Effects of Reservoir Heterogeneity on CO2 Dissolution Efficiency in Randomly Multilayered Formations
Abstract
1. Introduction
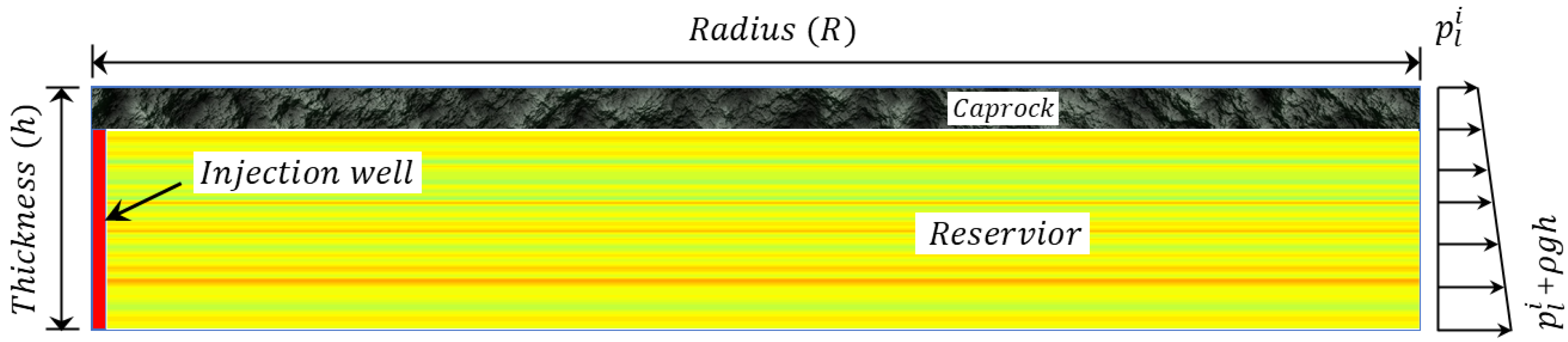
2. Mathematical Model
2.1. Extended Reaction System
2.2. Mass Transport Equations
2.3. Constitutive Equation
3. Numerical Implementation
Newton-Raphson Iteration
4. Numerical Simulation
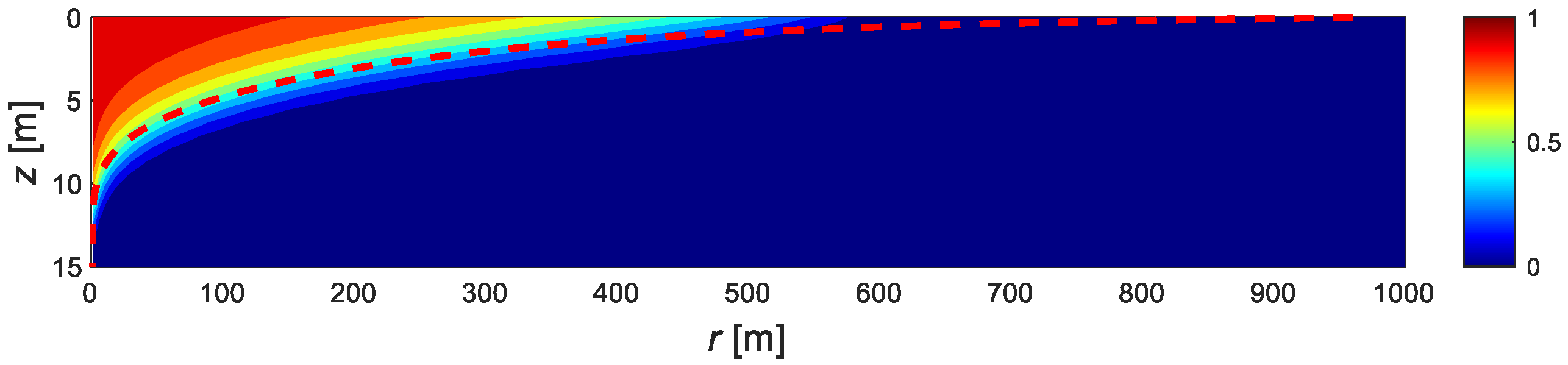
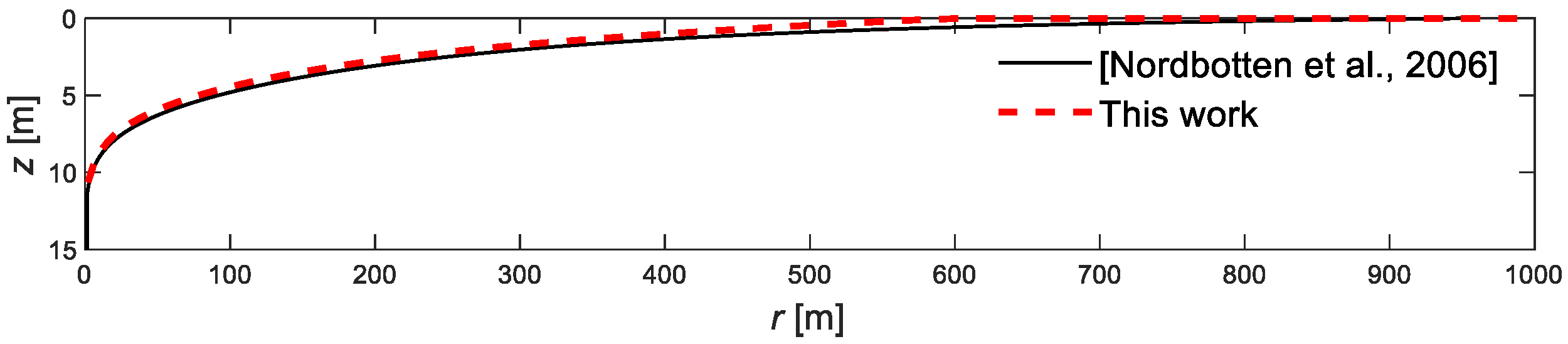
4.1. Model Validation
4.2. Effect of Pressure
4.3. Spatial Mobility and Distribution of CO2
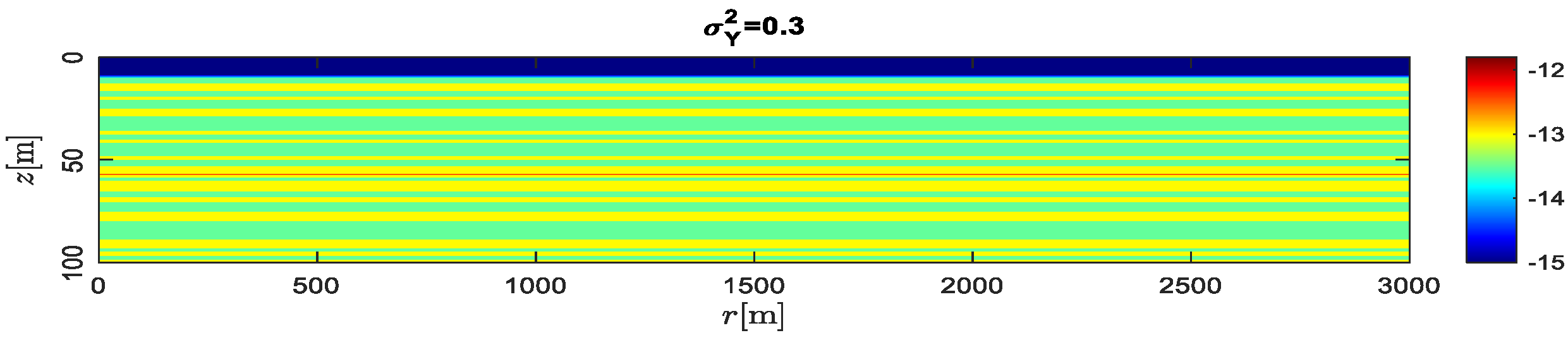
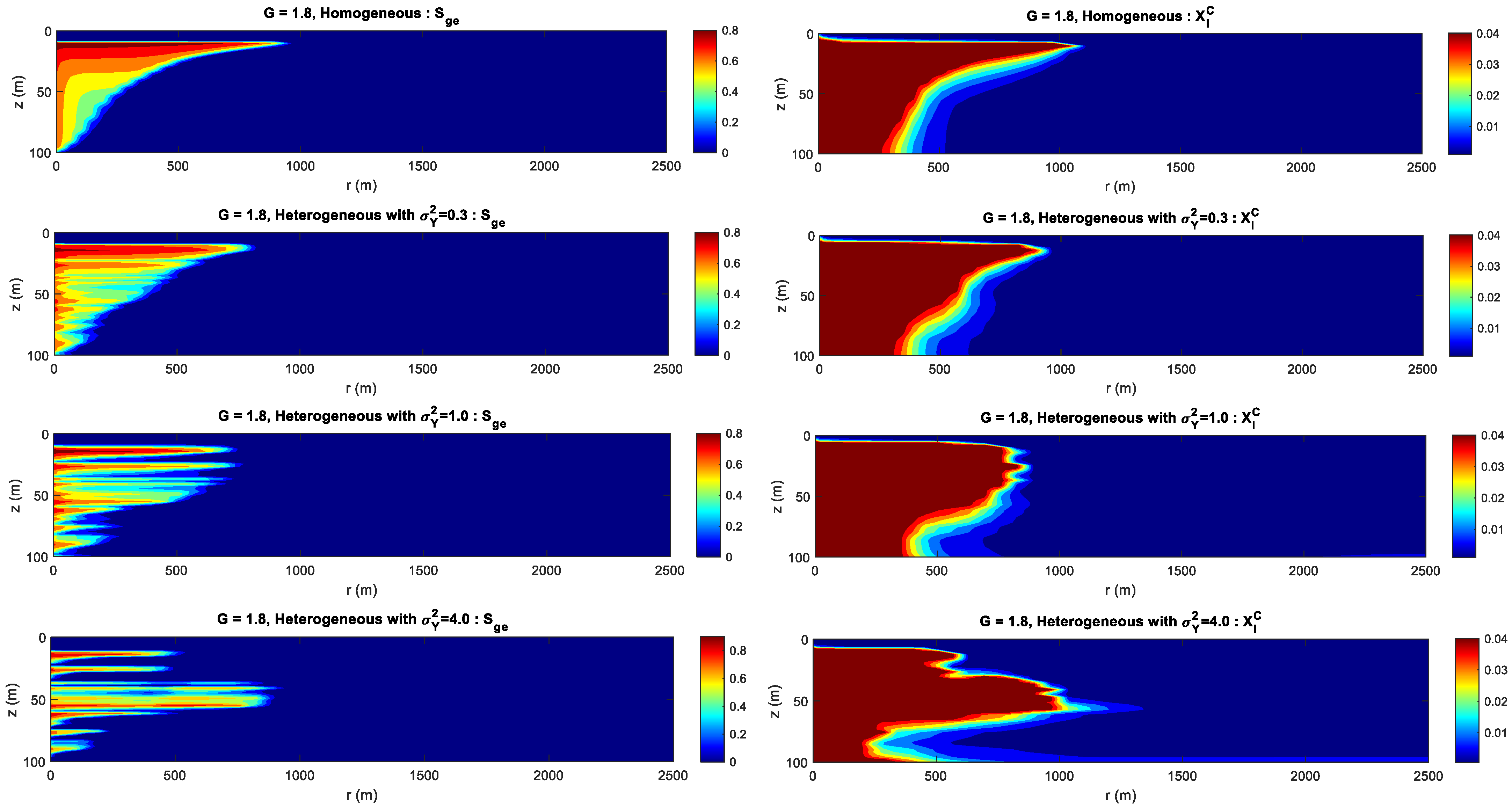
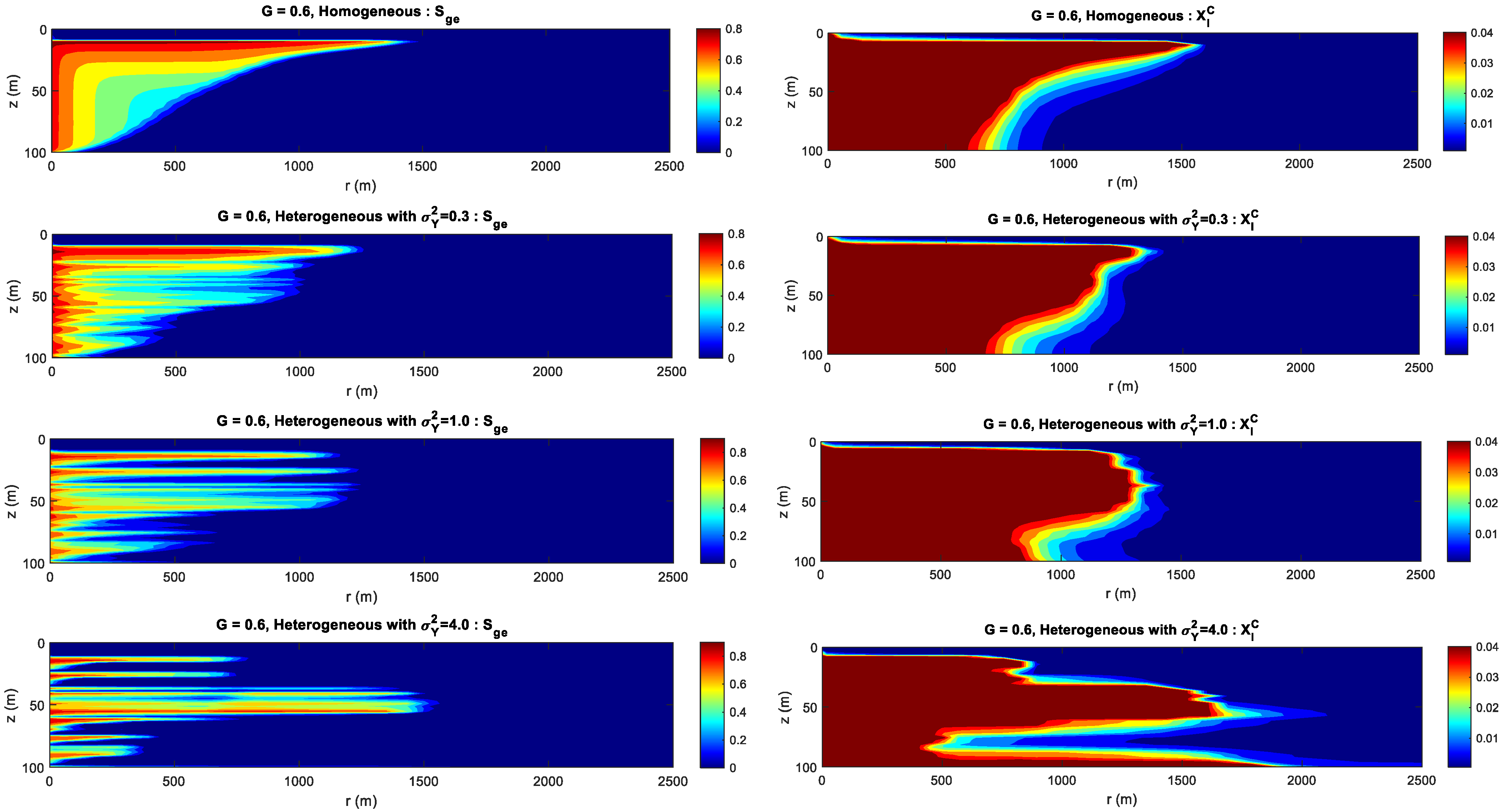
4.4. Effect of Heterogeneity and Gravity Index
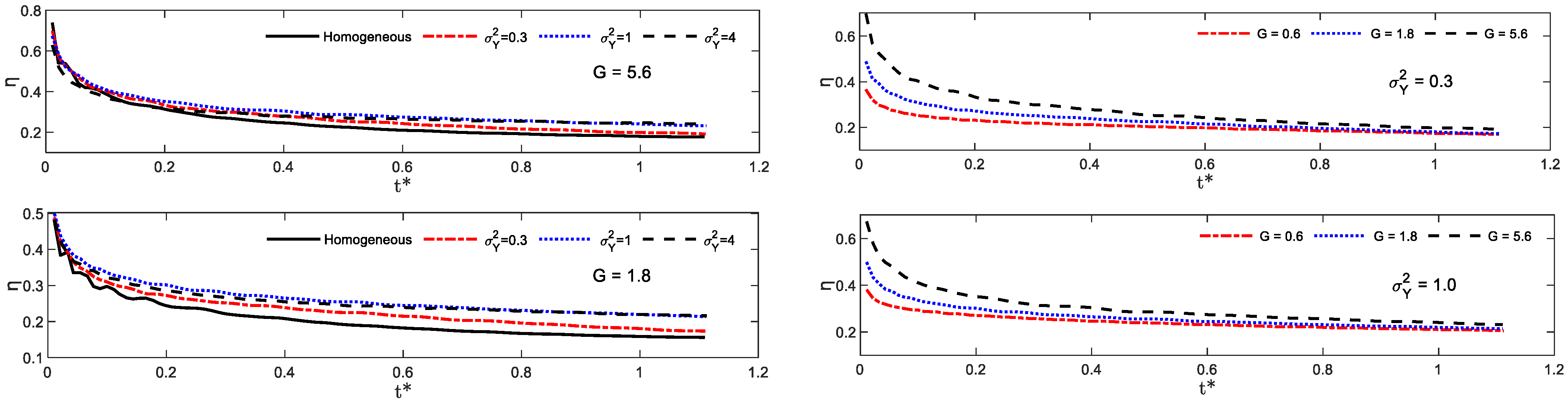
4.5. CO2 Dissolution Efficiency
5. Conclusions
- (1)
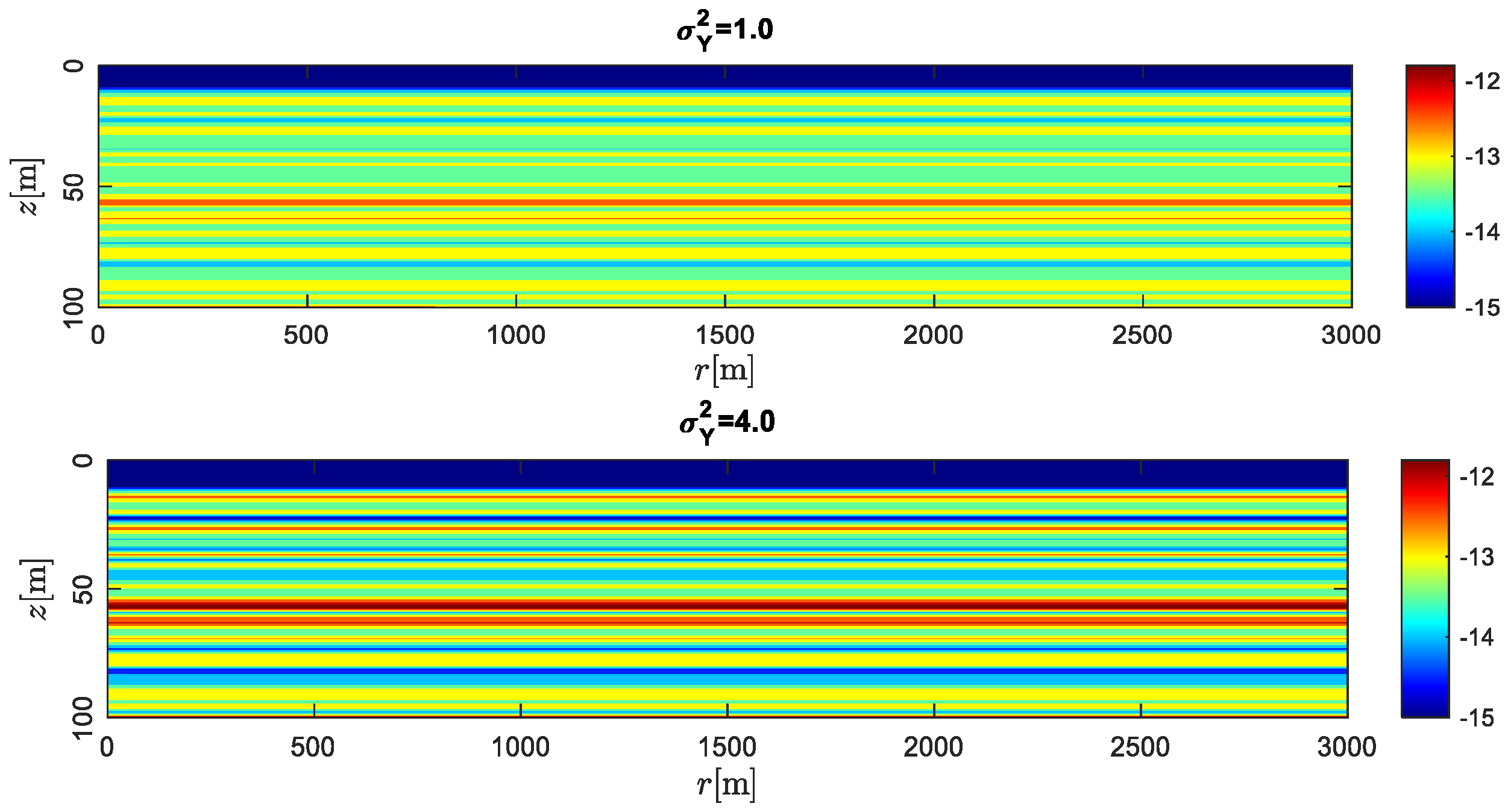
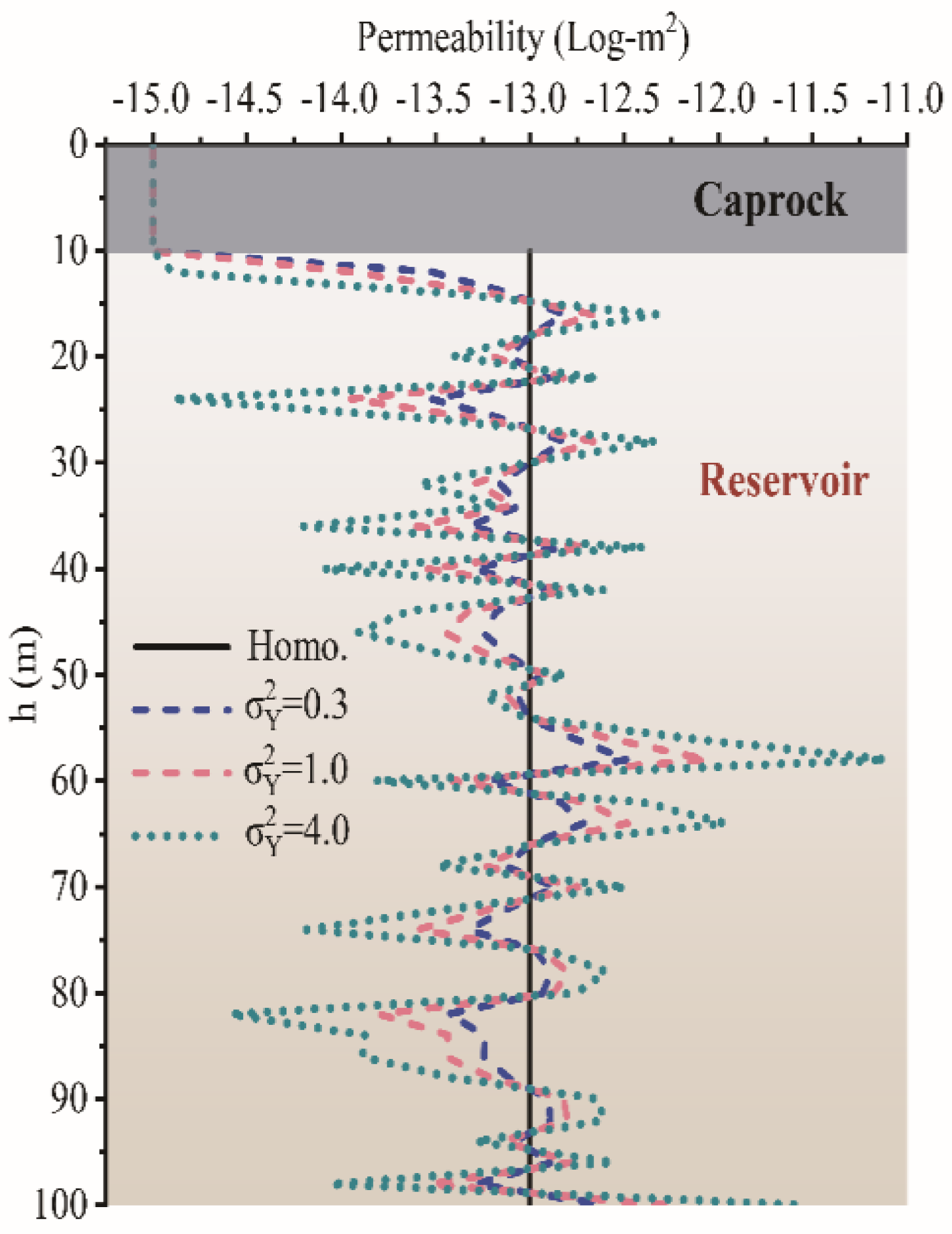
- The permeability heterogeneity is the primary factor influencing the spatial mobility and distribution of CO2 injection. Heterogeneity variances is considered to be an ideal representation of reservoir permeability.
- (2)
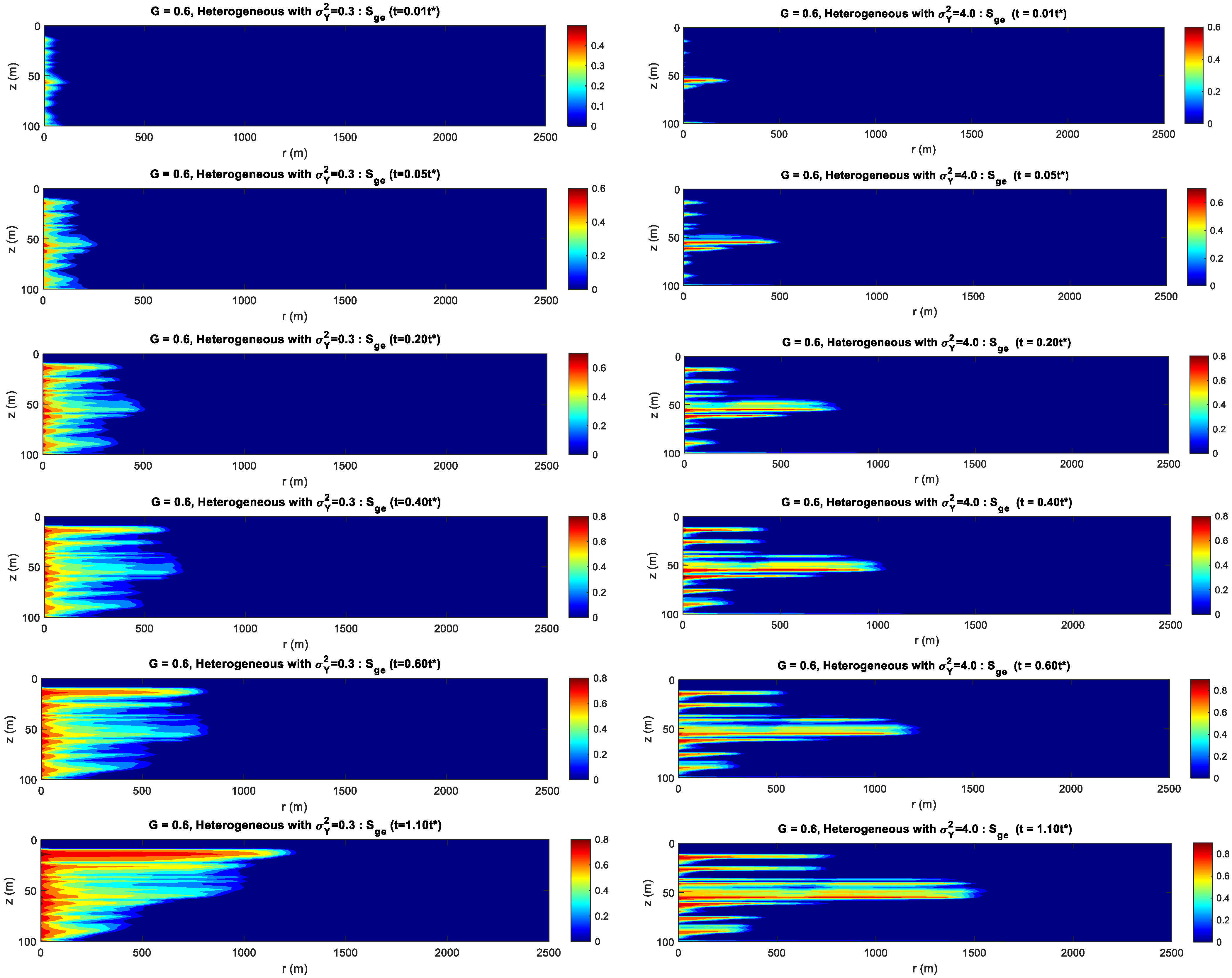
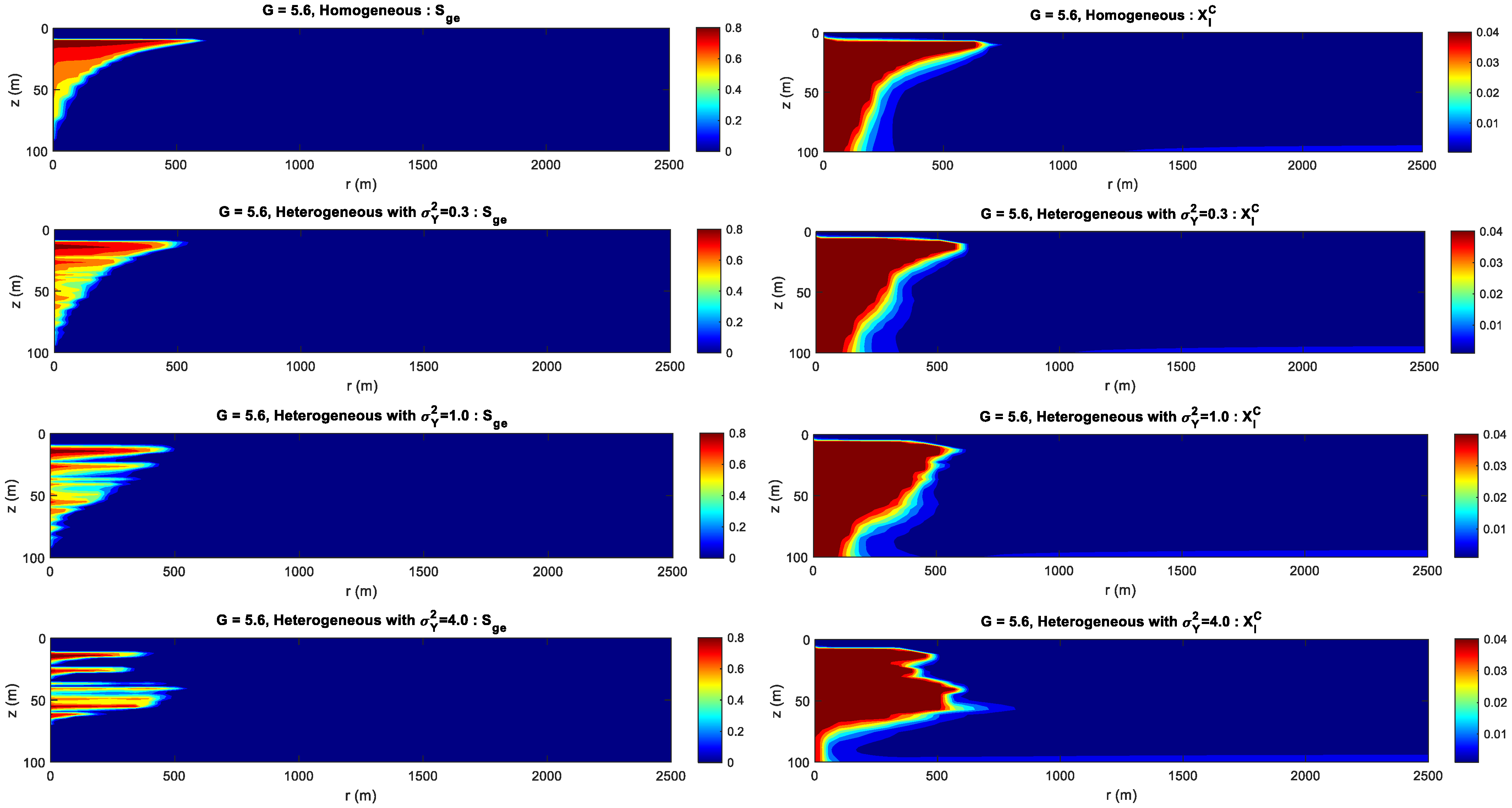
- For the formation with the smaller heterogeneity, the upwind and lateral migration of the CO2 plume is relatively more uniform around the injection well. For the bigger heterogeneity, CO2 preferentially migrates along the horizontal layer without accompanying the vertical migration.
- (3)
- For the formation with the bigger gravity index, gravity index is the dominant factor controlling CO2 migration. For the medium gravity index, the upward and lateral migration of the CO2 plume is determined by the gravity index and heterogeneity. For the smaller gravity index, formation heterogeneity is the key factor influencing CO2 distribution.
- (4)
- The dissolution curve shows a decreasing trend during CO2 injection. The dissolution curve with the higher gravity index is relatively higher at the initial injection period and the gap difference between dissolution curves approaches to a constant value. The permeability heterogeneity is the decisive factor in determining the finial CO2 dissolution efficiency.
- (5)
- From a practical point of view, most GCS field sites operate under . It is suggested to store CO2 in formations with relatively larger heterogeneity coefficient .
- (6)
- Reactive 3-Phase flow model for geological sequestration is considered for future research. It will be a more sophisticated analysis of the GCS, incorporating the chemical reaction among aqueous species and rock-forming minerals, as well as the partition between gaseous CO2 phase and liquid brine phase.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate change 2007: Mitigation of climate change: Contribution of working group III to the fourth assessment report of the intergovernmental panel on climate change. Choice Rev. Online 2008. [Google Scholar] [CrossRef]
- Haszeldine, R.S.; Flude, S.; Johnson, G.; Scott, V. Negative emissions technologies and carbon capture and storage to achieve the Paris agreement commitments. Phil. Trans. R. Soc. A 2018, 376, 20160447. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huang, G.; Li, T.; Zhang, Y.; Lou, Y. Analytical model of CO2 storage efficiency in saline aquifer with vertical heterogeneity. J. Nat. Gas Sci. Eng. 2014, 18, 77–89. [Google Scholar] [CrossRef]
- Vilarrasa, V.; Bolster, D.; Olivella, S.; Carrera, J. Coupled hydromechanical modeling of CO2 sequestration in deep saline aquifers. Int. J. Greenh. Gas Control 2010, 4, 910–919. [Google Scholar] [CrossRef]
- Riaz, A.; Hesse, M.; Tchelepi, H.A.; Orr, F.M. Onset of convection in a gravitationally unstable diffusive boundary layer in porous media. J. Fluid Mech. 2006, 548, 87–111. [Google Scholar] [CrossRef]
- Fang, Y.; Baojun, B.; Dazhen, T.; Dunn-Norman, S.; Wronkiewicz, D. Characteristics of CO2 sequestration in saline aquifers. Petrol. Sci. 2010, 7, 83–92. [Google Scholar]
- Kearns, J.; Teletzke, G.; Palmer, J.; Thomann, H.; Kheshgi, H.; Chen, Y.H.H.; Herzog, H. Developing a consistent database for regional geologic CO2 storage capacity worldwide. Energy Procedia 2017, 114, 4697–4709. [Google Scholar] [CrossRef]
- Zapata, Y.; Kristensen, M.R.; Huerta, N.; Brown, C.; Kabir, C.S.; Reza, Z. CO2 geological storage: Critical insights on plume dynamics and storage efficiency during long-term injection and post-injection periods. J. Nat. Gas Sci. Eng. 2020, 83, 103. [Google Scholar] [CrossRef]
- Singh, A.K.; Boettcher, N.; Wang, W.; Park, C.H.; Goerke, U.J.; Kolditz, O. Nonisothermal effects on two-phase flow in porous medium: CO2 disposal into a saline aquifer. Energy Proc. 2011, 4, 3889–3895. [Google Scholar] [CrossRef]
- Benson, S.M.; Cole, D.R. CO2 sequestration in deep sedimentary formations. Elements 2008, 4, 325–331. [Google Scholar] [CrossRef]
- Agartan, E.; Trevisan, L.; Cihan, A.; Birkholzer, J.; Zhou, Q.; Tissa, H. Illangasekare. Experimental study on effects of geologic heterogeneity in enhancing dissolution trapping of supercritical CO2. Water Resour. Res. 2015, 51, 1635–1648. [Google Scholar] [CrossRef]
- Kumar, S.; Foroozesh, J.; Edlmann, K.; Rezk, M.G.; Lim, C.Y. A comprehensive review of value-added CO2 sequestration in subsurface saline aquifers (Review). J. Nat. Gas Sci. Eng. 2020, 81, 103437. [Google Scholar] [CrossRef]
- Zhang, D.; Song, J. Mechanisms for Geological Carbon Sequestration. Procedia IUTAM 2014, 10, 319–327. [Google Scholar] [CrossRef]
- Saadatpoor, E.; Bryant, S.L.; Sepehrnoori, K. New trapping mechanism in carbon sequestration. Transp. Porous Media 2010, 82, 3–17. [Google Scholar] [CrossRef]
- Burnside, N.M.; Naylor, M. Review and implications of relative permeability of CO2/brine systems and residual trapping of CO2. Int. J. Greenh. Gas Control 2014, 23, 1–11. [Google Scholar] [CrossRef]
- Gershenzon, N.I.; Ritzi, R.W.; Dominic, D.F.; Mehnert, E.; Okwen, R.T. Comparison of CO2 trapping in highly heterogeneous reservoirs with Brooks-Corey and van Genuchten type capillary pressure curves. Adv. Water Resour. 2016, 96, 225–236. [Google Scholar] [CrossRef]
- Gershenzon, N.I.; Ritzi, R.W.; Dominic, D.F.; Mehnert, E.; Okwen, R.T. Capillary trapping of CO2 in heterogeneous reservoirs during the injection period. Int. J. Greenh. Gas Control 2017, 59, 13–23. [Google Scholar] [CrossRef]
- Adebayo, A.R. Sequential storage and in-situ tracking of gas in geological formations by a systematic and cyclic foam injection-A useful application for mitigating leakage risk during gas injection. J. Nat. Gas Sci. Eng. 2019, 62, 1–12. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Numerical simulation of CO2 disposal by mineral trapping in deep aquifers. Appl. Geochem. 2004, 19, 917–936. [Google Scholar] [CrossRef]
- Xu, T.; Apps, J.A.; Pruess, K. Mineral sequestration of carbon dioxide in a sandstone-shale system. Chem. Geol. 2005, 217, 295–318. [Google Scholar] [CrossRef]
- Gaus, I.; Audigane, P.; Andre, L.; Lions, J.; Jacquemet, N.; Durst, P.; Azaroual, M. Geochemical and solute transport modelling for CO2 storage, what to expect from it? Int. J. Greenh. Gas Control 2008, 2, 605–625. [Google Scholar] [CrossRef]
- De Silva, G.P.D.; Ranjith, P.G.; Perera, M.S.A. Geochemical aspects of CO2 sequestration in deep saline aquifers: A review. Fuel 2015, 155, 128–143. [Google Scholar] [CrossRef]
- Gilmore, K.A.; Neufeld, J.A.; Bickle, M.J. CO2 Dissolution Trapping Rates in Heterogeneous Porous Media. Geophys. Res. Lett. 2020, 47, e2020GL087001. [Google Scholar] [CrossRef]
- Sathaye, K.J.; Hesse, M.A.; Cassidy, M.; Stockli, D.F. Constraints on the magnitude and rate of CO2 dissolution at Bravo Dome natural gas field. Proc. Natl. Acad. Sci. USA 2014, 111, 15332–15337. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Kim, M.; Oh, J. Core-scale investigation of the effect of heterogeneity on the dynamics of residual and dissolution trapping of carbon dioxide. J. Hydrol. 2021, 596, 126109. [Google Scholar] [CrossRef]
- Sohal, M.A.; Le Gallo, Y.; Audigane, P.; de Dios, J.C.; Rigby, S.P. Effect of geological heterogeneities on reservoir storage capacity and migration of CO2 plume in a deep saline fractured carbonate aquifer. Int. J. Greenh. Gas Control 2021, 108, 103306. [Google Scholar] [CrossRef]
- Singh, M.; Chaudhuri, A.; Soltanian, M.R.; Stauffer, P.H. Coupled multiphase flow and transport simulation to model CO2 dissolution and local capillary trapping in permeability and capillary heterogeneous reservoir. Int. J. Greenh. Gas Control 2021, 108, 103329. [Google Scholar] [CrossRef]
- Onoja, M.U.; Williams, J.D.; Vosper, H.; Shariatipour, S.M. Effect of sedimentary heterogeneities in the sealing formation on predictive analysis of geological CO2 storage. Int. J. Greenh. Gas Control 2019, 82, 229–243. [Google Scholar] [CrossRef]
- Mouche, E.; Hayek, M.; Mugler, C. Upscaling of CO2 vertical migration through a periodic layered porous medium: The capillary-free and capillary-dominant cases. Adv. Water Resour. 2010, 33, 1164–1175. [Google Scholar] [CrossRef]
- Green, C.; Ennis-King, J.; Pruess, K. Effect of Vertical Heterogeneity on Long-Term Migration of CO2 in Saline Formations. Energy Procedia 2009, 1, 1823–1830. [Google Scholar] [CrossRef]
- Deng, H.L.; Stauffer, P.H.; Dai, Z.X.; Jiao, Z.S.; Surdam, R.C. Simulation of industrial-scale CO2 storage: Multi-scale heterogeneity and its impacts on storage capacity, injectivity and leakage. Int. J. Greenh. Gas Control 2012, 10, 397–418. [Google Scholar] [CrossRef]
- Kim, M.; Kim, K.-Y.; Han, W.S.; Oh, J.; Park, E. Density-driven convection in a fractured porous media: Implications for geological CO2 storage. Water Resour. Res. 2019, 55, 5852–5870. [Google Scholar] [CrossRef]
- Shafabakhsh, P.; Ataie-Ashtiani, B.; Simmons, C.T.; Younes, A.; Fahs, M. Convective-Reactive Transport of Dissolved CO2 in Fractured-Geological Formations. Int. J. Greenh. Gas Control 2021, 109, 103365. [Google Scholar] [CrossRef]
- Galkin, S.V.; Martyushev, D.A.; Osovetsky, B.M.; Kazymov, K.P.; Song, H. Evaluation of void space of complicated potentially oil-bearing carbonate formation using X-ray tomography and electron microscopy methods. Energy Rep. 2022, 8, 6245–6257. [Google Scholar] [CrossRef]
- Martyushev, D.A.; Ponomareva, I.N.; Chukhlov, A.S.; Davoodi, S.; Osovetsky, B.M.; Kazymov, K.P.; Yang, Y. Study of void space structure and its influence on carbonate reservoir properties: X-ray microtomography, electron microscopy, and well testing. Mar. Pet. Geol. 2023, 151, 106192. [Google Scholar] [CrossRef]
- Martyushev, D.A.; Govindarajan, S.K.; Li, Y.; Yang, Y. Experimental study of the influence of the content of calcite and dolomite in the rock on the efficiency of acid treatment. J. Pet. Sci. Eng. 2022, 208, 109770. [Google Scholar] [CrossRef]
- Martyushev, D.A. Improving the geological and hydrodynamic model a carbonate oil object by taking into account the permeability anisotropy parameter. J. Min. Inst. 2020, 243, 313–318. [Google Scholar] [CrossRef]
- Martyushev, D.A.; Ponomareva, I.N.; Osovetsky, B.M.; Kazymov, K.P.; Tomilina, E.M.; Lebedeva, A.S.; Chukhlov, A.S. Study of the structure and development of oil deposits in carbonate reservoirs using field data and X-ray microtomography. Georesursy 2022, 24, 114–124. [Google Scholar]
- Oh, J.; Kim, K.Y.; Han, W.S.; Park, E.; Kim, J.C. Migration behavior of supercritical and liquid CO2 in a stratified system: Experiments and numerical simulations. Water Resour. Res. 2015, 51, 7937–7958. [Google Scholar] [CrossRef]
- Rasmusson, K.; Tsang, C.-F.; Tsang, Y.; Rasmusson, M.; Pan, L.; Fagerlund, F.; Bensabat, J.; Niemi, A. Distribution of injected CO2 in a stratified saline reservoir accounting for coupled wellbore-reservoir flow. Greenh. Gases Sci. Technol. 2015, 5, 419–436. [Google Scholar] [CrossRef]
- Lie, K.-A. An Introduction to Reservoir Simulation Using MATLAB/GNU Octave: User Guide for the MATLAB Reservoir Simulation Toolbox (MRST); Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Spycher, N.; Pruess, K. CO2-H2O mixtures in the geological sequestration of CO2. II. Partitioning in chloride brines at 12-100 °C and up to 600 bar. Geochim. Cosmochim. Acta 2005, 69, 3309–3320. [Google Scholar] [CrossRef]
- Spycher, N.; Pruess, K.; Ennis-King, J. CO2-H2O mixtures in the geological sequestration of CO2. I. Assessment and calculation of mutual solubilities from 12 to 100 °C and up to 600 bar. Geochim. Cosmochim. Acta 2003, 67, 3015–3031. [Google Scholar] [CrossRef]
- Wang, Y.; Fernàndez-Garcia, D.; Saaltink, M.W. Carbon Dioxide (CO2) Dissolution Efficiency During Geological Carbon Sequestration (GCS) in Randomly Stratified Formations. Water Resour. Res. 2022, 58, e2022WR032325. [Google Scholar] [CrossRef]
- Leverett, M.C. Capillary behavior in porous solids. Trans. AIME 1941, 142, 159–172. [Google Scholar] [CrossRef]
- Buckley, S.E.; Leverett, M.C. Mechanism of fluid displacement in sands. Trans. AIME 1942, 146, 107–116. [Google Scholar] [CrossRef]
- Juanes, R.; Spiteri, E.J.; Orr, F.M.; Blunt, M.J. Impact of relative permeability hysteresis on geological CO2 storage. Water Resour. Res. 2006, 42, W12418. [Google Scholar] [CrossRef]
- Krevor, S.C.; Pini, R.; Zuo, L.; Benson, S.M. Relative permeability and trapping of CO2 and water in sandstone rocks at reservoir conditions. Water Resour. Res. 2012, 48, W02532. [Google Scholar] [CrossRef]
- McWhorter, D.B.; Sunada, D.K. Exact integral solutions for two-phase flow. Water Resour. Res. 1990, 26, 399–413. [Google Scholar] [CrossRef]
- Nordbotten, J.M.; Celia, M.A. Similarity solutions for fluid injection into confined aquifers. J. Fluid Mech. 2006, 51, 307–327. [Google Scholar] [CrossRef]
- Bai, T.; Tahmasebi, P. Sequential Gaussian simulation for geosystems modeling: A machine learning approach. Geosci. Front. 2022, 13, 101258. [Google Scholar] [CrossRef]

| Parameter | Value | Parameter | Value |
|---|---|---|---|
| 1.0 | 1.0 | ||
| 5.0 | 0.4 | ||
| 0.5 | 0.5 |
| Parameters | Symbol | Units | Values |
|---|---|---|---|
| Domain size | (R, b) | [m] | (3000, 100) |
| Grid discretization | (Nr, Nz) | [–] | (100, 100) |
| Porosity | ϕ | [–] | 0.1 |
| Mean permeability | [m2] | 10−13 | |
| Initial liquid pressure | pl | [bar] | 150 |
| Initial gas pressure | pg | [bar] | 1 |
| Well radius | rw | [m] | 0.1 |
| Total injection mass | Minj | [Mt] | 2.5 |
| Residual saturations | (Slr, Sgr) | [–] | (0.2, 0) |
| Hydrodynamic dispersivities | (αL, αT) | [m] | (5, 1) |
| Molecular diffusion coefficient | Dm | [m2⋅s−1] | 10−9 |
| Salinity | [molal] | 0.1 | |
| Temperature | Tc | [°C] | 60 |
| Simulation time | t | [s] | 3.15 × 107 |
| Case | ||||
|---|---|---|---|---|
| 1 | 7.5 | 10−13 | 0 | 0.6 |
| 2 | 2.5 | 10−13 | 0 | 1.8 |
| 3 | 0.8 | 10−13 | 0 | 5.6 |
| 4 | 0.8 | 10−13 | 0.3 | 5.6 |
| 5 | 0.8 | 10−13 | 1.0 | 5.6 |
| 6 | 0.8 | 10−13 | 4.0 | 5.6 |
| 7 | 2.5 | 10−13 | 0.3 | 1.8 |
| 8 | 2.5 | 10−13 | 1.0 | 1.8 |
| 9 | 2.5 | 10−13 | 4.0 | 1.8 |
| 10 | 7.5 | 10−13 | 0.3 | 0.6 |
| 11 | 7.5 | 10−13 | 1.0 | 0.6 |
| 12 | 7.5 | 10−13 | 4.0 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Lv, Y.; Yuan, C.; Zhu, X.; Guo, J.; Liu, W.; Li, H. Effects of Reservoir Heterogeneity on CO2 Dissolution Efficiency in Randomly Multilayered Formations. Energies 2023, 16, 5219. https://doi.org/10.3390/en16135219
Fang X, Lv Y, Yuan C, Zhu X, Guo J, Liu W, Li H. Effects of Reservoir Heterogeneity on CO2 Dissolution Efficiency in Randomly Multilayered Formations. Energies. 2023; 16(13):5219. https://doi.org/10.3390/en16135219
Chicago/Turabian StyleFang, Xiaoyu, Yanxin Lv, Chao Yuan, Xiaohua Zhu, Junyang Guo, Weiji Liu, and Haibo Li. 2023. "Effects of Reservoir Heterogeneity on CO2 Dissolution Efficiency in Randomly Multilayered Formations" Energies 16, no. 13: 5219. https://doi.org/10.3390/en16135219
APA StyleFang, X., Lv, Y., Yuan, C., Zhu, X., Guo, J., Liu, W., & Li, H. (2023). Effects of Reservoir Heterogeneity on CO2 Dissolution Efficiency in Randomly Multilayered Formations. Energies, 16(13), 5219. https://doi.org/10.3390/en16135219






