Experimental Study on Temperature Sensitivity of the State of Charge of Aluminum Battery Storage System
Abstract
1. Introduction
2. Materials and Methods
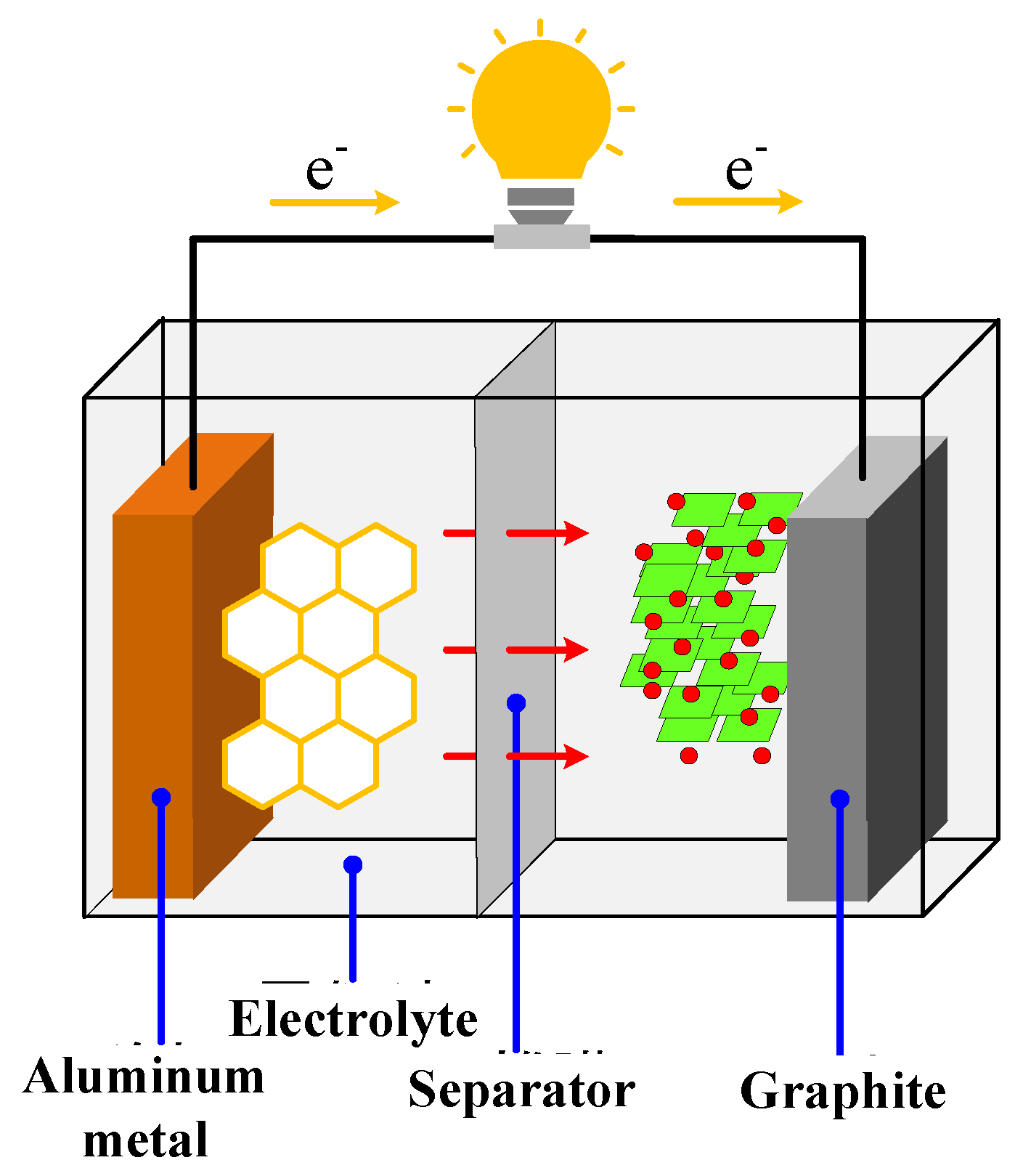
2.1. Principle and Introduction of Aluminum-Ion Batteries
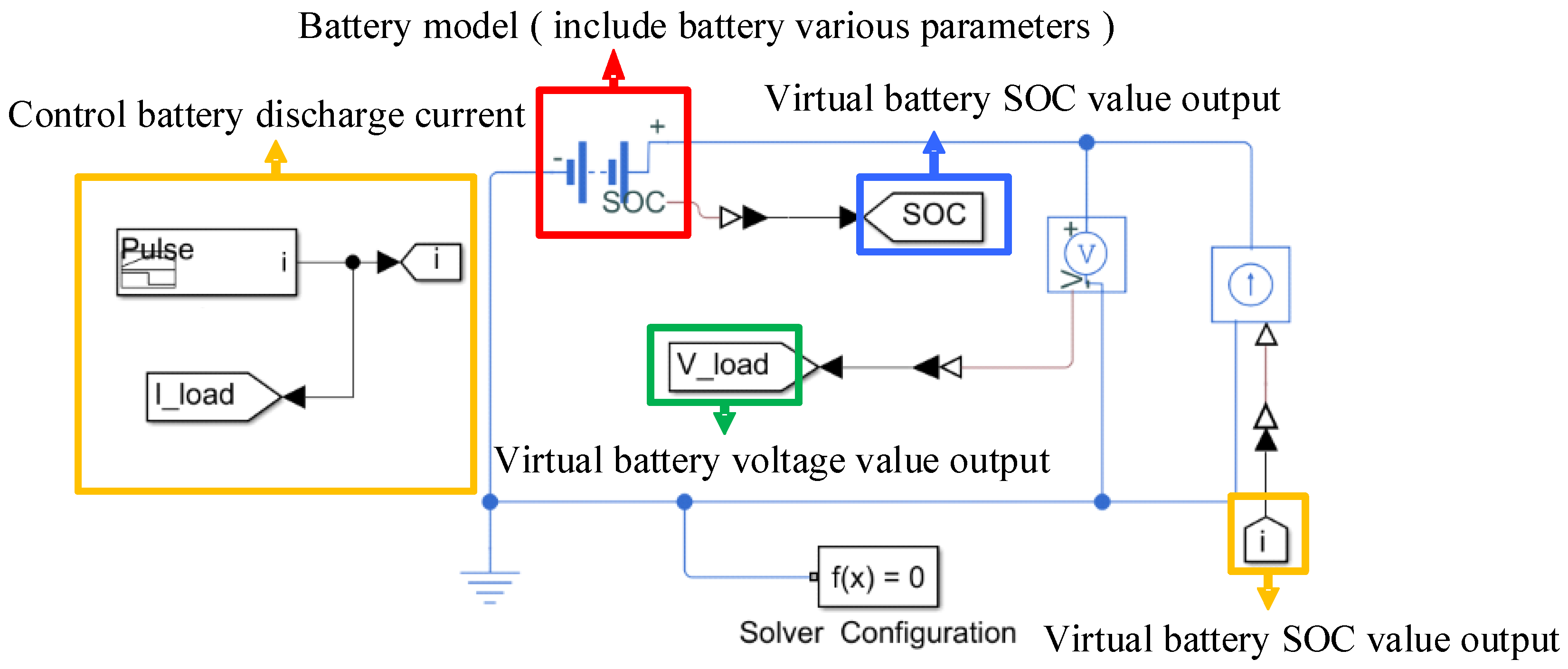
2.2. Thevenin Second-Order Equivalent Circuit Model
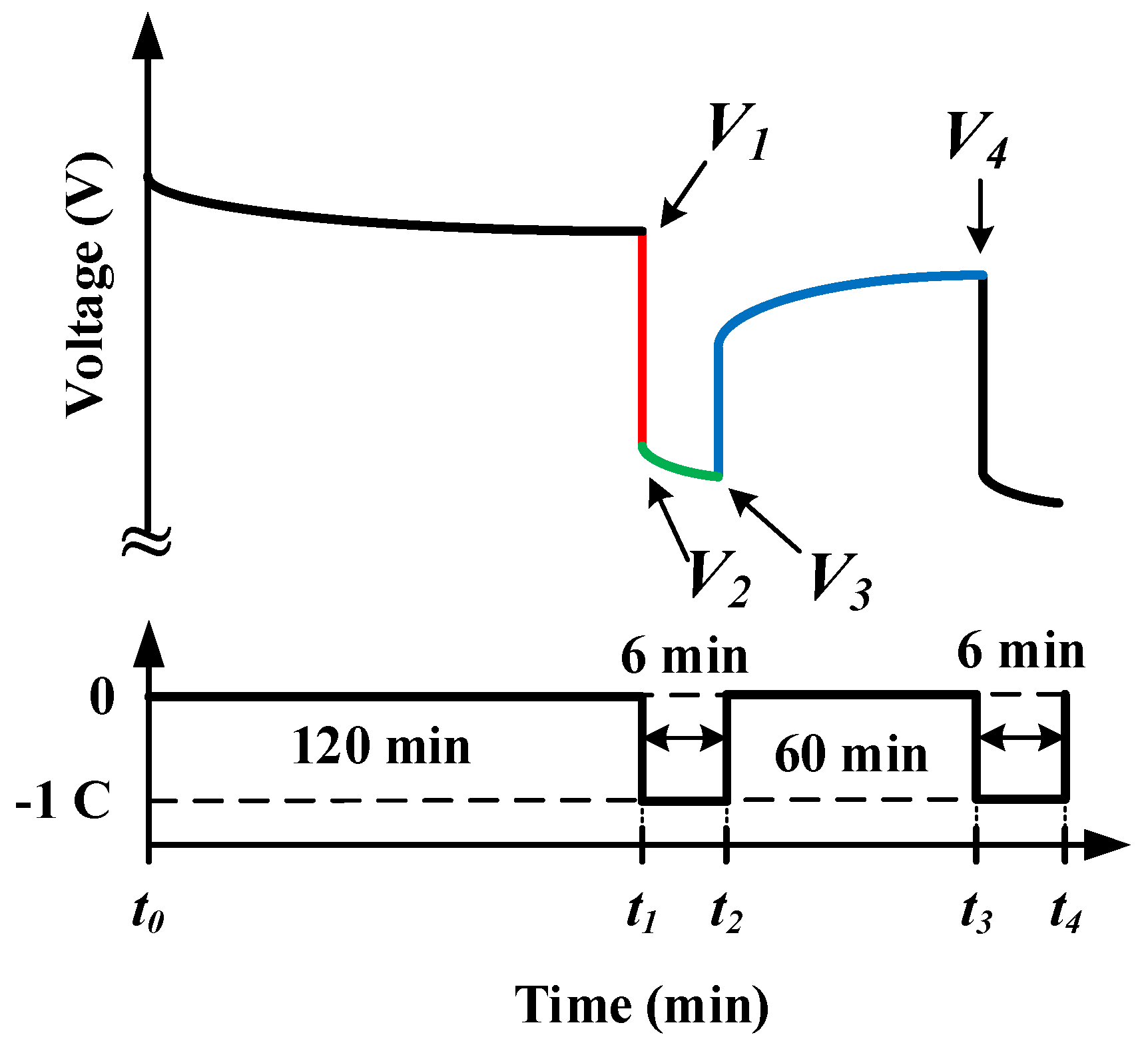
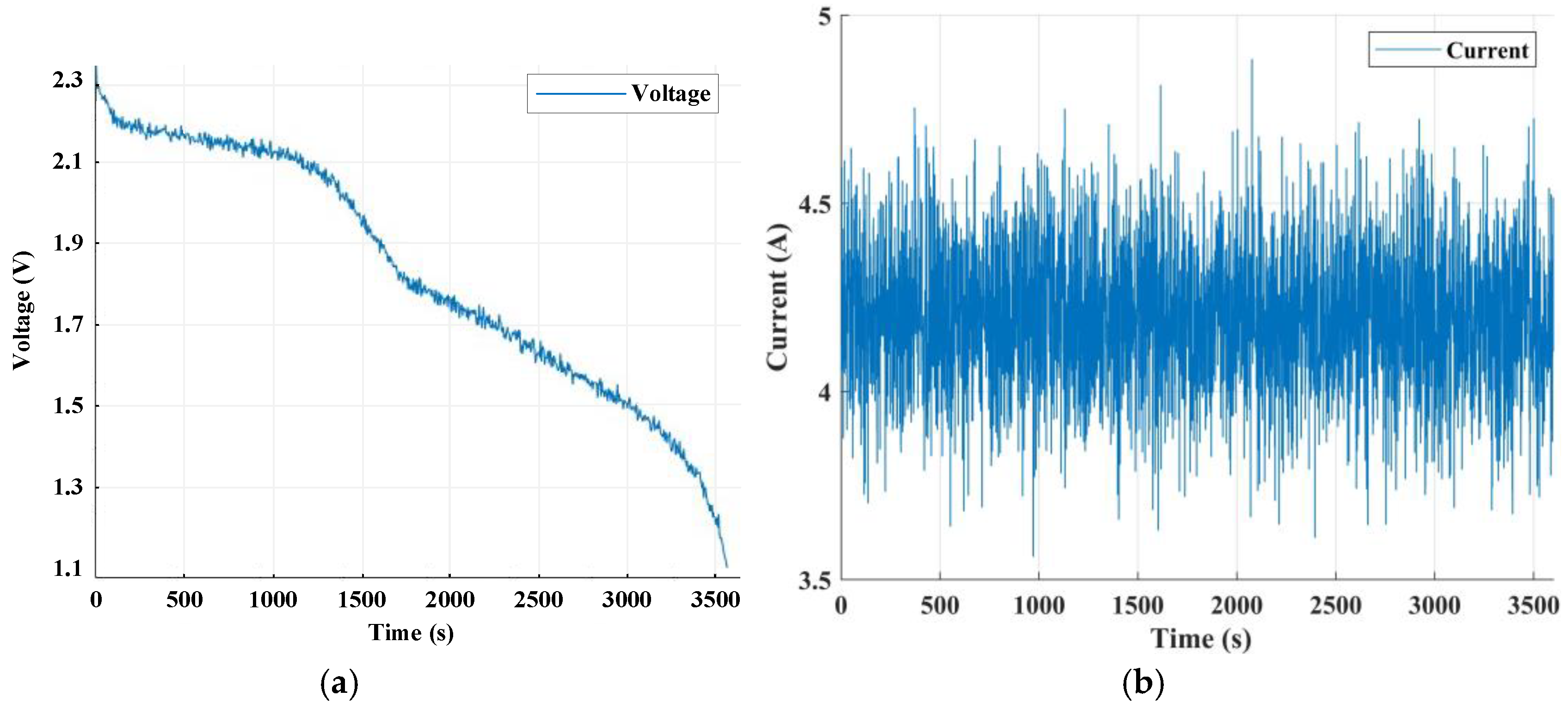
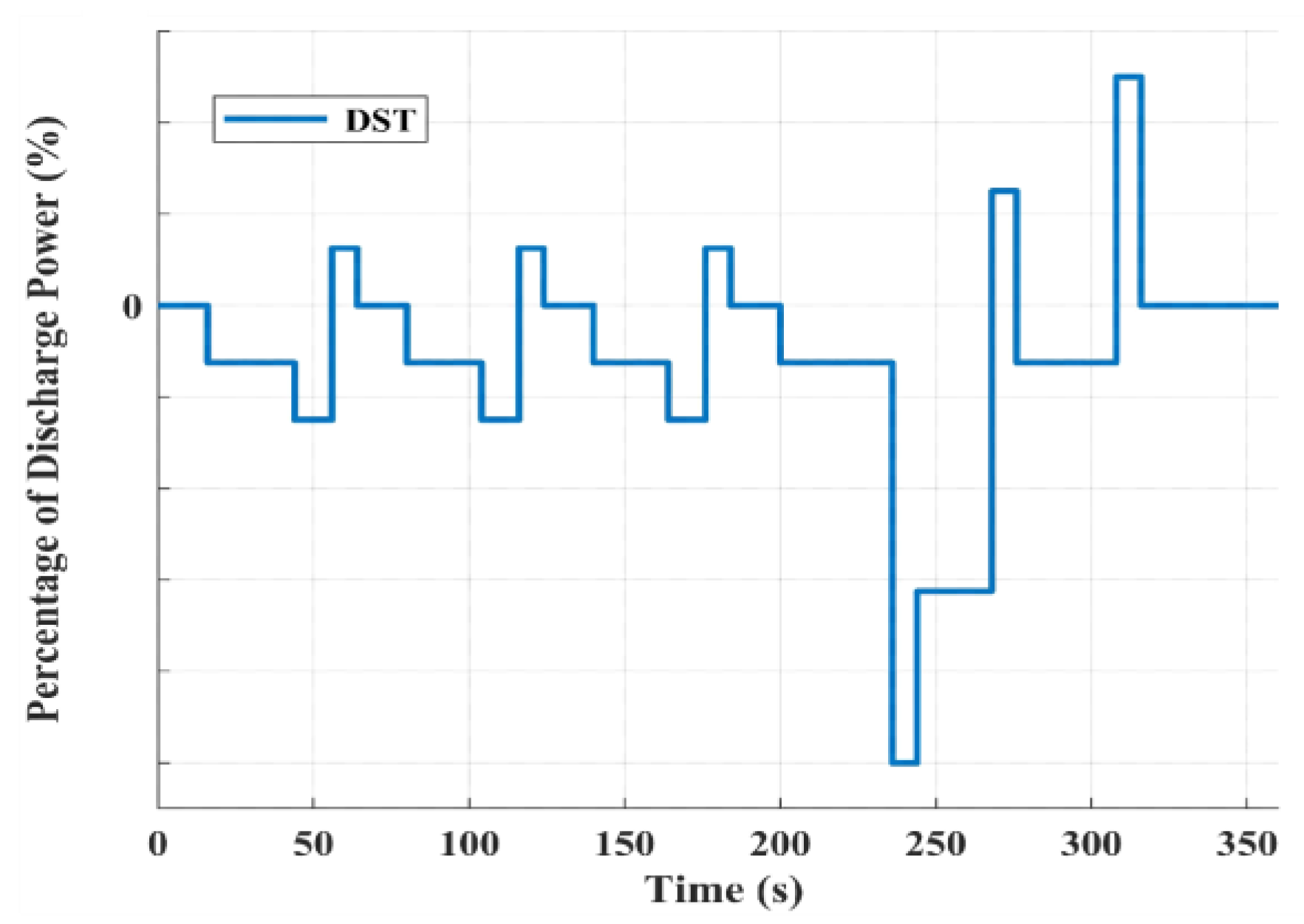
2.3. Battery Galvanostatic Intermittent Titration Technique Test
2.4. EKF Method for Al-Battery SOC Estimation
2.5. Governing Equations
- (1)
- Continuity Conservation Equation:where is the fluid density, is the fluid vector, and is the time, the above equation is expanded as follows:
- (2)
- Momentum Conservation Equations:Also known as Navier–Stokes Equations, among them , this equation is called Laplacian Operator. Where is the inertial force per unit volume, is the pressure per unit volume, is the body force per unit volume, is the viscous force per unit volume. is pressure, is the gravitational component in the , , directions in space. Since the direction of gravity in this study is only in the direction, only the gravity field in the direction is considered.
- (3)
- Energy Conservation Equation:Which can be written as:Among them, is the specific heat capacity, is the temperature item to be obtained, is the thermal conductivity coefficient of the fluid.
2.6. Turbulence Model
SST k-ω Model
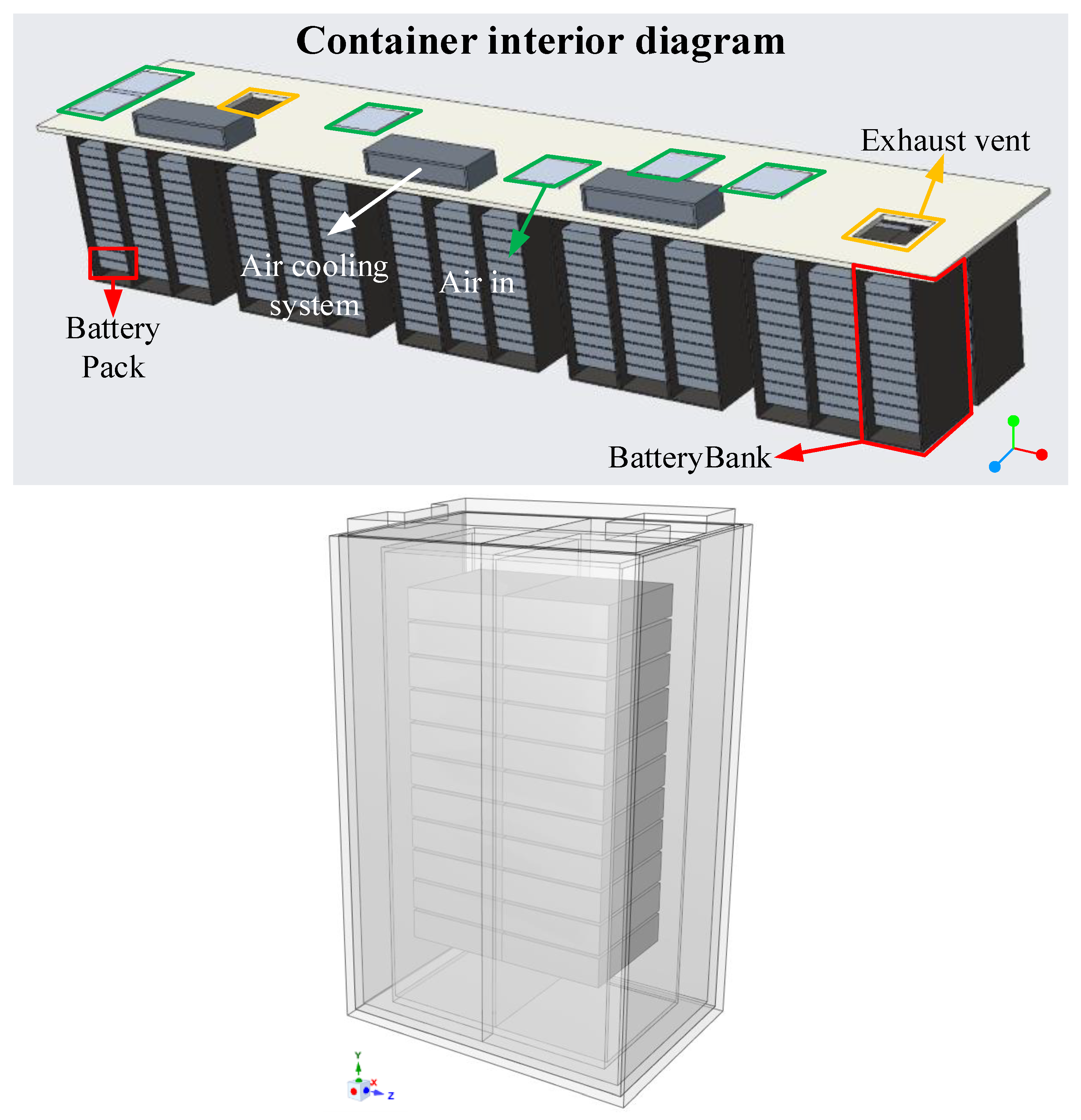
2.7. Thermal Analysis of Energy Storage Cabinet
2.8. Heat Generation of Al-Battery
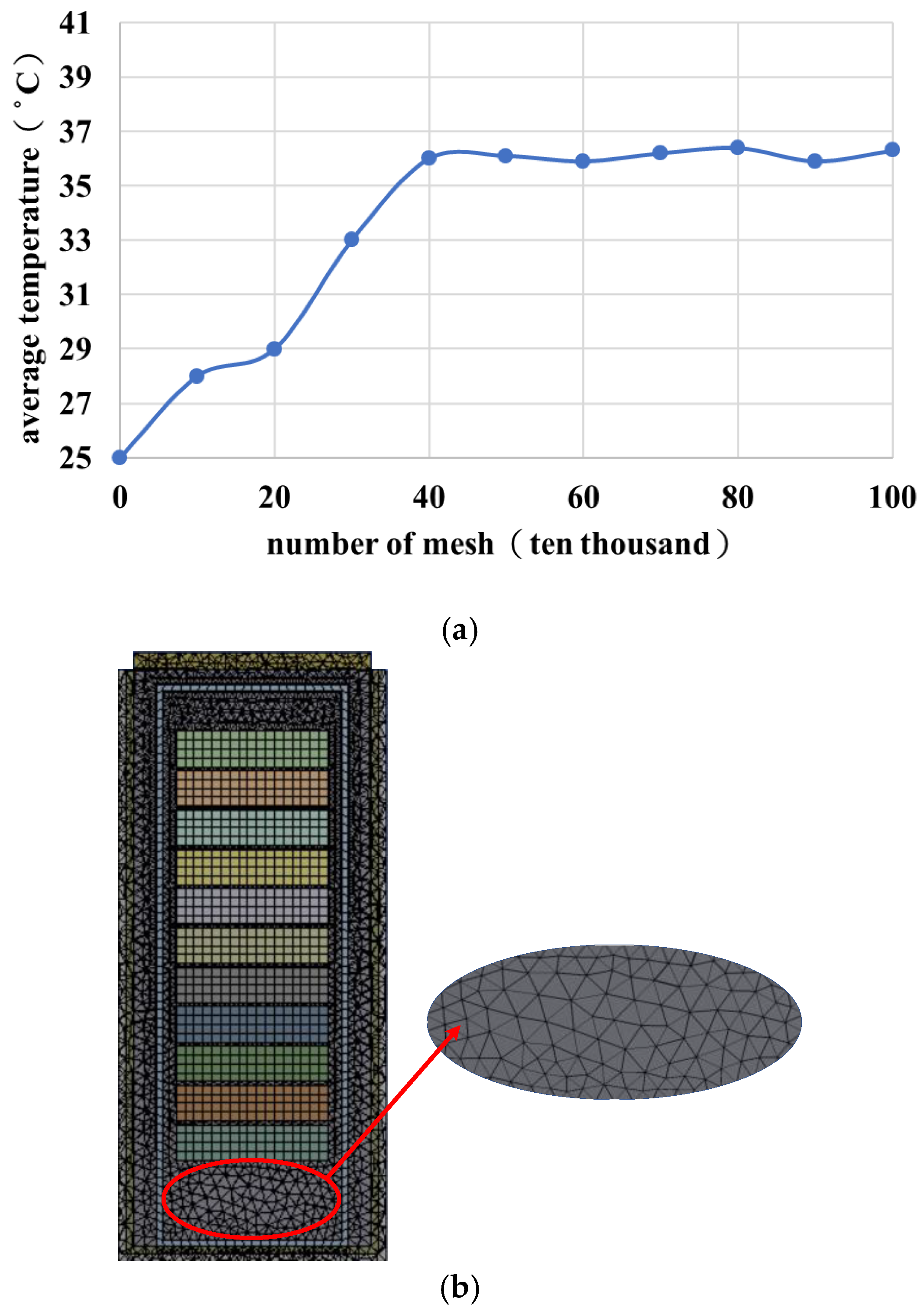
2.9. Mesh and Mesh Independence Test
3. Results and Discussion
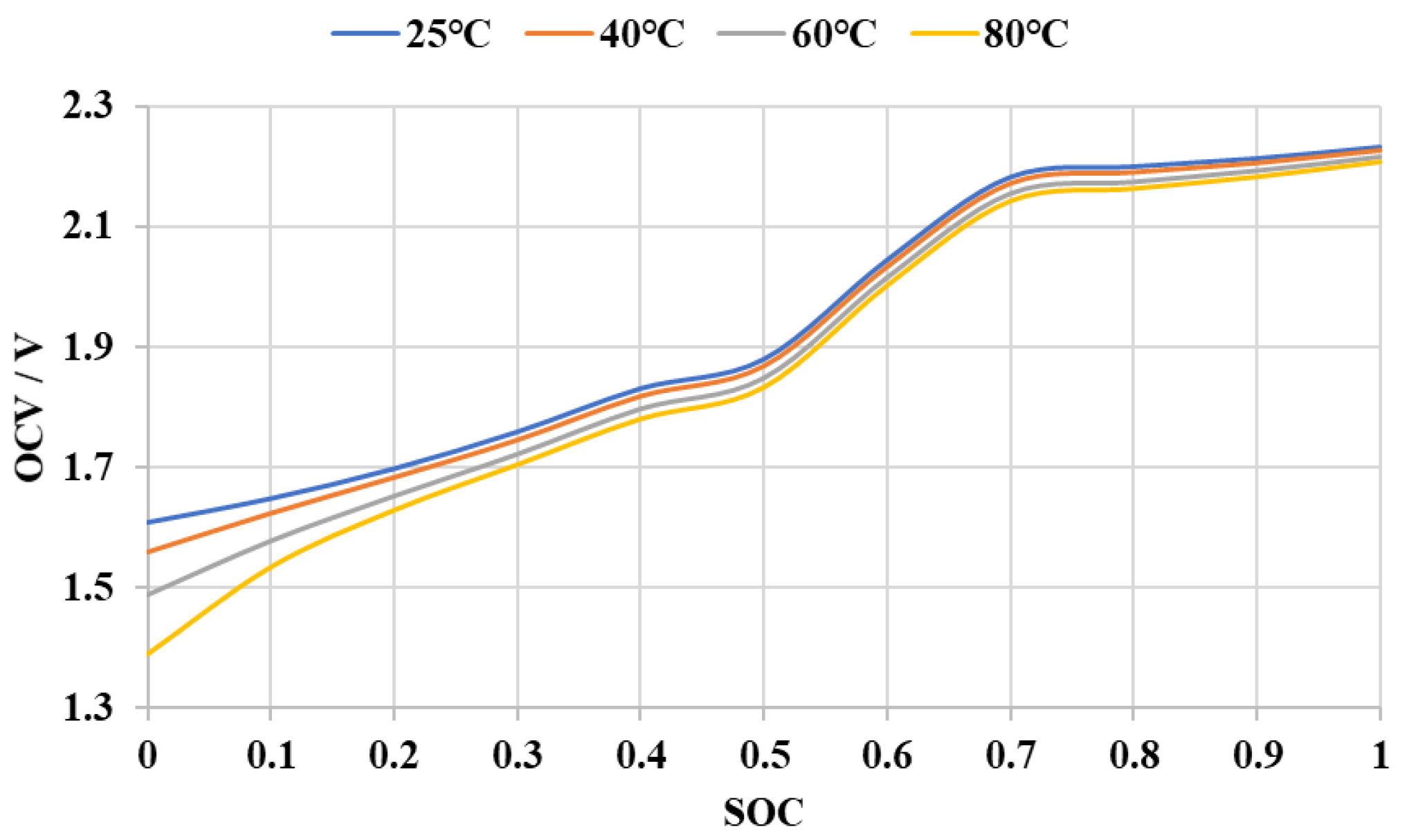
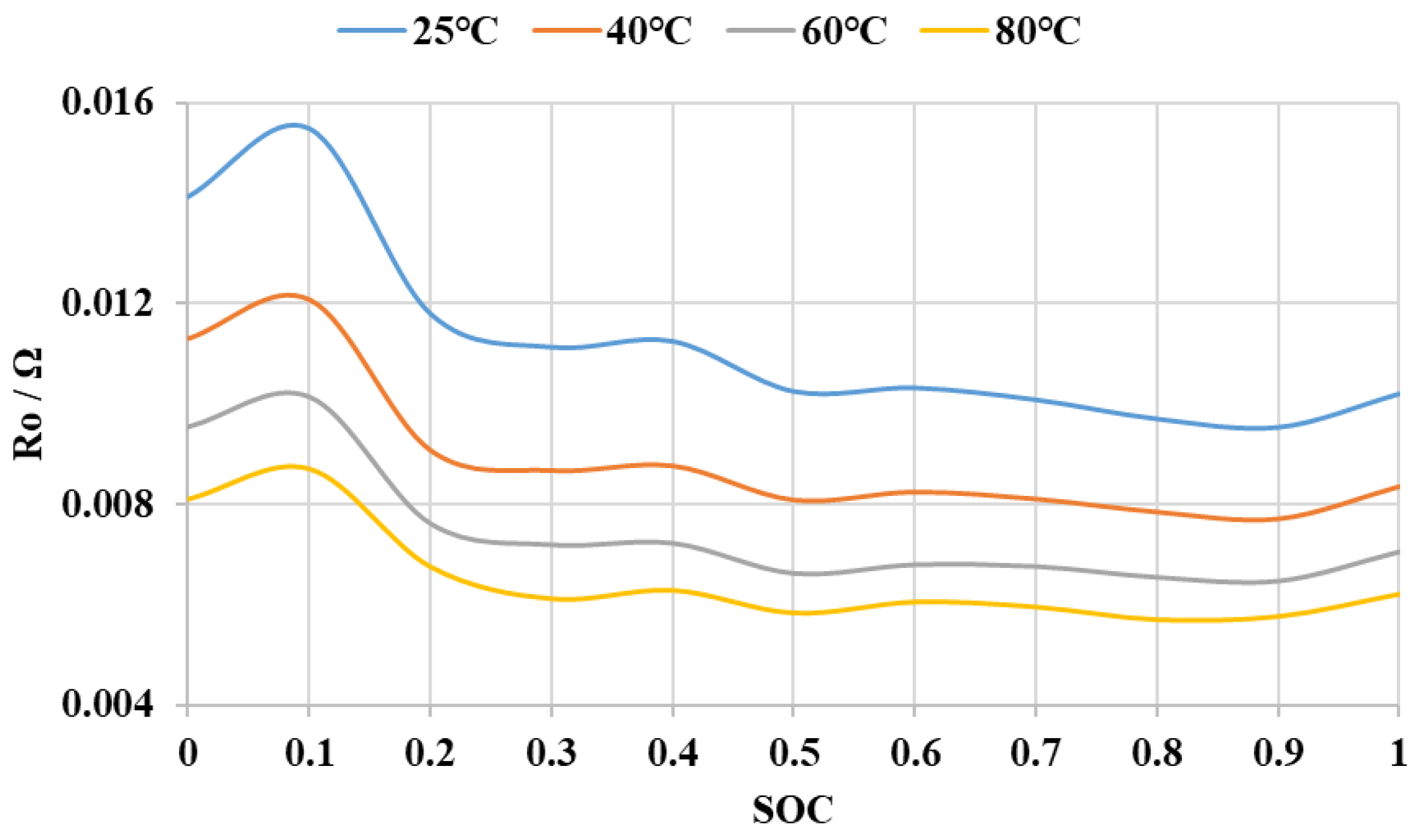
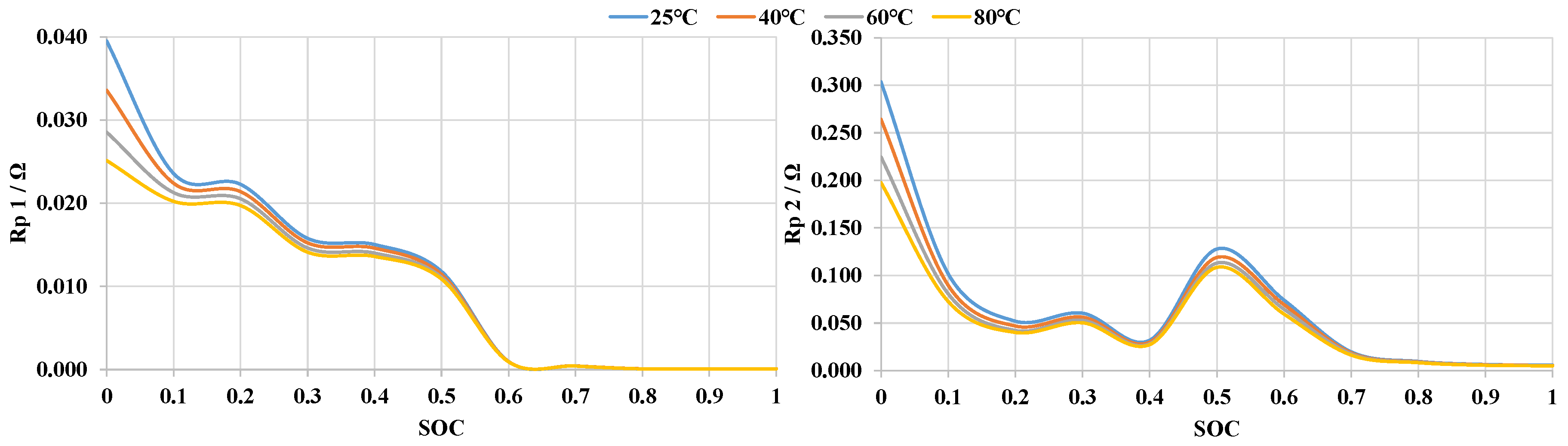
3.1. Establishment of Battery Equivalent Model
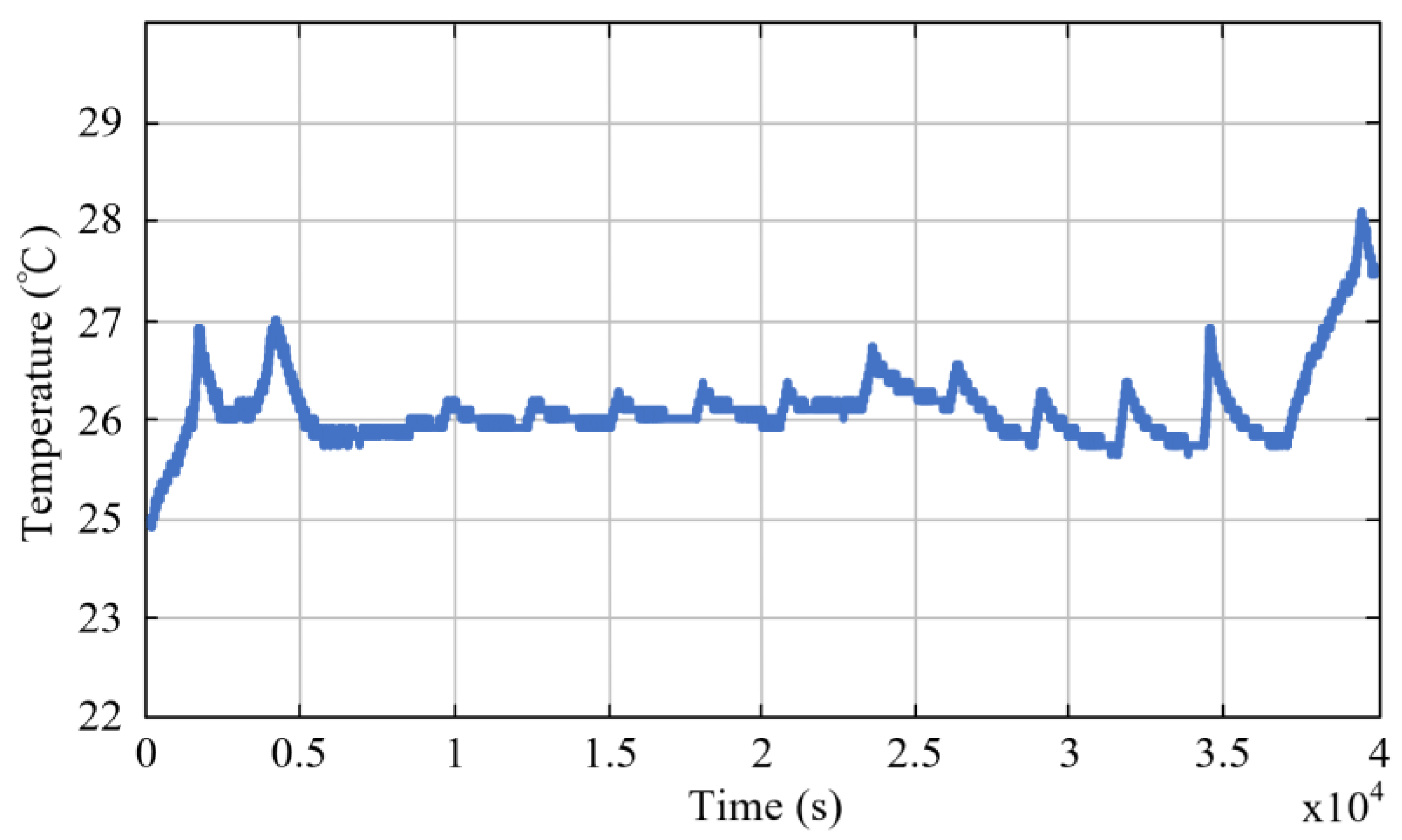
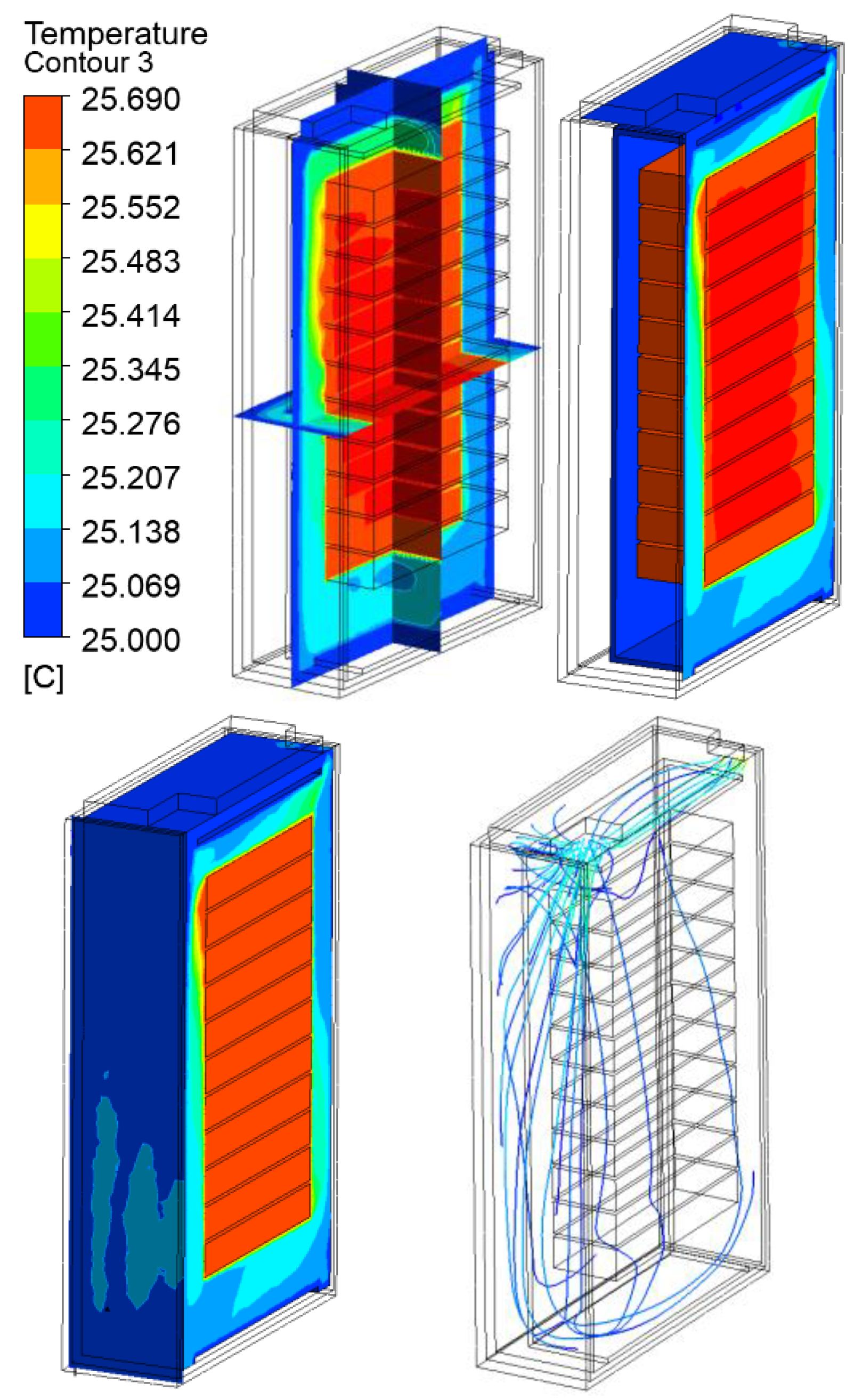
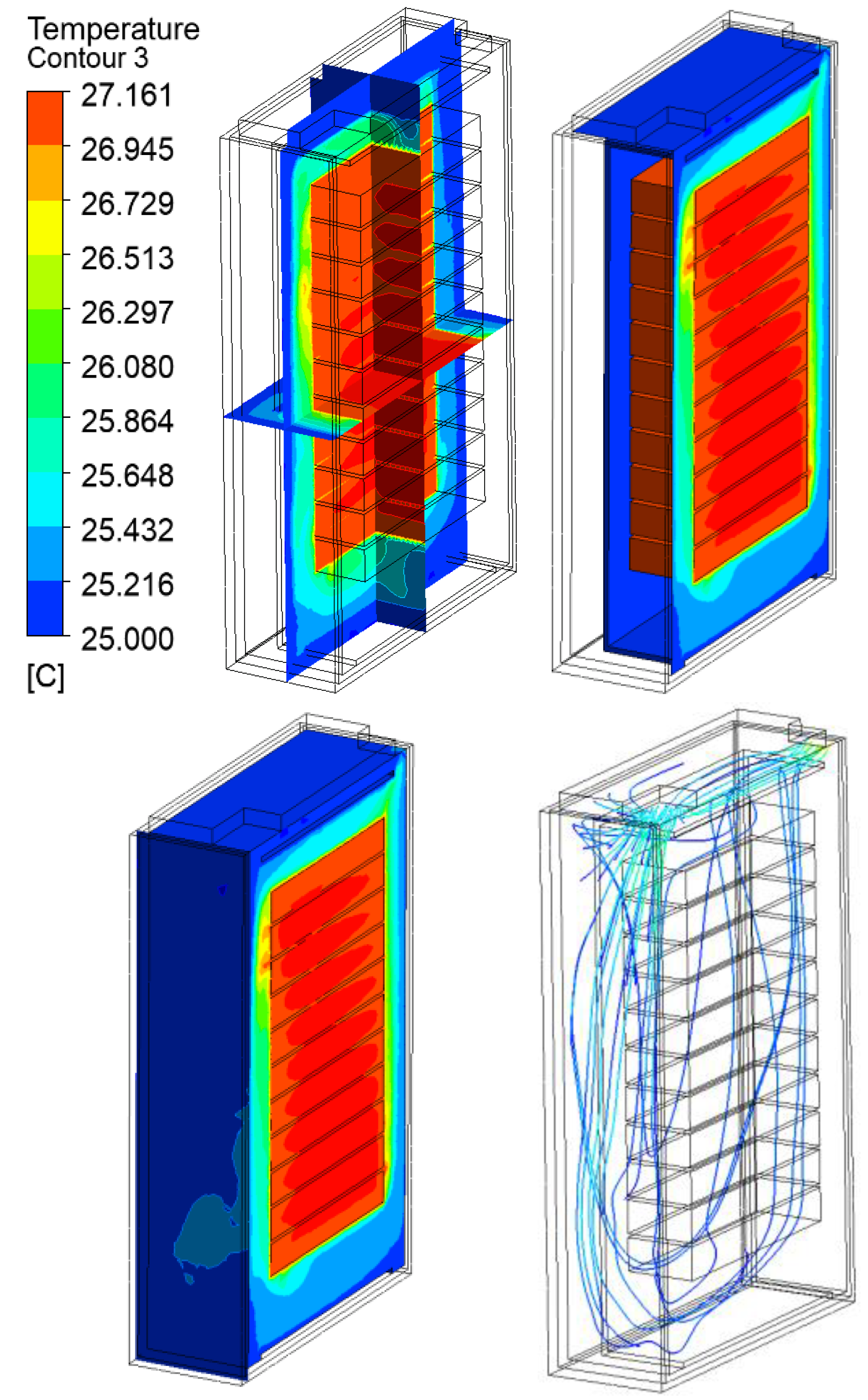
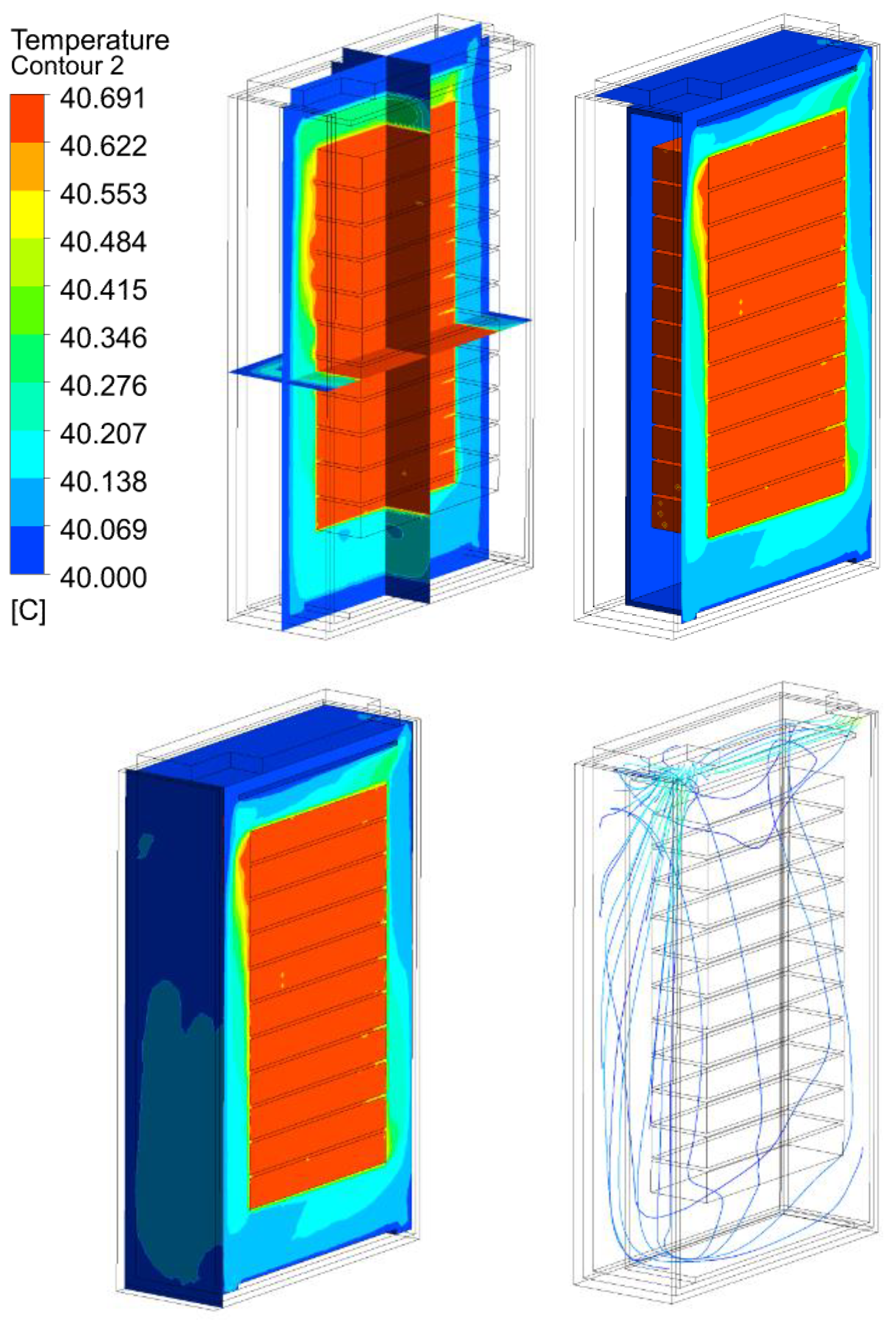
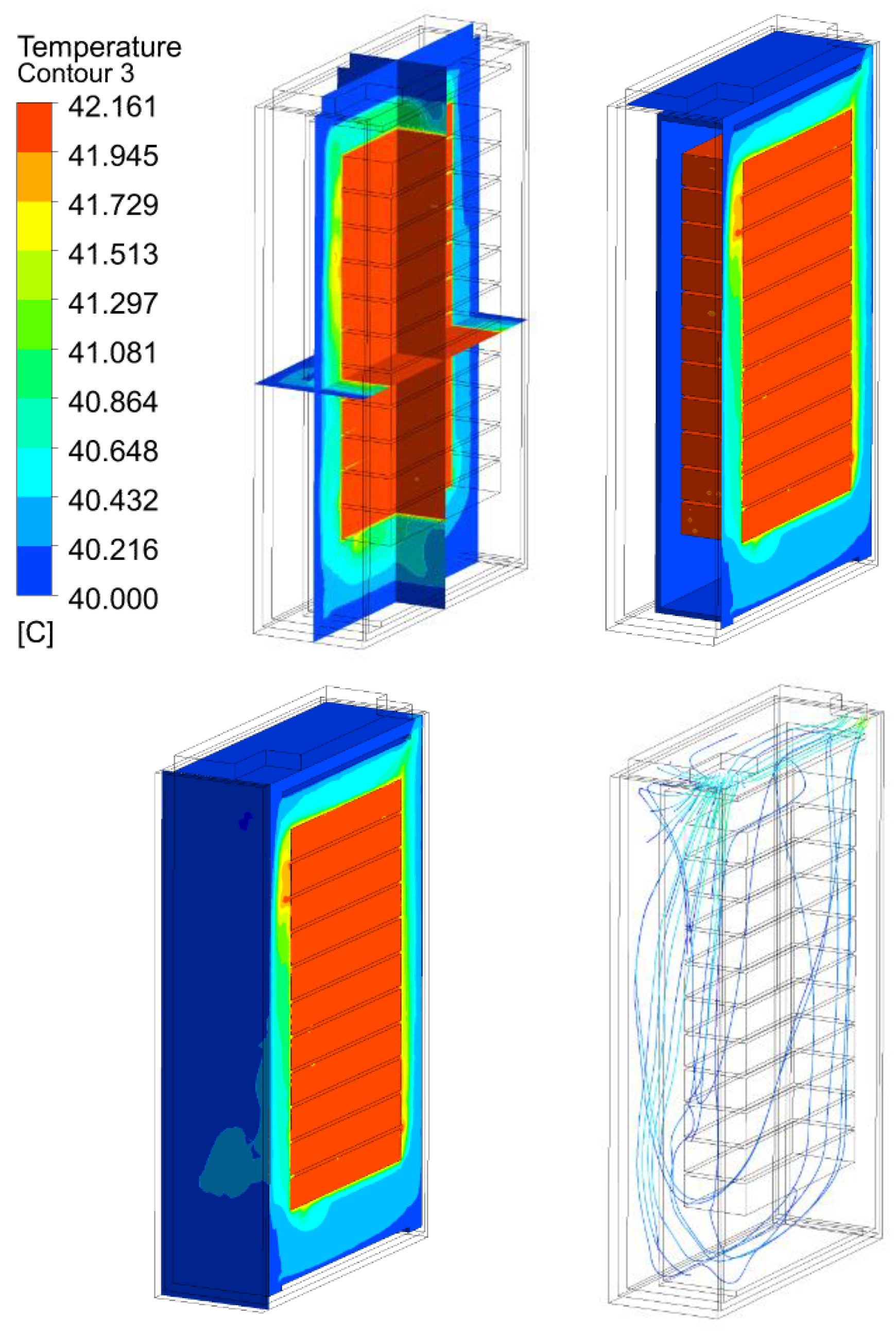
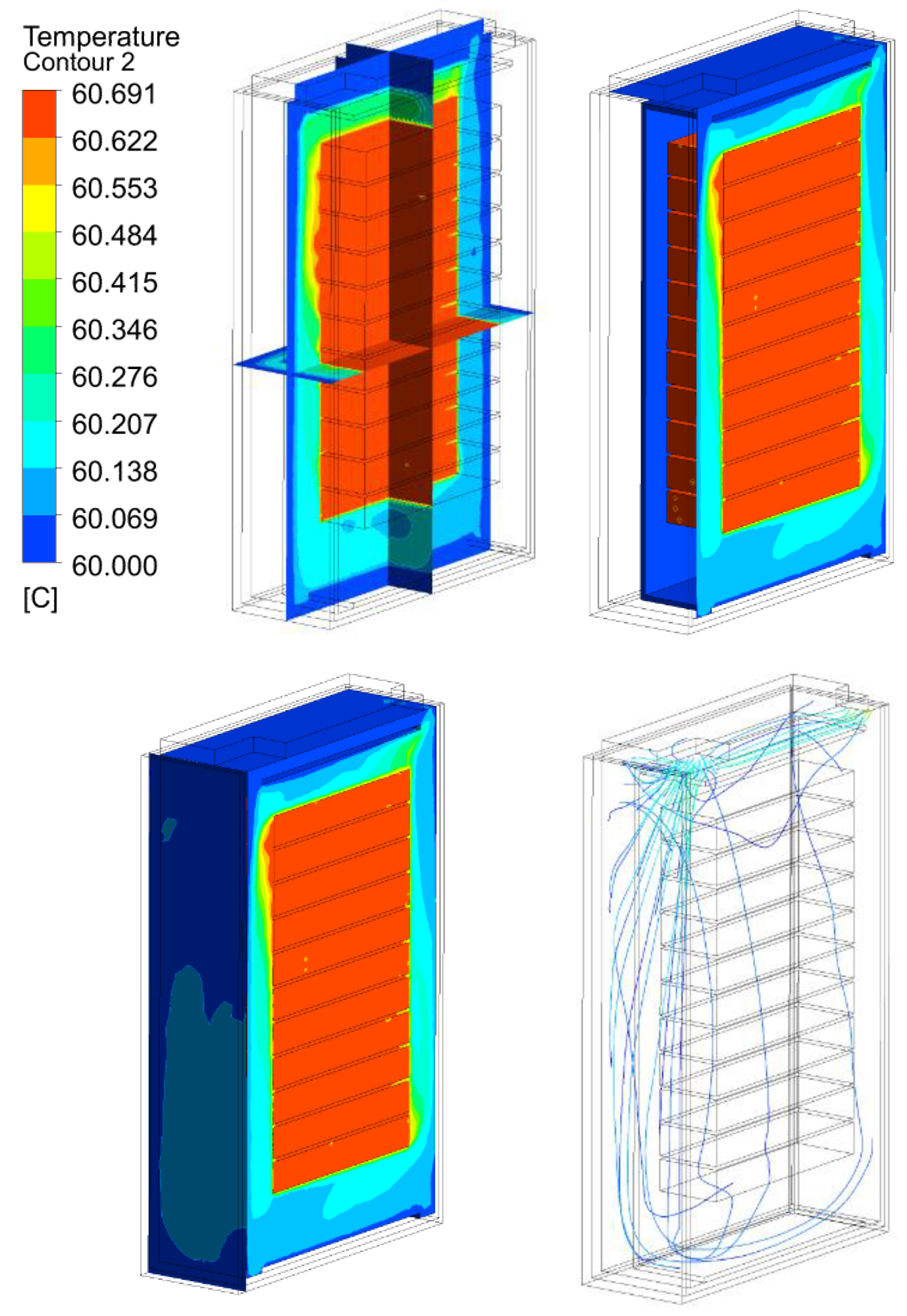
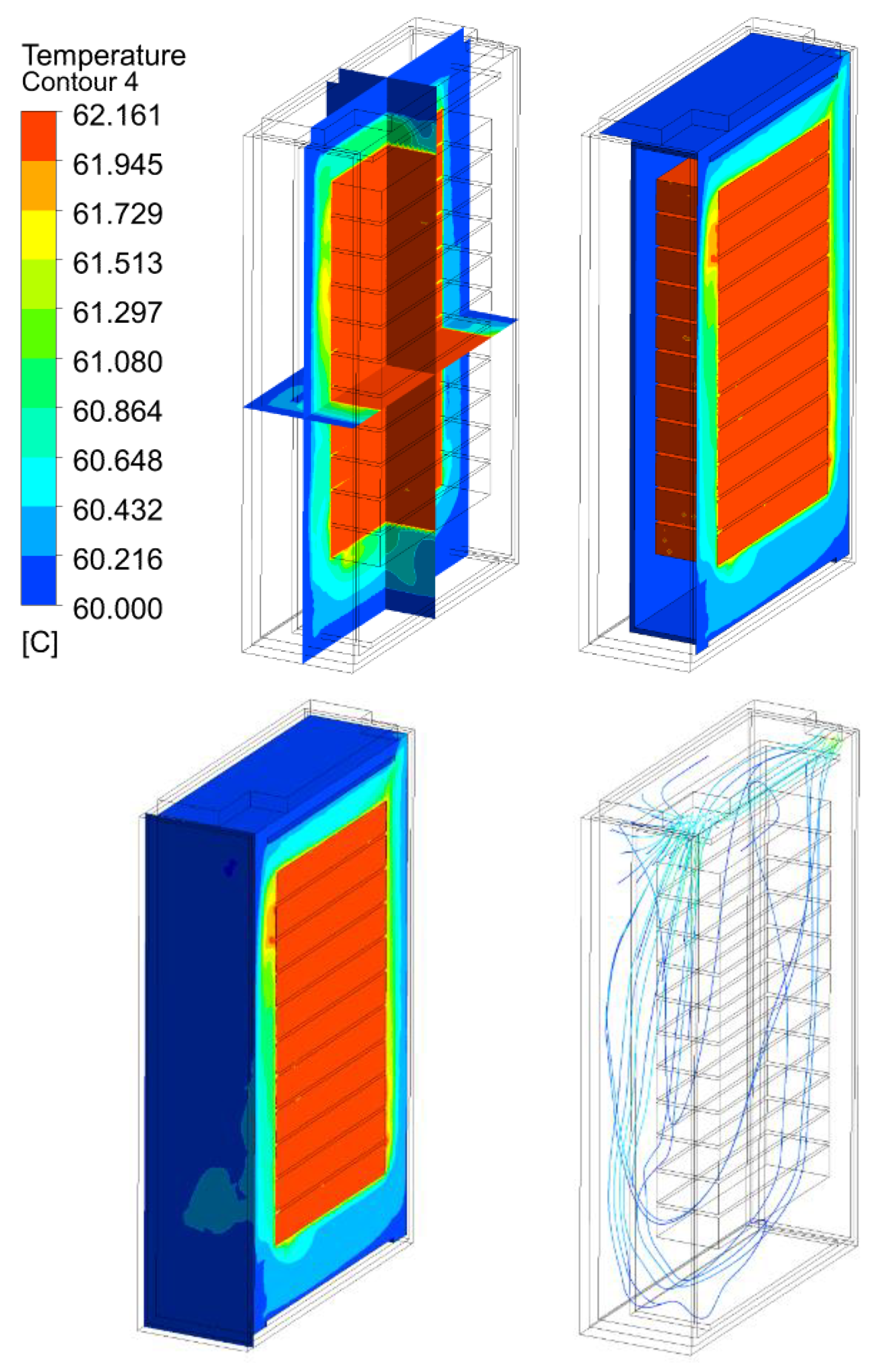
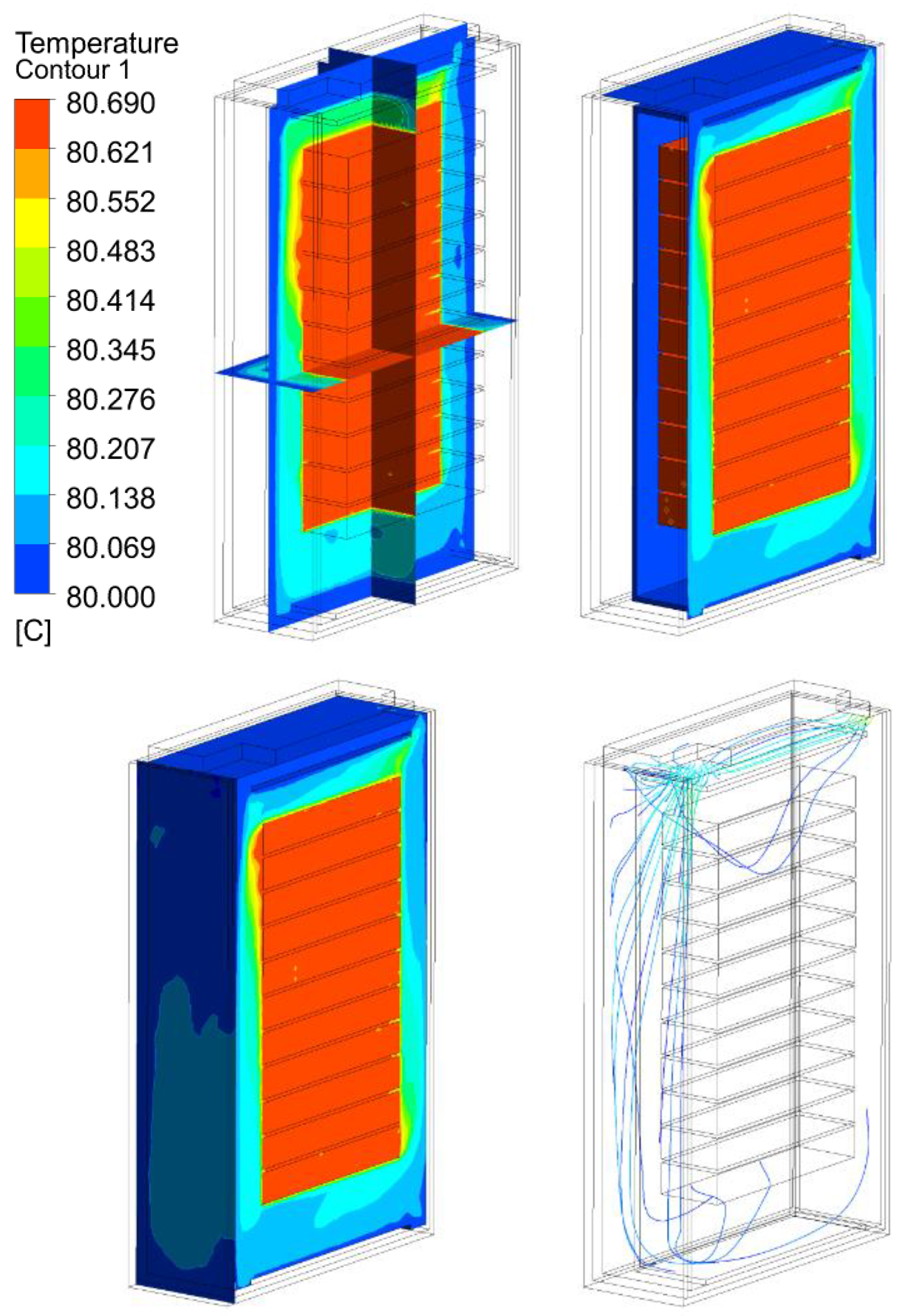
3.2. Thermal Analysis Results of Energy Storage Rack
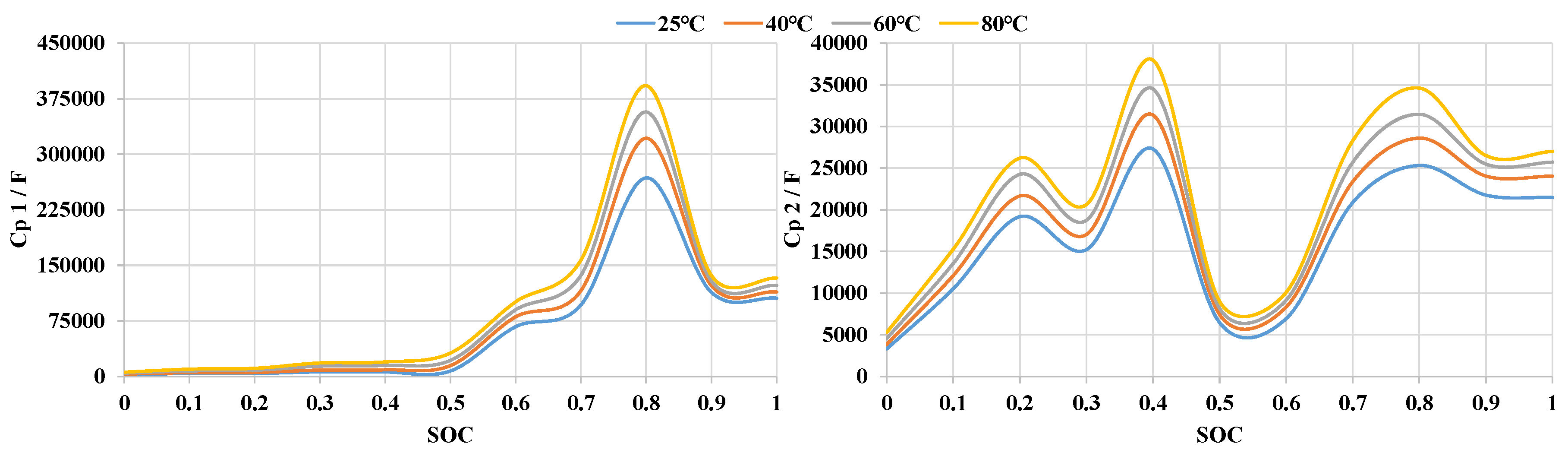
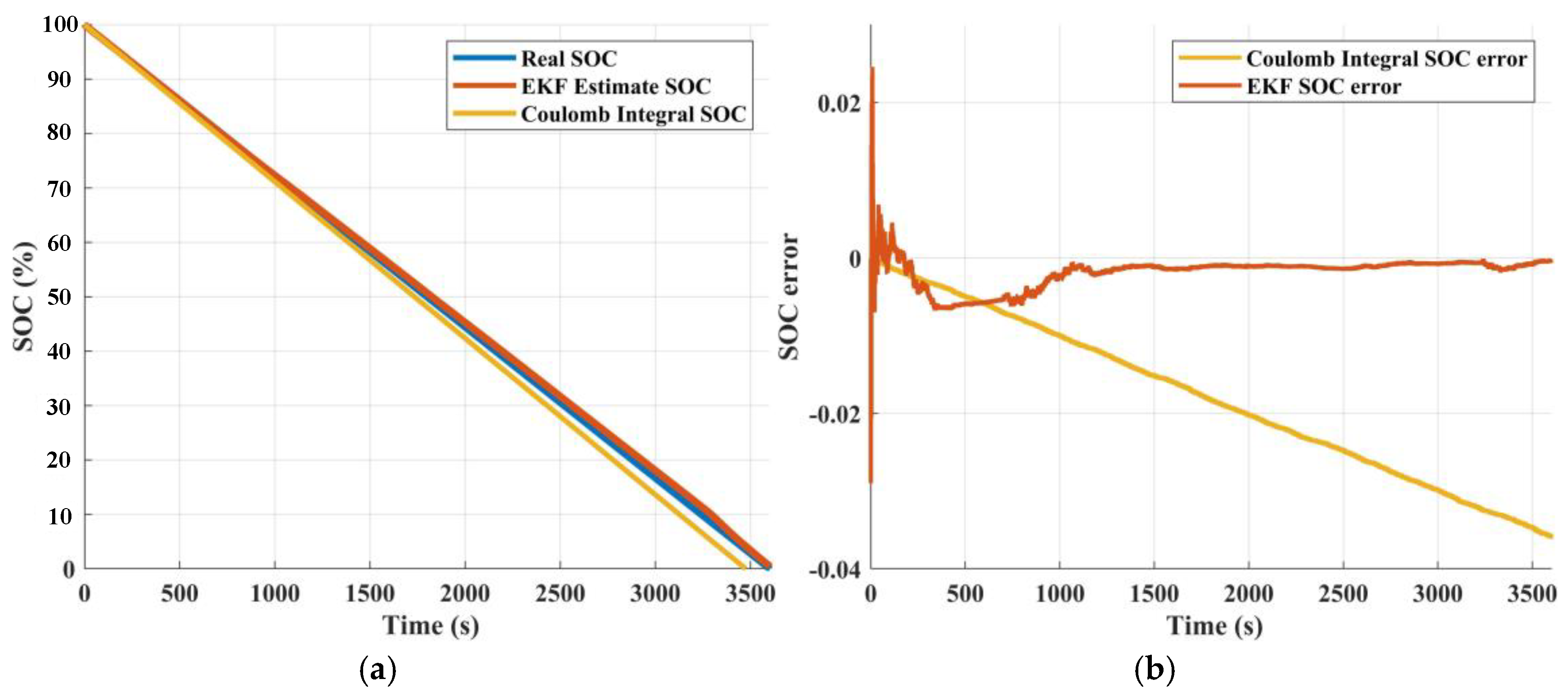
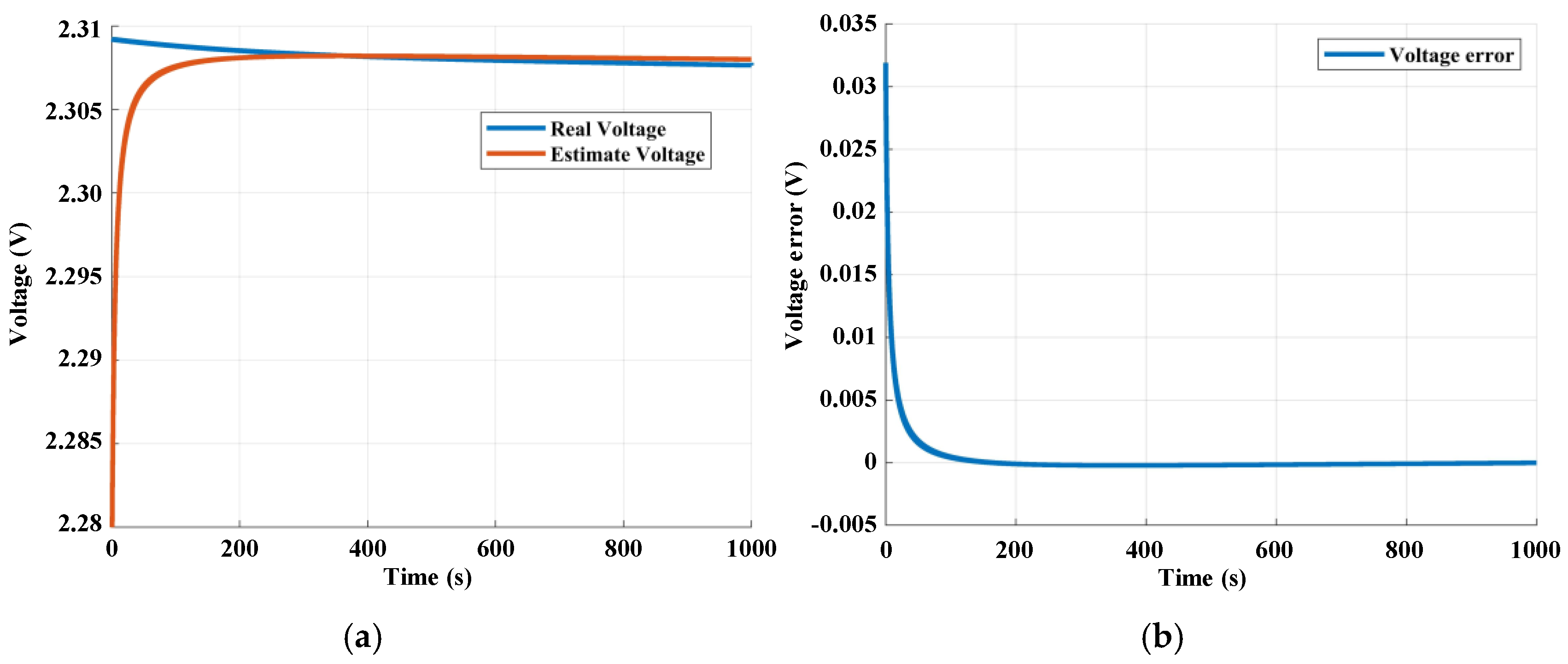
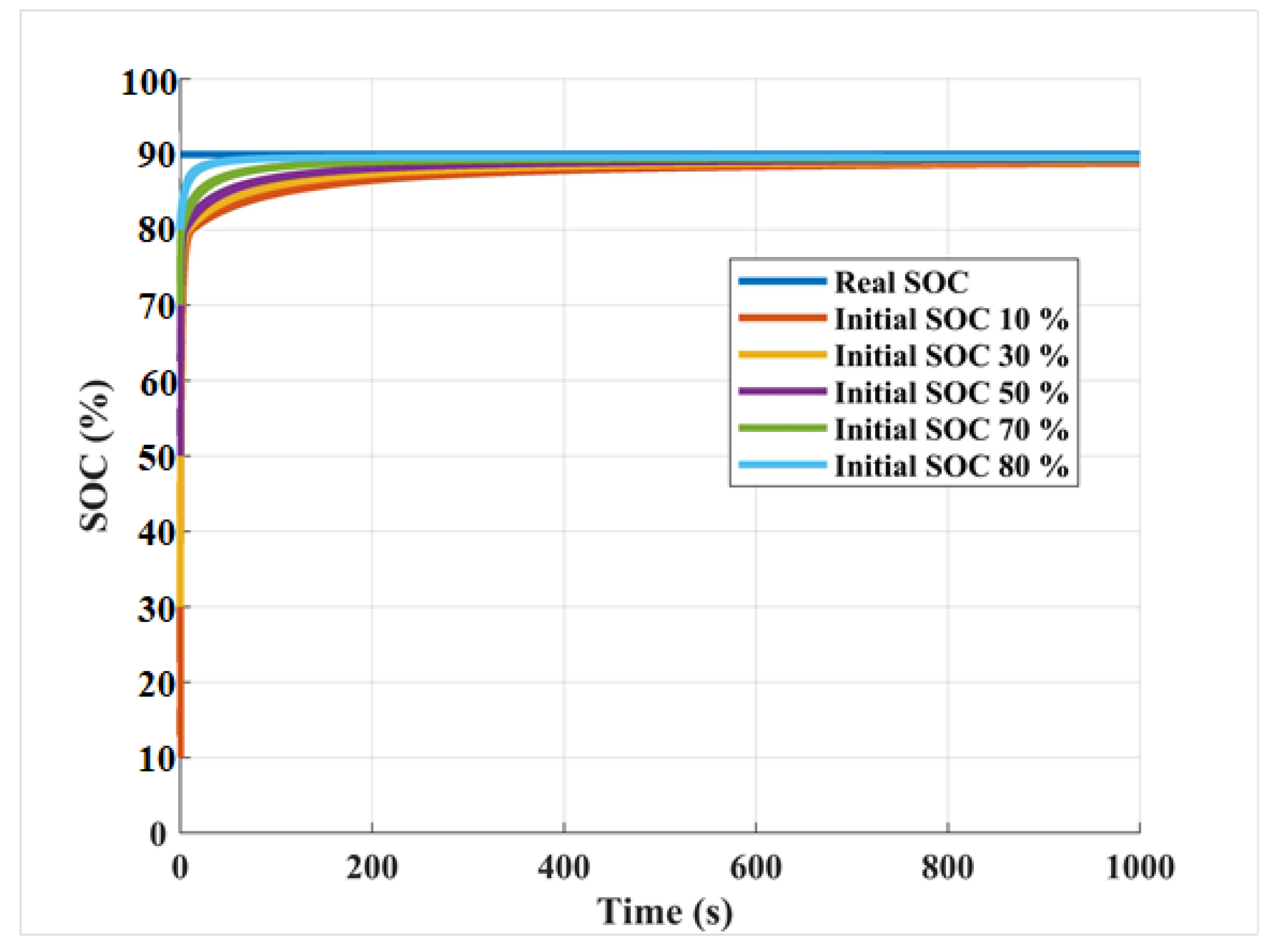
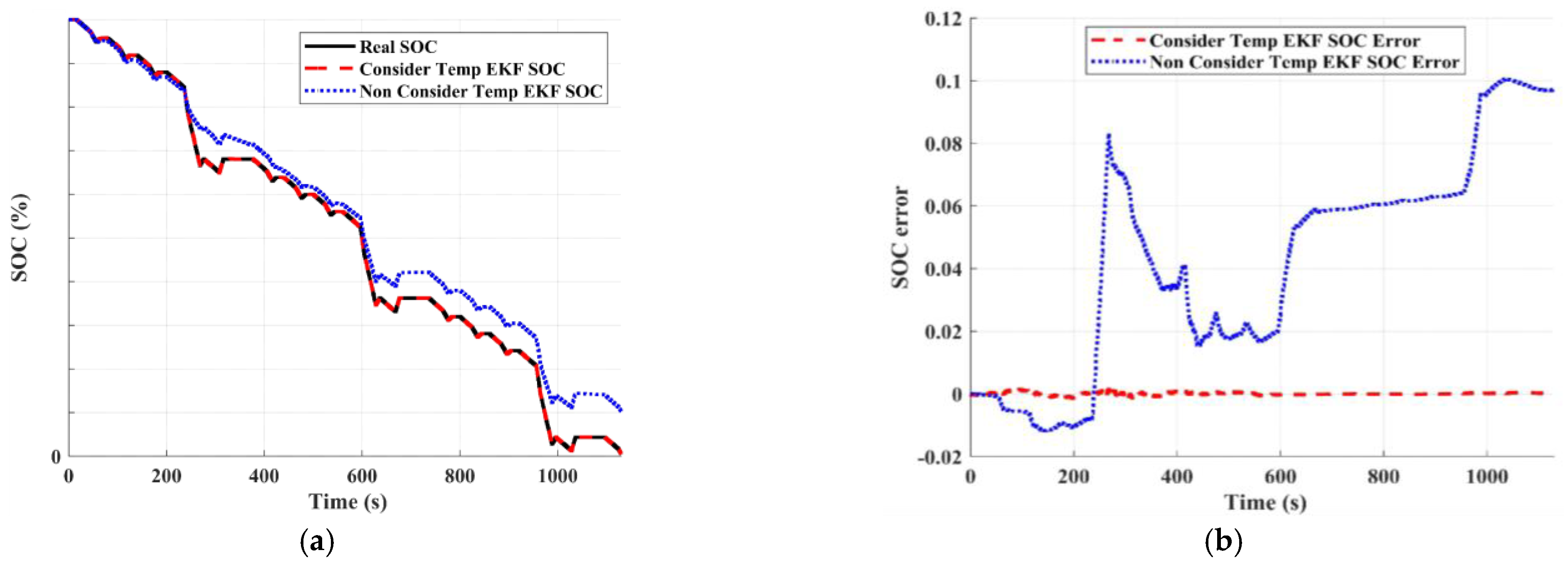
3.3. State of Charge Estimation Results
3.4. Temperature Sensitivity of AlB’s SOC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| State transition matrix | |
| State control matrix | |
| electrochemical polarization capacitance | |
| concentration polarization capacitance | |
| Specific heat | |
| The open circuit voltage of the battery | |
| Faraday constant | |
| Observation state transition matrix | |
| Heat convection coefficient | |
| Output current | |
| Kalman gain in state | |
| Thermal conductivity in the r direction | |
| Thermal conductivity in the direction | |
| Thermal conductivity in the r direction | |
| Charge quantity | |
| Covariance of prediction error in state | |
| Error common variance matrix | |
| Generation of turbulent kinetic energy | |
| Covariance of system process noise | |
| Total heat | |
| Irreversible heat | |
| Reversible heat | |
| Heat convection | |
| Covariance of system measurement noise | |
| electrochemical polarization resistance | |
| concentration polarization resistance | |
| the ohm’s internal resistance | |
| R | Covariance of system measurement noise |
| Time constant | |
| Temperature | |
| Battery temperature | |
| Ambient temperature | |
| Time | |
| System control matrix of state | |
| Battery working voltage | |
| Open circuit voltage | |
| terminal voltage of the internal resistance | |
| terminal voltage of the concentration polarization | |
| terminal voltage of the electrochemical polarization | |
| System state matrix of state | |
| System state matrix of state | |
| Zk | measured value of state |
| X axis velocity component | |
| Y axis velocity component | |
| Z axis velocity component | |
| Fluid velocity vector | |
| Dissipation of turbulent kinetic energy | |
| Generation of turbulent dissipation rate | |
| Dissipation of turbulent dissipation rate | |
| Re | Reynolds number |
| Greek symbols | |
| Gradient operator | |
| fluid density (kg/m3) | |
| Thermal conductivity (W/m-K) | |
| dynamic viscosity (kg/m·s) | |
| Prandtl number | |
| AIB | Aluminum-ion battery |
| C-rate | current discharge/ charge rate |
| CFD | Computational fluid dynamics |
| BESS | Battery energy storage system |
| 3D | Three dimensions |
| LIB | Lithium-ion battery |
| HEV | Hybrid electric vehicle |
| OCV | Open circuit voltage |
| DPPC | Discharge pulse power characterization |
| SOC | State of charge |
| EV | Electric vehicle |
| BMS | Battery management system |
| EMIC | 1-ethyl-3-methylimidazolium chloride |
| AlCl3 | Aluminum chloride |
References
- Giannelos, S.; Konstantelos, I.; Strbac, G. Option value of dynamic line rating and storage. In Proceedings of the 2018 IEEE International Energy Conference (ENERGYCON), Limassol, Cyprus, 3–7 June 2018; pp. 1–6. [Google Scholar]
- Ji, Y.; Zhang, Y.; Wang, C.-Y. Li-Ion Cell Operation at Low Temperatures. J. Electrochem. Soc. 2013, 160, A636–A649. [Google Scholar] [CrossRef]
- He, X.; Hu, Z.; Restuccia, F.; Fang, J.; Rein, G. Experimental study of the effect of the state of charge on self-heating ignition of large ensembles of lithium-ion batteries in storage. Appl. Therm. Eng. 2022, 212, 118621. [Google Scholar] [CrossRef]
- Ren, D.; Smith, K.; Guo, D.; Han, X.; Feng, X.; Lu, L.; Ouyang, M.; Li, J. Investigation of Lithium Plating-Stripping Process in Li-Ion Batteries at Low Temperature Using an Electrochemical Model. J. Electrochem. Soc. 2018, 165, A2167–A2178. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Q.; Li, S.; Mao, J. High-temperature effect on electrochemical performance of Li4Ti5O12 based anode material for Li-ion batteries. J. Alloys Compd. 2018, 753, 192–202. [Google Scholar] [CrossRef]
- Pang, C.; Dutta, P.; Kezunovic, M. BEVs/PHEVs as Dispersed Energy Storage for V2B Uses in the Smart Grid. IEEE Trans. Smart Grid 2011, 3, 473–482. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Temperature effect and thermal impact in lithium-ion batteries: A review. Prog. Nat. Sci. 2018, 28, 653–666. [Google Scholar] [CrossRef]
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.M.; Jervis, R.; Bailey, J.J.; Vo, N.T.; Magdysyuk, O.V.; Drakopoulos, M.; et al. Shearing, Thermal Runaway: Identifying the Cause of Rupture of Li-Ion Batteries during Thermal Runaway. Adv. Sci. 2018, 5, 1870003. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Z.; He, W.; Zhao, J. Thermal issues about Li-ion batteries and recent progress in battery thermal management systems: A review. Energy Convers. Manag. 2017, 150, 304–330. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.-Y.; Tang, X. Cycling degradation of an automotive LiFePO4 lithium-ion battery. J. Power Sources 2011, 196, 1513–1520. [Google Scholar] [CrossRef]
- Kim, J.; Oh, J.; Lee, H. Review on battery thermal management system for electric vehicles. J. Appl. Therm. Eng. 2019, 149, 192–212. [Google Scholar] [CrossRef]
- Wu, W.; Wang, S.; Wu, W.; Chen, K.; Hong, S.; Lai, Y. Acritical review of battery thermal performance and liquid based battery thermal management. Energy Convers. Manag. 2019, 182, 262–281. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, Y.; Liu, Y.; Li, H.; Gao, J.; Zhong, L.; Lou, X. Temperature Stability of Dielectric Constant and Energy Storage Properties of (Pb1-x,Lax)(Zr0.65,Ti0.35)O3 Relaxor Ferroelectric Thin Films. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 2052–2057. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Smith, B.D.; Wills, R.G.A.; Cruden, A.J. Aqueous Al-ion cells and supercapacitors—A comparison. Energy Rep. 2020, 6, 166–173. [Google Scholar] [CrossRef]
- Pozzato, G.; Müller, M.; Formentin, S.; Savaresi, S.M. Economic MPC for online least costly energy management of hybrid electric vehicles. Control. Eng. Pract. 2020, 102, 104534. [Google Scholar] [CrossRef]
- Fan, J.; Qin, Z.; Luo, Y.; Li, K.; Peng, H. A novel power management strategy for hybrid off-road vehicles. Control. Eng. Pract. 2020, 101, 104452. [Google Scholar] [CrossRef]
- Li, Q.; Bjerrum, N.J. Aluminum as anode for energy storage and conversion: A review. J. Power Sources 2002, 110, 1–10. [Google Scholar] [CrossRef]
- Ellingsen, L.A.W.; Holland, A.; Drillet, J.F.; Peters, W.; Eckert, M.; Concepcion, C.; Ruiz, O.; Colin, J.F.; Knipping, E.; Pan, Q.; et al. Environmental screening of electrode materials for a rechargeable aluminum battery with an AlCl3/EMIMCl electrolyte. Materials 2018, 11, 936. [Google Scholar] [CrossRef]
- Mckerracher, R.; Holland, A.; Cruden, A.; Wills, R. Comparison of carbon materials as cathodes for the aluminium-ion battery. Carbon 2018, 144, 333–341. [Google Scholar] [CrossRef]
- Holland, A.W.; Cruden, A.; Zerey, A.; Hector, A.; Wills, R.G.A. Electrochemical study of TiO2 in aqueous AlCl3 electrolyte via vacuum impregnation for superior high-rate electrode performance. BMC Energy 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Lin, M.-C.; Gong, M.; Lu, B.; Wu, Y.; Wang, D.-Y.; Guan, M.; Angell, M.; Chen, C.; Yang, J.; Hwang, B.-J.; et al. An ultrafast rechargeable aluminium-ion battery. Nature 2015, 520, 324–328. [Google Scholar] [CrossRef]
- Hu, Z.; He, X.; Restuccia, F.; Rein, G. Anisotropic and homogeneous model of heat transfer for self-heating ignition of large ensembles of lithium-ion batteries during storage. Appl. Therm. Eng. 2021, 197, 117301. [Google Scholar] [CrossRef]
- Du, S.; Jia, M.; Cheng, Y.; Tang, Y.; Zhang, H.; Ai, L.; Zhang, K.; Lai, Y. Study on the thermal behaviors of power lithium iron phosphate (LFP) aluminum-laminated battery with different tab configurations. Int. J. Therm. Sci. 2015, 89, 327–336. [Google Scholar] [CrossRef]
- Shen, X.; Sun, T.; Yang, L.; Krasnoslobodtsev, A.; Sabirianov, R.; Sealy, M.; Mei, W.-N.; Wu, Z.; Tan, L. Ultra-fast charging in aluminum-ion batteries: Electric double layers on active anode. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Saha, S.; Haque, M.E.; Arif, M.; Oo, A. A Parameter Extraction Method for the Thevenin Equivalent Circuit Model of Li-ion Batteries. In Proceedings of the IEEE Industry Applications Society Annual Meeting, Baltimore, MD, USA, 29 September 2019–3 October 2019; pp. 1–7. [Google Scholar] [CrossRef]
- Gurjer, L.; Chaudhary, P.; Verma, H.K. Detailed Modelling Procedure for Lithium-ion Battery Using Thevenin Equivalent. In Proceedings of the 2019 IEEE International Conference on Electrical, Computer and Communication Technologies (ICECCT), Coimbatore, India, 20–22 February 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Immonen, E.; Hurri, J. Incremental thermo-electric CFD modeling of a high-energy Lithium-Titanate Oxide battery cell in different temperatures: A comparative study. Appl. Therm. Eng. 2021, 197, 117260. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, B.; Li, B.; Cao, L.; Lai, Y.; Zheng, W.; Wang, H.; Wang, W. State of the Art of Lithium-Ion Battery SOC Estimation for Electrical Vehicles. Energies 2018, 11, 1820. [Google Scholar] [CrossRef]
- Wang, K.; Feng, X.; Pang, J.; Ren, J.; Duan, C.; Li, L. State of charge (SOC) estimation of lithium-ion battery based on adaptive square root unscented kalman filter. J. Electrochem. Sci. 2020, 15, 9499–9516. [Google Scholar]
- Li, W.; Yang, Y.; Wang, D.; Yin, S. The multi-innovation extended Kalman filter algorithm for battery SOC estimation. Ionics 2020, 26, 6145–6156. [Google Scholar] [CrossRef]
- Cai, Y.-S.; Ho, J.-R. Development of ANSYS-Fluent-based Numerical Approaches for Studies of Three-dimensional Gas Flows in a Planetary MOCVD Reactor. Electronic Theses & Dissertations. Master’s Thesis, National Central University, Taoyuan, Taiwan, 2015. [Google Scholar]
- Huang, C.; Tseng, C. Numerical Simulation and Analysis of Dynamic Stall on a Pitching Airfoil with Turbulence Models. Master’s Thesis, Department of Mechanical and Electro-Mechanical Engineering, 2017. [Google Scholar]
- Jiang, C.; Wang, S.; Wu, B.; Fernandez, C.; Xiong, X.; Coffie-Ken, J. A state-of-charge estimation method of the power lithium-ion battery in complex conditions based on adaptive square root extended Kalman filter. Energy 2020, 219, 119603. [Google Scholar] [CrossRef]
- Hu, Y.; Choe, S.-Y.; Garrick, T.R. Hybridized time-frequency method for the measurement of entropy coefficient of lithium-ion battery. Electrochim. Acta 2020, 362, 137124. [Google Scholar] [CrossRef]






| Characteristic | Type | Unit |
|---|---|---|
| Resistance at 25 °C | 10 K | Ω |
| Temperature Range | −40 to +120 °C | |
| R Tolerance | 1.0 | % |
| B Value | 3435 | |
| B Tolerance | 1.0 | % |
| Wire Type | Separate | |
| Package | DIP |
| Property | Value | Unit |
|---|---|---|
| ρ | 1.225 | kg/m3 |
| 1 | m/s | |
| 1.8 ×∙10−5 | Pa∙S | |
| Reynolds number (Re) | 14,544 | - |
| Material | Air | Cabinet Steel Frame | Foam Insulation | Battery Cell | |
|---|---|---|---|---|---|
| Material Property | |||||
| Density (kg/m3) | 1.184 | 8030 | 17.6 | 2697 | |
| Specific Heat (j/kg-k) | 1006.43 | 502.48 | 1501 | 823 | |
| Heat Conductivity (W/m-k) | 0.0242 | 16.27 | 0.0439 | Axial-28.5 Radial-3.4 | |
| Viscosity (kg/m-s) | 1.7894 × 10−5 | - | - | - | |
| Molecular Weight (kg/kmol) | 28.966 | - | - | - | |
| Property | Fluid Velocity (m/s) | Temperature (°C) | Pressure (atm) | Amount | |
|---|---|---|---|---|---|
| Boundary | |||||
| Air inlet | 1.05 | 25 | - | 6 | |
| Exhaust vent | - | 25 | 1 | 2 | |
| Wall boundary condition | No-slip wall boundary | ||||
| Battery Type | Aluminum-Ion Battery | |
|---|---|---|
| Term | ||
| Cell nominal voltage (V) | 2.35~1.1 | |
| Cell nominal capacity (Ah) | 0.764 | |
| Anode type | Aluminum | |
| Cathode type | 3D Graphite | |
| electrolyte | Ionic liquid (EMIC&AlCl3) | |
| Temperature | 25 °C | 40 °C | 60 °C | 80 °C | |
|---|---|---|---|---|---|
| Power Output/C-rate | |||||
| 30 kW/7C | 25.69 | 40.69 | 60.69 | 80.69 | |
| 120 kW/28C | 27.16 | 42.16 | 62.16 | 82.16 | |
30 kW/7C |  |  |  |  | |
120 kW/28C |  |  | 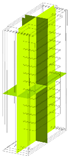 | 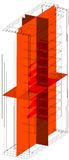 | |
| SOC Errors | EKFtemp | EKF | Coulomb’s Integral | Sensitivity | |
|---|---|---|---|---|---|
| Environment Temperature | |||||
| 25 °C | 0.0010 | 0.0010 | 0.07 | 0.000 | |
| 40 °C | 0.0034 | 0.0065 | 0.10 | 0.911 | |
| 60 °C | 0.0055 | 0.0120 | 0.24 | 1.181 | |
| 80 °C | 0.0060 | 0.0162 | 0.31 | 1.700 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.-H.; Hsieh, C.-H.; Teng, L.-T.; Huang, C.-C. Experimental Study on Temperature Sensitivity of the State of Charge of Aluminum Battery Storage System. Energies 2023, 16, 4270. https://doi.org/10.3390/en16114270
Chen B-H, Hsieh C-H, Teng L-T, Huang C-C. Experimental Study on Temperature Sensitivity of the State of Charge of Aluminum Battery Storage System. Energies. 2023; 16(11):4270. https://doi.org/10.3390/en16114270
Chicago/Turabian StyleChen, Bin-Hao, Chen-Hsiang Hsieh, Li-Tao Teng, and Chien-Chung Huang. 2023. "Experimental Study on Temperature Sensitivity of the State of Charge of Aluminum Battery Storage System" Energies 16, no. 11: 4270. https://doi.org/10.3390/en16114270
APA StyleChen, B.-H., Hsieh, C.-H., Teng, L.-T., & Huang, C.-C. (2023). Experimental Study on Temperature Sensitivity of the State of Charge of Aluminum Battery Storage System. Energies, 16(11), 4270. https://doi.org/10.3390/en16114270







