Microstructure and First Hydrogenation Properties of Ti16V60Cr24−xFex + 4 wt.% Zr Alloy for x = 0, 4, 8, 12, 16, 20, 24
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
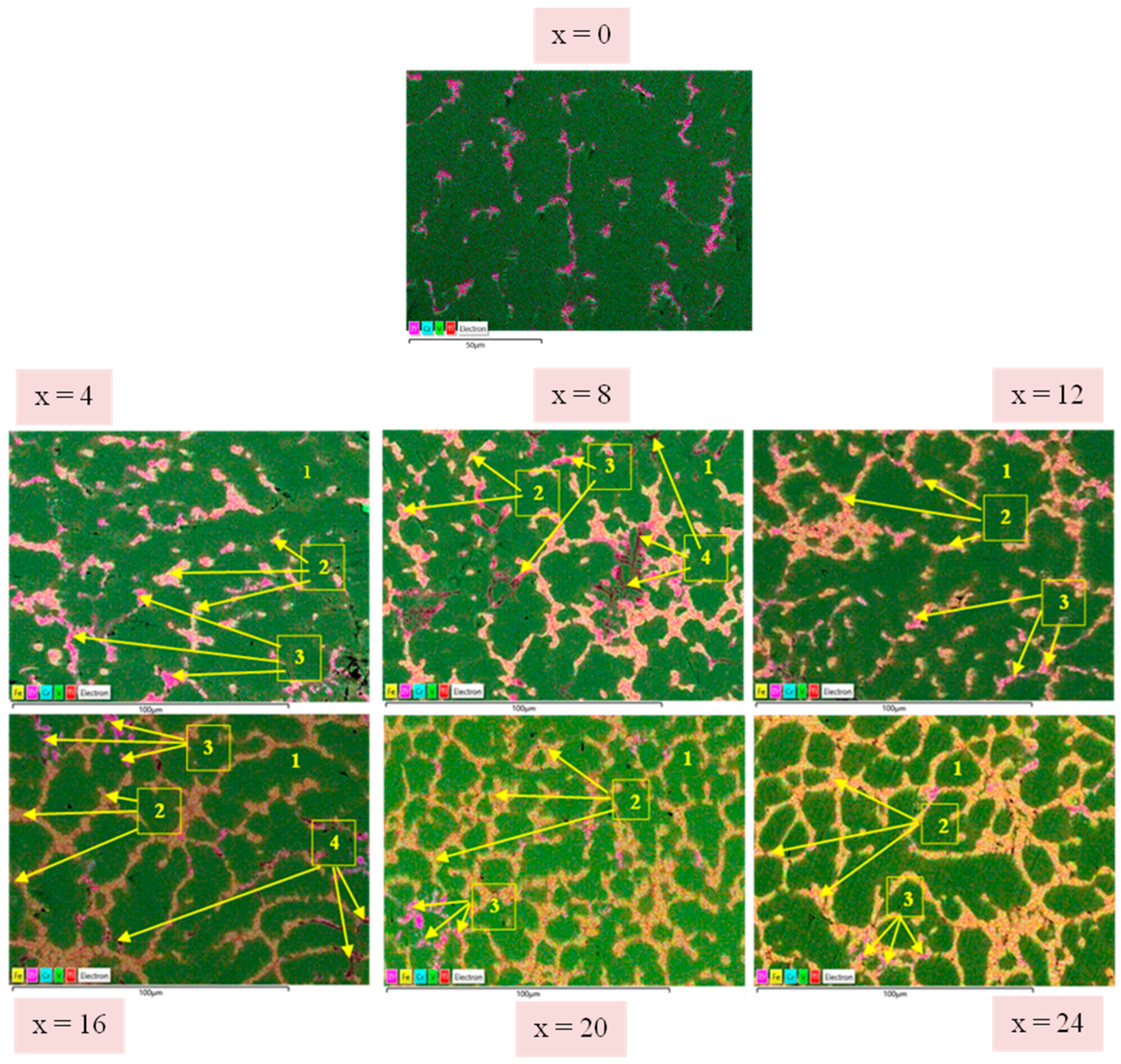
3.1. Microstructure
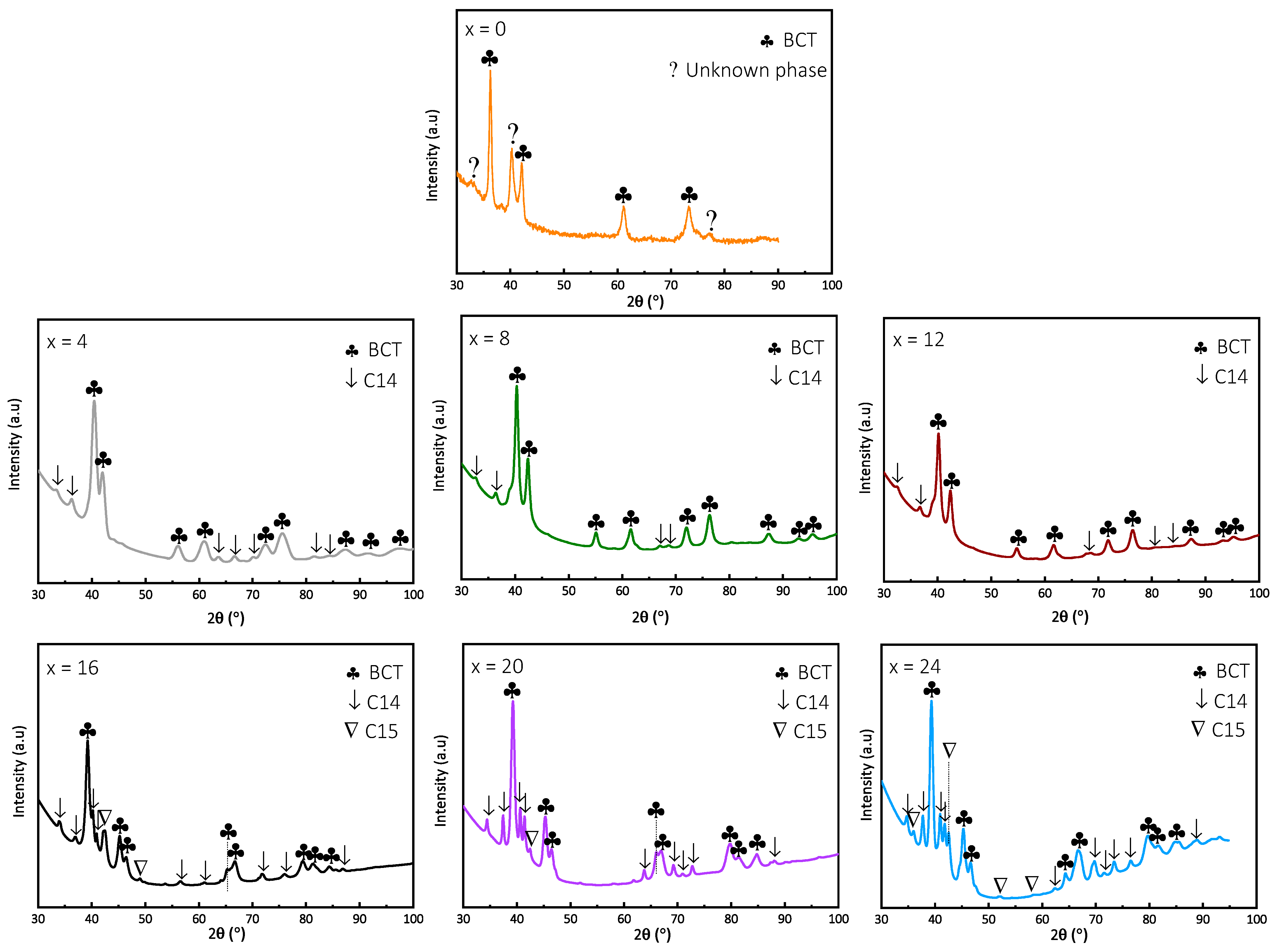
3.2. Crystal Structure
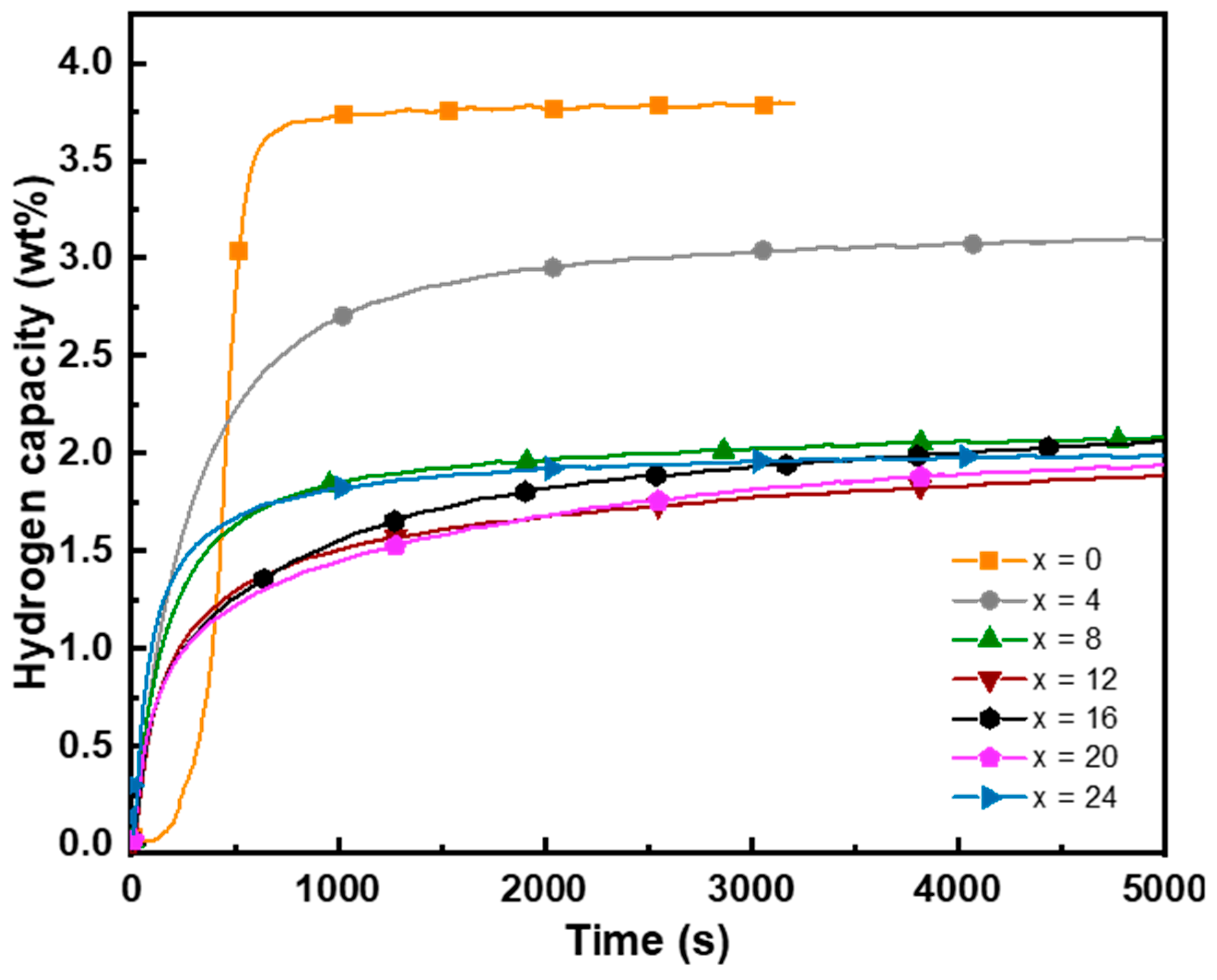
3.3. First Hydrogenation
4. Conclusions
- -
- All alloys presented a two-phase microstructure: a matrix and a secondary phase.
- -
- The as-cast XRD patterns showed a single BCC phase for x = 0 to 8 and a BCC with a C14 Laves phase for x = 12 to 24. The lattice parameter of the BCC phase linearly decreased with increasing Fe proportion. This is in accordance with Vegard’s law.
- -
- The first hydrogenation of all alloys at 298 K under 3 MPa revealed a decrease in the hydrogen capacity with an increasing proportion of Fe. This trend is explained by the reduction in the BCC lattice parameter and the increase in the electron-to-atom ratio due to the presence of Fe.
- -
- The XRD patterns of hydride alloys revealed a C14 phase in addition to the BCC phase for x = 4 and 8, and a C15 phase in addition to BCC and C14 for x = 16, 20, 24. The absence of Laves in the as-cast state is still misunderstood but neutron diffraction measurements are planned to better investigate and understand the crystal structure of all studied alloys.
- -
- According to the present work on the vanadium-rich Ti16V60Cr24−xFex for x = 0, 4, 8, 12, 16, 20, 24 alloys, we found that the presence of Fe enhanced the kinetic but lowered the maximum hydrogen capacity. For x higher than 4 at.%, there is a loss of almost 50% of the hydrogen capacity. Consequently, the reversible capacity will also be low. In the future, it would be interesting to only focus on x ≤ 4 alloys. Just using a small proportion of iron will stabilize the hydride while maintaining a high hydrogen capacity. Thermodynamics will be studied in a future investigation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Akiba, E. Hydrogen-Absorbing Alloys. Curr. Opin. Solid State Mater. Sci. 1999, 4, 267–272. [Google Scholar] [CrossRef]
- Edalati, K.; Akiba, E.; Horita, Z. High-Pressure Torsion for New Hydrogen Storage Materials. Sci. Technol. Adv. Mater. 2018, 19, 185–193. [Google Scholar] [CrossRef]
- Akiba, E.; Okada, M. M Etallic Hydrides III: Cubic Solid-Solution Alloys. MRS Bull. 2002, 27, 699–703. [Google Scholar] [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E.; Li, H. wen Hydrogen—A Sustainable Energy Carrier. Prog. Nat. Sci. Mater. Int. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- Bibienne, T.; Bobet, J.L.; Huot, J. Crystal Structure and Hydrogen Storage Properties of Body Centered Cubic 52Ti-12V-36Cr Alloy Doped with Zr7Ni10. J. Alloys Compd. 2014, 607, 251–257. [Google Scholar] [CrossRef]
- Towata, S.I.; Noritake, T.; Itoh, A.; Aoki, M.; Miwa, K. Cycle Durability of Ti-Cr-V Alloys Partially Substituted by Nb or Fe. J. Alloys Compd. 2013, 580, S226–S228. [Google Scholar] [CrossRef]
- Selvaraj, S.; Ichikawa, T.; Jain, A.; Kumar, S.; Zhang, T.; Miyaoka, H.; Kojima, Y.; Isobe, S. Study of Cyclic Performance of V-Ti-Cr Alloys Employed for Hydrogen Compressor. Int. J. Hydrogen Energy 2018, 43, 2881–2889. [Google Scholar] [CrossRef]
- Lototsky, M.V.; Yartys, V.A.; Zavaliy, I.Y. Vanadium-Based BCC Alloys: Phase-Structural Characteristics and Hydrogen Sorption Properties. J. Alloys Compd. 2005, 404–406, 421–426. [Google Scholar] [CrossRef]
- Shashikala, K.; Kumar, A.; Betty, C.A.; Banerjee, S.; Sengupta, P.; Pillai, C.G.S. Improvement of the Hydrogen Storage Properties and Electrochemical Characteristics of Ti0.85VFe0.15 Alloy by Ce Substitution. J. Alloys Compd. 2011, 509, 9079–9083. [Google Scholar] [CrossRef]
- Cao, Z.; Ouyang, L.; Wang, H.; Liu, J.; Sun, L.; Zhu, M. Composition Design of Ti-Cr-Mn-Fe Alloys for Hybrid High-Pressure Metal Hydride Tanks. J. Alloys Compd. 2015, 639, 452–457. [Google Scholar] [CrossRef]
- Cho, S.W.; Han, C.S.; Park, C.N.; Akiba, E. Hydrogen Storage Characteristics of Ti-Cr-V Alloys. J. Alloys Compd. 1999, 288, 294–298. [Google Scholar] [CrossRef]
- Yu, X.B.; Yang, Z.X.; Feng, S.L.; Wu, Z.; Xu, N.X. Influence of Fe Addition on Hydrogen Storage Characteristics of Ti-V-Based Alloy. Int. J. Hydrogen Energy 2006, 31, 1176–1181. [Google Scholar] [CrossRef]
- Luo, L.; Bian, X.; Wu, W.-Y.; Yuan, Z.-M.; Li, Y.-Z.; Zhai, T.-T.; Hu, F. Influence of annealing on microstructure and hydrogen storage properties of V48Fe12Ti15Cr25 alloy. J. Iron Steel Res. Int. 2020, 27, 217–227. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Ichikawa, T.; Kojima, Y.; Dey, G.K. Development of Vanadium Based Hydrogen Storage Material: A Review. Renew. Sustain. Energy Rev. 2017, 72, 791–800. [Google Scholar] [CrossRef]
- Shashikala, K.; Banerjee, S.; Kumar, A.; Pai, M.R.; Pillai, C.G.S. Improvement of Hydrogen Storage Properties of TiCrV Alloy by Zr Substitution for Ti. Int. J. Hydrogen Energy 2009, 34, 6684–6689. [Google Scholar] [CrossRef]
- Towata, S.I.; Noritake, T.; Itoh, A.; Aoki, M.; Miwa, K. Effect of Partial Niobium and Iron Substitution on Short-Term Cycle Durability of Hydrogen Storage Ti-Cr-V Alloys. Int. J. Hydrogen Energy 2013, 38, 3024–3029. [Google Scholar] [CrossRef]
- Bibienne, T.; Gosselin, C.; Bobet, J.L.; Huot, J. Replacement of Vanadium by Ferrovanadium in a Ti-Based Body Centred Cubic (BCC) Alloy: Towards a Low-Cost Hydrogen Storage Material. Appl. Sci. 2018, 8, 1151. [Google Scholar] [CrossRef]
- Dixit, V.; Huot, J. Investigation of the Microstructure, Crystal Structure and Hydrogenation Kinetics of Ti-V-Cr Alloy with Zr Addition. J. Alloys Compd. 2019, 785, 1115–1120. [Google Scholar] [CrossRef]
- Sleiman, S.; Huot, J. Microstructure and Hydrogen Storage Properties of Ti1V0.9Cr1.1 Alloy with Addition of x Wt % Zr (x = 0, 2, 4, 8, and 12). Inorganics 2017, 5, 86. [Google Scholar] [CrossRef]
- Abdul, J.M.; Chown, L.H.; Odusote, J.K.; Nei, J.; Young, K.H.; Olayinka, W.T. Hydrogen Storage Characteristics and Corrosion Behavior of Ti24V40C34Fe2 Alloy. Batteries 2017, 3, 19. [Google Scholar] [CrossRef]
- Tamura, T.; Tominaga, Y.; Matsumoto, K.; Fuda, T.; Kuriiwa, T.; Kamegawa, A.; Takamura, H.; Okada, M. Protium Absorption Properties of Ti-V-Cr-Mn Alloys with a b.c.c. Structure. J. Alloys Compd. 2002, 330–332, 522–525. [Google Scholar] [CrossRef]
- Taizhong, H.; Zhu, W.; Jinzhou, C.; Xuebin, Y.; Baojia, X.; Naixin, X. Dependence of Hydrogen Storage Capacity of TiCr1.8−X(VFe)X on V-Fe Content. Mater. Sci. Eng. A 2004, 385, 17–21. [Google Scholar] [CrossRef]
- Yu, X.B.; Wu, Z.; Xia, B.J.; Xu, N.X. Enhancement of Hydrogen Storage Capacity of Ti-V-Cr-Mn BCC Phase Alloys. J. Alloys Compd. 2004, 372, 272–277. [Google Scholar] [CrossRef]
- Hang, Z.; Xiao, X.; Tan, D.; He, Z.; Li, W.; Li, S.; Chen, C.; Chen, L. Microstructure and Hydrogen Storage Properties of Ti10V84−xFe6Zrx (x = 1–8) Alloys. Int. J. Hydrogen Energy 2010, 35, 3080–3086. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Jiang, X.; Li, S.; Li, X. Hydrogen Storage Performances, Kinetics and Microstructure of Ti1.02Cr1.0Fe0.7−XMn0.3Alx Alloy by Al Substituting for Fe. Renew. Energy 2020, 153, 1140–1154. [Google Scholar] [CrossRef]
- Charbonnier, V.; Enoki, H.; Asano, K.; Kim, H.; Sakaki, K. Tuning the Hydrogenation Properties of Ti1+yCr2−XMnx Laves Phase Compounds for High Pressure Metal-Hydride Compressors. Int. J. Hydrogen Energy 2021, 46, 36369–36380. [Google Scholar] [CrossRef]
- Yoo, J.H.; Shim, G.; Park, C.N.; Kim, W.B.; Cho, S.W. Influence of Mn or Mn plus Fe on the Hydrogen Storage Properties of the Ti-Cr-V Alloy. Int. J. Hydrogen Energy 2009, 34, 9116–9121. [Google Scholar] [CrossRef]
- Aoki, M.; Noritake, T.; Ito, A.; Ishikiriyama, M.; Towata, S.I. Improvement of Cyclic Durability of Ti-Cr-V Alloy by Fe Substitution. Int. J. Hydrogen Energy 2011, 36, 12329–12332. [Google Scholar] [CrossRef]
- Cho, S.W.; Enoki, H.; Akiba, E. Effect of Fe Addition on Hydrogen Storage Characteristics of Ti0.16Zr0.05Cr0.22V0.57 Alloy. J. Alloys Compd. 2000, 307, 304–310. [Google Scholar] [CrossRef]
- Nygård, M.M.; Sørby, M.H.; Grimenes, A.A.; Hauback, B.C. The Influence of Fe on the Structure and Hydrogen Sorption Properties of Ti-V-Based Metal Hydrides. Energies 2020, 13, 2874. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, P.; Xiao, X.; Zhan, L.; Li, Z.; Wang, S.; Chen, L. Investigation on Ti–Zr–Cr–Fe–V Based Alloys for Metal Hydride Hydrogen Compressor at Moderate Working Temperatures. Int. J. Hydrogen Energy 2021, 46, 21580–21589. [Google Scholar] [CrossRef]
- Ravalison, F.; Rabkin, E.; Huot, J. Methods to Improve the First Hydrogenation of the Vanadium-Rich BCC Alloy Ti16V60Cr24. Hydrogen 2022, 3, 303–311. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for Microscopy. BioTechniques 2007, 43, S25–S30. [Google Scholar] [CrossRef] [PubMed]
- Bruker, A.X. TOPAS V3: General Profile and Structure Analysis Software for Powder Diffraction Data. In User’s Manual Bruker AXS; Bruker: Karlsruhe, Germany, 2005. [Google Scholar]
- Broom, D.P. Hydrogen Storage Materials The Characterisation of Their Storage Properties, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 9780857292209. [Google Scholar]
- Pearson, W.B. The Crystal Chemistry and Physics of Metals and Alloys; Wiley Interscience: Hoboken, NJ, USA, 1972. [Google Scholar]
- Stein, F.; Leineweber, A. Laves Phases: A Review of Their Functional and Structural Applications and an Improved Fundamental Understanding of Stability and Properties. J. Mater. Sci. 2021, 56, 5321–5427. [Google Scholar] [CrossRef]
- Hynninen, A.P.; Filion, L.; Dijkstra, M. Stability of LS and L S2 Crystal Structures in Binary Mixtures of Hard and Charged Spheres. J. Chem. Phys. 2009, 131, 064902. [Google Scholar] [CrossRef]
- Grytsiv, A.; Chen, X.Q.; Witusiewicz, V.T.; Rogl, P.; Podloucky, R.; Pomjakushin, V.; Maccio, D.; Saccone, A.; Giester, G.; Sommer, F. Atom Order and Thermodynamic Properties of the Ternary Laves Phase Ti(TiyNixAl1−x−y)2. Z. Fur Krist. 2006, 221, 334–348. [Google Scholar] [CrossRef]
- Peisl, H. Lattice Strains Due to Hydrogen in Metals. In Hydrogen in Metals I, Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 1978; Volume 28, ISBN 978-3-540-08705-2. [Google Scholar]
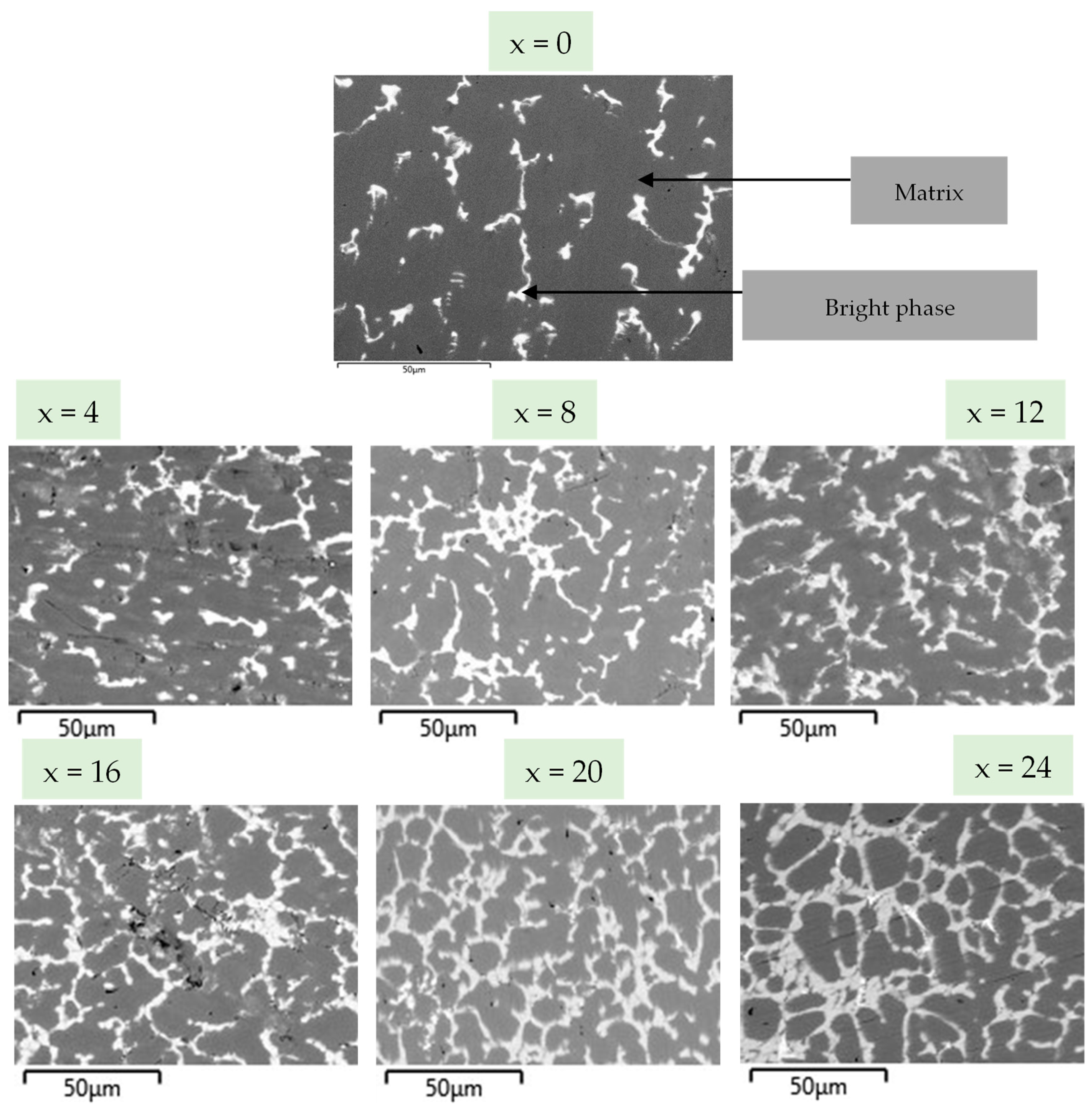


| x | 0 | 4 | 8 | 12 | 16 | 20 | 24 |
|---|---|---|---|---|---|---|---|
| Matrix | 93 | 87 | 82 | 83 | 81 | 66 | 72 |
| Secondary phase | 7 | 13 | 18 | 17 | 19 | 34 | 28 |
| x | Ti (at.%) | V (at.%) | Cr (at.%) | Fe (at.%) | Zr (at.%) |
|---|---|---|---|---|---|
| 0 | 14.2 | 62.4 | 22.9 | - | 0.5 |
| 4 | 15.9 | 60.2 | 19.3 | 4.1 | 0.5 |
| 8 | 15.2 | 60.2 | 16.1 | 7.9 | 0.6 |
| 12 | 14.1 | 60.2 | 13.1 | 12.4 | 0.2 |
| 16 | 13.5 | 61.7 | 8.3 | 16.2 | 0.3 |
| 20 | 14.2 | 60.7 | 4.2 | 20.6 | 0.3 |
| 24 | 10.4 | 70.4 | - | 19 | 0.2 |
| x | Ti (at.%) | V (at.%) | Cr (at.%) | Fe (at.%) | Zr (at.%) |
|---|---|---|---|---|---|
| 0 | 33.8 | 5.1 | 1.8 | - | 59.3 |
| 4 | 32.9 | 20.1 | 7.1 | 1.8 | 38.1 |
| 8 | 32.5 | 17.0 | 5.1 | 10.4 | 35.0 |
| 12 | 32.2 | 15.7 | 3.6 | 12.5 | 36.0 |
| 16 | 32.3 | 11.5 | 1.8 | 8.4 | 46.0 |
| 20 | 28.9 | 20.7 | 1.3 | 6.3 | 42.8 |
| 24 | 31.3 | 13.4 | - | 10 | 45.3 |
| x | Ti (at.%) | V (at.%) | Cr (at.%) | Fe (at.%) | Zr (at.%) |
|---|---|---|---|---|---|
| 4 | 19.2 | 28.7 | 16.5 | 15.4 | 20.2 |
| 8 | 23.7 | 27.9 | 10.4 | 20.5 | 17.5 |
| 12 | 33.7 | 20.6 | 7.5 | 25.6 | 12.6 |
| 16 | 28 | 29.3 | 4.1 | 28.2 | 10.4 |
| 20 | 25.1 | 30.8 | 2.3 | 32.4 | 9.4 |
| 24 | 26.1 | 31.5 | - | 33.8 | 8.6 |
| x | Ti (at.%) | V (at.%) | Cr (at.%) | Fe (at.%) | Zr (at.%) |
|---|---|---|---|---|---|
| 8 | 64 | 14.9 | 3.9 | 1.3 | 15.9 |
| 16 | 63.4 | 14.8 | 1.8 | 4.4 | 15.6 |
| x | 0 | 4 | 8 | 12 | 16 | 20 | 24 |
|---|---|---|---|---|---|---|---|
| BCC | 100 | 100 | 100 | 79 (2) | 77 (3) | 74 (2) | 72 (3) |
| C14 | - | - | - | 21 (4) | 23 (3) | 26 (2) | 28 (3) |
| BCC | Lattice Parameter (Å) | Crystallite Size (nm) | Microstrain (%) | Cell Volume (Å3) |
|---|---|---|---|---|
| x = 0 | 3.0331 (4) | 36 (2) | 1.08 (2) | 27.90 (2) |
| x = 4 | 3.0207 (1) | 23 (1) | 0.98 (3) | 27.56 (1) |
| x = 8 | 3.0162 (1) | 21 (1) | 0.71 (3) | 27.43 (2) |
| x = 12 | 3.0105 (1) | 22 (1) | 0.60 (4) | 27.28 (2) |
| x = 16 | 3.0052 (1) | 33 (2) | 0.78 (3) | 27.14 (1) |
| x = 20 | 2.9943 (1) | 29 (1) | 0.66 (3) | 26.98 (2) |
| x = 24 | 2.9990 (2) | 30 (1) | 0.46 (2) | 26.97 (5) |
| C14 | Lattice Parameter (Å) | Crystallite Size (nm) | Cell Volume (Å3) |
|---|---|---|---|
| x = 12 | a = 5.0280 (3) c = 8.1850 (2) | 22 (1) | 179.42 (4) |
| x = 16 | a = 4.9833 (2) c = 8.1426 (2) | 25 (2) | 175.10 (2) |
| x = 20 | a = 4.9629 (1) c = 8.1033 (2) | 46 (4) | 172.84 (2) |
| x = 24 | a = 4.9610 (1) c = 8.0987 (2) | 38 (2) | 172.62 (5) |
| Sample | Phase | Lattice Parameter (Å) | Crystallite Size (nm) | Microstrain (%) | Cell Volume (Å3) | Abundance (%) |
|---|---|---|---|---|---|---|
| x = 0 | BCT | a = 3.0350 (1) c = 4.2640 (2) | 40 (1) | 1.09 | 39.28 (3) | 100 |
| x = 4 | BCT | a = 3.0557 (1) c = 3.2896 (2) | 40 (1) | 1.09 | 30.64 (3) | 80 (2) |
| C14 | a = 5.3355 (1) c = 8.8310 (1) | 11 (1) | - | 217.72 (2) | 20 (1) | |
| x = 8 | BCT | a = 3.0068 (1) c = 3.2896 (2) | 17 (1) | 0.70 (1) | 30.08 (3) | 78 (1) |
| C14 | a = 5.3355 (1) c = 8.8310 (1) | 13 (1) | - | 222.85 (3) | 22 (1) | |
| x = 12 | BCT | a = 2.9943(1) c = 3.3338 (1) | 15 (1) | 0.70 (1) | 29.89 (1) | 76 (1) |
| C14 | a = 5.4525 (2) c = 8.4845 (1) | 12 (1) | - | 218.45 (2) | 24 (1) | |
| x = 16 | BCT | a = 2.8302 (1) c = 3.9134 (2) | 20 (1) | 1.07 (1) | 31.35 (2) | 58 (3) |
| C14 | a = 5.2568 (2) c = 8.600 (4) | 24 (1) | - | 205.81 (2) | 24 (2) | |
| C15 | a = 7.060 (2) | 16 (1) | 0.40 (2) | 352 (3) | 18 (2) | |
| x = 20 | BCT | a = 2.8279 (1) c = 3.9014 (2) | 28 (2) | 1.30 (2) | 31.20 (2) | 64 (3) |
| C14 | a = 5.1957 (2) c = 8.497 (3) | 30 (1) | - | 198.65 (1) | 23 (2) | |
| C15 | a = 7.159 (2) | 10 (2) | - | 367 (2) | 13 (3) | |
| x = 24 | BCT | a = 2.8292 (1) c = 3.8906 (2) | 40 (4) | 1.39 (4) | 31.14 (2) | 53 (4) |
| C14 | a = 5.1957 (2) c = 8.497 (3) | 30 (1) | - | 194.47 (5) | 37 (4) | |
| C15 | a = 7.042 (2) | 20 (3) | - | 349.2 (3) | 11 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravalison, F.; Huot, J. Microstructure and First Hydrogenation Properties of Ti16V60Cr24−xFex + 4 wt.% Zr Alloy for x = 0, 4, 8, 12, 16, 20, 24. Energies 2023, 16, 5360. https://doi.org/10.3390/en16145360
Ravalison F, Huot J. Microstructure and First Hydrogenation Properties of Ti16V60Cr24−xFex + 4 wt.% Zr Alloy for x = 0, 4, 8, 12, 16, 20, 24. Energies. 2023; 16(14):5360. https://doi.org/10.3390/en16145360
Chicago/Turabian StyleRavalison, Francia, and Jacques Huot. 2023. "Microstructure and First Hydrogenation Properties of Ti16V60Cr24−xFex + 4 wt.% Zr Alloy for x = 0, 4, 8, 12, 16, 20, 24" Energies 16, no. 14: 5360. https://doi.org/10.3390/en16145360
APA StyleRavalison, F., & Huot, J. (2023). Microstructure and First Hydrogenation Properties of Ti16V60Cr24−xFex + 4 wt.% Zr Alloy for x = 0, 4, 8, 12, 16, 20, 24. Energies, 16(14), 5360. https://doi.org/10.3390/en16145360







