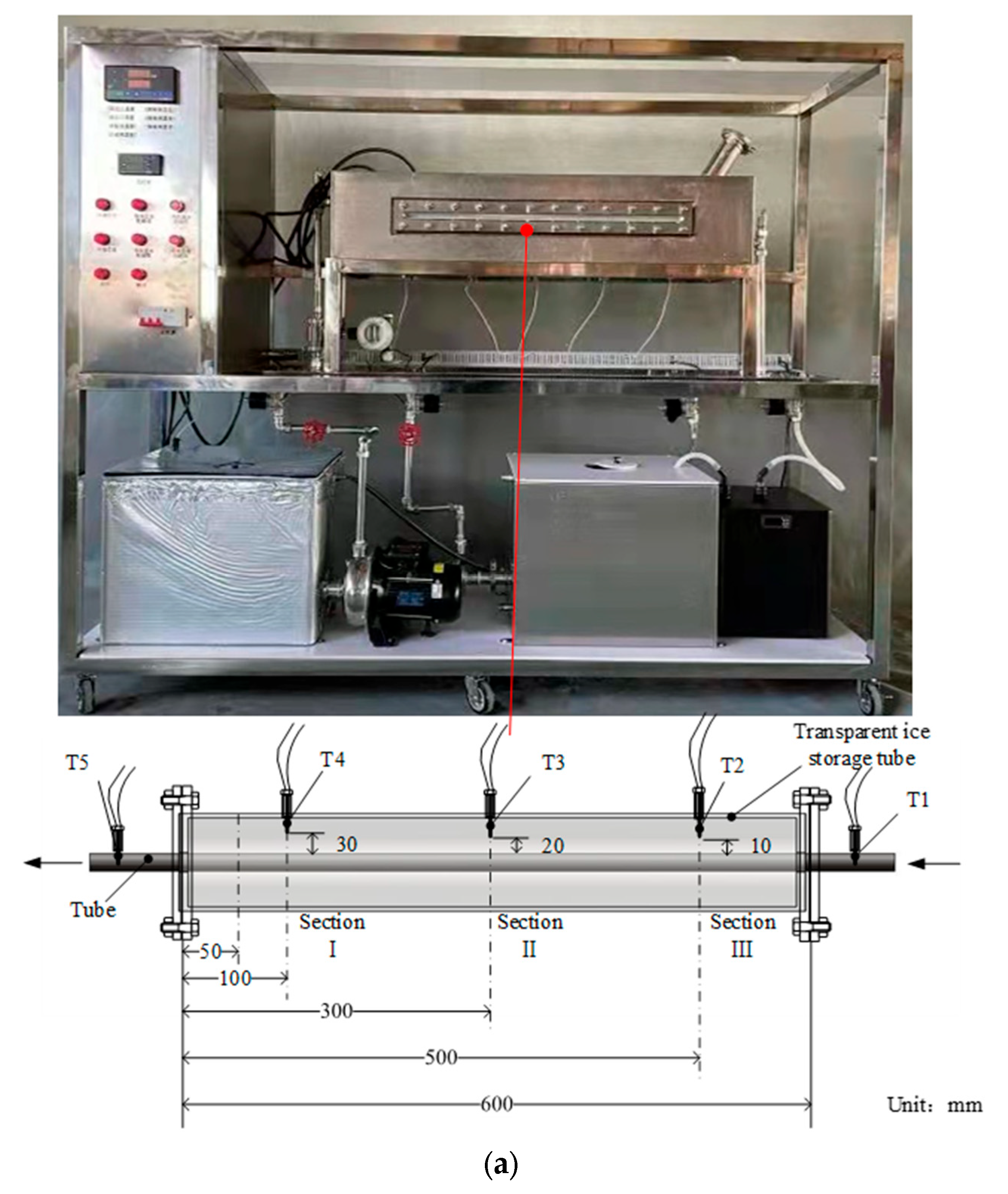
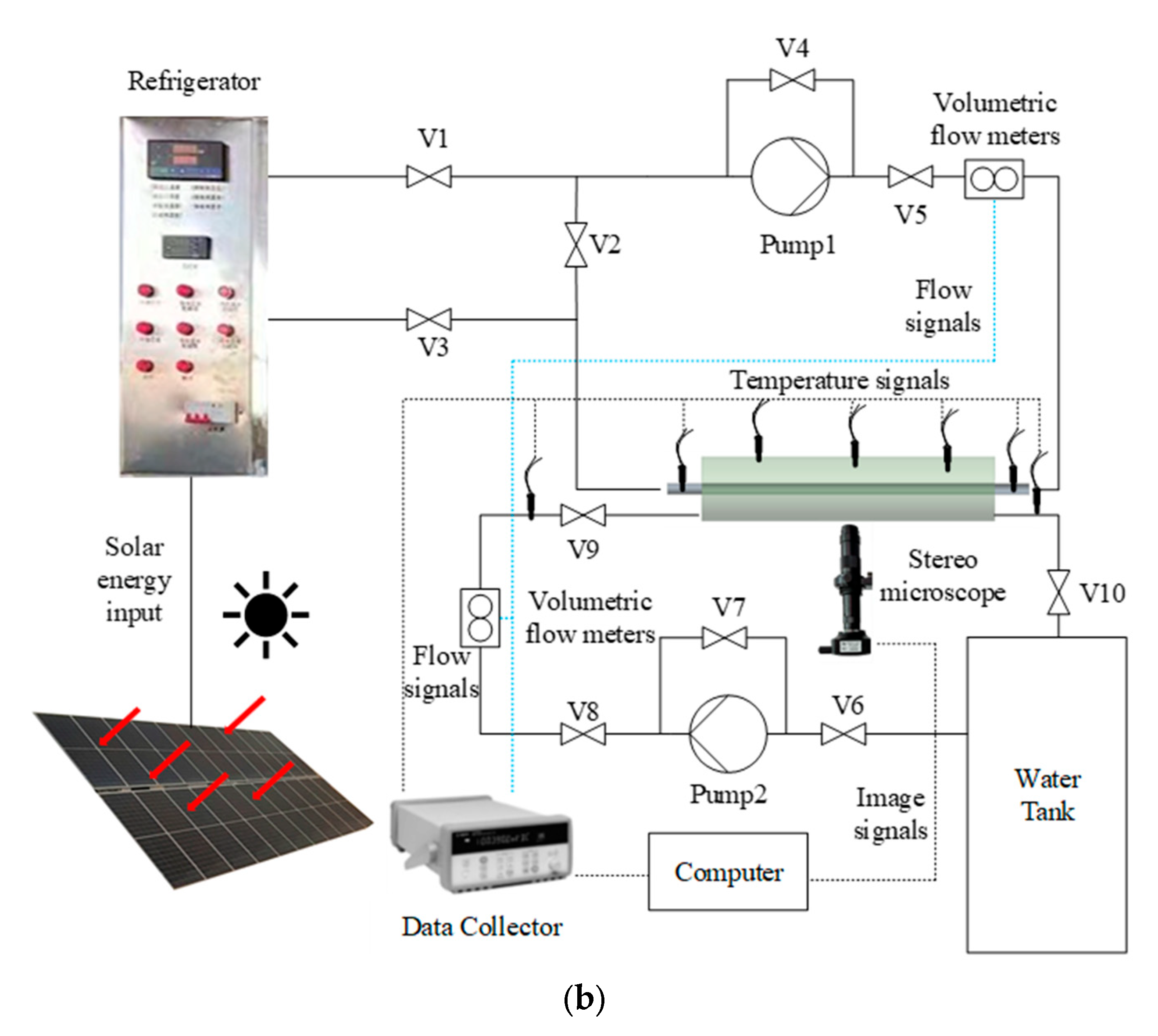
3.1. Experimental Setup
3.2. Experimental Operation Flow
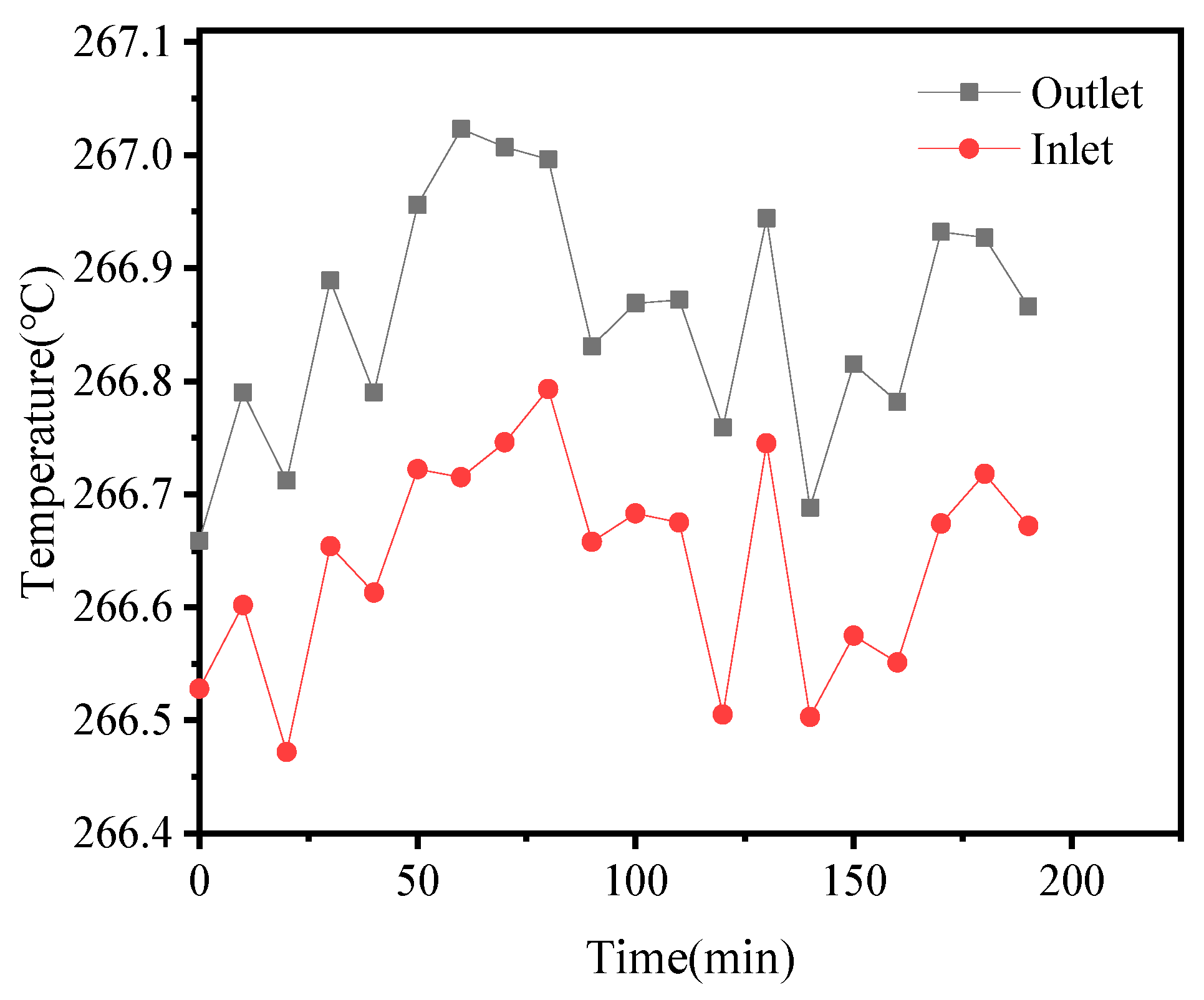
3.3. Experimental Error and Uncertainty
4. Results and Discussion
4.1. Mechanistic Analysis of the Icing Processes
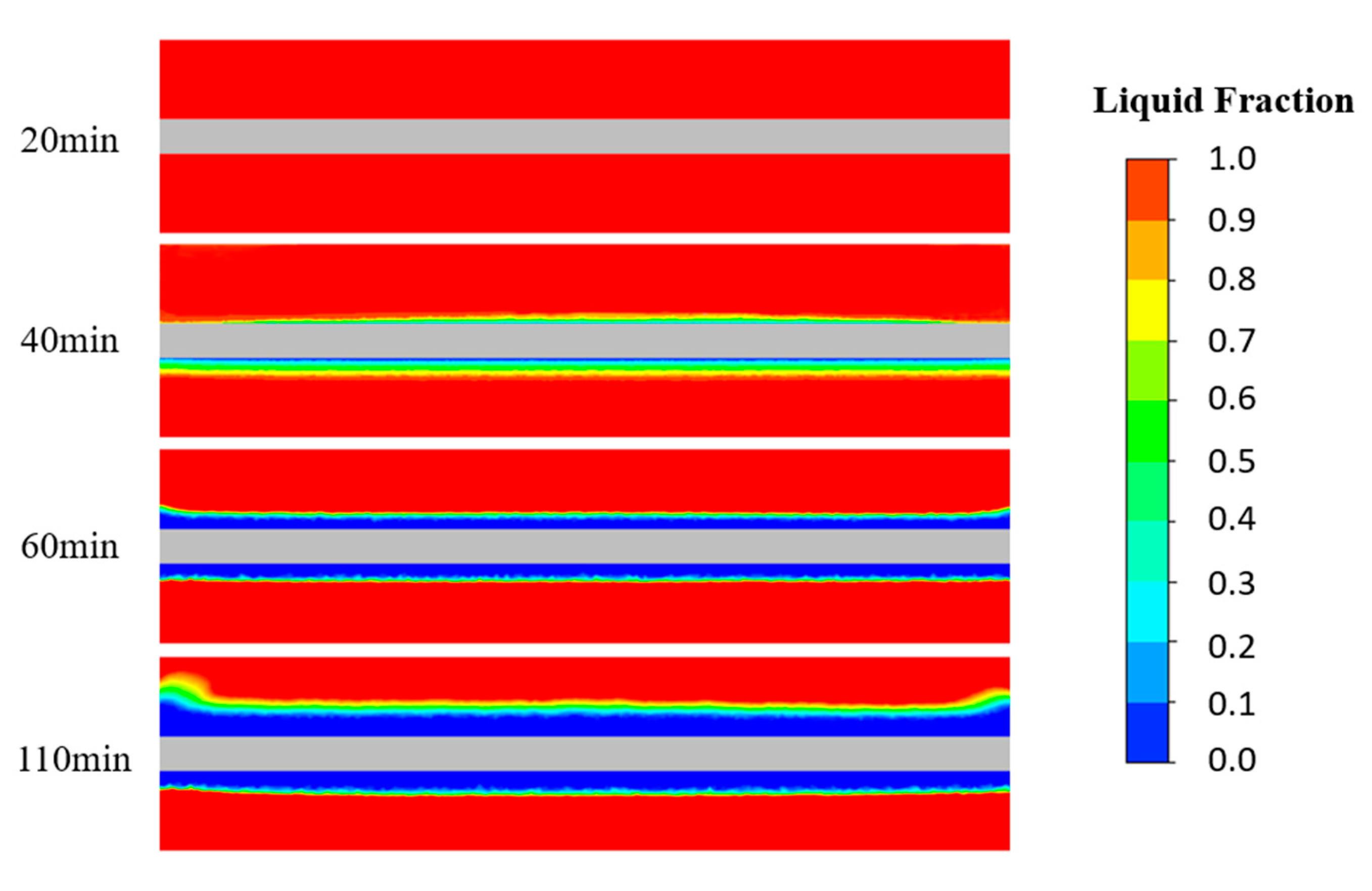
4.2. Liquid Phase Rate Cloud Analysis of the Ice Storage Process
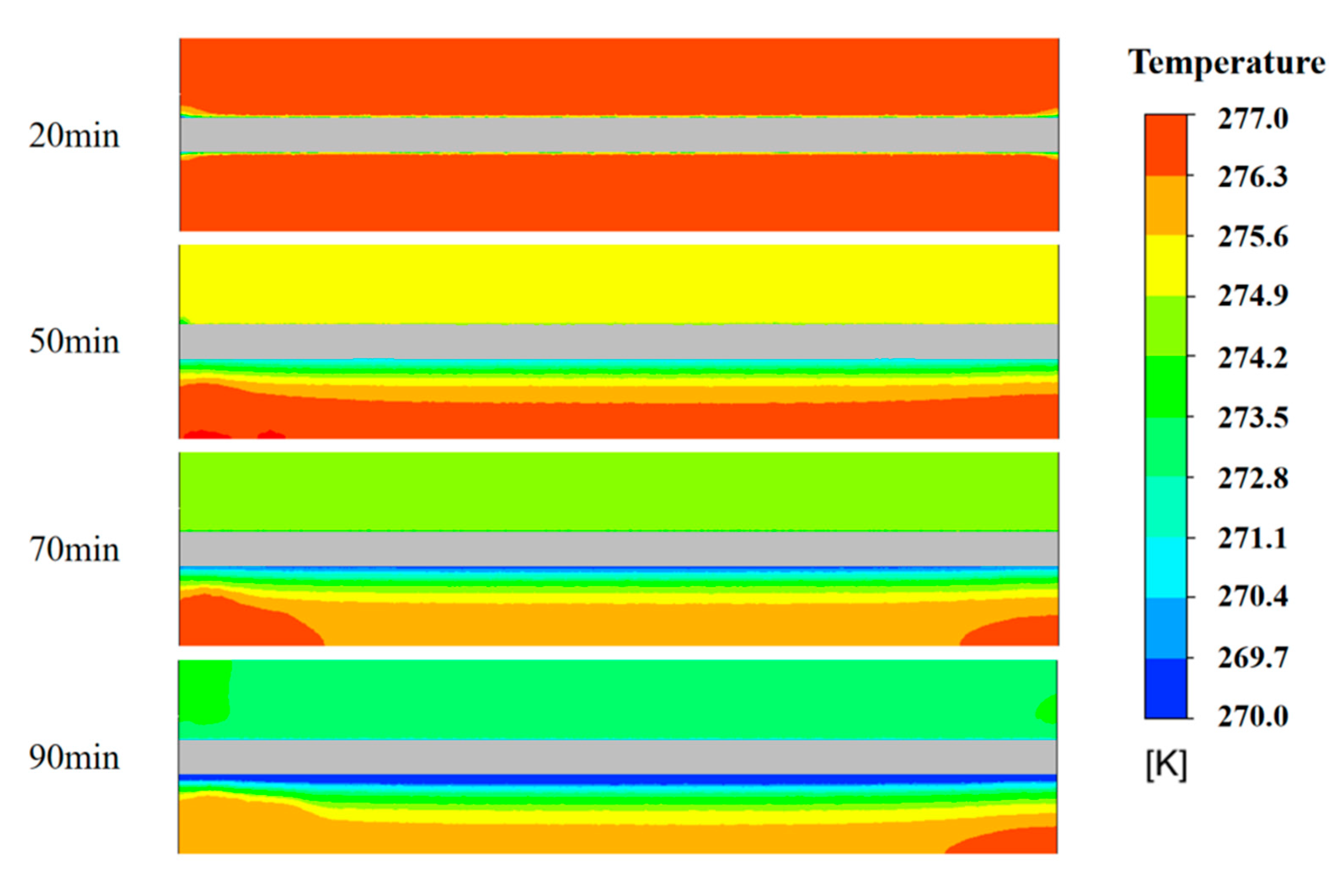
4.3. Temperature Cloud Analysis of the Ice Storage Process
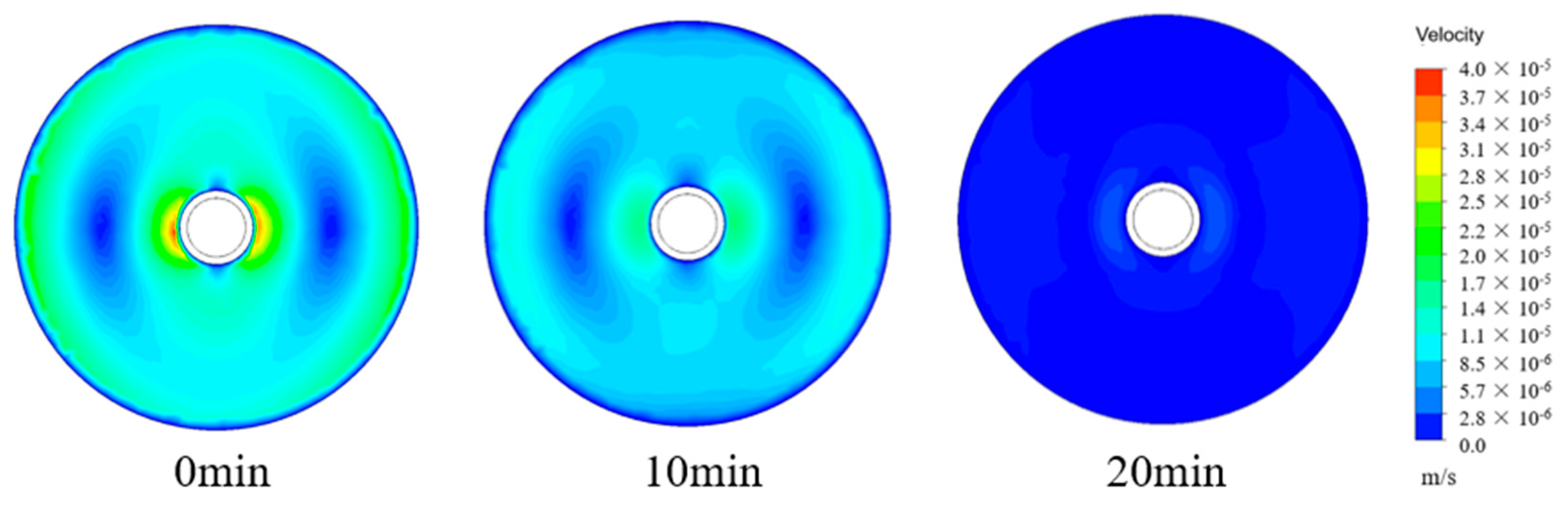
4.4. Velocity Cloud Analysis of the Ice Storage Process
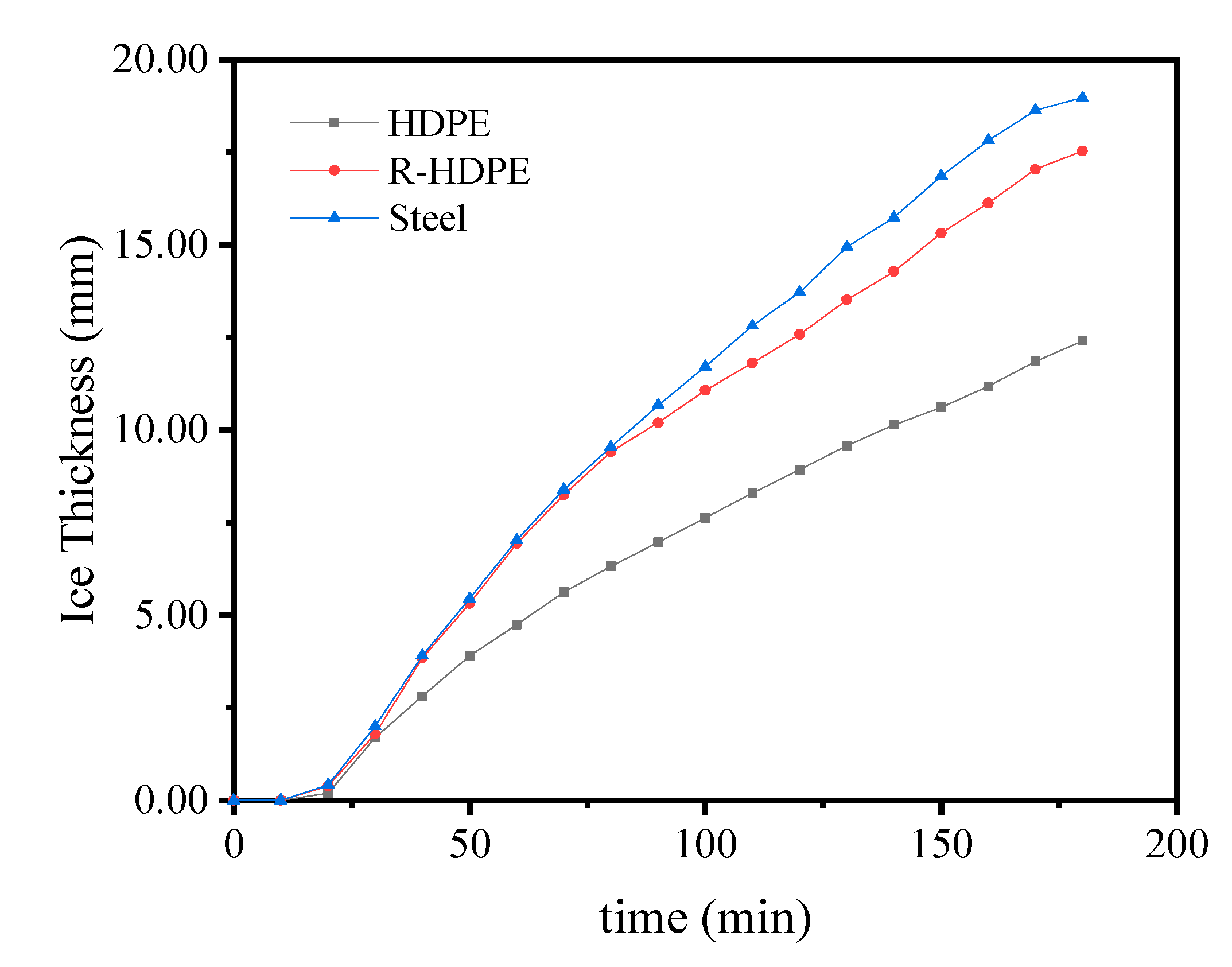
4.5. Effect of Thermal Conductivity of Ice Storage Process Tube
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbol | u | xdirection velocity-vector component [m/s] | |
| C | porosity constant [C = 1.6 × 103] | v | ydirection velocity-vector component [m/s] |
| Hm | latent heat [J/kg] | x,y,z | coordinate axes |
| h | sensible enthalpy [J/kg] | Greek symbols | |
| K0 | empirical constant in K–Carman equation | α | thermal diffusivity [m2/s] |
| K | permeability | μ | viscosity [Pa·s] |
| k | thermal conductivity [W/(m·K)] | ρ | density [kg/m3] |
| L | length [mm] | γ | liquid fraction |
| P | pressure [Pa] | δ | thickness [mm] |
| q | permeability [10−3] | Abbreviation | |
| r | radius [mm] | CFD | computational fluid dynamics |
| t | times [s] | HDPE | high-density polyethylene |
| T | temperature [K] | HTF | heat transfer fluid |
| Ts | freezing temperature [K] | PCM | phase change material |
| U | velocity [m/s] |
Appendix A
| Items | T [°C] | ρ [kg/m3] | cp [kJ/(kg·K)] | λ [W/(m·K)] | μ [Pa·s] | h [kJ/kg] |
|---|---|---|---|---|---|---|
| ice | −6.0~0.0 | 917 | / | 2.2000000 | / | 335 |
| water | 1.0 | 999.898 | 4.2176 | 0.5581080 | 0.00176 | 335 |
| 2.0 | 999.940 | 4.2176 | 0.5605883 | 0.00176 | 335 | |
| 3.0 | 999.964 | 4.2176 | 0.5630162 | 0.00176 | 335 | |
| 4.0 | 999.972 | 4.2176 | 0.5653938 | 0.00176 | 335 | |
| 5.0 | 999.964 | 4.2176 | 0.5677233 | 0.00176 | 335 | |
| 6.0 | 999.940 | 4.2176 | 0.5700065 | 0.00176 | 335 | |
| 7.0 | 999.901 | 4.2176 | 0.5722453 | 0.00176 | 335 | |
| 8.0 | 999.848 | 4.2176 | 0.5744415 | 0.00176 | 335 | |
| 9.0 | 999.781 | 4.2176 | 0.5765965 | 0.00176 | 335 | |
| 10.0 | 999.699 | 4.2176 | 0.5787119 | 0.00176 | 335 |
References
- Zeng, Y.; Zhang, R.W.; Wang, D.; Mu, Y.F.; Jia, H.J. A regional power grid operation and planning method considering renewable energy generation and load control. Appl. Energy 2019, 237, 304–313. [Google Scholar] [CrossRef]
- Chang, C.; Xu, X.Y.; Zheng, J.Y.; Dong, J.S.; Guo, X.X.; Zhao, M.Z. Research on Coil Type Ice Storage Air Conditioning Technology for Power Grid Peak Regulation. In Proceedings of the CSEE 2023 Congress Proceedings, Lisbon, Portugal, 29–31 March 2023; pp. 1–18. [Google Scholar]
- Sadi, M.; Arabkoohsar, A. Techno-economic analysis of off-grid solar-driven cold storage systems for preventing the waste of agricultural products in hot and humid climates. J. Clean. Prod. 2020, 275, 124143. [Google Scholar] [CrossRef]
- Chakravarty, K.H.; Sadi, M.; Chakravarty, H. A review on integration of renewable energy processes in vapor absorption chiller for sustainable cooling. Sustain. Energy Technol. Assess. 2022, 50, 101822. [Google Scholar] [CrossRef]
- Song, X.; Zhu, T.; Liu, L.X.; Cao, Z.J. Study on optimal ice storage capacity of ice thermal storage system and its influence factors. Energy Convers. Manag. 2018, 164, 288–300. [Google Scholar] [CrossRef]
- Thiem, S.; Born, A.; Danov, V.; Vandersickel, A.; Schafer, J.; Hamacher, T. Automated identification of a complex storage model and hardware implementation of a model-predictive controller for a cooling system with ice storage. Appl. Therm. Eng. 2017, 121, 922–940. [Google Scholar] [CrossRef]
- Salt, H.H. Experimental Study of Water Solidification Phenomenon for Ice-on-Coil Thermal Energy Storage Application Utilizing Falling Film. Appl. Therm. Eng. 2018, 146, 135–145. [Google Scholar]
- Cui, B.; Cao, D.; Xiao, F.; Wang, S.W. Model-based optimal design of active cool thermal energy storage for maximal life-cycle cost saving from demand management in commercial buildings. Appl. Energy 2017, 201, 382–396. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, P.; Li, Z.; Ni, W.D. An engineering approach to the optimal design of distributed energy systems in China. Appl. Therm. Eng. 2013, 53, 387–396. [Google Scholar] [CrossRef]
- Mousavi, A.S.S.; Poncet, S.; Sedighi, K.; Aghajani, M.D. Numerical modeling of the melting process in a shell and coil tube ice storage system for air-conditioning application. Appl. Sci. 2019, 9, 26–27. [Google Scholar]
- Chen, H.J.; Wang, W.P.; Chen, S.L. Optimization of an ice-storage air conditioning system using dynamic programming method. Refrigeration 2005, 25, 461–472. [Google Scholar] [CrossRef]
- Lu, Y.H.; Wang, S.W.; Sun, Y.J.; Yan, C.C. Optimal scheduling of buildings with energy generation and thermal energy storage under dynamic electricity pricing using mixed-integer nonlinear programming. Appl. Energy 2015, 147, 49–58. [Google Scholar] [CrossRef]
- Morgan, S. Experimental analysis of optimal control of passive and active building thermal storage inventory. Diss. Theses-Grad Work. 2007, 3, 1429–1432. [Google Scholar]
- Candanedo, J.A.; Dehkordi, V.R.; Stylianou, M. Model-based predictive control of an ice storage device in a building cooling system. Appl. Energy 2013, 111, 1032–1045. [Google Scholar] [CrossRef]
- Antoine, G.; Julien, E.; Matthieu, G.; Stéphane, G. Predictive control of multizone heating ventilation and air-conditioning systems in non-residential buildings. Appl. Soft Comput. 2015, 37, 847–862. [Google Scholar]
- Wang, B.L.; Li, X.T.; Zhang, M.Y.; Yang, X.D. Experimental Investigation of Discharge Performance and Temperature Distribution of an External Melt Ice-on-Coil Ice Storage Tank. Hvac. R. Res. 2003, 9, 291–308. [Google Scholar] [CrossRef]
- Hamzeh, H.A.; Miansari, M. Numerical study of tube arrangement and fin effects on improving the ice formation in ice-on-coil thermal storage systems. Int. Commun. Heat Mass Transf. 2020, 113, 104520. [Google Scholar] [CrossRef]
- Huang, Y.P.; Sun, Q.; Yao, F.; Zhang, C.B. Performance optimization of a finned shell-and-tube ice storage unit. Appl. Therm. Eng. 2020, 167, 114788. [Google Scholar] [CrossRef]
- Hosseinzadeh, K.; Alizadeh, M.; Tavakoli, M.H.; Ganji, D.D. Investigation of phase change material solidification process in a LHTESS in the presence of fins with variable thickness and hybrid nanoparticles. Appl. Therm. Eng. 2019, 152, 706–717. [Google Scholar] [CrossRef]
- Tay, N.; Bruno, F.; Belusko, M. Comparison of pinned and finned tubes in a phase change thermal energy storage system using CFD. Appl. Energy 2013, 104, 79–86. [Google Scholar] [CrossRef]
- Li, H.Y.; Hu, C.Z.; He, Y.C.; Tang, D.W.; Wang, K.M. Influence of fin parameters on the melting behavior in a horizontal shell-and-tube latent heat storage unit with longitudinal fins. J. Energy Storage 2021, 34, 102230. [Google Scholar] [CrossRef]
- Zhou, L.; Wu, Z.G. Analysis of HTFs,PCMs and fins effects on the thermal performance of shell-tube thermal energy storage units. Sol. Energy 2015, 122, 382–395. [Google Scholar]
- Scanlon, T.J.; Stickland, M.T. An experimental and numerical investigation of natural convection melting. Int. Commun. Heat Mass Transf. 2001, 28, 181–190. [Google Scholar] [CrossRef]
- Dekhil, M.A.; Tala, J.V.S.; Bulliard-Sauret, O.; Bougeard, D. Numerical analysis of the performance enhancement of a latent heat storage shell and tube unit using finned tubes during melting and solidification. Appl. Therm. Eng. 2021, 192, 116866. [Google Scholar] [CrossRef]
- Voller, V.R.; Prakash, C. A fixed grid numerical modelling methodology for convection-diffusion mushy region phase-change problems. Int. J. Heat Mass Transf. 1987, 30, 1709–1719. [Google Scholar] [CrossRef]
- Jannesari, H.; Abdollahi, N. Experimental and numerical study of thin ring and annular fin effects on improving the ice formation in ice-on-coil thermal storage systems. Appl. Energy 2017, 189, 369–384. [Google Scholar] [CrossRef]
- Chen, J.S.; Chen, J.T.; Liu, C.W.; Liang, C.P.; Lin, C.W. Analytical solutions to two-dimensional advection–dispersion equation in cylindrical coordinates in finite domain subject to first-and third-type inlet boundary conditions. J. Hydrol. 2011, 405, 522–531. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, G.F.; Wang, Y.; Gao, P.H.; Fan, C.G.; Wang, Z.F. Solidification of an annular finned tube ice storage unit. Appl. Therm. Eng. 2022, 212, 118567. [Google Scholar] [CrossRef]
- Sang, W.H.; Lee, Y.T.; Chung, J.D.; Kim, S.T.; Kim, T.; Lee, K.H. Efficient numerical approach for simulating a full-scale vertical ice-on-coil type latent thermal storage tank. Int. Commun. Heat Mass Transf. 2016, 78, 29–38. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Chang, C.; Xu, X.Y.; Dong, J.S.; Zhao, M.Z.; Zhang, S.G. Effect of material characteristics on ice storage performance of an external melting ice-on-coil tube. Energy Proc. 2021, 24, 1–5. [Google Scholar]






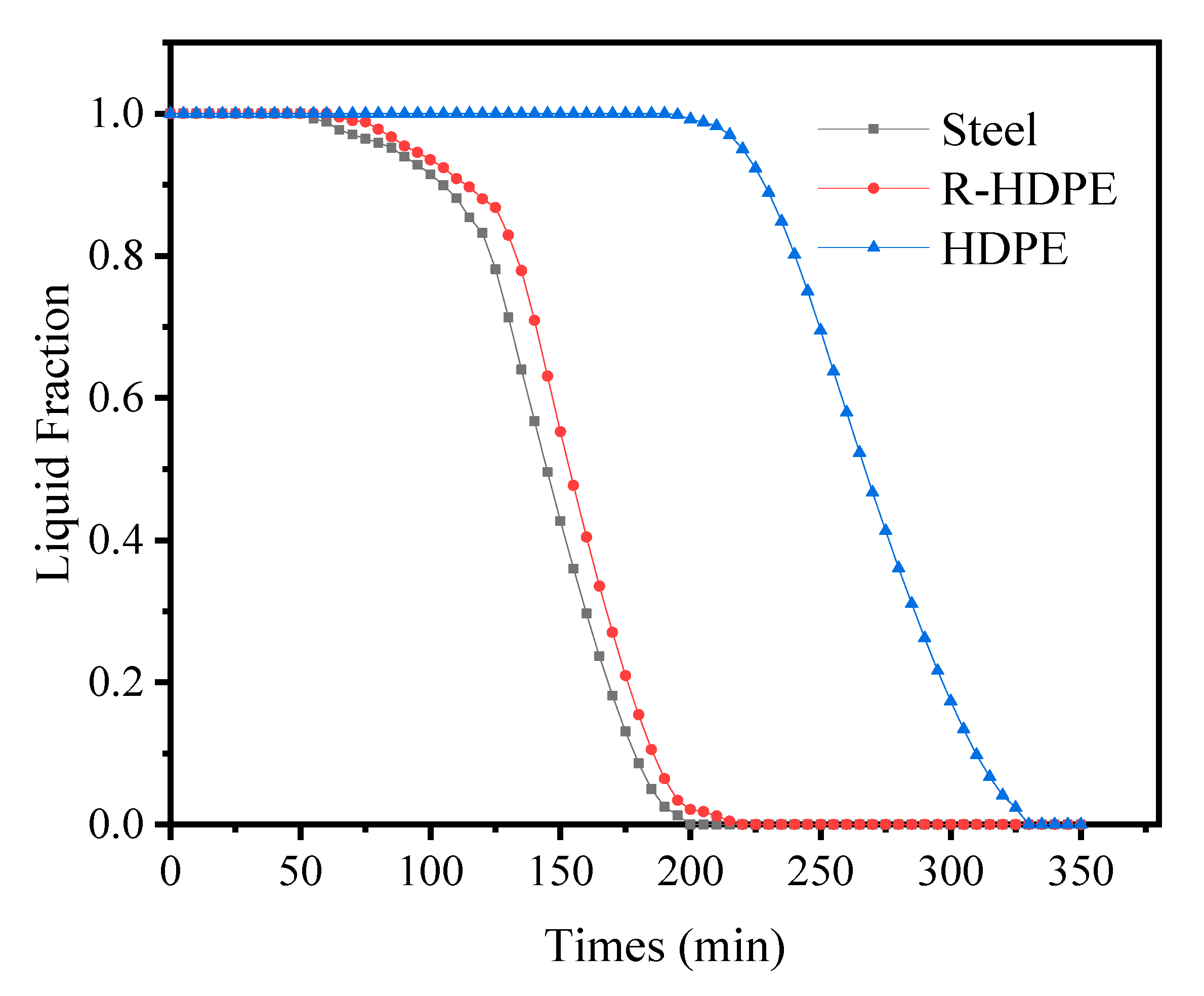
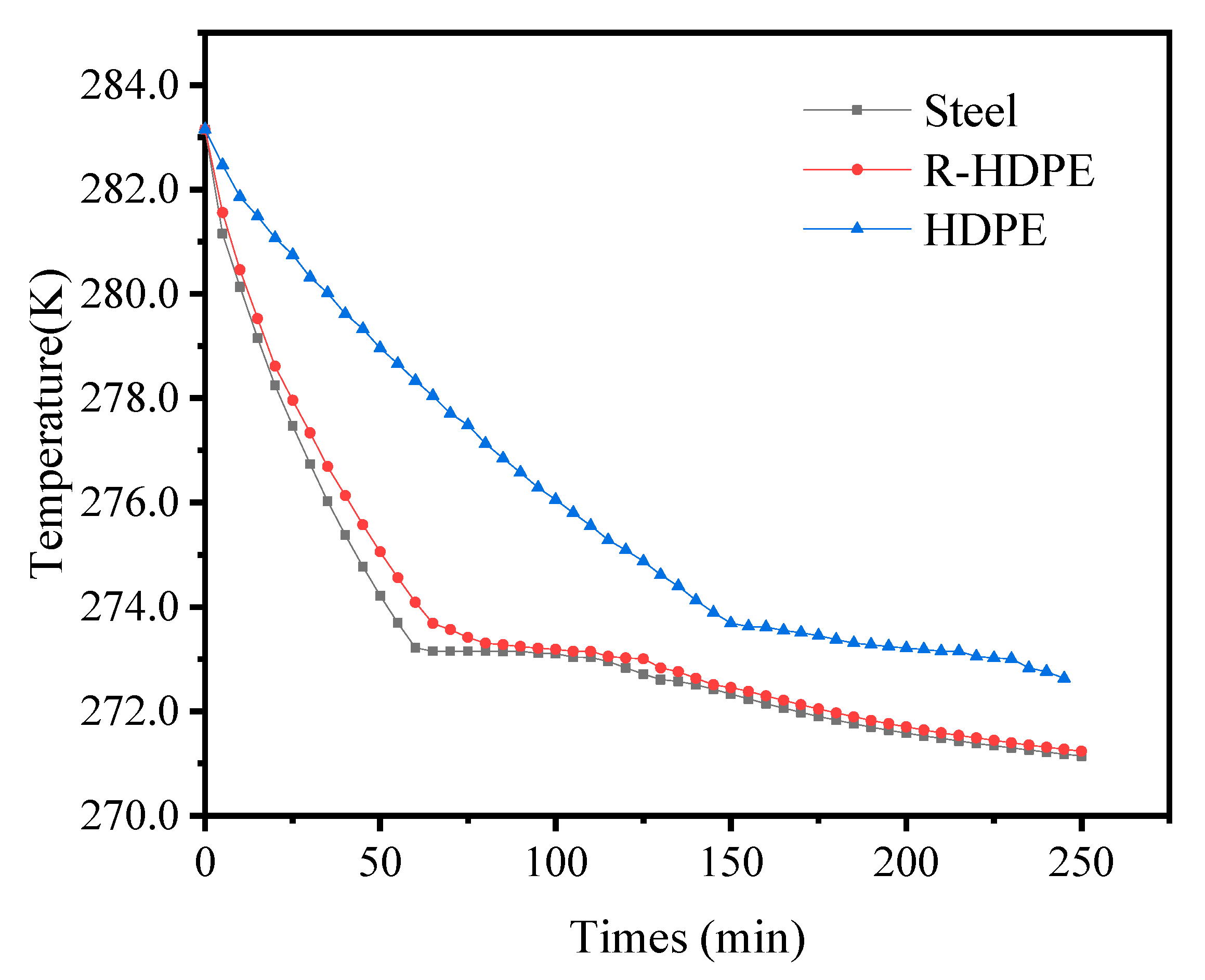
| Items | Symbols | Units | Values |
|---|---|---|---|
| Length of the ice storage | L | (mm) | 600.0 |
| Inner radius of the ice storage | r3 | (mm) | 50.0 |
| Outer radius of the coil | r2 | (mm) | 13.3 |
| Inner radius of the coil | r1 | (mm) | 11.2 |
| Initial temperature of ethylene glycol | T1 | (°C) | −6.0 |
| Flow rate of ethylene glycol | U | (m/s) | 0.5 |
| Initial water temperature | T2 | (°C) | 10.0 |
| Thickness of insulation layer | δ | (mm) | 20.0 |
| Items | λ[W/(m·K)] | Length [mm] | Thickness [mm] |
|---|---|---|---|
| HDPE | 0.2 | 600.00 | 2.00 |
| R-HDPE | 2.8 | 600.00 | 2.00 |
| Steel | 40.0 | 600.00 | 2.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Chang, C.; Guo, X.; Zhao, M. Experimental and Numerical Study of the Ice Storage Process and Material Properties of Ice Storage Coils. Energies 2023, 16, 5511. https://doi.org/10.3390/en16145511
Xu X, Chang C, Guo X, Zhao M. Experimental and Numerical Study of the Ice Storage Process and Material Properties of Ice Storage Coils. Energies. 2023; 16(14):5511. https://doi.org/10.3390/en16145511
Chicago/Turabian StyleXu, Xiaoyu, Chun Chang, Xinxin Guo, and Mingzhi Zhao. 2023. "Experimental and Numerical Study of the Ice Storage Process and Material Properties of Ice Storage Coils" Energies 16, no. 14: 5511. https://doi.org/10.3390/en16145511
APA StyleXu, X., Chang, C., Guo, X., & Zhao, M. (2023). Experimental and Numerical Study of the Ice Storage Process and Material Properties of Ice Storage Coils. Energies, 16(14), 5511. https://doi.org/10.3390/en16145511