Two-Stage Dry Reforming Process for Biomass Gasification: Product Characteristics and Energy Analysis
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.1.1. Biomass
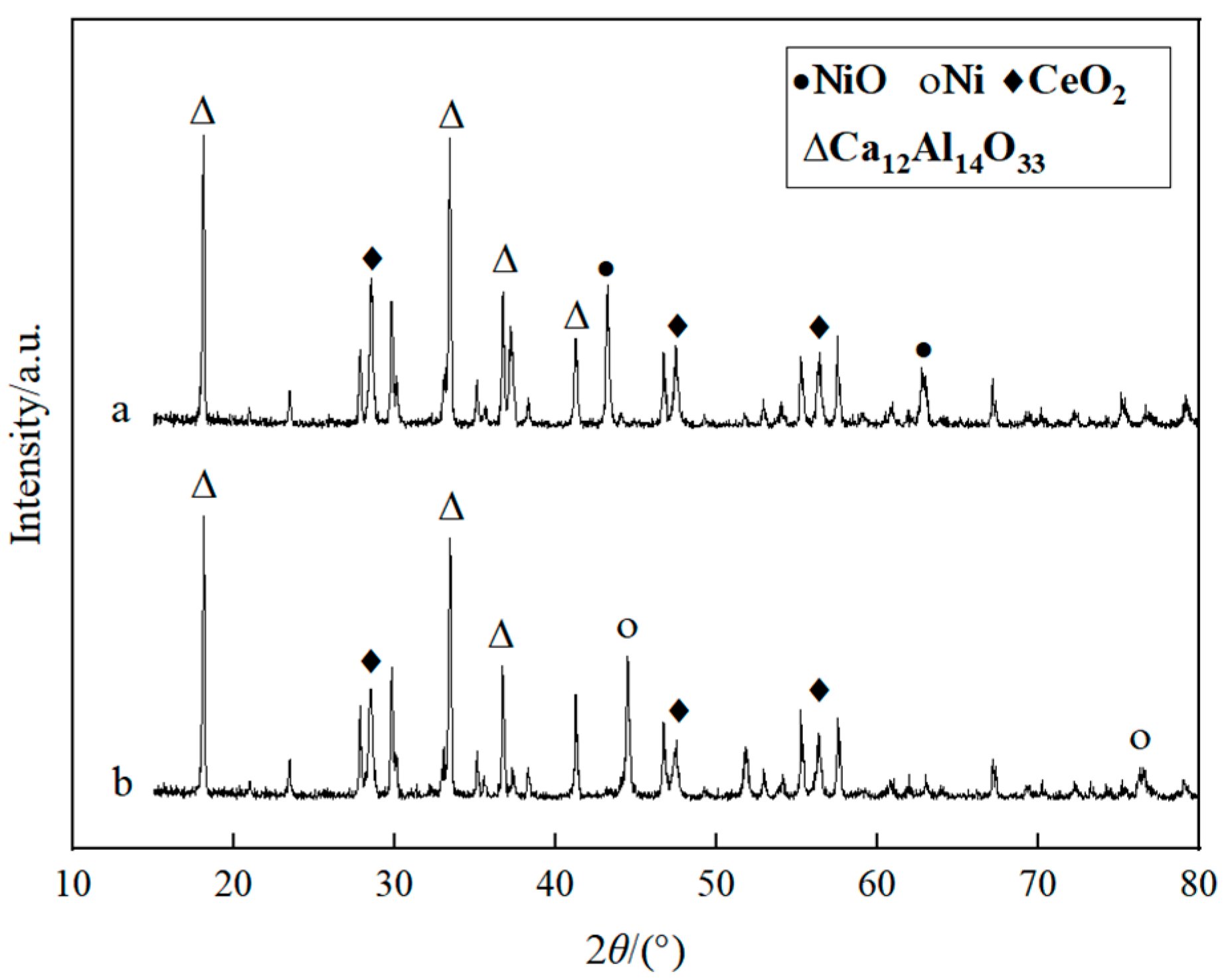
2.1.2. Catalyst
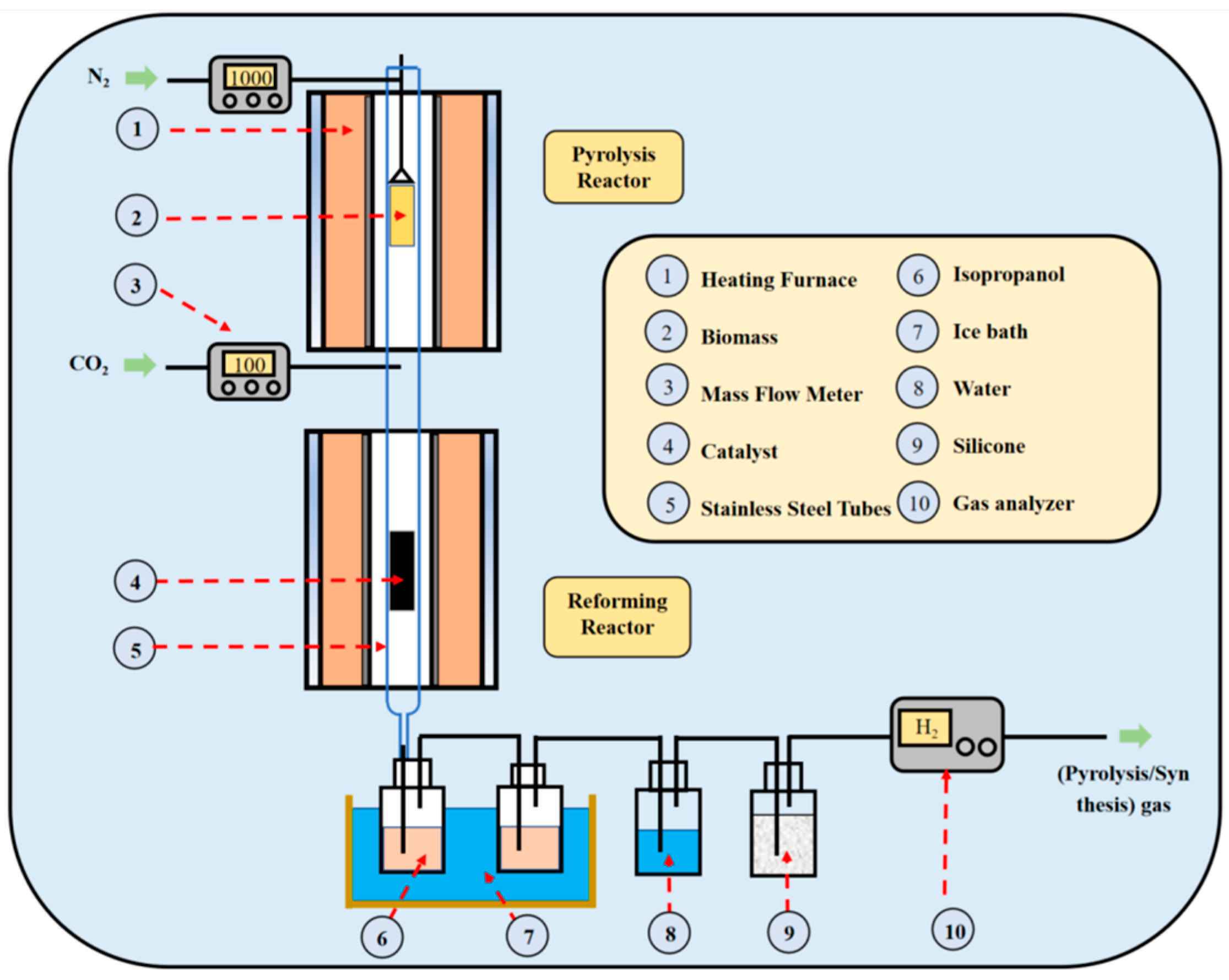
2.2. Biomass Pyrolysis Experiments
2.3. Experimental Process of Biomass Catalytic Dry Reforming
- H2/CO ratio, X. The ratio of the volume of H2 to CO produced by biomass gasification reforming:where is the volume of H2 produced by gasification reforming, Nm3; is the volume of CO produced by gasification reforming, Nm3.
- H2 and CO yield, . The total volume of H2 and CO gas produced by gasification reforming of biomass per unit mass:where is the H2 and CO yield, Nm3/kg; is the volume of H2 and CO produced by biomass gasification reforming, Nm3, respectively; and m is the mass of input biomass, kg.
- Calorific value of reformed gas, HHV. High level calorific value of syngas produced by gasification reforming of biomass per unit mass:where HHV is the calorific value of reformed gas MJ/kg; and is the calorific value of gas. is the volume of gas produced by biomass gasification reforming, Nm3; and m is the mass of input biomass, kg.
- CO2 utilization,. The volume of initial CO2 input minus the volume of CO2 produced by gasification reforming of unit mass of biomass:where is the CO2 utilization rate, Nm3/kg; is the initial input CO2 volume, Nm3; is the volume of CO2 produced by gasification reforming, Nm3; and m is the mass of the input biomass, kg.
3. Results and Discussions
3.1. Effect of Reforming Temperature
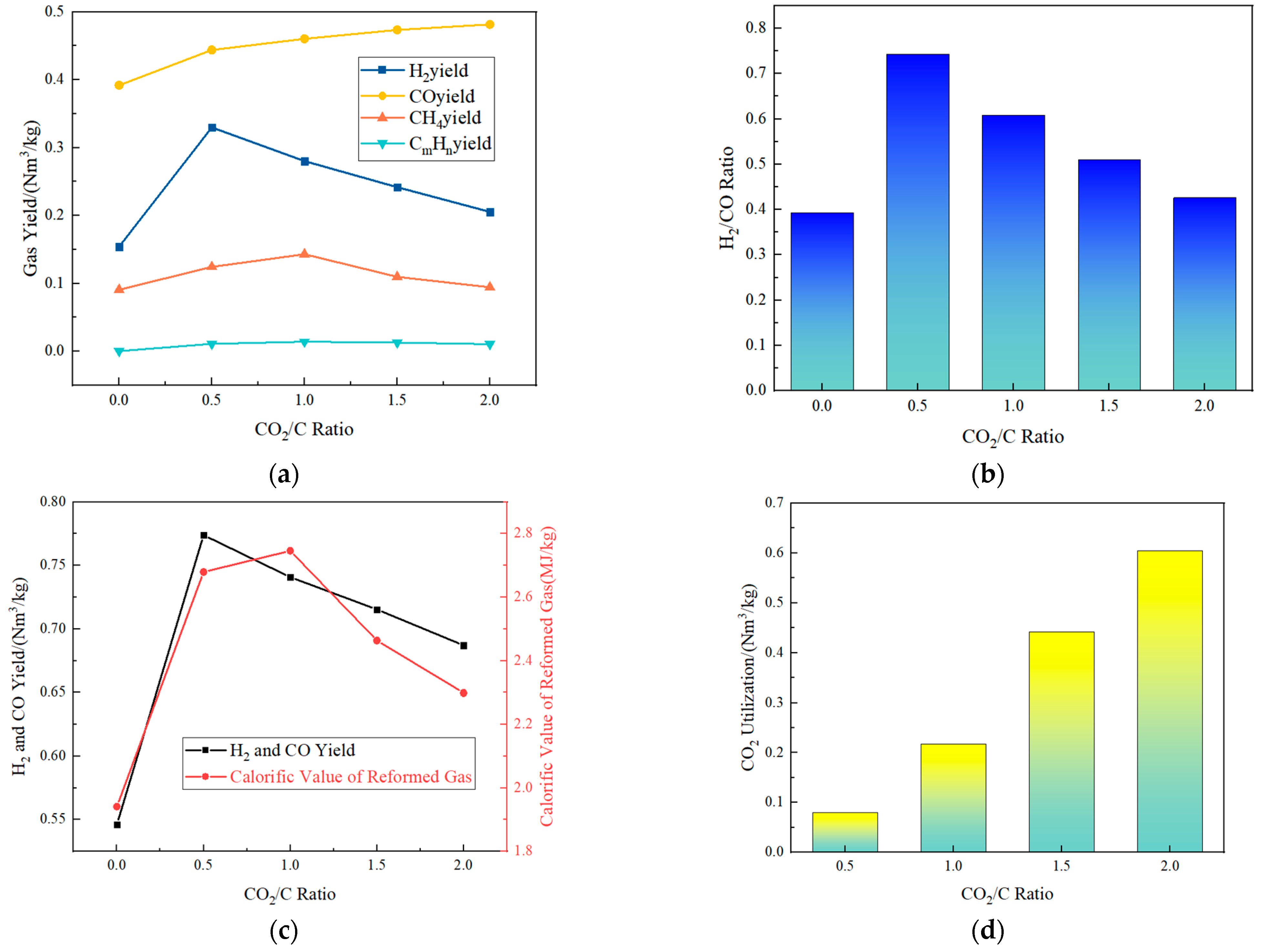
3.2. Effect of CO2/C Ratio
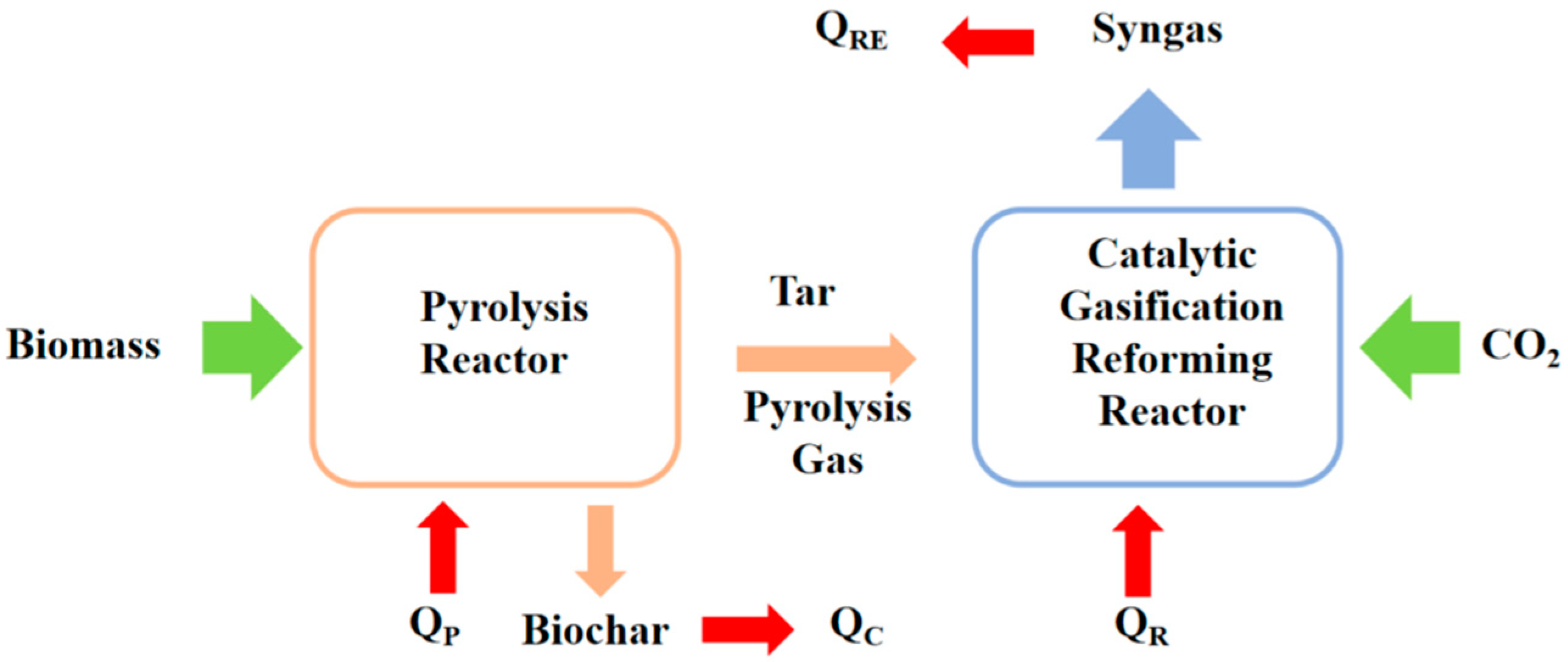
4. Energy Analysis of Biomass Catalytic Dry Reforming System
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. Global Energy Review; IEA: Paris, France, 2021.
- Ramos, J.L.; Pakuts, B.; Godoy, P.; García-Franco, A.; Duque, E. Addressing the energy crisis: Using microbes to make biofuels. Microb. Biotechnol. 2022, 15, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Pilar González-Vázquez, M.; Rubiera, F.; Pevida, C.; Pio, D.T.; Tarelho, L.A.C. Thermodynamic Analysis of Biomass Gasification Using Aspen Plus: Comparison of Stoichiometric and Non-Stoichiometric Models. Energies 2021, 14, 189. [Google Scholar] [CrossRef]
- Mathieu, P.; Dubuisson, R. Performance analysis of a biomass gasifier. Energy Convers. Manag. 2002, 43, 1291–1299. [Google Scholar] [CrossRef]
- Xia, S.; Zheng, A.; Zhao, K.; Zhao, Z.; Li, H. Evaluation of Pyrolysis Reactivity, Kinetics, and Gasification Reactivity of Corn Cobs after Torrefaction Pretreatment. Energies 2022, 15, 9277. [Google Scholar] [CrossRef]
- Brigagão, G.V.; de Queiroz Fernandes Araújo, O.; de Medeiros, J.L.; Mikulcic, H.; Duic, N. A Techno-Economic Analysis of Thermochemical Pathways for Corncob-to-Energy: Fast Pyrolysis to Bio-Oil, Gasification to Methanol and Combustion to Electricity. Fuel Process. Technol. 2019, 193, 102–113. [Google Scholar] [CrossRef]
- Lin, Y.C.; Huber, G.W. The critical role of heterogeneous catalysis in lignocellulosic biomass conversion. Energy Environ. Sci. 2009, 2, 68–80. [Google Scholar] [CrossRef]
- Mettler, M.S.; Vlachos, D.G.; Dauenhauer, P.J. Top ten fundamental challenges of biomass pyrolysis for biofuels. Energy Environ. Sci. 2012, 5, 7797–7809. [Google Scholar] [CrossRef]
- Mettler, M.S.; Mushrif, S.H.; Paulsen, A.D.; Javadekar, A.D.; Vlachos, D.G.; Dauenhauer, P.J. Revealing pyrolysis chemistry for biofuels production: Conversion of cellulose to furans and small oxygenates. Energy Environ. Sci. 2012, 5, 5414–5424. [Google Scholar] [CrossRef]
- Balat, M. Production of bioethanol from lignocellulosic materials via the biochemical pathway: A review. Energy Convers. Manag. 2011, 52, 858–875. [Google Scholar] [CrossRef]
- Wang, J.; Hou, D.; Liu, Z.; Tao, J.; Yan, B.; Liu, Z.; Yang, T.; Su, H.; Tahir, M.H.; Chen, G. Emergy Analysis of Agricultural Waste Biomass for Energy-Oriented Utilization in China: Current Situation and Perspectives. Sci. Total Environ. 2022, 849, 157798. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, R.; Yu, Z.; Wang, Z.; Yu, Q.; Qin, Q. Combined Steam/Dry Reforming of Bio-Oil for H2/CO Syngas Production with Blast Furnace Slag as Heat Carrier. Energy 2020, 200, 117481. [Google Scholar] [CrossRef]
- Fierro, V.; Klouz, V.; Akdim, O.; Mirodatos, C. Oxidative reforming of biomass derived ethanol for hydrogen production in fuel cell applications. Catal. Today 2002, 75, 141–144. [Google Scholar] [CrossRef]
- Rennard, D.; French, R.; Czernik, S.; Josephson, T.; Schmidt, L. Production of synthesis gas by partial oxidation and steam reforming of biomass pyrolysis oils. Int. J. Hydrogen Energy 2010, 35, 4048–4059. [Google Scholar] [CrossRef]
- Ashok, J.; Dewangan, N.; Das, S.; Hongmanorom, P.; Wai, M.H.; Tomishige, K.; Kawi, S. Recent progress in the development of catalysts for steam reforming of biomass tar model reaction. Fuel Process. Technol. 2020, 199, 106252. [Google Scholar] [CrossRef]
- Guan, G.; Kaewpanha, M.; Hao, X.; Abudula, A. Catalytic steam reforming of biomass tar: Prospects and challenges. Renew. Sustain. Energy Rev. 2016, 58, 450–461. [Google Scholar] [CrossRef]
- Courson, C.; Makaga, E.; Petit, C.; Kiennemann, A. Development of Ni catalysts for gas production from biomass gasification. Reactivity in steam-and dry-reforming. Catal. Today 2000, 63, 427–437. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Colmenares Quintero, R.F.; Pieta, I.S. Catalytic dry reforming for biomass-based fuels processing: Progress and future perspectives. Energy Technol. 2016, 4, 881–890. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, H.; Bartocci, P.; Fantozzi, F.; Mašek, O.; Agblevor, F.A.; Wei, Z.; Yang, H.; Chen, H.; Lu, X.; et al. Prospective contributions of biomass pyrolysis to China’s 2050 carbon reduction and renewable energy goals. Nat. Commun. 2021, 12, 1679–1698. [Google Scholar] [CrossRef] [PubMed]
- Pohořelý, M.; Jeremiáš, M.; Svoboda, K.; Kameníková, P.; Skoblia, S.; Beňo, Z. CO2 as moderator for biomass gasification. Fuel 2014, 117, 198–205. [Google Scholar] [CrossRef]
- Hu, X.; Lu, G. Bio-oil steam reforming, partial oxidation or oxidative steam reforming coupled with bio-oil dry reforming to eliminate CO2 emission. Int. J. Hydrogen Energy 2010, 35, 7169–7176. [Google Scholar] [CrossRef]
- Encinar, J.M.; Beltran, F.J.; Ramiro, A.; Gonzalez, J.F. Pyrolysis/gasification of agricultural residues by carbon dioxide in the presence of different additives: Influence of variables. Fuel Process. Technol. 1998, 55, 219–233. [Google Scholar] [CrossRef]
- Sadhwani, N.; Adhikari, S.; Eden, M.R. Biomass gasification using carbon dioxide: Effect of temperature, CO2/C ratio, and the study of reactions influencing the process. Ind. Eng. Chem. Res. 2016, 55, 2883–2891. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X. Role of Filamentous Coke in Deactivation of Ni/Bio-Char Catalyst during Dry Reforming of Non-Oxygenates Tar. J. Anal. Appl. Pyrolysis 2021, 159, 105314. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.-P.; Zhao, X.-Y.; Yang, F.-L.; Wei, X.-Y. Recent Advances in Syngas Production from Biomass Catalytic Gasification: A Critical Review on Reactors, Catalysts, Catalytic Mechanisms and Mathematical Models. Renew. Sustain. Energy Rev. 2019, 116, 109426. [Google Scholar] [CrossRef]
- Meng, J.; Zhao, Z.; Wang, X.; Wu, X.; Zheng, A.; Huang, Z.; Zhao, K.; Li, H. Effects of Catalyst Preparation Parameters and Reaction Operating Conditions on the Activity and Stability of Thermally Fused Fe-Olivine Catalyst in the Steam Reforming of Toluene. Int. J. Hydrogen Energy 2018, 43, 127–138. [Google Scholar] [CrossRef]
- Oh, G.; Park, S.Y.; Seo, M.W.; Kim, Y.K.; Ra, H.W.; Lee, J.-G.; Yoon, S.J. Ni/Ru–Mn/Al2O3 Catalysts for Steam Reforming of Toluene as Model Biomass Tar. Renew. Energy 2016, 86, 841–847. [Google Scholar] [CrossRef]
- de Castro, T.P.; Silveira, E.B.; Rabelo-Neto, R.C.; Borges, L.E.P.; Noronha, F.B. Study of the Performance of Pt/Al2O3 and Pt/CeO2/Al2O3 Catalysts for Steam Reforming of Toluene, Methane and Mixtures. Catal. Today 2018, 299, 251–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Ou, Z.; Qin, C.; Ran, J.; Wu, C. Roles of Alkali/Alkaline Earth Metals in Steam Reforming of Biomass Tar for Hydrogen Production over Perovskite Supported Ni Catalysts. Fuel 2019, 257, 116032. [Google Scholar] [CrossRef]
- Li, L.; Yang, Z.; Qin, X.; Chen, J.; Yan, K.; Zou, G.; Peng, Z.; Wang, F.; Song, Z.; Ma, C. Toluene Microwave-Assisted Reforming with CO2 or a Mixed Agent of CO2-H2O on Fe-Doped Activated Biochar. Energy 2019, 177, 358–366. [Google Scholar] [CrossRef]
- Yao, D.; Hu, Q.; Wang, D.; Yang, H.; Wu, C.; Wang, X.; Chen, H. Hydrogen Production from Biomass Gasification Using Biochar as a Catalyst/Support. Bioresour. Technol. 2016, 216, 159–164. [Google Scholar] [CrossRef]
- Michel, R.; Rapagnà, S.; Di Marcello, M.; Burg, P.; Matt, M.; Courson, C.; Gruber, R. Catalytic Steam Gasification of Miscanthus X Giganteus in Fluidised Bed Reactor on Olivine Based Catalysts. Fuel Process. Technol. 2011, 92, 1169–1177. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Steam Gasification of Biomass in a Conical Spouted Bed Reactor with Olivine and γ-Alumina as Primary Catalysts. Fuel Process. Technol. 2013, 116, 292–299. [Google Scholar] [CrossRef]
- Tan, R.S.; Tuan Abdullah, T.A.; Johari, A.; Md Isa, K. Catalytic Steam Reforming of Tar for Enhancing Hydrogen Production from Biomass Gasification: A Review. Front. Energy 2020, 14, 545–569. [Google Scholar] [CrossRef]
- Hu, M.; Laghari, M.; Cui, B.; Xiao, B.; Zhang, B.; Guo, D. Catalytic Cracking of Biomass Tar over Char Supported Nickel Catalyst. Energy 2018, 145, 228–237. [Google Scholar] [CrossRef]
- Lin, F.; Xu, M.; Ramasamy, K.K.; Li, Z.; Klinger, J.L.; Schaidle, J.A.; Wang, H. Catalyst Deactivation and Its Mitigation during Catalytic Conversions of Biomass. ACS Catal. 2022, 12, 13555–13599. [Google Scholar] [CrossRef]
- Qingli, X.; Peng, F.; Wei, Q.; Kai, H.; Shanzhi, X.; Yongjie, Y. Catalyst Deactivation and Regeneration during CO2 Reforming of Bio-Oil. Int. J. Hydrogen Energy 2019, 44, 10277–10285. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Liao, S.; Yan, R. Hydrogen-Rich Gas Production by Air–Steam Gasification of Rice Husk Using Supported Nano-NiO/γ-Al2O3 Catalyst. Int. J. Hydrogen Energy 2010, 35, 7399–7404. [Google Scholar] [CrossRef]
- Di Carlo, A.; Borello, D.; Sisinni, M.; Savuto, E.; Venturini, P.; Bocci, E.; Kuramoto, K. Reforming of Tar Contained in a Raw Fuel Gas from Biomass Gasification Using Nickel-Mayenite Catalyst. Int. J. Hydrogen Energy 2015, 40, 9088–9095. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, W.; Yu, Z.; Xie, H.; Zhou, M.; Wang, Z. Enhanced Reforming of Tar Based on Double-Effect Ni/CaO–Ca12Al14O33 Catalysts: Modified by Ce, Mg, and Fe. Front. Energy Res. 2021, 9, 729919. [Google Scholar] [CrossRef]
- Cay, H.; Duman, G.; Yanik, J. Two-Step Gasification of Biochar for Hydrogen-Rich Gas Production: Effect of the Biochar Type and Catalyst. Energy Fuels 2019, 33, 7398–7405. [Google Scholar] [CrossRef]
- Li, B.; Fabrice Magoua Mbeugang, C.; Liu, D.; Zhang, S.; Wang, S.; Wang, Q.; Xu, Z.; Hu, X. Simulation of Sorption Enhanced Staged Gasification of Biomass for Hydrogen Production in the Presence of Calcium Oxide. Int. J. Hydrogen Energy 2020, 45, 26855–26864. [Google Scholar] [CrossRef]
- Yang, H.; Cui, Y.; Han, T.; Sandström, L.; Jönsson, P.; Yang, W. High-Purity Syngas Production by Cascaded Catalytic Reforming of Biomass Pyrolysis Vapors. Appl. Energy 2022, 322, 119501. [Google Scholar] [CrossRef]
- Wagenaar, B.M.; Prins, W.; van Swaaij, W.P.M. Pyrolysis of Biomass in the Rotating Cone Reactor: Modelling and Experimental Justification. Chem. Eng. Sci. 1994, 49, 5109–5126. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen production from steam gasification of biomass: Influence of process parameters on hydrogen yield—A review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Cheng, Y.; Thow, Z.; Wang, C.H. Biomass gasification with CO2 in a fluidized bed. Powder Technol. 2016, 296, 87–101. [Google Scholar] [CrossRef]

| Raw Materials | Ultimate Analysis( %) | Proximate Analysis (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Corn cob | C | H | O | N | S | Moisture | Ash | Volatile | Fixed Carbon |
| 46.78 | 5.96 | 46.76 | 0.48 | 0.03 | 1.39 | 3.76 | 79.00 | 15.85 | |
| Groups | Hemicellulose | Cellulose | Lignin | NDS | Ash |
|---|---|---|---|---|---|
| Content/% | 25.40 | 62.11 | 9.90 | 1.56 | 2.40 |
| Composition | Gases | Tar | Water | Biochar |
|---|---|---|---|---|
| Content (kg/kg) | 0.18 | 0.14 | 0.44 | 0.24 |
| Composition | H2 Yield | CO Yield | CH4 Yield | CmHn Yield | CO2 Yield |
|---|---|---|---|---|---|
| Content (Nm3/kg) | 0 | 0.042 | 0.011 | 0.005 | 0.057 |
| Relative Molecular Mass | <150 | 150~200 | >200 |
|---|---|---|---|
| Relative content (%) | 38.01 | 43.18 | 6.80 |
| Serial Number | Name | Molecular Formula | Relative Molecular Mass | Relative Content (%) |
|---|---|---|---|---|
| 1 | N-Methyl-1,3-propanediamine | C4H12N2 | 88.15 | 3.20 |
| 2 | p-Methyl phenol | C7H8O | 108.14 | 0.53 |
| 3 | 1,4-Benzenediol | C6H6O2 | 110.11 | 4.15 |
| 4 | 2,3-Dihydrobenzofuran | C8H8O | 120.15 | 10.32 |
| 5 | 4-Hydroxybenzaldehyde | C7H6O2 | 122.12 | 1.17 |
| 6 | p-Ethyl phenol | C8H10O | 122.16 | 4.15 |
| 7 | 2,5-Dihydroxytoluene | C7H8O2 | 124.14 | 5.35 |
| 8 | Glycerol monoacetate | C5H10O4 | 134.13 | 1.17 |
| 9 | 3,4,5-Trimethylphenol | C9H12O | 136.19 | 1.70 |
| 10 | 3-Methoxy-4-methylaniline | C8H11NO | 137.18 | 1.12 |
| 11 | 4-Methylguaiacol | C8H10O2 | 138.16 | 3.24 |
| 12 | 3-Methoxycatechol | C7H8O3 | 140.14 | 0.99 |
| 13 | P-Vinyl Guaiacol | C9H10O2 | 150.17 | 6.83 |
| 14 | Vanillin | C8H8O3 | 152.15 | 4.17 |
| 15 | Lilac Alcohol | C8H10O3 | 154.16 | 3.92 |
| 16 | 1,6-Anhydroglucopyranose | C6H10O5 | 162.14 | 2.68 |
| 17 | Isoeugenol | C10H12O2 | 164.20 | 2.83 |
| 18 | Diglycerin | C6H14O5 | 166.17 | 1.28 |
| 19 | Acetosyringone | C10H12O4 | 196.20 | 3.30 |
| 20 | Supramolecular | — | >200 | 6.80 |
| Reaction | Equation | Numbering |
|---|---|---|
| Pyrolytic reaction | CH1.591O0.821→C + CO + H2 + CO2 + H2O + CH4 + Tar | (1) |
| Tar CO2 reforming reaction | CHxOy + aCO2→bCO + cH2 + dCH4 + eH2O | (2) |
| Methane CO2 reforming reaction | CH4 + CO2 ↔ 2CO + 2H2 | (3) |
| Water gas reaction | C + H2O ↔ CO + H2 | (4) |
| C + 2H2O ↔ CO2 + 2H2 | ||
| Boudouard reaction | C + CO2 ↔ 2CO | (5) |
| Water gas shift reaction | CO + H2O ↔ H2 + CO2 | (6) |
| Methanation of carbon | C + 2H2 ↔ CH4 | (7) |
| Reaction Condition | (MJ/kg) | (MJ/kg) | (MJ/kg) | (MJ/kg) | (MJ/kg) | |
|---|---|---|---|---|---|---|
| Name | Number | |||||
| Reforming Temperature/°C (CO2/C ratio = 1.0) | 750 | 2.37 | 7.55 | 0.83 | 1.92 | −6.27 |
| 800 | 3.12 | 2.01 | −4.07 | |||
| 850 | 5.49 | 2.09 | −1.78 | |||
| 900 | 8.03 | 2.17 | 0.68 | |||
| 950 | 8.34 | 2.30 | 0.86 | |||
| CO2/C Ratio (reforming temperature = 900 °C) | 0 | 0.98 | 1.38 | −7.53 | ||
| 0.5 | 5.19 | 1.67 | −1.66 | |||
| 1.0 | 8.03 | 2.17 | 0.68 | |||
| 1.5 | 9.72 | 2.71 | 1.83 | |||
| 2.0 | 10.99 | 3.31 | 2.49 | |||
| Reaction Condition | (MJ/kg) | (MJ/kg) | (MJ/kg) | (MJ/kg) | |
|---|---|---|---|---|---|
| Name | Number | ||||
| Reforming Temperature/°C (CO2/C ratio = 1.0) | 750 | 0.41 | 1.26 | −0.84 | 0.83 |
| 800 | 0.49 | 1.36 | 1.27 | 3.12 | |
| 850 | 0.58 | 1.46 | 3.45 | 5.49 | |
| 900 | 0.67 | 1.57 | 5.80 | 8.03 | |
| 950 | 0.76 | 1.67 | 5.92 | 8.34 | |
| CO2/C Ratio (reforming temperature = 900 °C) | 0 | 0.67 | 0.00 | −1.64 | −0.98 |
| 0.5 | 0.67 | 0.73 | 3.80 | 5.19 | |
| 1.0 | 0.67 | 1.57 | 5.80 | 8.03 | |
| 1.5 | 0.67 | 2.40 | 6.65 | 9.72 | |
| 2.0 | 0.67 | 3.24 | 7.08 | 10.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Xie, H.; Yu, Z.; Qin, M.; Wu, Z.; Wang, P.; Zhao, X.; Zhang, S. Two-Stage Dry Reforming Process for Biomass Gasification: Product Characteristics and Energy Analysis. Energies 2023, 16, 4783. https://doi.org/10.3390/en16124783
Gao Y, Xie H, Yu Z, Qin M, Wu Z, Wang P, Zhao X, Zhang S. Two-Stage Dry Reforming Process for Biomass Gasification: Product Characteristics and Energy Analysis. Energies. 2023; 16(12):4783. https://doi.org/10.3390/en16124783
Chicago/Turabian StyleGao, Yang, Huaqing Xie, Zhenyu Yu, Mengxin Qin, Zhenguo Wu, Panlei Wang, Xi Zhao, and Shiyi Zhang. 2023. "Two-Stage Dry Reforming Process for Biomass Gasification: Product Characteristics and Energy Analysis" Energies 16, no. 12: 4783. https://doi.org/10.3390/en16124783
APA StyleGao, Y., Xie, H., Yu, Z., Qin, M., Wu, Z., Wang, P., Zhao, X., & Zhang, S. (2023). Two-Stage Dry Reforming Process for Biomass Gasification: Product Characteristics and Energy Analysis. Energies, 16(12), 4783. https://doi.org/10.3390/en16124783







