Capacity Degradation and Aging Mechanisms Evolution of Lithium-Ion Batteries under Different Operation Conditions
Abstract
1. Introduction
- A high-fidelity simulation model by coupling the P2D model and four aging mechanisms were developed to emulate the battery aging behaviors under different operation conditions.
- The battery capacity degradation under different DODs, SOC swing ranges and temperatures was analyzed systematically based on the simulation experiment.
- The aging mechanisms and the interaction effects between them under these usage conditions were analyzed systematically, based on which some new discoveries were made.
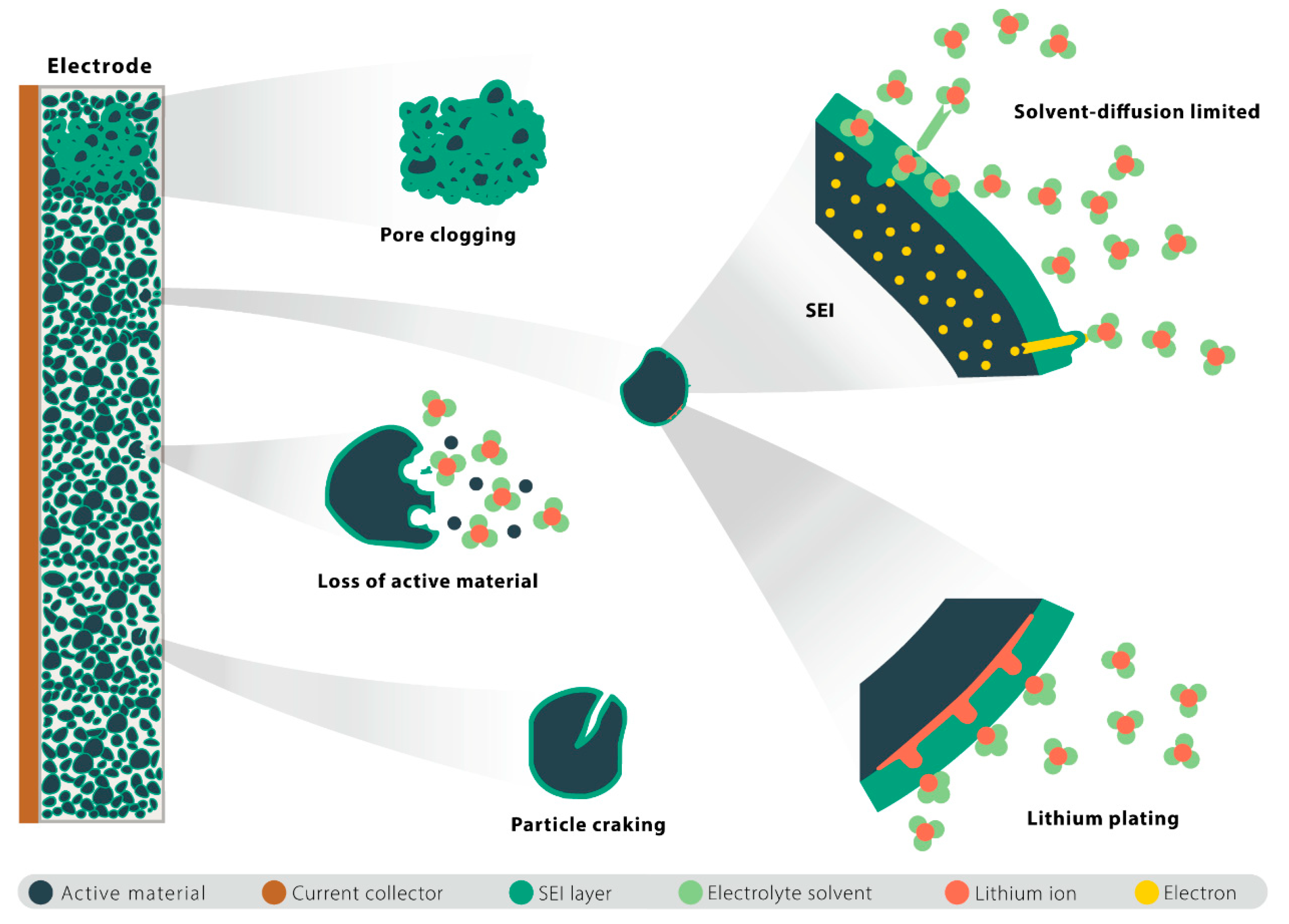
2. Model Development
2.1. SEI Layer Growth Model
- A decrease in the negative electrode porosity:
- An increase in the overpotential of the SEI layer caused by an internal resistance increase in the SEI layer:where is the negative electrode porosity, is the specific surface area. For spherical particles, , is the negative electrode spherical particle radius. is the overpotential of the SEI layer, is the total current density in negative charge conservation and is the conductivity of the SEI layer.
2.2. Lithium Plating Model
2.3. Particle Cracking Model
2.4. Loss of Active Material Model
3. Simulation Experiment
3.1. Battery Specification
3.2. Simulation Tool and Parameterization
3.3. Cycling Protocols
4. Results and Discussion
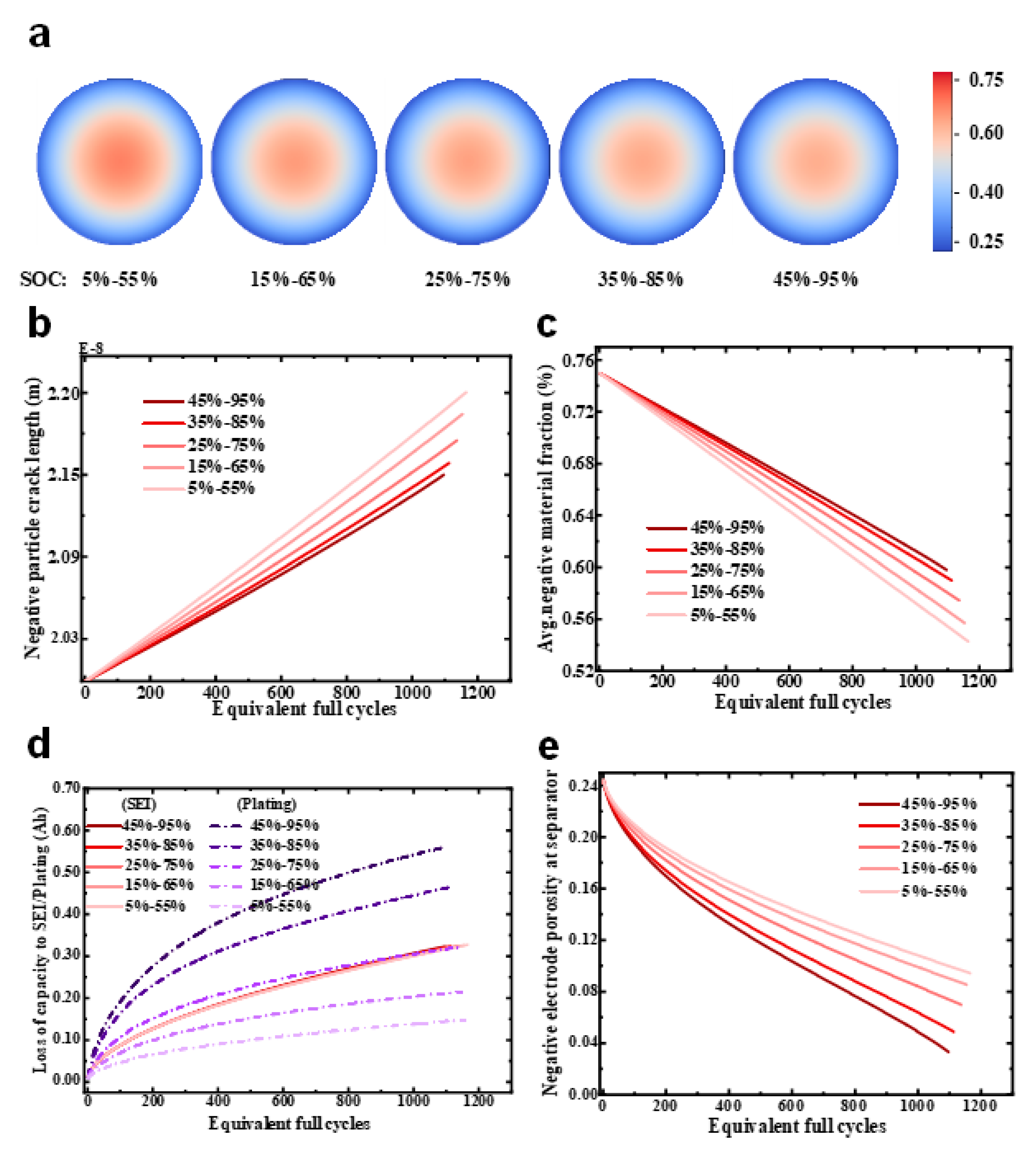
4.1. Simulation Results with Different SOC Swing Ranges
4.1.1. Aging Mechanisms Analysis under Different SOC Ranges
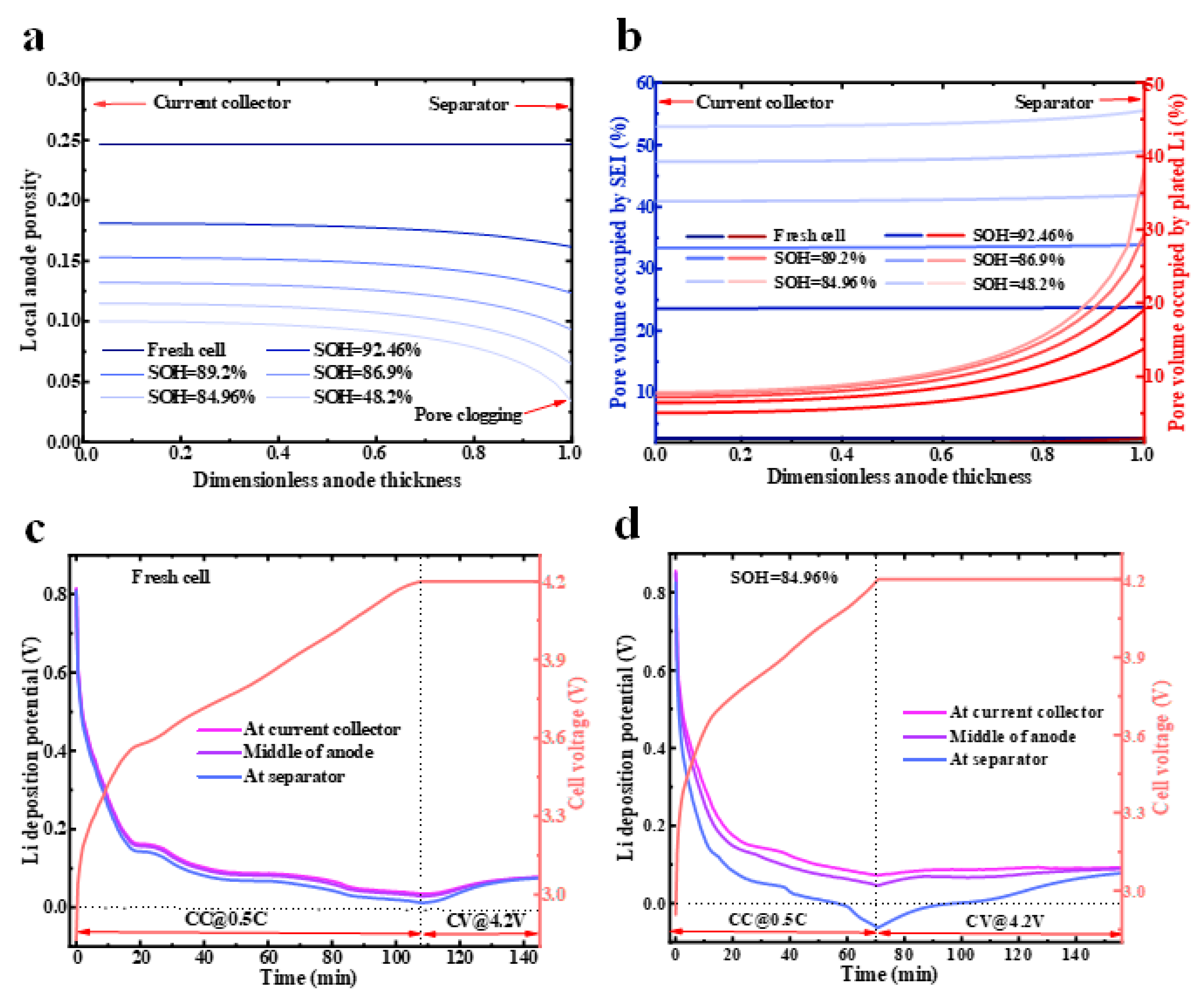
4.1.2. Nonlinear Degradation Due to Pore Clogging
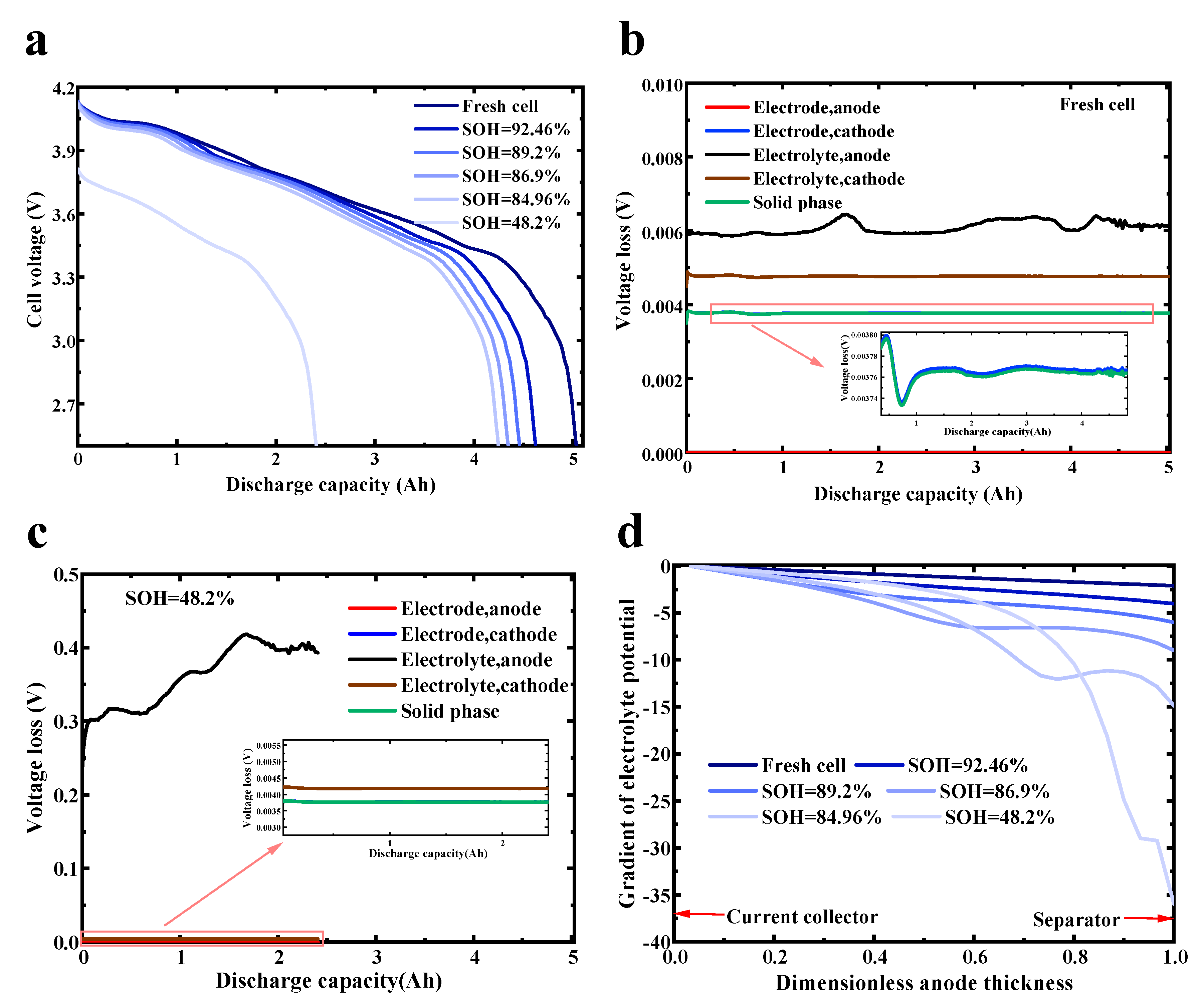
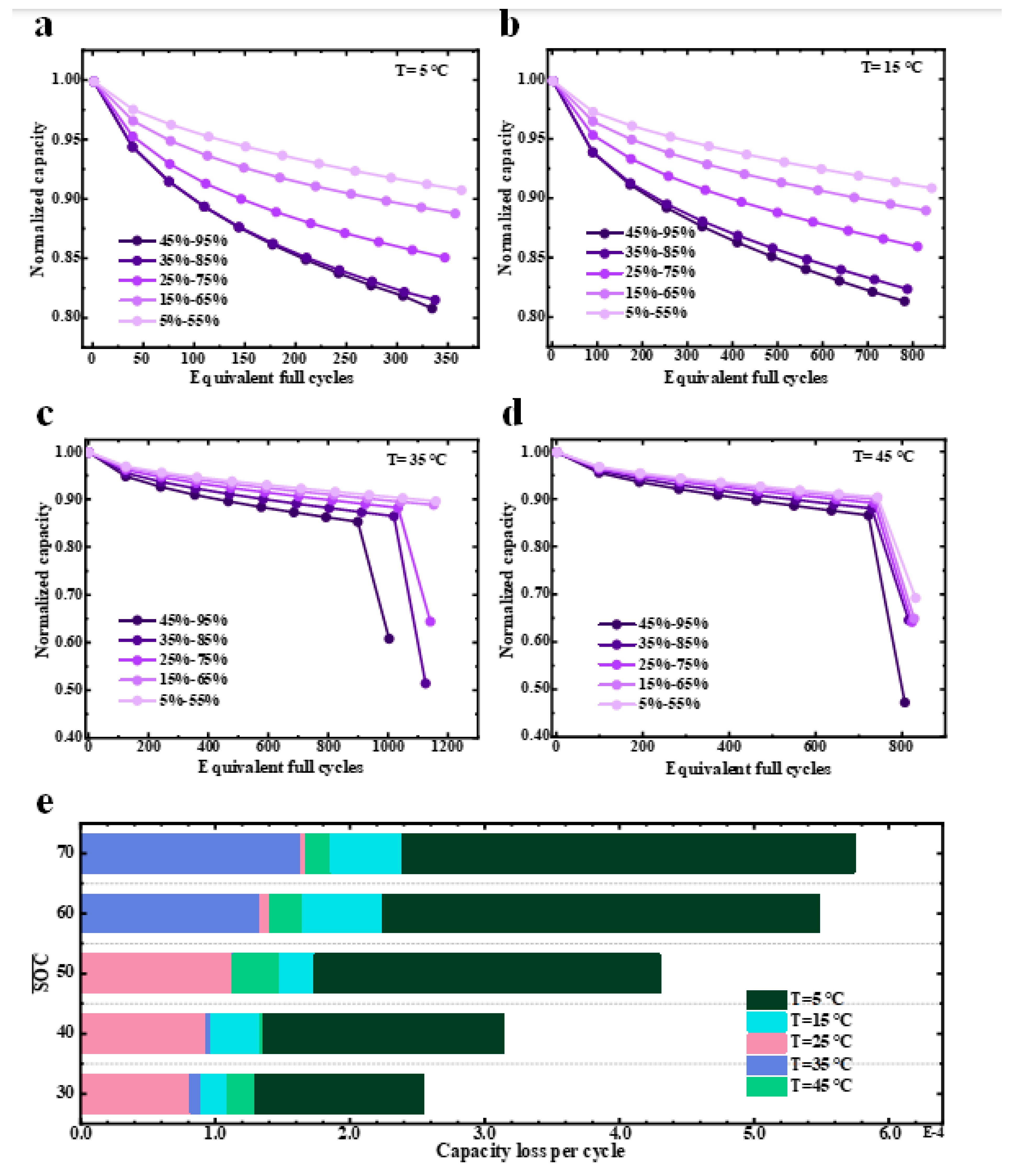
4.2. Simulation Results with Different Temperatures
Aging Mechanisms with Different Temperatures
5. Conclusions
- There is an aging-minimal operation state at a specific SOC swing range, DOD and temperature for the battery. This minimal aging state of the battery was determined by the trade-off between different aging mechanisms under specific operating conditions. For example, the optimal operating temperature range for this cell was around 25–35 °C, and the cell degraded faster under temperatures outside this range, necessitating the health conscious-control of battery operations for an extended lifetime in practice.
- There was a negative feedback mechanism between the SEI growth and Li plating. By consuming cyclable lithium, SEI growth could prevent the negative electrode from becoming highly lithiated during Li plating, thereby restraining Li plating. Furthermore, SEI growth could inhibit the formation of dead lithium. However, particle cracking and LAM clearly had a positive feedback mechanism between each other. That is, LAM could reduce the interface area between the active material and the electrolyte, increasing the interface current density, the internal concentration of the particles and the mechanical stress, thus further promoting active particle cracking.
- Lithium plating is highly sensitive to ambient temperatures and SOC swing ranges, while SEI growth is mainly sensitive to ambient temperatures. Low temperatures caused accelerated battery capacity fading and an increase in resistance. The main cause was that lithium plating occurred relatively easily at low temperatures. Furthermore, low temperatures can also speed up particle cracking, resulting in increased LAM. If the temperature is too high, lithium plating is inhibited, and the SEI layer growth is accelerated. At different temperatures, the battery capacity decline rate can be ranked as T = 5 °C > T = 15 °C > T = 45 °C > T = 25 °C ≈ T = 35 °C.
- The clogging of the anode pores near the separator caused a significant accumulation of the lithium ions there. These circumstances aggravate the increase in the local electrolyte potential gradient in the anode, which, in turn, causes a sudden increase in the anode electrolyte’s resistance and, thus, nonlinear capacity degradation.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bresser, D.; Hosoi, K.; Howell, D.; Li, H.; Zeisel, H.; Amine, K.; Passerini, S. Perspectives of automotive battery R&D in China, Germany, Japan, and the USA. J. Power Sources 2018, 382, 176–178. [Google Scholar]
- Zheng, Y.; Ouyang, M.; Lu, L.; Li, J.; Han, X.; Xu, L.; Ma, H.; Dollmeyer, T.A.; Freyermuth, V. Cell state-of-charge inconsistency estimation for LiFePO4 battery pack in hybrid electric vehicles using mean-difference model. Appl. Energy 2013, 111, 571–580. [Google Scholar] [CrossRef]
- Ouyang, M.; Feng, X.; Han, X.; Lu, L.; Li, Z.; He, X. A dynamic capacity degradation model and its applications considering varying load for a large format Li-ion battery. Appl. Energy 2016, 165, 48–59. [Google Scholar] [CrossRef]
- Matsuda, T.; Ando, K.; Myojin, M.; Matsumoto, M.; Sanada, T.; Takao, N.; Imai, H.; Imamura, D. Investigation of the influence of temperature on the degradation mechanism of commercial nickel manganese cobalt oxide-type lithium-ion cells during long-term cycle tests. J. Energy Storage 2019, 21, 665–671. [Google Scholar] [CrossRef]
- Wang, J.; Purewal, J.; Liu, P.; Hicks-Garner, J.; Soukazian, S.; Sherman, E.; Sorenson, A.; Vu, L.; Verbrugge, M.W. Degradation of lithium ion batteries employing graphite negatives and nickel–cobalt–manganese oxide+ spinel manganese oxide positives: Part 1, aging mechanisms and life estimation. J. Power Sources 2014, 269, 937–948. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.R.; Veit, C.; Möller, K.-C.; Besenhard, J.O.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. Thermal stability of LiPF6/EC+ DEC electrolyte with charged electrodes for lithium ion batteries. Thermochim. Acta 2005, 437, 12–16. [Google Scholar] [CrossRef]
- Petzl, M.; Kasper, M.; Danzer, M.A. Lithium plating in a commercial lithium-ion battery–A low-temperature aging study. J. Power Sources 2015, 275, 799–807. [Google Scholar] [CrossRef]
- Yang, X.G.; Leng, Y.; Zhang, G.; Ge, S.; Wang, C.-Y. Modeling of lithium plating induced aging of lithium-ion batteries: Transition from linear to nonlinear aging. J. Power Sources 2017, 360, 28–40. [Google Scholar] [CrossRef]
- Han, X.; Ouyang, M.; Lu, L.; Li, J.; Zheng, Y.; Li, Z. A comparative study of commercial lithium ion battery cycle life in electrical vehicle: Aging mechanism identification. J. Power Sources 2014, 251, 38–54. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Lin, C.K.; Ren, Y.; Amine, K.; Qin, Y.; Chen, Z. In situ high-energy X-ray diffraction to study overcharge abuse of 18650-size lithium-ion battery. J. Power Sources 2013, 230, 32–37. [Google Scholar] [CrossRef]
- Edge, J.S.; O’Kane, S.; Prosser, R.; Kirkaldy, N.D.; Patel, A.N.; Hales, A.; Ghosh, A.; Ai, W.; Chen, J.; Yang, J.; et al. Lithium ion battery degradation: What you need to know. Phys. Chem. Chem. Phys. 2021, 23, 8200–8221. [Google Scholar] [CrossRef]
- Wang, J.; Liu, P.; Hicks-Garner, J.; Sherman, E.; Soukiazian, S.; Verbrugge, M.; Tataria, H.; Musser, J.; Finamore, P. Cycle-life model for graphite-LiFePO4 cells. J. Power Sources 2011, 196, 3942–3948. [Google Scholar] [CrossRef]
- Watanabe, S.; Kinoshita, M.; Hosokawa, T.; Morigaki, K.; Nakura, K. Capacity fade of LiAlyNi1−x−yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (surface analysis of LiAlyNi1−x−yCoxO2 cathode after cycle tests in restricted depth of discharge ranges). J. Power Sources 2014, 258, 210–217. [Google Scholar] [CrossRef]
- Wikner, E.; Björklund, E.; Fridner, J.; Brandell, D.; Thiringer, T. How the utilised SOC window in commercial Li-ion pouch cells influence battery ageing. J. Power Sources Adv. 2021, 8, 100054. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Zhang, C.; Zhang, W.; Jiang, Y. Aging mechanisms under different state-of-charge ranges and the multi-indicators system of state-of-health for lithium-ion battery with Li (NiMnCo)O2 cathode. J. Power Sources 2018, 400, 641–651. [Google Scholar] [CrossRef]
- Zhu, J.; Knapp, M.; Sørensen, D.R.; Heere, M.; Darma, M.S.; Müller, M.; Mereacre, L.; Dai, H.; Senyshyn, A.; Wei, X.; et al. Investigation of capacity fade for 18650-type lithium-ion batteries cycled in different state of charge (SoC) ranges. J. Power Sources 2021, 489, 229422. [Google Scholar] [CrossRef]
- Wikner, E.; Thiringer, T. Extending battery lifetime by avoiding high SOC. Appl. Sci. 2018, 8, 1825. [Google Scholar] [CrossRef]
- Saxena, S.; Hendricks, C.; Pecht, M. Cycle life testing and modeling of graphite/LiCoO2 cells under different state of charge ranges. J. Power Sources 2016, 327, 394–400. [Google Scholar] [CrossRef]
- Preger, Y.; Barkholtz, H.M.; Fresquez, A.; Campbell, D.L.; Juba, B.W.; Romàn-Kustas, J.; Ferreira, S.R.; Chalamala, B.R. Degradation of commercial lithium-ion cells as a function of chemistry and cycling conditions. J. Electrochem. Soc. 2020, 167, 120532. [Google Scholar] [CrossRef]
- Fuller, T.F.; Doyle, M.; Newman, J. Simulation and optimization of the dual lithium ion insertion cell. J. Electrochem. Soc. 1994, 141, 1. [Google Scholar] [CrossRef]
- Single, F.; Latz, A.; Horstmann, B. Identifying the mechanism of continued growth of the solid–electrolyte interphase. ChemSusChem 2018, 11, 1950–1955. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.; Morcrette, M.; Teyssot, A.; Delacourt, C. Multimodal physics-based aging model for life prediction of Li-ion batteries. J. Electrochem. Soc. 2008, 156, A145. [Google Scholar] [CrossRef]
- O’Kane SE, J.; Campbell, I.D.; Marzook, M.W.; Offer, G.J.; Marinescu, M. Physical origin of the differential voltage minimum associated with lithium plating in Li-ion batteries. J. Electrochem. Soc. 2020, 167, 090540. [Google Scholar] [CrossRef]
- O’Kane SE, J.; Ai, W.; Madabattula, G.; Alonso-Alvarez, D.; Timms, R.; Sulzer, V.; Edge, J.S.; Wu, B.; Offer, J.G.; Marinescu, M. Lithium-ion battery degradation: How to model it. Phys. Chem. Chem. Phys. 2022, 24, 7909–7922. [Google Scholar] [CrossRef]
- Reniers, J.M.; Mulder, G.; Howey, D.A. Review and performance comparison of mechanical-chemical degradation models for lithium-ion batteries. J. Electrochem. Soc. 2019, 166, A3189. [Google Scholar] [CrossRef]
- Purewal, J.; Wang, J.; Graetz, J.; Soukiazian, S.; Tataria, H.; Verbrugge, M.W. Degradation of lithium ion batteries employing graphite negatives and nickel–cobalt–manganese oxide+ spinel manganese oxide positives: Part 2, chemical–mechanical degradation model. J. Power Sources 2014, 272, 1154–1161. [Google Scholar] [CrossRef]
- Sulzer, V.; Marquis, S.G.; Timms, R.; Robinson, M.; Chapman, S.J. Python battery mathematical modelling (PyBaMM). J. Open Res. Softw. 2021, 9, 14. [Google Scholar] [CrossRef]
- Andersson JA, E.; Gillis, J.; Horn, G.; Rawlings, J.B.; Diehl, M. CasADi: A software framework for nonlinear optimization and optimal control. Math. Program. Comput. 2019, 11, 1–36. [Google Scholar] [CrossRef]
- Ai, W.; Kraft, L.; Sturm, J.; Jossen, A.; Wu, B. Electrochemical thermal-mechanical modelling of stress inhomogeneity in lithium-ion pouch cells. J. Electrochem. Soc. 2019, 167, 013512. [Google Scholar] [CrossRef]




| Battery Type | LGM 50 |
|---|---|
| Anode/Cathode material | Graphite and SiOy/LiNi0.8Co0.1MnO0.1 |
| Cut-off voltages (V) | 2.5–4.2 |
| Nominal voltage (V) | 3.63 |
| Nominal capacity (Ah) | 5 |
| Operating temperature (°C) | 0–50 |
| Max discharge current (A) | 1.5 C |
| Dimensions (mm) | 20 × 70 |
| Battery mass (g) | 68.3 |
| Negative Electrode | Positive Electrode | ||||
|---|---|---|---|---|---|
| Symbol | Definition | Default Value | Reference | Default Value | Reference |
| Solvent diffusivity in SEI | 2 × 10−21 | [23] | - | - | |
| Li plating/stripping rate constant | 7.5 × 10−5 | [26] | - | - | |
| Rate constant for dead Li formation | 1 × 10−9 | [26] | - | - | |
| Paris’ law cracking rate | 3.9 × 10−20 | [28] | 3.9 × 10−20 | [28] | |
| Loss of active material proportional term | 2.78 × 10−8 | [31] | 2.78 × 10−8 | [31] | |
| Tests | Procedure |
| Reference performance test (RPT) | (1) Constant current (CC) charge at 0.5 C to 4.2 V |
| (2) Constant voltage (CV) charge at 4.2 V until current < C/100 | |
| (3) Rest for 1 h | |
| (4) CC discharge at 0.3 C to 2.5 V | |
| (5) Rest for 1 h | |
| Cycling test | (1) CC charge at 0.5 C to SOC upper limit |
| (2) Rest for 60 s | |
| (3) CC discharge at 1 C to SOC lower limit | |
| (4) Rest for 60 s | |
| (5) Loop Step (1) to (4) for 100 equivalent full cycles |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Zhang, Y.; Tang, A. Capacity Degradation and Aging Mechanisms Evolution of Lithium-Ion Batteries under Different Operation Conditions. Energies 2023, 16, 4232. https://doi.org/10.3390/en16104232
Luo G, Zhang Y, Tang A. Capacity Degradation and Aging Mechanisms Evolution of Lithium-Ion Batteries under Different Operation Conditions. Energies. 2023; 16(10):4232. https://doi.org/10.3390/en16104232
Chicago/Turabian StyleLuo, Guoqing, Yongzhi Zhang, and Aihua Tang. 2023. "Capacity Degradation and Aging Mechanisms Evolution of Lithium-Ion Batteries under Different Operation Conditions" Energies 16, no. 10: 4232. https://doi.org/10.3390/en16104232
APA StyleLuo, G., Zhang, Y., & Tang, A. (2023). Capacity Degradation and Aging Mechanisms Evolution of Lithium-Ion Batteries under Different Operation Conditions. Energies, 16(10), 4232. https://doi.org/10.3390/en16104232








